-
PDF
- Split View
-
Views
-
Cite
Cite
Kathleen Abu-Saad, Drora Fraser, Maternal Nutrition and Birth Outcomes, Epidemiologic Reviews, Volume 32, Issue 1, April 2010, Pages 5–25, https://doi.org/10.1093/epirev/mxq001
Close - Share Icon Share
Abstract
In this review, the authors summarize current knowledge on maternal nutritional requirements during pregnancy, with a focus on the nutrients that have been most commonly investigated in association with birth outcomes. Data sourcing and extraction included searches of the primary resources establishing maternal nutrient requirements during pregnancy (e.g., Dietary Reference Intakes), and searches of Medline for “maternal nutrition”/[specific nutrient of interest] and “birth/pregnancy outcomes,” focusing mainly on the less extensively reviewed evidence from observational studies of maternal dietary intake and birth outcomes. The authors used a conceptual framework which took both primary and secondary factors (e.g., baseline maternal nutritional status, socioeconomic status of the study populations, timing and methods of assessing maternal nutritional variables) into account when interpreting study findings. The authors conclude that maternal nutrition is a modifiable risk factor of public health importance that can be integrated into efforts to prevent adverse birth outcomes, particularly among economically developing/low-income populations.
INTRODUCTION
Nutrition plays a major role in maternal and child health. Poor maternal nutritional status has been related to adverse birth outcomes; however, the association between maternal nutrition and birth outcome is complex and is influenced by many biologic, socioeconomic, and demographic factors, which vary widely in different populations (1). Understanding the relation between maternal nutrition and birth outcomes may provide a basis for developing nutritional interventions that will improve birth outcomes and long-term quality of life and reduce mortality, morbidity, and health-care costs.
Although the importance of maternal nutrition to fetal development and birth outcomes has been clearly demonstrated in experimental animal studies, the findings of studies in humans are much less consistent, due, to some extent, to secondary factors that differ from study to study (e.g., baseline maternal nutritional status, socioeconomic status (SES) of the study population, timing and methods of assessing or manipulating maternal nutritional variables). In addition, most of the studies and literature reviews dealing with maternal nutrition and birth outcomes have approached the issue by investigating single nutrients in isolation. On one level, this is necessary for an in-depth study of the complex issues involved. However, nutrient deficiencies are generally found in low-SES populations, where they are more likely to involve multiple rather than single deficiencies (2); and studies that address and bring together the broader picture of multiple nutrient intakes or deficiencies are lacking.
In this review, our intention is to provide a broad multinutrient and multifactorial overview of the literature regarding maternal nutrition and birth outcomes. We summarize current knowledge on maternal nutritional requirements during pregnancy and review studies of the nutrients/nutrient combinations that have been most commonly investigated in association with birth outcomes, including energy, protein, essential fatty acids (specifically omega-3 fatty acids), iron, folate, and multinutrient supplements. Other nutrients which have been studied in conjunction with birth/pregnancy outcomes (e.g., magnesium, zinc, calcium, vitamin C) but for which there is less evidence are not included because of space limitations. Given the breadth of the topic, we limit our focus to the 3 major adverse birth outcomes: low birth weight, preterm birth, and intrauterine growth restriction (IUGR). These adverse birth outcomes represent the leading causes of neonatal death among children born without congenital anomalies (3, 4) and often result in short- and long-term health problems/disabilities (5), including a possible predisposition to chronic disease in adult life (6). In addition, they have been researched extensively with regard to nutritional causation/mechanisms and may be modifiable through nutritional interventions. We do not cover pregnancy complications (e.g., preeclampsia and gestational diabetes) (7), which are outside of the scope of this review as we have defined it, or other adverse birth outcomes (e.g., congenital anomalies) that have been linked to maternal nutrition and have been quite extensively reviewed in the literature (8–10).
Data sourcing and extraction included searches of the primary resources establishing maternal nutrient requirements during pregnancy (e.g., Dietary Reference Intakes, determined by the National Academy of Sciences) and Medline (National Library of Medicine) searches encompassing “maternal nutrition”/[specific nutrient of interest]/“dietary intake” and “birth/pregnancy outcomes”/[specific adverse outcome of interest, e.g., preterm birth or birth weight]. We included primarily studies published from 2000 onward; however, where we deemed it important, occasionally studies published earlier than 2000 were also included.
We focused mainly upon the evidence from observational studies of maternal dietary intake and birth outcomes, because reviews of randomized controlled trials (RCTs) are plentiful and need not be replicated. The observational literature, however, has been less extensively reviewed. Furthermore, as we discuss below in the “Conceptual Models” section, because of the infeasibility of taking into account or controlling for factors and effect modifiers that precede or extend beyond the duration of most RCTs (or that differ from trial to trial in meta-analyses of RCTs), a number of scholars have cautioned that evidence from RCTs regarding nutrition and disease/health outcomes should not be taken in isolation but rather should be considered together with evidence from observational and experimental studies (11–14). Thus, our intention in this review is to bring together the main observational evidence in this field to provide a parallel resource that can be viewed together with the evidence from RCTs, in an effort to better understand associations between maternal nutrition and birth outcomes. We also discuss secondary factors, many of a methodological or study-design nature, that may lead to inconsistent findings, as well as the theory and evidence regarding the role of SES factors in the maternal nutrition-birth outcome association.
ADVERSE BIRTH OUTCOMES AND THEIR CONSEQUENCES
The adverse birth outcomes covered in this review—namely, low birth weight, preterm birth, and IUGR—can have lifelong consequences for development, quality of life, and health care costs. Low birth weight is defined as a birth weight less than 2,500 g; it can result from premature delivery, intrauterine growth failure or disruption, or a combination of the two (5). Low birth weight is an important secondary factor in 40%–80% of neonatal deaths, 98% of which occur in developing countries (3). In both developed and developing countries, low birth weight is strongly associated with perinatal morbidity and increased risk of long-term disability (5). Preterm birth, which is defined as a gestational age less than 37 completed weeks, contributes substantially to the incidence of low birth weight and is the leading underlying cause of infant mortality among infants with nonlethal congenital anomalies (4). The costs of postpartum hospitalization and treatment are extremely high for low birth weight and preterm infants. Studies conducted in countries with technologically advanced medical systems indicate that average neonatal hospitalization costs per low-birth-weight and preterm infant rise exponentially as birth weight and gestational age at delivery decrease (15, 16). In a large, population-based study in California, the total costs of hospitalization during the neonatal period (first 4 weeks of life) for the 266 infants with a birth weight of 500–750 g were nearly the same (∼$60,000,000) as the total costs of neonatal hospitalization for the 48,610 infants with a birth weight of 2,750–3,000 g, a group that was over 182 times larger (17). Hospitalization costs during the first 10 years of life continue to be 4–10 times higher for low-birth-weight and preterm infants than for normal-birth-weight and term infants (15–20). In addition, costs for physical, educational, and social developmental services to children born low birth weight or preterm are 2–10 times higher than those for their normal-birth-weight and term counterparts (15, 21, 22).
IUGR carries increased risks of perinatal and infant mortality and morbidity in the short run and increased risks of disorders/disruptions of child growth and development (e.g., neurologic disorders, learning disabilities, childhood psychiatric disorders, mental retardation) in the long run (4, 5, 23). Infants with birth weights below the 10th percentile for their gestational age are classified as small for gestational age, and research shows that, even if they are born at term, these infants are at increased risk of neonatal mortality (24–26). It is hypothesized that several major adult diseases, such as coronary heart disease, hypertension, and type 2 diabetes, originate in impaired intrauterine growth and development, especially when combined with rapid or excessive growth/weight gain in childhood or adulthood, and may even have transgenerational effects (6, 27–31). The biologic plausibility of this hypothesis has been well-established with animal studies (32, 33); however, most human studies have been observational and thus not appropriate for drawing causal inferences. A review of the evidence for developmental origins of hypertension shows a fairly consistent association between fetal undernutrition (as measured by low birth weight) and elevated risk of adult hypertension, even though very few of the studies were conducted in non-Western, developing/transitional populations (27). Such developing/transitional contexts theoretically provide the optimal conditions for expression of the developmental-origins-of-adult-disease phenomenon, if they are characterized by a high prevalence of inadequate prenatal nutrition, followed by exposure to improving nutritional conditions that facilitate overnutrition in childhood and adulthood.
CONCEPTUAL MODELS FOR STUDYING THE MATERNAL NUTRITION–ADVERSE BIRTH OUTCOME ASSOCIATION
The assumptions underlying studies of maternal nutrition and birth outcomes are often inherently determined by feasibility and study design and may not be explicitly examined or discussed. We will briefly consider these underlying assumptions and then propose a conceptual model for researching the maternal nutrition–birth outcome relation and interpreting study results.
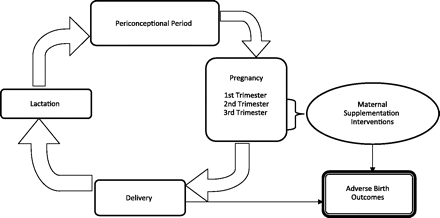
Well-designed RCT results are generally ranked as the highest level of evidence for use in evidence-based medical practice, because they are the only type of study from which causal inferences can be made without concerns about comparability between the study groups. However, the use of RCTs to explore nutrition and most health outcomes is limited, because dietary intervention trials running from baseline to a health/disease endpoint (which may require decades) are unfeasible (11, 12). One of the most common uses of RCTs in nutrition research has been the study of maternal nutrition and birth outcomes, since the “outcome” occurs within a predictable and relatively short time period. These RCTs, with few exceptions, manipulate the intake of 1–2 nutrients or test the effect of a multinutrient supplement during the course of 1 (or, more commonly, part of 1) pregnancy (Figure 1).
Design of most clinical trials evaluating associations between maternal nutrition and adverse birth outcomes (preterm birth, low birth weight, and/or intrauterine growth restriction) within the context of the complete reproductive cycle.
There have been extensive reviews of evidence from RCTs with regard to the question of whether or not maternal nutrition affects or can be changed to modify adverse birth outcomes (1, 34–39). In most reviews of RCTs, meta-analysis is employed, bringing together findings from a range of studies with differing baseline population characteristics, as well as supplementation protocols with differing starting points, durations, and amounts/formulations—all of which further complicates the interpretation of results. Table 1 summarizes findings from reviews of RCTs for the nutrients and birth outcomes of interest in this article and highlights the ranges of populations and supplement timing, duration, and dosage they encompass.
Results From Published Reviews of Randomized/Quasi-Randomized Clinical Trials on Associations Between Maternal Nutrition and Adverse Birth Outcomes
| First Author, Year (Reference No.) | Review Design | Baseline Maternal Nutritional Status | Nutrient(s) Targeted | Gestational Age at Initiation, weeks | Supplement Amount, per day | Conclusions |
| Kramera, 2003 (34) | Meta-analysis of controlled trials | Evaluated on the basis of prepregnancy/early pregnancy maternal weight. Studies included both well-nourished and undernourished women; analysis was stratified by adequate and inadequate maternal nutrition only for the outcome of mean birth weight. | Energy/protein: | |||
| Advice | <20–27 | Unlikely to confer major health benefits. | ||||
| Balanced energy/protein supplement | From previous birth to ≤30 | 273–1,017 kcal; 6–44 g of protein; 1 unspecified | Improves fetal growth (finding due largely to 1 Gambian study with the highest supplement dose; significance disappears when this study is removed from meta-analysis). | |||
| High-protein supplement | ≤30 | 470 kcal; 40 g of protein | Not beneficial/may harm fetus (based on 1 study of women with adequate dietary protein intake who received a high-protein supplement throughout pregnancy (1, 91)). | |||
| Isotopic protein supplement | From first trimester to 28 | 273–425 kcal; 8–11 g of protein; 1 unspecified | Not beneficial/may harm fetus. | |||
| Energy/protein restriction | ≤28; 1 unspecified | 1,250–2,000 kcal | Not beneficial/may harm fetus. | |||
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron alone | 20–60 mg | No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. | |
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron-folate | ∼11–23 | 60–65 mg of iron and 0.25–0.35 mg of folate | Risk of SGA birth was significantly reduced by 12%. No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. |
| Makridesa, 2006 (36) | Meta-analysis of RCTs | Analyses were stratified by gestational age and risk level at trial entry and by supplement type; there were not enough data to conduct subgroup analysis by baseline dietary intake. | Marine oil (omega-3 fatty acids) | 12–30 | 133–3,000 mg | No effect upon PTB, LBW, or intrauterine growth restriction. Risk of early PTB (<34 weeks) was reduced by 30% among high-risk women. |
| Szajewska, 2006 (37) | Meta-analysis of RCTs | Women with low-risk pregnancies (based on obstetric history). | Omega-3 fatty acids | 15–30; not reported in 1 study | 137–1,183 mg of docosahexaenoic acid and 803 mg of eicosapentaenoic acid | No effect on rates of PTB and LBW. |
| Haidera, 2007 (38) | Meta-analysis of RCTs | All studies had been conducted in low-income countries. | Multinutrient | From first trimester to 36 | Combinations of 3–15 vitamins and minerals in differing doses | 17% reduction in LBW birth and 8% reduction in SGA birth when compared with no supplementation; no significant reduction in PTB. When compared with iron-folate supplementation, no added benefit for reducing LBW, SGA birth, or PTB outcomes. |
| Shah, 2009 (39) | Meta-analysis of RCTs | Mostly low-income/developing-country populations. | Multinutrient | All stages of pregnancy, from early detection to 37 | 8–16 micronutrients of varying dosages | Multimicronutrient supplements significantly reduced risk of LBW and increased birth weight in comparison with placebo or iron-folic acid supplements alone. No associations with PTB or SGA birth. |
| First Author, Year (Reference No.) | Review Design | Baseline Maternal Nutritional Status | Nutrient(s) Targeted | Gestational Age at Initiation, weeks | Supplement Amount, per day | Conclusions |
| Kramera, 2003 (34) | Meta-analysis of controlled trials | Evaluated on the basis of prepregnancy/early pregnancy maternal weight. Studies included both well-nourished and undernourished women; analysis was stratified by adequate and inadequate maternal nutrition only for the outcome of mean birth weight. | Energy/protein: | |||
| Advice | <20–27 | Unlikely to confer major health benefits. | ||||
| Balanced energy/protein supplement | From previous birth to ≤30 | 273–1,017 kcal; 6–44 g of protein; 1 unspecified | Improves fetal growth (finding due largely to 1 Gambian study with the highest supplement dose; significance disappears when this study is removed from meta-analysis). | |||
| High-protein supplement | ≤30 | 470 kcal; 40 g of protein | Not beneficial/may harm fetus (based on 1 study of women with adequate dietary protein intake who received a high-protein supplement throughout pregnancy (1, 91)). | |||
| Isotopic protein supplement | From first trimester to 28 | 273–425 kcal; 8–11 g of protein; 1 unspecified | Not beneficial/may harm fetus. | |||
| Energy/protein restriction | ≤28; 1 unspecified | 1,250–2,000 kcal | Not beneficial/may harm fetus. | |||
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron alone | 20–60 mg | No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. | |
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron-folate | ∼11–23 | 60–65 mg of iron and 0.25–0.35 mg of folate | Risk of SGA birth was significantly reduced by 12%. No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. |
| Makridesa, 2006 (36) | Meta-analysis of RCTs | Analyses were stratified by gestational age and risk level at trial entry and by supplement type; there were not enough data to conduct subgroup analysis by baseline dietary intake. | Marine oil (omega-3 fatty acids) | 12–30 | 133–3,000 mg | No effect upon PTB, LBW, or intrauterine growth restriction. Risk of early PTB (<34 weeks) was reduced by 30% among high-risk women. |
| Szajewska, 2006 (37) | Meta-analysis of RCTs | Women with low-risk pregnancies (based on obstetric history). | Omega-3 fatty acids | 15–30; not reported in 1 study | 137–1,183 mg of docosahexaenoic acid and 803 mg of eicosapentaenoic acid | No effect on rates of PTB and LBW. |
| Haidera, 2007 (38) | Meta-analysis of RCTs | All studies had been conducted in low-income countries. | Multinutrient | From first trimester to 36 | Combinations of 3–15 vitamins and minerals in differing doses | 17% reduction in LBW birth and 8% reduction in SGA birth when compared with no supplementation; no significant reduction in PTB. When compared with iron-folate supplementation, no added benefit for reducing LBW, SGA birth, or PTB outcomes. |
| Shah, 2009 (39) | Meta-analysis of RCTs | Mostly low-income/developing-country populations. | Multinutrient | All stages of pregnancy, from early detection to 37 | 8–16 micronutrients of varying dosages | Multimicronutrient supplements significantly reduced risk of LBW and increased birth weight in comparison with placebo or iron-folic acid supplements alone. No associations with PTB or SGA birth. |
Abbreviations: LBW, low birth weight; PTB, preterm birth; RCTs, randomized controlled trials; SGA, small-for-gestational-age.
