-
PDF
- Split View
-
Views
-
Cite
Cite
Shinsuke Uchida, Yukihiro Yoshida, Yuichiro Ohe, Yuko Nakayama, Noriko Motoi, Aki Kobayashi, Keisuke Asakura, Kazuo Nakagawa, Shun-ichi Watanabe, Trimodality therapy for superior sulcus tumour: experience of a single institution over 19 years, European Journal of Cardio-Thoracic Surgery, Volume 56, Issue 1, July 2019, Pages 167–173, https://doi.org/10.1093/ejcts/ezy480
Close - Share Icon Share
Abstract
Induction chemoradiotherapy followed by surgery is the standard treatment for superior sulcus tumours (SSTs). However, the protocols, chemotherapy agents and cycles used as well as the mode and intensity of radiotherapy vary between institutions. Thus, the objective of the study was to investigate the effects of trimodality therapy on the outcomes of patients with SSTs.
Sixty patients with SSTs were enrolled between January 1999 and December 2017. Induction therapy consisted primarily of 2 cycles of mitomycin–vindesine–cisplatin or cisplatin–vinorelbine delivered concurrently to the tumour with 40–45 Gy of radiation. Surgery was performed 2–6 weeks after completion of induction therapy.
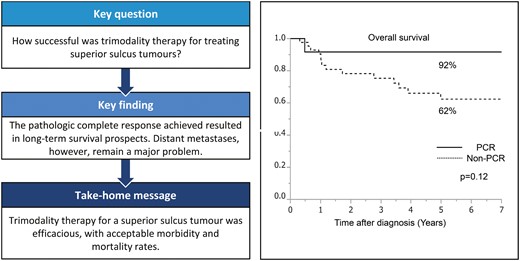
Fifty-four (90%) patients underwent radical surgical resection. Complete pathological resection was achieved in 44 patients (81%). There was no 30-day mortality. After a median follow-up of 57.0 months, 19 (35%) patients experienced recurrence, and 8 (15%) patients showed brain metastasis. A pathological complete response (PCR) was observed in 12 (22%) patients. The 5-year survival rate for the entire population (n = 54) was 69% (95% confidence interval 55–81%). The survival rate was better for patients who underwent complete resection than for those who underwent incomplete resection (73% vs 51%, P = 0.46). A better survival rate was evident in patients with PCR than in those without PCR (92% vs 62%, P = 0.12).
Trimodality therapy for SSTs was efficacious and associated with favourable outcomes, with acceptable morbidity and mortality. PCR in patients with resected SSTs reveals promising long-term survival prospects with the trimodality therapy.
INTRODUCTION
Superior sulcus tumours (SSTs) are a small subset of non-small-cell lung cancer (NSCLC). Initially, SSTs were considered incurable. However, surgical resection could be facilitated after preoperative radiation therapy, with a 5-year survival rate of 30% [1]. The Southwestern Oncology Group (SWOG)/North American Intergroup reported a prospective, multi-institutional, phase II trial (SWOG 9416/Intergroup Trial 0160) and the Japan Clinical Oncology Group (JCOG) reported the 9806 trial with an improved 5-year overall survival (OS) for those undergoing chemoradiotherapy (CRT) followed by R0 resection compared with the earlier results reported in trials using radiation alone followed by surgery [2, 3]. Based on these data, induction CRT followed by surgery was adopted as the standard of care [4]. However, the induction CRT protocols, chemotherapy agents and cycles used, as well as the mode and intensity of radiotherapy, vary between institutions. Accordingly, the OS rates are different between institutions [2, 3, 5–15]. Thus, the aim of the current study was to investigate the effects of trimodality therapy on the short- and long-term outcomes of patients with SSTs at a single institution over 19 years.
MATERIALS AND METHODS
Eligibility
This study was approved by the National Cancer Center Review Board (Research number 2018-045). Among 7011 patients who underwent lung resection for primary lung cancers at the National Cancer Center Hospital between January 1999 and December 2017, 60 patients (0.8%) with SSTs were enrolled in this study. An SST was defined as an NSCLC located on the apex of the lung, with radiographic invasion of the first rib or structures of the apical chest wall. We retrospectively searched the prospective database of our department to collect data including clinical, surgical and pathological findings.
Clinical stage evaluation
The clinical extent of SST invasion was evaluated by computed tomography (CT) scan and/or magnetic resonance imaging (MRI) scan of the neck and chest. Positron emission tomography/CT was used to review systemic metastasis from 2006 to 2017. Before 2006, systemic metastatic status was evaluated by CT or MRI. Eligible patients had histologically confirmed resectable or marginally resectable NSCLC of the superior sulcus. Endobronchial ultrasonography or mediastinoscopy was performed for mediastinal lymph nodes, which were suspected to be nodal positive. Patients with clinical N2 disease were excluded from the induction treatment. However, those with ipsilateral supraclavicular nodal involvement (N3) were eligible based on the JCOG 9806 protocol, unless the N3 was accompanied by mediastinal nodal metastasis. All patients were evaluated for eligibility by multidisciplinary teams consisting of a radiation oncologist, medical oncologist and thoracic surgeon.
