-
PDF
- Split View
-
Views
-
Cite
Cite
Nicoline Jochmann, Karl Stangl, Edeltraut Garbe, Gert Baumann, Verena Stangl, Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases, European Heart Journal, Volume 26, Issue 16, August 2005, Pages 1585–1595, https://doi.org/10.1093/eurheartj/ehi397
Close - Share Icon Share
Abstract
Differences in pharmacokinetics, pharmacodynamics, and physiology contribute to the phenomenon that women and men frequently respond differently to cardiovascular drugs. Hormonal influences, in addition, can play an important role: for example, the menstrual cycle, menopause, and pregnancy—as a result of fluctuations in concentrations of sexual steroids, and of changes in total body water—can be associated with gender-specific differences in the plasma levels of cardiovascular drugs. Clinical relevance accordingly results, especially for substances with a narrow therapeutic margin. This review treats the most important pharmacodynamic gender-relevant differences in this context, and surveys available evidence on the benefits of therapy of chronic cardiovascular diseases in women. On the whole, the study situation for women is appreciably less favourable than for men: owing to the fact that women are under-represented in most studies, and that few gender-specific analyses have been conducted.
See page 1571 for the editorial comment on this article (doi:10.1093/eurheartj/ehi428)
Introduction
In accordance with the growing knowledge of the relevance of gender-specific differences in cardiovascular diseases, gender-adapted diagnosis and therapy has gained significance during recent years. Although there is widespread call today for evidence-based pharmacotherapy, the lack of solid data from studies often restricts these possibilities in women.1,2 The percentage of women participating in studies on coronary heart disease has risen since the mid-1980s, with the result that the percentage of women covered by such investigations now coincides with the actual prevalence of CHD in women. Women are still under-represented in studies on arterial hypertension and heart failure.2 It is not rare that women respond to cardiovascular medication differently from men. The causes may be related to differences in physiology, pharmacokinetics, and pharmacodynamics.
Gender-specific physiological differences include lower body mass index (BMI) and smaller organ size in women compared with men, resulting in larger distribution volumes in men. Women have a higher proportion of body fat which may increase the distribution volume for lipophilic drugs.3 In women, the percentage of tissue-water fluctuates throughout the menstrual cycle, as high estradiol concentrations are associated with sodium and water retention. Women have a lower glomerular filtration rate and lower creatinine clearance. In men, testosterone-induced increase in muscle metabolism is associated with augmented creatinine clearance.4
In the cardiovascular system, women and men likewise demonstrate differences that can explain dissimilarities in the therapeutic response to various drugs.5 Heart size is less in women.6 On the average, resting heart rate in women is three to five beats higher than in men.7,8 Length of the cardiac cycle in men is longer. In women, length of the cardiac cycle varies throughout the menstrual cycle and is prolonged during menstruation. These cyclic fluctuations no longer appear following complete autonomous blockade.7 Women have a longer corrected QT interval and a shorter sinus node recovery time.9,10
From pharmacokinetic studies there is evidence of gender-specific differences for a number of drugs. Drug absorption, either orally or transdermally, does not differ significantly between women and men. The same applies for plasma-protein binding of drugs. Relevant differences between women and men in the unbound fraction of highly plasma-protein-bound drugs have not been shown.11 Gender-specific differences in the activity of drug-metabolizing enzymes are possibly of clinical relevance. Many cardiovascular drugs are metabolized by enzymes of the cytochrome P450 (CYP) system. Endogenous hormones, including estrogens and progestins are also metabolized via these enzymes. Whereas men seem to have higher activities of the CYP450 isoenzymes CYP1A2, CYP2D6, and possibly CYP2E1, women appear to have a higher clearance of CYP3A4 substrates, although findings are not consistent.3,11–14
In human liver biopsies a higher expression of CYP3A4 messenger RNA and two-fold higher CYP3A4 levels were found in women compared with men.14 CYP3A4 contributes to first-pass metabolism of >50% of commonly used drugs. Substrates of this enzyme include the following: atorvastatin, diltiazem, estradiol, lovastatin, nimlodipine, nisoldipine, quinidine, verapamil, and simvastatin. For CYP2C19, which in part metabolizes propranolol, relevant gender-specific differences are not known. For CYP2C9 (substrates such as fluvastatin, torasemide, and in part losartan and irbesartan), only limited data are available.11 For CYP2D6 (substrates such as encainide, flecainide, mexiletine, propafenone, metoprolol, timolol, and in part propanolol), no differences in the activity between women and men,15 lower activity, and even higher activity have been reported for women.16–18 The earlier stated differences become clinically relevant primarily for substances with a narrow therapeutic margin, as is the case for most antiarrhythmics.
Few data are available on gender differences with respect to drug transporters, whose expression and activity are regulated partially by genetic elements and partially by sex hormones.11,19,20 As the isoenzyme CYP3A4 and the multidrug efflux transporter p-glycoprotein demonstrate appreciable substrate overlap, it has been hypothesized that gender-specific differences in the clearance of CYP3A4 substrates may be attributable to lower expression of p-glycoprotein in the liver of women.21 A recently published study, however, did not observe a differential hepatic expression of this transporter between women and men.14
Genetic variants are known for a considerable number of drug metabolizing enzymes, and also for drug transporters. These variants can lead to diminished enzyme activity, which is of documented clinical relevance e.g. for CYP2D6, CYP2C19, CYP2C9, or they may lead to increased enzyme activity, as in the case of CYP2D6. A reduction in enzyme activity can play a key role in the development of adverse drug reactions as a result of relative ‘overdosing’.22 There are no solid data that confirm that the frequency of these genetic variants differs between women and men.