Cochrane review.
Results From Published Reviews of Randomized/Quasi-Randomized Clinical Trials on Associations Between Maternal Nutrition and Adverse Birth Outcomes
| First Author, Year (Reference No.) | Review Design | Baseline Maternal Nutritional Status | Nutrient(s) Targeted | Gestational Age at Initiation, weeks | Supplement Amount, per day | Conclusions |
| Kramera, 2003 (34) | Meta-analysis of controlled trials | Evaluated on the basis of prepregnancy/early pregnancy maternal weight. Studies included both well-nourished and undernourished women; analysis was stratified by adequate and inadequate maternal nutrition only for the outcome of mean birth weight. | Energy/protein: | |||
| Advice | <20–27 | Unlikely to confer major health benefits. | ||||
| Balanced energy/protein supplement | From previous birth to ≤30 | 273–1,017 kcal; 6–44 g of protein; 1 unspecified | Improves fetal growth (finding due largely to 1 Gambian study with the highest supplement dose; significance disappears when this study is removed from meta-analysis). | |||
| High-protein supplement | ≤30 | 470 kcal; 40 g of protein | Not beneficial/may harm fetus (based on 1 study of women with adequate dietary protein intake who received a high-protein supplement throughout pregnancy (1, 91)). | |||
| Isotopic protein supplement | From first trimester to 28 | 273–425 kcal; 8–11 g of protein; 1 unspecified | Not beneficial/may harm fetus. | |||
| Energy/protein restriction | ≤28; 1 unspecified | 1,250–2,000 kcal | Not beneficial/may harm fetus. | |||
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron alone | 20–60 mg | No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. | |
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron-folate | ∼11–23 | 60–65 mg of iron and 0.25–0.35 mg of folate | Risk of SGA birth was significantly reduced by 12%. No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. |
| Makridesa, 2006 (36) | Meta-analysis of RCTs | Analyses were stratified by gestational age and risk level at trial entry and by supplement type; there were not enough data to conduct subgroup analysis by baseline dietary intake. | Marine oil (omega-3 fatty acids) | 12–30 | 133–3,000 mg | No effect upon PTB, LBW, or intrauterine growth restriction. Risk of early PTB (<34 weeks) was reduced by 30% among high-risk women. |
| Szajewska, 2006 (37) | Meta-analysis of RCTs | Women with low-risk pregnancies (based on obstetric history). | Omega-3 fatty acids | 15–30; not reported in 1 study | 137–1,183 mg of docosahexaenoic acid and 803 mg of eicosapentaenoic acid | No effect on rates of PTB and LBW. |
| Haidera, 2007 (38) | Meta-analysis of RCTs | All studies had been conducted in low-income countries. | Multinutrient | From first trimester to 36 | Combinations of 3–15 vitamins and minerals in differing doses | 17% reduction in LBW birth and 8% reduction in SGA birth when compared with no supplementation; no significant reduction in PTB. When compared with iron-folate supplementation, no added benefit for reducing LBW, SGA birth, or PTB outcomes. |
| Shah, 2009 (39) | Meta-analysis of RCTs | Mostly low-income/developing-country populations. | Multinutrient | All stages of pregnancy, from early detection to 37 | 8–16 micronutrients of varying dosages | Multimicronutrient supplements significantly reduced risk of LBW and increased birth weight in comparison with placebo or iron-folic acid supplements alone. No associations with PTB or SGA birth. |
| First Author, Year (Reference No.) | Review Design | Baseline Maternal Nutritional Status | Nutrient(s) Targeted | Gestational Age at Initiation, weeks | Supplement Amount, per day | Conclusions |
| Kramera, 2003 (34) | Meta-analysis of controlled trials | Evaluated on the basis of prepregnancy/early pregnancy maternal weight. Studies included both well-nourished and undernourished women; analysis was stratified by adequate and inadequate maternal nutrition only for the outcome of mean birth weight. | Energy/protein: | |||
| Advice | <20–27 | Unlikely to confer major health benefits. | ||||
| Balanced energy/protein supplement | From previous birth to ≤30 | 273–1,017 kcal; 6–44 g of protein; 1 unspecified | Improves fetal growth (finding due largely to 1 Gambian study with the highest supplement dose; significance disappears when this study is removed from meta-analysis). | |||
| High-protein supplement | ≤30 | 470 kcal; 40 g of protein | Not beneficial/may harm fetus (based on 1 study of women with adequate dietary protein intake who received a high-protein supplement throughout pregnancy (1, 91)). | |||
| Isotopic protein supplement | From first trimester to 28 | 273–425 kcal; 8–11 g of protein; 1 unspecified | Not beneficial/may harm fetus. | |||
| Energy/protein restriction | ≤28; 1 unspecified | 1,250–2,000 kcal | Not beneficial/may harm fetus. | |||
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron alone | 20–60 mg | No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. | |
| Peña-Rosasa, 2009 (35) | Meta-analysis of RCTs and quasi-RCTs | Mixed, studies conducted in high- and low-income countries/populations; analysis was stratified by gestational age and hemoglobin level at trial entry and by supplement dose. | Iron-folate | ∼11–23 | 60–65 mg of iron and 0.25–0.35 mg of folate | Risk of SGA birth was significantly reduced by 12%. No effect on PTB or LBW. Great heterogeneity between studies makes interpretation of results difficult; pooled analysis may not be appropriate. |
| Makridesa, 2006 (36) | Meta-analysis of RCTs | Analyses were stratified by gestational age and risk level at trial entry and by supplement type; there were not enough data to conduct subgroup analysis by baseline dietary intake. | Marine oil (omega-3 fatty acids) | 12–30 | 133–3,000 mg | No effect upon PTB, LBW, or intrauterine growth restriction. Risk of early PTB (<34 weeks) was reduced by 30% among high-risk women. |
| Szajewska, 2006 (37) | Meta-analysis of RCTs | Women with low-risk pregnancies (based on obstetric history). | Omega-3 fatty acids | 15–30; not reported in 1 study | 137–1,183 mg of docosahexaenoic acid and 803 mg of eicosapentaenoic acid | No effect on rates of PTB and LBW. |
| Haidera, 2007 (38) | Meta-analysis of RCTs | All studies had been conducted in low-income countries. | Multinutrient | From first trimester to 36 | Combinations of 3–15 vitamins and minerals in differing doses | 17% reduction in LBW birth and 8% reduction in SGA birth when compared with no supplementation; no significant reduction in PTB. When compared with iron-folate supplementation, no added benefit for reducing LBW, SGA birth, or PTB outcomes. |
| Shah, 2009 (39) | Meta-analysis of RCTs | Mostly low-income/developing-country populations. | Multinutrient | All stages of pregnancy, from early detection to 37 | 8–16 micronutrients of varying dosages | Multimicronutrient supplements significantly reduced risk of LBW and increased birth weight in comparison with placebo or iron-folic acid supplements alone. No associations with PTB or SGA birth. |
Abbreviations: LBW, low birth weight; PTB, preterm birth; RCTs, randomized controlled trials; SGA, small-for-gestational-age.
Cochrane review.
Some of the more recent Cochrane reviews have tried to control for or reduce the effects of variation within these parameters by stratifying subgroup analyses by broad categories of gestational age, baseline nutritional or risk status at trial entry, type/amount of supplement use, etc. (35, 36). However, potentially important differences in design that may lead to different findings can still be obscured. For example, in a Cochrane meta-analysis of the effect of iron supplementation on rates of low birth weight (35), the group receiving supplements in the 1 study that began iron supplementation very early in pregnancy (mean gestational age of 11 weeks at trial entry) exhibited significantly lower rates of low birth weight (40), but this effect was obscured in the meta-analysis of all other trials and even in the subanalysis of trials beginning at less than 20 weeks of gestational age. Likewise, in the evaluation of iron-folate supplementation and low birth weight, a trial from a developing country demonstrated a significant reduction in low birth weight (41), but this effect was neutralized in the meta-analysis that included 1 other trial from an industrialized country (42).
In most meta-analyses of the association between maternal nutrition and birth outcomes, researchers have concluded that the nutritional interventions tested had no effect upon adverse birth outcomes, and the variation in study designs and populations included is likely to have biased the results toward the null hypothesis. In light of these issues, a number of scholars have cautioned that RCTs and meta-analyses of RCTs testing nutritional change and health/disease outcomes should not be taken in isolation as definitive evidence of the presence or absence of a diet–health/disease outcome relation but rather should be assessed and interpreted in combination with other available evidence (e.g., biochemical, experimental, epidemiologic) (11–14).
A growing body of evidence indicates that important nutrition-related influences on birth outcomes are not captured within the second-to-third trimester, the period usually examined in RCTs. The impact of maternal nutrition on birth outcomes may be attenuated by socioeconomic/environmental factors in various ways. For example, SES levels that influence the quality of habitual and pregnancy dietary intake can result in chronic undernutrition, as well as in multiple rather than single nutrient deficiencies, that cannot be overcome by a few months of supplementation during a single pregnancy (1, 2). Cultural/environmental factors may influence parameters such as maternal age at initiation of childbearing (32) and length of the interpregnancy interval and of the entire reproductive cycle, including lactation (43–45). Life-cycle and intergenerational factors, such as the mother's nutrition and growth during childhood and the intrauterine environment she experienced, may also influence reproductive outcomes (46–49). The association between maternal nutrition measures and birth outcomes is further complicated by the indirect link between maternal and fetal nutrition, which is mediated by the mother's habitual dietary intake; her intermediary metabolism and endocrine status; partitioning of nutrients among storage, use, and circulation; the capacity of circulating transport proteins; and cardiovascular adaptations to pregnancy which determine uterine blood flow (2).
Maternal nutritional deficiencies are also likely to have different effects depending upon the stage of fetal development at which they occur. A number of experimental animal studies and observational human studies point to the importance of nutritional insults that occur at the very earliest embryonic stages to subsequent fetal growth and birth outcomes (44, 45). Evidence from animal studies indicates that fetal growth is most affected by maternal dietary nutrient deficiencies (particularly deficiencies of protein and micronutrients) during the peri-implantation stage and the stage of rapid placental development (50, 51). Thus, researchers need to move beyond treating diet during pregnancy in isolation and begin focusing on maternal nutritional status throughout the periconceptional, pregnancy, and lactation periods as a continuum that affects maternal, fetal, and infant health (43). This approach has critical implications for when and how maternal dietary intake is assessed, when interventions are begun, and how study results are then interpreted.
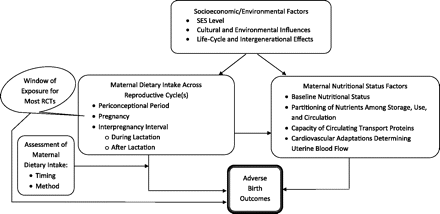
These factors formed the conceptual framework for this review. We use this broader conceptual model, which takes into account the factors, timing, and time period evaluated by a study when interpreting its results (Figure 2). Because of the breadth of the subject and the body of literature, we focus primarily on evidence from observational studies of maternal dietary intake and birth outcomes, which have received less attention in reviews than have RCTs and which provide additional information for consideration of these issues. Studies of maternal dietary intake and studies using other measures of maternal nutritional status (e.g., anthropometric, biochemical) are examined, since these parameters are interrelated and are all relevant to understanding associations between maternal nutrition and adverse birth outcomes.
Conceptual framework for studying associations between maternal nutrition and adverse birth outcomes. This framework 1) takes into account the influence of socioeconomic status (SES)/environmental factors on maternal dietary intake across single and multiple reproductive cycles and on maternal nutritional status as possible mediators of the association with adverse birth outcomes; 2) interprets the effects of randomized controlled trial (RCT) interventions on adverse birth outcomes in light of their timing/duration within the reproductive cycle(s) and of the broader socioeconomic/environmental context; and 3) accounts for the effect of the timing and method of dietary assessment as a potential mediator of the association between maternal dietary intake and adverse birth outcomes.
SES AS AN ANTECEDENT DETERMINANT OF ADVERSE BIRTH OUTCOMES
Adverse birth outcomes have been strongly associated with socioeconomic factors (52–58). Rates of preterm birth, low birth weight, and IUGR are higher in developing countries than in developed countries and, within developed countries, are higher among low-SES groups (55).
SES is a complex construct that has been used to define social inequality and usually includes measures of income, occupation, and/or educational attainment. Educational level has been the strongest and most consistent SES predictor of health. A low educational level limits access to jobs and other social resources, especially in industrialized countries, and thus increases the risk of poverty. Kramer et al. (55) used the conceptual model of causal pathways to explain the effects of social disparities on health. Society-level determinants (e.g., poverty, income inequality) are considered antecedent to, or “upstream” from, individual-level exposures and behaviors.
With regard to birth outcomes, low SES levels do not directly affect fetal growth but rather lead to unhealthy exposures that increase the risk of adverse birth outcomes. The exposures or mediating variables that have been considered in the literature include maternal anthropometric factors and nutrition, substance use/abuse, genitourinary tract infections, physically demanding work, lack of access to quality prenatal care, and psychosocial factors (e.g., stress, anxiety, and depression) (55). A study of SES gradients and low birth weight (58) confirmed that, although psychosocial variables played a role in SES gradients, most of the relations were due to the material conditions associated with income and material inputs.
One of the pathways though which SES may influence birth outcomes is its impact on diet quality. Improved maternal nutrition has been associated with increased fetal growth and a reduction in adverse birth outcomes in developing countries and in populations with nutrient deficiencies, but not in well-nourished populations (1, 2, 23). The authors of a comprehensive review of nutritional interventions during pregnancy raised the issue of the duration/amount of nutritional supplementation and suggested that 2 or 3 decades of chronic undernutrition among women in developing populations were not likely to be overcome by a few months of extra nutrient intake during the course of a single pregnancy (1). Taking a longer-term approach, Villar and Rivera (45) observed a biologically significant increase in birth weight (301 g) after nutritional supplementation was provided to a sample of chronically yet moderately malnourished Guatemalan women during 2 consecutive pregnancies and the interim lactation period. Studies of maternal dietary intake have also confirmed the importance of SES. In a study of the diet quality of pregnant Kenyan women, Kamau-Mbuthia and Elmadfa (59) reported that SES factors (e.g., education and employment) were important predictors of nutrient intake and diet quality. Among rural Indian women, intake of dairy products was strongly associated with SES and was also associated with birth size (23).
A number of researchers have concluded that maternal nutrition is not associated with adverse birth outcomes in industrialized populations (60, 61). Mathews et al.’s study of dietary intake during pregnancy and birth weights in England found no associations (60); however, the sample included only white, nulliparous mothers of term infants, among whom the lower SES categories were underrepresented (62), and median dietary intakes met the US Recommended Daily Allowances for most nutrients other than iron. In another study that found no association between the pregnancy dietary intake of low-income ethnic groups in the United States and adverse birth outcomes, Cohen et al. (61) reported sufficiently high mean daily intakes of most nutrients (including protein, iron and folate) to meet pregnancy Recommended Daily Allowances. Neither study considered long-term or periconceptional nutritional intake or explored the possibility of coexisting multinutrient deficiencies among persons with below-median/mean nutrient intakes. In their review of low birth weight in the United States, Goldenberg and Culhane (5) concluded that virtually all nutritional interventions aimed at reducing rates of adverse birth outcomes had failed but did not distinguish between groups with differing SES characteristics.
In contrast to these studies, Doyle et al. (63) found a dose-response relation between nutrient intake and birth weight in a low-SES population in East London, United Kingdom; and Scholl et al. (64) found that a nutrition intervention among low-income US women produced reductions in the incidences of preterm birth and low birth weight. Studies of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) have produced mixed results (58, 65, 66). In 1 study of WIC participants measuring diet quality, the mean score (53.9 out of a possible total of 100) still fell into the lower end of the “needs improvement” category (scores of 50–79), indicating that the WIC subsidies were not sufficient to fully overcome the negative effects of low SES on diet quality (67).
Kramer et al. (55) observed that the countries which had achieved the lowest rates of adverse birth outcomes had done so not through health-care interventions but rather by reducing the prevalence of socioeconomic disadvantage. In concluding their review of socioeconomic disparities in pregnancy outcome, they stated, “It may not be possible to eliminate the higher risks of IUGR and preterm birth among the poor without eliminating poverty itself” (55, p. 205). Similarly, in a review of the nutritional coping strategies of low-income mothers in the United Kingdom, Attree (68) concluded that interventions aimed at encouraging individual lifestyle changes must also include measures to improve families’ socioeconomic circumstances. On the basis of the finding that low-income mothers’ efforts to manage poverty often had negative effects on their nutrient and health status, Attree recommended a shift in emphasis in health policy toward giving a higher priority to measures that deal with the underlying determinants of health (68). Pragmatically, the best approach may be a double-pronged effort to promote proven nutrition/supplementation interventions that are economically and logistically feasible in resource-poor countries, while continuing to draw attention to and advocate for improvements in the underlying determinants of poverty. This approach is exemplified by Bhutta et al. (69) in their review of effective interventions for addressing maternal and child undernutrition.
OTHER MAJOR FACTORS ASSOCIATED WITH ADVERSE BIRTH OUTCOMES AND LINKS TO MATERNAL NUTRITION
There are a number of well-established risk factors for adverse birth outcomes, such as smoking, use of alcohol and other substances, maternal infections, and a history of preterm birth. In most cases there has been little or no research about how maternal nutrition may interface with these risk factors to either elevate or reduce risks of adverse birth outcomes. The few extant studies of smoking, maternal energy intake, and IUGR seemed to suggest a lack of association, but no other aspect of the diet was evaluated (70). Other studies have suggested that smoking and alcohol use may interact with maternal micronutrient status and deficiencies to impair fetal development (71); however, the evidence is insufficient for drawing firm conclusions. Studies investigating links between maternal nutrition and maternal infection and preterm birth or repeated preterm births have also been few (33, 72, 73), and further research is warranted in both of these areas.
SPECIFIC NUTRITIONAL REQUIREMENTS DURING PREGNANCY AND BIRTH OUTCOMES
Optimal maternal and fetal pregnancy outcomes are dependent upon the intake of sufficient nutrients to meet maternal and fetal requirements (31). Malnutrition results from inadequate dietary intake, is synonymous with growth failure, and was conventionally attributed to protein-energy malnutrition generally, especially during the rapid growth phases in the life cycle, such as gestation. It was subsequently recognized that poor growth results not only from a deficiency of protein and energy but also from inadequate intake of micronutrients that are vital during rapid growth phases (31–33). Here we summarize current knowledge of maternal requirements for the nutrients that play a critical role during pregnancy and have been studied in conjunction with birth outcomes. Table 2 contains a brief synopsis of recommendations for the nutrients targeted in this review, taken from Dietary Reference Intakes (74) and expert consultations, which provide general summaries of maternal requirements and have been widely used for evaluating the adequacy of maternal nutrient intakes during pregnancy. Further discussion of each recommendation in Table 2 is included in specific nutrient subsections below.
Selected Nutritional Requirements for Adult Women (Aged 19–50 Years) During Pregnancy
| Nutrient | Daily Requirement | Comments | Source |
| Energy, kcal | 2,200–2,900 | Dependent upon maternal body mass index, age, physiologic appetite, and rate of weight gain. | American Dietetic Association (75) |
| Protein, g | 71 | Refers to intake of complete proteins (containing all 9 indispensable amino acids); reflects maternal requirements for maintaining nitrogen equilibrium, plus the protein deposition requirements of pregnancy. | Recommended Daily Allowance from DRI (74) |
| Lysinea, mg/kg | 51 | Plays critical role in protein synthesis. | |
| Omega-3 fatty acids, g | Very important in the development of the brain and central nervous system. Essential to the formation of new tissues, which occurs at an elevated rate during pregnancy and fetal development. | DRI (74) | |
| Total | 1.4 | International Society for the Study of Fatty Acids and Lipids (99) | |
| Docosahexaenoic acid | 0.3 | ||
| Eicosapentaenoic acid | 0.2 | ||
| Iron, mg | 27 | Based on the assumption that 75% of the iron comes from heme sources (e.g., meat and poultry). | DRI (116, 117) |
| Folate, μg | 600 | Required for cellular reactions, including DNA and nucleic acid synthesis, and for widespread, sustained cell division. | DRI (124) |
| Nutrient | Daily Requirement | Comments | Source |
| Energy, kcal | 2,200–2,900 | Dependent upon maternal body mass index, age, physiologic appetite, and rate of weight gain. | American Dietetic Association (75) |
| Protein, g | 71 | Refers to intake of complete proteins (containing all 9 indispensable amino acids); reflects maternal requirements for maintaining nitrogen equilibrium, plus the protein deposition requirements of pregnancy. | Recommended Daily Allowance from DRI (74) |
| Lysinea, mg/kg | 51 | Plays critical role in protein synthesis. | |
| Omega-3 fatty acids, g | Very important in the development of the brain and central nervous system. Essential to the formation of new tissues, which occurs at an elevated rate during pregnancy and fetal development. | DRI (74) | |
| Total | 1.4 | International Society for the Study of Fatty Acids and Lipids (99) | |
| Docosahexaenoic acid | 0.3 | ||
| Eicosapentaenoic acid | 0.2 | ||
| Iron, mg | 27 | Based on the assumption that 75% of the iron comes from heme sources (e.g., meat and poultry). | DRI (116, 117) |
| Folate, μg | 600 | Required for cellular reactions, including DNA and nucleic acid synthesis, and for widespread, sustained cell division. | DRI (124) |
Abbreviation: DRI, Dietary Reference Intakes.
Primary limiting amino acid in diets based on cereal proteins.
Selected Nutritional Requirements for Adult Women (Aged 19–50 Years) During Pregnancy
| Nutrient | Daily Requirement | Comments | Source |
| Energy, kcal | 2,200–2,900 | Dependent upon maternal body mass index, age, physiologic appetite, and rate of weight gain. | American Dietetic Association (75) |
| Protein, g | 71 | Refers to intake of complete proteins (containing all 9 indispensable amino acids); reflects maternal requirements for maintaining nitrogen equilibrium, plus the protein deposition requirements of pregnancy. | Recommended Daily Allowance from DRI (74) |
| Lysinea, mg/kg | 51 | Plays critical role in protein synthesis. | |
| Omega-3 fatty acids, g | Very important in the development of the brain and central nervous system. Essential to the formation of new tissues, which occurs at an elevated rate during pregnancy and fetal development. | DRI (74) | |
| Total | 1.4 | International Society for the Study of Fatty Acids and Lipids (99) | |
| Docosahexaenoic acid | 0.3 | ||
| Eicosapentaenoic acid | 0.2 | ||
| Iron, mg | 27 | Based on the assumption that 75% of the iron comes from heme sources (e.g., meat and poultry). | DRI (116, 117) |
| Folate, μg | 600 | Required for cellular reactions, including DNA and nucleic acid synthesis, and for widespread, sustained cell division. | DRI (124) |
| Nutrient | Daily Requirement | Comments | Source |
| Energy, kcal | 2,200–2,900 | Dependent upon maternal body mass index, age, physiologic appetite, and rate of weight gain. | American Dietetic Association (75) |
| Protein, g | 71 | Refers to intake of complete proteins (containing all 9 indispensable amino acids); reflects maternal requirements for maintaining nitrogen equilibrium, plus the protein deposition requirements of pregnancy. | Recommended Daily Allowance from DRI (74) |
| Lysinea, mg/kg | 51 | Plays critical role in protein synthesis. | |
| Omega-3 fatty acids, g | Very important in the development of the brain and central nervous system. Essential to the formation of new tissues, which occurs at an elevated rate during pregnancy and fetal development. | DRI (74) | |
| Total | 1.4 | International Society for the Study of Fatty Acids and Lipids (99) | |
| Docosahexaenoic acid | 0.3 | ||
| Eicosapentaenoic acid | 0.2 | ||
| Iron, mg | 27 | Based on the assumption that 75% of the iron comes from heme sources (e.g., meat and poultry). | DRI (116, 117) |
| Folate, μg | 600 | Required for cellular reactions, including DNA and nucleic acid synthesis, and for widespread, sustained cell division. | DRI (124) |
Abbreviation: DRI, Dietary Reference Intakes.
Primary limiting amino acid in diets based on cereal proteins.
Energy
Energy is the chief nutritional determinant of gestational weight gain; however, the strength of the relation is confounded by a number of intervening factors (e.g., changes in basal metabolism and levels of physical activity, the composition of accumulated maternal and fetal tissue) (75–77). In addition, deficiencies of other specific nutrients may limit or restrict gestational and fetal weight gain (76). During pregnancy, additional energy is required for the growth and maintenance of the fetus, the placenta, and maternal tissues. Energy metabolism changes during the course of pregnancy and differs considerably among women (74, 77–79). Maternal basal metabolism increases because of the increased mass of metabolically active tissues; maternal cardiovascular, renal, and respiratory work; and new tissue synthesis. The available evidence suggests that the efficiency of energy metabolism may increase during pregnancy, but the mechanisms involved are not well understood.
On the basis of theoretical calculations, the Food and Agriculture Organization/World Health Organization/United Nations University recommended that during pregnancy women increase their energy intake by 85 kcal/day in the first trimester, 285 kcal/day in the second trimester, and 475 kcal/day in the third trimester (78). The more generic energy intake recommendation of the American Dietetic Association (75), 2,200–2,900 kcal/day, is included in Table 2. Studies of well-nourished pregnant women in Scotland, Denmark, Australia, and the United States have generally indicated a slight, though not always statistically significant or universal, increase in energy intake during pregnancy (76). However, studies of well-nourished pregnant women exposed to the Dutch Famine during World War II showed that severe calorie restriction in certain stages of pregnancy, which led to low maternal weight gain or weight loss in the third trimester, was associated with reduced birth weight (80).
The results of energy intake studies among low-income women in developing countries have been inconsistent. If the energy intake of chronically undernourished women does not increase during pregnancy, fetal and maternal tissue growth may be limited to that which can be attained by adjustments in nutrient utilization (76). In these populations, environmental factors, such as seasonality (which affects food availability), dietary intake, and workload/energy expenditures, have been shown to be associated with birth weight (33). A large retrospective cohort study in rural Gambia showed rates of small-for-gestational-age birth to be highest at the end of the “hungry” season and to be negatively associated with maternal weight gain (81). Similarly, a study in rural India found higher maternal food intake coupled with lower levels of strenuous activity in late gestation to be associated with increased birth size (82).
The impact of maternal energy intake on birth outcomes has mainly been researched in energy/balanced-energy-protein supplementation trials, which have been comprehensively evaluated in a Cochrane review (including only RCTs of sufficient methodological quality) (34) and a review of community-based interventions (including additional supplementation trials and prospective cohort studies) (3). In the Cochrane review, Kramer and Kakuma (34) employed meta-analysis combining results on a broad range of population types and supplement dosage, initiation, and duration (Table 1); they concluded that since the benefits for fetal growth/birth outcomes were modest-to-negligible, future investigators should study outcomes other than fetal growth.
After considering a broader evidence base and examining studies on an individual basis, Bhutta et al. (3) concluded that administration of energy supplementation to chronically undernourished populations in sufficient quantity and/or duration did lead to significant increases in birth weight and decreases in rates of low-birth-weight and small-for-gestational-age birth and merited further study, implementation, and evaluation in these populations. Thus, the evidence from undernourished/low-SES populations tends to support an association between chronically inadequate energy intake and adverse birth outcomes. However, issues related to other cultural/environmental factors, such as length of the interpregnancy interval and lactation periods and life-cycle/intergenerational effects of an insufficient intrauterine energy supply, have not been adequately addressed. In addition, since “energy intake” may also serve as an indirect indicator of the overall quantity and quality of food intake, the possibility that maternal energy intake indirectly reflects other diet characteristics, such as nutrient density and dietary diversity (67, 83), merits more attention.
Protein
The average requirement for the additional protein needed by pregnant women is based on calculations of the amount needed for initial deposition of pregnancy-related tissue and the amount needed to maintain new tissue (Table 2).
A large proportion of the world's population who are low-SES at the household and/or population level subsist on diets based predominantly on cereals, which also serve as their main source of protein. Lysine, which is the primary limiting amino acid in most cereal proteins, is needed in greater quantities during gestation because of its critical role in protein synthesis (74). The importance of lysine to normal growth has been established in animal models, which have consistently found a poorer rate of weight gain in rats fed on a lysine-deficient diet than in control rats fed on a lysine-adequate diet (84). Among humans, there have been no studies of lysine intake among pregnant women; however, in 2 recent RCTs of lysine-fortified flour in low-SES populations with wheat-based diets (85, 86), growth rates among children in the treatment group were significantly higher than those in the controls, even after only 3 months of exposure. This issue merits further exploration among pregnant women with cereal-based diets in low-SES populations, among whom the rates of low birth weight and IUGR are high.
Cohort studies, which vary in terms of the baseline nutritional status of study populations and dietary assessment method and timing, have produced mixed results regarding the association between dietary protein intake and birth outcomes (Table 3). Associations between protein intake and birth outcomes were unlikely to be found in well-nourished populations, especially if diet was assessed in the second trimester or later and was not evaluated for type or quality of protein intake (60, 61). However, maternal protein intake (specifically that from dairy sources) was found to be associated with increased birth weight (23, 87–89), particularly among studies that assessed maternal intake periconceptionally and in very early pregnancy, in both developed (87–89) and developing/low-income (23, 90) populations. In a study of US women (primarily WIC recipients), Sloan et al. (90) found both low and high protein intakes in the second and third trimesters to be associated with decreased birth weight but also found protein intake to be adequate among most women, even in this low-income cohort. Among chronically undernourished Indian women with little or no intake of protein from animal sources, those who ate dairy products at least every other day in early pregnancy had infants with significantly higher birth weights (23).
Results From Individual Studies of Associations Between Maternal Protein Intake and Adverse Birth Outcomes
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean or Median Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Sloan, 2001 (90) | Prospective cohort | 2,163 | Low-income, healthy US women with singleton pregnancies | 2 24-hour recalls | 16–24 and 32 | 78.2 (25.3)a | Birth weight | Mean protein intakes of <50 g/day (12% of sample) and ≥85 g/day (36% of sample) were associated with 77-g and 71-g decreases in birth weight, respectively, compared with intermediate intake (50–84.9 g/day). Nonpregnancy protein intake is typically high among US women, and increased protein intake during pregnancy may be detrimental to birth weight in this population. |
| Mathews, 1999 (60) | Prospective cohort | 693 | Well-nourished, white, nulliparous British women with term births | 7-day diary | 13–19 | 73 [62–85]b | Birth weight | No association between protein intake and birth weight. |
| FFQ | 28 | 87 [71–105] | ||||||
| Cohen, 2001 (61) | Prospective cohort | 4,054 | Adequately nourished, low- SES nulliparous US women of different ethnicities | 1 24-hour recall | 13–21 | (In all ethnic groups, over 70% met or exceeded the Recommended Daily Allowance for protein.) | Birth weight, preterm birth, SGA birth | No associations between protein intake and any of the birth outcomes. |
| Cucó, 2006 (88) | Prospective cohort | 77 | Well-nourished Spanish women with singleton, full-term fetuses | 7-day diaries | Periconception and 6, 10, 26, and 38 | ∼80 [67–93] (70%–75% from animal proteins) | Birth weight (only 1.5% LBW outcomes) | Periconceptionally and at the 6th, 10th, 26th, and 38th weeks of gestation, a 1-g increase in maternal protein intake led to a significant 8- to 14-g increase in birth weight. All macronutrients were evaluated; only protein intake was significantly associated with birth weight throughout the study. |
| Moore, 2004 (87) | Prospective cohort | 557 | Healthy Caucasian Australian women | FFQ | <16 | 89 [67–112] | Birth weight | Proportion of energy derived from protein in early pregnancy was positively associated with birth weight (1-g increase was associated with 16-g increase in birth weight); among “reliable dietary reporters” (n = 429), isoenergic 1% increase in dairy protein was associated with a 25-g increase in birth weight; no detrimental effects of high protein intake. |
| 30–34 (covering past 3 months) | 86 [71–108] | |||||||
| Olsen, 2007 (89) | Prospective cohort | 50,117 | Danish National Birth Cohort women with singleton, term births | FFQ (covering previous 4 weeks) with interest in protein from dairy products | Midpregnancy | 3.1 (2.0) glasses (200 mL) of milk per day | Birth weight, SGA birth | Women consuming more than 6 glasses of milk daily had a 49% lower risk of SGA and a 108-g increase in birth weight compared with those consuming no milk. There was also increased risk of large-for-gestational-age birth. |
| Rao, 2001 (23) | Prospective cohort | 797 | Chronically undernourished rural Indian women | 24-hour recall, FFQ covering previous 3 months | 18 and 28 | 44–45 (14) | Birth weight | Higher dairy-product intake in early pregnancy (6–18 weeks) was positively associated with birth weight. |
| (0%–15% from animal proteins) | ||||||||
| Lechting, 1975 (92) | Prospective cohort | 405 | Chronically malnourished Guatemalan women | Protein-rich or no-protein supplement during 2 consecutive pregnancies and the lactation period 24- and 72-hour recalls | Daily supplement intake recordedEach trimester of pregnancy | ∼40 g/day (unsupplemented) | Birth weight, LBW | Difference of 115 g in birth weight between protein-rich group and the group with no protein supplementation (P < 0.025). |
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean or Median Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Sloan, 2001 (90) | Prospective cohort | 2,163 | Low-income, healthy US women with singleton pregnancies | 2 24-hour recalls | 16–24 and 32 | 78.2 (25.3)a | Birth weight | Mean protein intakes of <50 g/day (12% of sample) and ≥85 g/day (36% of sample) were associated with 77-g and 71-g decreases in birth weight, respectively, compared with intermediate intake (50–84.9 g/day). Nonpregnancy protein intake is typically high among US women, and increased protein intake during pregnancy may be detrimental to birth weight in this population. |
| Mathews, 1999 (60) | Prospective cohort | 693 | Well-nourished, white, nulliparous British women with term births | 7-day diary | 13–19 | 73 [62–85]b | Birth weight | No association between protein intake and birth weight. |
| FFQ | 28 | 87 [71–105] | ||||||
| Cohen, 2001 (61) | Prospective cohort | 4,054 | Adequately nourished, low- SES nulliparous US women of different ethnicities | 1 24-hour recall | 13–21 | (In all ethnic groups, over 70% met or exceeded the Recommended Daily Allowance for protein.) | Birth weight, preterm birth, SGA birth | No associations between protein intake and any of the birth outcomes. |
| Cucó, 2006 (88) | Prospective cohort | 77 | Well-nourished Spanish women with singleton, full-term fetuses | 7-day diaries | Periconception and 6, 10, 26, and 38 | ∼80 [67–93] (70%–75% from animal proteins) | Birth weight (only 1.5% LBW outcomes) | Periconceptionally and at the 6th, 10th, 26th, and 38th weeks of gestation, a 1-g increase in maternal protein intake led to a significant 8- to 14-g increase in birth weight. All macronutrients were evaluated; only protein intake was significantly associated with birth weight throughout the study. |
| Moore, 2004 (87) | Prospective cohort | 557 | Healthy Caucasian Australian women | FFQ | <16 | 89 [67–112] | Birth weight | Proportion of energy derived from protein in early pregnancy was positively associated with birth weight (1-g increase was associated with 16-g increase in birth weight); among “reliable dietary reporters” (n = 429), isoenergic 1% increase in dairy protein was associated with a 25-g increase in birth weight; no detrimental effects of high protein intake. |
| 30–34 (covering past 3 months) | 86 [71–108] | |||||||
| Olsen, 2007 (89) | Prospective cohort | 50,117 | Danish National Birth Cohort women with singleton, term births | FFQ (covering previous 4 weeks) with interest in protein from dairy products | Midpregnancy | 3.1 (2.0) glasses (200 mL) of milk per day | Birth weight, SGA birth | Women consuming more than 6 glasses of milk daily had a 49% lower risk of SGA and a 108-g increase in birth weight compared with those consuming no milk. There was also increased risk of large-for-gestational-age birth. |
| Rao, 2001 (23) | Prospective cohort | 797 | Chronically undernourished rural Indian women | 24-hour recall, FFQ covering previous 3 months | 18 and 28 | 44–45 (14) | Birth weight | Higher dairy-product intake in early pregnancy (6–18 weeks) was positively associated with birth weight. |
| (0%–15% from animal proteins) | ||||||||
| Lechting, 1975 (92) | Prospective cohort | 405 | Chronically malnourished Guatemalan women | Protein-rich or no-protein supplement during 2 consecutive pregnancies and the lactation period 24- and 72-hour recalls | Daily supplement intake recordedEach trimester of pregnancy | ∼40 g/day (unsupplemented) | Birth weight, LBW | Difference of 115 g in birth weight between protein-rich group and the group with no protein supplementation (P < 0.025). |
Abbreviations: FFQ, food frequency questionnaire; LBW, low birth weight; SGA, small-for-gestational-age.
Mean value and (in parentheses) standard deviation.
Median value and (in brackets) interquartile range (25th–75th percentiles).
Results From Individual Studies of Associations Between Maternal Protein Intake and Adverse Birth Outcomes
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean or Median Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Sloan, 2001 (90) | Prospective cohort | 2,163 | Low-income, healthy US women with singleton pregnancies | 2 24-hour recalls | 16–24 and 32 | 78.2 (25.3)a | Birth weight | Mean protein intakes of <50 g/day (12% of sample) and ≥85 g/day (36% of sample) were associated with 77-g and 71-g decreases in birth weight, respectively, compared with intermediate intake (50–84.9 g/day). Nonpregnancy protein intake is typically high among US women, and increased protein intake during pregnancy may be detrimental to birth weight in this population. |
| Mathews, 1999 (60) | Prospective cohort | 693 | Well-nourished, white, nulliparous British women with term births | 7-day diary | 13–19 | 73 [62–85]b | Birth weight | No association between protein intake and birth weight. |
| FFQ | 28 | 87 [71–105] | ||||||
| Cohen, 2001 (61) | Prospective cohort | 4,054 | Adequately nourished, low- SES nulliparous US women of different ethnicities | 1 24-hour recall | 13–21 | (In all ethnic groups, over 70% met or exceeded the Recommended Daily Allowance for protein.) | Birth weight, preterm birth, SGA birth | No associations between protein intake and any of the birth outcomes. |
| Cucó, 2006 (88) | Prospective cohort | 77 | Well-nourished Spanish women with singleton, full-term fetuses | 7-day diaries | Periconception and 6, 10, 26, and 38 | ∼80 [67–93] (70%–75% from animal proteins) | Birth weight (only 1.5% LBW outcomes) | Periconceptionally and at the 6th, 10th, 26th, and 38th weeks of gestation, a 1-g increase in maternal protein intake led to a significant 8- to 14-g increase in birth weight. All macronutrients were evaluated; only protein intake was significantly associated with birth weight throughout the study. |
| Moore, 2004 (87) | Prospective cohort | 557 | Healthy Caucasian Australian women | FFQ | <16 | 89 [67–112] | Birth weight | Proportion of energy derived from protein in early pregnancy was positively associated with birth weight (1-g increase was associated with 16-g increase in birth weight); among “reliable dietary reporters” (n = 429), isoenergic 1% increase in dairy protein was associated with a 25-g increase in birth weight; no detrimental effects of high protein intake. |
| 30–34 (covering past 3 months) | 86 [71–108] | |||||||
| Olsen, 2007 (89) | Prospective cohort | 50,117 | Danish National Birth Cohort women with singleton, term births | FFQ (covering previous 4 weeks) with interest in protein from dairy products | Midpregnancy | 3.1 (2.0) glasses (200 mL) of milk per day | Birth weight, SGA birth | Women consuming more than 6 glasses of milk daily had a 49% lower risk of SGA and a 108-g increase in birth weight compared with those consuming no milk. There was also increased risk of large-for-gestational-age birth. |
| Rao, 2001 (23) | Prospective cohort | 797 | Chronically undernourished rural Indian women | 24-hour recall, FFQ covering previous 3 months | 18 and 28 | 44–45 (14) | Birth weight | Higher dairy-product intake in early pregnancy (6–18 weeks) was positively associated with birth weight. |
| (0%–15% from animal proteins) | ||||||||
| Lechting, 1975 (92) | Prospective cohort | 405 | Chronically malnourished Guatemalan women | Protein-rich or no-protein supplement during 2 consecutive pregnancies and the lactation period 24- and 72-hour recalls | Daily supplement intake recordedEach trimester of pregnancy | ∼40 g/day (unsupplemented) | Birth weight, LBW | Difference of 115 g in birth weight between protein-rich group and the group with no protein supplementation (P < 0.025). |
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean or Median Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Sloan, 2001 (90) | Prospective cohort | 2,163 | Low-income, healthy US women with singleton pregnancies | 2 24-hour recalls | 16–24 and 32 | 78.2 (25.3)a | Birth weight | Mean protein intakes of <50 g/day (12% of sample) and ≥85 g/day (36% of sample) were associated with 77-g and 71-g decreases in birth weight, respectively, compared with intermediate intake (50–84.9 g/day). Nonpregnancy protein intake is typically high among US women, and increased protein intake during pregnancy may be detrimental to birth weight in this population. |
| Mathews, 1999 (60) | Prospective cohort | 693 | Well-nourished, white, nulliparous British women with term births | 7-day diary | 13–19 | 73 [62–85]b | Birth weight | No association between protein intake and birth weight. |
| FFQ | 28 | 87 [71–105] | ||||||
| Cohen, 2001 (61) | Prospective cohort | 4,054 | Adequately nourished, low- SES nulliparous US women of different ethnicities | 1 24-hour recall | 13–21 | (In all ethnic groups, over 70% met or exceeded the Recommended Daily Allowance for protein.) | Birth weight, preterm birth, SGA birth | No associations between protein intake and any of the birth outcomes. |
| Cucó, 2006 (88) | Prospective cohort | 77 | Well-nourished Spanish women with singleton, full-term fetuses | 7-day diaries | Periconception and 6, 10, 26, and 38 | ∼80 [67–93] (70%–75% from animal proteins) | Birth weight (only 1.5% LBW outcomes) | Periconceptionally and at the 6th, 10th, 26th, and 38th weeks of gestation, a 1-g increase in maternal protein intake led to a significant 8- to 14-g increase in birth weight. All macronutrients were evaluated; only protein intake was significantly associated with birth weight throughout the study. |
| Moore, 2004 (87) | Prospective cohort | 557 | Healthy Caucasian Australian women | FFQ | <16 | 89 [67–112] | Birth weight | Proportion of energy derived from protein in early pregnancy was positively associated with birth weight (1-g increase was associated with 16-g increase in birth weight); among “reliable dietary reporters” (n = 429), isoenergic 1% increase in dairy protein was associated with a 25-g increase in birth weight; no detrimental effects of high protein intake. |
| 30–34 (covering past 3 months) | 86 [71–108] | |||||||
| Olsen, 2007 (89) | Prospective cohort | 50,117 | Danish National Birth Cohort women with singleton, term births | FFQ (covering previous 4 weeks) with interest in protein from dairy products | Midpregnancy | 3.1 (2.0) glasses (200 mL) of milk per day | Birth weight, SGA birth | Women consuming more than 6 glasses of milk daily had a 49% lower risk of SGA and a 108-g increase in birth weight compared with those consuming no milk. There was also increased risk of large-for-gestational-age birth. |
| Rao, 2001 (23) | Prospective cohort | 797 | Chronically undernourished rural Indian women | 24-hour recall, FFQ covering previous 3 months | 18 and 28 | 44–45 (14) | Birth weight | Higher dairy-product intake in early pregnancy (6–18 weeks) was positively associated with birth weight. |
| (0%–15% from animal proteins) | ||||||||
| Lechting, 1975 (92) | Prospective cohort | 405 | Chronically malnourished Guatemalan women | Protein-rich or no-protein supplement during 2 consecutive pregnancies and the lactation period 24- and 72-hour recalls | Daily supplement intake recordedEach trimester of pregnancy | ∼40 g/day (unsupplemented) | Birth weight, LBW | Difference of 115 g in birth weight between protein-rich group and the group with no protein supplementation (P < 0.025). |
Abbreviations: FFQ, food frequency questionnaire; LBW, low birth weight; SGA, small-for-gestational-age.
