-
PDF
- Split View
-
Views
-
Cite
Cite
Stavros V Konstantinides, Guy Meyer, Cecilia Becattini, Héctor Bueno, Geert-Jan Geersing, Veli-Pekka Harjola, Menno V Huisman, Marc Humbert, Catriona Sian Jennings, David Jiménez, Nils Kucher, Irene Marthe Lang, Mareike Lankeit, Roberto Lorusso, Lucia Mazzolai, Nicolas Meneveau, Fionnuala Ní Áinle, Paolo Prandoni, Piotr Pruszczyk, Marc Righini, Adam Torbicki, Eric Van Belle, José Luis Zamorano, ESC Scientific Document Group , 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC), European Heart Journal, Volume 41, Issue 4, 21 January 2020, Pages 543–603, https://doi.org/10.1093/eurheartj/ehz405
Close - Share Icon Share
 For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the Guidelines see https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehz405#supplementary-data
For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the Guidelines see https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehz405#supplementary-data
Table of contents
Abbreviations and acronyms 546
1 Preamble 547
2 Introduction 548
2.1 Why do we need new Guidelines on the diagnosis and management of pulmonary embolism? 548
2.2 What is new in the 2019 Guidelines? 549
2.2.1 New/revised concepts in 2019 549
2.2.2 Changes in recommendations 2014–19 549
2.2.3 Main new recommendations 2019 550
3 General considerations 550
3.1 Epidemiology 550
3.2 Predisposing factors 551
3.3 Pathophysiology and determinants of outcome 552
4 Diagnosis 554
4.1 Clinical presentation 554
4.2 Assessment of clinical (pre-test) probability 554
4.3 Avoiding overuse of diagnostic tests for pulmonary embolism 555
4.4 D-dimer testing 555
4.4.1 Age-adjusted D-dimer cut-offs 555
4.4.2 D-dimer cut-offs adapted to clinical probability 555
4.4.3 Point-of-care D-dimer assays 555
4.5 Computed tomographic pulmonary angiography 555
4.6 Lung scintigraphy 556
4.7 Pulmonary angiography 557
4.8 Magnetic resonance angiography 557
4.9 Echocardiography 557
4.10 Compression ultrasonography 558
4.12 Computed tomography venography 560
5 Assessment of pulmonary embolism severity and the risk of early death 560
5.1 Clinical parameters of pulmonary embolism severity 560
5.2 Imaging of right ventricular size and function 560
5.2.1 Echocardiography 560
5.2.2 Computed tomographic pulmonary angiography 561
5.3 Laboratory biomarkers 561
5.3.1 Markers of myocardial injury 561
5.3.2 Markers of right ventricular dysfunction 561
5.3.3 Other laboratory biomarkers 561
5.4 Combined parameters and scores for assessment of pulmonary embolism severity 562
5.5 Integration of aggravating conditions and comorbidity into risk assessment of acute pulmonary embolism 562
5.6 Prognostic assessment strategy 562
6 Treatment in the acute phase 564
6.1 Haemodynamic and respiratory support 564
6.1.1 Oxygen therapy and ventilation 564
6.1.2 Pharmacological treatment of acute right ventricular failure 564
6.1.3 Mechanical circulatory support and oxygenation 565
6.1.4 Advanced life support in cardiac arrest 565
6.2 Initial anticoagulation 565
6.2.1 Parenteral anticoagulation 565
6.2.2 Non-vitamin K antagonist oral anticoagulants 566
6.2.3 Vitamin K antagonists 566
6.3 Reperfusion treatment 566
6.3.1 Systemic thrombolysis 566
6.3.2 Percutaneous catheter-directed treatment 567
6.3.3 Surgical embolectomy 567
6.4 Multidisciplinary pulmonary embolism teams 568
6.5 Vena cava filters 568
7 Integrated risk-adapted diagnosis and management 570
7.1 Diagnostic strategies 570
7.1.1 Suspected pulmonary embolism with haemodynamic instability 571
7.1.2 Suspected pulmonary embolism without haemodynamic instability 572
7.1.2.1 Strategy based on computed tomographic pulmonary angiography 572
7.1.2.2 Strategy based on ventilation/perfusion scintigraphy 572
7.2 Treatment strategies 572
7.2.1 Emergency treatment of high-risk pulmonary embolism 572
7.2.2 Treatment of intermediate-risk pulmonary embolism 572
7.2.3 Management of low-risk pulmonary embolism: triage for early discharge and home treatment 572
8 Chronic treatment and prevention of recurrence 574
8.1 Assessment of venous thromboembolism recurrence risk 575
8.2 Anticoagulant-related bleeding risk 576
8.3 Regimens and treatment durations with non-vitamin K antagonist oral anticoagulants, and with other non-vitamin K antagonist antithrombotic drugs 576
8.5 Management of pulmonary embolism in patients with cancer 578
9 Pulmonary embolism and pregnancy 579
9.1 Epidemiology and risk factors for pulmonary embolism in pregnancy 579
9.2 Diagnosis of pulmonary embolism in pregnancy 579
9.2.1 Clinical prediction rules and D-dimers 579
9.2.2 Imaging tests 579
9.3 Treatment of pulmonary embolism in pregnancy 581
9.3.1 Role of a multidisciplinary pregnancy heart team 582
9.4 Amniotic fluid embolism 582
10 Long-term sequelae of pulmonary embolism 583
10.1 Persisting symptoms and functional limitation after pulmonary embolism 583
10.2 Chronic thromboembolic pulmonary hypertension 583
10.2.1 Epidemiology, pathophysiology, and natural history 583
10.2.2 Clinical presentation and diagnosis 584
10.2.3 Surgical treatment 584
10.2.4 Balloon pulmonary angioplasty 585
10.2.5 Pharmacological treatment 585
10.3 Strategies for patient follow-up after pulmonary embolism 586
11 Non-thrombotic pulmonary embolism 587
12 Key messages 587
13 Gaps in the evidence 588
14 ‘What to do’ and ‘what not to do’ messages from the Guidelines 589
15 Supplementary data 590
16 Appendix 590
17 References 591
Recommendations
4.11 Recommendations for diagnosis 559
5.7 Recommendations for prognostic assessment 564
6.6 Recommendations for acute-phase treatment of high-risk pulmonary embolism 568
6.7 Recommendations for acute-phase treatment of intermediate- or low-risk pulmonary embolism 569
6.8 Recommendations for multidisciplinary pulmonary embolism teams 569
6.9 Recommendations for inferior vena cava filters 569
6.10 Recommendations for early discharge and home treatment 569
8.4 Recommendations for the regimen and the duration of anticoagulation after pulmonary embolism in patients without cancer 577
8.6 Recommendations for the regimen and the duration of anticoagulation after pulmonary embolism in patients with active cancer 579
9.5 Recommendations for pulmonary embolism in pregnancy 582
10.4 Recommendations for follow-up after acute pulmonary embolism 587
List of tables
Table 1 Classes of recommendation 548
Table 2 Levels of evidence 548
Table 3 Predisposing factors for venous thromboembolism 552
Table 4 Definition of haemodynamic instability, which delineates acute high-risk pulmonary embolism 553
Table 5 The revised Geneva clinical prediction rule for pulmonary embolism 554
Table 6 Imaging tests for diagnosis of pulmonary embolism 556
Table 7 Original and simplified Pulmonary Embolism Severity Index 562
Table 8 Classification of pulmonary embolism severity and the risk of early (in-hospital or 30-day) death 563
Table 9 Treatment of right ventricular failure in acute high-risk pulmonary embolism 565
Table 10 Thrombolytic regimens, doses, and contra indications 567
Table 11 Categorization of risk factors for venous thromboembolism based on the risk of recurrence over the long-term 575
Table 12 Estimated radiation absorbed in procedures used for diagnosing pulmonary embolism (based on various references) 581
Table 13 Risk factors and predisposing conditions for Chronic thromboembolic pulmonary hypertension 584
List of figures
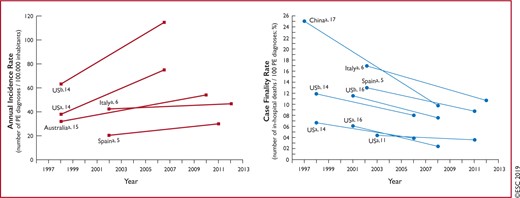
Figure 1 Trends in annual incidence rates and case fatality rates of pulmonary embolism around the world, based on data retrieved from various references 551
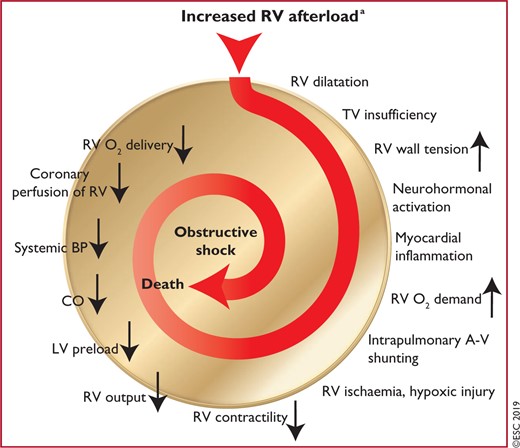
Figure 2 Key factors contributing to haemodynamic collapse and death in acute pulmonary embolism 553
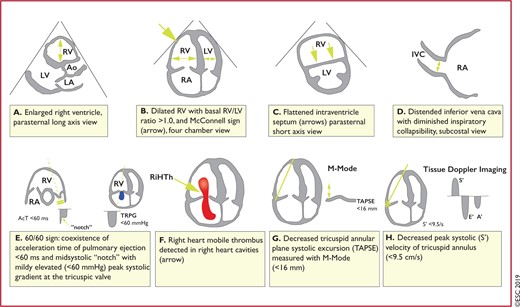
Figure 3 Graphic representation of transthoracic echocardiographic parameters in the assessment of right ventricular pressure overload 558
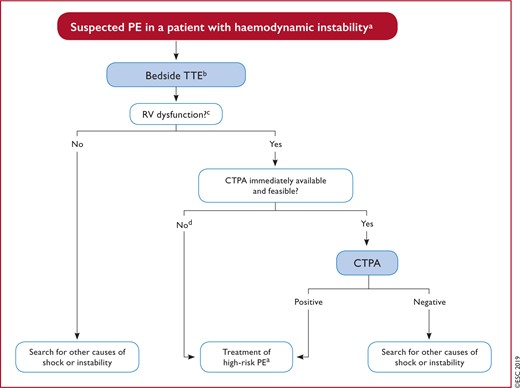
Figure 4 Diagnostic algorithm for patients with suspected high-risk pulmonary embolism, presenting with haemodynamic instability 570
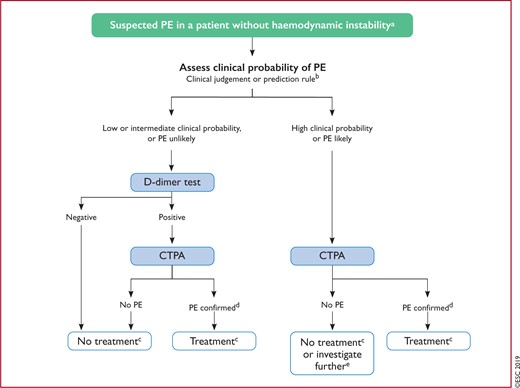
Figure 5 Diagnostic algorithm for patients with suspected pulmonary embolism without haemodynamic instability 571
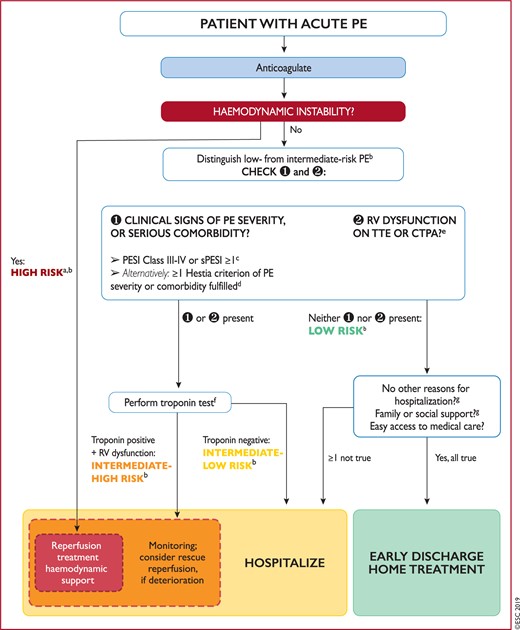
Figure 6 Risk-adjusted management strategy for acute pulmonary embolism 573
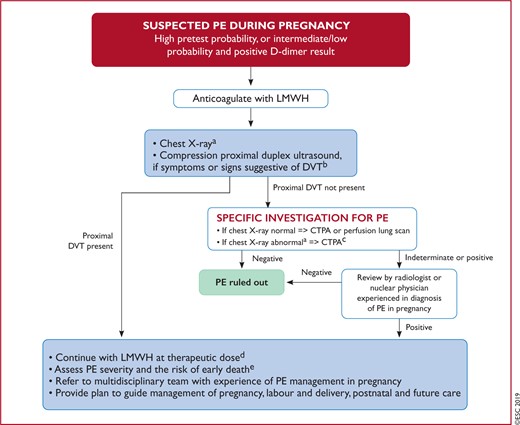
Figure 7 Diagnostic workup for suspected pulmonary embolism during pregnancy and up to 6 weeks post-partum 580
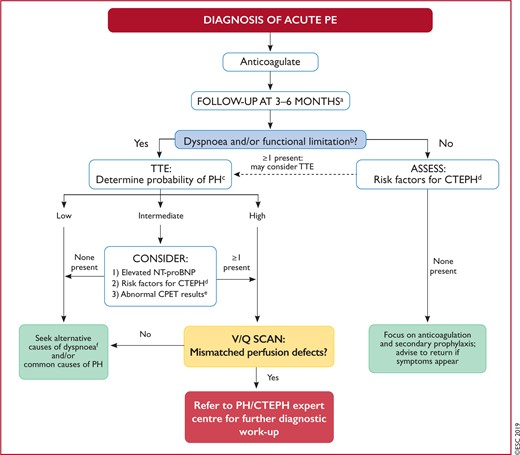
Figure 8 Follow-up strategy and diagnostic workup for long-term sequelae of pulmonary embolism 586
Abbreviations and acronyms
- AcT
Right ventricular outflow Doppler acceleration time
- AFE
Amniotic fluid embolism
- ALT
Alanine aminotransferase
- AMPLIFY
Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-line Therapy
- ASPIRE
Aspirin to Prevent Recurrent Venous Thromboembolism trial
- AV
Arteriovenous
- b.i.d
Bis in die (twice a day)
- BNP
B-type natriuretic peptide
- BP
Blood pressure
- BPA
Balloon pulmonary angioplasty
- b.p.m
Beats per minute
- CI
Confidence interval
- CO
Cardiac output
- CPET
Cardiopulmonary exercise testing
- CPG
Committee for Practice Guidelines
- CrCl
Creatinine clearance
- CRNM
Clinically relevant non-major (bleeding)
- CT
Computed tomogram/tomographic/tomography
- CTED
Chronic thromboembolic disease
- CTEPH
Chronic thromboembolic pulmonary hypertension
- CTPA
Computed tomography pulmonary angiography/angiogram
- CUS
Compression ultrasonography
- CYP3A4
Cytochrome 3A4
- DAMOVES
D-dimer, Age, Mutation, Obesity, Varicose veins, Eight [coagulation factor VIII], Sex
- DASH
D-dimer, Age, Sex, Hormonal therapy
- DVT
Deep vein thrombosis
- ECMO
Extracorporeal membrane oxygenation
- ELISA
Enzyme-linked immunosorbent assay
- EMA
European Medicines Agency
- ERS
European Respiratory Society
- ESC
European Society of Cardiology
- FAST
H-FABP, Syncope, Tachycardia (prognostic score)
- FDA
US Food and Drug Administration
- GUSTO
Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries
- HAS-BLED
Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly
- HERDOO2
Hyperpigmentation, Edema, or Redness in either leg; D-dimer level ≥250 μg/L; Obesity with body mass index ≥30 kg/m2; or Older age, ≥65 years
- H-FABP
Heart-type fatty acid-binding protein
- HIV
Human immunodeficiency virus
- HR
Hazard ratio
- INR
International normalized ratio
- IU
International units
- i.v
Intravenous
- IVC
Inferior vena cava
- LA
Left atrium
- LMWH
Low-molecular weight heparin(s)
- LV
Left ventricle/ventricular
- MRA
Magnetic resonance angiography
- NCT
National clinical trial
- NOAC(s)
Non-vitamin K antagonist oral anticoagulant(s)
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- NYHA
New York Heart Association
- OBRI
Outpatient Bleeding Risk Index
- o.d
Omni die (once a day)
- OR
Odds ratio
- PAH
Pulmonary arterial hypertension
- PAP
Pulmonary artery pressure
- PE
Pulmonary embolism
- PEA
Pulmonary endarterectomy
- PEITHO
Pulmonary Embolism Thrombolysis trial
- PERC
Pulmonary Embolism Rule-out Criteria
- PERT
Pulmonary Embolism Response Team
- PESI
Pulmonary Embolism Severity Index
- P-gp
P-glycoprotein
- PH
Pulmonary hypertension
- PIOPED
Prospective Investigation On Pulmonary Embolism Diagnosis
- PISAPED
Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis
- PREPIC
Prevention of Recurrent Pulmonary Embolism by Vena Cava Interruption
- PVR
Pulmonary vascular resistance
- RA
Right atrium/atrial
- RCT
Randomized controlled trial
- RIETE
Registro Informatizado de la Enfermedad Thromboembolica venosa
- RR
Relative risk
- rtPA
Recombinant tissue-type plasminogen activator
- RV
Right ventricle/ventricular
- SaO2
Arterial oxygen saturation
- SPECT
Single-photon emission computed tomography
- sPESI
Simplified Pulmonary Embolism Severity Index
- SURVET
Sulodexide in Secondary Prevention of Recurrent Deep Vein Thrombosis study
- TAPSE
Tricuspid annular plane systolic excursion
- TOE
Transoesophageal echocardiography/echocardiogram
- TTE
Transthoracic echocardiography/echocardiogram
- TV
Tricuspid valve
- U
Unit
- UFH
Unfractionated heparin
- VKA(s)
Vitamin K antagonist(s)
- V/Q
Ventilation/perfusion (lung scintigraphy)
- VTE
Venous thromboembolism
- VTE-BLEED
ActiVe cancer, male with uncontrolled hyperTension at baseline, anaEmia, history of BLeeding, agE ≥60 years, rEnal Dysfunction
- WARFASA
Warfarin and Aspirin study
1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC), as well as by other societies and organisations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
The ESC carries out a number of registries which are essential to assess, diagnostic/therapeutic processes, use of resources and adherence to Guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on data collected during routine clinical practice.
The guidelines are developed together with derivative educational material addressing the cultural and professional needs for cardiologists and allied professionals. Collecting high-quality observational data, at appropriate time interval following the release of ESC Guidelines, will help evaluate the level of implementation of the Guidelines, checking in priority the key end points defined with the ESC Guidelines and Education Committees and Task Force members in charge.
The Members of this Task Force were selected by the ESC, including representation from its relevant ESC sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk–benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined in Tables 1 and 2.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period were notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the Guidelines are approved by all the experts involved in the Task Force. The finalized document is approved by the CPG for publication in the European Heart Journal. The Guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC Guidelines also includes the creation of educational tools and implementation programmes for the recommendations including condensed pocket guideline versions, summary slides, booklets with essential messages, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access to the full text version of the Guidelines, which is freely available via the ESC website and hosted on the EHJ website. The National Societies of the ESC are encouraged to endorse, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription.
2 Introduction
2.1 Why do we need new Guidelines on the diagnosis and management of pulmonary embolism?
This document follows the previous ESC Guidelines focusing on the clinical management of pulmonary embolism (PE), published in 2000, 2008, and 2014. Many recommendations have been retained or their validity has been reinforced; however, new data have extended or modified our knowledge in respect of the optimal diagnosis, assessment, and treatment of patients with PE. These new aspects have been integrated into previous knowledge to suggest optimal and—whenever possible—objectively validated management strategies for patients with suspected or confirmed PE. To limit the length of the printed text, additional information, tables, figures, and references are available as supplementary data on the ESC website (www.escardio.org).
These Guidelines focus on the diagnosis and management of acute PE in adult patients. For further details specifically related to the diagnosis and management of deep vein thrombosis (DVT), the reader is referred to the joint consensus document of the ESC Working Groups of Aorta and Peripheral Vascular Diseases, and Pulmonary Circulation and Right Ventricular Function.1
2.2 What is new in the 2019 Guidelines?
2.2.1 New/revised concepts in 2019
| Diagnosis |
| D-dimer cut-off values adjusted for age or clinical probability can be used as an alternative to the fixed cut-off value. |
| Updated information is provided on the radiation dosage when using CTPA and a lung scan to diagnose PE (Table 6). |
| Risk assessment |
| A clear definition of haemodynamic instability and high-risk PE is provided (Table 4). |
| Assessment of PE severity and early PE-related risk is recommended, in addition to comorbidity/aggravating conditions and overall death risk. |
| A clear word of caution that RV dysfunction may be present, and affect early outcomes, in patients at ‘low risk’ based on clinical risk scores. |
| Treatment in the acute phase |
| Thoroughly revised section on haemodynamic and respiratory support for high-risk PE (Section 6.1). |
| A dedicated management algorithm is proposed for high-risk PE (Supplementary Figure 1). |
| NOACs are recommended as the first choice for anticoagulation treatment in a patient eligible for NOACs; VKAs are an alternative to NOACs. |
| The risk-adjusted management algorithm (Figure 6) was revised to take into consideration clinical PE severity, aggravating conditions/comorbidity, and the presence of RV dysfunction. |
| Chronic treatment after the first 3 months |
| Risk factors for VTE recurrence have been classified according to high, intermediate, or low recurrence risk (Table 11). |
| Potential indications for extended anticoagulation are discussed, including the presence of a minor transient or reversible risk factor for the index PE, any persisting risk factor, or no identifiable risk factor. |
| Terminology such as ‘provoked’ vs. ‘unprovoked’ PE/VTE is no longer supported by the Guidelines, as it is potentially misleading and not helpful for decision-making regarding the duration of anticoagulation. |
| VTE recurrence scores are presented and discussed in parallel with bleeding scores for patients on anticoagulation treatment (Supplementary Tables 13 and 14 respectively). |
| A reduced dose of apixaban or rivaroxaban for extended anticoagulation should be considered after the first 6 months of treatment. |
| PE in cancer |
| Edoxaban or rivaroxaban should be considered as an alternative to LMWH, with a word of caution for patients with gastrointestinal cancer due to the increased bleeding risk with NOACs. |
| PE in pregnancy |
| A dedicated diagnostic algorithm is proposed for suspected PE in pregnancy (Figure 7). |
| Updated information is provided on radiation absorption related to procedures used for diagnosing PE in pregnancy (Table 12). |
| Long-term sequelae |
| An integrated model of patient care after PE is proposed to ensure optimal transition from hospital to community care. |
| Recommendations on patient care have been extended to the entire spectrum of post-PE symptoms and functional limitation, not only CTEPH. |
| A new comprehensive algorithm is proposed for patient follow-up after acute PE (Figure 8). |
| Diagnosis |
| D-dimer cut-off values adjusted for age or clinical probability can be used as an alternative to the fixed cut-off value. |
| Updated information is provided on the radiation dosage when using CTPA and a lung scan to diagnose PE (Table 6). |
| Risk assessment |
| A clear definition of haemodynamic instability and high-risk PE is provided (Table 4). |
| Assessment of PE severity and early PE-related risk is recommended, in addition to comorbidity/aggravating conditions and overall death risk. |
| A clear word of caution that RV dysfunction may be present, and affect early outcomes, in patients at ‘low risk’ based on clinical risk scores. |
| Treatment in the acute phase |
| Thoroughly revised section on haemodynamic and respiratory support for high-risk PE (Section 6.1). |
| A dedicated management algorithm is proposed for high-risk PE (Supplementary Figure 1). |
| NOACs are recommended as the first choice for anticoagulation treatment in a patient eligible for NOACs; VKAs are an alternative to NOACs. |
| The risk-adjusted management algorithm (Figure 6) was revised to take into consideration clinical PE severity, aggravating conditions/comorbidity, and the presence of RV dysfunction. |
| Chronic treatment after the first 3 months |
| Risk factors for VTE recurrence have been classified according to high, intermediate, or low recurrence risk (Table 11). |
| Potential indications for extended anticoagulation are discussed, including the presence of a minor transient or reversible risk factor for the index PE, any persisting risk factor, or no identifiable risk factor. |
| Terminology such as ‘provoked’ vs. ‘unprovoked’ PE/VTE is no longer supported by the Guidelines, as it is potentially misleading and not helpful for decision-making regarding the duration of anticoagulation. |
| VTE recurrence scores are presented and discussed in parallel with bleeding scores for patients on anticoagulation treatment (Supplementary Tables 13 and 14 respectively). |
| A reduced dose of apixaban or rivaroxaban for extended anticoagulation should be considered after the first 6 months of treatment. |
| PE in cancer |
| Edoxaban or rivaroxaban should be considered as an alternative to LMWH, with a word of caution for patients with gastrointestinal cancer due to the increased bleeding risk with NOACs. |
| PE in pregnancy |
| A dedicated diagnostic algorithm is proposed for suspected PE in pregnancy (Figure 7). |
| Updated information is provided on radiation absorption related to procedures used for diagnosing PE in pregnancy (Table 12). |
| Long-term sequelae |
| An integrated model of patient care after PE is proposed to ensure optimal transition from hospital to community care. |
| Recommendations on patient care have been extended to the entire spectrum of post-PE symptoms and functional limitation, not only CTEPH. |
| A new comprehensive algorithm is proposed for patient follow-up after acute PE (Figure 8). |
CTEPH = Chronic thromboembolic pulmonary hypertension; CTPA = computed tomography pulmonary angiography; LMWH = low-molecular weight heparin; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; RV = right ventricular; VKA(s) = vitamin K antagonist(s); VTE = venous thromboembolism.
| Diagnosis |
| D-dimer cut-off values adjusted for age or clinical probability can be used as an alternative to the fixed cut-off value. |
| Updated information is provided on the radiation dosage when using CTPA and a lung scan to diagnose PE (Table 6). |
| Risk assessment |
| A clear definition of haemodynamic instability and high-risk PE is provided (Table 4). |
| Assessment of PE severity and early PE-related risk is recommended, in addition to comorbidity/aggravating conditions and overall death risk. |
| A clear word of caution that RV dysfunction may be present, and affect early outcomes, in patients at ‘low risk’ based on clinical risk scores. |
| Treatment in the acute phase |
| Thoroughly revised section on haemodynamic and respiratory support for high-risk PE (Section 6.1). |
| A dedicated management algorithm is proposed for high-risk PE (Supplementary Figure 1). |
| NOACs are recommended as the first choice for anticoagulation treatment in a patient eligible for NOACs; VKAs are an alternative to NOACs. |
| The risk-adjusted management algorithm (Figure 6) was revised to take into consideration clinical PE severity, aggravating conditions/comorbidity, and the presence of RV dysfunction. |
| Chronic treatment after the first 3 months |
| Risk factors for VTE recurrence have been classified according to high, intermediate, or low recurrence risk (Table 11). |
| Potential indications for extended anticoagulation are discussed, including the presence of a minor transient or reversible risk factor for the index PE, any persisting risk factor, or no identifiable risk factor. |
| Terminology such as ‘provoked’ vs. ‘unprovoked’ PE/VTE is no longer supported by the Guidelines, as it is potentially misleading and not helpful for decision-making regarding the duration of anticoagulation. |
| VTE recurrence scores are presented and discussed in parallel with bleeding scores for patients on anticoagulation treatment (Supplementary Tables 13 and 14 respectively). |
| A reduced dose of apixaban or rivaroxaban for extended anticoagulation should be considered after the first 6 months of treatment. |
| PE in cancer |
| Edoxaban or rivaroxaban should be considered as an alternative to LMWH, with a word of caution for patients with gastrointestinal cancer due to the increased bleeding risk with NOACs. |
| PE in pregnancy |
| A dedicated diagnostic algorithm is proposed for suspected PE in pregnancy (Figure 7). |
| Updated information is provided on radiation absorption related to procedures used for diagnosing PE in pregnancy (Table 12). |
| Long-term sequelae |
| An integrated model of patient care after PE is proposed to ensure optimal transition from hospital to community care. |
| Recommendations on patient care have been extended to the entire spectrum of post-PE symptoms and functional limitation, not only CTEPH. |
| A new comprehensive algorithm is proposed for patient follow-up after acute PE (Figure 8). |
| Diagnosis |
| D-dimer cut-off values adjusted for age or clinical probability can be used as an alternative to the fixed cut-off value. |
| Updated information is provided on the radiation dosage when using CTPA and a lung scan to diagnose PE (Table 6). |
| Risk assessment |
| A clear definition of haemodynamic instability and high-risk PE is provided (Table 4). |
| Assessment of PE severity and early PE-related risk is recommended, in addition to comorbidity/aggravating conditions and overall death risk. |
| A clear word of caution that RV dysfunction may be present, and affect early outcomes, in patients at ‘low risk’ based on clinical risk scores. |
| Treatment in the acute phase |
| Thoroughly revised section on haemodynamic and respiratory support for high-risk PE (Section 6.1). |
| A dedicated management algorithm is proposed for high-risk PE (Supplementary Figure 1). |
| NOACs are recommended as the first choice for anticoagulation treatment in a patient eligible for NOACs; VKAs are an alternative to NOACs. |
| The risk-adjusted management algorithm (Figure 6) was revised to take into consideration clinical PE severity, aggravating conditions/comorbidity, and the presence of RV dysfunction. |
| Chronic treatment after the first 3 months |
| Risk factors for VTE recurrence have been classified according to high, intermediate, or low recurrence risk (Table 11). |
| Potential indications for extended anticoagulation are discussed, including the presence of a minor transient or reversible risk factor for the index PE, any persisting risk factor, or no identifiable risk factor. |
| Terminology such as ‘provoked’ vs. ‘unprovoked’ PE/VTE is no longer supported by the Guidelines, as it is potentially misleading and not helpful for decision-making regarding the duration of anticoagulation. |
| VTE recurrence scores are presented and discussed in parallel with bleeding scores for patients on anticoagulation treatment (Supplementary Tables 13 and 14 respectively). |
| A reduced dose of apixaban or rivaroxaban for extended anticoagulation should be considered after the first 6 months of treatment. |
| PE in cancer |
| Edoxaban or rivaroxaban should be considered as an alternative to LMWH, with a word of caution for patients with gastrointestinal cancer due to the increased bleeding risk with NOACs. |
| PE in pregnancy |
| A dedicated diagnostic algorithm is proposed for suspected PE in pregnancy (Figure 7). |
| Updated information is provided on radiation absorption related to procedures used for diagnosing PE in pregnancy (Table 12). |
| Long-term sequelae |
| An integrated model of patient care after PE is proposed to ensure optimal transition from hospital to community care. |
| Recommendations on patient care have been extended to the entire spectrum of post-PE symptoms and functional limitation, not only CTEPH. |
| A new comprehensive algorithm is proposed for patient follow-up after acute PE (Figure 8). |
CTEPH = Chronic thromboembolic pulmonary hypertension; CTPA = computed tomography pulmonary angiography; LMWH = low-molecular weight heparin; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; RV = right ventricular; VKA(s) = vitamin K antagonist(s); VTE = venous thromboembolism.
2.2.2 Changes in recommendations 2014–19
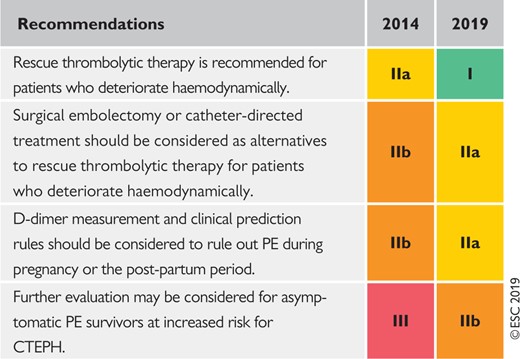 |
 |
CTEPH = Chronic thromboembolic pulmonary hypertension; PE = pulmonary embolism.
Coloured columns indicate classes of recommendation (see Table 1 for colour coding).
 |
 |
CTEPH = Chronic thromboembolic pulmonary hypertension; PE = pulmonary embolism.
Coloured columns indicate classes of recommendation (see Table 1 for colour coding).
2.2.3 Main new recommendations 2019
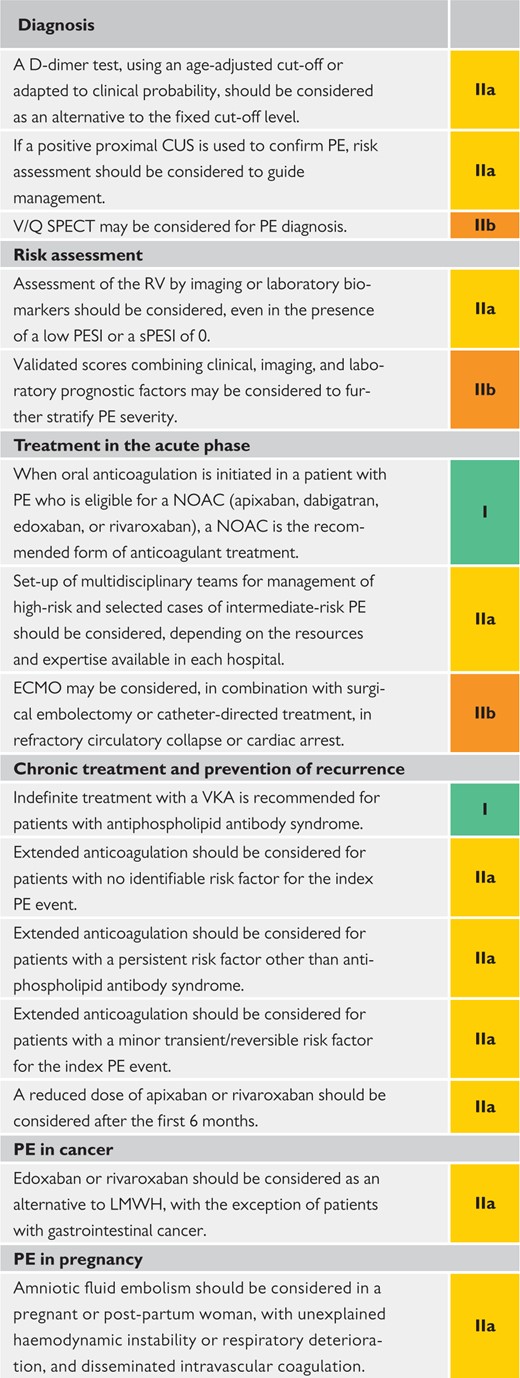 |
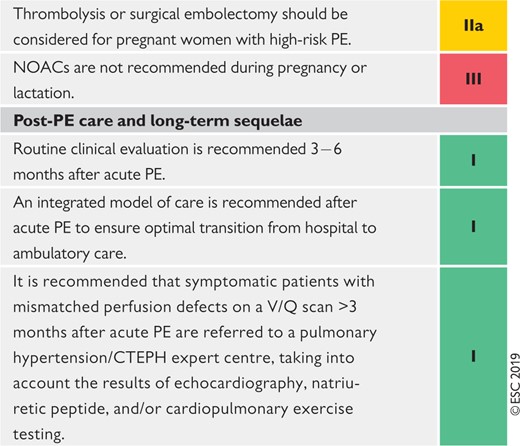 |
 |
 |
CPET = cardiopulmonary exercise testing; CTEPH = Chronic thromboembolic pulmonary hypertension; CUS = compression ultrasonography; ECMO = extracorporeal membrane oxygenation; LMWH = low-molecular weight heparin; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; SPECT = single-photon emission computed tomography; sPESI = simplified Pulmonary Embolism Severity Index; VKA(s) = vitamin K antagonist(s); V/Q = ventilation/perfusion (lung scintigraphy).
Coloured columns indicate classes of recommendation (see Table 1 for colour coding).
 |
 |
 |
 |
CPET = cardiopulmonary exercise testing; CTEPH = Chronic thromboembolic pulmonary hypertension; CUS = compression ultrasonography; ECMO = extracorporeal membrane oxygenation; LMWH = low-molecular weight heparin; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; SPECT = single-photon emission computed tomography; sPESI = simplified Pulmonary Embolism Severity Index; VKA(s) = vitamin K antagonist(s); V/Q = ventilation/perfusion (lung scintigraphy).
Coloured columns indicate classes of recommendation (see Table 1 for colour coding).
3 General considerations
3.1 Epidemiology
Venous thromboembolism (VTE), clinically presenting as DVT or PE, is globally the third most frequent acute cardiovascular syndrome behind myocardial infarction and stroke.2 In epidemiological studies, annual incidence rates for PE range from 39–115 per 100 000 population; for DVT, incidence rates range from 53–162 per 100 000 population.3,4 Cross-sectional data show that the incidence of VTE is almost eight times higher in individuals aged ≥80 years than in the fifth decade of life.3 In parallel, longitudinal studies have revealed a rising tendency in annual PE incidence rates4–7 over time. Together with the substantial hospital-associated, preventable, and indirect annual expenditures for VTE (an estimated total of up to €8.5 billion in the European Union),8 these data demonstrate the importance of PE and DVT in ageing populations in Europe and other areas of the world. They further suggest that VTE will increasingly pose a burden on health systems worldwide in the years to come.
PE may cause ≤300 000 deaths per year in the US, ranking high among the causes of cardiovascular mortality.3 In six European countries with a total population of 454.4 million, more than 370 000 deaths were related to VTE in 2004, as estimated on the basis of an epidemiological model.9 Of these patients, 34% died suddenly or within a few hours of the acute event, before therapy could be initiated or take effect. Of the other patients, death resulted from acute PE that was diagnosed after death in 59% and only 7% of patients who died early were correctly diagnosed with PE before death.9
Time trend analyses in European, Asian, and North American populations suggest that case fatality rates of acute PE may be decreasing.4–7,10,11 Increased use of more effective therapies and interventions, and possibly better adherence to guidelines,12,13 has most likely exerted a significant positive effect on the prognosis of PE in recent years. However, there is also a tendency towards overdiagnosis of (subsegmental or even non-existent) PE in the modern era,14 and this might in turn lead to a false drop in case fatality rates by inflating the denominator, i.e. the total number of PE cases.
Figure 1 summarizes the existing data on global trends in PE, highlighting increasing incidence rates in parallel with decreasing case fatality rates over an ∼15 year period.
In children, studies have reported an annual incidence of VTE of between 53–57 per 100 000 among hospitalized patients,19,20 and between 1.4–4.9 per 100 000 in the community overall.21,22
3.2 Predisposing factors
There is an extensive collection of predisposing environmental and genetic factors for VTE; a list of predisposing (risk) factors is shown in Table 3. VTE is considered to be a consequence of the interaction between patient-related—usually permanent—risk factors and setting-related—usually temporary—risk factors. Since categorization of temporary and permanent risk factors for VTE is important for assessing the risk of recurrence, and consequently for decision-making on chronic anticoagulation, it is discussed in more detail in section 8 of these Guidelines.
| Strong risk factors (OR > 10) . |
|---|
|
| Moderate risk factors (OR 2–9) |
|
| Weak risk factors (OR < 2) |
|
| Strong risk factors (OR > 10) . |
|---|
|
| Moderate risk factors (OR 2–9) |
|
| Weak risk factors (OR < 2) |
|
HIV = human immunodeficiency virus; OR = odds ratio; VTE = venous thromboembolism.
| Strong risk factors (OR > 10) . |
|---|
|
| Moderate risk factors (OR 2–9) |
|
| Weak risk factors (OR < 2) |
|
| Strong risk factors (OR > 10) . |
|---|
|
| Moderate risk factors (OR 2–9) |
|
| Weak risk factors (OR < 2) |
|
HIV = human immunodeficiency virus; OR = odds ratio; VTE = venous thromboembolism.
Major trauma, surgery, lower-limb fractures and joint replacements, and spinal cord injury are strong provoking factors for VTE.23,24 Cancer is a well-recognized predisposing factor for VTE. The risk of VTE varies with different types of cancer;25,26 pancreatic cancer, haematological malignancies, lung cancer, gastric cancer, and brain cancer carry the highest risk.27,28 Moreover, cancer is a strong risk factor for all-cause mortality following an episode of VTE.29
Trends in annual incidence rates (left panel) and case fatality rates (right panel) of pulmonary embolism around the world, based on data retrieved from various references.5,6,11,14–17 Reproduced with permission from JACC 2016;67:976-90. PE = pulmonary embolism; US = United States. aPE listed as principal diagnosis. bAny listed code for PE was considered.
Oestrogen-containing oral contraceptive agents are associated with an elevated VTE risk, and contraceptive use is the most frequent VTE risk factor in women of reproductive age.30–32 More specifically, combined oral contraceptives (containing both an oestrogen and a progestogen) are associated with an approximately two- to six-fold increase in VTE risk over baseline.32,33 In general, the absolute VTE risk remains low in the majority of the >100 million combined oral contraceptive users worldwide;34 however, VTE risk factors, including severe inherited thrombophilia (discussed in section 8),35 increase this risk. Third-generation combined oral contraceptives, containing progestogens such as desogestrel or gestodene, are associated with a higher VTE risk than the second-generation combined oral contraceptives, which contain progestogens such as levonorgestrel or norgestrel.36,37 On the other hand, hormone-releasing intrauterine devices and some progesterone-only pills (used at contraceptive doses) are not associated with a significant increase in VTE risk;33,38 consequently, and following counselling and full risk assessment, these options are often proposed to women with a personal or strong family history of VTE.
In post-menopausal women who receive hormone replacement therapy, the risk of VTE varies widely depending on the formulation used.39
Infection is a common trigger for VTE.23,40,41 Blood transfusion and erythropoiesis-stimulating agents are also associated with an increased risk of VTE.23,42
In children, PE is usually associated with DVT and is rarely unprovoked. Serious chronic medical conditions and central venous lines are considered likely triggers of PE.43
VTE may be viewed as part of the cardiovascular disease continuum, and common risk factors—such as cigarette smoking, obesity, hypercholesterolaemia, hypertension, and diabetes mellitus44–47—are shared with arterial disease, notably atherosclerosis.48–51 However, this may be an indirect association mediated, at least in part, by the complications of coronary artery disease and, in the case of smoking, cancer.52,53 Myocardial infarction and heart failure increase the risk of PE.54,55 Conversely, patients with VTE have an increased risk of subsequent myocardial infarction and stroke, or peripheral arterial embolization.56
3.3 Pathophysiology and determinants of outcome
Acute PE interferes with both circulation and gas exchange. Right ventricular (RV) failure due to acute pressure overload is considered the primary cause of death in severe PE. Pulmonary artery pressure (PAP) increases if >30–50% of the total cross-sectional area of the pulmonary arterial bed is occluded by thromboemboli.57 PE-induced vasoconstriction, mediated by the release of thromboxane A2 and serotonin, contributes to the initial increase in pulmonary vascular resistance (PVR) after PE.58 Anatomical obstruction and hypoxic vasoconstriction in the affected lung area lead to an increase in PVR, and a proportional decrease in arterial compliance.59
The abrupt increase in PVR results in RV dilation, which alters the contractile properties of the RV myocardium via the Frank–Starling mechanism. The increase in RV pressure and volume leads to an increase in wall tension and myocyte stretch. The contraction time of the RV is prolonged, while neurohumoral activation leads to inotropic and chronotropic stimulation. Together with systemic vasoconstriction, these compensatory mechanisms increase PAP, improving flow through the obstructed pulmonary vascular bed and thus temporarily stabilizing systemic blood pressure (BP). However, the extent of immediate adaptation is limited, as a non-preconditioned, thin-walled RV is unable to generate a mean PAP >40 mmHg.
Prolongation of RV contraction time into early diastole in the left ventricle (LV) leads to leftward bowing of the interventricular septum.60 The desynchronization of the ventricles may be exacerbated by the development of right bundle branch block. As a result, LV filling is impeded in early diastole, and this may lead to a reduction in the cardiac output (CO), and contribute to systemic hypotension and haemodynamic instability.61
As described above, excessive neurohumoral activation in PE can be the result of both abnormal RV wall tension and circulatory shock. The finding of massive infiltrates of inflammatory cells in the RV myocardia of patients who died within 48 h of acute PE may be explained by high levels of epinephrine released as a result of the PE-induced ‘myocarditis’.62 This inflammatory response might explain the secondary haemodynamic destabilization that sometimes occurs 24–48 h after acute PE, although early recurrence of PE may be an alternative explanation in some of these cases.
Finally, the association between elevated circulating levels of biomarkers of myocardial injury and an adverse early outcome indicates that RV ischaemia is of pathophysiological significance in the acute phase of PE.63,64 Although RV infarction is uncommon after PE, it is likely that the imbalance between oxygen supply and demand can result in damage to cardiomyocytes, and further reduce contractile forces. Systemic hypotension is a critical element in this process, leading to impairment of the coronary driving pressure to the overloaded RV.
The detrimental effects of acute PE on the RV myocardium and the circulation are summarized in Figure 2.
Respiratory failure in PE is predominantly a consequence of haemodynamic disturbances.66 Low CO results in desaturation of the mixed venous blood. Zones of reduced flow in obstructed pulmonary arteries, combined with zones of overflow in the capillary bed served by non-obstructed pulmonary vessels, result in ventilation/perfusion mismatch, which contributes to hypoxaemia. In about one-third of patients, right-to-left shunting through a patent foramen ovale can be detected by echocardiography; this is caused by an inverted pressure gradient between the right atrium (RA) and left atrium, and may lead to severe hypoxaemia, and an increased risk of paradoxical embolization and stroke.67 Finally, even if they do not affect haemodynamics, small distal emboli may create areas of alveolar haemorrhage resulting in haemoptysis, pleuritis, and pleural effusion, which is usually mild. This clinical presentation is known as ‘pulmonary infarction’. Its effect on gas exchange is normally mild, except in patients with pre-existing cardiorespiratory disease.
Key factors contributing to haemodynamic collapse and death in acute pulmonary embolism (modified from Konstantinides et al.65 with permission). A-V = arterio-venous; BP = blood pressure; CO = cardiac output; LV - left ventricular; O2 = oxygen; RV = right ventricular; TV = tricuspid valve. aThe exact sequence of events following the increase in RV afterload is not fully understood.
In view of the above pathophysiological considerations, acute RV failure, defined as a rapidly progressive syndrome with systemic congestion resulting from impaired RV filling and/or reduced RV flow output,68 is a critical determinant of clinical severity and outcome in acute PE. Accordingly, clinical symptoms, and signs of overt RV failure and haemodynamic instability, indicate a high risk of early (in-hospital or 30 day) mortality. High-risk PE is defined by haemodynamic instability and encompasses the forms of clinical presentation shown in Table 4.
As an immediately life-threatening situation, high-risk PE requires an emergency diagnostic (upon suspicion) and therapeutic (upon confirmation or if the level of suspicion is sufficiently high) strategy, as outlined in section 7. However, the absence of haemodynamic instability does not exclude beginning (and possibly progressing) RV dysfunction, and thus an elevated PE-related early risk. In this large population, further assessment (outlined in sections 5 and 7) is necessary to determine the level of risk and adjust management decisions accordingly.
Definition of haemodynamic instability, which delineates acute high-risk pulmonary embolism (one of the following clinical manifestations at presentation)
| (1) Cardiac arrest . | (2) Obstructive shock68–70 . | (3) Persistent hypotension . |
|---|---|---|
| Need for cardiopulmonary resuscitation | Systolic BP < 90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite adequate filling status | Systolic BP < 90 mmHg or systolic BP drop ≥40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolaemia, or sepsis |
| And | ||
| End-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate) |
| (1) Cardiac arrest . | (2) Obstructive shock68–70 . | (3) Persistent hypotension . |
|---|---|---|
| Need for cardiopulmonary resuscitation | Systolic BP < 90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite adequate filling status | Systolic BP < 90 mmHg or systolic BP drop ≥40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolaemia, or sepsis |
| And | ||
| End-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate) |
BP = blood pressure.
Definition of haemodynamic instability, which delineates acute high-risk pulmonary embolism (one of the following clinical manifestations at presentation)
| (1) Cardiac arrest . | (2) Obstructive shock68–70 . | (3) Persistent hypotension . |
|---|---|---|
| Need for cardiopulmonary resuscitation | Systolic BP < 90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite adequate filling status | Systolic BP < 90 mmHg or systolic BP drop ≥40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolaemia, or sepsis |
| And | ||
| End-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate) |
| (1) Cardiac arrest . | (2) Obstructive shock68–70 . | (3) Persistent hypotension . |
|---|---|---|
| Need for cardiopulmonary resuscitation | Systolic BP < 90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite adequate filling status | Systolic BP < 90 mmHg or systolic BP drop ≥40 mmHg, lasting longer than 15 min and not caused by new-onset arrhythmia, hypovolaemia, or sepsis |
| And | ||
| End-organ hypoperfusion (altered mental status; cold, clammy skin; oliguria/anuria; increased serum lactate) |
BP = blood pressure.
4 Diagnosis
The increased awareness of venous thromboembolic disease and the ever-increasing availability of non-invasive imaging tests, mainly computed tomography (CT) pulmonary angiography (CTPA), have generated a tendency for clinicians to suspect and initiate a diagnostic workup for PE more frequently than in the past. This changing attitude is illustrated by the rates of PE confirmation among patients undergoing diagnostic workup: these were as low as 5% in recent North American diagnostic studies, in sharp contrast to the approximately 50% prevalence reported back in the early 1980s.71 Therefore, it is critical that, when evaluating non-invasive diagnostic strategies for PE in the modern era, it is ensured that they are capable of safely excluding PE in contemporary patient populations with a rather low pre-test probability of the disease.72 Conversely, a positive test should have an adequate specificity to set the indication for anticoagulant treatment.
4.1 Clinical presentation
The clinical signs and symptoms of acute PE are non-specific. In most cases, PE is suspected in a patient with dyspnoea, chest pain, pre-syncope or syncope, or haemoptysis.73–75 Haemodynamic instability is a rare but important form of clinical presentation, as it indicates central or extensive PE with severely reduced haemodynamic reserve. Syncope may occur, and is associated with a higher prevalence of haemodynamic instability and RV dysfunction.76 Conversely, and according to the results of a recent study, acute PE may be a frequent finding in patients presenting with syncope (17%), even in the presence of an alternative explanation.77
In some cases, PE may be asymptomatic or discovered incidentally during diagnostic workup for another disease.
Dyspnoea may be acute and severe in central PE; in small peripheral PE, it is often mild and may be transient. In patients with pre-existing heart failure or pulmonary disease, worsening dyspnoea may be the only symptom indicative of PE. Chest pain is a frequent symptom of PE and is usually caused by pleural irritation due to distal emboli causing pulmonary infarction.78 In central PE, chest pain may have a typical angina character, possibly reflecting RV ischaemia, and requiring differential diagnosis from an acute coronary syndrome or aortic dissection.
In addition to symptoms, knowledge of the predisposing factors for VTE is important in determining the clinical probability of the disease, which increases with the number of predisposing factors present; however, in 40% of patients with PE, no predisposing factors are found.79 Hypoxaemia is frequent, but ≤40% of patients have normal arterial oxygen saturation (SaO2) and 20% have a normal alveolar–arterial oxygen gradient.80,81 Hypocapnia is also often present. A chest X-ray is frequently abnormal and, although its findings are usually non-specific in PE, it may be useful for excluding other causes of dyspnoea or chest pain.82 Electrocardiographic changes indicative of RV strain—such as inversion of T waves in leads V1–V4, a QR pattern in V1, a S1Q3T3 pattern, and incomplete or complete right bundle branch block—are usually found in more severe cases of PE;83 in milder cases, the only abnormality may be sinus tachycardia, present in 40% of patients. Finally, atrial arrhythmias, most frequently atrial fibrillation, may be associated with acute PE.
4.2 Assessment of clinical (pre-test) probability
The combination of symptoms and clinical findings with the presence of predisposing factors for VTE allows the classification of patients with suspected PE into distinct categories of clinical or pre-test probability, which correspond to an increasing actual prevalence of confirmed PE. This pre-test assessment can be done either by implicit (empirical) clinical judgement or by using prediction rules. As the post-test (i.e. after an imaging test) probability of PE depends not only on the characteristics of the diagnostic test itself but also on the pre-test probability, this is a key step in all diagnostic algorithms for PE.
The value of empirical clinical judgement has been confirmed in several large series.84,85 Clinical judgement usually includes commonplace tests such as chest X-rays and electrocardiograms for differential diagnosis. However, as clinical judgement lacks standardization, several explicit clinical prediction rules have been developed. Of these, the most frequently used prediction rules are the revised Geneva rule (Table 5) and the Wells rule (see Supplementary Data Table 1).86 Both prediction rules have been simplified in an attempt to increase their adoption into clinical practice;87,88 the simplified versions have been externally validated.89,90
The revised Geneva clinical prediction rule for pulmonary embolism
| Items . | Clinical decision rule points . | |
|---|---|---|
| . | Original version91 . | Simplified version87 . |
| Previous PE or DVT | 3 | 1 |
| Heart rate | ||
| 75–94 b.p.m. | 3 | 1 |
| ≥95 b.p.m. | 5 | 2 |
| Surgery or fracture within the past month | 2 | 1 |
| Haemoptysis | 2 | 1 |
| Active cancer | 2 | 1 |
| Unilateral lower-limb pain | 3 | 1 |
| Pain on lower-limb deep venous palpation and unilateral oedema | 4 | 1 |
| Age >65 years | 1 | 1 |
| Clinical probability | ||
| Three-level score | ||
| Low | 0–3 | 0–1 |
| Intermediate | 4–10 | 2–4 |
| High | ≥11 | ≥5 |
| Two-level score | ||
| PE-unlikely | 0–5 | 0–2 |
| PE-likely | ≥6 | ≥3 |
| Items . | Clinical decision rule points . | |
|---|---|---|
| . | Original version91 . | Simplified version87 . |
| Previous PE or DVT | 3 | 1 |
| Heart rate | ||
| 75–94 b.p.m. | 3 | 1 |
| ≥95 b.p.m. | 5 | 2 |
| Surgery or fracture within the past month | 2 | 1 |
| Haemoptysis | 2 | 1 |
| Active cancer | 2 | 1 |
| Unilateral lower-limb pain | 3 | 1 |
| Pain on lower-limb deep venous palpation and unilateral oedema | 4 | 1 |
| Age >65 years | 1 | 1 |
| Clinical probability | ||
| Three-level score | ||
| Low | 0–3 | 0–1 |
| Intermediate | 4–10 | 2–4 |
| High | ≥11 | ≥5 |
| Two-level score | ||
| PE-unlikely | 0–5 | 0–2 |
| PE-likely | ≥6 | ≥3 |
b.p.m. = beats per minute; DVT = deep vein thrombosis; PE = pulmonary embolism.
The revised Geneva clinical prediction rule for pulmonary embolism
| Items . | Clinical decision rule points . | |
|---|---|---|
| . | Original version91 . | Simplified version87 . |
| Previous PE or DVT | 3 | 1 |
| Heart rate | ||
| 75–94 b.p.m. | 3 | 1 |
| ≥95 b.p.m. | 5 | 2 |
| Surgery or fracture within the past month | 2 | 1 |
| Haemoptysis | 2 | 1 |
| Active cancer | 2 | 1 |
| Unilateral lower-limb pain | 3 | 1 |
| Pain on lower-limb deep venous palpation and unilateral oedema | 4 | 1 |
| Age >65 years | 1 | 1 |
| Clinical probability | ||
| Three-level score | ||
| Low | 0–3 | 0–1 |
| Intermediate | 4–10 | 2–4 |
| High | ≥11 | ≥5 |
| Two-level score | ||
| PE-unlikely | 0–5 | 0–2 |
| PE-likely | ≥6 | ≥3 |
| Items . | Clinical decision rule points . | |
|---|---|---|
| . | Original version91 . | Simplified version87 . |
| Previous PE or DVT | 3 | 1 |
| Heart rate | ||
| 75–94 b.p.m. | 3 | 1 |
| ≥95 b.p.m. | 5 | 2 |
| Surgery or fracture within the past month | 2 | 1 |
| Haemoptysis | 2 | 1 |
| Active cancer | 2 | 1 |
| Unilateral lower-limb pain | 3 | 1 |
| Pain on lower-limb deep venous palpation and unilateral oedema | 4 | 1 |
| Age >65 years | 1 | 1 |
| Clinical probability | ||
| Three-level score | ||
| Low | 0–3 | 0–1 |
| Intermediate | 4–10 | 2–4 |
| High | ≥11 | ≥5 |
| Two-level score | ||
| PE-unlikely | 0–5 | 0–2 |
| PE-likely | ≥6 | ≥3 |
b.p.m. = beats per minute; DVT = deep vein thrombosis; PE = pulmonary embolism.