Further female-specific aspects must be considered in the administration of drugs. Menstrual cycle, pregnancy, and menopause can be associated with changes in the pharmacokinetics of drugs, mostly as a result of changes in sex steroid concentrations and alterations in total body water (e.g. expansion of total body water, increase of renal plasma flow, and glomerular filtration during pregnancy). It has been reported that menstruation, pregnancy, and ovariectomy can modulate CYP2D6 activity.23–25 The clinical relevance of these changes is not clear. In addition, interactions with exogenous hormone therapy such as oral contraception and hormone replacement therapy must be taken into account. Estrogens and progestins interact with a number of cardiovascular drugs, possibly by inhibiting CYP enzymes or increasing drug glucuronidation. In vivo data have shown that oral contraceptives can increase or decrease drug concentrations of co-administered medications.11
Female gender has been shown to be a risk factor for the development of adverse drug reactions. Women have a 50–70% greater risk of suffering adverse drug reactions than men. Although the underlying reasons have to be elucidated, hormonal and immunological factors, in addition to differences in pharmacokinetics and pharmacodynamics, have been discussed.13
Methods
A literature review of articles on female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases was performed using the Medline databases. Medical subject headings and free-text searches were used, with entry of the search terms ‘women’, ‘gender’, ‘sex-specific’, ‘female-specific’, and ‘gender-specific’, combined with the terms ‘cardiovascular’, ‘cardiac’, as well as ‘pharmacotherapy’, ‘medication’, ‘drugs’, and ‘pharmacokinetics’. The resulting article titles or abstracts were scanned for relevance. Those regarding female or gender-specific aspects in chronic cardiovascular pharmacotherapy were included in the review, and the bibliographies of all studies identified were searched for additional relevant studies. In addition, major randomized cardiovascular studies with beta-blockers, renin–angiotensin system (RAS) antagonists, calcium channel blockers, digitalis, antiarrhythmics, acetylsalicylic acid, clopidogrel, and statins were checked individually with regard to female-specific aspects.
Cardiovascular pharmacotherapy
Beta-blockers
Experimental evidence has indicated that myocardial β1-receptors are up-regulated in case of estrogen deficiency, without effects on binding affinity.26,27 Hormone supplementation with estrogens and progestins can prevent such up-regulation.27 Reduced cardiac sympathetic response to catecholamines results, on the whole, under endogenous estrogens. Since sex hormones can modulate the regulation of β-adrenergic receptors in heart and vessels, gender-specific differences in the pharmacodynamics of β-receptor blockers are to be expected.
Gender-specific differences with respect to pharmacokinetic properties have been described for cardioselective and non-selective beta-blockers. The β1-selective blocker metoprolol is primarily metabolized via CYP2D6. Evidence exists that men have a greater activity of this enzyme and a faster clearance of metoprolol.18,28 Women have a significantly lower peripheral volume of distribution for metoprolol even after normalization for body-weight. Consequently, significantly higher plasma levels of metoprolol have been observed in women: maximum concentrations lay ∼100% above those for men.28 Drug exposure to metoprolol is further increased under therapy with oral contraceptives.29
For the non-selective beta-blocker propanolol, plasma concentrations in women are ∼80% higher than in men.30 Increased enzyme expression of CYP2D6 through increased testosterone levels in men has been discussed as the cause of greater clearance in men.28 Corresponding to the higher beta-blocker plasma levels in women, they demonstrate a more pronounced decrease in heart rate and systolic blood pressure under beta-blocker therapy than men. Women, in addition, experience significantly smaller increases in exercise heart rates than do men under these conditions.28,31,32
Major clinical endpoint studies of beta-blocker therapy in secondary prevention after myocardial infarction have revealed contradictory findings with respect to gender-specific differences. These studies, however, have not included sufficient numbers of women to enable significant findings.32,33 A meta-analysis of five randomized studies including a total of 5474 patients (4353 men and 1121 women) for investigating the effects of metoprolol on mortality after myocardial infarction, revealed a reduction in cardiovascular death that was comparable in women and men.34
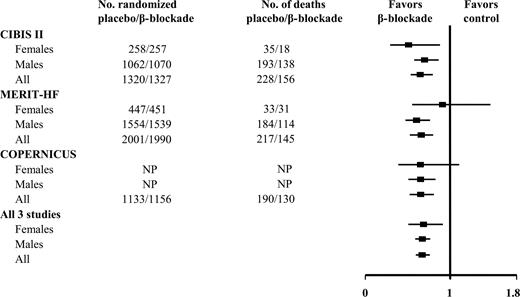
Recent investigations on the significance of beta-blocker therapy for heart failure support the assumption that beta-blocker therapy in men tends to be associated with a more favourable prognosis than in women. These studies included appreciably fewer women than men. In both the MERIT-HF (Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure)35 as well as the COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival) study,36 the mortality reduction for women in the subgroup analysis was not significant. It was only in the post-hoc analysis of CIBIS II (Cardiac Insufficiency BIsoprolol Study II)37 that the prognostic advantage for women was significant: it actually lay above that for men. On the whole, the data for women seem to be less favourable than for men. A finding moreover attributed not only to the smaller proportion of women in the studies, but also to the fact that the women studied were older and sicker than the comparable male cohort. Once the findings of these three major beta-blocker studies of heart failure were pooled in a meta-analysis (>8900 female patients), results also revealed significant reduction of mortality in women (Figure 1).38
Antagonists of the RAS
Estrogens elevate the angiotensin II plasma levels and consecutively reduce, via negative feedback regulation, ACE and renin activity, as well as expression of the angiotensin II Type-1 receptor.39–42 Estrogen-induced inhibition of the RAS results as a net effect.39 Accordingly, pre-menopausal women demonstrate lower ACE activity than post-menopausal women: a difference abolished by hormone replacement therapy.42 The cardioprotective effects of endogenous estrogens may result in part from inhibition of the RAS. It has not been established, whether these hormonal influences on the RAS modulate effectiveness of therapy with ACE-inhibitors. Relevant gender-specific pharmacokinetic differences have not been described for the ACE-inhibitors captopril and lisinopril.43,44 With a fixed 5 mg dose of ramipril, however, higher plasma levels (AUC) occurred in women than in men, due to women's lower body weight.45 Blood-pressure reduction with different ACE-inhibitors is comparable in women and men.46
ACE-inhibitors are part of evidence-based therapy of heart failure; numerous endpoint studies have verified their prognostic benefits. Data are less well founded for women, as women are under-represented in most of these studies. Two meta-analyses that pooled results of ACE-inhibitor therapy for chronic heart failure have described a trend towards benefits of ACE-inhibitor therapy for women that are less than for men.47,48 The combined analysis of 30 studies on ACE-inhibitor therapy in heart failure established a reduction of 37% in men in mortality and/or hospitalization due to heart failure, but only 22% in women.47 A further meta-analysis investigating the effects of ACE-inhibitor therapy early after myocardial infarction complicated by left-ventricular dysfunction, found comparable favourable effects for both genders with respect to prognosis and hospitalization rate.49 Women with asymptomatic left-ventricular dysfunction appear not to profit from ACE-inhibitor therapy with regard to morbidity and mortality.48
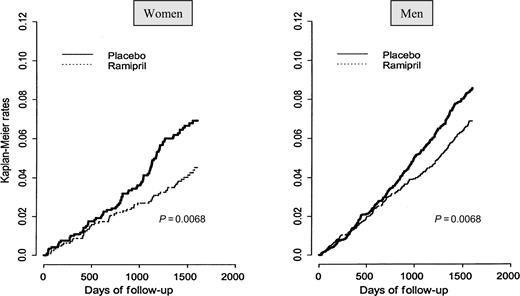
The question of whether ACE-inhibitors have a favourable impact in primary and secondary prevention of vascular diseases, without the presence of left-ventricular dysfunction, has been examined by HOPE (Heart Outcomes Prevention Evaluation Study), EUROPA (EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease), and PEACE (Prevention of Events with Angiotensin Converting Enzyme Inhibition).50–52 HOPE disclosed that the administration of ACE-inhibitors for high-risk female patients was associated with mortality reduction (coronary event) of 38%, results similar to those for men (Figure 2).50 EUROPA confirmed the results from HOPE for men; on the basis of the low number of women included (14.5%), the findings for women were not significant.51 The PEACE study investigated the benefits of trandolapril in 8290 male and female patients with established coronary heart disease. Their recently published findings revealed no significant differences between ACE-inhibitor therapy and placebo with respect to cardiovascular death. This study, which included 18% women, reported no gender-specific differences.52 It is under discussion why PEACE, unlike HOPE and EUROPA, produced negative results: in addition to possible substance-specific differences between the individual ACE-inhibitors, it has been assumed that the lack of reported advantages results from the lower risk profile of the PEACE patients, and from the fact that a higher percentage of them had received preliminary treatment with statins.53 On the basis of the small proportion of women included in ACE-inhibitor studies, data for women are less advantageous than for men. The question of whether women basically profit less from ACE-inhibitor therapy has not been definitely elucidated.