Mean value and (in parentheses) standard deviation.
Median value and (in brackets) interquartile range (25th–75th percentiles).
The Cochrane review of balanced energy/protein supplementation RCTs (34) showed a benefit to fetal growth, due primarily to a Gambian study with the highest supplement level (Table 1). The high-protein supplement meta-analysis (1) found possibly detrimental effects, due largely to 1 study of low-income US women (91) with adequate protein intake in their unsupplemented diet.
In a longitudinal cohort study in a chronically undernourished Guatemalan population, pregnant mothers and children up to age 7 years were offered a protein-rich or energy-only supplement. Birth weights were modestly yet significantly higher for infants of mothers receiving the protein-rich supplement (92). Follow-up studies on the children who received the protein-rich supplement from birth to age 3 years have shown significantly improved growth, intellectual development, and wage levels. This unique longitudinal cohort study has provided valuable insights into the mechanisms and pathways through which intrauterine and early childhood nutrition may affect biologic and SES parameters and thus have lifelong and intergenerational ramifications (93–95).
In many studies evaluating maternal protein intake and birth outcomes (though not all), investigators have described the SES characteristics of their samples, and the findings suggest that SES plays a mediating role in this association. The timing of the dietary assessment points to the importance of protein intake in the periconceptional and early pregnancy periods. Little or no attention has been given to cultural/environmental and life-cycle factors, and therefore these aspects warrant further study.
Essential fatty acids
Certain polyunsaturated fatty acids, omega-6 and omega-3 fatty acids, are essential for human development and health but cannot be synthesized by the human body, so they must be obtained through the diet (96, 97). Being important structural elements of cell membranes, these fatty acids are essential to the formation of new tissues, which occurs at an elevated rate during pregnancy and fetal development (96–99) (Table 2).
The diet and body stores of essential polyunsaturated fatty acids in pregnant women need to meet the polyunsaturated fatty acid requirements of both mother and fetus, because the developing fetus depends upon maternal fatty acids and polyunsaturated fatty acids for its supply. The omega-6 and omega-3 fatty acid status of mothers has been found to decline during pregnancy, and while normalization occurs after delivery, it appears to take more than 6 months (96, 100).
There is some evidence from biochemical studies among populations with high marine-food intakes suggesting that higher intakes of long-chain omega-3 fatty acids during pregnancy may result in an increased duration of gestation and may also improve fetal growth (101–104) (Table 4). Studies assessing maternal dietary intake and birth outcomes have produced mixed results (105–113) (Table 5). The studies were conducted in different populations (though all in developed countries) with differing baseline maternal nutritional status, risk levels, and outcome variables; and the ways in which omega-3 fatty acid intake was measured or characterized (e.g., number of fish meals per week, fish intake (g/day), individual or total omega-3 fatty acid intake (g/day), etc.) also differed. Despite this, both the biomarker studies and the dietary intake studies identified a threshold effect only, below which intake or erythrocyte/plasma omega-3 fatty acid levels were positively associated with fetal growth measures or length of gestation. In addition, the studies that considered relative (omega-3:omega-6 fatty acid ratio) or overall (104) fatty acid profiles were more likely to detect associations with birth size/length of gestation than those exploring direct relations between omega-3 fatty acids and birth outcomes.
Results From Individual Studies of Associations Between Maternal Omega-3/Fatty Acid Status Biomarkers and Birth Outcomes
| First Author, Year (Reference No.) | Subjects | Nutrient | Biomarker | Outcome(s) | Results | Conclusions |
| Olsen, 1991 (101) | 62 Faroese women and 37 Danish women | Marine (long-chain) n-3 fatty acids | n-3 and n-6 fatty acids quantified in erythrocytes within 2 days of delivery | Length of gestation | Mean n-3:n-6 fatty acid ratio was 0.73 (SD, 0.11) in Faroese women and 0.61 (SD, 0.12) in Danish women (P < 0.001). | The longer gestation in Faroese women than in Danish women may be due to long-chain n-3 fatty acids down-regulating the formation of prostaglandins. Higher n-3:n-6 fatty acid ratios in Danish women led to significantly longer gestation. The hypothesized exposure-effect relation was not found in Faroese women, perhaps because of their higher level of long-chain n-3 fatty acid exposure. |
| 20% increase in n-3:n-6 ratio was associated with 5.7-day longer gestation in Danish women (P = 0.02) but not in Faroese women. | ||||||
| Grandjean, 2001 (102) | 182 healthy Faroe Island women with singleton pregnancies (population with high fish intake) | Marine (long-chain) n-3 fatty acids and contaminants | Maternal serum at 34 weeks, umbilical cord whole blood and serum at delivery | Length of gestation and birth weight | n-3 fatty acid serum concentrations were higher than most previously published values; mean birth weights were relatively high (boys: 3,801 g, girls: 3,537 g), and only 7% had birth weights below 3,000 g. | Increased intake of n-3 marine fatty acids may increase gestation length, but birth weight adjusted for gestational age may decrease at high intake levels (but apparently not due to contaminants). |
| Birth weight adjusted for gestational age decreased by 246 g (95% CI: 16, 476) for each 1% increase of EPA in cord serum. | ||||||
| Lucas, 2004 (103) | 454 newborns in Nunavik region of northern Quebec and 26 newborns in southern Quebec, Canada | Marine (long-chain) n-3 fatty acids | Fatty acid concentrations in umbilical cord plasma | Length of gestation and birth weight | In Nunavik newborns, docosahexaenoic acid concentration, n-3:n-6 ratio, and long-chain n-3:PUFA ratio were 3 times higher than in southern Quebec newborns. Gestational age in the third tertile of long-chain n-3:PUFA ratio was 5.9 days longer than gestational age in the first tertile (P = 0.02). | In the Nunavik population, a population with a high intake of seafood, a higher n-3:n6 ratio was associated with significantly longer gestation. |
| van Eijsden, 2008 (104) | Low-to-moderate-risk multiethnic sample of 3,704 women in Amsterdam, the Netherlands | Maternal fatty acids | Plasma phospholipids at ∼12 weeks’ gestation | Birth weight and SGA at term | In multivariate analysis, EPA and dihomo-γ-linolenic acid were positively associated with birth weight and arachidonic acid was negatively associated with birth weight; associations with SGA birth were similar. The 7% of women with most adverse fatty acid profiles had infants 125 g lighter and with a 2.12 times’ higher SGA risk (95% CI: 1.44, 3.13) than women with the best fatty acid profiles. | Results suggest that maternal fatty acid profile in early pregnancy affects fetal growth. |
| First Author, Year (Reference No.) | Subjects | Nutrient | Biomarker | Outcome(s) | Results | Conclusions |
| Olsen, 1991 (101) | 62 Faroese women and 37 Danish women | Marine (long-chain) n-3 fatty acids | n-3 and n-6 fatty acids quantified in erythrocytes within 2 days of delivery | Length of gestation | Mean n-3:n-6 fatty acid ratio was 0.73 (SD, 0.11) in Faroese women and 0.61 (SD, 0.12) in Danish women (P < 0.001). | The longer gestation in Faroese women than in Danish women may be due to long-chain n-3 fatty acids down-regulating the formation of prostaglandins. Higher n-3:n-6 fatty acid ratios in Danish women led to significantly longer gestation. The hypothesized exposure-effect relation was not found in Faroese women, perhaps because of their higher level of long-chain n-3 fatty acid exposure. |
| 20% increase in n-3:n-6 ratio was associated with 5.7-day longer gestation in Danish women (P = 0.02) but not in Faroese women. | ||||||
| Grandjean, 2001 (102) | 182 healthy Faroe Island women with singleton pregnancies (population with high fish intake) | Marine (long-chain) n-3 fatty acids and contaminants | Maternal serum at 34 weeks, umbilical cord whole blood and serum at delivery | Length of gestation and birth weight | n-3 fatty acid serum concentrations were higher than most previously published values; mean birth weights were relatively high (boys: 3,801 g, girls: 3,537 g), and only 7% had birth weights below 3,000 g. | Increased intake of n-3 marine fatty acids may increase gestation length, but birth weight adjusted for gestational age may decrease at high intake levels (but apparently not due to contaminants). |
| Birth weight adjusted for gestational age decreased by 246 g (95% CI: 16, 476) for each 1% increase of EPA in cord serum. | ||||||
| Lucas, 2004 (103) | 454 newborns in Nunavik region of northern Quebec and 26 newborns in southern Quebec, Canada | Marine (long-chain) n-3 fatty acids | Fatty acid concentrations in umbilical cord plasma | Length of gestation and birth weight | In Nunavik newborns, docosahexaenoic acid concentration, n-3:n-6 ratio, and long-chain n-3:PUFA ratio were 3 times higher than in southern Quebec newborns. Gestational age in the third tertile of long-chain n-3:PUFA ratio was 5.9 days longer than gestational age in the first tertile (P = 0.02). | In the Nunavik population, a population with a high intake of seafood, a higher n-3:n6 ratio was associated with significantly longer gestation. |
| van Eijsden, 2008 (104) | Low-to-moderate-risk multiethnic sample of 3,704 women in Amsterdam, the Netherlands | Maternal fatty acids | Plasma phospholipids at ∼12 weeks’ gestation | Birth weight and SGA at term | In multivariate analysis, EPA and dihomo-γ-linolenic acid were positively associated with birth weight and arachidonic acid was negatively associated with birth weight; associations with SGA birth were similar. The 7% of women with most adverse fatty acid profiles had infants 125 g lighter and with a 2.12 times’ higher SGA risk (95% CI: 1.44, 3.13) than women with the best fatty acid profiles. | Results suggest that maternal fatty acid profile in early pregnancy affects fetal growth. |
Abbreviations: CI, confidence interval; EPA, eicosapentaenoic acid; n-3, omega-3; n-6, omega-6; PUFA, polyunsaturated fatty acid; SD, standard deviation; SGA, small-for-gestational-age.