Induction chemotherapy
Chemotherapy schemes changed over the years. From 1999 to 2008, the patients received 2 cycles of mitomycin–vindesine– cisplatin (MVP) combination chemotherapy with a 4-week interval in between. Cisplatin at 80 mg/m2 and mitomycin at 8 mg/m2 were administered on chemotherapy day 1, and vindesine was administered at 3 mg/m2 on days 1 and 8. From 2009 to 2017, the patients were concurrently administered chemotherapy comprising 2 cycles of intravenous injection of cisplatin at 80 mg/m2 and vinorelbine at 20 mg/m2 on day 1 and bolus vinorelbine at 20 mg/m2 on day 8 (PV) during radiation therapy. The toxicity of induction therapy was scored according to the Common Terminology Criteria for Adverse Events version 4.0 [16].
Induction radiotherapy
Thoracic radiotherapy was started on chemotherapy day 2. The first session was scheduled to be administered with the first chemotherapy cycle at 27 Gy in 15 fractions over 3 weeks. The second session was started after an interval of a week until day 2 of the second course of chemotherapy with the cycle of MVP, which was administered at 18 Gy in 10 fractions over 2 weeks. The total radiation dose administered was thus 45 Gy in 25 fractions. After 2009, radiotherapy concurrently started with chemotherapy. The total dose administered was 45 Gy in 25 fractions. After the CT simulator was installed in our hospital at the end of 2011, 3-dimensional radiotherapy was used instead of 2-dimensional radiotherapy. Intensity-modulated radiation therapy was not used. The radiation field basically included the primary tumour and the ipsilateral supraclavicular nodes. The mediastinal and hilar lymph nodes were not irradiated, even in cases with hilar nodal involvement. Tumour responses were assessed radiographically according to the response evaluation criteria in solid tumours (RECIST) and were classified as complete response, partial response, stable disease or progressive disease (PD) [17].
Surgery
After the induction CRT, each case was reevaluated to determine the clinical response and resectability. For all patients, restaging was performed according to the 7th edition of the TNM Classification of Malignant Tumors. Surgery was performed 2–6 weeks after the completion of the induction therapy. Patients underwent surgery with en bloc resection of the involved chest wall and structures. Reconstruction of vascular structures was typically performed when necessary to achieve an R0 resection. The resected margin was evaluated by intraoperative rapid diagnosis to determine the extent of resection.
Pathological findings
The pathological response was divided into 3 categories: pathological complete response (PCR, no residual microscopic tumour), minimal microscopic residual disease (few scattered viable tumour foci within mostly necrotic or fibrotic masses, ≤10% residual viable tumour) and gross residual disease (mostly or entirely viable tumour, >10% residual viable tumour).
Boost therapy
For cases with incomplete resection, boost radiotherapy of 21.6 Gy was administered in 12 fractions. Patients who were judged to have undergone complete resection were followed up without additional therapy until there was clinical evidence of recurrence.
Follow-up
After curative resection, the patients were followed up with chest and abdominal CT every 6 months or 1 year for the first 5 years at least. We obtained follow-up information from the patients’ electronic medical records and through telephone interviews and questionnaires.
Statistical analysis
Statistical analysis was performed using JMP® 14 (SAS Institute Inc., Cary, NC, USA). OS was defined as the interval from the date of diagnosis to the date of death or was censored at the date of the last follow-up evaluation and estimated from the date of surgery using the Kaplan–Meier survival analysis method. Survival differences were compared by using the log-rank test. A P-value of <0.05 was considered significant.
RESULTS
From January 1999 to December 2017, 60 patients were enrolled into this study. Six patients were excluded (4 patients developed PD, 1 patient underwent surgical resection at another hospital and the remaining patient underwent wedge resection due to unexpected pleural dissemination). Fifty-four (90%) patients underwent radical surgical resection. These patients comprised 51 men and 3 women, with a median age of 53 years (range 33–75 years). The preoperative status of the lymph nodes was cN0 in 42 (78%) patients, cN1 in 9 (17%) patients and cN3 in 3 (5%) patients. Data of 54 patients were analysed to determine the surgical procedures, pathological results, recurrence free survival and OS. The patient characteristics are shown in Table 1.
