-
PDF
- Split View
-
Views
-
Cite
Cite
Jan-Philip Molkentin, Matthias P. Nägele, Michelle Frank, Isabella Sudano, Frank Enseleit, Markus J. Wilhelm, Thomas F. Lüscher, Francesco Maisano, Frank Ruschitzka, Andreas J. Flammer, Prognostic value of mean pulmonary artery pressure in the stable phase after heart transplantation, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 4, October 2017, Pages 775–780, https://doi.org/10.1093/ejcts/ezx180
Close - Share Icon Share
Abstract
In heart transplant recipients, elevated mean pulmonary artery pressure (mPAP) shortly before or after transplantation represents a powerful predictor for an adverse short-term outcome. Less is known on cardiac and pulmonary pressures measured in the stable phase after heart transplantation. The aim of this study was to assess the predictive value of mPAP, mean pulmonary capillary wedge pressure and mean central venous pressure in the stable phase after transplantation.
All patients (n = 260, mean age 47.4 ± 12.7 years, 224 males) who received a cardiac allograft at the University Hospital Zurich between September 1985 and August 2014 and who had undergone at least 1 right heart catheterization after transplantation (median 358 days after transplantation) were included and survival analysis was performed (median follow-up 11.9 years).
The median mPAP, mean pulmonary capillary wedge pressure and mean central venous pressure were 15 mmHg (interquartile range 12–19 mmHg), 8 mmHg (interquartile range 6–11 mmHg) and 3 mmHg (interquartile range 1–5 mmHg), respectively. In mPAP median split survival analysis, patients with an mPAP above the median had a significantly lower long-term survival than patients with or below median mPAP (P = 0.012). mPAP but not mean central venous pressure or mean pulmonary capillary wedge pressure was independently associated with long-term mortality in multivariable Cox-hazard survival analysis (hazard ratio 1.10, confidence interval 1.04–1.16, P = 0.001). Other factors independently associated with mortality were age at transplantation (hazard ratio 1.03 per year, confidence interval 1.01–1.04, P = 0.002) and serum creatinine (μmol/l) (hazard ratio 1.003, confidence interval 1.001–1.010, P = 0.021).
Our results demonstrate that mPAP measured in the stable phase after heart transplantation is an independent prognostic factor for long-term mortality.
INTRODUCTION
Heart transplantation is the most effective treatment for patients suffering from end-stage heart failure [1–3]. Numerous factors influence short-term and long-term survival after heart transplantation. These include acute and chronic rejection reactions, side effects of immunosuppressant drugs but also alterations in central haemodynamics or volume status. Several invasive and non-invasive markers and tests are available for risk stratification and optimization of post-transplant care. Among those, right heart catheterization (RHC) which is often performed along endomyocardial biopsy represents one of the most common assessments in heart transplant recipients. RHC is used to invasively determine pressures of the right side of the heart and the pulmonary circulation. Important parameters are mean central venous pressure (mCVP), mean pulmonary artery pressure (mPAP) and mean pulmonary wedge pressure (mPCWP) which are indicators not only of left- and right-sided allograft function and the pulmonary vasculature but also the systemic haemodynamic state [4–7].
In prior studies, particular focus was placed on the evaluation of mPAP shortly before and after heart transplantation [8–12]. These studies indicated that pulmonary hypertension in the immediate pre- or post-transplant period, defined as mPAP equal to or greater than 25 mmHg is a marker for a worse prognosis in the short term. However, cardiac and pulmonary haemodynamics are known to change significantly after heart transplantation and less is known on the predictive impact of mPAP and other RHC pressures obtained in the stable phase after heart transplantation. In particular, no studies have been performed so far evaluating the association of RHC pressures with long-term mortality after heart transplantation.
Hence, the aim of this study was to assess mPAP and other RHC pressures in the stable phase after heart transplantation and their respective association with mortality using a contemporary patient cohort with available long-term follow-up data.
METHODS
Patient population
The study followed a retrospective observational design. Patients who received a cardiac allograft at the University Hospital Zurich between September 1985 and August 2014 and who had undergone at least 1 RHC after transplantation were eligible and included in the study. Date of transplantation served as time point for the survival analysis. The decision when to perform RHC was left to the treating physician. Patients were treated with standard combination immunosuppressant therapy according to medical guidelines [13]. Basic clinical parameters were obtained from medical records and included general demographic parameters, medical history, vital parameters, concomitant medication and laboratory parameters. Patients receiving ventricular assist device therapy prior to transplantation were not included in this analysis.
Right heart catheterization
The obtained first RHC pressure values included pulmonary artery wedge pressure, pulmonary artery pressure, right ventricular pressure and central venous pressure. RHC was performed using a balloon-tipped pulmonary artery catheter with preferred access through the right internal jugular vein according to standard medical practice. Pulmonary capillary wedge pressure was obtained in expiration. Survival of patients was followed up until August 2014. No patients were lost to follow up, as they had to be seen by a specialist in heart transplantation. The study was approved by the local ethics committee (Ethics Committee of the Canton of Zurich, Project No. KEK-ZH-2010–0211). Due to the use of retrospective anonymized data, the ethics committee did not deem obtaining informed consent necessary.
Data analysis and statistics
Differences between groups, as defined in the results section, were compared using the unpaired t-test. For the comparison of categorical data, we employed the Fisher’s exact test. For survival plots, a Kaplan–Meier survival curve starting from the date of transplantation was plotted. Differences in survival were compared for significance using a multivariable Cox-hazard survival analysis. The proportional hazards assumption were evaluated using a smoothed scaled Schoenfeld residual plot as well as the Grambsch–Therneau test to assess for the non-zero slope in the model of the Schoenfeld residuals. Covariates were chosen based on univariable pre-screening with the information provided from the patient history and laboratory results as well as clinical judgment by the treating physicians. These included namely lactate dehydrogenase (LDH) and pro-brain natriuretic peptide (BNP). Cut-off values for mPAP for mortality were analysed using a receiver operating curve. Missing data were encountered on several occasions for several variables. Nonetheless these variables were included despite missing data. Using the below mentioned statistical packages and employing multiple imputations, the uncertainty derived from missing data was propagated into the effect calculations.
All statistical tests were 2-sided and P-values of <0.05 were considered to be statistically significant. Data were analysed using IBM SPSS Statistics for Macintosh, Version 22.0. and JMP®, Version 10. SAS Institute Inc., Cary, NC, USA, 1989–2007.
RESULTS
Patient characteristics
Of all heart transplant patients at the University Hospital Zurich, 260 patients (mean age 47.4 ± 12.7 years) were eligible and included in the study. Baseline characteristics of the study population, including laboratory parameters and concomitant immunosuppressant therapy are shown in Table 1. The majority of patients were male (n = 224, 86%). The 2 main reasons for heart transplantation were advanced dilated or ischaemic cardiomyopathy.
Baseline characteristics at first RHC and cause of death at follow-up
| Characteristics . | Study population (n = 260) . |
|---|---|
| Mean age (years) | 47.4 ± 12.7 |
| Female patients | 36 (13.8%) |
| Cardiomyopathy before TPL | |
| Dilated cardiomyopathy | 121 (46.5%) |
| Ischaemic heart disease | 97 (37.3%) |
| Valvular heart disease | 12 (4.6%) |
| Congenital heart disease | 11 (4.2%) |
| Other | 19 (7.3%) |
| Laboratory parameters | |
| Haemoglobin (g/dl) (n = 218) | 11.6 ± 9.5 |
| Thrombocytes (x103/μl) (n = 217) | 252 ± 94 |
| Leukocytes (x103/μl) (n = 217) | 7.2 ± 3.8 |
| Potassium (mmol/l) (n = 218) | 3.8 ± 0.9 |
| Creatinine (μmol/l) (n = 218) | 121.8 ± 64.2 |
| CK (μg/l) (n = 124) | 55.5 ± 83.6 |
| LDH (U/l) (n = 211) | 377.8 ± 274.6 |
| Total cholesterol (mmol/l) (n = 157) | 5.8 ± 1.6 |
| Immunosuppressant therapy | |
| Prednisone | 207 (79.6%) |
| Tacrolimus | 106 (40.8%) |
| Azathioprine | 160 (61.5%) |
| Cyclosporine | 192 (73.8%) |
| Mycophenolate | 88 (33.8%) |
| Right heart catheterization | |
| mCVP (mmHg) (n = 254) | 3.9 ± 5.9 |
| mPAP (mmHg) (n = 260) | 16.1 ± 5.6 |
| mPCWP (mmHg) (n = 259) | 8.9 ± 5.6 |
| Mean aortic pressure (mmHg) (n = 252) | 99.7 ± 14.9 |
| Heart rate (beats per minute) (n = 259) | 90.7 ± 13.5 |
| Systolic BP (mmHg) (n = 253) | 133.3 ± 17.1 |
| Diastolic BP (mmHg) (n = 253) | 85.4 ± 11.9 |
| Cause of death at follow-up | |
| Cancer | 36.4% (n = 32) |
| Cardiac allograft vasculopathy | 10.2% (n = 9) |
| Heart failure | 5.7% (n = 5) |
| Infection | 7.9% (n = 7) |
| Acute rejection | 1.1% (n = 1) |
| Other | 38.6% (n = 34) |
| Characteristics . | Study population (n = 260) . |
|---|---|
| Mean age (years) | 47.4 ± 12.7 |
| Female patients | 36 (13.8%) |
| Cardiomyopathy before TPL | |
| Dilated cardiomyopathy | 121 (46.5%) |
| Ischaemic heart disease | 97 (37.3%) |
| Valvular heart disease | 12 (4.6%) |
| Congenital heart disease | 11 (4.2%) |
| Other | 19 (7.3%) |
| Laboratory parameters | |
| Haemoglobin (g/dl) (n = 218) | 11.6 ± 9.5 |
| Thrombocytes (x103/μl) (n = 217) | 252 ± 94 |
| Leukocytes (x103/μl) (n = 217) | 7.2 ± 3.8 |
| Potassium (mmol/l) (n = 218) | 3.8 ± 0.9 |
| Creatinine (μmol/l) (n = 218) | 121.8 ± 64.2 |
| CK (μg/l) (n = 124) | 55.5 ± 83.6 |
| LDH (U/l) (n = 211) | 377.8 ± 274.6 |
| Total cholesterol (mmol/l) (n = 157) | 5.8 ± 1.6 |
| Immunosuppressant therapy | |
| Prednisone | 207 (79.6%) |
| Tacrolimus | 106 (40.8%) |
| Azathioprine | 160 (61.5%) |
| Cyclosporine | 192 (73.8%) |
| Mycophenolate | 88 (33.8%) |
| Right heart catheterization | |
| mCVP (mmHg) (n = 254) | 3.9 ± 5.9 |
| mPAP (mmHg) (n = 260) | 16.1 ± 5.6 |
| mPCWP (mmHg) (n = 259) | 8.9 ± 5.6 |
| Mean aortic pressure (mmHg) (n = 252) | 99.7 ± 14.9 |
| Heart rate (beats per minute) (n = 259) | 90.7 ± 13.5 |
| Systolic BP (mmHg) (n = 253) | 133.3 ± 17.1 |
| Diastolic BP (mmHg) (n = 253) | 85.4 ± 11.9 |
| Cause of death at follow-up | |
| Cancer | 36.4% (n = 32) |
| Cardiac allograft vasculopathy | 10.2% (n = 9) |
| Heart failure | 5.7% (n = 5) |
| Infection | 7.9% (n = 7) |
| Acute rejection | 1.1% (n = 1) |
| Other | 38.6% (n = 34) |
Values are presented as mean ± standard deviation or n (%).
BP: blood pressure; CK: creatine kinase; LDH: lactate dehydrogenase; RHC: right heart catheterization; mCVP: mean central venous pressure; mPAP: mean pulmonary artery pressure; mPCWP: mean pulmonary capillary wedge pressure; RHC: right heart catheterization; TPL: transplantation.
