-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Czerny, Jürg Schmidli, Sabine Adler, Jos C van den Berg, Luca Bertoglio, Thierry Carrel, Roberto Chiesa, Rachel E Clough, Balthasar Eberle, Christian Etz, Martin Grabenwöger, Stephan Haulon, Heinz Jakob, Fabian A Kari, Carlos A Mestres, Davide Pacini, Timothy Resch, Bartosz Rylski, Florian Schoenhoff, Malakh Shrestha, Hendrik von Tengg-Kobligk, Konstantinos Tsagakis, Thomas R Wyss, EACTS/ESVS scientific document group , Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS), European Journal of Cardio-Thoracic Surgery, Volume 55, Issue 1, January 2019, Pages 133–162, https://doi.org/10.1093/ejcts/ezy313
Close - Share Icon Share
TABLE OF CONTENTS
ABBREVIATIONS AND ACRONYMS 134
1. INTRODUCTION 135
1.1. Purpose 135
1.2. Classes of recommendations and levels of evidence 135
1.3. Terminology 135
1.3.1 Categorization of tears in aortic dissection 136
1.3.2 Phases of acute aortic dissection 136
1.3.3 Type A, type B and non-A-non-B aortic dissection 136
1.3.4 Definition of complications in acute aortic dissection 136
1.3.5 Aortic arch replacement of various extents 136
1.3.6 Residual dissection after type A repair 137
1.3.7 Chimneys, snorkels, periscopes 137
1.4. Organization 137
1.4.1 Aortic team definition 137
2. NATURAL COURSE OF THE DISEASE AND UNDERLYING PATHOLOGIES 137
2.1 Natural course of the disease 138
2.2 Underlying pathologies: aortic arch dissection 138
2.3 Type A aortic dissection 139
2.4. Type B aortic dissection 140
2.5 Non-A-non-B aortic dissection: type B dissection involving the aortic arch 140
2.6 Aortic intramural haematoma 140
2.7. Penetrating aortic ulcer 141
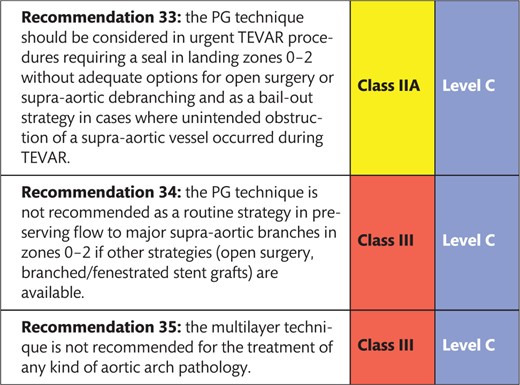
2.8 Recommendations for open and endovascular interventions based on aortic diameter 141
3. IMAGING AND DIAGNOSTIC WORK-UP 141
3.1 Computed tomography angiography 141
3.2 Magnetic resonance imaging 142
3.3 Ultrasound 142
3.4 Diagnostic work-up in aortic arch disease: emergency repair setting 142
4. RISK STRATIFICATION, PATIENT SELECTION AND TREATMENT APPROACH 143
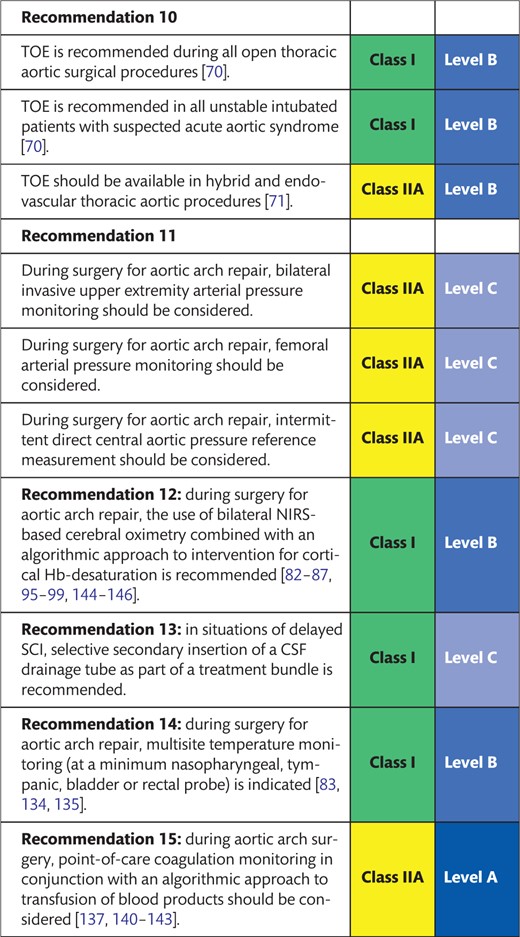
5. MONITORING DURING AORTIC ARCH REPAIR 143
5.1 Transoesophageal echocardiography 143
5.2 Invasive arterial pressure monitoring 143
5.3 Near-infrared spectroscopy-based regional oxygenation monitoring 144
5.4 Central nervous system electrophysiological function monitoring 144
5.5 Spinal cord perfusion pressure monitoring and lumbar cerebrospinal fluid drainage 145
5.6 Multisite temperature monitoring 145
5.7 Point-of-care coagulation monitoring 145
6. THERAPEUTIC OPTIONS 146
6.1 Open aortic arch replacement 146
6.2 Frozen elephant trunk technique 147
6.3 Transposition (debranching) of supra-aortic vessels and thoracic endovascular aortic repair and the importance of the left subclavian artery in spinal cord blood supply 148
6.3.1 Importance of the subclavian arteries in supplying blood to the spinal cord 150
6.4 Total endovascular aortic arch repair 150
6.5 Alternative approaches 152
7. TEN POINTS DESCRIBING WHEN TO CHOOSE WHAT KIND OF APPROACH 153
8. RARE PATHOLOGIES 153
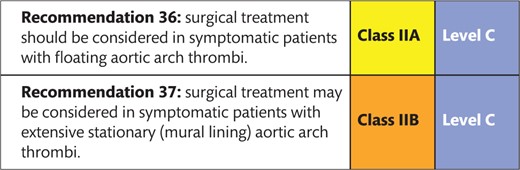
8.1 Thrombus 153
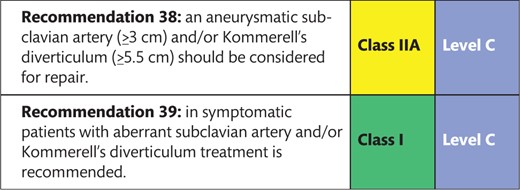
8.2 Aberrant subclavian artery and Kommerell’s diverticulum 153
8.3 Trauma 154
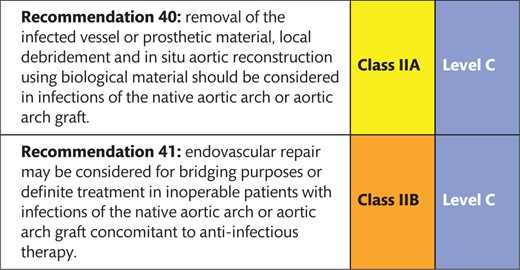
8.4 Infection 154
9. AORTITIS OF THE AORTIC ARCH 154
9.1 Giant cell arteritis 154
9.2 Diagnostic approach 155
9.3 Therapy 155
9.4 Complications and outlook 155
9.5 Takayasu’s arteritis 155
9.6 Diagnostic approach 155
9.7 Therapeutic approach 155
9.8 Complications 155
9.9 Conclusion 155
10. DURABILITY AND REPORTING STANDARDS AND QUALITY INDICATORS 155
11. GAPS IN EVIDENCE 156
ACKNOWLEDGEMENTS 156
Conflict of interest 156
REFERENCES 156
Abbreviations and acronyms
- 3D
Three-dimensional
- AHA
American Heart Association
- CE
Contrast enhanced
- CPB
Cardiopulmonary bypass
- CPM
Clinical prediction models
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- CTA
Computed tomography angiography
- DTA
Descending thoracic aorta
- EACTS
European Association for Cardio-Thoracic Surgery
- ECG
Electrocardiography
- EEG
Electroencephalography
- ESC
European Society of Cardiology
- ESVS
European Society for Vascular Surgery
- ET
Elephant trunk
- FA
Femoral artery
- FET
Frozen elephant trunk
- GCA
Giant cell arteritis
- Gd
Gadolinium
- Hb
Haemoglobin
- HCA
Hypothermic circulatory arrest
- IA
Innominate artery
- IMH
Intramural haematoma
- LDS
Loeys–Dietz syndrome
- LSA
Left subclavian artery
- MEP
Motor evoked potentials
- MFS
Marfan syndrome
- MPR
Multiplanar reconstruction
- MRA
Magnetic resonance angiography
- MRI
Magnetic resonance imaging
- NIRS
Near-infrared spectroscopy
- PAU
Penetrating aortic ulcer
- PET
Positron emission tomography
- PG
Parallel graft
- rSO2
Regional cerebral oxygen saturation
- SACP
Selective antegrade cerebral perfusion
- SCI
Spinal cord injury
- SSEP
Somatosensory evoked potentials
- STS
Society of Thoracic Surgeons
- STS PROM
Society of Thoracic Surgeons Predicted Risk of Mortality
- TA
Thoraco-abdominal
- TAA
Thoracic aortic aneurysm
- TAA/TAAA
Thoracic/Thoraco-abdominal aortic aneurysm
- TAR
Total arch replacement
- TOE
Transoesophageal echocardiography
- TEVAR
Thoracic endovascular aortic repair
- UFH
Unfractionated heparin
- US
Ultrasound
- WC
Writing committee
1. INTRODUCTION
1.1 Purpose
The last decade has substantially broadened treatment options for patients with thoracic aortic pathology involving the aortic arch. Traditionally, treatment of aortic arch pathology was a domain of open cardiac surgery. The advent of combined vascular and endovascular procedures opened a new field thereby enabling treatment in previously operated on and in less fit patients. As a subsequent technological leap, branched arch stent grafts became available and are currently gaining acceptance in the community. Also, open surgery has substantially improved, and the increased use of right subclavian artery cannulation and selective antegrade cerebral perfusion (SACP) at warmer lower body circulatory arrest times together with improved monitoring of organ function has substantially contributed to excellent results in these still major operations. Still, neurological complications remain a major concern of all procedures addressing aortic arch pathology irrespective if open surgery or endovascular repair. The reduction of neurological complications to a minimum will be one of the major tasks of the future.
Cross linking between cardiac and vascular surgery has amplified knowledge. Interestingly enough, although dividing cardiac and vascular surgery into separate units was popular for a time, in many institutions they are being combined again to create aortic centres, a trend which should be interpreted as a plea to work together without creating borders between specialties.
Our hope is that, in the future, treatment portfolios will be designed by a single group of people working together to understand the natural course of the disease where physicians are doing the right things when it comes to treatment and the entire aortic team follows an anticipative strategy to remain ahead of the disease process.
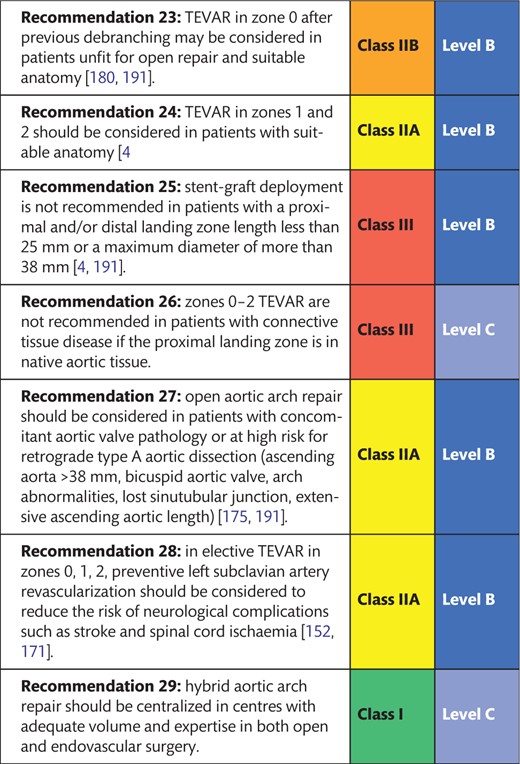
The purpose of this combined effort of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society for Vascular Surgery (ESVS) was to develop an expert consensus document covering all aspects of aortic arch disease and to provide the community with a pragmatic guide to understand the natural history of the various disease processes, to aid in indicating treatment and to provide support in choosing the right treatment modality in the right patient at the right point in time. Finally, this document aims to harmonize terminology in acute and chronic proximal thoracic aortic pathology.
1.2 Classes of recommendations and levels of evidence
The recommendation grade indicates the strength of a recommendation. Definitions of the classes of recommendations and levels of evidence are shown in Tables 1 and 2.
1.3 Terminology
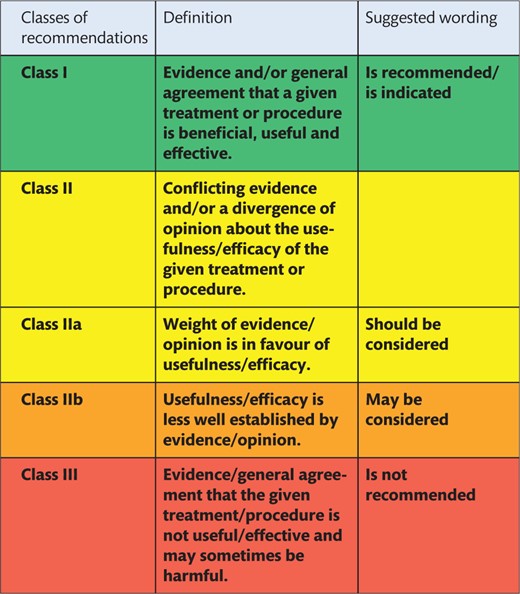
The Writing Committee (WC) refers to and recommends the use of the definition of attachment zones as provided by ‘Reporting standards for thoracic endovascular aortic repair’, which are also known as ‘Ishimaru zones’ in the aortic arch [1] (Fig. 1).
Definition of attachment zones, also known as Ishimaru zones (printed with permission from © Campbell Medical Illustration).