Characteristics of all eligible patients
| Characteristics . | . |
|---|---|
| Age (years), median (range) | 53 (33–75) |
| Sex, n (%) | |
| Male | 51 (94) |
| Female | 3 (6) |
| ECOG performance status, n (%) | |
| 0–1 | 46 (85) |
| 2 | 8 (15) |
| Smoking history, n (%) | 54 (100) |
| Primary site, n (%) | |
| Right | 36 (67) |
| Left | 18 (33) |
| Clinical T stage, n (%) | |
| T3 | 46 (85) |
| T4 | 8 (15) |
| Clinical N stage, n (%) | |
| N0 | 42 (78) |
| N1 | 9 (17) |
| N2 | 0 (0) |
| N3 | 3 (5) |
| Histology, n (%) | |
| Adenocarcinoma | 36 (67) |
| Squamous cell carcinoma | 14 (26) |
| Others | 4 (7) |
| Induction chemotherapy, n (%) | |
| MVP | 35 (65) |
| PV | 19 (35) |
| Radiological response, n (%) | |
| Complete response | 0 (0) |
| Partial response | 20 (37) |
| Stable disease | 34 (63) |
| Characteristics . | . |
|---|---|
| Age (years), median (range) | 53 (33–75) |
| Sex, n (%) | |
| Male | 51 (94) |
| Female | 3 (6) |
| ECOG performance status, n (%) | |
| 0–1 | 46 (85) |
| 2 | 8 (15) |
| Smoking history, n (%) | 54 (100) |
| Primary site, n (%) | |
| Right | 36 (67) |
| Left | 18 (33) |
| Clinical T stage, n (%) | |
| T3 | 46 (85) |
| T4 | 8 (15) |
| Clinical N stage, n (%) | |
| N0 | 42 (78) |
| N1 | 9 (17) |
| N2 | 0 (0) |
| N3 | 3 (5) |
| Histology, n (%) | |
| Adenocarcinoma | 36 (67) |
| Squamous cell carcinoma | 14 (26) |
| Others | 4 (7) |
| Induction chemotherapy, n (%) | |
| MVP | 35 (65) |
| PV | 19 (35) |
| Radiological response, n (%) | |
| Complete response | 0 (0) |
| Partial response | 20 (37) |
| Stable disease | 34 (63) |
ECOG: Eastern Cooperative Oncology Group; MVP: mitomycin–vindesine–cisplatin; PV: cisplatin–vinorelbine.
Characteristics of all eligible patients
| Characteristics . | . |
|---|---|
| Age (years), median (range) | 53 (33–75) |
| Sex, n (%) | |
| Male | 51 (94) |
| Female | 3 (6) |
| ECOG performance status, n (%) | |
| 0–1 | 46 (85) |
| 2 | 8 (15) |
| Smoking history, n (%) | 54 (100) |
| Primary site, n (%) | |
| Right | 36 (67) |
| Left | 18 (33) |
| Clinical T stage, n (%) | |
| T3 | 46 (85) |
| T4 | 8 (15) |
| Clinical N stage, n (%) | |
| N0 | 42 (78) |
| N1 | 9 (17) |
| N2 | 0 (0) |
| N3 | 3 (5) |
| Histology, n (%) | |
| Adenocarcinoma | 36 (67) |
| Squamous cell carcinoma | 14 (26) |
| Others | 4 (7) |
| Induction chemotherapy, n (%) | |
| MVP | 35 (65) |
| PV | 19 (35) |
| Radiological response, n (%) | |
| Complete response | 0 (0) |
| Partial response | 20 (37) |
| Stable disease | 34 (63) |
| Characteristics . | . |
|---|---|
| Age (years), median (range) | 53 (33–75) |
| Sex, n (%) | |
| Male | 51 (94) |
| Female | 3 (6) |
| ECOG performance status, n (%) | |
| 0–1 | 46 (85) |
| 2 | 8 (15) |
| Smoking history, n (%) | 54 (100) |
| Primary site, n (%) | |
| Right | 36 (67) |
| Left | 18 (33) |
| Clinical T stage, n (%) | |
| T3 | 46 (85) |
| T4 | 8 (15) |
| Clinical N stage, n (%) | |
| N0 | 42 (78) |
| N1 | 9 (17) |
| N2 | 0 (0) |
| N3 | 3 (5) |
| Histology, n (%) | |
| Adenocarcinoma | 36 (67) |
| Squamous cell carcinoma | 14 (26) |
| Others | 4 (7) |
| Induction chemotherapy, n (%) | |
| MVP | 35 (65) |
| PV | 19 (35) |
| Radiological response, n (%) | |
| Complete response | 0 (0) |
| Partial response | 20 (37) |
| Stable disease | 34 (63) |
ECOG: Eastern Cooperative Oncology Group; MVP: mitomycin–vindesine–cisplatin; PV: cisplatin–vinorelbine.
Induction chemoradiation toxicity
For 54 patients, the median radiation dose was 45 Gy (40–45 Gy). A total of 35 (65%) patients received the MVP therapy, and 19 (35%) patients received the PV therapy. Grade 3/4 toxicities associated with chemoradiation therapy were observed in 33 (61%) patients, including leucopenia in 30 (56%) patients, neutropenia in 29 (54%) patients, nausea in 1 (2%) patient, vomiting in 1 (2%) patient and lung infection in 1 (2%) patient. The patients who received the MVP regimen showed more toxicities than those who received the PV regimen. Table 2 shows the summary of toxicities.
