-
PDF
- Split View
-
Views
-
Cite
Cite
George C M Siontis, Pavel Overtchouk, Thomas J Cahill, Thomas Modine, Bernard Prendergast, Fabien Praz, Thomas Pilgrim, Tatjana Petrinic, Adriani Nikolakopoulou, Georgia Salanti, Lars Søndergaard, Subodh Verma, Peter Jüni, Stephan Windecker, Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis, European Heart Journal, Volume 40, Issue 38, 7 October 2019, Pages 3143–3153, https://doi.org/10.1093/eurheartj/ehz275
Close - Share Icon Share
Abstract
Owing to new evidence from randomized controlled trials (RCTs) in low-risk patients with severe aortic stenosis, we compared the collective safety and efficacy of transcatheter aortic valve implantation (TAVI) vs. surgical aortic valve replacement (SAVR) across the entire spectrum of surgical risk patients.
The meta-analysis is registered with PROSPERO (CRD42016037273). We identified RCTs comparing TAVI with SAVR in patients with severe aortic stenosis reporting at different follow-up periods. We extracted trial, patient, intervention, and outcome characteristics following predefined criteria. The primary outcome was all-cause mortality up to 2 years for the main analysis. Seven trials that randomly assigned 8020 participants to TAVI (4014 patients) and SAVR (4006 patients) were included. The combined mean STS score in the TAVI arm was 9.4%, 5.1%, and 2.0% for high-, intermediate-, and low surgical risk trials, respectively. Transcatheter aortic valve implantation was associated with a significant reduction of all-cause mortality compared to SAVR {hazard ratio [HR] 0.88 [95% confidence interval (CI) 0.78–0.99], P = 0.030}; an effect that was consistent across the entire spectrum of surgical risk (P-for-interaction = 0.410) and irrespective of type of transcatheter heart valve (THV) system (P-for-interaction = 0.674). Transcatheter aortic valve implantation resulted in lower risk of strokes [HR 0.81 (95% CI 0.68–0.98), P = 0.028]. Surgical aortic valve replacement was associated with a lower risk of major vascular complications [HR 1.99 (95% CI 1.34–2.93), P = 0.001] and permanent pacemaker implantations [HR 2.27 (95% CI 1.47–3.64), P < 0.001] compared to TAVI.
Compared with SAVR, TAVI is associated with reduction in all-cause mortality and stroke up to 2 years irrespective of baseline surgical risk and type of THV system.
Introduction
Within the last decade, transcatheter aortic valve implantation (TAVI) has emerged as a valuable alternative to surgical aortic valve replacement (SAVR) in an increasingly wide spectrum of patients with severe symptomatic aortic stenosis (AS).1 , 2 The safety and efficacy of TAVI was initially established in patients at high surgical risk [Society of Thoracic Surgeons Predicted Risk of Mortality (STS) score ≥8–15%] in the PARTNER 1A3–5 and US CoreValve high-risk trials6–9 showing comparable clinical outcomes compared to SAVR. A role for TAVI in patients at intermediate surgical risk (STS score 4–8%) has been subsequently investigated in the PARTNER 2A10 and SURTAVI11 trials, demonstrating non-inferiority of TAVI in this patient population. Furthermore, these trials demonstrated a signal for the superiority of TAVI over SAVR when performed via transfemoral approach.11 , 12 Surgical aortic valve replacement has remained the standard of care in patients at low surgical risk (STS score <4%) and the role of TAVI in this group has only been recently explored.13 – 15 Meanwhile, SAVR has established durable long-term outcomes and a low risk of periprocedural adverse events (including mortality).5 , 9 , 16
Newly available evidence from randomized controlled trials (RCTs) comparing TAVI with SAVR among low-risk patients with severe symptomatic aortic stenosis provide the stimulus for a broad and comprehensive review of all randomized trials across the entire spectrum of surgical risk with a focus on clinically important outcomes In this study, we update our previously published meta-analysis from 201612 and compare the collective safety and efficacy of TAVI vs. SAVR as assessed in RCTs across the entire spectrum of surgical risk and important subgroups.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)17 and is registered with PROSPERO (CRD42016037273). Ethical approval was not required.
Data sources and search strategy
A systematic literature search of Medline, Embase, and the Cochrane Library Central Register of Controlled Trials (CENTRAL) was conducted from 15 April 15 2016 (the latest search in our previously published meta-analysis)12 through 19 March 2019. We focused on peer-reviewed publications of RCTs. Details of the search algorithm are provided in Supplementary material online, Section S1. The reference lists of trials and meta-analyses identified in the search were screened for additional eligible studies and no language or sample size restrictions were applied.
Study selection
All RCTs comparing TAVI vs. SAVR in patients with severe symptomatic AS and with outcomes reported over a period of at least 1 year or longer of follow-up were considered, irrespective of baseline surgical risk. Individual reports of the same trial providing outcome data at different follow-up periods were included separately. We excluded trials that solely examined non-arterial (transthoracic) access for TAVI, head-to-head trials of different transcatheter heart valve (THV) systems, and those that compared TAVI with medical therapy. Observational studies were excluded owing to the inherent risk of bias.
After removal of duplicates, the titles and abstracts of the search results were screened for relevance by two authors (P.O. and T.C.). Full texts of the remaining studies were individually and independently assessed for inclusion based on predefined criteria. The final list of included trials was agreed by discussion between all authors, with full agreement required before inclusion. Disagreement amongst reviewers was resolved through consensus.
Data extraction and management in study level
A standardized form recording key items was used for data extraction performed by one author (F.P.) and verified by a second (G.C.M.S.). We extracted the following information for each study: publication characteristics (including authors, publication year, and journal); study design (trial design, clinical setting, funding source, period of recruitment, duration of follow-up, number of patients randomized, and number analysed for each outcome); population characteristics (eligibility criteria, age, gender, body mass index, comorbidities, surgical risk of patients in each group, and other relevant baseline data); intervention (SAVR, transfemoral, or transthoracic TAVI) and comparator characteristics; and outcome data, including reported outcome definitions and summary data related to treatment effects. A trial enrolling >50% of recruited patients with an STS score <4% was considered to represent a low surgical risk population.
Assessment of risk of bias
We used the Cochrane ‘Risk of Bias’ tool18 to categorize several domains for each individual trial: sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting. Blinding of participants and physicians was deemed irrelevant owing to the interventional nature of both TAVI and SAVR. The overall risk of bias for each trial was then judged to be low, unclear, or high based on whether the level of bias in individual domains could have resulted in material biases in the risk estimates.