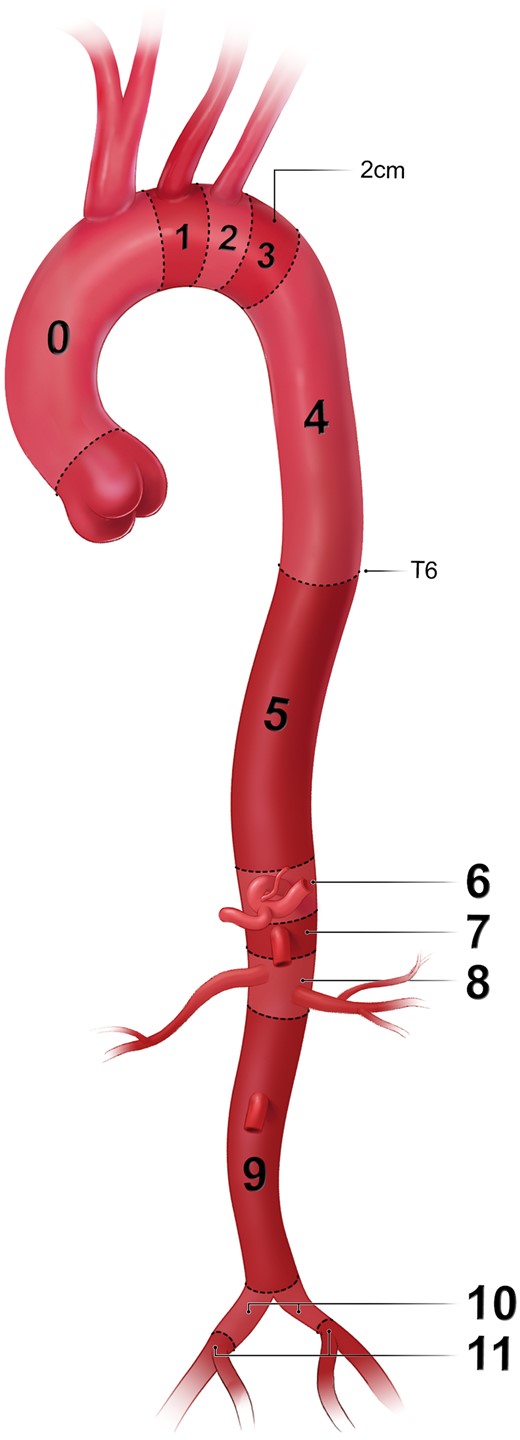
Regarding anatomical characteristics of the aortic arch, we refer to the classifications of type I, type II and type III aortic arch configurations [2]. There are 3 types of aortic arches, and they are based on the relationship of the innominate artery (IA) to the aortic arch [3]. In a type I aortic arch, all 3 great vessels originate in the same horizontal plane as the outer curvature of the aortic arch. In a type II aortic arch, the IA originates between the horizontal planes of the outer and inner curvatures of the aortic arch. In a type III aortic arch, the IA originates below the horizontal plane of the inner curvature of the aortic arch (Fig. 2).
Aortic arch configurations (printed with permission from © Emily McDougall Art).
Regarding the use of descriptive terms of specific arch configurations such as gothic arch, steep arch angulation and aortic arch radius, no least common denominator could be identified to add a meaningful definition. Therefore, the use of these terms to describe a specific morphology remains subjective.
1.3.1 Categorization of tears in aortic dissection
The WC suggests that the terms ‘multiple entries and re-entries’ be removed from clinical use and be replaced by the wording ‘most proximal tear’, ‘communications between lumina’ and ‘most distal tear’ in addition to the term ‘primary entry tear’. This proposed wording should help create a better understanding of the pathophysiology as well as help standardize communication between physicians describing the pathology.
1.3.2 Phases of acute aortic dissection
The WC suggests use of the term ‘acute’ for any dissection between the onset of symptoms and 14 days, ‘subacute’ between 15 days and 90 days and ‘chronic’ thereafter.
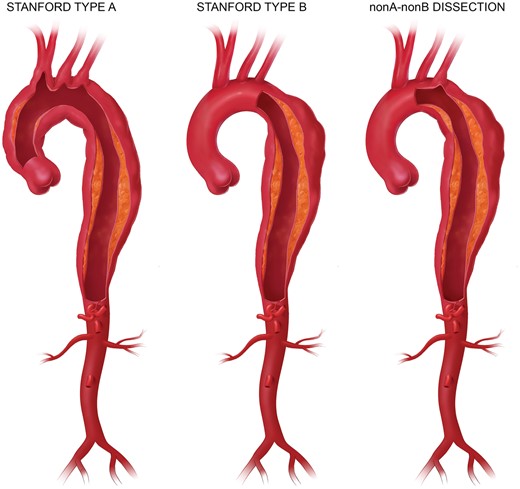
1.3.3 Type A, type B and non-A-non-B aortic dissection
The WC refers to the original proposal from Stanford that defines type A aortic dissection as any dissection involving the ascending aorta but refers to type B aortic dissection when only the descending thoracic aorta (DTA) is involved. Arch involvement either by the most proximal tear or by retrograde extension is referred to as non-A-non-B aortic dissection.
1.3.4 Definition of complications in acute aortic dissection
The WC uses the wording of the ESVS clinical practice guidelines on the management of DTA diseases, which define complicated type B aortic dissection as ‘the presence of rapid aortic expansion, aortic rupture and/or hypotension/shock, visceral, renal or limb malperfusion, paraplegia/paraparesis (spinal malperfusion), periaortic haematoma, recurrent or refractory pain and refractory hypertension despite adequate medical therapy’ [4].
The WC also applies this wording for complications in acute type A as well as in acute non-A-non-B aortic dissection and adds pericardial tamponade, acute aortic valve regurgitation, coronary and cerebral malperfusion to the one with either type A or non-A-non-B aortic dissection [5].
1.3.5 Aortic arch replacement of various extents
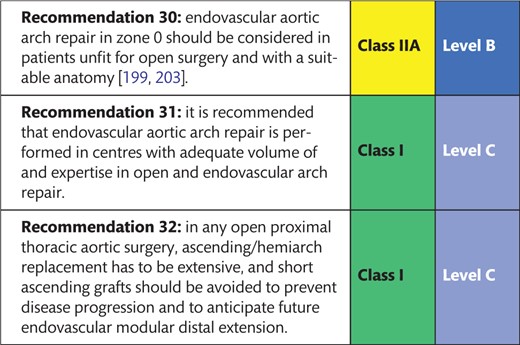
When referring to aortic arch treatment, qualitative and semiquantitative statements should be avoided. Given the rising number of patients receiving open and endovascular therapy, it seems reasonable to refer to the treatment-based classification using the terminology ‘zones 0–4’ when describing surgery on the aortic arch. Again, ‘distal arch aneurysm’ covers a wide range of anatomical variations. Replacing the arch using a frozen elephant trunk (FET) with an anastomosis proximal to the left carotid artery and selective reimplantation using separate grafts is not adequately covered in the current definitions.
One notable exception is the term ‘hemiarch’, which has been widely used for decades even if it also covers a wide range of surgical strategies from just replacing the ascending aorta and performing an open distal anastomosis to resecting the entire concavity of the arch down to the proximal DTA.
For the purpose of this paper, total arch replacement (TAR) is defined as replacing the entire aortic arch—or excluding it from circulation as is the case when using the FET technique—from the offspring of the IA to a point beyond the offspring of the left subclavian artery (LSA). Reimplantation or revascularization of the supra-aortic branches can be performed in many ways, and the method used is not part of the definition of TAR. To facilitate communication and to harmonize the standards of reporting, defining TAR as replacing (or excluding from circulation) aortic zones 0–2 (or beyond) seems reasonable. All other procedures on the arch should be named partial arch replacement.
1.3.6 Residual dissection after type A repair
The chronic dissected state of aortic segments distal to the proximal repair is defined as ‘residual dissection after type A repair’.
1.3.7 Chimneys, snorkels, periscopes
The WC refers to chimneys, snorkels and periscopes using the term ‘parallel grafts’.
1.4 Organization
1.4.1 Aortic team definition
The WC advocates that an aortic team should be closely involved from diagnosis to treatment and finally follow-up and should be led by members from cardiac and vascular surgery in collaboration with anaesthesiology, cardiology, radiology and genetics. A major advantage of surgery as the leading specialty is that surgeons have experience linking radiographic findings to tissue quality, which is a major component when opting for open surgery or endovascular treatment.
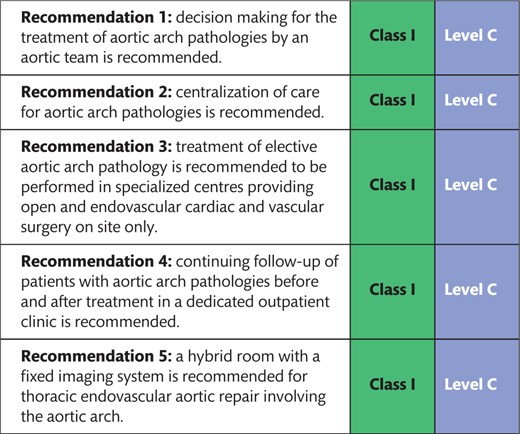
Additionally, centralization of care of aortic arch pathologies in large centres is recommended because it is the only way to effectively understand the natural course of the disease, provide the entire range of treatment options under one umbrella and treat potential complications of each individual therapy [6]. A streamlined emergent care pathway (24/7 availability without diversion), adequate transportation and transfer capabilities as well as rapid activation of the multidisciplinary team must be available.
There is growing evidence that there is a clear correlation between numbers and outcome in aortic medicine [7–12]. With regard to imaging, it is clear that the ability to obtain a hybrid-room setting is limited in many hospitals. However, few trade-offs should be made because adequate intraoperative imaging forms the basis of reliable delivery of quality.
Finally, a structured surveillance of all patients, either before they reach the criteria for treatment or after treatment, is strongly emphasized. One reason is quality control; another is the potential to develop aortic pathology in non-treated upstream or downstream aortic segments.
2. NATURAL COURSE OF THE DISEASE AND UNDERLYING PATHOLOGIES
The vast majority of aortic arch pathologies are based on either aneurysm formation or dissection. Although dissection on the basis of previous aneurysm formation is rare, it is the main driver for accelerated growth during follow-up. An isolated aneurysm of the aortic arch is rare, and most arch aneurysms that ultimately lead to surgical intervention are caused by aneurysms or dissections of either the ascending or the DTA, which at some point extend into the arch or by penetrating aortic ulcers (PAU).
2.1 Natural course of the disease
Population-based studies have shown that 60% of thoracic aortic aneurysms (TAA) occur in the root or the ascending aorta, 40% in the DTA and 10% include the aortic arch with some extending into more than 1 thoracic aortic segment [13]. There is no controlled trial that specifically looked at the natural history of aortic arch disease. Several papers discussing the fate of the aortic arch do so by almost exclusively citing data that were derived from either observations on the ascending or the DTA. Moreover, contemporary observational studies and registries are heavily biased by the fact that many patients with aneurysm diameters exceeding the threshold for surgery recommended by the current guidelines do in fact undergo surgery [14]. Therefore, there is a tendency towards facing dissection in patients with smaller diameters that had not yet reached the threshold for surgery. Conversely, some patients present with large aneurysms that exceed by far the current recommendations for surgery but have not yet dissected. Most papers dealing with aortic diameters and risk for dissection base their conclusions on post-dissection diameters. Due to the formation of intra- and periaortic haematomas, measuring the post-dissection diameter is not reliable. A study looking at patients with acute type A dissection who for some reason previously underwent imaging of the aorta has shown that the aortic diameter increases by about 30% at the time of dissection [15]. This clearly indicates that diameter at the time of presentation itself is not the sole predictor of the risk of dissection.
The 2010 American Heart Association (AHA) [2] and 2014 European Society for Cardiology (ESC) [16] guidelines refer to various publications that focused on interventions in arch aneurysms or dissections, especially regarding hybrid procedures, but the 2014 ESC guidelines do not cite a single paper on the natural history of the arch aneurysm, and the 2010 AHA guidelines refer only to the 1997 paper from the Yale cohort [17]. Data from the Yale aortic database have demonstrated an average annual growth rate of 1 mm for ascending aortic aneurysms and 2.9 mm for descending aortic aneurysms. Nevertheless, growth rates vary according to the underlying disease and the absolute size of the aneurysm. Larger aneurysms tend to grow faster. It is important to realize that 95% of patients with TAA are asymptomatic until the first event. Calculating the risk for dissection or rupture is difficult, but a large study including 721 patients with TAA demonstrated an annual risk for dissection or rupture of 6.9% in patients with an aneurysm diameter greater than 60 mm. The 5-year survival rate in patients with TAA not undergoing intervention was only 54% [18, 19].
There are only a few reports that focus specifically on the aortic arch. In a small study including 45 patients over a 14-year period with a mean follow-up of 37 months, the average annual growth rate was 2.5 mm per year but varied widely between 0 and 16 mm. During the study period, 22% of patients suffered from a rupture. The authors calculated that aneurysms with an annual growth rate of >5.5 mm per year have a 67% likelihood of rupture compared with 8.3% in patients with a growth rate of <5.5 mm per year. Furthermore, in their study, an aneurysm size >6.5 cm and hyperlipidaemia correlated with more rapid expansion. In a multivariate analysis, growth rate was the sole independent risk factor for aneurysm rupture (OR 1.43; 95% confidence interval, 1.06–1.92; P = 0.018) [20]. Although the current evidence is minimal, there seems to be no justification to conduct a prospective randomized trial comparing natural history to treatment.
It has been shown that 21% of patients with TAA have a relative with an already known aneurysm and that patients with familial occurrence of TAA have aneurysms that grow faster than those in patients with sporadic forms (2.1 mm per year vs 1.6 mm per year; ascending and DTA combined) [21]. This is an important aspect of thoracic aortic disease, and rapid progress is currently being made in identifying genetic mutations causing TAA. Over the past decade, the medical community has slowly accepted the idea that patients presenting with aortic aneurysm and/or dissection are part of a wide spectrum of genetically mediated diseases that present in syndromic as well as non-syndromic forms. Marfan syndrome (MFS) has long been the only seriously considered differential diagnosis in terms of a heritable disorder of connective tissue in patients with an aortic aneurysm. It has been shown that aneurysm formation in MFS is driven by excessive levels of transforming growth factor-β, a ubiquitous cytokine in most mammalian cells that is involved in cellular proliferation and differentiation. Loeys and Dietz identified a subset of patients sharing certain features such as a bifid uvula, hypertelorism and marked tortuosity of the vessels that had not been typically associated with MFS. The group identified mutations in the gene encoding for the transforming growth factor-β receptors 1 and 2 as the causative mutation [22, 23]. Identifying Loeys–Dietz syndrome (LDS) as a separate entity was important because patients with LDS suffered from acute aortic dissection at aortic diameters that had not been considered a cut-off to proceed to surgery in patients with MFS. Meanwhile several different mutations in patients within the spectrum of LDS have been identified. Preliminary data suggest significant differences in the risk of acute dissection in these patients. Data from the Johns Hopkins group showed that a significant number of LDS patients had to undergo interventions on the aortic arch after elective root replacement, something that has been rarely seen in Marfan patients.