Regardless of the score used, the proportion of patients with confirmed PE can be expected to be ∼10% in the low-probability category, 30% in the moderate-probability category, and 65% in the high-probability category.92 When the two-level classification is used, the proportion of patients with confirmed PE is ∼12% in the PE-unlikely category and 30% in the PE-likely category.92 A direct prospective comparison of these rules confirmed a similar diagnostic performance.89
4.3 Avoiding overuse of diagnostic tests for pulmonary embolism
Searching for PE in every patient with dyspnoea or chest pain may lead to high costs and complications of unnecessary tests. The Pulmonary Embolism Rule-out Criteria (PERC) were developed for emergency department patients with the purpose of selecting, on clinical grounds, patients whose likelihood of having PE is so low that diagnostic workup should not even be initiated.93 They comprise eight clinical variables significantly associated with an absence of PE: age < 50 years; pulse < 100 beats per minute; SaO2 >94%; no unilateral leg swelling; no haemoptysis; no recent trauma or surgery; no history of VTE; and no oral hormone use. The results of a prospective validation study,94 and those of a randomized non-inferiority management study,95 suggested safe exclusion of PE in patients with low clinical probability who, in addition, met all criteria of the PERC rule. However, the low overall prevalence of PE in these studies94,95 does not support the generalizability of the results.
4.4 D-dimer testing
D-dimer levels are elevated in plasma in the presence of acute thrombosis because of simultaneous activation of coagulation and fibrinolysis. The negative predictive value of D-dimer testing is high, and a normal D-dimer level renders acute PE or DVT unlikely. On the other hand, the positive predictive value of elevated D-dimer levels is low and D-dimer testing is not useful for confirmation of PE. D-dimer is also more frequently elevated in patients with cancer,96,97 in hospitalized patients,89,98 in severe infection or inflammatory disease, and during pregnancy.99,100 Accordingly, the number of patients in whom D-dimer must be measured to exclude one PE (number needed to test) rises from 3 in the general population of an emergency department to ≥10 in the specific situations listed above.
As a number of D-dimer assays are available, clinicians should become aware of the diagnostic performance of the test used in their own hospital. The quantitative enzyme-linked immunosorbent assay (ELISA) or ELISA-derived assays have a diagnostic sensitivity of ≥95%, and can be used to exclude PE in patients with either low or intermediate pre-test probability. In the emergency department, a negative ELISA D-dimer can, in combination with clinical probability, exclude the disease without further testing in ∼30% of patients with suspected PE.101–103 Outcome studies have shown that the 3 month thrombo-embolic risk was <1% in patients with low or intermediate clinical probability who were left untreated on the basis of a negative test result.104
4.4.1 Age-adjusted D-dimer cut-offs
The specificity of D-dimer in suspected PE decreases steadily with age to ∼10% in patients >80 years of age.105 The use of age-adjusted cut-offs may improve the performance of D-dimer testing in the elderly. A multinational prospective management study evaluated a previously validated age-adjusted cut-off (age × 10 µg/L, for patients aged >50 years) in a cohort of 3346 patients.106 Patients with a normal age-adjusted D-dimer value did not undergo CTPA; they were left untreated and followed for a 3 month period. Among the 766 patients who were ≥75 years of age, 673 had a non-high clinical probability. Use of the age-adjusted (instead of the ‘standard’ 500 µg/L) D-dimer cut-off increased the number of patients in whom PE could be excluded from 6.4 to 30%, without additional false-negative findings.106
4.4.2 D-dimer cut-offs adapted to clinical probability
A prospective management trial used the ‘YEARS’ clinical decision rule, which consists of three clinical items of the Wells score (see Supplementary Data Table 1)—namely signs of DVT, haemoptysis, and PE more likely than an alternative diagnosis—plus D-dimer concentrations.107 PE was considered to be excluded in patients without clinical items and D-dimer levels <1000 ng/mL, or in patients with one or more clinical items and D-dimer levels <500 ng/mL. All other patients underwent CTPA. Of the 2946 patients (85%) in whom PE was ruled out at baseline and who were left untreated, 18 [0.61%, 95% confidence interval (CI) 0.36–0.96%] were diagnosed with symptomatic VTE during the 3 month follow-up. CTPA was avoided in 48% of the included patients using this algorithm, compared to 34% if the Wells rule and a fixed D-dimer threshold of 500 ng/mL would have been applied.107
4.4.3 Point-of-care D-dimer assays
In certain situations, notably in community or primary care medicine, ‘on-the-spot’ D-dimer testing may have advantages over referring a patient to a central laboratory for D-dimer testing. This may particularly apply to remote areas where access to healthcare is limited.108,109 However, point-of-care assays have a lower sensitivity and negative predictive value compared with laboratory-based D-dimer tests. In a systematic review and meta-analysis, sensitivity of point-of-care D-dimer assays was 88% (95% CI 83–92%) whereas conventional laboratory-based D-dimer testing had a sensitivity of at least 95%.110 As a result, point-of-care D-dimer assays should only be used in patients with a low pre-test probability. In these situations, PE could be ruled out in 46% of patients with suspected PE without proceeding to imaging tests (with a failure rate of 1.5%), as suggested by a prospective study in Dutch primary care.111
4.5 Computed tomographic pulmonary angiography
Multidetector CTPA is the method of choice for imaging the pulmonary vasculature in patients with suspected PE. It allows adequate visualization of the pulmonary arteries down to the subsegmental level.112–114 The Prospective Investigation On Pulmonary Embolism Diagnosis (PIOPED) II study observed a sensitivity of 83% and a specificity of 96% for (mainly four-detector) CTPA in PE diagnosis.115 PIOPED II also highlighted the influence of pre-test clinical probability on the predictive value of multidetector CTPA. In patients with a low or intermediate clinical probability of PE, a negative CTPA had a high negative predictive value for PE (96 and 89%, respectively), but its negative predictive value was only 60% if the pre-test probability was high. Conversely, the positive predictive value of a positive CTPA was high (92–96%) in patients with an intermediate or high clinical probability, but much lower (58%) in patients with a low pre-test likelihood of PE.115 Therefore, clinicians should consider further testing in case of discordance between clinical judgement and the CTPA result.
Several studies have provided evidence in favour of CTPA as a stand-alone imaging test for excluding PE. Taken together, the available data suggest that a negative CTPA result is an adequate criterion for the exclusion of PE in patients with low or intermediate clinical probability of PE. On the other hand, it remains controversial whether patients with a negative CTPA and a high clinical probability should be further investigated.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a potentially fatal late sequela of PE, but pre-existing CTEPH should not be missed in patients investigated for suspected acute PE. Signs of pre-existing CTEPH on CTPA are listed in Supplementary Data Table 2; the diagnosis and management of CTEPH is discussed in section 10.
The major strengths, weaknesses/limitations, and radiation issues related to the use of CTPA in the diagnosis of PE are summarized in Table 6.
Imaging tests for diagnosis of pulmonary embolism
| . | Strengths . | Weaknesses/limitations . | Radiation issuesa . |
|---|---|---|---|
| CTPA |
|
|
|
| Planar V/Q scan |
|
|
|
| V/Q SPECT |
|
|
|
| Pulmonary angiography |
|
|
|
| . | Strengths . | Weaknesses/limitations . | Radiation issuesa . |
|---|---|---|---|
| CTPA |
|
|
|
| Planar V/Q scan |
|
|
|
| V/Q SPECT |
|
|
|
| Pulmonary angiography |
|
|
|
CTPA = computed tomographic pulmonary angiography; mGy = milligray; mSv = millisieverts; PE = pulmonary embolism; SPECT = single-photon emission computed tomography; V/Q = ventilation/perfusion (lung scintigraphy).
In this section, effective radiation dose is expressed in mSv [dose in mSv = absorbed dose in mGy × radiation weighting factor (1.0 for X-rays) × tissue weighting factor]. This reflects the effective doses of all organs that have been exposed, that is, the overall radiation dose to the body from the imaging test. Compare with Table 12, in which the absorbed radiation dose is expressed in mGy to reflect the radiation exposure to single organs or to the foetus.
For comparison, the whole-body effective dose of a chest X-ray examination is 0.1 mSv.141
Imaging tests for diagnosis of pulmonary embolism
| . | Strengths . | Weaknesses/limitations . | Radiation issuesa . |
|---|---|---|---|
| CTPA |
|
|
|
| Planar V/Q scan |
|
|
|
| V/Q SPECT |
|
|
|
| Pulmonary angiography |
|
|
|
| . | Strengths . | Weaknesses/limitations . | Radiation issuesa . |
|---|---|---|---|
| CTPA |
|
|
|
| Planar V/Q scan |
|
|
|
| V/Q SPECT |
|
|
|
| Pulmonary angiography |
|
|
|
CTPA = computed tomographic pulmonary angiography; mGy = milligray; mSv = millisieverts; PE = pulmonary embolism; SPECT = single-photon emission computed tomography; V/Q = ventilation/perfusion (lung scintigraphy).
In this section, effective radiation dose is expressed in mSv [dose in mSv = absorbed dose in mGy × radiation weighting factor (1.0 for X-rays) × tissue weighting factor]. This reflects the effective doses of all organs that have been exposed, that is, the overall radiation dose to the body from the imaging test. Compare with Table 12, in which the absorbed radiation dose is expressed in mGy to reflect the radiation exposure to single organs or to the foetus.
For comparison, the whole-body effective dose of a chest X-ray examination is 0.1 mSv.141
4.6 Lung scintigraphy
The planar ventilation/perfusion [V/Q (lung scintigraphy)] scan is an established diagnostic test for suspected PE. Perfusion scans are combined with ventilation studies, for which multiple tracers such as xenon-133 gas, krypton-81 gas, technetium-99m-labelled aerosols, or technetium-99m-labelled carbon microparticles (Technegas) can be used. The purpose of the ventilation scan is to increase specificity: in acute PE, ventilation is expected to be normal in hypoperfused segments (mismatched). Being a lower-radiation and contrast medium-sparing procedure, the V/Q scan may preferentially be applied in outpatients with a low clinical probability and a normal chest X-ray, in young (particularly female) patients, in pregnant women, in patients with history of contrast medium-induced anaphylaxis, and patients with severe renal failure.116
Planar lung scan results are frequently classified according to the criteria established in the PIOPED study.117 These criteria were the subject of debate and have been revised.118,119 To facilitate communication with clinicians, a three-tier classification is preferable: normal scan (excluding PE), high-probability scan (considered diagnostic of PE in most patients), and non-diagnostic scan.120–122 Prospective clinical outcome studies suggested that it is safe to withhold anticoagulant therapy in patients with a normal perfusion scan. This was confirmed by a randomized trial comparing the V/Q scan with CTPA.122 An analysis from the PIOPED II study suggested that a high-probability V/Q scan could confirm PE, although other sources suggest that the positive predictive value of a high-probability lung scan is not sufficient to confirm PE in patients with a low clinical probability.123,124
Performing only a perfusion scan might be acceptable in patients with a normal chest X-ray; any perfusion defect in this situation would be considered a mismatch. The high frequency of non-diagnostic scans is a limitation because they indicate the necessity for further diagnostic testing. Various strategies to overcome this problem have been proposed, notably the incorporation of clinical probability. Although the use of perfusion scanning and chest X-ray with the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISAPED) criteria may be associated with a low rate of inconclusive results, the sensitivity appears too low to exclude PE and thus this approach may be less safe than CTPA.123,125
Several studies suggest that data acquisition in single-photon emission CT (SPECT) imaging, with or without low-dose CT, may decrease the proportion of non-diagnostic scans to as low as 0–5%.121,126–128 However, most studies reporting on the accuracy of SPECT are limited by their retrospective design129,130 or the inclusion of SPECT itself in the reference standard,127 and only one study used a validated diagnostic algorithm.131 The diagnostic criteria for SPECT also varied; most studies defined PE as one or two subsegmental perfusion defects without ventilation defects, but these criteria are infrequently used in clinical practice. In addition, the optimal scanning technique (perfusion SPECT, V/Q SPECT, perfusion SPECT with non-enhanced CT, or V/Q SPECT with non-enhanced CT) remains to be defined. Finally, few outcome studies are available, and with incomplete follow-up.132 Large-scale prospective studies are needed to validate SPECT techniques.
The major strengths, weaknesses/limitations, and radiation issues related to the use of V/Q scan and V/Q SPECT in the diagnosis of PE are summarized in Table 6.
4.7 Pulmonary angiography
For several decades, pulmonary angiography was the ‘gold standard’ for the diagnosis or exclusion of acute PE, but it is now rarely performed as less-invasive CTPA offers similar diagnostic accuracy.133 The diagnosis of acute PE is based on direct evidence of a thrombus in two projections, either as a filling defect or as amputation of a pulmonary arterial branch.134 Thrombi as small as 1–2 mm within the subsegmental arteries can be visualized by digital subtraction angiography, but there is substantial interobserver variability at this level.135,136
Pulmonary angiography is not free of risk. In a study of 1111 patients, procedure-related mortality was 0.5%, major non-fatal complications occurred in 1%, and minor complications in 5%.137 The majority of deaths occurred in patients with haemodynamic compromise or respiratory failure. The amount of contrast agent should be reduced and non-selective injections avoided in patients with haemodynamic compromise.138
The major strengths, weaknesses/limitations, and radiation issues related to the use of pulmonary angiography in the diagnosis of PE are summarized in Table 6.
4.8 Magnetic resonance angiography
Magnetic resonance angiography (MRA) has been evaluated for several years regarding suspected PE. However, the results of large-scale studies139,140 show that this technique, although promising, is not yet ready for clinical practice due to its low sensitivity, the high proportion of inconclusive MRA scans, and its low availability in most emergency settings. The hypothesis that a negative MRA, combined with the absence of proximal DVT on compression ultrasonography (CUS), may safely rule out clinically significant PE is currently being investigated in an ongoing multicentre outcome study [Clinicaltrials.gov National Clinical Trial (NCT) number 02059551].
4.9 Echocardiography
Acute PE may lead to RV pressure overload and dysfunction, which can be detected by echocardiography. Given the peculiar geometry of the RV, there is no individual echocardiographic parameter that provides fast and reliable information on RV size or function. This is why echocardiographic criteria for the diagnosis of PE have differed between studies. Because of the reported negative predictive value of 40–50%, a negative result cannot exclude PE.124,142,143 On the other hand, signs of RV overload or dysfunction may also be found in the absence of acute PE, and may be due to concomitant cardiac or respiratory disease.144
Echocardiographic findings of RV overload and/or dysfunction are graphically presented in Figure 3. RV dilation is found in ≥25% of patients with PE on transthoracic echocardiography (TTE) and is useful for risk stratification of the disease.145 More specific echocardiographic findings were reported to retain a high positive predictive value for PE even in the presence of pre-existing cardiorespiratory disease. Thus, the combination of a pulmonary ejection acceleration time (measured in the RV outflow tract) <60 ms with a peak systolic tricuspid valve gradient <60 mmHg (‘60/60’ sign), or with depressed contractility of the RV free wall compared to the ‘echocardiographic’ RV apex (McConnell sign), is suggestive of PE.146 However, these findings are present in only ∼12 and 20% of unselected PE patients, respectively.145 Detection of echocardiographic signs of RV pressure overload helps to distinguish acute PE from RV free wall hypokinesia or akinesia due to RV infarction, which may mimic the McConnell sign.147 It should be noted that in ∼10% of PE patients, echocardiography can show potentially misleading incidental findings such as significant LV systolic dysfunction or valvular heart disease.145 Decreased tricuspid annular plane systolic excursion (TAPSE) may also be present in PE patients.148,149 Echocardiographic parameters of RV function derived from Doppler tissue imaging and wall strain assessment may also be affected by the presence of acute PE (Figure 3). However, they probably have low sensitivity as stand-alone findings, as they were reported to be normal in haemodynamically stable patients despite the presence of PE.150,151
Graphic representation of transthoracic echocardiographic parameters in the assessment of right ventricular pressure overload. A′ = peak late diastolic (during atrial contraction) velocity of tricuspid annulus by tissue Doppler imaging; AcT = right ventricular outflow Doppler acceleration time; Ao = aorta; E′ = peak early diastolic velocity of tricuspid annulus by tissue Doppler imaging; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; RA = right atrium; RiHTh = right heart thrombus (or thrombi); RV = right ventricle/ventricular; S′ = peak systolic velocity of tricuspid annulus by tissue Doppler imaging; TAPSE = tricuspid annular plane systolic excursion; TRPG = tricuspid valve peak systolic gradient.
Echocardiographic examination is not mandatory as part of the routine diagnostic workup in haemodynamically stable patients with suspected PE,124 although it may be useful in the differential diagnosis of acute dyspnoea. This is in contrast to suspected high-risk PE, in which the absence of echocardiographic signs of RV overload or dysfunction practically excludes PE as the cause of haemodynamic instability. In the latter case, echocardiography may be of further help in the differential diagnosis of the cause of shock, by detecting pericardial tamponade, acute valvular dysfunction, severe global or regional LV dysfunction, aortic dissection, or hypovolaemia.152 Conversely, in a haemodynamically compromised patient with suspected PE, unequivocal signs of RV pressure overload, especially with more specific echocardiographic findings (60/60 sign, McConnell sign, or right-heart thrombi), justify emergency reperfusion treatment for PE if immediate CT angiography is not feasible in a patient with high clinical probability and no other obvious causes for RV pressure overload.152
Mobile right-heart thrombi are detected by TTE or transoesophageal echocardiography (TOE), or by CT angiography, in <4% of unselected patients with PE.153–155 Their prevalence may reach 18% among PE patients in the intensive care setting.156 Mobile right-heart thrombi essentially confirm the diagnosis of PE and are associated with high early mortality, especially in patients with RV dysfunction.155,157–159
In some patients with suspected acute PE, echocardiography may detect increased RV wall thickness or tricuspid insufficiency jet velocity beyond values compatible with acute RV pressure overload (>3.8 m/s or a tricuspid valve peak systolic gradient >60 mmHg).160 In these cases, chronic thromboembolic (or other) pulmonary hypertension (PH) should be included in the differential diagnosis.
4.10 Compression ultrasonography
In the majority of cases, PE originates from DVT in a lower limb, and only rarely from upper-limb DVT (mostly following venous catheterization). In a study using venography, DVT was found in 70% of patients with proven PE.161 Nowadays, lower-limb CUS has largely replaced venography for diagnosing DVT. CUS has a sensitivity >90% and a specificity of ∼95% for proximal symptomatic DVT.162,163 CUS shows a DVT in 30–50% of patients with PE,162–164 and finding a proximal DVT in patients suspected of having PE is considered sufficient to warrant anticoagulant treatment without further testing.165 However, patients in whom PE is indirectly confirmed by the presence of a proximal DVT should undergo risk assessment for PE severity and the risk of early death.
In the setting of suspected PE, CUS can be limited to a simple four-point examination (bilateral groin and popliteal fossa). The only validated diagnostic criterion for DVT is incomplete compressibility of the vein, which indicates the presence of a clot, whereas flow measurements are unreliable. A positive proximal CUS result has a high positive predictive value for PE. The high diagnostic specificity (96%) along with a low sensitivity (41%) of CUS in this setting was shown by a recent meta-analysis.165,166 CUS is a useful procedure in the diagnostic strategy of patients with CT contraindications. The probability of a positive proximal CUS in suspected PE is higher in patients with signs and symptoms related to the leg veins than in asymptomatic patients.162,163
In patients admitted to the emergency department with haemodynamic instability and suspicion of PE, a combination of venous ultrasound with cardiac ultrasound may further increase specificity. Conversely, an echocardiogram without signs of RV dysfunction and a normal venous ultrasound excluded PE with a high (96%) negative predictive value in one study.167
For further details on the diagnosis and management of DVT, the reader is referred to the joint consensus document of the ESC Working Groups of Aorta and Peripheral Vascular Diseases, and Pulmonary Circulation and Right Ventricular Function.1
4.11 Recommendations for diagnosis
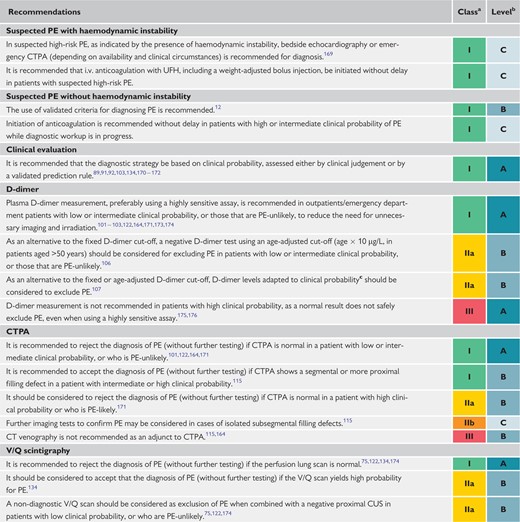 |
 |
 |
 |
CT = computed tomographic; CTPA = computed tomography pulmonary angiography/angiogram; CUS = compression ultrasonography; DVT = deep vein thrombosis; i.v. = intravenous; MRA = magnetic resonance angiography; PE = pulmonary embolism; SPECT = single-photon emission computed tomography; UFH = unfractionated heparin; V/Q = ventilation/perfusion (lung scintigraphy); VTE = venous thromboembolism.
Class of recommendation.
Level of evidence.
D-dimer cut-off levels adapted to clinical probability according to the YEARS model (signs of DVT, haemoptysis, and whether an alternative diagnosis is less likely than PE) may be used. According to this model, PE is excluded in patients without clinical items and D-dimer levels <1000 µg/L, or in patients with one or more clinical items and D-dimer levels <500 µg/L.107
Low level of recommendation in view of the limitations summarized in Table 5.
 |
 |
 |
 |
CT = computed tomographic; CTPA = computed tomography pulmonary angiography/angiogram; CUS = compression ultrasonography; DVT = deep vein thrombosis; i.v. = intravenous; MRA = magnetic resonance angiography; PE = pulmonary embolism; SPECT = single-photon emission computed tomography; UFH = unfractionated heparin; V/Q = ventilation/perfusion (lung scintigraphy); VTE = venous thromboembolism.
Class of recommendation.
Level of evidence.
D-dimer cut-off levels adapted to clinical probability according to the YEARS model (signs of DVT, haemoptysis, and whether an alternative diagnosis is less likely than PE) may be used. According to this model, PE is excluded in patients without clinical items and D-dimer levels <1000 µg/L, or in patients with one or more clinical items and D-dimer levels <500 µg/L.107
Low level of recommendation in view of the limitations summarized in Table 5.
4.12 Computed tomography venography
When using CTPA, it is possible to image the deep veins of the legs during the same acquisition.115 However, this approach has not been widely validated and the added value of venous imaging is limited.164 Moreover, using CT venography is associated with increased radiation doses.168
5 Assessment of pulmonary embolism severity and the risk of early death
Risk stratification of patients with acute PE is mandatory for determining the appropriate therapeutic management approach. As described in section 3.3, initial risk stratification is based on clinical symptoms and signs of haemodynamic instability (Table 4), which indicate a high risk of early death. In the large remaining group of patients with PE who present without haemodynamic instability, further (advanced) risk stratification requires the assessment of two sets of prognostic criteria: (i) clinical, imaging, and laboratory indicators of PE severity, mostly related to the presence of RV dysfunction; and (ii) presence of comorbidity and any other aggravating conditions that may adversely affect early prognosis.
5.1 Clinical parameters of pulmonary embolism severity
Acute RV failure, defined as a rapidly progressive syndrome with systemic congestion resulting from impaired RV filling and/or reduced RV flow output,68 is a critical determinant of outcome in acute PE. Tachycardia, low systolic BP, respiratory insufficiency (tachypnoea and/or low SaO2), and syncope, alone or in combination, have been associated with an unfavourable short-term prognosis in acute PE.
5.2 Imaging of right ventricular size and function
5.2.1 Echocardiography
Echocardiographic parameters used to stratify the early risk of patients with PE are graphically presented in Figure 3, and their prognostic values are summarized in Supplementary Data Table 3. Of these, an RV/LV diameter ratio ≥1.0 and a TAPSE <16 mm are the findings for which an association with unfavourable prognosis has most frequently been reported.148
Overall, evidence for RV dysfunction on echocardiography is found in ≥25% of unselected patients with acute PE.145 Systematic reviews and meta-analyses have suggested that RV dysfunction on echocardiography is associated with an elevated risk of short-term mortality in patients who appear haemodynamically stable at presentation,180,181 but its overall positive predictive value for PE-related death was low (<10%) in a meta-analysis.180 This weakness is partly related to the fact that echocardiographic parameters have proved difficult to standardize.148,180 Nevertheless, echocardiographic assessment of the morphology and function of the RV is widely recognized as a valuable tool for the prognostic assessment of normotensive patients with acute PE in clinical practice.
In addition to RV dysfunction, echocardiography can identify right-to-left shunt through a patent foramen ovale and the presence of right heart thrombi, both of which are associated with increased mortality in patients with acute PE.67,158 A patent foramen ovale also increases the risk of ischaemic stroke due to paradoxical embolism in patients with acute PE and RV dysfunction.182,183
5.2.2 Computed tomographic pulmonary angiography
CTPA parameters used to stratify the early risk of patients with PE are summarized in Supplementary Data Table 3. Four-chamber views of the heart by CT angiography can detect RV enlargement (RV end-diastolic diameter and RV/LV ratio measured in the transverse or four-chamber view) as an indicator of RV dysfunction. The prognostic value of an enlarged RV is supported by the results of a prospective multicentre cohort study in 457 patients.184 In that study, RV enlargement (defined as an RV/LV ratio ≥0.9) was an independent predictor of an adverse in-hospital outcome, both in the overall population with PE [hazard ratio (HR) 3.5, 95% CI 1.6–7.7] and in haemodynamically stable patients (HR 3.8, 95% CI 1.3–10.9).184 A meta-analysis of 49 studies investigating >13 000 patients with PE confirmed that an increased RV/LV ratio of ≥1.0 on CT was associated with a 2.5-fold increased risk for all-cause mortality [odds ratio (OR) 2.5, 95% CI 1.8–3.5], and with a five-fold risk for PE-related mortality (OR 5.0, 95% CI 2.7–9.2).185
Mild RV dilation (RV/LV slightly above 0.9) on CT is a frequent finding (>50% of haemodynamically stable PE patients186), but it probably has minor prognostic significance. However, increasing RV/LV diameter ratios are associated with rising prognostic specificity,187,188 even in patients considered to be at ‘low’ risk on the basis of clinical criteria.186 Thus, RV/LV ratios ≥ 1.0 (instead of 0.9) on CT angiography may be more appropriate to indicate poor prognosis.
Apart from RV size and the RV/LV ratio, CT may provide further prognostic information based on volumetric analysis of the heart chambers189–191 and assessment of contrast reflux to the inferior vena cava (IVC).185,192,193
5.3 Laboratory biomarkers
5.3.1 Markers of myocardial injury
Elevated plasma troponin concentrations on admission may be associated with a worse prognosis in the acute phase of PE. Cardiac troponin I or T elevation are defined as concentrations above the normal limits, and thresholds depend on the assay used; an overview of the cut-off values has been provided by a meta-analysis.194 Of patients with acute PE, between 30 (using conventional assays)194,195 and 60% (using high-sensitivity assays)196,197 have elevated cardiac troponin I or T concentrations. A meta-analysis showed that elevated troponin concentrations were associated with an increased risk of mortality, both in unselected patients (OR 5.2, 95% CI 3.3–8.4) and in those who were haemodynamically stable at presentation (OR 5.9, 95% CI 2.7–13.0).195
On their own, increased circulating levels of cardiac troponins have relatively low specificity and positive predictive value for early mortality in normotensive patients with acute PE. However, when interpreted in combination with clinical and imaging findings, they may improve the identification of an elevated PE-related risk and the further prognostic stratification of such patients (Supplementary Data Table 4). At the other end of the severity spectrum, high-sensitivity troponin assays possess a high negative predictive value in the setting of acute PE.197 For example, in a prospective multicentre cohort of 526 normotensive patients, high-sensitivity troponin T concentrations <14 pg/mL had a negative predictive value of 98% for excluding an adverse in-hospital clinical outcome.63 Age-adjusted high-sensitivity troponin T cut-off values (≥14 pg/mL for patients aged <75 years and ≥45 pg/mL for those ≥75 years) may further improve the negative predictive value of this biomarker.196
Heart-type fatty acid-binding protein (H-FABP), an early and sensitive marker of myocardial injury, provides prognostic information in acute PE, both in unselected198,199 and normotensive patients.200,201 In a meta-analysis investigating 1680 patients with PE, H-FABP concentrations ≥6 ng/mL were associated with an adverse short-term outcome (OR 17.7, 95% CI 6.0–51.9) and all-cause mortality (OR 32.9, 95% CI 8.8–123.2).202
5.3.2 Markers of right ventricular dysfunction
RV pressure overload due to acute PE is associated with increased myocardial stretch, which leads to the release of B-type natriuretic peptide (BNP) and N-terminal (NT)-proBNP. Thus, the plasma levels of natriuretic peptides reflect the severity of RV dysfunction and haemodynamic compromise in acute PE.203 A meta-analysis found that 51% of 1132 unselected patients with acute PE had elevated BNP or NT-proBNP concentrations on admission; these patients had a 10% risk of early death (95% CI 8.0–13%) and a 23% (95% CI 20–26%) risk of an adverse clinical outcome.204
Similar to cardiac troponins (see above), elevated BNP or NT-proBNP concentrations possess low specificity and positive predictive value (for early mortality) in normotensive patients with PE,205 but low levels of BNP or NT-proBNP are capable of excluding an unfavourable early clinical outcome, with high sensitivity and a negative predictive value.180 In this regard, an NT-proBNP cut-off value <500 pg/mL was used to select patients for home treatment in a multicentre management study.206 If emphasis is placed on increasing the prognostic specificity for an adverse early outcome, higher cut-off values ≥600 pg/mL might be more appropriate.207
5.3.3 Other laboratory biomarkers
Lactate is a marker of imbalance between tissue oxygen supply and demand, and consequently of severe PE with overt or imminent haemodynamic compromise. Elevated arterial plasma levels ≥2 mmol/L predict PE-related complications, both in unselected208 and in initially normotensive209,210 PE patients.
