-
PDF
- Split View
-
Views
-
Cite
Cite
Tina Torbati, Chrisandra Shufelt, Janet Wei, C Noel Bairey Merz, Premature menopause and cardiovascular disease: can we blame estrogen?, European Heart Journal, Volume 43, Issue 40, 21 October 2022, Pages 4158–4160, https://doi.org/10.1093/eurheartj/ehac321
Close - Share Icon Share
Women who experience premature or early menopause are at increased risk for adverse cardiovascular outcomes.
Women who experience premature or early menopause are at increased risk for adverse cardiovascular outcomes.
This editorial refers to ‘Age at menopause and risk of heart failure and atrial fibrillation: a nationwide cohort study’, J. Shin et al., https://doi.org/10.1093/eurheartj/ehac364.
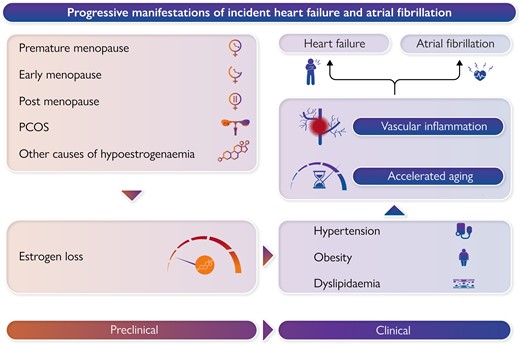
Cardiovascular disease (CVD) is the leading cause of death in women at all ages, with the incidence increasing related to age at and around the time of the menopause transition (average age 52 years).1 With the aging population, it is estimated 6000 women will be entering menopause every day in the USA, totaling nearly 2 million women a year.2 The American College of Cardiology and American Heart Association guidelines have recognized that premature menopause (<40 years) is a risk enhancer for atherosclerotic cardiovascular disease due to the cardiometabolic changes that occur earlier.1 However, European cardiovascular guidelines have not endorsed this and the association with early menopause (<45 years) is less clear.3 While 1% of women undergo premature menopause, up to 10% of women experience early menopause, defined as <45 years. Prior meta-analyses have found that early menopause is associated with a 33% higher risk of heart failure (HF) with reduced ejection fraction when compared to women with non-early menopause.4 Conversely, prior studies have not found an association between earlier age of menopause and risk of atrial fibrillation (AF).5,6
In this issue of European Heart Journal, Shin et al. investigate the association between premature menopause (<40 years) and early age of menopause with the incidence of HF and AF as individual disease entities.7 HF was defined as one or more hospitalizations or one or more outpatient visits with a diagnosis of ICD-10 code 150, while AF was defined as one hospitalization or more than two outpatient visits with ICD-10 code 148. Using the Korean National Health Insurance System, over 1.4 million post-menopausal women were categorized into a history of premature menopause and those without a history of premature menopause. Following 9.1 years of mean follow-up, the study found a greater incidence of HF and AF in women with a history of premature menopause (both log rank P < 0.001). After adjusting for CVD risk factors including hypertension, diabetes, and dyslipidemia, menopause hormone therapy (HT) and age of menarche, women with premature menopause had a higher risk of HF (1.33, 95% CI: 1.26–1.40) and higher risk of AF (1.09, 95% CI: 1.02–1.16) compared to women without premature menopause. The authors further stratified premature menopause by natural vs. surgical, finding that premature natural menopause was associated with greater risk of HF (HR: 1.33, 95% CI: 1.25–1.40) and AF (HR: 1.09, 95% CI: 1.03–1.16), while premature surgical menopause was not significantly associated with a risk of HF (1.25, 95% CI: 0.72–2.15) or AF (0.87, 95% CI: 0.53–1.45). Furthermore, premature menopause was significantly associated with coronary heart disease (1.16, 95% CI: 1.09–1.22) compared to all other categories of age at menopause.
In addition to analyzing by premature menopause, Shin and colleagues further evaluated the association of HF and AF by the age a woman entered menopause (i.e. <40 years, 40–44 years, 45–49 years, ≥50 years). As age of menopause decreased, the incidence of both HF and AF increased (both log rank P < 0.001). Specifically, early menopause at the threshold of <45 years had an increased risk of HF (1.23, 95% CI: 1.19–1.28) and increased risk of AF (1.10, 95% CI: 1.06–1.14) compared to all other age-at-menopause categories. There were no significant interactions between traditional cardiovascular risk factors, menopausal hormone therapy, and age of menarche.
This new study adds to the growing body of evidence that a woman’s reproductive history is important for assessing cardiovascular risk later in life. Similar to prior studies, the current study supports consideration of menopausal age as a risk predictor for HF.4,8 Notably, the reported association with AF in the current study is novel, with two prior studies not finding a relationship between menopause and AF.5,6 The current study extends the prior literature by investigating a larger and broader cohort of post-menopausal women, including women with surgical and natural menopause.
While the menopause transition contributes to activation of the renin-angiotensin-aldosterone system as a result of estrogen loss, the current analysis controlled for HT and menopause HT suggesting the mechanism of increased risk cannot be blamed on estrogen alone. However, inflammation may mediate relations between estrogen loss and CV risk, which can predispose these women to AF and HF.9 It has been shown that a decline in ovarian function as a result of menopause is associated with an increase in proinflammatory cytokines including IL-1, IL-6, and TNF-α. Furthermore, we have previously reported in the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study of women undergoing coronary angiography for suspected ischemia, the premenopausal women with premature obstructive coronary artery disease (CAD) had higher serologic Th1 cytokines relative to Th2/hematopoietic regulatory cytokines.10 Further work to understand reproductive health and vascular inflammation is needed.
Given that CVD is age-related, premature and early menopause may simply be markers of accelerated aging. Recent studies have found that early menopause is associated with the DNA damage repair pathway, with the rapid accumulation of DNA damage leading to accelerated aging in affected organs and tissues such as the ovary and eventually aging throughout the whole body.10 Thus, genetic factors and aging biological markers associated between earlier menopause and CVD may also contribute to the development of AF and HF and should be further studied.
The association between NT-proBNP and heart failure with reduced ejection fraction has been reported as greater among women with early menopause when compared to those that did not enter early menopause; however, there was not a significant association between NT-proBNP and incident heart failure with preserved ejection fraction (HFpEF).11 We have previously reported from the WISE that HFpEF, characterized by left ventricular diastolic dysfunction and related to the coronary microvascular dysfunction, is prevalent among women with no obstructive CAD.12,13 Again, vascular inflammation may influence the development of coronary microvascular dysfunction and subsequent HFpEF via endothelial dysfunction, impaired angiogenesis, perivascular fibrosis, and blood vessel rarefaction. Given the rising incidence of HFpEF among women, these findings may identify novel inflammatory and ischemic treatment targets for prevention of HFpEF.
Accordingly, as Shin et al. conclude, women-specific risk factors such as reproductive history should be considered for HF and AF risk prediction and preventive strategies. Women who experience premature or early menopause are at increased risk for adverse cardiovascular outcomes—it does not appear that estrogen alone can be blamed. We commend the authors for adding to the existing and growing body of literature in regard to this topic. Further work investigating reproductive history including the menopause transition for CVD prediction and prevention in women is needed.
Funding
This work was supported by the National Institutes of Health (R01HL124649, U54 AG065141), the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, DC, the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
Conflict of interest: C.N.B.M. serves as Board of Director for iRhythm, fees paid through CSMC from Abbott Diagnostics andSanofi. J.W. served on an advisory board for Abbott Vascular. All other authors have no conflict of interest to report.