With the advent of high-throughput sequencing techniques, more and more causative mutations in non-syndromic forms of type A aortic dissection have been identified. It has been shown that 11–19% of patients without (known) genetic defects have a first-degree relative with type A aortic dissection. Identifying the causative mutation in patients presenting with type A aortic dissection has a direct impact on the indication for surgery, the extent of surgery, and the prognosis of the patient and of his or her relatives.
2.2 Underlying pathologies: aortic arch dissection
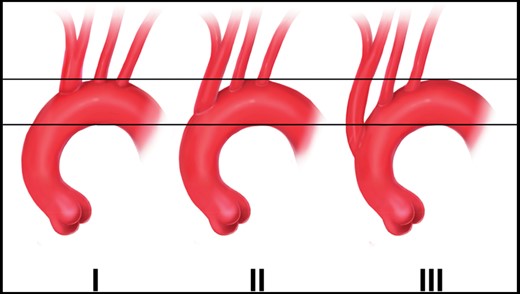
According to the Stanford classification of aortic dissection, a dissection is considered to be a type A dissection if the ascending aorta is involved, regardless of the location of the primary entry tear. According to this definition, a dissection in the aortic arch is generally considered a type B dissection. But as 90% of the type B dissections occur distally to the LSA, the majority of data on type B dissection does not apply to aortic arch dissection [4]. Nevertheless, the notion of ‘non-A-non-B’ dissections needs to be established (Fig. 3).
Definitions of aortic dissections (printed with permission from © Campbell Medical Illustration).
Some studies have implicated anatomical variants as predisposing factors for dissections with entries in the aortic arch. In a study including 157 patients [24] who underwent surgery for acute type A aortic dissection, 14% of the patients had either a common origin of the IA and the left common carotid artery (LCCA) or an origin of the LCCA from the IA, and the rate of arch entries in this group was significantly higher compared to that in patients without this pattern (59% vs 13%, P < 0.001). Furthermore, the presence of this arch pattern was associated with a higher rate of postoperative neurological injury (odds ratio 4.9, 95% confidence interval 1.635–14.734; P = 0.005).
2.3 Type A aortic dissection
The fate of the aortic arch in patients with type A aortic dissection is strongly correlated with the extent of the initial surgery. It has been clearly shown that not replacing the entire ascending aorta results in a high rate of reoperations. Therefore, performing at least a primary entry tear-oriented hemiarch replacement is recommended.
The additional burden of replacing the entire aortic arch as an adjunct to elective or emergent proximal repair is not very well defined and makes comparison with patients undergoing secondary TAR difficult. Most papers reporting on outcomes after surgery for type A dissection or those dealing with reintervention after proximal repair do not discuss arch-related morbidity and mortality separately [25, 26]. The major risk factor for the need of reintervention on the aortic arch and distal aorta after repaired type A dissection is a patent false lumen. Furthermore, pseudoaneurysm or dehiscence at the level of the distal anastomosis has been described as a frequent cause for reoperation. Therefore, several groups began to advocate TAR and implantation of an FET in addition to proximal repair in type A dissection.
Interestingly, Asian groups tend to favour a more aggressive approach and mostly recommend TAR during initial surgery for type A dissection. It has been discussed whether this is also due to a more favourable anatomy in the Asian population and a more pronounced atherosclerotic burden in Western countries, which increases the risk for stroke during TAR. In 2009, a Japanese group published one of the very few reports comparing hemiarch replacement with an open distal anastomosis to TAR with implantation of an FET [27]. In 120 patients presenting with acute type A dissection, mortality was only 4% with no new cerebral events and a survival of 95% at 5 years in the FET group compared to 69% in the hemiarch group.
A Chinese-American collaboration focusing specifically on patients with type A dissection and an entry tear in the arch analysed 104 patients who underwent FET and TAR and compared them with 728 patients undergoing surgery for type A dissection with entry tears elsewhere. Operative mortality was 8.6% with a 2.9% paraplegia rate. The stroke rate was surprisingly low at 1.9%. In this series, survival and freedom from late adverse events was 89% and 85% at 8 years, respectively, after a mean follow-up of 5.6 ± 2.6 years. Compared to other series, the time from onset of symptoms to surgery of 4.7 ± 3.5 days was quite long. Furthermore, computed tomography (CT) results after a mean of 4.6 ± 2.9 years postoperatively were only available in 65 patients but showed complete false lumen obliteration in 63 patients. The authors concluded that type A dissection with entry in the arch can be treated safely by FET and TAR and provides durable results [28]. Unfortunately, a true comparison with patients undergoing less extensive surgery was not performed.
Data from patients with MFS have shown that the extent of arch surgery during the initial intervention did not influence the need for thoraco-abdominal (TA) repair during follow-up. These data suggest that it is the dissection itself that drives the need for reoperations in these patients and that the aortic arch is only one of many segments that have to be repaired over the years [29]. In a large series of patients with MFS, it was shown that there was no significant difference regarding the rate of reoperation in patients with persisting dissection in the DTA after TAR compared to those without [30]. The rate for reinterventions was 50% in both groups at 10 years. Nevertheless, the rate of reoperation was higher in patients with a dissection in the aortic arch where only the ascending aorta was replaced compared to those patients without a dissected arch. Therefore, in the rare cases where the dissection is confined to the aortic arch, complete exclusion of the dissection may reduce the need for reinterventions and should be attempted. The principal importance of closing the primary entry tear during the index procedure and the differences in the natural history of the disease if the primary entry tear has been effectively closed or not have been previously described [31].
2.4 Type B aortic dissection
The International Registry of Acute Aortic Dissection investigators compared patients with and without retrograde extension of type B dissection [32]. Retrograde extension into the aortic arch occurred in 16.5% of patients. There was no difference in the rate of patients presenting with complicated type B dissection. In this registry, there was no difference regarding choice of treatment by the participating centres. Patients with and without arch involvement received best medical treatment only in 53.7% vs 56.5% (P = 0.68), endovascular treatment in 32.8% vs 31.1% (P = 0.78), open operation in 11.9% vs 9.5% (P = 0.54), or hybrid approach in 1.5% vs 3.0% (P = 0.70), respectively. Furthermore, there was no difference in in-hospital mortality rates in patients with (10.7%) or without (10.4%) retrograde arch extension (P = 0.96). Five-year survival was similar with 78.3% and 77.8%, respectively (P = 0.27). Unfortunately, this study did not look at those patients who not only had arch involvement but also had their primary entry tear in the arch.
A few years ago, it was proposed that patients with an entry at the inner curvature of the distal aortic arch have a higher risk of having a complicated type B dissection compared to those with an entry on the outer curvature [33, 34]. At that time it was speculated that the LSA may represent a natural barrier for progress of the dissection into the aortic arch. In this series, the incidence of primary complicated type B aortic dissection was 3 times higher in patients with an entry in the lesser curvature compared to those with an entry in the outer curvature (61% vs 21%, P = 0.003). Interestingly, a Japanese study with a total of 224 patients with type B dissection found that in multivariate analysis an entry at the outer curvature of the distal aortic arch was associated with a greater need for late open aortic surgery, aortic interventions and aortic events after a mean follow-up of 6.0 ± 4.1 years [35]. However, it has to be stated that there are several clinical scenarios where the location of the primary entry tear remains either unclear or a matter of discussion, e.g. in combination with an intramural haematoma (IMH). This finding might be attributed to the quality of the imaging or simply to a masked disease process. Serial adequate imaging may unmask the exact location of the primary entry tear within the first days after the acute event, just as transoesophageal echocardiography (TOE) can help in elucidating the exact location [36].
2.5 Non-A-non-B aortic dissection: type B dissection involving the aortic arch
Both Stanford and DeBakey classifications do not address the clinical scenario whereby the aortic arch but not the ascending aorta is dissected [37]. In the ESC 2014 aortic guidelines the comments on Stanford classification regarding arch dissection in patients with non-dissected ascending aorta are missing [16]. The 2010 AHA guidelines recommend that patients with descending aortic dissection and entry within the arch be categorized as proximal type B dissection [2]. Distal type B dissection refers to descending aortic dissection and entry distal to the LSA [2]. The evolution of the term non-A-non-B aortic dissection can be seen more as a kind of evolution of the understanding of the pathophysiological process, which was initially described in 1994 [38]. In a recent study including 43 patients with descending aortic dissection and dissection components in the aortic arch, the authors found 21 patients with entry in the DTA and 22 patients with entry within the aortic arch [39]. The incidence of non-A-non-B dissection was 11% among all patients with acute aortic dissection. Patients with non-A-non-B dissection presented with a common origin of the IA and LCCA in 28% and an arch origin of the left vertebral artery in 16%. The overwhelming majority of patients underwent aortic repair. Emergency aortic repair due to malperfusion or aortic rupture was necessary in 29% of patients with descending entry and in 36% of patients with arch entry. Another 43% of patients with descending entry and 36% of patients with arch entry required aortic repair within 2 weeks after dissection onset due to rapid aortic growth, aortic rupture, new organ malperfusion or persisting pain. All patients, except for 1, required repair for the aneurysm at follow-up. Overall in-hospital mortality in patients with acute non-A-non-B dissection was 9%. The highest in-hospital mortality rate of 37% was observed in patients with an arch entry who underwent emergency surgery.
Clinical presentation, treatment and outcome in non-A-non-B dissection patients are different from those commonly reported for patients with acute type B dissection. The involvement of the arch in the dissection process of the DTA seems to have an important impact on clinical course and outcome; therefore it is reasonable not to categorize these patients as type B, but as non-A-non-B aortic dissection.
2.6 Aortic intramural haematoma
The ESC guidelines define aortic IMH as a circular or crescent-shaped thickening >5 mm of the aortic wall with the absence of a dissecting membrane, intimal disruption or false lumen flow [16]. The ESVS guidelines define intramural haematoma as the presence of blood within the aortic wall without intimal disruption or an identifiable entry point on imaging [4]. Whereas current guidelines see IMH as a separate entity, distinguishing between IMH and dissection may not always be possible in clinical practice. There is certainly a time-dependent variable with regard to diagnosis because patients frequently present with new intimal lesions 24–48 h after the initial imaging studies were performed. The current definition of IMH may be challenged as more sophisticated imaging methods will be able to identify more primary entry tears and therefore identify more IMH as a precursor of acute aortic dissection.
Some of the predictive factors for disease progression that have been proposed for patients with IMH without associated ulcer or intimal erosion include involvement of the ascending aorta, aortic diameter >50 mm in initial imaging and persistent pain. Predictors of disease progression in patients with IMH and an associated aortic ulcer or intimal erosion include increase of associated pleural effusion, recurrent pain, ulcer located in the ascending aorta or arch with initial maximum ulcer diameter >20 mm or more and initial maximum ulcer depth >10 mm [40, 41]. In a German multicentre study, 60% of IMH patients revealed evidence of significant progression and 20% developed overt dissection within 30 days of hospital admission [42].
Data are particularly scarce on IMH in the aortic arch. In a 2012 publication on IMH from the International Registry of Acute Aortic Dissection investigators, the authors analysed 178 patients, 42% of whom presented with type A and 58% with type B IMH. In 24 (13%) of these the most proximal extent was in the aortic arch. Separate analysis of these patients showed that 16 were medically managed, 4 underwent surgery, 2 received endovascular treatment and 2 had hybrid interventions. There were 3 deaths (12.5%) in the population, and the authors concluded that this group had a slightly higher mortality rate and an increased need for interventions than patients presenting with type B IMH [43].
2.7 Penetrating aortic ulcer
The current ESC guidelines on aortic disease define PAU as an ulceration of an aortic atherosclerotic plaque penetrating through the internal elastic lamina into the media. It is thought that PAU occurs in 2–7% of all patients with acute aortic syndromes. Although there are no controlled studies regarding the natural history of PAU in different settings, reports have shown that PAU can result in the development of a true aortic aneurysm, IMH or an aortic dissection. Patients presenting with PAU frequently have a high atherosclerotic burden. Risk factors for PAU include advanced age, male gender, tobacco smoking, hypertension, coronary artery disease, chronic obstructive pulmonary disease and presence of abdominal aneurysm. In a study from the Mayo Clinic including 105 patients, ulcerations were located in the DTA in 94 patients, in the aortic arch in 11 patients and 10% presented with PAUs in multiple locations. Interestingly, the rate of PAUs located in the arch was significantly higher in the group of patients who were asymptomatic compared to those who were symptomatic (20% vs 5%, P = 0.03) [44].
These data are in line with those from a large series of 388 patients from the Philadelphia group presenting with PAU where 6.8% of patients had PAUs located in the aortic arch. The authors reported a higher number of open repairs in this patient group but there are no data regarding specific outcome parameters [45]. Indications for intervention according to the current guidelines include persistent or recurrent pain, contained rupture, rapid growth, periaortic haematoma and pleural effusion. It is thought that in asymptomatic patients with PAU, a diameter >20 mm and a neck >10 mm have a higher risk of progression and early intervention should be evaluated.
2.8 Recommendations for open and endovascular interventions based on aortic diameter
Given the paucity of data on the natural history as well as on the varying results of open surgery, there are few recommendations regarding the optimal timing of surgery solely based on the diameter of the arch. The current guidelines recommend surgery in isolated arch aneurysms at a diameter of 55 mm. Both the AHA and the ESC guidelines acknowledge the fact that the indication for surgery in arch aneurysm is strongly influenced by the overall vascular situation, especially the diameter of the adjacent ascending and descending aortic segments. In the majority of patients, this will determine the threshold for intervention.