Outcomes
The primary endpoint was all-cause mortality up to 2 years (and for the longest available follow-up period in each individual trial). Secondary endpoints were stroke and disabling stroke (categorized separately), cardiovascular death, myocardial infarction (MI), acute kidney injury (AKI), new-onset atrial fibrillation (NOAF), major bleeding (as defined by individual studies), major vascular complications, valve endocarditis, and permanent pacemaker implantation, up to 2-year follow-up.
The number of events [with accompanying hazard ratios (HRs) and 95% confidence intervals (CIs)] was extracted for each outcome at 30-day and up to 2-year follow-up according to Valve Academic Research Consortium (VARC) or more recent VARC-2 endpoint definitions for consistency across the trials.19 We used the intention-to-treat (ITT) principle and utilized as-treated data, if ITT data were unavailable. Hazard ratios took precedence over risk ratios (RRs) to incorporate time-to-event data and allow for censoring. We derived RR using the number of events and participants in each treatment group when HR were unavailable. Disagreements between reviewers were resolved through consensus or third-party adjudication.
Data synthesis
Bayesian adaptive statistical methods were used within two trials.11 , 15 As these studies used non-informative (uniform) priors, we approximated 95% CIs from the reported 95% credible intervals for the difference in incidence rates and derived corresponding standard errors and z-scores. Assuming that the z-score for the log incidence rate ratio approximates to that of the incidence rate difference, we then derived a standard error for the log incidence rate ratio. Available data were synthesized using DerSimonian and Laird random-effects meta-analysis.20 We assessed the extent of heterogeneity in each meta-analysis using τ 2 as estimated with the restricted maximum likelihood method. Values around 0.04, 0.16, and 0.36 were considered to represent low, moderate, and high heterogeneity, respectively.21 We performed stratified meta-analyses for the primary outcome according to surgical risk (high, intermediate, or low), access route (transfemoral or transthoracic), and THV system (balloon- or self-expandable). All analyses were performed using Stata 15.0 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
Study search and study characteristics
Our systematic electronic literature search identified 333 studies and after removal of records according to pre-specified criteria, 11 full text reports were reviewed for eligibility. We also identified three additional trials fulfilling the inclusion criteria for this update that had been published since our last meta-analysis12 (Supplementary material online, Section S2). In total therefore, 14 articles reporting on seven trials (PARTNER 1A,3 – 5 US CoreValve High Risk,6 – 9 NOTION,13 , 22 , 23 PARTNER 2A,10 SURTAVI,11 PARTNER 3,14 and Evolut Low Risk15) were deemed eligible and included in the meta-analysis (Supplementary material online, Section S2 and Table 1).
Characteristics of trials included in the meta-analysis
| . | PARTNER 1A . | US CoreValve high risk . | NOTION . | PARTNER 2A . | SURTAVI . | PARTNER 3 . | Evolut low risk . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . |
| Trials characteristics | ||||||||||||||
| Number of centres | 25 | 45 | 3 | 57 | 87 | 71 | 86 | |||||||
| Recruitment period | 2007–09 | 2011–12 | 2009–13 | 2011–13 | 2012–16 | 2016–17 | 2016–18 | |||||||
| Year of publication | 2011 | 2014 | 2015 | 2016 | 2017 | 2019 | 2019 | |||||||
| Longest follow-up (years) | 5 | 5 | 5 | 2 | 2 | 1 | 2 | |||||||
| Design | Non-inferiority | Non-inferiority | Superiority | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority | |||||||
| Funding source | Edwards Lifesciences | Medtronic | Danish Heart Foundation | Edwards Lifesciences | Medtronic | Edwards Lifesciences | Medtronic | |||||||
| ITT patients, n | 348 | 351 | 394 | 401 | 145 | 135 | 1011 | 1021 | 879 | 867 | 503 | 497 | 734 | 734 |
| As-treated patients, n | 344 | 313 | 391 | 359 | 142 | 134 | 994 | 944 | 864 | 796 | 496 | 454 | 725 | 678 |
| Target population | High surgical risk | High surgical risk | Low surgical riska | Intermediate surgical risk | Intermediate surgical risk | Low surgical risk | Low surgical risk | |||||||
| Patients characteristics | ||||||||||||||
| Age (years), mean (SD) | 84 ± 7 | 85 ± 6 | 83 ± 7 | 84 ± 6 | 79 ± 5 | 79 ± 5 | 82 ± 7 | 82 ± 7 | 80 ± 6 | 80 ± 6 | 73 ± 6 | 74 ± 6 | 74 ± 6 | 74 ± 6 |
| Women, n (%) | 147 (42) | 153 (44) | 183 (46) | 189 (47) | 67 (46) | 64 (47) | 463 (46) | 461 (45) | 366 (42) | 358 (41) | 161 (32) | 131 (26) | 266 (36) | 246 (34) |
| STS-PROM, mean (SD) | 11.8 ± 3.3 | 11.7 ± 3.5 | 7.3 ± 3.0 | 7.5 ± 3.2 | 2.9 ± 1.6 | 3.1 ± 1.7 | 5.8 ± 2.1 | 5.8 ± 1.9 | 4.4 ± 1.5 | 4.5 ± 1.6 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.7 | 1.9 ± 0.7 |