Toxicity due to induction therapy (grade III–IV) (n = 33)
| . | n (%) . |
|---|---|
| Grade 3/4 morbidity | 33 (61) |
| Leucopenia | 30 (56) |
| Neutropenia | 29 (54) |
| Nausea | 1 (2) |
| Vomiting | 1 (2) |
| Lung infection | 1 (2) |
| Chemotherapy regimen | |
| MVP | 26 (79) |
| PV | 7 (21) |
| . | n (%) . |
|---|---|
| Grade 3/4 morbidity | 33 (61) |
| Leucopenia | 30 (56) |
| Neutropenia | 29 (54) |
| Nausea | 1 (2) |
| Vomiting | 1 (2) |
| Lung infection | 1 (2) |
| Chemotherapy regimen | |
| MVP | 26 (79) |
| PV | 7 (21) |
MVP: mitomycin–vindesine–cisplatin; PV: cisplatin–vinorelbine.
Toxicity due to induction therapy (grade III–IV) (n = 33)
| . | n (%) . |
|---|---|
| Grade 3/4 morbidity | 33 (61) |
| Leucopenia | 30 (56) |
| Neutropenia | 29 (54) |
| Nausea | 1 (2) |
| Vomiting | 1 (2) |
| Lung infection | 1 (2) |
| Chemotherapy regimen | |
| MVP | 26 (79) |
| PV | 7 (21) |
| . | n (%) . |
|---|---|
| Grade 3/4 morbidity | 33 (61) |
| Leucopenia | 30 (56) |
| Neutropenia | 29 (54) |
| Nausea | 1 (2) |
| Vomiting | 1 (2) |
| Lung infection | 1 (2) |
| Chemotherapy regimen | |
| MVP | 26 (79) |
| PV | 7 (21) |
MVP: mitomycin–vindesine–cisplatin; PV: cisplatin–vinorelbine.
Restaging and clinical response
The clinical response was evaluated according to RECIST and classified as complete response, partial response, stable disease and PD. No patient showed complete response. A total of 20 (37%) patients showed partial response, and 34 (63%) patients showed stable disease. Four patients with PD did not enter this study. After induction CRT, no patient showed N3 disease whereas 4 (7%) patients showed persistent N1 disease. Down-staging of nodal status was observed in 8 (15%) patients.
Surgical findings
Complete pathological resection was achieved in 44 (81%) patients. Fifty-three (98%) of the lung resections were lobectomies and 1 (2%) was a pneumonectomy, which was required due to extra-nodal tumour invasion to the central bronchus. Chest wall resection was performed in 46 (85%) patients. Of them, 9 (17%) patients required reconstruction owing to identified defects. The chest wall reconstruction was performed with a 2-mm GORE-TEX Soft Tissue Patch (W.L. Gore and Associates, Inc., Flagstaff, AZ, USA). Eight patients who underwent only lobectomy without chest wall resection had tumour regression after the induction therapy; therefore, chest wall resection was no longer considered necessary according to the surgeon. Data regarding surgical procedures are summarized in Table 3. The most frequently used approach was the posterior approach (n = 49; 91%). Anterior approaches such as neck–clavicular incision and hemiclamshell incision were used in cases with subclavian vessel involvement or anterior chest wall infiltration (n = 5; 9%). A total of 10 (19%) patients underwent extended resections including resection of the Th1 root, pulmonary artery and subclavian vessels. A total of 3 (6%) patients underwent reconstruction of vascular structures. Vascular reconstructions were performed by continuous suture using 4-0 or 5-0 monofilament. One (2%) patient underwent partial vertebrectomy. Five (9%) patients had partial resection of the Th1 brachial plexus. The C8 root was preserved in all patients.
Details of surgical procedures and pathological results
| Characteristics . | . |
|---|---|
| Approach, n (%) | |
| Anterior approach | 5 (9) |
| Posterior approach | 49 (91) |
| Surgical procedure, n (%) | |
| Right upper lobectomy | 36 (67) |
| Left upper lobectomy | 17 (31) |
| Left pneumonectomy | 1 (2) |
| Combined resection, n (%) | |
| Chest wall resection | 46 (85) |
| Vertebral body | 1 (2) |
| Subclavian artery | 1 (2) |
| Subclavian vein | 2 (4) |
| Th1 root | 5 (9) |
| Pulmonary artery | 2 (4) |
| Chest wall reconstruction, n (%) | 9 (17) |
| Operative time (min), median (range) | 253 (142–505) |
| Blood loss (ml), median (range) | 530 (35–1474) |
| Completeness of resection, n (%) | |
| Complete resection (R0) | 44 (81) |
| Incomplete resection (R1) | 10 (19) |
| Pathological N stage, n (%) | |
| N0 | 49 (90) |
| N1 | 3 (6) |
| N2 | 2 (4) |
| Pathological findings, n (%) | |
| Complete response | 12 (22) |
| Minimal microscopic residual disease | 32 (59) |
| Gross residual disease | 10 (19) |
| Characteristics . | . |
|---|---|
| Approach, n (%) | |
| Anterior approach | 5 (9) |
| Posterior approach | 49 (91) |
| Surgical procedure, n (%) | |
| Right upper lobectomy | 36 (67) |
| Left upper lobectomy | 17 (31) |
| Left pneumonectomy | 1 (2) |
| Combined resection, n (%) | |
| Chest wall resection | 46 (85) |
| Vertebral body | 1 (2) |
| Subclavian artery | 1 (2) |
| Subclavian vein | 2 (4) |
| Th1 root | 5 (9) |
| Pulmonary artery | 2 (4) |
| Chest wall reconstruction, n (%) | 9 (17) |
| Operative time (min), median (range) | 253 (142–505) |
| Blood loss (ml), median (range) | 530 (35–1474) |
| Completeness of resection, n (%) | |
| Complete resection (R0) | 44 (81) |
| Incomplete resection (R1) | 10 (19) |
| Pathological N stage, n (%) | |
| N0 | 49 (90) |
| N1 | 3 (6) |
| N2 | 2 (4) |
| Pathological findings, n (%) | |
| Complete response | 12 (22) |
| Minimal microscopic residual disease | 32 (59) |
| Gross residual disease | 10 (19) |
Details of surgical procedures and pathological results
| Characteristics . | . |
|---|---|
| Approach, n (%) | |
| Anterior approach | 5 (9) |
| Posterior approach | 49 (91) |
| Surgical procedure, n (%) | |
| Right upper lobectomy | 36 (67) |
| Left upper lobectomy | 17 (31) |
| Left pneumonectomy | 1 (2) |
| Combined resection, n (%) | |
| Chest wall resection | 46 (85) |
| Vertebral body | 1 (2) |
| Subclavian artery | 1 (2) |
| Subclavian vein | 2 (4) |
| Th1 root | 5 (9) |
| Pulmonary artery | 2 (4) |
| Chest wall reconstruction, n (%) | 9 (17) |
| Operative time (min), median (range) | 253 (142–505) |
| Blood loss (ml), median (range) | 530 (35–1474) |
| Completeness of resection, n (%) | |
| Complete resection (R0) | 44 (81) |
| Incomplete resection (R1) | 10 (19) |
| Pathological N stage, n (%) | |
| N0 | 49 (90) |
| N1 | 3 (6) |
| N2 | 2 (4) |
| Pathological findings, n (%) | |
| Complete response | 12 (22) |
| Minimal microscopic residual disease | 32 (59) |
| Gross residual disease | 10 (19) |
| Characteristics . | . |
|---|---|
| Approach, n (%) | |
| Anterior approach | 5 (9) |
| Posterior approach | 49 (91) |
| Surgical procedure, n (%) | |
| Right upper lobectomy | 36 (67) |
| Left upper lobectomy | 17 (31) |
| Left pneumonectomy | 1 (2) |
| Combined resection, n (%) | |
| Chest wall resection | 46 (85) |
| Vertebral body | 1 (2) |
| Subclavian artery | 1 (2) |
| Subclavian vein | 2 (4) |
| Th1 root | 5 (9) |
| Pulmonary artery | 2 (4) |
| Chest wall reconstruction, n (%) | 9 (17) |
| Operative time (min), median (range) | 253 (142–505) |
| Blood loss (ml), median (range) | 530 (35–1474) |
| Completeness of resection, n (%) | |
| Complete resection (R0) | 44 (81) |
| Incomplete resection (R1) | 10 (19) |
| Pathological N stage, n (%) | |
| N0 | 49 (90) |
| N1 | 3 (6) |
| N2 | 2 (4) |
| Pathological findings, n (%) | |
| Complete response | 12 (22) |
| Minimal microscopic residual disease | 32 (59) |
| Gross residual disease | 10 (19) |
Perioperative outcome and pathological findings
The patients’ chest tubes were removed after a median of 3.5 postoperative days (range 1–16). The median length of hospital stay from operation to discharge was 9 days (range 4–59 days). Postoperative complications of grade IIIa–V of the Clavien–Dindo classification were observed in 7 (13%) patients. Table 4 shows the summary of morbidity and mortality. Three (6%) patients underwent reoperation. Reoperations were performed for chylothorax in 2 cases and empyema in 1 case. There was no 30-day mortality. However, 1 (2%) patient died due to interstitial pneumonitis after pneumonectomy at postoperative day 54. Complication rates among those with T3 and T4 tumours were 31% (n = 17) and 2% (n = 1), respectively.
Postoperative complications and mortality
| Characteristics . | n (%) . |
|---|---|
| Complication | |
| Pneumonia | 2 (4) |
| Chylothorax | 2 (4) |
| Empyema and interstitial pneumonitis | 1 (2) |
| Cerebral infarction and interstitial pneumonitis | 1 (2) |
| Prolonged air leakage | 1 (2) |
| Mortality | |
| 30-Day mortality | 0 (0) |
| 90-Day mortality | 1 (2) |
| Characteristics . | n (%) . |
|---|---|
| Complication | |
| Pneumonia | 2 (4) |
| Chylothorax | 2 (4) |
| Empyema and interstitial pneumonitis | 1 (2) |
| Cerebral infarction and interstitial pneumonitis | 1 (2) |
| Prolonged air leakage | 1 (2) |
| Mortality | |
| 30-Day mortality | 0 (0) |
| 90-Day mortality | 1 (2) |
Postoperative complications and mortality
| Characteristics . | n (%) . |
|---|---|
| Complication | |
| Pneumonia | 2 (4) |
| Chylothorax | 2 (4) |
| Empyema and interstitial pneumonitis | 1 (2) |
| Cerebral infarction and interstitial pneumonitis | 1 (2) |
| Prolonged air leakage | 1 (2) |
| Mortality | |
| 30-Day mortality | 0 (0) |
| 90-Day mortality | 1 (2) |
| Characteristics . | n (%) . |
|---|---|
| Complication | |
| Pneumonia | 2 (4) |
| Chylothorax | 2 (4) |
| Empyema and interstitial pneumonitis | 1 (2) |
| Cerebral infarction and interstitial pneumonitis | 1 (2) |
| Prolonged air leakage | 1 (2) |
| Mortality | |
| 30-Day mortality | 0 (0) |
| 90-Day mortality | 1 (2) |
A total of 44 (81%) patients had complete resection (R0), and 10 patients had incomplete resection (R1). The Supplementary Material, Table S1 summarizes the detailed characteristics of patients with incomplete resection; 6 patients received postoperative boost radiotherapy. There was no case of macroscopically incomplete resection (R2). The pathological nodal status was pN0 in 49 (90%) patients, pN1 in 3 (6%) patients and pN2 in 2 (4%) patients. A pathological response with <10% viable tumour cells was observed in 44 (81%) patients. A PCR was observed in 12 (22%) patients (Table 3).
Follow-up, survival and prognostic factors
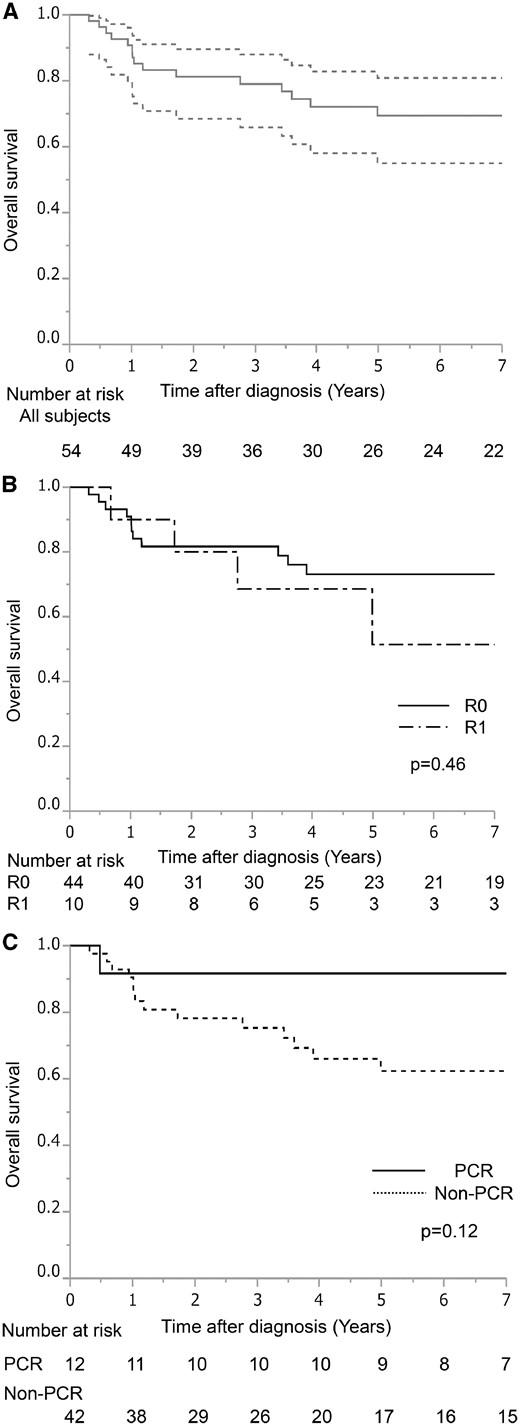
After a median follow-up of 57.0 months (range 3.8–227.5 months), 19 (35%) patients experienced recurrence. Tumour recurrences were as follows: distant in 16 (30%) patients and locoregional in 3 (5%) patients. The most frequent recurrence site was the brain (n = 8, 15%). Table 5 summarizes the detailed recurrences and sites of metastasis. The 5-year survival rate for the entire population (n = 54) was 69% [95% confidence interval (CI) 55–81%] (Fig. 1A). Although not statistically significant, the 5-year survival rate was higher in patients who underwent complete rather than incomplete resection (73% vs 51%, P = 0.46) (Fig. 1B). Furthermore, the 5-year survival rate was better in patients with a PCR compared to that in patients with an incomplete pathological response (5-year survival: 92% vs 62%, respectively; P = 0.12) (Fig. 1C). There was no statistically significant difference between >10% (gross residual tumour group) and ≤10% residual viable tumour (minimal microscopic residual tumour group). Forty-four patients had minimal residual tumour. Ten patients had gross residual tumours. The 5-year survival rate was similar between the minimal residual tumour group and the gross residual tumour group (69% vs 70%, P = 0.58). Patients with adenocarcinoma had worse prognosis than non-adenocarcinoma patients (61% vs 83%, P = 0.32). After follow-up of the 54 patients, 17 (31%) patients died (13 due to recurrent disease). Among 44 patients with complete resection, 10 patients died of lung cancer, 2 patients died of secondary cancer and 1 patient died postoperatively. Among 10 patients with incomplete resection, 3 patients died due to lung cancer and 1 patient died due to cerebral infarction.
(A) Survival curves of all 54 patients who underwent trimodality treatment for superior sulcus tumours. (B) Survival curves according to the completeness of resection. (C) Survival curves according to the pathological response. PCR: pathological complete response.
Recurrences and sites of metastasis (n = 19)
| Sites . | n (%) . |
|---|---|
| Distant | 16 (30) |
| Brain | 7 (13) |
| Bone | 3 (5) |
| Contralateral lung | 2 (4) |
| Meningitis | 1 (2) |
| Liver and brain | 1 (2) |
| Liver and kidney | 1 (2) |
| Adrenal | 1 (2) |
| Locoregional | 3 (5) |
| Supraclavicular lymph node | 1 (2) |
| Mediastinal lymph node | 1 (2) |
| Pleural dissemination | 1 (2) |
| Sites . | n (%) . |
|---|---|
| Distant | 16 (30) |
| Brain | 7 (13) |
| Bone | 3 (5) |
| Contralateral lung | 2 (4) |
| Meningitis | 1 (2) |
| Liver and brain | 1 (2) |
| Liver and kidney | 1 (2) |
| Adrenal | 1 (2) |
| Locoregional | 3 (5) |
| Supraclavicular lymph node | 1 (2) |
| Mediastinal lymph node | 1 (2) |
| Pleural dissemination | 1 (2) |
Recurrences and sites of metastasis (n = 19)
| Sites . | n (%) . |
|---|---|
| Distant | 16 (30) |
| Brain | 7 (13) |
| Bone | 3 (5) |
| Contralateral lung | 2 (4) |
| Meningitis | 1 (2) |
| Liver and brain | 1 (2) |
| Liver and kidney | 1 (2) |
| Adrenal | 1 (2) |
| Locoregional | 3 (5) |
| Supraclavicular lymph node | 1 (2) |
| Mediastinal lymph node | 1 (2) |
| Pleural dissemination | 1 (2) |
| Sites . | n (%) . |
|---|---|
| Distant | 16 (30) |
| Brain | 7 (13) |
| Bone | 3 (5) |
| Contralateral lung | 2 (4) |
| Meningitis | 1 (2) |
| Liver and brain | 1 (2) |
| Liver and kidney | 1 (2) |
| Adrenal | 1 (2) |
| Locoregional | 3 (5) |
| Supraclavicular lymph node | 1 (2) |
| Mediastinal lymph node | 1 (2) |
| Pleural dissemination | 1 (2) |
DISCUSSION
Based on the results of 2 novel phase II trials for SST, the SWOG 9416/Intergroup Trial 0160 and the JCOG 9806 trial, induction CRT followed by surgery is currently considered the standard of care. However, a remarkable variability in chemotherapy agents and cycles, mode and intensity of radiotherapy can be observed in the protocols followed by different institutions. The mortality rates associated with induction chemoradiation were reported to be 2.7% and 1.3%, respectively, in the SWOG 9416 and JCOG 9806 trials [2, 3]. In this study, the mortality rate associated with induction chemoradiation was 0%. The morbidity and mortality rates after surgical resection of SSTs were 10–55% and 0–9%, respectively, in previous studies [2, 3, 5–15] (Supplementary Material, Table S2). In our institution, major postoperative complications occurred in 7 (13%) patients. The 30-day mortality rate was 0%. These relatively low morbidity and mortality rates reflect the safety of the trimodality therapy employed in our hospital. ‘The 5-year survival rate for the entire population (n = 54) in this study was relatively better than that in other studies [2, 3, 5–15] (Supplementary Material, Table S2)’. Furthermore, we analysed the survival rate for 60 patients who underwent induction therapy. As a result, the 5-year OS rate in the 60 patients was 64% (95% CI 50–76%).
