-
PDF
- Split View
-
Views
-
Cite
Cite
Authors/Task Force Members, Lars Rydén, Peter J. Grant, Stefan D. Anker, Christian Berne, Francesco Cosentino, Nicolas Danchin, Christi Deaton, Javier Escaned, Hans-Peter Hammes, Heikki Huikuri, Michel Marre, Nikolaus Marx, Linda Mellbin, Jan Ostergren, Carlo Patrono, Petar Seferovic, Miguel Sousa Uva, Marja-Riita Taskinen, Michal Tendera, Jaakko Tuomilehto, Paul Valensi, Jose Luis Zamorano, ESC Committee for Practice Guidelines (CPG), Jose Luis Zamorano, Stephan Achenbach, Helmut Baumgartner, Jeroen J. Bax, Héctor Bueno, Veronica Dean, Christi Deaton, Çetin Erol, Robert Fagard, Roberto Ferrari, David Hasdai, Arno W. Hoes, Paulus Kirchhof, Juhani Knuuti, Philippe Kolh, Patrizio Lancellotti, Ales Linhart, Petros Nihoyannopoulos, Massimo F. Piepoli, Piotr Ponikowski, Per Anton Sirnes, Juan Luis Tamargo, Michal Tendera, Adam Torbicki, William Wijns, Stephan Windecker, Document Reviewers, Guy De Backer, Per Anton Sirnes, Eduardo Alegria Ezquerra, Angelo Avogaro, Lina Badimon, Elena Baranova, Helmut Baumgartner, John Betteridge, Antonio Ceriello, Robert Fagard, Christian Funck-Brentano, Dietrich C. Gulba, David Hasdai, Arno W. Hoes, John K. Kjekshus, Juhani Knuuti, Philippe Kolh, Eli Lev, Christian Mueller, Ludwig Neyses, Peter M. Nilsson, Joep Perk, Piotr Ponikowski, Željko Reiner, Naveed Sattar, Volker Schächinger, André Scheen, Henrik Schirmer, Anna Strömberg, Svetlana Sudzhaeva, Juan Luis Tamargo, Margus Viigimaa, Charalambos Vlachopoulos, Robert G. Xuereb, ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD)., European Heart Journal, Volume 34, Issue 39, 14 October 2013, Pages 3035–3087, https://doi.org/10.1093/eurheartj/eht108
Close - Share Icon Share
Abbreviations and acronyms
- 2hPG
2-hour post-load plasma glucose
- ABI
ankle–brachial index
- ACCOMPLISH
Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACE-I
angiotensin converting enzyme inhibitor
- ACS
acute coronary syndrome
- ACTIVE
Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events
- ACTIVE A
Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events Aspirin
- ACTIVE W
Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events Warfarin
- ADA
American Diabetes Association
- ADDITION
Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care
- ADP
adenosine diphosphate
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- AF
atrial fibrillation
- AGEs
advanced glycation end-products
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes
- ALTITUDE
Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints
- Apo
apolipoprotein
- ARB
angiotensin receptor blocker
- ARIC
Atherosclerosis Risk In Communities
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- ASCOT
Anglo-Scandinavian Cardiac Outcomes Trial
- ATLAS
Assessment of Treatment with Lisinopril And Survival
- AVERROES
Apixaban VERsus acetylsalicylic acid to pRevent strOkES
- AWESOME
Angina With Extremely Serious Operative Mortality Evaluation
- BARI 2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- BEST
BEta blocker STroke trial
- BMS
bare-metal stent
- BP
blood pressure
- CABG
coronary artery bypass graft surgery
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CAN
cardiac autonomic neuropathy
- CAPRIE
Clopidogrel vs. Aspirin in Patients at Risk of Ischaemic Events
- CARDia
Coronary Artery Revascularization in Diabetes
- CARDS
Collaborative Atorvastatin Diabetes Study
- CETP
cholesterylester transfer protein
- CHA2DS2-VASc
cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)-vascular disease, age 65–74 and sex category (female)
- CHADS2
cardiac failure, hypertension, age, diabetes, stroke (doubled)
- CHARISMA
Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance
- CHARM
Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity
- CI
confidence interval
- CIBIS
Cardiac Insufficiency Bisoprolol Study
- CLI
critical limb ischaemia
- COMET
Carvedilol Or Metoprolol European Trial
- COPERNICUS
Carvedilol Prospective Randomized Cumulative Survival
- COX-1 and 2
cyclo-oxygenase 1 and 2
- CTT
Cholesterol Treatment Trialists
- CVD
cardiovascular disease
- DCCT
Diabetes Control and Complications Trial
- DECODE
Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe
- DES
drug-eluting stent
- DETECT-2
The Evaluation of Screening and Early Detection Strategies for T2DM and IGT
- DIABHYCAR
Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events and Ramipril
- DIAMOND
Danish Investigations and Arrhythmia ON Dofetilide
- DIG
Digitalis Investigation Group
- DIGAMI
Diabetes and Insulin–Glucose Infusion in Acute Myocardial Infarction
- DIRECT
DIabetic REtinopathy Candesartan Trials
- DM
diabetes mellitus
- DPP-4
dipeptidylpeptidase-4
- ECG
electrocardiogram
- EDIC
Epidemiology of Diabetes Interventions and Complications
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cells
- ERFC
Emerging Risk Factor Collaboration
- EUROASPIRE
European Action on Secondary Prevention through Intervention to Reduce Events
- EUROPA
EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery disease
- FDA
Food and Drug Administration
- FFA
free fatty acid
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- FINDRISC
FINnish Diabetes RIsk SCore
- FPG
fasting plasma glucose
- FREEDOM
Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease
- GFR
glomerular filtration rate
- GIK
glucose-insulin-potassium
- GLP-1
glucagon-like peptide-1
- GLUT-4
glucose transporter 4
- HAS-BLED
Hypertension, Abnormal renal/liver function (1 point each), Stroke, Bleeding history or predisposition, Labile INR, Elderly (>65), Drugs/alcohol concomitantly (1 point each)
- HbA1c
glycated haemoglobin A1C
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- HI-5
Hyperglycaemia: Intensive Insulin Infusion in Infarction
- HOMA-IR
Homeostasis Model Assessment of Insulin Resistance
- HOPE
Heart Outcomes Prevention Evaluation
- HOT
Hypertension Optimal Treatment
- HPS
Heart Protection Study
- HPS-2-THRIVE
Heart Protection Study 2 Treatment of HDL to Reduce the Incidence of Vascular Events
- HR
hazard ratio
- HSP
hexosamine pathway
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- IMMEDIATE
Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency Care
- IMPROVE-IT
IMProved Reduction of Outcomes: Vytorin Efficacy International Trial
- INR
international normalized ratio
- IR
insulin resistance
- IRS-1
insulin receptor substrate-1
- ISAR-REACT
Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment
- ITA
internal thoracic artery
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein cholesterol
- LEAD
lower extremity artery disease
- Lp a
lipoprotein a
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MACCE
major adverse cardiac and cerebrovascular events
- MAIN COMPARE
Revascularization for unprotected left main coronary artery stenosis: comparison of percutaneous
- MERIT-HF
Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure
- MetS
metabolic syndrome
- MI
myocardial infarction
- MRA
mineralocorticoid receptor antagonists
- N-ER
niacin
- NAPDH
nicotinamide adenine dinucleotide phosphate hydrogen
- NDR
National Diabetes Register
- NHANES
National Health and Nutrition Examination Survey
- NICE
National Institute for Health and Clinical Excellence (UK)
- NNT
number needed to treat
- NO
nitric oxide
- NOAC
new oral anticoagulants
- NYHA
New York Heart Association
- OAT
Occluded Artery Trial
- OGTT
oral glucose tolerance test
- OMT
optimal medical treatment
- ONTARGET
ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial
- OR
odds ratio
- ORIGIN
Outcome Reduction with an Initial Glargine Intervention trial
- PAD
peripheral artery disease
- PAI-1
plasminogen activator inhibitor-1
- PCI
percutaneous coronary intervention
- PG
plasma glucose
- PI3K
phosphatidylinositol 3-kinases
- PKC
protein kinase C
- PLATO
PLATelet inhibition and patient Outcomes trial
- PPARα
peroxisome proliferator-activated receptor alpha
- PPARγ
peroxisome proliferator-activated receptor gamma
- PREDIMED
Primary Prevention of Cardiovascular Disease with a Mediterranean Diet
- PROActive
PROspective pioglitAzone Clinical Trial In macroVascular Events
- PROCAM
Prospective Cardiovascular Münster
- RAAS
renin-angiotensin-aldosterone system
- RAGE
receptor for advanced glycation end products
- RCT
randomized controlled trial
- RE-LY
Randomized Evaluation of the Long-term anticoagulant therapy with dabigatran etexilate
- REGICOR
Myocardial Infarction Population Registry of Girona
- RESOLVE
Safety and Efficacy of Ranibizumab in Diabetic Macular Edema Study
- RESTORE
Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema
- RIDE
Ranibizumab Injection in Subjects With Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus
- RISE
Ranibizumab Injection in Subjects With Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus
- ROCKET
Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition, compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation
- ROS
reactive oxygen species
- RRR
relative risk reduction
- SCORE®
The European Systematic Coronary Risk Evaluation
- SGLT2
sodium–glucose co-transporter-2
- SHARP
Study of Heart and Renal Protection
- SMI
silent myocardial ischaemia
- SR-B
scavenger receptor B
- SOLVD
Studies Of Left Ventricular Dysfunction
- STEMI
ST-elFevation myocardial infarction
- SYNTAX
SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TACTICS-TIMI 18
Treat angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis In Myocardial Infarction
- TG
triglyceride
- TIA
transient ischaemic attack
- tPA
tissue plasminogen activator
- TRL
triglyceride-rich lipoprotein
- UKPDS
United Kingdom Prospective Diabetes Study
- VADT
Veterans Administration Diabetes Trial
- VEGF
vascular endothelial growth factor
- VKA
vitamin K antagonist
- VLDL
very low-density lipoprotein
- WHO
World Health Organization
1. Preamble
This is the second iteration of the European Society of Cardiology (ESC) and European Association for the Study of Diabetes (EASD) joining forces to write guidelines on the management of diabetes mellitus (DM), pre-diabetes, and cardiovascular disease (CVD), designed to assist clinicians and other healthcare workers to make evidence-based management decisions. The growing awareness of the strong biological relationship between DM and CVD rightly prompted these two large organizations to collaborate to generate guidelines relevant to their joint interests, the first of which were published in 2007. Some assert that too many guidelines are being produced but, in this burgeoning field, five years in the development of both basic and clinical science is a long time and major trials have reported in this period, making it necessary to update the previous Guidelines.
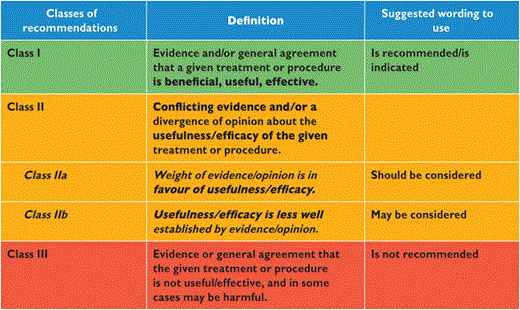
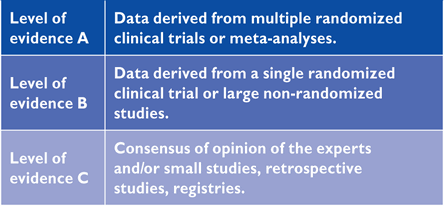
The processes involved in generating these Guidelines have been previously described and can be found at http://www.escardio.org/guidelines-surveys/esc-guidelines/about/Pages/rules-writing.aspx. In brief, the EASD and the ESC appointed Chairs to represent each organization and to direct the activities of the Task Force. Its members were chosen for their particular areas of expertise relevant to different aspects of the guidelines, for their standing in the field, and to represent the diversity that characterizes modern Europe. Each member agreed to produce—and regularly update—conflicts of interest, the details of which are held at the European Heart House and available at the following web address: http://www.escardio.org/guidelines. Members of the Task Force generally prepared their contributions in pairs and the ESC recommendations for the development of guidelines were followed, using the standard classes of recommendation, shown below, to provide consistency to the committee's recommendations (Tables 1 and 2).
Initial editing and review of the manuscripts took place at the Task Force meetings, with systematic review and comments provided by the ESC Committee for Practice Guidelines and the EASD Panel for Overseeing Guidelines and Statements.
These Guidelines are the product of countless hours of hard work, time given freely and without complaint by the Task Force members, administrative staff and by the referees and supervisory committees of the two organizations. It is our hope that this huge effort has generated guidelines that will provide a greater understanding of the relationship between these two complex conditions and an accessible and useful adjunct to the clinical decision-making process that will help to provide further clarity and improvements in management.
The task of developing Guidelines covers not only the integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations.
To implement the Guidelines, condensed pocket guidelines, summary slides, booklets with essential messages and an electronic version for digital applications (smartphones, etc.) are produced. These versions are abridged; thus, if needed, one should always refer to the full text version, which is freely available on the ESC website.
2. Introduction
The increasing prevalence of DM worldwide has led to a situation where approximately 360 million people had DM in 2011, of whom more than 95% would have had type 2 DM (T2DM). This number is estimated to increase to 552 million by 2030 and it is thought that about half of those will be unaware of their diagnosis. In addition, it is estimated that another 300 million individuals had features indicating future risk of developing T2DM, including fasting hyperglycaemia, impaired glucose tolerance (IGT), gestational DM and euglycaemic insulin resistance (IR).1 The majority of new cases of T2DM occur in the context of westernized lifestyles, high-fat diets and decreased exercise, leading to increasing levels of obesity, IR, compensatory hyperinsulinaemia and, ultimately, beta-cell failure and T2DM. The clustering of vascular risk seen in association with IR, often referred to as the metabolic syndrome, has led to the view that cardiovascular risk appears early, prior to the development of T2DM, whilst the strong relationship between hyperglycaemia and microvascular disease (retinopathy, nephropathy, neuropathy) indicates that this risk is not apparent until frank hyperglycaemia appears. These concepts highlight the progressive nature of both T2DM and associated cardiovascular risk, which pose specific challenges at different stages of the life of an individual with DM. The effects of advancing age, co-morbidities and problems associated with specific groups all indicate the need to manage risk in an individualized manner, empowering the patient to take a major role in the management of his or her condition.
As the world in general—and Europe in particular—changes in response to demographic and cultural shifts in societies, so the patterns of disease and their implications vary. The Middle East, the Asia–Pacific rim and parts of both North and South America have experienced massive increases in the prevalence of DM over the past 20 years, changes mirrored in European populations over the same period. Awareness of specific issues associated with gender and race and, particularly, the effects of DM in women—including epigenetics and in utero influences on non-communicable diseases—are becoming of major importance. In 2011 approximately 60 million adult Europeans were thought to have DM, half of them diagnosed, and the effects of this condition on the cardiovascular health of the individual and their offspring provide further public health challenges that agencies are attempting to address worldwide.
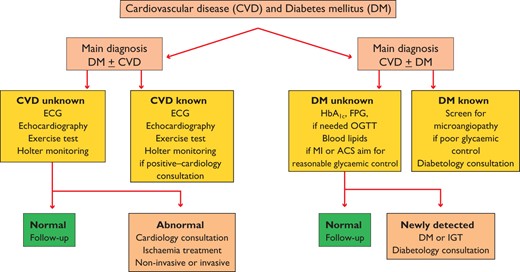
DM and CVD develop in concert with metabolic abnormalities mirroring and causing changes in the vasculature. More than half the mortality and a vast amount of morbidity in people with DM is related to CVD, which caused physicians in the fields of DM and cardiovascular medicine to join forces to research and manage these conditions (Figure 1). At the same time, this has encouraged organizations such as the ESC and EASD to work together and these guidelines are a reflection of that powerful collaboration.
Investigational algorithm outlining the principles for the diagnosis and management of cardiovascular disease (CVD) in diabetes mellitus (DM) patients with a primary diagnosis of DM or a primary diagnosis of CVD. The recommended investigations should be considered according to individual needs and clinical judgement and are not meant as a general recommendation to be undertaken by all patients.
The emphasis in these Guidelines is to provide information on the current state of the art in how to prevent and manage the diverse problems associated with the effects of DM on the heart and vasculature in a holistic manner. In describing the mechanisms of disease, we hope to provide an educational tool and, in describing the latest management approaches, an algorithm for achieving the best care for patients in an individualized setting. It should be noted that these guidelines are written for the management of the combination of CVD (or risk of CVD) and DM, not as a separate guideline for each condition. This is important considering that those who, in their daily practice, manage these patients frequently have their main expertise in either DM or CVD or general practice. If there is a demand for a more intricate analysis of specific issues discussed in the present Guidelines, further information may be derived from detailed guidelines issued by various professional organizations such as ESC, the European Atherosclerosis Society and EASD, e.g. on acute coronary care, coronary interventions, hyperlipidaemia or glucose lowering therapy, to mention only a few.
It has been a privilege for the Chairs to have been trusted with the opportunity to develop these guidelines whilst working with some of the most widely acknowledged experts in this field. We want to extend our thanks to all members of the Task Force who gave so much of their time and knowledge, to the referees who contributed a great deal to the final manuscript, and to members of the ESC and EASD committees that oversaw this project. Finally, we express our thanks to the guidelines team at the European Heart House, in particular Catherine Després, Veronica Dean and Nathalie Cameron, for their support in making this process run smoothly.
Stockholm and Leeds, April 2014
Lars Ryden Peter Grant
3. Abnormalities of glucose metabolism and cardiovascular disease
3.1 Definition, classification and diagnosis
DM is a condition defined by an elevated level of blood glucose. The classification of DM is based on recommendations from the World Health Organization (WHO) and the American Diabetes Association (ADA).2–6 Glycated haemoglobin A1c (HbA1c) has been recommended as a diagnostic test for DM,7,8 but there remain concerns regarding its sensitivity in predicting DM and HbA1c values <6.5% do not exclude DM that may be detected by blood glucose measurement,7–10 as further discussed in Section 3.3. Four main aetiological categories of DM have been identified: type 1 diabetes (T1DM), T2DM, ‘other specific types’ of DM and ‘gestational DM’ (Table 3).2
Comparison of 2006 World Health Organization (WHO) and 2003/2011 and 2012 American Diabetes Association (ADA) diagnostic criteria
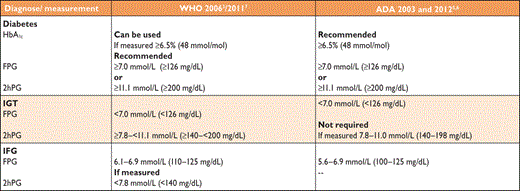 |
 |
FPG = fasting plasma glucose; IGT = impaired glucose tolerance; IFG = impaired fasting glucose; 2hPG = 2-h post-load plasma glucose.
Comparison of 2006 World Health Organization (WHO) and 2003/2011 and 2012 American Diabetes Association (ADA) diagnostic criteria
 |
 |
FPG = fasting plasma glucose; IGT = impaired glucose tolerance; IFG = impaired fasting glucose; 2hPG = 2-h post-load plasma glucose.
Type 1 diabetes is characterized by deficiency of insulin due to destruction of pancreatic beta-cells, progressing to absolute insulin deficiency. Typically, T1DM occurs in young, slim individuals presenting with polyuria, thirst and weight loss, with a propensity to ketosis. However, T1DM may occur at any age,11 sometimes with slow progression. In the latter condition, latent auto-immune DM in adults (LADA), insulin dependence develops over a few years. People who have auto-antibodies to pancreatic beta-cell proteins, such as glutamic-acid-decarboxylase, protein tyrosine phosphatase, insulin or zinc transporter protein, are likely to develop either acute-onset or slowly progressive insulin dependence.12,13 Auto-antibodies targeting pancreatic beta-cells are a marker of T1DM, although they are not detectable in all patients and decrease with age, compared with other ethnicities and geographic regions, T1DM is more common in Caucasian individuals.14
Type 2 diabetes is characterized by a combination of IR and beta-cell failure, in association with obesity (typically with an abdominal distribution) and sedentary lifestyle—major risk factors for T2DM. Insulin resistance and an impaired first-phase insulin secretion causing post-prandial hyperglycaemia characterize the early stage of T2DM. This is followed by a deteriorating second-phase insulin response and persistent hyperglycaemia in the fasting state.15,16 T2DM typically develops after middle age and comprises over 90% of adults with DM. However, with increasing obesity in the young and in non-Europid populations, there is a trend towards a decreasing age of onset.
Gestational diabetes develops during pregnancy. After delivery, most return to a euglycaemic state, but they are at increased risk for overt T2DM in the future. A meta-analysis reported that subsequent progression to DM is considerably increased after gestational DM.17 A large Canadian study found that the probability of DM developing after gestational DM was 4% at 9 months and 19% at 9 years after delivery.18
Other specific types of diabetes include: (i) single genetic mutations that lead to rare forms of DM such as maturity-onset DM of the young; (ii) DM secondary to other pathological conditions or diseases (pancreatitis, trauma or surgery of the pancreas) and (iii) drug- or chemically induced DM.
Disorders of glucose metabolism, impaired fasting glucose (IFG) and IGT, often referred to as ‘pre-diabetes’, reflect the natural history of progression from normoglycaemia to T2DM. It is common for such individuals to oscillate between different glycaemic states, as can be expected when the continuous variable PG is dichotomized. IGT can only be recognized by the results of an oral glucose tolerance test (OGTT): 2-hour post-load plasma glucose (2hPG) ≥7.8 and <11.1 mmol/L (≥140 and <200 mg/dL). A standardized OGTT is performed in the morning after an overnight fast (8–14 h). One blood sample should be taken before and one 120 min after intake, over 5 min, of 75 g glucose dissolved in 250–300 mL water (note that the timing of the test begins when the patient starts to drink).
Current clinical criteria issued by the World Health organization and American Diabetes Association.3,8 The WHO criteria are based on fasting plasma glucose (FPG) and 2hPG concentrations. They recommend use of an OGTT in the absence of overt hyperglycaemia.3 The ADA criteria encourage the use of HbA1c, fasting glycaemia and OGTT, in that order.8 The argument for FPG or HbA1c over 2hPG is primarily related to feasibility. The advantages and disadvantages of using glucose testing and HbA1c testing are summarized in a WHO report from 2011,7 and are still the subject of some debate (see Section 3.3). The diagnostic criteria adopted by WHO and ADA (Table 3) for the intermediate levels of hyperglycaemia are similar for IGT but differ for IFG. The ADA lower threshold for IFG is 5.6 mmol/L (101 mg/dL),8 while WHO recommends the original cut-off point of 6.1 mmol/L (110 mg/dL).3
To standardize glucose determinations, venous plasma measures have been recommended.3,8 Measurements based on venous whole blood tend to give results 0.5 mmol/L (9 mg/dL) lower than plasma values. Since capillary blood is often used for point-of-care testing, it is important to underline that capillary values may differ from plasma values more in the post-load than in the fasting state. Therefore, a recent comparative study suggests that the cut-off points for DM, IFG and IGT differ when venous blood and capillary blood are used as outlined in Table 4.19
Cut-points for diagnosing DM, impaired glucose tolerance, and impaired fasting glucose based on other blood specimens than the recommended standard, venous plasma
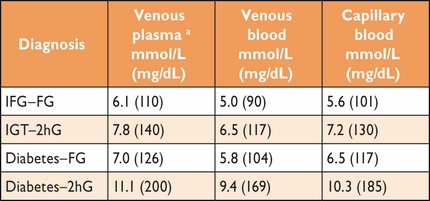 |
 |
FPG = fasting plasma glucose; FG = Fasting Glucose; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; 2hG = 2-h post-load glucose; 2hPG = 2-h post-load plasma glucose.
aStandard.
Cut-points for diagnosing DM, impaired glucose tolerance, and impaired fasting glucose based on other blood specimens than the recommended standard, venous plasma
 |
 |
FPG = fasting plasma glucose; FG = Fasting Glucose; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; 2hG = 2-h post-load glucose; 2hPG = 2-h post-load plasma glucose.
aStandard.
Classification depends on whether only FPG is measured or if it is combined with 2hPG. An individual with IFG in the fasting state may have IGT or even DM if investigated with an OGTT. A normal FPG reflects an ability to maintain adequate basal insulin secretion, in combination with hepatic insulin sensitivity sufficient to control hepatic glucose output. A post-load glucose level within the normal range requires an appropriate insulin secretory response and adequate insulin sensitivity in peripheral tissues. It is important to pay attention to the analytical method when interpreting samples. This applies to both glucose and HbA1c determinations.
3.2 Epidemiology
The International Diabetes Federation's global estimates for 2011 (Table 5) suggest that 52 million Europeans aged 20–79 years have DM and that this number will increase to over 64 million by 2030.1 In 2011, 63 million Europeans had IGT. A total of 281 million men and 317 million women worldwide died with DM in 2011, most from CVD. The healthcare expenditure for DM in Europe was about 75 billion Euros in 2011 and is projected to increase to 90 billion by 2030.
Burden of DM in Europe in 2011 and predictions for 20301
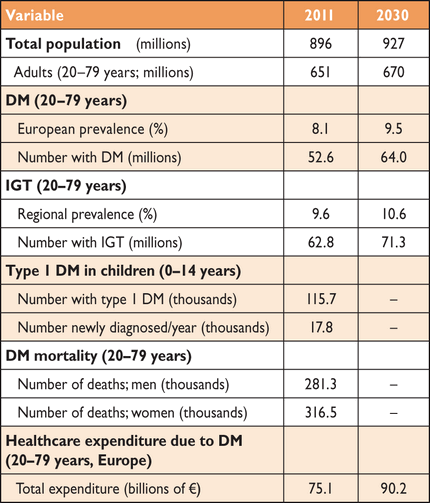 |
 |
DM = diabetes mellitus; IGT = impaired glucose tolerance.
Burden of DM in Europe in 2011 and predictions for 20301
 |
 |
DM = diabetes mellitus; IGT = impaired glucose tolerance.