| CKD 4 or 5, n (%) | 38 (11) | 24 (7) | 48 (12) | 52 (13) | 2 (1) | 1 (1) | 51 (5) | 53 (5) | 14 (2) | 17 (2) | 1 (0.2) | 1 (0.2) | 3 (0.4) | 1 (0.1) |
| PAD, n (%) | 148 (43) | 142 (40) | 163 (41) | 169 (42) | 6 (4) | 9 (7) | 282 (28) | 336 (33) | 266 (30) | 238 (27) | 34 (7) | 33 (7) | 55 (7) | 62 (8) |
| Known AF or flutter, n (%) | 80 (23) | 73 (21) | 161 (41) | 190 (47) | 40 (28) | 34 (25) | 313 (31) | 359 (35) | 243 (28) | 211 (24) | 78 (16) | 85 (12) | 113 (15) | 109 (15) |
| Prior pacemaker, n (%) | 69 (20) | 76 (22) | 92 (23) | 83 (21) | 5 (3) | 6 (4) | 118 (12) | 123 (12) | 84 (10) | 72 (8) | 12 (2) | 13 (3) | 25 (3) | 28 (4) |
| LVEF, mean (SD) | 53 ± 14 | 53 ± 13 | 57 ± 13 | 56 ± 12 | 57 ± 10 | 55 ± 10 | 56 ± 11 | 55 ± 12 | No data | No data | 66 ± 9 | 66 ± 9 | 62 ± 8 | 62 ± 8 |
| Interventions characteristics | ||||||||||||||
| THV system | Sapien | NA | CoreValve | NA | CoreValve | NA | Sapien XT | NA | CoreValve, Evolut R | NA | Sapien 3 | NA | CoreValve, Evolut R, Evolut PRO | NA |
| Surgical aortic valve prosthesis | NA | No data | NA | Biological 98.6%; mechanical 1.4% | NA | Bioprosthesis 100% | NA | No data | NA | No data | NA | No data | NA | Bioprosthesis 100% |
| Access site, n (%) | ||||||||||||||
| Transfemoral | 244 (70) | NA | 394 (100) | NA | 145 (100) | NA | 775 (77) | NA | 864 (100) | NA | 503 (100) | NA | 731 (99.6)b | NA |
| Transthoracic | 104 (30) | NA | 0 (0) | NA | 0 (0) | NA | 236 (23) | NA | 0 (0) | NA | 0 (0) | NA | 3 (0.4)c | NA |
| . | PARTNER 1A . | US CoreValve high risk . | NOTION . | PARTNER 2A . | SURTAVI . | PARTNER 3 . | Evolut low risk . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . |
| Trials characteristics | ||||||||||||||
| Number of centres | 25 | 45 | 3 | 57 | 87 | 71 | 86 | |||||||
| Recruitment period | 2007–09 | 2011–12 | 2009–13 | 2011–13 | 2012–16 | 2016–17 | 2016–18 | |||||||
| Year of publication | 2011 | 2014 | 2015 | 2016 | 2017 | 2019 | 2019 | |||||||
| Longest follow-up (years) | 5 | 5 | 5 | 2 | 2 | 1 | 2 | |||||||
| Design | Non-inferiority | Non-inferiority | Superiority | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority | |||||||
| Funding source | Edwards Lifesciences | Medtronic | Danish Heart Foundation | Edwards Lifesciences | Medtronic | Edwards Lifesciences | Medtronic | |||||||
| ITT patients, n | 348 | 351 | 394 | 401 | 145 | 135 | 1011 | 1021 | 879 | 867 | 503 | 497 | 734 | 734 |
| As-treated patients, n | 344 | 313 | 391 | 359 | 142 | 134 | 994 | 944 | 864 | 796 | 496 | 454 | 725 | 678 |
| Target population | High surgical risk | High surgical risk | Low surgical riska | Intermediate surgical risk | Intermediate surgical risk | Low surgical risk | Low surgical risk | |||||||
| Patients characteristics | ||||||||||||||
| Age (years), mean (SD) | 84 ± 7 | 85 ± 6 | 83 ± 7 | 84 ± 6 | 79 ± 5 | 79 ± 5 | 82 ± 7 | 82 ± 7 | 80 ± 6 | 80 ± 6 | 73 ± 6 | 74 ± 6 | 74 ± 6 | 74 ± 6 |
| Women, n (%) | 147 (42) | 153 (44) | 183 (46) | 189 (47) | 67 (46) | 64 (47) | 463 (46) | 461 (45) | 366 (42) | 358 (41) | 161 (32) | 131 (26) | 266 (36) | 246 (34) |
| STS-PROM, mean (SD) | 11.8 ± 3.3 | 11.7 ± 3.5 | 7.3 ± 3.0 | 7.5 ± 3.2 | 2.9 ± 1.6 | 3.1 ± 1.7 | 5.8 ± 2.1 | 5.8 ± 1.9 | 4.4 ± 1.5 | 4.5 ± 1.6 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.7 | 1.9 ± 0.7 |
| CKD 4 or 5, n (%) | 38 (11) | 24 (7) | 48 (12) | 52 (13) | 2 (1) | 1 (1) | 51 (5) | 53 (5) | 14 (2) | 17 (2) | 1 (0.2) | 1 (0.2) | 3 (0.4) | 1 (0.1) |
| PAD, n (%) | 148 (43) | 142 (40) | 163 (41) | 169 (42) | 6 (4) | 9 (7) | 282 (28) | 336 (33) | 266 (30) | 238 (27) | 34 (7) | 33 (7) | 55 (7) | 62 (8) |
| Known AF or flutter, n (%) | 80 (23) | 73 (21) | 161 (41) | 190 (47) | 40 (28) | 34 (25) | 313 (31) | 359 (35) | 243 (28) | 211 (24) | 78 (16) | 85 (12) | 113 (15) | 109 (15) |
| Prior pacemaker, n (%) | 69 (20) | 76 (22) | 92 (23) | 83 (21) | 5 (3) | 6 (4) | 118 (12) | 123 (12) | 84 (10) | 72 (8) | 12 (2) | 13 (3) | 25 (3) | 28 (4) |
| LVEF, mean (SD) | 53 ± 14 | 53 ± 13 | 57 ± 13 | 56 ± 12 | 57 ± 10 | 55 ± 10 | 56 ± 11 | 55 ± 12 | No data | No data | 66 ± 9 | 66 ± 9 | 62 ± 8 | 62 ± 8 |
| Interventions characteristics | ||||||||||||||
| THV system | Sapien | NA | CoreValve | NA | CoreValve | NA | Sapien XT | NA | CoreValve, Evolut R | NA | Sapien 3 | NA | CoreValve, Evolut R, Evolut PRO | NA |
| Surgical aortic valve prosthesis | NA | No data | NA | Biological 98.6%; mechanical 1.4% | NA | Bioprosthesis 100% | NA | No data | NA | No data | NA | No data | NA | Bioprosthesis 100% |
| Access site, n (%) | ||||||||||||||
| Transfemoral | 244 (70) | NA | 394 (100) | NA | 145 (100) | NA | 775 (77) | NA | 864 (100) | NA | 503 (100) | NA | 731 (99.6)b | NA |
| Transthoracic | 104 (30) | NA | 0 (0) | NA | 0 (0) | NA | 236 (23) | NA | 0 (0) | NA | 0 (0) | NA | 3 (0.4)c | NA |
Greater than 50% with STS score <4.
0.6% subclavian approach.