3. IMAGING AND DIAGNOSTIC WORK-UP
3.1 Computed tomography angiography
Computed tomography angiography (CTA) is the most commonly used imaging modality to assess the aorta and has many advantages over other imaging modalities. Currently, it remains the modality of first choice [46]. It is able to quickly acquire high spatial resolution 3-dimensional (3D) images of the aorta and surrounding structures, enable diagnosis and aid in planning treatment.
The acquisition should start cranially to the aortic arch and include the supra-aortic branches—ideally the circle of Willis—and extend caudally to the level of the femoral heads. A scan prior to contrast administration (‘native’) is performed in some institutions for some questions, e.g. to rule out IMH. A total of 50–120 ml of contrast medium is generally needed (0.5–0.7 g of iodine per kilogram of body weight) [47, 48]. At the CT console a region-of-interest marker is placed in the thoracic aorta. When the contrast enhancement reaches a certain density threshold (e.g. 120 Hounsfield units) within the chosen region of interest, the start of the scan is delayed for a few seconds (depending on the scanners speed) to perform data acquisition at the correct position in the ideal moment of the arterial phase. If needed for evaluation of e.g. organ perfusion, a second scan in the venous phase may be acquired after a delay of 60–90 s upon arrival of contrast.
CTA data can be acquired with reference to the electrocardiogram (ECG) signal to provide images of each phase of the cardiac cycle in order to minimize the artefacts from cardiac pulsation and aortic wall motion, which requires a low pitch down to 0.2, i.e. a slow-moving table. There are 2 techniques to obtain an ECG-gated CTA scan pro- or retrospectively [49]. Artefacts from an incompliant patient or from a bowel or breathing motion are not compensated. To describe cardiac or vessel motion during an R-R’-interval, a maximum of twenty 3D data sets of the entire cardiac cycle can be gained using retrospective triggering. This allows reconstruction of a maximum of twenty 3D CTA scans. This dynamic CTA provides information on aortic movement and dynamic changes in aortic perfusion. However, a radiation dose of retrospectively triggered or gated CTA is much higher in comparison to that of conventional CTA [49, 50]. The use of dual source technology and the high pitch that can be achieved with this technique (up to 3.4) may overcome the need for ECG triggering and thus reduce the radiation dose, without loss of diagnostic accuracy [51].
Post-processing of axial CT data is possible using multiplanar reformation (MPR), maximal intensity projection and volume rendering technique [49, 52]. MPR allows for generation of an arbitrarily angled cross-section within the entire 3D data set. Such MPRs allow a better visualization and appreciation of anatomical and pathological structures [49, 53]. Semi- or full automatic centreline analysis is used to improve length measurement accuracy and to determine the diameter perpendicular to the centreline [53]. Aortic diameter measurements must always be obtained using MPR reconstruction on planes perpendicular to the aortic flow direction (‘double-oblique’ technique) [54].
3.2 Magnetic resonance imaging
Magnetic resonance imaging (MRI) can provide 3D images of the aorta and surrounding structures with high contrast enhancement and high spatial resolution. MRI has obvious advantages over CT including superior soft tissue contrast, the absence of ionizing radiation, and the ability to depict and quantify functional parameters. Combining anatomical and functional information in a single acquisition means that MRI can potentially provide a more comprehensive evaluation of thoracic aortic disease. The relatively long acquisition times, however, limit its use in the acute setting.
Magnetic resonance angiography (MRA) is the most commonly used MRI technique for both pre- and post-procedural imaging of the thoracic aorta. CE (contrast enhanced) MRA techniques rely on the T1 shortening effect of gadolinium (Gd)-chelate contrast agents in blood to generate a high intravascular signal instead of exploiting the inherent motion of blood flow as in the flow-based time-of-flight and phase-contrast techniques. Thanks to this different approach, the vascular signal generated with CE-MRA is not hampered by the numerous flow-related artefacts that can degrade the flow-based MRA techniques [55, 56]. One of the more effective compounds for vascular contrast is gadobenate dimeglumine, which has been proven to perform better than the standard compounds due to weak binding to serum albumin [57]. Some issues were raised regarding the occurrence of a syndrome named nephrogenic systemic fibrosis, which limits the applicability of CE-MRA in patients with renal insufficiency [58]. There is active research going on investigating the relevance of Gd-deposition in the human body after CE exams, especially in the brain [59, 60]. Today, no clinical symptoms have been described as associated with intracerebral Gd-deposition.
The use of phased array coils provides the additional benefit of markedly shortening image acquisition times or, with the use of parallel imaging schemes, of acquiring higher spatial resolution image sets in the same time period [61, 62]. As with CTA, the vascular enhancement is a transient and dynamic process; hence the critical element to be set for a CE-MRA is the proper timing for the image acquisition.
Dynamic MRA provides temporal information during the heart cycle that can be visualized as a dynamic display, thereby adding a fourth dimension, 4-dimensional CE-MRA. Its acquisition is typically combined with a Gd-based contrast medium injection while a sequence of 3D volumes is acquired over time including fat suppression [49, 63, 64]. Fast gradient echo-sequence covers the entire aorta allowing high temporal resolution of e.g. 2–4 s/volume and an interpolated spatial resolution of 1 mm3 at a static magnetic field strength of 3 Tesla. These fastest, time-resolved MRA techniques are available, with 2 common acronyms for this approach: TWIST (time-resolved angiography with interleaved stochastic trajectories) and TREAT (time-resolved echo-shared angiographic technique) [65].
Cranial MRI can be used in addition to intracranial Doppler ultrasound (US) to assess circle of Willis completeness, which helps predict the risk of insufficient cross-flow and stroke. Time-resolved MRA of the thoracic aorta is the optimal method to study the mobility, stiffness and dynamics of dissection membranes, as well as the resulting static or dynamic large vessel occlusion mechanisms. Similar to intracardiac flow dynamics in valvular disease, true and false lumen antegrade, retrograde and turbulent flows should be imaged using MRA as the ‘gold standard’.
3.3 Ultrasound
US techniques have a small field of view compared with CT and MRI. US is also constrained by not being able to image through bone or gasses/air, but US can provide functional information with high temporal resolution. CE US is currently being performed using microbubbles as intravenous exogenous contrast medium, e.g. for endoleak detection during endovascular aortic repair follow-up [66]. Both TOE and transthoracic echocardiography can be performed bedside with a low incidence of complications. Using a variety of imaging projections, the aorta and its major branches can be visualized. More recently 3D techniques have been developed that can provide further information regarding the aorta and valve function, although their clinical incremental value has not yet been fully assessed. US can add important dynamic and functional insights to the disease process at several levels, also with regard to aortic branches of the first order such as the supra-aortic, visceral, renal and iliac/femoral vessels.
Intravascular US provides dynamic information regarding both the true and false lumens and allows detection of false lumen thrombosis with higher sensitivity and specificity than TOE. Because of its invasiveness, the use of intravascular ultrasound is limited to intraoperative guidance.
3.4 Diagnostic work-up in aortic arch disease: emergency repair setting
The diagnostic workup in preparation of emergency aortic arch repair, in most cases acute Stanford type A or non-A-non-B dissections, also focuses on selecting the most effective, most durable, and safest operative and perfusion strategy, however with less time available and a limited diagnostic workup of supra-aortic and intracranial collateral flow. CTA, TOE in the operating theatre and sometimes supra-aortic Duplex US of carotid arteries can possibly provide sufficient information to be able to plan and to treat.
4. RISK STRATIFICATION, PATIENT SELECTION AND TREATMENT APPROACH
Risk constellations and case mix in patients with aortic disease are no less heterogeneous than in the cardiac surgical population. Currently available modalities for perioperative risk assessment like the Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) [67] or EuroSCORE I and II [68] have been well validated for cardiac surgery but not for aortic disease and its surgical and endovascular treatment options. Thus, STS PROM and EuroSCORE are inappropriate risk prediction tools for patients with aortic arch pathologies and procedures. The same holds true for other, unmodelled severity scores.
Since clinical prediction models (CPM) are indispensable for any risk stratification in patients undergoing invasive procedures, their lack for aortic arch pathologies hampers comparisons of prospective study results, database analyses, therapies and the performances of institutional and health care systems. In this field, development of a dedicated CPM and risk score remains therefore an unmet need. In recognition of the increasing frequency and complexity of thoracic aortic medicine, the STS recently formed a task force on aortic surgery and added aortic pathology as a module in order to collect data for CPM development and further research [69].
Aortic arch pathology of various degrees of complexity that do not involve the rest of the cardiovascular system are the exception rather than the rule. Despite the presence of several underlying pathologies that lead to the final common path of aneurysmal formation/lesion development, the algorithm to diagnose concomitant cardiac and vascular conditions should be standardized in all patients being evaluated for treatment. Finally, the outcome of this diagnostic algorithm should also have an impact on the final treatment strategy.
Each patient should undergo transthoracic echocardiography or in case of remaining need, TOE. Coronary angiography is recommended in all patients who need open surgery whereas non-invasive testing might be regarded as sufficient in selected cases scheduled for endotherapy in the absence of symptoms indicative of coronary artery disease. In candidates for endovascular treatment with a medical history of coronary artery disease, additional diagnostics should be considered to quantify the severity of the underlying concomitant condition.
Supra-aortic branches should be evaluated by US and there is definitive need for evaluating cerebral cross-flow and the patency of the circle of Willis. Finally, a CTA should evaluate the entire aorta including the branches of first order. Harmonization of the aforementioned diagnostic modalities should then lead to a recommendation for treatment, be it open surgery, combined vascular and endovascular procedures, a full endovascular approach or a recommendation for conservative treatment in cases in which the remaining risk of concomitant conditions outweighs the potential benefit of treatment.
5. MONITORING DURING AORTIC ARCH REPAIR
As for any major cardiovascular surgery, standard monitoring includes non-invasive and invasive haemodynamic, respiratory, anaesthesia, temperature, coagulation and laboratory monitoring. Additional monitoring techniques for aortic arch procedures should be selected according to the specific requirements of the patient, the surgeon and the interventionist in order to help preserve haemodynamics and organ function and to support procedural management [2].
5.1 Transoesophageal echocardiography
TOE offers real-time 2-dimensional and 3D morphological and functional cardiovascular assessment as a semi-invasive imaging modality. Echocardiography systems used in aortic arch programs should include options and probes for epiaortic and epicardial US, for Doppler and 2-dimensional interrogation of supra-aortic and peripheral vessels, as well as for US-guided vascular access. There is consensus in current guidelines that perioperative TOE is recommended for all adult open thoracic aortic surgical procedures, i.e. also those involving the aortic arch [70]. Also, TOE is indicated in patients with suspected acute aortic syndrome who are unstable and are already intubated [70].
During hybrid and endovascular thoracic aortic procedures, TOE should at least be available. Use of TOE may be considered, e.g. in dissection cases and when general anaesthesia is provided, for purposes of procedural and instrumentation guidance, i.e. guidewire placement via the dissected aorta [71], endoleak assessment [71–74] or detection of cannulation injury. The benefit of TOE in these scenarios compared with its perioperative use is less well supported by evidence. Since some endovascular procedures may be performed with the patient under local anaesthesia, anaesthesia or increased sedation requirements for purposes of TOE monitoring are to be weighed against its incremental diagnostic benefit.
5.2 Invasive arterial pressure monitoring
During endovascular or surgical repair of aortic arch pathology, continuous monitoring of invasive arterial blood pressure is indicated. Selection of the monitoring site should take the vessel pathology into account (e.g. dissection, stenosis, fistula, atheroma, anatomical variants) and must not interfere with vascular access and branch vessel manipulation. In endovascular procedures involving the aortic arch, multiple arterial access sites via the lower and upper extremities are usually required. The arterial site dedicated to anaesthesia monitoring must therefore be chosen carefully in consultation with the performing team and on an individualized basis.
Open surgical repair of the aortic arch requires periods of occlusion and selective perfusion of supra-aortic branches at least temporarily and often sequentially. A single-site arterial line is not sufficient for uninterrupted monitoring of vital organ perfusion pressures. Bilateral invasive radial artery pressure measurement allows monitoring of the cerebral perfusion pressure without interruption during direct subclavian cannulation or during subclavian cross-clamping for cannulation and during repair. When right axillary antegrade cerebral perfusion is performed via a cannulated graft sewn to the artery, simultaneous monitoring of the right axillary antegrade cerebral perfusion inflow pressure and the resulting left radial pressure is possible. This may provide information about functional integrity of the circle of Willis [75] and/or run-off blood flow from the LSA to the DTA. Nevertheless, bilateral radial pressure monitoring is used in aortic arch surgery only by about 50% of the European centres surveyed [76].
Additional femoral arterial (FA) pressure monitoring (preferably at the non-surgical or non-dissected FA) allows assessment of the efficacy of distal body perfusion before and after hypothermic circulatory arrest (HCA) and detection of the post-repair pressure gradients across the arch. Particularly during rewarming from HCA and for several hours after termination of prolonged cardiopulmonary bypass (CPB) runs, radial pressure often underestimates central aortic pressure, which is better approximated by the FA pressure. Due to vasodilatory arteriovenous shunting in the distal upper extremities, radial pressure may underestimate central aortic pressure (measured by direct needle transduction) by up to 20 mmHg mean and 35 mmHg systolic pressure [77, 78]. Overdiagnosis of ‘vasoplegic syndrome’ or ‘vasodilatory shock’, with inadequate dosing of vasopressor agents, may be avoided by central aortic pressure verification and use of the FA for early postoperative pressure monitoring.
For surgical repair of the aortic arch, the bilateral invasive upper extremity arterial pressure should therefore be monitored routinely. In this type of surgery with prolonged CPB and periods of HCA, consideration should also be given to intermittent direct central aortic pressure reference measurement and/or additional FA pressure monitoring.
5.3 Near-infrared spectroscopy-based regional oxygenation monitoring
Near-infrared spectroscopy (NIRS) of haemoglobin (Hb) fractions can be used to continuously monitor the balance of oxygen supply and demand in superficial cortical regions of the brain, i.e. by bifrontal NIRS-derived cerebral oximetry [79, 80]. The potential and the limitations have extensively been studied during carotid endarterectomy where the evidence to define clear cut-off points for the presence of perioperative cerebral ischaemia still is limited [81]. During aortic arch procedures, cerebral tissue Hb may desaturate for a large variety of reasons, e.g. global or unilateral hypoperfusion [82] or cerebrovenous congestion; aortic or SACP cannula malposition, vessel dissection or malperfusion; systemic hypotension, hypoxaemia, hypocapnia, haemodilution, anaemia or low cardiac output; insufficient levels of hypothermia or anaesthesia; aggressive rewarming [83]; or other causes of regional or global ischaemia. If this monitoring modality is used, differential diagnosis and the use of an algorithmic approach to intervention for regional cerebral tissue Hb desaturation are recommended [84, 85].