The most frequent adverse reaction to ACE-inhibitor therapy, coughing, arises more frequently in women than men: by a factor of ∼1.5–2.54,55 No gender-specific differences with respect to the occurrence of angioneurotic edemas or urticaria have been described under ACE-inhibitor therapy.56
Besides ACE-inhibitors, angiotensin II Type-1 (AT1) receptor antagonists play a key role in the therapy of cardiovascular diseases. Relevant gender-specific pharmacokinetic differences have not been observed for most of the AT1 receptor antagonists.57–59 For losartan and telmisartan, maximum plasma concentrations are twice as high in women compared with men; however, dose modifications for women have not been recommended.58
Major recent studies have investigated the effects of AT1 receptor antagonists: for hypertension, LIFE (Losartan Intervention for Endpoint Reduction in Hypertension), and VALUE (Valsartan Antihypertensive Long-term Use Evaluation);60,61 for heart failure, ELITE II (Evaluation of Losartan In The Elderly), Val-HeFT (Valsartan Heart Failure Trial), and CHARM (Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity);62–64 and for therapy after myocardial infarction, VALIANT (VALsartan In Acute myocardial iNfarcTion), and OPTIMAAL (OPtimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan).65,66 These studies determined no gender-specific differences. With the exception of LIFE (with a share of women of 54%), however, these studies included appreciably fewer women than men: VALUE 42, ELITE II 30, Val-HeFT 20, CHARM overall 31, VALIANT 31, OPTIMAAL 29%.60–66
A further component of the RAS, aldosterone, is a prime target of cardiovascular therapy. Pharmacokinetics of the selective aldosterone receptor blocker eplerenone did not differ significantly between women and men.67 To the best of our knowledge, there have been no studies on gender differences with regard to pharmacokinetics for the non-selective aldosterone blocker spironolactone.
Clinical studies have provided no gender-specific differences. Neither RALES (Randomized ALdactone Evaluation Study), which revealed the prognostic advantage of the non-specific aldosterone blockade with aldactone in 822 patients with severe ischaemic and non-ischaemic heart failure (NYHA III–IV), nor EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study), with the selective aldosterone receptor antagonist eplerenone for left ventricular dysfunction (EF <40%) after myocardial infarction, revealed gender-specific differences in subgroup analyses, with 27% women in RALES and 28% women in EPHESUS.68,69
Calcium channel blockers
Pharmacokinetic studies have described gender-specific differences for some,70–72 but not for all calcium channel blockers.73 These substances are subject to considerable first-pass metabolism in the liver, and are substrates of CYP3A4,71 for which higher activities have been described in women than in men.3,11,12,14 Accordingly, women have shown faster clearance and lower serum levels of calcium channel blockers, e.g. nifedipine, than do men.72 Women also demonstrate faster clearance of verapamil after intravenous administration, which suggests gender-dependent differences in hepatic metabolism. Although after oral administration of a single dose of verapamil women showed slower clearance than men,71 clearance after oral administration in the steady state appears to be faster in women than in men.70 Despite these gender-specific pharmacokinetic differences, impact on pharmacodynamics is apparently slight: although there is evidence of greater verapamil-induced blood-pressure reduction in women.71 Verapamil clearance slows with increasing age in women: which explains why older women (>60 years) experience more effective blood-pressure reduction than do younger women (from 20 to 30 years).74
It has not been conclusively determined whether the pharmacokinetic differences among calcium channel blockers have relevant clinical impact. The Amlodipine Cardiovascular Community Trial (ACCT) determined that therapy with amlodipine, after dose–body weight adjustment, resulted in blood-pressure reduction more pronounced in women than in men. This effect depended on whether women were using hormone replacement therapy.75 The HOT (Hypertension Optimal Treatment) study investigated the effectiveness of aspirin and intensive blood-pressure reduction with felodipine on cardiovascular events, if necessary, in combination with other antihypertensives. HOT described that the rate of myocardial infarction significantly fell in women with the lowest diastolic target blood pressure compared with women with higher blood pressure target values. This trend likewise became apparent in men, but the results were not significant.76 The major hypertension trials with calcium channel blockers have included comparable numbers of women and men or have even included more women than men. These studies have revealed no evidence for gender-specific differences in outcomes.77–81
Digitalis
A post-hoc analysis of the DIG (Digitalis Investigation Group) trial, which investigated the effects of digoxin in patients with heart failure, determined clear gender-specific differences. In contrast to men, women experienced significantly elevated mortality under digoxin in comparison to placebo: 33.1 vs. 28.9%.82 The cause here has been attributed to relatively excessive dosage in women: despite lower administered digoxin doses, women demonstrated higher serum levels than did men. An additional retrospective analysis of the DIG trial emphasizes the importance of the digitalis serum levels in this context, as higher levels, in men as well, were associated with elevated mortality, whereas lower levels were related to more favourable prognosis.83 It has been discussed whether the elevated mortality with high digitalis levels in both sexes is attributable to arrhythmogenic events. Blaustein et al.84 have suspected gender-specific differences in cellular sodium and calcium handling that could explain the different effects of glycosides in women and men. Women demonstrate lower Na+ concentrations and fewer Na+ pumps in erythrocytes than do men.85 For women suffering from heart failure, studies have also determined fewer Na+ pumps in skeletal muscle than in men.86 Extrapolation of these data to cardiomyocytes could explain that lower number of active Na+ pumps localized in the plasma membrane would predispose heart failure patients to fatal arrhythmias.