0.4% direct aortic approach.
AF, atrial fibrillation; CKD, chronic kidney disease; ITT, intention-to-treat; LVEF, left ventricular ejection fraction; NA, not applicable; PAD, peripheral artery disease; SAVR, surgical aortic valve replacement; SD, standard deviation; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve.
Characteristics of trials included in the meta-analysis
| . | PARTNER 1A . | US CoreValve high risk . | NOTION . | PARTNER 2A . | SURTAVI . | PARTNER 3 . | Evolut low risk . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . |
| Trials characteristics | ||||||||||||||
| Number of centres | 25 | 45 | 3 | 57 | 87 | 71 | 86 | |||||||
| Recruitment period | 2007–09 | 2011–12 | 2009–13 | 2011–13 | 2012–16 | 2016–17 | 2016–18 | |||||||
| Year of publication | 2011 | 2014 | 2015 | 2016 | 2017 | 2019 | 2019 | |||||||
| Longest follow-up (years) | 5 | 5 | 5 | 2 | 2 | 1 | 2 | |||||||
| Design | Non-inferiority | Non-inferiority | Superiority | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority | |||||||
| Funding source | Edwards Lifesciences | Medtronic | Danish Heart Foundation | Edwards Lifesciences | Medtronic | Edwards Lifesciences | Medtronic | |||||||
| ITT patients, n | 348 | 351 | 394 | 401 | 145 | 135 | 1011 | 1021 | 879 | 867 | 503 | 497 | 734 | 734 |
| As-treated patients, n | 344 | 313 | 391 | 359 | 142 | 134 | 994 | 944 | 864 | 796 | 496 | 454 | 725 | 678 |
| Target population | High surgical risk | High surgical risk | Low surgical riska | Intermediate surgical risk | Intermediate surgical risk | Low surgical risk | Low surgical risk | |||||||
| Patients characteristics | ||||||||||||||
| Age (years), mean (SD) | 84 ± 7 | 85 ± 6 | 83 ± 7 | 84 ± 6 | 79 ± 5 | 79 ± 5 | 82 ± 7 | 82 ± 7 | 80 ± 6 | 80 ± 6 | 73 ± 6 | 74 ± 6 | 74 ± 6 | 74 ± 6 |
| Women, n (%) | 147 (42) | 153 (44) | 183 (46) | 189 (47) | 67 (46) | 64 (47) | 463 (46) | 461 (45) | 366 (42) | 358 (41) | 161 (32) | 131 (26) | 266 (36) | 246 (34) |
| STS-PROM, mean (SD) | 11.8 ± 3.3 | 11.7 ± 3.5 | 7.3 ± 3.0 | 7.5 ± 3.2 | 2.9 ± 1.6 | 3.1 ± 1.7 | 5.8 ± 2.1 | 5.8 ± 1.9 | 4.4 ± 1.5 | 4.5 ± 1.6 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.7 | 1.9 ± 0.7 |
| CKD 4 or 5, n (%) | 38 (11) | 24 (7) | 48 (12) | 52 (13) | 2 (1) | 1 (1) | 51 (5) | 53 (5) | 14 (2) | 17 (2) | 1 (0.2) | 1 (0.2) | 3 (0.4) | 1 (0.1) |
| PAD, n (%) | 148 (43) | 142 (40) | 163 (41) | 169 (42) | 6 (4) | 9 (7) | 282 (28) | 336 (33) | 266 (30) | 238 (27) | 34 (7) | 33 (7) | 55 (7) | 62 (8) |
| Known AF or flutter, n (%) | 80 (23) | 73 (21) | 161 (41) | 190 (47) | 40 (28) | 34 (25) | 313 (31) | 359 (35) | 243 (28) | 211 (24) | 78 (16) | 85 (12) | 113 (15) | 109 (15) |
| Prior pacemaker, n (%) | 69 (20) | 76 (22) | 92 (23) | 83 (21) | 5 (3) | 6 (4) | 118 (12) | 123 (12) | 84 (10) | 72 (8) | 12 (2) | 13 (3) | 25 (3) | 28 (4) |
| LVEF, mean (SD) | 53 ± 14 | 53 ± 13 | 57 ± 13 | 56 ± 12 | 57 ± 10 | 55 ± 10 | 56 ± 11 | 55 ± 12 | No data | No data | 66 ± 9 | 66 ± 9 | 62 ± 8 | 62 ± 8 |
| Interventions characteristics | ||||||||||||||
| THV system | Sapien | NA | CoreValve | NA | CoreValve | NA | Sapien XT | NA | CoreValve, Evolut R | NA | Sapien 3 | NA | CoreValve, Evolut R, Evolut PRO | NA |
| Surgical aortic valve prosthesis | NA | No data | NA | Biological 98.6%; mechanical 1.4% | NA | Bioprosthesis 100% | NA | No data | NA | No data | NA | No data | NA | Bioprosthesis 100% |
| Access site, n (%) | ||||||||||||||
| Transfemoral | 244 (70) | NA | 394 (100) | NA | 145 (100) | NA | 775 (77) | NA | 864 (100) | NA | 503 (100) | NA | 731 (99.6)b | NA |
| Transthoracic | 104 (30) | NA | 0 (0) | NA | 0 (0) | NA | 236 (23) | NA | 0 (0) | NA | 0 (0) | NA | 3 (0.4)c | NA |
| . | PARTNER 1A . | US CoreValve high risk . | NOTION . | PARTNER 2A . | SURTAVI . | PARTNER 3 . | Evolut low risk . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . | TAVI . | SAVR . |
| Trials characteristics | ||||||||||||||
| Number of centres | 25 | 45 | 3 | 57 | 87 | 71 | 86 | |||||||
| Recruitment period | 2007–09 | 2011–12 | 2009–13 | 2011–13 | 2012–16 | 2016–17 | 2016–18 | |||||||
| Year of publication | 2011 | 2014 | 2015 | 2016 | 2017 | 2019 | 2019 | |||||||
| Longest follow-up (years) | 5 | 5 | 5 | 2 | 2 | 1 | 2 | |||||||
| Design | Non-inferiority | Non-inferiority | Superiority | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority | |||||||
