-
PDF
- Split View
-
Views
-
Cite
Cite
Elisabet Suades-González, Mireia Gascon, Mònica Guxens, Jordi Sunyer, Air Pollution and Neuropsychological Development: A Review of the Latest Evidence, Endocrinology, Volume 156, Issue 10, 1 October 2015, Pages 3473–3482, https://doi.org/10.1210/en.2015-1403
Close - Share Icon Share
For the last decade, literature on the detrimental impacts of air pollution on brain, cognition and behavior has exponentially increased. Our aim is to review the latest epidemiologic literature on the association between outdoor air pollution and neuropsychological developmental in children. Two independent researchers searched for published studies between January 1, 2012 and June 12, 2015 in MEDLINE, Web of Science, and Science direct using defined keywords on outdoor air pollution and neuropsychological development. Selection of articles was based on study eligibility criteria. We encountered sufficient evidence of detrimental effects of pre- or postnatal exposure to polycyclic aromatic hydrocarbons on global intelligence quotient. The evidence was also sufficient for the association between pre- or postnatal exposure to fine particulate matter (PM2.5) and autism spectrum disorder, and limited evidence was encountered between nitrogen oxides and autism spectrum disorder. For other exposure-outcome associations reviewed, the evidence was inadequate or insufficient. Although evidence is not yet conclusive and further research is needed, the latest epidemiological studies support the hypothesis that pre- or postnatal exposure to ambient pollution, particularly polycyclic aromatic hydrocarbons, PM2.5, and nitrogen oxides has a negative impact on the neuropsychological development of children. The public health impact of air pollutants cannot be ignored and the precautionary principle should be applied to protect children.
Ambient particulate matter (PM) pollution was the ninth leading risk factor in 2010 for global disease burden (1). Air pollution occurs anywhere and has become a global public health concern (2). Common sources of outdoor air pollution are combustion of fossil fuels, and industrial and agricultural processes. Air pollutants of major public health concern include PM (eg, organic and elemental carbon [EC], metals and polycyclic aromatic hydrocarbons [PAHs]), carbon monoxide (CO), ozone (O3), nitrogen dioxide (NO2), and sulfur dioxide (SO2). The cardiovascular and respiratory health effects of air pollution have been well documented (3–6), and there is increasing evidence of its hazards on the central nervous system (CNS) (7). Furthermore, air pollution has recently been considered as a suspected neurodevelopmental toxicant (8). Neural development (eg, proliferation, migration, differentiation, synaptogenesis, myelination, and apoptosis) extends from the embryonic period through adolescence (9). Therefore, this period is a critical developmental window for CNS development. There are studies reporting neuropsychological delays related to ambient pollutants exposure during pregnancy or childhood (10). Previous reviews evaluated the epidemiological evidence of the brain effects of air pollution (7, 10). The aim in the current work is to complement and update these reviews with the latest literature on air pollution and neuropsychological development in children.
Materials and Methods
Search strategy
This review was done following the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (11). The databases searched between January 1, 2012 and June 12, 2015 for relevant studies were MEDLINE (National Library of Medicine), Web of Science, and Science Direct using the next keywords: air pollution keywords (“air pollution,” “particulate matter,” “environmental pollution,” “environmental pollutants,” “black carbon,” “elemental carbon,” “polycyclic aromatic hydrocarbons,” “PAH,” “nitrogen dioxide,” “PM2.5,” “urban pollution,” or “traffic”) combined with the next keywords for cognition (“cognition,” “cognitive,” “intelligence,” “brain,” “behavior,” “neurobehavioral,” “neurodevelopment,” “neurotoxicity,” “autism,” “attention,” “attention deficit hyperactivity disorder,” or “ADHD”). Limits were: humans, all children 0–18 years, English language. Identification and first screening of the studies was performed using the information available in the title and the abstract.
Study selection
Two reviewers independently screened titles and abstracts of all studies identified. Potentially relevant studies were retrieved in full text and assessed for eligibility; any discrepancies were resolved by discussion between the 2 independent researchers or by discussion with a third review author. The selection criteria were that the eligible study had to: 1) include humans as study subjects; 2) conduct exposure assessment to outdoor air pollution during pregnancy, around birth or during childhood using direct or indirect approaches; 3) include health outcomes related to cognition, behavior, neurodevelopmental disorders; 4) be an original research article; 5) include a case-control, cohort or cross-sectional design; and 6) be written in English.
Evaluation of evidence
We classified as good quality studies those studies with: 1) a cohort or a case-control design; 2) a minimum sample size of 100 children; 3) exposure assessment using standardized and validated methods; 4) outcome assessment using standardized and validated neuropsychological tools; and 5) accounting for confounding in the analysis (Supplemental Table 1). The strength of evidence for associations between air pollutants exposure and outcomes was based on the levels of evidence used by the International Agency for Research on Cancer (2006) (12). The 2 independent reviewers classified the evidence and in case of disagreement a third reviewer gave the decision. Evidence for a causal relationship for each air pollutant-outcome combination was classified as sufficient: if most of the studies, including good quality studies, report an association, but evidence is not yet conclusive enough to conclude that there is a causal relationship; limited: several good quality, independent studies report an association but evidence is not yet conclusive enough; inadequate: if associations are reported in 1 or more studies, but insufficient quality, insufficient number of studies, lack of consistency between studies, and/or lack of statistical power preclude a conclusion regarding the presence or absence of an association; insufficient: if no associations are reported in 1 or more studies, but insufficient quality, insufficient number of studies, lack of consistency between studies, and/or lack of statistical power preclude a conclusion regarding the presence or absence of an association; and evidence for lack of association: several good quality studies are consistent in not showing an association.
We organized the review by outcome and then by the time of exposure (pre- or postnatal) and air pollutant.
Results
Search results
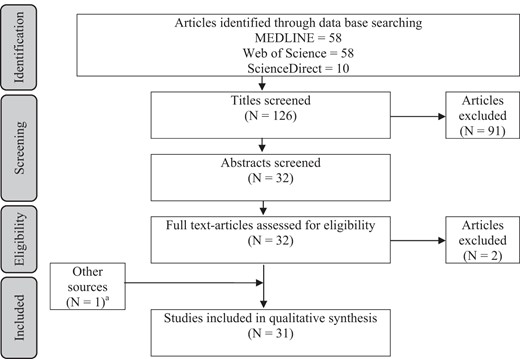
A total of 126 articles were identified in MEDLINE, Web of Science, and Science Direct. Initial title and abstract screening identified 32 candidate studies, and they were all assessed for eligibility: one study was excluded because of indoor exposure (13) and another one because it only provided interaction values between prenatal exposure to airborne PAHs and maternal psychological distress during pregnancy on subsequent behavioral problems in children (14). An additional study was found in MEDLINE but not through the keyword search (15). Figure 1 shows the selection process of the 31 final studies included in this review.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram
a, One study was also found through MEDLINE but not through the standard search.
Exposure assessment
PM (n = 16 studies), nitrogen oxides (NOx) (n = 13 studies), and PAHs (n = 8 studies) were the most evaluated pollutants. Other air toxics were evaluated to a lesser extent: 7 studies assessed O3, 4 assessed CO, 3 assessed SO2, 3 assessed lead, 2 assessed black carbon (BC), 2 assessed EC, and 2 assessed other compounds such as antimony, arsenic, cadmium, chromium, copper, manganese, or mercury. The exposure assessment methods differed depending on the toxics: for the PAHs, most studies (n = 6) used adducts in cord blood; concentrations of other pollutants were estimated by land-use regression models in 8 of the studies (Supplemental Table 2).
Air pollutants and cognitive and psychomotor development
Several cognitive and psychomotor outcomes were assessed in the studies included in this review, being global intelligence quotient (IQ) the most evaluated one. Overall, the neuropsychological tests used were mostly administered by psychologists/physicians. Other studies used computerized testing or questionnaires answered by the parents. The instruments used were heterogeneous among studies (Table 1).