Elevated serum creatinine levels and a decreased (calculated) glomerular filtration rate are related to 30 day all-cause mortality in acute PE.211 Elevated neutrophil gelatinase-associated lipocalin and cystatin C, both indicating acute kidney injury, are also of prognostic value.212
A recent meta-analysis investigating 18 616 patients with acute PE found that hyponatraemia predicted in-hospital mortality (OR 5.6, 95% CI 3.4–9.1).213
Vasopressin is released upon endogenous stress, hypotension, and low CO. Its surrogate marker, copeptin, has been reported to be useful for risk stratification of patients with acute PE.214,215 In a single-centre derivation study investigating 268 normotensive PE patients, copeptin levels ≥24 pmol/L were associated with a 5.4-fold (95% CI 1.7–17.6) increased risk of an adverse outcome.216 These results were confirmed in 843 normotensive PE patients prospectively included in three European cohorts.217
5.4 Combined parameters and scores for assessment of pulmonary embolism severity
In patients who present without haemodynamic instability, individual baseline findings may not suffice to determine and further classify PE severity and PE-related early risk when used as stand-alone parameters. As a result, various combinations of the clinical, imaging, and laboratory parameters described above have been used to build prognostic scores, which permit a (semi)quantitative assessment of early PE-related risk of death. Of these, the Bova218–221 and the H-FABP (or high-sensitivity troponin T), Syncope, Tachycardia (FAST) scores219,222,223 have been validated in cohort studies (see Supplementary Data Table 4). However, their implications for patient management remain unclear. To date, only a combination of RV dysfunction on an echocardiogram (or CTPA) with a positive cardiac troponin test has directly been tested as a guide for early therapeutic decisions (anticoagulation plus reperfusion treatment vs. anticoagulation alone) in a large randomized controlled trial (RCT) of PE patients presenting without haemodynamic instability.224
5.5 Integration of aggravating conditions and comorbidity into risk assessment of acute pulmonary embolism
In addition to the clinical, imaging, and laboratory findings, which are directly linked to PE severity and PE-related early death, baseline parameters related to aggravating conditions and comorbidity are necessary to assess a patient’s overall mortality risk and early outcome. Of the clinical scores integrating PE severity and comorbidity, the Pulmonary Embolism Severity Index (PESI) (Table 7) is the one that has been most extensively validated to date.225–228 The principal strength of the PESI lies in the reliable identification of patients at low risk for 30 day mortality (PESI classes I and II). One randomized trial employed a low PESI as the principal inclusion criterion for home treatment of acute PE.178
In view of the complexity of the original PESI, which includes 11 differently weighed variables, a simplified version (sPESI; Table 7) has been developed and validated.229–231 As with the original version of the PESI, the strength of the sPESI lies in the reliable identification of patients at low risk for 30 day mortality. The prognostic performance of the sPESI has been confirmed in observational cohort studies,227,228 although this index has not yet been prospectively used to guide therapeutic management of low-risk PE patients.
Original and simplified Pulmonary Embolism Severity Index
| Parameter . | Original version226 . | Simplified version229 . |
|---|---|---|
| Age | Age in years | 1 point (if age >80 years) |
| Male sex | +10 points | – |
| Cancer | +30 points | 1 point |
| Chronic heart failure | +10 points | 1 point |
| Chronic pulmonary disease | +10 points | |
| Pulse rate ≥110 b.p.m. | +20 points | 1 point |
| Systolic BP <100 mmHg | +30 points | 1 point |
| Respiratory rate >30 breaths per min | +20 points | – |
| Temperature <36°C | +20 points | – |
| Altered mental status | +60 points | – |
| Arterial oxyhaemoglobin saturation <90% | +20 points | 1 point |
| Risk strataa | ||
|
| |
| ≥1 point(s) = 30 day mortality risk 10.9% (95% CI 8.5–13.2%) | |
| Parameter . | Original version226 . | Simplified version229 . |
|---|---|---|
| Age | Age in years | 1 point (if age >80 years) |
| Male sex | +10 points | – |
| Cancer | +30 points | 1 point |
| Chronic heart failure | +10 points | 1 point |
| Chronic pulmonary disease | +10 points | |
| Pulse rate ≥110 b.p.m. | +20 points | 1 point |
| Systolic BP <100 mmHg | +30 points | 1 point |
| Respiratory rate >30 breaths per min | +20 points | – |
| Temperature <36°C | +20 points | – |
| Altered mental status | +60 points | – |
| Arterial oxyhaemoglobin saturation <90% | +20 points | 1 point |
| Risk strataa | ||
|
| |
| ≥1 point(s) = 30 day mortality risk 10.9% (95% CI 8.5–13.2%) | |
BP = blood pressure; b.p.m. = beats per minute; CI = confidence interval.
Based on the sum of points.
Original and simplified Pulmonary Embolism Severity Index
| Parameter . | Original version226 . | Simplified version229 . |
|---|---|---|
| Age | Age in years | 1 point (if age >80 years) |
| Male sex | +10 points | – |
| Cancer | +30 points | 1 point |
| Chronic heart failure | +10 points | 1 point |
| Chronic pulmonary disease | +10 points | |
| Pulse rate ≥110 b.p.m. | +20 points | 1 point |
| Systolic BP <100 mmHg | +30 points | 1 point |
| Respiratory rate >30 breaths per min | +20 points | – |
| Temperature <36°C | +20 points | – |
| Altered mental status | +60 points | – |
| Arterial oxyhaemoglobin saturation <90% | +20 points | 1 point |
| Risk strataa | ||
|
| |
| ≥1 point(s) = 30 day mortality risk 10.9% (95% CI 8.5–13.2%) | |
| Parameter . | Original version226 . | Simplified version229 . |
|---|---|---|
| Age | Age in years | 1 point (if age >80 years) |
| Male sex | +10 points | – |
| Cancer | +30 points | 1 point |
| Chronic heart failure | +10 points | 1 point |
| Chronic pulmonary disease | +10 points | |
| Pulse rate ≥110 b.p.m. | +20 points | 1 point |
| Systolic BP <100 mmHg | +30 points | 1 point |
| Respiratory rate >30 breaths per min | +20 points | – |
| Temperature <36°C | +20 points | – |
| Altered mental status | +60 points | – |
| Arterial oxyhaemoglobin saturation <90% | +20 points | 1 point |
| Risk strataa | ||
|
| |
| ≥1 point(s) = 30 day mortality risk 10.9% (95% CI 8.5–13.2%) | |
BP = blood pressure; b.p.m. = beats per minute; CI = confidence interval.
Based on the sum of points.
The diagnosis of concomitant DVT has been identified as an adverse prognostic factor, being independently associated with death within the first 3 months after acute PE.232 In a meta-analysis investigating 8859 patients with PE, the presence of concomitant DVT was confirmed as a predictor of 30 day all-cause mortality (OR 1.9, 95% CI 1.5–2.4), although it did not predict PE-related adverse outcomes at 90 days.233 Thus, concomitant DVT can be regarded as an indicator of significant comorbidity in acute PE.
5.6 Prognostic assessment strategy
The classification of PE severity and the risk of early (in-hospital or 30 day) death is summarized in Table 8. Risk assessment of acute PE begins upon suspicion of the disease and initiation of the diagnostic workup. At this early stage, it is critical to identify patients with (suspected) high-risk PE. This clinical setting necessitates an emergency diagnostic algorithm (Figure 4) and immediate referral for reperfusion treatment, as explained in section 7, and displayed in Figure 6 and Supplementary Data Figure 1. Testing for laboratory biomarkers such as cardiac troponins or natriuretic peptides is not necessary for immediate therapeutic decisions in patients with high-risk PE.
Classification of pulmonary embolism severity and the risk of early (in-hospital or 30 day) death
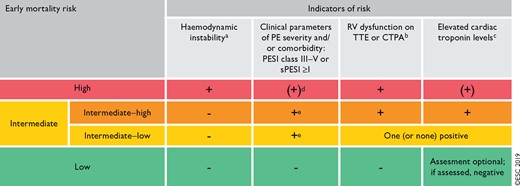 |
 |
BP = blood pressure; CTPA = computed tomography pulmonary angiography; H-FABP = heart-type fatty acid-binding protein; NT-proBNP = N-terminal pro B-type natriuretic peptide; PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; sPESI = simplified Pulmonary Embolism Severity Index; TTE = transthoracic echocardiogram.
One of the following clinical presentations (Table 4): cardiac arrest, obstructive shock (systolic BP <90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite an adequate filling status, in combination with end-organ hypoperfusion), or persistent hypotension (systolic BP <90 mmHg or a systolic BP drop ≥40 mmHg for >15 min, not caused by new-onset arrhythmia, hypovolaemia, or sepsis).
Prognostically relevant imaging (TTE or CTPA) findings in patients with acute PE, and the corresponding cut-off levels, are graphically presented in Figure 3, and their prognostic value is summarized in Supplementary Data Table 3.
Elevation of further laboratory biomarkers, such as NT-proBNP ≥600 ng/L, H-FABP ≥6 ng/mL, or copeptin ≥24 pmol/L, may provide additional prognostic information. These markers have been validated in cohort studies but they have not yet been used to guide treatment decisions in randomized controlled trials.
Haemodynamic instability, combined with PE confirmation on CTPA and/or evidence of RV dysfunction on TTE, is sufficient to classify a patient into the high-risk PE category. In these cases, neither calculation of the PESI nor measurement of troponins or other cardiac biomarkers is necessary.
Signs of RV dysfunction on TTE (or CTPA) or elevated cardiac biomarker levels may be present, despite a calculated PESI of I–II or an sPESI of 0.234 Until the implications of such discrepancies for the management of PE are fully understood, these patients should be classified into the intermediate-risk category.
Classification of pulmonary embolism severity and the risk of early (in-hospital or 30 day) death
 |
 |
BP = blood pressure; CTPA = computed tomography pulmonary angiography; H-FABP = heart-type fatty acid-binding protein; NT-proBNP = N-terminal pro B-type natriuretic peptide; PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; sPESI = simplified Pulmonary Embolism Severity Index; TTE = transthoracic echocardiogram.
One of the following clinical presentations (Table 4): cardiac arrest, obstructive shock (systolic BP <90 mmHg or vasopressors required to achieve a BP ≥90 mmHg despite an adequate filling status, in combination with end-organ hypoperfusion), or persistent hypotension (systolic BP <90 mmHg or a systolic BP drop ≥40 mmHg for >15 min, not caused by new-onset arrhythmia, hypovolaemia, or sepsis).
Prognostically relevant imaging (TTE or CTPA) findings in patients with acute PE, and the corresponding cut-off levels, are graphically presented in Figure 3, and their prognostic value is summarized in Supplementary Data Table 3.
Elevation of further laboratory biomarkers, such as NT-proBNP ≥600 ng/L, H-FABP ≥6 ng/mL, or copeptin ≥24 pmol/L, may provide additional prognostic information. These markers have been validated in cohort studies but they have not yet been used to guide treatment decisions in randomized controlled trials.
Haemodynamic instability, combined with PE confirmation on CTPA and/or evidence of RV dysfunction on TTE, is sufficient to classify a patient into the high-risk PE category. In these cases, neither calculation of the PESI nor measurement of troponins or other cardiac biomarkers is necessary.
Signs of RV dysfunction on TTE (or CTPA) or elevated cardiac biomarker levels may be present, despite a calculated PESI of I–II or an sPESI of 0.234 Until the implications of such discrepancies for the management of PE are fully understood, these patients should be classified into the intermediate-risk category.
In the absence of haemodynamic instability at presentation, further risk stratification of PE is recommended, as it has implications for early discharge vs. hospitalization or monitoring of the patient (explained in section 7). Table 8 provides an overview of the clinical, imaging, and laboratory parameters used to distinguish intermediate- and low-risk PE. The PESI is—in its original or simplified form—the most extensively validated and most broadly used clinical score to date, as it integrates baseline indicators of the severity of the acute PE episode with aggravating conditions and the comorbidity of the patient. Overall, a PESI of class I–II or an sPESI of 0 is a reliable predictor of low-risk PE.
In addition to clinical parameters, patients in the intermediate-risk group who display evidence of both RV dysfunction (on echocardiography or CTPA) and elevated cardiac biomarker levels in the circulation (particularly a positive cardiac troponin test) are classified into the intermediate−high-risk category. As will be discussed in more detail in section 7, close monitoring is recommended in these cases to permit the early detection of haemodynamic decompensation or collapse, and consequently the need for rescue reperfusion therapy.179 Patients in whom the RV appears normal on echocardiography or CTPA, and/or who have normal cardiac biomarker levels, belong to the intermediate−low-risk category. As an alternative approach, use of further prognostic scores combining clinical, imaging, and laboratory parameters may be considered to semi-quantitatively assess the severity of the PE episode, and distinguish intermediate−high-risk and intermediate−low-risk PE. Supplementary Data Table 4 lists the scores most frequently investigated for this purpose in observational (cohort) studies; however, none of them has been used in RCTs to date.
A recent meta-analysis included 21 cohort studies with a total of 3295 patients with ‘low-risk’ PE based on a PESI of I–II or an sPESI of 0.234 Overall, 34% (95% CI 30–39%) of them were reported to have signs of RV dysfunction on echocardiography or CTPA. Data on early mortality were provided in seven studies (1597 patients) and revealed an OR of 4.19 (95% CI 1.39–12.58) for death from any cause in the presence of RV dysfunction; elevated cardiac troponin levels were associated with a comparable magnitude of risk elevation.234 Early all-cause mortality rates (1.8% for RV dysfunction and 3.8% for elevated troponin levels234) were in the lower range of those previously reported for patients with intermediate-risk PE.235 Until the clinical implications of such discrepancies are clarified, patients with signs of RV dysfunction or elevated cardiac biomarkers, despite a low PESI or an sPESI of 0, should be classified into the intermediate−low-risk category.
5.7 Recommendations for prognostic assessment
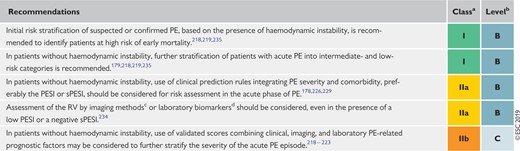 |
 |
PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricle; sPESI = simplified Pulmonary Embolism Severity Index.
Class of recommendation.
Level of evidence.
Transthoracic echocardiography or computed tomography pulmonary angiography.
Cardiac troponins or natriuretic peptides.
 |
 |
PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricle; sPESI = simplified Pulmonary Embolism Severity Index.
Class of recommendation.
Level of evidence.
Transthoracic echocardiography or computed tomography pulmonary angiography.
Cardiac troponins or natriuretic peptides.
6 Treatment in the acute phase
6.1 Haemodynamic and respiratory support
6.1.1 Oxygen therapy and ventilation
Hypoxaemia is one of the features of severe PE, and is mostly due to the mismatch between ventilation and perfusion. Administration of supplemental oxygen is indicated in patients with PE and SaO2 <90%. Severe hypoxaemia/respiratory failure that is refractory to conventional oxygen supplementation could be explained by right-to-left shunt through a patent foramen ovale or atrial septal defect.67 Further oxygenation techniques should also be considered, including high-flow oxygen (i.e. a high-flow nasal cannula)236,237 and mechanical ventilation (non-invasive or invasive) in cases of extreme instability (i.e. cardiac arrest), taking into consideration that correction of hypoxaemia will not be possible without simultaneous pulmonary reperfusion.
Patients with RV failure are frequently hypotensive or are highly susceptible to the development of severe hypotension during induction of anaesthesia, intubation, and positive-pressure ventilation. Consequently, intubation should be performed only if the patient is unable to tolerate or cope with non-invasive ventilation. When feasible, non-invasive ventilation or oxygenation through a high-flow nasal cannula should be preferred; if mechanical ventilation is used, care should be taken to limit its adverse haemodynamic effects. In particular, positive intrathoracic pressure induced by mechanical ventilation may reduce venous return and worsen low CO due to RV failure in patients with high-risk PE; therefore, positive end-expiratory pressure should be applied with caution. Tidal volumes of approximately 6 mL/kg lean body weight should be used in an attempt to keep the end-inspiratory plateau pressure <30 cm H2O. If intubation is needed, anaesthetic drugs more prone to cause hypotension should be avoided for induction.
6.1.2 Pharmacological treatment of acute right ventricular failure
Acute RV failure with resulting low systemic output is the leading cause of death in patients with high-risk PE. The principles of acute right heart failure management have been reviewed in a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the ESC.68 An overview of the current treatment options for acute RV failure is provided in Table 9.
Treatment of right ventricular failure in acute high-risk pulmonary embolism
| Strategy . | Properties and use . | Caveats . |
|---|---|---|
| Volume optimization | ||
| Cautious volume loading, saline, or Ringer's lactate, ≤500 mL over 15–30 min | Consider in patients with normal–low central venous pressure (due, for example, to concomitant hypovolaemia) | Volume loading can over-distend the RV, worsen ventricular interdependence, and reduce CO239 |
| Vasopressors and inotropes | ||
| Norepinephrine, 0.2–1.0 µg/kg/mina 240 | Increases RV inotropy and systemic BP, promotes positive ventricular interactions, and restores coronary perfusion gradient | Excessive vasoconstriction may worsen tissue perfusion |
| Dobutamine, 2–20 µg/kg/min241 | Increases RV inotropy, lowers filling pressures | May aggravate arterial hypotension if used alone, without a vasopressor; may trigger or aggravate arrhythmias |
| Mechanical circulatory support | ||
| Veno–arterial ECMO/extracorporeal life support251,252,258 | Rapid short-term support combined with oxygenator | Complications with use over longer periods (>5–10 days), including bleeding and infections; no clinical benefit unless combined with surgical embolectomy; requires an experienced team |
| Strategy . | Properties and use . | Caveats . |
|---|---|---|
| Volume optimization | ||
| Cautious volume loading, saline, or Ringer's lactate, ≤500 mL over 15–30 min | Consider in patients with normal–low central venous pressure (due, for example, to concomitant hypovolaemia) | Volume loading can over-distend the RV, worsen ventricular interdependence, and reduce CO239 |
| Vasopressors and inotropes | ||
| Norepinephrine, 0.2–1.0 µg/kg/mina 240 | Increases RV inotropy and systemic BP, promotes positive ventricular interactions, and restores coronary perfusion gradient | Excessive vasoconstriction may worsen tissue perfusion |
| Dobutamine, 2–20 µg/kg/min241 | Increases RV inotropy, lowers filling pressures | May aggravate arterial hypotension if used alone, without a vasopressor; may trigger or aggravate arrhythmias |
| Mechanical circulatory support | ||
| Veno–arterial ECMO/extracorporeal life support251,252,258 | Rapid short-term support combined with oxygenator | Complications with use over longer periods (>5–10 days), including bleeding and infections; no clinical benefit unless combined with surgical embolectomy; requires an experienced team |
CO = cardiac output; BP = blood pressure; ECMO = extracorporeal membrane oxygenation; RV = right ventricle/ventricular.
Epinephrine is used in cardiac arrest.
Treatment of right ventricular failure in acute high-risk pulmonary embolism
| Strategy . | Properties and use . | Caveats . |
|---|---|---|
| Volume optimization | ||
| Cautious volume loading, saline, or Ringer's lactate, ≤500 mL over 15–30 min | Consider in patients with normal–low central venous pressure (due, for example, to concomitant hypovolaemia) | Volume loading can over-distend the RV, worsen ventricular interdependence, and reduce CO239 |
| Vasopressors and inotropes | ||
| Norepinephrine, 0.2–1.0 µg/kg/mina 240 | Increases RV inotropy and systemic BP, promotes positive ventricular interactions, and restores coronary perfusion gradient | Excessive vasoconstriction may worsen tissue perfusion |
| Dobutamine, 2–20 µg/kg/min241 | Increases RV inotropy, lowers filling pressures | May aggravate arterial hypotension if used alone, without a vasopressor; may trigger or aggravate arrhythmias |
| Mechanical circulatory support | ||
| Veno–arterial ECMO/extracorporeal life support251,252,258 | Rapid short-term support combined with oxygenator | Complications with use over longer periods (>5–10 days), including bleeding and infections; no clinical benefit unless combined with surgical embolectomy; requires an experienced team |
| Strategy . | Properties and use . | Caveats . |
|---|---|---|
| Volume optimization | ||
| Cautious volume loading, saline, or Ringer's lactate, ≤500 mL over 15–30 min | Consider in patients with normal–low central venous pressure (due, for example, to concomitant hypovolaemia) | Volume loading can over-distend the RV, worsen ventricular interdependence, and reduce CO239 |
| Vasopressors and inotropes | ||
| Norepinephrine, 0.2–1.0 µg/kg/mina 240 | Increases RV inotropy and systemic BP, promotes positive ventricular interactions, and restores coronary perfusion gradient | Excessive vasoconstriction may worsen tissue perfusion |
| Dobutamine, 2–20 µg/kg/min241 | Increases RV inotropy, lowers filling pressures | May aggravate arterial hypotension if used alone, without a vasopressor; may trigger or aggravate arrhythmias |
| Mechanical circulatory support | ||
| Veno–arterial ECMO/extracorporeal life support251,252,258 | Rapid short-term support combined with oxygenator | Complications with use over longer periods (>5–10 days), including bleeding and infections; no clinical benefit unless combined with surgical embolectomy; requires an experienced team |
CO = cardiac output; BP = blood pressure; ECMO = extracorporeal membrane oxygenation; RV = right ventricle/ventricular.
Epinephrine is used in cardiac arrest.
If the central venous pressure is low, modest (≤500 mL) fluid challenge can be used as it may increase the cardiac index in patients with acute PE.238 However, volume loading has the potential to over-distend the RV and ultimately cause a reduction in systemic CO.239 Experimental studies suggest that aggressive volume expansion is of no benefit and may even worsen RV function.240 Cautious volume loading may be appropriate if low arterial pressure is combined with an absence of elevated filling pressures. Assessment of central venous pressure by ultrasound imaging of the IVC (a small and/or collapsible IVC in the setting of acute high-risk PE indicates low volume status) or, alternatively, by central venous pressure monitoring may help guide volume loading. If signs of elevated central venous pressure are observed, further volume loading should be withheld.
Use of vasopressors is often necessary, in parallel with (or while waiting for) pharmacological, surgical, or interventional reperfusion treatment. Norepinephrine can improve systemic haemodynamics by bringing about an improvement in ventricular systolic interaction and coronary perfusion, without causing a change in PVR.240 Its use should be limited to patients in cardiogenic shock. Based on the results of a small series, the use of dobutamine may be considered for patients with PE, a low cardiac index, and normal BP; however, raising the cardiac index may aggravate the ventilation/perfusion mismatch by further redistributing flow from (partly) obstructed to unobstructed vessels.241 Although experimental data suggest that levosimendan may restore RV–pulmonary arterial coupling in acute PE by combining pulmonary vasodilation with an increase in RV contractility,242 no evidence of clinical benefit is available.
Vasodilators decrease PAP and PVR, but may worsen hypotension and systemic hypoperfusion due to their lack of specificity for the pulmonary vasculature after systemic [intravenous (i.v.)] administration. Although small clinical studies have suggested that inhalation of nitric oxide may improve the haemodynamic status and gas exchange of patients with PE,243–245 no evidence for its clinical efficacy or safety is available to date.246
6.1.3 Mechanical circulatory support and oxygenation
The temporary use of mechanical cardiopulmonary support, mostly with veno–arterial extracorporeal membrane oxygenation (ECMO), may be helpful in patients with high-risk PE, and circulatory collapse or cardiac arrest. Survival of critically ill patients has been described in a number of case series,247–252 but no RCTs testing the efficacy and safety of these devices in the setting of high-risk PE have been conducted to date. Use of ECMO is associated with a high incidence of complications, even when used for short periods, and the results depend on the experience of the centre as well as patient selection. The increased risk of bleeding related to the need for vascular access should be considered, particularly in patients undergoing thrombolysis. At present, the use of ECMO as a stand-alone technique with anticoagulation is controversial247,252 and additional therapies, such as surgical embolectomy, have to be considered.
A few cases suggesting good outcomes with use of the Impella® catheter in patients in shock caused by acute PE have been reported.253,254
6.1.4 Advanced life support in cardiac arrest
Acute PE is part of the differential diagnosis of cardiac arrest with non-shockable rhythm against a background of pulseless electrical activity. In cardiac arrest presumably caused by acute PE, current guidelines for advanced life support should be followed.255,256 The decision to treat for acute PE must be taken early, when a good outcome is still possible. Thrombolytic therapy should be considered; once a thrombolytic drug is administered, cardiopulmonary resuscitation should be continued for at least 60–90 min before terminating resuscitation attempts.257
6.2 Initial anticoagulation
6.2.1 Parenteral anticoagulation
In patients with high or intermediate clinical probability of PE (see section 4), anticoagulation should be initiated while awaiting the results of diagnostic tests. This is usually done with subcutaneous, weight-adjusted low-molecular weight heparin (LMWH) or fondaparinux (Supplementary Data Table 5), or i.v. unfractionated heparin (UFH). Based on pharmacokinetic data (Supplementary Data Table 6),259 an equally rapid anticoagulant effect can also be achieved with a non-vitamin K antagonist oral anticoagulant (NOAC), and phase III clinical trials have demonstrated the non-inferior efficacy of a single-oral drug anticoagulation strategy using higher doses of apixaban for 7 days or rivaroxaban for 3 weeks.259–261
LMWH and fondaparinux are preferred over UFH for initial anticoagulation in PE, as they carry a lower risk of inducing major bleeding and heparin-induced thrombocytopenia.262–265 Neither LMWH nor fondaparinux need routine monitoring of anti-Xa levels. Use of UFH is nowadays largely restricted to patients with overt haemodynamic instability or imminent haemodynamic decompensation in whom primary reperfusion treatment will be necessary. UFH is also recommended for patients with serious renal impairment [creatinine clearance (CrCl) ≤30 mL/min] or severe obesity. If LMWH is prescribed in patients with CrCl 15 − 30 mL/min, an adapted dosing scheme should be used. The dosing of UFH is adjusted based on the activated partial thromboplastin time (Supplementary Data Table 7).266
6.2.2 Non-vitamin K antagonist oral anticoagulants
NOACs are small molecules that directly inhibit one activated coagulation factor, which is thrombin for dabigatran and factor Xa for apixaban, edoxaban, and rivaroxaban. The characteristics of NOACs used in the treatment of acute PE are summarized in Supplementary Data Table 6. Owing to their predictable bioavailability and pharmacokinetics, NOACs can be given at fixed doses without routine laboratory monitoring. Compared with vitamin K antagonists (VKAs), there are fewer interactions when NOACs are given concomitantly with other drugs.259 In the phase III VTE trials, the dosages of dabigatran, rivaroxaban, and apixaban were not reduced in patients with mild–moderate renal dysfunction (CrCl between 30–60 mL/min), whereas edoxaban was given at a 30 mg dose in these patients. Patients with CrCl <25 mL/min were excluded from the trials testing apixaban, whereas patients with CrCl <30 mL/min were excluded from those investigating rivaroxaban, edoxaban, and dabigatran (Supplementary Data Table 8).
Phase III trials on the treatment of acute VTE (Supplementary Data Table 8), as well as those on extended treatment beyond the first 6 months (see section 8), demonstrated the non-inferiority of NOACs compared with the combination of LMWH with VKA for the prevention of symptomatic or lethal VTE recurrence, along with significantly reduced rates of major bleeding.267 The different drug regimens tested in these trials are displayed in Supplementary Data Table 8. In a meta-analysis, the incidence rate of the primary efficacy outcome was 2.0% for NOAC-treated patients and 2.2% for VKA-treated patients [relative risk (RR) 0.88, 95% CI 0.74–1.05].268 Major bleeding occurred in 1.1% of NOAC-treated patients and 1.7% of VKA-treated patients for an RR of 0.60 (95% CI 0.41–0.88). Compared with VKA-treated patients, critical site major bleeding occurred less frequently in NOAC-treated patients (RR 0.38, 95% CI 0.23– 0.62); in particular, there was a significant reduction in intracranial bleeding (RR 0.37, 95% CI 0.21–0.68) and in fatal bleeding (RR 0.36, 95% CI 0.15–0.87) with NOACs compared with VKAs.268
Suggestions for the anticoagulation management of PE in specific clinical situations, for which conclusive evidence is lacking, are presented in Supplementary Data Table 9.