A problem when diagnosing T2DM is the lack of a unique biological marker—besides post-prandial plasma glucose (PG)—that would separate IFG, IGT, or T2DM from normal glucose metabolism. T2DM develops following a prolonged period of euglycaemic IR, which progresses with the development of beta-cell failure to frank DM with increased risk of vascular complications. The present definition of DM is based on the level of glucose at which retinopathy occurs, but macrovascular complications such as coronary, cerebrovascular and peripheral artery disease (PAD) appear earlier and, using current glycaemic criteria, are often present at the time when T2DM is diagnosed. Over 60% of people with T2DM develop CVD, a more severe and costly complication than retinopathy. Thus, CVD risk should be given a higher priority when cut-points for hyperglycaemia are defined and should be re-evaluated based on the CVD risk.
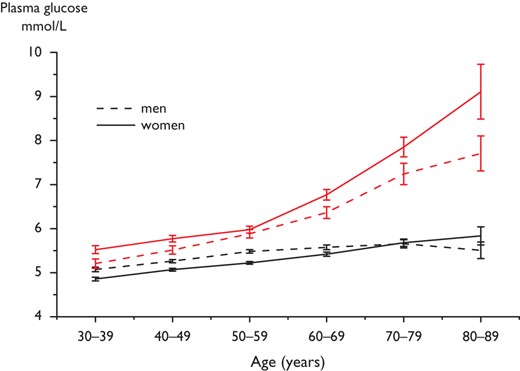
The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe (DECODE) study (Figure 2) reported data on disorders of glucose metabolism in European populations.20 The limited data on HbA1c in these populations indicate major discrepancies, compared with results from an OGTT,21 although this was not confirmed in the Evaluation of Screening and Early Detection Strategies for T2DM and IGT (DETECT-2) as further elaborated upon in Section 3.3.22 In Europeans, the prevalence of DM rises with age in both genders. Thus <10% of people below 60 years, 10–20% between 60 and 69 years and 15–20% above 70 years have previously known DM and in addition similar proportions have screen-detected asymptomatic DM.20 This means that the lifetime risk for DM is 30–40% in European populations. Similarly, the prevalence of IGT increases linearly from about 15% in middle aged to 35–40% in elderly Europeans. Even HbA1c increases with age in both genders.23
Mean FPG fasting (two lower lines) and 2hPG (two upper lines) concentrations (95% confidence intervals shown by vertical bars) in 13 European population-based cohorts included in the DECODE study.20 Mean 2hPG increases particularly after the age of 50 years. Women have significantly higher mean 2hPG concentrations than men, a difference that becomes more pronounced above the age of 70 years. Mean FPG increases only slightly with age. FPG = fasting plasma glucose; 2hPG = 2-h post-load plasma glucose.
3.3 Screening for disorders of glucose metabolism
Type 2 diabetes mellitus does not cause specific symptoms for many years, which explains why approximately half of the cases of T2DM remain undiagnosed at any time.20,23 Population testing of blood glucose to determine CV risk is not recommended, due to the lack of affirmative evidence that the prognosis of CVD related to T2DM can be improved by early detection and treatment.24,25 Screening of hyperglycaemia for CV risk purposes should therefore be targeted to high-risk individuals. The Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (ADDITION) study provided evidence that the risk of CVD events is low in screen-detected people with T2DM. Screening may, however, facilitate CV risk reduction and early detection may benefit progression of microvascular disease, which may make screening for T2DM beneficial.26 In addition, there is an interest in identifying people with IGT, since most will progress to T2DM and this progression can be retarded by lifestyle interventions.27–31 The diagnosis of DM has traditionally been based on the level of blood glucose that relates to a risk of developing micro- rather than macrovascular disease. The DETECT-2 study analysed results from 44 000 persons in nine studies across five countries.22 It was concluded that a HbA1c of >6.5% (48 mmol/L) and an FPG of >6.5 mmol/L (117 mg/dL) together gave a better discrimination in relation to the view—adopted by the ADA6 and WHO7—that, for general population, screening an HbA1c >6.5% is diagnostic of DM, but between 6.0–6.5%, an FPG needs to be measured to establish a diagnosis. Caveats exist in relation to this position, as extensively reviewed by Hare et al.32 Problems exist in relation to pregnancy, polycystic ovary syndrome,33 haemoglobinopathies and acute illness mitigating against its use under such circumstances. Moreover, the probability of a false negative test result, compared with the OGTT, is substantial when attempting to detect DM by measuring only FPG and/or HbA1c in an Asian population.34 A study in Spanish people with high risk, i.e. >12/26 points in the FINnish Diabetes RIsk SCore (FINDRISC) study, revealed that 8.6% had undiagnosed T2DM by the OGTT, whilst only 1.4% had an HbA1c >6.5%, indicating a further need to evaluate the use of HbA1c as the primary diagnostic test in specific populations.9 There remains controversy regarding the approach of using HbA1c for detecting undiagnosed DM in the setting of coronary heart disease and CV risk management,7–10,32 although advocates argue that HbA1c in the range 6.0–6.5% requires lifestyle advice and individual risk factor management alone, and that further information on 2hPG does not alter such management.
The approaches for early detection of T2DM and other disorders of glucose metabolism are: (i) measuring PG or HbA1c to explicitly determine prevalent T2DM and impaired glucose regulation; (ii) using demographic and clinical characteristics and previous laboratory tests to determine the likelihood for T2DM and (iii) collecting questionnaire-based information that provides information on the presence of aetiological risk factors for T2DM. The last two approaches leave the current glycaemic state ambiguous and glycaemia testing is necessary in all three approaches, to accurately define whether T2DM and other disorders of glucose metabolism exist. However, the results from such a simple first-level screening can markedly reduce the numbers who need to be referred for further testing of glycaemia and other CVD risk factors. Option two is particularly suited to those with pre-existing CVD and women with previous gestational DM, while the third option is better suited to the general population and also for overweight/obese people.
Several DM risk scores for DM have been developed. Most perform well and it does not matter which one is used, as underlined by a recent systematic review.35 The FINnish Diabetes RIsk SCore (www.diabetes.fi/english) is the most commonly used to screen for DM risk in Europe (Figure 3).
FINnish Diabetes RIsk SCore (FINDRISC) to assess the 10-year risk of type 2 diabetes in adults. (Modified from Lindstrom et al.36 available at: www.diabetes.fi/english).
This tool, available in almost all European languages, predicts the 10-year risk of T2DM—including asymptomatic DM and IGT—with 85% accuracy.36,37 It has been validated in most European populations. It is necessary to separate individuals into three different scenarios: (i) the general population; (ii) people with assumed abnormalities (e.g. obese, hypertensive, or with a family history of DM) and (iii) patients with prevalent CVD. In the general population and people with assumed abnormalities, the appropriate screening strategy is to start with a DM risk score and to investigate individuals with a high value with an OGTT or a combination of HbA1c and FPG.36,37 In CVD patients, no diabetes risk score is needed but an OGTT is indicated if HbA1c and/or FPG are inconclusive, since people belonging to these groups may often have DM revealed only by an elevated 2hPG.38–41
3.4 Disorders of glucose metabolism and cardiovascular disease
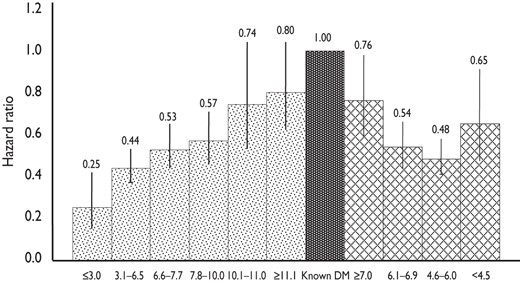
Both undiagnosed T2DM and other disorders of glucose metabolism are risk factors for CVD. The most convincing evidence for such relationship was provided by the collaborative DECODE study, analysing several European cohort studies with baseline OGTT data.42–44 Increased mortality was observed in people with DM and IGT, identified by 2hPG, but not in people with IFG. A high 2hPG predicted all-cause and CVD mortality after adjustment for other major cardiovascular risk factors, while a high FPG alone was not predictive once 2hPG was taken into account. The highest excess CVD mortality in the population was observed in people with IGT, especially those with normal FPG.44 The relationship between 2hPG and mortality was linear, but this relationship was not observed with FPG (Figure 4).
Hazard ratios and 95% confidence intervals (vertical bars) for CVD mortality for FPG (hatched bars) and 2hPG (dotted bars) intervals using previously diagnosed DM (dark bar) as the common reference category. Data are adjusted for age, sex, cohort, body mass index, systolic blood pressure, total cholesterol, and smoking. (Adapted from refs.42,43).
Several studies have shown that increasing HbA1c is associated with increasing CVD risk.45–47 Studies that compared all three glycaemic parameters—FPG, 2hPG and HbA1c —simultaneously for mortality and CVD risk revealed that the association is strongest for 2hPG and that the risk observed with FPG and HbA1c is no longer significant after controlling for the effect of 2hPG.48,49
Women with newly diagnosed T2DM have a higher relative risk for CVD mortality than their male counterparts.20,50–52 A review on the impact of gender on the occurrence of coronary artery disease (CAD) mortality reported that the overall relative risk (the ratio of risk in women to risk in men) was 1.46 (95% CI 1.21–1.95) in people with DM and 2.29 (95% CI 2.05–2.55) in those without, suggesting that the well-known gender differential in CAD is reduced in DM.53 A meta-analysis of 37 prospective cohort studies (n = 447 064 DM patients) aimed at estimating sex-related risk of fatal CAD, reported higher mortality in patients with DM than those without (5.4 vs. 1.6%, respectively).54 The relative risk, or hazard ratio (HR), among people with and without DM was significantly greater among women (HR 3.50; 95% CI 2.70–4.53) than in men (HR 2.06; 95% CI 1.81–2.34). Thus the gender difference in CVD risk seen in the general population is much smaller in people with DM and the reason for this is still unclear. A recent British study revealed a greater adverse influence of DM per se on adiposity, Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and downstream blood pressure, lipids, endothelial dysfunction and systemic inflammation in women, compared with men, which may contribute to their greater relative risk of CAD.55 Also, it seems that, compared with men, women have to put on more weight—and therefore undergo bigger changes in their risk factor status—to develop DM.56
3.5 Delaying conversion to type 2 diabetes mellitus
Unhealthy dietary habits and a sedentary lifestyle are of major importance in the development of T2DM.57,58 As reviewed in the European evidence-based guideline for the prevention of T2DM,59 randomized clinical trials (RCTs) demonstrate that lifestyle modification, based on modest weight loss and increased physical activity, prevents or delays progression in high-risk individuals with IGT. Thus, those at high risk for T2DM and those with established IGT should be given appropriate lifestyle counselling (Table 6). A tool kit, including practical advice for healthcare personnel, has recently been developed.60 The seemingly lower risk reduction in the Indian and Chinese trials was due to the higher incidence of T2DM in these populations and the absolute risk reductions were strikingly similar between all trials: approximately 15–20 cases per 100 person-years. It was estimated that lifestyle intervention has to be provided to 6.4 high-risk individuals for an average of 3 years to prevent one case of DM. Thus the intervention is highly efficient.31 A 12-year follow-up of men with IGT who participated in the Malmö Feasibility Study61 revealed that all-cause mortality among men in the former lifestyle intervention group was lower (and similar to that in men with normal glucose tolerance) than that among men who had received ‘routine care’ (6.5 vs. 14.0 per 1000 person years; P = 0.009). Participants with IGT in the 6-year lifestyle intervention group in the Chinese Da Qing study had, 20 years later, a persistent reduction in the incidence of T2DM and a non-significant reduction of 17% in CVD death, compared with control participants.62 Moreover, the adjusted incidence of severe retinopathy was 47% lower in the intervention than in the control group, which was interpreted as being related to the reduced incidence of T2DM.63 During an extended 7-year follow-up of the Finnish DPS study,27 there was a marked and sustained reduction in the incidence of T2DM in people who had participated in the lifestyle intervention (for an average of 4 years). In the 10-year follow-up, total mortality and CVD incidence were not different between the intervention and control groups but the DPS participants, who had IGT at baseline, had lower all-cause mortality and CVD incidence, compared with a Finnish population-based cohort of people with IGT.64 During the 10-year overall follow-up of the US Diabetes Prevention Programme Outcomes Study, the incidence of T2DM in the original lifestyle intervention group remained lower than in the control group.65
Prevention of T2DM by lifestyle intervention – the evidence
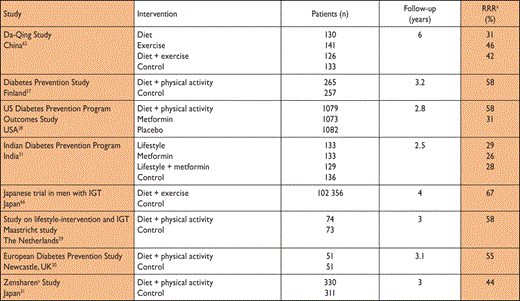 |
 |
IGT = impaired glucose tolerance; RRR = relative risk reduction; SLIM = Study on lifestyle-intervention and IGT Maastricht.
aAbsolute risk reduction numbers would have added value but could not be reported since such information is lacking in several of the studies.
bThe Zensharen study recruited people with IFG, while other studies recruited people with IGT.
Prevention of T2DM by lifestyle intervention – the evidence
 |
 |
IGT = impaired glucose tolerance; RRR = relative risk reduction; SLIM = Study on lifestyle-intervention and IGT Maastricht.
aAbsolute risk reduction numbers would have added value but could not be reported since such information is lacking in several of the studies.
bThe Zensharen study recruited people with IFG, while other studies recruited people with IGT.
3.6 Recommendations for diagnosis of disorders of glucose metabolism
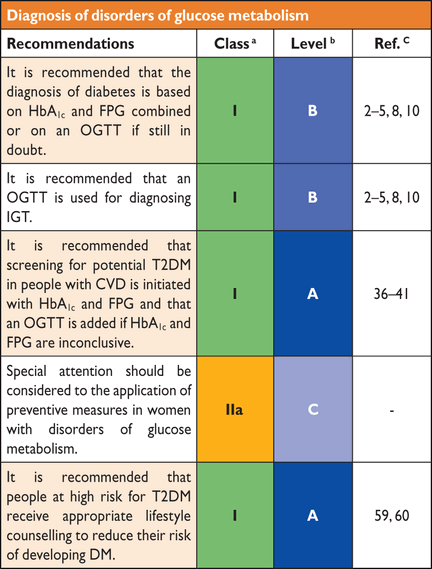 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; FPG = fasting plasma glucose; HbA1c = glycated haemoglobin A1c; IGT = impaired glucose tolerance; OGTT = oral glucose tolerance test; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
4. Molecular basis of cardiovascular disease in diabetes mellitus
4.1 The cardiovascular continuum in diabetes mellitus
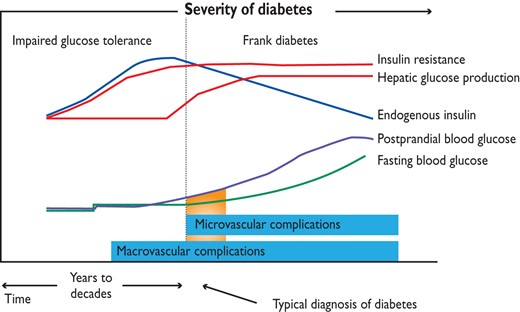
Type 2 diabetes mellitus is characterized by a state of long-standing IR, compensatory hyperinsulinaemia and varying degrees of elevated PG, associated with clustering of cardiovascular risk and the development of macrovascular disease prior to diagnosis (Figure 5). The early glucometabolic impairment is characterized by a progressive decrease in insulin sensitivity and increased glucose levels that remain below the threshold for a diagnosis of T2DM, a state known as IGT.
The pathophysiological mechanisms supporting the concept of a ‘glycaemic continuum’ across the spectrum of IFG, IGT, DM and CVD will be addressed in the following sections. The development
of CVD in people with IR is a progressive process, characterized by early endothelial dysfunction and vascular inflammation leading to monocyte recruitment, foam cell formation and subsequent development of fatty streaks. Over many years, this leads to atherosclerotic plaques, which, in the presence of enhanced inflammatory content, become unstable and rupture to promote occlusive thrombus formation. Atheroma from people with DM has more lipid, inflammatory changes and thrombus than those free from DM. These changes occur over a 20–30 year period and are mirrored by the molecular abnormalities seen in untreated IR and T2DM.
4.2 Pathophysiology of insulin resistance in type 2 diabetes mellitus
Insulin resistance has an important role in the pathophysiology of T2DM and CVD and both genetic and environmental factors facilitate its development. More than 90% of people with
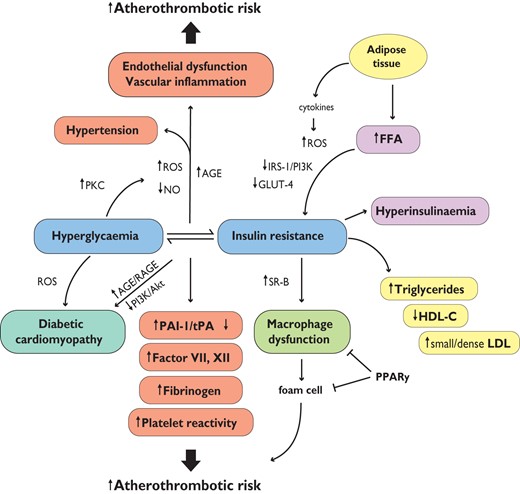
T2DM are obese,67 and the release of free fatty acids (FFAs) and cytokines from adipose tissue directly impairs insulin sensitivity (Figure 6). In skeletal muscle and adipose tissue, FFA-induced reactive oxygen species (ROS) production blunts activation of insulin receptor substrate 1 (IRS-1) and PI3K-Akt signalling, leading to down-regulation of insulin responsive glucose transporter 4 (GLUT-4).68,69
Hyperglycaemia, insulin resistance, and cardiovascular disease. AGE = advanced glycated end-products; FFA = free fatty acids; GLUT-4 = glucose transporter 4; HDL-C = high-density lipoprotein cholesterol; LDL = low-density lipoprotein particles; NO = nitric oxide; PAI-1 = plasminogen activator inhibitor-1; PKC = protein kinase C; PPARy = peroxisome proliferator-activated receptor y; PI3K = phosphatidylinositide 3-kinase; RAGE = AGE receptor; ROS = reactive oxygen species; SR-B = scavenger receptor B; tPA = tissue plasminogen activator.
4.3 Endothelial dysfunction, oxidative stress and vascular inflammation
FFA-induced impairment of the PI3K pathway blunts Akt activity and phosphorylation of endothelial nitric oxide synthase (eNOS) at Ser1177, resulting in decreased production of nitric oxide (NO), endothelial dysfunction,70 and vascular remodelling (increased intima-media thickness), important predictors of CVD (Figure 6).71,72. In turn, accumulation of ROS activates transcription factor NF-κB, leading to increased expression of inflammatory adhesion molecules and cytokines.69 Chronic IR stimulates pancreatic secretion of insulin, generating a complex phenotype that includes progressive beta cell dysfunction,68 decreased insulin levels and increased PG. Evidence supports the concept that hyperglycaemia further decreases endothelium-derived NO availability and affects vascular function via a number of mechanisms, mainly involving overproduction of ROS (Figure 6).73 The mitochondrial electron transport chain is one of the first targets of high glucose, with a direct net increase in superoxide anion (O2−) formation. A further increase in O2− production is driven by a vicious circle involving ROS-induced activation of protein kinase C (PKC).74 Activation of PKC by glucose leads to up-regulation of NADPH oxidase, mitochondrial adaptor p66Shc and COX-2 as well as thromboxane production and impaired NO release (Figure 6).75–77. Mitochondrial ROS, in turn, activate signalling cascades involved in the pathogenesis of cardiovascular complications, including polyol flux, advanced glycation end-products (AGEs) and their receptors (RAGEs), PKC and hexosamine pathway (HSP) (Figure 6). Recent evidence suggests that hyperglycaemia-induced ROS generation is involved in the persistence of vascular dysfunction despite normalization of glucose levels. This phenomenon has been called 'metabolic memory' and may explain why macro- and microvascular complications progress, despite intensive glycaemic control, in patients with DM. ROS-driven epigenetic changes are particularly involved in this process.74,78
4.4 Macrophage dysfunction
The increased accumulation of macrophages occurring in obese adipose tissue has emerged as a key process in metabolic inflammation and IR.79 In addition, the insulin-resistant macrophage increases expression of the oxidized low-density lipoprotein (LDL) scavenger receptor B (SR-B), promoting foam cell formation and atherosclerosis. These findings are reversed by peroxisome proliferator-activated receptor gamma (PPARγ) activation, which enhances macrophage insulin signalling (Figure 6). In this sense it seems that macrophage abnormalities provide a cellular link between DM and CVD by both enhancing IR and by contributing to the development of fatty streaks and vascular damage.
4.5 Atherogenic dyslipidaemia
Insulin resistance results in increased FFA release to the liver due to lipolysis. Therefore, enhanced hepatic very low-density lipoprotein (VLDL) production occurs due to increased substrate availability, decreased apolipoprotein B-100 (ApoB) degradation and increased lipogenesis. In T2DM and the metabolic syndrome, these changes lead to a lipid profile characterized by high triglycerides (TGs), low high-density lipoprotein cholesterol (HDL-C), increased remnant lipoproteins, apolipoprotein B (ApoB) synthesis and small, dense LDL particles (Figure 6).80 This LDL subtype plays an important role in atherogenesis being more prone to oxidation. On the other hand, recent evidence suggests that the protective role of HDL may be lost in T2DM patients due to alterations of the protein moiety, leading to a pro-oxidant, inflammatory phenotype.81 In patients with T2DM, atherogenic dyslipidaemia is an independent predictor of cardiovascular risk, stronger than isolated high triglycerides or a low HDL cholesterol.80
4.6 Coagulation and platelet function
In T2DM patients, IR and hyperglycaemia participate to the pathogenesis of a prothrombotic state characterized by increased plasminogen activator inhibitor-1(PAI-1), factor VII and XII, fibrinogen and reduced tissue plasminogen activator (tPA) levels (Figure 6).82 Among factors contributing to the increased risk of coronary events in DM, platelet hyper-reactivity is of major relevance.83 A number of mechanisms contribute to platelet dysfunction, affecting the adhesion and activation, as well as aggregation, phases of platelet-mediated thrombosis. Hyperglycaemia alters platelet Ca2+ homeostasis, leading to cytoskeleton abnormalities and increased secretion of pro-aggregant factors. Moreover, hyperglycaemia-induced up-regulation of glycoproteins (Ib and IIb/IIIa), P-selectin and enhanced P2Y12 signalling are key events underlying atherothrombotic risk in T1DM and T2DM (Figure 6).
4.7 Diabetic cardiomyopathy
In patients with T2DM, reduced IS predisposes to impaired myocardial structure and function and partially explains the exaggerated prevalence of heart failure in this population. Diabetic cardiomyopathy is a clinical condition diagnosed when ventricular dysfunction occurs in the absence of coronary atherosclerosis and hypertension. Patients with unexplained dilated cardiomyopathy were 75% more likely to have DM than age-matched controls.84 Insulin resistance impairs myocardial contractility via reduced Ca2+ influx through L-type Ca2+ channels and reverse mode Na2+/Ca2+ exchange. Impairment of phosphatidylinositol 3-kinases (PI3K)/Akt pathway subsequent to chronic hyperinsulinaemia is critically involved in cardiac dysfunction in T2DM.85
Together with IR, hyperglycaemia contributes to cardiac- and structural abnormalities via ROS accumulation, AGE/RAGE signalling and hexosamine flux.84,86 Activation of ROS-driven pathways affects the coronary circulation, leads to myocardial hypertrophy and fibrosis with ventricular stiffness and chamber dysfunction (Figure 6).86
4.8 The metabolic syndrome
The metabolic syndrome (MetS) is defined as a cluster of risk factors for CVD and T2DM, including raised blood pressure, dyslipidaemia (high triglycerides and low HDL cholesterol), elevated PG and central obesity. Although there is agreement that the MetS deserves attention, there has been an active debate concerning the terminology and diagnostic criteria related to its definition.87 However, the medical community agrees that the term ‘MetS’ is appropriate to represent the combination of multiple risk factors. Although MetS does not include established risk factors (i.e. age, gender, smoking) patients with MetS have a two-fold increase of CVD risk and a five-fold increase in development of T2DM.
4.9 Endothelial progenitor cells and vascular repair
Circulating cells derived from bone marrow have emerged as critical to endothelial repair. Endothelial progenitor cells (EPCs), a sub-population of adult stem cells, are involved in maintaining endothelial homeostasis and contribute to the formation of new blood vessels. Although the mechanisms whereby EPCs protect the cardiovascular system are unclear, evidence suggests that impaired function and reduced EPCs are features of T1DM and T2DM. Hence, these cells may become a potential therapeutic target for the management of vascular complications related to DM.88
4.10 Conclusions
Oxidative stress plays a major role in the development of micro- and macrovascular complications. Accumulation of free radicals in the vasculature of patients with DM is responsible for the activation of detrimental biochemical pathways, leading to vascular inflammation and ROS generation. Since the cardiovascular risk burden is not eradicated by intensive glycaemic control associated with optimal multifactorial treatment, mechanism-based therapeutic strategies are needed. Specifically, inhibition of key enzymes involved in hyperglycaemia-induced vascular damage, or activation of pathways improving insulin sensitivity, may represent promising approaches.
5. Cardiovascular risk assessment in patients with dysglycaemia
The aim of risk assessment is to categorize the population into those at low, moderate, high and very-high CVD risk, to intensify preventive approaches in the individual. The 2012 Joint European Society guidelines on CVD prevention recommended that patients with DM, and at least one other CV risk factor or target organ damage, should be considered to be at very high risk and all other patients with DM to be at high risk.89 Developing generally applicable risk scores is difficult, because of confounders associated with ethnicity, cultural differences, metabolic and inflammatory markers—and, importantly, CAD and stroke scores are different. All this underlines the great importance of managing patients with DM according to evidence-based, target-driven approaches, tailored to the individual needs of the patient.