A survey of 144 European cardiac centres found that NIRS oximetry is used in aortic arch surgery by 65% of institutions [76]. It also showed that NIRS oximetry has largely replaced invasive jugular bulb oximetry [76, 86]. An analysis of open arch surgical strategies at 12 large European centres reported NIRS use for neuromonitoring in all centres [87]. The limitation remains that uneventful intraoperative bifrontal regional cerebral oximetry saturation (rSO2) tracings do not rule out focal cerebral ischaemia, which may occur outside the limited field of view of current NIRS devices. Transcranial Doppler monitoring presents another option to monitor changes in cerebral perfusion but is more complex with regard to the setup and the application during aortic arch surgery.
So far there is only low-grade evidence in adult cardiac surgery [85, 88–94] and moderate-grade evidence in thoracic aortic surgery that link intraoperative cerebral rSO2 desaturation to postoperative new neurological morbidity [83, 95–97]. Nevertheless, with its perceivably favourable risk-benefit ratio, the routine use of non-invasive continuous NIRS monitoring during the thoracic aortic procedures is increasing [76, 87, 98–100]. For surgical and hybrid repair of aortic arch pathology, NIRS-based continuous monitoring of rSO2 is recommended in combination with an algorithmic approach to intervention for desaturation events [84, 96]. Good evidence for a benefit of NIRS monitoring in endovascular arch repair is still lacking. Indications for its use are pragmatically inferred from surgical (carotid, arch) and stroke populations [99, 101, 102]. NIRS-based continuous monitoring of rSO2 should therefore be considered as an opinion-based level of evidence.
5.4 Central nervous system electrophysiological function monitoring
Electroencephalography (EEG) (raw or, more commonly, processed to parametric display) has been widely used in aortic arch surgery to ensure electrical and cerebral metabolic suppression to a level of complete electrocerebral inactivity prior to HCA. This appears helpful in view of the considerable interindividual variability in cooling efficacy and ischaemic risk [103]. Cooling time to cortical isoelectricity is not precisely predictable from tympanic or nasopharyngeal temperature trends, since electrocerebral inactivity may ensue within a wide range of temperatures, i.e. a nasopharyngeal temperature between 27.2°C and 12.5°C [104]. The strategy of HCA with hypothermia-induced electrocerebral inactivity has produced increasingly good neurological and survival outcomes over time [105], but evidence as to the incremental benefit from EEG monitoring per se remains scarce.
Nowadays, the strategy of open aortic arch surgery increasingly shifts to using moderate HCA (>/= 28°C systemic) combined with hypothermic SACP [106–111], with comparably good major outcomes and lower stroke rates [109]. With this strategy, hypothermic EEG silence is no longer targeted during cooling, and EEG monitoring refocuses on the detection of ischaemia and inadequate anaesthetic levels as in other surgical fields. Still, the choice of lower core temperatures should be considered as having a sufficient safety margin according to the expected lower body circulatory arrest time.
European and German surveys report that EEG is monitored in aortic arch surgery by a third of the polled centres (16–38%) [76, 98]. Bilateral EEG has been shown anecdotally to indicate the inefficacy of SACP during moderate HCA [112]. Further evidence is lacking so far that EEG monitoring improves the major outcomes of aortic arch surgery with SACP or of hybrid or endovascular arch repair. Since its incremental benefit in the surgical or endovascular repair of aortic arch pathology is established only by opinion and low-grade evidence, EEG or processed EEG monitoring may be considered according to institutional preferences (e.g. use of HCA) and concomitant indications (carotid cross-clamping, monitoring of anaesthetics effect).
Monitoring of motor [motor evoked potentials (MEP)] or somatosensory evoked potentials (SSEP) can be useful in TAA and TA aortic surgery or endovascular repair in order to guide therapy and to allow early intervention in the anaesthetized patient [113–116]. A meta-analysis confirmed the good performance of MEP monitoring in detecting postoperative paraplegia in thoracic and/or thoraco-abdominal open repair [117]. Both MEP and the less well investigated SSEP neuromonitoring have been found useful in the prevention and prediction of paraplegia [118, 119]. In a retrospective analysis, MEP was found useful in simultaneous arch and TA aortic surgery as part of a protocolized brain and spinal cord protection bundle [120]. The selective use of MEP and SSEP monitoring in aortic arch surgical or endovascular repair may therefore be considered based on the requirements of the individual patient, surgery or procedure, on the urgency of the procedure and on institutional resources [2].
During hybrid arch repair, considerations of extracranial cerebrovascular surgery in anaesthetized patients apply, whereas aortic arch debranching is performed without CPB. During this period, monitoring for cerebral ischaemia according to institutional preferences (EEG or SSEP and/or NIRS) should be considered [99, 101, 121]. Subsequent thoracic endovascular aortic repair (TEVAR) deployment may compromise spinal cord collateral perfusion. Depending on the extent of coverage and the compromise of collateral flow, MEP or SSEP monitoring during this period should be considered in selected patients to assess the integrity of spinal cord function [103].
5.5 Spinal cord perfusion pressure monitoring and lumbar cerebrospinal fluid drainage
Distal aortic arch repair involving the DTA and use of the FET may compromise the collateral vascular network and hence the perfusion of the spinal cord. Segmental spinal artery inflow may become impaired depending on the flow characteristics of the dissection and the extent of coverage by stent grafts [122, 123]. Known contributors to spinal cord injury (SCI) are perioperative arterial hypotension, previous abdominal aortic aneurysm repair and loss of LSA inflow [124]. A systematic review reported a SCI incidence of 5.1% following FET deployment [125]. To date, evidence is insufficient for a recommendation to use prophylactic MEP and/or cerebrospinal fluid (CSF) pressure monitoring and drainage in aortic arch repair with the use of FET [125, 126]. However, the use of lumbar CSF pressure monitoring and drainage may be considered based on individualized risk assessment for spinal cord ischaemia [127, 128]. In situations of delayed SCI, selective secondary insertion of drainage as part of a treatment bundle is recommended [126, 129]. As imaging is still not able to provide us with a detailed description of intraspinal collateralization, which might be the answer to who is at increased risk for SCI, risk prediction models remain approximations, e.g. the collateral network concept and, developed on that basis, the 4-territory concept [130, 131].
CSF drainage management—CSF pressure is measured in mmHg in the majority of settings (since invention of electronic pressure transducers): cm H2O and mmHg are not ‘close in numbers’ but enjoy a firm relationship (1 cm H2O = 0.735 mmHg). Spinal perfusion pressure (SPP = MAP −CSFP, or −CVP whichever is higher) can only be determined correctly if arterial and CSF pressure transducers are referenced to the same level (phlebostatic axis = right atrial level) and unit of measurement. Hence, mmHg makes more sense, too, although some drainage systems give parallel scales in mmHg and cm H2O (e.g. Medtronic Duet® External Drainage and Monitoring System). After placement, a normal CSF opening pressure is 5–18 mmHg, and CSF may be drained to a target CSFP of 10–12 mmHg, as long as there is no SCI.
Some institutions target the normal preoperative opening pressure, measured on catheter placement, as the individual baseline pressure [124] unless there is reason to suspect SCI. Drainage should always occur slowly; large bolus CSF withdrawals must be avoided. If SCI occurs, reasonable CSF pressure targets are 8–10 mmHg, with limits on ‘volume’ flow at 40 ml/4 h, although some groups drain even lower to 7 or 5 mmHg, and larger volumes (≤20 ml/h) [132]. But there is a clear risk (approximately 1%) of overdraining, intracranial hypotension and consecutive brain damage (subdural haematoma or hygroma, intracranial haemorrhage, brain herniation).
5.6 Multisite temperature monitoring
During CPB, temperature gradients between different monitoring sites (nasopharyngeal, bilateral tympanic, bladder or rectal) develop temporarily during cooling and rewarming and have to be taken into consideration [133]. During open aortic arch surgery, monitoring of nasopharyngeal and tympanic temperatures is recommended to ensure adequate brain cooling and to prevent cerebral hyperthermia and associated central nervous system injury during rewarming [83, 134, 135]. Additionally, bladder core temperature provides the best information available for the protection of the viscerals, renals, lower extremities and finally the spinal cord.
5.7 Point-of-care coagulation monitoring
Surgical as well as endovascular aortic arch repair requires reversible anticoagulation with unfractionated heparin (UFH). Although open surgery on CPB carries a substantially higher risk of major blood loss and transfusion, bleeding complications increase morbidity and mortality with either approach. Both procedural anticoagulation and postoperative haemostasis require laboratory monitoring to minimize both haemorrhagic and thrombotic complications. The whole-blood activated clotting time test is a functional point-of-care method, which is recommended to guide UFH anticoagulation, as well as its reversal with protamine and is indicated as a minimum requirement during surgical, hybrid or endovascular aortic arch repair.
Activated clotting time is not highly specific for UFH activity, however, and may be confounded by hypothermia, haemodilution, loss of platelets and loss of coagulation factors [136], all of which typically occur during aortic arch open surgery. Therefore, and in accordance with the 2017 EACTS/EACTA (European Association of Cardiothoracic Anaesthesiology) Guidelines for Patient Blood Management, when using heparin for arch surgery with prolonged CPB and HCA, the management team should consider using quantitative monitoring of circulating UFH concentrations rather than simple serial activated clotting time measurement [137].
Whole-blood viscoelastic coagulation test systems (thromboelastography rotational thromboelastometry) provide point-of-care analysis of clot generation and stability with short response times. In conjunction with treatment algorithms, they have been shown to be helpful in differential diagnosis and treatment of post-CPB bleeding [138]. Moderate-level evidence from trials of elective cardiac surgery with CPB indicates that the use of thromboelastography or rotational thromboelastometry-guided transfusion strategies may reduce exposure to allogeneic blood products [139–141] and possibly surgical re-exploration for bleeding [137, 142, 143]. In aortic arch open surgery, viscoelastic point-of-care testing should be considered, in conjunction with perioperative treatment algorithms for bleeding patients, in order to reduce allogeneic transfusion exposure and cost.
 |
 |
CSF: cerebrospinal fluid; Hb: haemoglobin; NIRS: near-infrared spectroscopy; SCI: spinal cord injury; TOE: transoesophageal echocardiography.
 |
 |
CSF: cerebrospinal fluid; Hb: haemoglobin; NIRS: near-infrared spectroscopy; SCI: spinal cord injury; TOE: transoesophageal echocardiography.
6. THERAPEUTIC OPTIONS
6.1 Open aortic arch replacement
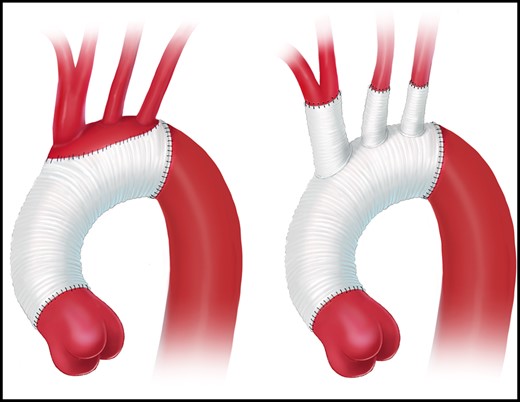
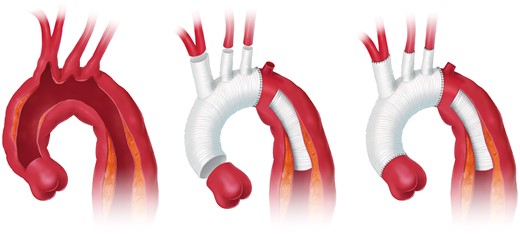
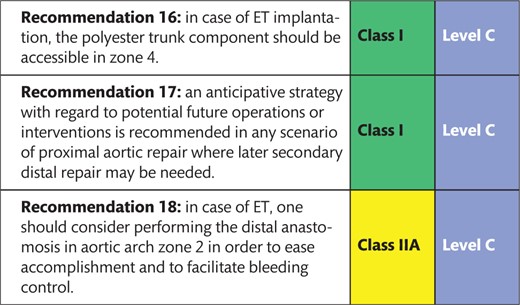
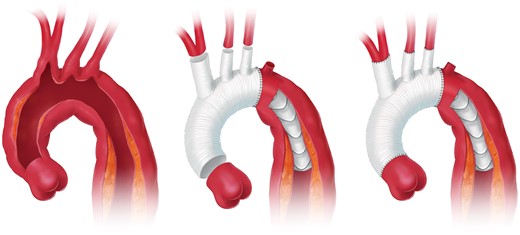
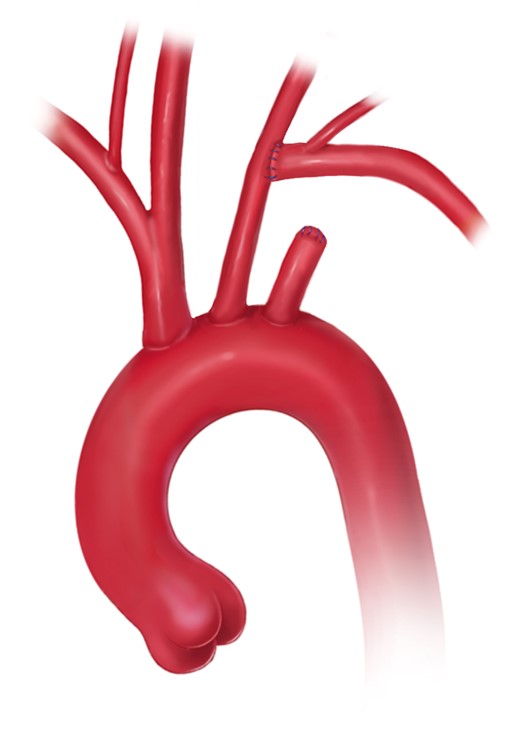
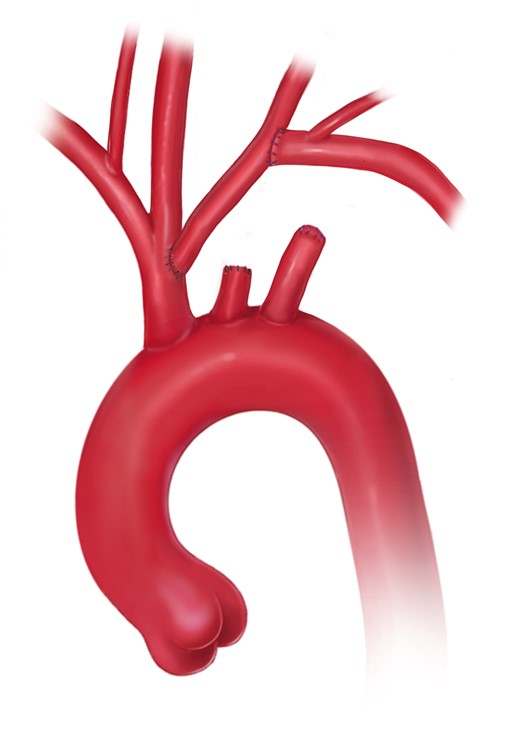
Open aortic arch replacement involving all 3 supra-aortic branches without the adjunct of either elephant trunk (ET) repair or in combination with the FET technique has become rare [147, 148] (Figs 4 and 5). The ET technique should be applied when the FET technique remains debatable. For instance, in large aneurysmal formations involving several TA segments and in very small true lumina with the risk of inducing pseudocoarctation), a FET procedure is not recommended.