Practical guidance for clinicians regarding the handling of NOACs and the management of emergency situations related to their use are regularly updated by the European Heart Rhythm Association.259
6.2.3 Vitamin K antagonists
VKAs have been the gold standard in oral anticoagulation for more than 50 years. When VKAs are used, anticoagulation with UFH, LMWH, or fondaparinux should be continued in parallel with the oral anticoagulant for ≥5 days and until the international normalized ratio (INR) value has been 2.0–3.0 for 2 consecutive days. Warfarin may be started at a dose of 10 mg in younger (e.g. aged <60 years) otherwise healthy patients and at a dose ≤5 mg in older patients.269 The daily dose is adjusted according to the INR over the next 5–7 days, aiming for an INR level of 2.0–3.0. Pharmacogenetic testing may increase the precision of warfarin dosing.270,271 When used in addition to clinical parameters, pharmacogenetic testing improves anticoagulation control and may be associated with a reduced risk of bleeding, but does not reduce the risk of thromboembolic events or mortality.272
The implementation of a structured anticoagulant service (most commonly, anticoagulant clinics) appears to be associated with increased time in the therapeutic range and improved clinical outcome, compared with control of anticoagulation by the general practitioner.273,274 Finally, in patients who are selected and appropriately trained, self-monitoring of VKA is associated with fewer thrombo-embolic events and increased time in the therapeutic range compared with usual care.275
6.3 Reperfusion treatment
6.3.1 Systemic thrombolysis
Thrombolytic therapy leads to faster improvements in pulmonary obstruction, PAP, and PVR in patients with PE, compared with UFH alone; these improvements are accompanied by a reduction in RV dilation on echocardiography.276–279 The greatest benefit is observed when treatment is initiated within 48 h of symptom onset, but thrombolysis can still be useful in patients who have had symptoms for 6–14 days.280 Unsuccessful thrombolysis, as judged by persistent clinical instability and unchanged RV dysfunction on echocardiography after 36 h, has been reported in 8% of high-risk PE patients.281
A meta-analysis of thrombolysis trials that included (but were not confined to) patients with high-risk PE, defined mainly as the presence of cardiogenic shock, indicated a significant reduction in the combined outcome of mortality and recurrent PE (Supplementary Data Table 10). This was achieved with a 9.9% rate of severe bleeding and a 1.7% rate of intracranial haemorrhage.282
In normotensive patients with intermediate-risk PE, defined as the presence of RV dysfunction and elevated troponin levels, the impact of thrombolytic treatment was investigated in the Pulmonary Embolism Thrombolysis (PEITHO) trial.179 Thrombolytic therapy was associated with a significant reduction in the risk of haemodynamic decompensation or collapse, but this was paralleled by an increased risk of severe extracranial and intracranial bleeding.179 In the PEITHO trial, 30 day death rates were low in both treatment groups, although meta-analyses have suggested a reduction in PE-related and overall mortality of as much as 50–60% following thrombolytic treatment in the intermediate-risk category (Supplementary Data Table 10).282,283
The approved regimens and doses of thrombolytic agents for PE, as well as the contraindications to this type of treatment, are shown in Table 10. Accelerated i.v. administration of recombinant tissue-type plasminogen activator (rtPA; 100 mg over 2 h) is preferable to prolonged infusions of first-generation thrombolytic agents (streptokinase and urokinase). Preliminary reports on the efficacy and safety of reduced-dose rtPA284,285 need confirmation by solid evidence before any recommendations can be made in this regard. UFH may be administered during continuous infusion of alteplase, but should be discontinued during infusion of streptokinase or urokinase.65 Reteplase,286 desmoteplase,287 or tenecteplase179,278,279 have also been investigated; at present, none of these agents are approved for use in acute PE.
Thrombolytic regimens, doses, and contraindications
| Molecule . | Regimen . | Contraindications to fibrinolysis . |
|---|---|---|
| rtPA | 100 mg over 2 h |
|
| 0.6 mg/kg over 15 min (maximum dose 50 mg)a | ||
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h | |
| Accelerated regimen: 1.5 million IU over 2 h | ||
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h | |
| Accelerated regimen: 3 million IU over 2 h |
| Molecule . | Regimen . | Contraindications to fibrinolysis . |
|---|---|---|
| rtPA | 100 mg over 2 h |
|
| 0.6 mg/kg over 15 min (maximum dose 50 mg)a | ||
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h | |
| Accelerated regimen: 1.5 million IU over 2 h | ||
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h | |
| Accelerated regimen: 3 million IU over 2 h |
BP = blood pressure; IU = international units; rtPA, recombinant tissue-type plasminogen activator.
This is the accelerated regimen for rtPA in pulmonary embolism; it is not officially approved, but it is sometimes used in extreme haemodynamic instability such as cardiac arrest.
Thrombolytic regimens, doses, and contraindications
| Molecule . | Regimen . | Contraindications to fibrinolysis . |
|---|---|---|
| rtPA | 100 mg over 2 h |
|
| 0.6 mg/kg over 15 min (maximum dose 50 mg)a | ||
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h | |
| Accelerated regimen: 1.5 million IU over 2 h | ||
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h | |
| Accelerated regimen: 3 million IU over 2 h |
| Molecule . | Regimen . | Contraindications to fibrinolysis . |
|---|---|---|
| rtPA | 100 mg over 2 h |
|
| 0.6 mg/kg over 15 min (maximum dose 50 mg)a | ||
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h | |
| Accelerated regimen: 1.5 million IU over 2 h | ||
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h | |
| Accelerated regimen: 3 million IU over 2 h |
BP = blood pressure; IU = international units; rtPA, recombinant tissue-type plasminogen activator.
This is the accelerated regimen for rtPA in pulmonary embolism; it is not officially approved, but it is sometimes used in extreme haemodynamic instability such as cardiac arrest.
It remains unclear whether early thrombolysis for (intermediate- or high-risk) acute PE has an impact on clinical symptoms, functional limitation, or CTEPH at long-term follow-up. A small randomized trial of 83 patients suggested that thrombolysis might improve functional capacity at 3 months compared with anticoagulation alone.278 In the PEITHO trial,179 mild persisting symptoms, mainly dyspnoea, were present in 33% of the patients at long-term (at 41.6 ± 15.7 months) clinical follow-up.288 However, the majority of patients (85% in the tenecteplase arm and 96% in the placebo arm) had a low or intermediate probability—based on the ESC Guidelines definition289—of persisting or new-onset PH at echocardiographic follow-up.288 Consequently, the findings of this study do not support a role for thrombolysis with the aim of preventing long-term sequelae (section 10) after intermediate-risk PE, although they are limited by the fact that clinical follow-up was available for only 62% of the study population.
6.3.2 Percutaneous catheter-directed treatment
Mechanical reperfusion is based on the insertion of a catheter into the pulmonary arteries via the femoral route. Different types of catheters (summarized in Supplementary Data Table 11) are used for mechanical fragmentation, thrombus aspiration, or more commonly a pharmacomechanical approach combining mechanical or ultrasound fragmentation of the thrombus with in situ reduced-dose thrombolysis.
Most knowledge about catheter-based embolectomy is derived from registries and pooled results from case series.290,291 The overall procedural success rates (defined as haemodynamic stabilization, correction of hypoxia, and survival to hospital discharge) of percutaneous catheter-based therapies reported in these studies have reached 87%;292 however, these results may be subject to publication bias. One RCT compared conventional heparin-based treatment and a catheter-based therapy combining ultrasound-based clot fragmentation with low-dose in situ thrombolysis in 59 patients with intermediate-risk PE. In that study, ultrasound-assisted thrombolysis was associated with a larger decrease in the RV/LV diameter ratio at 24 h, without an increased risk of bleeding.293 Data from two prospective cohort studies294,295 and a registry,296 with a total of 352 patients, support the improvement in RV function, lung perfusion, and PAP in patients with intermediate- or high-risk PE using this technique. Intracranial haemorrhage was rare, although the rate of Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) severe and moderate bleeding complications was 10% in one of these cohorts.294 These results should be interpreted with caution, considering the relatively small numbers of patients treated, the lack of studies directly comparing catheter-directed with systemic thrombolytic therapy, and the lack of data from RCTs on clinical efficacy outcomes.
6.3.3 Surgical embolectomy
Surgical embolectomy in acute PE is usually carried out with cardiopulmonary bypass, without aortic cross-clamping and cardioplegic cardiac arrest, followed by incision of the two main pulmonary arteries with the removal or suction of fresh clots. Recent reports have indicated favourable surgical results in high-risk PE, with or without cardiac arrest, and in selected cases of intermediate-risk PE.297–300 Among 174 322 patients hospitalized between 1999 and 2013 with a diagnosis of PE in New York state, survival and recurrence rates were compared between patients who underwent thrombolysis (n = 1854) or surgical embolectomy (n = 257) as first-line therapy.297 Overall, there was no difference between the two types of reperfusion treatment regarding 30 day mortality (15 and 13%, respectively), but thrombolysis was associated with a higher risk of stroke and re-intervention at 30 days. No difference was found in terms of 5 year actuarial survival, but thrombolytic therapy was associated with a higher rate of recurrent PE requiring readmission compared with surgery (7.9 vs. 2.8%). However, the two treatments were not randomly allocated in this observational retrospective study, and the patients referred for surgery may have been selected. An analysis of the Society of Thoracic Surgery Database with multicentre data collection, including 214 patients submitted for surgical embolectomy for high- (n = 38) or intermediate-risk (n = 176) PE, revealed an in-hospital mortality rate of 12%, with the worst outcome (32%) in the group experiencing pre-operative cardiac arrest.299
Recent experience appears to support combining ECMO with surgical embolectomy, particularly in patients with high-risk PE with or without the need for cardiopulmonary resuscitation. Among patients who presented with intermediate-risk PE (n = 28), high-risk PE without cardiac arrest (n = 18), and PE with cardiac arrest (n = 9), the in-hospital and 1 year survival rates were 93 and 91%, respectively.300
6.4 Multidisciplinary pulmonary embolism teams
The concept of multidisciplinary rapid-response teams for the management of ‘severe’ (high-risk and selected cases of intermediate-risk) PE emerged in the USA, with increasing acceptance by the medical community and implementation in hospitals in Europe and worldwide. Set-up of PE response teams (PERTs) is encouraged, as they address the needs of modern systems-based healthcare.301 A PERT brings together a team of specialists from different disciplines including, for example, cardiology, pulmonology, haematology, vascular medicine, anaesthesiology/intensive care, cardiothoracic surgery, and (interventional) radiology. The team convenes in real time (face-to-face or via web conference) to enhance clinical decision-making. This allows the formulation of a treatment plan and facilitates its immediate implementation.301 The exact composition and operating mode of a PERT are not fixed, depending on the resources and expertise available in each hospital for the management of acute PE.
6.5 Vena cava filters
The aim of vena cava interruption is to mechanically prevent venous clots from reaching the pulmonary circulation. Most devices in current use are inserted percutaneously and can be retrieved after several weeks or months, or left in place over the long-term, if needed. Potential indications include VTE and absolute contraindication to anticoagulant treatment, recurrent PE despite adequate anticoagulation, and primary prophylaxis in patients with a high risk of VTE. Other potential indications for filter placement, including free-floating thrombi, have not been confirmed in patients without contraindications to therapeutic anticoagulation.
Only two phase III randomized trials have compared anticoagulation with or without vena cava interruption in patients with proximal DVT, with or without associated PE.302–304 In the Prevention of Recurrent Pulmonary Embolism by Vena Cava Interruption (PREPIC) study, insertion of a permanent vena cava filter was associated with a significant reduction in the risk of recurrent PE and a significant increase in the risk of DVT, without a significant difference in the risk of recurrent VTE or death.303,304 The PREPIC-2 trial randomized 399 patients with PE and venous thrombosis to receive anticoagulant treatment, with or without a retrievable vena cava filter. In this study, the rate of recurrent VTE was low in both groups and did not differ between groups.302 A systematic review and meta-analysis of published reports on the efficacy and safety of vena cava filters included 11 studies, with a total of 2055 patients who received a filter vs. 2149 controls.305 Vena cava filter placement was associated with a 50% decrease in the incidence of PE and an ∼70% increase in the risk of DVT over time. Neither all-cause mortality nor PE-related mortality differed between patients with or without filter placement.
The broad indication for placement of a venous filter in patients with recent (<1 month) proximal DVT and an absolute contraindication to anticoagulant treatment is based mainly on the perceived high risk of recurrent PE in this setting, and the lack of other treatment options.
Complications associated with vena cava filters are common and can be serious. A systematic literature review revealed penetration of the venous wall in 1699 (19%) of 9002 procedures; of these cases, 19% showed adjacent organ involvement and ≥8% were symptomatic.306 Lethal complications were rare (only two cases), but 5% of the patients required major interventions such as surgical removal of the filter, endovascular stent placement or embolization, endovascular retrieval of the permanent filter, or percutaneous nephrostomy or ureteral stent placement.306 Further reported complications include filter fracture and/or embolization, and DVT occasionally extending up to the vena cava.303,307,308
6.6 Recommendations for acute-phase treatment of high-risk pulmonary embolisma
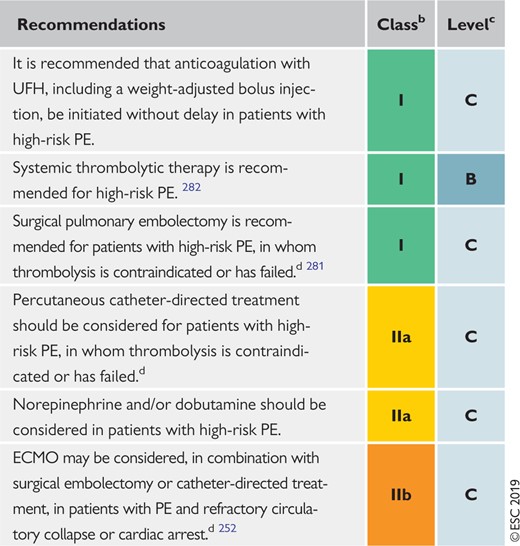 |
 |
ECMO = extracorporeal membrane oxygenation; PE = pulmonary embolism; UFH = unfractionated heparin.
See Table 4 for definition of high-risk PE. After haemodynamic stabilization of the patient, continue with anticoagulation treatment as in intermediate- or low-risk PE (section 6.7).
Class of recommendation.
Level of evidence.
If appropriate expertise and resources are available on-site.
 |
 |
ECMO = extracorporeal membrane oxygenation; PE = pulmonary embolism; UFH = unfractionated heparin.
See Table 4 for definition of high-risk PE. After haemodynamic stabilization of the patient, continue with anticoagulation treatment as in intermediate- or low-risk PE (section 6.7).
Class of recommendation.
Level of evidence.
If appropriate expertise and resources are available on-site.
6.7 Recommendations for acute-phase treatment of intermediate- or low-risk pulmonary embolism
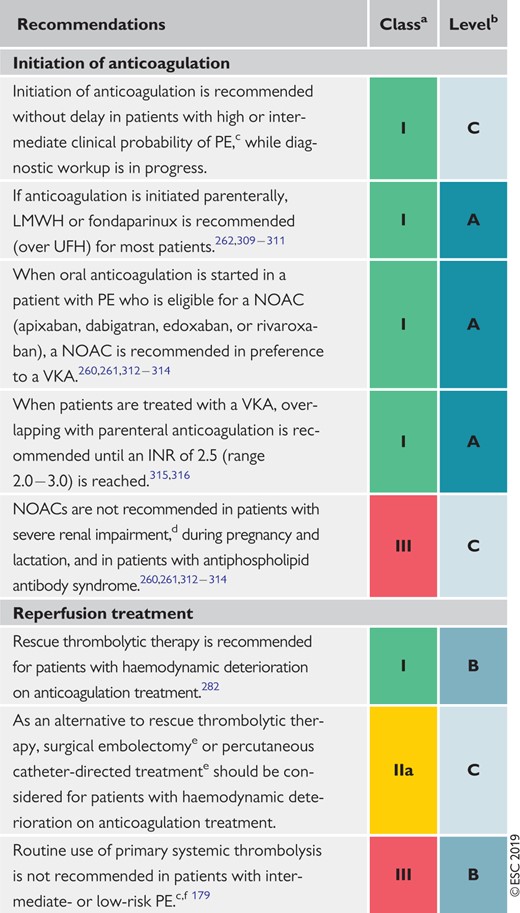 |
 |
CrCl = creatinine clearance; INR = international normalized ratio; LMWH = low-molecular weight heparin; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; UFH = unfractionated heparin; VKA = vitamin K antagonist.
Class of recommendation.
Level of evidence.
See Table 8 for definition of the PE severity and PE-related risk.
Dabigatran is not recommended in patients with CrCl <30 mL/min. Edoxaban should be given at a dose of 30 mg once daily in patients with CrCl of 15 − 50 mL/min and is not recommended in patients with CrCl <15 mL/min. Rivaroxaban and apixaban are to be used with caution in patients with CrCl 15 − 29 mL/min, and their use is not recommended in patients with CrCl <15 mL/min.
If appropriate expertise and resources are available on-site.
The risk‐to‐benefit ratios of surgical embolectomy or catheter‐directed procedures have not yet been established in intermediate- or low‐risk PE.
 |
 |
CrCl = creatinine clearance; INR = international normalized ratio; LMWH = low-molecular weight heparin; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; UFH = unfractionated heparin; VKA = vitamin K antagonist.
Class of recommendation.
Level of evidence.
See Table 8 for definition of the PE severity and PE-related risk.
Dabigatran is not recommended in patients with CrCl <30 mL/min. Edoxaban should be given at a dose of 30 mg once daily in patients with CrCl of 15 − 50 mL/min and is not recommended in patients with CrCl <15 mL/min. Rivaroxaban and apixaban are to be used with caution in patients with CrCl 15 − 29 mL/min, and their use is not recommended in patients with CrCl <15 mL/min.
If appropriate expertise and resources are available on-site.
The risk‐to‐benefit ratios of surgical embolectomy or catheter‐directed procedures have not yet been established in intermediate- or low‐risk PE.
6.8 Recommendations for multidisciplinary pulmonary embolism teams
6.9 Recommendations for inferior vena cava filters
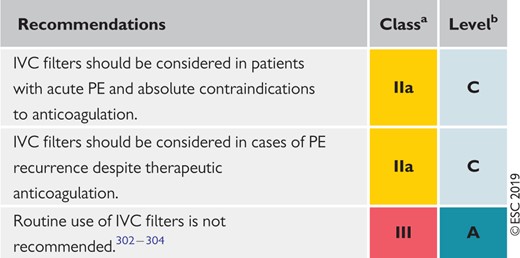 |
 |
IVC = inferior vena cava; PE = pulmonary embolism.
Class of recommendation.
Level of evidence.
 |
 |
IVC = inferior vena cava; PE = pulmonary embolism.
Class of recommendation.
Level of evidence.
6.10 Recommendations for early discharge and home treatment
 |
 |
PE = pulmonary embolism.
Class of recommendation.
Level of evidence.
See section 7 and Figure 6 for further guidance on defining low-risk PE and decision-making.
 |
 |
PE = pulmonary embolism.
Class of recommendation.
Level of evidence.
See section 7 and Figure 6 for further guidance on defining low-risk PE and decision-making.
7 Integrated risk-adapted diagnosis and management
7.1 Diagnostic strategies
Various combinations of clinical assessments, plasma D-dimer measurements, and imaging tests have been proposed and validated for PE diagnosis. These strategies have been tested in patients presenting with suspected PE in the emergency department or during their hospital stay,101,164,171,320 and more recently in the primary care setting.111 Withholding of anticoagulation without adherence to evidence-based diagnostic strategies was associated with a significant increase in the number of VTE episodes and sudden cardiac death at 3 month follow-up.12 The most straightforward diagnostic algorithms for suspected PE—with and without haemodynamic instability—are presented in Figures 4 and 5, respectively. However, it is recognized that the diagnostic approach for suspected PE may vary, depending on the availability of, and expertise in, specific tests in various hospitals and clinical settings.
Diagnostic algorithm for patients with suspected high-risk pulmonary embolism presenting with haemodynamic instability. CTPA = computed tomography pulmonary angiography; CUS = compression ultrasonography; DVT = deep vein thrombosis; LV = left ventricle; PE = pulmonary embolism; RV = right ventricle; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiogram. aSee Table 4 for definition of haemodynamic instability and high-risk PE. bAncillary bedside imaging tests may include TOE, which may detect emboli in the pulmonary artery and its main branches; and bilateral venous CUS, which may confirm DVT and thus VTE. cIn the emergency situation of suspected high-risk PE, this refers mainly to a RV/LV diameter ratio >1.0; the echocardiographic findings of RV dysfunction, and the corresponding cut-off levels, are graphically presented in Figure 3, and their prognostic value summarized in Supplementary Data Table 3. dIncludes the cases in which the patient's condition is so critical that it only allows bedside diagnostic tests. In such cases, echocardiographic findings of RV dysfunction confirm high-risk PE and emergency reperfusion therapy is recommended
Diagnostic algorithm for patients with suspected pulmonary embolism without haemodynamic instability. CTPA = computed tomography pulmonary angiography/angiogram; PE = pulmonary embolism. aThe proposed diagnostic strategy for pregnant women with suspected acute PE is discussed in section 9. bTwo alternative classification schemes may be used for clinical probability assessment, i.e. a three-level scheme (clinical probability defined as low, intermediate, or high) or a two-level scheme (PE unlikely or PE likely). When using a moderately sensitive assay, D-dimer measurement should be restricted to patients with low clinical probability or a PE-unlikely classification, while highly sensitive assays may also be used in patients with intermediate clinical probability of PE due to a higher sensitivity and negative predictive value. Note that plasma D-dimer measurement is of limited use in suspected PE occurring in hospitalized patients. cTreatment refers to anticoagulation treatment for PE. dCTPA is considered diagnostic of PE if it shows PE at the segmental or more proximal level. eIn case of a negative CTPA in patients with high clinical probability, investigation by further imaging tests may be considered before withholding PE-specific treatment.
The diagnostic strategy for suspected acute PE in pregnancy is discussed in section 9.
7.1.1 Suspected pulmonary embolism with haemodynamic instability
The proposed strategy is shown in Figure 4. The clinical probability is usually high and the differential diagnosis includes cardiac tamponade, acute coronary syndrome, aortic dissection, acute valvular dysfunction, and hypovolaemia. The most useful initial test in this situation is bedside TTE, which will yield evidence of acute RV dysfunction if acute PE is the cause of the patient's haemodynamic decompensation. In a highly unstable patient, echocardiographic evidence of RV dysfunction is sufficient to prompt immediate reperfusion without further testing. This decision may be strengthened by the (rare) visualization of right heart thrombi.155,157,321,322 Ancillary bedside imaging tests include TOE, which may allow direct visualization of thrombi in the pulmonary artery and its main branches, especially in patients with RV dysfunction. TOE should be cautiously performed in hypoxaemic patients. Moreover, bedside CUS can detect proximal DVT. As soon as the patient is stabilized using supportive treatment, final confirmation of the diagnosis by CT angiography should be sought.
For unstable patients admitted directly to the catheterization laboratory with suspected acute coronary syndrome, pulmonary angiography may be considered as a diagnostic procedure after the acute coronary syndrome has been excluded, provided that PE is a probable diagnostic alternative and particularly if percutaneous catheter-directed treatment is a therapeutic option.
7.1.2 Suspected pulmonary embolism without haemodynamic instability
7.1.2.1 Strategy based on computed tomographic pulmonary angiography
The proposed strategy based on CTPA is shown in Figure 5. In patients admitted to the emergency department, measurement of plasma D-dimer is the logical first step following the assessment of clinical probability and allows PE to be ruled out in ∼30% of outpatients. D-dimer should not be measured in patients with a high clinical probability of PE, owing to a low negative predictive value in this population.323 It is also less useful in hospitalized patients because the number that needs to be tested to obtain a clinically relevant negative result is high.
In most centres, multidetector CTPA is the second-line test in patients with an elevated D-dimer level and the first-line test in patients with a high clinical probability of PE. CTPA is considered to be diagnostic of PE when it shows a clot at least at the segmental level of the pulmonary arterial tree. False-negative results of CTPA have been reported in patients with a high clinical probability of PE;115 however, such discrepancies are infrequent and the 3 month thromboembolic risk was low in these patients.171 Accordingly, both the necessity of performing further tests and the nature of these tests remain controversial in these clinical situations.
7.1.2.2 Strategy based on ventilation/perfusion scintigraphy
In hospitals in which V/Q scintigraphy is readily available, it is a valid option for patients with an elevated D-dimer and a contraindication to CTPA. Also, V/Q scintigraphy may be preferred over CTPA to avoid unnecessary radiation, particularly in younger patients and in female patients in whom thoracic CT might raise the lifetime risk of breast cancer.324 V/Q lung scintigraphy is diagnostic (with either normal- or high-probability findings) in ∼30–50% of emergency ward patients with suspected PE.75,122,134,325 The proportion of diagnostic V/Q scans is higher in patients with a normal chest X-ray, and this might support the use of a V/Q scan as a first-line imaging test for PE in younger patients, depending on local availability.326 The number of patients with inconclusive findings may further be reduced by taking into account clinical probability. Thus, patients with a non-diagnostic lung scan and low clinical probability of PE have a low prevalence of confirmed PE,124,325 and the negative predictive value of this combination is further increased by the absence of a DVT on lower-limb CUS. If a high-probability lung scan is obtained from a patient with low clinical probability of PE, confirmation by other tests should be considered.
7.2 Treatment strategies
7.2.1 Emergency treatment of high-risk pulmonary embolism
The algorithm for a risk-adjusted therapeutic approach to acute PE is shown in Figure 6; an emergency management algorithm specifically for patients with suspected acute high-risk PE is proposed in Supplementary Data Figure 1. Primary reperfusion treatment, in most cases systemic thrombolysis, is the treatment of choice for patients with high-risk PE. Surgical pulmonary embolectomy or percutaneous catheter-directed treatment are alternative reperfusion options in patients with contraindications to thrombolysis, if expertise with either of these methods and the appropriate resources are available on-site.
Following reperfusion treatment and haemodynamic stabilization, patients recovering from high-risk PE can be switched from parenteral to oral anticoagulation. As patients belonging to this risk category were excluded from the phase III NOAC trials, the optimal time point for this transition has not been determined by existing evidence but should instead be based on clinical judgement. The specifications concerning the higher initial dose of apixaban or rivaroxaban (for 1 and 3 weeks after PE diagnosis, respectively), or the minimum overall period (5 days) of heparin anticoagulation before switching to dabigatran or edoxaban, must be followed (see Supplementary Data Table 8 for tested and approved regimens).
7.2.2 Treatment of intermediate-risk pulmonary embolism
For most cases of acute PE without haemodynamic compromise, parenteral or oral anticoagulation (without reperfusion techniques) is adequate treatment. As shown in Figure 6, normotensive patients with at least one indicator of elevated PE-related risk, or with aggravating conditions or comorbidity, should be hospitalized. In this group, patients with signs of RV dysfunction on echocardiography or CTPA (graphically presented in Figure 3), accompanied by a positive troponin test, should be monitored over the first hours or days due to the risk of early haemodynamic decompensation and circulatory collapse.179 Routine primary reperfusion treatment, notably full-dose systemic thrombolysis, is not recommended, as the risk of potentially life-threatening bleeding complications appears too high for the expected benefits from this treatment.179 Rescue thrombolytic therapy or, alternatively, surgical embolectomy or percutaneous catheter-directed treatment should be reserved for patients who develop signs of haemodynamic instability. In the PEITHO trial, the mean time between randomization and death or haemodynamic decompensation was 1.79 ± 1.60 days in the placebo (heparin-only) arm.179 Therefore, it appears reasonable to leave patients with intermediate−high-risk PE on LMWH anticoagulation over the first 2 − 3 days and ensure that they remain stable before switching to oral anticoagulation. As mentioned in the previous section, the specifications concerning the increased initial dose of apixaban or rivaroxaban, or the minimum overall period of heparin anticoagulation before switching to dabigatran or edoxaban, must be followed.
Suggestions for the anticoagulation and overall management of acute PE in specific clinical situations, for which conclusive evidence is lacking, are presented in Supplementary Data Table 9.
7.2.3 Management of low-risk pulmonary embolism: triage for early discharge and home treatment
As a general rule, early discharge of a patient with acute PE and continuation of anticoagulant treatment at home should be considered if three sets of criteria are fulfilled: (i) the risk of early PE-related death or serious complications is low (section 5); (ii) there is no serious comorbidity or aggravating condition(s) (see section 5) that would mandate hospitalization; and (iii) proper outpatient care and anticoagulant treatment can be provided, considering the patient’s (anticipated) compliance, and the possibilities offered by the healthcare system and social infrastructure.
Randomized trials and prospective management cohort studies that investigated the feasibility and safety of early discharge, and home treatment, of PE adhered to these principles, even though slightly different criteria or combinations thereof were used to ensure the above three requirements.
Central Illustration. Risk-adjusted management strategy for acute pulmonary embolism. CTPA = computed tomography pulmonary angiography/angiogram; PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; sPESI = simplified Pulmonary Embolism Severity Index; TTE = transthoracic echocardiogram. aSee also emergency management algorithm shown in the online Supplementary Data. bRefer to Table 8 for definition of high, intermediate-high-, intermediate-low-, and low-risk PE. cCancer, heart failure and chronic lung disease are included in the PESI and sPESI (Table 7). dSee Supplementary Data Table 12 for the Hestia criteria. ePrognostically relevant imaging (TTE or CTPA) findings in patients with acute PE, are graphically presented in Figure 3. fA cardiac troponin test may already have been performed during initial diagnostic work-up. gIncluded in the Hestia criteria.
The Hestia exclusion criteria (Supplementary Data Table 12) represent a checklist of clinical parameters or questions that can be obtained/answered at the bedside. They integrate aspects of PE severity, comorbidity, and the feasibility of home treatment. If the answer to one or more of the questions is ‘yes’, then the patient cannot be discharged early. In a single-arm management trial that used these criteria to select candidates for home treatment, the 3 month rate of recurrent VTE was 2.0% (0.8–4.3%) in patients with acute PE who were discharged within 24 h.317 In a subsequent non-inferiority trial that randomized 550 patients to direct discharge based on the Hestia criteria alone vs. additional NT-proBNP testing and discharge if levels were ≤500 pg/mL, the primary outcome (30 day PE- or bleeding-related mortality, cardiopulmonary resuscitation, or admission to an intensive care unit) was very low in both arms. The results suggest no incremental value of natriuretic-peptide testing in patients who are eligible for home treatment based on the Hestia criteria, although the study was not powered to exclude this possibility.318
The PESI and its simplified form, the sPESI (Table 7), also integrate clinical parameters of PE severity and comorbidity to permit assessment of overall 30 day mortality. Compared with the Hestia criteria, the PESI is more standardized, but it contains a less-comprehensive list of aggravating conditions; moreover, the sPESI excludes all patients with cancer from the low-risk category (compare Table 7 with Supplementary Data Table 12). The PESI was not primarily developed as a tool to select candidates for home treatment, but it has been used—in combination with additional feasibility criteria—in a trial of 344 patients randomized to inpatient vs. outpatient treatment of PE.178 One (0.6%) patient in each treatment group died within 90 days.178
In patients who were included in prospective cohort studies and treated at home, with or without a short hospitalization period, the 3 month rates of thromboembolic recurrence, major bleeding, and death were 1.75, 1.43, and 2.83%, respectively.327
In summary, the currently available evidence indicates that both the Hestia rule and the PESI or sPESI appear capable of reliably identifying patients who are (i) at low PE-related risk, and (ii) free of serious comorbidity. Consequently, either may be used for clinical triage according to local experience and preference. If a PESI- or sPESI-based approach is chosen, it must be combined with assessment of the feasibility of early discharge and home treatment; this assessment is already integrated into the Hestia criteria.
A more difficult decision related to immediate or early discharge is whether the exclusion of intermediate-risk PE on clinical grounds alone is adequate, or whether the assessment of RV dysfunction or myocardial injury (see section 5) by an imaging test or a laboratory biomarker is necessary to provide maximal safety for the patient in this ‘vulnerable’ early period. A systematic review and meta-analysis of cohort studies suggested that the prognostic sensitivity is increased further when clinical criteria (e.g. PESI or sPESI) are combined with imaging findings, or laboratory biomarker levels.234 A multicentre prospective management trial tested this hypothesis, investigating the efficacy and safety of early discharge, and ambulatory rivaroxaban treatment, in patients selected by clinical criteria and an absence of RV dysfunction. Overall, ∼20% of the screened unselected patients with PE were included. At the predefined interim analysis of 525 patients (50% of the planned population), the 3 month rate of symptomatic or fatal recurrent VTE was 0.6% (one-sided upper 99.6% CI 2.1%), permitting the early rejection of the null hypothesis and termination of the trial. Major bleeding occurred in six (1.2%) of the patients in the safety population. There were no PE-related deaths.319 In view of the existing evidence—and taking into consideration (i) the catastrophic scenario of early death if a patient with acute PE is falsely judged to be at low risk on clinical grounds alone and discharged ‘too early’ (as described in a prematurely terminated trial328), and (ii) the ease and minimal additional effort of assessing RV size and function at presentation by echocardiography, or on the CTPA performed to diagnose the PE event itself329 (section 5)—it is wise to exclude RV dysfunction and right heart thrombi if immediate or early (within the first 24–48 h) discharge of the patient is planned.
8 Chronic treatment and prevention of recurrence
The aim of anticoagulation after acute PE is to complete the treatment of the acute episode and prevent recurrence of VTE over the long-term. Current drugs and regimens for the initial phase, and the first months of anticoagulant treatment, are described insection 6.
Most of the randomized studies focusing on long-term anticoagulation for VTE have included patients with DVT, with or without PE; only two randomized studies have specifically focused on patients with PE.330,331 The incidence of recurrent VTE does not appear to depend on the clinical manifestation of the first event (i.e. it is similar after PE and after proximal DVT). However, in patients who have had a PE, VTE more frequently recurs as PE, while in patients who have had a DVT, it tends to recur more frequently as DVT.332 As a consequence, the case fatality rate of recurrent VTE in patients who have previously had a PE is twice as high as that of VTE recurrence after DVT.333,334
Landmark clinical trials have evaluated various durations of anticoagulant treatment with VKAs for VTE.330,331,335–337 The findings of these studies permit the following conclusions. First, all patients with PE should receive ≥3 months of anticoagulant treatment. Second, after withdrawal of anticoagulant treatment, the risk of recurrence is expected to be similar if anticoagulants are stopped after 3–6 months compared with longer treatment periods (e.g. 12–24 months). Third, extended oral anticoagulant treatment reduces the risk for recurrent VTE by ≤90%, but this benefit is partially offset by the risk of bleeding.
Oral anticoagulants are highly effective in preventing recurrent VTE during treatment, but they do not eliminate the risk of subsequent recurrence after the discontinuation of treatment.330,331 Based on this fact on the one hand, and considering the bleeding risk of anticoagulation treatment on the other, the clinically important question is how to best select candidates for extended or indefinite anticoagulation. Involvement of the patient in the decision-making process is crucial to optimize and maintain treatment adherence.
8.1 Assessment of venous thromboembolism recurrence risk
The risk for recurrent VTE after discontinuation of treatment is related to the features of the index PE (or, in the broader sense, VTE) event. A study, which followed patients after a first episode of acute PE, found that the recurrence rate after discontinuation of treatment was ∼2.5% per year after PE associated with transient risk factors, compared with 4.5% per year after PE occurring in the absence of known cancer, known thrombophilia, or any transient risk factor.331 Similar observations were made in other prospective studies in patients with DVT.337 Advancing the concept further, randomized anticoagulation trials over the past 15 years, which have focused on secondary VTE prevention, have classified patients into distinct groups based on their risk of VTE recurrence after discontinuation of anticoagulant treatment. In general, these groups are: (i) patients in whom a strong (major) transient or reversible risk factor, most commonly major surgery or trauma, can be identified as being responsible for the acute (index) episode; (ii) patients in whom the index episode might be partly explained by the presence of a weak (minor) transient or reversible risk factor, or if a non-malignant risk factor for thrombosis persists; (iii) patients in whom the index episode occurred in the absence of any identifiable risk factor (the present Guidelines avoid terms such as ‘unprovoked’ or ‘idiopathic’ VTE); (iv) patients with one or more previous episodes of VTE, and those with a major persistent pro-thrombotic condition such as antiphospholipid antibody syndrome; and (v) patients with active cancer.338
Table 11 shows examples of transient/reversible and persistent risk factors for VTE, classified by the risk of long-term recurrence. As active cancer is a major risk factor for recurrence of VTE, but also for bleeding while on anticoagulant treatment,339 section 8.4 is specifically dedicated to the management of PE in patients with cancer.
Categorization of risk factors for venous thromboembolism based on the risk of recurrence over the long-term
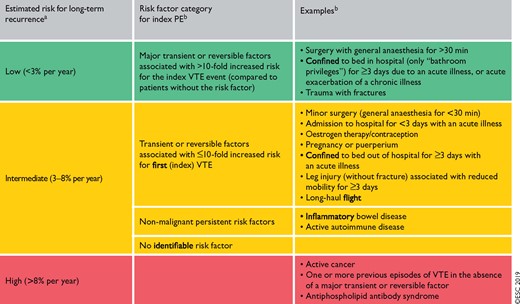 |
 |
PE = pulmonary embolism; VTE = venous thromboembolism.
If anticoagulation is discontinued after the first 3 months (based on data from Baglin et al.340 and Iorio et al.341).
The categorization of risk factors for the index VTE event is in line with that proposed by the International Society on Thrombosis and Haemostasis.338 The present Guidelines avoid terms such as ‘provoked’, ‘unprovoked’, or ‘idiopathic’ VTE.
Categorization of risk factors for venous thromboembolism based on the risk of recurrence over the long-term
 |
 |
PE = pulmonary embolism; VTE = venous thromboembolism.
If anticoagulation is discontinued after the first 3 months (based on data from Baglin et al.340 and Iorio et al.341).
The categorization of risk factors for the index VTE event is in line with that proposed by the International Society on Thrombosis and Haemostasis.338 The present Guidelines avoid terms such as ‘provoked’, ‘unprovoked’, or ‘idiopathic’ VTE.
Overall, assessment of the VTE recurrence risk after acute PE, in the absence of a major transient or reversible risk factor, is a complex issue. Beyond the examples listed in Table 11, patients who are carriers of some forms of hereditary thrombophilia, notably those with confirmed deficiency of antithrombin, protein C, or protein S, and patients with homozygous factor V Leiden or homozygous prothrombin G20210A mutation, are often candidates for indefinite anticoagulant treatment after a first episode of PE occurring in the absence of a major reversible risk factor. In view of these possible implications, testing for thrombophilia (including antiphospholipid antibodies and lupus anticoagulant)342 may be considered in patients in whom VTE occurs at a young age (e.g. aged <50 years) and in the absence of an otherwise identifiable risk factor, especially when this occurs against the background of a strong family history of VTE. In such cases, testing may help to tailor the regimen and dose of the anticoagulant agent over the long-term. On the other hand, no evidence of a clinical benefit of extended anticoagulant treatment is currently available for carriers of heterozygous factor V Leiden or prothrombin 20210A mutation.
A number of risk prediction models have been developed for the assessment of the risk of recurrence in an individual patient (Supplementary Data Table 13).343,344 The clinical value and, in particular, the possible therapeutic implications of these models in the NOAC era are unclear.
8.2 Anticoagulant-related bleeding risk
Incidence estimates from cohort studies conducted more than 15 years ago reported an ∼3% annual incidence of major bleeding in patients treated with VKAs.345 Meta-analyses of phase III studies focusing on the first 3 − 12 months of anticoagulant treatment showed an ∼40% reduction in the risk for major bleeding with NOACs compared with VKAs.346 The risk of major bleeding is higher in the first month of anticoagulant treatment, and then declines and remains stable over time. Based on currently available evidence, risk factors include: (i) advanced age (particularly >75 years); (ii) previous bleeding (if not associated with a reversible or treatable cause) or anaemia; (iii) active cancer; (iv) previous stroke, either haemorrhagic or ischaemic; (v) chronic renal or hepatic disease; (vi) concomitant antiplatelet therapy or non-steroidal anti-inflammatory drugs (to be avoided, if possible); (vii) other serious acute or chronic illness; and (viii) poor anticoagulation control.
Existing bleeding risk scores and their current validation status are reviewed in Supplementary DataTable 14. The patient’s bleeding risk should be assessed, either by implicit judgement after evaluating individual risk factors or by the use of a bleeding risk score, at the time of initiation of anticoagulant treatment. It should be reassessed periodically (e.g. once a year in patients at low risk, and every 3 or 6 months in patients at high risk for bleeding). Bleeding risk assessment should be used to identify and treat modifiable bleeding risk factors, and it may influence decision-making on the duration and regimen/dose of anticoagulant treatment after acute PE.
8.3 Regimens and treatment durations with non-vitamin K antagonist oral anticoagulants, and with other non-vitamin K antagonist antithrombotic drugs
All patients with PE should be treated with anticoagulants for ≥3 months.347 Beyond this period, the balance between the risk of VTE recurrence and that of bleeding, which has been used to select candidates for extended anticoagulation after a first VTE event in the VKA era, is currently being revisited based on the lower bleeding rates with NOACs. However, despite the improved safety of these drugs compared with VKAs, treatment with NOACs is not without risk. Phase III clinical trials on the extended treatment of VTE have shown that the rate of major bleeding may be ∼1%, and that of clinically relevant non-major (CRNM) bleeding as high as 6%. Bleeding rates may be higher in everyday clinical practice.348,349
The NOAC trials that focused on extended VTE treatment are summarized in Supplementary Data Table 15. In all studies, patients with PE made up approximately one-third of the entire study population, while the remaining two-thirds were patients with proximal DVT but no clinically overt PE. Patients needed to have completed the initial and long-term anticoagulation phase to be included in the extended studies.
Dabigatran was compared with warfarin or placebo in two different studies (Supplementary Data Table 15).350 In these studies, dabigatran was non-inferior to warfarin for the prevention of confirmed recurrent symptomatic VTE or VTE-related death, and more effective than placebo for the prevention of symptomatic recurrent VTE or unexplained death.350 The rate of major bleeding was 0.9% with dabigatran compared to 1.8% with warfarin (HR 0.52, 95% CI 0.27–1.02).350
Rivaroxaban was compared with placebo or aspirin in two different studies in patients who had completed 6–12 months of anticoagulation treatment for a first VTE event (Supplementary Data Table 15). Treatment with rivaroxaban [20 mg once a day (o.d.)] reduced recurrent VTE by ∼80%, with a 6.0% incidence of major or CRNM bleeding as compared to 1.2% with placebo.351 Rivaroxaban given at a dose of 20 or 10 mg o.d. was compared with aspirin (100 mg o.d.) in 3365 patients.352 Both doses of rivaroxaban reduced symptomatic recurrent fatal or non-fatal VTE by ∼70% in comparison with aspirin. No significant differences in the rates of major or CRNM bleeding were shown between either dose of rivaroxaban and aspirin.352
Patients with VTE were randomized to receive two different doses of apixaban [2.5 or 5 mg twice a day (bis in die: b.i.d.)] or placebo after 6–12 months of initial anticoagulation (Supplementary Data Table 15).353 Both doses of apixaban reduced the incidence of symptomatic recurrent VTE or death from any cause compared with placebo, with no safety concerns.353
Patients at high bleeding risk—based on the investigator’s judgement, the patient’s medical history, and the results of laboratory examinations—were excluded from the extension studies mentioned above; this was also the case for studies on extended anticoagulation with VKAs.330,331 This fact should be taken into account during triage of a patient for extended anticoagulation with one of the above regimens.
In a randomized, open-label study in high-risk patients with antiphospholipid syndrome (testing triple positive for lupus anticoagulant, anticardiolipin, and anti-β2-glycoprotein I), rivaroxaban was associated with an increased rate of thromboembolic and major bleeding events compared with warfarin (HR for the composite primary outcome 6.7; 95% CI 1.5–30.5).354 At present, NOACs are not an alternative to VKAs for patients with antiphospholipid syndrome.
In two trials with a total of 1224 patients, extended therapy with aspirin (after termination of standard oral anticoagulation) was associated with a 30–35% reduction in the risk of recurrence compared with placebo (Supplementary Data Table 15).355,356 However, more recently, another trial demonstrated the superiority of anticoagulation with rivaroxaban, either 20 or 10 mg o.d., over aspirin for secondary prophylaxis of VTE recurrence.352
A randomized, placebo controlled study evaluated sulodexide (2 × 250 lipasemic unit capsules b.i.d.) for the prevention of recurrence in 615 patients with a first VTE event without an identifiable risk factor, who had completed 3–12 months of oral anticoagulant treatment (Supplementary Data Table 15).357 Sulodexide reduced the risk of recurrence by ∼50% with no apparent increase in bleeding events. However, only 8% of patients in this study had PE as the index VTE event.357
8.4 Recommendations for the regimen and duration of anticoagulation after pulmonary embolism in patients without cancer
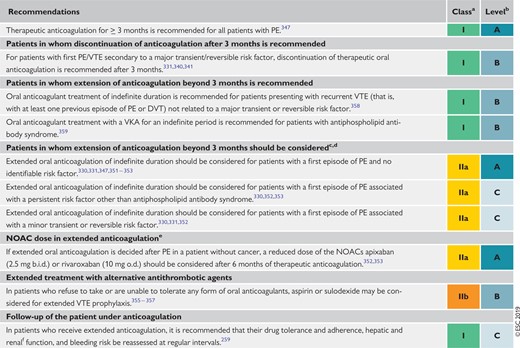 |
 |
b.i.d. = bis in die (twice a day); DVT = deep vein thrombosis; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); o.d. = omni die (once a day); PE = pulmonary embolism; VKA = vitamin K antagonist; VTE = venous thromboembolism.
Class of recommendation.
Level of evidence.
The patient’s bleeding risk should be assessed (see Supplementary Data Table 14 for prediction models) to identify and treat modifiable bleeding risk factors, and it may influence decision-making on the duration and regimen/dose of anticoagulant treatment.
Refer to Supplementary Data Table 9 for therapeutic decisions in specific clinical situations.
If dabigatran or edoxaban is chosen for extended anticoagulation after PE, the dose should remain unchanged, as reduced-dose regimens were not investigated in dedicated extension trials.313,350
Especially for patients receiving NOACs.
 |
 |
b.i.d. = bis in die (twice a day); DVT = deep vein thrombosis; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); o.d. = omni die (once a day); PE = pulmonary embolism; VKA = vitamin K antagonist; VTE = venous thromboembolism.
Class of recommendation.
Level of evidence.
The patient’s bleeding risk should be assessed (see Supplementary Data Table 14 for prediction models) to identify and treat modifiable bleeding risk factors, and it may influence decision-making on the duration and regimen/dose of anticoagulant treatment.
Refer to Supplementary Data Table 9 for therapeutic decisions in specific clinical situations.
If dabigatran or edoxaban is chosen for extended anticoagulation after PE, the dose should remain unchanged, as reduced-dose regimens were not investigated in dedicated extension trials.313,350
Especially for patients receiving NOACs.
8.5 Management of pulmonary embolism in patients with cancer
Five RCTs compared LMWH vs. conventional VTE treatment (heparin followed by VKA) in the treatment of VTE in cancer-associated thrombosis.360–364 In 2003, a significant reduction in VTE recurrence was reported with LMWH compared with conventional (VKA) treatment without an increase in bleeding complications.362 In a more recent trial, long-term administration of tinzaparin failed to achieve a statistically significant reduction in overall VTE recurrence over conventional treatment (HR 0.65, 95% CI 0.41–1.03); however, the overall rate of recurrent VTE in the control arm was lower than that previously observed, probably as a result of the recruitment of patients with a lower cancer-specific thrombotic risk.360 Overall, LMWHs were found to decrease the risk of recurrent VTE by 40% with a risk of major bleeding complications similar to that of VKAs.365 Accordingly, LMWHs have become the standard of care. However, these agents are associated with a relevant cost and burden for patients. In addition, the absolute rate of recurrent VTE while on LMWH remains high (7–9%) compared with that observed in non-cancer patients with VTE on conventional treatment (1.5–3%).365
NOACs could make the treatment of VTE easier and more convenient in patients with cancer, due to their oral administration in fixed-dose regimens and their lower cost compared with LMWH. However, only 3–9% of patients included in phase III studies with NOACs for the treatment of VTE had concomitant cancer.260,261,312,314,351 A randomized, open-label trial compared edoxaban with LMWH in the secondary prevention of VTE in 1050 patients with cancer-associated thrombosis (mostly symptomatic or asymptomatic PE).366 Edoxaban (60 mg o.d., reduced to 30 mg in subjects with moderate renal impairment, low body weight, or concomitant need for strong inhibitors of glycoprotein-P) was started after 5 days of LMWH and treatment was given for ≥6 months. Edoxaban was non-inferior to dalteparin in the prevention of VTE recurrence or major bleeding over 12 months after randomization (HR 0.97, 95% CI 0.70–1.36). Major bleeding occurred in 6.9% of the patients in the edoxaban arm and 4.0% in the dalteparin arm (difference in risk 2.9 percentage points, 95% CI 0.1–5.6). This difference appears to have been mainly accounted for by the high rate of bleeding in patients with gastrointestinal cancer allocated to the edoxaban group.366 Similar results were reported by a randomized, open-label pilot trial comparing rivaroxaban with dalteparin in 406 patients with VTE and cancer, 58% of whom had metastases.367 A significant decrease in the risk of recurrent VTE was observed with rivaroxaban (HR 0.43, 95% CI 0.19–0.99). The 6 month cumulative rate of major bleeding, which was mostly gastrointestinal, was 6% (95% CI 3–11%) for rivaroxaban and 4% (95% CI 2–8%) for dalteparin (HR 1.83, 95% CI 0.68–4.96). Corresponding rates of CRNM bleeds were 13% (95% CI 9–19%) and 4% (95% CI 2–9%), respectively (HR 3.76, 95% CI 1.63–8.69).367
Based on the currently available evidence, as described above, patients with acute PE and cancer, particularly those with gastrointestinal cancer, should be encouraged to continue LMWH for ≥ 3–6 months. This also applies to patients in whom oral treatment is unfeasible due to problems of intake or absorption, and to those with severe renal impairment. In all other cases, especially in patients with an anticipated low risk of bleeding and without gastrointestinal tumours, the choice between LMWH and edoxaban or rivaroxaban is left to the discretion of the physician, and the patient’s preference.
Owing to the high risk for recurrence, patients with cancer should receive indefinite anticoagulation after a first episode of VTE. Although existing evidence is limited, it is conceivable that once cancer is cured the risk for recurrence decreases and anticoagulation can be stopped. However, the definition of cured cancer is not always clear. The risk of recurrence of PE in cancer was assessed in a cohort study of 543 patients and was validated in an independent set of 819 patients.368 The proposed score to predict the risk of recurrence included breast cancer (minus 1 point), Tumour Node Metastasis stage I or II (minus 1 point), and female sex, lung cancer, and previous VTE (plus 1 point each). Patients with a score ≤0 were at low risk (≤4.5%) and those with a score ≥1 were at high (≥19%) risk of VTE recurrence over the first 6 months.368
After the first 3–6 months, extended anticoagulation may consist of continuation of LMWH or transition to an oral anticoagulant. Two cohort studies have assessed the safety of extended treatment with LMWH (≤12 months) in cancer-associated thrombosis.369,370 In both studies, the incidence of bleeding complications was higher in the first months and then reached a plateau that remained unchanged after the sixth month. In the absence of conclusive evidence, the decision to continue with LMWH or to change to VKA or a NOAC should be made on a case-by-case basis after consideration of the success of anticancer therapy, the estimated risk of recurrence of VTE, the bleeding risk, and the preference of the patient. Periodic reassessment of the risk-to-benefit ratio of continued anticoagulant treatment is mandatory.
As mentioned in section 5, venous filters are principally indicated when anticoagulation is impossible due to active haemorrhage or an excessive bleeding risk. However, the risk of VTE recurrence in the absence of anticoagulation is particularly high in patients with cancer, and the insertion of a filter should not delay the initiation of anticoagulation as soon as it is safe to do so. There is no evidence to support the use of venous filters as an adjunct to anticoagulation treatment in patients with cancer.
A number of studies have reported that a proportion of patients presenting with PE in the absence of identifiable risk factors develop cancer within the first year after diagnosis.371 Consequently, the optimal strategy to achieve early diagnosis of these occult cancers was investigated. Two large randomized trials failed to show that comprehensive CT of the abdomen or 18F deoxy-fluoro-glucose positron emission tomography were able to detect more cancers than limited screening in patients with an unprovoked VTE.372,373 Therefore, based on current evidence, the search for occult cancer after an episode of VTE may be restricted to careful history taking, physical examination, basic laboratory tests, and a chest X-ray (if no CTPA was performed to diagnose PE).372,374,375
In patients with cancer, incidental PE should be managed in the same manner as symptomatic PE, whether it involves segmental or more proximal branches, multiple subsegmental vessels, or a single subsegmental vessel in association with detectable DVT.376,377
8.6 Recommendations for the regimen and the duration of anticoagulation after pulmonary embolism in patients with active cancer
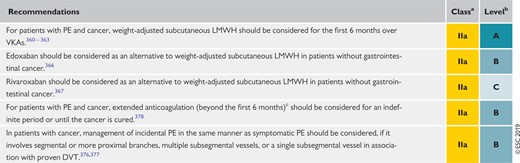 |
 |
DVT = deep vein thrombosis; LMWH = low-molecular weight heparin; PE = pulmonary embolism; VKAs = vitamin K antagonists.
Class of recommendation.
Level of evidence.
Refer to Supplementary Data Table 9 for further guidance on therapeutic decisions after the first 6 months.
 |
 |
DVT = deep vein thrombosis; LMWH = low-molecular weight heparin; PE = pulmonary embolism; VKAs = vitamin K antagonists.
Class of recommendation.
Level of evidence.
Refer to Supplementary Data Table 9 for further guidance on therapeutic decisions after the first 6 months.
9 Pulmonary embolism and pregnancy
9.1 Epidemiology and risk factors for pulmonary embolism in pregnancy
Acute PE remains one of the leading causes of maternal death in high-income countries.379,380 For example, in the UK and Ireland, thrombosis and thromboembolism were the most common causes of direct maternal death (death resulting from the pregnancy rather than pre-existing conditions) in the triennium 2013–15, resulting in 1.13 mortalities per 100 000 maternities (https://www.npeu.ox.ac.uk/mbrrace-uk). VTE risk is higher in pregnant women compared with non-pregnant women of similar age; it increases during pregnancy and reaches a peak during the post-partum period.381 The baseline pregnancy-related risk increases further in the presence of additional VTE risk factors, including in vitro fertilization: in a cross-sectional study derived from a Swedish registry, the HR for VTE following in vitro fertilization was 1.77 (95% CI 1.41–2.23) overall and 4.22 (95% CI 2.46–7.20) during the first trimester.382 Other important and common risk factors include prior VTE, obesity, medical comorbidities, stillbirth, pre-eclampsia, post-partum haemorrhage, and caesarean section; documented risk assessment is therefore essential.383
The recommendations provided in these Guidelines are in line with those included in the 2018 ESC Guidelines on the management of cardiovascular diseases during pregnancy.384
9.2 Diagnosis of pulmonary embolism in pregnancy
9.2.1 Clinical prediction rules and D-dimers
Diagnosis of PE during pregnancy can be challenging as symptoms frequently overlap with those of normal pregnancy. The overall prevalence of confirmed PE is low among women investigated for the disease, between 2 and 7%.385–388 D-dimer levels continuously increase during pregnancy,389,390 and levels are above the threshold for VTE ‘rule-out’ in almost one-quarter of pregnant women in the third trimester.390 The results of a multinational prospective management study of 441 pregnant women presenting to emergency departments with clinically suspected PE suggest that a diagnostic strategy–based on the assessment of clinical probability, D-dimer measurement, CUS, and CTPA–may safely exclude PE in pregnancy.388 In that study, PE exclusion on the basis of a negative D-dimer result (without imaging) was possible in 11.7% of the 392 women with a non-high pre-test probability (Geneva) score, a rate that was reduced to 4.2% in the third trimester.388 A further prospective management study evaluated a combination of a pregnancy-adapted YEARS algorithm with D-dimer levels in 498 women with suspected PE during pregnancy. PE was ruled out without CTPA in women deemed to be at low PE risk according to the combination of the algorithm and D-dimer results. At 3 months, only one woman with PE excluded on the basis of the algorithm developed a popliteal DVT (0.21%, 95% CI 0.04–1.2) and no women developed PE.391
9.2.2 Imaging tests
A proposed algorithm for the investigation of suspected PE in women who are pregnant, or ≤6 weeks post-partum, is shown in Figure 7. Both maternal and foetal radiation exposure are low using modern imaging techniques (Table 12).385,392–398 For V/Q scans and CTPA, foetal radiation doses are well below the threshold associated with foetal radiation complications (which is 50–100 mSv).399,400 In the past, CTPA has been reported to cause high radiation exposure to the breast;395,401 however, CT technology has evolved, and several techniques can now reduce radiation exposure without compromising image quality. These include reducing the anatomical coverage of the scan,393 reducing the kilovoltage, using iterative reconstructive techniques, and reducing the contrast-monitoring component of the CTPA.392,393 Modern CTPA imaging techniques may therefore expose the maternal breast to median doses as low as 3–4 mGy (Table 12).392 The effect on maternal cancer risk with modern CTPA techniques is negligible (lifetime cancer risk is reportedly increased by a factor of 1.0003–1.0007); avoiding CTPA on the grounds of maternal cancer risk is therefore not justified.394
Diagnostic workup and management of suspected pulmonary embolism during pregnancy, and up to 6 weeks post-partum. CTPA = computed tomography pulmonary angiography; CUS = compression ultrasonography; DVT = deep vein thrombosis; LMWH = low-molecular-weight heparin; PE = pulmonary embolism. aIf chest X-ray abnormal, consider also alternative cause of chest symptoms. bDVT in pelvic veins may not be ruled out by CUS. If the entire leg is swollen, or there is buttock pain or other symptoms suggestive of pelvic thrombosis, consider magnetic resonance venography to rule out DVT. cCTPA technique must ensure very low foetal radiation exposure (see Table 12). dPerform full blood count (to measure haemoglobin and platelet count) and calculate creatinine clearance before administration. Assess bleeding risk and ensure absence of contra-indications. eSee Table 8.
| Test . | Estimated foetal radiation exposure (mGy)a . | Estimated maternal radiation exposure to breast tissue (mGy)a . |
|---|---|---|
| Chest X-ray | <0.01 | <0.1 |
|
|
|
| Ventilation lung scan | 0.10–0.30 | <0.01 |
| CTPA | 0.05–0.5 | 3–10 |
| Test . | Estimated foetal radiation exposure (mGy)a . | Estimated maternal radiation exposure to breast tissue (mGy)a . |
|---|---|---|
| Chest X-ray | <0.01 | <0.1 |
|
|
|
| Ventilation lung scan | 0.10–0.30 | <0.01 |
| CTPA | 0.05–0.5 | 3–10 |
CTPA = computed tomography pulmonary angiography; mGy = milligray; MBq = megabecquerel; PE = pulmonary embolism.
In this section, absorbed radiation dose is expressed in mGy to reflect the radiation exposure to single organs, or the foetus, as a result of various diagnostic techniques. Compare with Table 6, in which effective radiation dose is expressed in millisieverts to reflect the effective doses of all organs that have been exposed.
| Test . | Estimated foetal radiation exposure (mGy)a . | Estimated maternal radiation exposure to breast tissue (mGy)a . |
|---|---|---|
| Chest X-ray | <0.01 | <0.1 |
|
|
|
| Ventilation lung scan | 0.10–0.30 | <0.01 |
| CTPA | 0.05–0.5 | 3–10 |
| Test . | Estimated foetal radiation exposure (mGy)a . | Estimated maternal radiation exposure to breast tissue (mGy)a . |
|---|---|---|
| Chest X-ray | <0.01 | <0.1 |
|
|
|
| Ventilation lung scan | 0.10–0.30 | <0.01 |
| CTPA | 0.05–0.5 | 3–10 |
CTPA = computed tomography pulmonary angiography; mGy = milligray; MBq = megabecquerel; PE = pulmonary embolism.
In this section, absorbed radiation dose is expressed in mGy to reflect the radiation exposure to single organs, or the foetus, as a result of various diagnostic techniques. Compare with Table 6, in which effective radiation dose is expressed in millisieverts to reflect the effective doses of all organs that have been exposed.
A normal perfusion scan and a negative CTPA appear equally safe for ruling out PE in pregnancy, as suggested by retrospective series.385,386,402–404 Inconclusive results can be a problem (4–33% of investigations),385,386,405 especially late in pregnancy.405 A recent survey of 24 sites in the UK, representing a population of 15.5 million, revealed a similar rate of inadequate or indeterminate CTPA and scintigraphy scans, suggesting that the initial choice of imaging is best determined by local expertise and resources.406
V/Q SPECT is associated with low foetal and maternal radiation exposure, and has promise in PE diagnosis in pregnancy.407 However, further evaluation of this technique is required before its widespread incorporation into diagnostic algorithms. For MRA, the long-term effects of gadolinium contrast on the foetus are not known. In non-pregnant patients, technically inadequate images are frequently obtained and the rate of inconclusive scan results is high.140 Therefore, use of this technique for diagnosing or ruling out PE during pregnancy cannot be recommended at present. Conventional pulmonary angiography involves significantly higher radiation exposure of the foetus (2.2–3.7 mSv) and should be avoided during pregnancy.400
Overdiagnosis of PE is a potential pitfall that can have significant, lifelong implications for a pregnant woman, including the risk of bleeding at the time of delivery, the withholding of oestrogen contraception, and the requirement for thromboprophylaxis during future pregnancies. Consequently, avoiding PE overdiagnosis in pregnancy is as important as not missing a PE diagnosis.
9.3 Treatment of pulmonary embolism in pregnancy
LMWH is the treatment of choice for PE during pregnancy.384 In contrast to VKAs and NOACs, LMWH does not cross the placenta, and consequently does not confer a risk of foetal haemorrhage or teratogenicity. Moreover, while UFH is also safe in pregnancy, LMWH has more predictable pharmacokinetics and a more favourable risk profile.408–411 Although no RCT has evaluated the optimal dose of LMWH for the treatment of PE during pregnancy, currently published data favour similar dosing to non-pregnant patients, either with o.d. or b.i.d. regimens based on early pregnancy weight.408,410 For the majority of patients receiving LMWH treatment for PE during pregnancy,412,413 it remains uncertain whether using serial measurements of plasma anti-activated coagulation factor X activity to guide dosing may be of clinical benefit. It is important to bear in mind that: (i) LMWH has a predictable pharmacokinetic profile, (ii) data on optimal anti-activated coagulation factor levels are lacking, and (iii) the assay itself has limitations.414 In addition, there are no solid data on the clinical benefit vs. harm of frequent, weight-based dose adjustments of LMWH during pregnancy. Thus, anti-activated coagulation factor X monitoring may be reserved for specific high-risk circumstances such as recurrent VTE, renal impairment, and extremes of body weight.
The use of UFH has been associated with heparin-induced thrombocytopenia and bone loss. It remains uncertain whether, and to what extent, the risk of bone loss is increased with LMWH use. In a recent observational cohort study, in which bone mineral density was measured by dual-energy X-ray absorptiometry 4–7 years after the last delivery in 152 women (92 of whom received prolonged LMWH during pregnancy), lumbar spine bone mineral density was similar in LWMH-treated women and controls following adjustment for potential confounders. No osteoporosis or osteoporotic fractures were reported.415
Fondaparinux may be considered if there is an allergy or adverse response to LMWH, although solid data are lacking and minor transplacental passage has been demonstrated.416 VKAs cross the placenta and are associated with a well-defined embryopathy during the first trimester. Administration of VKAs in the third trimester can result in foetal and neonatal haemorrhage, as well as placental abruption. Warfarin may be associated with central nervous system anomalies in the foetus throughout pregnancy. NOACs are contraindicated in pregnant patients.417
The management of labour and delivery requires particular attention. In women receiving therapeutic LMWH, strong consideration should be given to planned delivery in collaboration with the multidisciplinary team to avoid the risk of spontaneous labour while fully anticoagulated. The incidence of spinal haematoma after regional anaesthesia is unknown in pregnant women under anticoagulation treatment. If regional analgesia is considered for a woman receiving therapeutic LMWH, ≥24 h should have elapsed since the last LMWH dose before insertion of a spinal or epidural needle (assuming normal renal function and including risk assessment at extremes of body weight).
In high-risk situations, for example in patients with recent PE, it is recommended that LMWH be converted to UFH ≥36 h prior to delivery. The UFH infusion should be stopped 4 − 6 h prior to anticipated delivery and the activated partial thromboplastin time should be normal (i.e. not prolonged) prior to regional anaesthesia.418
Data are limited on the optimal timing of post-partum reinitiation of LMWH.419,420 Timing will depend upon the mode of delivery and an assessment of the thrombotic vs. bleeding risk by a multidisciplinary team. LMWH should not be given for ≥4 h after removal of the epidural catheter; the decision on timing and dose should consider whether the epidural insertion was traumatic, and take into account the risk profile of the woman. For example, an interim dose of a prophylactic LMWH dose may be considered post-operatively (after caesarean section), once at least 4 h have elapsed since epidural catheter removal, and allowing for an interval of ≥8–12 h between the prophylactic and the next therapeutic dose. Close collaboration between the obstetrician, the anaesthesiologist, and the attending physician is recommended.
Anticoagulant treatment should be administered for ≥6 weeks after delivery and with a minimum overall treatment duration of 3 months. LMWH and warfarin can be given to breastfeeding mothers; the use of NOACs is not recommended.417
High-risk, life-threatening PE during pregnancy is a rare, but potentially devastating, event. A recent systematic review included 127 cases of severe PE during pregnancy (and until 6 weeks post-partum) treated with thrombolysis, thrombectomy, and/or ECMO.421 Both high- and intermediate-risk PE cases were included, and 23% of women experienced cardiac arrest. Reported survival rates were 94 and 86% following thrombolysis and surgical thrombectomy, respectively; however, these favourable rates may reflect reporting bias. Following thrombolysis, major bleeding occurred in 18 and 58% of cases during pregnancy and in the post-partum period, respectively.421 Finally, foetal deaths occurred in 12 and 20% of the cases following thrombolysis and thrombectomy, respectively.421 Thrombolytic treatment should not be used peri-partum, except in the setting of life-threatening PE. Typically, UFH is used in the acute treatment of high-risk PE.
Although the indications for vena cava filters are similar to those for non-pregnant patients (discussed in section 6), there is limited experience with their use in pregnancy and the risk associated with the procedure may be increased.
Suggestions for the anticoagulation management of PE in specific clinical situations (also) related to pregnancy, for which conclusive evidence is lacking, are presented in Supplementary Data Table 9.
9.3.1 Role of a multidisciplinary pregnancy heart team
A team of multidisciplinary colleagues should collaborate in the planning of ante-, peri-, and post-partum care pathways for women with cardiovascular diseases, including PE. As many members as possible of this team should have expertise in the management of PE during pregnancy and the post-partum period. Jointly agreed, written care pathways should be available (if timelines permit) for effective communication (an example is shown in Figure 7).
9.4 Amniotic fluid embolism
Amniotic fluid embolism (AFE) is a rare condition that occurs during pregnancy or shortly after delivery. It remains one of the leading causes of direct maternal death (i.e. death resulting from the pregnancy rather than from pre-existing conditions) in high-income countries.422 Diagnosis of AFE is challenging, being primarily a clinical diagnosis of exclusion. Awareness of AFE, prompt diagnosis, and aggressive life support are of critical importance. AFE is characterized by unexplained sudden cardiovascular or respiratory deterioration, often accompanied by disseminated intravascular coagulation,422 and occurring during pregnancy or after delivery.423,424 The reported incidence is approximately 2–7 per 100 000 maternities, with a mortality rate of 0.5–6 deaths per 100 000 deliveries.422,425,426 Reported case fatality rates vary, reflecting the challenges in making the diagnosis and the rarity of AFE. In a retrospective Californian study including more than 3.5 million deliveries, a case fatality rate of 13% was reported, as in other US and Canadian studies.425 Similarly, a case fatality rate of 19% was reported in a recent prospective UK population-based study with validated case criteria.422 Recent literature have suggested that risk factors for AFE may include pre-existing cardiac, cerebrovascular, and renal disorders, placenta previa, polyhydramnios, stillbirth, chorioamnionitis, hypertensive disorders, instrumental delivery, and caesarean section.422,425 Management of AFE is supportive, and based on high-quality emergency care following the recognition and diagnosis of the condition, with prompt treatment of bleeding and coagulopathy.423 Awareness of AFE should be integral to the education of involved physicians and to emergency algorithms.
9.5 Recommendations for pulmonary embolism in pregnancy
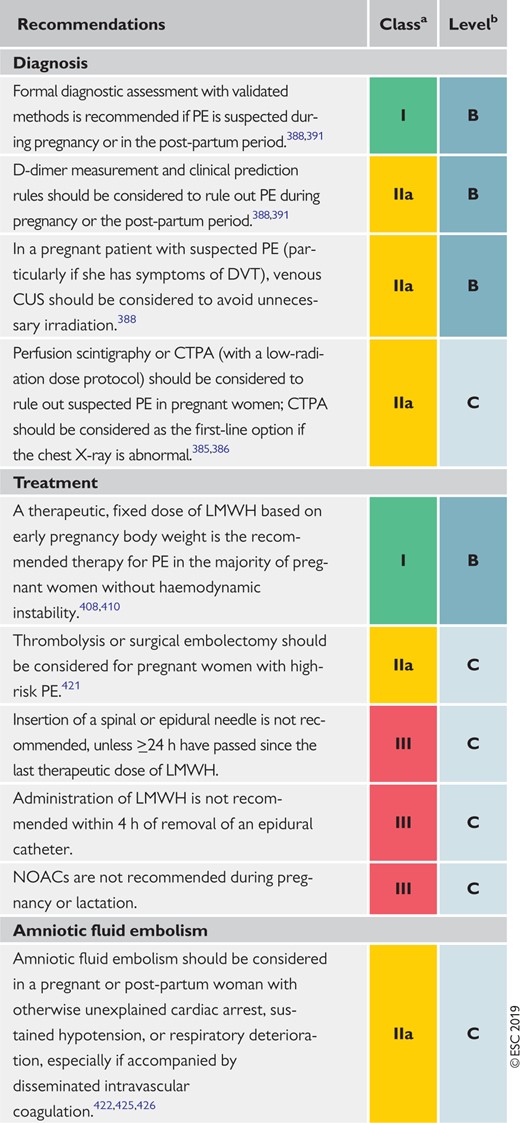 |
 |
CTPA = computed tomography pulmonary angiography; CUS = compression ultrasonography; DVT = deep vein thrombosis; LMWH = low-molecular weight heparin; NOACs = non-vitamin K antagonist oral anticoagulants; PE = pulmonary embolism.
Class of recommendation.
Level of evidence.
 |
 |
CTPA = computed tomography pulmonary angiography; CUS = compression ultrasonography; DVT = deep vein thrombosis; LMWH = low-molecular weight heparin; NOACs = non-vitamin K antagonist oral anticoagulants; PE = pulmonary embolism.
Class of recommendation.
Level of evidence.
10 Long-term sequelae of pulmonary embolism
The patency of the pulmonary arterial bed is restored in the majority of PE survivors within the first few months following the acute episode; therefore, no routine follow-up CTPA imaging is needed in such patients treated for PE.427 However, in other patients, thrombi become persistent and organized, which in rare cases may result in CTEPH, a potentially life-threatening obstructing vasculopathy. The rarity of this condition is in contrast to the relatively large number of patients who report persisting dyspnoea or poor physical performance over several months after acute PE. Thus, the aims of an efficient follow-up strategy after PE should be to: (i) provide appropriate care (exercise rehabilitation, treatment of comorbidity, behavioural education, and modification of risk factors) to patients with persisting symptoms, and (ii) ensure early detection of CTEPH to refer the patient for further diagnostic workup and specific treatment.
10.1 Persisting symptoms and functional limitation after pulmonary embolism
Cohort studies conducted over the past decade (summarized in Klok et al.428) have revealed that persisting or deteriorating dyspnoea, and poor physical performance, are frequently present 6 months to 3 years after an acute PE episode. The proportion of patients claiming that their health status is worse at 6 month follow-up than it was at the time of PE diagnosis varies widely, ranging between 20 and 75%.429–431 The following baseline parameters and findings could be identified as predictors of exertional dyspnoea at long-term follow-up after PE: advanced age, cardiac or pulmonary comorbidity, higher body mass index, and history of smoking;429 higher systolic PAP and RV dysfunction at diagnosis;430,432,433 and residual pulmonary vascular obstruction at discharge.434
More recently, a prospective cohort study enrolled 100 patients at five Canadian hospitals between 2010 and 2013, and followed them over 1 year.435 As many as 47% of the patients had reduced maximal aerobic capacity, defined as peak oxygen consumption <80% of the predicted value on cardiopulmonary exercise testing (CPET). This functional outcome was associated with significantly worse generic health-related quality of life and dyspnoea scores, as well as with a significantly reduced 6 min walk distance.435 Independent predictors of reduced functional exercise capacity and quality of life over time included female sex, higher body mass index, history of lung disease, higher pulmonary artery systolic pressures on the 10 day echocardiogram, and higher main pulmonary artery diameter on the baseline CTPA.436 Of note, pulmonary function tests and echocardiographic results at follow-up were largely within normal limits, both in patients with and without reduced maximal aerobic capacity.435 Lack of an association between exercise impairment, and persistent RV dilation or dysfunction, was also reported by a study of 20 survivors of massive or submassive PE.437
Taken together, older and more recent cohort studies have suggested that muscle deconditioning, particularly in the presence of excess body weight and cardiopulmonary comorbidity, is largely responsible for the frequently reported dyspnoea and signs of exercise limitation after acute PE. This also means that, at least in the majority of cases, poor physical performance after PE does not appear to be attributable to ‘large’ residual thrombi, or persisting/progressive PH and RV dysfunction. Ongoing prospective studies in large numbers of patients may help to better identify predictors of functional and/or haemodynamic impairment after acute PE, and their possible implications for shaping follow-up programmes.438
As mentioned in section 6, it remains unclear whether early reperfusion treatment, notably thrombolysis, has an impact on clinical symptoms, functional limitation, or persistent (or new-onset) PH at long-term follow-up after PE. Consequently, prevention of long-term PE sequelae is, at present, no justification for thrombolytic treatment in the acute phase of PE.
10.2 Chronic thromboembolic pulmonary hypertension
10.2.1 Epidemiology, pathophysiology, and natural history
CTEPH is a disease caused by the persistent obstruction of pulmonary arteries by organized thrombi, leading to flow redistribution and secondary remodelling of the pulmonary microvascular bed. CTEPH has been reported with a cumulative incidence of between 0.1 and 9.1% in the first 2 years after a symptomatic PE event; the large margin of error is due to referral bias, the paucity of early symptoms, and the difficulty of differentiating acute PE from symptoms of pre-existing CTEPH.439,440 A prospective, multicentre, observational screening survey for the detection of CTEPH included patients with acute PE from 11 centres in Switzerland, from March 2009 to November 2016. Screening for possible CTEPH was performed at 6, 12, and 24 months using a stepwise algorithm that included a phone-based dyspnoea survey, TTE, right heart catheterization, and radiological confirmation of CTEPH. Of 508 patients assessed for CTEPH screening over 2 years, CTEPH incidence following PE was 3.7 per 1000 patient-years, with a 2 year cumulative incidence of 0.79%.441 In Germany, the incidence of CTEPH in 2016 was estimated at 5.7 per million adult population.442
The hallmark of CTEPH is fibrotic transformation of a pulmonary arterial thrombus, causing fixed mechanical obstruction of pulmonary arteries and leading to overflow of the open pulmonary arterial bed. Together with collateral supply from systemic arteries downstream of pulmonary arterial occlusions, this contributes to microvascular remodelling causing a progressive increase in PVR.443 Owing to this complex pathophysiology, there is no clear correlation between the degree of mechanical obstruction found at imaging and haemodynamics, which can deteriorate in the absence of recurrent PE.444
Two historical trials assessed survival in patients with CTEPH before the availability of surgical treatment. In both studies, mean PAP >30 mmHg was related to poor survival, similar to that reported for idiopathic pulmonary arterial hypertension.445,446
The most frequently cited risk factors and predisposing conditions for CTEPH are shown in Table 13. In an international registry, a history of acute PE was reported by 75% of patients.447 Associated conditions and comorbidities included thrombophilic disorders, particularly antiphospholipid antibody syndrome and high coagulation factor VIII levels, cancer, a history of splenectomy, inflammatory bowel disease, ventriculo-atrial shunts, and infection of chronic i.v. lines and devices such as implantable pacemakers.
| Findings related to the acute PE event (obtained at PE diagnosis) . | Concomitant chronic diseases and conditions predisposing to CTEPH (documented at PE diagnosis or at 3–6 month follow-up) . |
|---|---|
| Previous episodes of PE or DVT | Ventriculo-atrial shunts |
| Large pulmonary arterial thrombi on CTPA | Infected chronic i.v. lines or pacemakers |
| Echocardiographic signs of PH/RV dysfunctiona | History of splenectomy |
| CTPA findings suggestive of pre-existing chronic thromboembolic diseaseb | Thrombophilic disorders, particularly antiphospholipid antibody syndrome and high coagulation factor VIII levels |
| Non-O blood group | |
| Hypothyroidism treated with thyroid hormones | |
| History of cancer | |
| Myeloproliferative disorders | |
| Inflammatory bowel disease | |
| Chronic osteomyelitis |
| Findings related to the acute PE event (obtained at PE diagnosis) . | Concomitant chronic diseases and conditions predisposing to CTEPH (documented at PE diagnosis or at 3–6 month follow-up) . |
|---|---|
| Previous episodes of PE or DVT | Ventriculo-atrial shunts |
| Large pulmonary arterial thrombi on CTPA | Infected chronic i.v. lines or pacemakers |
| Echocardiographic signs of PH/RV dysfunctiona | History of splenectomy |
| CTPA findings suggestive of pre-existing chronic thromboembolic diseaseb | Thrombophilic disorders, particularly antiphospholipid antibody syndrome and high coagulation factor VIII levels |
| Non-O blood group | |
| Hypothyroidism treated with thyroid hormones | |
| History of cancer | |
| Myeloproliferative disorders | |
| Inflammatory bowel disease | |
| Chronic osteomyelitis |
CTEPH = Chronic thromboembolic pulmonary hypertension; CTPA = computed tomographic pulmonary angiography; DVT = deep vein thrombosis; i.v. = intravenous; LV = left ventricular; PE = pulmonary embolism; PH = pulmonary hypertension; RV = right ventricular.
Echocardiographic criteria of RV dysfunction are graphically presented in Figure 3, and their prognostic value summarized in Supplementary Data Table 3. On CTPA (four-chamber views of the heart), RV dysfunction is defined as RV/LV diameter ratio >1.0.
Direct and indirect vascular signs, as well as lung parenchymal findings, are summarized in Supplementary Data Table 2.
| Findings related to the acute PE event (obtained at PE diagnosis) . | Concomitant chronic diseases and conditions predisposing to CTEPH (documented at PE diagnosis or at 3–6 month follow-up) . |
|---|---|
| Previous episodes of PE or DVT | Ventriculo-atrial shunts |
| Large pulmonary arterial thrombi on CTPA | Infected chronic i.v. lines or pacemakers |
| Echocardiographic signs of PH/RV dysfunctiona | History of splenectomy |
| CTPA findings suggestive of pre-existing chronic thromboembolic diseaseb | Thrombophilic disorders, particularly antiphospholipid antibody syndrome and high coagulation factor VIII levels |
| Non-O blood group | |
| Hypothyroidism treated with thyroid hormones | |
| History of cancer | |
| Myeloproliferative disorders | |
| Inflammatory bowel disease | |
| Chronic osteomyelitis |
| Findings related to the acute PE event (obtained at PE diagnosis) . | Concomitant chronic diseases and conditions predisposing to CTEPH (documented at PE diagnosis or at 3–6 month follow-up) . |
|---|---|
| Previous episodes of PE or DVT | Ventriculo-atrial shunts |
| Large pulmonary arterial thrombi on CTPA | Infected chronic i.v. lines or pacemakers |
| Echocardiographic signs of PH/RV dysfunctiona | History of splenectomy |
| CTPA findings suggestive of pre-existing chronic thromboembolic diseaseb | Thrombophilic disorders, particularly antiphospholipid antibody syndrome and high coagulation factor VIII levels |
| Non-O blood group | |
| Hypothyroidism treated with thyroid hormones | |
| History of cancer | |
| Myeloproliferative disorders | |
| Inflammatory bowel disease | |
| Chronic osteomyelitis |
CTEPH = Chronic thromboembolic pulmonary hypertension; CTPA = computed tomographic pulmonary angiography; DVT = deep vein thrombosis; i.v. = intravenous; LV = left ventricular; PE = pulmonary embolism; PH = pulmonary hypertension; RV = right ventricular.
Echocardiographic criteria of RV dysfunction are graphically presented in Figure 3, and their prognostic value summarized in Supplementary Data Table 3. On CTPA (four-chamber views of the heart), RV dysfunction is defined as RV/LV diameter ratio >1.0.
Direct and indirect vascular signs, as well as lung parenchymal findings, are summarized in Supplementary Data Table 2.
10.2.2 Clinical presentation and diagnosis
Diagnosing CTEPH is difficult. Algorithms for predicting450 or ruling out CTEPH451,452 are limited by a lack of specificity. The clinical characteristics of patients enrolled in an international CTEPH registry have shown that the median age at diagnosis is 63 years and that both sexes are equally affected; paediatric cases are rare.447 Clinical symptoms and signs are non-specific or absent in early CTEPH, with signs of right heart failure only becoming evident in advanced disease. Thus, early diagnosis remains a challenge in CTEPH, with a median time of 14 months between symptom onset and diagnosis in expert centres.453 When present, the clinical symptoms of CTEPH may resemble those of acute PE or of pulmonary arterial hypertension; in the latter context, oedema and haemoptysis occur more often in CTEPH, while syncope is more common in pulmonary arterial hypertension.453
The diagnosis of CTEPH is based on findings obtained after at least 3 months of effective anticoagulation, to distinguish this condition from acute PE. The diagnosis requires a mean PAP of ≥25 mmHg along with a pulmonary arterial wedge pressure of ≤15 mmHg, documented at right heart catheterization in a patient with mismatched perfusion defects on V/Q lung scan. Specific diagnostic signs for CTEPH on multidetector CT angiography or conventional pulmonary cineangiography include ring-like stenoses, webs, slits, and chronic total occlusions.289
Some patients may present with normal pulmonary haemodynamics at rest despite symptomatic disease. If other causes of exercise limitation are excluded, these patients are considered as having chronic thromboembolic disease (CTED). Identification of patients with chronic thromboembolism without PH, who may have an indication for surgical or interventional treatment, requires particular expertise and should be done in CTEPH referral centres. Among 1019 patients who were submitted to pulmonary endarterectomy (PEA) in a UK referral centre, 42 patients did not have pulmonary hypertension at rest but showed functional improvement after the operation.454
Planar V/Q lung scan is a suitable first-line imaging modality for CTEPH as it has 96–97% sensitivity and 90–95% specificity for the diagnosis.455 SPECT seems less sensitive than planar V/Q scanning if assessed at a level of individual segmental arteries, but it is unlikely to miss clinically relevant CTEPH in an individual patient. In contrast to CTEPH, abnormal mismatched perfusion defects sometimes found in pulmonary arterial hypertension and pulmonary veno-occlusive disease typically have a non-segmental pattern.
CTPA is gaining ground as a diagnostic modality in CTEPH,456 but it should not be used as a stand-alone test to exclude the disease.455 Newer diagnostic tests include dual-energy CT, which allows the simultaneous assessment of patency of the pulmonary arteries and of lung perfusion, probably at a cost of some increase in radiation delivered to the patient. Magnetic resonance imaging of the pulmonary vasculature is still considered inferior to CT.457 Cone-beam CT,458 angioscopy,459 intravascular ultrasound, and optical coherence tomography are more suitable for the characterization of lesions during interventional treatment than for diagnosis. High-resolution CT scan of the chest may assist in the differential diagnosis of CTEPH, showing emphysema, bronchial, or interstitial lung disease, as well as infarcts, and vascular and thoracic wall malformations. Perfusion inequalities manifesting as a mosaic parenchymal pattern are frequently found in CTEPH, but may also be observed in ≤12% of patients with other causes of PH. Differential diagnosis of CTEPH should also include pulmonary arteritis, pulmonary angiosarcoma, tumour embolism, parasites (hydatid cyst), foreign body embolism, and congenital or acquired pulmonary artery stenoses.289
10.2.3 Surgical treatment
Surgical PEA is the treatment of choice for operable CTEPH. In contrast to surgical embolectomy for acute PE, treatment of CTEPH necessitates a true bilateral endarterectomy through the medial layer of the pulmonary arteries. It requires deep hypothermia and intermittent circulatory arrest, without a need for cerebral perfusion.460,461 In-hospital mortality is currently as low as 4.7%462 and is even lower in high-volume single centres.463 The majority of patients experience substantial relief from symptoms and near-normalization of haemodynamics.461–464 Owing to the complexity of both the surgical technique and peri-procedural management, PEA is performed in specialized centres. Eligibility for surgery requires a decision taken during a dedicated meeting of a multidisciplinary CTEPH team including experienced surgeons for PEA, interventional radiologists or cardiologists, radiologists experienced in pulmonary vascular imaging, and clinicians with expertise in PH. The CTEPH team should confirm the diagnosis, assess the surgical accessibility of chronic post-thrombotic obstructions (‘surgical operability’), and consider the risks related to comorbidities (‘medical operability’). The operability of patients with CTEPH is determined by multiple factors that cannot easily be standardized. These are related to the suitability of the patient, the expertise of the surgical team, and available resources. General criteria include pre-operative New York Heart Association (NYHA) functional class and the surgical accessibility of thrombi in the main, lobar, or segmental pulmonary arteries.462 Advanced age per se is no contraindication for surgery. There is no haemodynamic threshold or measure of RV dysfunction that can be considered to preclude PEA.
Data from the international CTEPH registry, set up in 27 centres to evaluate the long-term outcome and outcome predictors in 679 operated and not-operated patients, showed estimated survival at 3 years of 89% in operated and 70% in not-operated patients.465 Mortality was associated with NYHA functional class, RA pressure, and a history of cancer.465 In this prospective registry, the long-term prognosis of operated patients was better than the outcome of not-operated patients.465 Additional correlates of mortality were bridging therapy with pulmonary vasodilators, post-operative PH, surgical complications, and additional cardiac procedures in operated patients, and comorbidities such as coronary disease, left heart failure, and chronic obstructive pulmonary disease in not-operated patients.465 A recent report identified mean PAP ≥38 mmHg and PVR ≥425 dyn*s*cm−5 as determinants of poor prognosis in survivors of surgical treatment for CTEPH.466
Post-operative ECMO is recommended as the standard of care in PEA centres.461 Early post-operative reperfusion oedema may require veno-arterial ECMO, and severe persistent PH may be bridged to emergency lung transplantation with ECMO. After PEA, patients should be followed in CTEPH centres to exclude persistent or recurrent PH, with at least one haemodynamic assessment to be considered at 6–12 months after the intervention.
10.2.4 Balloon pulmonary angioplasty
Over the past decade, balloon pulmonary angioplasty (BPA) has emerged as an effective treatment for technically inoperable CTEPH. It allows dilatation of obstructions down to subsegmental vessels, which are inaccessible to surgery. BPA is a stepwise procedure requiring several (usually 4–10) separate sessions. This is necessary to engage all under-perfused lung segments, while limiting the contrast burden and radiation delivered per session. Navigation in distal pulmonary arteries requires particular expertise, as the complexity and individual variability of the pulmonary arterial tree greatly exceeds that of other vascular beds. Complications include wire- and balloon-induced injury, which may result in intrapulmonary bleeding, haemoptysis, and reperfusion lung injury. Usually, bleeding resolves spontaneously, but sometimes it has to be controlled by transient balloon inflation proximal to the site of perforation; in rare cases it requires embolization. Mild hypoxaemia is frequent and can be controlled by oxygen delivery. Mechanical ventilation or ECMO is rarely needed.
The largest published registry to date included 249 patients with a mean age of 61.5 years, who were treated with BPA between 2004 and 2013 in seven Japanese centres.467 Mean PAP decreased from 43 to 24 mmHg after terminating BPA sessions, and this result was maintained in 196 patients who underwent follow-up right heart catheterization. Complications occurred in 36% of the patients, including pulmonary injury (18%), haemoptysis (14%), and pulmonary artery perforation (2.9%). After BPA, 30 day mortality was 2.6% and overall survival was 97% at 1 year.467
While most of the BPA procedures are performed in technically inoperable patients, this method has also been used for sequential treatment for PH persisting after PEA. Few ‘rescue’ BPA interventions performed in unstable patients remaining on ECMO after PEA were ineffective.468
10.2.5 Pharmacological treatment
Optimal medical treatment for CTEPH consists of anticoagulants, as well as diuretics and oxygen in cases of heart failure or hypoxaemia. Lifelong oral anticoagulation with VKAs is recommended, and also after successful PEA or BPA. No data exist on the efficacy and safety of NOACs.
Pulmonary microvascular disease in CTEPH has provided the rationale for also testing drugs that have been approved for pulmonary arterial hypertension for this indication. Based on available data, medical treatment of CTEPH with targeted therapy is now justified for technically inoperable patients,469,470 as well as for patients with PH persisting after PEA.469 To date, the only drug approved for inoperable CTEPH or persistent/recurrent PH after PEA is riociguat, an oral stimulator of soluble guanylate cyclase.469 In a prospective randomized trial of 261 patients with inoperable CTEPH or persistent/recurrent PH after PEA, treatment with riociguat significantly increased 6 min walking distance and reduced PVR.469 In a similar population of 157 patients, the dual endothelin antagonist bosentan showed a positive effect on haemodynamics, but no improvement was observed in exercise capacity and the primary outcome was not met.471 Another dual endothelin antagonist, macitentan, was found to significantly improve PVR and 6 min walking distance compared to placebo in a phase II trial focusing on inoperable patients with CTEPH.470 Currently, riociguat is being tested in trials addressing its efficacy and safety: (i) as bridging therapy for patients scheduled to undergo PEA (NCT 03273257) and (ii) in comparison to BPA (NCT 02634203).
Overall, the effects on clinical worsening of drugs tested with RCTs in patients with CTEPH have not yet been clarified. Furthermore, no data exist on medical treatment in technically operable patients with prohibitive comorbidities or those refusing surgery. Off-label combination of drugs approved for pulmonary arterial hypertension has been proposed for CTEPH patients presenting with severe haemodynamic compromise, but only limited prospective data are available to date.470
Medical therapy is not indicated in symptomatic survivors of acute PE with documented post-thrombotic obstructions but an absence of PH at right heart catheterization at rest (CTED).
10.3 Strategies for patient follow-up after pulmonary embolism
Figure 8 displays a proposed follow-up strategy for survivors of acute PE following discharge from hospital. Evaluation of the patients 3–6 months after the acute PE episode is recommended to assess the persistence (or new onset) and severity of dyspnoea or functional limitation, and to check for possible signs of VTE recurrence, cancer, or bleeding complications of anticoagulation. The severity of dyspnoea can be assessed using the Medical Research Council scale;160 alternatively, the World Health Organization functional class can be determined (Supplementary Data Table 16).289
Follow-up strategy and diagnostic workup for long-term sequelae of pulmonary embolism. CPET = cardiopulmonary exercise testing; CTEPH = chronic thromboembolic pulmonary hypertension; NT-proBNP = N-terminal pro B-type natriuretic peptide; PE = pulmonary embolism; PH = pulmonary hypertension; TTE = transthoracic echocardiography/echocardiogram; V/Q = ventilation/perfusion (lung scintigraphy). aAssess the persistence (or new onset) and severity of dyspnoea or functional limitation, and also check for possible signs of VTE recurrence, cancer, or bleeding complications of anticoagulation. bThe Medical Research Council scale can be used to standardize the evaluation of dyspnoea;160 alternatively, the World Health Organization functional class can be determined (Supplementary Data Table16).289 cAs defined by the ESC/ERS guidelines on the diagnosis and treatment of Pulmonary Hypertension (Supplementary Data Tables 17 and 18).289 dRisk factors and predisposing conditions for CTEPH are listed in Table 13. eCardiopulmonary exercise testing, if appropriate expertise and resources are available on site; abnormal results include, among others, reduced maximal aerobic capacity (peak oxygen consumption), increased ventilatory equivalent for carbon dioxide, and reduced end-tidal carbon dioxide pressure. fConsider CPET in the diagnostic work-up.
In patients complaining of persisting dyspnoea and poor physical performance, TTE should be considered as the next step to assess the probability of (chronic) PH and thus possible CTEPH. The criteria and levels of PH probability are defined by current ESC Guidelines,289 and are listed in Supplementary Data Tables 17 and 18. Patients with a high echocardiographic probability of PH, or those with intermediate probability combined with elevated NT-proBNP levels or risk factors/predisposing conditions for CTEPH, such as those listed in Table 13, should be considered for a V/Q scan.
If mismatched perfusion defects are found on the V/Q scan, referral to a PH or CTEPH expert centre for further diagnostic workup is indicated. If, on the other hand, the V/Q scan is normal and the patient’s symptoms remain unexplained, CPET may be performed. By providing evidence of reduced maximal aerobic capacity, CPET supports the need for further follow-up visits and helps to identify candidates for pulmonary rehabilitation, exercise, or weight-reduction programmes.435,436 CPET may also be helpful in patients with suspected CTEPH and coexisting left heart and/or respiratory disease; in such cases, it can help to establish the main limiting factor and thus set priorities for the treatment strategy.472
For patients who report as free of dyspnoea or functional limitation at 3–6-month follow-up after acute PE but have risk factors/predisposing conditions for CTEPH (Table 13), further follow-up visits may be scheduled and the patient must be advised to return if symptoms appear. Alternatively, TTE may be considered to assess the probability of PH (Figure 8).
Apart from the recommended screening and diagnostic measures, an integrated model of patient care after PE should be provided, taking into consideration the infrastructure and possibilities offered by each country’s health system. The model should include appropriately qualified nurses, interdisciplinary working with physicians in the care of both in-hospital and ambulatory PE patients, standardized treatment protocols adapted to the capacities of each hospital, and bidirectional referral pathways between general practice and the hospital. Such models ensure smooth transitions between hospital specialists and practitioners; provide continuity, and easy access to care along with information and education; and respect the patients’ preferences, and those of their families and social environment. In this context, nurse-led care models to deliver follow-up have been shown to be effective after acute coronary syndrome,473 in primary care-based management of chronic diseases,474 and in community based self-management initiatives.475 A recently published study investigated the care of 42 patients followed at a pulmonary arterial hypertension (PAH)/CTEPH nurse-led outpatient clinic and showed positive results.476 During patient follow-up visits, appropriately qualified nurses screen for signs and symptoms indicating VTE recurrence or complications of treatment, and assess adherence to medication. Nurses work collaboratively with patients using behavioural frameworks and motivational interviewing, to identify and modify associated risk factors (smoking cessation, diet, physical activity, and exercise). In addition, they promote self-management skills such as the use of compression stockings, safe increase in mobility, increased awareness of signs of recurrence, or complications.
10.4 Recommendations for follow-up after acute pulmonary embolism
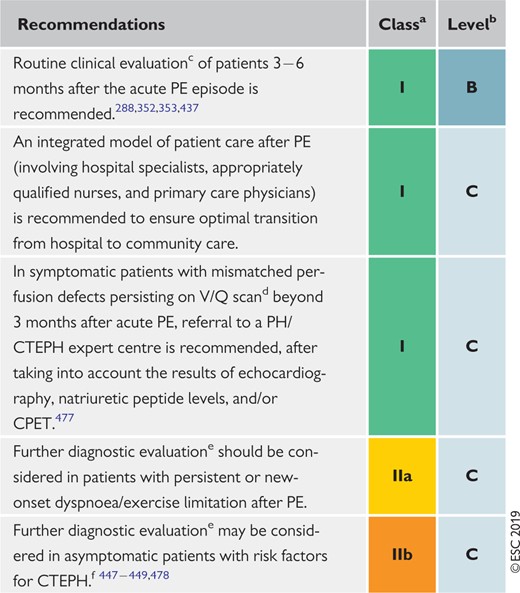 |
 |
CPET = cardiopulmonary exercise testing; CT = computed tomography; CTEPH = Chronic thromboembolic pulmonary hypertension; PE = pulmonary embolism; PH = pulmonary hypertension; V/Q = ventilation/perfusion (lung scintigraphy).
Class of recommendation.
Level of evidence.
For symptoms suggesting recurrence, bleeding, malignancy, or persistent or new-onset exercise limitation, and to decide on extension of anticoagulant treatment.
Alternatively, dual-energy CT may be used, if appropriate expertise and resources are available on-site.
As proposed in the algorithm shown in Figure 8.
Risk factors and predisposing conditions for CTEPH are listed in Table 13.
 |
 |
CPET = cardiopulmonary exercise testing; CT = computed tomography; CTEPH = Chronic thromboembolic pulmonary hypertension; PE = pulmonary embolism; PH = pulmonary hypertension; V/Q = ventilation/perfusion (lung scintigraphy).
Class of recommendation.
Level of evidence.
For symptoms suggesting recurrence, bleeding, malignancy, or persistent or new-onset exercise limitation, and to decide on extension of anticoagulant treatment.
Alternatively, dual-energy CT may be used, if appropriate expertise and resources are available on-site.
As proposed in the algorithm shown in Figure 8.
Risk factors and predisposing conditions for CTEPH are listed in Table 13.
11 Non-thrombotic pulmonary embolism
This section is included in the Supplementary Data available online on the EHJ and ESC websites (www.escardio.org/guidelines).
12 Key messages
The ESC Task Force has selected 10 simple key messages and rules to guide physicians in the diagnosis and management of PE:
In patients presenting with haemodynamic instability, perform bedside TTE as a fast, immediate step to differentiate suspected high-risk PE from other acute life-threatening situations.
If you suspect acute PE, institute anticoagulation therapy as soon as possible, while the diagnostic workup is ongoing, unless the patient is bleeding or has absolute contraindications to this therapy.
Use recommended, validated diagnostic algorithms for PE, including standardized assessment of (pre-test) clinical probability and D-dimer testing. They help to avoid unnecessary, expensive, and potentially harmful imaging tests and exposure to ionizing radiation.
If the CTPA report suggests single subsegmental PE, consider the possibility of a false-positive finding. Discuss the findings again with the radiologist and/or seek a second opinion to avoid misdiagnosis, and unnecessary, potentially harmful anticoagulation treatment.
Confirmation of PE in a patient, without haemodynamic instability, must be followed by further risk assessment involving clinical findings, evaluation of the size and/or function of the RV, and laboratory biomarkers as appropriate. This information will help you to decide on the need for reperfusion treatment or monitoring for patients at elevated risk, or consider the option of early discharge and continuation of anticoagulation on an ambulatory basis for patients at low risk.
As soon as you diagnose (or strongly suspect) high-risk PE, select the best reperfusion option (systemic thrombolysis, surgical embolectomy, or catheter-directed treatment) considering the patient’s risk profile, and the resources and expertise available at your hospital. For patients with intermediate−high-risk PE, reperfusion is not first-line treatment, but you should prospectively plan the management strategy with your team to have a contingency plan ready if the situation deteriorates.
Prefer anticoagulation with a NOAC over the ‘traditional’ LMWH−VKA regimen unless the patient has contraindication(s) to this type of drug.
Always remember that, with the exception of acute PE provoked by a strong transient/reversible risk factor, there is a lifelong risk of VTE recurrence after a first episode of PE. Consequently, re-examine the patient after the first 3 − 6 months of anticoagulation, weigh the benefits vs. risks of continuing treatment, and decide on the extension and dose of anticoagulant therapy, also considering the patient’s preference. Remember to recommend regular follow-up examinations, e.g. at yearly intervals.
If you suspect PE in a pregnant patient, consider diagnostic pathways and algorithms including CTPA or V/Q lung scan, which can be used safely during pregnancy.
After acute PE, patients should not be lost to follow-up. Apart from checking for possible signs of VTE recurrence, cancer, or bleeding complications of anticoagulation, ask the patient if there is persisting or new-onset dyspnoea or functional limitation. If yes, implement a staged diagnostic workup to exclude CTEPH or chronic thromboembolic disease, and to detect/treat comorbidity or ‘simple’ deconditioning. Follow-up imaging is not routinely recommended in an asymptomatic patient, but it may be considered in patients with risk factors for development of CTEPH.
13 Gaps in the evidence
Diagnosis
The optimal method to adjust (based on the patient’s age or in combination with clinical probability) the D-dimer threshold, permitting the exclusion of PE while reducing the number of unnecessary imaging tests to a minimum, remains to be determined.
The diagnostic value and clinical significance of isolated subsegmental contrast-filling defects in the modern CTPA era remain controversial.
No robust data exist to guide the decision on whether to treat incidental PE with anticoagulants compared with a strategy of watchful waiting.
For patients presenting with non-traumatic chest pain, the benefits vs. risks of ‘triple rule-out’ (for coronary artery disease, PE, and aortic dissection) CT angiography need further evaluation before such an approach can be routinely recommended.
Assessment of pulmonary embolism severity and the risk of early death
The optimal, clinically most relevant combination (and cut-off levels) of clinical and biochemical predictors of early PE-related death remain to be determined, particularly with regard to identifying possible candidates for reperfusion treatment among patients with intermediate-risk PE.
The need for assessment of the RV status in addition to clinical parameters, to classify a patient with acute symptomatic PE as being at low vs. intermediate risk, needs to be confirmed by further prospective management (cohort) studies.
Treatment in the acute phase
The clinical benefits vs. risks of reduced-dose thrombolysis and catheter-based reperfusion modalities in patients with intermediate−high-risk PE should be evaluated in prospective randomized trials.
The place of ECMO in the management of acute high-risk PE awaits support by additional evidence from prospective management (cohort) studies.
The optimal anticoagulant drug(s) and regimen in patients with renal insufficiency and CrCl <30 mL/min remain unclear.
The criteria for selecting patients for early discharge and outpatient treatment of PE, and particularly the need for assessment of the RV status with imaging methods and/or laboratory markers in addition to calculating a clinical score, need to be further validated in prospective cohort studies.
Chronic treatment and prevention of recurrence
The clinical value and the possible therapeutic implications of models or scores assessing the risk of VTE recurrence, and the risk of bleeding under anticoagulation, need to be revisited in the NOAC era.
The effectiveness of extended treatment with a reduced dose, or apixaban or rivaroxaban, should be confirmed in patients with a high risk of recurrent PE.
The evidence supporting the efficacy and safety of NOACs for the treatment of PE in patients with cancer needs to be extended by further studies.
In patients with cancer, the anticoagulant regimen and dose after the first 6 months should be clarified and prospectively tested.
The optimal time for discontinuing anticoagulant treatment after an episode of acute PE in patients with cancer is yet to be determined.
Pulmonary embolism and pregnancy
Diagnostic algorithms for PE in pregnancy, using modern radiological imaging techniques and low radiation doses, need to be prospectively tested in adequately powered cohort studies.
Controversy persists on the optimal LMWH dose and regimen for the treatment of PE during pregnancy.
NOACs are not allowed in pregnancy. However, if exposure to these drugs occurs during pregnancy despite this warning, any possible effects on the foetus should be recorded to provide more precise information on the risks and complications of these drugs, and adapt the instructions to physicians in the future.
Long-term sequelae of pulmonary embolism
The optimal follow-up strategy, including the spectrum of diagnostic tests that may be necessary, in patients with persisting symptoms and functional limitation after acute PE needs to be defined and prospectively validated.
In the absence of persisting symptoms or functional limitation after acute PE, the criteria for identifying patients whose risk of developing CTEPH may be sufficiently high to justify further diagnostic workup require further elaboration and validation in prospective cohort studies.
14 ‘What to do’ and ‘what not to do’ messages from the Guidelines
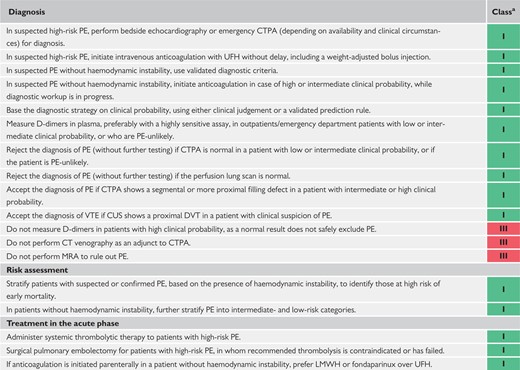 |
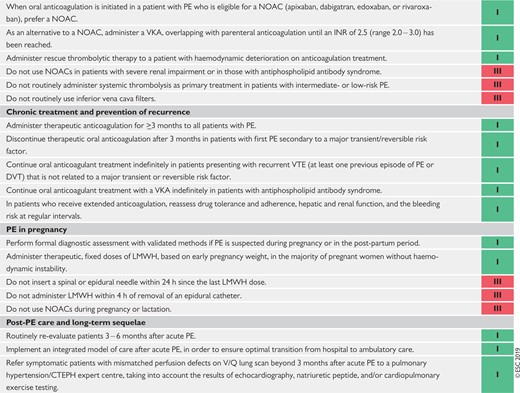 |
 |
 |
CT = computed tomography; CTPA = computed tomographic pulmonary angiography/angiogram; CTEPH = Chronic thromboembolic pulmonary hypertension; CUS = compression ultrasonography; DVT = deep vein thrombosis; INR = international normalized ratio; LMWH = low-molecular weight heparin; MRA = magnetic resonance angiography; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; UFH = unfractionated heparin; VKA = vitamin K antagonist; V/Q = ventilation/perfusion (lung scintigraphy); VTE = venous thromboembolism.
Class of recommendation.
 |
 |
 |
 |
CT = computed tomography; CTPA = computed tomographic pulmonary angiography/angiogram; CTEPH = Chronic thromboembolic pulmonary hypertension; CUS = compression ultrasonography; DVT = deep vein thrombosis; INR = international normalized ratio; LMWH = low-molecular weight heparin; MRA = magnetic resonance angiography; NOAC(s) = non-vitamin K antagonist oral anticoagulant(s); PE = pulmonary embolism; UFH = unfractionated heparin; VKA = vitamin K antagonist; V/Q = ventilation/perfusion (lung scintigraphy); VTE = venous thromboembolism.
Class of recommendation.
15 Supplementary data
Supplementary Data with additional Web Supplementary Tables complementing the full text, as well as section 11 on non-thrombotic PE, are available on the European Heart Journal website and via the ESC website at www.escardio.org/guidelines.
The disclosure forms of all experts involved in the development of these Guidelines are available on the ESC website www.escardio.org/guidelines
Author/Task Force Member Affiliations: listed in the Appendix.
ESC Committee for Practice Guidelines (CPG) and National Cardiac Societies document reviewers: listed in the Appendix.
ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing & Allied Professions (ACNAP), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI), Heart Failure Association (HFA).
Councils: Council on Cardiovascular Primary Care.
Working Groups: Aorta and Peripheral Vascular Diseases, Cardiovascular Surgery, Pulmonary Circulation and Right Ventricular Function, Thrombosis.
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC (journals.permissions@oxfordjournals.org).
Disclaimer. The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge, and the evidence available at the time of their publication. The ESC is not responsible in the event of any contradiction, discrepancy, and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic, or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and, where appropriate and/or necessary, the patient’s caregiver. Nor do the ESC Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
16 Appendix
Author/Task Force Member Affiliations:
Cecilia Becattini, Internal and Cardiovascular Medicine, University of Perugia, Perugia, Italy; Héctor Bueno, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain; and Cardiology, Hospital Universitario 12 de Octubre & i+12 Research Institute, Madrid, Spain; CIBERCV, Madrid, Spain; Geert-Jan Geersing, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands; Veli-Pekka Harjola, Emergency Medicine, Department of Emergency Medicine and Services, Helsinki University, Helsinki University Hospital, Helsinki, Finland; Menno V. Huisman, Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Netherlands; Marc Humbert, Service de Pneumologie, Hôpital Bicêtre, Assistance Publique-Hôpitaux de Paris, Univ. Paris-Sud, Université Paris-Saclay, Le Kremlin-Bicêtre, France; Catriona Sian Jennings, National Heart and Lung Institute (NHLI), Imperial College London, London, United Kingdom; David Jiménez, Respiratory Department, Ramón y Cajal Hospital and Alcala University, IRYCIS, Madrid, Spain; Nils Kucher, Angiology, University Hospital, Zurich, Switzerland; Irene Marthe Lang, Cardiology, Medical University of Vienna, Vienna, Austria; Mareike Lankeit, Department of Internal Medicine and Cardiology, Campus Virchow Klinikum, Charité–University Medicine Berlin, Berlin, Germany; and Center for Thrombosis and Hemostasis, University Medical Center Mainz, Mainz, Germany; Clinic of Cardiology and Pneumology, University Medical Center Göttingen, Göttingen, Germany; Roberto Lorusso, Cardio-Thoracic Surgery Department, Heart and Vascular Centre, Maastricht University Medical Centre (MUMC), Cardiovascular Research Institute Maastricht (CARIM), Maastricht, Netherlands; Lucia Mazzolai, Department of Angiology, CHUV, Lausanne, Switzerland; Nicolas Meneveau, Department of Cardiology, University Hospital Jean Minjoz and EA3920, University of Franche-Comté, Besançon, France; Fionnuala Ní Áinle, Haematology, Rotunda and Mater University Hospitals, Dublin, University College Dublin, Dublin, Ireland; Paolo Prandoni, Arianna Foundation on Anticoagulation, Bologna, Italy; Piotr Pruszczyk, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Warsaw, Poland; Marc Righini, Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland; Adam Torbicki, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, Warsaw, ECZ-Otwock, Poland; Eric Van Belle, Cardiology, Institut Coeur Poumon CHU de Lille and INSERM U1011 Lille, Lille, France; José Luis Zamorano, Cardiology, Hospital Ramón y Cajal, Madrid, Spain.
ESC Committee for Practice Guidelines (CPG): Stephan Windecker (Chairperson) (Switzerland), Victor Aboyans (France), Colin Baigent (United Kingdom), Jean-Philippe Collet (France), Veronica Dean (France), Victoria Delgado (Netherlands), Donna Fitzsimons (United Kingdom), Chris P. Gale (United Kingdom), Diederick E. Grobbee (Netherlands), Sigrun Halvorsen (Norway), Gerhard Hindricks (Germany), Bernard Iung (France), Peter Jüni (Canada), Hugo A. Katus (Germany), Ulf Landmesser (Germany), Christophe Leclercq (France), Maddalena Lettino (Italy), Basil S. Lewis (Israel), Bela Merkely (Hungary), Christian Mueller (Switzerland), Steffen E. Petersen (United Kingdom), Anna Sonia Petronio (Italy), Dimitrios J. Richter (Greece), Marco Roffi (Switzerland), Evgeny Shlyakhto (Russian Federation), Iain A. Simpson (United Kingdom), Miguel Sousa-Uva (Portugal), Rhian M. Touyz (United Kingdom).
ESC National Cardiac Societies actively involved in the review process of the 2019 ESC Guidelines on the diagnosis and management of acute pulmonary embolism:
Algeria: Algerian Society of Cardiology, Naima Hammoudi; Armenia: Armenian Cardiologists Association, Hamlet Hayrapetyan; Austria: Austrian Society of Cardiology, Julia Mascherbauer; Azerbaijan: Azerbaijan Society of Cardiology, Firdovsi Ibrahimov; Belarus: Belorussian Scientific Society of Cardiologists, Oleg Polonetsky; Belgium: Belgian Society of Cardiology, Patrizio Lancellotti; Bulgaria: Bulgarian Society of Cardiology, Mariya Tokmakova; Croatia: Croatian Cardiac Society, Bosko Skoric; Cyprus: Cyprus Society of Cardiology, Ioannis Michaloliakos; Czech Republic: Czech Society of Cardiology, Martin Hutyra; Denmark: Danish Society of Cardiology, Søren Mellemkjaer; Egypt: Egyptian Society of Cardiology, Mansour Mostafa; Estonia: Estonian Society of Cardiology, Julia Reinmets; Finland: Finnish Cardiac Society, Pertti Jääskeläinen; France: French Society of Cardiology, Denis Angoulvant; Germany: German Cardiac Society, Johann Bauersachs; Greece: Hellenic Society of Cardiology, George Giannakoulas; Hungary: Hungarian Society of Cardiology, Endre Zima; Italy: Italian Federation of Cardiology, Carmine Dario Vizza; Kazakhstan: Association of Cardiologists of Kazakhstan, Akhmetzhan Sugraliyev; Kosovo (Republic of): Kosovo Society of Cardiology, Ibadete Bytyçi; Latvia: Latvian Society of Cardiology, Aija Maca; Lithuania: Lithuanian Society of Cardiology, Egle Ereminiene; Luxembourg: Luxembourg Society of Cardiology, Steve Huijnen; Malta: Maltese Cardiac Society, Robert Xuereb; Moldova (Republic of): Moldavian Society of Cardiology, Nadejda Diaconu; Montenegro: Montenegro Society of Cardiology, Nebojsa Bulatovic; Morocco: Moroccan Society of Cardiology, Ilyasse Asfalou; North Macedonia: North Macedonian Society of Cardiology, Marijan Bosevski; Norway: Norwegian Society of Cardiology, Sigrun Halvorsen; Poland: Polish Cardiac Society, Bożena Sobkowicz; Portugal: Portuguese Society of Cardiology, Daniel Ferreira; Romania: Romanian Society of Cardiology, Antoniu Octavian Petris; Russian Federation: Russian Society of Cardiology, Olga Moiseeva; San Marino: San Marino Society of Cardiology, Marco Zavatta; Serbia: Cardiology Society of Serbia, Slobodan Obradovic; Slovakia: Slovak Society of Cardiology, Iveta Šimkova; Slovenia: Slovenian Society of Cardiology, Peter Radsel; Spain: Spanish Society of Cardiology, Borja Ibanez; Sweden: Swedish Society of Cardiology, Gerhard Wikström; Switzerland: Swiss Society of Cardiology, Drahomir Aujesky; Turkey: Turkish Society of Cardiology, Cihangir Kaymaz; Ukraine: Ukrainian Association of Cardiology, Alexander Parkhomenko; United Kingdom of Great Britain and Northern Ireland: British Cardiovascular Society, Joanna Pepke-Zaba.
17 References
Investigators P.
PREPIC Study Group.
Society for Maternal-Fetal Medicine;
Author notes
Marc Humbert, Marion Delcroix, Sean Gaine, Olivier Sanchez, and Anton Vonk Noordegraaf: representing the ERS.