5.1 Risk scores developed for people without diabetes
Framingham Study risk equations based on age, sex, blood pressure, cholesterol (total and HDL) and smoking, with DM status as a categorical variable,90 have been validated prospectively in several populations.91,92 In patients with DM, results are inconsistent, underestimating CVD risk in a UK population and overestimating it in a Spanish population.93,94 Recent results from the Framingham Heart Study demonstrate that standard risk factors, including DM measured at baseline, are related to the incidence of CVD events after 30 years of follow-up.95
The European Systematic Coronary Risk Evaluation (SCORE®) for fatal coronary heart disease and CVD was not developed for application in patients with DM.89,93
The DECODE Study Group developed a risk equation for cardiovascular death, incorporating glucose tolerance status and FPG.96 This risk score was associated with an 11% underestimation of cardiovascular risk.93
The Prospective Cardiovascular Münster (PROCAM)97 scoring scheme had poor calibration, with an observed/predicted events ratio of 2.79 for CVD and 2.05 for CAD.98
The Myocardial Infarction Population Registry of Girona (REGICOR)99 tables, applied to a Mediterranean (Spanish) population, underestimated CVD risk.94
5.2 Evaluation of cardiovascular risk in people with pre-diabetes
Data from the DECODE study showed that high 2hPG, but not FPG, predicted all-cause mortality, CVD and CAD, after adjustment for other major cardiovascular risk factors (for further details see Section 3.2).43,100
5.3 Risk engines developed for people with diabetes
The United Kingdom Prospective Diabetes Study (UKPDS) risk score for CAD has a good sensitivity (90%) in a UK population,101,102 overestimated risk in a Spanish population,94 and had moderate specificity in a Greek population.103 Moreover, this risk score was developed before the advent of modern strategies for CVD prevention.
The Swedish National Diabetes Register (NDR) was applied in a homogeneous Swedish population and reported a good calibration.104
The Framingham Study. Stroke has only undergone validation in a Spanish group of 178 patients and overestimated the risk.105,106
The UKPDS for stroke underestimated the risk of fatal stroke in a US population.107
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) is a contemporary model for cardiovascular risk prediction, developed from the international ADVANCE cohort.108 This model, which incorporates age at diagnosis, known duration of DM, sex, pulse pressure, treated hypertension, atrial fibrillation, retinopathy, HbA1c, urinary albumin/creatinine ratio and non-HDL cholesterol at baseline, displayed an acceptable discrimination and good calibration during internal validation. The external applicability of the model was tested on an independent cohort of individuals with T2DM, where similar discrimination was demonstrated.
A recent meta-analysis reviewed 17 risk scores, 15 from predominantly white populations (USA and Europe) and two from Chinese populations (Hong Kong). There was little evidence to suggest that using risk scores specific to DM provides a more accurate estimate of CVD risk.109 Risk scores for the evaluation of DM have good results in the populations in which they were developed, but validation is needed in other populations.
5.4 Risk assessment based on biomarkers and imaging
The Atherosclerosis Risk In Communities (ARIC) study prospectively evaluated whether adding C-reactive protein or 18 other novel risk factors individually to a basic risk model would improve prediction of incident CAD in middle-aged men and women. None of these novel markers added to the risk score.110 A Dutch study involving 972 DM patients evaluated baseline UKPDS risk score and the accumulation of advanced glycation end-products (AGEs) in skin111 using auto-fluorescence. The addition of skin AGEs to the UKPDS risk engine resulted in re-classification of 27% of the patients from the low- to the high-risk group. The 10-year cardiovascular event rate was higher in patients with a UKPDS score >10% when skin AGEs were above the median (56 vs. 39%).112 This technique may become a useful tool in risk stratification in DM but further information is needed for this to be verified.
In patients with T2DM, albuminuria is a risk factor for future CV events, CHF and all-cause, even after adjusting for other risk factors.113 Elevated circulating NT-proBNP is also a strong predictor of excess overall and cardiovascular mortality, independent of albuminuria and conventional risk factors.114
Subclinical atherosclerosis, measured by coronary artery calcium (CAC) imaging, has been found superior to established risk factors for predicting silent myocardial ischaemia and short-term outcome. CAC and myocardial perfusion scintigraphy findings were synergistic for the prediction of short-term cardiovascular events.115
Ankle-brachial index (ABI),116 carotid intima-media thickness and detection of carotid plaques,117 arterial stiffness by pulse wave velocity,118 and cardiac autonomic neuropathy (CAN) by standard reflex tests119 may be considered as useful cardiovascular markers, adding predictive value to the usual risk estimate.
Coronary artery disease (CAD) is often silent in DM patients and up to 60% of myocardial infarctions (MI) may be asymptomatic, diagnosed only by systematic electrocardiogram (ECG) screening.120 Silent myocardial ischaemia (SMI) may be detected by ECG stress test, myocardial scintigraphy or stress echocardiography. Silent myocardial ischaemia affects 20–35% of DM patients who have additional risk factors, and 35–70% of patients with SMI have significant coronary stenoses on angiography whereas, in the others, SMI may result from alterations of coronary endothelium function or coronary microcirculation. SMI is a major cardiac risk factor, especially when associated with coronary stenoses on angiography, and the predictive value of SMI and silent coronary stenoses added to routine risk estimate.121 However, in asymptomatic patients, routine screening for CAD is controversial. It is not recommended by the ADA, since it does not improve outcomes as long as CV risk factors are treated.122 This position is, however, under debate and the characteristics of the patients who should be screened for CAD need to be better defined.123 Further evidence is needed to support screening for SMI in all high-risk patients with DM. Screening may be performed in patients at a particularly high risk, such as those with evidence of peripheral artery disease (PAD) or high CAC score or with proteinuria, and in people who wish to start a vigorous exercise programme.124
Cardiovascular target organ damage, including low ABI, increased carotid intima-media thickness, artery stiffness or CAC score, CAN and SMI may account for a part of the cardiovascular residual risk that remains, even after control of conventional risk factors. The detection of these disorders contributes to a more accurate risk estimate and should lead to a more intensive control of modifiable risk factors, particularly including a stringent target for LDL-cholesterol (LDL-C) of <1.8 mmol/L (∼70 mg/dL).125 In patients with SMI, medical treatment or coronary revascularization may be proposed on an individual basis. However the cost-effectiveness of this strategy needs to be evaluated.
5.5 Gaps in knowledge
There is a need to learn how to prevent or delay T1DM.
There is a need for biomarkers and diagnostic strategies useful for the early detection of CAD in asymptomatic patients.
Prediction of CV risk in people with pre-diabetes is poorly understood.
5.6 Recommendations for cardiovascular risk assessment in diabetes
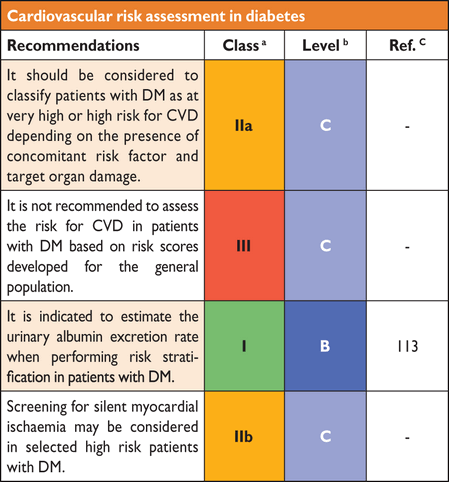 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
6. Prevention of cardiovascular disease in patients with diabetes
6.1 Lifestyle
A joint scientific statement from the ADA and EASD advocates lifestyle management (including healthy eating, physical activity and cessation of smoking) as a first measure for the prevention and/or management of T2DM, with targets of weight loss and reduction of cardiovascular risk.126 An individualized approach to T2DM is also recommended by other organizations.127 A recent Cochrane review concluded that data on the efficacy of dietary intervention in T2DM are scarce and of relatively poor quality.128 The ADA position statement, Nutrition Recommendations and Interventions for Diabetes provides a further review of these issues.129,130
Most European people with T2DM are obese, and weight control has been considered a central component of lifestyle intervention. 'Look AHEAD (Action for Health in Diabetes)' was a large clinical trial of the effects of long-term weight loss on glycaemia and prevention of CVD events in T2DM. One-year results of the intensive lifestyle intervention showed an average 8.6% weight loss, a significant reduction in HbA1c and a reduction in several CVD risk factors—benefits that were sustained after four years.131,132 The trial was, however, stopped for reasons of futility in 2012, since no difference in CVD events was detected between groups. Weight reduction—or at least stabilization in overweight or moderately obese people—will still be an important component in a lifestyle programme and may have pleiotropic effects. In very obese individuals, bariatric surgery causes long-term weight loss and reduces the rate of incident T2DM and mortality.133
6.1.1 Diet
Dietary interventions recommended by the EASD Diabetes and Nutrition Study Group are less prescriptive than many earlier sets of dietary advice.57 They acknowledge that several dietary patterns can be adopted and emphasize that an appropriate intake of total energy and a diet in which fruits, vegetables, wholegrain cereals and low-fat protein sources predominate are more important than the precise proportions of total energy provided by the major macronutrients. It is also considered that salt intake should be restricted.
It has been suggested that there is no benefit in a high-protein- over a high-carbohydrate diet in T2DM.134 Specific dietary recommendations include limiting saturated and trans fats and alcohol intake, monitoring carbohydrate consumption and increasing dietary fibre. Routine supplementation with antioxidants, such as vitamins E and C and carotene, is not advised because of lack of efficacy and concern related to long-term safety.135 For those who prefer a higher intake of fat, a Mediterranean-type diet is acceptable, provided that fat sources are derived primarily from monounsaturated oils—as shown by the Primary Prevention of Cardiovascular Disease with a Mediterranean Diet (PREDIMED) study using virgin olive oil.136
Recommended distributions of macronutrients:57
Proteins: 10–20% of total energy in patients without nephropathy (if nephropathy, less protein).
Saturated and transunsaturated fatty acids: combined <10% of the total daily energy. A lower intake, <8%, may be beneficial if LDL-C is elevated.
Oils rich in monounsaturated fatty acids are useful fat sources and may provide 10–20% total energy, provided that total fat intake does not exceed 35% of total energy.
Polyunsaturated fatty acids: up to 10% total daily energy.
Total fat intake should not exceed 35% of total energy. For those who are overweight, fat intake <30% may facilitate weight loss. Consumption of two to three servings of—preferably—oily fish each week and plant sources of n-3 fatty acids (e.g. rapeseed oil, soybean oil, nuts and some green leafy vegetables) are recommended to ensure an adequate intake of n-3 fatty acids. Cholesterol intake should be <300 mg/day and be further reduced if LDL-C is elevated. The intake of trans fatty acids should be as small as possible, preferably none from industrial origin and limited to <1% of total energy intake from natural origin.
Carbohydrate may range from 45–60% of total energy. Metabolic characteristics suggest that the most appropriate intakes for individuals with DM are within this range. There is no justification for the recommendation of very low carbohydrate diets in DM. Carbohydrate quantities, sources and distribution should be selected to facilitate near-normal long-term glycaemic control. In those treated with insulin or oral hypoglycaemic agents, timing and dosage of the medication should match quantity and nature of carbohydrate. When carbohydrate intake is at the upper end of the recommended range, it is important to emphasize foods rich in dietary fibre and with a low glycaemic index.
Vegetables, legumes, fruits and wholegrain cereals should be part of the diet.
Dietary fibre intake should be >40 g/day (or 20 g/1000 Kcal/day), about half of which should be soluble. Daily consumption of ≥5 servings of fibre-rich vegetables or fruit and ≥4 servings of legumes per week can provide minimum requirements for fibre intake. Cereal-based foods should be wholegrain and high in fibre.
Alcohol drinking in moderate amounts, not exceeding two glasses or 20 g/day for men and one glass or 10 g/day for women,89 is associated with a lower risk of CVD, compared with teetotallers and heavy alcohol drinkers, both in individuals with and without DM.137 Excessive intake is associated with hypertriglyceridaemia and hypertension.89
Coffee drinking: >4 cups/day is associated with a lower risk of CVD in people with T2DM,138 but it should be noted that boiled coffee without filtering raises LDL-C and should be avoided.139
6.1.2 Physical activity
Physical activity is important in the prevention of the development of T2DM in people with IGT and and for the control of glycaemia and related CVD complications.140,141 Aerobic and resistance training improve insulin action and PG, lipids, blood pressure and cardiovascular risk.142 Regular exercise is necessary for continuing benefit.
Little is known about the best way to promote physical activity; however, data from a number of RCTs support the need for reinforcement by healthcare workers.143–145 Systematic reviews143,144 found that structured aerobic exercise or resistance exercise reduced HbA1c by about 0.6% in T2DM. Since a decrease in HbA1c is associated with a long-term decrease in CVD events and a reduction in microvascular complications,146 long-term exercise regimens that lead to an improvement in glycaemic control may ameliorate the appearance of vascular complications. Combined aerobic and resistance training has a more favourable impact on HbA1c than aerobic or resistance training alone.147 In a recent meta-analysis of 23 studies, structured exercise training was associated with a 0.7% fall in HbA1c, compared with controls.143 Structured exercise of >150 min/week was associated with a fall in HbA1c of 0.9% <150 min/week with a fall of 0.4%. Overall, interventions of physical activity advice were associated with lower HbA1c levels only when combined with dietary advice.147
6.1.3 Smoking
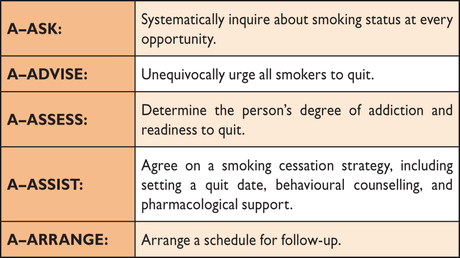
Smoking increases the risk of T2DM,148 CVD and premature death,149 and should be avoided. Stopping smoking decreases risk of CVD.150 People with DM who are current smokers should be offered a structured smoking cessation programme including pharmacological support with, for example, buproprion and varenicline if needed. Detailed instruction on smoking cessation should be given according to the five A principles (Table 7) as is further elaborated in the 2012 Joint European Prevention guidelines.89
6.1.4 Gaps in knowledge
Lifestyles that influence the risk of CVD among people with DM are constantly changing and need to be followed.
The CVD risk, caused by the increasing prevalence of T2DM in young people due to unhealthy lifestyles, is unknown.
It is not known whether the remission in T2DM seen after bariatric surgery will lead to a reduction in CVD risk.
6.1.5 Recommendations on life style modifications in diabetes
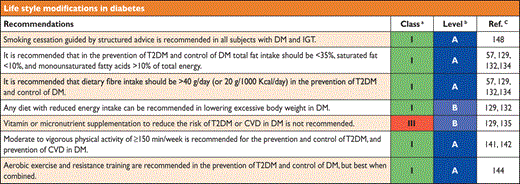 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
6.2 Glucose control
Randomized controlled trials provide compelling evidence that the microvascular complications of DM are reduced by tight glycaemic control,151–153 which also exerts a favourable, although smaller, influence on CVD that becomes apparent after many years.154,155 However, intensive glucose control, combined with effective blood pressure control and lipid lowering, appear to markedly shorten the time needed to make improvements in the rate of cardiovascular events.156
6.2.1 Microvascular disease (retinopathy, nephropathy and neuropathy)
Intensified glucose lowering, targeting an HbA1c of 6.0–7.0%, (42–53 mmol/mol),157 has consistently been associated with a decreased frequency and severity of microvascular complications. This applies to both T1DM and T2DM, although the outcomes are less apparent in T2DM with established complications, for which the number needed to treat (NNT) is high.158–162 Analyses from the Diabetes Control and Complications Trial (DCCT) and the UKPDS demonstrated a continuous relationship between increasing HbA1c and microvascular complications, without an apparent threshold.146,163 In the DCCT, a decrease in HbA1c of 2% (21.9 mmol/mol) significantly lowered the risk of the development and progression of retinopathy and nephropathy,151 although the absolute reduction was low at HbA1c <7.5% (58 mmol/mol). The UKPDS reported a similar relationship in people with T2DM.146,152
6.2.2 Macrovascular disease (cerebral, coronary and peripheral artery disease)
Although there is a strong relationship between glycaemia and microvascular disease, the situation regarding macrovascular disorders is less clear. Hyperglycaemia in the high normal range, with minor elevations in HbA1c,164,165 has been associated with increased cardiovascular risk in a dose-dependent fashion. However, the effects of improving glycaemia on cardiovascular risk remain uncertain and recent RCTs have not provided clear evidence in this area.159–162 The reasons, of which there are several, include the presence of multiple co-morbidities in long-standing T2DM and the complex risk phenotype generated in the presence of IR (for further details see Section 4).
6.2.3 Medium-term effects of glycaemic control
Action to Control Cardiovascular Risk in Diabetes (ACCORD). A total of 10 251 T2DM participants at high cardiovascular risk were randomized to intensive glucose control achieving an HbA1c of 6.4% (46 mmol/mol), or to standard treatment achieving an HbA1c of 7.5% (58 mmol/mol).159 After a mean follow-up of 3.5 years the study was terminated due to higher mortality in the intensive arm (14/1000 vs. 11/1000 patient deaths/year), which was pronounced in those with multiple cardiovascular risk factors and driven mainly by cardiovascular mortality. As expected, the rate of hypoglycaemia was higher under intensive treatment and in patients with poorer glycaemic control, although the role of hypoglycaemia in the CVD outcomes is not entirely clear. Further analysis revealed that the higher mortality may have been due to fluctuations in glucose, in combination with an inability to control glucose according to target, despite aggressive glucose lowering treatment.166 A recent extended follow-up of ACCORD did not support the hypothesis that severe symptomatic hypoglycaemia was related to the higher mortality.167
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE). A total of 11 140 T2DM participants at high cardiovascular risk were randomized to intensive or conventional glucose-lowering therapy.160 The intensive arm achieved an HbA1c of 6.5% (48 mmol/mol), compared with 7.3% (56 mmol/mol) in the standard arm. The primary endpoint (major macrovascular or microvascular complications) was reduced in the intensive arm (HR 0.90; 95% CI 0.82–0.98) due to a reduction in nephropathy. Intensive glycaemic control failed to influence the macrovascular component of the primary endpoint (HR 0.94; 95% CI 0.84–1.06). In contrast to ACCORD, there was no increase in mortality (HR 0.93; 95% CI 0.83–1.06) despite a similar decrease in HbA1c. Severe hypoglycaemia was reduced by two thirds in the intensive arm of ADVANCE, compared with ACCORD, and HbA1c lowering to target was achieved at a slower rate than in ACCORD. In addition, the studies had a different baseline CVD risk, with a higher rate of events in the control group of ADVANCE.
Veterans Administration Diabetes Trial (VADT). In this trial, 1791 T2DM patients were randomized to intensive or standard glucose control, achieving an HbA1c of 6.9% (52 mmol/mol) in the intensive therapy group, compared with 8.4% (68 mmol/mol) in the standard therapy group.161 There was no significant reduction of the primary composite cardiovascular endpoint in the intensive therapy group (HR 0.88; 95% CI 0.74–1.05).
Outcome Reduction with an Initial Glargine Intervention Trial (ORIGIN). This study randomized 12 537 people (mean age, 63.5 years) at high CVD risk plus IFG, IGT or T2DM to receive insulin glargine (with a target fasting blood glucose level of 5.3 mmol/L (≤95 mg/dL) or to standard care. After a median follow-up of 6.2 years, the rates of incident CV outcomes were similar in the insulin glargine and standard care groups. Rates of severe hypoglycaemia were 1.00 vs. 0.31 per 100 person-years. Median weight increased by 1.6 kg in the insulin glargine group and fell by 0.5 kg in the standard care group. There was no indication that insulin glargine was associated with cancer.168
Conclusion.A meta-analysis of cardiovascular outcomes based on VADT, ACCORD and ADVANCE suggested that an HbA1c reduction of ∼1% was associated with a 15% relative risk reduction (RRR) in non-fatal MI but without benefits on stroke or all-cause mortality.169 However, patients with a short duration of T2DM, lower baseline HbA1c at randomization, and without a history of CVD seemed to benefit from more-intensive glucose-lowering strategies. This interpretation is supported by ORIGIN, which did not demonstrate benefit or detriment on cardiovascular end-points by early institution of insulin-based treatment, even though insulin glargine was associated with increased hypoglycaemia. This suggests that intensive glycaemic control should be appropriately applied in an individualized manner, taking into account age, duration of T2DM and history of CVD.
6.2.4 Long-term effects of glycaemic control
Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC). In DCCT, the rate of cardiovascular events was not significantly altered in the intensive-treatment group.151 After termination of the study, 93% of the cohort were followed for an additional 11 years under EDIC, during which the differences in HbA1c disappeared.154 During the combined 17-year follow-up, the risk of any cardiovascular event was reduced in the intensive group by 42% (9–63%; P < 0.01).
United Kingdom Prospective Diabetes Study (UKPDS). Although a clear reduction in microvascular complications was evident, the reduction in MI was only 16% (P = 0.052). In the extension phase of the study, a risk reduction in MI remained at 15%, which became significant as the number of cases increased. Furthermore, the beneficial effects persisted for any DM-related end point; MI and death from any cause was reduced by 13%.155 It should be noted that this study was performed when lipid lowering and blood pressure were less effectively managed, partially due to the lack of availability of potent, currently available drugs. Thus UKPDS was performed when other important parts of a multifactorial management were less efficient. One may speculate that it may have been easier to verify a beneficial effect of glucose-lowering agents at that time, than in subsequently performed trials.
Conclusion. DCCT and UKPDS showed that, in T1DM and T2DM: (i) glycaemic control is important for reducing long-term macrovascular complications; (ii) a very long follow-up period is required to demonstrate an effect and (iii) early glucose control is important (metabolic memory).
6.2.5 Glycaemic targets
An HbA1c target of <7.0% (<53 mmol/mol) to reduce microvascular disease is a generally accepted level.151–153,155,159 The evidence for an HbA1c target in relation to macrovascular risk is less compelling, in part due to the complexities surrounding the chronic, progressive nature of DM and the effects of metabolic memory.153,155,169 Consensus indicates that an HbA1c of ≤7% should be targeted, but with acknowledgement of the need to pay attention to the individual requirements of the patient. Ideally, tight control should be instigated early in the course of the disorder in younger people and without attendant co-morbidities. Fasting plasma glucose (FPG) should be <7.2 mmol/L (<120 mg/dL) and post-prandial <9–10 mmol/L (<160–180 mg/dL) on an individualized basis. Successful glucose-lowering therapy is assisted by self-monitoring of blood glucose, most notably in patients with insulin-treated DM.170 When near-normoglycaemia is the objective, post-prandial glycaemia needs to be taken into account in addition to fasting glycaemia. However, although post-prandial hyperglycaemia is associated with an increased incidence of CVD events (see section 3:4) it remains controversial as to whether treatment targets addressing post-prandial hyperglycaemia are of added benefit to CVD outcomes.171–174
More stringent targets (e.g. HbA1c 6.0–6.5% (42–48 mmol/mol]) might be considered in selected patients with short disease duration, long life expectancy and no significant CVD, if it can be achieved without hypoglycaemia or other adverse effects. As discussed above, the accumulated results from T2DM cardiovascular trials suggest that not everyone benefits from aggressive glucose management. It follows that it is important to individualize treatment targets.126
6.2.6 Glucose-lowering agents
The choice of pharmacological agent, the combinations employed and the potential side-effects are related to the mode of action of the drug. The choice of agent, the conditions of their use and the role of combination therapy is beyond the scope of this document and has been extensively reviewed in the joint ADA/EASD guidelines.126 In brief, therapeutic agents for managing hyperglycaemia can be broadly characterized as belonging to one of three groups: (i) insulin providers [insulin, sulphonylureas, meglitinides, glucagon-like peptide-1(GLP-1) receptor agonists, dipeptidylpeptidase-4 (DPP-4) inhibitors]; (ii) insulin sensitizers (metformin, pioglitazone) and (iii) glucose absorption inhibitors [alpha-glucosidase inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors]. The sulphonylureas, meglitinides and incretin mimetics (GLP-1 receptor agonists and DPP-4 inhibitors) all act by stimulating the pancreatic beta-cell to increase endogenous insulin secretion. The GLP-1 receptor agonists and the DPP-4 inhibitors have additional actions on the gastro-intestinal tract and brain, which have a beneficial effect on satiety (weight neutral for DPP-4 inhibitors, weight loss-associated with GLP-1 receptor agonists), although transient nausea occurring in about 20% of those treated may persist for 4–6 weeks after initiation of therapy. Pioglitazone is a PPARγ agonist with partial peroxisome proliferator-activated receptor alpha (PPARα) effects, which lowers glucose by ameliorating insulin resistance, while metformin is a biguanide that exerts similar effects through AMP kinase activation. Both agents tend to reduce insulin requirements in insulin-treated T2DM and, in the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROActive) study, pioglitazone use was associated with prolonged reductions in insulin requirements.175 Acarbose reduces glucose absorption from the gastro-intestinal tract, whilst the SGLT2 inhibitors act on the proximal renal tubule to reduce glucose absorption. The expected decrease in HbA1c with each of the oral treatments, or with subcutaneous administration of GLP-1 agonists as monotherapy, is generally about 0.5–1.0%, although this can vary between individuals, depending on the duration of DM and other factors. Triple therapy—metformin plus two from pioglitazone, sulphonylurea, incretin mimetics, meglitinides and glucose absorption inhibitors—is commonly required as the disorder progresses.
In T1DM, intensive glucose-lowering therapy using a basal-bolus regimen, delivered either by multiple insulin injections or using an insulin pump, is the 'gold standard'.151 In T2DM, metformin is the first-line drug treatment, especially in overweight patients.126 A concern over the use of metformin has been the risk of lactic acidosis, especially in patients with impaired renal function and hepatic disease. In systematic reviews of trial data with selected patients, lactic acidosis is not over-represented.176 Despite this, metformin is not recommended if the estimated eGFR is <50 mL/min.177 There is an ongoing debate as to whether these thresholds are too restrictive. The UK National Institute for Health and Clinical Excellence (NICE) guidelines are more flexible, allowing use down to a eGFR of 30 mL/min, with dose reduction advised at 45 mL/min.127
To attain glucose targets, a combination of glucose-lowering drugs is often required soon after diagnosis. Early aggressive therapy seems to have a role in reducing cardiovascular complications, but has not been formally tested in prospective trials.