Results From Individual Studies of Associations Between Maternal Omega-3/Fatty Acid Status Biomarkers and Birth Outcomes
| First Author, Year (Reference No.) | Subjects | Nutrient | Biomarker | Outcome(s) | Results | Conclusions |
| Olsen, 1991 (101) | 62 Faroese women and 37 Danish women | Marine (long-chain) n-3 fatty acids | n-3 and n-6 fatty acids quantified in erythrocytes within 2 days of delivery | Length of gestation | Mean n-3:n-6 fatty acid ratio was 0.73 (SD, 0.11) in Faroese women and 0.61 (SD, 0.12) in Danish women (P < 0.001). | The longer gestation in Faroese women than in Danish women may be due to long-chain n-3 fatty acids down-regulating the formation of prostaglandins. Higher n-3:n-6 fatty acid ratios in Danish women led to significantly longer gestation. The hypothesized exposure-effect relation was not found in Faroese women, perhaps because of their higher level of long-chain n-3 fatty acid exposure. |
| 20% increase in n-3:n-6 ratio was associated with 5.7-day longer gestation in Danish women (P = 0.02) but not in Faroese women. | ||||||
| Grandjean, 2001 (102) | 182 healthy Faroe Island women with singleton pregnancies (population with high fish intake) | Marine (long-chain) n-3 fatty acids and contaminants | Maternal serum at 34 weeks, umbilical cord whole blood and serum at delivery | Length of gestation and birth weight | n-3 fatty acid serum concentrations were higher than most previously published values; mean birth weights were relatively high (boys: 3,801 g, girls: 3,537 g), and only 7% had birth weights below 3,000 g. | Increased intake of n-3 marine fatty acids may increase gestation length, but birth weight adjusted for gestational age may decrease at high intake levels (but apparently not due to contaminants). |
| Birth weight adjusted for gestational age decreased by 246 g (95% CI: 16, 476) for each 1% increase of EPA in cord serum. | ||||||
| Lucas, 2004 (103) | 454 newborns in Nunavik region of northern Quebec and 26 newborns in southern Quebec, Canada | Marine (long-chain) n-3 fatty acids | Fatty acid concentrations in umbilical cord plasma | Length of gestation and birth weight | In Nunavik newborns, docosahexaenoic acid concentration, n-3:n-6 ratio, and long-chain n-3:PUFA ratio were 3 times higher than in southern Quebec newborns. Gestational age in the third tertile of long-chain n-3:PUFA ratio was 5.9 days longer than gestational age in the first tertile (P = 0.02). | In the Nunavik population, a population with a high intake of seafood, a higher n-3:n6 ratio was associated with significantly longer gestation. |
| van Eijsden, 2008 (104) | Low-to-moderate-risk multiethnic sample of 3,704 women in Amsterdam, the Netherlands | Maternal fatty acids | Plasma phospholipids at ∼12 weeks’ gestation | Birth weight and SGA at term | In multivariate analysis, EPA and dihomo-γ-linolenic acid were positively associated with birth weight and arachidonic acid was negatively associated with birth weight; associations with SGA birth were similar. The 7% of women with most adverse fatty acid profiles had infants 125 g lighter and with a 2.12 times’ higher SGA risk (95% CI: 1.44, 3.13) than women with the best fatty acid profiles. | Results suggest that maternal fatty acid profile in early pregnancy affects fetal growth. |
| First Author, Year (Reference No.) | Subjects | Nutrient | Biomarker | Outcome(s) | Results | Conclusions |
| Olsen, 1991 (101) | 62 Faroese women and 37 Danish women | Marine (long-chain) n-3 fatty acids | n-3 and n-6 fatty acids quantified in erythrocytes within 2 days of delivery | Length of gestation | Mean n-3:n-6 fatty acid ratio was 0.73 (SD, 0.11) in Faroese women and 0.61 (SD, 0.12) in Danish women (P < 0.001). | The longer gestation in Faroese women than in Danish women may be due to long-chain n-3 fatty acids down-regulating the formation of prostaglandins. Higher n-3:n-6 fatty acid ratios in Danish women led to significantly longer gestation. The hypothesized exposure-effect relation was not found in Faroese women, perhaps because of their higher level of long-chain n-3 fatty acid exposure. |
| 20% increase in n-3:n-6 ratio was associated with 5.7-day longer gestation in Danish women (P = 0.02) but not in Faroese women. | ||||||
| Grandjean, 2001 (102) | 182 healthy Faroe Island women with singleton pregnancies (population with high fish intake) | Marine (long-chain) n-3 fatty acids and contaminants | Maternal serum at 34 weeks, umbilical cord whole blood and serum at delivery | Length of gestation and birth weight | n-3 fatty acid serum concentrations were higher than most previously published values; mean birth weights were relatively high (boys: 3,801 g, girls: 3,537 g), and only 7% had birth weights below 3,000 g. | Increased intake of n-3 marine fatty acids may increase gestation length, but birth weight adjusted for gestational age may decrease at high intake levels (but apparently not due to contaminants). |
| Birth weight adjusted for gestational age decreased by 246 g (95% CI: 16, 476) for each 1% increase of EPA in cord serum. | ||||||
| Lucas, 2004 (103) | 454 newborns in Nunavik region of northern Quebec and 26 newborns in southern Quebec, Canada | Marine (long-chain) n-3 fatty acids | Fatty acid concentrations in umbilical cord plasma | Length of gestation and birth weight | In Nunavik newborns, docosahexaenoic acid concentration, n-3:n-6 ratio, and long-chain n-3:PUFA ratio were 3 times higher than in southern Quebec newborns. Gestational age in the third tertile of long-chain n-3:PUFA ratio was 5.9 days longer than gestational age in the first tertile (P = 0.02). | In the Nunavik population, a population with a high intake of seafood, a higher n-3:n6 ratio was associated with significantly longer gestation. |
| van Eijsden, 2008 (104) | Low-to-moderate-risk multiethnic sample of 3,704 women in Amsterdam, the Netherlands | Maternal fatty acids | Plasma phospholipids at ∼12 weeks’ gestation | Birth weight and SGA at term | In multivariate analysis, EPA and dihomo-γ-linolenic acid were positively associated with birth weight and arachidonic acid was negatively associated with birth weight; associations with SGA birth were similar. The 7% of women with most adverse fatty acid profiles had infants 125 g lighter and with a 2.12 times’ higher SGA risk (95% CI: 1.44, 3.13) than women with the best fatty acid profiles. | Results suggest that maternal fatty acid profile in early pregnancy affects fetal growth. |
Abbreviations: CI, confidence interval; EPA, eicosapentaenoic acid; n-3, omega-3; n-6, omega-6; PUFA, polyunsaturated fatty acid; SD, standard deviation; SGA, small-for-gestational-age.
Results From Individual Studies of Associations Between Maternal Omega-3 Fatty Acid Intake and Birth Outcomes
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Olsen, 1995 (106) | Population-based prospective cohort | 965 | Well-nourished Danish women | FFQ covering previous 3 months | ∼30 | 0.25 | Length of gestation, intrauterine growth restriction | Within the intake range of this population, n-3 fatty acid intake in the second and third trimesters of pregnancy did not predict length of gestation or fetal growth rate. |
| Olsen, 2002 (107) and Olsen, 2006 (108) | Prospective cohort | 8,729 | Danish women, SES level not described | FFQ | 16th and 30th | 0.18 (standard deviation, 0.16) | PTB, LBW | Women who ate no seafood during the first and second trimesters were at 3.0 times’ (95% CI: 1.2, 11.2) higher risk of PTB and 3.6 times’ (95% CI: 1.1, 11.1) higher risk of LBW than women who ate fish at least once per week. Dose-response range was mainly 0–0.15 g of n-3 fatty acids (96). Results of the 2006 analysis were similar (97). |
| Oken, 2004 (109) | Prospective cohort | 2,109 | Well-nourished, well-educated ethnically diverse US women | FFQ | Early pregnancy (26–28) | Combined eicosapentaenoic acid + docosahexaenoic acid: from 0.02 (mean of first quartile) to 0.38 (mean of fourth quartile) | PTB, LBW, SGA birth, birth weight, fetal growth | Long chain n-3 fatty acids were not associated with length of gestation or PTB risk but were associated with reduced fetal growth. (A warning was issued during the study against pregnant women's eating fish because of contaminants; researchers were not able to evaluate the effects of the contaminants.) |
| Rogers, 2004 (110) | Geographically based prospective cohort | 11,585 | Women in southwest England with singleton pregnancies; representative of United Kingdom population in terms of most demographic characteristics, but SES was higher than average | FFQ | 32 | Fish: 0.147 | SGA birth, length of gestation, birth weight | In adjusted analysis, women who ate no fish had a 1.37 times’ (95% CI: 1.02, 1.84) higher risk of SGA birth than women in the highest fish intake group. There was no association with length of gestation, PTB, or LBW. |
| Quantilea 0: 0.0 | ||||||||
| Quantile 5: 0.40 | ||||||||
| Guldner, 2007 (111) | Prospective cohort | 2,398 | French women with singleton pregnancies with low baseline rates of adverse birth outcomes | FFQ (covering periconceptional intake) | First trimester | Fish: 20.4 | PTB, LBW, SGA birth | Different categories of seafood were differently associated with birth outcomes: fish intake increased length of gestation; intake of large crustaceans decreased fetal growth (possibly due to the high level of contaminants found in large crustaceans in France). |
| Shellfish: 19.7 | ||||||||
| Halldorsson, 2007 (112) | Prospective cohort | 44,824 | National Danish birth cohort | FFQ | ∼25 | Not reported (6.6% ate ≥4 fatty fish meals per month) | SGA birth | Fatty fish intake of >60 g/day was associated with higher SGA birth risk (odds ratio = 1.24, 95% CI: 1.03, 1.49) in comparison with ≤5 g/day. There were no associations with intake of lean fish. Fatty fish intake may be associated with reduced fetal growth due to exposure to persistent organic pollutants. |
| Haugen, 2008 (113) | Prospective cohort | 26,125 | Norwegian women; SES level not described | FFQ (covering intake since becoming pregnant) | 18–22 | Not reported (66% ate fish ≥2 times per week) | Gestational length, PTB | Intake of fish ≥2 times per week was associated with lower PTB risk (odds ratio = 0.84, 95% CI: 0.74, 0.95) in comparison with <2 times per week. |
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Olsen, 1995 (106) | Population-based prospective cohort | 965 | Well-nourished Danish women | FFQ covering previous 3 months | ∼30 | 0.25 | Length of gestation, intrauterine growth restriction | Within the intake range of this population, n-3 fatty acid intake in the second and third trimesters of pregnancy did not predict length of gestation or fetal growth rate. |
| Olsen, 2002 (107) and Olsen, 2006 (108) | Prospective cohort | 8,729 | Danish women, SES level not described | FFQ | 16th and 30th | 0.18 (standard deviation, 0.16) | PTB, LBW | Women who ate no seafood during the first and second trimesters were at 3.0 times’ (95% CI: 1.2, 11.2) higher risk of PTB and 3.6 times’ (95% CI: 1.1, 11.1) higher risk of LBW than women who ate fish at least once per week. Dose-response range was mainly 0–0.15 g of n-3 fatty acids (96). Results of the 2006 analysis were similar (97). |
| Oken, 2004 (109) | Prospective cohort | 2,109 | Well-nourished, well-educated ethnically diverse US women | FFQ | Early pregnancy (26–28) | Combined eicosapentaenoic acid + docosahexaenoic acid: from 0.02 (mean of first quartile) to 0.38 (mean of fourth quartile) | PTB, LBW, SGA birth, birth weight, fetal growth | Long chain n-3 fatty acids were not associated with length of gestation or PTB risk but were associated with reduced fetal growth. (A warning was issued during the study against pregnant women's eating fish because of contaminants; researchers were not able to evaluate the effects of the contaminants.) |
| Rogers, 2004 (110) | Geographically based prospective cohort | 11,585 | Women in southwest England with singleton pregnancies; representative of United Kingdom population in terms of most demographic characteristics, but SES was higher than average | FFQ | 32 | Fish: 0.147 | SGA birth, length of gestation, birth weight | In adjusted analysis, women who ate no fish had a 1.37 times’ (95% CI: 1.02, 1.84) higher risk of SGA birth than women in the highest fish intake group. There was no association with length of gestation, PTB, or LBW. |
| Quantilea 0: 0.0 | ||||||||
| Quantile 5: 0.40 | ||||||||
| Guldner, 2007 (111) | Prospective cohort | 2,398 | French women with singleton pregnancies with low baseline rates of adverse birth outcomes | FFQ (covering periconceptional intake) | First trimester | Fish: 20.4 | PTB, LBW, SGA birth | Different categories of seafood were differently associated with birth outcomes: fish intake increased length of gestation; intake of large crustaceans decreased fetal growth (possibly due to the high level of contaminants found in large crustaceans in France). |
| Shellfish: 19.7 | ||||||||
| Halldorsson, 2007 (112) | Prospective cohort | 44,824 | National Danish birth cohort | FFQ | ∼25 | Not reported (6.6% ate ≥4 fatty fish meals per month) | SGA birth | Fatty fish intake of >60 g/day was associated with higher SGA birth risk (odds ratio = 1.24, 95% CI: 1.03, 1.49) in comparison with ≤5 g/day. There were no associations with intake of lean fish. Fatty fish intake may be associated with reduced fetal growth due to exposure to persistent organic pollutants. |
| Haugen, 2008 (113) | Prospective cohort | 26,125 | Norwegian women; SES level not described | FFQ (covering intake since becoming pregnant) | 18–22 | Not reported (66% ate fish ≥2 times per week) | Gestational length, PTB | Intake of fish ≥2 times per week was associated with lower PTB risk (odds ratio = 0.84, 95% CI: 0.74, 0.95) in comparison with <2 times per week. |
Abbreviations: CI, confidence interval; FFQ, food frequency questionnaire; LBW, low birth weight; n-3, omega-3; n-6, omega-6; PTB, preterm birth; SES, socioeconomic status; SGA, small-for-gestational-age.
Participants were divided into 6 groups according to their calculated weekly intake of omega-3 fatty acids (quantile 0 to quantile 5), with participants in quantile 0 consuming no seafood and the other 5 groups being fifths of the remaining women. Values in the table represent mean omega-3 fatty acid intakes (g/day) for the quantiles.