A major challenge of surgical treatment for SSTs is to achieve complete resection because of the technical difficulties associated with the anatomical structures and approaches of the thoracic inlet [1, 18, 19]. R0 resection was an important predictor of survival in previous studies [10, 14]. In the JCOG 9806 trial, the 5-year OS for patients with R0 resection was 70% compared to 24% in patients with R1 or R2 resection. In our department, the surgical approach is decided based on whether the tumour involves anterior or posterior structures in the thoracic inlet. The 5-year survival rate for patients with R0 resection was better than that for patients with R1 resection (73% vs 51%). Intraoperative frozen sections of the tissue around the tumour were evaluated in 40 of the 54 patients (74%). In 36 (67%) patients, frozen sections of the surgical margin were diagnosed negative for malignancy. The diagnosis based on frozen section evaluation was correct in 31 (n = 31/36, 86%) patients. In 5 of the 36 patients (14%), a few viable cells were observed around the resected specimen. The frozen section evaluation was useful to decide the extent of surgical resection. Tumour invaded to the C8/Th1 root of the brachial plexus in 5 patients with incomplete resection. Among them, 4 patients received the boost radiotherapy and 3 patients are still alive with no recurrence. The survival rate was better even in patients with an incomplete resection. The complete resection rate (81%) was slightly lower than that in other reports. The accuracy of the pathological examination in assessing a complete resection is questionable between institutions. According to the review of our pathological findings, a few viable cells were found on the surface of resected specimens. Five patients with incomplete resections had intraoperative frozen sections, which were negative for malignancy. Therefore, we believe that some cases of ‘incomplete resection’ were the same as ‘complete resection’. Additionally, among 6 patients who received boost therapy, no one had local recurrence. Stereotactic body radiation boost therapy after concurrent chemoradiation for locally advanced NSCLC was feasible resulting in improvements in local control [20]. The boost therapy might be effective for patients with incomplete resection.
PCR was noted in 22% of the patients; these findings were similar to those in the JCOG 9806 trial (21%) and others (range 11–62%). A better survival rate was evident in patients with a PCR (5-year survival: 92%). The lack of statistical difference may have been a result of the limited length of follow-up time. PCR was an important prognostic factor as it was in previous reports [8, 14]. The patient with a higher dose of radiotherapy had a higher PCR rate [5]. Among 12 patients with PCR, 1 patient died due to brain metastasis. The pattern of recurrence was characterized by 5% of local recurrence and 30% of distant metastasis, showing better local control. These results are in agreement with the data of the SWOG 9416/Intergroup Trial 0160 and JCOG 9806 trials, which showed a 2-fold higher incidence of distant metastasis than that of local recurrence [2, 3]. The current challenge in SST management is the prevention of brain metastases. All patients with brain metastasis were diagnosed as having adenocarcinoma in our study, and the 5-year survival rate for the patients with adenocarcinoma was worse than in patients with non-adenocarcinoma. Approaches to improve survival in such patients included the use of prophylactic cranial irradiation [21]. However, the results of a phase III trial by the Radiation Therapy Oncology Group (RTOG 0214) showed no statistically significant differences in OS for patients who underwent prophylactic cranial irradiation versus those who did not undergo prophylactic cranial irradiation [22]. Future strategies to improve outcomes for SSTs are needed to control brain metastasis. Recently, agents targeting molecular pathways such as tyrosine kinase inhibitors and immune checkpoint inhibitors have been developed for patients with brain metastasis [23, 24].
Antibodies that block the programmed death 1 protein improve survival in patients with advanced NSCLC [25]. Neoadjuvant therapy for resectable NSCLC has been reported by Forde et al. [26]. In their study, nivolumab was administered intravenously every 2 weeks, with surgery planned 4 weeks after the first dose. Of the 21 eligible patients, 20 (95%) patients underwent complete resection. A major pathological response was noted in 9 of 20 patients (45%). Furthermore, 3 patients achieved PCR. Neoadjuvant nivolumab was associated with few side effects and induced a major pathological response in patients with resected tumours [26]. Larger studies are needed to determine the most effective duration of neoadjuvant therapy. Quadramodality treatment for SSTs including immune checkpoint blockade might be considered in the future.
Limitations
This study has some limitations. The study was retrospectively conducted in a single institution using a small population. The length of follow-up after surgery was limited, and a selected group of patients who underwent trimodality therapy was included. On the other hand, this study offers the advantage of being a single-institution study with a homogenous induction regimen and surgical approach to SSTs.
CONCLUSION
In conclusion, trimodality therapy was efficacious in patients with SSTs, with acceptable morbidity and mortality. The PCR achieved resulted in long-term survival prospects. Distant metastasis, however, remains a major problem.
Conflict of interest: none declared.
Presented at the 32nd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 18–20 October 2018.
REFERENCES
Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (24 April 2018, date last accessed).
- polymerase chain reaction
- radiation therapy
- metastatic malignant neoplasm to brain
- chemotherapy regimen
- cisplatin
- follow-up
- objective (goal)
- neoadjuvant therapy
- surgical procedures, operative
- survival rate
- vindesine
- mitomycin
- morbidity
- mortality
- neoplasms
- surgery specialty
- radiochemotherapy
- pancoast tumor
- excision
- complete remission
- cisplatin/vinorelbine