-
PDF
- Split View
-
Views
-
Cite
Cite
Ole Ahlehoff, Gunnar H. Gislason, Casper H. Jørgensen, Jesper Lindhardsen, Mette Charlot, Jonas B. Olesen, Steen Z. Abildstrøm, Lone Skov, Christian Torp-Pedersen, Peter Riis Hansen, Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study, European Heart Journal, Volume 33, Issue 16, August 2012, Pages 2054–2064, https://doi.org/10.1093/eurheartj/ehr285
Close - Share Icon Share
Abstract
Psoriasis is a chronic inflammatory disease and inflammation contributes to the pathogenesis of atrial fibrillation (AF) and ischaemic stroke. We therefore investigated the risk of these endpoints in patients with psoriasis.
Cohort study of the entire Danish population followed from 1997 to 2006 by individual-level-linkage of nationwide prospectively recorded registers. Multivariable Poisson's regression and sensitivity analyses were used to assess the psoriasis-related risk of AF and ischaemic stroke. A total of 36 765 patients with mild psoriasis and 2793 with severe psoriasis were compared with 4 478 926 individuals, i.e. the reference population. In patients with mild psoriasis, the adjusted rate ratios (RRs) for AF were 1.50 (1.21–1.86) and 1.16 (1.08–1.24) in patients aged <50 and ≥50 years, respectively. Patients with severe psoriasis had a higher risk of AF with RRs 2.98 (1.80–4.92) in patients aged <50 years and 1.29 (1.01–1.65) in patients aged ≥50 years. Patients with psoriasis also demonstrated a disease severity-dependent increased risk of ischaemic stroke, i.e. RRs 1.97 (1.66–2.34) and 2.80 (1.81–4.34) in patients aged <50 years with mild and severe psoriasis, and RRs 1.13 (1.04–1.21) and 1.34 (1.04–1.71) in patients aged ≥50 years with mild and severe psoriasis, respectively. A range of sensitivity analyses yielded comparable results.
Psoriasis is associated with increased risk of AF and ischaemic stroke. These novel results add to a growing body of evidence, suggesting that patients with psoriasis could be considered at increased cardiovascular risk.
See page 1989 for the editorial comment on this article (doi:10.1093/eurheartj/ehr425)
Introduction
Psoriasis is a type 1 helper cell (Th1)/Th17 chronic inflammatory disorder affecting ∼2% of the population.1,2 Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia and is associated with increased risk of stroke, heart failure, coronary artery disease, and cardiovascular mortality.3,4 It is well-established that atherosclerosis is also a chronic inflammatory disease and more recent evidence has linked inflammatory mechanisms to the pathogenesis of AF, e.g. with atrial infiltration of inflammatory cells and raised circulating levels of inflammatory markers and markers of oxidative stress.5–10 The relationship between psoriasis and AF is unknown and although psoriasis has been linked to acute myocardial infarction and cardiovascular death, less evidence is available regarding the risk of stroke in these patients.11–20 We therefore used Danish nationwide registers of hospitalization and drug dispensing from pharmacies to determine the risk of AF and ischaemic stroke in patients with psoriasis.
Methods
Data sources and study population
The study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.21 In Denmark, all citizens have a unique personal civil registration number that enables individual-level-linkage of information across nationwide registers. All medications dispensed from pharmacies were obtained from the National Prescription Registry (the Danish Registry of Medicinal Product Statistics), where all dispensed prescriptions from Danish pharmacies have been recorded since 1995. All deaths were identified from the Central Population Register, in which deaths are recorded within 2 weeks. Causes of death were obtained from the National Causes of Death Register, in which causes of death were recorded using the International Classification of Diseases (ICD). Data on death, co-morbidity, and concomitant medication were linked on individual case level and were also linked to socioeconomic data. Morbidity was obtained from the Danish National Patient Register in which all hospital admissions, diagnoses, and invasive procedures have been recorded since 1978 according to ICD (ICD-8 until 1994 and ICD-10 thereafter). Co-morbidity at study entry was described by valvular heart disease (ICD-10 I05–I07 and I33–I36) and Charlson's index, as defined by 19 pre-specified diagnoses at study entry and up to 1 year previously, and modified to ICD-10.22 Socioeconomic status was defined by the individual average yearly gross income during a 5-year period prior to study inclusion and patients were divided into quintiles according to their income.
The entire Danish population as of 1 January 1997 was followed until 31 December 2006, emigration, or death. Patients with previous AF, stroke, and/or psoriasis were excluded. Patients with psoriasis were identified by dispensed prescriptions of topical vitamin D derivatives, i.e. first-line treatment used exclusively for psoriasis.2 To validate the use of vitamin D derivatives as the criteria for identifying mild psoriasis, we contacted LEO Pharma, the manufacturer of topical vitamin D derivatives, and were allowed to disclose results from a survey done by the company marketing division. Here, 44 Danish dermatologists and 93 general practitioners indicated that vitamin D derivatives were the most commonly used treatment for new patients with reported use in 48–54% of newly diagnosed patients and in 71% of patients receiving continuing topical treatment (data on file, LEO Pharma, Denmark). To further validate these findings, we examined the hospital records of 155 randomly selected patients with psoriasis as determined by a hospital dermatologist. A total of 114 patients (73.5%) had a history of vitamin D derivative use. Patients were identified when claiming their second such prescription to ensure persistent medical treatment for psoriasis and classified as severe psoriasis by hospitalizations or outpatient hospital consultations for psoriasis (ICD-10 L40) or psoriatic arthritis (M070–M073) at the time of their third in- or outpatient diagnosis as accepted previously.20
Medical treatment
The medications examined in the current study were dispensed on prescription in Denmark, and the National Prescription Register that is directly linked to the system for reimbursement of drug expenses has been shown to be accurate.23 Drugs are registered according to the international Anatomical Therapeutical Chemical (ATC) classification system. Patients with psoriasis were identified by their claimed prescriptions of topical vitamin D derivates (ATC D05AX). Baseline treatment with anti-depressive medication (N06A), non-steroid anti-inflammatory drugs (M01A), anti-thyroid medication, i.e. propylthiouracil and imidazole derivatives (H03B), platelet inhibitors (B01AC), glucose-lowering drugs (A10), statins (C10A), β-blockers (C07), angiotensin-converting enzyme inhibitors/angiotensin 2-receptor antagonists (C09), vitamin K antagonists (B01AA), loop diuretics (C03C), and spironolactone (C03D) was defined by dispensed prescriptions up to 6 months prior to the study inclusion date.
Study endpoints
The following pre-specified endpoints were assessed: AF (ICD-8 codes 427.94 and 427.95, and ICD-10 code I48) and ischaemic stroke (ICD-8 codes 432–436, and ICD-10 codes I63 and I64), respectively. We only studied the risk of first-time occurrence of an event and therefore did not evaluate AF burden. The diagnoses of AF and ischaemic stroke in the Danish National Patient Registry have been validated previously with 99% of AF diagnoses confirmed and the positive predictive value of ischaemic stroke (I63) was 97%, whereas haemorrhagic strokes only comprised 5.8% of the unspecified strokes (I64).24,25
Statistical analysis
Baseline characteristics were presented as means with standard deviations, frequencies, and percentages. The rate ratio (RR) for each study endpoint was estimated by Poisson's regression models stratified for age and adjusted for confounding factors including calendar year, concomitant medication, co-morbidity, socioeconomic data, and gender. Cox's regression is routinely used for epidemiological cohort studies of censored data but is very inefficient and time-consuming when large data sets are analysed. In contrast, Poisson's regression is an accurate and efficient alternative to Cox's regression analyses of cohort studies with varying lengths of follow-up, censoring, multiple time scales, time-varying covariates, and need for multivariable adjustments. Indeed, the interpretation of differences in RRs provided by Poisson's regression and hazard ratios provided by Cox's regression is similar. Exposure to psoriasis was included as a time-dependent variable so that patients were only considered at risk from the time they fulfilled the criteria for the psoriasis diagnosis. Age and calendar year were also included as time-dependent variables. Co-morbidity and concomitant medication were included as fixed variables obtained at baseline at 1 January 1997. Unadjusted event rates were summarized as events per 1000 patient-years and the Nelson–Aalen cumulative hazard curves were drawn. To examine the potential impact of competing risks on the estimates obtained in the primary model, we calculated the subhazard ratios of the study endpoints by the use of multivariable-adjusted competing risk regression considering competing risk from all-cause mortality. Subhazard ratios were interpreted as standard hazard ratios of proportional hazard regression or RRs from Poisson's regression (see Supplementary Data).