Epidemiological Studies on Air Pollution and Neuropsychological Development
| Author (y) . | Country and Study Designa . | n . | Ageb . | Neuropsychological Assessment Cognition or Behavior . | Test . | Air Pollution Exposure Period . | Pollutant . | Main Findingsc . |
|---|---|---|---|---|---|---|---|---|
| Cognitive and psychomotor development | ||||||||
| Calderón-Garcidueñas et al (2012) | Mexico Prospective cohort (I) | 20 | 7 y | Global IQ | WISC-R | Postnatal (livelong residency in Mexico City) | CO, NO, O3, PM, SO2, lead: | ↑ Cognitive performance on measures related to temporal/parietal/frontal neurocognitive networks |
| Clark et al (2012) | United Kingdom Cross-sectional | 960 | 9–10 y | Reading comprehension | Suffolk reading scale | Postnatal | NO2: | — |
| Episodic memory | Child memory scale | |||||||
| Working memory | “The search and the memory task” | |||||||
| Health | SDQ | |||||||
| Self-rated health | ||||||||
| Perera, Li et al (2012) | China Birth cohort (I) | 100 | 5 y | Global IQ | WPPSI | Prenatal (entire pregnancy) | PAH: | — Interaction with environmental tobacco smoke: ↓ global and verbal scores |
| van Kempen et al (2012) | The Netherlands Cross-sectional | 553 | 9–11 y | Reaction speed, attention, coordination, perceptual coding, span length | Neurobehavioral evaluation system: DMST HECT SAT SDST SRTT | Postnatal | NO2: | At school: ↓ span length At home: - At home and road traffic noise: ↓ reaction time |
| Chiu et al (2013) | United States Birth cohort (I) | 174 | 7–14 y | Attention | CPT | Postnatal (from birth to cognitive assessment) | BC: | ↑ Commission errors and ↓ reaction time among boys |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | Learning disability, special education | Parental report | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | — — — ↑ Special education, particularly in males — — — |
| Guxens et al (2014) | The Netherlands (I), Germany (I), France (II), Italy (I), Greece (I), Spain (V) Birth cohort | 9482 | 1–6 y | Cognitive and psychomotor development | ASQ BSID I-II-III DDST II MCDI MIDI MSCA | Prenatal (entire pregnancy) | NOx, PM2.5, PM2.5–10, PM10: | ↓ Psychomotor development, particularly NO2 No association with general cognition or language development |
| Kim et al (2014) | South Korea Birth cohort (I) | 520 | 6, 12, and 24 mo | Cognitive development | K-BSID-II | Prenatal (entire pregnancy) | NO2: | ↓ Psychomotor development |
| Psychomotor development | PM10: | ↓ Cognitive and psychomotor development | ||||||
| Lin et al (2014) | China Birth cohort (I) | 533 | 6 and 18 mo | Cognitive development Psychomotor development | 2 neurobehavioral development parental-reported scales | Prenatal and postnatal (beginning of gestational period to 18 mo of age) | CO: Hydrocarbons: NO2: O3: PM10: SO2: | — (2nd and 3rd trimesters): ↓ Gross motor scores at 6 mo of age — — — (all pregnancy to 12 months of age): ↓ Fine motor development at 18 mo of age |
| Lovasi et al (2014) | United States Birth cohort (I) | 277 | 5 y | Global IQ | WPPSI-R | Prenatal (from 20th wk of pregnancy) | PAH: | ↓ Global and verbal scores |
| Tang, Lee et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global, motor and adaptive scores |
| Tang, Li et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global and motor scores |
| Calderón-Garcidueñas et al (2015) | Mexico Cross-sectional | 50 | 13.4 (4.8) y | The impact of the APOE status (ε4 vs ε3) on global IQ | WISC-R | Lifelong residency in Mexico City (including pregnancy) | O3, PM: | (APOE ε4 children): ↓ Short and working memory, reasoning and knowledge and a negative difference for verbal and global IQ |
| Harris MH et al (2015) | United States Birth cohort (I) | 1109 | 6.6–10.9 y | Global IQ, visual motor abilities, visual memory | KBIT-2, WRAML2 (visual memory index), WRAVMA (visual-motor subtest) | Prenatal (3rd trimester of pregnancy) and postnatal (the year before each cognitive assessment, the first 6 y of life) | BC: PM2.5: Prenatal roadway proximity: | — — ↓ Nonverbal IQ and↓ visual motor abilities |
| Jedrychowski et al (2015) | Poland Birth cohort (I) | 170 | 7 y | Global IQ | WISC-R | Prenatal (from 8th to 13th wk of pregnancy) | PAH: | Depressed verbal IQ compared with nonverbal IQ |
| Kicinski et al (2015) | Belgium Cross-sectional | 606 | 13.6–17 y | Attention, manual motor speed | Neurobehavioral evaluation system: Continuous performance test, Digit span test, Finger tapping test | Postnatal | Traffic exposure: | ↓ Sustained attention |
| Sunyer et al (2015) | Spain Prospective cohort (I) | 2715 | 7–10 y | Working memory, attention | ANT N-back task | Postnatal (throughout 1 y of follow-up) | EC, NO2, UFP: | ↓ Working memory and attention |
| Neurobehavior | ||||||||
| Perera, Tang et al (2012)f | United States Birth cohort (I) | 253 | 6–7 y | Behavior (anxiety/depression and attention problems) | CBCL | Prenatal (3rd trimester of pregnancy) | PAH: | ↑ Symptoms of anxiety/depression and attention problems |
| Becerra et al (2013) | United States Case-control | 83 229 7594 cases 75 635 controls | 3–5 y | ASD | DSM-IV-R | Prenatal (entire pregnancy) | CO: NO: NO2: O3: PM2.5: PM10: | — ↑ ASD ↑ ASD ↑ ASD ↑ ASD — |
| Jung et al (2013) | China Prospective cohort (I) | 49 073 | 6.26 (2.91) y | ASD | ICD-9-CM | Postnatal (preceding 1–4 y newly diagnostic ASD) | CO: NO2: O3: PM10: SO2: | ↑ ASD ↑ ASD ↑ ASD — ↑ ASD |
| Newman et al (2013) | United States Birth cohort (I) | 576 | 7 y | Behavior | BASC-2 | Postnatal (1st year of life) | ECAT: | ↑ hyperactivity scores (stronger association in children whose mothers had higher education) |
| Roberts et al (2013)g | United States Birth cohort (I) | 22 426 | Not provided | ASD | Parental report ADI-R to 50 randomly selected cases | Perinatal | Antimony: Arsenic: Cadmium: Chromium: Diesel particulate: Lead: Manganese: Mercury: Methylene chloride: Nickel: Quinoline: Styrene: Trichloro-ethylene: Vinyl chloride: | — — — — ↑ ASD ↑ ASD ↑ ASD — — ↑ ASD — — — — |
| Volk et al (2013) | United States Case-control | 524 279 cases 245 controls | 24–60 mo | ASD | ADI-R ADOS Mullen scales of early learning. Vineland adaptive behavior scales | Prenatal and postnatal (entire pregnancy and 1st year of life) | NO2: O3: PM2.5: PM10: | ↑ ASD — ↑ ASD ↑ ASD |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | ADHD | Parental report (of ever-doctor diagnosed ADHD) | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | ↓ ADHD and learning disability among females — ↓ ADHD — — — — |
| Gong et al (2014) | Sweden Birth cohort (I) | 3426 | 9–12 y | ASD, ADHD | A-TAC | Prenatal and postnatal (entire pregnancy, 1st y and 9th y) | NOx: PM10: | — — |
| Perera et al (2014)f | United States Birth cohort (I) | 250 | 9 y | ADHD | CBCL CPRS | Prenatal (cord and maternal blood) | PAH: | ↑ CPRS-DSM oriented ADHD problems |
| Volk et al (2014) | United States Case-control | 407 251 cases 156 controls | 2–5 y | The relationship of air pollution exposure and genotype (MET rs1858830 CC) with ASD | ADI-R ADOS | Prenatal (entire pregnancy) | PM2.5: PM10: O3: NO2: | ↑ ASD ↑ ASD — ↑ ASD MET rs1858830 CC genotype and high air pollutant exposure: ↑ ASD |
| Von Ehrenstein et al (2014) | United States Birth cohort (I) | 768 | 31–71 mo | ASD | DSM-IV-R | Prenatal (entire pregnancy) | 24 toxics, including aromatic solvents, chlorinated solvents, volatile organics, total PAHs, and metals | ↑ ASD; particularly, exposure during 1st trimester |
| Guxens et al (2015) | Sweden (I), the Netherlands (I), Italy (I), Spain (III) Birth cohorts and child cohort | 8079 | 4–10 y | Autistic traits | A-TAC CAST CBCL SRS | Prenatal (entire pregnancy) | NOx: PM2.5: PM10: PM2.5–10: PM2.5absorbance | — — — — — |
| Kalkbrenner et al (2015) | United States Case-control | 15 645 979 cases 14 666 controls | 8 y | ASD | DSM-IV-R | Prenatal and postnatal (1 y before birth through 1st birthday) | PM10: | ↑ ASD; particularly exposure during 3rd trimester of pregnancy |
| Raz et al (2015) g | United States Nested Case-control | 1767 245 cases 1522 controls | Not provided | ASD | ADOS Maternal report SRS | Prenatal and postnatal (9 mo before pregnancy, entire pregnancy and 9 mo after birth) | PM2.5: PM10–2.5: | ↑ ASD; stronger association for males; exposure during 3rd trimester of pregnancy and 9 mo after birth Little association with ASD |
| Talbott et al (2015) | United States Case-control | 430 211 cases 219 controls | 3–7 y | ASD | ADOS SCQ | Prenatal and postnatal (3 mo before, entire pregnancy, 1 and 2 y after) | PM2.5: | ↑ ASD; particularly, postnatal y 2 and prepregnancy through pregnancy |
| Author (y) . | Country and Study Designa . | n . | Ageb . | Neuropsychological Assessment Cognition or Behavior . | Test . | Air Pollution Exposure Period . | Pollutant . | Main Findingsc . |
|---|---|---|---|---|---|---|---|---|
| Cognitive and psychomotor development | ||||||||
| Calderón-Garcidueñas et al (2012) | Mexico Prospective cohort (I) | 20 | 7 y | Global IQ | WISC-R | Postnatal (livelong residency in Mexico City) | CO, NO, O3, PM, SO2, lead: | ↑ Cognitive performance on measures related to temporal/parietal/frontal neurocognitive networks |
| Clark et al (2012) | United Kingdom Cross-sectional | 960 | 9–10 y | Reading comprehension | Suffolk reading scale | Postnatal | NO2: | — |
| Episodic memory | Child memory scale | |||||||
| Working memory | “The search and the memory task” | |||||||
| Health | SDQ | |||||||
| Self-rated health | ||||||||
| Perera, Li et al (2012) | China Birth cohort (I) | 100 | 5 y | Global IQ | WPPSI | Prenatal (entire pregnancy) | PAH: | — Interaction with environmental tobacco smoke: ↓ global and verbal scores |
| van Kempen et al (2012) | The Netherlands Cross-sectional | 553 | 9–11 y | Reaction speed, attention, coordination, perceptual coding, span length | Neurobehavioral evaluation system: DMST HECT SAT SDST SRTT | Postnatal | NO2: | At school: ↓ span length At home: - At home and road traffic noise: ↓ reaction time |
| Chiu et al (2013) | United States Birth cohort (I) | 174 | 7–14 y | Attention | CPT | Postnatal (from birth to cognitive assessment) | BC: | ↑ Commission errors and ↓ reaction time among boys |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | Learning disability, special education | Parental report | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | — — — ↑ Special education, particularly in males — — — |
| Guxens et al (2014) | The Netherlands (I), Germany (I), France (II), Italy (I), Greece (I), Spain (V) Birth cohort | 9482 | 1–6 y | Cognitive and psychomotor development | ASQ BSID I-II-III DDST II MCDI MIDI MSCA | Prenatal (entire pregnancy) | NOx, PM2.5, PM2.5–10, PM10: | ↓ Psychomotor development, particularly NO2 No association with general cognition or language development |
| Kim et al (2014) | South Korea Birth cohort (I) | 520 | 6, 12, and 24 mo | Cognitive development | K-BSID-II | Prenatal (entire pregnancy) | NO2: | ↓ Psychomotor development |
| Psychomotor development | PM10: | ↓ Cognitive and psychomotor development | ||||||
| Lin et al (2014) | China Birth cohort (I) | 533 | 6 and 18 mo | Cognitive development Psychomotor development | 2 neurobehavioral development parental-reported scales | Prenatal and postnatal (beginning of gestational period to 18 mo of age) | CO: Hydrocarbons: NO2: O3: PM10: SO2: | — (2nd and 3rd trimesters): ↓ Gross motor scores at 6 mo of age — — — (all pregnancy to 12 months of age): ↓ Fine motor development at 18 mo of age |
| Lovasi et al (2014) | United States Birth cohort (I) | 277 | 5 y | Global IQ | WPPSI-R | Prenatal (from 20th wk of pregnancy) | PAH: | ↓ Global and verbal scores |
| Tang, Lee et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global, motor and adaptive scores |
| Tang, Li et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global and motor scores |
| Calderón-Garcidueñas et al (2015) | Mexico Cross-sectional | 50 | 13.4 (4.8) y | The impact of the APOE status (ε4 vs ε3) on global IQ | WISC-R | Lifelong residency in Mexico City (including pregnancy) | O3, PM: | (APOE ε4 children): ↓ Short and working memory, reasoning and knowledge and a negative difference for verbal and global IQ |
| Harris MH et al (2015) | United States Birth cohort (I) | 1109 | 6.6–10.9 y | Global IQ, visual motor abilities, visual memory | KBIT-2, WRAML2 (visual memory index), WRAVMA (visual-motor subtest) | Prenatal (3rd trimester of pregnancy) and postnatal (the year before each cognitive assessment, the first 6 y of life) | BC: PM2.5: Prenatal roadway proximity: | — — ↓ Nonverbal IQ and↓ visual motor abilities |
| Jedrychowski et al (2015) | Poland Birth cohort (I) | 170 | 7 y | Global IQ | WISC-R | Prenatal (from 8th to 13th wk of pregnancy) | PAH: | Depressed verbal IQ compared with nonverbal IQ |
| Kicinski et al (2015) | Belgium Cross-sectional | 606 | 13.6–17 y | Attention, manual motor speed | Neurobehavioral evaluation system: Continuous performance test, Digit span test, Finger tapping test | Postnatal | Traffic exposure: | ↓ Sustained attention |
| Sunyer et al (2015) | Spain Prospective cohort (I) | 2715 | 7–10 y | Working memory, attention | ANT N-back task | Postnatal (throughout 1 y of follow-up) | EC, NO2, UFP: | ↓ Working memory and attention |
| Neurobehavior | ||||||||
| Perera, Tang et al (2012)f | United States Birth cohort (I) | 253 | 6–7 y | Behavior (anxiety/depression and attention problems) | CBCL | Prenatal (3rd trimester of pregnancy) | PAH: | ↑ Symptoms of anxiety/depression and attention problems |
| Becerra et al (2013) | United States Case-control | 83 229 7594 cases 75 635 controls | 3–5 y | ASD | DSM-IV-R | Prenatal (entire pregnancy) | CO: NO: NO2: O3: PM2.5: PM10: | — ↑ ASD ↑ ASD ↑ ASD ↑ ASD — |
| Jung et al (2013) | China Prospective cohort (I) | 49 073 | 6.26 (2.91) y | ASD | ICD-9-CM | Postnatal (preceding 1–4 y newly diagnostic ASD) | CO: NO2: O3: PM10: SO2: | ↑ ASD ↑ ASD ↑ ASD — ↑ ASD |
| Newman et al (2013) | United States Birth cohort (I) | 576 | 7 y | Behavior | BASC-2 | Postnatal (1st year of life) | ECAT: | ↑ hyperactivity scores (stronger association in children whose mothers had higher education) |
| Roberts et al (2013)g | United States Birth cohort (I) | 22 426 | Not provided | ASD | Parental report ADI-R to 50 randomly selected cases | Perinatal | Antimony: Arsenic: Cadmium: Chromium: Diesel particulate: Lead: Manganese: Mercury: Methylene chloride: Nickel: Quinoline: Styrene: Trichloro-ethylene: Vinyl chloride: | — — — — ↑ ASD ↑ ASD ↑ ASD — — ↑ ASD — — — — |
| Volk et al (2013) | United States Case-control | 524 279 cases 245 controls | 24–60 mo | ASD | ADI-R ADOS Mullen scales of early learning. Vineland adaptive behavior scales | Prenatal and postnatal (entire pregnancy and 1st year of life) | NO2: O3: PM2.5: PM10: | ↑ ASD — ↑ ASD ↑ ASD |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | ADHD | Parental report (of ever-doctor diagnosed ADHD) | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | ↓ ADHD and learning disability among females — ↓ ADHD — — — — |
| Gong et al (2014) | Sweden Birth cohort (I) | 3426 | 9–12 y | ASD, ADHD | A-TAC | Prenatal and postnatal (entire pregnancy, 1st y and 9th y) | NOx: PM10: | — — |
| Perera et al (2014)f | United States Birth cohort (I) | 250 | 9 y | ADHD | CBCL CPRS | Prenatal (cord and maternal blood) | PAH: | ↑ CPRS-DSM oriented ADHD problems |