Results From Individual Studies of Associations Between Maternal Omega-3 Fatty Acid Intake and Birth Outcomes
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Olsen, 1995 (106) | Population-based prospective cohort | 965 | Well-nourished Danish women | FFQ covering previous 3 months | ∼30 | 0.25 | Length of gestation, intrauterine growth restriction | Within the intake range of this population, n-3 fatty acid intake in the second and third trimesters of pregnancy did not predict length of gestation or fetal growth rate. |
| Olsen, 2002 (107) and Olsen, 2006 (108) | Prospective cohort | 8,729 | Danish women, SES level not described | FFQ | 16th and 30th | 0.18 (standard deviation, 0.16) | PTB, LBW | Women who ate no seafood during the first and second trimesters were at 3.0 times’ (95% CI: 1.2, 11.2) higher risk of PTB and 3.6 times’ (95% CI: 1.1, 11.1) higher risk of LBW than women who ate fish at least once per week. Dose-response range was mainly 0–0.15 g of n-3 fatty acids (96). Results of the 2006 analysis were similar (97). |
| Oken, 2004 (109) | Prospective cohort | 2,109 | Well-nourished, well-educated ethnically diverse US women | FFQ | Early pregnancy (26–28) | Combined eicosapentaenoic acid + docosahexaenoic acid: from 0.02 (mean of first quartile) to 0.38 (mean of fourth quartile) | PTB, LBW, SGA birth, birth weight, fetal growth | Long chain n-3 fatty acids were not associated with length of gestation or PTB risk but were associated with reduced fetal growth. (A warning was issued during the study against pregnant women's eating fish because of contaminants; researchers were not able to evaluate the effects of the contaminants.) |
| Rogers, 2004 (110) | Geographically based prospective cohort | 11,585 | Women in southwest England with singleton pregnancies; representative of United Kingdom population in terms of most demographic characteristics, but SES was higher than average | FFQ | 32 | Fish: 0.147 | SGA birth, length of gestation, birth weight | In adjusted analysis, women who ate no fish had a 1.37 times’ (95% CI: 1.02, 1.84) higher risk of SGA birth than women in the highest fish intake group. There was no association with length of gestation, PTB, or LBW. |
| Quantilea 0: 0.0 | ||||||||
| Quantile 5: 0.40 | ||||||||
| Guldner, 2007 (111) | Prospective cohort | 2,398 | French women with singleton pregnancies with low baseline rates of adverse birth outcomes | FFQ (covering periconceptional intake) | First trimester | Fish: 20.4 | PTB, LBW, SGA birth | Different categories of seafood were differently associated with birth outcomes: fish intake increased length of gestation; intake of large crustaceans decreased fetal growth (possibly due to the high level of contaminants found in large crustaceans in France). |
| Shellfish: 19.7 | ||||||||
| Halldorsson, 2007 (112) | Prospective cohort | 44,824 | National Danish birth cohort | FFQ | ∼25 | Not reported (6.6% ate ≥4 fatty fish meals per month) | SGA birth | Fatty fish intake of >60 g/day was associated with higher SGA birth risk (odds ratio = 1.24, 95% CI: 1.03, 1.49) in comparison with ≤5 g/day. There were no associations with intake of lean fish. Fatty fish intake may be associated with reduced fetal growth due to exposure to persistent organic pollutants. |
| Haugen, 2008 (113) | Prospective cohort | 26,125 | Norwegian women; SES level not described | FFQ (covering intake since becoming pregnant) | 18–22 | Not reported (66% ate fish ≥2 times per week) | Gestational length, PTB | Intake of fish ≥2 times per week was associated with lower PTB risk (odds ratio = 0.84, 95% CI: 0.74, 0.95) in comparison with <2 times per week. |
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Assessment | Mean Intake, g/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Olsen, 1995 (106) | Population-based prospective cohort | 965 | Well-nourished Danish women | FFQ covering previous 3 months | ∼30 | 0.25 | Length of gestation, intrauterine growth restriction | Within the intake range of this population, n-3 fatty acid intake in the second and third trimesters of pregnancy did not predict length of gestation or fetal growth rate. |
| Olsen, 2002 (107) and Olsen, 2006 (108) | Prospective cohort | 8,729 | Danish women, SES level not described | FFQ | 16th and 30th | 0.18 (standard deviation, 0.16) | PTB, LBW | Women who ate no seafood during the first and second trimesters were at 3.0 times’ (95% CI: 1.2, 11.2) higher risk of PTB and 3.6 times’ (95% CI: 1.1, 11.1) higher risk of LBW than women who ate fish at least once per week. Dose-response range was mainly 0–0.15 g of n-3 fatty acids (96). Results of the 2006 analysis were similar (97). |
| Oken, 2004 (109) | Prospective cohort | 2,109 | Well-nourished, well-educated ethnically diverse US women | FFQ | Early pregnancy (26–28) | Combined eicosapentaenoic acid + docosahexaenoic acid: from 0.02 (mean of first quartile) to 0.38 (mean of fourth quartile) | PTB, LBW, SGA birth, birth weight, fetal growth | Long chain n-3 fatty acids were not associated with length of gestation or PTB risk but were associated with reduced fetal growth. (A warning was issued during the study against pregnant women's eating fish because of contaminants; researchers were not able to evaluate the effects of the contaminants.) |
| Rogers, 2004 (110) | Geographically based prospective cohort | 11,585 | Women in southwest England with singleton pregnancies; representative of United Kingdom population in terms of most demographic characteristics, but SES was higher than average | FFQ | 32 | Fish: 0.147 | SGA birth, length of gestation, birth weight | In adjusted analysis, women who ate no fish had a 1.37 times’ (95% CI: 1.02, 1.84) higher risk of SGA birth than women in the highest fish intake group. There was no association with length of gestation, PTB, or LBW. |
| Quantilea 0: 0.0 | ||||||||
| Quantile 5: 0.40 | ||||||||
| Guldner, 2007 (111) | Prospective cohort | 2,398 | French women with singleton pregnancies with low baseline rates of adverse birth outcomes | FFQ (covering periconceptional intake) | First trimester | Fish: 20.4 | PTB, LBW, SGA birth | Different categories of seafood were differently associated with birth outcomes: fish intake increased length of gestation; intake of large crustaceans decreased fetal growth (possibly due to the high level of contaminants found in large crustaceans in France). |
| Shellfish: 19.7 | ||||||||
| Halldorsson, 2007 (112) | Prospective cohort | 44,824 | National Danish birth cohort | FFQ | ∼25 | Not reported (6.6% ate ≥4 fatty fish meals per month) | SGA birth | Fatty fish intake of >60 g/day was associated with higher SGA birth risk (odds ratio = 1.24, 95% CI: 1.03, 1.49) in comparison with ≤5 g/day. There were no associations with intake of lean fish. Fatty fish intake may be associated with reduced fetal growth due to exposure to persistent organic pollutants. |
| Haugen, 2008 (113) | Prospective cohort | 26,125 | Norwegian women; SES level not described | FFQ (covering intake since becoming pregnant) | 18–22 | Not reported (66% ate fish ≥2 times per week) | Gestational length, PTB | Intake of fish ≥2 times per week was associated with lower PTB risk (odds ratio = 0.84, 95% CI: 0.74, 0.95) in comparison with <2 times per week. |
Abbreviations: CI, confidence interval; FFQ, food frequency questionnaire; LBW, low birth weight; n-3, omega-3; n-6, omega-6; PTB, preterm birth; SES, socioeconomic status; SGA, small-for-gestational-age.
Participants were divided into 6 groups according to their calculated weekly intake of omega-3 fatty acids (quantile 0 to quantile 5), with participants in quantile 0 consuming no seafood and the other 5 groups being fifths of the remaining women. Values in the table represent mean omega-3 fatty acid intakes (g/day) for the quantiles.
However, in some dietary studies, investigators reported negative associations between fish intake and birth size, possibly due to contaminants found in certain types of seafood (109, 111, 112). Trials of omega-3 fatty acid supplementation have had mixed results. However, in recent reviews combining observational and experimental evidence, investigators have concluded that adequate omega-3 fatty acid intake and status is associated with both maternal and fetal benefits, although issues regarding possible negative effects from marine-food contaminants (109, 111, 114) or excess omega-3 fatty acid intake have been raised and require further research and consensus as to the implications for marine food intake during pregnancy (105). As we noted above, all of the studies were conducted in developed countries, and SES level (assessed primarily using educational level as a proxy) was taken into account in the analyses. However, in many cases, the range of SES levels within the study populations was limited, and the role of SES was not explicitly discussed, although seafood intake has been shown to be associated with SES in several developed countries (112, 115).
Cultural and environmental factors that affect seafood/omega-3 intake, other than contaminant levels, have also been given little attention, aside from the observation that populations with traditionally high seafood intakes tend to have better birth outcomes than populations characterized by low seafood intakes. One large, ongoing cohort study (110) was designed as a longitudinal study and has the potential to provide future information on associations between seafood intake during pregnancy and life-cycle and intergenerational developmental parameters.
Iron
Nutritional iron deficiency is highest in segments of the population that are experiencing peak growth rates, such as infants, young children, and pregnant women (116). The risk of developing iron deficiency is greatest during pregnancy (especially for low-SES women and ethnic minority groups), since maternal iron requirements are substantially higher than average absorbable iron intakes (44, 117) (Table 2). If a woman's diet does not contain enough iron to meet these needs, the body can meet fetal requirements only by drawing upon maternal iron stores. The demands of the developing fetus may cause the mother to develop iron-deficiency anemia if she had inadequate iron stores at the beginning of the pregnancy (118). Estimates from the World Health Organization indicate that, on average, 56% of pregnant women in developing countries are anemic, as are 18% of pregnant women in developed countries (119). In developing countries where malaria, hookworm infestations, and helminth infections are endemic, these may be the primary causes of anemia, rather than iron deficiency, or they may compound the effects of iron-deficiency anemia; thus, they must be treated along with iron deficiency in order to reduce rates of anemia (120–122).
We found no studies focusing on dietary iron intake (iron from food sources, not supplements) and birth outcomes, and studies that assessed overall pregnancy nutrient intakes and birth outcomes did not observe associations between dietary iron intake and adverse birth outcomes (60, 61). However, many investigators have evaluated associations between anemia and adverse birth outcomes. There is substantial observational evidence showing that maternal iron-deficiency anemia prior to and in early pregnancy places the mother at increased risk of preterm birth or low-birth-weight delivery (44, 119). Severe anemia (hemoglobin level <80 g/L) is associated with the birth of small babies, as a consequence of both preterm labor and growth restriction. The minimum incidence of low birth weight and preterm birth is found when hemoglobin concentrations are 95–105 g/L (118).
Public health polices recommending/providing iron supplements to pregnant women are widely in place throughout the world (119); however, the extent of coverage and compliance with these policies varies. There is strong evidence that iron deficiency in the first trimester of pregnancy results in significant decrements in fetal growth and is generally more damaging to pregnancy outcome than iron-deficiency anemia in the second and third trimesters. On the basis of available observational and experimental data, Scholl (44) suggested that iron supplementation should be started in early pregnancy, if not periconceptionally, in order to reduce the incidence of preterm birth, and that supplementation beginning in midpregnancy, as it does for many women, is unlikely to reduce preterm birth risk. Thus, special emphasis should be placed upon improving maternal iron nutritional status prior to, as well as early in, pregnancy and throughout the period of lactation (43, 116). A number of randomized intervention studies of iron supplementation beginning in early pregnancy have shown reductions in risk of low birth weight and/or preterm birth, particularly among women with insufficient iron stores (44) or women of low SES (40). Conversely, women who have high iron stores and elevated ferritin levels (>41 ng/mL), particularly in the third trimester of pregnancy, are at greatly increased risk of preterm birth. This association has been attributed either to intrauterine infection (which causes elevated serum ferritin levels) or to the failure of the maternal plasma volume to expand (44).
Given the differences in rates of anemia between developed and developing countries, SES levels are likely to affect both the amount and the quality/bioavailability of dietary iron intake. In a Cochrane review of iron supplementation during pregnancy, Peña-Rosas and Viteri (35) did not stratify their meta-analyses by SES, and they acknowledged that pooled analysis might not be appropriate, given the heterogeneity of the studies. Cultural/environmental factors, ranging from dietary sources of iron to attitudes toward and availability of iron supplements, age at initiation of childbearing, and length of interpregnancy intervals, have not been sufficiently investigated. Given the importance of maternal iron status to infant and childhood growth and development (123), longitudinal studies that investigate the life-cycle and intergenerational implications of maternal iron deficiency are also needed.
Folate
Folate, a water-soluble B-complex vitamin, is considered an essential nutrient, since it cannot be synthesized in the human body. Folate is critical to fetal development because it is a cofactor for many essential cellular reactions, including DNA and nucleic acid synthesis (4). The need for folic acid increases during times of rapid tissue growth, which during pregnancy includes an increase in red blood cell mass, enlargement of the uterus, and the growth of the placenta and fetus (124) (Table 2).
Insufficient maternal folate intake has been linked to low birth weight, IUGR, and preterm birth (4, 125). Marginal maternal folate intake/status can impair cellular growth in the fetus or placenta. In several studies in rats and mice, low maternal dietary folate intake resulted in an increase in the incidence of IUGR (126, 127). In human studies, the findings have been mixed (128–132) (Table 6). In several large-scale studies, low folate intake assessed periconceptionally through midpregnancy was associated with a more than 2- to 4-fold increase in risk of infant low birth weight and/or preterm birth, particularly in low-income populations (128–130). In a mixed-SES sample of US women, periconceptional use of folic acid supplements for 1 year or more significantly reduced preterm birth rates prior to 32 weeks (131). However, in a large RCT among pregnant women in rural Nepal, folic acid supplementation alone initiated in early pregnancy (∼11 weeks) did not reduce preterm birth rates or have a significant effect upon rates of low birth weight in comparison with no supplementation (41). Nevertheless, low circulating levels of folate during pregnancy have been associated with increased rates of IUGR among low-income populations in both developed and developing countries (4, 133, 134). Thus, the body of research suggests the importance of folate in the periconceptional stage, and a mediating role for SES. As with the other nutrients reviewed, little attention has been given to the role of cultural/environmental or intergenerational factors in the association between maternal folate and birth outcome.
Results From Individual Studies of Associations Between Maternal Dietary Folate Intake and Birth Outcomes
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Intake or Supplementation Use Assessment | Mean Intake, μg/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Scholl, 1996 (128) | Prospective cohort | 832 | Low-income urban US women with overall inadequate diet and poor nutritional status | 3 24-hour recalls | At initiation of prenatal care, 28, and 36 | 284 (234)a from food and 172 (172) from supplements | PTB, LBW | In multivariate analyses, women with low mean folate intake (≤240 μg/day) had twice the risk of PTB and LBW as women with high folate intake (>240 μg/day) |
| Neggers, 1997 (129) | Prospective cohort | 1,398 | Low-income US African-American and white women | 2 24-hour recalls | 18 and 30 | (Women were offered a prenatal supplement containing 1 mg of folic acid and 60 mg of iron) | PTB, LBW | Infant birth weight (47.6 g) for mothers whose dietary folate intake was above the 90th percentile was significantly higher (P < 0.05) than that for mothers whose dietary folate intake was below the 10th percentile. |
| Siega-Riz, 2004 (130) | Prospective cohort | 2,314 | Lower- to middle-income US women receiving prenatal care | Food frequency questionnaire covering second trimester of pregnancy | 24–29 | 463 (248) | PTB | Folate intake ≤500 μg/day was associated with increased risk of PTB (relative risk = 1.8, 95% confidence interval: 1.4, 2.6) in multivariate analysis. |
| Bukowski, 2009 (131) | Multicenter prospective cohort | 34,480 | Multiethnic, mixed-socioeconomic-status sample of US pregnant women with singleton pregnancies of <14 weeks’ gestation at enrollment | Self-reported periconceptional use of folate supplements | 10–13 (completed weeks) | Periconceptional supplement use: | PTB, SGA birth | In multivariate analysis, periconceptional folate supplementation for ≥1 year significantly reduced risk of PTB by 69% during weeks 20–28 and by 47% during weeks 28–32 but did not reduce PTB risk after 32 weeks. An association between supplementation for <1 year and reduced PTB risk disappeared after adjustment for maternal characteristics. No associations with SGA birth were found. |
| None, 44% | ||||||||
| <1 year, 36% | ||||||||
| ≥1 year, 20% | ||||||||
| Wantanabe, 2008 (132) | Prospective cohort | 197 | Well-nourished Japanese women with singleton pregnancies | Diet history questionnaire | 12 | 249 (113) | Birth weight | Dietary folate intake was not a significant predictor of birth weight, perhaps because of the small sample size. (Folic acid food fortification was not endorsed, and supplement intake was low; only in the 90th percentile did dietary folate intakes reach 400 μg/day). |
| 20 | 262 (94) | |||||||
| 32 | 275 (100) | |||||||
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Intake or Supplementation Use Assessment | Mean Intake, μg/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Scholl, 1996 (128) | Prospective cohort | 832 | Low-income urban US women with overall inadequate diet and poor nutritional status | 3 24-hour recalls | At initiation of prenatal care, 28, and 36 | 284 (234)a from food and 172 (172) from supplements | PTB, LBW | In multivariate analyses, women with low mean folate intake (≤240 μg/day) had twice the risk of PTB and LBW as women with high folate intake (>240 μg/day) |
| Neggers, 1997 (129) | Prospective cohort | 1,398 | Low-income US African-American and white women | 2 24-hour recalls | 18 and 30 | (Women were offered a prenatal supplement containing 1 mg of folic acid and 60 mg of iron) | PTB, LBW | Infant birth weight (47.6 g) for mothers whose dietary folate intake was above the 90th percentile was significantly higher (P < 0.05) than that for mothers whose dietary folate intake was below the 10th percentile. |
| Siega-Riz, 2004 (130) | Prospective cohort | 2,314 | Lower- to middle-income US women receiving prenatal care | Food frequency questionnaire covering second trimester of pregnancy | 24–29 | 463 (248) | PTB | Folate intake ≤500 μg/day was associated with increased risk of PTB (relative risk = 1.8, 95% confidence interval: 1.4, 2.6) in multivariate analysis. |
| Bukowski, 2009 (131) | Multicenter prospective cohort | 34,480 | Multiethnic, mixed-socioeconomic-status sample of US pregnant women with singleton pregnancies of <14 weeks’ gestation at enrollment | Self-reported periconceptional use of folate supplements | 10–13 (completed weeks) | Periconceptional supplement use: | PTB, SGA birth | In multivariate analysis, periconceptional folate supplementation for ≥1 year significantly reduced risk of PTB by 69% during weeks 20–28 and by 47% during weeks 28–32 but did not reduce PTB risk after 32 weeks. An association between supplementation for <1 year and reduced PTB risk disappeared after adjustment for maternal characteristics. No associations with SGA birth were found. |
| None, 44% | ||||||||
| <1 year, 36% | ||||||||
| ≥1 year, 20% | ||||||||
| Wantanabe, 2008 (132) | Prospective cohort | 197 | Well-nourished Japanese women with singleton pregnancies | Diet history questionnaire | 12 | 249 (113) | Birth weight | Dietary folate intake was not a significant predictor of birth weight, perhaps because of the small sample size. (Folic acid food fortification was not endorsed, and supplement intake was low; only in the 90th percentile did dietary folate intakes reach 400 μg/day). |
| 20 | 262 (94) | |||||||
| 32 | 275 (100) | |||||||
Abbreviations: LBW, low birth weight; PTB, preterm birth; SD, standard deviation; SGA, small-for-gestational-age.
Numbers in parentheses, standard deviation.
Results From Individual Studies of Associations Between Maternal Dietary Folate Intake and Birth Outcomes
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Intake or Supplementation Use Assessment | Mean Intake, μg/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Scholl, 1996 (128) | Prospective cohort | 832 | Low-income urban US women with overall inadequate diet and poor nutritional status | 3 24-hour recalls | At initiation of prenatal care, 28, and 36 | 284 (234)a from food and 172 (172) from supplements | PTB, LBW | In multivariate analyses, women with low mean folate intake (≤240 μg/day) had twice the risk of PTB and LBW as women with high folate intake (>240 μg/day) |
| Neggers, 1997 (129) | Prospective cohort | 1,398 | Low-income US African-American and white women | 2 24-hour recalls | 18 and 30 | (Women were offered a prenatal supplement containing 1 mg of folic acid and 60 mg of iron) | PTB, LBW | Infant birth weight (47.6 g) for mothers whose dietary folate intake was above the 90th percentile was significantly higher (P < 0.05) than that for mothers whose dietary folate intake was below the 10th percentile. |
| Siega-Riz, 2004 (130) | Prospective cohort | 2,314 | Lower- to middle-income US women receiving prenatal care | Food frequency questionnaire covering second trimester of pregnancy | 24–29 | 463 (248) | PTB | Folate intake ≤500 μg/day was associated with increased risk of PTB (relative risk = 1.8, 95% confidence interval: 1.4, 2.6) in multivariate analysis. |
| Bukowski, 2009 (131) | Multicenter prospective cohort | 34,480 | Multiethnic, mixed-socioeconomic-status sample of US pregnant women with singleton pregnancies of <14 weeks’ gestation at enrollment | Self-reported periconceptional use of folate supplements | 10–13 (completed weeks) | Periconceptional supplement use: | PTB, SGA birth | In multivariate analysis, periconceptional folate supplementation for ≥1 year significantly reduced risk of PTB by 69% during weeks 20–28 and by 47% during weeks 28–32 but did not reduce PTB risk after 32 weeks. An association between supplementation for <1 year and reduced PTB risk disappeared after adjustment for maternal characteristics. No associations with SGA birth were found. |
| None, 44% | ||||||||
| <1 year, 36% | ||||||||
| ≥1 year, 20% | ||||||||
| Wantanabe, 2008 (132) | Prospective cohort | 197 | Well-nourished Japanese women with singleton pregnancies | Diet history questionnaire | 12 | 249 (113) | Birth weight | Dietary folate intake was not a significant predictor of birth weight, perhaps because of the small sample size. (Folic acid food fortification was not endorsed, and supplement intake was low; only in the 90th percentile did dietary folate intakes reach 400 μg/day). |
| 20 | 262 (94) | |||||||
| 32 | 275 (100) | |||||||
| First Author, Year (Reference No.) | Study Design | No. of Subjects | Participant Characteristics | Dietary Intake or Supplementation Use Assessment | Mean Intake, μg/day | Outcome Measure(s) | Conclusions | |
| Method | Timing, weeks | |||||||
| Scholl, 1996 (128) | Prospective cohort | 832 | Low-income urban US women with overall inadequate diet and poor nutritional status | 3 24-hour recalls | At initiation of prenatal care, 28, and 36 | 284 (234)a from food and 172 (172) from supplements | PTB, LBW | In multivariate analyses, women with low mean folate intake (≤240 μg/day) had twice the risk of PTB and LBW as women with high folate intake (>240 μg/day) |
| Neggers, 1997 (129) | Prospective cohort | 1,398 | Low-income US African-American and white women | 2 24-hour recalls | 18 and 30 | (Women were offered a prenatal supplement containing 1 mg of folic acid and 60 mg of iron) | PTB, LBW | Infant birth weight (47.6 g) for mothers whose dietary folate intake was above the 90th percentile was significantly higher (P < 0.05) than that for mothers whose dietary folate intake was below the 10th percentile. |
| Siega-Riz, 2004 (130) | Prospective cohort | 2,314 | Lower- to middle-income US women receiving prenatal care | Food frequency questionnaire covering second trimester of pregnancy | 24–29 | 463 (248) | PTB | Folate intake ≤500 μg/day was associated with increased risk of PTB (relative risk = 1.8, 95% confidence interval: 1.4, 2.6) in multivariate analysis. |
| Bukowski, 2009 (131) | Multicenter prospective cohort | 34,480 | Multiethnic, mixed-socioeconomic-status sample of US pregnant women with singleton pregnancies of <14 weeks’ gestation at enrollment | Self-reported periconceptional use of folate supplements | 10–13 (completed weeks) | Periconceptional supplement use: | PTB, SGA birth | In multivariate analysis, periconceptional folate supplementation for ≥1 year significantly reduced risk of PTB by 69% during weeks 20–28 and by 47% during weeks 28–32 but did not reduce PTB risk after 32 weeks. An association between supplementation for <1 year and reduced PTB risk disappeared after adjustment for maternal characteristics. No associations with SGA birth were found. |
| None, 44% | ||||||||
| <1 year, 36% | ||||||||
| ≥1 year, 20% | ||||||||
| Wantanabe, 2008 (132) | Prospective cohort | 197 | Well-nourished Japanese women with singleton pregnancies | Diet history questionnaire | 12 | 249 (113) | Birth weight | Dietary folate intake was not a significant predictor of birth weight, perhaps because of the small sample size. (Folic acid food fortification was not endorsed, and supplement intake was low; only in the 90th percentile did dietary folate intakes reach 400 μg/day). |
| 20 | 262 (94) | |||||||
| 32 | 275 (100) | |||||||
Abbreviations: LBW, low birth weight; PTB, preterm birth; SD, standard deviation; SGA, small-for-gestational-age.
Numbers in parentheses, standard deviation.
Multiple nutrient deficiencies and public health considerations
The literature on maternal nutrition and birth outcomes has been dominated by studies of single macro- or micronutrients. Studies of maternal dietary intake and birth outcomes usually assess outcomes for each nutrient separately because of the high intercorrelations between most nutrient intakes, and such studies tend not to find associations, particularly in industrialized populations (60, 61). Public health policy-makers have tended to take a similar approach, and in both developed and developing countries, they most commonly recommend only that pregnant women take iron or iron/folate supplements routinely because of the difficulties of reaching the recommended intakes of these 2 micronutrients through diet alone (35, 119).
However, undernutrition is most likely to exist in developing countries/low-SES populations in which diets are inadequate in high-quality, nutrient-dense foods (e.g., animal-source foods) because of their expense. In such settings, women of childbearing age are often at risk of multiple nutrient deficiencies, so the reductionist approach of studying a single nutrient in isolation is illogical. However, the more logical approach of studying multiple micronutrient supplements or improved overall diet quality has not been adequately tested or researched (1, 2, 38, 43, 135).
RCTs of multinutrient supplementation have generated mixed results and have not generally produced substantial improvements over iron-folic acid supplementation (Table 1). Thus, they have not been adopted in public health programs. Additional reasons are related to concerns about the possible adverse effects of excessive amounts of some micronutrients and of interactions between micronutrients in a multinutrient supplement (38). However, in a meta-analysis of the most recent trials, including primarily very vulnerable populations, Shah and Ohlsson (39) found a significant reduction in low birth weight among women receiving a multimicronutrient supplement as compared with those receiving an iron-folic acid supplement alone (relative risk = 0.83, 95% confidence interval: 0.74, 0.93). On the basis of these results, Shah and Ohlsson advocated a change in public health policies globally, from recommending only iron-folic acid supplementation to recommending multimicronutrient supplementation for pregnant women (39). Bhutta and Haider (135), however, called for further research to determine whether the above benefits are found across all levels of maternal nutritional status and to ensure that perinatal outcomes are not negatively affected before multimicronutrient supplementation is made a universal policy. In addition, they recommended that optimal maternal nutritional status be achieved through multiple interventions, including those aimed at reducing the burden of infection, providing fortified food supplements, and reducing household food insecurity, rather than through maternal multimicronutrient supplementation alone (135).
Allen (136) has argued that the global focus on supplementation programs is a “top-down” orientation toward inadequate nutrient intakes, which has diverted attention from considering sustainable food-based approaches. In the limited number of observational food-based intervention studies that have been conducted, investigators have reported associations between a higher-quality maternal diet or one supplemented with nutrient-dense foods and reduced risk of adverse birth outcomes (23, 43, 65). Cohort studies on the effects of the most long-standing of these interventions, the WIC program in the United States, have demonstrated reduced risks of preterm birth and low birth weight, particularly among women in the highest risk groups (e.g., women with a history of abortion and inadequate prenatal care) (65) and those who participated in the program for a minimum of 6 months during pregnancy (58).
A food supplementation intervention carried out among pregnant women in Guatemala for the duration of 2 pregnancies and the intervening lactation period significantly increased birth weights (45, 92); however, the researchers acknowledged the limitations and disadvantages of such programs, in terms of their expense, time consumption, and sustainability for large populations over long periods of time. In addition, such programs were prone to the creation of “dependent” populations, and where needs at the household level were great, the food supplements did not necessarily go to the intended recipient, unless the program was carefully controlled (33, 92). Thus, Lechtig et al. (92) suggested that more emphasis should be placed upon the development, implementation, and evaluation of programs aimed at improving specific SES factors (e.g., family income) as a more effective means than food supplementation of breaking the cycle of socioeconomic deprivation, maternal undernutrition, and adverse birth outcomes.
Allen (136) highlighted the option of increasing the production and consumption of animal-source foods as another method of increasing overall diet quality at the household level, which some models have shown to be both economically beneficial and sustainable. At the same time, with food-based approaches to meeting the nutrient needs of pregnant women, it is important to understand and avoid the possible negative effects of foodborne contaminants on fetal growth and birth outcomes, for which seafood, dairy products, poultry, meat, fruits, and vegetables may carry a risk (137–139). In a recent review, Bhutta et al. (69) considered food-based approaches to have potential but to have been inadequately developed and tested thus far.
CONCLUSION
Maternal nutrition plays a crucial role in influencing fetal growth and birth outcomes. It is a modifiable risk factor of public health importance in the effort to prevent adverse birth outcomes, particularly among developing/low-income populations. The existing intervention studies, which primarily have involved single-nutrient interventions conducted for a limited period of time during a single pregnancy, have shown a positive effect on birth outcomes in some cases; but the evidence is far from consistent. While RCTs provide the best evidence of causal relations, they are hampered by practical issues such as restraints on sample size, the length and timing of the intervention, and costs. In view of these limitations, we will have to rely on additional information from observational studies. These observational and experimental studies, where practical, should take maternal nutritional/multinutrient status into account, starting in the periconceptional period and/or persisting for the duration of more than 1 pregnancy/reproductive cycle. While associations between maternal dietary intake and adverse birth outcomes and between SES/environmental factors and adverse birth outcomes have been demonstrated separately, they are clearly interrelated. New approaches are needed to take these interrelations, including their life-cycle and intergenerational effects, into account. Such approaches have the potential to further our understanding of maternal dietary/nutritional influences on birth outcomes and to advance the effort to reduce adverse birth outcomes.
Abbreviations
- IUGR
intrauterine growth restriction
- RCTs
randomized controlled trials
- SES
socioeconomic status
- WIC
Women, Infants, and Children
Author affiliations: Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Tel Hashomer, Israel (Kathleen Abu-Saad); and Department of Epidemiology and Health Services Evaluation, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel (Drora Fraser).
Conflict of interest: none declared.