Sensitivity analyses were performed with exclusion of patients at high risk of cardiovascular events prior to inclusion because of a history of myocardial infarction, and after censoring of patients with incident AF prior to ischaemic stroke, respectively. To address the potential impact of surveillance bias caused by the increased health-care consumption associated with our study criteria for the psoriasis diagnosis, we also conducted analyses where patients with psoriasis were identified by their first vitamin D prescription claim and classified as having severe disease at the time of their first in- or outpatient hospitalization with this diagnosis, i.e. a definition of psoriasis considerably less conservative and less related to recurrent physician and hospital visits than the one used in the primary analysis. Furthermore, analyses where subjects were censored at the time of a surgical procedure, newly diagnosed valvular heart disease, or start of anti-thyroid treatment (a proxy for hyperthyroidism) were performed to evaluate the impact of these potentially important predictors of AF. Finally, using the Greedy matching macro (last accessed 20 February 2010, at www.mayosearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas), patients with psoriasis were matched for age and gender with four controls from the general population to conduct a matched cohort study, and in multivariate Poisson's models, adjusted for baseline characteristics obtained at the time of incident psoriasis, we assessed the impact of changes in baseline characteristics on risk estimates of study endpoints. In addition, analyses were performed with the use of propensity score-matched models conditional of baseline covariates (see Supplementary Data). A propensity score for the likelihood of receiving topical vitamin D derivatives was quantified by multivariate logistic regression, and the C-statistic was used to assess the discriminative power. A matched cohort study was done using the Greedy matching macro, each case was matched to one control based on the propensity score.
Model assumptions, including the absence of interactions between model covariates and the proportional hazard/risk assumption, were tested and found to be valid unless otherwise indicated. Estimation of the impact of an unmeasured confounder was made according to the ‘rule out’ approach for all reported results.26 All statistical analyses were performed with the SAS statistical software version 9.1 and STATA software version 10.1.
Results
Significant interaction between severe psoriasis and age (P < 0.05) were observed and therefore all analyses were stratified by age (<50 or ≥50 years, respectively). The proportional risk assumption was tested by a likelihood ratio test of the statistical model with and without allowing for the interaction terms between exposure and calendar year (P > 0.05), indicating no violation of the assumption. We found no evidence of overdispersion and the overall appropriateness of the Poisson regression was tested by the goodness-of-fit test, indicating that the data set was suitable for the use of thePoisson regression.
Baseline characteristics and event rates
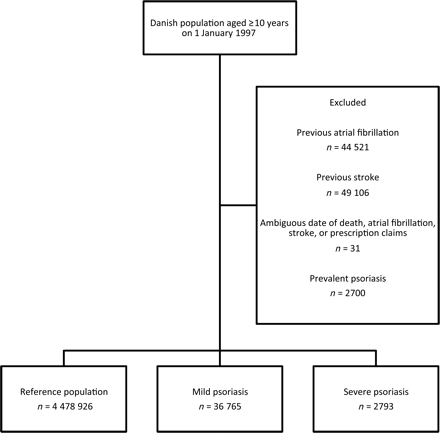
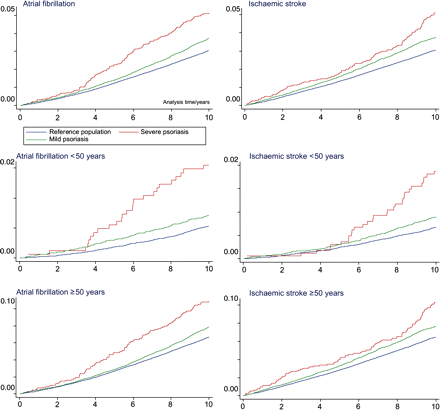
The study comprised 4 518 484 Danish citizens (49% men) and a total of 41 543 090 observational years with a maximum follow-up of 10 years. Loss to follow-up was less than 4% of the subjects included. A flowchart of the population selection is presented in Figure 1. A total of 36 765 patients with mild psoriasis and 2793 with severe psoriasis were identified during the study period and compared with 4 478 926 individuals comprising the reference population. Baseline characteristics are presented in Table 1. The incidence rates of AF and ischaemic stroke were higher in patients with psoriasis than in the reference population (Table 2). Overall and age-stratified cumulative hazard curves of the examined endpoints are presented in Figure 2.
Baseline characteristics of study population
| Characteristic . | Reference population (n = 4 478 926) . | Mild psoriasis (n = 36 765) . | Severe psoriasis (n = 2793) . |
|---|---|---|---|
| Age, years (SD) | 43.7 (19,7) | 46.1 (16.9) | 46.0 (16.4) |
| Age <50, years (SD) | 34.6 (11.9) | 38.8 (10.4) | 39.8 (10.0) |
| Age ≥50, years (SD) | 68.8 (9.4) | 67.3 (8.5) | 67.1 (8.4) |
| Men (%) | 2 282 954 (51.0) | 18 526 (50.4) | 1364 (48.8) |
| Women (%) | 2 195 972 (49.0) | 18 239 (49.6) | 1429 (51.2) |
| Mean follow-up time, years | 9.2 | 5.0 | 4.7 |
| No. of patient-years | 41 345 205 | 184 624 | 13 261 |
| Co-morbidity (%) | |||
| Valvular heart disease | 7201 (0.16) | 64 (0.17) | 2 (0.07) |
| Peripheral vascular disease | 5155 (0.12) | 37 (0.11) | 5 (0.18) |
| Cerebrovascular disease | 2705 (0.06) | 24 (0.07) | 1 (0.04) |
| Ischaemic heart disease | 16 551 (0.37) | 170 (0.46) | 20 (0.72) |
| Congestive heart failure | 7327 (0.16) | 41 (0.11) | 9 (0.32) |
| Previous myocardial infarction | 6594 (0.15) | 56 (0.15) | 6 (0.21) |
| Chronic obstructive pulmonary disease | 10 072 (0.22) | 54 (0.15) | 6 (0.21) |
| Cardiac dysrhythmia | 3027 (0.07) | 24 (0.07) | 40 (0.14) |
| Chronic renal failure | 2183 (0.05) | 9 (0.02) | 4 (0.14) |
| Cancer | 24 062 (0.54) | 162 (0.44) | 17 (0.61) |
| Treatment (%) | |||
| NSAID | 85 503 (1.91) | 830 (2.26) | 77 (2.76) |
| Anti-depressive drug | 85 554 (1.91) | 822 (2.24) | 74 (2.65) |
| Anti-thyroid drug | 12 433 (0.28) | 105 (0.29) | 3 (0.11) |
| Platelet inhibitor | 85 554 (1.91) | 822 (2.24) | 75 (2.69) |
| β-Blocker | 126 554 (2.83) | 1426 (3,88) | 110 (3.94) |
| ACE-I/ARB | 109 181 (2,44) | 1201 (3.27) | 89 (3.19) |
| Vitamin K antagonist | 10 454 (0.23) | 93 (0.25) | 6 (0.21) |
| Loop diuretic | 110 539 (2.47) | 823 (2.55) | 91 (3.26) |
| Statin | 26 702 (0.60) | 358 (0.97) | 21 (0.75) |
| Spironolactone | 13 104 (0.29) | 104 (0.28) | 11 (0.39) |
| Glucose-lowering drug (medically managed diabetes mellitus) | 67 956 (1.52) | 640 (1.74) | 65 (2.33) |
| Characteristic . | Reference population (n = 4 478 926) . | Mild psoriasis (n = 36 765) . | Severe psoriasis (n = 2793) . |
|---|---|---|---|
| Age, years (SD) | 43.7 (19,7) | 46.1 (16.9) | 46.0 (16.4) |
| Age <50, years (SD) | 34.6 (11.9) | 38.8 (10.4) | 39.8 (10.0) |
| Age ≥50, years (SD) | 68.8 (9.4) | 67.3 (8.5) | 67.1 (8.4) |
| Men (%) | 2 282 954 (51.0) | 18 526 (50.4) | 1364 (48.8) |
| Women (%) | 2 195 972 (49.0) | 18 239 (49.6) | 1429 (51.2) |
| Mean follow-up time, years | 9.2 | 5.0 | 4.7 |
| No. of patient-years | 41 345 205 | 184 624 | 13 261 |
| Co-morbidity (%) | |||
| Valvular heart disease | 7201 (0.16) | 64 (0.17) | 2 (0.07) |
| Peripheral vascular disease | 5155 (0.12) | 37 (0.11) | 5 (0.18) |
| Cerebrovascular disease | 2705 (0.06) | 24 (0.07) | 1 (0.04) |
| Ischaemic heart disease | 16 551 (0.37) | 170 (0.46) | 20 (0.72) |
| Congestive heart failure | 7327 (0.16) | 41 (0.11) | 9 (0.32) |
| Previous myocardial infarction | 6594 (0.15) | 56 (0.15) | 6 (0.21) |
| Chronic obstructive pulmonary disease | 10 072 (0.22) | 54 (0.15) | 6 (0.21) |
| Cardiac dysrhythmia | 3027 (0.07) | 24 (0.07) | 40 (0.14) |
| Chronic renal failure | 2183 (0.05) | 9 (0.02) | 4 (0.14) |
| Cancer | 24 062 (0.54) | 162 (0.44) | 17 (0.61) |
| Treatment (%) | |||
| NSAID | 85 503 (1.91) | 830 (2.26) | 77 (2.76) |
| Anti-depressive drug | 85 554 (1.91) | 822 (2.24) | 74 (2.65) |
| Anti-thyroid drug | 12 433 (0.28) | 105 (0.29) | 3 (0.11) |
| Platelet inhibitor | 85 554 (1.91) | 822 (2.24) | 75 (2.69) |
| β-Blocker | 126 554 (2.83) | 1426 (3,88) | 110 (3.94) |
| ACE-I/ARB | 109 181 (2,44) | 1201 (3.27) | 89 (3.19) |
| Vitamin K antagonist | 10 454 (0.23) | 93 (0.25) | 6 (0.21) |
| Loop diuretic | 110 539 (2.47) | 823 (2.55) | 91 (3.26) |
| Statin | 26 702 (0.60) | 358 (0.97) | 21 (0.75) |
| Spironolactone | 13 104 (0.29) | 104 (0.28) | 11 (0.39) |
| Glucose-lowering drug (medically managed diabetes mellitus) | 67 956 (1.52) | 640 (1.74) | 65 (2.33) |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin-2 receptor antagonists; NSAID, non-steroid anti-inflammatory drugs.
Baseline characteristics of study population
| Characteristic . | Reference population (n = 4 478 926) . | Mild psoriasis (n = 36 765) . | Severe psoriasis (n = 2793) . |
|---|---|---|---|
| Age, years (SD) | 43.7 (19,7) | 46.1 (16.9) | 46.0 (16.4) |
| Age <50, years (SD) | 34.6 (11.9) | 38.8 (10.4) | 39.8 (10.0) |
| Age ≥50, years (SD) | 68.8 (9.4) | 67.3 (8.5) | 67.1 (8.4) |
| Men (%) | 2 282 954 (51.0) | 18 526 (50.4) | 1364 (48.8) |
| Women (%) | 2 195 972 (49.0) | 18 239 (49.6) | 1429 (51.2) |
| Mean follow-up time, years | 9.2 | 5.0 | 4.7 |
| No. of patient-years | 41 345 205 | 184 624 | 13 261 |
| Co-morbidity (%) | |||
| Valvular heart disease | 7201 (0.16) | 64 (0.17) | 2 (0.07) |
| Peripheral vascular disease | 5155 (0.12) | 37 (0.11) | 5 (0.18) |
| Cerebrovascular disease | 2705 (0.06) | 24 (0.07) | 1 (0.04) |
| Ischaemic heart disease | 16 551 (0.37) | 170 (0.46) | 20 (0.72) |
| Congestive heart failure | 7327 (0.16) | 41 (0.11) | 9 (0.32) |
| Previous myocardial infarction | 6594 (0.15) | 56 (0.15) | 6 (0.21) |
| Chronic obstructive pulmonary disease | 10 072 (0.22) | 54 (0.15) | 6 (0.21) |
| Cardiac dysrhythmia | 3027 (0.07) | 24 (0.07) | 40 (0.14) |
| Chronic renal failure | 2183 (0.05) | 9 (0.02) | 4 (0.14) |
| Cancer | 24 062 (0.54) | 162 (0.44) | 17 (0.61) |
| Treatment (%) | |||
| NSAID | 85 503 (1.91) | 830 (2.26) | 77 (2.76) |
| Anti-depressive drug | 85 554 (1.91) | 822 (2.24) | 74 (2.65) |
| Anti-thyroid drug | 12 433 (0.28) | 105 (0.29) | 3 (0.11) |
| Platelet inhibitor | 85 554 (1.91) | 822 (2.24) | 75 (2.69) |
| β-Blocker | 126 554 (2.83) | 1426 (3,88) | 110 (3.94) |
| ACE-I/ARB | 109 181 (2,44) | 1201 (3.27) | 89 (3.19) |
| Vitamin K antagonist | 10 454 (0.23) | 93 (0.25) | 6 (0.21) |
| Loop diuretic | 110 539 (2.47) | 823 (2.55) | 91 (3.26) |
| Statin | 26 702 (0.60) | 358 (0.97) | 21 (0.75) |
| Spironolactone | 13 104 (0.29) | 104 (0.28) | 11 (0.39) |
| Glucose-lowering drug (medically managed diabetes mellitus) | 67 956 (1.52) | 640 (1.74) | 65 (2.33) |
| Characteristic . | Reference population (n = 4 478 926) . | Mild psoriasis (n = 36 765) . | Severe psoriasis (n = 2793) . |
|---|---|---|---|
| Age, years (SD) | 43.7 (19,7) | 46.1 (16.9) | 46.0 (16.4) |
| Age <50, years (SD) | 34.6 (11.9) | 38.8 (10.4) | 39.8 (10.0) |
| Age ≥50, years (SD) | 68.8 (9.4) | 67.3 (8.5) | 67.1 (8.4) |
| Men (%) | 2 282 954 (51.0) | 18 526 (50.4) | 1364 (48.8) |
| Women (%) | 2 195 972 (49.0) | 18 239 (49.6) | 1429 (51.2) |
| Mean follow-up time, years | 9.2 | 5.0 | 4.7 |
| No. of patient-years | 41 345 205 | 184 624 | 13 261 |
| Co-morbidity (%) | |||
| Valvular heart disease | 7201 (0.16) | 64 (0.17) | 2 (0.07) |
| Peripheral vascular disease | 5155 (0.12) | 37 (0.11) | 5 (0.18) |
| Cerebrovascular disease | 2705 (0.06) | 24 (0.07) | 1 (0.04) |
| Ischaemic heart disease | 16 551 (0.37) | 170 (0.46) | 20 (0.72) |
| Congestive heart failure | 7327 (0.16) | 41 (0.11) | 9 (0.32) |
| Previous myocardial infarction | 6594 (0.15) | 56 (0.15) | 6 (0.21) |
| Chronic obstructive pulmonary disease | 10 072 (0.22) | 54 (0.15) | 6 (0.21) |
| Cardiac dysrhythmia | 3027 (0.07) | 24 (0.07) | 40 (0.14) |
| Chronic renal failure | 2183 (0.05) | 9 (0.02) | 4 (0.14) |
| Cancer | 24 062 (0.54) | 162 (0.44) | 17 (0.61) |
| Treatment (%) | |||
| NSAID | 85 503 (1.91) | 830 (2.26) | 77 (2.76) |
| Anti-depressive drug | 85 554 (1.91) | 822 (2.24) | 74 (2.65) |
| Anti-thyroid drug | 12 433 (0.28) | 105 (0.29) | 3 (0.11) |
| Platelet inhibitor | 85 554 (1.91) | 822 (2.24) | 75 (2.69) |
| β-Blocker | 126 554 (2.83) | 1426 (3,88) | 110 (3.94) |
| ACE-I/ARB | 109 181 (2,44) | 1201 (3.27) | 89 (3.19) |
| Vitamin K antagonist | 10 454 (0.23) | 93 (0.25) | 6 (0.21) |
| Loop diuretic | 110 539 (2.47) | 823 (2.55) | 91 (3.26) |
| Statin | 26 702 (0.60) | 358 (0.97) | 21 (0.75) |
| Spironolactone | 13 104 (0.29) | 104 (0.28) | 11 (0.39) |
| Glucose-lowering drug (medically managed diabetes mellitus) | 67 956 (1.52) | 640 (1.74) | 65 (2.33) |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin-2 receptor antagonists; NSAID, non-steroid anti-inflammatory drugs.
Overall and age-stratified event rates and 95% confidence intervals per 1000 observational years
| . | Reference population . | Mild psoriasis . | Severe psoriasis . |
|---|---|---|---|
| Atrial fibrillation | |||
| Overall | 3.03 (3.02–3.05) | 4.67 (4.37–4.99) | 5.96 (4.80–7.42) |
| Aged <50 years | 0.26 (0.25–0.27) | 0.36 (0.24–0.53) | 0.59 (0.19–1.82) |
| Aged ≥50 years | 6.10 (6.05–6.15) | 7.21 (6.73–7.73) | 9.10 (7.2–11.40) |
| Ischaemic stroke | |||
| Overall | 3.06 (3.04–3.08) | 4.54 (4.24–4.85) | 6.82 (5.03–7.70) |
| Aged <50 years | 0.23 (0.22–0.23) | 0.61 (0.45–0.81) | 1.56 (0.78–3.13) |
| Aged ≥50 years | 5.94 (5.91–5.98) | 6.74 (6.28–7.24) | 8.88 (7.06–11.17) |
| . | Reference population . | Mild psoriasis . | Severe psoriasis . |
|---|---|---|---|
| Atrial fibrillation | |||
| Overall | 3.03 (3.02–3.05) | 4.67 (4.37–4.99) | 5.96 (4.80–7.42) |
| Aged <50 years | 0.26 (0.25–0.27) | 0.36 (0.24–0.53) | 0.59 (0.19–1.82) |
| Aged ≥50 years | 6.10 (6.05–6.15) | 7.21 (6.73–7.73) | 9.10 (7.2–11.40) |
| Ischaemic stroke | |||
| Overall | 3.06 (3.04–3.08) | 4.54 (4.24–4.85) | 6.82 (5.03–7.70) |
| Aged <50 years | 0.23 (0.22–0.23) | 0.61 (0.45–0.81) | 1.56 (0.78–3.13) |
| Aged ≥50 years | 5.94 (5.91–5.98) | 6.74 (6.28–7.24) | 8.88 (7.06–11.17) |
Overall and age-stratified event rates and 95% confidence intervals per 1000 observational years
| . | Reference population . | Mild psoriasis . | Severe psoriasis . |
|---|---|---|---|
| Atrial fibrillation | |||
| Overall | 3.03 (3.02–3.05) | 4.67 (4.37–4.99) | 5.96 (4.80–7.42) |
| Aged <50 years | 0.26 (0.25–0.27) | 0.36 (0.24–0.53) | 0.59 (0.19–1.82) |
| Aged ≥50 years | 6.10 (6.05–6.15) | 7.21 (6.73–7.73) | 9.10 (7.2–11.40) |
| Ischaemic stroke | |||
| Overall | 3.06 (3.04–3.08) | 4.54 (4.24–4.85) | 6.82 (5.03–7.70) |
| Aged <50 years | 0.23 (0.22–0.23) | 0.61 (0.45–0.81) | 1.56 (0.78–3.13) |
| Aged ≥50 years | 5.94 (5.91–5.98) | 6.74 (6.28–7.24) | 8.88 (7.06–11.17) |
| . | Reference population . | Mild psoriasis . | Severe psoriasis . |
|---|---|---|---|
| Atrial fibrillation | |||
| Overall | 3.03 (3.02–3.05) | 4.67 (4.37–4.99) | 5.96 (4.80–7.42) |
| Aged <50 years | 0.26 (0.25–0.27) | 0.36 (0.24–0.53) | 0.59 (0.19–1.82) |
| Aged ≥50 years | 6.10 (6.05–6.15) | 7.21 (6.73–7.73) | 9.10 (7.2–11.40) |
| Ischaemic stroke | |||
| Overall | 3.06 (3.04–3.08) | 4.54 (4.24–4.85) | 6.82 (5.03–7.70) |
| Aged <50 years | 0.23 (0.22–0.23) | 0.61 (0.45–0.81) | 1.56 (0.78–3.13) |
| Aged ≥50 years | 5.94 (5.91–5.98) | 6.74 (6.28–7.24) | 8.88 (7.06–11.17) |
Overall and age-stratified Nelson–Aalen cumulative hazard curves of the examined endpoints.
Risk of atrial fibrillation and ischaemic stroke
Psoriasis was associated with increased risk of AF and ischaemic stroke in unadjusted analyses as well as in the fully adjusted statistical model. Overall (i.e. not age stratified) RRs for AF and ischaemic stroke are presented in Tables 3 and 4. In patients with mild psoriasis, the adjusted RRs for AF were 1.50 (1.21–1.86) and 1.16 (1.08–1.24) in patients aged <50 and ≥50 years, respectively. Patients with severe psoriasis had a higher risk of AF with RRs 2.98 (1.80–4.92) in patients aged <50 years and 1.29 (1.01–1.65) in patients aged ≥50 years. Patients with psoriasis also demonstrated a disease severity-dependent increased risk of ischaemic stroke, i.e. RRs 1.97 (1.66–2.34) and 2.80 (1.81–4.34) in patients aged <50 years with mild and severe psoriasis, and RRs 1.13 (1.04–1.21) and 1.34 (1.04–1.71) in patients aged ≥50 years with mild and severe psoriasis, respectively.