| Volk et al (2014) | United States Case-control | 407 251 cases 156 controls | 2–5 y | The relationship of air pollution exposure and genotype (MET rs1858830 CC) with ASD | ADI-R ADOS | Prenatal (entire pregnancy) | PM2.5: PM10: O3: NO2: | ↑ ASD ↑ ASD — ↑ ASD MET rs1858830 CC genotype and high air pollutant exposure: ↑ ASD |
| Von Ehrenstein et al (2014) | United States Birth cohort (I) | 768 | 31–71 mo | ASD | DSM-IV-R | Prenatal (entire pregnancy) | 24 toxics, including aromatic solvents, chlorinated solvents, volatile organics, total PAHs, and metals | ↑ ASD; particularly, exposure during 1st trimester |
| Guxens et al (2015) | Sweden (I), the Netherlands (I), Italy (I), Spain (III) Birth cohorts and child cohort | 8079 | 4–10 y | Autistic traits | A-TAC CAST CBCL SRS | Prenatal (entire pregnancy) | NOx: PM2.5: PM10: PM2.5–10: PM2.5absorbance | — — — — — |
| Kalkbrenner et al (2015) | United States Case-control | 15 645 979 cases 14 666 controls | 8 y | ASD | DSM-IV-R | Prenatal and postnatal (1 y before birth through 1st birthday) | PM10: | ↑ ASD; particularly exposure during 3rd trimester of pregnancy |
| Raz et al (2015) g | United States Nested Case-control | 1767 245 cases 1522 controls | Not provided | ASD | ADOS Maternal report SRS | Prenatal and postnatal (9 mo before pregnancy, entire pregnancy and 9 mo after birth) | PM2.5: PM10–2.5: | ↑ ASD; stronger association for males; exposure during 3rd trimester of pregnancy and 9 mo after birth Little association with ASD |
| Talbott et al (2015) | United States Case-control | 430 211 cases 219 controls | 3–7 y | ASD | ADOS SCQ | Prenatal and postnatal (3 mo before, entire pregnancy, 1 and 2 y after) | PM2.5: | ↑ ASD; particularly, postnatal y 2 and prepregnancy through pregnancy |
Cr, Chromium; ECAT, EC attributed to traffic; Mn, manganese; Ni, nickel; WMH, White matter hyperintensities; ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedules; ANT, Attentional Network Test; ASQ, Ages and Stages Questionnaire; A-TAC, Autism-Tics, ADHD, and other Comorbidities inventory; BASC-2, Behavioral Assessment System for Children, Parent Rating Scale, 2nd Edition; BSID, Bayley Scales of Infant Development (I-first edition, II-second-edition, III-3rd Edition); CBCL, Child Behavior Checklist; CPRS, Conners Parent Rating Scale-Revised; CPT, Conner's Continuous Performance Test; DDST II, Denver Development Screening Test II; DMST, the Digit Memory Span Test; DSM-IV-R, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; GDS, Gesell Developmental Scale; HECT, the Hand-Eye Coordination Test; ICD-9_CM, International Classification of Diseases, 9th Revision, Clinical Modification; KBIT-2, Kaufman Brief Intelligence Test; K-BSID-II, Korean version of Bayley Scales of Infant Development II; MCDI, McArthur Communicative Development Inventory; MIDI, Minnesota Infant Development Inventory; MRI, Magnetic Resonance Imaging; MRS, Brain magnetic spectroscopy imaging; MSCA, McCarthy Scales of Children's Abilities; SCQ, Social Communication Questionnaire; SDQ, Strengths and Difficulties Questionnaire; SAT, the Switching Attention Test; SDST, the Symbol Digit Substitution Test; SRS, Social Responsiveness Scale; SRTT, the Simple Reaction Time Test; WISC-R, Wechsler Intelligence Scale for Children-Revised; WPPSI-R, Wechsler Preschool and Primary Scale of Intelligence-Revised; WRAML2, Wide Range Assessment of Memory and Learning; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Different study populations coming from a same country are indicated with (I), (II), (III), (IV), or (V). For instance, (III) means 3 different study populations from the same country.
At the time of outcome assessment.
Statistically significant main findings. Cognitive and psychomotor development outcomes: (↑) increased development with increasing exposure, (↓) decreased development with increasing exposure, and (-) association was not observed. Neurobehavior outcomes: (↑) Increasing odds with increasing exposure level, (↓) decreasing odds with increasing exposure level, and (-) association was not observed.
This is the same article including cognitive and neurobehavior outcomes.
The same cohort.
The same cohort evaluated at different ages.
The same cohort.
Epidemiological Studies on Air Pollution and Neuropsychological Development
| Author (y) . | Country and Study Designa . | n . | Ageb . | Neuropsychological Assessment Cognition or Behavior . | Test . | Air Pollution Exposure Period . | Pollutant . | Main Findingsc . |
|---|---|---|---|---|---|---|---|---|
| Cognitive and psychomotor development | ||||||||
| Calderón-Garcidueñas et al (2012) | Mexico Prospective cohort (I) | 20 | 7 y | Global IQ | WISC-R | Postnatal (livelong residency in Mexico City) | CO, NO, O3, PM, SO2, lead: | ↑ Cognitive performance on measures related to temporal/parietal/frontal neurocognitive networks |
| Clark et al (2012) | United Kingdom Cross-sectional | 960 | 9–10 y | Reading comprehension | Suffolk reading scale | Postnatal | NO2: | — |
| Episodic memory | Child memory scale | |||||||
| Working memory | “The search and the memory task” | |||||||
| Health | SDQ | |||||||
| Self-rated health | ||||||||
| Perera, Li et al (2012) | China Birth cohort (I) | 100 | 5 y | Global IQ | WPPSI | Prenatal (entire pregnancy) | PAH: | — Interaction with environmental tobacco smoke: ↓ global and verbal scores |
| van Kempen et al (2012) | The Netherlands Cross-sectional | 553 | 9–11 y | Reaction speed, attention, coordination, perceptual coding, span length | Neurobehavioral evaluation system: DMST HECT SAT SDST SRTT | Postnatal | NO2: | At school: ↓ span length At home: - At home and road traffic noise: ↓ reaction time |
| Chiu et al (2013) | United States Birth cohort (I) | 174 | 7–14 y | Attention | CPT | Postnatal (from birth to cognitive assessment) | BC: | ↑ Commission errors and ↓ reaction time among boys |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | Learning disability, special education | Parental report | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | — — — ↑ Special education, particularly in males — — — |
| Guxens et al (2014) | The Netherlands (I), Germany (I), France (II), Italy (I), Greece (I), Spain (V) Birth cohort | 9482 | 1–6 y | Cognitive and psychomotor development | ASQ BSID I-II-III DDST II MCDI MIDI MSCA | Prenatal (entire pregnancy) | NOx, PM2.5, PM2.5–10, PM10: | ↓ Psychomotor development, particularly NO2 No association with general cognition or language development |
| Kim et al (2014) | South Korea Birth cohort (I) | 520 | 6, 12, and 24 mo | Cognitive development | K-BSID-II | Prenatal (entire pregnancy) | NO2: | ↓ Psychomotor development |
| Psychomotor development | PM10: | ↓ Cognitive and psychomotor development | ||||||
| Lin et al (2014) | China Birth cohort (I) | 533 | 6 and 18 mo | Cognitive development Psychomotor development | 2 neurobehavioral development parental-reported scales | Prenatal and postnatal (beginning of gestational period to 18 mo of age) | CO: Hydrocarbons: NO2: O3: PM10: SO2: | — (2nd and 3rd trimesters): ↓ Gross motor scores at 6 mo of age — — — (all pregnancy to 12 months of age): ↓ Fine motor development at 18 mo of age |
| Lovasi et al (2014) | United States Birth cohort (I) | 277 | 5 y | Global IQ | WPPSI-R | Prenatal (from 20th wk of pregnancy) | PAH: | ↓ Global and verbal scores |
| Tang, Lee et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global, motor and adaptive scores |
| Tang, Li et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global and motor scores |
| Calderón-Garcidueñas et al (2015) | Mexico Cross-sectional | 50 | 13.4 (4.8) y | The impact of the APOE status (ε4 vs ε3) on global IQ | WISC-R | Lifelong residency in Mexico City (including pregnancy) | O3, PM: | (APOE ε4 children): ↓ Short and working memory, reasoning and knowledge and a negative difference for verbal and global IQ |
| Harris MH et al (2015) | United States Birth cohort (I) | 1109 | 6.6–10.9 y | Global IQ, visual motor abilities, visual memory | KBIT-2, WRAML2 (visual memory index), WRAVMA (visual-motor subtest) | Prenatal (3rd trimester of pregnancy) and postnatal (the year before each cognitive assessment, the first 6 y of life) | BC: PM2.5: Prenatal roadway proximity: | — — ↓ Nonverbal IQ and↓ visual motor abilities |
| Jedrychowski et al (2015) | Poland Birth cohort (I) | 170 | 7 y | Global IQ | WISC-R | Prenatal (from 8th to 13th wk of pregnancy) | PAH: | Depressed verbal IQ compared with nonverbal IQ |
| Kicinski et al (2015) | Belgium Cross-sectional | 606 | 13.6–17 y | Attention, manual motor speed | Neurobehavioral evaluation system: Continuous performance test, Digit span test, Finger tapping test | Postnatal | Traffic exposure: | ↓ Sustained attention |
| Sunyer et al (2015) | Spain Prospective cohort (I) | 2715 | 7–10 y | Working memory, attention | ANT N-back task | Postnatal (throughout 1 y of follow-up) | EC, NO2, UFP: | ↓ Working memory and attention |
| Neurobehavior | ||||||||
| Perera, Tang et al (2012)f | United States Birth cohort (I) | 253 | 6–7 y | Behavior (anxiety/depression and attention problems) | CBCL | Prenatal (3rd trimester of pregnancy) | PAH: | ↑ Symptoms of anxiety/depression and attention problems |
| Becerra et al (2013) | United States Case-control | 83 229 7594 cases 75 635 controls | 3–5 y | ASD | DSM-IV-R | Prenatal (entire pregnancy) | CO: NO: NO2: O3: PM2.5: PM10: | — ↑ ASD ↑ ASD ↑ ASD ↑ ASD — |
| Jung et al (2013) | China Prospective cohort (I) | 49 073 | 6.26 (2.91) y | ASD | ICD-9-CM | Postnatal (preceding 1–4 y newly diagnostic ASD) | CO: NO2: O3: PM10: SO2: | ↑ ASD ↑ ASD ↑ ASD — ↑ ASD |
| Newman et al (2013) | United States Birth cohort (I) | 576 | 7 y | Behavior | BASC-2 | Postnatal (1st year of life) | ECAT: | ↑ hyperactivity scores (stronger association in children whose mothers had higher education) |
| Roberts et al (2013)g | United States Birth cohort (I) | 22 426 | Not provided | ASD | Parental report ADI-R to 50 randomly selected cases | Perinatal | Antimony: Arsenic: Cadmium: Chromium: Diesel particulate: Lead: Manganese: Mercury: Methylene chloride: Nickel: Quinoline: Styrene: Trichloro-ethylene: Vinyl chloride: | — — — — ↑ ASD ↑ ASD ↑ ASD — — ↑ ASD — — — — |
| Volk et al (2013) | United States Case-control | 524 279 cases 245 controls | 24–60 mo | ASD | ADI-R ADOS Mullen scales of early learning. Vineland adaptive behavior scales | Prenatal and postnatal (entire pregnancy and 1st year of life) | NO2: O3: PM2.5: PM10: | ↑ ASD — ↑ ASD ↑ ASD |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | ADHD | Parental report (of ever-doctor diagnosed ADHD) | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | ↓ ADHD and learning disability among females — ↓ ADHD — — — — |
| Gong et al (2014) | Sweden Birth cohort (I) | 3426 | 9–12 y | ASD, ADHD | A-TAC | Prenatal and postnatal (entire pregnancy, 1st y and 9th y) | NOx: PM10: | — — |
| Perera et al (2014)f | United States Birth cohort (I) | 250 | 9 y | ADHD | CBCL CPRS | Prenatal (cord and maternal blood) | PAH: | ↑ CPRS-DSM oriented ADHD problems |
| Volk et al (2014) | United States Case-control | 407 251 cases 156 controls | 2–5 y | The relationship of air pollution exposure and genotype (MET rs1858830 CC) with ASD | ADI-R ADOS | Prenatal (entire pregnancy) | PM2.5: PM10: O3: NO2: | ↑ ASD ↑ ASD — ↑ ASD MET rs1858830 CC genotype and high air pollutant exposure: ↑ ASD |
| Von Ehrenstein et al (2014) | United States Birth cohort (I) | 768 | 31–71 mo | ASD | DSM-IV-R | Prenatal (entire pregnancy) | 24 toxics, including aromatic solvents, chlorinated solvents, volatile organics, total PAHs, and metals | ↑ ASD; particularly, exposure during 1st trimester |
| Guxens et al (2015) | Sweden (I), the Netherlands (I), Italy (I), Spain (III) Birth cohorts and child cohort | 8079 | 4–10 y | Autistic traits | A-TAC CAST CBCL SRS | Prenatal (entire pregnancy) | NOx: PM2.5: PM10: PM2.5–10: PM2.5absorbance | — — — — — |
| Kalkbrenner et al (2015) | United States Case-control | 15 645 979 cases 14 666 controls | 8 y | ASD | DSM-IV-R | Prenatal and postnatal (1 y before birth through 1st birthday) | PM10: | ↑ ASD; particularly exposure during 3rd trimester of pregnancy |
| Raz et al (2015) g | United States Nested Case-control | 1767 245 cases 1522 controls | Not provided | ASD | ADOS Maternal report SRS | Prenatal and postnatal (9 mo before pregnancy, entire pregnancy and 9 mo after birth) | PM2.5: PM10–2.5: | ↑ ASD; stronger association for males; exposure during 3rd trimester of pregnancy and 9 mo after birth Little association with ASD |
| Talbott et al (2015) | United States Case-control | 430 211 cases 219 controls | 3–7 y | ASD | ADOS SCQ | Prenatal and postnatal (3 mo before, entire pregnancy, 1 and 2 y after) | PM2.5: | ↑ ASD; particularly, postnatal y 2 and prepregnancy through pregnancy |
| Author (y) . | Country and Study Designa . | n . | Ageb . | Neuropsychological Assessment Cognition or Behavior . | Test . | Air Pollution Exposure Period . | Pollutant . | Main Findingsc . |
|---|---|---|---|---|---|---|---|---|
| Cognitive and psychomotor development | ||||||||
| Calderón-Garcidueñas et al (2012) | Mexico Prospective cohort (I) | 20 | 7 y | Global IQ | WISC-R | Postnatal (livelong residency in Mexico City) | CO, NO, O3, PM, SO2, lead: | ↑ Cognitive performance on measures related to temporal/parietal/frontal neurocognitive networks |
| Clark et al (2012) | United Kingdom Cross-sectional | 960 | 9–10 y | Reading comprehension | Suffolk reading scale | Postnatal | NO2: | — |
| Episodic memory | Child memory scale | |||||||
| Working memory | “The search and the memory task” | |||||||
| Health | SDQ | |||||||
| Self-rated health | ||||||||
| Perera, Li et al (2012) | China Birth cohort (I) | 100 | 5 y | Global IQ | WPPSI | Prenatal (entire pregnancy) | PAH: | — Interaction with environmental tobacco smoke: ↓ global and verbal scores |
| van Kempen et al (2012) | The Netherlands Cross-sectional | 553 | 9–11 y | Reaction speed, attention, coordination, perceptual coding, span length | Neurobehavioral evaluation system: DMST HECT SAT SDST SRTT | Postnatal | NO2: | At school: ↓ span length At home: - At home and road traffic noise: ↓ reaction time |
| Chiu et al (2013) | United States Birth cohort (I) | 174 | 7–14 y | Attention | CPT | Postnatal (from birth to cognitive assessment) | BC: | ↑ Commission errors and ↓ reaction time among boys |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | Learning disability, special education | Parental report | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | — — — ↑ Special education, particularly in males — — — |
| Guxens et al (2014) | The Netherlands (I), Germany (I), France (II), Italy (I), Greece (I), Spain (V) Birth cohort | 9482 | 1–6 y | Cognitive and psychomotor development | ASQ BSID I-II-III DDST II MCDI MIDI MSCA | Prenatal (entire pregnancy) | NOx, PM2.5, PM2.5–10, PM10: | ↓ Psychomotor development, particularly NO2 No association with general cognition or language development |
| Kim et al (2014) | South Korea Birth cohort (I) | 520 | 6, 12, and 24 mo | Cognitive development | K-BSID-II | Prenatal (entire pregnancy) | NO2: | ↓ Psychomotor development |
| Psychomotor development | PM10: | ↓ Cognitive and psychomotor development | ||||||
| Lin et al (2014) | China Birth cohort (I) | 533 | 6 and 18 mo | Cognitive development Psychomotor development | 2 neurobehavioral development parental-reported scales | Prenatal and postnatal (beginning of gestational period to 18 mo of age) | CO: Hydrocarbons: NO2: O3: PM10: SO2: | — (2nd and 3rd trimesters): ↓ Gross motor scores at 6 mo of age — — — (all pregnancy to 12 months of age): ↓ Fine motor development at 18 mo of age |
| Lovasi et al (2014) | United States Birth cohort (I) | 277 | 5 y | Global IQ | WPPSI-R | Prenatal (from 20th wk of pregnancy) | PAH: | ↓ Global and verbal scores |
| Tang, Lee et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global, motor and adaptive scores |
| Tang, Li et al (2014)e | China Birth cohort (II) | 308 | 2 y | Global IQ | GDS | Prenatal (cord and maternal blood) | PAH: | ↓ Global and motor scores |
| Calderón-Garcidueñas et al (2015) | Mexico Cross-sectional | 50 | 13.4 (4.8) y | The impact of the APOE status (ε4 vs ε3) on global IQ | WISC-R | Lifelong residency in Mexico City (including pregnancy) | O3, PM: | (APOE ε4 children): ↓ Short and working memory, reasoning and knowledge and a negative difference for verbal and global IQ |
| Harris MH et al (2015) | United States Birth cohort (I) | 1109 | 6.6–10.9 y | Global IQ, visual motor abilities, visual memory | KBIT-2, WRAML2 (visual memory index), WRAVMA (visual-motor subtest) | Prenatal (3rd trimester of pregnancy) and postnatal (the year before each cognitive assessment, the first 6 y of life) | BC: PM2.5: Prenatal roadway proximity: | — — ↓ Nonverbal IQ and↓ visual motor abilities |
| Jedrychowski et al (2015) | Poland Birth cohort (I) | 170 | 7 y | Global IQ | WISC-R | Prenatal (from 8th to 13th wk of pregnancy) | PAH: | Depressed verbal IQ compared with nonverbal IQ |
| Kicinski et al (2015) | Belgium Cross-sectional | 606 | 13.6–17 y | Attention, manual motor speed | Neurobehavioral evaluation system: Continuous performance test, Digit span test, Finger tapping test | Postnatal | Traffic exposure: | ↓ Sustained attention |
| Sunyer et al (2015) | Spain Prospective cohort (I) | 2715 | 7–10 y | Working memory, attention | ANT N-back task | Postnatal (throughout 1 y of follow-up) | EC, NO2, UFP: | ↓ Working memory and attention |
| Neurobehavior | ||||||||
| Perera, Tang et al (2012)f | United States Birth cohort (I) | 253 | 6–7 y | Behavior (anxiety/depression and attention problems) | CBCL | Prenatal (3rd trimester of pregnancy) | PAH: | ↑ Symptoms of anxiety/depression and attention problems |
| Becerra et al (2013) | United States Case-control | 83 229 7594 cases 75 635 controls | 3–5 y | ASD | DSM-IV-R | Prenatal (entire pregnancy) | CO: NO: NO2: O3: PM2.5: PM10: | — ↑ ASD ↑ ASD ↑ ASD ↑ ASD — |
| Jung et al (2013) | China Prospective cohort (I) | 49 073 | 6.26 (2.91) y | ASD | ICD-9-CM | Postnatal (preceding 1–4 y newly diagnostic ASD) | CO: NO2: O3: PM10: SO2: | ↑ ASD ↑ ASD ↑ ASD — ↑ ASD |
| Newman et al (2013) | United States Birth cohort (I) | 576 | 7 y | Behavior | BASC-2 | Postnatal (1st year of life) | ECAT: | ↑ hyperactivity scores (stronger association in children whose mothers had higher education) |
| Roberts et al (2013)g | United States Birth cohort (I) | 22 426 | Not provided | ASD | Parental report ADI-R to 50 randomly selected cases | Perinatal | Antimony: Arsenic: Cadmium: Chromium: Diesel particulate: Lead: Manganese: Mercury: Methylene chloride: Nickel: Quinoline: Styrene: Trichloro-ethylene: Vinyl chloride: | — — — — ↑ ASD ↑ ASD ↑ ASD — — ↑ ASD — — — — |
| Volk et al (2013) | United States Case-control | 524 279 cases 245 controls | 24–60 mo | ASD | ADI-R ADOS Mullen scales of early learning. Vineland adaptive behavior scales | Prenatal and postnatal (entire pregnancy and 1st year of life) | NO2: O3: PM2.5: PM10: | ↑ ASD — ↑ ASD ↑ ASD |
| Abid et al (2014)d | United States Cross-sectional | 1257 | 6–15 y | ADHD | Parental report (of ever-doctor diagnosed ADHD) | Postnatal | PAH 1-pyrene: 1-napthol: 2-napthol: 2-fluorene and 3-fluorene: 1-phenanthrene: 2-phenanthrene: 3-phenanthrene: | ↓ ADHD and learning disability among females — ↓ ADHD — — — — |
| Gong et al (2014) | Sweden Birth cohort (I) | 3426 | 9–12 y | ASD, ADHD | A-TAC | Prenatal and postnatal (entire pregnancy, 1st y and 9th y) | NOx: PM10: | — — |
| Perera et al (2014)f | United States Birth cohort (I) | 250 | 9 y | ADHD | CBCL CPRS | Prenatal (cord and maternal blood) | PAH: | ↑ CPRS-DSM oriented ADHD problems |
| Volk et al (2014) | United States Case-control | 407 251 cases 156 controls | 2–5 y | The relationship of air pollution exposure and genotype (MET rs1858830 CC) with ASD | ADI-R ADOS | Prenatal (entire pregnancy) | PM2.5: PM10: O3: NO2: | ↑ ASD ↑ ASD — ↑ ASD MET rs1858830 CC genotype and high air pollutant exposure: ↑ ASD |
| Von Ehrenstein et al (2014) | United States Birth cohort (I) | 768 | 31–71 mo | ASD | DSM-IV-R | Prenatal (entire pregnancy) | 24 toxics, including aromatic solvents, chlorinated solvents, volatile organics, total PAHs, and metals | ↑ ASD; particularly, exposure during 1st trimester |
| Guxens et al (2015) | Sweden (I), the Netherlands (I), Italy (I), Spain (III) Birth cohorts and child cohort | 8079 | 4–10 y | Autistic traits | A-TAC CAST CBCL SRS | Prenatal (entire pregnancy) | NOx: PM2.5: PM10: PM2.5–10: PM2.5absorbance | — — — — — |
| Kalkbrenner et al (2015) | United States Case-control | 15 645 979 cases 14 666 controls | 8 y | ASD | DSM-IV-R | Prenatal and postnatal (1 y before birth through 1st birthday) | PM10: | ↑ ASD; particularly exposure during 3rd trimester of pregnancy |
| Raz et al (2015) g | United States Nested Case-control | 1767 245 cases 1522 controls | Not provided | ASD | ADOS Maternal report SRS | Prenatal and postnatal (9 mo before pregnancy, entire pregnancy and 9 mo after birth) | PM2.5: PM10–2.5: | ↑ ASD; stronger association for males; exposure during 3rd trimester of pregnancy and 9 mo after birth Little association with ASD |
| Talbott et al (2015) | United States Case-control | 430 211 cases 219 controls | 3–7 y | ASD | ADOS SCQ | Prenatal and postnatal (3 mo before, entire pregnancy, 1 and 2 y after) | PM2.5: | ↑ ASD; particularly, postnatal y 2 and prepregnancy through pregnancy |
Cr, Chromium; ECAT, EC attributed to traffic; Mn, manganese; Ni, nickel; WMH, White matter hyperintensities; ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedules; ANT, Attentional Network Test; ASQ, Ages and Stages Questionnaire; A-TAC, Autism-Tics, ADHD, and other Comorbidities inventory; BASC-2, Behavioral Assessment System for Children, Parent Rating Scale, 2nd Edition; BSID, Bayley Scales of Infant Development (I-first edition, II-second-edition, III-3rd Edition); CBCL, Child Behavior Checklist; CPRS, Conners Parent Rating Scale-Revised; CPT, Conner's Continuous Performance Test; DDST II, Denver Development Screening Test II; DMST, the Digit Memory Span Test; DSM-IV-R, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; GDS, Gesell Developmental Scale; HECT, the Hand-Eye Coordination Test; ICD-9_CM, International Classification of Diseases, 9th Revision, Clinical Modification; KBIT-2, Kaufman Brief Intelligence Test; K-BSID-II, Korean version of Bayley Scales of Infant Development II; MCDI, McArthur Communicative Development Inventory; MIDI, Minnesota Infant Development Inventory; MRI, Magnetic Resonance Imaging; MRS, Brain magnetic spectroscopy imaging; MSCA, McCarthy Scales of Children's Abilities; SCQ, Social Communication Questionnaire; SDQ, Strengths and Difficulties Questionnaire; SAT, the Switching Attention Test; SDST, the Symbol Digit Substitution Test; SRS, Social Responsiveness Scale; SRTT, the Simple Reaction Time Test; WISC-R, Wechsler Intelligence Scale for Children-Revised; WPPSI-R, Wechsler Preschool and Primary Scale of Intelligence-Revised; WRAML2, Wide Range Assessment of Memory and Learning; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Different study populations coming from a same country are indicated with (I), (II), (III), (IV), or (V). For instance, (III) means 3 different study populations from the same country.
At the time of outcome assessment.
Statistically significant main findings. Cognitive and psychomotor development outcomes: (↑) increased development with increasing exposure, (↓) decreased development with increasing exposure, and (-) association was not observed. Neurobehavior outcomes: (↑) Increasing odds with increasing exposure level, (↓) decreasing odds with increasing exposure level, and (-) association was not observed.
This is the same article including cognitive and neurobehavior outcomes.
The same cohort.
The same cohort evaluated at different ages.
The same cohort.
Global IQ
Eleven studies evaluated the association of air pollutants with global IQ or infant cognitive development (Table 1). Five studies reported delayed global, verbal and/or psychomotor development in relation to prenatal exposure to PAHs (16–20). However, in 1 study, it was the interaction between adducts and environmental tobacco smoke that adversely affected global and verbal development (18). Two studies found an association between increasing NO2 exposure during pregnancy and delayed psychomotor development (21, 22); although in Kim et al (22), the association observed at 6 months did not persist at the age of 12 and 24 months. One study reported that increasing maternal exposure to PM10 reduced cognitive and psychomotor development (22). In Tang et al (20), exposure to PHAs was assessed pre- and postnatally in 2 birth cohorts before and after the shutdown of a coal-fired power plant; the authors encountered global and motor developmental delays. In another study, pre- and postnatal exposure to SO2 and hydrocarbons associated to psychomotor development delay (23). Pre- and postnatal exposure to PM and O3 associated with below-average scores in global and verbal development in children carrying the apolipoprotein E ϵ4 allele, the most prevalent genetic risk for Alzheimer's disease (24). Another study reported no association between pre- and postnatal exposure to PM2.5 or BC and neuropsychological development, although roadway proximity associated with detrimental nonverbal IQ and visual motor abilities (25). On the other hand, a prospective cohort study found an improvement in cognitive performance in relation to increasing postnatal exposure to air pollutants (CO, NO, O3, PM, SO2, and lead) (26).
Executive functions
None of the studies included in this review evaluated the effects of prenatal exposure to air pollutants on executive functions. Five studies investigated the effects of postnatal exposure to different air pollutants (NO2, EC, BC, and ultrafine particle [UFP]) and executive functions (attention and working memory); 4 of them reported adverse health effects of these pollutants (27–30), although in Chiu et al (27), the association was only observed in boys. One cross-sectional study did not find any association between NO2 and working memory (31).
Memory
An American birth cohort study with pre- and postnatal measures of exposure to BC and PM2.5 did not find any association with visual memory (25). Furthermore, in the cross-sectional study by Clark et al (31), no associations were reported between postnatal exposure to NO2 and episodic memory.
Visual motor abilities
Prenatal exposure to PM2.5 or BC did not associate with a poorer visual motor performance, although children prenatally living less than 50 m away from a major roadway did show lower visual motor abilities (25). Other studies did not report any association between postnatal exposure to NO2 and visual motor abilities (30) or lower manual motor speed associated with postnatal traffic exposure (28).
Academic skills
A cross-sectional study carried out in the United States found a positive association between postnatal exposure to PAHs and special education needs in males (32). Clark et al (31) did not find any associations between postnatal exposure to NO2 and reading comprehension.
In summary, the evidence of an association between pre- or postnatal exposure to PAHs and decrements in global IQ is sufficient. For other exposures the evidence is inadequate or insufficient. There is inadequate or insufficient evidence for associations on other specific cognitive functions such as executive function, memory, or visual motor abilities. Furthermore, it was not possible to distinguish a critical period of exposure due to the heterogeneity of the analysis approaches and results encountered among studies (Table 2).
Summary of the Evidence
| . | PM . | NOx . | O3 . | CO . | SO2 . | Other Air Toxics . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| UFP . | PM2.5 . | PM10 . | PAHs . | BC, EC . | ||||||
| Cognitive and psychomotor development | ||||||||||
| Global IQ | — | Inadequate | Inadequate | Sufficient | Insufficient | Inadequate | Inadequate | Insufficient | Inadequate | Inadequate |
| Executive functions | Inadequate | — | — | — | Inadequate | Inadequate | — | — | — | — |
| Memory | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Visual motor abilities | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Academic skills | — | — | — | Inadequate | — | Insufficient | — | — | — | — |
| Neurobehavior | ||||||||||
| ASD | — | Sufficient | Inadequate | — | — | Limited | Inadequate | Inadequate | Inadequate | Inadequate |
| ADHD | — | — | Insufficient | Inadequate | — | Insufficient | — | — | — | — |
| Behavioral problems | — | — | — | Inadequate | Inadequate | — | — | — | — | — |
| . | PM . | NOx . | O3 . | CO . | SO2 . | Other Air Toxics . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| UFP . | PM2.5 . | PM10 . | PAHs . | BC, EC . | ||||||
| Cognitive and psychomotor development | ||||||||||
| Global IQ | — | Inadequate | Inadequate | Sufficient | Insufficient | Inadequate | Inadequate | Insufficient | Inadequate | Inadequate |
| Executive functions | Inadequate | — | — | — | Inadequate | Inadequate | — | — | — | — |
| Memory | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Visual motor abilities | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Academic skills | — | — | — | Inadequate | — | Insufficient | — | — | — | — |
| Neurobehavior | ||||||||||
| ASD | — | Sufficient | Inadequate | — | — | Limited | Inadequate | Inadequate | Inadequate | Inadequate |
| ADHD | — | — | Insufficient | Inadequate | — | Insufficient | — | — | — | — |
| Behavioral problems | — | — | — | Inadequate | Inadequate | — | — | — | — | — |
Sufficient, if most of the studies, including good quality studies, report an association, but evidence is not yet conclusive enough to conclude that there is a causal relationship; limited, several good quality, independent, studies report an association, but evidence is not yet conclusive enough; inadequate, if associations are reported in 1 or more studies, but insufficient quality, insufficient number of studies, lack of consistency between studies, and/or lack of statistical power preclude a conclusion regarding the presence or absence of an association; insufficient, if no associations are reported in 1 or more studies, but insufficient quality, insufficient number of studies, lack of consistency between studies, and/or lack of statistical power preclude a conclusion regarding the presence or absence of an association; and evidence for lack of association, several good quality studies are consistent in not showing an association.