An additional crucial aspect is hormone replacement therapy. A subgroup analysis of HERS (Heart and Estrogen–Progestin Replacement Study), which investigated the effects of post-menopausal hormone replacement therapy in secondary prevention of cardiovascular diseases, evidenced that women under hormone replacement therapy who additionally received digitalis, experienced elevated incidence of coronary events in the first year of the study. This prognostically unfavourable effect of hormone replacement therapy did not occur in women who took no digitalis.87 As digitalis therapy in this study had not been randomized, it remains to be elucidated whether women taking digitalis had been sicker and whether this explains the higher incidence of coronary events. The mean age of women in the DIG trial was 66; most of them were therefore post-menopausal. In this study, hormone replacement therapy was not assessed, which renders impossible any statement on whether hormone therapy could explain the gender-specific unfavourable effects of digitalis.
Antiarrhythmics
Gender-specific differences in myocardial repolarization have been long known. In 1920, Bazett9 described that women demonstrate a longer corrected QT interval than do men. Although several explanations have been discussed in this context—e.g. differences in the autonomic nervous system and myocardial effects of sex hormones—the fundamental mechanisms have not been definitely elucidated. The fact that QT time in childhood is of equal length in both sexes, and that it shortens after puberty in young men with elevated androgen levels, speaks for effects of sex steroids.88,89
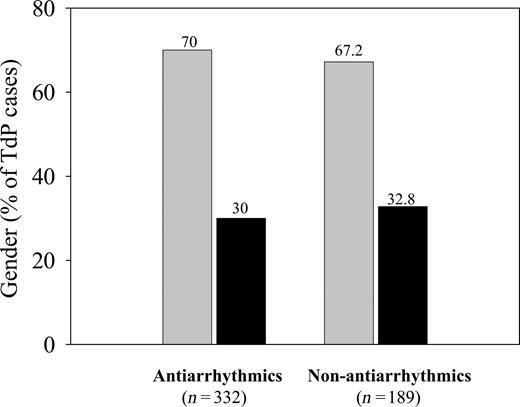
Incidence of the acquired long-QT syndrome is appreciably higher in women than in men. This syndrome can be induced not only by antiarrhythmics, but by a great number of other drugs, including psychotropic drugs and antibiotics such as erythromycin (Figure 3).90–93 Class I and III antiarrhythmics, potentially associated with prolongation of the QT interval, more often lead to torsades de pointes tachycardia in women.90–92 In addition to the significance of women's QT intervals that are already basally prolonged, other possible explanations for this phenomenon include hormonal influences as well as high dosage relative to body weight. Thus, modifications of ion channels by sex hormones may play a particular role in this context.90 Discussion has centred on whether gender-specific differences in the intrinsic activity and density of potassium channels of the female myocardium lead to increased vulnerability with use of drugs that prolong QT intervals.6
There is some evidence for gender-specific differences in pharmacokinetics and/or pharmacodynamics of some antiarrhythmics which may account for the increased incidence of QT interval prolongations. For the Class IA antiarrhythmic procainamide, serum levels are around 30% higher in women than in men. This finding has been primarily attributed to the lower BMI and consequently lower volume of distribution in women.94 Chinidin has led to more pronounced QT prolongation in women, although serum concentrations in both sexes were comparable.95 One study with the Class III antiarrhythmic ibutilide in healthy women found longer QT intervals in women than in men; in this study, cyclic fluctuations of the female QT time became apparent, with maxima during ovulation and menstruation.96 Reports have described more pronounced repolarization prolongations for d,l-sotalol in women, which extends repolarization via blockade of the fast potassium channels.91 Although numerous studies failed to perform gender-specific analyses and included insufficient numbers of women, there is definite evidence that these gender-specific differences in the effects of antiarrhythmics on the repolarization phase do gain clinical relevance.
In 1991, the Cardiac Arrhythmia Suppression Trial (CAST) with the Class IC antiarrhythmics encainide and flecainide called into question the application of antiarrhythmics for suppression of ventricular events in the post-infarction phase. Although this trial included 19% women, it did not conduct gender-specific assessment of results.97 The analysis of a large database with more than 3000 persons who had taken d,l-sotalol for treatment of atrial or ventricular arrhythmias disclosed that women suffered an elevated risk (4.1%) in comparison to men (1%) of developing torsades de pointes tachycardia.91 These gender-specific differences were observed independently of dosage and basal corrected QT interval.98 The Survival With ORal D-sotalol (SWORD) study, which examined the effectiveness of d-sotalol in patients with previous infarction, was prematurely terminated because of elevated mortality in the verum group. Female sex was a primary risk factor for excess mortality in the group treated with d-sotalol.99 The DIAMOND (Danish Investigations of Arrhythmia and Mortality on Dofetilide) study likewise revealed female sex as a significant risk factor for the development of torsades de pointes tachycardia, with an odds ratio of 3.2.100
Although amiodarone is associated with fewer pro-arrhythmias, women apparently develop torsades de pointes tachycardia twice as frequently as do men under this Class III antiarrhythmic drug.92 At present, there are no valid statements available on whether amiodarone has less favourable prognostic effects in women. The CHF Stat (Congestive Heart Failure Survival trial of antiarrhythmic therapy) study with amiodarone in patients with heart failure and asymptomatic ventricular arrhythmias, for example, included only 1% women.101 GESICA (Grupo de Estudio de la Sobrevida en la Insufficiencia Cardiaca en Argentina) investigated the effects of low-dose amiodarone on mortality in patients with chronic heart failure (EF ≤35%) without symptomatic arrhythmias. Although only 19% of the patients studied here were women, the subgroup analysis determined comparable reduction of mortality and hospitalization rates in men and women.102 EMIAT (European Myocardial Infarct Amiodarone Trial) and CAMIAT (Canadian Amiodarone Myocardial Infarction Arrhythmia Trial), which studied the effect of amiodarone after myocardial infarction, likewise included only a small percentage of women: 16 and 18%, respectively. These studies did not conduct gender-specific analyses.103,104 A retrospective analysis of the Multicenter UnSustained Tachycardia Trial (MUSTT) compared prophylactic defibrillator implantation with antiarrhythmic therapy for inducible patients. The study determined no gender-specific differences with respect to the risk of arrhythmogenic death, cardiac arrest, or overall mortality for female (14% included) and male (86% included) patients with coronary heart disease, reduced ejection fraction (<40%), and spontaneous, non-sustained ventricular tachycardia.105
Although data available support the general conclusion that women more frequently develop antiarrhythmic-induced pro-arrhythmias than do men, the impact on prognosis of this finding is not definitely clear. This situation highlights the importance of gender-specific analysis, as well as the inclusion of sufficient numbers of women, in studies investigating antiarrhythmic drugs.