Atrial fibrillation: rate ratios and 95% confidence intervals for psoriasis and individual covariates
| Atrial fibrillation . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.31 | 23.7 | 1.22–1.40 |
| Severe psoriasis | 1.63 | 38.7 | 1.30–2.02 |
| Age (per 10 years) | 1.09 | 1.09–1.09 | |
| Sex (male vs. female) | 1.62 | 1.60–1.64 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.22 | 18.0 | 1.14–1.30 |
| Severe psoriasis | 1.53 | 34.6 | 1.23–1.91 |
| Socioeconomic (per level increase) | 0.98 | 0.98–0.99 | |
| Previous myocardial infarction | 1.19 | 1.15–1.22 | |
| Congestive heart failure | 1.17 | 1.10–1.25 | |
| Valvular heart disease | 2.02 | 1.91–2.14 | |
| Chronic obstructive pulmonary disease | 1.77 | 1.67–1.89 | |
| Chronic renal failure | 1.78 | 1.49–2.13 | |
| Platelet inhibitor | 0.79 | 0.61–1.03 | |
| NSAID | 1.10 | 0.97–1.24 | |
| Anti-depressive drug | 1.24 | 0.98–1.57 | |
| Anti-thyroid drug | 1.61 | 1.52–1.70 | |
| β-Blocker | 1.62 | 1.59–1.65 | |
| ACE-I/ARB | 1.52 | 1.49–1.55 | |
| Vitamin K antagonist | 2.34 | 2.23–2.46 | |
| Loop diuretic | 1.52 | 1.49–1.55 | |
| Spironolactone | 1.17 | 1.11–1.24 | |
| Statin | 1.16 | 1.11–1.21 | |
| Glucose-lowering drug | 1.30 | 1.27–1.34 | |
| Atrial fibrillation . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.31 | 23.7 | 1.22–1.40 |
| Severe psoriasis | 1.63 | 38.7 | 1.30–2.02 |
| Age (per 10 years) | 1.09 | 1.09–1.09 | |
| Sex (male vs. female) | 1.62 | 1.60–1.64 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.22 | 18.0 | 1.14–1.30 |
| Severe psoriasis | 1.53 | 34.6 | 1.23–1.91 |
| Socioeconomic (per level increase) | 0.98 | 0.98–0.99 | |
| Previous myocardial infarction | 1.19 | 1.15–1.22 | |
| Congestive heart failure | 1.17 | 1.10–1.25 | |
| Valvular heart disease | 2.02 | 1.91–2.14 | |
| Chronic obstructive pulmonary disease | 1.77 | 1.67–1.89 | |
| Chronic renal failure | 1.78 | 1.49–2.13 | |
| Platelet inhibitor | 0.79 | 0.61–1.03 | |
| NSAID | 1.10 | 0.97–1.24 | |
| Anti-depressive drug | 1.24 | 0.98–1.57 | |
| Anti-thyroid drug | 1.61 | 1.52–1.70 | |
| β-Blocker | 1.62 | 1.59–1.65 | |
| ACE-I/ARB | 1.52 | 1.49–1.55 | |
| Vitamin K antagonist | 2.34 | 2.23–2.46 | |
| Loop diuretic | 1.52 | 1.49–1.55 | |
| Spironolactone | 1.17 | 1.11–1.24 | |
| Statin | 1.16 | 1.11–1.21 | |
| Glucose-lowering drug | 1.30 | 1.27–1.34 | |
Atrial fibrillation: rate ratios and 95% confidence intervals for psoriasis and individual covariates
| Atrial fibrillation . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.31 | 23.7 | 1.22–1.40 |
| Severe psoriasis | 1.63 | 38.7 | 1.30–2.02 |
| Age (per 10 years) | 1.09 | 1.09–1.09 | |
| Sex (male vs. female) | 1.62 | 1.60–1.64 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.22 | 18.0 | 1.14–1.30 |
| Severe psoriasis | 1.53 | 34.6 | 1.23–1.91 |
| Socioeconomic (per level increase) | 0.98 | 0.98–0.99 | |
| Previous myocardial infarction | 1.19 | 1.15–1.22 | |
| Congestive heart failure | 1.17 | 1.10–1.25 | |
| Valvular heart disease | 2.02 | 1.91–2.14 | |
| Chronic obstructive pulmonary disease | 1.77 | 1.67–1.89 | |
| Chronic renal failure | 1.78 | 1.49–2.13 | |
| Platelet inhibitor | 0.79 | 0.61–1.03 | |
| NSAID | 1.10 | 0.97–1.24 | |
| Anti-depressive drug | 1.24 | 0.98–1.57 | |
| Anti-thyroid drug | 1.61 | 1.52–1.70 | |
| β-Blocker | 1.62 | 1.59–1.65 | |
| ACE-I/ARB | 1.52 | 1.49–1.55 | |
| Vitamin K antagonist | 2.34 | 2.23–2.46 | |
| Loop diuretic | 1.52 | 1.49–1.55 | |
| Spironolactone | 1.17 | 1.11–1.24 | |
| Statin | 1.16 | 1.11–1.21 | |
| Glucose-lowering drug | 1.30 | 1.27–1.34 | |
| Atrial fibrillation . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.31 | 23.7 | 1.22–1.40 |
| Severe psoriasis | 1.63 | 38.7 | 1.30–2.02 |
| Age (per 10 years) | 1.09 | 1.09–1.09 | |
| Sex (male vs. female) | 1.62 | 1.60–1.64 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.22 | 18.0 | 1.14–1.30 |
| Severe psoriasis | 1.53 | 34.6 | 1.23–1.91 |
| Socioeconomic (per level increase) | 0.98 | 0.98–0.99 | |
| Previous myocardial infarction | 1.19 | 1.15–1.22 | |
| Congestive heart failure | 1.17 | 1.10–1.25 | |
| Valvular heart disease | 2.02 | 1.91–2.14 | |
| Chronic obstructive pulmonary disease | 1.77 | 1.67–1.89 | |
| Chronic renal failure | 1.78 | 1.49–2.13 | |
| Platelet inhibitor | 0.79 | 0.61–1.03 | |
| NSAID | 1.10 | 0.97–1.24 | |
| Anti-depressive drug | 1.24 | 0.98–1.57 | |
| Anti-thyroid drug | 1.61 | 1.52–1.70 | |
| β-Blocker | 1.62 | 1.59–1.65 | |
| ACE-I/ARB | 1.52 | 1.49–1.55 | |
| Vitamin K antagonist | 2.34 | 2.23–2.46 | |
| Loop diuretic | 1.52 | 1.49–1.55 | |
| Spironolactone | 1.17 | 1.11–1.24 | |
| Statin | 1.16 | 1.11–1.21 | |
| Glucose-lowering drug | 1.30 | 1.27–1.34 | |
Ischaemic stroke: rate ratios and 95% confidence intervals for psoriasis and individual covariates
| Ischaemic stroke . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.69 | 40.8 | 1.36–2.09 |
| Age (per 10 years) | 1.08 | 1.08–1.08 | |
| Sex (male vs. female) | 1.44 | 1.42–1.45 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.65 | 39.4 | 1.33–2.05 |
| Socioeconomic (per level increase) | 0.95 | 0.94–0.96 | |
| Previous myocardial infarction | 1.27 | 1.23–1.30 | |
| Congestive heart failure | 1.00 | 0.92–1.09 | |
| Valvular heart disease | 1.28 | 1.18–1.38 | |
| Chronic obstructive pulmonary disease | 1.21 | 1.11–1.31 | |
| Chronic renal failure | 2.06 | 1.71–2.48 | |
| Platelet inhibitor | 0.76 | 0.58–1.00 | |
| NSAID | 1.19 | 1.05–1.35 | |
| Anti-depressive drug | 1.18 | 0.92–1.50 | |
| Anti-thyroid drug | 1.01 | 0.94–1.09 | |
| β-Blocker | 1.40 | 1.37–1.43 | |
| ACE-I/ARB | 1.42 | 1.40–1.46 | |
| Vitamin K antagonist | 1.40 | 1.32–1.49 | |
| Loop diuretic | 1.02 | 0.99–1.04 | |
| Spironolactone | 1.02 | 0.95–1.18 | |
| Statin | 1.12 | 1.07–1.18 | |
| Glucose-lowering drug | 2.13 | 2.08–2.18 | |
| Ischaemic stroke . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.69 | 40.8 | 1.36–2.09 |
| Age (per 10 years) | 1.08 | 1.08–1.08 | |
| Sex (male vs. female) | 1.44 | 1.42–1.45 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.65 | 39.4 | 1.33–2.05 |
| Socioeconomic (per level increase) | 0.95 | 0.94–0.96 | |
| Previous myocardial infarction | 1.27 | 1.23–1.30 | |
| Congestive heart failure | 1.00 | 0.92–1.09 | |
| Valvular heart disease | 1.28 | 1.18–1.38 | |