Aortic arch replacement using either the island technique or the selective reimplantation technique (printed with permission from © Emily McDougall Art).
Aortic arch replacement using the elephant trunk technique with the descending anastomosis in zone 2 (printed with permission from © Campbell Medical Illustration).
The ET technique with and without sewing collar solutions is an optimal solution when secondary surgical TA replacement is anticipated. The woven polyester is an ideal fabric to be clamped and to be sewn to with a downstream aortic graft for open descending thoracic or TA replacement. On the other hand, the ET can serve as an ideal landing zone for TEVAR extension if the ET is long enough. Therefore, a sufficient length is advisable. A clip at the end of the polyester graft can simplify cannulation during fluoroscopy. Retrograde perfusion of an ET via the femoral artery is not recommended as this might push the ET into the aortic arch and potentially obstruct supra-aortic vessels. Therefore antegrade perfusion via the right subclavian/axillary artery or via a side branch is recommended. In residual dissection after type A repair, the dissection membrane is usually removed as distal as possible at least for the length of the ET so that the ET floats in the common proximal lumen.
However, it should be mentioned that the ET portion should be left as long as possible to be accessible in zone 4 in order to serve as a platform for either open surgical or endovascular extension. Regarding the level of the descending aortic anastomosis, in parallel to the FET technique, a proximalization of the anastomosis into zone 2 eases accomplishment as well as bleeding control. Additionally, the risk of left laryngeal nerve palsy is reduced. Finally, a double-layer running suture or a strip of tissue will reinforce the anastomosis and will reduce the need for correction stitches for haemostasis.
With regard to supra-aortic vessels, selective replantation has the advantage of eliminating the largest amount of native tissue, thereby potentially reducing the risk for recurrence. A variety of branched grafts is available and should be used according to experience and preference.
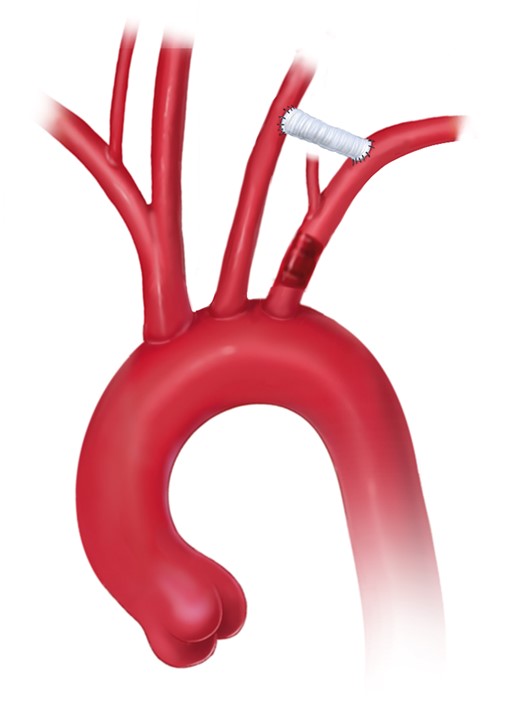
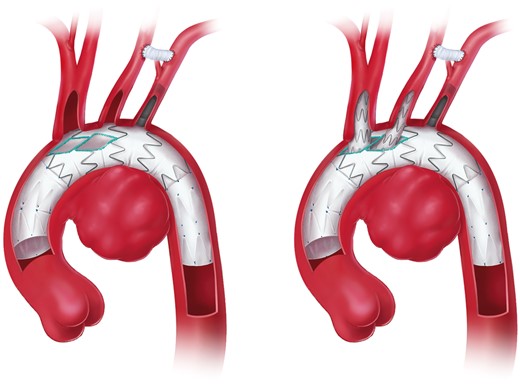
6.2 Frozen elephant trunk technique
FET combines the principles of open arch surgery and endovascular DTA repair (Fig. 6). The extension of arch replacement into the DTA by a separated stent graft was first introduced clinically by Masaaki Kato in October 1994. However, it was not until November 1996 that Kato et al. reported his experience with this technique in 10 patients [149, 150]. The technique has been used in Europe since 2001 [151, 152].
Aortic arch replacement using the frozen elephant trunk technique with the descending anastomosis in zone 2 (printed with permission from © Campbell Medical Illustration).
The technique is called FET following the development of a vascular and a stent-graft prosthesis combined into one entity [153]. Similar to the ET technique, a stent graft is introduced through the opened arch into the DTA, enabling the exclusion of distal arch pathologies in one step. The proximal part of the graft is used for conventional arch replacement. The breakthrough for the widespread application of this technique occurred in 2005 with the development of the first commercially available hybrid prosthesis, the so-called E-vita open™ [154]. The vascular graft, fabricated as a tube, is invaginated into a stent graft according to the principle of the modified ET technique [155] and the whole graft is delivered and deployed into the DTA with an endovascular introducer. The FET armamentarium is completed by a branched hybrid graft, so called Thoraflex™, which enables the reimplantation of the supra-aortic vessels separately using 3 prefabricated vascular branches [156]. A side graft allows direct cannulation for antegrade distal perfusion during the arch replacement. There are 2 other commercially available FETs: the Cronus (MicroPort, Shanghai, China) and the J graft (now Frozenix) (Japan Lifeline, Tokyo, Japan) [157, 158].
The FET is potentially indicated for all pathologies of the aortic arch, aneurysm and dissection [159–161]. Different from endovascular aortic repair, the fixation of the FET is performed by a circumferential suture, which eliminates the risk of a proximal endoleak. The endoluminal sealing of the surgical suture line by the stent graft improves haemostasis and makes FET ideal to fix the fragile aortic tissue. This combination of surgical suture and endovascular sealing enables the durable exclusion of antegrade false lumen perfusion in acute and chronic aortic dissection as well as aneurysmal cavities without excessive oversizing of the stent graft. Particularly in acute aortic dissection, a progressive false lumen thrombosis, seen in more than 90% of patients, followed by shrinkage and positive remodelling, has been reported from several studies [162, 163]. The potential exclusion of the downstream aortic pathology occurs predominantly up to the distal end of the stent graft, so that FET can be applied curatively only in association with the extension of thoraco-abdominal aortic disease in many scenarios. Patients with residual aortic pathology beyond the FET remain at risk for secondary treatment. However, shifting the treatment level with the stent graft to at least a mid-thoracic level facilitates secondary treatment by using the stent graft as a landing zone for endovascular or as a docking place for open surgical repair. In the case of an open TA repair, the capability of the stent graft to be clamped provides easier surgical access to perform the anastomosis beyond the arch with less necessity of rib resection and HCA and no risk for laryngeal nerve injury [164, 165]. However, the texture of the fabric of the endovascular/FET devices is by nature thinner and prone to fabric tears when an anastomosis is directly performed to a conventional polyester graft. Therefore, the suture should include the aortic wall as well as possible.
In the case of endovascular reintervention, the stent-graft component provides a safe landing zone for the distal extension. Thus, the FET can be used in type I and II TAAAs as a first-stage procedure when primary proximal sealing cannot be achieved adequately by endovascular means. In this case, the sizing and length of the FET should be planned considering the requirements of the second endovascular procedure in order to avoid excessive mismatch and a multicomponent secondary endovascular intervention. Generally, FET deployment beyond the transition zones 4–5 provides a safe length for additional stent-graft deployment and easier retrograde access in case of severe aortic tortuosity. However, care has to be taken in order to avoid extensive covering, which is reported to be associated with an increased risk for SCI [166, 167].
The technique of FET is similar to that of the classic ET and represents major surgery. Sophisticated cannulation and perfusion techniques have been introduced to make antegrade selective cerebral perfusion as safe as possible, to reduce lower body HCA times to a minimum and to improve organ protection in general. Considering the sealing properties of the stent graft, the proximalization of FET fixation from zone 3 to zone 2 facilitates the distal anastomosis and reduces the duration of lower body HCA as well as the risk for laryngeal nerve injury [168, 169]. Combining FET with LSA debranching minimizes the duration of arch repair and allows the perfusion of all 3 arch vessels for additional cerebral and spinal cord protection. The implementation of selective distal perfusion during arch repair using a side graft or balloon cannulas as an endoclamp within the FET reduces lower-body circulatory arrest times and thereby improves distal organ protection. In addition, selective myocardial perfusion during arch repair (‘heart beating’ concept) is used to reduce cardioplegic arrest times and to allow more extensive proximal surgical procedures [170].
To secure the FET treatment, the use of a guidewire, preferably via the FA under angiographic or echocardiographic control, may be of help. In aortic dissections, the wire secures FET deployment within the true lumen. In aneurysms, it facilitates the guidance of the FET over the thrombus formation and aortic tortuosity, thereby avoiding debris mobilization and distal embolization. Angioscopy represents an additional intraoperative tool in visualizing the landing zone and endoluminal obstacles and in controlling the deployment downstream [171]. Fluoroscopy during FET introduction is usually not needed ‘but can be helpful’.
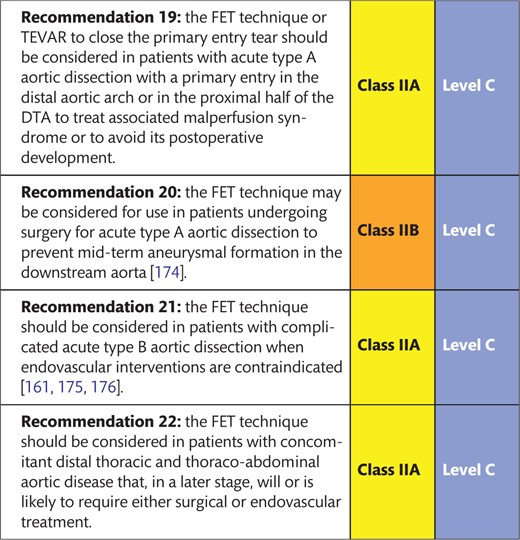
Usage of FET in acute and chronic aortic dissection with completely dependent visceral and renal artery perfusion from false lumen is possible but should be critically evaluated in advance. In these scenarios, preoperative verification of patent communications between lumina is recommended to avoid malperfusion. In connective tissue disease, the use of stent grafts is controversial and basically discouraged; in any case, avoidance of oversizing is recommended. In DTA rupture, a safe distal landing zone for definitive sealing is a prerequisite for FET treatment. The TEVAR component of the FET prosthesis cannot be equally interpreted as a ‘TEVAR-alone’ approach in patients with connective tissue disease because the remaining risk of a distal stent-graft-induced new entry is different in clinical weight and need for correction from a proximal stent-graft-induced new entry or, in other words, a retrograde type A aortic dissection [172, 173]. Recently, EACTS has formulated recommendations for use of the FET technique [174].
 |
 |
DTA: descending thoracic aorta; FET: frozen elephant trunk; TEVAR: thoracic endovascular aortic repair.
 |
 |
DTA: descending thoracic aorta; FET: frozen elephant trunk; TEVAR: thoracic endovascular aortic repair.
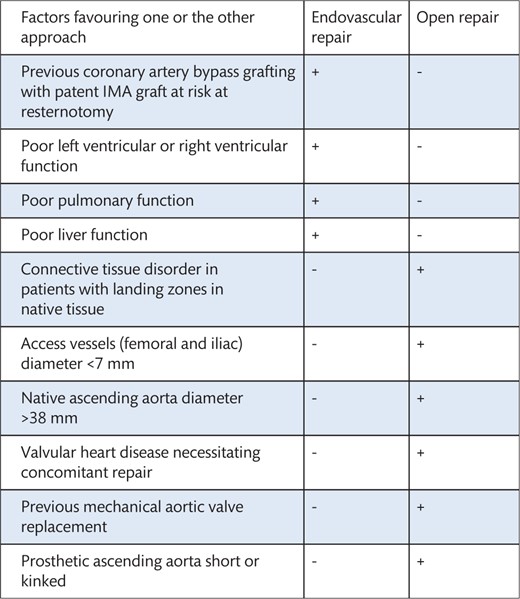
6.3 Transposition (debranching) of supra-aortic vessels and thoracic endovascular aortic repair and the importance of the subclavian arteries in maintaining the blood supply to the spinal cord
Hybrid arch repair (or combined vascular and endovascular treatment) is a combination of both open and endovascular procedures designed to treat aortic arch disease. The core principle behind this treatment relies on endovascular exclusion of the pathology following the creation of an adequate proximal landing zone (in zones 0, 1 and 2) [1] by means of supra-aortic transposition (debranching) of 1 (LSA), 2 (and LCCA) or 3 (and IA, i.e. total aortic arch debranching) arch vessels (Figs 7–9).
Subclavian-to-carotid transposition (printed with permission from © Emily McDougall Art).
Subclavian-to-carotid bypass and Amplatzer plug insertion in the proximal left subclavian artery (printed with permission from © Emily McDougall Art).
Autologous double transposition of the supra-aortic branches (printed with permission from © Emily McDougall Art).
Debranching options are multiple and can be performed by means of anatomical or extra-anatomical revascularization, with extrathoracic or intrathoracic approaches. The techniques presented in the literature are pleiomorphic: from aortic patch reimplantation to branched or simple grafts interposition and autologous transposition [177]. Open and endovascular procedures can be performed simultaneously or with a staged approach (open debranching first and endovascular exclusion as a second stage) according to need and preference [178, 179]. TEVAR in the aortic arch should be performed preferrably with a fixed imaging system.
The main potential advantage of the hybrid approach is the avoidance of aortic cross-clamping, HCA and CPB with the potential risk reduction in higher risk patients with proximal thoracic aortic pathology (zone 0 proximal neck). For patients at a higher risk of stroke, open aortic arch surgery remains the best therapeutic option because extensive manipulation during debranching as well as during TEVAR might cause embolization [178, 180, 181]. Patients presenting with distal arch pathology (zones 1 and 2 proximal neck) should be considered for an endovascular approach with prior LSA and/or LCCA revascularization, if anatomically suitable.
The devices employed for aneurysm exclusion are commercially available stent grafts, mostly designed for the treatment of DTA pathology. The instructions for the use of these devices require deployment in a proximal and a distal landing zone (native aorta or pre-existing graft) with a length ≥25 mm, measured on the inner curvature, and a diameter <38 mm, measured according to the manufacturer's recommendations (inner/inner versus outer/outer diameter) [182]. Application of such devices in patients affected by connective tissue disease is contraindicated unless both landing zones are within a previous surgical/endovascular graft [183]. Moreover, at least 1 adequate (>7 mm) access vessel is required for successful stent-graft insertion, and the aortic lumen characteristics should be taken into consideration to decrease the risk of embolization during advancement of the device in the aortic arch (e.g. shaggy aortas, floating thrombi, severe calcifications) [184]. Possible limitations of the hybrid approach are the lack of an inflow vessel for debranching (i.e. calcific/aneurysmatic ascending aorta), and the presence of landing zones of unsuitable length/diameter or narrow access vessels, inadequate for stent-graft introduction. Open repair should be considered in these cases as well as in cases at high risk of retrograde dissection (ascending aorta >38 mm, bicuspid aortic valve, arch abnormalities, lost sinotubular junction, and extended ascending aortic length).
Furthermore, the hybrid approach carries a risk of SCI due to the covered length of the DTA. For this reason, CSF drainage should be employed in patients with increased risk (e.g. previous aortic surgery, occluded hypogastric/subclavian arteries) [185–187]. Also in hybrid procedures, the current literature supports centralization in centres with adequate volume and expertise [7].
6.3.1 Importance of the left subclavian artery in supplying blood to the spinal cord
The main reason for prophylactic LSA revascularization prior to TEVAR is maintaining posterior cerebellar perfusion as well as maintaining upper inflow into the anterior spinal artery and thereby the spinal cord. There is convincing evidence that the combination of LSA occlusion and extensive coverage of thoracic segmental arteries by TEVAR is associated with an increased risk of SCI, which is significantly lower when the LSA is preserved. This becomes clear when the collateral network concept and consecutively the 4-territory concept are conceptually applied [130, 131, 188–190].
6.4 Total endovascular aortic arch repair
The development of new endovascular techniques to treat aortic arch aneurysms has mitigated the risks associated with open surgery and offers repair to patients who historically could not have undergone open repair. In the early years, because patients receiving external branch endografts experienced high rates of strokes, those endografts were not adopted in the global market [192–195]. The subsequent development in recent years of arch endografts with specific delivery systems, preloaded fenestrations and inner branches has improved results to a level that endovascular arch repair has today become a viable option for patients with increased risk for open repair (Fig. 10).
Total endovascular aortic arch repair using the double branch technique (printed with permission from © Campbell Medical Illustration).
In contrast to more stable segments of the aorta, where endovascular treatment has become the standard of care, the ascending aorta is characterized by high velocity and consequent shear stresses, 4-dimensional pulsatile and rotational movements during the cardiac and respiratory cycles and the proximity of the coronary ostia and aortic valve. Endovascular arch repair requires a stable proximal landing zone within a surgical graft or native ascending aorta with a diameter of 38 mm or less. Larger diameters are prone to retrograde dissection and thus should be avoided [196]. The proximal sealing zone should preferably have a length of 30 mm or more measured at the inner curvature that is free of excess calcification and thrombus and an angulation >60°.
Stroke remains a major concern during endovascular arch repair, with rates between 0 and 14% [197–199]. The mechanism of stroke includes solid emboli released by manipulation in the arch, air emboli released from the delivery system and coverage of the target vessels [193]. To minimize the risk of stroke, temporary carotid artery occlusion, filter placement and carbon dioxide flushing of the delivery system have been proposed [193, 194, 200].
For endovascular aortic arch repair, there are 2 general graft designs: fenestrated and branched arch endografts. Both designs are currently available as custom-made devices only, so a manufacturing time of between 4 and 8 weeks precludes their use in urgent and emergency situations. The current 2 inner branches design may, however, become a future platform of an ‘off-the-shelf’ branched stent graft that will be used in emergency situations.
Fenestrated arch endografts can incorporate multiple fenestrations or a combination of fenestrations and scallops. Graft apposition to the aortic wall at the level of the fenestrations is required for endovascular seal. The sealing zone therefore is usually in the mid-arch at the level of the branch vessels. Due to the distance from the femoral access vessels and the curvature of the arch, rotation of the fenestrated graft cannot be controlled, so precision of placement relies on meticulous preoperative planning and the use of precurved delivery systems and preloaded catheters that allow wires to be passed via these catheters and snared from the upper extremity access.
The largest cohort of fenestrated arch repair from Japan used a precurved fenestrated stent graft (Najuta graft) without preloaded wires in 363 patients with a landing zone in the ascending aorta and reported a 1.6% 30-day mortality and 1.8% stroke rate [201]. This system does not use bridging stents to fixate the fenestrations at the target-vessel ostia. The Zenith fenestrated arch endograft (Cook Medical, Brisbane, Australia) uses a preloaded wire system combining usually a fenestration and a scallop using a covered bridging stent to fixate the fenestration to the left LCCA or LSA as the target vessel. Small published series representing early experience have reported mortality up to 20% and stroke rate up to 14% [192, 202]. The Relay scalloped endograft (Terumo Aortic) does not include fenestrations, preloaded catheters or the use of covered stents for the target-vessels and is mainly used for zones 2 and 3. In a single reported small series, the mortality rate was 5% and the stroke rate 14% [197]. Stroke remains a major concern in any kind of open or endovascular aortic arch treatment strategy and can be seen as the major important challenge to address in the years to come.
Branched arch endografts currently include antegrade or retrograde internal side branches along the outer curve of the stent graft. The 2 currently available platforms in Europe aiming at a seal in the ascending aorta both use 2 antegrade inner branches to be connected to the IA and to the LCCA while the LSA is usually debranched in a staged procedure [193]. This design requires less precision in placement compared to fenestrated arch endografts because the distance between the branch openings and the target vessels allows for continued perfusion of the supra-aortic vessels after deployment of the main graft and a simplified catheterization of the inner branches.
The Zenith branched arch endograft (Cook Medical) includes a staged proximal release mechanism. Early experience has been reported with no 30-day death and a stroke rate of 11% [199]. The Relay branched arch endograft (Bolton Medical, Barcelona, Spain) is built using 2 parallel inner branches on the Relay NBS platform using a proximal tip-capture. Early experience in a small series collecting global experience showed a 7% mortality rate and a 7% disabling stroke rate [203].
Branched endovascular arch repair is today increasingly used in patients after previous open ascending repair for type A aortic dissection. The presence of a prosthetic graft in the ascending aorta acts as a favourable proximal landing zone for an arch endograft, excluding the risk of retrograde dissection, and >70% of patients have a proximal landing zone in the previous ascending aortic graft suitable for branched endovascular arch repair [204].
The TAG® single sidebranch endograft (Gore® Medical, Flagstaff, USA) and the Valiant™ Mona LSA single sidebranch stent graft (Medtronic Inc., Santa Rosa, CA, USA) are arch endografts with a single side branch designed to preserve the LSA in zone 2 TEVAR [205]. Both endografts are currently used in limited numbers of centres with no clinical data published so far.
At the present time, with careful patient selection and operator experience, early use of this technology presents an alternative to open aortic arch repair or conservative therapy, respectively.
6.5 Alternative approaches
Alternative approaches to the treatment of aortic arch pathologies are endovascular techniques that use the chimney graft, the periscope and sandwich technique [summarized as parallel grafts (PGs)] and in situ fenestration. PGs are bare or covered stents deployed in 1 or more supra-aortic vessels parallel to the main aortic arch stent graft. This allows the extension of the sealing zone of the aortic stent graft beyond the origin of the respective supra-aortic vessel. One of the first reported PGs in the literature was used in 2003 [206] in a patient undergoing endovascular aortic repair to secure a renal artery with a very short proximal landing zone. The first PG used in aortic arch treatment was reported 2 years later [207]. There are several modifications of the PG technology. The standard PG is proximally oriented and allows antegrade flow up to an aortic branch. The periscope PG is distally oriented and blood flow is retrograde. The sandwich technique includes an aortic stent graft deployed first as an artificial landing zone to implant the PGs. After PG implantation, another aortic stent graft is deployed to exclude the entire pathology. The PGs are located between both aortic stent grafts. Furthermore, PGs can be used only to compress the graft edge to secure the flow into the vessel where the PG was implanted. PGs are used as a bailout when target vessels are incidentally covered to allow very aggressive stent-graft placement in case of short landing zones 2 and 3.
There are several advantages of PG techniques compared with fenestrated or branched stent grafts. First, PGs are available off the shelf. Fenestrated and branched stent grafts are mostly customized and the time to manufacture them is 1 to 3 months. They are clearly not an option in patients requiring emergent or urgent aortic arch repair. Second, PGs are less expensive than fenestrated and branched stent grafts. Furthermore, there is a large experience of visceral CGs available in the literature with acceptable results, especially in patients requiring urgent aortic repair [208]. However, articles on supra-aortic PGs are scarce. The results of visceral PGs are probably not representative of the expected results of supra-aortic PGs.
PG techniques carry a risk of endoleak type I due to the so-called gutters, which are channels between the PG and the main aortic stent graft. Those gutters are per definition inevitable; however, not all of them lead to endoleaks detectable in CTA. Even thrombosed lesions can still remain under pressure, if there are gaps in the sealing zone. It may lead to endotension, which is defined as pressure within the aneurysm sac without evidence of endoleak as the cause. Endotension raises the risk of aneurysm rupture [209]. Gutters caused by PGs are specifically relevant if pathology at the outer curvature is treated like most cases of type B aortic dissection, where the gutters may cause a type 1A endoleak. If pathologies affecting the inner curvature are treated, gutters caused by PGs on the outer curvature are less prone to cause a type 1A endoleak.
Furthermore, stent grafts were not designed for the PG approach. The radial force, elasticity, shape and even length of currently used stent grafts in PG cases are not optimal. There are no covered stent grafts dedicated for PG techniques. Additionally, numerical studies suggest worse haemodynamic performances in PG models compared with surgical or hybrid arch repair models [210]. Finally, to avoid a type 1 endoleak, aortic stent-graft oversizing is necessary and, for larger aortas, aortic stent grafts with appropriate diameters are not available [211].
There are several reports of PG in the treatment of the aortic arch or a proximal DTA. The largest multicentre series include up to 95 patients [212]. The 30-day mortality rate ranges between 0 and 29% (including elective and emergency cases) [213–215]. The overall early patency rate of PGs ranges between 92% and 100% [178–180]. Early endoleak type I was reported in a meta-analysis of 314 cases at the level of 11% (range 0–44%) [216]. Forty-five percent of early endoleaks in this report sealed spontaneously. The follow-up in currently available reports ranges between 1 and 30 months [212–216]. There are no long-term follow-up data for these patients. The number of re-interventions is provided in most reports; however in the vast majority of reports there are no data on the numbers of patients who require aortic arch repair due to the failure of PGs. Finally and most importantly, in the vast majority of reports, there are no data on sac dynamics at follow-up.
PG technology is a useful treatment option in patients requiring emergent or urgent aortic arch repair. PGs can also be used as a bailout technique in case of accidental covering of a supra-aortic vessel. PGs should be avoided in elective cases with anatomy suitable for branched or fenestrated devices or open surgery until more data of better quality become available.
In situ fenestration of standard stent grafts is another option to extend the proximal landing zone by covering the supra-aortic branches and performing a fenestration via a retrograde access in vivo [217, 218]. Graft perforations can be performed by laser or mechanical means. This technique is new, and long-term data in humans are missing. Currently in situ fenestration is an off-label procedure that can be used only as an emergent bailout technique or in the setting of investigational studies. Recent work demonstrates that both laser and mechanical in situ fenestrations cause substantial damage to all available stent-graft fabrics [219].
The multilayer (or flow modulator) technique has recently been advocated for the treatment of various thoracic and abdominal aortic pathology including the aortic arch. The principle of the technique is formed by a self-expanding multilayered stent constructed of cobalt alloy wires interconnected in 5 layers. Thereby, blood flow through the stent is laminated, thereby reducing turbulence in the aneurysmal sac, which leads to sac thrombosis. The evidence regarding the mechanisms and efficacy currently remains conflicted [220–224].