Cardiovascular safety of glucose-lowering agents(Table 8). Concerns initiated by possible adverse cardiovascular effects of rosiglitazone178 raised questions as to the cardiovascular safety of glucose-lowering drugs, particularly when used in combination. A 10-year post-trial follow-up of UKPDS revealed that patients treated with sulphonylurea-insulin had a risk reduction (RR) for MI of 0.85 (95% CI 0.74–0.97; P = 0.01) and for death of 0.87 (95% CI 0.79–0.96; P < 0.007).153,155 The corresponding RRs for metformin in overweight patients were 0.67 (95% CI 0.51–0.89; P = 0.005) and 0.73 (95% CI 0.59–0.89; P = 0.002). Although UKPDS indicated that metformin has a beneficial effect on CVD outcomes—which led to metformin being adopted as first line treatment in overweight T2DM—it is important to underline that, overall, there is no clear evidence to support this view and there is a suggestion that, in combination with sulphonylurea, there may be detrimental effects related to both morbidity and mortality. However, the results of this meta-analysis also suggest a benefit after a long duration of treatment in younger patients.179 Pioglitazone reduced a secondary composite of all-cause mortality, fatal MI and stroke in the PROactive study (HR 0.84; 95% CI 0.72–0.98; P = 0.027) in T2DM patients at high risk of macrovascular disease.175 However, because the primary outcome in PROactive did not achieve statistical significance, the interpretation of these results remains contentious. The use of pioglitazone is associated with fluid retention secondary to renal effects, and this is associated with peripheral oedoma and worsening of established heart failure in susceptible individuals. Diuretic therapy can be initiated to ameliorate these side-effects. In the STOP-NIDDM trial, acarbose, when given to patients with IGT, reduced the number of CVD events, including cardiovascular mortality.172 Meglitinides have not been formally tested in T2DM but, in high-risk patients with IGT nateglinide, did not reduce either fatal or non-fatal cardiovascular events.180 No outcome data from RCTs have so far been published for glucagon-like peptide 1 agonists, DPP-4 inhibitors or SGLT-2 inhibitors, but large prospective trials with cardiovascular outcomes are in progress for GLP-1 receptor agonists and DPP-4 inhibitors and for SGLT2 inhibitors.
Pharmacological treatment options for T2DM
 |
 |
eGFR = estimated glomerular filtration rate; GLP-1 = glucagon-like peptide-1; DDP = Diabetes Prevention Program; SGLT2 = sodium glucose co-transporter 2.
Pharmacological treatment options for T2DM
 |
 |
eGFR = estimated glomerular filtration rate; GLP-1 = glucagon-like peptide-1; DDP = Diabetes Prevention Program; SGLT2 = sodium glucose co-transporter 2.
6.2.7 Special considerations
Hypoglycaemia. Intensive glucose lowering increases the incidence of severe hypoglycaemia three- to four-fold in both T1DM and T2DM.151,162 Impaired hypoglycaemic awareness increases with duration of DM and is a significant risk factor for hypoglycaemia, which must be taken into account when glucose-lowering therapy is considered.181 In addition to the short-term risks of cardiac arrhythmia and cardiovascular events, longer-term risks include dementia and cognitive dysfunction.182,183 The outcome of glucose-lowering studies has raised the question as to whether hypoglycaemia is an important risk factor for MI in patients with DM. Frier et al.182 have extensively reviewed this topic, providing evidence for a number of adverse effects of hypoglycaemia on the CV system, particularly in the presence of autonomic neuropathy. Insulin, meglitinides and sulphonylureas are particularly associated with hypoglycaemia, which is a common occurrence in both T1 and T2DM. Attention should be paid to avoidance of hypoglycaemia, whilst achieving glycaemic goals in an individualized manner.
Glucose lowering agents in chronic kidney disease. Around 25% of people with T2DM have chronic kidney disease (CKD) stages 3–4 (eGFR <50 mL/min). Aside from the increased CV risk associated with this condition, the use of glucose-lowering agents may need to be modified, either because a particular agent is contra-indicated in CKD or because the dosage needs to be altered.184 Metformin, acarbose and most sulphonylureas should be avoided in stage 3–4 CKD, whilst insulin therapy and pioglitazone can be used in their place as required. The DPP-4 inhibitors require dose adjustment with progressive CKD with the exception of linagliptin, which is well tolerated in these circumstances. The SGLT2 inhibitors have not been evaluated in CKD.
Elderly people. Older people have a higher atherosclerotic disease burden, reduced renal function and greater co-morbidity. Life expectancy is reduced, especially in the presence of long-term complications. Glycaemic targets for elderly people with long-standing or more complicated disease should be less ambitious than for younger, healthier individuals. If lower targets cannot be achieved with simple interventions, an HbA1c of <7.5–8.0% (<58–64 mmol/mol) may be acceptable, transitioning upwards as age increases and capacity for self-care, cognitive, psychological and economic status and support systems decline.126
Individualized care. The influences on quality of life, adverse effects of polypharmacy and inconvenience of intensified glucose-lowering regimens have to be carefully evaluated for each individual with DM (for further information see Section 9). From a public health perspective, even minor decreases in mean glycaemia may prove advantageous. On the other hand, the intensified glucose-lowering treatment may impose a considerable burden and possible harm on the individual. Each individual should be encouraged to achieve the best compromise between glucose control and vascular risk and, if intensified therapy is instituted, the patients must be informed and understand the benefits and risks.
6.2.8 Gaps in knowledge
Long-term CVD outcomes for most glucose-lowering treatments are not known.
The consequences of polypharmacy for quality of life and the most appropriate choice of treatment in DM-patients with comorbidities, particularly in the elderly, are unclear.
The level of glycaemia (FPG, 2hPG, HbA1c) at which CV benefits can be seen in T2DM is not known, since no studies with this aim have been carried out.
6.2.9 Recommendations for glycaemic control in diabetes
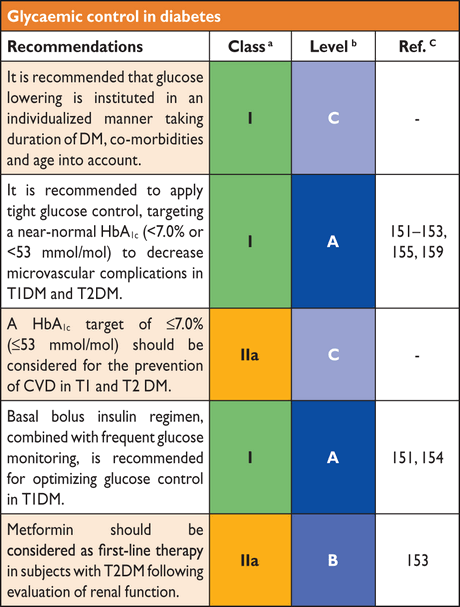 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1c; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1c; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
6.3 Blood pressure
The prevalence of hypertension is higher in patients with T1DM than in the general population (up to 49% in DCCT/EDIC)185,186 and more than 60% of patients diagnosed with T2DM have arterial hypertension.187 According to current pathophysiological considerations, this is related to: (i) hyperinsulinaemia linked to increased renal reabsorption of sodium; (ii) increased sympathetic tone and (iii) increased renin-angiotensin-aldosterone system activity.188 Obesity, aging and the appearance of renal disease further increase the prevalence of hypertension. DM and hypertension are additive risk factors for CVD. While the development of T2DM doubles the cardiovascular risk in men and more than triples the risk in women, hypertension causes a four-fold increase in cardiovascular risk in people with DM.189,190 Although treatment targets are presented, it should be recognised that blood pressure management needs to be implemented on an individualized basis. For example, multiple co-morbidities, increasing age, drug interactions and the pattern of vascular disease may all influence the therapeutic approach and individual target.
6.3.1 Treatment targets
In DM, the recommended level of blood pressure has been debated. In general, measures to lower elevated blood pressure should be applied in all patients with DM, due to the substantially enhanced cardiovascular risk associated with increased blood pressure levels in such patients. RCTs in T2DM have shown the positive effects on cardiovascular outcomes of lowering blood pressure at least below 140 mm Hg systolic and 85 mm Hg diastolic.191–194 The Hypertension Optimal Treatment (HOT) trial demonstrated that risk decreased when the diastolic target was below 80 mm Hg.195 However, the mean diastolic blood pressure in this group was still above 80 and the systolic pressure was as high as 144 mm Hg. The UKPDS showed that ‘tight’ (mean 144/82), compared with ‘less tight’ (154/87) control reduced macrovascular events by 24%. In a post-hoc observational analysis of the UKPDS trial, DM-related mortality decreased 15% with each 10 mm Hg drop, down to a systolic blood pressure of 120 mm Hg, with no indication of a threshold.196 In the more recent ACCORD trial, more than 4700 patients were assigned to intensive- (achieved mean systolic blood pressure 119 mm Hg) or standard treatment [mean systolic blood pressure (BP) 134 mm Hg] over a mean follow-up of 4.7 years. The relative reduction of the composite endpoint (non-fatal MI, non-fatal stroke, or CVD death) by the intensive treatment did not reach statistical significance.192 The average number of blood pressure-reducing drugs was 3.5 in the intensive group, against 2.1 in the standard group. The proportion of patients with serious side-effect—such as hypotension and declining renal function—increased from 1.3 to 3.3% with aggressive treatment. Since the risk–benefit ratio tipped towards harm, this study does not support a reduction of systolic blood pressure below 130 mm Hg. Bangalore et al.197 reported a meta-analysis of 13 RCTs with 37 736 patients with DM, IFG or IGT who, in the intensive group, had a systolic pressure ≤135 mm Hg and, in the standard group, ≤140 mm Hg. The more intensive control related to a 10% reduction in all-cause mortality (95% CI 0.83–0.98), a 17% reduction in stroke but a 20% increase in serious adverse events. Systolic BP ≤130 mm Hg was related to a greater reduction in stroke but did not affect other cardiovascular events.
In summary, present evidence makes it reasonable to reduce blood pressure in patients with DM to <140/85 mm Hg. It should be noted that further reduction might be associated with an increased risk of serious adverse events, especially in patients of advanced age and with longer duration of T2DM. Thus the risks and benefits of more intensive blood pressure management need to be carefully considered on an individual basis.
6.3.2 Managing blood pressure-lowering
Lifestyle intervention including salt restriction and weight loss is the therapeutic basis for all patients with hypertension; however, it is usually insufficient for adequate blood pressure control (for details see Section 6.1).
Pharmacological treatment has only been tested in a few RCTs comparing cardiovascular outcomes with blood pressure-lowering agents and specifically targeting patients with DM.191,198,199 However, several RCTs with sizeable DM subgroups reported specifically on the outcome in this subgroup.200–207 It appears that blockade of the renin-angiotensin-aldosterone system (RAAS), by means of an angiotensin converting enzyme inhibitor (ACE-I ) or an angiotensin-receptor-blocker (ARB), is of particular value, especially when treating hypertension in patients with DM at high cardiovascular risk.200,201,205–207 Evidence also supports the efficacy of an ACE-I, rather than a calcium channel blocker, as initial therapy when the intention is to prevent or retard the occurrence of microalbuminuria in hypertensive patients with DM.208 Dual RAAS blockade combining an ACE-I with an ARB did not show any further benefit in the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET), but was associated with more adverse events. In the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) trial, the addition of aliskiren to RAAS blockade in patients with T2DM at high risk for cardiovascular and renal events did not result in a decrease in cardiovascular events and may even have been harmful.209,210 Since DM patients tend to have high blood pressure during the night, administration of antihypertensive drugs at bedtime should be considered —ideally after evaluation of the 24-ambulatory blood pressure profile of the patient.
A matter that has been intensively discussed over the past decades is whether the metabolic actions of various blood pressure-lowering drugs are important for long-term cardiovascular outcome. It is well established that the use of thiazides and beta-blockers is associated with an increased risk of developing T2DM, compared with treatment with calcium channel blockers and inhibitors of the RAAS.211 It is not known whether treatment with beta-blockers and/or thiazides or thiazide-like diuretics in patients with established T2DM has any metabolic adverse events of clinical importance. The observation from UKPDS, that control of hyperglycaemia—in contrast to an effective blood pressure control—had a relatively minor influence on cardiovascular outcome, indicates that negative metabolic effects may be less important when treating hypertension in patients with DM, at least as regards macrovascular complications. Thus, while drugs with negative metabolic effects—especially the combination of a diuretic and a beta-blocker—should be avoided as first-line treatment in hypertensive patients with metabolic syndrome, the objective of lowering blood pressure seems more important than minor alterations in metabolic status in patients with established DM. A recent meta-analysis emphasized the priority of blood pressure lowering over choice of drug class.212 In the absence of cardiac co-morbidity, beta-blockers are not the first choice for the treatment of hypertension.205,206 Appropriate blood pressure control does often require combined therapy with a RAAS inhibitor and a calcium channel blocker or a diuretic. The Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial indicated that the calcium channel antagonist amlodipine is superior to hydrochlorothiazide in combination treatment with an ACE-I.207 In 6946 patients with DM, the number of primary events was 307 in the group treated with amlodipine and 383 in the group treated with hydrochlorothiazide as the add-on to benazepril (P = 0.003), despite a similar reduction of blood pressure in both groups.
6.3.3 Conclusion
The main aim when treating hypertension in patients with DM should be to lower blood pressure to <140/85 mm Hg. To achieve this goal, a combination of blood pressure-lowering drugs is needed in most patients. In patients with hypertension and nephropathy with overt proteinuria, an even lower BP (SBP <130 mm Hg) may be considered if tolerated by the patient (see Section 8). All available blood pressure-lowering drugs can be used, but evidence strongly supports the inclusion of an inhibitor of the RAAS (ACE-I/ARB) in the presence of proteinuria. It should be borne in mind that many DM patients do not reach the recommended BP target.213 It is also noteworthy that, in contrast to that reported with glycaemic control and statins,155 there is no hypertensive legacy or memory effect.194 As a consequence, sustained control and monitoring and consistent medical adjustment are recommended.
These main conclusions regarding treatment of patients with DM and hypertension are consistent with the Re-appraisal of the European Guidelines on Hypertension (2009)214 and the updated European Guidelines for hypertension 2013.215
6.3.4 Gaps in knowledge
The consequences of blood pressure-lowering multi-drug combinations in the elderly are poorly understood.
The evidence base for efficacy or harm for microvascular complications for both individual blood pressure-lowering drugs alone or in combination is weak.
The understanding of the role of arterial stiffness in predicting CV risk in patients with DM, over and above the role of conventional risk factors is poor.
Optimal blood pressure targets are unknown.
Are the metabolic side-effects of beta-blockers or diuretics clinically relevant?
6.3.5 Recommendations for blood pressure control in diabetes
 |
 |
ACE-I = angiotensin converting enzyme-inhibitors; ARB = angiotensin receptor blockers; DM = diabetes mellitus; RAAS = renin angiotensin aldosterone system.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
6.4 Dyslipidaemia
6.4.1 Pathophysiology
In individuals with T1DM and good glycaemic control, the pattern of lipid abnormalities contrasts with that of T2DM since, in T1DM, serum TG is normal and high-density lipoprotein cholesterol (HDL-C) is within the upper normal range or slightly elevated. This pattern is linked to insulin therapy, which increases lipoprotein lipase activity in adipose tissue, and the turnover rate of very low-density lipoprotein (VLDL) particles. However, qualitative changes in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) particles may potentially be atherogenic.
A cluster of lipid and apoprotein abnormalities accompanies T2DM, affecting all lipoprotein classes (Table 9). The two core components are a moderate elevation of fasting and non-fasting triglycerides (TGs) and low HDL-C. Other features comprise elevations of TG-rich lipoprotein (TRLs), including chylomicron and VLDL remnants, small dense LDL particles.
Characteristics of dyslipidaemia in type 2 diabetes mellitus
 |
 |
Apo = apolipoprotein; CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; LDL = low-density lipoprotein; TG = triglyceride; TRL = triglyceride-rich lipoprotein.
Characteristics of dyslipidaemia in type 2 diabetes mellitus
 |
 |
Apo = apolipoprotein; CVD = cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; LDL = low-density lipoprotein; TG = triglyceride; TRL = triglyceride-rich lipoprotein.
These components are not isolated abnormalities but are metabolically linked. Overproduction of large VLDL particles with increased secretion of both TGs and Apo B 100 leads to the generation of small, dense LDL particles and lowering of HDL-C. As VLDL, remnant and LDL particles carry a single Apo B 100 molecule, the dyslipidaemia is characterized by elevation of the Apo B concentration. Therefore, the malignant nature of dyslipidaemia in T2DM is not always revealed by routine lipid measures, as LDL-C remains within a normal range and it may often be better-characterized by using non-HDL-C. Substantial evidence indicates that an imbalance between the hepatic import and export of lipids results in excess liver fat accumulation (non-alcoholic fatty liver disease). Increased flux of FFA comes from both the systemic FFA pools and de novo lipogenesis in the setting of IR.216,217 Thus the content of liver fat and hepatic IR seem to be driving the overproduction of large VLDL particles in people with T2DM.
Impaired clearance of large VLDL particles, linked to increased concentration of Apo C, contributes to a more robust hypertriglyceridaemia.218 Thus dual metabolic defects contribute to the hypertriglyceridaemia in people with T2DM. Recent data suggest that part of the lipid oversupply to the liver in the presence of obesity may be due to a maladaptive response of adipose tissue to store circulating FFAs, leading to ectopic fat deposition and lipotoxicity that underlies dyslipidaemia in DM and IR.219
6.4.2 Epidemiology
The European Action on Secondary Prevention through Intervention to Reduce Events (EUROASPIRE III)220,221 survey reported that the overall prevalence of high TG and low HDL–C has almost doubled, compared with the prevalence seen by EUROASPIRE II, due to the increase in T2DM and obesity. A population-based survey of 75 048 patients with T2DM in the National Diabetes register in Sweden reported that 49% of patients did not receive lipid-lowering drugs. Fifty-five per cent of those treated had a TG <1.7 mmol/L and around two-thirds a normal HDL-C.222 Data from the same survey revealed that two-thirds of patients on lipid-lowering drugs achieved an LDL-C <2.5 mmol/L.223 However, in those with a history of CVD, more than 70% had LDL-C >1.8 mmol/L. Notably, only moderate doses of the different statins were used, highlighting the need for intensification of therapy and better management of the existing treatment gap.
Dyslipidaemia and vascular risk in type 2 diabetes mellitus. A wealth of data from case-control, mechanistic, genetic and large observational studies indicate that a causal association exists between elevation of triglyceride-rich particles and their remnants, low HDL-C and CVD risk.224,225 Data from statin trials strengthen the position of low HDL as an independent CVD risk marker, even in patients with an LDL-C level that is not elevated.226,227 Data from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study and ACCORD demonstrated that cardiovascular event rates were significantly higher in those with dyslipidaemia (LDL-C 2.6 mmol/L (100 mg/dL), TG ≥2.3 mmol/L and HDL-C ≤0.88 mmol/L).228,229 In FIELD,230 the baseline variables best predicting CVD events over a 5-year follow-up were lipid ratios (non-HDL/HDL-C and total/HDL-C). Apo B–Apo A is related to CVD outcomes, but this ratio was not superior to traditional lipid ratios. Of the single baseline lipid and lipoprotein concentrations, HDL-C, Apo A, non-HDL-C and Apo B individually predicted CVD events, although Apo A and Apo B did not perform better than HDL-C or non-HDL-C. The power of serum TG to predict CVD events was attenuated by adjustment for HDL-C. These results were unexpected, since the dyslipidaemia in DM is a cluster of abnormalities featuring elevations of Apo B and small dense LDL particles. The data are, however, in full agreement with results from the Emerging Risk Factor Collaboration (ERFC) study,231 based on 68 studies that included 302 430 participants without a history of CVD. In this analysis, non-HDL-C and Apo B each had very similar association with coronary heart disease irrespective of the presence of DM. The ERFC study reported that an increase of one standard deviation in HDL-C (0.38 mmol/L or 15 mg/dL) was associated with a 22% reduction in risk of coronary heart disease. HRs for non-HDL and HDL-C were similar to those observed for Apo B and Apo A and non-HDL-C was the best tool to capture the risk associated with elevation of triglyceride rich proteins in clinical practice. The use of Apo B and Apo B–Apo A are also advocated as CVD risk markers in T2DM.
6.4.3 Management of dyslipidaemia
Type 2 diabetes mellitus. Comprehensive and consistent data exist on the mechanism of action and efficacy of statins in the prevention of CVD events in T2DM.232 The benefits of statin therapy in lowering LDL-C and reducing CVD events are seen in all subgroup analyses of major RCTs.233 In a meta-analysis of 14 RCTs covering 18 686 people with DM, the mean duration of follow-up was 4.3 years, with 3247 major vascular events. The study reported a 9% reduction in all-cause mortality and a 21% reduction in the incidence of major vascular outcomes per mmol/L of LDL-C lowering (RR 0.79; 99% Cl 0.72–0.87; P < 0.0001), similar to that seen in non-DM. The magnitude of the benefit was associated with the absolute reduction in LDL-C, highlighting a positive relationship between LDL-C and CVD risk, and was seen at a starting LDL-C as low as 2.6 mmol/L.234
The results of the first meta-analyses of cardiovascular events of intensive vs. moderate statin therapy show a 16% risk reduction of coronary death or MI.235 Data from 10 RCTs, studying 41 778 patients followed for 2.5 years, showed that intensive statin dosage reduced the composite endpoint of CAD by 10% (95% Cl 0.84–0.96; P < 0.0001), but did not reduce CVD mortality.232 In a subgroup of patients with ACS, intensive statin therapy reduced both all-cause and CVD mortality. Intensive lowering of LDL-C by statins had a beneficial effect on progression of atheroma in DM and non-DM.236
Intensification of LDL-C lowering can also be achieved by adding ezetimibe to a statin, however, there are still no data from an RCT that this combination has a significant impact on CVD outcome. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT: ClinicalTrials.gov: NCT00202878) is, however, under way. An analysis of pooled safety data comparing the efficacy and safety profile of combination therapy with ezetimibe/statin vs. statin monotherapy in DM and non-DM (n = 21 794)237 reported that combination therapy provided larger effects on all major lipid measures. The Study of Heart and Renal Protection (SHARP) trial reported a 17% reduction of major atherosclerotic events in chronic kidney disease treated with simvastatin plus ezetimibe daily vs. placebo.238 In this context it should be emphasized that, although relative reduction of events may be similar for people with and without DM, the absolute benefit is greater in DM-patients due to their higher risk.
Type 1 diabetes mellitus. The Cholesterol Treatment Trialists (CTT) analysis included 1466 T1DM patients with an average age of 55 years and a majority with prior CVD events. This analysis showed a similar reduction of risk of CVD events (RR 0.79; 95% CI 0.62–1.01) to that seen in T2DM and with a P value for interaction of 1.0, verifying the result despite only a borderline significance in the subgroup.234 It should be recognized that no trial data exist on the efficacy of statin therapy in a younger population with T1DM. However, in T1DM, statin therapy should be considered on an individual basis in those at high risk for CVD events, irrespective of LDL-C concentration—for example T1DM patients with renal impairment.
Primary prevention. The Collaborative Atorvastatin Diabetes Study (CARDS) evaluated the benefits of a statin in patients with T2DM and at least one of the following risk factors: hypertension, current smoking, retinopathy, or albuminuria.239 In CARDS, 2838 T2DM patients were randomized to atorvastatin 10 mg/day or placebo. The study was terminated prematurely, due to a 37% reduction (95% CI -52 to -17; P = 0.0001) in the primary endpoint (first acute coronary heart disease event). The Heart Protection Study (HPS) recruited 2912 patients (mainly T2DM) without pre-existing CVD. Simvastatin (40 mg/day) reduced the composite primary endpoint by 33% (P = 0.0003; 95% Cl 17–46).240 In the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) subgroup analyses of DM patients free from CVD, 10 mg of atorvastatin reduced the rate of major CVD events and procedures by 23% (95% Cl 0.61–0.98; P = 0.04).241
Safety of statin therapy. Reports from major RCTs demonstrate that statins are safe and well-tolerated.242 The frequency of adverse events, except for muscle symptoms, is rare. In the majority of cases of myopathy or rhabdomyolysis there are drug interactions with a higher-than-standard dose of statin.243 The combination of gemfibrozil and statins should be avoided due to pharmacokinetic interaction, but there are no safety issues with fenofibrate and statins.228,229
A meta-analysis including 91 140 participants reported that statin therapy was associated with risk of new-onset T2DM (OR 1.09; 95% Cl 1.0–1.2; I2 = 11%), which increased with age.244 The data translate to one case of T2DM when 255 patients have been treated for 4 years. Over the same time, statins would prevent 5.4 CVD events for each mmol/L reduction in LDL-C. A meta-analysis of five statin trials reported that the risk of new onset DM increased with intensive statin (atorvastatin or simvastatin 80 mg daily) therapy (OR 1.12; 95% Cl 1.04–1.22; I2 = 0%), compared with moderate (simvastatin 20 mg or pravastatin 40 mg) doses.245 In the intensive group, two additional cases of new-onset DM per 1000 patient years were observed, whereas the number of CVD events was 6.5 cases fewer. Recently the Food and Drug Administration (FDA) of the USA approved label changes on increases of blood glucose and HbA1c for the statin class of drugs (www.fda.gov/downloads/Drugs/DrugSafety/UCM293474.pdf). The FDA still considers that the small risk of developing DM is clearly outweighed by the reduction of cardiovascular events.245,246 Further support for the safety of statins comes from a meta-analysis of 27 randomized trials that demonstrated that, in individuals with a five-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL-C produced an absolute reduction in major vascular events of about 11 per 1000 over five years, without an increase in incidence of cancer or deaths from other causes. This benefit greatly exceeds any known hazards of statin therapy.247
Residual risk in people on LDL-lowering therapy. T2DM patients at the LDL-C target remain at high risk of CVD events,224 and this residual risk is linked to many factors including elevation of TG-rich proteins, low HDL-C and small, dense LDL particles. It has been suggested that targeting elevated TG (>2.2 mmol/L) and/or low HDL-C (<1.0 mmol/L) may provide further benefits. In the FIELD study, fenofibrate therapy did not reduce the primary endpoint (CAD-related death and non-fatal MI), but total CVD events were reduced from 14 to 12.5% (HR 0.9; 95% Cl 0.80–0.99; P = 0.035).228,248 In the ACCORD trial, 5518 patients were assigned to fenofibrate plus simvastatin (20–40 mg daily) or placebo without any additional effect on the primary endpoint. In a pre-specified subgroup analysis of people with TG >2.3 mmol/L (>204 mg/dL) and HDL-C <0.9 mmol/L (<34 mg/dL), cardiovascular risk was reduced by 31% in the fenofibrate-plus-simvastatin group (for interaction between patients with this lipid profile vs. those without, P = 0.06).229 A subgroup analysis of dyslipidaemic people (TG >2.3 mmol/L and HDL-C <0.9 mmol/L) in the FIELD study revealed a 27% reduction in CVD risk.228 In both FIELD and ACCORD, fenofibrate therapy was associated with robust reduction of TG (22%), whereas elevation of HDL-C remained less than expected (+2% and +2.4%, respectively). Meta-analyses have confirmed the clinical benefits of fibrates on major CVD events but not on cardiovascular mortality.249,250 The effects seem to be linked to improvement in TGs.250
Strategies to elevate high-density lipoprotein cholesterol. The level of HDL-C is inversely related to CVD in epidemiological studies, as well as in many statin trials.218 Low levels of HDL-C are associated with increased levels of triglycerides and are often seen in patients with metabolic syndrome and/or DM. Targeting low HDL-C for CVD prevention is, however, not supported by evidence. Two recently reported RCTs, using the cholesterylester transfer protein (CETP) inhibitors torcetrapib and dalcetrapib,251,252 failed to reduce cardiovascular events despite a 30–40% increase in HDL-C. One explanation for these findings may relate to abnormal functional characteristics of HDL particles. If this is true, merely increasing the number of such particles without any improvement of their function may not alter CVD risk.
The pharmacological tools currently available to raise HDL-C in DM patients remain limited. Fenofibrate has trivial efficacy in this regard, while niacin (N-ER) has potentially useful properties, increasing HDL-C by 15–30%, with an associated increase in Apo A-1,224,253 besides lowering TG (up to 35%), LDL-C (about 20%) and Apo B and lipoprotein a (Lp a) (about 30%). Although a study showed favourable effects on angiographic measures, and on reduction of carotid wall area quantified with magnetic resonance imaging after one year of therapy,254 two recent clinical studies did not confirm the usefulness of N-ER for cardiovascular prevention. The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study showed no additional benefit of N-ER in patients with metabolic syndrome.255 In the Heart Protection Study 2 Treatment of HDL to Reduce the Incidence of Vascular Events (HPS-2 THRIVE) trial, 25 673 patients with known vascular disease were randomized to placebo or N-ER/laropiprant on a background of statin or statin/ezetimibe therapy. The trial was stopped prematurely after a median follow up of 3.9 years. At that time, 15.0% of patients in the control arm and 14.5% in the N-ER/laropiprant arm (ns) had reached the primary endpoint, a composite of coronary death, non-fatal MI, stroke, or coronary revascularization. Moreover, there was a significant 3.7% absolute excess risk of DM complications and a significant 1.8% excess risk of new-onset DM. In addition, N-ER treatment caused a 1.4% higher risk of infection and a 0.7% higher risk of bleeding, including an increased risk of haemorrhagic stroke.256. Based on these results, the EMA has withdrawn the marketing licence for N-ER/laropiprant.
So far, lifestyle intervention with smoking cessation, increased physical activity, weight reduction and decreased consumption of fast-absorbed carbohydrates remains the cornerstone of HDL-increasing therapy.
In patients with high TG (>5.4 mmol/L) lifestyle advice (with a focus on weight reduction and alcohol abuse if relevant) and improved glucose control are the main targets. Risks associated with TG are acute pancreatitis and polyneuropathy. In a pooled analysis of randomized trial data, use of statins was associated with a lower risk of pancreatitis in patients with normal or mildly elevated triglyceride levels. Fibrates were not protective and may even have enhanced the risk.257 Omega-3 fatty acids (2–4 g/day) may be used for TG-lowering in people with high levels.258 There is, however, no evidence that such supplements are of cardiovascular benefit in patients with DM.
6.4.4 Gaps in current knowledge
The role of HDL particles in the regulation of insulin secretion in beta-cells needs further exploration.
Efficiency and safety of drugs increasing or improving HDL-C particles is unclear.
The relative contributions of HDL function and plasma HDL concentration in the pathogenesis of CVD should be clarified.
6.4.5 Recommendations on management of dyslipidaemia in diabetes
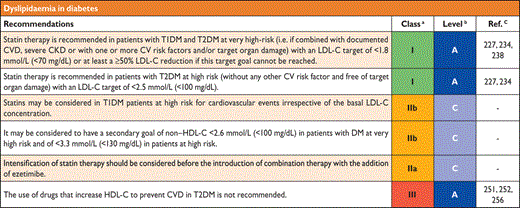 |
 |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; HDL-C = high density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; T1DM = type 1 diabetes mellitus, T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
 |
 |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; HDL-C = high density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; T1DM = type 1 diabetes mellitus, T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
6.5. Platelet function
Platelet activation plays a pivotal role in the initiation and progression of atherothrombosis.259 Abnormalities in the aggregation of platelets in DM ex vivo have been described by numerous groups,260 and both post-prandial and persistent hyperglycaemia have been identified as major determinants of in vivo platelet activation in the early and late phases of the natural history of T2DM.261,262
6.5.1 Aspirin
Aspirin inhibits thromboxane (TX) A2-dependent platelet activation and aggregation through irreversible inactivation of platelet cyclo-oxygenase 1 (COX-1) activity.263 No formal studies have specifically examined the dose- and time-dependence of its antiplatelet effect in patients with T2DM and aspirin is currently recommended at 75–162 mg once daily, i.e. at the same dose and dosing interval used in people without DM.263,264 However, once-daily administration of low-dose aspirin may be associated with incomplete inhibition of platelet COX-1 activity and TXA2-dependent platelet function,265–267 perhaps due to increased platelet turnover in DM.268 Evidence to support this view indicates the potentially beneficial effects of sustained efficacy using twice-daily aspirin in people with DM and CVD.268,269
Secondary prevention. The first collaborative overview of the Antiplatelet Trialists' Collaboration found that antiplatelet therapy (mostly with aspirin) is similarly effective among patients with pre-existing symptomatic CVD, regardless of the presence of DM.270 They analysed individual data on ‘serious vascular events’ (non-fatal MI, non-fatal stroke or vascular death) from approximately 4500 patients with DM in the randomized trials and found that treatment with antiplatelet drugs produced a proportional reduction of about one quarter.270 Therefore there is no apparent reason to treat patients with DM and CVD differently from non-DM patients and low-dose aspirin is uniformly recommended for both the acute treatment of ischaemic syndromes and their secondary prevention.263
Primary prevention. Low-dose aspirin is recommended by several North American organizations for the primary prevention of cardiovascular events in adults with DM.264,271 However, direct evidence of its efficacy and safety in this setting is lacking or, at best, inconclusive.272,273 Thus, in the most up-to-date meta-analysis, which includes three trials conducted specifically in patients with DM and six other trials in which such patients represent a subgroup within a broader population, aspirin was found to be associated with a non-significant 9% decrease in the risk of coronary events (RR 0.91; 95% CI 0.79–1.05) and a non-significant 15% reduction in the risk of stroke (RR 0.85; 95% CI 0.66–1.11).264 It should be emphasized that the total number of patients with DM enrolled in these nine trials was 11 787, with 10-year extrapolated coronary event rates ranging from as low as 2.5% to as high as 33.5%.264 These results have been interpreted as suggesting that aspirin probably produces a modest reduction in the risk of cardiovascular events, but the limited amount of available data precludes a precise estimate of the effect size. Consistent with this uncertainty, antiplatelet therapy with aspirin in adults at a low CVD risk is not recommended by the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on CVD Prevention in Clinical Practice.89
The risk–benefit ratio of aspirin. Based on data from a meta-analysis of the six primary prevention trials, aspirin was associated with a 55% increase in the risk of extracranial (mainly gastro-intestinal) bleeding, both in people without- (the majority) and with DM.274 In terms of the balance between the potential benefit and hazard of aspirin in primary prevention, these results probably represent a best-case scenario, as people at increased risk of gastro-intestinal bleeding were excluded and elderly people were under-represented.274 In the same analyses, the presence of DM at baseline was associated with a two-fold increase in vascular events but also with a 50% increased risk of major extracranial bleeds during follow-up.274
Both the Endocrine Society Clinical Practice Guideline and the ADA/AHA/ACCF Scientific Statement favour aspirin use in adults with DM when the 10-year risk of cardiovascular events is >10%.271,264 However, relatively little emphasis is placed in either statement on the need to evaluate the variable bleeding risk of the patient. While the annual risk of cardiovascular events can vary approximately 10-fold in DM,264 the annual risk of upper gastro-intestinal bleeding has been estimated to vary by up to 100-fold in the general population, depending on age and history of peptic ulcer disease.263,275
6.5.2 P2Y12 receptor blockers
Clopidogrel, an irreversible blocker of the adenosine diphosphate (ADP) receptor P2Y12, provides a valid alternative for patients who are aspirin-intolerant or have symptomatic peripheral vascular disease, because it has broad indications for long-term secondary prevention similar to aspirin.276,277 Moreover, clopidogrel (75 mg once daily) produced additive cardio-protective effects when combined with low-dose aspirin (75–160 mg once daily) in patients with ACS and those undergoing percutaneous coronary intervention (PCI).276 There is, however, evidence from the Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance (CHARISMA) study to indicate that clopidogrel, added to background aspirin, may have deleterious effects in patients with advanced nephropathy.278 More effective P2Y12 blockers include prasugrel and ticagrelor, a reversible P2Y12 blocker.276 In the TRITON-TIMI 38 trial, prasugrel (60 mg loading dose, followed by 10 mg daily) showed clear superiority over clopidogrel (300 mg loading dose, followed by 75 mg daily) in the prevention of recurrent ischaemic events post-acute coronary syndrome (ACS): however, in the general cohort, this benefit carried a risk of increased thrombolysis in myocardial infarction (TIMI) major bleeding.279 In a DM sub-study, a similar reduction in recurrent ischaemic events was seen, but in the DM cohort this was not accompanied by an increase in bleeding.280 Ticagrelor (180 mg loading dose, followed by 90 mg twice daily), was also more effective than clopidogrel (300–600 mg loading dose, followed by 75 mg daily) in reducing death from CV causes and total mortality at 12 months in a general post-ACS cohort,281 and decreased ischaemic events in DM patients without causing increased bleeding.282 Importantly, ticagrelor was shown to be superior to clopidogrel in ACS patients with renal impairment.283 There is no convincing evidence that either clopidogrel or the newer drugs are any more or less effective in people with DM than in those without.276 For the use of these drugs in connection to PCI, see Section 7.2.
6.5.3 Gaps in knowledge
The optimal antithrombotic regimen for the primary prevention of CVD in DM is not established.
6.5.4 Recommendations for antiplatelet therapy in patients with diabetes
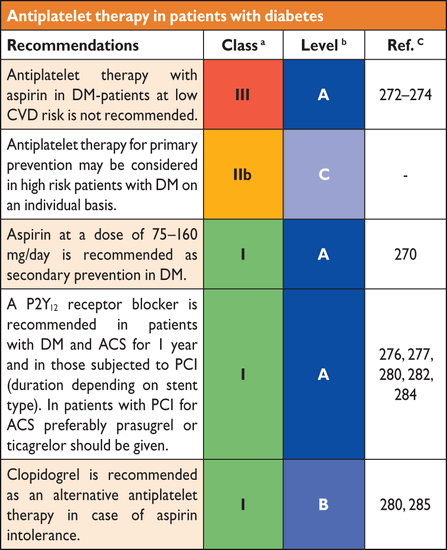 |
 |
ACS = acute coronary syndrome; CVD = cardiovascular disease; DM = diabetes mellitus; PCI = percutaneous coronary intervention.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
6.6 Multifactorial approaches
6.6.1 Principles of multifactorial management
Patients with glucose perturbations are in need of early risk assessment to identify co-morbidities and factors that increase cardiovascular risk. This includes evaluation of: (i) risk factors (e.g. lifestyle habits including smoking, hypertension and dyslipidaemia); (ii) microvascular and macrovascular disease and autonomic dysfunction; (iii) co-morbidities (e.g. heart failure and arrhythmias); (iv) inducible ischaemia by means of exercise testing, stress echocardiography, or myocardial scintigraphy and (v) myocardial viability and LV function by means of echo-Doppler and/or magnetic resonance imaging.286 The reliability of exercise testing, stress echocardiography, or myocardial scintigraphy is of a particular concern in the detection of ischaemia in DM. Confounders are a high threshold for pain due to autonomic dysfunction, the multi-vessel nature of coronary disease, ECG abnormalities, co-existence of PAD and use of multiple medications.
The total risk for cardiovascular complications is, to a large extent, related to synergistic interactions between IR, beta-cell dysfunction and subsequent hyperglycaemia but also the accumulation of cardiovascular risk factors. Accordingly, successful risk prevention depends on a comprehensive detection and management of all modifiable risk factors, as can be visualized by the use of risk engines (e.g. the UKPDS).101 It should be noted, however, that such engines need to be continuously updated.287 Further information can be obtained in Section 5.
The feasibility of intensified, multifactorial treatment for patients with T2DM in general practice was studied in the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen Detected Diabetes in Primary Care (ADDITION).288 The incidence of a first cardiovascular event was 7.2% (13.5 per 1000 person-years) in the intensive care group and 8.5% (15.9 per 1000 person-years) in the routine care group (HR 0·83; 95% CI 0·65–1·05), and incidence of all-cause mortality was 6.2% (11.6 per 1000 person-years) and 6.7% (12.5 per 1000 person-years), respectively (HR 0.91; 95% CI 0.69–1.21). It was concluded that an intervention to promote early intensive management of patients with T2DM was associated with a small but non-significant reduction in the incidence of cardiovascular events and death.26,289 A caveat in respect of ADDITION was the only slightly better control of important cardiovascular risk factors (HbA1c, cholesterol concentrations and blood pressure) in the intensive group. In contrast, the value of a multifactorial intervention in patients with DM and established microalbuminuria was demonstrated by the STENO 2 study which, in a highly specialized setting, randomized 160 participants to an intensive, target-driven multifactorial therapy or to conventional management. The targets in the intensively treated group were HbA1c <6.5%, total cholesterol <4.5 mmol/L (175 mg/dL) and blood pressure <130/80 mm Hg. All patients in this group received RAAS blockers and low-dose aspirin. Although treatment targets were not always attained in the intensive-treatment group, their overall management was considerably better than in routinely handled patients. This resulted in a reduction in microvascular and macrovascular events of about 50% after 7.8 years of follow-up. The target most successfully attained was that for cholesterol, probably making crucial the role of statins in the overall prevention strategy.290,291 Subsequently, target-driven therapy was recommended to patients in both groups. They were followed for 13 years after randomization. By that time, patients originally allocated to the intensively managed group had an absolute mortality reduction of 20% and the HR for death, compared with that in the conventional group, was 0.54 (95% CI 0.3–0.9; P < 0.02). The absolute risk reduction in cardiovascular events was 29%. In addition, there was a substantial reduction in diabetic nephropathy (relative risk 0.4; 95% CI 0.3–0.8; P < 0.004) and progression of retinopathy (relative risk 0.6; 95% CI 0.4–0.9; P = 0.01).156 In a health-economic analysis, intensive patient management was reported as more cost-effective than conventional care. Since increased expenses relating to intensive care were driven by pharmacy and consultation costs, such treatment would be dominant (i.e. cost- and life-saving with the use of generic drugs in a primary care setting).292
Data from the Euro Heart Survey on Diabetes and the Heart support a multifactorial approach as a cornerstone of patient management. Among 1425 patients with known T2DM and CAD, 44% received evidence-based pharmacological therapy, defined as a combination of aspirin, beta-blockade, RAAS inhibitors and statins in the absence of contra-indications. Patients on such drug combination had a significantly lower all-cause mortality (3.5 vs. 7.7%; P = 0.001) and fewer combined cardiovascular events (11.6 vs. 14.7%; P = 0.05) after one year of follow up, compared with those who did not receive a full combination of such drugs.213 The adjusted HR for the interaction between DM and treatment revealed that the use of evidence-based treatment in T2DM had an independent protective effect (HR for death: 0.4). An example of the inadequacy of a single drug approach to decrease the incidence of CVD originates from a study that randomized 37 overweight/obese insulin-resistant participants, still without DM, to fenofibrate, rosiglitazone, or a calorie-restricted diet. None of the tested treatments appeared to be a therapeutic intervention that, in isolation, had the capacity to normalize all—or at least a majority—of the metabolic disturbances (e.g. weight, insulin sensitivity, cholesterol, TG, post-load PG) in these patients at a greatly increased cardiovascular risk.293
Treatment targets are summarized in Table 10.
Summary of treatment targets for managing patients with diabetes mellitus or impaired glucose tolerance and coronary artery disease
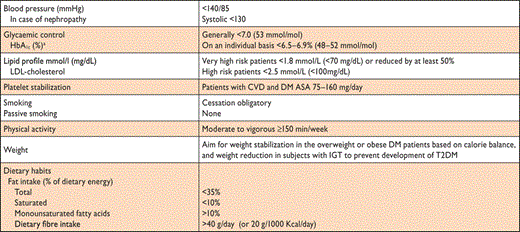 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1c; IGT = impaired glucose tolerance; LDL = low density lipoprotein;
T2DM = type 2 diabetes mellitus.
aDiabetes Control and Complication Trial standard.
Summary of treatment targets for managing patients with diabetes mellitus or impaired glucose tolerance and coronary artery disease
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1c; IGT = impaired glucose tolerance; LDL = low density lipoprotein;
T2DM = type 2 diabetes mellitus.
aDiabetes Control and Complication Trial standard.
6.6.2 Gaps in knowledge
Pleiotropic effects of glucose-lowering therapies on CVD outcomes are not fully understood.
6.6.3 Recommendations for multifactorial risk management in diabetes
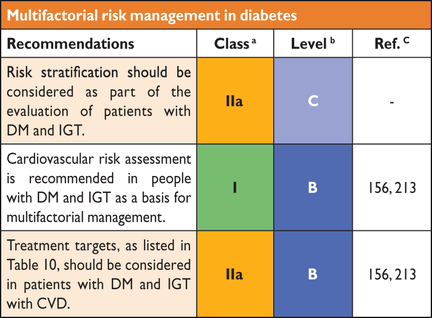 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1c; IGT = impaired glucose tolerance; LDL = low density lipoprotein; T2DM = type 2 diabetes mellitus.
aDiabetes Control and Complication Trial standard.
7. Management of stable and unstable coronary artery disease in patients with diabetes
7.1. Optimal medical treatment for patients with chronic coronary artery disease and diabetes
DM is associated with a poorer prognosis in patients with acute and stable CAD.294–296 This is apparent in patients with newly detected DM and IGT,297 and although the absolute risk is higher in men, the proportionate increase in risk is higher in women, in whom loss of cardioprotection occurs with DM.298 All patients with CAD, without previously known glucose perturbations, should, for the purpose of risk stratification and adapted management, have their glycaemic state evaluated. Elevated levels of HbA1c and FPG may establish the diagnosis of DM,299 but a normal value does not exclude glucose abnormalities. Accordingly, and as detailed in Section 3.3, the appropriate screening method is an oral glucose tolerance test (OGTT),3,38 which should not be performed earlier than 4–5 days after an acute coronary event (ACS) (i.e. acute MI or unstable angina) to minimize false positive results.300,301
In-hospital and long-term mortality after MI has declined, but the outcome is still poor amongst patients with DM. The reasons are partially unexplained but a higher prevalence of complications, in combination with lack of appropriate evidence-based treatment, contributes.302,303
Since very few pharmacological trials have been directed towards patients with DM, information on treatment efficacy is frequently based on subgroup analyses from existing trials. A disadvantage is the risk of looking at groups of patients with DM considered suitable for the trial but in which the DM phenotypes are not well defined. Moreover, patients with CVD often have a metabolic syndrome or undetected DM. With these limitations, available information favours a proportionately similar efficacy of cardiovascular risk management in DM and non-DM patients. Considering the higher risk for cardiovascular events, the absolute benefit is considerably higher in DM, and the NNT to avoid one cardiovascular event is lower in this population.213
7.1.1 Beta-adrenergic blockers
As outlined in current European guidelines on patients with CAD, beta-blockers are advocated for the whole spectrum of CAD, with different levels of recommendations and different levels of evidence.304–308 Beta-blockers relieve symptoms of myocardial ischaemia (angina pectoris) in patients with stable CAD and they may provide prognostic benefits, as suggested from retrospective analysis of placebo-controlled trials.305 Beta-blockers are particularly effective in improving prognosis in post-MI patients with DM by reducing the likelihood of reinfarction, sudden death and ventricular arrhythmias.309,310 Beta-blockers may have negative metabolic effects—for example, by increasing IR and masking hypoglycaemic symptoms—and there seems to be a difference between non-vasodilating, beta 1-antagonists (e.g. metoprolol and atenolol) and beta-blockers with vasodilating properties (e.g. the ß/α-adrenoblockers carvedilol and labetalol, and ß1-blockers with modulation synthesis of NO, nebivolol), with the latter advocated as having a better glucometabolic profile.311 Overall the positive effects of beta-blockade on prognosis outweigh the negative glucometabolic effects.
7.1.2 Blockers of the renin-angiotensin-aldosterone system
Treatment with ACE-I or ARB should be started during hospitalization for ACS and continued thereafter in patients with DM and left ventricular ejection fraction (LVEF) <40%, hypertension, or chronic kidney disease,304,306,307 and considered in all patients with ST-elevation MI (STEMI). Patients with DM and stable CAD are also recommended an ACE-I.305 The Heart Outcomes Prevention Evaluation (HOPE) study showed a 25% reduction in MI, stroke, or cardiovascular death for patients with known vascular disease or DM randomized to placebo or ramipril. This finding was consistent in the pre-specified subgroup of patients with DM.312 A proportionately similar trend to benefit was observed in the subgroup of patients with DM in the EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery disease (EUROPA) trial, recruiting a population at lower cardiovascular risk.313 The ONTARGET trial compared the ACE-I ramipril and the ARB telmisartan in a high-risk population similar to that in HOPE. In this head-to-head comparison, telmisartan was found to be equivalent to ramipril as regards the primary outcome—a composite of death from cardiovascular causes, MI, stroke or hospitalization for heart failure—while a combination of the two drugs caused adverse events without any increase in benefit.210
7.1.3 Lipid-lowering drugs
The beneficial effect of statins in patients with CAD and DM is firmly established. Details on lipid-lowering therapy are outlined in Section 6.4.
7.1.4 Nitrates and calcium channel blockers
There is no evidence for a prognostic impact of nitrates but they may be used for symptomatic relief.304,306,307 Calcium channel blockers are efficacious in relieving ischaemic symptoms, and verapamil and diltiazem may prevent re-infarction and death.304–307 These drugs may be appropriate for long-term use in patients without heart failure, as an alternative to beta-blockers or when beta-blockers may be a less attractive choice, e.g. due to obstructive airways disease. The combination of these drugs and beta-blockers should be avoided, considering the risk for bradycardia, atrio-ventricular conduction disturbances or compromised LV function. An alternative is the use of a dihydropyridine calcium channel blocker, such as amlodipine, felodipine or nicardipine.
7.1.5 Ivabradine
The specific, heart-rate lowering, anti-anginal drug ivabradine inhibits the If current—the primary modulator of spontaneous diastolic depolarization in the sinus node. Ivabradine is indicated in the treatment of chronic stable angina in CAD patients with a contra-indication or intolerance to beta-blockers, or in combination with beta-blockers if the patient remains symptomatic or has a heart rate >70 bpm, especially if there is also left ventricular (LV) dysfunction. It can be used in selected patients with non-ST elevation ACS in the event of beta-blocker intolerance, or insufficient heart rate reduction despite maximal tolerated beta-blocker dose.305,306 High heart rate is associated with a worse outcome in patients with DM,314 and ivabradine is effective in preventing angina in these patients without any safety concerns or adverse effects on glucose metabolism.315
7.1.6 Antiplatelet and antithrombotic drugs (see also Sections 6.5 and 7.2)
In secondary prevention, antiplatelet therapy in the form of low-dose aspirin (75–160 mg) or clopidogrel (separately or in combination) reduces the risk of stroke, MI or vascular death, although the benefits are somewhat less in DM.316 In patients with ACS without ST-segment elevation, glycoprotein IIb/IIIa receptor inhibitors seemed to be especially effective in patients with DM but this was not confirmed in the recent Early-ACS trial.317
Other antiplatelet drugs, such as thienopyridines (ticlopidine, clopidogrel, prasugrel and ticagrelor) reduce the risk of cardiovascular events when added to aspirin in patients with ACS.284,304,307 The incidence of cardiovascular death, MI or stroke decreased from 11.4 to 9.3% (RR 0.80; 95% CI 0.72–0.90) an effect that was sustained in patients with DM.282 In the Clopidogrel vs. Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) study—recruiting patients with recent ischaemic stroke, recent MI or established PAD—those with DM and vascular disease were provided better protection from serious cardiovascular events by clopidogrel than by aspirin. The annual event rate in patients with DM was 15.6% in those randomized to clopidogrel and 17.7% in those who received aspirin, i.e. an absolute risk reduction of 2.1% (P = 0.042), which corresponds to an RRR of 13% (RR 0.87; 95% CI 0.77–0.88) and with fewer bleeding complications. Due to the elevated event rates in patients with DM, the absolute benefit of clopidogrel is amplified in this clinical setting.285 In a subgroup analysis of the TRITON trial, patients with DM tended to have a greater reduction in ischaemic events, without an observed increase in major bleeding, with prasugrel than with clopidogrel.280 It is important to acknowledge that many trials do not separately report outcomes for patients with DM and recommendations are based on available evidence from trials including patients with and without DM.318
7.1.7 Glucose control in acute coronary syndromes
Elevated plasma glucose (PG) during an ACS is associated with a more serious prognosis in patients with DM than without.319–323 Hyperglycaemia may relate to previously undetected glucose perturbations, but also to stress-induced catecholamine release increasing FFA concentrations, decreased insulin production and increasing IR and glycogenolysis,301 with a negative impact on myocardial metabolism and function (for details see Section 4). Two strategies have been tested in an attempt to improve the prognosis in patients with an ACS.