| Funding source | Edwards Lifesciences | Medtronic | Danish Heart Foundation | Edwards Lifesciences | Medtronic | Edwards Lifesciences | Medtronic | |||||||
| ITT patients, n | 348 | 351 | 394 | 401 | 145 | 135 | 1011 | 1021 | 879 | 867 | 503 | 497 | 734 | 734 |
| As-treated patients, n | 344 | 313 | 391 | 359 | 142 | 134 | 994 | 944 | 864 | 796 | 496 | 454 | 725 | 678 |
| Target population | High surgical risk | High surgical risk | Low surgical riska | Intermediate surgical risk | Intermediate surgical risk | Low surgical risk | Low surgical risk | |||||||
| Patients characteristics | ||||||||||||||
| Age (years), mean (SD) | 84 ± 7 | 85 ± 6 | 83 ± 7 | 84 ± 6 | 79 ± 5 | 79 ± 5 | 82 ± 7 | 82 ± 7 | 80 ± 6 | 80 ± 6 | 73 ± 6 | 74 ± 6 | 74 ± 6 | 74 ± 6 |
| Women, n (%) | 147 (42) | 153 (44) | 183 (46) | 189 (47) | 67 (46) | 64 (47) | 463 (46) | 461 (45) | 366 (42) | 358 (41) | 161 (32) | 131 (26) | 266 (36) | 246 (34) |
| STS-PROM, mean (SD) | 11.8 ± 3.3 | 11.7 ± 3.5 | 7.3 ± 3.0 | 7.5 ± 3.2 | 2.9 ± 1.6 | 3.1 ± 1.7 | 5.8 ± 2.1 | 5.8 ± 1.9 | 4.4 ± 1.5 | 4.5 ± 1.6 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.7 | 1.9 ± 0.7 |
| CKD 4 or 5, n (%) | 38 (11) | 24 (7) | 48 (12) | 52 (13) | 2 (1) | 1 (1) | 51 (5) | 53 (5) | 14 (2) | 17 (2) | 1 (0.2) | 1 (0.2) | 3 (0.4) | 1 (0.1) |
| PAD, n (%) | 148 (43) | 142 (40) | 163 (41) | 169 (42) | 6 (4) | 9 (7) | 282 (28) | 336 (33) | 266 (30) | 238 (27) | 34 (7) | 33 (7) | 55 (7) | 62 (8) |
| Known AF or flutter, n (%) | 80 (23) | 73 (21) | 161 (41) | 190 (47) | 40 (28) | 34 (25) | 313 (31) | 359 (35) | 243 (28) | 211 (24) | 78 (16) | 85 (12) | 113 (15) | 109 (15) |
| Prior pacemaker, n (%) | 69 (20) | 76 (22) | 92 (23) | 83 (21) | 5 (3) | 6 (4) | 118 (12) | 123 (12) | 84 (10) | 72 (8) | 12 (2) | 13 (3) | 25 (3) | 28 (4) |
| LVEF, mean (SD) | 53 ± 14 | 53 ± 13 | 57 ± 13 | 56 ± 12 | 57 ± 10 | 55 ± 10 | 56 ± 11 | 55 ± 12 | No data | No data | 66 ± 9 | 66 ± 9 | 62 ± 8 | 62 ± 8 |
| Interventions characteristics | ||||||||||||||
| THV system | Sapien | NA | CoreValve | NA | CoreValve | NA | Sapien XT | NA | CoreValve, Evolut R | NA | Sapien 3 | NA | CoreValve, Evolut R, Evolut PRO | NA |
| Surgical aortic valve prosthesis | NA | No data | NA | Biological 98.6%; mechanical 1.4% | NA | Bioprosthesis 100% | NA | No data | NA | No data | NA | No data | NA | Bioprosthesis 100% |
| Access site, n (%) | ||||||||||||||
| Transfemoral | 244 (70) | NA | 394 (100) | NA | 145 (100) | NA | 775 (77) | NA | 864 (100) | NA | 503 (100) | NA | 731 (99.6)b | NA |
| Transthoracic | 104 (30) | NA | 0 (0) | NA | 0 (0) | NA | 236 (23) | NA | 0 (0) | NA | 0 (0) | NA | 3 (0.4)c | NA |
Greater than 50% with STS score <4.
0.6% subclavian approach.
0.4% direct aortic approach.
AF, atrial fibrillation; CKD, chronic kidney disease; ITT, intention-to-treat; LVEF, left ventricular ejection fraction; NA, not applicable; PAD, peripheral artery disease; SAVR, surgical aortic valve replacement; SD, standard deviation; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve.
Across the seven trials, 8020 participants were enrolled (4014 randomized to TAVI, 4006 randomized to SAVR). All but one were designed for non-inferiority of TAVI against SAVR (the NOTION trial13 was designed for superiority). Industry funding was obtained for the majority of the trials (six out of seven)3 , 6 , 10 , 11 , 14 , 15 and only one (NOTION13) was conducted without industry support. Two trials recruited patients at high surgical risk,3 , 6 two at intermediate,10 , 11 and three at low surgical risk.13 – 15 Men were predominantly enrolled (TAVI: 59%, 2361/4014 patients; SAVR: 61%, 2404/4006 patients). The combined mean STS score for the TAVI arm across the trials was 9.4% for high-risk trials,3 , 6 5.1% for intermediate risk,10 , 11 and 2.0% for low-risk trials.13 – 15 Different generations of two widely used balloon- and self-expandable THV systems were implanted in three and four trials, respectively (Table 1). Transfemoral access was the preferred route of THV delivery (3656/4014 patients). Allocation concealment was unclear in six trials3 , 6 , 10 , 11 , 14 , 15 and two trials13 , 15 were deemed at high risk of bias because of unblinded outcome assessment (Supplementary material online, Section S3).