Acetylsalicylic acid
Gender-specific differences in the pharmacokinetics of acetylsalicylic acid have been known for many years. The bioavailability of acetylsalicylic acid is greater in women than in men, owing to slower clearance and, in turn, significant prolongation of half life.106 This gender-specific difference is assumably the result of greater activity of the degradation pathway via conjugation with glycine and glucuronic acid in men. As oral contraceptives can stimulate these degradation pathways, the difference in bioavailability of acetylsalicylic acid disappears in women under hormonal contraception.107In vitro data suggest that aspirin results in greater inhibition of thrombocyte aggregation in men than in women. Testosterone in vitro intensified aspirin-induced inhibition of platelet aggregation, whereas oestradiol showed no relevant influence.108
It is questionable whether these gender-specific differences possess clinical relevance. The benefits of acetylsalicylic acid for women and men are well documented for secondary prevention of cardiovascular disease. Aspirin reduces the incidence of myocardial infarction, stroke, and cardiovascular death by 25% in women and men.109 Similarly, the benefits of acetylsalicylic acid are equally well confirmed for women and men in acute therapy of myocardial infarction.110
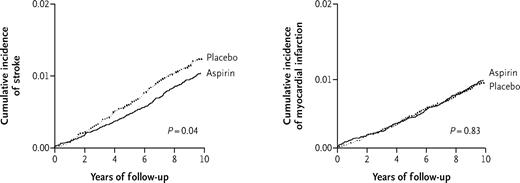
In contrast to men, the significance of aspirin in primary prevention in women is less clear. There is some evidence of protective benefits of aspirin for women as well: the epidemiological Nurses' Health Study revealed significant reduction in the risk of myocardial infarction among women who took one to six aspirin per week.111 Of the randomized studies on acetylsalicylic acid in primary prevention, only HOT and Primary Prevention Project (PPP) included women. In the HOT study, acetylsalicylic acid reduced fatal and non-fatal myocardial infarction by 36%; the gender-specific analysis disclosed no significant reduction in myocardial infarction in women.76 In the PPP study, aspirin reduced cardiovascular events by 23% in the total group of men and women.112 A recent analysis of the Women's Health Study, including 39 876 women over the age of 45, described that aspirin, although it significantly reduced the risk of stroke by about 24%, did not influence the risk of myocardial infarction or mortality. It was only in the subgroup of older women (>65 years) that aspirin was effective as well in the primary prevention of cardiac events (Figure 4).113
Neither HOT nor PPP evaluated the risk of gastrointestinal haemorrhage associated with aspirin by gender. The Framingham Heart Study examined acetylsalicylic acid (325 mg per day) as a predictor for severe haemorrhage and did not show a significant difference in haemorrhage risk between women and men.114
Clopidogrel
Clopidogrel, which prevents ADP-mediated thrombocyte activation, is a pro-drug. The active metabolite, a thiol derivative, is formed from oxidation of clopidogrel by the hepatic isoenzymes CYP2B6 and CYP3A4, and by subsequent hydrolysis. Production of the inactive primary metabolite, a derivative of carboxyl acid, takes place predominantly via CYP1A.115
No significant differences have been observed in plasma levels of the main circulating metabolite between women and men.67 Some variability, however, has been described with regard to clopidogrel-induced inhibition of platelet aggregation.116 In a small study, there was less inhibition of ADP-induced platelet aggregation in women, but no gender-specific difference in prolongation of bleeding time was observed.67 With the exception of CREDO (Clopidogrel for the Reduction of Events During Observation), in which subgroup analyses revealed beneficial effects of a ‘loading dose’ of clopidogrel that was comparable for women and men (29% women),117 few clinical studies with clopidogrel have presented gender-specific analyses. Neither CAPRIE (Clopidogrel vs. Aspirin in Patients at Risk of Ischaemic Events), which showed a prognostic advantage of clopidogrel over aspirin in 19 185 patients (28% women) with a high risk of ischaemic events, nor CLASSICS (The Clopidogrel Aspirin Stent International Cooperative Study) (23% women), which revealed that aspirin plus ticlopidin was equally effective after stent implantation compared with aspirin plus clopidogrel, presented evidence of gender-specific differences.118,119
Statins
Statins only show slight gender-specific differences in pharmacokinetics. With the exception of pravastatin, rosuvastatin (both without significant CYP metabolization), and fluvastatin (predominantly CYP2C9 metabolization), all statins are primarily subject to hepatic metabolism via CYP3A4 and cerivastatin additionally to metabolism via CYP2C8.120–125 Consequently, drug interactions with substances also metabolized via CYP3A4 have to be considered. In general, plasma concentrations of statins in women are higher than in men; these differences have been judged so slight that recommendations for dose adjustment have not been considered necessary.120–125 Nevertheless, the risk of adverse drug reactions appears greater in women. Administration of cerivastatin (since taken off the market) was associated with unacceptable frequencies of myopathy and rhabdomyolysis, especially in older, thin women.126 In pharmacokinetic studies with cerivastatin, older women had 30% higher maximum plasma levels than older men.122 Although this difference may not be relevant in healthy volunteers, the risk of adverse drug reactions increases with comorbidity and comedication in patients. One could speculate that, in such situations, even only slight to moderate differences in pharmacokinetic parameters could result in clinical relevance.