Metabolic modulation by means of glucose-insulin-potassium (GIK), regardless of the presence of DM or PG, is based on the assumption that an increase in intracellular potassium stabilizes the cardiomyocyte and facilitates glucose transportation into the cells.324 Other potential benefits are decreased beta oxidation of FFAs, improved use of glucose for energy production and improved endothelial function and fibrinolysis.301 RCTs failed to show mortality or morbidity benefits, as reviewed by Kloner and Nesto.324 This lack of effect may be due to increased PG or negative effects of the fluid load induced by the GIK-infusion. The Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency Care (IMMEDIATE) trial, randomizing patients after a median time of 90 minutes of suspected ACS to out-of-hospital emergency medical service administration of GIK or placebo, demonstrated a reduction of the composite outcome of cardiac arrest or in-hospital mortality with GIK treatment, but did not impact the pre-specified primary endpoint, i.e. progression of ACS to MI within 24 h.325
Glycaemic control has been tested in the RCTs 'Diabetes and Insulin–Glucose Infusion in Acute Myocardial Infarction' (DIGAMI)326,327 1 and 2 and 'Hyperglycaemia: Intensive Insulin Infusion in Infarction' (HI-5).328 The first DIGAMI trial randomized 620 patients with DM and acute MI to a ≥24-h insulin–glucose infusion, followed by multi-dose insulin, or to routine glucose-lowering therapy.326 Mortality after 3.4 years was 33% in the insulin group and 44% (P = 0.011) in the control group.329 DIGAMI 2 failed to demonstrate prognostic benefits. The most plausible reason for this discrepancy is that, in DIGAMI 1,326,330 admission HbA1c decreased more (1.5%), from a higher level (9.1%), compared with 0.5% from 8.3% in DIGAMI 2.327 In addition, the use of beta-blockade, statins and revascularization was more extensive in DIGAMI 2.
The difference in glucose levels between the control and insulin groups in the HI-5 study was small and there was no reduction in mortality among patients treated with insulin.328 Pooled data from the three studies confirmed that insulin–glucose infusion did not reduce mortality in the absence of glucose control in patients with acute MI and DM (RR 1.07; 95% CI 0.85–1.36; P = 0.547).331. Since neither DIGAMI 2 nor HI-5 achieved a difference in glucose control between the intensively treated and the control groups, it is still an open question as to whether glucose lowering is beneficial.
The Heart2D compared the effects of prandial (pre-meal insulin three times daily; n = 557) vs. basal glycaemic control (long-acting insulin once or twice daily; n = 558) on cardiovascular events in patients with T2DM. Glucose targets were a PPG of 7.5 mmol/L (135 mg/dL) and an FPG of 6.7 mmol/L (121 mg/dL) respectively. The basal group had a lower mean FPG (7.0 vs. 8.1 mmol/l; P < 0.001) but a similar daily fasting/pre-meal blood glucose (7.7 vs. 7.3 mmol/l; P = 0.233) vs. the prandial group and a similar level of HbA1c. The study was stopped after an average follow-up of 963 days, due to lack of efficacy.173
Some registry studies have suggested that there is a J- or U-shaped relationship between PG and prognosis,320,322,323 with the implication that hypoglycaemia, as well as hyperglycaemia, may be prognostically unfavourable. Compensatory mechanisms induced by hypoglycaemia, such as enhanced catecholamine release, may aggravate myocardial ischaemia and provoke arrhythmias.332,333 Recent data indicate that hypoglycaemic episodes identify patients at risk for other reasons (e.g. heart failure, renal dysfunction and malnutrition) and hypoglycaemia does not remain as an independent risk factor when correcting for such variables.334,335
A reasonable conclusion, from DIGAMI 1,326,330 is that DM and acute MI will benefit from glycaemic control if hyperglycaemia is significant (>10 mmol/L or >180 mg/dL). An approximation towards normoglycaemia, with less stringent targets in those with severe co-morbidities, is a reasonable goal but exact targets are still to be defined. Insulin infusion is the most efficient way to achieve rapid glucose control. Glucose management in the long-term perspective is presented elsewhere in these guidelines (Section 6.2).
7.1.8 Gaps in knowledge
The role and optimum level of glycaemic control in the outcome in ACS patients remain to be established.
Is it possible to reduce final infarct size by means of very early GIK administration after symptoms indicating MI?
7.1.9 Recommendations for the management of patients with stable and unstable coronary artery disease and diabetes
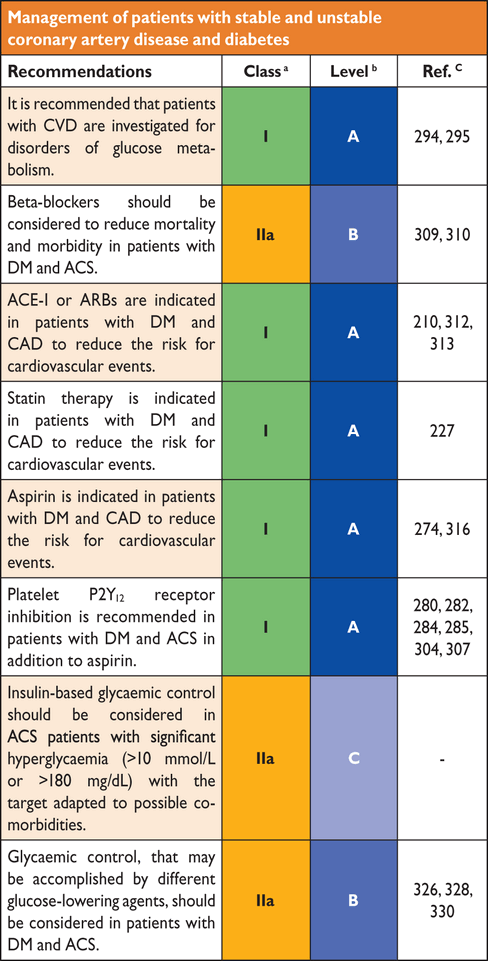 |
 |
ACE-I = angiotensin converting enzyme inhibitor; ACS = acute coronary syndrome; ADP = adenosine diphosphate; ARB = angiotensin receptor blockers; CAD = coronary artery disease; CVD = cardiovascular disease; DM = diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
7.2. Revascularization
A quarter of myocardial revascularization procedures are performed in patients with DM. Revascularization in these patients is challenged by a more diffuse atherosclerotic involvement of epicardial vessels, a higher propensity to develop re-stenosis after PCI and saphenous graft occlusion after coronary artery bypass graft surgery (CABG) and unremitting atherosclerotic progression causing new stenosis.336 This results in a higher risk, including long-term mortality, than seen in patients without DM, irrespective of revascularization modality (Figure 7).337 Evidence on the effect of myocardial revascularization in patients with DM has been obtained in the shifting context of a continued development of PCI, CABG and pharmacological treatments, making it difficult to establish adequate comparisons.308,338
7.2.1 Myocardial revascularization in stable and unstable coronary artery disease
Stable coronary artery disease. A randomized comparison of myocardial revascularization, either with CABG or PCI, vs. optimal medical treatment (OMT)—in DM patients considered eligible for either PCI or CABG—was performed in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial.339 Once PCI or CABG had been chosen as the most adequate potential revascularization technique, patients were randomized to OMT alone or to revascularization plus OMT. After five years, no significant differences were noted in the combined endpoint of death, MI or stroke between the OMT (12%) and revascularization (12%) arms. In the surgical group, freedom from major adverse cardiac and cerebrovascular events (MACCE) was significantly higher with CABG (78%) than with OMT alone (70%, P = 0.01), but there was no difference in survival (CABG 86%; OMT 84%; P = 0.33). In the PCI group, made up of patients with less-extensive CAD than in the CABG stratum, there were no significant differences in MACCE or survival between PCI and OMT. During subsequent follow-up, 38% of the patients assigned to OMT underwent at least one revascularization for symptomatic reasons, compared with 20% in the revascularization stratum, showing that an initial conservative strategy with OMT saved about 80% of interventions over the next five years. Overall, except in specific situations such as left main coronary artery stenosis ≥50%, proximal LAD stenosis or triple vessel disease with impaired LV function, myocardial revascularization in patients with DM did not improve survival when compared with medical treatment. When transferring these results into general practice, it should be kept in mind that the results were obtained in a selected population. Patients were excluded if they required immediate revascularization or had left main coronary disease, a creatinine level >2.0 mg/dL (>177 µmol/L), HbA1c >13.0%, class III–IV heart failure or if they had undergone PCI or CABG within the previous 12 months.
Acute coronary syndromes. No interaction between the effect of myocardial revascularization and the presence of DM has been documented in trials on non-ST-elevation ACS management.340–342 An early invasive strategy improved outcomes in the overall population of these studies,303,340,342 with a greater benefit in patients with DM in the Treat angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis In Myocardial Infarction (TACTICS-TIMI 18) trial.342 In STEMI patients, a pooled analysis of individual patient data (n = 6315) from 19 RCTs comparing primary PCI with fibrinolysis showed that patients with DM (n = 877; 14%) treated with reperfusion had an increased mortality, compared with those without DM. The benefits of a primary PCI, compared with fibrinolysis were, however, consistent in patients with and without DM.343 Patients with DM had significantly delayed initiation of reperfusion treatments and longer ischaemic times, probably related to atypical symptoms causing significant delays in the time for reperfusion treatment. However, the reduction in 30-day mortality observed in PCI-treated patients was most pronounced in this group. Owing to a higher absolute risk, the NNT to save one life at 30 days was significantly lower for DM (NNT 17; 95% CI 11–28) than for non-DM patients (NNT 48; 95% CI 37–60). A subgroup analysis of DM patients included in the Occluded Artery Trial (OAT) confirmed that, as in non-DM, revascularization of an occluded infarct-related artery 3–28 days after MI does not improve outcome.344
7.2.2 Type of intervention: coronary bypass graft vs. percutaneous intervention
Higher repeat revascularization rates after PCI have been consistently found in DM patients included in RCTs comparing CABG and PCI. A meta-analysis based on individual data from 10 RCTs (7812 patients) comparing both types of revascularizations suggests a distinct survival advantage for CABG in DM patients (Figure 7:1).337 Five-year mortality was 20% with PCI, compared with 12% with CABG (odds ratio 0.7; 95% CI 0.6–0.9), whereas no difference was found for patients without DM; the interaction between the presence of DM and type of revascularization was significant. A specific comparison of the efficacy and safety of PCI and CABG in patients with DM was performed in the Coronary Artery Revascularization in Diabetes (CARDia) trial.345 The introduction of drug-eluting stents (DES) coincided with the enrolment period, leading to a mixed use of bare-metal stents (BMS) (31%) and DES (69%). After one year there was a non-significantly higher rate of the composite of death, MI and stroke (driven by a higher rate of MI) and significantly higher rates of repeat revascularization in the PCI group (2 vs. 12%, P < 0.001). The conclusions of the study were hampered by the limited size of the study population (n = 510).
Mortality in patients assigned to coronary artery bypass graft or percutaneous coronary intervention by diabetes status in an analysis of 10 randomized trials. Reproduced with permission from Hlatky et al.337
The literature on CABG vs. PCI is confused by confounder bias in registries, the ongoing development of DES and, apart from the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) trial, a lack of prospective RCTs. The implication is that much of the available information has to be derived from subgroup analyses in trials in populations in which patients with DM may be relatively few or selected. As a consequence of increased repeat revascularization in the SYNTAX trial,346 performed in the DES era (using paclitaxel-eluting stents), the rate of MACCE after one year was twice as high with PCI as it was with CABG. In the pre-specified subgroup with DM, the relative risk for repeat revascularization after one year was even higher (RR 3.2; 95% CI 1.8–5.7; P < 0.001). In patients with DM and complex lesions, i.e. high SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) scores, one-year mortality was higher in the paclitaxel-eluting stent group (14% vs. 4%; P = 0.04).347 After five years of follow-up, the rates of MACCE were significantly higher in patients with DM, when comparing PCI with CABG (PCI: 46.5% vs. CABG: 29.0%; P < 0.001) as well as for repeat revascularization (PCI: 35.3% vs. CABG: 14.6%; P < 0.001). There was no difference in the composite of all-cause death/stroke/MI (PCI: 23.9% vs. CABG: 19.1%; P = 0.26). Similar results were seen— but with somewhat fewer events—among patients without DM. It was concluded that, although PCI is a potential treatment option in patients with less complex lesions, CABG should be the revascularization choice for patients with complex anatomic disease, especially with concurrent DM.348
In contrast, an analysis of DM patients included in the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME) randomized trial and registry, which included high-risk patients for CABG (prior CABG, recent MI, LVEF <30% or intra-aortic balloon pump treatment), showed no significant difference in three-year mortality between revascularization techniques.349 Data obtained in recent registries support a better outcome in patients with DM treated with CABG, compared with DES, even in terms of mortality, at the expense of a higher stroke rate.350 In an analysis of 86 244 patients ≥65 years of age undergoing CABG and 103 549 patients undergoing PCI from 2004 to 2008, four-year survival was significantly higher with surgery and the association of surgery with improved survival was most marked in insulin-treated DM.351 The Revascularization for unprotected left main coronary artery stenosis: comparison of percutaneous (MAIN COMPARE) study reported on the long-term outcome of 1474 patients with unprotected left main stenosis, treated with DES or CABG. In this specific setting, there was a similar rate of the composite endpoint death, Q-wave MI or stroke in the PCI and CABG arms and a significantly higher rate of repeat revascularizations in the DES arm. A subgroup analysis of the study comparing patients with (n = 507; 34%) and without DM did not reveal significant interactions between treatment outcomes and the presence or absence of DM after adjustment for co-variates.352 In an observational study from real-world patients in the Swedish Coronary Angiography and Angioplasty Registry, comprising 94 384 consecutive stent implantations, PCI with new generation DES was associated with a 38% reduced risk for clinically meaningful re-stenosis and a 23% lower death rate, compared with older DES.353 These findings are supported by the outcome of a meta-analysis of 49 randomized controlled trials, including 50 844 patients, comparing different drug-eluting stents or drug elution with bare-metal stents.354 The FREEDOM trial randomized 1900 patients—a majority with three-vessel disease—to treatment with CABG or PCI with sirolimus-eluting and paclitaxel-eluting stents. Newer-generation stents could be used as long as the FDA approved them. All patients were prescribed currently recommended medical therapies for the control of LDL-C, systolic BP and HbA1c. The primary results were a composite of total mortality and non-fatal MI or stroke. After a median of 3.8 years, the primary outcome occurred more frequently in the PCI group (P = 0.005), with a five-year rate of 26.6%, compared with 18.7% in the CABG group. The benefit of CABG was driven by differences in both MI (P < 0.001) and mortality (P = 0.049; Figure 7:2).
Kaplan-Meier estimates of the primary outcome and death. A: rates of the composite primary outcome of death, myocardial infarction or stroke and B: death from any cause truncated at five years after randomization. The P-value was calculated by means of the log-rank test on the basis of all available follow-up data. Reproduced by permission from Farkouh et al.355
It was concluded that CABG is superior to PCI for patients with DM and advanced CAD. There was no significant interaction based on SYNTAX score, since the absolute difference in the primary end points between PCI and CABG were similar in patients with low, intermediate and high SYNTAX scores. Given the wide variability of the patients enrolled in FREEDOM, the trial represents real-world practice. Further analysis revealed that CABG was a cost-effective strategy, compared with PCI.355,356 It can be concluded that a discussion with the patient, explaining the mortality benefit with CABG surgery, and an individualized risk assessment should be mandatory before the type of intervention is decided.308
7.2.3 Specific aspects of percutaneous and surgical revascularization in diabetes mellitus
The DIABETES trial demonstrated a 75% reduction in target vessel revascularization in DM patients treated with sirolimus-eluting stents (7%) vs. BMS (31%).357 This finding received further support from a meta-analysis of 35 trials comparing DES with BMS,358 which revealed a similar efficacy of sirolimus-eluting and paclitaxel-eluting stents in this regard (OR 0.29 for sirolimus; 0.38 for paclitaxel), provided that dual antiplatelet therapy after DES implantation was continued for >6 months. The risk of death associated with sirolimus-eluting stents was more than twice that associated with BMS in eight trials employing dual antiplatelet therapy for period of less than six months. In contrast, there was no increased risk associated with the use of DES in 27 trials with dual antiplatelet therapy maintained for more than six months. An analysis of registry data from the National Heart, Lung and Blood Institute Dynamic Registry revealed that, compared with BMS, DES were associated with fewer repeat revascularizations—to a similar extent in insulin-treated or non-insulin-treated DM.359 Finally, the second-generation everolimus-eluting stents were not superior in terms of target lesion failure after one year of follow-up in a head-to-head comparison with paclitaxel-eluting stents, while zotarolimus-eluting stents were inferior to sirolimus-eluting stents in patients with DM.360,361
Antithrombotic treatment in DM patients undergoing coronary revascularization for stable angina or ACS is no different from those without DM.317,362,363 Initial trials in glycoprotein IIb/IIIa inhibitors reported an interaction with DM, but this was not confirmed in the recent Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 2) trial performed in the clopidogrel era.364 Prasugrel is superior to clopidogrel in reducing the composite endpoint of cardiovascular death or MI or stroke without excess major bleeding. Similarly ticagrelor, in comparison with clopidogrel in the PLATelet inhibition and patient Outcomes (PLATO) trial, reduced the rate of ischaemic events in ACS patients, irrespective of the presence or absence of DM and glycaemic control, without an increase in major bleeding events.280,282
Patients with DM who undergo CABG usually have extensive CAD and require multiple grafts. There is no randomized evidence regarding the use of one vs. two internal thoracic artery (ITA) conduits in DM. Although observational evidence suggests that using bilateral ITA conduits improves patient outcome without compromising sternal stability, their use is still under debate, given a higher prevalence of wound infection and mediastinitis with DM.365 A recent meta-analysis has shown that ITA harvesting by skeletonization (without the satellite veins and fascia) reduces the risk of sternal wound infection, in particular in DM patients undergoing bilateral ITA grafting,366 although there are no randomized studies on this subject. A single-centre non-randomized study comparing CABG with bilateral ITA and PCI in DM reported improved outcomes (freedom from angina, re-intervention, or composite major adverse cardiac events) in the surgical group, but no difference in six-year survival (86% for CABG and 81% for PCI).367 Finally, more than 50% of patients with moderate-to-poor blood glucose control after cardiac surgery may not have been diagnosed as having DM during pre-operative assessment.368 This may lead to inadequate peri-operative glycaemic control, which is a predictor of in-hospital mortality and morbidity.
7.2.4 Myocardial revascularization and glucose-lowering treatments
Although hypoglycaemic medications may influence the safety of coronary angiography, as well as early and late outcomes of revascularization with PCI or CABG, few trials have addressed interactions with myocardial revascularization in DM.
The plasma half-life of metformin is 6.2 h. There is no adequate scientific support for the frequent practice of stopping metformin 24 to 48 h prior to angiography or PCI because of a potential risk of lactic acidosis, followed by restarting treatment 48 h later. More recent recommendations are less restrictive.308 Rather than stopping metformin treatment in all patients, a reasonable approach is to carefully monitor renal function after the procedure and to withhold metformin for 48 h if it deteriorates and until renal function has resumed its previous level.
Observational data reported concern over the use of sulphonylureas in patients treated with primary PCI for acute MI: this has not been confirmed by a post hoc analysis of the DIGAMI-2 trial, although the number of patients undergoing primary PCI in this trial was low.369 Arrhythmias and ischaemic complications were also less frequent in patients receiving gliclazide/glimepiride.370 Thiazolidinediones might be associated with lower re-stenosis rates after PCI with BMS,371 but carry an increased risk of heart failure due to water retention in the kidney (see also Section 6.2.6).
No trial has demonstrated that the administration of insulin or GIK improves PCI outcome after STEMI. Observational data in patients undergoing CABG suggest that use of continuous intravenous insulin infusion to achieve moderately tight glycaemic control (6.6–9.9 mmol/L or 120–180 mg/dL) is independently associated with lower mortality and major complications than that observed after tighter (<6.6 mmol/L or <120 mg/dL) or more lenient (>9.9 mmol/L or >180 mg/dL) glycaemic control.372 In the BARI 2D trial, outcomes were similar in patients receiving insulin sensitization vs. insulin provision to control blood glucose. In the CABG stratum, administration of insulin was associated with more cardiovascular events than insulin-sensitization medications.339,373
7.2.5 Gaps in knowledge
The optimal policy on metformin treatment in patients undergoing PCI is still uncertain.
The role and optimum level of glycaemic control in the outcome during and after myocardial revascularization remain to be established.
7.2.6 Recommendations for coronary revascularization of patients with diabetes
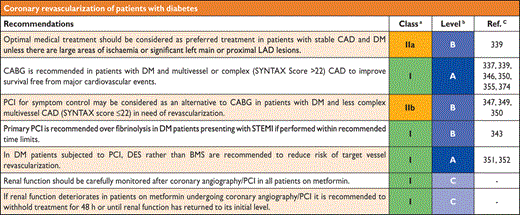 |
 |
BMS = bare-metal stent; CABG = coronary artery bypass grafting; CAD = coronary artery disease; DES = drug-eluting stent; DM = diabetes mellitus; LAD = left anterior descending coronary artery; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
 |
 |
BMS = bare-metal stent; CABG = coronary artery bypass grafting; CAD = coronary artery disease; DES = drug-eluting stent; DM = diabetes mellitus; LAD = left anterior descending coronary artery; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
8. Heart failure and diabetes
Heart failure and T2DM frequently co-exist, each adversely affecting the natural course of the other. The prevalence of risk factors for heart failure is common in patients with DM, among which CAD and hypertension are the most important. In addition, dysglycaemia may in itself have an unfavourable effect on the myocardium. This has led to recognition of a clinical entity labelled as DM cardiomyopathy, in which compromised diastolic function is an early feature. An analysis of 987 patients with heart failure and preserved LVEF, enrolled in the Digitalis Investigation Group (DIG) ancillary study,375 revealed that T2DM was associated with significantly increased risk of developing adverse heart failure outcomes. The clinical approach to cardiomyopathy includes echocardiographic assessment of LV diastolic dysfunction, which can worsen during physical exercise.376 Insulin resistance, which characterizes the heart failure syndrome, regardless of aetiology, seems to be an important factor behind the elevated risk of DM development among heart failure patients. Despite strong evidence linking heart failure and DM, an optimal management of these co-existing conditions is still not fully evidence-based owing to a lack of clinical trials specifically addressing such patient populations.
8.1 Prevalence and incidence of heart failure in type 2 diabetes mellitus, and type 2 diabetes mellitus in heart failure
Prevalence and incidence of heart failure in diabetes mellitus. The prevalence of heart failure in a general population is 1–4% and 0.3–0.5% of the patients have both heart failure and T2DM. Studies in heart failure populations reveal a prevalence of T2DM from 12–30%, rising with age.377,378 T2DM is a major independent risk factor for the development of heart failure. In the Framingham study, the relative risk of heart failure in patients with T2DM (age 45–74 years) was doubled for men and six times as high in women.379 The high incidence of heart failure in patients with T2DM was also confirmed in the National Health and Nutrition Examination Survey, which revealed T2DM as an independent risk factor for heart failure, with an HR of 1.85 (95% CI 1.51–2.28) in T2DM, compared with non-DM.380 Boonman-de Winter et al.381 who studied a Dutch group of 581 T2DM patients (aged >60 years) reported that 28% (95% CI 24–31%) had previously unknown heart failure; 5% with reduced LVEF and 23% with preserved LVEF. The prevalence increased rapidly with age, and heart failure with preserved LVEF was more common in women than men. Left ventricular dysfunction was diagnosed in 26% (95% CI 22–29%), and 25% (95% CI 22–29%) had diastolic dysfunction. This underlines the importance of looking for signs and symptoms of compromised myocardial function in patients with T2DM.
Several clinical correlates are independent risk factors for the development of heart failure in T2DM, including high HbA1c, increased body mass index, advancing age, associated CAD, retinopathy, nephropathy and insulin use. Also, in recent studies, end-stage renal disease, nephropathy, proteinuria and albuminuria, retinopathy and duration of T2DM were associated with heart failure and its progression.382
Prevalence and incidence of diabetes mellitus in heart failure. the prevalence of DM in a general population is 6– 8% but, as reviewed by McDonald et al., it is higher in people with symptomatic heart failure (12–30%) increasing towards 40% among hospitalized patients.1,383 However, the heart failure populations are older than the general population. It should be noted that the prevalence of DM patients is lower in heart failure trials, indicating a selection bias towards younger and/or less sick DM patients. Information on the incidence of DM in heart failure populations is sparse but, in an elderly Italian population, new-onset DM occurred in 29% during three years of follow-up, compared with 18% in controls without heart failure.384 When people with two or more visits in the Reykjavik study (n = 7060) were followed over 30 years, DM and heart failure did not predict each other independently, although fasting glucose and BMI were significant risk factors, both for glucose disturbances and heart failure.385
Diabetes cardiomyopathy. Long-standing hyperglycaemia may—even in the absence of other risk factors such as CAD, valvular disease or hypertension—affect the myocardial tissue, increasing the risk of dysfunction. A reduction of LV compliance—an early sign of DM cardiomyopathy—may indeed already be detectable early in the course of DM.386 The frequent co-existence of hypertension and DM makes the contribution of the glucometabolic state to the diastolic dysfunction difficult to isolate. The pathogenic mechanisms involve accumulation of advanced glycation products, collagen formation and interstitial fibrosis, leading to impaired calcium homeostasis and impaired myocardial insulin signalling (See Section 4 for further details and references). These perturbations increase myocardial stiffness and reduce myocardial compliance.387,388 According to the recommendations of the ESC, LV diastolic dysfunction is identified by quantitative estimation of LV diastolic properties, using conventional Doppler parameters of the transmitral inflow of blood and tissue Doppler imaging of the mitral annulus. Deteriorating diastolic dysfunction is associated with a progressive increase in LV filling pressure which, in turn, has an impact on the transmitral flow pattern.389 It has been claimed—but not verified in longitudinal studies—that myocardial dysfunction may progress in a time-dependent fashion after the onset of diastolic dysfunction, leading to systolic dysfunction and the classical features of heart failure. Due to the frequent co-existence of DM, hypertension and CAD, it has been debated whether the myocardial dysfunction is primarily triggered by the glucometabolic disorder itself, rather than by the synergistic action of these factors. From a clinical perspective, prevention of the development of LV systolic dysfunction and subsequent heart failure is currently focussed on pharmacological treatment of the co-morbidities. It may also explain why meticulous blood pressure-lowering seems to be particularly effective in people with DM.
8.2 Diabetes mellitus and heart failure: morbidity and mortality
Heart failure was a major cause of hospitalization in patients with T2DM in the Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events and Ramipril (DIABHYCAR) trial, investigating hospitalizations in T2DM patients with albuminuria.382 Conversely T2DM increased the risk of hospitalization in patients with heart failure in the BEta blocker STroke trial (BEST) trial390 (RR 1.16; 95% CI 1.02–1.32; P = 0.027). In Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF),391 patients with heart failure and T2DM had one-year hospitalization of 31%, compared with 24% for those free from DM.
In the DIABHYCAR study, the combination of heart failure and T2DM resulted in a 12-fold higher annual mortality than among patients with T2DM but without heart failure (36 vs. 3%).382 BEST and Studies Of Left Ventricular Dysfunction (SOLVD) reported T2DM as an independent predictor of mortality, mostly in ischaemic heart failure.390,392 Also, the Danish Investigations and Arrhythmia ON Dofetilide (DIAMOND) and Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) trials reported DM as an independent predictor of mortality, irrespective of aetiology.393,394
8.3 Pharmacological management of heart failure in type 2 diabetes mellitus
Three neurohormonal antagonists—an ACE-I or ARB, a beta-blocker and a mineralocorticoid receptor antagonist (MRA)—comprise the important pharmacological agents for the treatment of all patients with systolic heart failure, including those with DM. They are usually combined with a diuretic for relieving congestion and may also be supplemented by ivabradine.389
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. ACE-I is indicated in T2DM and heart failure, since it improves symptoms and reduces mortality. The SOLVD trial, using enalapril, showed a significant mortality reduction in DM with heart failure.392 Mortality risk reduction in the high-dose vs. low-dose lisinopril groups was 14% in DM and 6% in non-DM in the Assessment of Treatment with Lisinopril And Survival (ATLAS) trial.395 In a meta-analysis, the risk ratio for death was the same in the ACE-I treated group as in the placebo-treated group in T2DM (n = 2398) and non-T2DM (n = 10 188).396
Subgroup analyses of clinical trials indicate that the beneficial effects of ARBs are equivalent to those of ACE-I.397–400 An ARB can therefore be used as an alternative in ACE-I-intolerant patients. ACE-I and ARBs should not be combined in patients with an LVEF <40%, who are symptomatic despite optimal treatment with an ACE-I in combination with a beta-blocker. According to the 2012 ESC heart failure Guidelines, such patients should be prescribed an MRA (see below), which causes a larger morbidity and mortality reduction than that following addition of an ARB.389
When ACE-I and ARBs are used in patients with DM, surveillance of kidney function and potassium is mandatory, since nephropathy is a frequent occurrence.
Beta-blockers. In addition to an ACE-I (or, if not tolerated, an ARB) a beta-blocker should be given to all patients with an LVEF ≤40%. As an example, a subgroup analysis of the MERIT-HF trial shows that beta-blockers reduce mortality and hospital admission and improve symptoms without significant differences between T2DM and non-DM.391 Further, two meta-analyses of major heart failure trials indicate that the RR of mortality in patients with DM receiving a beta-blocker was significantly improved (0.84 vs. 0.72).396,401 Beta-blockers also reduce hospitalizations for heart failure in both DM and non-DM.390,391,402,403 Despite this, people with T2DM are less likely to be discharged from hospital on a beta-blocker (OR 0.72; 95% CI 0.55–0.94) than non-DM with heart failure.404 The following beta-blockers are recommended in heart failure and T2DM: metoprolol succinate in the slow release form (MERIT-HF), bisoprolol [Cardiac Insufficiency Bisoprolol Study (CIBIS II)] and carvedilol [Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) and Carvedilol Or Metoprolol European Trial (COMET)].402,403,405,406
Unwanted effects of beta-blockers in patients with DM and heart failure:
a) Hypoglycaemia. Evidence indicates that beta-blockers in DM alter counter-regulatory responses to hypoglycaemia with decreased tremor and palpitations but increased sweating.407 Prolonged hypoglycaemia has been described with non-cardio-selective beta-blockade (propranolol), but not with beta-1-selective agents or with carvedilol.408,409 Elderly DM patients on insulin (n = 13 559), without heart failure, experienced an increased risk of serious hypoglycaemia with non-selective beta-blockade (RR 2.16; 95% CI 1.15–4.02) but not with beta-1-selective drugs (RR 0.86; 95% CI 0.36–1.33).410
b) Negative metabolic effects. In hypertensive patients without heart failure, different beta-blockers may have varying effects on glycaemic indices, decreasing insulin sensitivity and increasing the risk of T2DM.410 The marked clinical benefits of beta-blockers in patients with DM and heart failure outweigh the risks of hypoglycaemia and dyslipidaemia or decreased insulin sensitivity.
Mineralocorticoid receptor antagonists. To reduce the risk of hospitalization and premature death, a low-dose MRA is indicated in all patients with persisting symptoms [New York Heart Association (NYHA) Class II–IV] and an LVEF ≤35%, despite treatment with an ACE-I (or, if not tolerated, an ARB) and a beta-blocker.411 The mortality benefit of spironolactone412 and eplerenone413 did not differ between patients with and without T2DM and heart failure. Surveillance of kidney function and potassium is mandatory, considering the increased risk of nephropathy in patients with DM.
Diuretics. The effect of diuretics on mortality and morbidity has not been investigated, but these drugs are useful for the relief of dyspnoea and oedema in heart failure with fluid overload, irrespective of the EF. Loop diuretics are recommended, rather than thiazides, which have been shown to promote hyperglycaemia.
Ivabradine. In a large, randomized, double-blind, placebo-controlled trial involving 6558 patients with heart failure in sinus rhythm and heart rate ≥70 bpm (3241 on ivabradine; 30% with T2DM), ivabradine demonstrated a significant reduction in composite endpoints of cardiovascular death and hospital admission for worsening heart failure. The beneficial difference was similar in a pre-specified subgroup analysis of patients with and without DM.414
8.4 Non-pharmacological therapies for heart failure in diabetes mellitus
Cardiac resynchronization therapy and implantable cardioverter defibrillators. Cardiac resynchronization therapy is a guideline-recommended heart failure treatment, proved to reduce mortality in patients in NYHA function class III – IV, an LVEF ≤35% despite optimal pharmacological treatment, in sinus rhythm and with a prolonged QRS duration (≥120–130 ms).415 Despite a lack of subgroup analyses, there is no reason to believe that the effect of resynchronization therapy should be any different in patients with or without DM. Also, there is no additional benefit from implantable cardioverter defibrillators in a subgroup of patients with T2DM and heart failure, compared with patients free from this disease.416
Cardiac transplantation is an accepted treatment for end-stage heart failure. The presence of DM is not a contra-indication, but the stringent selection criteria have to be acknowledged. The higher likelihood of cerebrovascular disease, decreased renal function and increased risk of infection has to be considered and may contra-indicate heart transplantation more often in patients with- than in those without DM.417 DM was an independent risk factor for decreased 10-year survival in a large registry study of patients (n = 22 385) transplanted between 1987 and 1999.418
8.5 Glucose-lowering treatment in patients with heart failure
The impact of various glucose-lowering drugs on T2DM patients with heart failure was systematically reviewed by Gitt et al.419 They noted that the only drugs addressed in RCTs were thiazolidinediones, while evidence on other compounds is largely based on subgroup analyses of larger intervention studies in systolic heart failure, observational studies or on registries.
The use of metformin, the recommended first-hand glucose lowering treatment, has previously been contra-indicated in patients with heart failure because of concerns regarding lactic acidosis. This drug has, however, been reported to be associated with lower mortality rates, lower rates of all-cause hospital admission and fewer adverse events,420,421 and an accumulation of lactic acidosis was not verified in a study by Masoudi et al., who reported that 2.3% of metformin users had metabolic acidosis, in comparison with 2.6% in those not treated with metformin.422 In a nested case-control study including patients with newly diagnosed heart failure and DM, who were either exposed to glucose-lowering drugs or not, the use of metformin [adjusted OR 0.65 (0.48–0.87)] or metformin with or without other agents [OR 0.72 (0.59–0.90)] was associated with lower mortality, while other oral glucose-lowering agents or insulin were neutral in this respect.423
Recommendations on sulphonylureas and heart failure are based on observational data. No relationship was seen between sulphonylurea and heart failure mortality in UKPDS,152 but in a large number of patients (n = 12 272) in the Saskatchewan Health database, mortality (52 vs. 33%) and hospitalizations (85 vs. 77%) were higher among patients treated with sulphonylureas than with metformin during an average of 2.5 years of follow-up.424 A similar difference, to the disadvantage of sulphonylureas, was not confirmed in a study on Medicare beneficiaries, concluding that there was no association with such treatment (HR = 0.99; 95% CI 0.91–1.08) or insulin (HR = 0.96; 95% CI 0.88–1.05) and mortality.422
The PPARγ-activating thiazolidinediones induce sodium retention and plasma volume expansion. The resulting fluid retention may provoke or worsen heart failure and cause increased numbers of hospitalizations.175,425,426 In the review by Gitt et al.,419 it was stated that thiazolidinediones should not be used because of an increased event rate in patients with T2DM and established heart failure and a large increase in incident heart failure. Accordingly, this class of glucose-lowering drugs is discouraged when treating T2DM patients with heart failure.
There is a lack of information on the impact of GLP-1 analogues or DPP-4 inhibitors in patients with heart failure, although experimental and early clinical observations indicate favourable effects on myocardial performance.427
Regarding the use of insulin, a retrospective cohort study of 16 417 patients with DM and a primary diagnosis of heart failure did not reveal any association between the use of insulin and mortality (HR 0.96; 95% CI 0.88–1.05), in comparison with several other classes of glucose-lowering drugs.422 In the ORIGIN trial, people at high CVD risk plus IFG, IGT or T2DM received insulin glargine or standard care, which mainly included metformin and sulphonylurea treatment. During the 6.2-year-long follow-up period there was no difference in hospitalizations for heart failure.168
8.6 Gaps in knowledge
The impact of glucose-lowering drugs including metformin, GLP-1 analogues and DPP-IV inhibitors on the prevention of heart failure is unknown.
8.7 Recommendations for management of heart failure in diabetes
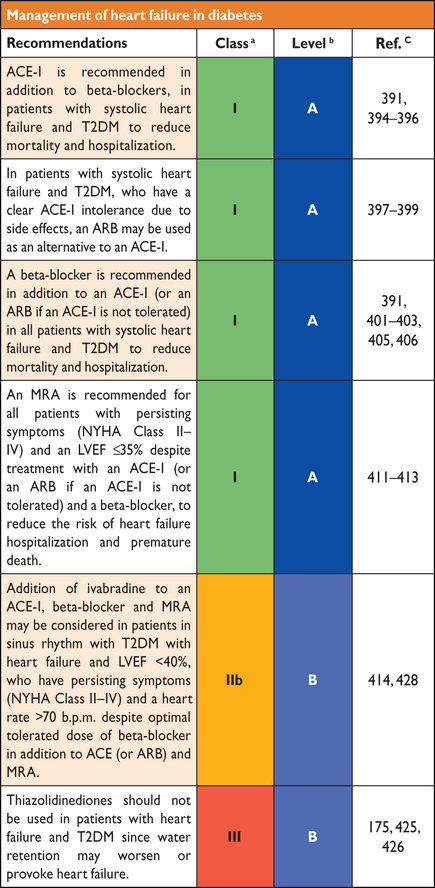 |
 |
ACE-I = angiotensin converting inhibitor; ARB = angiotensin receptor blocker; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
9. Arrhythmias: atrial fibrillation and sudden cardiac death
9.1 Diabetes mellitus and atrial fibrillation
Individuals with atrial fibrillation (AF) are at substantially increased risk of stroke and have twice the mortality rate from CVD as those in sinus rhythm.429,430 Diabetes mellitus is frequent in patients with AF. Community studies demonstrate the presence of DM in 13% of patients with AF.431 DM and AF share common antecedents, such as hypertension, atherosclerosis and obesity: however, the independent role of DM as a risk factor for AF has not been established.
The Manitoba Follow-up Study estimated the age-specific incidence of AF in 3983 men.432 DM was significantly associated with AF with a relative risk of 1.82 in univariate analysis. However, in the multivariable model, the association with DM was insignificant, suggesting that the increased risk may relate to ischaemic heart disease, hypertension or heart failure. In the Framingham Heart Study,433 DM was significantly associated with AF in both genders, even after adjustment for age and other risk factors (OR 1.4 for men and 1.6 for women). When developing a risk score for AF, the Framingham Heart study did not include DM as a significant predictor of AF.434 In another recent study, Nicholas et al. reported that DM was an independent predictor of AF in women only.435
A recent multi-centre study enrolling 11 140 DM patients confirmed that AF is relatively common in T2DM and demonstrated that when T2DM and AF co-exist, there is a substantially higher risk of all-cause mortality, cardiovascular death, stroke and heart failure.436 These findings suggest that AF identifies DM patients who are likely to obtain greater benefits from aggressive management of all cardiovascular risk factors. Because AF is asymptomatic—or only mildly symptomatic—in a substantial proportion of patients (about 30%), screening for AF can be recommended in selected patient groups with T2DM with any suspicion of paroxysmal or permanent AF by pulse palpation, routine 12-lead ECG, or Holter recordings.
Diabetes and risk of stroke in atrial fibrillation. Two recent systematic reviews have addressed the evidence base for stroke risk factors in AF and concluded that prior stroke/TIA/thrombo-embolism, age, hypertension, DM and structural heart disease are important risk factors.437,438
Diabetes and stroke risk stratification schemes. The simplest scheme is the CHADS2 [cardiac failure, hypertension, age, DM, stroke (doubled)] risk index. The 2010 ESC Guidelines for the management of AF, updated 2012, proposed a new scheme. The use of ‘low’, ‘moderate’ and ‘high’ risk has been re-emphasized, recognizing that risk is a continuum.439,440 The new scheme is expressed as an acronym CHA2DS2-VASc [cardiac failure, hypertension, age ≥75 (doubled), DM, stroke (doubled)-vascular disease, age 65–74 and sex category (female)]. It is based on a points system in which two points are assigned for history of stroke or TIA, or age ≥75 years and one point for the other variables. Heart failure is defined either as clinical heart failure or LV systolic dysfunction (EF <40%) and vascular disease as a history of MI, complex aortic plaque, or PAD.
Antithrombotic therapy in diabetes patients. A meta-analysis of 16 RCTs in 9874 patients was performed to characterize the efficacy of anticoagulant and antiplatelet agents for the prevention of stroke in AF.441 Oral anticoagulation was effective for primary and secondary prevention of stroke in studies comprising 2900 patients, with an overall 62% reduction of relative risk (95% CI 48–72). The absolute risk reduction was 2.7% per year for primary prevention and 8.4% per year for secondary prevention. Major extracranial bleeds were increased by anticoagulant therapy by 0.3% per year. Aspirin reduced risk of stroke by only 22% (95% CI 2–38), with an absolute risk reduction of 1.5% per year for primary prevention and 2.5% per year for secondary prevention. In five trials comparing anticoagulant therapy with antiplatelet agents in 2837 patients, warfarin was more effective than aspirin, with an RRR of 36% (95% CI 14–52). These responses were observed in both permanent and paroxysmal AF.
Supported by the results of several trials and the 2010 and in 2012 updated ESC Guidelines for management of AF,439,440 oral anticoagulation with vitamin K antagonists (VKAs)—or one of the new oral anticoagulants (NOAC; for further details see below)—are recommended in patients with AF. The choice of antithrombotic therapy should be based upon the absolute risk of stroke/thromboembolism and bleeding and the net clinical benefit for a given patient. Aspirin alone is not recommended for the prevention of thromboembolic disease in patients with DM and AF but, in patients unable or unwilling to use either VKAs or NOAC, the combination of aspirin and clopidogrel should be considered.442 VKA or NOAC should be used if there are one or more stroke risk factors, provided there are no contra-indications following careful assessment of the risk–benefit ratio and an appreciation of the patient's values and preferences.439,440 It can be concluded that VKA or NOAC should be used in all AF patients with DM unless contra-indicated, and if accepted by the patient. With the use of VKA, an international normalized ratio (INR) of 2.0–3.0 is the optimal range for prevention of stroke and systemic embolism in patients with DM. A lower target INR (1.8–2.5) has been proposed for the elderly but this is not based on evidence.
In the ACTIVE W warfarin was superior to clopidogrel plus aspirin (RRR 0.40; 95% CI 18–56), with no difference in rates of bleeding.442 The aspirin arm ACTIVE A aspirin found that major vascular events were reduced in patients receiving aspirin plus clopidogrel, compared with aspirin monotherapy (RR 0.89; 95% CI 0.81–0.98; P = 0.01).443 Thus, aspirin-plus-clopidogrel therapy may be considered as an interim measure if a VKA is unsuitable, but not as an alternative in patients at high bleeding risk. Combinations of VKA with antiplatelet therapy do not offer added beneficial effects on ischaemic stroke or vascular events and lead to more bleeding events,439 and such combinations should be avoided.
Two new classes of anticoagulants have been developed: oral direct thrombin inhibitors (e.g. dabigatran etexilate) and oral factor Xa inhibitors (e.g. rivaroxaban, apixaban, edoxiban, betrixiban). In the Randomized Evaluation of the Long-term anticoagulant therapy with dabigatran etexilate (RE-LY) study,444 dabigatran 110 mg b.i.d. was non-inferior to VKA for stroke prevention and systemic embolism with lower rates of major bleedings. Dabigatran 150 mg b.i.d. was associated with lower rates of stroke and systemic embolism with similar rates of major haemorrhages, compared with VKA therapy. The Apixaban VERsus acetylsalicylic acid to pRevent strOkES (AVERROES) study was stopped early, due to clear evidence of a reduction in stroke and systemic embolism with apixaban 5 mg b.i.d., compared with aspirin 81–324 mg once daily.445 A recent study, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), comparing warfarin with apixaban in patients with AF with a median CHADS2 score of 2.1, showed that apixaban 5 mg b.i.d. was superior to warfarin in preventing stroke or systemic embolism, caused less bleeding and resulted in lower mortality.446 Twenty-four per cent of the patients had DM. The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET) trial, comparing warfarin with rivaroxaban, showed the non-inferiority of rivaroxaban to warfarin in preventing stroke, systemic embolism or major bleeding among the AF patients with a relatively high CHADS2 score (median 3.5).447 These new drugs have the potential to be used as an alternative to warfarin, especially in patients intolerant to—or unsuitable for—VKAs. In analyses of pre-specified subgroups in the ROCKET trial, patients with DM had a level of protection similar to the overall study populations.
An assessment of bleeding risk should carried out before starting anticoagulation. Using a real-world cohort of 3978 European patients with AF from the Euro Heart Survey, a new simple bleeding score known as 'Hypertension, Abnormal renal/liver function (1 point each), Stroke, Bleeding history or predisposition, Labile INR, Elderly (>65), Drugs/alcohol concomitantly (1 point each)' (HAS-BLED) was developed,448 which includes hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (>65 years), drugs/alcohol, as risk factors of bleeding. A score ≥3 indicates high risk and some caution and regular review of the patients is needed following the initiation of antithrombotic therapy.
9.2 Sudden cardiac death
Clinical studies of sudden cardiac death in diabetes mellitus. Sudden cardiac death accounts for approximately 50% of all cardiovascular deaths. The majority are caused by ventricular tachyarrhythmia, often triggered by an ACS, which may occur without known cardiac disease or in association with structural heart disease.449,450 The published epidemiological studies in general population samples have shown that people with DM are at higher risk of sudden cardiac death. In the Framingham study, DM was associated with an increased risk of sudden cardiac death in all ages (almost four-fold) and was consistently greater in women than in men.451 The Nurses' Health Study,452 which included 121 701 women aged 30–55 years, followed for 22 years, reported that sudden cardiac death occurred as the first sign of heart disease in 69% of cases. DM was a strong risk factor, associated with three-fold increased risk of sudden death, while hypertension was associated with a 2.5-fold and obesity with a 1.6-fold increased risk. DM increases the RR for sudden cardiac death in different ethnic groups.453–455 A recent report from the ARIC investigators demonstrated that the magnitude of the relative increase in risk associated with DM was similar for sudden cardiac death and non-sudden cardiac death. In this study, DM attenuated the gender difference in absolute risk of sudden cardiac death.456
DM increases the cardiovascular mortality in patients with heart failure and in survivors of MI. In an analysis of the CHARM programme, DM was an independent predictor of mortality—including sudden cardiac death–in patients with heart failure independent of EF.457 In a series of 3276 post-infarction patients from Germany and Finland, the incidence of sudden cardiac death was higher in T2DM with an HR of 3.8 (95% CI 2.4–5.8; P < 0.001).458 The incidence of sudden cardiac death in post-infarction patients with DM and a LVEF >35% was equal to that of non-DM patients with an EF ≤35%. The incidence of sudden cardiac death was substantially increased among DM patients with an EF <35%, supporting the concept that a prophylactic implantable cardioverter defibrillator should be used in all symptomatic (NYHA Class II–IV) DM patients with an LVEF <35% unless contra-indicated. T2DM patients with congestive heart failure or post MI should have their LVEF measured, to identify candidates for prophylactic implantable cardioverter defibrillator therapy. Similarly, secondary prophylaxis with implantable cardioverter defibrillator therapy is indicated in DM patients resuscitated from ventricular fibrillation or sustained ventricular tachycardia, as recommended in the Guidelines.459 All post-infarction patients with heart failure should also be treated with beta-blocking drugs, which are well established as reducing sudden cardiac death.449,450
Pathophysiology of sudden cardiac death in diabetes mellitus. The causes underlying the increased vulnerability of the electrical substrate in DM are unclear and are likely to be consequent on several concomitant factors: (i) acute coronary occlusion and the presence and extent of CAD; (ii) myocardial fibrosis resulting in impaired LV filling (diastolic dysfunction) and systolic heart failure; (iii) microvascular disease and DM nephropathy; (iv) DM autonomic neuropathy; (v) abnormalities in electrical propagation in the myocardium reflected in ECG re-polarization and de-polarization abnormalities and (vi) obstructive sleep apnoea.459–466 Experimentally induced hypoglycaemia can also cause changes in cardiac electrophysiological properties. ‘Dead in bed’ syndrome is a term used to describe the unexpected death of young individuals with T1DM while sleeping, suggesting that hypoglycaemia may contribute to sudden cardiac death in DM.467
Jouven et al.,455 studied the RR of sudden cardiac death in groups of patients with different degrees of dysglycaemia and showed that higher values of glycaemia led to higher risk. Following adjustment for age, smoking habits, systolic blood pressure, heart disease and glucose-lowering treatment, even patients with borderline DM, defined as non-fasting glycaemia between 7.7 and 11.1 mmol/L (140 and 200 mg/dL), had an increased risk of sudden cardiac death (OR 1.24 compared with patients with normoglycaemia). The presence of microvascular disease, defined as retinopathy or proteinuria and female gender, increased risk in all groups. This study emphasizes that glucose intolerance seems to be a continuous variable directly related to the risk of sudden cardiac death, rather than supporting the previous view of risk being related to a specific threshold of glucose intolerance. This fits with the present concept that cardiovascular risk increases below present thresholds for DM already at glucose levels that have been considered fairly normal.
The Framingham investigators468 demonstrated, in a large community-based population that, after adjusting for co-variates, indices of reduced heart rate variability were influenced by plasma glucose. Hyperglycaemia—even mild—may be associated with lower heart rate variability.469 Similar findings were reported by the ARIC study,470 which showed that even patients with pre-diabetes have abnormalities of autonomic cardiac function and heart rate variability. These studies further confirm that glucose levels should be considered as a continuous variable influencing autonomic control of the heart. Unfortunately these studies were not designed to answer the question of whether reduced heart rate variability in DM is an independent predictor of sudden cardiac death. A recent study showed that measurement of autonomic markers, such as heart rate turbulence and deceleration capacity from 24-h Holter recordings, predicts the occurrence of cardiac death and sudden cardiac death among T2DM patients with recent MI.471
Cardiovascular autonomic neuropathy was significantly associated with subsequent mortality in people with DM in a meta-analysis of 15 studies.472 The Rochester DM neuropathy study was designed to define the risk factors for sudden cardiac death and the role of DM autonomic neuropathy in a population of 462 DM patients followed for 15 years.473 These data suggested that kidney dysfunction and atherosclerotic heart disease are the most important determinants of the risk of sudden cardiac death, whereas neither autonomic neuropathy nor QTc were independent predictors. This study did not include heart rate variability or other risk variables among the parameters introduced in multivariable analysis. In contrast, the results of the MONICA/KORA study reported that QTc was an independent predictor of sudden death, associated with a three-fold increase in patients with DM and a two-fold increase in those without.474 Measurements of heart rate variability and QTc may become valuable as predictors of sudden cardiac death in DM patients but evidence to support this as a general recommendation is still lacking.
On the basis of available evidence, it seems that all levels of glucose intolerance are associated with progressive development of a variety of abnormalities that adversely affect survival and predispose to sudden cardiac death. The identification of independent predictors of sudden cardiac death in DM has not progressed to a stage where it is possible to devise a risk stratification scheme for prevention.
Conclusions. Sudden cardiac death is a major cause of mortality in DM patients. While there are some risk factors for sudden cardiac death that may be specifically related to DM, such as microvascular disease and autonomic neuropathy, the focus should be on primary prevention of DM, atherosclerosis and CAD and secondary prevention of the cardiovascular consequences of these common conditions.
9.3 Gaps in knowledge
Information is lacking on the long-term impact of glycaemic control on the QTc interval.
What is the role of hypoglycaemia and other predictors in sudden cardiac death?
9.4 Recommendations for the management of arrhythmias in patients with diabetes mellitus
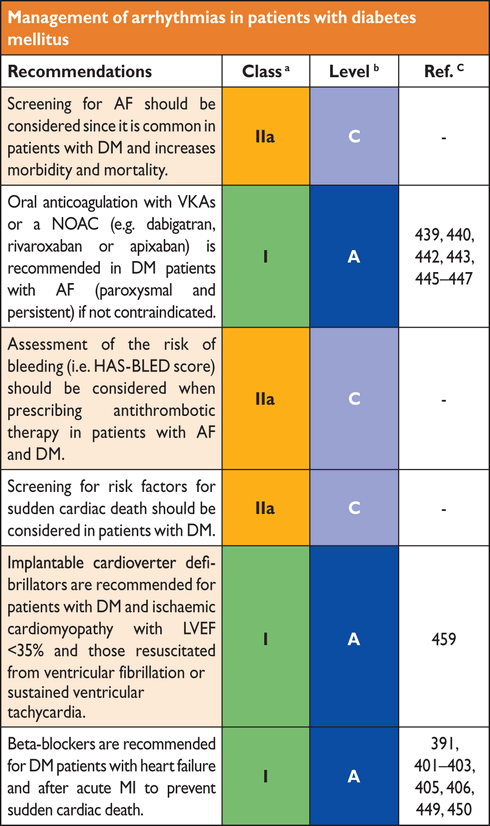 |
 |
AF = atrial fibrillation; DM = diabetes mellitus; EF = ejection fraction; LV = left ventricular; NOAC = new oral anticoagulants; VKA = vitamin K antagonist.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
10. Peripheral- and cerebrovascular disease
The definition of PAD used by the current ESC Guidelines includes atherosclerotic lesions in the extracranial carotid and vertebral, upper and lower extremity, mesenteric and renal arteries.475 The same definition will be used in the present document. Although abdominal aortic aneurysm is frequent in patients with DM, it is not included in the current PAD definition. Moreover, diagnosis and management of abdominal aortic aneurysm are carried out independent of the presence or absence of DM.
10.1 Peripheral artery disease
Diabetes mellitus is a risk factor for the development of atherosclerosis at any vascular site, but particularly for lower extremity artery disease (LEAD), for which it increases risk two- to four-fold and for carotid artery disease. In LEAD, cigarette smoking, DM and hypertension are important risk factors. Although the association of DM with LEAD is inconsistent on multivariable analysis, it appears that the duration and severity of DM particularly influence the risk of gangrene and ulceration.476,477 In population studies, the presence of carotid artery stenosis was associated with DM and other classical risk factors, irrespective of age.478–480 DM is present in a significant proportion of patients with multi-site atherosclerosis, who have a worse prognosis than those with a single disease location.481,482 Patients with DM should undergo comprehensive screening for the presence of PAD at different vascular sites. Medical history and physical examination (Tables 11 and 12) are the cornerstones of diagnostic workup and should include a review of the different vascular beds and their specific symptoms,475 although many patients remain asymptomatic. Further diagnostic evaluation and treatment should be applied according to the ESC Guidelines on PAD.475 Briefly, in all DM patients, clinical screening to detect PAD should be performed annually and beneficial lifestyle changes encouraged.483 All patients with PAD should receive adequate lipid-lowering, antihypertensive and antiplatelet treatment,125,274,484,485 with optimal glycaemic control.154,291,48611
Physical examination relevant to peripheral artery disease475
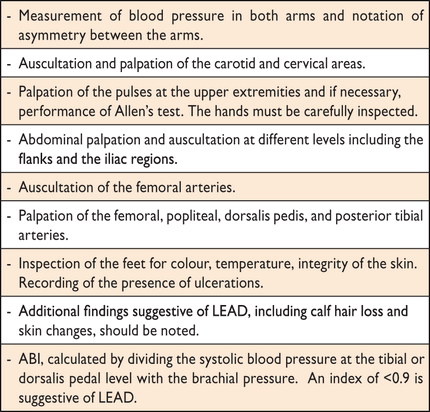 |
 |
ABI = ankle-brachial index; LEAD = lower extremity artery disease.
Physical examination relevant to peripheral artery disease475
 |
 |
ABI = ankle-brachial index; LEAD = lower extremity artery disease.
10.2 Lower extremity artery disease
Vascular obstructions are often located distally in patients with DM and typical lesions occur in the popliteal artery or in the vessels of the lower leg. In a cohort of 6880 patients over 65 years, one in five patients had LEAD, though only 10% were symptomatic.487 The incidence and prevalence of LEAD increase with age and duration of DM. The National Health and Nutrition Examination Survey (NHANES II) determined pulse amplitudes in adults and diminished or absent pulsation of the dorsalis pedis artery was found in 16% of adults with DM aged 35–54 years and in 24% of those aged 55–74 years.488 In many older patients, LEAD is already present at the time of diagnosis of DM. Progression of LEAD may result in foot ulceration, gangrene and ultimate amputation of part of the affected extremity. DM accounts for approximately 50% of all non-traumatic amputations in the United States and a second amputation is common. Mortality is increased in patients with LEAD and three-year survival after an amputation is less than 50%.485 Early diagnosis of LEAD in patients with DM is important for the prevention of progression of LEAD, as well as for prediction of the overall cardiovascular risk.
Diagnosis. Symptoms suggestive of claudication are walking impairment, e.g. fatigue, aching, cramping, or pain with localization to buttock, thigh, calf, or foot, particularly when symptoms are quickly relieved at rest. Palpation of pulses and visual inspection of the feet are essential. Dependent rubor, pallor when the foot is elevated, delayed hyperaemia when the foot is lowered, absence of hair growth and dystrophic toenails are signs of limb ischaemia. An objective measure of LEAD is the ABI, calculated by dividing the systolic blood pressure at the posterior tibial or dorsalis pedal level with the brachial systolic blood pressure. An index of <0.9 is suggestive of LEAD, particularly in the presence of symptoms or clinical findings such as bruits or absent pulses. An ABI <0.8 indicates PAD, regardless of symptoms. Sensitivity of ABI measurement may be increased after exercise. Post-exercise ABI may identify significant LEAD, even in people with a normal resting ABI.489 An ABI >1.40 indicates poorly compressible vessels as a result of stiff arterial walls (medial calcinosis) that can impede the correct estimation of pressure in the artery, even in severe ischaemia of the extremities.
Primary and secondary prevention of LEAD in patients with DM consists of lifestyle changes (addressing obesity, smoking and lack of exercise) and control of risk factors, including hyperglycaemia, hyperlipidaemia and hypertension.
Treatment. In a systematic review of RCTs of exercise programmes in symptomatic claudication, supervised exercise therapy was effective in increasing walking time, compared with standard care.490 Combination therapy including drugs and exercise is often used. Although several drugs such as cilostazol, naftidrofuryl and pentoxifylline increase walking distance in patients with intermittent claudication, their role remains uncertain. In addition, statin therapy has been reported to be of benefit by increasing walking distance in patients with PAD.475,491 If conservative therapy is unsuccessful, revascularization should be considered. In case of disabling claudication with culprit lesions located at aorta/iliac arteries, revascularization should be the first choice, along with management of risk factors.475 An algorithm for the treatment of intermittent claudication is shown in Figure 8.
Algorithm for treatment of intermittent claudication (from Tendera et al.475 with permission). CV = cardiovascular.
Critical limb ischaemia (CLI) is defined by the presence of ischaemic pain at rest and ischaemic lesions or gangrene attributable to arterial occlusive disease that is chronic and distinguishable from acute limb ischaemia. An algorithm for the management of CLI is provided in Figure 9.
Algorithm for the management of critical limb ischaemia (from Tendera et al.475 with permission). CVD = cardiovascular disease.
Importantly, beta-blockers are not contra-indicated in patients with LEAD and DM. A meta-analysis of 11 RCTs found that beta-blockers do not adversely affect walking capacity or symptoms of intermittent claudication in patients with mild-to-moderate PAD.492 At 32-month follow-up of 490 patients with PAD and prior MI, beta-blockers caused a 53% significant and independent decrease in new coronary events.493
Comprehensive management requires multidisciplinary care to control atherosclerotic risk factors, provision of revascularization where possible, optimization of wound care, wearing of appropriate shoes, treatment of infection and rehabilitation.475 The cornerstone of management is arterial reconstruction and limb salvage, which should be attempted without delay in all patients with critical limb ischaemia (CLI) when technically possible. The screening for—or assessment of—coronary or cerebrovascular diseases should not delay management of patients with CLI if clinically stable. Medical baseline therapy, including platelet inhibitors and statins, should be initiated according to principles outlined elsewhere in this document.475,494,495
The choice of revascularization strategy depends primarily on the anatomy of the arterial lesion. Outcomes of endovascular iliac artery repair in DM have been reported as similar to or worse than those without DM, and long-term patency is lower.496 Long-term patency rates of intravascular interventions in the tibio-peroneal region are low in patients with and without DM, but may be sufficient in the short term to facilitate healing of foot ulcers.496
The diabetic foot is a specific clinical entity that may involve neuropathy, trauma, arterial disease, infection and inflammation, often in combination. The serious consequences are ulceration, gangrene and high rates of amputation. Typically, in DM patients, LEAD is diffuse and particularly severe in distal vessels. When arterial disease is suspected, clinical examination of pulses with measurement of ABI is indicated to assess ischaemia. When, due to a heavily calcified arterial wall, the ABI is inconclusive, toe pressure, distal Doppler waveform analyses, or transcutaneous oxygen can assess the arterial status. When ischaemia is present, imaging should be performed to plan revascularization, which should be applied by the same criteria as for CLI. It is important to have direct flow to the foot to improve healing of ulcerations. Sufficient amputation is necessary in order to achieve adequate perfusion which, in combination with revascularization, will contain the ischaemic, inflammatory and infective process.
Follow-up should include patient education, smoking cessation, protective shoes, periodic foot care and reconstructive foot surgery as needed. The management of risk factors including glycaemic control and revascularization surveillance are mandatory.497
10.3 Carotid artery disease
Cerebrovascular disease is one of the leading causes of morbidity and mortality in Europe. DM is an independent risk factor for ischaemic stroke with an incidence 2.5–3.5 times higher than in people without DM.498,499 In this document, the discussion of stroke and transient ischaemic attack (TIA) prevention will be limited to the aspects related to carotid artery disease. It should be noted that only about 20% of all ischaemic strokes can be causally related to carotid artery stenosis.500 Although the presence of DM increases the likelihood of carotid artery disease, its presence does not change the general diagnostic and therapeutic approach.
Diagnosis. Carotid bruits are common in patients with carotid artery stenosis, although many remain asymptomatic regardless of lesion severity. Although the spectrum of symptoms is wide, only those who have suffered a stroke or TIA within the past six months are regarded as symptomatic.501,502 In this group of patients, the probability of recurrent stroke or TIA is high,503 therefore urgent imaging of the brain and supra-aortic vessels is mandatory in patients presenting with TIA or stroke. Duplex ultrasonography, computed tomography angiography and magnetic resonance imaging are indicated to evaluate carotid artery stenosis.
Treatment. Management depends on symptoms, severity of the lesion, prognosis for 5-year survival and the outcome of revascularization procedures. A management algorithm is shown in Figure 10.
Algorithm for the management of extra cranial carotid artery disease (from Tendera et al.,475 with permission).
Whilst carotid endarterectomy seems to offer a clear advantage over conservative treatment in patients with symptomatic carotid artery disease, the role of revascularization in asymptomatic patients remains less clear.475 It needs to be emphasized that most data in patients with no symptoms were collected before statins and antiplatelet agents became standard therapy. On the other hand, the results of both endarterectomy and carotid stenting have improved over time and the role of revascularization in this cohort needs to be reassessed.
10.4 Gaps in knowledge
In comparison with aspirin and clopidogrel, the efficacy of new antiplatelet drugs in patients with DM and PAD is not well known.
There is a need for comparisons of endovascular and surgical interventions in different subsets of patients with DM and concomitant carotid or lower extremity artery disease.
10.5 Recommendations for management of peripheral artery disease in diabetes
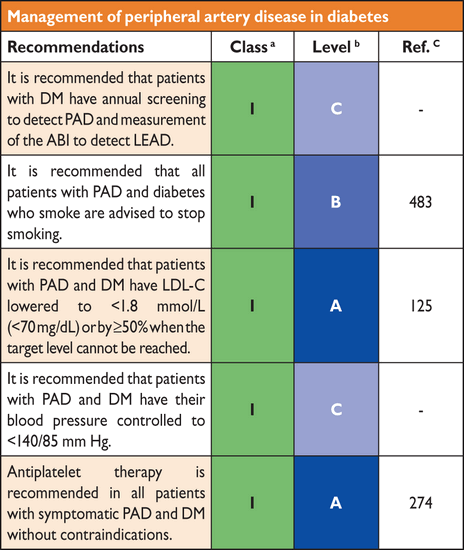 |
 |
ABI = ankle-brachial index; DM = diabetes mellitus; LDL-C = low-density lipoprotein cholesterol; LEAD = lower extremity artery disease; PAD = peripheral artery disease.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
11. Microvascular disease in the eyes and kidneys
Diabetes mellitus is an important risk factor for both renal and cardiovascular outcomes and renal impairment—in the form of elevated urinary albumin excretion and/or impaired GFR—is itself an independent predictor of cardiovascular outcomes.161,504,505 Urinary albumin excretion and loss of glomerular filtration rate (GFR) are to some extent beneficially modifiable by interventions that lower blood glucose and blood pressure.
Retinopathy is the most frequent microvascular complication in DM. Although the incidence has declined slowly following the implementation of intensive treatment regimens, vision-threatening proliferative retinopathy affects 50% of people with T1DM and 29% with T2DM develop vision-threatening macular oedema.506–508 Rapidly progressive retinopathy indicates increased cardiovascular risk and the combination of retinopathy and nephropathy predicts excess cardiovascular morbidity and mortality. In T2DM, advanced retinopathy more than doubles the risk of cardiovascular outcomes.509
11.1 Pathophysiology of microvascular disease
Renal neuropathic and ocular microvascular complications share some pathophysiological mechanisms that also affect the macrovascular endothelium. Chronic hyperglycaemia induces biochemical abnormalities causing protein glycation and overproduction of ROS, leading to vascular damage and responsive activation of tissue-specific growth/repair systems.510 The phenotypic characteristics of microvascular damage in DM are progressive vascular occlusion and increased vascular permeability. In the retina, progressive vascular occlusion promotes aberrant responsive neovascularization, causing proliferative retinopathy as an advanced complication. At any stage of progressive vasoregression, increased vascular permeability causes retinal thickening, which is clinically significant when affecting the central macula.
In the kidney, endothelial dysfunction and increased vascular permeability are clinically represented by microalbuminuria, and vascular occlusion corresponds to a progressive decline in renal function as measured by GFR.
11.2 Treatment and treatment targets
Lifestyle intervention. There are no trials proving that lifestyle interventions alone have an effect on the prevention of nephropathy, neuropathy or retinopathy.
Glycaemic control. (see section 6.2.1) As primary intervention, strict glycaemic control prevents both microvascular and cardiovascular outcomes with a long-term beneficial effect, both in T1DMand T2DM.151,152,154,155 In secondary prevention, strict glycaemic control prevents progression of renal impairment in both groups.160,511
Retinopathy. The recommended target for HbA1c in both T1DM and T2DM is <7% (<53 mmol/mol).152,512–514 Beyond a certain level of retinal damage, euglycaemia no longer provides a benefit against progression of retinopathy. For T1DM, this level of damage is precisely defined (i.e. moderate non-proliferative diabetic retinopathy), while in T2DM the point of no return is unknown.515 In T1DM, transient worsening of retinopathy due to euglycaemic re-entry (i.e. intensified insulin therapy after a prolonged period of insufficient glucose control) is outweighed by the long-term benefit of good glycaemic control.515 In contrast, in T2DM, a similar deterioration is not a consistent feature of improved glycaemic control. Progressing retinopathy benefits from multifactorial treatment.156 For further details, see Section 7.1.
Blood pressure – nephropathy. As a primary intervention, intensified blood pressure control using RAAS blockers prevents the onset of microalbuminuria in T2DM,191,193 but not in T1DM.516–518 As a secondary intervention, intensified blood pressure control using ACE-I to block the RAAS slowed progression of kidney disease in T1DM and reduced end-stage renal failure.519,520 A concomitant reduction in cardiovascular events was not demonstrated in these young patients, although it should be expected, considering the renal effects of ACE-I. In T2DM, high doses of ramipril prevented both renal and cardiovascular events.521 ARBs reduced progression from microalbuminuria to proteinuria and prevented renal events but not cardiovascular death.522,523 The currently recommended blood pressure target is <140/85 mm Hg but in patients with hypertension and nephropathy with overt proteinuria an even lower SBP (<130 mm Hg) may be considered if tolerated by the patient (see even Section 6.3.3).523
Blood pressure – retinopathy. Blood pressure control has beneficial effects on the progression of retinopathy. The recommended threshold is <140/85 mm Hg191,524 although other concomitant conditions, such as nephropathy, may require more intensive blood pressure control (systolic <130 mm Hg). Lowering blood pressure to this target does not adversely affect retinopathy. The DIabetic REtinopathy Candesartan Trials (DIRECT) studies investigated the effects of blood pressure-lowering with candesartan on the development and progression of retinopathy. There was a non-significant trend towards reduced progression of retinopathy, both in T1DM and T2DM.524,525
Lipid-lowering and antiplatelet therapy – nephropathy. Interventions on blood lipids and platelet aggregation have not been documented as altering renal disease in DM. Fibrates and PPARα agonists may reduce kidney function.526 In the FIELD study, fenofibrate reduced albuminuria and slowed estimated glomerular filtration rate (eGFR) loss over 5 years, despite initially and reversibly increasing plasma creatinine in T2DM.527
Recently, statin-plus-ezetimibe treatment provided cardiovascular protection in people with reduced kidney function including those with DM.238
Lipid-lowering and antiplatelet therapy – retinopathy. There are no clear target levels of lipids (cholesterol, triglycerides) for the prevention or retardation of retinopathy. In T2DM, the FIELD study reported that fenofibrate was associated with a reduction in requirement for laser therapy, although this effect appeared to be independent of effects on lipid levels. The ACCORD trial tested the outcome of lipid lowering, using combined statins and fenofibrate, on progression of retinopathy. Progression was defined as a three-step increase of the retinopathy level on to the Early Treatment of Diabetic Retinopathy Study severity scale, assessed by fundus photography from baseline, to the four-year study endpoint or pre-specified treatment events (photocoagulation or vitrectomy). The OR for reduction in progression of retinopathy by lipid treatment was 0.60 (95% CI 0.42–0.86; P < 0.0056). After 4 years the rates of progression of retinopathy were 7.3% with intensive glycaemia treatment, against 10.4% with standard therapy (adjusted OR 0.67; 95% CI 0.51–0.87; P = 0.003).513
Patients with T2DM require antiplatelet agents for secondary prevention of CVD. There is no specific contra-indication against the use of aspirin or other antiplatelet agents, as they do not increase the incidence of intravitreal haemorrhages.528 At doses given for secondary prevention of CVD, aspirin is unlikely to improve retinopathy outcome. Erythropoietin treatment in patients with diabetic kidney disease warrants close monitoring for retinopathy progression and for cardiovascular risk.528,529
Vision-threatening retinopathy. Severe non-proliferative or proliferative retinopathy or any level of DM-related macular oedema should immediately be referred to an experienced ophthalmologist. Vision-threatening proliferative retinopathy and macular oedema are treated by laser photocoagulation.528,530 In selected cases of severe non-proliferative DM-related retinopathy, laser photocoagulation may also be indicated. Selected cases of macular oedema with sub-foveal oedema and vision impairment <20/40 may benefit from intravitreal administration of ranibizumab, an inhibitor of vascular endothelial growth factor (VEGF). In four RCTs [Safety and Efficacy of Ranibizumab in Diabetic Macular Edema Study (RESOLVE), Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema (RESTORE), Ranibizumab Injection in Subjects With Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus (RIDE) and Ranibizumab Injection in Subjects With Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus (RISE)], one to two years of treatment with ranibizumab was more effective than sham or focal/grid laser therapy in improving best corrected visual acuity and reducing central retinal thickness in patients with visual impairment associated with diabetic macular oedema.531–533
11.3 Gaps in knowledge
The balance between the benefit to microvascular risk associated with tightening of glycaemic control and the risk of adverse CV outcomes is not understood.
11.4 Recommendations for management of microvascular disease in diabetes
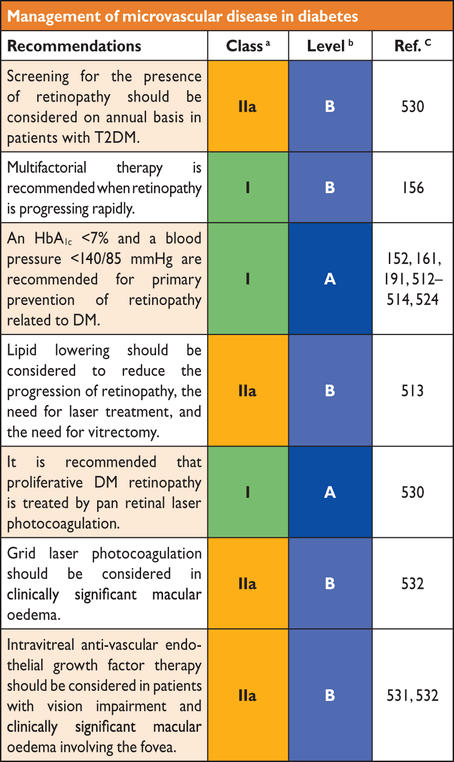 |
 |
BP = blood pressure; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1C; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
 |
 |
BP = blood pressure; DM = diabetes mellitus; HbA1c = glycated haemoglobin A1C; T2DM = type 2 diabetes mellitus.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
12. Patient-centred care
12.1 General aspects
The importance of multifactorial risk assessment and lifestyle management, including diet and exercise, in the prevention and treatment of DM and CVD has been emphasized in earlier sections. However, supporting patients in achieving and maintaining lifestyle changes on an individualized basis, using defined therapeutic goals and strategies, continues to be a substantial challenge. The intensive approach used successfully in clinical trials to prevent and treat DM and CVD is difficult to replicate in practice. Once intensive intervention stops, positive changes in lifestyle and risk factors may end, although ongoing booster sessions at intervals can maintain the effects.65
Effective strategies for supporting patients in achieving positive lifestyle changes and improving self-management can be recommended. Patient-centred care is an approach that facilitates shared control and decision-making between patient and provider; it emphasizes a focus on the whole person and their experiences of illness within social contexts, rather than a single disease or organ system, and it develops a therapeutic alliance between patient and provider.534 Patient-centred care fosters a multifactorial approach, working within the context of patient priorities and goals, and allows for lifestyle changes and treatments to be adapted and implemented within cultural beliefs and behaviours. Providers should take into account age, ethnic and gender differences in DM and CVD, including lifestyle, disease prevalence and presentation, response to treatment and outcomes.
Understanding the patient's perspective and priorities enables providers and patients to jointly develop realistic and acceptable goals and programmes for behavioural change and self-management. A Cochrane Collaboration systematic review of 11 clinical trials (n = 1532) concluded that group-based (≥6 participants), patient-centred education resulted in clinically relevant, significant improvements in glycaemic control, DM knowledge, triglyceride concentrations, blood pressure, medication reduction and self-management for 12–14 months. Benefits for 2–4 years, including decreased DM-related retinopathy, were apparent when group classes were provided on an annual basis.535 Cognitive behavioural strategies, including problem-solving, goal-setting, self-monitoring, ongoing support and feedback/positive reinforcement in individual or group-based sessions are effective in facilitating behavioural change, especially when multiple strategies are used.536–538 However, a systematic review of studies on increasing physical activity found the positive effect of these strategies to be short-term (six months) and to decline thereafter;538 this may simply indicate the need for subsequent booster sessions beginning around six months. Similar patient-centred cognitive educational strategies, along with simplification of dosing regimens and increasing convenience, can be effective in improving medication adherence.539–541 Research is still needed regarding the most effective strategy combinations and the duration, intensity and timing of sessions.
For patients with greater reluctance or resistance towards making behavioural changes, motivational interviewing is patient-centred counselling with the purpose of working through ambivalence and fostering a patient-driven agenda. Motivational interviewing has been effective in helping patients to decrease body mass index and systolic blood pressure and increase physical activity and fruit and vegetable consumption.542 Motivational interviewing techniques are often adapted and incorporated within prevention programmes.537
Multifaceted strategies are most effectively delivered through multidisciplinary teams. The International Diabetes Federation, Diabetes Roundtable and Global Partnership for Effective Diabetes Management are advocates for multidisciplinary team care in DM,543 and such teams are essential components of successful disease-management programmes for CVD.544 Nurse-led multidisciplinary programmes, including nurse case-management, have been effective in improving multiple cardiovascular risk factors and adherence in patients with CVD and DM within primary and secondary care.536,537,545,546
Patient-centred care emphasizes the person, their experiences, priorities and goals in managing various conditions, and the partnership between providers and patients. When this approach is used by a multidisciplinary team with skills in cognitive behavioural strategies, there will be increased success in supporting patients in achieving lifestyle changes and effectively self-managing their conditions. It is also important to recognise that single or limited interventions or sessions on behavioural change are not sufficient to maintain lifestyle changes and that ongoing support and booster sessions will be necessary for sustained change.
12.2 Gaps in Knowledge
Effects of patient-centred interventions on outcome measures, including micro- and macrovascular complications, are not known.
12.3 Recommendations for patient-centred care in diabetes
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting levels of evidence.
13. References
Author notes
Other ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), European Association of Cardiovascular Imaging (EACVI), European Association for Cardiovascular Prevention & Rehabilitation (EACPR), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA)
Working Groups: Coronary Pathophysiology and Microcirculation, Thrombosis, Cardiovascular Surgery
Councils: Cardiovascular Nursing and Allied Professions, Council for Cardiology Practice, Council on Cardiovascular Primary Care, Cardiovascular Imaging
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC.
Disclaimer. The ESC Guidelines represent the views of the ESC and EASD and were arrived at after careful consideration of the available evidence at the time they were written. Health professionals are encouraged to take them fully into account when exercising their clinical judgement. The guidelines do not, however, override the individual responsibility of health professionals to make appropriate decisions in the circumstances of the individual patients, in consultation with that patient and, where appropriate and necessary, the patient's guardian or carer. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.