All-cause mortality
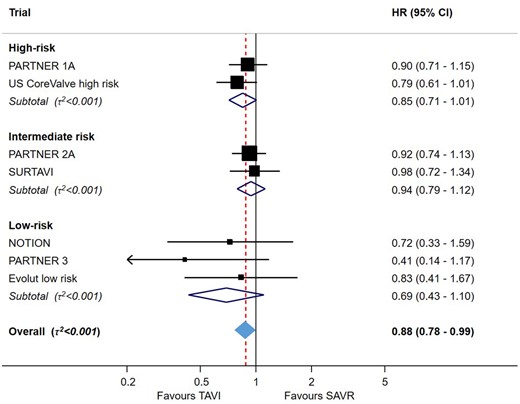
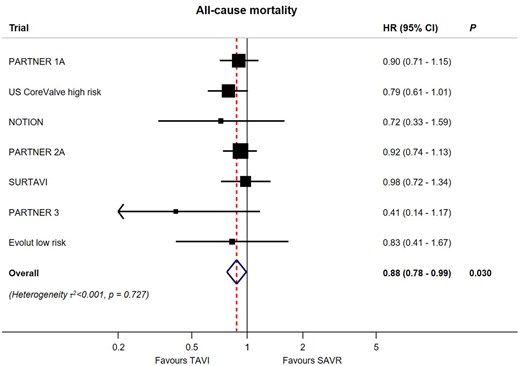
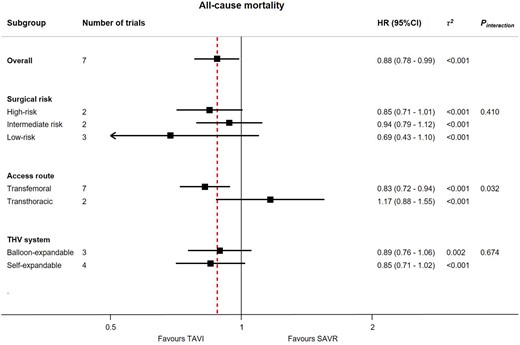
All seven trials contributed to the primary outcome of all-cause mortality up to 2-year follow-up (Figure 1). The available events and risk estimates in individual trials are provided in Supplementary material online, Section S4. Transcatheter aortic valve implantation was associated with reduced mortality compared to SAVR [HR 0.88 (95% CI 0.78–0.99), P = 0.030], with low heterogeneity across the trials (τ 2 < 0.001). In a subgroup analysis according to baseline surgical risk (high, intermediate, and low risk), we found little evidence for a treatment-by-subgroup interaction (P-for-interaction = 0.41) (Figure 2). Survival benefit was particularly evident in patients undergoing transfemoral TAVI, with a 17% relative reduction in the risk of all-cause mortality [HR 0.83 (95% CI 0.72–0.94)]; whereas there was no advantage of transthoracic TAVI over SAVR [HR 1.17 (95% CI 0.88–1.55)] with a P-for-interaction = 0.032 for the two alternative routes of access (Figure 2). There was no difference between the two THV systems used in the trials (P-for-interaction = 0.674). Finally, the summary estimate showed no difference between TAVI and SAVR when considering the longest available follow-up period for each trial [HR 0.96 (95% CI 0.87–1.06), P = 0.402, τ 2 < 0.001] including three trials with 5-year follow-up data5 , 9 , 23 (Supplementary material online, Section S7).
Meta-analysis for the primary outcome of all-cause mortality for transcatheter aortic valve implantation vs. surgical aortic valve replacement up to 2-year follow-up. For each trial, boxes and horizontal lines correspond to the respective point estimate and accompanying 95% confidence interval. The size of each box is proportional to the weight of that trial result. The vertical solid line on the forest plot represents the point estimate of hazard ratio = 1. The vertical dashed line on the plot represents the point estimate of overall hazard ratio derived from random-effect meta-analysis. The diamond represents the 95% confidence interval of the summary pooled estimate of the effect and is centred on pooled hazard ratios. Heterogeneity estimate of τ 2 accompanies the summary estimate. Details of data used from individual trials are available in Supplementary material online, Section S4. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Subgroup analyses for the primary outcome of all-cause mortality for transcatheter aortic valve implantation vs. surgical aortic valve replacement up to 2-year follow-up. The analysis by surgical risk included two trials (PARTNER 1A and US CoreValve high risk) which only included high-risk patients, two trials (PARTNER 2A and SURTAVI) which only included intermediate-risk patients, and three trials (NOTION, PARTNER 3, and Evolut low risk) included low-risk patients. The analysis by access route included five trials (US CoreValve high risk, NOTION, SURTAVI, PARTNER 3, and Evolut low risk) and two subgroups of trials (PARTNER 1A and PARTNER 2A), which compared transcatheter aortic valve implantation with transfemoral access against surgical aortic valve replacement, and two subgroups of trials (PARTNER 1A and PARTNER 2A), which compared transcatheter aortic valve implantation with transthoracic access against surgical aortic valve replacement. The analysis by transcatheter heart valve system included three trials (PARTNER 1A, PARTNER 2A, and PARTNER 3) with balloon-expandable system exclusively and four trials (US CoreValve high risk, NOTION, SURTAVI, and Evolut low risk) in which a self-expandable system was only used. For each subgroup, boxes and horizontal lines correspond to the respective point summary estimate and accompanying 95% confidence interval based on random-effects meta-analysis. The vertical solid line on the forest plot represents the point estimate of hazard ratio = 1. The vertical dashed line on the plot represents the point estimate of overall hazard ratio derived from random-effect meta-analysis for the primary outcome of interest of all-cause mortality. Heterogeneity estimate of τ 2 accompanies the summary estimates for each subgroup. Details of the data used from individual trials are available in Supplementary material online, Section S5. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve.
Secondary outcomes
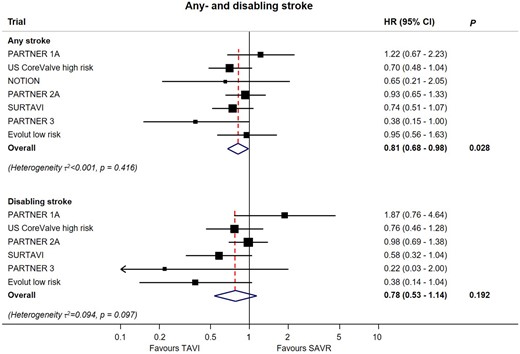
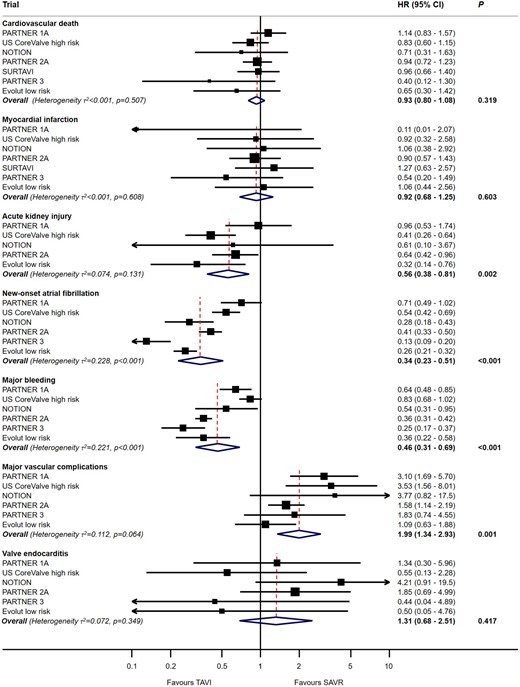
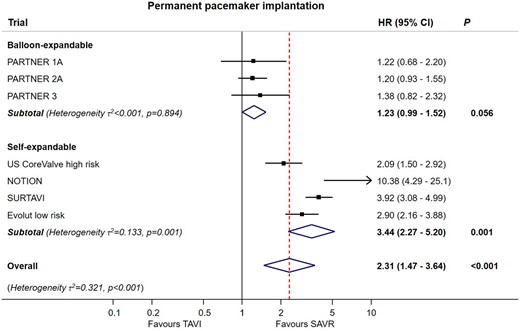
Numbers of events and extracted estimates are provided in Supplementary material online, Section S6. As shown in Figure 3, TAVI was associated with a significant reduction in stroke [HR 0.81 (95% CI 0.68–0.98), P = 0.028], but not disabling stroke [HR 0.78 (95% CI 0.53–1.14), P = 0.192], with low heterogeneity for both (τ 2 < 0.001 and τ 2 = 0.094, respectively). TAVI was associated with reduced risk of AKI, atrial fibrillation, or major bleeding (all P < 0.01) but not cardiovascular death, MI, or valve endocarditis (Figure 4). Conversely, SAVR was associated with reduced risk of major vascular complications [HR 1.99 (95% CI 1.34–2.93), P = 0.001] and need for permanent pacemaker implantation [HR 2.27 (95% CI 1.47–3.64), P < 0.001] with considerable heterogeneity for both (τ 2 = 0.112 and τ 2 = 0.321, respectively) (Figures 4 and 5). There was significant interaction between the two THV systems resulting from the higher risk of permanent pacemaker requirement after self-expandable valve implantation (P-for-interaction <0.001).
Meta-analyses for the outcomes of any or disabling stroke for transcatheter aortic valve implantation vs. surgical aortic valve replacement up to 2-year follow-up. For each subgroup, boxes and horizontal lines correspond to the respective point summary estimate and accompanying 95% confidence interval based on random-effects meta-analyses. The size of each box is proportional to weight of that trial result. The vertical solid line on the forest plot represents the point estimate of hazard ratio = 1. Heterogeneity estimate of τ 2 accompanies the summary estimates for each subgroup. Details of the data used from individual trials are available in Supplementary material online, Section S6. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Meta-analyses for the secondary outcomes for transcatheter aortic valve implantation vs. surgical aortic valve replacement up to 2-year follow-up. For each subgroup, boxes and horizontal lines correspond to the respective point summary estimate and accompanying 95% confidence interval based on random-effects meta-analysis. The size of each box is proportional to weight of that trial result. The vertical solid line on the forest plot represents the point estimate of hazard ratio = 1. Heterogeneity estimate of τ 2 accompanies the summary estimates for each subgroup. Details of the data used from individual trials are available in Supplementary material online, Section S6. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Meta-analysis for permanent pacemaker implantation for transcatheter aortic valve implantation vs. surgical aortic valve replacement stratified according to transcatheter heart valve system up to 2-year follow-up. For each subgroup, boxes and horizontal lines correspond to the respective point summary estimate and accompanying 95% confidence interval based on random-effect meta-analysis. The size of each box is proportional to weight of that trial result. The vertical solid line on the forest plot represents the point estimate of hazard ratio = 1. The vertical dashed line on the plot represents the point estimate of overall hazard ratio derived from random-effects meta-analysis for the outcome of permanent pacemaker implantation. Heterogeneity estimate of τ 2 accompanies the summary estimates for each subgroup. Details of the data used from individual trials are available in Supplementary material online, Section S6. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Discussion
The key findings of this meta-analysis of clinical outcomes of patients with symptomatic, severe AS randomized to TAVI or SAVR across all risk categories are:
all-cause mortality was lower after TAVI (12% relative risk reduction up to 2 years compared with SAVR);
mortality was even lower when TAVI was performed via the transfemoral route (17% relative risk reduction up to 2 years compared with SAVR);
these mortality benefits of TAVI were consistent across the entire spectrum of baseline surgical risk and irrespective of Food and Drug Administration (FDA) approved THV systems;
risk of stroke was lower after TAVI (19% relative risk reduction up to 2 years compared with SAVR); and
TAVI was linked to a higher risk of permanent pacemaker implantation and major vascular complications, but a reduced risk of major bleeding, NOAF, and AKI.
In this meta-analysis of aggregated data of seven randomized trials in 8020 patients on symptomatic patients with severe AS at low, intermediate, or high procedural risk, we found evidence for a significant survival benefit of TAVI when compared with SAVR, irrespective of baseline surgical risk (Take home figure). The individual studies within this meta-analysis were not powered to compare mortality in patients randomized to TAVI or SAVR, and only the US CoreValve High Risk trial has previously demonstrated a survival benefit of TAVI.6 The present analysis is therefore the first demonstration that there is a statistically significant survival advantage associated with TAVI over 2-year follow-up across the entire spectrum of surgical risk. Furthermore, when TAVI is performed via transfemoral route (which is feasible in >90% of patients in contemporary practice), there is an even greater mortality benefit (relative risk reduction 17%).
Meta-analysis for the primary outcome all-cause mortality comparing transcatheter aortic valve implantation vs. surgical aortic valve replacement up to 2-year follow-up stratified by baseline surgical risk at study level. For each trial, boxes and horizontal lines correspond to the respective point estimate and accompanying 95% confidence interval. A summary estimate is provided for each subgroup of trials according to surgical risk. The size of each box is proportional to the weight of the individual trial result. The vertical solid line on the forest plot represents the point estimate of hazard ratio = 1. The vertical dashed line on the plot represents the point estimate of overall hazard ratio derived from random-effect meta-analysis [hazard ratio 0.88 (95% confidence interval 0.78–0.99), P = 0.030]. The diamond represents the 95% confidence interval of the summary pooled estimate of the effect and is centred on pooled hazard ratios. Heterogeneity estimate of τ 2 accompanies the summary estimate. The P-value for linear trend from random-effects meta-regression is P = 0.983.
The second key finding of our meta-analysis is a significant reduction in the risk of stroke in patients undergoing TAVI compared with SAVR. Stroke is an infrequent but devastating complication of aortic valve replacement but individual trials of TAVI vs. SAVR have been underpowered to detect a difference in the incidence of procedural stroke.24 Herein, the pooled analysis provided demonstrates that TAVI is associated with a lower mid-term risk of all stroke (relative risk reduction 19%) when compared with SAVR, regardless of baseline risk. A benefit of TAVI in reducing the incidence of major stroke at 30 days was previously suggested by a pooled analysis of patients undergoing transfemoral TAVI in the PARTNER 1A and 2A trials (SAVR 3.9% vs. transfemoral TAVI 2.2%; P = 0.018).25 In addition, a previous meta-analysis of high- and intermediate-risk trials suggested that transcatheter aortic valve replacement was associated with a reduction in the composite of death or disabling stroke at 1 year.26 Our finding will have critical implications for decision-making in lower risk, younger patients who may be suitable for either TAVI or SAVR.
Comparison of secondary endpoints demonstrates differing risk profiles associated with TAVI and SAVR, which provides insights into the possible mechanism of benefit of TAVI. Although TAVI was associated with a reduction in overall mortality, cardiovascular mortality was comparable. However, patients undergoing TAVI had a striking reduction in the rate of non-cardiac complications, specifically a reduced risk of AKI and major bleeding, both previously identified as predictors of adverse outcome including mortality following aortic valve replacement.27 , 28 Moreover, the large difference in NOAF may also contribute to the difference in all-cause mortality in view of the increased risk of stroke.29 Transcatheter aortic valve implantation carries an increased risk of post-procedural permanent pacemaker (PPM) implantation and major vascular complications.12 , 30 The likelihood of post-procedural PPM implantation differs according to valve design (significantly higher for self-expandable valves compared to SAVR, marginally elevated for balloon-expandable valves).12 , 30 Further technical refinements are warranted to further improve the safety of TAVI given the significant impact of conduction abnormalities and major vascular complications on duration of hospital stay and prognosis.31
There are wider implications of our findings. Surgical risk scoring (using STS or EuroSCORE) remains an important aspect of decision-making between TAVI and SAVR, as recommended by the European Society of Cardiology and American Heart Association/American College of Cardiology.32 , 33 Our finding that the mortality benefits of TAVI extend across all risk categories suggests that there is no longer a requirement for surgical risk stratification among patients considered to undergo TAVI. Instead, TAVI should be considered the first-line interventional strategy for isolated AS in patients aged greater than 65 years. Surgical aortic valve replacement should be reserved for patients with complex anatomy precluding a good outcome from TAVI, concomitant conditions warranting surgery (e.g. aortic root aneurysm or complex coronary artery disease) or active infective endocarditis.
Additional studies of TAVI in younger, low-risk populations, and all-comers are underway (NCT02825134, NCT03112980). Further research is required to investigate the long-term (>5 year) THV durability, and to develop strategies for the optimal management of transcatheter and surgical bioprosthetic valve degeneration. In a sensitivity analysis, considering the longest available follow-up period (5-year follow-up data for PARTNER 1A,5 US CoreValve high risk,9 and NOTION23), TAVI was non-inferior to SAVR. It is important to note that this additional analysis is dominated by the high mortality rates of the PARTNER 1A (67.8% in TAVI and 62.4% in SAVR arm) and US CoreValve high risk (55.3% in TAVI and 55.4% in SAVR arm) at 5 years, suggesting that competing causes of death during follow-up camouflage any earlier treatment differences. Therefore, the intermediate 2-year follow-up provides a clinically meaningful outcome window in this elderly patient population with comorbidities before competing causes of death would exert a major influence on estimates, a known concern as observed in randomized trials with long-term follow-up.34
Limitations
Our study has several intrinsic limitations. The, definitions of low, intermediate, and high risk based on STS score used in the included trials are poorly predictive and overestimate procedural risk.35 Notwithstanding these considerations, the lack of significant interaction between baseline risk and clinical outcomes is robust, suggesting that the benefits of TAVI over SAVR relate to the procedure itself rather than patient characteristics. Second, there have been important changes in valve design and technical aspects of the TAVI procedure over the time period of study—the reported effect size may not fully account for these refinements. There have also been similar advances in surgical technique, as signified by the significantly improved transvalvular gradient and effective orifice area among patients undergoing SAVR in the PARTNER 3 trial.14 Third, the duration of follow-up in our main analysis was limited up to 2 years—longer follow-up will be important to confirm the durability of TAVI valves, which is of particular importance in younger patients. However, non-valve related mortality results in regression to no difference between TAVI and SAVR in older patients because of competing risks over long-term follow-up, which was also confirmed in our secondary analysis by including the longest available follow-up data from three trials. Finally, a lack of individual patient-level data and inconsistent reporting across trials precluded meta-analysis of other patient subgroups or additional outcomes of interest, such as valve gradient, valve area, or paravalvular regurgitation.
Conclusions
In this meta-analysis of seven landmark trials comparing TAVI with SAVR in patients with symptomatic, severe AS, TAVI was associated with a reduction in all-cause mortality and stroke up to 2 years. The mortality benefit of TAVI was observed consistently in patients at low, intermediate, and high procedural risk and irrespective of FDA approved THV type.
Conflict of interest: T.M. has served as consultant for Medtronic, Abbott, Microport, and Edwards Lifesciences. B.P. has received unrestricted education and research grants Edwards Lifesciences Speaker fees Edwards Lifesciences. F.P. has served as consultant for Edwards Lifesciences (consulting fees to the Institution). T.P. has received grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, and speaker fees from Boston Scientific and Biotronik. L.S. has received consultant fee and institutional research grant from Edwards Lifesciences and Medtronic. S.V. holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and/or speaking honoraria from Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Servier, and Valeant. P.J. is a Tier 1 Canada Research Chair in Clinical Epidemiology of Chronic Diseases, has received research grants to the institution from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly, and The Medicines Company, and serves as unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St. Jude Medical, and The Medicines Company. S.W. has received educational and research contracts to the institution from Amgen, Abbott, BMS, Bayer, Boston Scientific, Biotronik, Medtronic, Edwards Lifesciences, CSL, Polares, and Sinomed. All other authors have no conflict of interest.
See page 3154 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz696)
References
Author notes
George C.M. Siontis, Pavel Overtchouk and Thomas J. Cahill authors contributed equally to this article.