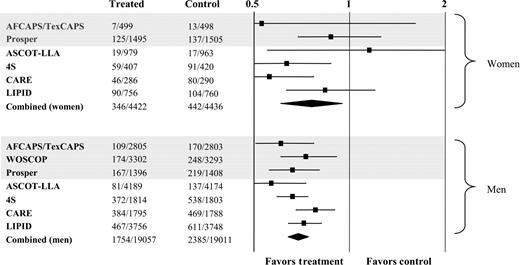
Major primary and secondary prevention studies have revealed that statins reduce the risks of cardiovascular events to a degree comparable between women and men.127 The percentage of women examined in these studies, however, was only ∼25%. A major meta-analysis, which has covered 10 endpoint studies with a total of 79 494 persons, determined a relative risk for severe coronary events of 0.73 for men and 0.77 for women taking statins (Figure 5).128 Despite the beneficial effects, statins are employed less frequently in women than in men in primary and secondary prevention of cardiovascular diseases.129
Summary
Gender-specific differences have not been investigated for many cardiovascular drugs. If such gender-specific analyses have been performed, pharmacokinetic differences for women and men became apparent. Administration of fixed doses, not adapted to body weight, frequently result in higher plasma concentrations in women, owing to their lower distribution volume compared with men. Further influencing factors in women are hormonal changes and different activities of a number of drug metabolizing enzymes. The higher plasma concentrations in women may be one explanation why female sex is associated with a greater risk of adverse drug reactions.
Despite these often relevant pharmacokinetic differences between female and male patients, the impact on pharmacodynamics is generally moderate. There are only slight differences concerning the prognostic significance of primary and secondary preventive cardiovascular therapeutic strategies for women and men. It must be emphasized, however, that women are often under-represented in endpoint studies. Statements for women are mostly reached via subgroup, post hoc, or meta-analyses. In order to assure sufficiently eloquent statements for women as well, studies should to an increasing degree take the following aspects into sufficient consideration in study design and in analysis of findings: The sample sizes must be adequately large to assure an appropriate inclusion rate for women, with statistically sufficient power. In addition, it is essential to determine and analyse data on hormonal aspects such as concomitant hormone therapy (e.g. oral contraceptives and hormone replacement). Owing to the problem of relative overdosing for women in cases of fixed doses, dosage should to an increasing extent include dose adaptation on the basis of patient weight. Finally, already known gender-specific differences in pharmacodynamics and in complication rates (e.g. torsades de pointes tachycardia) should be systematically incorporated into the analysis carried out in such studies.
The following is a summary of relevant gender-specific aspects of chronic cardiovascular therapy:
Women demonstrate higher plasma levels than do men under β-blockade. Accordingly, reduction in blood pressure is more pronounced in women, with a smaller increase in exercise heart rate. With respect to mortality reduction after myocardial infarction or heart failure, beta-blocker therapy exhibits similar benefits for women and men.
Meta-analyses of ACE-inhibitor therapy in heart failure allow assumption of a tendency in women towards less effective mortality and morbidity reduction under ACE-inhibitor therapy. Adverse drug reactions in the form of ACE-inhibitor cough occur twice as frequently in women.
Despite appreciable gender-specific pharmacokinetic differences under calcium channel blockers, the impact on pharmacodynamics is slight. Reduction in blood pressure is more pronounced in women than in men. Clinical endpoint studies have revealed no relevant differences between women and men with regard to mortality and morbidity for cardiovascular diseases.
For digitalis, there is evidence of higher mortality in female patients with chronic heart failure. The cause is assumed to be excessive dosage for women.
Pro-arrhythmic effects in the form of torsades de pointes tachycardia, as the expression of an acquired long-QT syndrome, occur in women under antiarrhythmic therapy significantly more frequently than in men. The significance of these more frequent pro-arrhythmias on prognosis of women has not been fully elucidated.
In secondary prevention of cardiovascular diseases, therapy with acetylsalicylic acid is equally well documented for women and men. The benefit of aspirin in primary prevention of myocardial infarction is less clear for women.
Pharmacokinetic gender-specific differences with respect to statins are slight. Despite higher plasma concentrations in women for a number of statins, there have been no recommendations for dose adjustment in women. Primary and secondary prevention studies have revealed beneficial effects that are comparable for women and men.
Figure 1 Relative risk ratios and 95% confidence intervals for total mortality in women and men, in studies evaluating the impact of β-blockade in heart failure (modified with permission from Ghali et al.38).
Figure 2 Effects of long-term therapy with the ACE-inhibitor ramipril on cardiovascular deaths in high-risk women and men in the HOPE trial (with permission from Lonn et al.50).
Figure 3 Relation between female (grey bars) and male (black bars) genders and torsades de pointe tachycardia for antiarrhythmic and non-antiarrhythmic drugs in a database search (modified with permission from Bednar et al.93).
Figure 4 Cumulative incidence of stroke and myocardial infarction under therapy with aspirin (100 mg) vs. control in 39 876 initially healthy women in Women's Health Study (with permission from Ridker et al.113).
Figure 5 Relative risk ratios and 95% confidence intervals for major coronary events in women and men in outcome studies, in evaluation of the impact of statins on major coronary events. Grey bars indicate primary prevention studies. WOSCOP included only men (modified with permission from Cheung et al.128).
References
Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute.
Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics.
Gross JL, Friedman R, Azevedo MJ, Silveiro SP, Pecis M. Effect of age and sex on glomerular filtration rate measured by 51Cr-EDTA.
Conte MR. Gender differences in the neurohumoral control of the cardiovascular system.
Legato M. Gender and the heart: sex-specific differences in normal anatomy and physiology.
Burke JH, Goldberger JJ, Ehlert FA, Kruse JT, Parker MA, Kadish AH. Gender differences in heart rate before and after autonomic blockade: evidence against an intrinsic gender effect.
Liu K, Ballew C, Jacobs DR Jr, Sidney S, Savage PJ, Dyer A, Hughes G, Blanton MM. Ethnic differences in blood pressure, pulse rate, and related characteristics in young adults. The CARDIA study.
Villareal RP, Woodruff AL, Massumi A. Gender and cardiac arrhythmias.
Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates.
Wolbold R, Klein K, Burk O, Nussler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM. Sex is a major determinant of CYP3A4 expression in human liver.
Walle T, Walle UK, Cowart TD, Conradi EC. Pathway-selective sex differences in the metabolic clearance of propranolol in human subjects.
Hamelin BA, Desgagnés V, Rail J, Bogaty P, Morin J, Turgeon J. CYP2D6 ultrarapid metabolism and response to metoprolol.
Kashuba AD, Nafziger AN. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs.
Labbé L, Sirois C, Pilote S, Arseneault M, Robitaille NM, Turgeon J, Hamelin BA. Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates.
Fröhlich M, Albermann N, Sauer A, Walter-Sack I, Haefeli WE, Weiss J. In vitro and ex vivo evidence for modulation of p-glycoprotein activity by progestins.
Cummins CL, Wu CY, Benet LZ. Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by p-glycoprotein.
Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics.
Hogstedt S, Lindberg B, Rane A. Increased oral clearance of metoprolol in pregnancy.
Llerena A, Cobaleda J, Martinez C, Benitz J. Interethnic differences in drug metabolism: influence of genetic and environmental factors on desrisoquine hydroxylation phenotype.
Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy.
Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of β1-adrenoceptor upon ischemic insult in the rat heart.
Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of β1-adrenergic receptors in ovariectomized rat hearts.
Luzier AB, Killian A, Wilton JH, Wilson MF, Forrest A, Kazierad DJ. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers.
Kendall MJ, Quarterman CP, Jack DB, Beeley L. Metoprolol pharmacokinetics and the oral contraceptive pill.
Walle T, Walle K, Mathur RS, Palesh YY, Conradi EC. Propranolol metabolism in normal subjects: association with steroid hormones.
Gilmore DA, Gal J, Gerber JG, Nies AS. Age and gender influence the stereoselective pharmacokinetics of propranolol.
Walle T, Byington RP, Furberg CD, McIntyre KM, Vokonas PS. Biologic determinants of propranolol disposition: results from 1308 patients in the beta-Blocker Heart Attack Trial.
The beta-Blocker Heart Attack Trial (BHAT). A randomized trial of propanolol in patients with acute myocardial infarction. I. Mortality results.
Olsson G, Wikstrand J, Warnold I, Manger Cats V, McBoyle D, Herlitz J, Hjalmarson A, Sonneblick EH. Metoprolol-induced reduction in postinfarction mortality: pooled results from five double-blind randomized trials.
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF).
Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study.
Tabassome S, Mary-Krause M, Funck-Brentano C, Jaillon P; on behalf of the CIBIS II Investigators. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II).
Ghali JK, Pina IL, Gottlieb SS, Deedwania PC, Wikstrand JC. Metoprolol CR/XL in female patients with heart failure: analysis of the experience in metoprolol extended-release randomized intervention trial in heart failure (MERIT-HF).
Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system.
Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity.
Harvey PJ, Morris BL, Miller JA, Floras JS. Estradiol induces discordant angiotensin and blood pressure responses to orthostasis in healthy postmenopausal women.
Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women.
Massana E, Barbanoj MJ, Moros C, Morte A, Gich I, Jane F. No sex-related pharmacokinetic and pharmacodynamic differences of captopril.
Saenz-Campos D, Bayes MC, Massana E, Martin S, Barbanoj M, Jane F. Sex-related pharmacokinetic and pharmacodynamic variations of lisinopril.
Vree TB, Dammers E, Ulc I, Horkovics-Kovats S, Ryska M, Merkx I. Lack of male–female differences in disposition and esterase hydrolysis of ramipril to ramiprilat in healthy volunteers after a single oral dose.
Wing LMH, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GLR, Johnston CI, McNeil JJ, MacDonald GJ, Marley JE, Morgan TO, West MJ, for the Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly.
Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials.
Shekelle PG, Rich MW, Morton SC, Atkinson W, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner Stevenson L. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status.
Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, Torp-Pedersen C, Ball S, Pogue J, Moye L, Braunwald E, for the ACE inhibitor myocardial infarction collaborative group. Long-term ACE inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients.
Lonn E, Roccaforte R, Yi Q, Dagenais G, Sleight P, Bosch J, Suhan P, Micks M, Probstfield J, Bernstein V, Yusuf S. Effect of long-term therapy with ramipiril in high-risk women.
Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study).
PEACE Trial Investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease.
Pitt B. ACE inhibitors for patients with vascular disease without left ventricular dysfunction—may they rest in PEACE?
Mackay FJ, Pearce GL, Mann RD. Cough and angiotensin II receptor antagonists: cause or confounding?
Strocchi E, Malini PL, Valtancoli G, Ricci C, Bassein L, Ambrosioni E. Cough during treatment with angiotensin-converting enzyme inhibitors. Analysis of predisposing factors.
Pillans PI, Coulter DM, Black P. Angiooedema and urticaria with angiotensin converting enzyme inhibitors.
Gleiter CH, Mörike KE. Clinical pharmacokinetics of candesartan.
Israili ZH. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension.
Vachharajani NN, Shyu WC, Smith RA, Greene DS. The effects of age and gender on the pharmacokinetics of irbesartan.
Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A; VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial.
Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the Losartan Heart Failure Survival Study ELITE II.
Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure.
Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Östergren J, Yusuf S; CHARM investigators and committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme.
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM; Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both.
Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone in morbidity and mortality in patients with severe heart failure.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.
Kang D, Verotta D, Krecic-Shepard ME, Modi NB, Gupta SK, Schwartz JB. Population analyses of sustained-release verapamil in patients: effects of sex, race, and smoking.
Krecic-Shepard ME, Barnas CR, Slimko J, Schwartz JB. Faster clearance of sustained release verapamil in men versus women: continuing observations on sex-specific differences after oral administration of verapamil.
Krecic-Shepard ME, Park K, Barnas C, Slimko J, Kerwin DR, Schwartz JB. Race and sex influence clearance of nifedipine: results of a population study.
Abad-Santos F, Novalbos J, Galvez-Mugica MA, Gallego-Sandin S, Almeida S, Vallee F, Garcia AG. Assessment of sex differences in pharmacokinetics and pharmacodynamics of amlodipine in a bioequivalence study.
Schwartz JB, Capili H, Daugherty J. Aging of women alters s-verapamil pharmacokinetics and pharmacodynamics.
Kloner RA, Sowers JR, DiBona GF, Gaffney M, Wein M, for the Amlodipine Cardiovascular Community Trial Study Group. Sex- and age-related antihypertensive effects of amlodipine.
Kjeldsen SE, Kolloch RE, Leonetti G, Malliond J-M, Zanchetti A, Elmfeldt D, Warnold I, Hansson L for the HOT Study Group. Influence of gender and age on the preventing cardiovascular disease by antihypertensive treatment and acetysalicyl acid. The HOT Study.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT).