Summary of the Evidence
| . | PM . | NOx . | O3 . | CO . | SO2 . | Other Air Toxics . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| UFP . | PM2.5 . | PM10 . | PAHs . | BC, EC . | ||||||
| Cognitive and psychomotor development | ||||||||||
| Global IQ | — | Inadequate | Inadequate | Sufficient | Insufficient | Inadequate | Inadequate | Insufficient | Inadequate | Inadequate |
| Executive functions | Inadequate | — | — | — | Inadequate | Inadequate | — | — | — | — |
| Memory | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Visual motor abilities | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Academic skills | — | — | — | Inadequate | — | Insufficient | — | — | — | — |
| Neurobehavior | ||||||||||
| ASD | — | Sufficient | Inadequate | — | — | Limited | Inadequate | Inadequate | Inadequate | Inadequate |
| ADHD | — | — | Insufficient | Inadequate | — | Insufficient | — | — | — | — |
| Behavioral problems | — | — | — | Inadequate | Inadequate | — | — | — | — | — |
| . | PM . | NOx . | O3 . | CO . | SO2 . | Other Air Toxics . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| UFP . | PM2.5 . | PM10 . | PAHs . | BC, EC . | ||||||
| Cognitive and psychomotor development | ||||||||||
| Global IQ | — | Inadequate | Inadequate | Sufficient | Insufficient | Inadequate | Inadequate | Insufficient | Inadequate | Inadequate |
| Executive functions | Inadequate | — | — | — | Inadequate | Inadequate | — | — | — | — |
| Memory | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Visual motor abilities | — | Insufficient | — | — | Insufficient | Insufficient | — | — | — | — |
| Academic skills | — | — | — | Inadequate | — | Insufficient | — | — | — | — |
| Neurobehavior | ||||||||||
| ASD | — | Sufficient | Inadequate | — | — | Limited | Inadequate | Inadequate | Inadequate | Inadequate |
| ADHD | — | — | Insufficient | Inadequate | — | Insufficient | — | — | — | — |
| Behavioral problems | — | — | — | Inadequate | Inadequate | — | — | — | — | — |
Sufficient, if most of the studies, including good quality studies, report an association, but evidence is not yet conclusive enough to conclude that there is a causal relationship; limited, several good quality, independent, studies report an association, but evidence is not yet conclusive enough; inadequate, if associations are reported in 1 or more studies, but insufficient quality, insufficient number of studies, lack of consistency between studies, and/or lack of statistical power preclude a conclusion regarding the presence or absence of an association; insufficient, if no associations are reported in 1 or more studies, but insufficient quality, insufficient number of studies, lack of consistency between studies, and/or lack of statistical power preclude a conclusion regarding the presence or absence of an association; and evidence for lack of association, several good quality studies are consistent in not showing an association.
Air pollution and neurobehavior
Autism spectrum disorder (ASD)
Overall, 11 studies evaluated the association of ambient air pollution with ASD or autistic traits (Table 1). The case definition was based on validated instruments, meeting diagnostic criteria and/or parental reports, although in 2 studies screening tools were used. Three studies found an association between prenatal exposure to air pollutants and ASD; Becerra et al (33) reported an increased risk of ASD with increasing NOx, O3, and PM2.5 exposure. ASD development was related with increasing levels of several toxics (including 1,3-butadiene, meta/para-xylene, lead) during pregnancy in Von Ehrenstein et al (15). Volk et al (34) reported a gene-environment interaction between increasing air pollutant (NO2, PM2.5, and PM10) exposure and ASD in subjects with the MET rs1858830 CC genotype. Another study observed an increased odds of ASD in relation to perinatal exposure to diesel particulate, lead, manganese and nickel (35). Four other studies assessed pre- and postnatal exposure to NO2, PM2.5, and PM10 and associations with ASD were reported in all of them (36–39), particularly for PM2.5 (37–39). An increased odds of ASD diagnosis was reported in Jung et al in relation to postnatal exposure to CO, NO2, O3, and SO2 (40). However, 2 other studies in Europe encountered no association between prenatal exposure to NO2 and PM2.5 or PM10 with autistic traits (41) or between pre- and postnatal exposure to NOx, PM10, and ASD (42).
Attention deficit hyperactivity disorder (ADHD)
An American birth cohort study reported that increasing prenatal levels of PAHs associated with an increased odds of ADHD behavior problems (43). However, another birth cohort study from Sweden (42) and a cross-sectional American study (32) could not find any association between pre- and postnatal exposure to NOx and PM10 or postnatal exposure to PAH and ADHD, respectively.
Behavioral problems
Perera et al (44) reported an association between increasing prenatal exposure to PAH and increasing symptoms of anxiety/depression and attention problems in an American birth cohort. Newman et al (45) reported that increasing postnatal exposure to EC was associated with increasing hyperactivity scores, and this association was stronger in children whose mothers had higher education.
In summary, the evidence of an association between pre- or postnatal exposure to PM2.5 and ASD is sufficient. Furthermore, limited evidence was found for NOx and ASD. For the rest of the exposures, the evidence is inadequate. Current studies do not allow disentangling the critical period of exposure (pre- or postnatal) in the occurrence of ASD. There is inadequate or insufficient evidence of an association between air pollutants and ADHD or behavioral problems (Table 2).
Discussion
This review of the latest epidemiological studies found sufficient evidence for pre- or postnatal PAHs exposure to decreased global IQ. Results of available studies also indicate sufficient evidence between PM2.5 and ASD and limited evidence between NOx and ASD. For the other combinations of exposures and outcomes evidence is inadequate or insufficient due to the reduced number of studies, deficient quality or low consistency of the results between studies. Heterogeneity between studies in exposure (eg, methods used, applying individual vs multipollutant models to disentangle possible synergistic effects) and outcome assessment (eg, definition of cases, instruments used, screening vs diagnostic tools), hampers the possibility of conducting a metaanalysis.
Mechanisms
One of the first histopathological evidence of neuropathology associated to air pollution in animals was reported by Calderón-Garcidueñas and coworker (46) and Calderón-Garcidueñas et al (47, 48); in postmortem studies conducted on canines exposed to air pollutants, an accelerated Alzheimer's type pathology (chronic inflammation, neurodegeneration, and DNA damage in various brain regions) was observed. Evidence on the adverse CNS effects of air pollution in human and particularly animal studies has accumulated for over a decade (7, 49). Inhaled pollutants deposit in the respiratory tract and can translocate to the CNS via the olfactory epithelium, via the blood brain barrier or via the sensory afferents found in the gastrointestinal tract (7). The potential cellular mechanisms identified as responsible for CNS damage are neuroinflammation, oxidative stress, glial activation, and white matter injury (4, 46). A better understanding of the specific components of air pollution responsible for CNS damage and the molecular mechanisms involved in humans still needs further research.
Limitations of the current review
Articles published before 2012 were not included in the current review. However, our aim was to complement and update previous reviews with the latest evidence to date on the effects of air pollution on neuropsychological development in children. Publication bias cannot be discarded, because studies with no significant findings are less likely to be published. Finally, only articles published in English were included in this review.
Recommendations and conclusions
The future research should be addressed to identify the individual contribution of various air toxicants by applying multiple pollutant models. However, potential synergistic effects cannot be discarded. Biological pathways related to neuropsychological development also need further investigation. Studies including a more detailed exposure assessment are needed: PM composition, sources apportionment, long-term evaluations and identification of critical windows of exposure. Also, studies should account for residual confounding (eg, individual socioeconomic characteristics, neighborhood deprivation, exposure to environmental tobacco smoke, and traffic noise, because it could be associated with impaired cognitive development) (30, 31). Furthermore, structural and functional brain scans can help to understand better which brain areas and cognitive functions are the most vulnerable and affected. The gene-environment interactions should be further explored to examine the air pollution effects on the brain of genetically susceptible populations. Finally, the role of gender in the effects of air pollution deserves more investigation; preliminary findings in animal and human studies seem to support the hypothesis that males may be more susceptible to air pollution neurotoxicity than females (29, 49, 50).
In conclusion, the present review suggests an association between outdoor air pollution, particularly PAH, PM2.5, and NOx, and neuropsychological development in children. Together with the well-documented effects of air pollution on cardiovascular and pulmonary diseases, the public health impact cannot be ignored. The precautionary principle should be applied to protect the general population, and specially children given their vulnerability and the potential long-term effects of accumulated toxic exposure across life stages.
Acknowledgments
The research leading to these results has received funding from the European Research Council under the ERC Grant Agreement number 268479 – the BREATHE project and from the Spanish Instituto de Salud Carlos III (MS13/00054).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- BC
black carbon
- CNS
central nervous system
- CO
carbon monoxide
- EC
elemental carbon
- IQ
intelligence quotient
- NO2
nitrogen dioxide
- NOx
nitrogen oxides
- O3
ozone
- PAH
polycyclic aromatic hydrocarbon
- PM
particulate matter
- SO2
sulfur dioxide
- UFP
ultrafine particle.
References