7. TEN POINTS DESCRIBING WHEN TO CHOOSE WHAT KIND of APPROACH
8. RARE PATHOLOGIES
8.1 Thrombus
An aortic thrombus is a rare entity [225]. The aortic arch and the DTA have been recognized as predilection sites for aortic thrombi [226]. An aortic arch thrombus bears the risk of life-threatening stroke and peripheral embolization [227]. The morphological form of the thrombus should be taken into consideration by distinguishing a mobile (i.e. floating, bulging into the lumen) from a stationary (mural lining) thrombus. Symptomatic patients (i.e. ischaemia due to embolization: stroke, limb ischaemia, visceral or renal ischaemia) often require urgent treatment. In asymptomatic patients the diagnosis is mostly a chance finding in imaging studies performed for other reasons. There is a high prevalence of hypercoagulation and a haematologic disorders including malignancy in patients with an aortic thrombus [226]. These conditions have to be considered when establishing individual treatment strategies. Other possible sources of embolization have to be ruled out preoperatively in symptomatic patients. Treatment options include conservative management (anticoagulation) and surgery (thrombectomy, local resection of the attachment site, aortic arch replacement or debranching and thoracic endovascular repair). However, endovascular treatment requires an adequate landing zone in the ascending and DTA. Furthermore, guidewire and stent-graft manipulation in the thrombotic aortic arch bear an additional risk for embolization. A hybrid approach with supra-aortic debranching and antegrade stent-graft implantation has been reported [228]. A recently published case series reported excellent outcome with regard to survival and freedom from recurrence of thrombus formation with surgical thrombectomy [227]. The value of minimally invasive approaches including transarterial balloon thrombectomy or catheter-based percutaneous thrombus aspiration remains unclear. Follow-up imaging is recommended in patients under conservative treatment to assess for thrombus dissolution.
8.2 Aberrant subclavian artery and Kommerell’s diverticulum
The prevalence of aberrant subclavian artery and Kommerell’s diverticulum is 0.4–2.3% [229]. Anatomically, the aberrant subclavian artery passes in 80% posterior to the oesophagus, in 15% between the oesophagus and the trachea, and in 5% anterior to the trachea [229]. Symptomatic patients suffering from dysphagia, dyspnoea, coughing, chest pain, aspiration, or recurrent pulmonary infection represent only 5%. Asymptomatic patients can be managed conservatively. Aneurysmatic aberrant subclavian arteries ≥3 cm in diameter and Kommerell’s diverticula with a diameter ≥5.5 should be considered for repair due to their risk of rupture and dissection. But, actual size measurement of the Kommerell’s diverticulum is highly controversial with no clear consensus. Tanaka et al. [230] recommend measuring Kommerell’s diverticulum from the wall next to the trachea to the opposite aortic wall or from the tip of the diverticulum to the opposite aortic wall. Additionally, they measure the subclavian artery diameter at its orifice. Operative treatment modalities include resection and ligation of the symptomatic or aneurysmatic aberrant subclavian artery to release compression (important in symptomatic patients) and subclavian-carotid transposition or bypass to re-establish arterial circulation to the right arm. Resection of the offspring of the aberrant subclavian artery is not necessary in asymptomatic patients. Kommerell’s diverticulum can be treated by stent-graft implantation or DTA replacement. TEVAR might be challenging due to steep arches, which are often present in these patients.
8.3 Trauma
Aortic injury is highly lethal, representing the second most common cause of death in blunt trauma after brain injury. A lesion at the aortic isthmus in loco typico is present in up to 90% of deceleration trauma patients admitted to hospital alive. An autopsy study of 242 fatal blunt aortic injuries showed that isthmus lesions represented 58% and aortic arch lesions were rare (3%) [231]. Iatrogenic lesions associated with catheter manipulation in the arch is another possible source of trauma. Timing of repair conforms to the extent of the lesion. Classification of traumatic aortic injury according to Azizzadeh et al. [232] includes 4 grades of lesions: I intimal flap, II intramural haematoma, III pseudoaneurysm and IV rupture. Whereas grade I and II lesions permit conservative management with serial imaging controls, grades III–IV should be repaired. Operative treatment modalities include a hybrid approach with supra-aortic debranching and stent-graft implantation or aortic arch replacement. Endovascular management is preferred when feasible. Timing, type and extent of treatment also strongly depend on concomitant injuries (e.g. traumatic brain injury).
8.4 Infection
Infection of the native aorta or, more often, of an aortic graft encompasses considerable morbidity and mortality. For diagnostic purposes a positron emission tomography (PET) scan may add value to differentiate general inflammation (e.g. postoperatively) from infection. However, metabolic activity on PET-CT is only a minor criterium. The Management of Aortic Graft Infection Collaboration criteria offer support in the diagnosis of aortic graft infection [233]. Summarizing, the diagnosis of native aortic or prosthetic aortic infection includes clinical/surgical, radiological and laboratory data [233].
Operative treatment modalities include removal of the infected material, local debridement and in situ aortic reconstruction. Conservative treatment may be considered in selected cases [234]. TEVAR as emergency therapy despite suspected aortic infection is feasible and may well serve as a definite treatment option in selected cases [235].
Specific antibiotic and antimycotic treatment according to microbiological analyses has to be established for all patients. The appropriate type of material for aortic reconstruction is under discussion: prosthetic (plain, antibiotics or silver coated) or biological (homograft, autologous veins, xenopericardial material) grafts are available. The required treatment urgency has an influence on preoperative diagnostic features (imaging and microbiological sampling) and the availability of the specific replacement material. Xenopericardial material (self-made tube grafts) due to permanent off-the-shelf availability, ease of handling and good clinical results is favoured [236, 237]. In addition, antibiotic therapy may be withdrawn in many cases during follow-up, which is the exception in patients after alloplastic replacement.
9. AORTITIS OF THE AORTIC ARCH
Immune-mediated vasculitis represents a frequent and possibly organ- or life-threatening condition.
Large-vessel vasculitis is the most frequent cause of vasculitis. It is encountered mostly either in young women, known as Takayasu’s arteritis, or in people over the age of 50 years, known as giant cell arteritis (GCA) [238]. Both entities share the possible involvement of the aortic arch, which is usually detected late. The risk of ongoing inflammation leads to stenosis and dilatation, finally resulting in the risk of aortic rupture.
9.1 Giant cell arteritis
GCA might present with a sudden onset of temporal headache, malaise with signs of a systemic inflammatory response syndrome of unknown origin, weakness of the shoulder and hip girdle and weight loss. GCA was formerly known as Horton’s disease and represented a segmental vasculitic involvement of the temporal arteries. Novel diagnostic methods have allowed us to broaden our understanding of the disease. Meanwhile, CTA as well as MRA and/or PET-CT enable detection of additional vasculitic involvement of the aorta that is mainly located in the region of the aortic arch and the DTA and, in part, in the abdominal aortic or iliac sections.
9.2 Diagnostic approach
Further diagnostic evaluation of possible aortic involvement is reasonable in order not to miss concomitant large vessel vasculitis [239]. Glucocorticoid treatment should be withheld until after the procedure if medically justifiable: 3–5 days after start of glucocorticoid treatment, vessel wall signals mostly disappear, resulting in negative results despite underlying inflammation [239, 240].
9.3 Therapy
In case of temporal arteritis immediate initiation of therapy is warranted for fear of further vasculitic involvement of the vasculature supplying the optic nerve, with a possibly rapid onset of mostly irreversible blindness. In case of additional or isolated large vessel vasculitis, rapid reduction of vessel wall inflammation is supposed to reduce further sequelae. GCs still represent the mainstay of therapy. Current investigations demonstrated interleukin-6 as being mainly involved in orchestrating disease onset as well as the course of disease: meanwhile, therapeutic strategies targeting interleukin-6 and its specific receptor have proven beneficial in inducing and maintaining remission [241, 242].
9.4 Complications and outlook
Rupture of the aorta and/or its associated branches appears to represent a rare complication, yet true incidences are difficult to depict because e.g. ‘silent’ GCA is not routinely followed clinically and/or radiographically. Furthermore, the former routine for histological evaluation of the resected vasculature in order to prove immunologically driven inflammation has unfortunately lost importance. Follow-up of patients focusses on clinical and serological signs of relapse and/or remission. For the time being, radiographic diagnostics are not reliable for determining ongoing or recurrent vascular inflammation: despite clinical and serological remission persistent MRA signals within the vessel wall might represent either persistent low disease activity or formation of new vasculature or even display some kind of vascular repair. Nevertheless, MRA and/or PET-CT might prove useful in the early detection of vascular damage and should therefore be performed repeatedly.
9.5 Takayasu’s arteritis
Takayasu’s arteritis manifestations are rarely suspicious of an underlying immune-mediated vascular process but rather point to vascular damage after the development of stenosis. Because mostly supra-aortic branches of the aortic arch are affected, patients often present with pulselessness of the upper extremities, arm claudication, dizziness or suspicion of cerebral ischaemia.
9.6 Diagnostic approach
The diagnostic procedure comprises the same imaging methods as in GCA with US being relevant for supra-aortic branches, and CTA, MRA and PET-CT being reserved for screening for remaining aortic involvement. Active lesions are probably being detected in phases of serological inflammation.
9.7 Therapeutic approach
As in GCA, initial therapy comprises the use of GCs aiming at induction of remission. Therapeutic strategies for maintenance of remission are not well characterized for this rare disease. Nonspecific immunosuppression targeting the involved lymphocytic subgroups by using e.g. azathioprine or methotrexate has empirically proven beneficial. Therapeutic strategies aiming at tumour necrosis factor-alpha and anti-interleukin-6 yield positive results and suggest further adaptations for future therapies [243, 244].
9.8 Complications
Vascular reconstruction might be indicated in the late phase of the disease when symptomatic stenoses occur leading to reduced perfusion in the connected arterial segment. Vascular interventions such as dilatations appear of only little benefit due to the inflammatory nature of the disease, with prompt re-stenoses occurring quite frequently. Stenting and/or vascular repair has proven more beneficial.
9.9 Conclusion
Overall, suspicion of large-vessel vasculitis due to autoimmune pathophysiology should be considered in either young women presenting with mostly late complications of upper extremity claudication and supra-aortic malperfusion (Takayasu’s arteritis) and in people older than 50 years with sudden onset of an inflammatory syndrome of unknown origin, temporal headache and constitutional symptoms (GCA). Prompt diagnosis and therapy especially in GCA will help to minimize the initial risk of permanent loss of vision and to reduce the occurrence of long-term vascular complications.
10. DURABILITY AND REPORTING STANDARDS AND QUALITY INDICATORS
Although reporting standards in classical adult cardiac or vascular surgery have widely been established, there is work to do in the aortic sector in particular when it comes to treatment of the aortic arch. As long as no preoperative risk stratification score for aortic disease has been established, currently available risk score systems like STS PROM [67] or EuroSCORE I and II [68] may help in predicting risk with their known limitations when applied to patients with aortic disease. However, the main advantage when using them in their current form is the potential comparability between studies where currently there is no least common denominator available.
The results of endovascular repair should be reported according to current Society of Vascular Surgery guidelines that consider both technical and clinical end points in order to evaluate the performance of the devices combined with the clinical outcomes of their application. Clinical outcomes for aortic arch treatment should clearly include 30-day mortality rates as well as neurological outcomes (stroke and spinal cord ischaemia). Moreover, the completeness of follow-up information is of paramount importance and cannot be overemphasized [245]. Neurological outcomes should be reported according to current recommendations [246]. Currently, there is no robust evidence to recommend minimum caseloads for aortic arch procedures, both open surgery and endovascular repair, either for centres or for individual physicians but a clear volume-outcome correlation like in many other cardiovascular procedures supports centralization and specialization [16, 87, 247].
11. GAPS IN EVIDENCE
The supportive evidence level in the foregoing for the management of aortic arch diseases is mostly ‘C’, for several reasons. The patient population requiring aortic arch procedures is small compared to other cardiovascular patient populations, although growing. The caseload is low in many centres, and published series tend to be small in numbers. Also, there is much heterogeneity in presentations, patients and treatment approaches. In particular, therapies for aortic arch pathologies are driven by rapid innovations in technology as well as by institutional preference. Therefore it is very clear that close international scientific and clinical collaboration is required to solve these issues.
The following unmet needs and gaps in evidence are identified, as topics of future clinical research in the field:
An increase of evidence in the pathophysiology and in the prevention of perioperative stroke
An increase of evidence in selecting the best treatment option in patients with acute and chronic aortic arch disease
A need for further international standardization of terminology
A need for standardized surveillance and for follow-up after treatment
A need to develop prospectively maintained, large multicentric clinical databases for aortic arch pathologies.
In recognition of the shortcomings of current cardiovascular surgical risk scoring systems in this field [248], as an initiative, the STS Task Force on Aortic Surgery has already developed new sections pertaining to aortic root and thoracic aortic surgery to reflect technical advances in open and endovascular aortic procedures [69]. An exemplary set of pertinent variables is given in the STS Aorta Surgery Worksheet V2.9 [249]:
Data-driven development and continuous adaptation of dedicated CPM for aortic arch repair
Accrual of more evidence on effects of caseload and centralization of care on outcome of aortic arch repair
A need to address frailty [250–252] and gender differences in outcome research
A need to define differences in the risk of acute dissection among genetically mediated aortic disease syndromes
In type A dissection, to better define the extent of index surgery
Improving the evidence for measures to reduce lower body circulatory arrest time and for selective myocardial perfusion during open aortic arch repair
To resolve the controversy over the use of stent grafts in connective tissue disease
ACKNOWLEDGEMENTS
We thank Tilo Kölbel for his contribution to sections 6.4 Therapeutic options: Total endovascular aortic arch repair and 6.5 Therapeutic options: Alternative approaches. We thank Tim Berger and Jeannie Wurz for their extensive support in editing this paper.
Conflict of interest: Luca Bertoglio: Consultant for Cook Medical, travel grants, speaking fees from Cook Medical, Gore, Jotec, Medtronic and Cordis. Roberto Chiesa: Travel grants, speaking fees from Cook Medical, Gore, Jotec, Medtronic and Cordis. Rachel E. Clough: Grants from Cook Medical outside of this work. Martin Czerny: Consultant for Terumo Aortic, Medtronic and Cryolife. Balthasar Eberle: Speaking fees from Medtronic. Stephan Haulon: Consultant for Cook Medical, GE Healthcare and Medtronic. Heinz Jakob: Lecture fee and royalties from Cryolife and Jotec. Carlos A Mestres: reports “other” from Edwards outside of this work. Timothy Resch: Consultant for Cook Medical. Bartosz Rylski: Consultant for Terumo Aortic. Jürg Schmidli: Shareholder of LeMaitre. Malakh Shrestha: Consultant for Vascutek. Konstantinos Tsagakis: Consultant for Jotec and W.L. Gore Associates. The other authors have nothing to disclose in relation to this work.
REFERENCES
www.sts.org/sites/default/files/documents/ACSD_AorticWorksheet_Final080817.pdf (20 May 2004, date last accessed).
Author notes
Representing the European Association for Cardio-Thoracic Surgery (EACTS).
Representing the European Society for Vascular Surgery (ESVS).