-
PDF
- Split View
-
Views
-
Cite
Cite
Edith Lubos, Renate Schnabel, Hans J. Rupprecht, Christoph Bickel, Claudia M. Messow, Susanne Prigge, François Cambien, Laurence Tiret, Thomas Münzel, Stefan Blankenberg, Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study, European Heart Journal, Volume 27, Issue 2, January 2006, Pages 150–156, https://doi.org/10.1093/eurheartj/ehi582
Close - Share Icon Share
Abstract
Aims Metalloproteinases are proteolytic enzymes, which decompose the extracellular matrix, influence cardiac remodelling, and are inhibited by tissue inhibitor of metalloproteinases (TIMPs). Little is known about the prognostic impact of the TIMP-1/matrix metalloproteinase complex in patients with future cardiovascular death.
Methods and results In 1979 patients with suspected coronary artery disease (CAD), TIMP-1 has been determined at baseline. Among 1945 (98.4%) patients with a mean follow-up period of 2.6±1.2 years, 75 patients died because of cardiovascular causes.
Mean concentrations of TIMP-1 were higher among patients who experienced a fatal cardiovascular event than among those who did not (820 vs. 692 ng/mL; P<0.001). Age and sex adjusted hazard ratio of future cardiovascular death associated with one standard deviation of TIMP-1 level, was 1.37 (95% CI: 1.17–1.61; P<0.001). The hazard ratio remained nearly identical after adjustment for clinical and therapeutic confounders. B-type natriuretic peptide (2.75, 95% CI: 1.94–3.89; P<0.001), C-reactive protein (1.79, 95% CI: 1.43–2.24; P<0.001), and TIMP-1 (1.30, 95% CI: 1.07–1.58; P=0.008) were independently associated with future cardiovascular death.
Conclusion In patients with CAD, TIMP-1 proves as an independent predictor for future cardiovascular death.
See page 121 for the editorial comment on this article (doi:10.1093/eurheartj/ehi639)
Introduction
Atherosclerosis and constrictive arterial remodelling probably occur as a result of an alteration in the local balance of the extracellular matrix. Atherosclerotic plaque evolution as well as structural changes of infarcted tissues are influenced by specific tissue matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases and their endogenous tissue inhibitors which decisively control extracellular matrix turnover.1,2 Several studies have shown that extracellular matrix degradation by MMPs, specifically MMP-9, is involved in the pathogenesis of a wide spectrum of cardiovascular disorders, including atherosclerosis, restenosis, cardiomyopathy, congestive heart failure, myocardial infarction, and aortic aneurysm.3,4 Increased activity and concentration of MMP-9 in plaques might contribute to vulnerability as well as to increased levels of circulating MMP-9, which has been demonstrated to be associated with future cardiovascular events.5
MMP activity is controlled by endogenous tissue inhibitors of metalloproteinases (TIMPs). There are four known family members which bind with varying affinities to different MMPs and thereby inhibit active forms of most MMPs.6,7 Regulation and maintenance of extracellular matrix homeostasis is the primary physiological role of TIMPs. However, these multi-functional proteins activate growth factor and inhibit angiogenesis and apoptosis.8,9 TIMP-1 is one of the best characterized TIMPs which binds with a high affinity to activated MMPs, and has complex roles in physiological and pathological tissue remodelling.10,11 In TIMP-1 knock-out mice, TIMP-1 deficiency has been shown to amplify adverse left ventricular (LV) remodelling after myocardial infarction.12 TIMP-1 appears to play an important role in regulation of LV structure and systolic function.13,14 Plasma TIMP-1 levels are elevated in patients with coronary disease.15 In the Framingham heart study, total TIMP-1 was related to major cardiovascular risk factors, in particular hypertension which may influence vascular and cardiac remodelling via extracellular matrix degradation.16 However, the direct mechanistic role of MMPs and TIMPs in the post-MI remodelling process has not been completely established and clinical studies evaluating the role of plasma TIMP-1 levels on future cardiovascular events are scant.
The aim of the present study was to investigate whether plasma TIMP-1 concentrations might constitute a risk biomarker for future cardiovascular death in a large cohort of patients with angiographically proven coronary artery disease (CAD). In particular, we examined the prognostic role of TIMP-1 in context of the novel biomarkers B-type natriuretic peptide (BNP) and C-reactive protein.
Methods
Study population
In all eligible patients with chest pain, coronary angiography was performed and relevant CAD was defined by >30% stenosis in at least one major coronary artery. Between June 1999 and February 2004, 1979 patients admitted to the Department of Medicine II of the Johannes Gutenberg-University, Mainz, Germany or the Bundeswehrzentralkrankenhaus, Koblenz, Germany with suspected CAD were enrolled in the AtheroGene study.
A description of the design of the AtheroGene study can be found in detail elsewhere.17 For this study, we excluded patients without evidence of CAD as defined earlier and patients with evidence of significant concomitant diseases, in particular haemodynamically significant valvular heart disease, surgery or trauma within the previous month, known cardiomyopathy, known malignant diseases, febrile conditions, or use of oral anticoagulant therapy within the previous 4 weeks. Each patient completed a questionnaire that provided information about cardiovascular risk factors. Diabetes mellitus was diagnosed in patients with a history of dietary treatment, or medication for diabetes, or whose current fasting blood glucose level was >125 mg/dL. For measures of lipids and hypertension, we considered the absolute continuous values of high-density cholesterol, low density cholesterol, and triglycerides as well as systolic, diastolic, and mean arterial blood pressure. We used the systolic blood pressure values in below and above 125 mmHg according to the practice guidelines of the European Society of Hypertension for clinic, ambulatory, and self blood pressure measurement.18
Patients were classified as currently smoking, as having smoked in the past (if they had stopped >4 weeks and <40 years earlier), or as never having smoked (if they had never smoked or had stopped 40 or more years earlier).
Follow-up information was available for 1945 (98.4%) from 1979 patients and median follow-up time was 2.6 years (maximum 5.0). There were 75 deaths from cardiovascular causes, 33 deaths from other causes, and 47 non-fatal myocardial infarctions, and 49 non-fatal strokes. Information about the causes of death and clinical events was obtained from hospital and general-practitioner charts.
Study participants had German nationality. Participation was voluntary, and each patient gave written informed consent. The Ethics Committee of the University of Mainz approved the study.
Laboratory methods
In all study subjects' blood was drawn under standardized conditions before coronary angiography was performed. Samples were centrifuged at 4000 g for 10 min, divided into aliquots and frozen at −80°C until analysis. Serum TIMP-1 was measured with a commercially available enzyme linked immunosorbent assay (Human, Biotrak, ELISA System, Amersham Biosciences, USA).
Plasma BNP was determined using a fluorescence immunoassay (Biosite Diagnostics Inc., San Diego, CA, USA). The detection limit reported is <5 pg/mL. The assay has an inter-assay coefficient of variation of near 10% and a recovery of 100% of added peptide was found. Cross-reactivity with other natriuretic peptides is negligible.19
C-reactive protein was determined by a highly sensitive, latex particle-enhanced immunoassay (detection range of 0–20 mg/L, Roche Diagnostics, Mannheim, Germany). Lipid serum levels were measured immediately by routine methods.
Statistical analysis
Mean and/or median levels and proportions of baseline cardiovascular risk factors were calculated for all patients.
Data presented are percentage of patients or mean±SD or median and 25th/75th percentile for skewed variables. Association of baseline characteristics and survival is assessed by univariate Cox regression for continuous variables and by log-rank test for categorial variables. To investigate the association between TIMP-1 and survival, we used the Kaplan–Meier method and the log-rank test. Survival rates were calculated using the Kaplan–Meier method. The primary endpoint was death from cardiovascular causes. Data from patients who died from other causes were censored at the time of death. To normalize the distribution of skewed variables log transformation was performed. Additionally, to indicate predictive value of TIMP-1 level on the risk of cardiovascular death, various Cox regression models were carried out. The first model was adjusted for age and sex and the second, additionally, for traditional risk factors (body mass index, mean arterial blood pressure, diabetes, smoking status, and high density lipoprotein) as well as clinical and therapeutic variables [extent of vessel disease, presence or absence of acute coronary syndrome, angiotensin-converting enzyme (ACE)-inhibitors, statin and beta-blocker therapy, and serum creatinine level]. Further adjustment was performed for ejection fraction (Model 3) and finally for other cardiovascular biomarkers (BNP and C-reactive protein) (Model 4). Analyses were performed by constructing Cox regression models comparing one standard deviation and the thirds of TIMP-1 level. In order to clarify if the influence of TIMP-1 is different in patients presenting traditional risk factors from the influence in patients without, several subgroup analyses were carried out. Hazard ratios and 95% confidence intervals were reported.
As P-values were not adjusted for multiple testing they have to be considered as descriptive. All computations were carried out using the SPSS Version 11.05 programme and R2.0.1. [R Development Core Team (2004). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.r-project.org.]
Results
Baseline characteristics
Table 1 provides the baseline characteristics of the 1945 study participants and TIMP-1 level was normally distributed. It ranged from 6.0–2667 ng/mL, with a mean (±SD) of 697±223, a median of 676, an interquartile range of 561–802 ng/mL. The mean baseline level of TIMP-1 activity was higher among those who died from cardiovascular causes than among those who did not (820 vs. 692 ng/mL; P<0.001). There was no difference in TIMP-1 concentration between those individuals who developed and who did not develop a non-fatal myocardial infarction (675 vs. 698 ng/mL; P=0.49). As expected, in patients with future cardiovascular death BNP (P<0.001), C-reactive protein (P<0.001), and creatinine (P<0.001) were elevated.
Predictors of TIMP-1
Table 2 outlines the mean, respectively, median levels or correlation coefficients of inflammatory markers, such as TIMP-1, C-reactive protein, and BNP according to the prevalence of traditional risk factors and clinical variables. TIMP-1 was higher among patients with diabetes, and severe vessel disease. Patients receiving statin medication had lower TIMP-1 levels. Interestingly, TIMP-1 levels correlated inversely with smoking history. No difference in TIMP-1 plasma levels was observed between patients presenting with stable angina or acute coronary syndrome.
TIMP-1 and future cardiovascular events
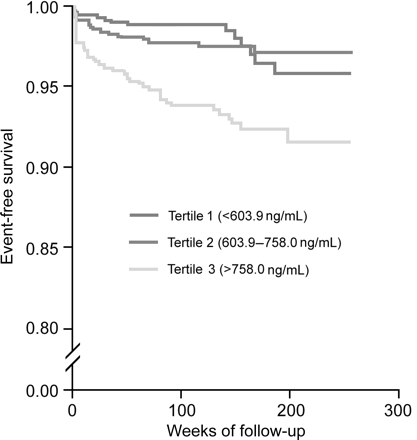
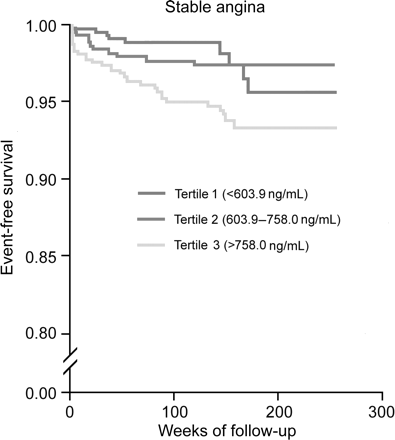
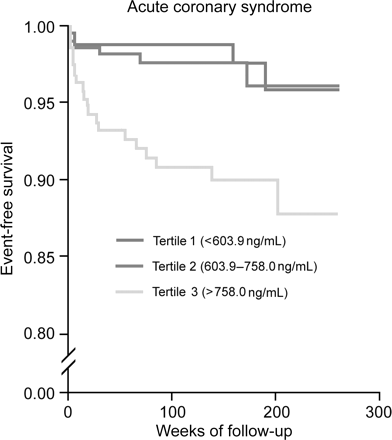
Figure 1 displays the Kaplan–Meier curves for eventfree survival according to thirds of TIMP-1 level. The unadjusted rate of cardiovascular death was highest in patients within the upper third of TIMP-1 level. Importantly, similar results have been achieved if analysis was stratified according to patients presenting with stable angina (Figure 2) and acute coronary syndrome (Figure 3).
Table 3 presents the hazard ratios for cardiovascular death associated with one standard deviation and thirds of TIMP-1 level. To assess the predictive value of TIMP-1 additional to other risk factors, we used various Cox regression models. The hazard ratio for future cardiovascular death increased with increasing thirds (P=0.001) such as patients within the highest third of TIMP-1 level had a 2.54-fold (95% CI: 1.38–4.68; P=0.001) risk compared with individuals within the lowest third in an age and sex adjusted model. The hazard ratios remained nearly unchanged after adjustment for cardiovascular risk factors and clinical features (Table 3, Model 2). Further adjustment for ejection fraction (Table 3, Model 3) as well as C-reactive protein and BNP (Table 3, Model 4) also did not attenuate the hazard ratio within the highest third of TIMP-1 level compared with the lowest third.
In Table 4 shows hazard ratios of one standard deviation of TIMP-1 adjusted for age and sex obtained in subgroup analysis in groups according to categorized risk factors. In particular, TIMP-1 levels were strongly predictive in patients with systolic blood pressure over 125 mmHg.
Comparative analyses of cardiovascular biomarkers
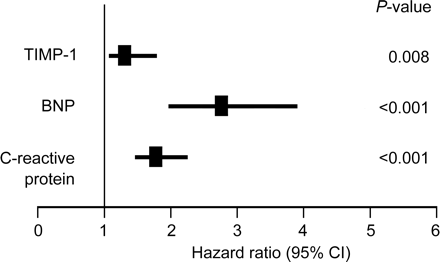
To finally place the predictive power of TIMP-1 into the context of that obtained from the novel biological markers BNP and C-reactive protein (Figure 4), we performed a series of Cox regression analyses. If introduced separately into a model adjusting for age, sex, and traditional risk factors, BNP revealed the strongest association for future cardiovascular death (2.75, 95% CI: 1.94–3.89; P<0.001), whereas the predictive power of TIMP-1 and C-reactive protein was of similar magnitude.
Discussion
The current prospective data suggest TIMP-1 as a risk predictor for cardiovascular death in a large cohort of patients with angiographically documented CAD. This association does not change appreciably after adjustment for most potential confounders and is present in all subgroups evaluated, in particular in hypertensive individuals.
Many pathophysiological mechanisms contribute to the rupture of an unstable plaque, such as inflammation, pro-thrombotic and thrombotic activity, shear stress, endothelial responsiveness to dilatation, collagen degradation, intraplaque angiogenesis, and the morphological characteristics of the plaque itself.20,21 The vulnerability of atherosclerotic plaques is the result of a dynamic process between production and degradation of collagen from smooth muscle cells.22 In addition, derangements in the dynamic balance of cardiac extracellular matrix accumulation and breakdown are additionally associated with LV remodelling, which can cause LV dilatation and LV hypertrophy (LVH).23 The dynamic process of matrix collagen is mainly controlled by locally produced extracellular MMPs and their inhibitors, TIMPs. TIMPs are proteins which regulate the connective tissue metabolism by forming a high affinity, irreversible complexes with the active form of the MMPs, rendering them inactive.24
The MMPs family of enzymes contributes in both normal and pathological tissue remodelling. Inappropriate remodelling underlies the pathogenesis of advanced atherosclerotic plaques, the substrate of acute cardiovascular syndromes. In this context, serum levels of MMP-9 and TIMP-1 are significantly higher in patients with stable and unstable angina and myocardial infarction than those of healthy controls and are raised with or without a rise in cardiac enzymes.25,26 Serum MMPs, TIMPs, and pro-inflammatory cytokines play an important role in the pathophysiology of the acute coronary syndromes.27 The presence of pro-inflammatory cytokines and other inflammatory mediators within the micro-environment of an atherosclerotic plaque leads to an imbalance between the MMPs and TIMPs, which might consequently lead to plaque rupture. Recent experimental data suggest that TIMP-1, in certain settings, may be pro-atherosclerotic.28 An increased plasma TIMP-1 level may be an epiphenomenon or an adaptive response to an increase in MMP activity, the latter being promoted by cardiovascular risk factors.
Independent of its impact on atherosclerotic plaque stability, TIMPs seem to substantially contribute to the maintenance of LV geometry and function.29 In response to significant haemodynamic alterations, LV remodelling is characterized by changes of myocardial extracellular matrix.16 Plasma TIMP-1 level may be a marker of cardiovascular extracellular matrix remodelling, a process that may be enhanced with increasing cardiovascular risk.
Several traditional cardiovascular risk factors, in particular hypertension are known to influence LVH.30,31 In particular, we note an association between circulating TIMP-1 levels and the prevalence of hypertension. Hypertension leads to structural changes to the cardiac and vascular extracellular matrix, and plasma TIMP-1 levels are increased and associated with LVH and LV diastolic impairment in some,14,32 but not all studies.33 Thus, TIMP-1 apparently represents a marker of vascular remodelling, which in turn may lead to increased LV afterload with subsequent LVH, if the balance of enzymes responsible for matrix composition is disturbed.2 Alternatively, elevated TIMP-1 levels may reflect increased collagen content of the cardiac extracellular matrix, which adversely affects LV systolic function. Taken together, the plasma TIMP-1 level can be considered as an important marker of the cardiovascular extracellular matrix remodelling process, that may increase with exaggerating cardiovascular risk, in particular in hypertensive individuals.
Treatment of hypertension with ACE-inhibitors decreases TIMP-1 levels34 and exerts positive effects in LV remodelling process.27 Hence, TIMP-1 might not only be a cardiovascular risk biomarker but could also be considered as a marker for close monitoring of antihypertensive therapy, a hypothesis which should be addressed prospectively.
Limitations
The AtheroGene register allows to determine different types of novel biomarkers in a prospective manner. It has to be kept in mind that this is an observational study. Results have to be confirmed in further prospective studies, which directly address TIMP as a cardiovascular predictor.
Measurement of all inflammatory markers was performed on samples that were stored deep-frozen until analysis. We, therefore, cannot exclude the possibility of protein degradation. However, this should not affect the validity of our results, as all patients have been handled identically. Furthermore, as expected, only a relatively small number of events has been registered during follow-up in this intermediate risk population which may lead to unstable results especially in the multi-variate survival analysis.
MMP-activity, especially MMP-9 activity, has not been determined. As the current measure indicated the MMP-9/TIMP-1 complex and MMP-9 raises in parallel to TIMP-1, measurement of TIMP-1 alone might indicate the system activity.
Patients suffering from CAD and elevated levels of TIMP-1 are at increased risk for future cardiovascular death. Although TIMP-1 related to the presence of various cardiovascular risk factors, in particular hypertension, it remains as a risk predictor giving important additional information to other risk factors. Thus, TIMP-1 might influence LV geometric processes, acts as a prognostic marker of cardiovascular risk, and might be modulated by antihypertensive treatment.
Acknowledgements
The AtheroGene study is supported by a grant of the ‘Stiftung Rheinland-Pfalz für Innovation’, Ministry for Science and Education (AZ 15202–386261/545), Mainz. We thank Biosite Inc., for providing test material (BNP) and Roche Diagnostics for also providing test material (C-reactive protein). We are indebted to Margot Neuser for graphical work.
Appendix
The AtheroGene group
Stefan Blankenberg, Hans-Jürgen Rupprecht, Christoph Bickel, Christine Espinola-Klein, Jürgen Meyer, Renate Schnabel, Edith Lubos, Department of Medicine II, Johannes Gutenberg-University Mainz, Germany; Laurence Tiret, Odette Poirier, Viviane Nicaud, David Tregouet, Jean-Louis Georges, François Cambien, INSERM U525, Paris, France.
Conflict of interest: none declared.
Figure 1 Cumulative incidence of cardiovascular death according to TIMP-1 tertiles in all patients.
Figure 2 Cumulative incidence of cardiovascular death according to TIMP-1 tertiles in patients with stable angina.
Figure 3 Cumulative incidence of cardiovascular death according to TIMP-1 tertiles in patients with acute coronary syndrome.
Figure 4 Hazard ratio and 95% CI of cardiovascular death of each biomarker. Hazard ratio and 95% CI of cardiovascular death associated with increase of one standard deviation to account for the different ranges of the values of the different variables. Each biomarker was entered separately into a model along with the classical risk factors comprising age, sex, body mass index, mean arterial blood pressure, diabetes, smoking status, and HDL. Age, body mass index, mean arterial blood pressure, and HDL entered the model as continuous variables. BNP and C-reactive protein were log-transformed.
Baseline characteristics of the study population
| Characteristic . | Total group (n=1945) . |
|---|---|
| Age (years) | 61.2±9.7 |
| Male sex (%) | 78.9 |
| Classical risk factors | |
| Body mass index, (kg/m2) | 27.7±3.9 |
| Diabetes (%) | |
| None | 83.1 |
| Oral medication | 8.6 |
| Insulin-dependent | 8.2 |
| Blood pressure (mmHg) | |
| Systolica | 130 (115/148) |
| Diastolicb | 69.9±12.2 |
| Mean arterial pressureb | 94.6±15.6 |
| Smoking status (%) | |
| Never smoked | 35.5 |
| Formerly smoked | 44.5 |
| Currently smoke | 20.0 |
| Lipid variables | |
| LDL cholesterol (mg/dL) | 127±41.2 |
| HDL cholesterol (mg/dL) | 49.3±13.6 |
| Triglycerides (mg/dL) | 131 (96/184) |
| Family history (%) | 37.3 |
| Cardiac medication | |
| Beta-blocker medication (%) | 64.5 |
| Statin medication (%) | 55.7 |
| ACE-inhibitor medication (%) | 49.1 |
| Clinical variables | |
| Disease in one or more vessels (%) | |
| One | 27.3 |
| Two | 31.1 |
| Three | 41.4 |
| History of myocardial infarction (%) | 37.5 |
| Left ventricular ejection fraction (%)c | 62.8±15.4 |
| Biomarkers | |
| TIMP-1 (ng/mL) | 697±223 676 (561/802) |
| BNP (pg/mL)d | 50.9 (16.7/155) |
| C-reactive protein (mg/L)e | 3.2 (1.5/8.0) |
| Creatinine (mg/dL)f | 0.94 (0.82/1.07) |
| Characteristic . | Total group (n=1945) . |
|---|---|
| Age (years) | 61.2±9.7 |
| Male sex (%) | 78.9 |
| Classical risk factors | |
| Body mass index, (kg/m2) | 27.7±3.9 |
| Diabetes (%) | |
| None | 83.1 |
| Oral medication | 8.6 |
| Insulin-dependent | 8.2 |
| Blood pressure (mmHg) | |
| Systolica | 130 (115/148) |
| Diastolicb | 69.9±12.2 |
| Mean arterial pressureb | 94.6±15.6 |
| Smoking status (%) | |
| Never smoked | 35.5 |
| Formerly smoked | 44.5 |
| Currently smoke | 20.0 |
| Lipid variables | |
| LDL cholesterol (mg/dL) | 127±41.2 |
| HDL cholesterol (mg/dL) | 49.3±13.6 |
| Triglycerides (mg/dL) | 131 (96/184) |
| Family history (%) | 37.3 |
| Cardiac medication | |
| Beta-blocker medication (%) | 64.5 |
| Statin medication (%) | 55.7 |
| ACE-inhibitor medication (%) | 49.1 |
| Clinical variables | |
| Disease in one or more vessels (%) | |
| One | 27.3 |
| Two | 31.1 |
| Three | 41.4 |
| History of myocardial infarction (%) | 37.5 |
| Left ventricular ejection fraction (%)c | 62.8±15.4 |
| Biomarkers | |
| TIMP-1 (ng/mL) | 697±223 676 (561/802) |
| BNP (pg/mL)d | 50.9 (16.7/155) |
| C-reactive protein (mg/L)e | 3.2 (1.5/8.0) |
| Creatinine (mg/dL)f | 0.94 (0.82/1.07) |
Data presented are percentage of patients or mean±SD or median and 25th/75th percentile for skewed variables.
LDL, low density lipoprotein; HDL, high density lipoprotein; TIMP, tissue inhibitor of metalloproteinase; BNP, B-type natriuretic peptide; ACE, angiotensin-coverting enzyme.
aSystolic blood pressure was available for 1717 patients.
bDiastolic and mean arterial blood pressure was available for 1714 patients.
cLeft ventricular ejection fraction determined by left ventricular angiography was available for 1341 patients.
dB-type natriuretic peptide was available for 1827 patients.
eC-reactive protein was available for 1973 patients.
fCreatinine was available for 1961 patients.
Baseline characteristics of the study population
| Characteristic . | Total group (n=1945) . |
|---|---|
| Age (years) | 61.2±9.7 |
| Male sex (%) | 78.9 |
| Classical risk factors | |
| Body mass index, (kg/m2) | 27.7±3.9 |
| Diabetes (%) | |
| None | 83.1 |
| Oral medication | 8.6 |
| Insulin-dependent | 8.2 |
| Blood pressure (mmHg) | |
| Systolica | 130 (115/148) |
| Diastolicb | 69.9±12.2 |
| Mean arterial pressureb | 94.6±15.6 |
| Smoking status (%) | |
| Never smoked | 35.5 |
| Formerly smoked | 44.5 |
| Currently smoke | 20.0 |
| Lipid variables | |
| LDL cholesterol (mg/dL) | 127±41.2 |
| HDL cholesterol (mg/dL) | 49.3±13.6 |
| Triglycerides (mg/dL) | 131 (96/184) |
| Family history (%) | 37.3 |
| Cardiac medication | |
| Beta-blocker medication (%) | 64.5 |
| Statin medication (%) | 55.7 |
| ACE-inhibitor medication (%) | 49.1 |
| Clinical variables | |
| Disease in one or more vessels (%) | |
| One | 27.3 |
| Two | 31.1 |
| Three | 41.4 |
| History of myocardial infarction (%) | 37.5 |
| Left ventricular ejection fraction (%)c | 62.8±15.4 |
| Biomarkers | |
| TIMP-1 (ng/mL) | 697±223 676 (561/802) |
| BNP (pg/mL)d | 50.9 (16.7/155) |
| C-reactive protein (mg/L)e | 3.2 (1.5/8.0) |
| Creatinine (mg/dL)f | 0.94 (0.82/1.07) |
| Characteristic . | Total group (n=1945) . |
|---|---|
| Age (years) | 61.2±9.7 |
| Male sex (%) | 78.9 |
| Classical risk factors | |
| Body mass index, (kg/m2) | 27.7±3.9 |
| Diabetes (%) | |
| None | 83.1 |
| Oral medication | 8.6 |
| Insulin-dependent | 8.2 |
| Blood pressure (mmHg) | |
| Systolica | 130 (115/148) |
| Diastolicb | 69.9±12.2 |
| Mean arterial pressureb | 94.6±15.6 |
| Smoking status (%) | |
| Never smoked | 35.5 |
| Formerly smoked | 44.5 |
| Currently smoke | 20.0 |
| Lipid variables | |
| LDL cholesterol (mg/dL) | 127±41.2 |
| HDL cholesterol (mg/dL) | 49.3±13.6 |
| Triglycerides (mg/dL) | 131 (96/184) |
| Family history (%) | 37.3 |
| Cardiac medication | |
| Beta-blocker medication (%) | 64.5 |
| Statin medication (%) | 55.7 |
| ACE-inhibitor medication (%) | 49.1 |
| Clinical variables | |
| Disease in one or more vessels (%) | |
| One | 27.3 |
| Two | 31.1 |
| Three | 41.4 |
| History of myocardial infarction (%) | 37.5 |
| Left ventricular ejection fraction (%)c | 62.8±15.4 |
| Biomarkers | |
| TIMP-1 (ng/mL) | 697±223 676 (561/802) |
| BNP (pg/mL)d | 50.9 (16.7/155) |
| C-reactive protein (mg/L)e | 3.2 (1.5/8.0) |
| Creatinine (mg/dL)f | 0.94 (0.82/1.07) |
Data presented are percentage of patients or mean±SD or median and 25th/75th percentile for skewed variables.
LDL, low density lipoprotein; HDL, high density lipoprotein; TIMP, tissue inhibitor of metalloproteinase; BNP, B-type natriuretic peptide; ACE, angiotensin-coverting enzyme.
aSystolic blood pressure was available for 1717 patients.
bDiastolic and mean arterial blood pressure was available for 1714 patients.
cLeft ventricular ejection fraction determined by left ventricular angiography was available for 1341 patients.
dB-type natriuretic peptide was available for 1827 patients.
eC-reactive protein was available for 1973 patients.
fCreatinine was available for 1961 patients.
Correlation coefficients and levels of biomarkers according to cardiovascular risk factors
| Variable . | TIMP-1 (ng/mL) . | C-reactive protein (mg/L) . | BNP (pg/mL) . |
|---|---|---|---|
| Age | 0.19 | 0.07 | 0.31 |
| Body mass index (kg/m2) | 0.08 | 0.14 | −0.02 |
| Systolic blood pressure (mmHg) | 0.05 | −0.01 | 0.08 |
| Diastolic blood pressure (mmHg) | −0.004 | 0.02 | −0.05 |
| Mean arterial pressure (mmHg) | 0.02 | −0.009 | 0.02 |
| HDL cholesterol (mg/dL) | −0.12 | −0.17 | −0.06 |
| LDL cholesterol (mg/dL) | −0.02 | 0.06 | −0.09 |
| Triglycerides (mg/dL) | 0.12 | 0.09 | −0.1 |
| Glucose (mmol/L) | 0.14 | 0.15 | 0.05 |
| TIMP-1 (ng/mL) | 1 | 0.16 | 0.18 |
| C-reative protein (mg/L) | 0.1 | 1 | 0.31 |
| Female | 710 (224) | 4.5 (2.1/11.7) | 74.4 (29.9/211) |
| Male | 695 (225) | 2.9 (1.4/7.3) | 45.5 (14.6/141) |
| P-value | 0.23 | <0.001 | <0.001 |
| No diabetes | 684 (221) | 3.1 (1.4/7.8) | 48.4 (16.3/143) |
| Diabetes | 752 (230) | 3.7 (1.8/9.2) | 62.6 (18.6/200) |
| P-value | <0.001 | 0.004 | 0.006 |
| Never smoking | 723 (232) | 3.1 (1.4/7.5) | 55.9 (20.2/171) |
| Formerly smoking | 694 (216) | 2.8 (1.4/7.5) | 47.0 (15.4/142) |
| Current smoke | 664 (226) | 4.1 (1.8/11.7) | 49.4 (11.6/155) |
| P-value | <0.001 | <0.001 | 0.03 |
| One-vessel disease | 680 (219) | 2.8 (1.3/7.2) | 36.3 (7.8/111) |
| Two-vessel disease | 686 (224) | 3.4 (1.5/8.0) | 50.2 (17.5/157) |
| Three-vessel disease | 721 (227) | 3.4 (1.5/9.1) | 62.3 (20.9/177) |
| P-value | 0.001 | 0.03 | <0.001 |
| Without statin medication | 721 (242) | 3.8 (1.6/9.6) | 56.5 (16.6/163) |
| Statin medication | 677 (205) | 2.7 (1.4/7.0) | 46.9 (17.1/144) |
| P-value | <0.001 | <0.001 | 0.09 |
| Variable . | TIMP-1 (ng/mL) . | C-reactive protein (mg/L) . | BNP (pg/mL) . |
|---|---|---|---|
| Age | 0.19 | 0.07 | 0.31 |
| Body mass index (kg/m2) | 0.08 | 0.14 | −0.02 |
| Systolic blood pressure (mmHg) | 0.05 | −0.01 | 0.08 |
| Diastolic blood pressure (mmHg) | −0.004 | 0.02 | −0.05 |
| Mean arterial pressure (mmHg) | 0.02 | −0.009 | 0.02 |
| HDL cholesterol (mg/dL) | −0.12 | −0.17 | −0.06 |
| LDL cholesterol (mg/dL) | −0.02 | 0.06 | −0.09 |
| Triglycerides (mg/dL) | 0.12 | 0.09 | −0.1 |
| Glucose (mmol/L) | 0.14 | 0.15 | 0.05 |
| TIMP-1 (ng/mL) | 1 | 0.16 | 0.18 |
| C-reative protein (mg/L) | 0.1 | 1 | 0.31 |
| Female | 710 (224) | 4.5 (2.1/11.7) | 74.4 (29.9/211) |
| Male | 695 (225) | 2.9 (1.4/7.3) | 45.5 (14.6/141) |
| P-value | 0.23 | <0.001 | <0.001 |
| No diabetes | 684 (221) | 3.1 (1.4/7.8) | 48.4 (16.3/143) |
| Diabetes | 752 (230) | 3.7 (1.8/9.2) | 62.6 (18.6/200) |
| P-value | <0.001 | 0.004 | 0.006 |
| Never smoking | 723 (232) | 3.1 (1.4/7.5) | 55.9 (20.2/171) |
| Formerly smoking | 694 (216) | 2.8 (1.4/7.5) | 47.0 (15.4/142) |
| Current smoke | 664 (226) | 4.1 (1.8/11.7) | 49.4 (11.6/155) |
| P-value | <0.001 | <0.001 | 0.03 |
| One-vessel disease | 680 (219) | 2.8 (1.3/7.2) | 36.3 (7.8/111) |
| Two-vessel disease | 686 (224) | 3.4 (1.5/8.0) | 50.2 (17.5/157) |
| Three-vessel disease | 721 (227) | 3.4 (1.5/9.1) | 62.3 (20.9/177) |
| P-value | 0.001 | 0.03 | <0.001 |
| Without statin medication | 721 (242) | 3.8 (1.6/9.6) | 56.5 (16.6/163) |
| Statin medication | 677 (205) | 2.7 (1.4/7.0) | 46.9 (17.1/144) |
| P-value | <0.001 | <0.001 | 0.09 |
For the association of TIMP-1, C-reactive protein and, BNP with categorical variables, data are presented as mean (SD) or median and 25th/75th percentile for skewed variables. For normally distributed variables, P-values were computed with t-tests; for skewed variables, P-values were computed with the Mann–Whitney U-test. For the association of TIMP-1, C-reactive protein and BNP with continuous variables, Spearman correlation coefficients are presented.
Correlation coefficients and levels of biomarkers according to cardiovascular risk factors
| Variable . | TIMP-1 (ng/mL) . | C-reactive protein (mg/L) . | BNP (pg/mL) . |
|---|---|---|---|
| Age | 0.19 | 0.07 | 0.31 |
| Body mass index (kg/m2) | 0.08 | 0.14 | −0.02 |
| Systolic blood pressure (mmHg) | 0.05 | −0.01 | 0.08 |
| Diastolic blood pressure (mmHg) | −0.004 | 0.02 | −0.05 |
| Mean arterial pressure (mmHg) | 0.02 | −0.009 | 0.02 |
| HDL cholesterol (mg/dL) | −0.12 | −0.17 | −0.06 |
| LDL cholesterol (mg/dL) | −0.02 | 0.06 | −0.09 |
| Triglycerides (mg/dL) | 0.12 | 0.09 | −0.1 |
| Glucose (mmol/L) | 0.14 | 0.15 | 0.05 |
| TIMP-1 (ng/mL) | 1 | 0.16 | 0.18 |
| C-reative protein (mg/L) | 0.1 | 1 | 0.31 |
| Female | 710 (224) | 4.5 (2.1/11.7) | 74.4 (29.9/211) |
| Male | 695 (225) | 2.9 (1.4/7.3) | 45.5 (14.6/141) |
| P-value | 0.23 | <0.001 | <0.001 |
| No diabetes | 684 (221) | 3.1 (1.4/7.8) | 48.4 (16.3/143) |
| Diabetes | 752 (230) | 3.7 (1.8/9.2) | 62.6 (18.6/200) |
| P-value | <0.001 | 0.004 | 0.006 |
| Never smoking | 723 (232) | 3.1 (1.4/7.5) | 55.9 (20.2/171) |
| Formerly smoking | 694 (216) | 2.8 (1.4/7.5) | 47.0 (15.4/142) |
| Current smoke | 664 (226) | 4.1 (1.8/11.7) | 49.4 (11.6/155) |
| P-value | <0.001 | <0.001 | 0.03 |
| One-vessel disease | 680 (219) | 2.8 (1.3/7.2) | 36.3 (7.8/111) |
| Two-vessel disease | 686 (224) | 3.4 (1.5/8.0) | 50.2 (17.5/157) |
| Three-vessel disease | 721 (227) | 3.4 (1.5/9.1) | 62.3 (20.9/177) |
| P-value | 0.001 | 0.03 | <0.001 |
| Without statin medication | 721 (242) | 3.8 (1.6/9.6) | 56.5 (16.6/163) |
| Statin medication | 677 (205) | 2.7 (1.4/7.0) | 46.9 (17.1/144) |
| P-value | <0.001 | <0.001 | 0.09 |
| Variable . | TIMP-1 (ng/mL) . | C-reactive protein (mg/L) . | BNP (pg/mL) . |
|---|---|---|---|
| Age | 0.19 | 0.07 | 0.31 |
| Body mass index (kg/m2) | 0.08 | 0.14 | −0.02 |
| Systolic blood pressure (mmHg) | 0.05 | −0.01 | 0.08 |
| Diastolic blood pressure (mmHg) | −0.004 | 0.02 | −0.05 |
| Mean arterial pressure (mmHg) | 0.02 | −0.009 | 0.02 |
| HDL cholesterol (mg/dL) | −0.12 | −0.17 | −0.06 |
| LDL cholesterol (mg/dL) | −0.02 | 0.06 | −0.09 |
| Triglycerides (mg/dL) | 0.12 | 0.09 | −0.1 |
| Glucose (mmol/L) | 0.14 | 0.15 | 0.05 |
| TIMP-1 (ng/mL) | 1 | 0.16 | 0.18 |
| C-reative protein (mg/L) | 0.1 | 1 | 0.31 |
| Female | 710 (224) | 4.5 (2.1/11.7) | 74.4 (29.9/211) |
| Male | 695 (225) | 2.9 (1.4/7.3) | 45.5 (14.6/141) |
| P-value | 0.23 | <0.001 | <0.001 |
| No diabetes | 684 (221) | 3.1 (1.4/7.8) | 48.4 (16.3/143) |
| Diabetes | 752 (230) | 3.7 (1.8/9.2) | 62.6 (18.6/200) |
| P-value | <0.001 | 0.004 | 0.006 |
| Never smoking | 723 (232) | 3.1 (1.4/7.5) | 55.9 (20.2/171) |
| Formerly smoking | 694 (216) | 2.8 (1.4/7.5) | 47.0 (15.4/142) |
| Current smoke | 664 (226) | 4.1 (1.8/11.7) | 49.4 (11.6/155) |
| P-value | <0.001 | <0.001 | 0.03 |
| One-vessel disease | 680 (219) | 2.8 (1.3/7.2) | 36.3 (7.8/111) |
| Two-vessel disease | 686 (224) | 3.4 (1.5/8.0) | 50.2 (17.5/157) |
| Three-vessel disease | 721 (227) | 3.4 (1.5/9.1) | 62.3 (20.9/177) |
| P-value | 0.001 | 0.03 | <0.001 |
| Without statin medication | 721 (242) | 3.8 (1.6/9.6) | 56.5 (16.6/163) |
| Statin medication | 677 (205) | 2.7 (1.4/7.0) | 46.9 (17.1/144) |
| P-value | <0.001 | <0.001 | 0.09 |
For the association of TIMP-1, C-reactive protein and, BNP with categorical variables, data are presented as mean (SD) or median and 25th/75th percentile for skewed variables. For normally distributed variables, P-values were computed with t-tests; for skewed variables, P-values were computed with the Mann–Whitney U-test. For the association of TIMP-1, C-reactive protein and BNP with continuous variables, Spearman correlation coefficients are presented.
Hazard ratios for future cardiovascular death according to TIMP-1 level
| . | TIMP-1 ng/mL HR per increase standard deviation . | TIMP-1 ng/mL HR per third . | P-value (Type III test) for increasing thirds . | ||
|---|---|---|---|---|---|
| Survival rates | |||||
| 2 years | 0.967 | ||||
| 4 years | 0.947 | ||||
| Third | 1 | 2 | 3 | ||
| ng/mL | <603.9 | 603.9–758.0 | >758.0 | ||
| n | 1945 | 648 | 652 | 645 | |
| Cardiovascular death, n (%) | 75 (3.9) | ||||
| Adjusted for age and sex (Model 1) | 0.001 | ||||
| Hazard ratio | 1.37 | 1.0 | 1.09 | 2.54 | |
| 95% CI | 1.17–1.61 | — | 0.53–2.21 | 1.38–4.68 | |
| P-value | <0.001 | — | 0.82 | 0.003 | |
| Adjusted for risk factors and clinical variables (Model 2)a | 0.04 | ||||
| Hazard ratio | 1.25 | 1.0 | 1.02 | 2.04 | |
| 95% CI | 1.02–1.54 | — | 0.46–2.25 | 1.02–4.05 | |
| P-value | 0.03 | — | 0.97 | 0.04 | |
| Adjusted for risk factors and clinical variables and EF (Model 3)b | 0.02 | ||||
| Hazard ratio | 1.16 | 1.0 | 1.49 | 3.25 | |
| 95% CI | 0.91–1.47 | — | 0.51–4.38 | 1.29–8.20 | |
| P-value | 0.25 | — | 0.47 | 0.01 | |
| Fully adjusted (Model 4)c | 0.08 | ||||
| Hazard ratio | 1.08 | 1.0 | 1.51 | 2.76 | |
| 95% CI | 0.83–1.40 | — | 0.51–4.49 | 1.06–7.16 | |
| P-value | 0.58 | — | 0.46 | 0.04 | |
| . | TIMP-1 ng/mL HR per increase standard deviation . | TIMP-1 ng/mL HR per third . | P-value (Type III test) for increasing thirds . | ||
|---|---|---|---|---|---|
| Survival rates | |||||
| 2 years | 0.967 | ||||
| 4 years | 0.947 | ||||
| Third | 1 | 2 | 3 | ||
| ng/mL | <603.9 | 603.9–758.0 | >758.0 | ||
| n | 1945 | 648 | 652 | 645 | |
| Cardiovascular death, n (%) | 75 (3.9) | ||||
| Adjusted for age and sex (Model 1) | 0.001 | ||||
| Hazard ratio | 1.37 | 1.0 | 1.09 | 2.54 | |
| 95% CI | 1.17–1.61 | — | 0.53–2.21 | 1.38–4.68 | |
| P-value | <0.001 | — | 0.82 | 0.003 | |
| Adjusted for risk factors and clinical variables (Model 2)a | 0.04 | ||||
| Hazard ratio | 1.25 | 1.0 | 1.02 | 2.04 | |
| 95% CI | 1.02–1.54 | — | 0.46–2.25 | 1.02–4.05 | |
| P-value | 0.03 | — | 0.97 | 0.04 | |
| Adjusted for risk factors and clinical variables and EF (Model 3)b | 0.02 | ||||
| Hazard ratio | 1.16 | 1.0 | 1.49 | 3.25 | |
| 95% CI | 0.91–1.47 | — | 0.51–4.38 | 1.29–8.20 | |
| P-value | 0.25 | — | 0.47 | 0.01 | |
| Fully adjusted (Model 4)c | 0.08 | ||||
| Hazard ratio | 1.08 | 1.0 | 1.51 | 2.76 | |
| 95% CI | 0.83–1.40 | — | 0.51–4.49 | 1.06–7.16 | |
| P-value | 0.58 | — | 0.46 | 0.04 | |
CI denotes confidence interval.
aFurther adjusted for body mass index (continuous variable), mean arterial blood pressure (continuous variable), diabetes, smoking status, HDL (continuous variable). Additionally adjusted for extent of vessel disease, presence or absence of acute coronary syndrome, ACE-inhibitors, statin and beta-blocker therapy, and serum creatinine level (log-transformed variables). Because of missing data, this model considered only 63 cardiovascular deaths.
bModel 3 additionally adjusted for ejection fraction. Because of missing data, this model considered only 42 cardiovascular deaths.
c Model 4 additionally adjusted for BNP and C-reactive protein level (log-transformed variables). Because of missing data, this model considered only 40 cardiovascular deaths.
Hazard ratios for future cardiovascular death according to TIMP-1 level
| . | TIMP-1 ng/mL HR per increase standard deviation . | TIMP-1 ng/mL HR per third . | P-value (Type III test) for increasing thirds . | ||
|---|---|---|---|---|---|
| Survival rates | |||||
| 2 years | 0.967 | ||||
| 4 years | 0.947 | ||||
| Third | 1 | 2 | 3 | ||
| ng/mL | <603.9 | 603.9–758.0 | >758.0 | ||
| n | 1945 | 648 | 652 | 645 | |
| Cardiovascular death, n (%) | 75 (3.9) | ||||
| Adjusted for age and sex (Model 1) | 0.001 | ||||
| Hazard ratio | 1.37 | 1.0 | 1.09 | 2.54 | |
| 95% CI | 1.17–1.61 | — | 0.53–2.21 | 1.38–4.68 | |
| P-value | <0.001 | — | 0.82 | 0.003 | |
| Adjusted for risk factors and clinical variables (Model 2)a | 0.04 | ||||
| Hazard ratio | 1.25 | 1.0 | 1.02 | 2.04 | |
| 95% CI | 1.02–1.54 | — | 0.46–2.25 | 1.02–4.05 | |
| P-value | 0.03 | — | 0.97 | 0.04 | |
| Adjusted for risk factors and clinical variables and EF (Model 3)b | 0.02 | ||||
| Hazard ratio | 1.16 | 1.0 | 1.49 | 3.25 | |
| 95% CI | 0.91–1.47 | — | 0.51–4.38 | 1.29–8.20 | |
| P-value | 0.25 | — | 0.47 | 0.01 | |
| Fully adjusted (Model 4)c | 0.08 | ||||
| Hazard ratio | 1.08 | 1.0 | 1.51 | 2.76 | |
| 95% CI | 0.83–1.40 | — | 0.51–4.49 | 1.06–7.16 | |
| P-value | 0.58 | — | 0.46 | 0.04 | |
| . | TIMP-1 ng/mL HR per increase standard deviation . | TIMP-1 ng/mL HR per third . | P-value (Type III test) for increasing thirds . | ||
|---|---|---|---|---|---|
| Survival rates | |||||
| 2 years | 0.967 | ||||
| 4 years | 0.947 | ||||
| Third | 1 | 2 | 3 | ||
| ng/mL | <603.9 | 603.9–758.0 | >758.0 | ||
| n | 1945 | 648 | 652 | 645 | |
| Cardiovascular death, n (%) | 75 (3.9) | ||||
| Adjusted for age and sex (Model 1) | 0.001 | ||||
| Hazard ratio | 1.37 | 1.0 | 1.09 | 2.54 | |
| 95% CI | 1.17–1.61 | — | 0.53–2.21 | 1.38–4.68 | |
| P-value | <0.001 | — | 0.82 | 0.003 | |
| Adjusted for risk factors and clinical variables (Model 2)a | 0.04 | ||||
| Hazard ratio | 1.25 | 1.0 | 1.02 | 2.04 | |
| 95% CI | 1.02–1.54 | — | 0.46–2.25 | 1.02–4.05 | |
| P-value | 0.03 | — | 0.97 | 0.04 | |
| Adjusted for risk factors and clinical variables and EF (Model 3)b | 0.02 | ||||
| Hazard ratio | 1.16 | 1.0 | 1.49 | 3.25 | |
| 95% CI | 0.91–1.47 | — | 0.51–4.38 | 1.29–8.20 | |
| P-value | 0.25 | — | 0.47 | 0.01 | |
| Fully adjusted (Model 4)c | 0.08 | ||||
| Hazard ratio | 1.08 | 1.0 | 1.51 | 2.76 | |
| 95% CI | 0.83–1.40 | — | 0.51–4.49 | 1.06–7.16 | |
| P-value | 0.58 | — | 0.46 | 0.04 | |
CI denotes confidence interval.
aFurther adjusted for body mass index (continuous variable), mean arterial blood pressure (continuous variable), diabetes, smoking status, HDL (continuous variable). Additionally adjusted for extent of vessel disease, presence or absence of acute coronary syndrome, ACE-inhibitors, statin and beta-blocker therapy, and serum creatinine level (log-transformed variables). Because of missing data, this model considered only 63 cardiovascular deaths.
bModel 3 additionally adjusted for ejection fraction. Because of missing data, this model considered only 42 cardiovascular deaths.
c Model 4 additionally adjusted for BNP and C-reactive protein level (log-transformed variables). Because of missing data, this model considered only 40 cardiovascular deaths.
Hazard ratios of TIMP-1 adjusted for age and sex according to categorical risk factors
| Variable . | . | Hazard ratio (95% CI) per one standard deviation increase of TIMP-1 . | P-value . |
|---|---|---|---|
| Sexa | Female | 1.38 (0.92–2.09) | 0.12 |
| Male | 1.37 (1.15–1.62) | <0.001 | |
| Body mass index (kg/m2) | <30 | 1.35 (1.13–1.63) | 0.001 |
| ≥30 | 1.44 (1.00–2.06) | 0.05 | |
| Diabetes | No | 1.30 (1.05–1.60) | 0.02 |
| Yes | 1.59 (1.15–2.19) | 0.005 | |
| Systolic blood pressure (mmHg) | ≤125 | 1.27 (0.98–1.64) | 0.067 |
| >125 | 1.46 (1.11–1.92) | 0.006 | |
| Smoking | No | 1.15 (0.84–1.58) | 0.38 |
| Former/Current | 1.66 (1.32–2.10) | <0.001 | |
| N-vessel disease | One | 1.50 (0.93–2.42) | 0.10 |
| Multi-vessel | 1.36 (1.15–1.60) | <0.001 | |
| Statin medication | No | 1.22 (0.97–1.53) | 0.09 |
| Yes | 1.84 (1.36–2.48) | <0.001 | |
| Ejection fraction (%) | >40 | 1.37 (1.09–1.71) | 0.006 |
| ≤40 | 1.22 (0.82–1.81) | 0.33 |
| Variable . | . | Hazard ratio (95% CI) per one standard deviation increase of TIMP-1 . | P-value . |
|---|---|---|---|
| Sexa | Female | 1.38 (0.92–2.09) | 0.12 |
| Male | 1.37 (1.15–1.62) | <0.001 | |
| Body mass index (kg/m2) | <30 | 1.35 (1.13–1.63) | 0.001 |
| ≥30 | 1.44 (1.00–2.06) | 0.05 | |
| Diabetes | No | 1.30 (1.05–1.60) | 0.02 |
| Yes | 1.59 (1.15–2.19) | 0.005 | |
| Systolic blood pressure (mmHg) | ≤125 | 1.27 (0.98–1.64) | 0.067 |
| >125 | 1.46 (1.11–1.92) | 0.006 | |
| Smoking | No | 1.15 (0.84–1.58) | 0.38 |
| Former/Current | 1.66 (1.32–2.10) | <0.001 | |
| N-vessel disease | One | 1.50 (0.93–2.42) | 0.10 |
| Multi-vessel | 1.36 (1.15–1.60) | <0.001 | |
| Statin medication | No | 1.22 (0.97–1.53) | 0.09 |
| Yes | 1.84 (1.36–2.48) | <0.001 | |
| Ejection fraction (%) | >40 | 1.37 (1.09–1.71) | 0.006 |
| ≤40 | 1.22 (0.82–1.81) | 0.33 |
aAdjusted only for age.
Hazard ratios of TIMP-1 adjusted for age and sex according to categorical risk factors
| Variable . | . | Hazard ratio (95% CI) per one standard deviation increase of TIMP-1 . | P-value . |
|---|---|---|---|
| Sexa | Female | 1.38 (0.92–2.09) | 0.12 |
| Male | 1.37 (1.15–1.62) | <0.001 | |
| Body mass index (kg/m2) | <30 | 1.35 (1.13–1.63) | 0.001 |
| ≥30 | 1.44 (1.00–2.06) | 0.05 | |
| Diabetes | No | 1.30 (1.05–1.60) | 0.02 |
| Yes | 1.59 (1.15–2.19) | 0.005 | |
| Systolic blood pressure (mmHg) | ≤125 | 1.27 (0.98–1.64) | 0.067 |
| >125 | 1.46 (1.11–1.92) | 0.006 | |
| Smoking | No | 1.15 (0.84–1.58) | 0.38 |
| Former/Current | 1.66 (1.32–2.10) | <0.001 | |
| N-vessel disease | One | 1.50 (0.93–2.42) | 0.10 |
| Multi-vessel | 1.36 (1.15–1.60) | <0.001 | |
| Statin medication | No | 1.22 (0.97–1.53) | 0.09 |
| Yes | 1.84 (1.36–2.48) | <0.001 | |
| Ejection fraction (%) | >40 | 1.37 (1.09–1.71) | 0.006 |
| ≤40 | 1.22 (0.82–1.81) | 0.33 |
| Variable . | . | Hazard ratio (95% CI) per one standard deviation increase of TIMP-1 . | P-value . |
|---|---|---|---|
| Sexa | Female | 1.38 (0.92–2.09) | 0.12 |
| Male | 1.37 (1.15–1.62) | <0.001 | |
| Body mass index (kg/m2) | <30 | 1.35 (1.13–1.63) | 0.001 |
| ≥30 | 1.44 (1.00–2.06) | 0.05 | |
| Diabetes | No | 1.30 (1.05–1.60) | 0.02 |
| Yes | 1.59 (1.15–2.19) | 0.005 | |
| Systolic blood pressure (mmHg) | ≤125 | 1.27 (0.98–1.64) | 0.067 |
| >125 | 1.46 (1.11–1.92) | 0.006 | |
| Smoking | No | 1.15 (0.84–1.58) | 0.38 |
| Former/Current | 1.66 (1.32–2.10) | <0.001 | |
| N-vessel disease | One | 1.50 (0.93–2.42) | 0.10 |
| Multi-vessel | 1.36 (1.15–1.60) | <0.001 | |
| Statin medication | No | 1.22 (0.97–1.53) | 0.09 |
| Yes | 1.84 (1.36–2.48) | <0.001 | |
| Ejection fraction (%) | >40 | 1.37 (1.09–1.71) | 0.006 |
| ≤40 | 1.22 (0.82–1.81) | 0.33 |
aAdjusted only for age.
References
McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore!
Ikonomidis JS, Gibson WC, Butler JE, McClister DM, Sweterlitsch SE, Thompson RP, Mukherjee R, Spinale FG. Effects of deletion of the tissue inhibitor of matrix metalloproteinases-1 gene on the progression of murine thoracic aortic aneurysms.
Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease.
Benjamin IJ. Matrix metalloproteinases: from biology to therapeutic strategies in cardiovascular disease.
Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease.
Nagase H, Suzuki K, Itoh Y, Kan CC, Gehring MR, Huang W, Brew K. Involvement of tissue inhibitors of metalloproteinases (TIMPS) during matrix metalloproteinase activation.
Liu J, Xiong W, Baca-Regen L, Nagase H, Baxter BT. Mechanism of inhibition of matrix metalloproteinase-2 expression by doxycycline in human aortic smooth muscle cells.
Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior.
Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions.
Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer.
Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice.
Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart.
Timms PM, Wright A, Maxwell P, Campbell S, Dawnay AB, Srikanthan V. Plasma tissue inhibitor of metalloproteinase-1 levels are elevated in essential hypertension and related to left ventricular hypertrophy.
Hirohata S, Kusachi S, Murakami M, Murakami T, Sano I, Watanabe T, Komatsubara I, Kondo J, Tsuji T. Time dependent alterations of serum matrix metalloproteinase-1 and metalloproteinase-1 tissue inhibitor after successful reperfusion of acute myocardial infarction.
Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study.
Blankenberg S, Rupprecht HJ, Bickel C, Espinola-Klein C, Rippin G, Hafner G, Ossendorf M, Steinhagen K, Meyer J. Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease.
O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. Practice Guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement.
Cabanes L, Richaud-Thiriez B, Fulla Y, Heloire F, Vuillemard C, Weber S, Dusser D. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea.
Davies MJ. Reactive oxygen species, metalloproteinases, and plaque stability.
Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study.
Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions.
Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K. Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome.
Noji M, Takagi Y, Kimura N, Inoue K, Saito M, Horikoshi M, Saito F, Takahashi H, Saito K. Serine acetyltransferase involved in cysteine biosynthesis from spinach: molecular cloning, characterization and expression analysis of cDNA encoding a plastidic isoform.
Tziakas DN, Chalikias GK, Parissis JT, Hatzinikolaou EI, Papadopoulos ED, Tripsiannis GA, Papadopoulou EG, Tentes IK, Karas SM, Chatseras DI. Serum profiles of matrix metalloproteinases and their tissue inhibitor in patients with acute coronary syndromes. The effects of short-term atorvastatin administration.
Silence J, Collen D, Lijnen HR. Reduced atherosclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene.
Roten L, Nemoto S, Simsic J, Coker ML, Rao V, Baicu S, Defreyte G, Soloway PJ, Zile MR, Spinale FG. Effects of gene deletion of the tissue inhibitor of the matrix metalloproteinase-type 1 (TIMP-1) on left ventricular geometry and function in mice.
Hammond IW, Devereux RB, Alderman MH, Laragh JH. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults.
Sundstrom J, Lind L, Vessby B, Andren B, Aro A, Lithell H. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later.
Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension.
Li-Saw-Hee FL, Edmunds E, Blann AD, Beevers DG, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapy.