-
PDF
- Split View
-
Views
-
Cite
Cite
Sonia S. Anand, Shofiqul Islam, Annika Rosengren, Maria Grazia Franzosi, Krisela Steyn, Afzal Hussein Yusufali, Matyas Keltai, Rafael Diaz, Sumathy Rangarajan, Salim Yusuf, on behalf of the INTERHEART Investigators, Risk factors for myocardial infarction in women and men: insights from the INTERHEART study, European Heart Journal, Volume 29, Issue 7, April 2008, Pages 932–940, https://doi.org/10.1093/eurheartj/ehn018
Close - Share Icon Share
Abstract
Coronary heart disease (CHD) is a leading cause of death among men and women globally. Women develop CHD about 10 years later than men, yet the reasons for this are unclear. The purpose of this report is to determine if differences in risk factor distributions exist between women and men across various age categories to help explain why women develop acute MI later than men.
We used the INTERHEART global case–control study including 27 098 participants from 52 countries, 6787 of whom were women. The median age of first acute MI was higher in women than men (65 vs. 56 years; P < 0.0001). Nine modifiable risk factors were associated with MI in women and men. Hypertension [2.95(2.66 –3.28) vs. 2.32(2.16–2.48)], diabetes [4.26(3.68–4.94) vs. 2.67(2.43–2.94), physical activity [0.48(0.41–0.57) vs. 0.77(0.71–0.83)], and moderate alcohol use [0.41(0.34–0.50) vs. 0.88(0.82–0.94)] were more strongly associated with MI among women than men. The association of abnormal lipids, current smoking, abdominal obesity, high risk diet, and psychosocial stress factors with MI was similar in women and men. Risk factors associations were generally stronger among younger individuals compared to older women and men. The population attributable risk (PAR) of all nine risk factors exceeded 94%, and was similar among women and men (96 vs. 93%). Men were significantly more likely to suffer a MI prior to 60 years of age than were women, however, after adjusting for levels of risk factors, the sex difference in the probability of MI cases occurring before the age of 60 years was reduced by more than 80%.
Women experience their first acute MI on average 9 years later than men. Nine modifiable risk factors are significantly associated with acute MI in both men and women and explain greater than 90% of the PAR. The difference in age of first MI is largely explained by the higher risk factor levels at younger ages in men compared to women.
Introduction
Coronary heart disease (CHD) is the leading cause of death among women in developed and developing countries.1 The incidence of CHD is markedly lower among women than men prior to the age of 50 years after which time CHD increases and approaches that seen among men by the eighth decade.2,3 Although the Framingham study described risk factors for CHD in women, the study was limited to white Caucasians living in the USA, and was unable to explain the later age of first occurrence of myocardial infarction (MI) among women compared to men.4 This may be because Framingham only measured a limited number of risk factors. It is generally believed that the later age of MI in women is due to the protective effects of female sex hormones, but differences in diet and smoking may also be important.5,6
The INTERHEART study was a global case–control study including participants from 52 countries in which nine modifiable risk factors were found to explain greater than 90% of acute MI in young and older individuals, women and men, across all major ethnic groups.7 INTERHEART included 6787 women (including about 3000 cases), making it one of the largest studies of MI risk factors in women, and the only one to include large numbers of individuals from developing countries and of non-European ethnicity. The purpose of this report is to determine if differences in risk factor distributions exist between women and men across various age categories in order to help explain why women develop acute MI later than men.
Methods
INTERHEART was a standardized case–control study of acute MI in which 12 461 cases (3002 women) and 14 637 controls (3785 women) were enrolled between February 1999 and March 2003. Informed consent was obtained from all participants. Women and men participants were recruited from 262 centres from 52 countries in Asia, Europe, the Middle East, Africa, Australia, North America and South America and the details of selection and baseline characteristics have been previously reported.7 Briefly, cases of first acute MI presenting within 24 h of symptom onset were eligible. To identify the incident cases of acute MI, all patients, irrespective of age, admitted to the coronary care unit (or an equivalent cardiology ward) within 24 h of symptom onset were screened. Cases were eligible if they had characteristic symptoms plus electrocardiogram changes indicative of a new MI (new pathologic Q waves, at least 1 mm ST elevation in any 2 or more contiguous limb leads or a new left bundle branch block, or new persistent ST–T wave changes diagnostic of a non-Q wave MI) or a plasma level of cardiac troponin level above that considered normal in the hospital/institution where the patient was registered. At least one age-matched (up to 5 years older or younger) and sex-matched control was recruited per case from hospital- or community-based sources, using specific criteria. Criteria for inclusion or exclusion of study subjects have been described in detail before.8
Procedures
Information about demographic factors, lifestyle (smoking, leisure time physical activity, and dietary patterns), personal and family history of cardiovascular disease, and risk factors (hypertension, diabetes mellitus, and psychosocial factors) were obtained using structured questionnaires administered by study personnel. All physical examinations were undertaken in the same manner in cases and controls. Staff at each site were trained using standard manuals, videotapes, or instructions at study meetings or during site visits. Description of the measurement techniques have been published before.7,8 Although blood pressure at the time of the examination was recorded in both cases and controls, only self-reported history of hypertension is used in this analysis, because levels in cases would be systematically affected by the MI and its treatments.
Non-fasting blood samples (20 mL) were drawn from every individual and centrifuged within 2 h of admission, aliquotted and frozen immediately at −20 or −70°C after processing. Centres were instructed to draw blood from cases within 24 h of symptom onset. Blood samples were available in 21 508 (79%) of 27 098 cases and controls, 16 353 men and 5155 women. Samples were shipped in nitrogen vapour tanks by courier from every site to a blood storage site, where they were stored at −160°C in liquid nitrogen (Hamilton, Canada) or at −70°C in China. Blood samples from all countries other than China were analysed in Hamilton for total cholesterol, HDL cholesterol, and Apolipoproteins B (ApoB) and A1 (ApoA1). The methods for analyses have been reported previously.7 All data were transferred to the Population Health Research Institute, McMaster University and Hamilton Health Sciences, Canada, where quality-control checks and statistical analyses were done.
Definitions of risk factors
Abnormal lipids were defined using the ApoB/ApoA ratio comparing the upper tertile to the lowest tertile. Current smokers were defined as individuals who reported smoking cigarettes or other forms of tobacco (beedies, cigars, pipes, sheesha) in the previous year and included individuals who had quit within the previous year; former smokers were defined as smokers who had quit more than a year earlier,9 abdominal obesity was defined using the waist to hip ratio incorporating sex-specific cut-offs for women and men comparing the upper tertile to the lowest tertile.10 Hypertension and diabetes were defined by self-report. Participants were classified as physically active if they were involved in moderate (walking, cycling) or strenuous exercise (jogging, football, vigorous swimming) for 4 h or more per week. Regular alcohol use was defined as consumption of alcohol at least three times a week. A dietary risk score using seven food items (meats, fried foods, salty snacks, green leafy vegetables, other raw vegetables, other cooked vegetables, and fruits) compared individuals in the highest quartile to those in the lowest quartile, to compute the risk of MI.11 Psychosocial stress factors (depression, locus of control, global stress, financial stress, and life events including marital separation, job loss, family conflict) were systematically recorded and a combined psychosocial index was devised with a combination of the parameter estimates from the completely adjusted multivariate logistic regression model.12 Risk factors assessed in this analysis used similar definitions to those reported in the original INTERHEART publication7 with two exceptions: (i) in this analysis we defined used Apo B/A tertiles as compared to quintiles/deciles and (ii) we used an expanded dietary score to classify subjects as having a ‘high risk diet’ compared to only fruit and vegetable consumption.
Statistical analysis
The age standardized prevalence, odds ratios and their 95% confidence intervals (CIs), were calculated as described previously.7 The prevalence of risk factors was compared by age strata defined as <60 vs. ≥60 years. This cut-point was chosen because the incidence of MI increases sharply after the age of 60 years in women,4 and risk factors may also change in frequency after women have completed menopause. Unconditional logistic regression models were chosen over conditional models (i.e. matched analysis) because the results were approximately similar with both models, and undertaking a strict matched analysis would mean relevant loss of information because of the exclusion of unmatched cases and controls.7 To determine the association of risk factors with acute MI, each risk factor was entered into a logistic regression model in which the dependent variable was acute MI (case vs. non-case) and all models were adjusted for age, current smoking and region. To compare the strength of association for each risk factor between women and men or between age categories within each sex, interaction terms were entered and the P-value for the interaction term was calculated. These models were also adjusted for age and region. For interactions, a P-value of <0.01 was considered significant. Owing to the slight difference in the proportion of hospital-based controls among women (68.1%) as compared to men (61%), interactions between type of control (i.e. hospital based vs. community based) and sex were also examined, although no significant interactions were observed. Population attributable risks (PARs) and 95% CI were calculated for various risk factors in the study, using a method based on unconditional logistic regression.13 PAR estimates were calculated using the Interactive Risk Attributable Program (IRAP) software (US National Cancer Institute, 2002). Statistical analyses and graphics were produced using the SAS system Version 9.1 (SAS, Cary, NC), and S-Plus Version 7.0 (Insightful, Seattle Wash). The PARs presented were adjusted for confounders in a similar manner to the corresponding logistic regression models for odds ratio estimates, and where indicated stratified by subgroup of interest. For estimating variance, the reader is referred to Benichou and Gail.14 CI calculations were based on this method using a logit transformation approach. To keep all PAR calculations, positive alcohol was classified as lack of alcohol intake, and physical activity was defined as physical inactivity. The effect of combination of all exposures was estimated by summation of model coefficients and their anti-logs. We used a ‘case only’ approach to compute the probability of classifying cases of acute MI into a younger age group (<60 years) vs. an older age (≥60 years) for which we constructed a logistic regression model and both unadjusted and adjusted models (for all nine risk factors) were calculated.15
Role of funding sources
A list of the 41 funding sources has been published previously7 and none had any role in design, data collection, analysis, interpretation or writing of this report. Two authors (S.A. and S.Y.) had full access to all study data and had final responsibility for the decision to submit this manuscript for publication.
Results
A total of 27 098 subjects, 12 460 cases, and 14 634 controls are included in this report from 52 countries. Six thousand seven hundred and eighty-seven participants were women, made up of 3002 cases and 3785 controls. Approximately two-thirds of women with MI (n = 2032) were equal to or over the age of 60 years, compared to 40% (n = 3805) of men with MI (Table 1). Significant age differences were observed in regions where the mean age of cases was high (i.e. Western Europe aged 67 years) and where the mean age was lower (i.e. 57 years among Arabs from the Middle East) (see Supplementary Data).
Sex and age distribution of acute myocardial infarction cases and controls
| . | Overall . | Women . | Men . | |||
|---|---|---|---|---|---|---|
| . | Cases . | Controls . | Cases . | Controls . | Cases . | Controls . |
| Overall (n) | 12 460 | 14 634 | 3002 | 3785 | 9458 | 10 849 |
| <40 years | 752 (6.0)a | 1087 (7.4) | 68 (2.3) | 102 (2.7) | 684 (7.2) | 985 (9.1) |
| 40–50 | 2491 (20.0) | 3138 (21.4) | 285 (9.5) | 451 (11.9) | 2206 (23.3) | 2687 (24.8) |
| 50–59 | 3380 (27.6) | 4036 (27.6) | 617 (20.6) | 944 (24.9) | 2763 (29.2) | 3092 (28.5) |
| <60 years | 6623 (53.2) | 8261 (56.5) | 970 (32.3) | 1497 (39.6) | 5653 (59.8) | 6764 (62.3) |
| ≥60 years | 5837 (46.8) | 6373 (43.5) | 2032 (67.7) | 2288 (60.4) | 3805 (40.2) | 4085 (37.7) |
| . | Overall . | Women . | Men . | |||
|---|---|---|---|---|---|---|
| . | Cases . | Controls . | Cases . | Controls . | Cases . | Controls . |
| Overall (n) | 12 460 | 14 634 | 3002 | 3785 | 9458 | 10 849 |
| <40 years | 752 (6.0)a | 1087 (7.4) | 68 (2.3) | 102 (2.7) | 684 (7.2) | 985 (9.1) |
| 40–50 | 2491 (20.0) | 3138 (21.4) | 285 (9.5) | 451 (11.9) | 2206 (23.3) | 2687 (24.8) |
| 50–59 | 3380 (27.6) | 4036 (27.6) | 617 (20.6) | 944 (24.9) | 2763 (29.2) | 3092 (28.5) |
| <60 years | 6623 (53.2) | 8261 (56.5) | 970 (32.3) | 1497 (39.6) | 5653 (59.8) | 6764 (62.3) |
| ≥60 years | 5837 (46.8) | 6373 (43.5) | 2032 (67.7) | 2288 (60.4) | 3805 (40.2) | 4085 (37.7) |
aPercentage of column total.
Note: During recruitment controls were matched to cases based on age (±5 years) and sex.
Sex and age distribution of acute myocardial infarction cases and controls
| . | Overall . | Women . | Men . | |||
|---|---|---|---|---|---|---|
| . | Cases . | Controls . | Cases . | Controls . | Cases . | Controls . |
| Overall (n) | 12 460 | 14 634 | 3002 | 3785 | 9458 | 10 849 |
| <40 years | 752 (6.0)a | 1087 (7.4) | 68 (2.3) | 102 (2.7) | 684 (7.2) | 985 (9.1) |
| 40–50 | 2491 (20.0) | 3138 (21.4) | 285 (9.5) | 451 (11.9) | 2206 (23.3) | 2687 (24.8) |
| 50–59 | 3380 (27.6) | 4036 (27.6) | 617 (20.6) | 944 (24.9) | 2763 (29.2) | 3092 (28.5) |
| <60 years | 6623 (53.2) | 8261 (56.5) | 970 (32.3) | 1497 (39.6) | 5653 (59.8) | 6764 (62.3) |
| ≥60 years | 5837 (46.8) | 6373 (43.5) | 2032 (67.7) | 2288 (60.4) | 3805 (40.2) | 4085 (37.7) |
| . | Overall . | Women . | Men . | |||
|---|---|---|---|---|---|---|
| . | Cases . | Controls . | Cases . | Controls . | Cases . | Controls . |
| Overall (n) | 12 460 | 14 634 | 3002 | 3785 | 9458 | 10 849 |
| <40 years | 752 (6.0)a | 1087 (7.4) | 68 (2.3) | 102 (2.7) | 684 (7.2) | 985 (9.1) |
| 40–50 | 2491 (20.0) | 3138 (21.4) | 285 (9.5) | 451 (11.9) | 2206 (23.3) | 2687 (24.8) |
| 50–59 | 3380 (27.6) | 4036 (27.6) | 617 (20.6) | 944 (24.9) | 2763 (29.2) | 3092 (28.5) |
| <60 years | 6623 (53.2) | 8261 (56.5) | 970 (32.3) | 1497 (39.6) | 5653 (59.8) | 6764 (62.3) |
| ≥60 years | 5837 (46.8) | 6373 (43.5) | 2032 (67.7) | 2288 (60.4) | 3805 (40.2) | 4085 (37.7) |
aPercentage of column total.
Note: During recruitment controls were matched to cases based on age (±5 years) and sex.
Risk factor distribution among controls
The distribution of risk factors varied significantly between women and men controls. Overall, significantly fewer women compared to men had abnormal lipids (24.3 vs. 36.2%), were current (9.2 vs. 33.0%) or former (11.6 vs. 24.7%) smokers, consumed a high risk diet (17.9 vs. 23.3%), performed regular physical activity (16.5 vs. 20.3%), and drank alcohol (11.2 vs. 29.1%). The proportions of women and men controls with diabetes (7.9 vs. 7.4%), abdominal obesity (33.3 vs. 33.3%), and psychosocial stress (86.4 vs. 88.8%) were similar. However, women were significantly more likely to have hypertension compared to men (28.3 vs. 19.7%).
Sex differences in risk factor associations with acute myocardial infarction
All nine risk factors were significantly associated with acute MI in women and men, with some variations in odds ratios observed (Figure 1). Risk factors which were more strongly associated with MI in women compared to men included hypertension, diabetes, alcohol intake, and physical activity. Only former smoking was more strongly associated with MI in men compared to that in women. The association of current smoking, consumption of a high-risk diet, abdominal obesity, and psychosocial factors with MI did not vary significantly by sex. Generally risk factors were more strongly associated with acute MI in younger (<60 years) compared to older (≥60 years) women and men. Among women, ApoB/A levels, current smoking, hypertension, and diabetes were more strongly associated with MI in younger compared to older women (Figure 2A). Among men, ApoB/A, current and former smoking, hypertension, and abdominal obesity but not diabetes were more strongly associated with MI in younger compared to older men (Figure 2B). Interestingly, the protective effects of physical activity and MI and regular alcohol consumption and MI were stronger among older compared to younger men.
Comparison of risk factors related to acute MI between women and men. ApoB/A-1 ratio comparison of the upper tertile to the lowest tertile. Abdominal obesity: comparison of the sex-specific upper tertile the lowest tertile of waist to hip ratio. Psychosocial stress: individuals with at least one of the five psychosocial stress component factors [i.e. depression, global stress, financial stress, locus of control, or other stresses (including separation, job loss, and family conflict)]. High risk diet: comparison of the top quartile to the bottom quartile
(A) The impact of risk factors in younger vs. older women. (B) The impact of risk factors in younger vs. older men. ApoB/A-1 ratio comparison of the upper tertile to the lowest tertile. Abdominal obesity: comparison of the sex-specific upper tertile the lowest tertile of waist to hip ratio. Psychosocial stress: individuals with at least one of the five psychosocial stress component factors [i.e. depression, global stress, financial stress, locus of control, or other stresses (including separation, job loss, and family conflict)]. High risk diet: comparison of the top quartile to the bottom quartile
Sex differences in the population attributable risks
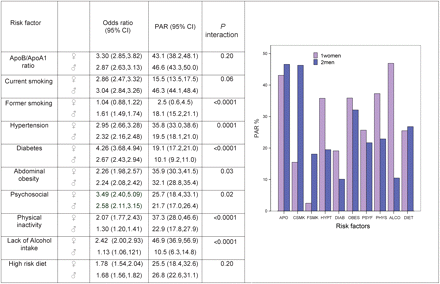
Considering all nine risk factors, the collective PAR on MI is 96% (95% CI: 94–98) in women compared to 93% (95% CI: 92–95) among men. Previous analyses of the INTERHEART data indicate that the PAR of all nine risk factors is greater in younger vs. older women and men.7 In addition, some regional variations in the PARs for women and men are also present as previously described (http://image.thelancet.com/extras/04art8001webtable3.pdf). The contribution of risk factors to the overall PAR varied between the sexes due to the differences in odds ratios and prevalences’ of the risk factors (Figure 3). The PARs of hypertension (35.8 vs. 19.5%) and diabetes (19.1 vs. 10.1%), physical inactivity (37.3 vs. 22.9), and alcohol use (46.9 vs. 10.5%) were significantly greater among women compared to men. Whereas among men, former smoking was associated with a higher PAR than it was among women (18.1 vs. 2.5). Interestingly, the metabolic syndrome-related risk factors (i.e. diabetes, hypertension, abnormal lipids, and abdominal obesity) contributed substantially to the risk of MI among women (73%; 95% CI: 69–78) and men (68%; 95% CI: 65–71). The combined PAR for lifestyle factors including smoking, low alcohol use, high risk diet and physical inactivity was significantly higher among women than men [74.3 (95% CI: 67.9–80.7 vs. 67.3 (95% CI: 63.9–70.8)]. However, after removing alcohol, the combined lifestyle PAR was slightly lower among women compared to men [55.2 (47.9–62.6) vs. 63.4 (60.1–66.8)]. The lifestyle PARs were quantitatively but not significantly greater among younger women and men compared to older women and men (data not shown).
Comparison of population attributable risks between women and men. APO, ApoB/A ratio; CSMK, current smoking; FSMK, former smoking; HYPT, hypertension; DIAB, diabetes; OBES, abdominal obesity; PSYF, psychosocial; PHYS, physical Inactivity; ALCO, lack of alcohol intake; DIET, high risk diet
Explaining age differences in distribution of myocardial infarction cases between men and women
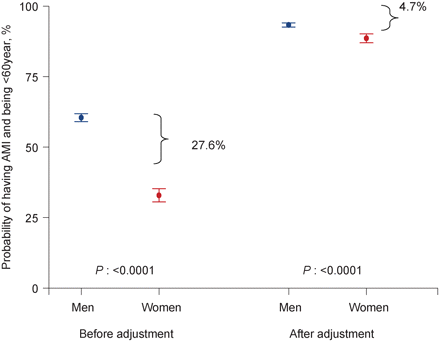
The predicted probability of being an acute MI case less than 60 years of age was substantially higher among men compared to women (60.6 vs. 33.0%, difference of 27.6%). This sex difference decreased to 23.2% after adjustment for regional differences, decreased to 18.2% after adjustment for region and smoking, and decreased to 4.7% after adjustment for all nine risk factors and region (Figure 4). Thus more than 80% of the earlier age of first MI in men compared to women is explained by the differences in the distribution of the nine risk factors. This suggests that the earlier age of acute MI in men can largely be explained by the higher levels of some risk factors men possess at younger ages.
Differences in predicted probability of infarction cases <60 years comparing men and women. The difference in the point estimate is shown graphically before and after adjustment for INTERHEART risk factors to show the effect of the risk factors in accounting for the predicted probability difference. Before adjustment for risk factor differences: the predicted probability of being a male case <60 years is 60.6% (95% CI: 59.0–61.9) and the predicted probability of being a female case <60 years is 33.0% (95% CI: 30.6–35.3). The difference between these estimates is 27.6%. After adjustment for all nine risk factors and region: the difference in the estimates between proportions of men (93.3%, 95% CI: 92.6–94.1) and women (88.6%, 95% CI: 87.0–90.2) cases <60 years is reduced to 4.7%
Discussion
Our study demonstrates that women experience their first MI on average 9 years later than men in all regions of the world. Nine modifiable risk factors are associated with incident MI and explain more than 95% of the PAR of acute MI among women and men from all regions of the world. Similar risk factor associations with MI are present in women and men for abnormal lipids, current smoking, abdominal obesity, dietary intake, and psychosocial stress factors. However, risk factor–MI associations for hypertension, diabetes, physical activity, alcohol use, and former smoking differ between the sexes. Further these associations are generally stronger, in younger individuals—both in women and men. The younger age of onset of acute MI in men is largely explained by higher levels of risk factors including abnormal lipids, and smoking, before the age of 60 years among men.
Our findings are consistent with previous investigators who have reported that the determinants of CHD among women are similar to those among men.2,16 Although the Framingham study reported that the difference between women and men in the incidence of CHD could not be explained by the risk factors they measured (cholesterol, blood pressure, diabetes, smoking),2 the nine risk factors measured in the diverse population of INTERHEART have led to new and expanded insights. In particular, the apolipoproteins and abdominal obesity are much stronger predictors of the risk of MI than blood lipid levels and body mass index.7,10 Furthermore, the Framingham analysis did not include dietary intake, physical activity, or psychosocial factors in their comparison of women and men. Using the INTERHEART data, it appears that the lower MI burden among women at younger ages is largely explained by a lower risk factor burden.
The reasons why the risk factors are lower in women at younger ages compared to men require further study. Tobacco use is heavily influenced by the historical context of communities and cultural norms, and in most societies women have smoked less than men. Tobacco use clearly explains part of the lower rate of MI in younger women compared to that in men.17 However, in some societies, smoking is increasing in young women which may remove some of the relative advantage that women have over men in avoiding or delaying CHD.18 At younger ages, women also have substantially lower rates of abnormal lipids compared to men, yet with advancing age the proportion of abnormal lipids among women increases, while decreasing among men. Although it is possible that elevated lipid levels among younger men are influenced by their adverse dietary profile (as we observed) and as suggested by Lawlor et al.5 in their ecological analysis of saturated fat intake and MI differences, it is also possible that this difference is explained by a direct effect of endogenous estrogen on apolipoprotein levels, or indirectly via its effect on fat distribution, or on genetic variants that regulate lipid levels.19–21
Our data question the common belief that MIs in young women (or men) are caused by novel risk factors, as we have demonstrated that the associations of a number of the conventional risk factors with acute MI are stronger in younger women and men. Several of the risk factors in young women are more strongly associated with acute MI compared to older women. For example, diabetes in women less than 60 years is more strongly associated with MI than the presence of diabetes in women over the age of 60 years [5.69 (95% CI: 4.36–7.42) vs. 3.71 (95% CI: 3.10–4.45)]. The same pattern is observed for abnormal lipids, hypertension, and smoking. This indicates that when these risk factors are present in young women they should be aggressively modified, and not ignored under the mistaken assumption that, because women are less likely to suffer from CHD than men at younger ages, modifying these risk factors is unimportant.22
The nine INTERHEART risk factors account for the majority of MI cases in women around the world with an overall PAR of >90%. These data facilitate the development of gender-specific policies for screening and surveillance of women at risk of MI. However, it should be noted that the PARs for MI associated with specific risk factors within a given region are likely to be dynamic as cultural and societal changes influence health behaviours such as diet, smoking and physical activity, such that the burden of risk factors may change over time. For example, at present, the PAR of smoking is substantially lower among women compared to men. This is likely due to cultural differences, but MI attributable to smoking would be expected to increase if smoking rates increased in women. Therefore, public health policies must continue to reinforce this behaviour especially among vulnerable subgroups.9,18 The PAR of low alcohol use in women is also substantial, and likely due to cultural forces resulting in fewer women consuming alcohol on a regular basis. However, from a prevention standpoint we do not advocate initiation or an increase in alcohol consumption among women, as this practice can be accompanied by smoking, and other adverse health consequences including strokes, cancers, and injuries.23–26 Our data suggest that lifestyle practices such as avoidance of smoking, regular physical activity, and optimal dietary practices have the potential to substantially reduce the risk of acute MI in women. Lifestyle factors (not including alcohol) appear to be particularly important as collectively they account for 55% of the PAR in women and slightly greater among men. This is supported by the prospective cohort data of Stampfer et al.27 who reported that among women, adherence to lifestyle guidelines involving body weight, diet, exercise, alcohol consumption, and abstinence from smoking is associated with an 80% lower risk of CHD. Although individuals must take some responsibility to curb their risk factors, the greatest gains will likely be made through policy changes which promote regular physical activity, easy and affordable access to healthy foods such as fruits and vegetables, and limited access to other dietary components such as salt and high fat energy dense foods.
The main strength of our study is the large number of cases and controls from different regions of the world (with over 3000 cases of MI in women), which allowed high power for the analyses by sex, age, and comparisons across regions. Further the inclusion of new markers such as apolipoproteins, abdominal obesity, and psychosocial factors expands the information collected per subject. All epidemiologic studies including case–control studies are inherently susceptible to biases. We have minimized biases by careful attention to the selection of cases and controls, other aspects of design and in the data analysis. We reduced the selection bias in enrolling cases by attempting to recruit cases of first MI, thereby eliminating potential biases resulting from changes in lifestyle that could be adopted by subjects already suffering from coronary artery disease. We selected both hospital- and community-based controls in approximately similar proportions among women and men, and their separate analysis yielded similar results indicating the absence of major selection biases. We minimized the measurement bias by employing uniform, standardized methods of data collection by trained research assistants in both cases and controls. Diabetes and hypertension were based on patients self-report. Therefore, the associations of diabetes and hypertension with MI from our study are likely underestimates of the true association with acute MI. It is possible that some of the psychosocial factors (e.g. self reported stress) may be affected by recall biases. However, this is less likely to apply to some of the other psychosocial measures such as life events, depression prior to the first MI and perhaps locus of control. Excluding the psychosocial variables from our analysis still results in a very high PAR in both men and women.
Conclusions
Women experience their first acute MI on average 9 years later than men. Nine modifiable risk factors are significantly associated with acute MI in both men and women and explain greater than 90% of the PAR. The difference in age of first MI is largely explained by the higher risk factor levels at younger ages in men compared to women. The approach to prevention of MI in men and women can be based on similar principles in all regions of the world.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The INTERHEART study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the International Clinical Epidemiology Network (INCLEN), and through unrestricted grants from several pharmaceutical companies (with major contributions from AstraZeneca, Novartis, Sanofi Aventis, Knoll Pharmaceuticals [now Abbott], Bristol Myers Squibb, and King Pharma), and by various national bodies in different countries Chile: Universidad de la Frontera, Sociedad Chilena de Cardiologia Filial Sur; Colombia: Colciencias, Ministerio de Salud; Croatia: Croatian Ministry of Science & Technology; Guatemala: Liga Guatemalteca del Corazon; Hungary: Astra Hassle, National Health Science Council, George Gabor Foundation; Iran: Iran Ministry of Health; Italy: Boehringer-Ingelheim, Japan: Sankyo Pharmaceutical Co., Banyu Pharmaceutical Co., Astra Japan; Kuwait: Endowment Fund for Health Development in Kuwait; Pakistan: ATCO Laboratories; Philippines: Philippine Council for Health Research & Dev., Pfizer Philippines Foundation, Inc., Astra Pharmacetuicals, Inc. & the Astra Fund for Clinical Research & Continuing Medical Education, Pharmacia & Upjohn Inc.; Poland: Foundation PROCLINICA; Singapore: Singapore National Heart Association; South Africa: MRC South Africa, Warner-Parke-Davis Pharmaceuticals, Aventis; Sweden: Grant from the Swedish State under LUA Agreement, Swedish Heart and Lung Foundation; Thailand: The Heart Association of Thailand, Thailand Research Fund.
Acknowledgements
We thank Kathy Stewart, Laura Joldersma, and Xiaohe Zhang for their assistance in tables and figure preparation, the WHO and the World Heart Federation for their endorsement, and all of our colleagues for help that led to the successful completion of this global study. S.S.A. holds a Canadian Institutes of Health Research (CIHR) Clinician Scientist Award (Phase 2) and the May Cohen Eli Lilly Chair in Women's Health Research, McMaster University. S.Y. holds an endowed chair of the Heart and Stroke Foundation of Ontario, and held a Senior Scientist Award from the CIHR.
Contributions of Authors: S.S.A. and S.Y. coordinated the data analysis and S.S.A. drafted the initial manuscript. S.I. performed the primary statistical analysis. S.R. coordinated the INTERHEART study. A.R., M.G.F., K.S., A.H.Y., M.K., and R.D. recruited subjects into the INTERHEART study and critically reviewed the manuscript. S.Y. initiated and supervised the conduct of the INTERHEART study.



![Comparison of risk factors related to acute MI between women and men. ApoB/A-1 ratio comparison of the upper tertile to the lowest tertile. Abdominal obesity: comparison of the sex-specific upper tertile the lowest tertile of waist to hip ratio. Psychosocial stress: individuals with at least one of the five psychosocial stress component factors [i.e. depression, global stress, financial stress, locus of control, or other stresses (including separation, job loss, and family conflict)]. High risk diet: comparison of the top quartile to the bottom quartile](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/29/7/10.1093_eurheartj_ehn018/4/m_ehn01801.gif?Expires=1716313951&Signature=ccF1M~cyHvKKCMh1K57LrlZi0~o-3qMg3a7wTQWlw2DK28aJJ-SdPhPY1F385SNq5KKpiwDyVtQqOSFVjdjipgMEPGYiLxsJviM7E~P4PKcgExtncLrJC0aTTucY1Zz2Y9MSeMys3NhT2TRUzMS8FQKVdrssGAYYFAhulcrVEzGsx9hAWuvKBk5TFRBInIc3d9o6G9q7nXwIlWiWd~q-GgSsKm0hwm0yyu-i9E5kfEwmBpF~h0p8gIx3il-Pw9w64l2mgjR6CmU~StE2k6tYgwV0JJYIv3iVF7h~JLfO2iNPrP7Nwz5VETvfrzW0ucHIIgWOb9WZgZRmAYTiou0ujQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![(A) The impact of risk factors in younger vs. older women. (B) The impact of risk factors in younger vs. older men. ApoB/A-1 ratio comparison of the upper tertile to the lowest tertile. Abdominal obesity: comparison of the sex-specific upper tertile the lowest tertile of waist to hip ratio. Psychosocial stress: individuals with at least one of the five psychosocial stress component factors [i.e. depression, global stress, financial stress, locus of control, or other stresses (including separation, job loss, and family conflict)]. High risk diet: comparison of the top quartile to the bottom quartile](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/29/7/10.1093_eurheartj_ehn018/4/m_ehn01802.gif?Expires=1716313951&Signature=TlRFvHhMx-WrlY7SEkWujgOqiv4S0rGKBg5KUjyNpWr97F-mX~SyyEGQXYRSVvG2buWWG~3Ta7ZTSnFStqmsKxy~Xhryqidj68D63sfO3AbOsQugz3SPcLyRgOvPggaIDZtjGtVtnqshifKLFyoO9~gj-DkPd270pSJCAzro4Leu6LPxI-clGydd6xEiLTTLd1zDvHdUtKaE9AinFFffWo1HDHfXA4QQspZe01xBw0QHAnuBwvsU3LqhkWz0EFD~J2eVE43lb-NoAcYhZLs-nh7uBpteYdKeWHLE0oIZoV7gBUGZ4oyO85Fqp-ltKNud~hNmzfGl3Z20UO3HCZg94Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)