Baseline characteristics at first RHC and cause of death at follow-up
| Characteristics . | Study population (n = 260) . |
|---|---|
| Mean age (years) | 47.4 ± 12.7 |
| Female patients | 36 (13.8%) |
| Cardiomyopathy before TPL | |
| Dilated cardiomyopathy | 121 (46.5%) |
| Ischaemic heart disease | 97 (37.3%) |
| Valvular heart disease | 12 (4.6%) |
| Congenital heart disease | 11 (4.2%) |
| Other | 19 (7.3%) |
| Laboratory parameters | |
| Haemoglobin (g/dl) (n = 218) | 11.6 ± 9.5 |
| Thrombocytes (x103/μl) (n = 217) | 252 ± 94 |
| Leukocytes (x103/μl) (n = 217) | 7.2 ± 3.8 |
| Potassium (mmol/l) (n = 218) | 3.8 ± 0.9 |
| Creatinine (μmol/l) (n = 218) | 121.8 ± 64.2 |
| CK (μg/l) (n = 124) | 55.5 ± 83.6 |
| LDH (U/l) (n = 211) | 377.8 ± 274.6 |
| Total cholesterol (mmol/l) (n = 157) | 5.8 ± 1.6 |
| Immunosuppressant therapy | |
| Prednisone | 207 (79.6%) |
| Tacrolimus | 106 (40.8%) |
| Azathioprine | 160 (61.5%) |
| Cyclosporine | 192 (73.8%) |
| Mycophenolate | 88 (33.8%) |
| Right heart catheterization | |
| mCVP (mmHg) (n = 254) | 3.9 ± 5.9 |
| mPAP (mmHg) (n = 260) | 16.1 ± 5.6 |
| mPCWP (mmHg) (n = 259) | 8.9 ± 5.6 |
| Mean aortic pressure (mmHg) (n = 252) | 99.7 ± 14.9 |
| Heart rate (beats per minute) (n = 259) | 90.7 ± 13.5 |
| Systolic BP (mmHg) (n = 253) | 133.3 ± 17.1 |
| Diastolic BP (mmHg) (n = 253) | 85.4 ± 11.9 |
| Cause of death at follow-up | |
| Cancer | 36.4% (n = 32) |
| Cardiac allograft vasculopathy | 10.2% (n = 9) |
| Heart failure | 5.7% (n = 5) |
| Infection | 7.9% (n = 7) |
| Acute rejection | 1.1% (n = 1) |
| Other | 38.6% (n = 34) |
| Characteristics . | Study population (n = 260) . |
|---|---|
| Mean age (years) | 47.4 ± 12.7 |
| Female patients | 36 (13.8%) |
| Cardiomyopathy before TPL | |
| Dilated cardiomyopathy | 121 (46.5%) |
| Ischaemic heart disease | 97 (37.3%) |
| Valvular heart disease | 12 (4.6%) |
| Congenital heart disease | 11 (4.2%) |
| Other | 19 (7.3%) |
| Laboratory parameters | |
| Haemoglobin (g/dl) (n = 218) | 11.6 ± 9.5 |
| Thrombocytes (x103/μl) (n = 217) | 252 ± 94 |
| Leukocytes (x103/μl) (n = 217) | 7.2 ± 3.8 |
| Potassium (mmol/l) (n = 218) | 3.8 ± 0.9 |
| Creatinine (μmol/l) (n = 218) | 121.8 ± 64.2 |
| CK (μg/l) (n = 124) | 55.5 ± 83.6 |
| LDH (U/l) (n = 211) | 377.8 ± 274.6 |
| Total cholesterol (mmol/l) (n = 157) | 5.8 ± 1.6 |
| Immunosuppressant therapy | |
| Prednisone | 207 (79.6%) |
| Tacrolimus | 106 (40.8%) |
| Azathioprine | 160 (61.5%) |
| Cyclosporine | 192 (73.8%) |
| Mycophenolate | 88 (33.8%) |
| Right heart catheterization | |
| mCVP (mmHg) (n = 254) | 3.9 ± 5.9 |
| mPAP (mmHg) (n = 260) | 16.1 ± 5.6 |
| mPCWP (mmHg) (n = 259) | 8.9 ± 5.6 |
| Mean aortic pressure (mmHg) (n = 252) | 99.7 ± 14.9 |
| Heart rate (beats per minute) (n = 259) | 90.7 ± 13.5 |
| Systolic BP (mmHg) (n = 253) | 133.3 ± 17.1 |
| Diastolic BP (mmHg) (n = 253) | 85.4 ± 11.9 |
| Cause of death at follow-up | |
| Cancer | 36.4% (n = 32) |
| Cardiac allograft vasculopathy | 10.2% (n = 9) |
| Heart failure | 5.7% (n = 5) |
| Infection | 7.9% (n = 7) |
| Acute rejection | 1.1% (n = 1) |
| Other | 38.6% (n = 34) |
Values are presented as mean ± standard deviation or n (%).
BP: blood pressure; CK: creatine kinase; LDH: lactate dehydrogenase; RHC: right heart catheterization; mCVP: mean central venous pressure; mPAP: mean pulmonary artery pressure; mPCWP: mean pulmonary capillary wedge pressure; RHC: right heart catheterization; TPL: transplantation.
Patient follow-up
The first RHC used for the study analyses were conducted at a median of 358 days [interquartile range (IQR) 184.5–408.1 days] after heart transplantation (Table 1). The median mPAP, mPCWP and mCVP were 15 mmHg (IQR 12–19 mmHg), 8 mmHg (IQR 6–11 mmHg) and 3 mmHg (IQR 1–5 mmHg), respectively. Patients were followed for up to a median of 11.9 years (range 0.3–27.9 years). Over the whole follow-up, 88 patients (33.84%) died. The most common causes of death were cancer and graft atherosclerosis amongst other various causes (Table 1).
Changes in pressure values over time
Pre-transplantation pressure values were not available for this study. mPAP, mPCWP and mCVP decreased significantly after the third month until the end of the third year after transplantation (mPAP from 18.3 ± 5.2 mmHg to 15.4 ± 6.1 mmHg; mCVP from 5.8 ± 3.3 mmHg to 4.7 ± 2.8 mmHg and mPCWP from 11.1 ± 5.3 mmHg to 7.6 ± 4.5 mmHg). Afterwards mPAP as well as mCVP and mPCWP consecutively increased until the fifth year (mPAP to 17.4 ± 4.8 mmHg; mCVP to 5.2 mmHg ± 4.1 mmHg and mPCWP to 10.4 ± 5.1 mmHg) and remained unchanged until the end of the follow-up period.
Association of pulmonary artery pressure with mortality
For the survival analysis, patients were split into 2 groups according to the median mPAP. We chose a dichotomization of pulmonary pressures as previous studies had suggested especially elevated pulmonary pressures were associated with poor outcome. Since our data stem from a steady period following transplantation, we opted for an evaluation of elevated normal pressure values on survival. Patients with an mPAP higher than the median (mPAP > 15 mmHg) were assigned into the higher mPAP group and those with a mPAP at or below the median (mPAP ≤ 15 mmHg) were assigned to the lower mPAP group. A study of patients with pulmonary hypertension versus those without could not be performed, because the number of patients with pulmonary hypertension was only 17 out of 260. Hence we decided to analyse the study population according to the median mPAP in our group, thus 15 mmHg. Differences in clinical parameters between the 2 groups are shown in Table 2. The 2 groups matched well except for age at transplantation, total follow-up time, serum LDH and creatinine. Significant differences were also noted for all right heart pressure values.
Characteristics and outcomes of patients split by median mPAP
| Characteristics . | mPAP ≤15 mmHg . | mPAP >15 mmHg . | P-value . |
|---|---|---|---|
| Number of patients (n) | 136 | 124 | 0.87 |
| Female patients (%) | 22 (16.2%) | 14 (11.3%) | 0.43 |
| Age at TPL (years) | 45.2 ± 1 3.2 | 50.9 ± 11.6 | 0.008 |
| Total mean follow-up time (years after TPL) | 14.2 ± 8.2 | 10.4 ± 6.5 | 0.012 |
| Number of deaths in follow-up period (n) | 41 (30.4%) | 47 (37.6%) | 0.014 |
| Number of patients with rejection at the time of the first RHC (ISHLT >2a) | 23 (17%) | 29 (23%) | 0.98 |
| Median number of rejection episodes before first RHC | 0 | 0 | 0.37 |
| IQR 0–2 | IQR 0–2 | ||
| Cardiomyopathy before TPL | |||
| Dilated cardiomyopathy | 60 (44%) | 61 (49%) | 0.72 |
| Ischaemic cardiomyopathy | 51 (38%) | 46 (37%) | |
| Valvular heart disease | 6 (4%) | 6 (5%) | |
| Congenital heart disease | 9 (7%) | 2 (1%) | |
| Other | 9 (7%) | 10 (8%) | |
| Right heart catheterization | |||
| Days since TPL at first RHC (IQR) | 358 (184-406) | 346 (142-404) | 0.06 |
| mPAP (mmHg) | 12.1 ± 2.3 | 20.5 ± 4.7 | 0.005 |
| mPCWP (mmHg) | 6.2 ± 2.8 | 11.8 ± 5.4 | 0.006 |
| Transpulmonary gradient (mmHg) | 5.8 ± 2.7 | 9.0 ± 3.9 | 0.011 |
| mCVP (mmHg) | 2.5 ± 1.3 | 5.3 ± 3.5 | 0.008 |
| Mean systolic blood pressure (mmHg) | 131.6 ± 15.8 | 135.2 ± 18.4 | 0.15 |
| Mean diastolic blood pressure (mmHg) | 84.9 ± 11.8 | 85.9 ± 12.0 | 0.46 |
| Heart rate (beats per minute) | 90.5 ± 13.8 | 90.8 ± 13.2 | 0.87 |
| Laboratory parameters | |||
| Haemoglobin (g/dl) | 12.7 ± 13.2 | 10.6 ± 1.9 | 0.31 |
| Thrombocytes (x103/μl) | 260.1 ± 104.6 | 244.5 ± 77.2 | 0.88 |
| Leukocytes (x103/μl) | 7.1 ± 3.2 | 7.3 ± 4.4 | 0.44 |
| Potassium (mmol/l) | 3.7 ± 0.7 | 3.9 ± 0.9 | 0.11 |
| Creatinine (μmol/l) | 113.4 ± 45.9 | 130.5 ± 78.1 | 0.029 |
| CK (μg/l) | 62.2 ± 104.5 | 46.2 ± 38.7 | 0.11 |
| LDH (U/l) | 306.6 ± 259.9 | 450.9 ± 271.2 | 0.012 |
| Immunosuppressant therapy | |||
| Prednisone | 106 (78.5%) | 101 (80.8%) | 0.28 |
| Tacrolimus | 47 (34.8%) | 59 (47.2%) | 0.99 |
| Azathioprine | 84 (62.2%) | 76 (60.8%) | 0.99 |
| Cyclosporine | 98 (72.6%) | 94 (75.2%) | 0.99 |
| Mycophenolate | 39 (28.9%) | 49 (39.29%) | 0.99 |
| Characteristics . | mPAP ≤15 mmHg . | mPAP >15 mmHg . | P-value . |
|---|---|---|---|
| Number of patients (n) | 136 | 124 | 0.87 |
| Female patients (%) | 22 (16.2%) | 14 (11.3%) | 0.43 |
| Age at TPL (years) | 45.2 ± 1 3.2 | 50.9 ± 11.6 | 0.008 |
| Total mean follow-up time (years after TPL) | 14.2 ± 8.2 | 10.4 ± 6.5 | 0.012 |
| Number of deaths in follow-up period (n) | 41 (30.4%) | 47 (37.6%) | 0.014 |
| Number of patients with rejection at the time of the first RHC (ISHLT >2a) | 23 (17%) | 29 (23%) | 0.98 |
| Median number of rejection episodes before first RHC | 0 | 0 | 0.37 |
| IQR 0–2 | IQR 0–2 | ||
| Cardiomyopathy before TPL | |||
| Dilated cardiomyopathy | 60 (44%) | 61 (49%) | 0.72 |
| Ischaemic cardiomyopathy | 51 (38%) | 46 (37%) | |
| Valvular heart disease | 6 (4%) | 6 (5%) | |
| Congenital heart disease | 9 (7%) | 2 (1%) | |
| Other | 9 (7%) | 10 (8%) | |
| Right heart catheterization | |||
| Days since TPL at first RHC (IQR) | 358 (184-406) | 346 (142-404) | 0.06 |
| mPAP (mmHg) | 12.1 ± 2.3 | 20.5 ± 4.7 | 0.005 |
| mPCWP (mmHg) | 6.2 ± 2.8 | 11.8 ± 5.4 | 0.006 |
| Transpulmonary gradient (mmHg) | 5.8 ± 2.7 | 9.0 ± 3.9 | 0.011 |
| mCVP (mmHg) | 2.5 ± 1.3 | 5.3 ± 3.5 | 0.008 |
| Mean systolic blood pressure (mmHg) | 131.6 ± 15.8 | 135.2 ± 18.4 | 0.15 |
| Mean diastolic blood pressure (mmHg) | 84.9 ± 11.8 | 85.9 ± 12.0 | 0.46 |
| Heart rate (beats per minute) | 90.5 ± 13.8 | 90.8 ± 13.2 | 0.87 |
| Laboratory parameters | |||
| Haemoglobin (g/dl) | 12.7 ± 13.2 | 10.6 ± 1.9 | 0.31 |
| Thrombocytes (x103/μl) | 260.1 ± 104.6 | 244.5 ± 77.2 | 0.88 |
| Leukocytes (x103/μl) | 7.1 ± 3.2 | 7.3 ± 4.4 | 0.44 |
| Potassium (mmol/l) | 3.7 ± 0.7 | 3.9 ± 0.9 | 0.11 |
| Creatinine (μmol/l) | 113.4 ± 45.9 | 130.5 ± 78.1 | 0.029 |
| CK (μg/l) | 62.2 ± 104.5 | 46.2 ± 38.7 | 0.11 |
| LDH (U/l) | 306.6 ± 259.9 | 450.9 ± 271.2 | 0.012 |
| Immunosuppressant therapy | |||
| Prednisone | 106 (78.5%) | 101 (80.8%) | 0.28 |
| Tacrolimus | 47 (34.8%) | 59 (47.2%) | 0.99 |
| Azathioprine | 84 (62.2%) | 76 (60.8%) | 0.99 |
| Cyclosporine | 98 (72.6%) | 94 (75.2%) | 0.99 |
| Mycophenolate | 39 (28.9%) | 49 (39.29%) | 0.99 |
Values are presented as mean ± standard deviation or n (%).
TPL: transplantation; RHC: right heart catheter; IQR: interquartile range; PAP: pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; CVP: central venous pressure; ISHLT: International Society for Heart and Lung Transplantation; CK: creatine kinase; LDH: lactate dehydrogenase.
Characteristics and outcomes of patients split by median mPAP
| Characteristics . | mPAP ≤15 mmHg . | mPAP >15 mmHg . | P-value . |
|---|---|---|---|
| Number of patients (n) | 136 | 124 | 0.87 |
| Female patients (%) | 22 (16.2%) | 14 (11.3%) | 0.43 |
| Age at TPL (years) | 45.2 ± 1 3.2 | 50.9 ± 11.6 | 0.008 |
| Total mean follow-up time (years after TPL) | 14.2 ± 8.2 | 10.4 ± 6.5 | 0.012 |
| Number of deaths in follow-up period (n) | 41 (30.4%) | 47 (37.6%) | 0.014 |
| Number of patients with rejection at the time of the first RHC (ISHLT >2a) | 23 (17%) | 29 (23%) | 0.98 |
| Median number of rejection episodes before first RHC | 0 | 0 | 0.37 |
| IQR 0–2 | IQR 0–2 | ||
| Cardiomyopathy before TPL | |||
| Dilated cardiomyopathy | 60 (44%) | 61 (49%) | 0.72 |
| Ischaemic cardiomyopathy | 51 (38%) | 46 (37%) | |
| Valvular heart disease | 6 (4%) | 6 (5%) | |
| Congenital heart disease | 9 (7%) | 2 (1%) | |
| Other | 9 (7%) | 10 (8%) | |
| Right heart catheterization | |||
| Days since TPL at first RHC (IQR) | 358 (184-406) | 346 (142-404) | 0.06 |
| mPAP (mmHg) | 12.1 ± 2.3 | 20.5 ± 4.7 | 0.005 |
| mPCWP (mmHg) | 6.2 ± 2.8 | 11.8 ± 5.4 | 0.006 |
| Transpulmonary gradient (mmHg) | 5.8 ± 2.7 | 9.0 ± 3.9 | 0.011 |
| mCVP (mmHg) | 2.5 ± 1.3 | 5.3 ± 3.5 | 0.008 |
| Mean systolic blood pressure (mmHg) | 131.6 ± 15.8 | 135.2 ± 18.4 | 0.15 |
| Mean diastolic blood pressure (mmHg) | 84.9 ± 11.8 | 85.9 ± 12.0 | 0.46 |
| Heart rate (beats per minute) | 90.5 ± 13.8 | 90.8 ± 13.2 | 0.87 |
| Laboratory parameters | |||
| Haemoglobin (g/dl) | 12.7 ± 13.2 | 10.6 ± 1.9 | 0.31 |
| Thrombocytes (x103/μl) | 260.1 ± 104.6 | 244.5 ± 77.2 | 0.88 |
| Leukocytes (x103/μl) | 7.1 ± 3.2 | 7.3 ± 4.4 | 0.44 |
| Potassium (mmol/l) | 3.7 ± 0.7 | 3.9 ± 0.9 | 0.11 |
| Creatinine (μmol/l) | 113.4 ± 45.9 | 130.5 ± 78.1 | 0.029 |
| CK (μg/l) | 62.2 ± 104.5 | 46.2 ± 38.7 | 0.11 |
| LDH (U/l) | 306.6 ± 259.9 | 450.9 ± 271.2 | 0.012 |
| Immunosuppressant therapy | |||
| Prednisone | 106 (78.5%) | 101 (80.8%) | 0.28 |
| Tacrolimus | 47 (34.8%) | 59 (47.2%) | 0.99 |
| Azathioprine | 84 (62.2%) | 76 (60.8%) | 0.99 |
| Cyclosporine | 98 (72.6%) | 94 (75.2%) | 0.99 |
| Mycophenolate | 39 (28.9%) | 49 (39.29%) | 0.99 |
| Characteristics . | mPAP ≤15 mmHg . | mPAP >15 mmHg . | P-value . |
|---|---|---|---|
| Number of patients (n) | 136 | 124 | 0.87 |
| Female patients (%) | 22 (16.2%) | 14 (11.3%) | 0.43 |
| Age at TPL (years) | 45.2 ± 1 3.2 | 50.9 ± 11.6 | 0.008 |
| Total mean follow-up time (years after TPL) | 14.2 ± 8.2 | 10.4 ± 6.5 | 0.012 |
| Number of deaths in follow-up period (n) | 41 (30.4%) | 47 (37.6%) | 0.014 |
| Number of patients with rejection at the time of the first RHC (ISHLT >2a) | 23 (17%) | 29 (23%) | 0.98 |
| Median number of rejection episodes before first RHC | 0 | 0 | 0.37 |
| IQR 0–2 | IQR 0–2 | ||
| Cardiomyopathy before TPL | |||
| Dilated cardiomyopathy | 60 (44%) | 61 (49%) | 0.72 |
| Ischaemic cardiomyopathy | 51 (38%) | 46 (37%) | |
| Valvular heart disease | 6 (4%) | 6 (5%) | |
| Congenital heart disease | 9 (7%) | 2 (1%) | |
| Other | 9 (7%) | 10 (8%) | |
| Right heart catheterization | |||
| Days since TPL at first RHC (IQR) | 358 (184-406) | 346 (142-404) | 0.06 |
| mPAP (mmHg) | 12.1 ± 2.3 | 20.5 ± 4.7 | 0.005 |
| mPCWP (mmHg) | 6.2 ± 2.8 | 11.8 ± 5.4 | 0.006 |
| Transpulmonary gradient (mmHg) | 5.8 ± 2.7 | 9.0 ± 3.9 | 0.011 |
| mCVP (mmHg) | 2.5 ± 1.3 | 5.3 ± 3.5 | 0.008 |
| Mean systolic blood pressure (mmHg) | 131.6 ± 15.8 | 135.2 ± 18.4 | 0.15 |
| Mean diastolic blood pressure (mmHg) | 84.9 ± 11.8 | 85.9 ± 12.0 | 0.46 |
| Heart rate (beats per minute) | 90.5 ± 13.8 | 90.8 ± 13.2 | 0.87 |
| Laboratory parameters | |||
| Haemoglobin (g/dl) | 12.7 ± 13.2 | 10.6 ± 1.9 | 0.31 |
| Thrombocytes (x103/μl) | 260.1 ± 104.6 | 244.5 ± 77.2 | 0.88 |
| Leukocytes (x103/μl) | 7.1 ± 3.2 | 7.3 ± 4.4 | 0.44 |
| Potassium (mmol/l) | 3.7 ± 0.7 | 3.9 ± 0.9 | 0.11 |
| Creatinine (μmol/l) | 113.4 ± 45.9 | 130.5 ± 78.1 | 0.029 |
| CK (μg/l) | 62.2 ± 104.5 | 46.2 ± 38.7 | 0.11 |
| LDH (U/l) | 306.6 ± 259.9 | 450.9 ± 271.2 | 0.012 |
| Immunosuppressant therapy | |||
| Prednisone | 106 (78.5%) | 101 (80.8%) | 0.28 |
| Tacrolimus | 47 (34.8%) | 59 (47.2%) | 0.99 |
| Azathioprine | 84 (62.2%) | 76 (60.8%) | 0.99 |
| Cyclosporine | 98 (72.6%) | 94 (75.2%) | 0.99 |
| Mycophenolate | 39 (28.9%) | 49 (39.29%) | 0.99 |
Values are presented as mean ± standard deviation or n (%).
TPL: transplantation; RHC: right heart catheter; IQR: interquartile range; PAP: pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; CVP: central venous pressure; ISHLT: International Society for Heart and Lung Transplantation; CK: creatine kinase; LDH: lactate dehydrogenase.
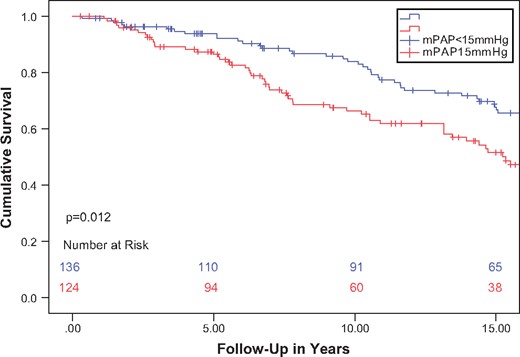
A Kaplan–Meier survival curve of higher versus lower than median mPAP was plotted (Fig. 1) and survival between the 2 groups was compared. Patients in the higher mPAP group showed a significantly higher all-cause mortality than patients in the lower mPAP group (all-cause mortality at 25 years 37.6% vs 30.4% vs respectively, P = 0.008). Because the 2 groups did not match for all factors (namely age at transplantation, serum creatinine and LDH), a multivariable approach was chosen to independently assess the role of RHC pressures on survival. When corrected for age at transplantation, serum creatinine and serum LDH, mPAP but not mCVP or mPCWP was independently and positively associated with all-cause mortality in the multivariable Cox-hazard survival analysis [hazard ratio 1.10, confidence interval (CI) 1.04–1.16, P = 0.001]. Other factors independently associated with mortality were age at transplantation (hazard ratio per 1.03, CI 1.01–1.04 per 1 year increase, P = 0.002) and serum creatinine (hazard ratio 1.003, CI 1.001–1.010 per 1 μmol/l creatinine increase, P = 0.021) measured at the time of the RHC (Table 3). The covariates were checked for collinearity, which was not found. Hence we are confident that even in the presence of an event per variable ratio of 5 that the results of the regression analysis are valid.
Multivariate regression analysis results
| Variable . | P-value . | 95% confidence interval . | Hazard ratio . |
|---|---|---|---|
| CVP | 0.022 | 1.0023–1.042 | 1.121 |
| PCWP | 0.020 | 1.002–1.083 | 1.023 |
| PAP | 0.003 | 1.021–1.105 | 1.104 |
| Systolic blood pressure | 0.203 | 0.995–1.025 | 1.230 |
| Diastolic blood pressure | 0.193 | 0.965–1.007 | 1.002 |
| Age at transplantation | 0.026 | 1.002–1.038 | 1.032 |
| Serum creatinine | 0.001 | 1.001–1.011 | 1.003 |
| Potassium | 0.399 | 0.950–1.139 | 0.879 |
| Calcium | 0.386 | 0.887–1.439 | 1.127 |
| pro-BNP | 0.055 | 0.090–1.987 | 0.867 |
| Leukocyte count | 0.104 | 0.870–1.013 | 0.576 |
| Thrombocyte count | 0.403 | 0.997–1.101 | 1.453 |
| Haemoglobin | 0.428 | 0.692–1.169 | 2.436 |
| Creatinine kinase | 0.981 | 0.257–3.757 | 0.498 |
| LDH | 0.991 | 0.254–3.875 | 0.889 |
| Cholesterol | 0.945 | 0.001–4.834 | 1.052 |
| Variable . | P-value . | 95% confidence interval . | Hazard ratio . |
|---|---|---|---|
| CVP | 0.022 | 1.0023–1.042 | 1.121 |
| PCWP | 0.020 | 1.002–1.083 | 1.023 |
| PAP | 0.003 | 1.021–1.105 | 1.104 |
| Systolic blood pressure | 0.203 | 0.995–1.025 | 1.230 |
| Diastolic blood pressure | 0.193 | 0.965–1.007 | 1.002 |
| Age at transplantation | 0.026 | 1.002–1.038 | 1.032 |
| Serum creatinine | 0.001 | 1.001–1.011 | 1.003 |
| Potassium | 0.399 | 0.950–1.139 | 0.879 |
| Calcium | 0.386 | 0.887–1.439 | 1.127 |
| pro-BNP | 0.055 | 0.090–1.987 | 0.867 |
| Leukocyte count | 0.104 | 0.870–1.013 | 0.576 |
| Thrombocyte count | 0.403 | 0.997–1.101 | 1.453 |
| Haemoglobin | 0.428 | 0.692–1.169 | 2.436 |
| Creatinine kinase | 0.981 | 0.257–3.757 | 0.498 |
| LDH | 0.991 | 0.254–3.875 | 0.889 |
| Cholesterol | 0.945 | 0.001–4.834 | 1.052 |
CVP: central venous pressure; PCWP: pulmonary capillary wedge pressure; PAP: pulmonary artery pressure; LDH: lactate dehydrogenase.
Multivariate regression analysis results
| Variable . | P-value . | 95% confidence interval . | Hazard ratio . |
|---|---|---|---|
| CVP | 0.022 | 1.0023–1.042 | 1.121 |
| PCWP | 0.020 | 1.002–1.083 | 1.023 |
| PAP | 0.003 | 1.021–1.105 | 1.104 |
| Systolic blood pressure | 0.203 | 0.995–1.025 | 1.230 |
| Diastolic blood pressure | 0.193 | 0.965–1.007 | 1.002 |
| Age at transplantation | 0.026 | 1.002–1.038 | 1.032 |
| Serum creatinine | 0.001 | 1.001–1.011 | 1.003 |
| Potassium | 0.399 | 0.950–1.139 | 0.879 |
| Calcium | 0.386 | 0.887–1.439 | 1.127 |
| pro-BNP | 0.055 | 0.090–1.987 | 0.867 |
| Leukocyte count | 0.104 | 0.870–1.013 | 0.576 |
| Thrombocyte count | 0.403 | 0.997–1.101 | 1.453 |
| Haemoglobin | 0.428 | 0.692–1.169 | 2.436 |
| Creatinine kinase | 0.981 | 0.257–3.757 | 0.498 |
| LDH | 0.991 | 0.254–3.875 | 0.889 |
| Cholesterol | 0.945 | 0.001–4.834 | 1.052 |
| Variable . | P-value . | 95% confidence interval . | Hazard ratio . |
|---|---|---|---|
| CVP | 0.022 | 1.0023–1.042 | 1.121 |
| PCWP | 0.020 | 1.002–1.083 | 1.023 |
| PAP | 0.003 | 1.021–1.105 | 1.104 |
| Systolic blood pressure | 0.203 | 0.995–1.025 | 1.230 |
| Diastolic blood pressure | 0.193 | 0.965–1.007 | 1.002 |
| Age at transplantation | 0.026 | 1.002–1.038 | 1.032 |
| Serum creatinine | 0.001 | 1.001–1.011 | 1.003 |
| Potassium | 0.399 | 0.950–1.139 | 0.879 |
| Calcium | 0.386 | 0.887–1.439 | 1.127 |
| pro-BNP | 0.055 | 0.090–1.987 | 0.867 |
| Leukocyte count | 0.104 | 0.870–1.013 | 0.576 |
| Thrombocyte count | 0.403 | 0.997–1.101 | 1.453 |
| Haemoglobin | 0.428 | 0.692–1.169 | 2.436 |
| Creatinine kinase | 0.981 | 0.257–3.757 | 0.498 |
| LDH | 0.991 | 0.254–3.875 | 0.889 |
| Cholesterol | 0.945 | 0.001–4.834 | 1.052 |
CVP: central venous pressure; PCWP: pulmonary capillary wedge pressure; PAP: pulmonary artery pressure; LDH: lactate dehydrogenase.
Kaplan–Meier curve of total survival according to mPAP median split.
The receiver operating characteristics (ROC) analysis for mPAP showed a significant association (C = 0.549, SE = 0.036, 95% CI: 0.478–0620).
Pulmonary hypertension as defined by mPAP ≥ 25 mmHg was found in 17 (7%) of the 260 patients. There was a numerically higher mortality rate in patients with pulmonary hypertension compared to patients with mPAP at or below 25 mmHg which did not gain statistical significance (hazard ratio 1.94, CI 0.66–4.66, P = 0.199).
DISCUSSION
In this cohort of 260 heart transplant patients with RHC data obtained in the stable phase after transplantation, an mPAP above the median of 15 mmHg was independently associated with a worse long-term prognosis compared to mPAP values at or below the median. In contrast, no significant predictive value was found for mCVP or mPCWP in multivariable adjusted survival analysis.
Prior studies indicated that elevated pulmonary artery pressure measured before heart transplantation is an indicator of poor short- to mid-term survival following transplantation [8–12]. However, little is known about post-transplantation pulmonary pressures and their respective association with mortality in longer term follow-up. RHC pressures including pulmonary artery pressure are known to change significantly in the first months after transplantation as part of the adaptation process of the allograft and the vasculature of the host [9–11]. Most of the RHC of this study were performed after this adaptive phase, in a period where major pressure changes are less likely to occur and pressures more closely reflect the steady state of the patient.
Sahar et al. [9] observed that patients with unchanged or elevated pulmonary artery pressures after heart transplantation had a significantly worse prognosis compared to patients in whom pulmonary artery pressures decreased after transplantation. Delgado et al. [10] found similar results and noted that the presence of pulmonary hypertension before transplantation is a risk factor for early postoperative mortality. These studies focused on the prognostic relevance of pulmonary hypertension (mPAP ≥ 25 mmHg) in the immediate pre- and postoperative period of heart transplant recipients. While there was a numerically higher long-term mortality in patients with pulmonary hypertension in our cohort as well, this result did not gain statistical significance. This is most likely explained by the low number of patients presenting with pulmonary hypertension in our cohort, resulting in insufficient power to evaluate this particular subpopulation. It is worth noting that the median baseline mPAP in our cohort was lower than reported in other centres [10], which may be due to differences in RHC methodology or patient characteristics such as concomitant therapy.
Our results indicate that mPAP values as low as 15 mmHg and higher, thus values in the normal range are already significantly associated with increased long-term mortality in heart transplant recipients. This suggests that there is a continuum of risk with increasing pressures in the lung circulation. Indeed, there is evidence from non-transplant cohorts that high-normal pulmonary artery pressures are associated with worse prognosis compared to lower pressures [14]. In addition to pulmonary pressures, other parameters such as pulmonary vascular resistance may be decisive in risk stratification as indicated by a study by Costard-Jäckle et al [12]. In their study, increased pulmonary vascular resistance but not pulmonary hypertension was associated with increased mortality over a follow-up period of 3 years. In our study, cardiac output was not available in a large subset of patients and thus pulmonary vascular resistance could not be calculated.
The reasons why elevated pulmonary artery pressure is associated with a worse long-term prognosis in heart transplant recipients are not entirely clear. A complex and multifactorial relationship is likely as pulmonary artery pressure is not only a marker for left and right ventricular function but also for structural and functional abnormalities of the pulmonary vasculature as well as systemic factors such as volume status or neurohormonal activation [15]. In heart failure, elevated pulmonary artery pressure is a secondary consequence of elevated left ventricular filling pressure which can be estimated using mPCWP [16]. Interestingly, in our cohort mPCWP and CVP did not show any significant correlation with long-term prognosis in multivariable Cox-hazard survival analysis, suggesting that the association of mPAP with mortality in our cohort is not mediated by a higher rate of heart failure-related events. Indeed, heart failure was the cause of death of only 6% of patients in our study during long-term follow-up. This was further emphasized by lack of correlation between RHC pressures and cause of death.
The most common single cause of death was cancer (36% of patients). Solid organ recipients are known to have an increased risk of cancer and the individual effect and intensity of immunosuppressant therapy may play a role in that regard [17]. Besides cancer, immunosuppressant drugs have the potential for significant cardiovascular and renal side effects. This is well documented for cyclosporine which is known to cause arterial hypertension and renal dysfunction via several pathways including sodium retention [18], endothelin-1-mediated systemic vasoconstriction [19] and impaired nitric oxide signalling [20]. Interestingly, endothelin-1 activation is also a central pathophysiological component of pulmonary arterial hypertension [21]. Thus, higher pulmonary artery pressure in heart transplant recipients may represent a marker for increased susceptibility to long-term side effects of immunosuppressant drugs. Larger studies are needed to assess the effects of individual immunosuppressant drugs on pulmonary artery pressure and mortality as our study lacked statistical power for relevant subgroup analyses. If this hypothesis holds true, pulmonary artery pressure-guided medical and immunosuppressant therapy could improve outcomes in heart transplant recipients.
STRENGTHS AND LIMITATIONS
Strengths of this study are the long follow-up period of up to 28 years, longer than any previous study, and the use of invasively obtained pulmonary pressures in the stable phase after heart transplantation. This allowed evaluation of the long-term prognostic impact of pulmonary pressures after the adaptation of the host to the allograft.
A potential limitation is the retrospective nature of the study, although data have been recorded prospectively in a specific database. Also, patients were selected only on the basis of having undergone a RHC regardless of age or comorbidities. Confounding by changes in immunosuppressant treatment after data collection (era-effect) cannot be completely ruled out. Residual confounding by other potentially important parameters is also possible. RHC pressures from before transplantation and/or during the operation were not available. Thus, the impact of pre-transplantation pulmonary hypertension and its reversibility after transplantation could not be analysed. Also, the degree of urgency or whether the patient underwent emergency transplantation was unknown. Finally, the majority of heart transplant patients were male, thus our findings may not be generally applicable to female patients.
CONCLUSIONS
In summary, our results suggest that long-term survival in heart transplant patients is significantly associated with mPAP, but not mPCWP or mCVP in the stable phase after transplantation. Higher mPAP represents an independent prognostic marker for a worse long-term prognosis after heart transplantation.
Funding
This work was supported in part by the Foundation for Cardiovascular Research—Zurich Heart House.
Conflict of interest: none declared.
REFERENCES
Author notes
The first two authors contributed equally to this study.
- heart transplantation
- allograft heart
- lung
- follow-up
- hospitals, university
- pulmonary wedge pressure
- central venous pressure, measurement of
- central venous pressure
- heart
- mortality
- pressure-physical agent
- patient prognosis
- transplantation
- pulmonary artery line
- creatinine tests, serum
- pulmonary artery mean pressure