| Chronic obstructive pulmonary disease | 1.21 | 1.11–1.31 | |
| Chronic renal failure | 2.06 | 1.71–2.48 | |
| Platelet inhibitor | 0.76 | 0.58–1.00 | |
| NSAID | 1.19 | 1.05–1.35 | |
| Anti-depressive drug | 1.18 | 0.92–1.50 | |
| Anti-thyroid drug | 1.01 | 0.94–1.09 | |
| β-Blocker | 1.40 | 1.37–1.43 | |
| ACE-I/ARB | 1.42 | 1.40–1.46 | |
| Vitamin K antagonist | 1.40 | 1.32–1.49 | |
| Loop diuretic | 1.02 | 0.99–1.04 | |
| Spironolactone | 1.02 | 0.95–1.18 | |
| Statin | 1.12 | 1.07–1.18 | |
| Glucose-lowering drug | 2.13 | 2.08–2.18 | |
Ischaemic stroke: rate ratios and 95% confidence intervals for psoriasis and individual covariates
| Ischaemic stroke . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.69 | 40.8 | 1.36–2.09 |
| Age (per 10 years) | 1.08 | 1.08–1.08 | |
| Sex (male vs. female) | 1.44 | 1.42–1.45 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.65 | 39.4 | 1.33–2.05 |
| Socioeconomic (per level increase) | 0.95 | 0.94–0.96 | |
| Previous myocardial infarction | 1.27 | 1.23–1.30 | |
| Congestive heart failure | 1.00 | 0.92–1.09 | |
| Valvular heart disease | 1.28 | 1.18–1.38 | |
| Chronic obstructive pulmonary disease | 1.21 | 1.11–1.31 | |
| Chronic renal failure | 2.06 | 1.71–2.48 | |
| Platelet inhibitor | 0.76 | 0.58–1.00 | |
| NSAID | 1.19 | 1.05–1.35 | |
| Anti-depressive drug | 1.18 | 0.92–1.50 | |
| Anti-thyroid drug | 1.01 | 0.94–1.09 | |
| β-Blocker | 1.40 | 1.37–1.43 | |
| ACE-I/ARB | 1.42 | 1.40–1.46 | |
| Vitamin K antagonist | 1.40 | 1.32–1.49 | |
| Loop diuretic | 1.02 | 0.99–1.04 | |
| Spironolactone | 1.02 | 0.95–1.18 | |
| Statin | 1.12 | 1.07–1.18 | |
| Glucose-lowering drug | 2.13 | 2.08–2.18 | |
| Ischaemic stroke . | Rate ratio . | Attributable risk percentage (%) . | 95% confidence interval . |
|---|---|---|---|
| Overall estimates adjusted for age and sex | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.69 | 40.8 | 1.36–2.09 |
| Age (per 10 years) | 1.08 | 1.08–1.08 | |
| Sex (male vs. female) | 1.44 | 1.42–1.45 | |
| Overall estimates adjusted for age, sex, calendar year, co-morbidity, medication, and socioeconomic status | |||
| Mild psoriasis | 1.25 | 20.0 | 1.17–1.34 |
| Severe psoriasis | 1.65 | 39.4 | 1.33–2.05 |
| Socioeconomic (per level increase) | 0.95 | 0.94–0.96 | |
| Previous myocardial infarction | 1.27 | 1.23–1.30 | |
| Congestive heart failure | 1.00 | 0.92–1.09 | |
| Valvular heart disease | 1.28 | 1.18–1.38 | |
| Chronic obstructive pulmonary disease | 1.21 | 1.11–1.31 | |
| Chronic renal failure | 2.06 | 1.71–2.48 | |
| Platelet inhibitor | 0.76 | 0.58–1.00 | |
| NSAID | 1.19 | 1.05–1.35 | |
| Anti-depressive drug | 1.18 | 0.92–1.50 | |
| Anti-thyroid drug | 1.01 | 0.94–1.09 | |
| β-Blocker | 1.40 | 1.37–1.43 | |
| ACE-I/ARB | 1.42 | 1.40–1.46 | |
| Vitamin K antagonist | 1.40 | 1.32–1.49 | |
| Loop diuretic | 1.02 | 0.99–1.04 | |
| Spironolactone | 1.02 | 0.95–1.18 | |
| Statin | 1.12 | 1.07–1.18 | |
| Glucose-lowering drug | 2.13 | 2.08–2.18 | |
Sensitivity analyses
Exclusion of patients with prior myocardial infarction
Exclusion of patients with prior myocardial infarction did not attenuate the observed association between psoriasis, AF, and ischaemic stroke, and in this analysis, the psoriasis severity dose–response and higher relative risks in patients <50 years were maintained. Indeed, patients with severe psoriasis had markedly increased risk of AF that was comparable to the results of the primary analyses [RRs 3.02 (1.83–4.98) and 1.32 (1.03–1.69) in patients aged <50 and ≥50 years, respectively]. Similar results were also apparent for the risk of ischaemic stroke [RRs 2.87 (confidence interval {CI} 1.85–4.44) and 1.31 (CI 1.02–1.68) in patients with severe psoriasis aged <50 and ≥50 years, respectively].
Censoring of patients at the time of a surgical procedure, valvular heart disease, or anti-thyroid treatment
After potentially strong predictors of AF, i.e. surgical procedures, newly diagnosed valvular heart disease and medically treated hyperthyroidism were controlled for the association between psoriasis and AF was strengthened. In this analysis, RRs for mild psoriasis were 1.67 (CI 1.38–2.07) and 1.41 (CI 1.31–1.51) for patients aged <50 and ≥50 years, respectively. The corresponding RRs for severe psoriasis were 3.15 (CI 1.96–5.05) and 1.30 (CI 1.38–2.04).
Censoring of patients with incident atrial fibrillation prior to ischaemic stroke
When patients who experienced an episode of AF prior to ischaemic stroke were censored, the risk estimates, including the disease severity dose-dependent association between psoriasis and elevated risk of ischaemic stroke remained significant (data not shown). For example, RRs for ischaemic stroke in patients <50 years were 1.98 (1.67–2.36) and 2.90 (1.87–4.50) in patients with mild and severe psoriasis, respectively.
Age- and gender-matched cohort analyses
A total of 36 765 patients with mild psoriasis and 2793 patients with severe psoriasis were matched on age and gender with 158 232 controls in order to update baseline characteristics to the time of psoriasis diagnosis for psoriasis patients and their controls. In these analyses, psoriasis continued to confer increased risk of AF and ischaemic stroke. The RRs for AF in patients <50 years were 1.11 (0.89–1.39) and 2.54 (1.54–4.19) for mild and severe psoriasis, respectively. In patients aged ≥50 years, the corresponding RRs were lower [1.37 (1.27–1.48) and 1.44 (1.13–1.84)]. The psoriasis severity- and age-dependent elevated risks of ischaemic stroke were comparable to the results obtained in the primary analyses.
Analysis with altered criteria for the psoriasis diagnosis
When the criteria for psoriasis were altered to the first vitamin D prescription claim and first hospitalization for severe psoriasis, a total of 56 633 with mild psoriasis and 6569 patients with severe psoriasis were identified. When the 22 018 patients with prevalent psoriasis according to the altered criteria for the psoriasis diagnosis were excluded, the new criteria yielded a psoriasis prevalence of 1.8%. In this population, event rates and RRs for AF and ischaemic stroke were comparable to results of the primary analyses, indicating that increased health-care consumption and attendant surveillance bias were unlikely to contribute significantly to the results.
Unmeasured confounding
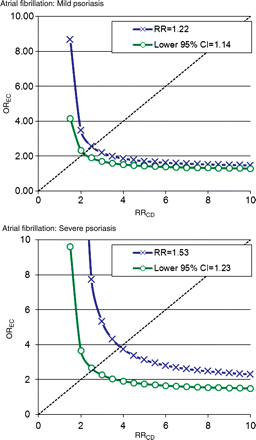
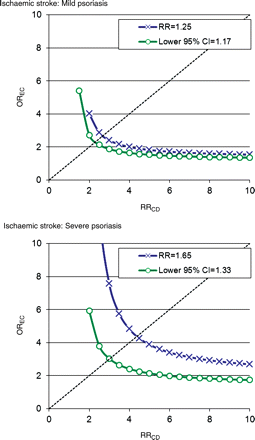
Assuming a 20% prevalence of an unmeasured confounder in the entire population, i.e. a prevalence exceeding that of heavy smoking (>20 cigarettes/day) or obesity (body mass index >30 kg/m2) and a prevalence of the exposure (psoriasis) of 1%, we calculated the hypothetical size of such an unmeasured confounder. The results (Figures 3 and 4) indicated that an unmeasured confounder should carry a very strong association with the outcome and, importantly, be very unevenly distributed between groups with a very strong association with psoriasis. Indeed, the estimated magnitude of a confounder that could nullify our results exceeded the effects and distribution of any measured confounder, e.g. valvular heart disease or previous myocardial infarction.
Atrial fibrillation: estimation of the impact of an unmeasured confounder assuming a prevalence of the confounder of 20% in the population and a prevalence of the exposure of 1%. OREC: association between the confounder and psoriasis. RRCD: association between the confounder and the outcome. The green line indicates the magnitude needed for an unmeasured confounder to render the results statistically insignificant at a given OREC and RRCD. The blue line indicates the corresponding magnitude of an unmeasured confounder needed to nullify the results.
Ischaemic stroke: estimation of the impact of an unmeasured confounder assuming a prevalence of the confounder of 20% in the population and a prevalence of the exposure of 1%. OREC: association between the confounder and psoriasis. RRCD: association between the confounder and the outcome. The green line indicates the magnitude needed for an unmeasured confounder to render the results statistically insignificant at a given OREC and RRCD. The blue line indicates the corresponding magnitude of an unmeasured confounder needed to nullify the results.
Discussion
The current study is, to our knowledge, the first to simultaneously examine the risk of AF and ischaemic stroke in a nationwide cohort of psoriasis patients. The main result of the study was that psoriasis was associated with AF and ischaemic stroke independent of age, gender, co-morbidity, concomitant medication, and socioeconomic status. This result was further supported by a consistent dose–response relationship between psoriasis severity and risk of AF and ischaemic stroke, and was corroborated by sensitivity analyses, including propensity score-matched analysis. These novel results add to mounting evidence of the role of psoriasis as a clinically relevant cardiovascular risk factor and they call for increased awareness of cardiovascular risk factor management in this large group of patients.
The main limitation of our study is inherent to the observational design and precludes conclusions on causality. Another important limitation was our inability to account for some important cardiovascular risk factors, e.g. lipid levels, obesity, and smoking. Although the incorporation of, for example, concomitant medication and socioeconomic status into our regression analyses captured some of these effects, there remains a possibility for unmeasured confounding. We calculated, however, that an unmeasured confounder would have to be highly prevalent, strongly associated with psoriasis, and carry a very high risk in order to nullify our findings.26 This supports the integrity of our results, but the calculations do not enable us to refute any impact of unmeasured confounders. Misclassification of cardiovascular diseases and risk factors due to, for example, untreated hypertension, diabetes, or dyslipidaemia represents another potential source of bias. Such misclassification of risk factors is, however, arguably likely to be most common in subjects without psoriasis and may have inflicted an uncertainty of the results towards the null. With regard to effects of competing risks, i.e. event(s), e.g. death, that alter or prevent the subject's risk of experiencing the outcome(s) of interest, this would tend to lead to overestimate the true risk of the outcome. Our demonstration of similar results (see Supplementary Data) when accounting for competing risk, however, indicates no significant impact of this phenomenon on the results.
Psoriasis and atherosclerosis are chronic inflammatory diseases with considerable overlap of inflammatory mechanisms.6,27 Psoriasis has previously been associated with cardiovascular risk factors, surrogate markers of atherothrombotic disease, and cardiovascular morbidity and mortality.12–20,28,29 Furthermore, reports of diminished cardiovascular risk in patients with psoriasis receiving systemic anti-inflammatory therapy with methotrexate and tumour necrosis factor-α inhibitors and, conversely, a reduction in psoriatic cutaneous plaque activity after treatment with statins, are suggestive of coincident inflammatory mechanisms.30–32 The association between psoriasis and AF has not been explored previously, but a small study has linked psoriasis to raised heart rate and increased frequency of supraventricular extrasystoles.33 Atrial fibrillation is the most prevalent arrhythmia and is associated with increased risks of ischaemic stroke, heart failure, coronary artery disease, and cardiovascular death.3,4,8 Inflammation and oxidative stress have been proposed to play a role in the induction, recurrence, and sustainability of AF.7,9 For example, atrial infiltration of inflammatory cells has been observed in patients with AF.10 Furthermore, hypertension, diabetes mellitus, obesity, and smoking increase the risk of AF and are associated with markers of inflammation and oxidative stress, and circulating levels of inflammatory markers (e.g. plasma C-reactive protein) appear to correlate with the success of electrical cardioversion and recurrence of AF following cardioversion.34–37 Less AF recurrences after cardioversion and reduction in postoperative AF after cardiac surgery by anti-inflammatory therapy with methylprednisolone and statins have also been reported.38,39 Along this line, our demonstration of a consistent disease severity-dependent association between psoriasis and AF supports the hypothesis of shared inflammatory mechanisms between the two diseases which was further supported by the sensitivity analysis with adjustments for surgical procedures and medically treated hyperthyroidism. It is also notable that exclusion of patients with prior myocardial infarction did not cancel out the observed association, suggesting that the risk of AF in patients with psoriasis was not mainly driven by concomitant coronary artery disease.
Ischaemic stroke is associated with other manifestations of atherosclerosis, e.g. coronary artery disease, in which inflammation plays a pivotal role from fatty streak formation to plaque instability and thrombosis.5,6,40 Indeed, patients with myocardial infarction often have morphologically unstable carotid atherosclerotic plaques and patients with cerebral ischaemic events often exhibit clinically significant coronary artery disease.41–43 Furthermore, endothelial dysfunction (a primary step in atherogenesis), which is an established predictor of adverse cardiovascular events including ischaemic stroke, is associated with inflammation and has been demonstrated in patients with psoriatic arthritis.29,44,45 Although psoriasis has previously been associated with increased risk of stroke, we are the first to report the magnitude of this risk after controlling for the possible confounding by AF, surgical procedures, hyperthyroidism, and coronary artery disease.13,15,16 Specifically, we found that risk estimates for ischaemic stroke were raised in all patients with psoriasis and not markedly attenuated after censoring patients with incident AF, suggesting that the ischaemic stroke risk exceeded the risk attributable to AF. Our results are therefore consistent with the role of psoriasis as an independent risk factor for ischaemic stroke, and inflammation is likely to be a common denominator underlying this association. Apart from the mechanistic correlates between psoriasis, AF, and ischaemic stroke in terms of inflammatory processes, the probability of a causal relation is increased by the consistent dose–response relationship between psoriasis severity and the examined endpoints. The present results are also corroborated by our previous findings of augmented risk of myocardial infarction, stroke (haemorrhagic and ischaemic), coronary revascularization, and cardiovascular death in the Danish population.20 Taken together, results from these studies and the present investigation of the entire Danish population indicate that patients with psoriasis are at an age- and psoriasis severity-dependent increased risk of a wide range of cardiovascular adverse outcomes. The adjusted risk estimates were generally higher in young patients, and this finding parallels reports on psoriasis-related risk of myocardial infarction, presumably mirroring, in part, the age- and co-morbidity-dependent increase in overall cardiovascular risk, i.e. with attenuation of the relative contribution of psoriasis-related risk in the elderly.
Some further strengths and limitations of the study need to be addressed. In Denmark, health care is readily accessible and essentially free of charge, and by including the entire Danish population, we avoided selection bias related to for example, subject gender, age, socioeconomic status, health-care insurance, and labour market participation. The use of validated study endpoints and the completeness of follow-up also strengthen the study conclusions. The use of nationwide registers of hospitalizations and dispensed prescriptions from all Danish pharmacies reduced potential surveillance bias. This was further supported by the adjustment for Charlson's co-morbidity index, which holds information on baseline health-care consumption. Furthermore, the results were robust to adjustments for relevant baseline characteristics and remained valid in a range of sensitivity analyses and propensity score-matched models. We were unable to identify patients with psoriasis treated with topical corticosteroids alone (due to the liberal indications for these agents), allowing for misclassification of some patients with psoriasis, including prevalent cases not treated with topical vitamin D, as reference population. Such misclassification, however, would have led to the underestimation of event rates in patients with psoriasis and attenuation of the true psoriasis-related risk of adverse events. Given the very large sample size, the impact of misclassifying patients with prevalent or incident psoriasis of varying severity, as reference population, is unlikely to have been major. Furthermore, relatively few patients probably used topical steroids alone, since we found a prevalence of psoriasis as determined by the first vitamin D prescription claim alone close to the expected overall psoriasis prevalence of ∼2%.1 Since the registration of systemic immunomodulatory treatment that is exclusively managed by dermatological hospital departments is, at present, incomplete, we were unable to use systemic treatment for psoriasis to define our cohort, and regrettably also unable to examine any impact of varying treatment regimens on the cardiovascular risk in patients with psoriasis. Finally, the Danish population predominantly consists of Caucasians and extrapolation of the results to other ethnicities should only be done with caution.
Conclusion
This large study of psoriasis-related risk of AF and ischaemic stroke in an unselected nationwide population suggests that psoriasis is a risk factor for these adverse cardiovascular events. The relative risks of AF and ischaemic stroke were highest in young patients with severe psoriasis. The results add to accumulating evidence, indicating that patients with psoriasis are at increased cardiovascular risk. Studies that more accurately account for important cardiovascular risk factors and immunomodulatory treatment are awaited to confirm this novel association and prospective randomized trials are needed to determine the value of intensive cardiovascular risk factor modification.
Ethics
This study was approved by The Danish Data Protection Agency (ref. 2007-41-1667), and data at the individual case level were made available by the national registers in an anonymized form. Registry studies do not require ethical approval in Denmark. The authors had full access to the data and take full responsibility for its integrity.
Supplementary material
Funding
The study was supported by Department of Cardiology, Copenhagen University Hospital Gentofte, DK-2900, Denmark. The funding source had no influence on study design, interpretation of results, or the decision to submit the article.
Conflict of interest: none declared.




Comments
We greatly appreciate the interest in our study (1). Karoline Adamzik et al. suggest excessive alcohol intake in patients with psoriasis as a possible explanation of the association between psoriasis and increased risk of atrial fibrillation. We agree that excessive alcohol intake is a risk factor for atrial fibrillation and fully acknowledge a potential impact of unmeasured confounders, e.g., alcohol use on the reported results. Given the modest impact of including strong predictors of atrial fibrillation, e.g., coronary artery disease and valvular heart disease on the strength of the association between psoriasis and risk of atrial fibrillation, however, we find it unlikely that the observed increased risk of atrial fibrillation in patients with psoriasis was mainly driven by increased alcohol consumption. Importantly, the covariates included in our statistical models, e.g., liver disease, chronic obstructive pulmonary disease, socioeconomic status, and use of anti-depressive medication, are also likely to capture major effects of unmeasured confounders including alcohol intake. The calculations presented in the paper on the potential impact of unmeasured confounding further support the integrity of our results (1).
1. Ahlehoff O, Gislason GH, Jorgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstrom SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2011 Aug 12.
Conflict of Interest:
None declared
Dear Sir,
We read with great interest the study examining psoriasis and the risk of atrial fibrillation and ischaemic stroke by Ahlehoff et al.(1) This nationwide cohort study in the Danish population found a significantly increased risk of atrial fibrillation (AF) and ischaemic stroke in psoriasis patients. As stated by the authors, psoriasis has been accepted as a systemic disease and furthermore, as an independent cardiovascular risk factor.(2) Psoriasis patients have been shown to have a higher risk of atherosclerosis, myocardial infarction and other cardiovascular diseases.(3) The confounders in this study as mentioned by the authors include obesity, hypertension, diabetes, dyslipidemia and cigarette smoking. All of these confounders have an increased prevalence in psoriasis patients with moderate to severe disease.(4) We would additionally consider excessive alcohol consumption as a confounding factor in psoriasis patients who develop AF and ischaemic stroke.
Patients with moderate to severe psoriasis appear to consume excess alcohol in comparison to controls. A large study of over 10,000 patients with moderate to severe psoriasis demonstrated a high relative risk for mortality from alcohol related causes in psoriasis of 4.46 for male patients and 5.60 for female patients.(5) A prospective study in American women in 2010, showed a relative risk of developing psoriasis of 1.72 (95% confidence interval [CI], 1.15-2.57) for an alcohol consumption of 2.3 drinks/week or more.(6) Up to 32% of patients with moderate to severe psoriasis have excessive alcohol consumption when screened using validated questionnaires such as the Alcohol Use Disorders Identification Test (AUDIT) and Michigan Alcohol Sreening Test MAST.(7,8) Seven per cent of our psoriasis patients have alcohol dependence (defined as physical or psychological symptoms of alcohol withdrawal on drinking cessation) as indicated by increased levels of carbohydrate-deficient transferring, the most sensitive and specific alcohol biomarker.8 We have reported previously that patients with alcoholic liver disease have a higher prevalence of psoriasis than the normal population.(9) Excessive alcohol intake is a known risk factor for cardiovascular diseases including AF and ischaemic stroke (10,11) and has been reported as a dose dependent risk factor for atrial fibrillation.(12,13)
We would suggest that excessive alcohol consumption may be one of the unmeasured confounders that contributed to the excessive rates of atrial fibrillation and ischaemic stroke reported in this excellent study.
References
1. Ahlehoff O, Gislason GH, J?rgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstr?m SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2011 Aug 12.
2. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
3. Tobin AM, Veale DJ, Fitzgerald O, Rogers S, Collins P, O'Shea D, Kirby B. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol. 2010;37:1386-94.
4. Kimball AB, Gu?rin A, Tsaneva M, Yu AP, Wu EQ, Gupta SR, Bao Y, Mulani PM. Economic burden of comorbidities in patients with psoriasis is substantial. J Eur Acad Dermatol Venereol. 2011;25:157-63.
5. Poikolainen K, Karvonen J, Pukkala E. Excess mortality related to alcohol and smoking among hospital-treated patients with psoriasis. Arch Dermatol 1999;135:1490-3.
6. Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol intake and risk of incident psoriasis in US women: a prospective study. Arch Dermatol 2010;146:1364-9.
7. Kirby B, McAleer MA, Mason DL, Fortune DG, Main CJ, Griffith CE. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol 2008; 158:138-40.
8. McAleer MA, Mason DL, Cunningham S, O'Shea SJ, McCormick PA, Stone C, Collins P, Rogers S, Kirby B. Alcohol misuse in patients with psoriasis: identification and relationship to disease severity and psychological distress Br J Dermatol 2011; 164:256-126
9. Tobin AM, Higgins EM, Norris S, Kirby B. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol 2009;34:698- 70.
10. Macfarlane PW, Murray H, Sattar N, Stott DJ, Ford I, BuckleyB, Jukema JW, Westendorp RG, Shepherd J. The incidence and risk factors for new onset atrial fibrillation in the PROSPER study. Europace. 2011;13:634- 9.
11. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671.
12. Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for atrial fibrillation: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57:427-36.
13. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. Eur J Cardiovasc Prev Rehabil. 2010;17:706-12.
Correspondence: Department of Dermatology, St Vincent's University Hospital, Elm Park, Dublin 4, Ireland Phone: 00353-1-2214189 Fax: 00353-1-2213717 E-mail: K.Adamzik@st-vincents.ie
Conflict of Interest:
None declared