Fagard RH, Staessen JA, Thijs L, Gasowski J, Bulpitt CJ, Clement D, de Leeuw PW, Dobovisek J, Jääskivi M, Leonetti G, O'Brian E, Palatini P, Parati G, Rodicio JL, Vanhanen H, Webster J, for the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Response to antihypertensive therapy in older patients with sustained and nonsustained systolic hypertension.
Hansson L, Lindholm LH, Ekborn T, Dahlöf B, Lanke J, Scherstén B, Wester P-Q, Hedner T, de Faire U, for the STOP-Hypertension-2 study group. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study.
Kjeldsen SE, Hedner T, Syvertsen JO, Lund-Johansen, Hansson L, Lanke J, Lindholm LH, de Faire U, Dahlhöf B, Karlberg BE, for the NORDIL Study Group. Influence of age, sex and blood pressure on the principal endpoints of the Nordic Diltiazem (NORDIL) Study.
Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure.
Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure.
Blaustein MP, Robinson SW, Gottlieb SS, Balke CW, Hamlyn JM. Sex, digitalis, and the sodium pump.
Smith JB, Wade MB, Fineberg NS, Weinberger MH. Influence of race, sex, and blood pressure on erythrocyte sodium transport in humans.
Green HJ, Duscha BD, Sullivan MJ, Keteyian SJ, Kraus WE. Normal skeletal muscle Na(+)-K(+) pump concentration in patients with chronic heart failure.
Furberg CD, Vittinghoff E, Davidson M, Herrington DM, Simon JA, Wenger NK, Hulley S. Subgroup interactions in the Heart and Estrogen/Progestin Replacement Study: lessons learned.
Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization.
Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age.
Drici MD, Clement N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome.
Lehmann MH, Hardy S, Archibald D, Quart B, MacNeil DJ. Sex differences in risk of torsades de pointes with d,l-sotalol.
Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs.
Bednar MM, Harrigan EP, Ruskin JN. Torsades de pointes associated with nonantiarrhythmic drugs and observations on gender and QTc.
Koup JR, Abel RB, Smithers JA, Eldon MA, de Vries TM. Effect of age, gender and race on steady state procainamide pharmacokinetics after administration of procanbid sustained-release tablets.
Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women.
Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle.
The Cardiac Arrhythmia Suppression Trial Investigators (CAST). Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction.
Wolbrette DL. Risk of proarrhythmia with class III antiarrhythmic agents: sex-based differences and other issues.
Pratt CM, Camm AJ, Cooper W, Friedman PL, MacNeil DJ, Moulton KM, Pitt B, Schwartz PJ, Veltri EP, Waldo AL, for the SWORD Investigators. Mortality in the Survival With ORal D-Sotalol (SWORD) trial: why did patients die?
Torp-Pederson C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm JA. Dofetilide in patients with congestive heart failure and left ventricular dysfunction.
Massie BM, Fisher SG, Radford M, Deedwania PC, Singh BN, Fletcher RD, Singh SN. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. CHF-Stat Investigators.
Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R for Grupo de Estudio de la Sobrevida en la Insufficiencia Cardiaca en Argentina (GESICA). Randomised trial of low-dose amiodarone in severe congestive heart failure.
Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, Simon P, for the European Myocardial Infarct Amiodarone Trial Investigators. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT.
Cairns JA, Connolly SJ, Roberts R, Gent M, for the Canadian Amiodarone Myocardial Infraction Arrhythmia Trial Investigators. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT.
Russo AM, Stamato NJ, Lehmann MH, Hafley GE, Lee KL, Pieper K, Buxton AE, and the MUSTT Investigators. Influence of gender on arrhythmia characteristics and outcome in the Multicenter UnSustained Tachycardia Trial.
Ho PC, Triggs EJ, Bourne DW, Heazlewood VJ. The effect of age and sex on the disposition of acetylsalicyl acid and its metabolites.
Miners JO, Grugrinovich N, Whitehead AG, Robson RA, Birkett DJ. Influence of gender and oral contraceptive steroids on the metabolism of salicylic acid and acetylsalicylic acid.
Spranger M, Aspey BS, Harrison MJ. Sex difference in antithrombotic effect of aspirin.
Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.
ISIS-2 (Second International Study of Infarct Survival) collaborative group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2.
Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. A prospective study of aspirin use and primary prevention of cardiovascular disease in women.
de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project.
Ridker PM, Cook NR, Lee I-M, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women.
Sam C, Massaro JM, D'Agostino RB Sr, Levy D, Lambert JW, Wolf PA, Benjamin EJ; Framingham Heart Study. Warfarin and aspirin use and the predictors of major bleeding complications in atrial fibrillation (the Framingham Heart Study).
Lenz T, Wilson A. Clinical pharmacokinetics of antiplatelet agents used in the secondary prevention of stroke.
Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals.
Steinhubl SR, Berger PB, Tift Mann J, Fry ETA, deLago A, Wilmer C, Topol EJ. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention.
Cannon CP; CAPRIE Investigators. Effectiveness of clopidogrel versus aspirin in preventing acute myocardial infarction in patients with symptomatic atherothrombosis (CAPRIE trial).
Bertrand ME, Rupprecht H-J, Urban P, Gershlick AH. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS).
Gibson DM, Bron NJ, Richens A, Hounslow NJ, Sedman AJ, Whitfield LR. Effect of age and gender on pharmacokinetics of atorvastatin in humans.
Cheng H, Rogers JD, Sweany AE, Dobrinska MR, Stein EA, Tate AC, Amin RD, Quan H. Influence of age and gender on the plasma profiles of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitory activity following multiple doses of lovastatin and simvastatin.
Isaacsohn J, Zinny M, Mazzu A, Lettieri J, Heller AH. Influence of gender on the pharmacokinetics, safety, and tolerability of cerivastatin in healthy adults.
Martin PD, Dane AL, Nwose OM, Schneck DW, Warwick MJ. No effect of age or gender on the pharmacokinetics of rosuvastatin: a new HMG-CoA reductase inhibitor.
Scripture CD, Pieper JA. Clinical pharmacokinetics of fluvastatin.
Smith HT, Jokubaitis LA, Troendle AJ, Hwang DS, Robinson WT. Pharmacokinetics of fluvastatin and specific drug interactions.
FDA. CDER. Report no.: http:www.fda.gov/cder/foi/label2001/207S6lbl.pdf.
Shepard DR, Jenid H, Thacker HL. Gender, hyperlipidemia, and coronary artery disease.
Cheung BMY, Lauder IJ, Lau C-P, Kumana CR. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes.