-
PDF
- Split View
-
Views
-
Cite
Cite
Alberico L Catapano, Ian Graham, Guy De Backer, Olov Wiklund, M John Chapman, Heinz Drexel, Arno W Hoes, Catriona S Jennings, Ulf Landmesser, Terje R Pedersen, Željko Reiner, Gabriele Riccardi, Marja-Riita Taskinen, Lale Tokgozoglu, W M Monique Verschuren, Charalambos Vlachopoulos, David A Wood, Jose Luis Zamorano, Marie-Therese Cooney, ESC Scientific Document Group , 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias, European Heart Journal, Volume 37, Issue 39, 14 October 2016, Pages 2999–3058, https://doi.org/10.1093/eurheartj/ehw272
Close - Share Icon Share
The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS)
Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR)
List of abbreviations
- ABI
ankle-brachial index
- ACC
American College of Cardiology
- ACCELERATE
Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High-Risk for Vascular Outcomes
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACS
acute coronary syndrome
- AFCAPS/TEXCAPS
Air Force/Texas Coronary Atherosclerosis Prevention Study
- AHA
American Heart Association
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes
- ALT
alanine aminotransferase
- Apo
apolipoprotein
- ART
antiretroviral treatment
- ASSIGN
CV risk estimation model from the Scottish Intercollegiate Guidelines Network
- ASTRONOMER
Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin
- AURORA
A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events
- BIP
Bezafibrate Infarction Prevention study
- BMI
body mass index
- CABG
coronary artery bypass graft surgery
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CARE
Cholesterol and Recurrent Events
- CETP
cholesteryl ester transfer protein
- CHD
coronary heart disease
- CIMT
carotid intima-media thickness
- CK
creatine kinase
- CKD
chronic kidney disease
- CTT
Cholesterol Treatment Trialists
- CV
cardiovascular
- CVD
cardiovascular disease
- CYP
cytochrome P450
- 4D
Die Deutsche Diabetes Dialyse
- DASH
Dietary Approaches to Stop Hypertension
- DGAT-2
diacylglycerol acyltransferase-2
- DHA
docosahexaenoic acid
- DLCN
Dutch Lipid Clinic Network
- EAS
European Atherosclerosis Society
- EMA
European Medicines Agency
- EPA
eicosapentaenoic acid
- ER
extended release
- ESC
European Society of Cardiology
- ESRD
end-stage renal disease
- EU
European Union
- FACE-BD
Fondamental Academic Centers of Expertise in Bipolar Disorders
- FATS
Familial Atherosclerosis Treatment Study
- FCH
familial combined hyperlipidaemia
- FDA
US Food and Drug Administration
- FDC
fixed-dose combination
- FH
familial hypercholesterolaemia
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- FOCUS
Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention
- GFR
glomerular filtration rate
- GISSI
Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico
- GP
general practitioner
- GWAS
genome-wide association studies
- HAART
highly active antiretroviral treatment
- HATS
HDL-Atherosclerosis Treatment Study
- HbA1C
glycated haemoglobin
- HeFH
heterozygous familial hypercholesterolaemia
- HDL-C
high-density lipoprotein cholesterol
- HF
heart failure
- HHS
Helsinki Heart Study
- HIV
human immunodeficiency virus
- HMG-CoA
hydroxymethylglutaryl-coenzyme A
- HPS
Heart Protection Study
- HPS2-THRIVE
Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events
- HoFH
homozygous familial hypercholesterolaemia
- HTG
hypertriglyceridaemia
- HR
hazard ratio
- hs-CRP
high-sensitivity C-reactive protein
- ICD
International Classification of Diseases
- IDEAL
Incremental Decrease In End-points Through Aggressive Lipid-lowering Trial
- IDL
intermediate-density lipoproteins
- ILLUMINATE
Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- JUPITER
Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
- KDIGO
Kidney Disease: Improving Global Outcomes
- LAL
lysosomal acid lipase
- LCAT
lecithin cholesterol acyltransferase
- LDL-C
low-density lipoprotein cholesterol
- LDLR
low-density lipoprotein receptor
- LEAD
lower extremities arterial disease
- LIPID
Long-Term Intervention with Pravastatin in Ischemic Disease
- LPL
lipoprotein lipase
- Lp
lipoprotein
- MetS
metabolic syndrome
- MI
myocardial infarction
- MTP
microsomal triglyceride transfer protein
- MUFA
monounsaturated fatty acid
- NICE
National Institute for Health and Care Excellence
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NNT
number needed to treat
- NPC1L1
Niemann-Pick C1-like protein 1
- NSTE-ACS
non-ST elevation acute coronary syndrome
- NYHA
New York Heart Association
- PAD
peripheral arterial disease
- PCI
percutaneous coronary intervention
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PPAR-α
peroxisome proliferator-activated receptor-α
- PROCAM
Prospective Cardiovascular Munster Study
- PROSPER
Prospective Study of Pravastatin in the Elderly at Risk
- PUFA
polyunsaturated fatty acid
- RAAS
renin–angiotensin–aldosterone system
- RCT
randomized controlled trial
- REACH
Reduction of Atherothrombosis for Continued Health
- REDUCE-IT
Reduction of Cardiovascular Events with EPA-Intervention Trial
- REVEAL
Randomized Evaluation of the Effects of Anacetrapib Through Lipid modification
- RR
relative risk
- RYR
red yeast rice
- 4S
Scandinavian Simvastatin Survival Study
- SALTIRE
Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression
- SAGE
Studies Assessing Goals in the Elderly
- SCORE
Systemic Coronary Risk Estimation
- SEAS
Simvastatin and Ezetimibe in Aortic Stenosis
- SFA
saturated fatty acid
- SHARP
Study of Heart and Renal Protection
- SLE
systemic lupus erythematosus
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- STEMI
ST elevation myocardial infarction
- STRENGTH
Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia
- TIA
transient ischaemic attack
- TC
total cholesterol
- T2DM
type 2 diabetes mellitus
- TG
triglyceride
- TNT
Treatment to new targets
- TRL
triglyceride-rich lipoprotein
- ULN
upper limit of normal
- UMPIRE
Use of a Multidrug Pill In Reducing cardiovascular Events
- VA-HIT
Veterans Affairs High-density lipoprotein Intervention Trial
- VLDL
very low-density lipoprotein
- WHO
World Health Organization
Preamble
Guidelines summarize and evaluate all available evidence on a particular issue at the time of the writing process, with the aim of assisting health professionals in selecting the best management strategies for an individual patient with a given condition, taking into account the impact on outcome as well as the risk–benefit ratio of particular diagnostic or therapeutic means. Guidelines and recommendations should help health professionals to make decisions in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC) and by the European Atherosclerosis Society (EAS), as well as by other societies and organisations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
Members of this Task Force were selected by the ESC, including representation from the European Association for Cardiovascular Prevention & Rehabilitation (EACPR), and EAS to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management (including diagnosis, treatment, prevention and rehabilitation) of a given condition according to ESC Committee for Practice Guidelines (CPG) policy and approved by the EAS. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk–benefit ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined in Tables 1 and 2
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period must be notified to the ESC and EAS and updated. The Task Force received its entire financial support from the ESC and EAS without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines produced by task forces, expert groups or consensus panels. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts, and in this case by EAS-appointed experts. After appropriate revisions the Guidelines are approved by all the experts involved in the Task Force. The finalized document is approved by the CPG and EAS for publication in the European Heart Journal and in Atherosclerosis. The Guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC and EAS Guidelines covers not only integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket guideline versions, summary slides, booklets with essential messages, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.) are produced. These versions are abridged and thus, if needed, one should always refer to the full text version, which is freely available on the ESC website. The National Societies of the ESC are encouraged to endorse, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Surveys and registries are needed to verify that real-life daily practice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, disseminating them and implementing them into clinical practice.
Health professionals are encouraged to take the ESC and EAS Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC and EAS Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
1. What is cardiovascular disease prevention?
1.1 Definition and rationale
Cardiovascular disease (CVD) kills >4 million people in Europe each year. It kills more women [2.2 million (55%)] than men [1.8 million (45%)], although cardiovascular (CV) deaths before the age of 65 years are more common in men (490 000 vs. 193 000).1 Prevention is defined as a coordinated set of actions, at the population level or targeted at an individual, aimed at eradicating, eliminating or minimizing the impact of CV diseases and their related disability. CVD remains a leading cause of morbidity and mortality, despite improvements in outcomes for CVD. More patients are surviving their first CVD event and are at high risk of recurrences. In addition, the prevalence of some risk factors, notably diabetes and obesity, is increasing. The importance of CVD prevention remains undisputed and should be delivered at different levels: (i) in the general population by promoting healthy lifestyle behaviour2 and (ii) at the individual level, in those at moderate to high risk of CVD or patients with established CVD, by tackling an unhealthy lifestyle (e.g. poor-quality diet, physical inactivity, smoking) and by reducing increased levels of CV risk factors such as increased lipid or blood pressure levels. Prevention is effective in reducing the impact of CVD; the elimination of health risk behaviours would make it possible to prevent at least 80% of CVD and even 40% of cancers, thus providing added value for other chronic diseases.3,4
1.2 Development of the Joint Task Force guidelines
The present guidelines represent an evidence-based consensus of the European Task Force including the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS).
By appraising the current evidence and identifying remaining knowledge gaps in managing the prevention of dyslipidaemias, the Task Force formulated recommendations to guide actions to prevent CVD in clinical practice by controlling elevated lipid plasma levels. The Task Force followed the quality criteria for development of guidelines, which can be found at http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines. Recommendations are graded in classes (Table 1) and in levels of evidence (Table 2).
This document has been developed for healthcare professionals to facilitate informed communication with individuals about their CV risk and the benefits of adopting and sustaining a healthy lifestyle and of early modification of their CV risk. In addition, the guidelines provide tools for healthcare professionals to promote up-to-date intervention strategies and integrate these strategies into national or regional prevention frameworks and to translate them into locally delivered healthcare services, in line with the recommendations of the World Health Organization (WHO) Global Status Report on Noncommunicable Diseases 2010.5
A lifetime approach to CV risk is considered.6 This implies that apart from improving lifestyle habits and reducing risk factor levels in patients with established CVD and in those at increased risk of developing CVD, healthy people of all ages should be encouraged to adopt or sustain a healthy lifestyle. Healthcare professionals play an important role in achieving this in their clinical practice.
1.3 Cost-effectiveness of prevention
In 2009, healthcare costs related to CVD in Europe amounted to €106 billion, representing ∼9% of the total healthcare expenditure across the European Union (EU).8 In the USA, direct annual costs of CVD are projected to triple between 2010 and 2030.9 Thus, CVD represents a considerable economic burden to society, and this necessitates an effective approach to CVD prevention. There is consensus in favour of an approach combining strategies to improve CV health across the population at large from childhood onwards, with actions to improve CV health in individuals at increased risk of CVD or with established CVD.
Most studies assessing the cost-effectiveness of prevention of CVD combine evidence from clinical research with simulation approaches, while data from randomized controlled trials (RCTs) are relatively scarce.7,10,11 Cost-effectiveness results strongly depend on parameters such as the target population's age, the overall population risk of CVD and the cost of interventions. Hence, results obtained in one country might not be valid in another. Furthermore, changes such as the introduction of generic drugs can considerably change cost-effectiveness.12 In general, lifestyle changes may be more cost effective at the population level than drug treatments (Table 3).
Suggestions for implementing healthy lifestyles
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Suggestions for implementing healthy lifestyles
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
More than half of the reduction in CV mortality in the last three decades has been attributed to population-level changes in CV risk factors, primarily reductions in cholesterol and blood pressure levels and smoking.13–16 This favourable trend is partly offset by increases in other major risk factors, such as obesity and type 2 diabetes.13–16 Ageing of the population also contributes to increasing the absolute number of CVD events.17
Several population-level interventions have proven to efficiently affect lifestyle in individuals, leading to this success: awareness and knowledge of how lifestyle risk factors lead to CVD increased in recent decades and undoubtedly contributed to the decline in smoking and cholesterol levels. Moreover, legislation promoting a healthy lifestyle, such as reduced salt intake and smoking bans, are cost effective in preventing CVD.18–22
Lowering blood cholesterol levels using statins10,11,23–25 and improving blood pressure control are also cost effective.26,27 Importantly, a sizable portion of patients on hypolipidaemic or antihypertensive drug treatment fail to take their treatment adequately or to reach their therapeutic goals,28,29 with clinical and economic consequences.30 Reinforcing measures aimed at improving adherence to treatment is cost effective.31,32
It has been suggested that the prescription to the whole population older than 55 years of age of a single pill containing a combination of CV drugs (the polypill) could prevent as much as 80% of CVD events33 and be cost effective.34 Part of the cost-effectiveness of the polypill is due to improvement in adherence to treatment, but which combination of drugs is most cost effective in which target population remains to be assessed.35
Considerable evidence has quantified the relative efforts and costs in relation to health impact. The efforts may be depicted in the health impact pyramid (Figure 1), where interventions with the broadest impact on populations represent the base and interventions with considerable individual effort are at the top.36
The cost-effectiveness of CVD prevention has been calculated in various contexts. According to the WHO, policy and environmental changes could reduce CVD in all countries for <US$1 per person per year, while interventions at the individual level are considerably more expensive.37. A report from the National Institute for Health and Care Excellence (NICE) estimated that a UK national programme reducing population CV risk by 1% would prevent 25 000 CVD cases and generate savings of €40 million per year.38 Coronary artery disease (CAD) mortality rates could be halved by only modest risk factor reduction,39 and it has been suggested that eight dietary priorities alone could halve CVD death.40
There is consensus that all the levels of the pyramid should be targeted but that emphasis should be put on the second level. Targeting lower levels in the health impact pyramid will also address the socio-economic divide in CV health, which has not diminished despite major improvements in the treatment of CVD in recent decades.9,10
2. Total cardiovascular risk
2.1 Total cardiovascular risk estimation
CV risk in the context of these guidelines means the likelihood of a person developing a fatal or non-fatal atherosclerotic CV event over a defined period of time.
2.1.1 Rationale for assessing total cardiovascular disease risk
All current guidelines on the prevention of CVD in clinical practice recommend the assessment of total CAD or CV risk, because atherosclerotic CVD is usually the product of a number of risk factors, and prevention of CVD in a given person should be adapted to his/her total CV risk: the higher the risk, the more intense the action should be.
Many risk assessment systems are available and have been comprehensively reviewed, including different Framingham models,41 Systemic Coronary Risk Estimation (SCORE),42 ASSIGN (CV risk estimation model from the Scottish Intercollegiate Guidelines Network),43 Q-Risk,44 Prospective Cardiovascular Munster Study (PROCAM),45 Reynolds,46,47 CUORE,48 the Pooled Cohort equations49 and Globorisk.50 Most guidelines use one of these risk estimation systems.50–52
One of the advantages of the SCORE system is that it can be recalibrated for use in different populations by adjustment for secular changes in CVD mortality and risk factor prevalences. Calibrated country-specific versions exist for Belgium, Cyprus, Czech Republic, Germany, Greece, Poland, Slovakia, Spain, Switzerland and Sweden, and country-specific electronic versions for Bosnia and Herzegovina, Croatia, Estonia, France, Romania, Russian Federation and Turkey can be found at http://www.heartscore.org. Other risk estimation systems can also be recalibrated, but the process is easier for mortality than for total events. The European Guidelines on CVD prevention in clinical practice (version 2012)6 recommend use of the SCORE system because it is based on large, representative European cohort datasets.
Risk charts such as SCORE are intended to facilitate risk estimation in apparently healthy persons with no documented CVD. Patients who have had a clinical event such as acute coronary syndrome (ACS) or a stroke are at very high risk of a further event and automatically qualify for risk factor evaluation and management (Table 6).
Simple principles of risk assessment, developed in these guidelines, can be defined as follows:
Persons with
documented CVD
type 1 or type 2 diabetes
very high levels of individual risk factors
chronic kidney disease (CKD) (refer to section 9.9)
For all other people, the use of a risk estimation system such as SCORE is recommended to estimate total CV risk since many people have several risk factors that, in combination, may result in unexpectedly high levels of total CV risk.
SCORE chart: 10-year risk of fatal cardiovascular disease (CVD) in populations at high CVD risk based on the following risk factors: age, gender, smoking, systolic blood pressure, and total cholesterol. To convert the risk of fatal CVD to risk of total (fatal + nonfatal) hard CVD, multiply by 3 in men and 4 in women, and slightly less in old people. Note: the SCORE chart is for use in people without overt CVD, diabetes, chronic kidney disease, familial hypercholesterolaemia or very high levels of individual risk factors because such people are already at high-risk and need intensive risk factor advice.
SCORE chart: 10-year risk of fatal cardiovascular disease (CVD) in populations at low CVD risk based on the following risk factors: age, gender, smoking, systolic blood pressure, and total cholesterol. To convert the risk of fatal CVD to risk of total (fatal + non-fatal) hard CVD, multiply by 3 in men and 4 in women, and slightly less in old people. Note: the SCORE chart is for use in people without overt CVD, diabetes, chronic kidney disease, familial hypercholesterolaemia, or very high levels of individual risk factors because such people are already at high-risk and need intensive risk factor advice.
The reasons for retaining a system that estimates fatal as opposed to total fatal + non-fatal events are that non-fatal events are dependent on definition, developments in diagnostic tests and methods of ascertainment, all of which can vary, resulting in very variable multipliers to convert fatal to total events. In addition, total event charts, in contrast to those based on mortality, cannot easily be recalibrated to suit different populations.
Naturally, the risk of total fatal and non-fatal events is higher, and clinicians frequently ask for this to be quantified. The SCORE data indicate that the total CVD event risk is about three times higher than the risk of fatal CVD for men, so that a SCORE risk of 5% translates into a CVD risk of ∼15% of total (fatal + non-fatal) hard CVD endpoints; the multiplier is ∼4 in women and lower in older persons.
Clinicians often ask for thresholds to trigger certain interventions. This is problematic since risk is a continuum and there is no threshold at which, for example, a drug is automatically indicated. This is true for all continuous risk factors such as plasma cholesterol or systolic blood pressure. Therefore, the goals that are proposed in this document reflect this concept.
A particular problem relates to young people with high levels of risk factors; a low absolute risk may conceal a very high relative risk requiring intensive lifestyle advice. To motivate young people not to delay changing their unhealthy lifestyle, an estimate of their relative risk, illustrating that lifestyle changes can reduce relative risk substantially, may be helpful (Figure 4).
Relative risk chart for 10-year cardiovascular mortality. Please note that this chart shows RELATIVE not absolute risk. The risks are RELATIVE to 1 in the bottom left. Thus, a person in the top right hand box has a relative risk that is 12 times higher than a person in the bottom left.
Another approach to this problem in young people is to use CV risk age. The risk age of a person with several CV risk factors is the age of a person with the same level of risk but with ideal levels of risk factors. Thus a high-risk 40-year-old may have a risk age ≥60 years. Risk age is an intuitive and easily understood way of illustrating the likely reduction in life expectancy that a young person with a low absolute but high relative risk of CVD will be exposed to if preventive measures are not adopted. Risk age can be estimated visually by looking at the SCORE chart (as illustrated in Figure 5). In this chart, the risk age is calculated compared with someone with ideal risk factor levels, which have been taken as non-smoking, total cholesterol of 4 mmol/L (155 mg/dL) and systolic blood pressure of 120 mmHg. Risk age is also automatically calculated as part of the latest revision of HeartScore (http://www.HeartScore.org).
Risk age has been shown to be independent of the CV endpoint used,51,52 which bypasses the dilemma of whether to use a risk estimation system based on CVD mortality or on the more attractive but less reliable endpoint of total CVD events. Risk age can be used in any population regardless of baseline risk or secular changes in mortality, and therefore avoids the need for recalibration. At present, risk age is recommended for helping to communicate about risk, especially to younger people with a low absolute risk but a high relative risk. It is not currently recommended to base treatment decisions on risk age.
Lifetime risk is another approach to illustrating the impact of risk factors that may be useful in younger people.53 The greater the burden of risk factors, the higher the lifetime risk. This approach produces higher risk figures for younger people because of their longer exposure times. It is therefore more useful as a way of illustrating risk than as a guide to treatment because therapeutic trials have been based on a fixed follow-up period and not on lifetime risk and such an approach would likely lead to excessive use of drugs in young people.
Another problem relates to old people. In some age categories the majority, especially of men, will have estimated CV death risks exceeding the 5–10% level, based on age (and gender) only, even when other CV risk factor levels are relatively low. This could lead to excessive use of drugs in the elderly and should be evaluated carefully by the clinician. Recent work has shown that β-coefficients are not constant with ageing and that SCORE overestimates risk in older people.54 This article includes illustrative charts in subjects older than 65 years of age. While such subjects benefit from smoking cessation and control of hypertension and hyperlipidaemia, clinical judgement is required to avoid side effects from overmedication.
SCORE charts are available for both total cholesterol (TC) and the TC:high-density lipoprotein cholesterol (HDL-C) ratio. However, subsequent work on the SCORE database has shown that HDL-C can contribute more to risk estimation if entered as a separate variable as opposed to the ratio. For example, HDL-C modifies risk at all levels of risk as estimated from the SCORE cholesterol charts.55 Furthermore, this effect is seen in both genders and in all age groups, including older women. This is particularly important at levels of risk just below the 5% threshold for intensive risk modification; many of these subjects will qualify for intensive advice if their HDL-C is low. Charts including HDL-C are available on the ESC website (http://www.escardio.org/guidelines). The additional impact of HDL-C on risk estimation is illustrated in Figures 6 and 7. In these charts, HDL-C is used categorically. The electronic version of SCORE, HeartScore (http://www.heartscore.org), has been modified to take HDL-C into account on a continuous basis, which is even better; we recommend its use in order to increase the accuracy of the risk evaluation. Overall, HDL-C has a modest but useful effect in refining risk estimation,56 but this may not be universal, as its effect may not be seen in some low-risk populations, particularly with a relatively high mean HDL-C level.57
Risk function without high-density lipoprotein-cholesterol (HDL-C) for women in populations at high cardiovascular disease risk, with examples of the corresponding estimated risk when different levels of HDL-C are included.
Risk function without high-density lipoprotein-cholesterol (HDL-C) for men in populations at high cardiovascular disease risk, with examples of the corresponding estimated risk when different levels of HDL-C are included.
2.1.2 How to use the risk estimation charts
When it comes to European countries and to countries with cardiology societies that belong to the ESC, the low-risk charts should be considered for use in Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, The Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland and the United Kingdom. While any cut-off point is arbitrary and open to debate, in these guidelines the cut-off points for calling a country ‘low risk’ are based on age-adjusted 2012 CVD mortality rates (<225/100 000 in men and <175/100 000 in women) (http://apps.who.int/gho/data/node.main.A865CARDIOVASCULAR?lang=en).
The high-risk charts should be considered in all other countries. Of these, some are at very high risk, and the high-risk chart may underestimate risk in these countries. These are countries with a CVD mortality rate more than double the cut-off of low-risk countries according to 2012 WHO statistics (http://apps.who.int/gho/data/node.main.A865CARDIOVASCULAR?lang=en): ≥450/100 000 for men or ≥350/100 000 for women (Albania, Algeria, Armenia, Azerbaijan, Belarus, Bulgaria, Egypt, Georgia, Kazakhstan, Kyrgyzstan, Latvia, FYR Macedonia, Republic of Moldova, Russian Federation, Syrian Arab Republic, Tajikistan, Turkmenistan, Ukraine and Uzbekistan). The remaining high-risk countries are Bosnia and Herzegovina, Croatia, Estonia, Hungary, Lithuania, Montenegro, Morocco, Poland, Romania, Serbia, Slovakia, Tunisia and Turkey. Note that several countries have undertaken national recalibrations to allow for time trends in mortality and risk factor distributions. Such charts are likely to represent current risk levels better.
Social deprivation and psychosocial stress set the scene for increased risk.57 For those at intermediate risk, other factors, including metabolic factors such as increased apolipoprotein B (apoB), lipoprotein(a) (Lp(a)), triglycerides (TGs) or high-sensitivity C-reactive protein (hs-CRP) or the presence of albuminuria, may improve risk classification. Many other biomarkers are also associated with increased CVD risk, although few of these have been shown to be associated with appreciable reclassification. Total CV risk will also be higher than indicated in the SCORE charts in asymptomatic persons with abnormal markers of subclinical atherosclerotic vascular damage detected by coronary artery calcium (CAC), ankle-brachial index (ABI), pulse wave velocity or carotid ultrasonography. In studies comparing these markers, CAC had the best reclassification ability.58–60
Subjects in need of reclassification are those belonging to the intermediate CV risk group. Therefore the use of methods to detect these markers should be of interest in that group (class IIa, level of evidence B). Cut-off values that should be used in considering these markers as modifiers of total CV risk are CAC score >400 Agatston units, ABI <0.9 or >1.40, aortic pulse wave velocity of 10 m/s and the presence of plaques on carotid ultrasonography. Some factors such as a high HDL-C or apoA1 and a family history of longevity can also reduce risk.
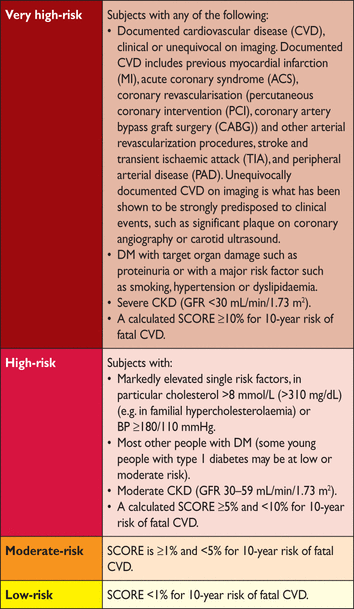
2.2 Risk levels
A total CV risk estimate is part of a continuum. The cut-off points that are used to define high risk are in part arbitrary and based on the risk levels at which benefit is evident in clinical trials. In clinical practice, consideration should be given to practical issues in relation to the local healthcare and health insurance systems. Not only should those at high risk be identified and managed, but those at moderate risk should also receive professional advice regarding lifestyle changes; in some cases drug therapy will be needed to control their plasma lipids.
In these subjects we realistically can
– prevent further increase in total CV risk,
– increase awareness of the danger of CV risk,
– improve risk communication and
– promote primary prevention efforts.
With these considerations one can propose the following levels of total CV risk (Table 4).
Risk categories
 |
 |
ACS = acute coronary syndrome; AMI = acute myocardial infarction; BP = blood pressure; CKD = chronic kidney disease; DM = diabetes mellitus; GFR = glomerular filtration rate; PAD = peripheral artery disease; SCORE = systematic coronary risk estimation; TIA = transient ischaemic attack.
Risk categories
 |
 |
ACS = acute coronary syndrome; AMI = acute myocardial infarction; BP = blood pressure; CKD = chronic kidney disease; DM = diabetes mellitus; GFR = glomerular filtration rate; PAD = peripheral artery disease; SCORE = systematic coronary risk estimation; TIA = transient ischaemic attack.
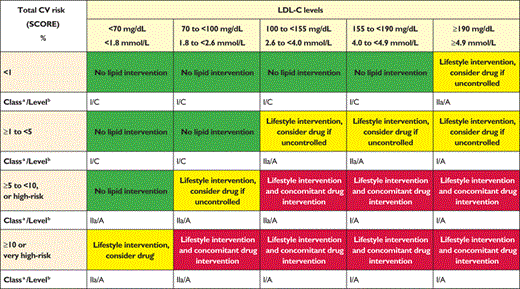
2.2.1 Risk-based intervention strategies
Table 5 presents suggested intervention strategies as a function of total CV risk and low-density lipoprotein cholesterol (LDL-C) level. This graded approach is based on evidence from multiple meta-analyses and individual RCTs, which show a consistent and graded reduction in CVD risk in response to reductions in TC and LDL-C levels.61–71 These trials are consistent in showing that the higher the initial LDL-C level, the greater the absolute reduction in risk, while the relative risk reduction remains constant at any given baseline LDL-C level. Advice on individual drug treatments is given in section 6.
Intervention strategies as a function of total cardiovascular risk and low-density lipoprotein cholesterol level
 |
 |
CV = cardiovascular; LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Estimation.
aClass of recommendation.
bLevel of evidence.
cIn patients with myocardial infarction, statin therapy should be considered irrespective of total cholesterol levels
Intervention strategies as a function of total cardiovascular risk and low-density lipoprotein cholesterol level
 |
 |
CV = cardiovascular; LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Estimation.
aClass of recommendation.
bLevel of evidence.
cIn patients with myocardial infarction, statin therapy should be considered irrespective of total cholesterol levels
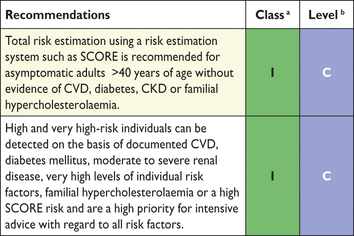
Recommendations for risk estimation
 |
 |
CVD = cardiovascular disease; SCORE = Systemic Coronary Risk Estimation.
aClass of recommendation.
bLevel of evidence.
Recommendations for risk estimation
 |
 |
CVD = cardiovascular disease; SCORE = Systemic Coronary Risk Estimation.
aClass of recommendation.
bLevel of evidence.
3. Evaluation of laboratory lipid and apolipoprotein parameters
Screening for dyslipidaemia is always indicated in subjects with clinical manifestations of CVD, in clinical conditions associated with increased risk for CVD and whenever risk factor screening is considered. In several clinical conditions, dyslipidaemia may contribute to an increased risk of developing CVD. Autoimmune chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus (SLE) and psoriasis are associated with increased CV risk and dyslipidaemia. Furthermore, in women, diabetes or hypertension during pregnancy are risk indicators, and in men, erectile dysfunction. Patients with CKD are also at increased risk for CVD events and should be screened for dyslipidaemias. Clinical manifestations of genetic dyslipidaemias, including xanthomas, xanthelasmas and premature arcus cornealis (<45 years), should be sought because they may signal the presence of a severe lipoprotein disorder, especially familial hypercholesterolaemia (FH), which is the most frequent monogenic disorder associated with premature CVD. Antiretroviral therapies may be associated with accelerated atherosclerosis. Screening for dyslipidaemias is also indicated in patients with peripheral arterial disease (PAD) or in the presence of increased carotid intima-media thickness (CIMT) or carotid plaques.
Screening for dyslipidaemias should be considered in all adult men ≥40 years of age and in women ≥50 years of age or post-menopausal, particularly in the presence of other risk factors (see section 2.2). It is also indicated to screen offspring of patients with severe dyslipidaemia and to follow them in specialized clinics if affected. Similarly, screening for significant lipoprotein disorders of family members of patients with premature CVD is recommended (see section 10) (Table 7).
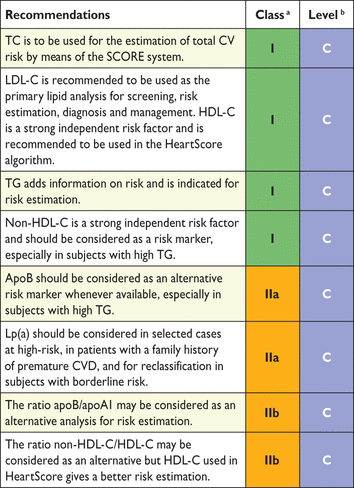
Recommendations for lipid analyses in cardiovascular disease risk estimation
 |
 |
Apo = apolipoprotein; CKD = chronic kidney disease; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; Lp = lipoprotein; SCORE = Systemic Coronary Risk Estimation; TC = total cholesterol; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
Recommendations for lipid analyses in cardiovascular disease risk estimation
 |
 |
Apo = apolipoprotein; CKD = chronic kidney disease; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; Lp = lipoprotein; SCORE = Systemic Coronary Risk Estimation; TC = total cholesterol; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
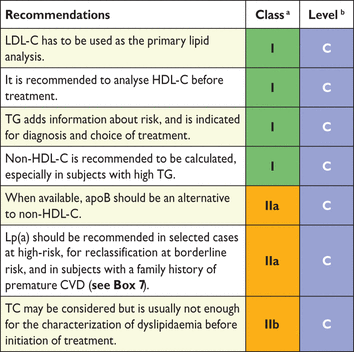
The suggested analyses used for baseline lipid evaluation are TC, TGs, HDL-C, LDL-C calculated with the Friedewald formula unless TGs are elevated (>4.5 mmol/L or >400 mg/dL) or with a direct method, and non-HDL-C. When available, apoB can be considered as an equivalent to non-HDL-C. Additional plasma lipid analyses that may be considered are Lp(a), apoB:apoA1 ratio and non-HDL-C:HDL-C ratio (Tables 7 and 8).
Recommendations for lipid analyses for characterization of dyslipidaemias before treatment
 |
 |
Apo = apolipoprotein; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; Lp = lipoprotein; TC = total cholesterol; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
Recommendations for lipid analyses for characterization of dyslipidaemias before treatment
 |
 |
Apo = apolipoprotein; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; Lp = lipoprotein; TC = total cholesterol; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
The direct methods for HDL-C and LDL-C analysis are currently widely used and are reliable in patients with normal lipid levels.72 However, in hypertriglyceridaemia (HTG) these methods have been found to be unreliable, with variable results and variations between the commercially available methods. Therefore, under these conditions, the values obtained with direct methods may be over- or underestimating the LDL-C and HDL-C levels. The use of non-HDL-C may overcome some of these problems, but it is still dependent on a correct analysis of HDL-C. An alternative to non-HDL-C may be the analysis of apoB. The analysis of apoB is accurate, with small variations, and is recommended as an alternative when available. Near patient testing is also available using dry chemistry methods. These methods may give a crude estimate, but should be verified by analysis in an established certified laboratory.
3.1 Fasting or non-fasting?
Traditionally blood samples for lipid analysis have been drawn in the fasting state. As recently shown, fasting and non-fasting sampling give similar results for TC, LDL-C and HDL-C. TGs are affected by food, resulting in, on average, an ∼0.3 mmol/L (27 mg/dL) higher plasma level, depending on the composition and the time frame of the last meal. For risk estimation, non-fasting has a prediction strength similar to fasting, and non-fasting lipid levels can be used in screening and in general risk estimation.73–76 It should be emphasized, however, that the risk may be underestimated in patients with diabetes, because in one study, patients with diabetes had up to 0.6 mmol/L lower LDL-C in non-fasting samples.77 Furthermore, to characterize severe dyslipidaemias further, and for follow-up of patients with HTG, fasting samples are recommended.
3.2 Intra-individual variation
There is a considerable intra-individual variation in plasma lipids. Variations of 5–10% for TC and >20% for TGs have been reported, particularly in those patients with HTG. This is to some extent due to analytical variation, but is also due to environmental factors such as diet and physical activity, and a seasonal variation, with higher levels of TC and HDL-C during the winter.78
3.3. Lipid and lipoprotein analyses
Throughout this section it should be noted that most risk estimation systems and virtually all drug trials are based on TC and LDL-C, and that clinical benefit from using other measures, including apoB, non-HDL-C and various ratios, while sometimes logical, has largely been based on post hoc analyses. Non-HDL-C has recently been proposed by locally developed guidelines such as NICE using the QRISK2 risk calculator.79,80 While the role of the alternative analyses is being established, traditional measures of risk such as TC, LDL-C and HDL-C remain robust and supported by a major evidence base. Furthermore, multiple clinical trials have established beyond all reasonable doubt that, at least in high-risk subjects, reduction of TC or LDL-C is associated with statistically and clinically significant reductions in CV events and mortality. Therefore, TC and LDL-C remain the primary targets recommended in these guidelines. However, for several reasons non-HDL-C and apoB are recommended as secondary targets. In patients with elevated TG levels, the extra risk carried with TG-rich lipoproteins is taken into account. Furthermore, some of the methodological problems with the direct methods for HDL-C and LDL-C may be reduced.
3.3.1 Total cholesterol
TC is recommended to be used to estimate total CV risk by means of the SCORE system. In individual cases, however, TC may be misleading. This is especially so in women, who often have higher HDL-C levels, and in subjects with diabetes or with high TGs, who often have low HDL-C levels. For an adequate risk analysis, at least LDL-C and HDL-C should be analysed. Note that assessment of total risk is not required in patients with familial hyperlipidaemia (including FH) or those with TC >7.5 mmol/L (290 mg/dL). These patients are always at high risk and should receive special attention.
3.3.2 Low-density lipoprotein cholesterol
In most clinical studies LDL-C has been calculated using the Friedewald formula.
Friedewald formula, in mmol/L: LDL-C = TC − HDL-C − (TG/2.2); in mg/dL: LDL-C = TC − HDL-C − (TG/5).
The calculated value of LDL-C is based on a number of assumptions:
Methodological errors may accumulate since the formula necessitates three separate analyses of TC, TGs and HDL-C.
A constant cholesterol:TG ratio in very low-density lipoprotein (VLDL) is assumed. With high TG values (>4.5 mmol/L or >400 mg/dL), the formula cannot be used.
The Friedewald formula may be unreliable when blood is obtained under non-fasting conditions. Under these conditions, non-HDL-C may be determined.
3.3.3 Non-high-density lipoprotein cholesterol
Non-HDL-C is used as an estimation of the total amount of atherogenic lipoproteins in plasma (VLDL, VLDL remnants, intermediate-density lipoprotein (IDL), LDL, Lp(a)) and relates well to apoB levels. Non-HDL-C is easily calculated from TC minus HDL-C. Some recent guidelines recommend non-HDL-C as a better risk indicator than LDL-C.82
Several analyses have been published comparing these variables in risk algorithms, but data are inconclusive. In some reports non-HDL-C is superior, but in others, LDL-C and non-HDL-C are reported to give similar information.83–85
Non-HDL-C has been shown to have a strong predictive power, and although the scientific background from randomized trials is weaker, there are practical aspects of using non-HDL-C instead of LDL-C in certain situations. Non-HDL-C is simple to calculate and does not require additional analyses. Both Friedewald's formula and direct LDL-C estimations have limitations in subjects with HTG and in subjects with very low LDL-C. Non-HDL-C also includes the atherogenic TG-rich lipoproteins (VLDL, IDL and remnants), which is essential considering the recent information from genome-wide association studies (GWASs) and Mendelian randomization76,86–89 supporting TGs and remnant particles as causative factors in atherogenesis.
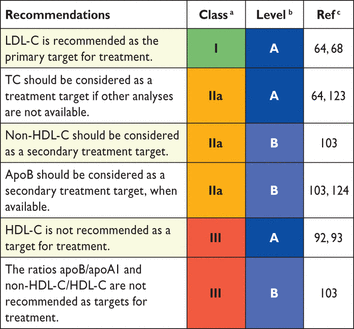
Since all trials use LDL-C, we still recommend this as the primary treatment target. However, non-HDL-C should be used as a secondary target when the LDL-C goal is reached. Goals for non-HDL-C are easily calculated as LDL-C goals plus 0.8 mmol/L (30 mg/dL).
3.3.4 High-density lipoprotein cholesterol
Low HDL-C has been shown to be a strong and independent risk factor in several studies and is included in most of the risk estimation tools available, including HeartScore. Very high levels of HDL-C have consistently not been found to be associated with atheroprotection.90 Based on epidemiological data, levels of HDL-C associated with increased risk for men are <1.0 mmol/L (40 mg/dL) and for women are <1.2 mmol/L (48 mg/dL). The causative role of HDL-C for protection against CVD has been questioned in several studies utilizing Mendelian randomization.87,89,91,92 Recent studies suggest that HDL has a complex role in atherogenesis and that the presence of dysfunctional HDL may be more relevant to the development of atherosclerosis than the HDL-C level.93–95 Most available assays are of high quality, but the method used should be evaluated against the available reference methods and controlled in international quality programmes. It should also be considered that HTG might interfere with the direct HDL-C assays.72
3.3.5 Triglycerides
TGs are determined by accurate enzymatic techniques. A rare error occurs in patients with hyperglycerolaemia, where falsely very high values for TGs are detected.
High TG levels are often associated with low HDL-C and high levels of small dense LDL particles. In a number of meta-analyses, TGs has been shown to be an independent risk factor.96,97 Furthermore, recent genetic data support the contention that elevated TG levels are a direct cause of CV disease.76,88
Recent studies suggest that non-fasting TGs may carry information regarding remnant lipoproteins associated with increased risk.76,86,98,99 For general screening and risk evaluation, non-fasting TGs can be used.
3.3.6 Apolipoproteins
From a technical point of view, there are advantages in the determination of apoB and apoA1. Good immunochemical methods are available and easily run in conventional autoanalysers. The analytical performance is good and the assays do not require fasting conditions and are not sensitive to markedly elevated TG levels.
Apolipoprotein B. ApoB is the major apolipoprotein of the atherogenic lipoprotein families (VLDL, IDL and LDL). ApoB is a good estimate of the number of these particles in plasma. This might be of special importance in the case of high concentrations of small dense LDL. Several prospective studies have shown that apoB is equal to LDL-C and non-HDL-C in risk prediction. ApoB has not been evaluated as a primary treatment target in clinical trials, but several post hoc analyses of clinical trials suggest that apoB may be not only a risk marker, but also a treatment target.100 A major disadvantage of apoB is that it is not included in algorithms for calculation of global risk, and it has not been a predefined treatment target in controlled trials. Recent data from a meta-analysis83,90 indicate that apoB does not provide any benefit beyond non-HDL-C or traditional lipid ratios.101 Likewise, apoB provided no benefit beyond traditional lipid markers in people with diabetes in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study.102 In contrast, in another meta-analysis of LDL-C, non-HDL-C and apoB, the latter was superior as a marker of CV risk.103 ApoB can be used as a secondary target, as suggested for non-HDL-C, when analysis for apoB is available.
Apolipoprotein A1. ApoA1 is the major protein of HDL-C and provides a satisfactory estimate of HDL-C concentration. However, each HDL particle may carry from one to five apoA1 molecules. Plasma apoA1 levels <120 mg/dL for men and <140 mg/dL for women correspond approximately to what is considered as low for HDL-C.
Apolipoprotein B:apolipoprotein A1 ratio, total cholesterol:high-density lipoprotein cholesterol ratio and non-high-density lipoprotein cholesterol:high-density lipoprotein cholesterol ratio. Ratios between atherogenic lipoproteins and HDL-C or apoA1 (TC:HDL-C, non-HDL-C:HDL-C, apoB:apoA1) are useful for risk estimation, but not for diagnosis or as treatment targets. The components of the ratio have to be considered separately.
Apolipoprotein CIII. ApoCIII has been identified as a potentially important new risk factor.104–106 ApoCIII is a key regulator of TG metabolism, and high apoCIII plasma levels are associated with high plasma VLDL and plasma TGs. Furthermore, loss of function mutations are associated with low TGs as well as with reduced risk for CVD.106,107 ApoCIII has been identified as a new potential therapeutic target that is currently being studied, but whether it has a role in clinical practice is unknown and its measurements on a routine basis are not encouraged.108
3.3.7 Lipoprotein(a)
Lp(a) has been found in several studies to be an additional independent risk marker; indeed, genetic data show it to be causal in the pathophysiology of atherosclerotic vascular disease and aortic stenosis.109–111 Lp(a) has properties in common with LDL, but it contains a unique protein, apolipoprotein(a) [apo(a)], that is structurally homologous to plasminogen. The plasma level of Lp(a) is to a major extent genetically determined. Several methods for determination of Lp(a) are available, but standardization between assays is needed.112 The measurement of Lp(a) is particularly stable over time. Plasma Lp(a) is not recommended for risk screening in the general population; however, Lp(a) measurement should be systematically considered in people with high CVD risk or a strong family history of premature atherothrombotic disease (Box 7).109 The risk is regarded as significant when Lp(a) is above the 80th percentile (50 mg/dL).109 Including Lp(a) in risk evaluation has been shown to give a correct reclassification113,114 and should be considered in patients on the borderline between high and moderate risk.
Reduction of Lp(a) has been shown with several of the emerging lipid-lowering drugs. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and nicotinic acid reduce Lp(a) by ∼30%.115–117 An effect on CVD events targeting Lp(a) has not been shown. Antisense drugs targeting the Lp(a) gene reduce the circulating levels of this protein by up to 80%. A reasonable option for patients at risk with high Lp(a) is an intensified treatment of the modifiable risk factors, including LDL-C.
3.3.8 Lipoprotein particle size
Lipoproteins are heterogeneous, and evidence suggests that subclasses of LDL and HDL may contribute differently to estimation of the risk of CVD.118 However, the causal relation of subclasses to atherosclerosis is unclear. Determination of small dense LDL may be regarded as an emerging risk factor and may be used in the future, but it is not currently recommended for risk estimation.119
3.3.9 Genotyping
Several genes have been associated with CVD. Large GWASs have been published for coronary heart disease (CHD), as well as for associated biomarkers and risk factors. At present, the use of genotyping for risk estimation is not recommended since known risk loci account for only a small proportion of risk.120 For the diagnosis of specific genetic hyperlipidaemias, genotyping of apolipoprotein E (apoE) and of genes associated with FH [low-density lipoprotein receptors (LDLRs), apoB and PCSK9] should be considered. In FH, a genetic diagnosis is important for family screening, to establish the diagnosis in patients with borderline LDL-C and to improve patient adherence to therapy.121
ApoE is present in three isoforms (apoE2, apoE3 and apoE4). ApoE genotyping is used primarily for the diagnosis of dysbetalipoproteinaemia (apoE2 homozygosity) and is indicated in cases with severe combined hyperlipidaemia. With increasing knowledge about common polymorphisms and lipoproteins, the importance of a polygenic background to familial hyperlipidaemias is emphasized.67,122
Table 7 lists recommendations for lipid analyses in CVD risk estimation, Table 8 lists recommendations for lipid analyses for characterization of dyslipidaemias before treatment and Table 9 lists recommendations for lipid analyses as treatment targets in the prevention of CVD.
Recommendations for lipid analyses as treatment targets in the prevention of cardiovascular disease
 |
 |
Apo = apolipoprotein; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TC = total cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for lipid analyses as treatment targets in the prevention of cardiovascular disease
 |
 |
Apo = apolipoprotein; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TC = total cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
4. Treatment targets
In both the 2011 EAS/ESC guidelines for the management of dyslipidaemias125 and the American Heart Association/American College of Cardiology (AHA/ACC) guidelines on the treatment of blood cholesterol to reduce atherosclerotic CV risk in adults,71 the importance of LDL-C lowering to prevent CVD is strongly emphasized. The approaches that are proposed to reach that LDL-C reduction are different. The task force charged with the development of the 2016 EAS/ESC updated guidelines on dyslipidaemias examined this issue in depth. It was recognized that the US expert panel confined itself to a simple, hard source of evidence coming from results in RCTs. Despite this, there has not been an RCT to support the AHA/ACC recommendation for the use of high-dose statins in all high-risk people regardless of baseline LDL-C level. The European Task Force felt that limiting the current knowledge on CV prevention only to results from RCTs reduces the exploitation of the potential that is available for prevention of CVD. It is the concordance of the conclusions from many different approaches (from basic science, clinical observations, genetics, epidemiology, RCTs, etc.) that contributes to the understanding of the causes of CVD and to the potential of prevention. The task force is aware of the limitations of some of the sources of evidence and accepts that RCTs have not examined different LDL-C goals systematically, but felt that it was appropriate to look at the totality of the evidence. Indeed, the task force accepts that the choice of any given target goal for LDL-C may be open to debate given the continuous nature of the relationship between LDL-C reduction and reduction in risk. Particular consideration was given to results from systematic reviews confirming the dose-dependent reduction in CVD with LDL-C lowering; the greater the LDL-C reduction, the greater the CV risk reduction.65,66 The benefits related to LDL-C reduction are not specific for statin therapy.63 No level of LDL-C below which benefit ceases or harm occurs has been defined.
There is considerable individual variability in the LDL-C response to dietary and drug treatments,61 which is traditionally taken to support a tailored approach to management. Total CV risk reduction should be individualized, and this can be more specific if goals are defined. The use of goals can also aid patient–doctor communication. It is judged likely that a goal approach may facilitate adherence to treatment, although this consensus opinion has not been fully tested. For all these reasons the European Task Force retains a goal approach to lipid management and treatment goals are defined, tailored to the total CV risk level. There is also evidence suggesting that lowering LDL-C beyond the goals that were set in the previous EAS/ESC guidelines is associated with fewer CVD events.126 Therefore, it seems appropriate to reduce LDL-C as low as possible, at least in patients at very high CV risk.
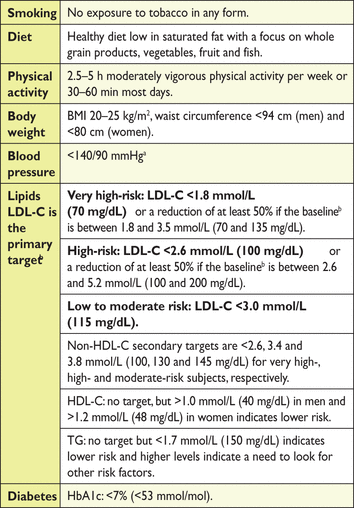
The lipid goals are part of a comprehensive CV risk reduction strategy, summarized in Table 10. The rationale for the non-lipid targets are given in the 2016 ESC Joint Prevention guidelines.485
Treatment targets and goals for cardiovascular disease prevention
 |
 |
BMI = body mass index; HbA1C = glycated haemoglobin; HDL-C = high-density
lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TG = triglycerides.
aThe BP target can be lower in some patients with type 2 diabetes127 and in some high-risk patients without diabetes who can tolerate multiple antihypertensive drugs.70
bThe term “baseline LDL-C“ refers to the level in a subject not taking any lipid lowering medication.
Treatment targets and goals for cardiovascular disease prevention
 |
 |
BMI = body mass index; HbA1C = glycated haemoglobin; HDL-C = high-density
lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TG = triglycerides.
aThe BP target can be lower in some patients with type 2 diabetes127 and in some high-risk patients without diabetes who can tolerate multiple antihypertensive drugs.70
bThe term “baseline LDL-C“ refers to the level in a subject not taking any lipid lowering medication.
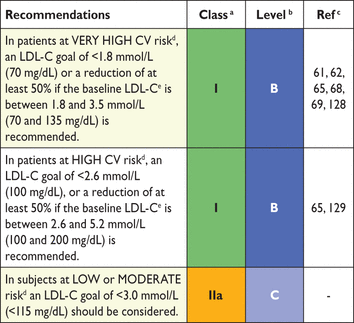
The targeted approach to lipid management is primarily aimed at reducing LDL-C. For patients at a very high total CV risk, the goal is an LDL-C <1.8 mmol/L (70 mg/dL). At least a 50% reduction from baseline (if >1.8 mmol/L) should also be achieved. For subjects at high total CV risk, the goal is an LDL-C level <2.6 mmol/L (100 mg/dL). At least a 50% reduction from baseline [if >2.6 mmol/L (100 mg/dL)] should also be achieved. In people at moderate total CV risk, the LDL-C goal is <3 mmol/L (115 mg/dL) (Table 11).
Recommendations for treatment goals for low-density lipoprotein-cholesterol
 |
 |
CV = cardiovascular; LDL-C = low-density lipoprotein-cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dFor definitions see section 2.2.
eThe term “baseline LDL-C” refers to the level in a subject not taking any lipid lowering medication.
Recommendations for treatment goals for low-density lipoprotein-cholesterol
 |
 |
CV = cardiovascular; LDL-C = low-density lipoprotein-cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dFor definitions see section 2.2.
eThe term “baseline LDL-C” refers to the level in a subject not taking any lipid lowering medication.
Recommendations for treatment goals for lowdensity lipoprotein-cholesterol (LDL-C)–examples
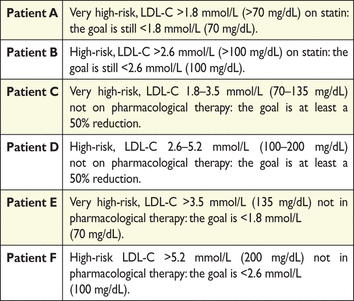 |
 |
Recommendations for treatment goals for lowdensity lipoprotein-cholesterol (LDL-C)–examples
 |
 |
When secondary targets are used the recommendations are
– non-HDL-C <2.6 mmol/L (100 mg/dL) and <3.4 mmol/L (130 mg/dL) in subjects at very high and high total CV risk, respectively (Class IIa, Level B).100,130
– apoB <80 mg/dL and <100 mg/dL in those at very high and high total CV risk, respectively (Class IIa, Level B).100,131
Clinicians should use clinical judgment when considering further treatment intensification in patients at high or very high total CV risk.
5. Lifestyle modifications to improve the plasma lipid profile
The role of nutrition in the prevention of CVD has been extensively reviewed.132–134 There is strong evidence showing that dietary factors may influence atherogenesis directly or through effects on traditional risk factors such as plasma lipids, blood pressure or glucose levels.
Results from RCTs relating dietary patterns to CVD have been reviewed.132 Some interventions resulted in significant CVD prevention, whereas others did not. In order to get an overall estimate of the impact of dietary modifications on the CV risk, different meta-analyses have been performed, sometimes with inconsistent outcomes.135,136 This is due not only to methodological problems, particularly inadequate sample size or the short duration of many trials included in the systematic revision, but also to the difficulty of evaluating the impact of a single dietary factor independently of any other changes in the diet. Such studies rarely allow attribution of reduction in CV risk to a single dietary component. These limitations suggest that caution is required in interpreting the results of meta-analyses of RCTs in relation to the impact of a single dietary change on CVD, particularly where they conflict with the existing global research, including clinical studies on risk factors and epidemiological observations. In this respect, it is relevant that a meta-analysis of the relationship between improvement of the plasma lipoprotein profile and the rate of CV events has demonstrated that non-HDL-C lowering translates into a reduction in risk independent of the mechanisms (statins, resins, diet and ileal bypass) involved.131
In summary, the available evidence from RCTs addressing the issue of how to modify the habitual diet in order to contribute to CVD prevention shows that the dietary patterns that have been more extensively evaluated are the Dietary Approaches to Stop Hypertension (DASH) diet, particularly in relation to blood pressure control, and the Mediterranean diet; both have been proven to be effective in reducing CV risk factors and, possibly, to contribute to CVD prevention.133 They are characterized by high consumption of fruits, vegetables and wholegrain cereal products; frequent intake of legumes, nuts, fish, poultry and low fat dairy products and limited intake of sweets, sugar-sweetened drinks and red meat. The DASH diet and the Mediterranean diet derive a large proportion of dietary fat from non-tropical vegetable oil rather than from animal sources; the most relevant difference between them is the emphasis on extra virgin olive oil given in the Mediterranean diet. This latter dietary pattern has been proven in RCTs to be effective in reducing CV diseases in primary and secondary prevention.137,138 In particular, the PREDIMED trial, a multicentre randomized intervention study conducted in Spain, evaluated the impact of a Mediterranean type of diet, supplemented with either extra-virgin olive oil or mixed nuts, on the rate of major CV events [myocardial infarction (MI), stroke or death from CV causes) in individuals at high CV risk but with no CVD at enrolment. The Mediterranean diet supplemented with extra-virgin olive oil or nuts significantly reduced the incidence of major CV events by almost 30%.137 However, despite the strong support of lifestyle intervention for CVD prevention coming from the PREDIMED and other intervention studies with CVD endpoints, most evidence linking nutrition to CVD is based on observational studies and investigations of the effects of dietary changes on CV risk factors.
The influence of lifestyle changes and functional foods on lipoproteins is evaluated and summarized in Table 12; in this table the magnitude of the effects and the levels of evidence refer to the impact of dietary modifications on the specific lipoprotein class and not to CVD endpoints.
Impact of specific lifestyle changes on lipid levels
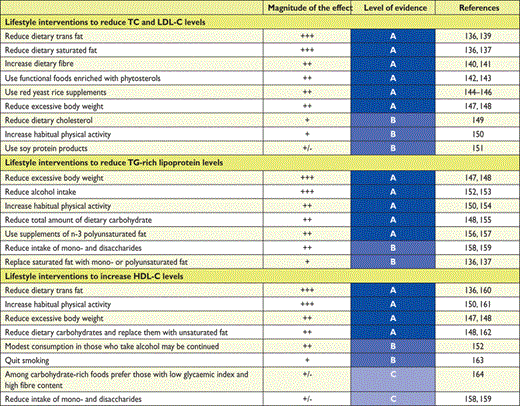 |
 |
HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TC = total cholesterol; TG = triglycerides.
The magnitude of the effect (+++ = marked effects, ++ = less pronounced effects, + = small effects, – = not effective) and the level of evidence refer to the impact of each dietary modification on plasma levels of a specific lipoprotein class.
Impact of specific lifestyle changes on lipid levels
 |
 |
HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TC = total cholesterol; TG = triglycerides.
The magnitude of the effect (+++ = marked effects, ++ = less pronounced effects, + = small effects, – = not effective) and the level of evidence refer to the impact of each dietary modification on plasma levels of a specific lipoprotein class.
5.1 The influence of lifestyle on total cholesterol and low-density lipoprotein cholesterol levels
Saturated fatty acids (SFAs) are the dietary factor with the greatest impact on LDL-C levels (0.02–0.04 mmol/L or 0.8–1.6 mg/dL of LDL-C increase for every additional 1% energy coming from saturated fat).165 Stearic acid, in contrast to other SFAs (lauric, myristic and palmitic), does not increase TC levels. Trans unsaturated fatty acids can be found in limited amounts (usually <5% of total fat) in dairy products and in meats from ruminants. ‘Partially hydrogenated fatty acids’ of industrial origin represent the major source of trans fatty acids in the diet; the average consumption of trans fatty acids ranges from 0.2% to 6.5% of the total energy intake in different populations.166 Quantitatively, dietary trans fatty acids have a similar elevating effect on LDL-C to that of SFAs; however, while SFAs increase HDL-C levels, trans fats decrease them.137 If 1% of the dietary energy derived from SFAs is replaced by n-6 polyunsaturated fatty acids (PUFAs), LDL-C decreases by 0.051 mmol/L (2.0 mg/dL); if replaced by monounsaturated fatty acids (MUFAs), the decrease would be 0.041 mmol/L (1.6 mg/dL); and if replaced by carbohydrate, it would be 0.032 mmol/L (1.2 mg/dL). PUFAs of the n-3 series have no hypocholesterolaemic effect; conversely, when they are used at high dosages (>3 g/day), the effect on LDL-C levels is either neutral or a slight increase [particularly with docosahexaenoic acid (DHA)] with a concomitant decrease of TGs.165
A positive relationship exists between dietary cholesterol and CAD mortality, which is partly independent of TC levels. Several experimental studies in humans have evaluated the effects of dietary cholesterol on cholesterol absorption and lipid metabolism and have revealed marked variability among individuals.167,168 Dietary carbohydrate is ‘neutral’ on LDL-C; therefore, carbohydrate-rich foods represent one of the possible options to replace saturated fat in the diet. However, the major drawback of their excessive consumption is represented by untoward effects on plasma TGs and on HDL-C levels.165 Dietary fibre (particularly of the soluble type), which is present in legumes, fruits, vegetables, and wholegrain cereals (oats, barley), has a direct hypocholesterolaemic effect. Therefore, carbohydrate foods rich in fibre represent a good dietary substitute for saturated fat in order to maximize the effects of the diet on LDL-C levels and to minimize the untoward effects of a high carbohydrate diet on other lipoproteins.140 Conversely, refined carbohydrate foods and beverages should not be recommended to replace saturated fat since they may contribute to elevated plasma TGs and lower HDL-C levels.
Body weight reduction also influences TC and LDL-C, but the magnitude of the effect is rather small; in grossly obese subjects, a decrease in LDL-C concentration of ∼0.2 mmol/L (8 mg/dL) is observed for every 10 kg of weight loss; the reduction of LDL-C is greater if weight loss is achieved with a low fat diet.147,148 Even smaller is the reduction of LDL-C levels induced by regular physical exercise.150,169 However, the beneficial effects of weight reduction and physical exercise on the CV risk profile go beyond LDL-C reduction and involve not only other lipoprotein classes but also other risk factors.
In Table 13, lifestyle interventions to lower TC and LDL-C are summarized. Given the cultural diversity of the European populations, they should be translated into practical behaviours, taking into account local habits and socio-economic factors.
Dietary recommendations to lower low-density lipoprotein-cholesterol and improve the overall lipoprotein profile
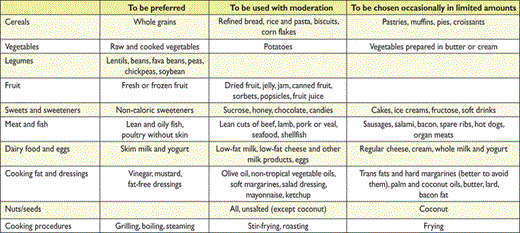 |
 |
Dietary recommendations to lower low-density lipoprotein-cholesterol and improve the overall lipoprotein profile
 |
 |
5.2 The influence of lifestyle on triglyceride levels
A high monounsaturated fat diet significantly improves insulin sensitivity compared with a high saturated fat diet.170 This goes in parallel with a reduction in TG levels, mostly in the post-prandial period.171 A more relevant hypotriglyceridaemic effect is observed when saturated fat is replaced by n-6 PUFA. A marked reduction of TGs can be obtained with a high dosage of long chain n-3 PUFAs; however, a dietary approach based exclusively on natural foods will seldom reach an intake adequate to achieve a clinically significant effect. To this aim, either pharmacological supplements or foods artificially enriched with n-3 PUFAs may be utilized.172 In people with severe HTG, in whom chylomicrons are equally present in the fasting state, it is appropriate to reduce the total amount of dietary fat as much as possible (<30 g/day). In these patients, the use of medium chain TGs (from C6 to C12) that avoid the formation of chylomicrons may be considered since they are directly transported and metabolized in the liver following transport in the portal vein.
Glucose and lipid metabolism are strongly related, and any perturbation of carbohydrate metabolism induced by a high carbohydrate diet will also lead to an increase in TG concentrations.148,165 The greater and more rapid this perturbation, the more pronounced are the metabolic consequences. Most detrimental effects of a high carbohydrate diet could be minimized if carbohydrate digestion and absorption were slowed down. The glycaemic index permits identification, among carbohydrate-rich foods, of those with ‘fast’ and ‘slow’ absorption. In particular, the detrimental effects of a high carbohydrate diet on TGs occur mainly when refined carbohydrate-rich foods are consumed, while they are much less prominent if the diet is based largely on fibre-rich, low glycaemic index foods. This applies particularly to people with diabetes or with metabolic syndrome (MetS).173,174
Habitual consumption of significant amounts (>10% energy) of dietary fructose contributes to TG elevations, particularly in people with HTG. These effects are dose dependent; with a habitual fructose consumption between 15 and 20% of the total energy intake, plasma TG increases as much as 30–40%. Sucrose, a disaccharide-containing glucose and fructose, represents an important source of fructose in the diet.158,175
Weight reduction improves insulin sensitivity and decreases TG levels. In many studies the reduction of TG levels due to weight reduction is between 20–30%; this effect is usually preserved as long as weight is not regained. Regular physical exercise reduces plasma TG levels over and above the effect of weight reduction.150,169,176
Alcohol intake has a major impact on TG levels. While in individuals with HTG even a small amount of alcohol can induce a further elevation of TG concentrations, in the general population alcohol exerts detrimental effects on TG levels only if the intake is excessive.152,177
5.3 The influence of lifestyle on high-density lipoprotein cholesterol levels
SFAs increase HDL-C levels in parallel with LDL-C; in contrast, trans fats decrease them.137 MUFA consumption as a replacement for SFAs has almost no effect on HDL-C, while n-6 PUFAs induce a slight decrease. In general, n-3 fatty acids have limited (<5%) or no effect on HDL-C levels.156,172
Increased carbohydrate consumption as an isocaloric substitution for fat is associated with a significant decrease in HDL-C [0.01 mmol/L (0.4 mg/dL) for every 1% energy substitution]. In this respect, both the glycaemic index and the fibre content do not seem to play a relevant role.178,179 The impact of fructose/sucrose intake on HDL-C does not seem different from that of other refined carbohydrates.158,159 Moderate alcohol consumption is associated with increased HDL-C levels as compared with abstainers, with a dose-response relationship. Weight reduction has a beneficial influence on HDL-C levels: a 0.01 mmol/L (0.4 mg/dL) increase is observed for every kilogram decrease in body weight when weight reduction has stabilized. Aerobic physical activity corresponding to a total energy expenditure of 1500–2200 kcal/week, such as 25–30 km of brisk walking per week (or any equivalent activity), may increase HDL-C levels by 0.08–0.15 mmol/L (3.1–6 mg/dL).176 Smoking cessation may also contribute to HDL-C elevation, provided that weight gain is prevented; this is often observed soon after quitting smoking.163
5.4 Lifestyle recommendations to improve the plasma lipid profile
LDL-C represents the primary lipoprotein target for reducing CV risk and therefore it deserves special emphasis in the evaluation of lifestyle measures useful for CVD prevention. However, it may be appropriate that the diet recommended to the general population, and particularly to people at increased CV risk, should not only lower LDL-C, but should also be able to improve plasma TG and HDL-C levels (Table 12). This section focuses on dietary and other lifestyle factors that have an effect on lipids. It has to be kept in mind that dietary components, other lifestyle factors and weight loss also contribute to reducing the overall CV risk through their influence on other risk factors, e.g. hypertension, subclinical inflammation or impaired insulin sensitivity.
5.4.1 Body weight and physical activity
Since overweight, obesity and abdominal adiposity often contribute to dyslipidaemia, caloric intake should be reduced and energy expenditure increased in those with excessive weight and/or abdominal adiposity. Overweight is defined as a body mass index (BMI) ≥25–30 kg/m2 and obesity as a BMI ≥30 kg/m2.
Abdominal adiposity can be detected easily by measuring waist circumference; this should be performed in all individuals who are either overweight, have dyslipidaemia or are at increased CV risk. Measurements of waist circumference >80 cm for women of any ethnicity and >94 cm for men of European ancestry or >90 cm for men of Asian origin indicate the presence of abdominal adiposity, even in people of normal weight (Table 14).180 Body weight reduction, even if modest (5–10% of basal body weight), improves lipid abnormalities and favourably affects the other CV risk factors often present in dyslipidaemic individuals.147 An even more marked hypolipidaemic effect occurs when weight reduction is more relevant, as observed in severely obese patients who undergo bariatric surgery. This treatment seems to induce beneficial effects not only on the overall risk factor profile, but also on CV events.181
Weight reduction can be achieved by decreasing the consumption of energy-dense foods, inducing a caloric deficit of 300–500 kcal/day. To be effective in the long run, this advice should be incorporated into structured, intensive lifestyle education programmes. In order to facilitate the maintenance of body weight close to the target, it is always appropriate to advise people with dyslipidaemia to engage in regular physical exercise of moderate intensity.150
Modest weight reduction and regular physical exercise of moderate intensity are very effective in preventing type 2 diabetes and improving all the metabolic abnormalities and CV risk factors clustering with insulin resistance, which are often associated with abdominal adiposity. Physical activity should be encouraged, with a goal of regular physical exercise for at least 30 min/day every day.169
5.4.2 Dietary fat
Limiting as much as possible the intake of trans fat is a key measure of the dietary prevention of CVD. Trying to avoid the consumption of foods made with processed sources of trans fats provides the most effective means of reducing the intake of trans fats to <1% of energy. Because the trans fatty acids produced in the partial hydrogenation of vegetable oils account for 80% of total intake, the food industry has an important role in decreasing the trans fatty acid content of the food supply. As for saturated fat, its consumption should be <10% of the total caloric intake and should be further reduced (<7% of energy) in the presence of hypercholesterolaemia. For most individuals, a wide range of total fat intakes is acceptable and will depend upon individual preferences and characteristics. However, fat intakes that >35% of calories are generally associated with increased intakes of both saturated fat and calories. Conversely, a low intake of fats and oils increases the risk of inadequate intakes of vitamin E and of essential fatty acids, and may contribute to unfavourable changes in HDL-C.165
Fat intake should predominantly come from sources of MUFAs and both n-6 and n-3 PUFAs. However, the intake of n-6 PUFAs should be limited to <10% of the energy intake, both to minimize the risk of lipid peroxidation of plasma lipoproteins and to avoid any clinically relevant HDL-C decrease.182 Not enough data are available to make a recommendation regarding the optimal n-3:n-6 fatty acid ratio.182,183 The cholesterol intake in the diet should be reduced (<300 mg/day), particularly in people with high plasma cholesterol levels.
5.4.3 Dietary carbohydrate and fibre
Carbohydrate intake should range between 45 and 55% of total energy intake. Consumption of vegetables, legumes, fruits, nuts and wholegrain cereals should be particularly encouraged, together with all the other foods rich in dietary fibre and/or with a low glycaemic index. A fat-modified diet that provides 25–40 g of total dietary fibre, including at least 7–13 g of soluble fibre, is well tolerated, effective and recommended for plasma lipid control; conversely, there is no justification for the recommendation of very low carbohydrate diets.164
Intake of sugars should not exceed 10% of total energy (in addition to the amount present in natural foods such as fruits and dairy products); more restrictive advice concerning sugars may be useful for those needing to lose weight or with high plasma TG values, MetS or diabetes. Soft drinks should be used with moderation by the general population and should be drastically limited in those individuals with elevated TG values.158,159
5.4.4 Alcohol
Moderate alcohol consumption [up to 20 g/day (2 units) for men and 10 g/day (1 unit) for women] is acceptable for those who drink alcoholic beverages, provided that TG levels are not elevated.
5.4.5 Smoking
Smoking cessation has clear benefits on the overall CV risk, and specifically on HDL-C, but special attention should be paid in order to prevent weight gain in people who stop smoking.163
5.5 Dietary supplements and functional foods for the treatment of dyslipidaemias
Innovative nutritional strategies to improve dyslipidaemias have been developed. They are based on either changing some ‘risky’ dietary components or encouraging the consumption of specifically targeted ‘healthy’ functional foods and/or dietary supplements; these so-called nutraceuticals can be used either as alternatives or in addition to lipid-lowering drugs.184 Nutritional evaluation of functional foods includes not only the search for clinical evidence of beneficial effects relevant to improved health or reduction of disease risk, but also the demonstration of good tolerability and the absence of major undesirable effects. The substantiation of health claims relevant for each food should be based on results from intervention studies in humans that are consistent with the proposed claims. Overall, the available evidence on functional foods so far identified in this field is incomplete; the major gap is the absence of diet-based intervention trials of sufficient duration to be relevant for the natural history of dyslipidaemia and CVD.
5.5.1 Phytosterols
The principal phytosterols are sitosterol, campesterol and stigmasterol; they occur naturally in vegetable oils and in smaller amounts in vegetables, fresh fruits, chestnuts, grains and legumes. The dietary intake of plant sterols ranges between an average of 250 mg/day in Northern Europe to ∼500 mg/day in Mediterranean countries. Phytosterols compete with cholesterol for intestinal absorption, thereby modulating TC levels.
Phytosterols have been added to spreads and vegetable oils (functional margarine, butter and cooking oils), as well as yoghurt and other foods; however, food matrices do not significantly influence the cholesterol-lowering efficacy of phytosterols at equivalent doses.142 The daily consumption of 2 g of phytosterols can effectively lower TC and LDL-C by 7–10% in humans (with a certain degree of heterogeneity among individuals), while it has little or no effect on HDL-C and TG levels.143 Although the effect of plant sterol consumption on TC levels has been clearly shown, no studies have been performed yet on the subsequent effect on CVD. However, the meta-analysis of Robinson et al.131 indicates that LDL-C reduction translates into CV benefits, independent of the mechanism involved. Long-term surveillance is also needed to guarantee the safety of the regular use of phytosterol-enriched products. The possible decrease in carotenoid and fat-soluble vitamin levels by sterols/stanols can be prevented with a balanced diet rich in these nutrients.185 Based on LDL-C lowering and the absence of adverse signals, functional foods with plant sterols/stanols (at least 2 g/day with the main meal) may be considered: (i) in individuals with high cholesterol levels at intermediate or low global CV risk who do not qualify for pharmacotherapy; (ii) as an adjunct to pharmacologic therapy in high- and very high-risk patients who fail to achieve LDL-C goals on statins or are statin intolerant; and (iii) in adults and children (>6 years) with FH, in line with current guidance.142
5.5.2 Monacolin and red yeast rice
Red yeast rice (RYR) is a source of fermented pigment that has been used in China as a food colorant and flavour enhancer for centuries. Hypocholesterolaemic effects of RYR are related to a statin-like mechanism, inhibition of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, of monacolins, which represent the bioactive ingredient. Different commercial preparations of RYR have different concentrations of monacolins, and lower TC and LDL-C to a variable extent,145 but the long-term safety of the regular consumption of these products is not fully documented. However, side effects similar to those observed with statins have been reported in some people using these nutraceuticals. Furthermore, their quality may vary widely.
In one RCT from China in patients with CAD, a partially purified extract of RYR reduced recurrent events by 45%.144 No other trial has been performed to confirm this finding. A clinically relevant hypocholesterolaemic effect (up to a 20% reduction) is observed with RYR preparations providing a daily dose of ∼2.5–10 mg monacolin K.146 Nutraceuticals containing purified RYR may be considered in people with elevated plasma cholesterol concentrations who do not qualify for treatment with statins in view of their global CV risk.
5.5.3 Dietary fibre
Available evidence consistently demonstrates a TC- and LDL-C-lowering effect of water-soluble fibre from oat and barley beta-glucan. Foods enriched with these fibres are well tolerated, effective and recommended for LDL-C lowering at a daily dose of at least 3 g/day.186,187
5.5.4 Soy protein
Soy protein has been indicated as being able to induce a modest LDL-C-lowering effect when replacing animal protein foods.151 However, this was not confirmed when changes in other dietary components were taken into account.
5.5.5 Policosanol and berberine
Policosanol is a natural mixture of long chain aliphatic alcohols extracted primarily from sugarcane wax.188 Studies show that policosanol from sugarcane, rice or wheat germ has no significant effect on LDL-C, HDL-C, TGs, apoB, Lp(a), homocysteine, hs-CRP, fibrinogen or blood coagulation factors.189
As for berberine, a recent meta-analysis has evaluated its effects on plasma lipids in humans; six trials were available for this purpose: the berberine group contained 229 patients and the control group contained 222 patients.190 The studies, showing a statistically significant heterogeneity, were all performed in China in people of Asian ethnicity. The comparative evaluation of berberine and lifestyle intervention or placebo indicated that in the berberine group, LDL-C and plasma TG levels were more effectively reduced than in the control group. However, due to the lack of high-quality randomized clinical trials, the efficacy of berberine for treating dyslipidaemia needs to be further validated.
5.5.6 n-3 unsaturated fatty acids
Observational evidence supports the recommendation that the intake of fish (at least twice a week) and long chain n-3 fatty acids supplements at low dosage may reduce the risk of CV death and stroke in primary prevention, but have no major effects on plasma lipoprotein metabolism.183 Pharmacological doses of n-3 fatty acids (2–3 g/day) reduce TG levels up to 30%, but a higher dosage may increase LDL-C. Alfa-linolenic acid (a medium chain n-3 fatty acid present in chestnuts, some vegetables and some seed oils) is less effective on TG levels. Long chain n-3 PUFAs also reduce the postprandial lipaemic response.156,172
5.6 Other features of a healthy diet contributing to cardiovascular disease prevention
The results of the PREDIMED trial are clearly in support of a diet inspired by the traditional Mediterranean diet as an effective approach to the lifestyle prevention of CVDs. This type of diet is characterized by the regular consumption of extra-virgin olive oil, fruits, nuts, vegetables and cereals; a moderate intake of fish and poultry and a low intake of dairy products, red meat, processed meats and sweets; wine is consumed in moderation with meals.137 Dietary choices inspired by this model should be recommended for both primary and secondary prevention of CVD.
One of the important features of this type of diet is represented by the consumption of large amounts of fruits and vegetables of different types providing a sufficient amount and variety of minerals, vitamins and antioxidants, particularly polyphenols. New evidence is accumulating on the possible beneficial effects of these compounds, which are also present in olive oil, red wine, coffee, tea and cocoa, on subclinical inflammation and endothelial function, as well as their beneficial influence on plasma TGs at fasting and particularly in the postprandial period.
As for the consumption of fish, at least two portions per week are recommended to the general population for the prevention of CVD, together with the regular consumption of other food sources of n-3 PUFAs (nuts, soy and flaxseed oil). For secondary prevention of CVD, the use of n-3 PUFA supplements is no longer recommended in view of the recent evidence showing no benefit on CVD of this supplementation in people who have already experienced a CV event. Previous RCTs where omega-3 supplementation was beneficial were not blinded or had low use of standard CV medications (such as statins).
Salt intake should be limited to <5 g/day, not only by reducing the amount of salt used for food seasoning, but especially by reducing the consumption of foods preserved by the addition of salt; this recommendation should be more stringent in people with hypertension or MetS.132–134
Food choices to lower TC and LDL-C are summarized in Table 13. Box 9 lists lifestyle measures and healthy food choices for managing total CV risk. All individuals should be advised on lifestyles associated with a lower CVD risk. High-risk subjects, in particular those with dyslipidaemia, should receive specialist dietary advice, if feasible.
Summary of lifestyle measures and healthy food choices for managing total cardiovascular risk
 |
 |
Summary of lifestyle measures and healthy food choices for managing total cardiovascular risk
 |
 |
6. Drugs for treatment of hypercholesterolaemia
6.1 Statins
6.1.1 Mechanism of action
Statins reduce the synthesis of cholesterol in the liver by competitively inhibiting HMG-CoA reductase activity. The reduction in intracellular cholesterol concentration induces an increased expression of LDLR on the surface of the hepatocytes, which results in increased uptake of LDL-C from the blood and a decreased plasma concentration of LDL-C and other apoB-containing lipoproteins, including TG-rich particles.
The degree of LDL-C reduction is dose dependent and varies between the different statins (supplementary Figure A and supplementary Table A).191 There is also considerable interindividual variation in LDL-C reduction with the same dose of drug.61 Poor response to statin treatment in clinical studies is to some extent caused by poor compliance, but may also be explained by a genetic background involving variations in genes of both cholesterol metabolism and of statin uptake and metabolism in the liver.192,193 Furthermore, conditions causing high cholesterol (e.g. hypothyroidism) should be considered. Indeed, interindividual variations in statin response warrant monitoring of individual response on initiation of therapy.
Percentage reduction of low-density lipoprotein cholesterol (LDL-C) requested to achieve goals as a function of the starting value.
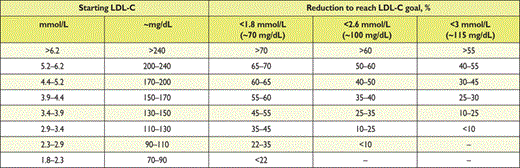 |
 |
Percentage reduction of low-density lipoprotein cholesterol (LDL-C) requested to achieve goals as a function of the starting value.
 |
 |
A systematic review and meta-analysis of the therapeutic equivalence of statins. ATOR = atorvastatin; FLUVA = fluvastatin; LOVA = lovastatin; PRAVA = pravastatin; SIMVA = simvastatin; ROSU = rosuvastatin; PITA = pitavastatin.
6.1.2 Efficacy of cardiovascular disease prevention in clinical studies
Statins are among the most studied drugs in CVD prevention, and dealing with single studies is beyond the scope of the present guidelines. A number of large-scale trials have demonstrated that statins substantially reduce CV morbidity and mortality in both primary and secondary prevention, in both genders and in all age groups. Statins have also been shown to slow the progression or even promote regression of coronary atherosclerosis.
Meta-analyses. A large number of meta-analyses have been performed to analyse the effects of statins in larger populations and in subgroups.64–66,68,129,194–200 In the large Cholesterol Treatment Trialists (CTT) analysis data, >170 000 participants and 26 RCTs with statins were included.64 A 10% proportional reduction in all-cause mortality and 20% proportional reduction in CAD death per 1.0 mmol/L (40 mg/dL) LDL-C reduction was reported. The risk of major coronary events was reduced by 23% and the risk of stroke was reduced by 17% per 1 mmol/L (40 mg/dL) LDL-C reduction. The benefits were similar in all subgroups examined. The benefits were significant within the first year, but were greater in subsequent years. There was no increased risk for any non-CV cause of death, including cancer, in those receiving statins. Other meta-analyses have confirmed these results, coming to essentially the same conclusions. Most of the meta-analyses include studies in primary as well as secondary prevention. The absolute benefit from statin treatment may be less evident in patients in primary prevention, who are typically at lower risk. Several meta-analyses have specifically studied statins in primary prevention.66,68,199 The largest of these was published as a Cochrane review in 2013.200 The analysis included 19 studies with different statins and with somewhat varying inclusion criteria. In this analysis, all-cause mortality was reduced by 14%, CVD events by 27%, fatal and non-fatal coronary events by 27% and stroke by 22% per 1 mmol/L (40 mg/dL) LDL-C reduction. The relative risk reduction in primary prevention is about the same as that observed in secondary prevention. Similar results were also observed in analyses of statin treatment in people with low risk of vascular disease.66 However, it should be emphasized that in subjects with lower risk, the absolute risk reduction is also lower.
Current available evidence from meta-analyses suggests that the clinical benefit is largely independent of the type of statin but depends on the extent of LDL-C lowering, therefore the type of statin used should reflect the LDL-C goal in a given patient.
The following scheme may be proposed.
Evaluate the total CV risk of the subject.
Involve the patient with decisions on CV risk management.
Identify the LDL-C goal for that risk level.
Calculate the percentage reduction of LDL-C required to achieve that goal.
Choose a statin and a dose that, on average, can provide this reduction.
Response to statin treatment is variable, therefore up-titration of the dose may be required.
If the highest tolerated statin dose does not reach the goal, consider drug combinations.
In addition, for subjects at very high and high risk, a ≥50% reduction in LDL-C should be achieved.
Other effects of statins. Although the reduction of LDL-C is the major effect of statins, a number of other, potentially important effects have been suggested (pleiotropic effects of statins).201,202 Among such effects that are potentially relevant for the prevention of CVD are anti-inflammatory and anti-oxidant effects of statin treatment. Effects have been shown in vitro and in experimental systems, but their clinical relevance remains controversial.203
Furthermore, the effects of statins on a number of other clinical conditions have been evaluated, including dementia,204 hepatic steatosis,205 cancer,206,207 venous thromboembolism208 and polycystic ovary syndrome.209 Available data are controversial and thus far no clinically relevant effect on these conditions has been demonstrated. Statins also reduce TGs by 30–50% and may increase HDL-C by 5–10%. For indications for statins in HTG, see section 7.4.
The suggested effect on Alzheimer's disease was recently reviewed in a Cochrane analysis reporting no conclusive positive effect from statins. Furthermore, case reports on neurocognitive side effects of statins have not been confirmed in analyses of larger patient populations or in meta-analyses.210
6.1.3 Adverse effects of statins
Statins differ in their absorption, bioavailability, plasma protein binding, excretion and solubility. Lovastatin and simvastatin are prodrugs, whereas the other available statins are administered in their active form. Their absorption rate varies between 20 and 98%. Many statins undergo significant hepatic metabolism via cytochrome P450 isoenzymes (CYPs), except pravastatin, rosuvastatin and pitavastatin. These enzymes are expressed mainly in the liver and gut wall. Although statins are generally well tolerated, there are adverse effects to be considered when statins are prescribed.
Muscle. Muscle symptoms are the most commonly described clinically relevant adverse effect of statin treatment.57 Rhabdomyolysis is the most severe form of statin-induced myopathy, characterized by severe muscular pain, muscle necrosis and myoglobinuria potentially leading to renal failure and death. In rhabdomyolysis, creatine kinase (CK) levels are elevated at least 10 times, often up to 40 times the upper limit of normal.211 The frequency of rhabdomyolysis has been estimated to represent 1–3 cases/100√000 patient-years.212 A more commonly described form of muscular adverse effect is muscular pain and tenderness (myalgia) without CK elevation or major functional loss. The actual frequency of this adverse effect, however, is unclear, and varies between different reports. In meta-analyses of RCTs, no increased frequency in statin-treated groups has been shown.213,214 On the other hand, the reported frequency varies between 10 and 15% in observational studies.215,216 One study, designed specifically to study the effects of statins on muscle symptoms, suggests that the frequency of muscle-related complaints is ∼5%.217 The diagnosis is based on the clinical observation and whether symptoms disappear after discontinuation of statins and recur with statin rechallenge. The symptoms are often vague and the association with statin treatment is often difficult to confirm. In patients with a high risk for CVD, it is essential to confirm the diagnosis before leaving the patient without the benefits of statin treatment. Risk factors for muscular adverse effects have been identified. Among these, the interaction with concomitant drug therapy should especially be considered (see below). Suggested practical management of muscular symptoms is given in supplementary material. In patients with high or very high risk for CVD, treatment with the highest tolerable dose of statin should be considered, in combination with a cholesterol absorption inhibitor, and if available a PCSK9 inhibitor may also be considered.218,219 Several studies have shown a considerable LDL-C lowering effect of alternative dosing such as every other day or twice a week with atorvastatin or rosuvastatin.57,220 Although no clinical endpoint trials are available, this treatment should be considered in high-risk patients who do not tolerate daily doses of statin treatment.
Liver. The activity of alanine aminotransferase (ALT) in plasma is commonly used to assess hepatocellular damage. Mild elevation of ALT occurs in 0.5–2.0% of patients on statin treatment, more commonly with potent statins or high doses. The common definition of clinically relevant ALT elevation has been an increase of three times the upper limit of normal (ULN) on two occasions. Mild elevation of ALT has not been shown to be associated with true hepatotoxicity or changes in liver function. Progression to liver failure is exceedingly rare, therefore routine monitoring of ALT during statin treatment is no longer recommended.221 Patients with mild ALT elevation due to steatosis have been studied during statin treatment and there is no indication that statins cause any worsening of liver disease.222–224
Diabetes. Patients on statin treatment have been shown to exhibit an increased risk of dysglycaemia and development of diabetes type 2. In a meta-analysis including 91 140 subjects, the relative risk was increased by 9% relative to placebo. The absolute risk was increased by 0.2%.
A minor, not clinically relevant elevation of glycated haemoglobin (HbA1C) has also been observed. The number needed to cause one case of diabetes was estimated at 255 over 4 years.225 However, the risk is higher with the more potent statins in high doses,226 and the risk for diabetes is higher in the elderly and in the presence of other risk factors for diabetes such as overweight or insulin resistance.227 Overall, the absolute reduction in the risk of CVD in high-risk patients outweighs the possible adverse effects of a small increase in the incidence of diabetes.
Kidney. The effect of statin treatment on renal function is still being debated. A recent Cochrane analysis could not find support for beneficial effects on renal function based on studies where creatinine clearance was available, and no deleterious effects were observed.228 An increased frequency of proteinuria has been reported for all statins, but has been analysed in more detail for rosuvastatin, probably due to the high frequency of proteinuria observed with a higher dose (80 mg). With a dose of 80 mg, a frequency of 12% was reported. With the approved doses up to 40 mg, the frequency is much lower and in line with the frequency for other statins. The proteinuria induced by statins is of tubular origin and is supposed to be due to reduced tubular reabsorption and not to glomerular dysfunction.229 In experimental systems, reduced pinocytosis has been shown in renal cells. The statin-induced reduced pinocytosis is directly related to the inhibition of cholesterol synthesis.230 In clinical trials the frequency of proteinuria is in general low and in most cases is not higher than for placebo.231
6.1.4 Interactions
A number of important drug interactions with statins have been described that may increase the risk of adverse effects. Inhibitors and inducers of enzymatic pathways involved in statin metabolism are summarized in Table 15. All currently available statins, except pravastatin, rosuvastatin and pitavastatin, undergo major hepatic metabolism via the CYPs. These isoenzymes are mainly expressed in the liver and intestine. Pravastatin does not undergo metabolism through the CYP system, but is metabolized by sulfation and conjugation. CYP3A isoenzymes are the most abundant, but other isoenzymes such as CYP2C8, CYP2C9, CYP2C19 and CYP2D6 are frequently involved in the metabolism of statins. Thus other pharmacological substrates of these CYPs may interfere with statin metabolism. Conversely, statin therapy may interfere with the catabolism of other drugs that are metabolized by the same enzymatic system.
Drugs potentially interacting with statins metabolized by CYP3A4 leading to increased risk of myopathy and rhabdomyolysis
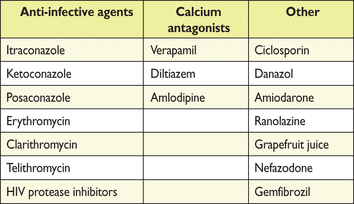 |
 |
Drugs potentially interacting with statins metabolized by CYP3A4 leading to increased risk of myopathy and rhabdomyolysis
 |
 |
Combinations of statins with fibrates may enhance the risk for myopathy. This risk is highest for gemfibrozil, and the association of gemfibrozil with statins should be avoided. The increased risk for myopathy when combining statins with other fibrates such as fenofibrate, bezafibrate or ciprofibrate seems to be small.234,235
The increased risk for myopathy with nicotinic acid has been debated, but in recent reviews no increased risk of myopathy was found with this agent.236,237
6.2 Bile acid sequestrants
6.2.1 Mechanism of action
Bile acids are synthesized in the liver from cholesterol and are released into the intestinal lumen, but most of the bile acid is returned to the liver from the terminal ileum via active absorption. The two older bile acid sequestrants, cholestyramine and colestipol, are both bile acid–binding exchange resins. Recently the synthetic drug colesevelam was introduced. The bile acid sequestrants are not systemically absorbed or altered by digestive enzymes, therefore the beneficial clinical effects are indirect. By binding the bile acids, the drugs prevent the entry of bile acids into the blood and thereby remove a large portion of the bile acids from the enterohepatic circulation. The liver, depleted of bile, synthesizes more from hepatic stores of cholesterol. The decrease in bile acid returned to the liver leads to upregulation of key enzymes responsible for bile acid synthesis from cholesterol, particularly CYP7A1. The increase in cholesterol catabolism to bile acids results in a compensatory increase in hepatic LDLR activity, clearing LDL-C from the circulation and thus reducing LDL-C levels. These agents also reduce glucose levels in hyperglycaemic patients. A recent Cochrane review found that colesevelam, when added to other antidiabetic agents, showed significant effects on glycaemic control; however, more research on the impact of CV risk is required.238
6.2.2 Efficacy in clinical studies
At the top dose of 24 g of cholestyramine, 20 g of colestipol or 4.5 g of colesevelam, a reduction in LDL-C of 18–25% has been observed. No major effect on HDL-C has been reported, while TGs may increase in some predisposed patients.
In clinical trials, bile acid sequestrants have contributed greatly to the demonstration of the efficacy of LDL-C lowering in reducing CV events in hypercholesterolaemic subjects, with a benefit proportional to the degree of LDL-C lowering. However, this study was performed before many of the modern treatment options were available.239–241
6.2.3 Adverse effects and interactions
Gastrointestinal adverse effects (most commonly flatulence, constipation, dyspepsia and nausea) are often present with these drugs, even at low doses, which limits their practical use. These adverse effects can be attenuated by beginning treatment at low doses and ingesting ample fluid with the drug. The dose should be increased gradually. Reduced absorption of fat-soluble vitamins has been reported. Furthermore, these drugs may increase circulating TG levels in certain patients.
Bile acid sequestrants have major drug interactions with many commonly prescribed drugs and should therefore be administered either 4 h before or 1 h after other drugs. Colesevelam represents a newer formulation of the bile acid sequestrant, which may be better tolerated than cholestyramine. Colesevelam has fewer interactions with other drugs and can be taken together with statins and several other drugs.242
6.3 Cholesterol absorption inhibitors
6.3.1 Mechanism of action
Ezetimibe is the first lipid-lowering drug that inhibits intestinal uptake of dietary and biliary cholesterol without affecting the absorption of fat-soluble nutrients. By inhibiting cholesterol absorption at the level of the brush border of the intestine [by interaction with the Niemann-Pick C1-like protein 1 (NPC1L1)], ezetimibe reduces the amount of cholesterol delivered to the liver. In response to reduced cholesterol delivery, the liver reacts by upregulating LDLR expression, which in turn leads to increased clearance of LDL-C from the blood.
6.3.2 Efficacy in clinical studies
In clinical studies, ezetimibe in monotherapy reduces LDL-C in hypercholesterolaemic patients by 15–22%. Combined therapy with ezetimibe and a statin provides an incremental reduction in LDL-C levels of 15–20%. The efficacy of ezetimibe in association with simvastatin has been addressed in subjects with aortic stenosis in the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study243 and in patients with CKD in the Study of Heart and Renal Protection (SHARP) (see sections 9.7.3 and 9.9.2). In both the SEAS and SHARP trials, a reduction in CV events was demonstrated in the simvastatin–ezetimibe arm vs. placebo.243,244
In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) ezetimibe was added to simvastatin (40 mg) in patients after ACS.63 A total of 18 144 patients were randomized and 5314 patients over 7 years experienced a CVD event; 170 fewer events (32.7 vs. 34.7%) were recorded in the group taking simvastatin plus ezetimibe (P = 0.016). The average LDL-C during the study was 1.8 mmol/L in the simvastatin group and 1.4 mmol/L in patients taking ezetimibe plus simvastatin. Also, ischaemic stroke was reduced by 21% in this trial (P = 0.008). There was no evidence of harm caused by the further LDL-C reduction. In this group of patients already treated with statins to reach goals, the absolute benefit from added ezetimibe was small, although significant. However, the study supports the proposition that LDL-C lowering by means other than statins is beneficial and can be performed without adverse effects. The beneficial effect of ezetimibe is also supported by genetic studies of mutations in NPC1L1. Naturally occurring mutations that inactivate the protein were found to be associated with reduced plasma LDL-C and reduced risk for CAD.245
Taken together with other studies such as the PRECISE-IVUS study,246 IMPROVE-IT supports the proposal that ezetimibe should be used as second-line therapy in association with statins when the therapeutic goal is not achieved at the maximal tolerated statin dose or in patients intolerant of statins or with contraindications to these drugs.
6.3.3 Adverse effects and interactions
Ezetimibe is rapidly absorbed and extensively metabolized to pharmacologically active ezetimibe glucuronide. The recommended dose of ezetimibe of 10 mg/day can be administered in the morning or evening without regard to food intake. There are no clinically significant effects of age, sex or race on ezetimibe pharmacokinetics, and no dosage adjustment is necessary in patients with mild hepatic impairment or mild to severe renal insufficiency. Ezetimibe can be co-administered with any dose of any statin. No major adverse effects have been reported; the most frequent adverse effects are moderate elevations of liver enzymes and muscle pain.
6.4 PCSK9 inhibitors
6.4.1 Mechanism of action
Recently a new class of drugs, PCSK9 inhibitors, has become available that targets a protein (PCSK9) involved in the control of the LDLR.247 Elevated levels/functions of this protein in plasma reduce LDLR expression by promoting, upon binding, the LDLR lysosomal catabolism and increase plasma LDL-C concentration, while lower levels/functions of PCSK9 are related to lower plasma LDL-C levels.248 Therapeutic strategies have been developed mainly using monoclonal antibodies that reduce LDL-C levels by ∼60% independent from the presence of a background lipid-lowering therapy. The mechanism of action relates to the reduction of plasma levels of PCSK9, which in turn is not available to bind the LDLR. Since this interaction triggers the intracellular degradation of the LDLR, lower levels of circulating PCSK9 will result in a higher expression of LDLRs at the cell surface and therefore in a reduction of circulating LDL-C levels.248
6.4.2 Efficacy in clinical studies
The European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have recently approved two monoclonal antibodies (Mabs) for the control of elevated plasma LDL-C. The efficacy at reducing LDL-C is in the range of 50–70%, independent of the presence of a background therapy (statins, ezetimibe, etc.); preliminary data from phase 3 trials suggest a reduction of CV events in line with the LDL-C reduction achieved.115,116 A recent meta-analysis confirmed these findings.249 No major effects are reported on HDL-C or plasma TGs. However, the TG effect must be reconfirmed in populations with higher starting plasma TG levels.
Given the mechanism of action, these drugs are effective at reducing LDL-C in all patients with the capability of expressing LDLRs in the liver. Therefore this pharmacological approach is effective in the vast majority of patients, including those with heterozygous FH (HeFH) and, albeit to a lower level, those with homozygous FH (HoFH) with residual LDLR expression. Receptor-deficient HoFH responds poorly to the therapy.
People at very high total CV risk, people with HeFH (and some with HoFH) on maximally tolerated doses of first- and second-line therapy and/or in apheresis and who are statin ‘intolerant’ with persistent high levels of LDL-C are reasonable candidates for the use of these drugs.
6.4.3 Adverse effects and interactions
Anti-PCSK9 Mabs are injected subcutaneously, usually every other week, at doses up to 150 mg. The potential for interaction with orally absorbed drugs is absent, as they will not interfere with pharmacokinetics or pharmacodynamics. Anti-PCSK9 Mabs do not modulate pathways involved in biotransformation or drug uptake/extrusion from cells. Among the most frequently reported side effects are itching at the site of injection and flu-like symptoms. In some studies an increase of patient-reported neurocognitive effects was described. This finding requires further scrutiny.250
6.5 Nicotinic acid
Nicotinic acid has broad lipid-modulating action, raising HDL-C in a dose-dependent manner by up to 25% and reducing LDL-C by 15–18% and TGs by 20–40% at the 2 g/day dose. Nicotinic acid is unique in lowering Lp(a) levels by up to 30% at this dose. After two large studies with nicotinic acid, one with extended-release niacin251 and one with niacin plus laropiprant,252 showed no beneficial effect, but rather an increased frequency of serious adverse effects, no medication containing nicotinic acid is currently approved in Europe. For the role of niacin in hypertriglyceridaemia, see section 7.6.
6.6 Drug combinations
Although the LDL-C goals are attained with monotherapy in many patients, a significant proportion of high-risk subjects or patients with very high LDL-C levels need additional treatment. There are also patients who are statin intolerant or are not able to tolerate higher statin doses. In these cases, combination therapy should be considered (Table 19). More information on statin intolerance is provided in Supplementary Figure C.
6.6.1 Statins and cholesterol absorption inhibitors
The combination of statins and ezetimibe is discussed above (see section 6.3.2).
6.6.2 Statins and bile acid sequestrants
The combination of a statin and cholestyramine, colestipol or colesevelam could be useful in achieving LDL-C goals. On average, the addition of a bile acid sequestrant to a statin reduces LDL-C by an additional 10–20%. However, there are no published clinical outcome trials with either conventional bile acid sequestrants or colesevelam in combination with other drugs. The combination has been found to reduce atherosclerosis, as evaluated by coronary angiography.253
6.6.3 Other combinations
In high-risk patients such as those with FH, or in cases of statin intolerance, other combinations may be considered. Co-administration of ezetimibe and bile acid sequestrants (colesevelam, colestipol or cholestyramine) resulted in an additional reduction of LDL-C levels without any additional adverse effects when compared with the stable bile acid sequestrant regimen alone.254 Clinical outcome studies with these combinations have not been performed.
Functional foods containing phytosterols as well as plant sterol–containing tablets additionally reduce LDL-C levels by up to 5–10% in patients taking a stable dose of a statin, and this combination is also well tolerated and safe.142,255 Phytosterols and plant sterols should be taken after a meal. However, it is still not known whether this could reduce the risk of CVD since no trials with plant sterols or stanols in combination with other lipid-lowering drugs are available for CVD outcomes. The combination of red yeast with statins is not recommended.
In patients at very high risk, with persistent high LDL-C despite being treated with a maximal statin dose in combination with ezetimibe, or in patients with statin intolerance, a PCSK9 inhibitor may be considered.
Recommendations for the treatment of hypercholesterolaemia are shown in Table 16.
Recommendations for the pharmacological treatment of hypercholesterolaemia
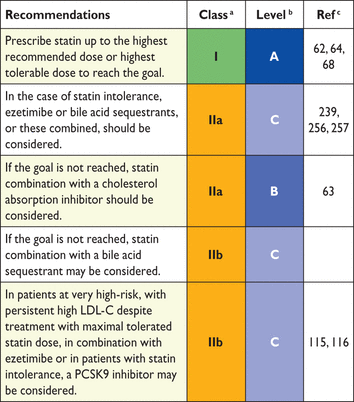 |
 |
LDL-C = low-density lipoprotein-cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for the pharmacological treatment of hypercholesterolaemia
 |
 |
LDL-C = low-density lipoprotein-cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
7. Drugs for treatment of hypertriglyceridaemia
7.1 Triglycerides and cardiovascular disease risk
Although the role of TGs as a risk factor for CVD has been strongly debated, recent data favour the role of TG-rich lipoproteins as a risk factor for CVD.87 Large prospective studies have reported that non-fasting TGs predict CAD risk more strongly than fasting TGs.98,99 Recent data from genetic studies utilizing a Mendelian randomization design have consistently linked both non-fasting TG levels as well as remnant cholesterol to increased risk of CVD events and all-cause mortality.86,107 Remnant cholesterol is a calculated parameter in these studies and equals TC − (HDL-C + LDL-C). These genetic data have strengthened the position of remnant cholesterol as a causal factor driving atherosclerosis and CVD events.75 Recently, remnant cholesterol has turned out to be a good surrogate marker of TGs and remnants.90 The burden of HTG as a CVD risk factor is highlighted by the fact that about one-third of adult individuals have TG levels >1.7 mmol/L (150 mg/dL).258 HTG can have different causes (Table 17), among which its polygenic nature is most important in relation to CVD prevention.
7.2 Definition of hypertriglyceridaemia
The definition of different categories for elevated fasting TG levels seems to be slightly variable in different guidelines and recommendations.67,259 According to the EAS consensus document, mild to moderate HTG is defined as TGs >1.7 mmol/L (150 mg/dL) and <10 mmol/L (880 mg/dL); TGs >10 mmol/L is defined as severe HTG.260 Age/gender, race/ethnicity and lifestyle are modulating factors at the population level for serum TGs. In the Copenhagen general population ∼27% had TGs >1.7 mmol/L.75 Severe HTG is rare and is typically associated with monogenic mutations. Severe HTG is associated with an increased risk for pancreatitis.
7.3 Strategies to control plasma triglycerides
A level of fasting TGs ≤1.7 mmol/L (150 mg/dL) is desirable. The first step is to consider possible causes of HTG and to evaluate the total CV risk. The primary goal is to achieve the LDL-C level recommended based on the total CV risk level. As compared with the overwhelming evidence for the benefits of LDL-C reduction, the evidence on the benefits of lowering elevated TG levels is still modest, and is primarily derived from subgroup or post hoc analyses. However, recent evidence of TGs as a causal risk factor may encourage TG lowering (Table 18).
Recommendations for drug treatments of hypertriglyceridaemia
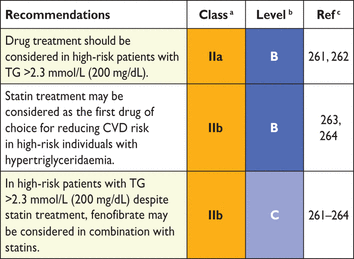 |
 |
CVD = cardiovascular disease; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for drug treatments of hypertriglyceridaemia
 |
 |
CVD = cardiovascular disease; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Although the CVD risk is increased if fasting TGs are >1.7 mmol/L (150 mg/dL),87 the use of drugs to lower TGs may only be considered in high-risk subjects when TGs are >2.3 mmol/L (200 mg/dL) and cannot be lowered by lifestyle measures. The available pharmacological interventions include statins, fibrates, PCSK9 inhibitors and n-3 PUFAs.
For information on lifestyle management, please refer to section 5.
7.4 Statins
Since statins have significant effects on mortality as well as most CVD outcome parameters, these drugs are the first choice to reduce both total CVD risk and moderately elevated TG levels. More potent statins (atorvastatin, rosuvastatin and pitavastatin) demonstrate a robust lowering of TG levels, especially at high doses and in patients with elevated TGs. In subgroup analyses from statin trials, the risk reduction is the same in subjects with HTG as in normotriglyceridaemic subjects.
7.5 Fibrates
7.5.1 Mechanism of action
Fibrates are agonists of peroxisome proliferator-activated receptor-α (PPAR-α), acting via transcription factors regulating various steps in lipid and lipoprotein metabolism. By interacting with PPAR-α, fibrates recruit different cofactors and regulate gene expression. As a consequence, fibrates have good efficacy in lowering fasting TG levels as well as post-prandial TGs and TG-rich lipoprotein (TRL) remnant particles. The HDL-C raising effects of fibrates are modest.263
7.5.2 Efficacy in clinical trials
The clinical effects of fibrates are primarily illustrated by five prospective RCTs: Helsinki Heart Study (HHS), Veterans Affairs High-density lipoprotein Intervention Trial (VA-HIT), Bezafibrate Infarction Prevention (BIP) study, Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, where fenofibrate was added to statin therapy.261,262,265–267
Although the Helsinki Heart Study reported a significant reduction in CVD outcomes with gemfibrozil, neither the FIELD nor the ACCORD study showed a reduction in total CVD outcomes. Decreases in the rates of non-fatal MI were reported, although often as a result of post hoc analyses. The effect was most evident in subjects with elevated TG/low HDL-C levels. However, the data on other outcome parameters have remained equivocal. Only one study, ACCORD, has analysed the effect of a fibrate as an add-on treatment to a statin. No overall benefit was reported in two recent meta-analyses.268,269 Results from other meta-analyses suggest reduced major CVD events in patients with high TGs and low HDL-C in fibrate-treated patients, but no decrease in CVD or total mortality.270–272 Thus the overall efficacy of fibrates on CVD outcomes is much less robust than that of statins. Overall, the possible benefits of fibrates require confirmation.
7.5.3 Adverse effects and interactions
Fibrates are generally well tolerated with mild adverse effects, gastrointestinal disturbances being reported in <5% of patients and skin rashes in 2%.273 In general, myopathy, liver enzyme elevations and cholelithiasis represent the most well-known adverse effects associated with fibrate therapy.273 In the FIELD study, small but significant increases in the incidence of pancreatitis (0.8% vs. 0.5%) and pulmonary embolism (1.1% vs. 0.7%) and a non-significant trend toward an increase in deep vein thrombosis (1.4% vs. 1.0%) were seen in those taking fenofibrate compared with placebo; this is in line with data from other fibrate studies.261 Elevations of both CK (>5 times above the ULN) and ALT (>3 times above the ULN) were reported more frequently for patients on fenofibrate than on placebo, but the incidence of these abnormalities remained <1% in both treatment groups. In the FIELD study, one case of rhabdomyolysis was reported in the placebo group and three cases in the fenofibrate group.261 The risk of myopathy has been reported to be 5.5-fold greater with fibrate use as a monotherapy compared with statin use. The risk of myopathy is greater in patients with CKD, and it varies with different fibrates and statins used in combination. This is explained by the pharmacological interaction between different fibrates and glucuronidation of statins. Gemfibrozil inhibits the metabolism of statins via the glucuronidation pathway, which leads to highly increased plasma concentrations of statins. As fenofibrate does not share the same pharmacokinetic pathways as gemfibrozil, the risk of myopathy is much less with this combination therapy.273
As a class, fibrates have been reported to raise both serum creatinine and homocysteine in both short-term and long-term studies. The increase of serum creatinine by fibrate therapy seems to be fully reversible when the drug is stopped. Data from meta-analyses suggest that a reduction of calculated glomerular filtration rate (GFR) does not reflect any adverse effects on kidney function.274 The increase in homocysteine by fibrates has been considered to be relatively innocent with respect to CVD risk. However, the fibrate-induced increase in homocysteine may blunt the increases in both HDL-C and apoA1, and this may contribute to the smaller than estimated benefits of fenofibrate in the outcome parameters.275 High homocysteine also promotes thrombosis, and the increased trend to deep vein thrombosis seen in the FIELD study was associated with the baseline homocysteine levels, but no interaction was observed between the increase of homocysteine by fibrate and venous thromboembolic events.276
7.6 Nicotinic acid
7.6.1 Mechanism of action
Nicotinic acid has been reported to decrease fatty acid influx to the liver and the secretion of VLDL by the liver. This effect appears to be mediated in part by the action on hormone-sensitive lipase in the adipose tissue. Nicotinic acid has key action sites in both liver and adipose tissue. In the liver, nicotinic acid inhibits diacylglycerol acyltransferase-2 (DGAT-2), resulting in decreased secretion of VLDL particles from the liver, which is also reflected in reductions of both IDL and LDL particles.277 Nicotinic acid raises HDL-C and apoA1 primarily by stimulating apoA1 production in the liver.277 The effects of nicotinic acid on lipolysis and fatty acid mobilization in adipocytes are well established.
7.6.2 Efficacy in clinical trials
Nicotinic acid has multiple effects on serum lipids and lipoproteins.277 Nicotinic acid effectively reduces not only TGs, but also LDL-C, reflecting its effect on all apoB-containing proteins. Nicotinic acid increases apoA1-containing lipoproteins, reflected in increases of HDL-C and apoA1. At a daily dose of 2 g it reduces TGs by 20–40% and LDL-C by 15–18% and increases HDL-C by 15–35%.257,277,278 The favourable effect on angiographic measures has been reported in the Familial Atherosclerosis Treatment Study (FATS) and in the HDL-Atherosclerosis Treatment Study (HATS).279
Two large randomized clinical trials [the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) and the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE)] using, respectively, extended release (ER) nicotinic acid vs. placebo in addition to simvastatin, and ER nicotinic acid/laropiprant vs. placebo in patients treated with simvastatin (plus, if indicated, ezetimibe), failed to report positive benefits of the therapies on CV outcomes and have challenged the position and benefits of niacin in lipid management.251,252 Furthermore, there was an increased frequency of severe adverse effects in the niacin groups. Since the EMA suspended ER nicotinic acid/laropiprant, this therapeutic option is unavailable in Europe.
7.7 n-3 fatty acids
7.7.1 Mechanism of action
n-3 fatty acids [eicosapentaenoic acid (EPA) and DHA] are used at pharmacological doses to lower TGs. n-3 fatty acids (2–4 g/day) affect serum lipids and lipoproteins, in particular VLDL concentration. The underlying mechanism is poorly understood, although it may be related, at least in part, to their ability to interact with PPARs and to a decreased secretion of apoB.
7.7.2 Efficacy in clinical trials
n-3 fatty acids reduce TGs, but their effects on other lipoproteins are trivial. More detailed data on clinical outcomes are needed to justify the use of prescription n-3 fatty acids.280 The recommended doses of total EPA and DHA to lower TGs have varied between 2 and 4 g/day. Three recent studies in subjects with high TGs using EPA reported a significant reduction in serum TG levels of up to 45% in a dose-dependent manner.281–283 The efficacy of omega-3 fatty acids to lower serum TGs has also been reported in meta-analyses.284 One meta-analysis included 63 030 subjects from 20 trials and reported no overall effect of omega-3 fatty acids on composite CV events {relative risk [RR] 0.96 [95% confidence interval (CI) 0.90, 1.02]; P = 0.24} or total mortality [RR 0.95 (95% Cl 0.86, 1.04); P = 0.28]. A major side effect was gastrointestinal disturbances.285 The FDA has approved the use of n-3 fatty acids (prescription products) as an adjunct to diet if TGs are >5.6 mmol/L (496 mg/dL). Although a recent Japanese study in patients with hypercholesterolaemia reported a 19% reduction in CVD outcome,286 the data remain inconclusive and their clinical efficacy appears to be related to non-lipid effects.287,288 Two randomized placebo-controlled trials [Reduction of Cardiovascular Events with EPA-Intervention Trial (REDUCE-IT) and Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia (STRENGTH)] to study the potential benefits of EPA on CVD outcomes in subjects with elevated serum TGs are ongoing. REDUCE-IT aims to recruit ∼8000 subjects and STRENGTH 13 000 subjects.
7.7.3 Safety and interactions
The administration of n-3 fatty acids appears to be safe and devoid of clinically significant interactions. However, the antithrombotic effects may increase the propensity to bleed, especially when given in addition to aspirin/clopidogrel. Recently the data from one study associated the risk of prostate cancer with high dietary intake of n-3 PUFAs.289
Summary of the efficacy of drug combinations for the management of mixed dyslipidaemias
 |
 |
TG = triglycerides.
Summary of the efficacy of drug combinations for the management of mixed dyslipidaemias
 |
 |
TG = triglycerides.
8. Drugs affecting high-density lipoprotein cholesterol (Table 20)
Low levels of HDL-C constitute a strong, independent and inverse predictor of the risk of premature development of atherosclerosis.83 Moreover, the increase in CV risk relative to low HDL-C levels is especially dramatic over the range of HDL-C from 0.65 to 1.17 mmol/L (25 to 45 mg/dL).260 Results from a meta-analysis of four intervention trials, which involved the use of intravascular ultrasound to evaluate changes in coronary atheroma volume, indicated that elevation ≥7.5% in HDL-C, together with a reduction in LDL-C to a target of 2.0 mmol/L (<80 mg/dL), represented the minimum requirement for plaque regression.290
Subjects with type 2 diabetes or those with mixed or combined dyslipidaemia, renal and hepatic insufficiency states or autoimmune diseases often present with low plasma concentrations of HDL-C. In addition to low HDL-C, these disease states feature a moderate or marked degree of HTG. The intravascular metabolism of TG-rich lipoproteins (principally VLDL) is intimately linked to that of HDL. Drug-induced elevation of HDL-C may lead to reductions in the cholesterol content of both VLDL and LDL. The magnitude of reduction in VLDL cholesterol (VLDL-C) and LDL-C under these circumstances tends to differ markedly as a function of the specific mechanism of action of the pharmacological agent concerned, as well as the dose employed and the baseline lipid phenotype. Furthermore, the percentage increase in HDL-C following treatment tends to be greater in subjects with the lowest baseline levels.
The available options for elevating low HDL-C levels are relatively few. While HDL-C levels may be increased by up to 10% by implementing therapeutic lifestyle changes, including weight reduction, exercise, smoking cessation and moderate alcohol consumption, many patients will also require pharmacological intervention if an HDL-C increase is sought. However, until now there has been no clear direct evidence that raising HDL-C really results in CVD prevention. Recent studies aimed at testing this hypothesis have failed to show any beneficial effect [ILLUMINATE (torcetrapib), Dalcetrapib Outcomes (dal-OUTCOMES), ACCELERATE (evacetrapib), HPS2-THRIVE (nicotinic acid plus statin), AIM-HIGH (nicotinic acid on background statin)], although the population selection in the last two studies may not have been optimal. The ongoing study with a cholesteryl ester transfer protein (CETP) inhibitor, the Randomized Evaluation of the Effects of Anacetrapib Through Lipid modification (REVEAL), will provide more information.
8.1 Statins
Statins produce modest elevations in HDL-C. In a meta-analysis291 of several intervention studies in dyslipidaemic patients, elevations in HDL-C varied with dose among the respective statins; such elevations were typically in the range of 5–10%. As a result of the marked reductions in atherogenic apoB-containing lipoproteins by statins, it is difficult to assess the extent to which the effect on HDL-C levels might contribute to the overall observed reductions in CV risk consistently seen in statin intervention trials. Despite such an effect, however, the elevated CV risk associated specifically with low HDL-C levels was only partially corrected by statin treatment in the Treatment to New Targets (TNT) trial.292
8.2 Fibrates
As a class, fibrates differ in their potential to modulate the atherogenic lipid profile by concomitantly lowering TG levels (up to 50%) and by raising those of HDL-C (up to 10–15% in short-term studies). However, the HDL-raising effect has been markedly less (∼5%) in the long-term intervention trials in people with type 2 diabetes261,262; such differences appear to reflect distinctions in their relative binding affinities for PPARs, and notably for PPAR-α.293
8.3 Nicotinic acid
Nicotinic acid appears to increase HDL-C by partially reducing HDL catabolism and by mainly increasing apoA1 synthesis by the liver. The latter effect is regarded as the most relevant for the HDL functions.263 Itsefficacy in clinical trials and adverse effects and drug interactions are described in section 7.6.
8.4 Cholesteryl ester transfer protein inhibitors
To date, the most efficacious pharmacological approach to elevation of low HDL-C levels has involved direct inhibition of CETP by small molecule inhibitors, which may induce an increase in HDL-C by ≥100% on a dose-dependent basis. Of the three CETP inhibitors developed originally (torcetrapib, dalcetrapib and anacetrapib), torcetrapib was withdrawn following an excess of mortality in the torcetrapib arm of the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial.294 The Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High-Risk for Vascular Outcomes (ACCELERATE) trial of evacetrapib in acute coronary syndrome subjects on statins was terminated due to futility.
Retrospectively, it appears that the deleterious effects of torcetrapib arose primarily from off-target toxicity related to activation of the renin–angiotensin–aldosterone system (RAAS). The results of the dalcetrapib trial (Dal-OUTCOMES) shows no effects in ACS patients. Phase III trials with anacetrapib (REVEAL) are ongoing.
8.5 Future perspectives
Major developments in the search for efficacious agents to raise HDL-C and apoA1 with concomitant benefit in atherosclerosis and CV events are on the horizon. Among them, major interest is focused on apoA1 mimetic peptides, which are not only active in cellular cholesterol efflux, but may also exert a vast array of biological activities including anti-inflammatory and immune-modulating effects. However, the genetic studies suggesting that low HDL-C is not causative for CVD may cast doubt on the possibilities of these treatment options.
Recommendations if drug treatment of low high-density lipoprotein cholesterol is considered
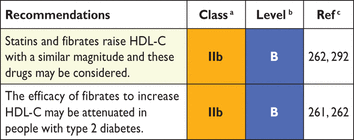 |
 |
HDL-C = high-density lipoprotein cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations if drug treatment of low high-density lipoprotein cholesterol is considered
 |
 |
HDL-C = high-density lipoprotein cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9. Management of dyslipidaemia in different clinical settings
9.1 Familial dyslipidaemias
Plasma lipid levels are to a very large extent determined by genetic factors. In its more extreme forms this is manifested as familial hyperlipidaemia. A number of monogenic lipid disorders have been identified; among these, FH is most common and strongly related to CVD. In general in patients with dyslipidaemia, most commonly the pattern of inheritance does not suggest that there is a major single gene disorder (monogenic) causing the abnormality, but rather that it stems from inheriting more than one lipoprotein gene variant that, on its own, might have relatively little effect, but in combination with another or others has a greater influence on TC, TGs or HDL-C. The pattern of inheritance is polygenic.295 It is common to find that high LDL-C, high TGs or low HDL-C affect several family members.
9.1.1 Familial combined hyperlipidaemia
Familial combined hyperlipidaemia (FCH) is a highly prevalent dyslipidaemia (1:100) and an important cause of premature CAD. FCH is characterized by elevated levels of LDL-C, TGs or both. The phenotype varies even among members of the same family. FCH shares considerable phenotype overlap with type 2 diabetes and MetS. FCH is a complex disease and the phenotype is determined by interaction of multiple susceptibility genes and the environment. The phenotype even within a family shows high inter- and intraperson variability based on lipid values (TGs, LDL-C, HDL-C and apoB). Therefore, the diagnosis is commonly missed in clinical practice; the combination of apoB >120 mg/dL + TGs >1.5 mmol/L (133 mg/dL) with a family history of premature CVD can be used to identify subjects who most probably have FCH.296 Currently, research is ongoing to define genetic markers; hopefully this approach will facilitate diagnosis of this frequent genetic dyslipidaemia.
The concept of FCH is also valuable clinically in assessing CV risk. It emphasizes both the importance of considering family history in deciding how rigorously to treat dyslipidaemia and that elevated LDL-C levels are riskier when HTG is also present. Statin treatment decreases CV risk by the same relative amount in people with HTG as in those without. Because the absolute risk is often greater in those with HTG, they may therefore benefit greatly from hypocholesterolaemic therapy.
9.1.2 Familial hypercholesterolaemia
9.1.2.1 Heterozygous familial hypercholesterolaemia
FH is a common monogenic dyslipidaemia causing premature CVD due to lifelong elevation of plasma levels of LDL-C. If left untreated, men and women with heterozygous FH (HeFH) typically develop CAD before the ages of 55 and 60 years, respectively. However, if diagnosed early and properly treated, the risk for CAD may be dramatically reduced, with some studies even suggesting a normal life expectancy.
The frequency of HeFH in the population has earlier been estimated at 1/500; however, recent studies from whole populations suggest that the frequency is higher, in some populations as high as 1/137.297 Based on extrapolations from available data, the frequency of HeFH can be estimated to be between 1/200 and 1/250, putting the total number of cases at between 14 and 34 million worldwide.121,298 Only a minor fraction of these are identified and properly treated. The risk of CHD among individuals with definite or probable HeFH is estimated to be increased at least 10-fold.
FH is a monogenic disease caused by loss of function mutations in the LDLR or apoB genes or a gain of function mutation in the PCSK9 gene; 95% of FH is caused by mutations in LDLR. More than a thousand different mutations have been identified in LDLR causing FH. The different mutations cause reduced function or complete loss of function. Complete loss of receptor function is associated with more severe disease. A total of 4–5% of FH is caused by mutations in apoB causing reduced binding to LDLR and ∼1% is caused by mutations in PCSK9 causing increased catabolism of LDLR.
The diagnosis of FH is in most cases based on the clinical picture. Different criteria for the diagnosis have been developed. The commonly used criteria from the Dutch Lipid Clinic Network (DLCN) are shown in Table 21. Other criteria are the Simon Broome register or the WHO criteria.299,300
Dutch Lipid Clinic Network diagnostic criteria for familial hypercholesterolaemia301
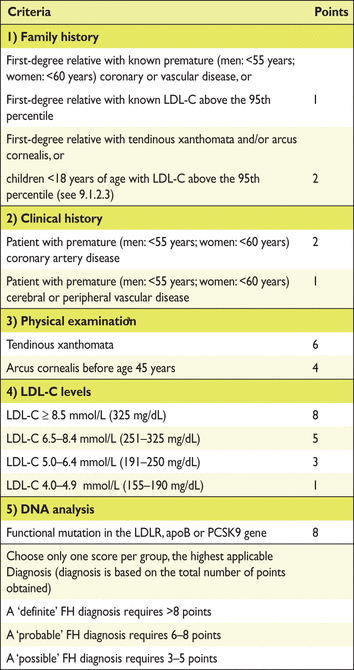 |
 |
FH = familial hypercholesterolaemia; LDL-C = low-density lipoproteincholesterol.
aExclusive of each other (i.e. maximum 6 points if both are present)
Dutch Lipid Clinic Network diagnostic criteria for familial hypercholesterolaemia301
 |
 |
FH = familial hypercholesterolaemia; LDL-C = low-density lipoproteincholesterol.
aExclusive of each other (i.e. maximum 6 points if both are present)
The clinical diagnosis of HeFH is based on family history of hypercholesterolaemia or premature CHD, clinical history of the patient regarding CVD and clinical signs. Finally, the diagnosis can be verified by showing causative mutations in the three pathogenic genes. However, in most studies the frequency of detectable mutations in patients with a clinically definite or probable HeFH is only 60–70%. This suggests that a considerable fraction of patients with FH have either a polygenic cause of the disease or that other genes, not yet identified, are involved.
Genetic testing and cascade screening. Probands (index cases) should be identified according to the following criteria:
plasma cholesterol ≥8 mmol/L (310 mg/dL) in an adult or adult family member (or >95th percentile by age and gender for country),
premature CHD in the subject or a family member,
tendon xanthomas in the subject or a family member or
sudden premature cardiac death in a family member
Cholesterol-lowering treatment should be initiated as soon as possible after the diagnosis has been made. To improve risk assessment, use of imaging techniques to detect asymptomatic atherosclerosis is recommended. The concept of cumulative cholesterol burden illustrates the importance of early treatment (for children, see below). Treatment should be initiated with high-intensity statin treatment, in most cases in combination with ezetimibe. LDL-C goals are <2.6 mmol/L (100 mg/dL) or <1.8 mmol/L (70 mg/dL) if CVD is present.
PCSK9 antibodies have recently been registered for use in FH patients. The drugs very efficiently lower LDL-C by up to 60% on top of the statin. Randomized controlled studies have yet to report clinical endpoints and therefore the use of these drugs should be limited. PCSK9 inhibitors should be considered in patients with FH at very high risk due to the presence of CVD, a family history of CAD at a very young age or an LDL-C level far from goal even on maximal other therapy. PCSK9 inhibitors should also be considered in HeFH patients who cannot tolerate statins and in FH patients with high Lp(a).
Recommendations for the detection and treatment of patients with HeFH are shown in Table 22.
Recommendations for the detection and treatment of patients with heterozygous familial hypercholesterolaemia
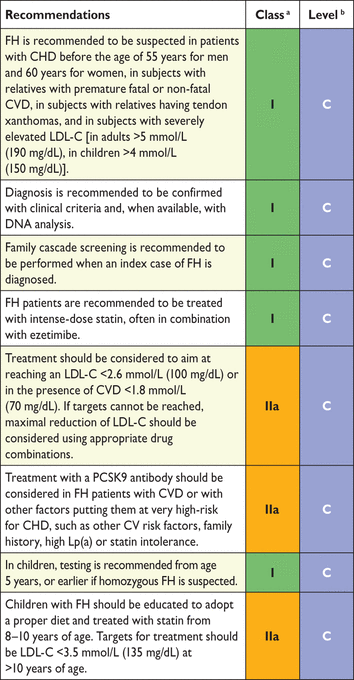 |
 |
CHD = coronary heart disease; CVD = cardiovascular disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein-cholesterol; Lp(a) = lipoprotein(a).
aClass of recommendation.
bLevel of evidence.
Recommendations for the detection and treatment of patients with heterozygous familial hypercholesterolaemia
 |
 |
CHD = coronary heart disease; CVD = cardiovascular disease; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein-cholesterol; Lp(a) = lipoprotein(a).
aClass of recommendation.
bLevel of evidence.
9.1.2.2 Homozygous familial hypercholesterolaemia
Homozygous FH (HoFH) is a rare and life-threatening disease. The clinical picture is characterized by extensive xanthomas, marked premature and progressive CVD and total cholesterol >13 mmol/L (500 mg/dL). Most patients develop CAD and aortic stenosis before the age of 20 years and die before 30 years of age. The frequency of HoFH is estimated to be 1/160 000–1/300 000. The early identification of these children and prompt referral to a specialized clinic is crucial. The patients should be treated with available cholesterol-lowering drugs and, when available, with lipoprotein apheresis. For a more detailed discussion of HoFH, including the role of PCSK9 inhibitors and the microsomal triglyceride transfer protein (MTP) inhibitor lomitapide, see the EAS consensus paper on HoFH.302
9.1.2.3 Familial hypercholesterolaemia in children
FH is diagnosed in children based on phenotypic criteria including elevated LDL-C plus a family history of elevated LDL-C, premature CAD and/or positive genetic testing.303 Testing during childhood is optimal to discriminate between FH and non-FH using LDL-C. LDL-C ≥5 mmol/L (190 mg/dL) is most probably FH. In children with a family history of high cholesterol or premature CHD, the cut-off point may be put at ≥4.0 mmol/L (160 mg/dL). If a parent has a known genetic defect, the diagnostic level for the child is ≥3.5 mmol/L (130 mg/dL).
Although placebo-controlled trials are missing in children, there are observational studies suggesting that early treatment can reduce LDL-C burden, improve endothelial function, substantially attenuate development of atherosclerosis and improve coronary outcomes.303 Treatment of children with FH includes a healthy lifestyle and statin treatment. A heart-healthy diet should be adopted early in life and statin treatment should be considered at 8–10 years of age. Statin treatment should be started with low doses and the dose should be increased to reach goals. The goal in children >10 years of age is an LDL-C <3.5 mmol/L (135 mg/dL) and at younger ages at least a 50% reduction of LDL-C.
9.1.3 Familial dysbetalipoproteinaemia
Familial dysbetalipoproteinaemia (i.e. type III hyperlipoproteinaemia; remnant removal disease) is rare and is generally inherited as an autosomal recessive disorder with variable penetrance. It is rare for it to be expressed in women before menopause. The majority of cases are homozygous for the E2 isoform of apoE. ApoE is important for hepatic clearance of chylomicron remnants and IDL. ApoE2 binds less readily than isoforms E3 or E4 to hepatic receptors. However, without some coincidental cause of dyslipidaemia, apoE2 homozygosity does not generally cause familial dysbetalipoproteinaemia syndrome. The syndrome often develops in the presence of dyslipidaemia associated with HTG, diabetes mellitus, obesity or hypothyroidism.
Familial dysbetalipoproteinaemia produces a characteristic clinical syndrome in which both TC and TGs are elevated before treatment, usually both in the range of 7–10 mmol/L. In severe cases, patients develop tuberoeruptive xanthomata, particularly over the elbows and knees, and palmar xanthomata in the skin creases of the hands and wrists. The risk of CAD is very high, and accelerated atherosclerosis of the femoral and tibial arteries is also prevalent.
The detection of apoE2 homozygosity in a dyslipidaemic patient is diagnostic and analysis of apoE isoforms is now available in most clinical laboratories. In older patients with xanthomata resembling those of familial dysbetalipoproteinaemia, who prove not to be homozygous for apoE2, a paraprotein should be sought. The treatment of familial dysbetalipoproteinaemia should be undertaken in a specialist clinic. Most cases respond well to treatment with a statin or, if dominated by high TGs, a fibrate; often a combination of a statin and a fibrate may be needed.
9.1.4 Genetic causes of hypertriglyceridaemia
The genetic aetiology for HTG seems to be very complex, with effects of both common and rare genetic variants.67,304 Moderate elevation of TG levels (between 2.0–10.0 mmol/L) is caused by the polygenic effect of multiple genes influencing both VLDL production and removal. In CVD prevention, polygenic moderate TG elevation is to be considered. Monogenic severe HTG causes pancreatitis and lipid deposits. Thus far, mutations in six genes (LPL, apoC2, apoA5, LMF1, GPIHBP1 and GPD1) with monogenic effect have been recognized to lead to severe elevation of serum TGs due to disruption of the chylomicron removal pathways. These mutations are inherited as an autosomal recessive trait and are rare. A profound defect in the catabolism of chylomicrons and VLDL results in chylomicronaemia and TG levels >11.2 mmol/L (1000 mg/dL), with turbid and milky serum. Severe HTG is seen in patients who are homozygous or compound heterozygous for mutations of the enzyme lipoprotein lipase (LPL) and in the other genes linked to catabolism of TG-rich lipoproteins. Recently, gene therapy for LPL deficiency has been developed and tested in clinical trials305 and the alipogene tiparvovec was approved by the EMA in 2013. A gain of function mutation in apoC3 leading to high apoC3 levels can also cause severe HTG by the inhibition of LPL activity, whereas loss of function mutations are associated with a favourable lipid profile with low TG levels.306 These findings have raised the possibility of apoC3 as a novel lipid drug target. In summary, the development of new therapeutic options for this rare disease raises the need for awareness and screening of these patients.
9.1.4.1 Action to prevent acute pancreatitis in severe hypertriglyceridaemia
The risk of pancreatitis is clinically significant if TGs exceed 10 mmol/L (880 mg/dL), and actions to prevent acute pancreatitis are mandatory. Notably, HTG is the cause of ∼10% of all cases with pancreatitis, and patients can develop pancreatitis even when their TG concentration is 5–10 mmol/L (440–880 mg/dL). Recent data from a prospective cohort study (n = 33 346) reported that the risk of acute pancreatitis increased significantly over the quartiles of serum TGs, highlighting that serum TGs as a risk factor may have been underestimated.307 Any factor that increases VLDL production can aggravate the risk of pancreatitis, with alcohol consumption being the most common contributing factor. The patient should be admitted to hospital if symptomatic, or careful and close follow-up of the patient's TG values should be undertaken. Restriction of calories and fat content (10–15% recommended) in the diet and alcohol abstinence are obligatory. Fibrate therapy (fenofibrate) should be initiated, with n-3 fatty acids (2–4 g/day) as adjunct therapy or nicotinic acid. Lomitapide may also be considered in severe cases.67 In patients with diabetes, insulin therapy should be initiated to achieve good glycaemic control. In general, a sharp decrease of TG values is seen within 2–5 days. In the acute setting, plasmapheresis is able to rapidly lower TG levels.308
9.1.5 Other genetic disorders of lipoprotein metabolism (Table 23)
Sometimes patients are encountered with extremely low levels of LDL-C or HDL-C. The most common genetic hypolipidaemia is hypobetalipoproteinaemia, which is dominantly inherited and often due to truncation of apoB. Serum LDL-C is typically between 0.5 and 1.5 mmol/L (20–60 mg/dL). It is generally of no medical significance. A more profound deficiency of apoB occurs in abetalipoproteinaemia when steatorrhoea and neurological or other complications require specialist treatment. Almost absent levels of HDL-C occur in Tangier disease (analphalipoproteinaemia) and very low levels of HDL-C occur in lecithin cholesterol acyltransferase (LCAT) deficiency. Both these conditions are associated with distinct clinical syndromes and require specialist investigation. Very high levels of HDL-C are detected in patients with CETP deficiency. In the heterozygous form, typically levels of 2.0–2.4 mmol/L (80–90 mg/dL) are observed, and levels ≥5 mmol/L (200 mg/dL) are observed in homozygotes. This is not associated with atherosclerotic disease and may be associated with reduced risk.
Lysosomal acid lipase (LAL) deficiency or cholesterol ester storage disease (in children with Wolman disease) is a rare cause (recessive transmission) of elevated LDL-C and low HDL-C accompanied by hepatomegaly and microvesicular hepatosteatosis. Statin treatment does decrease LDL-C, and therefore could prevent CVD in these patients, but it cannot stop the progression of liver damage. Enzyme replacement therapy with sebelipase alfa might offer a treatment solution in the near future.309
Genetic disorders of lipoprotein metabolism
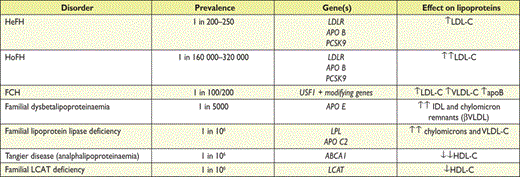 |
 |
apo = apolipoprotein; FCH = familial combined hyperlipidaemia; HeFH = heterozygous familial hypercholesterolaemia; HoFH = homozygous familial hypercholesterolaemia; HDL-C = high-density lipoprotein-cholesterol; IDL = intermediate-density lipoprotein; LCAT = lecithin cholesterol acyltransferase; LDL-C = low-density lipoproteincholesterol; VLDL = very low-density lipoprotein-cholesterol.
Genetic disorders of lipoprotein metabolism
 |
 |
apo = apolipoprotein; FCH = familial combined hyperlipidaemia; HeFH = heterozygous familial hypercholesterolaemia; HoFH = homozygous familial hypercholesterolaemia; HDL-C = high-density lipoprotein-cholesterol; IDL = intermediate-density lipoprotein; LCAT = lecithin cholesterol acyltransferase; LDL-C = low-density lipoproteincholesterol; VLDL = very low-density lipoprotein-cholesterol.
9.2 Children
Only children with FH should be considered for lipid-lowering drug treatment. In other cases of dyslipidaemia in children, focus should be on diet and treatment of underlying metabolic disorders. HoFH patients should be treated with lipid-lowering drugs as early as possible, and the same is true for HeFH patents with extremely high LDL-C, i.e. ≥400 mg/dL (∼10.3 mmol/L).310 In the case of other HeFH children, statin treatment is generally withheld until sometime between the ages of 8 and 10 years. There is evidence from carotid ultrasound measurements that increased CIMT in children with HeFH compared with siblings who have not inherited HeFH can be detected from the age of 6 years onwards, and that the progression of increasing CIMT can be ameliorated with statin therapy and/or apheresis.311 However, the exact age at which to start statin treatment is a matter of clinical judgement.
9.3 Women
Among several studies that have evaluated the impact of lipid-lowering therapy on primary and secondary prevention of CAD, only a few have included women, usually in small numbers, and the results have often not been separately reported by gender.312 The most recent CTT meta-analysis, however, indicates a similar relative benefit in men and women.65
9.3.1 Primary prevention
The benefit of statins in primary prevention is less well-established in women than in men. This may be because of their lower baseline risk and their underrepresentation in trials, and points to the need to include gender balance and sufficient numbers to detect modest absolute treatment effects in future trials.
The 2013 Cochrane analysis showed reductions of all-cause mortality, vascular events and revascularisations with statins in primary prevention. Effects in women were similar to those in men.200 In postmenopausal women, plaque rupture was found to be a more common cause of ACS than plaque erosion and is correlated with TC levels.313
A recent meta-analysis of statin trials in the CTT database compared the effects of statin therapy between men and women.65 The proportional (relative risk) reductions in major coronary events, coronary revascularisations and stroke did not differ significantly by gender. All-cause mortality reductions were seen in both women and men, showing that statin therapy is of similar effectiveness. Significant decreases in vascular events in primary prevention were seen in both women and men. Thus statin use should be considered for primary prevention in women at high CV risk with the same indications as for men.
9.3.2 Secondary prevention
More data coming from large RCTs of secondary prevention are available for women. The results of these trials concordantly show that lipid-lowering therapy substantially reduces CV events in these patients, although no reduction in total mortality risk could be demonstrated. The meta-analysis of Walsh and Pignone314 reported a 26% reduction of CV mortality, a 29% reduction of MI and a 20% reduction of total CAD events in a cohort of 8272 females with previous CVD mainly treated with statins. The CTT meta-analysis also indicates that the benefit overall is similar in men and women.65 Therefore, secondary prevention of CV events in women should routinely include a statin-based lipid-lowering regimen, with the same recommendations and therapeutic goals that are applied to men.
9.3.3 Non-statin lipid-lowering drugs
No definitive evidence of cardioprotective effects was available until recently. The IMPROVE-IT study63 included patients at least 50 years of age who had been hospitalized within the preceding 10 days for an ACS (24% women). The combination of simvastatin–ezetimibe was compared with simvastatin monotherapy. The rate of the composite endpoint of death from CV causes, MI or stroke was significantly lower, by 1.8 percentage points, in the combination group and the benefit of simvastatin–ezetimibe was consistent for women.63
The ACCORD lipid study showed less primary event reduction with combination therapy in women, but the recent FIELD study analysis showed consistent CV event reduction in women and men.315 Ezetimibe, or fibrates, alone or in combination with statins, can be used, depending on the type of dyslipidaemia and adverse effect profiles. Recent data with PCSK9 inhibitors indicate a similar efficacy in reducing LDL-C in women vs. men.115,116
9.3.4 Hormone therapy
Currently used third-generation low-dose oestrogen–progestin oral contraceptives do not appear to increase adverse coronary events316 and can be used, after baseline lipid profile assessment, in women with acceptable TC levels. In contrast, alternative contraceptive measures should be recommended in women with hypercholesterolaemia [LDL-C >4 mmol/L (160 mg/dL)] or with multiple risk factors and in those at high risk of thrombotic events.317 Oestrogen replacement therapy, despite some favourable effects on the lipid profile, has not been demonstrated to reduce CV risk and cannot be recommended for CVD prevention in women.318 No lipid-lowering drugs should be administered during pregnancy and the period of breastfeeding since data on possible adverse effects are lacking. However, bile acid sequestrants may be considered.
Box 10 lists the main measures in the management of dyslipidaemia in women.
9.4 Older persons
The proportion of older people in society is increasing and, as a consequence, >80% of individuals who die from CVD are >65 years of age. The proportion of patients with MI >85 years of age has increased several fold.319 Coronary care has also been improved for older adults, with improving prognosis after the first MI.320 To target the older population with risk reduction is important since CVD or subclinical atherosclerosis is common and dyslipidaemia is frequent.
Results from a meta-analysis on blood cholesterol and vascular mortality indicates that high TC is a significant risk factor for CAD mortality at all ages, but this association is attenuated in older adults; 1 mmol/L (38.7 mg/dL) lower TC was associated with about one-half [hazard ratio (HR) 0.44] lower CAD mortality in the age group 40–49 years compared with an HR of 0.85 in those 80–89 years old.321,322 However, although a relative risk reduction is seen in the oldest subjects, the increased frequency of CAD means that the absolute number of cases associated with cholesterol is highest in this group. Evidence for treatment in this group, particularly for those >80–85 years of age, is limited, and clinical judgement should guide decisions in the very old.
9.4.1 Primary prevention
The most important way to prevent CVD in older adults is to promote a healthy lifestyle and reduction of risk factors early in life. Several studies have shown that a healthy lifestyle early in life prevents CVD in older age and reduces lifetime risk for CVD.53,323–325 Lifetime prevention includes no smoking, control of blood pressure, healthy eating habits, regular exercise and controlling body weight. No primary prevention study has specifically targeted the older population.326 Available data are based on subgroup analyses from controlled studies. In a recent meta-analysis, subjects >65 years of age (n = 24 674) from eight studies were included.327 Statin treatment reduced MI (RR 0.61) and stroke (RR 0.76). The reduction in all-cause mortality was not significant (RR 0.94). In the Air Force/Texas Coronary Atherosclerosis Prevention (AFCAPS-TEXCAP) study, risk reduction was similar above and below the median age (57 years for men and 62 years for women).328 In the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial a post hoc analysis of subjects older and younger than 70 years showed that the relative risk reduction for a composite CVD endpoint was similar in the two groups. The number needed to treat for 4 years to prevent one major event was 24 in the older group and 36 in the younger age group.329
9.4.2 Secondary prevention
Also in secondary prevention, very few studies have targeted the older population. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) study included patients 70–82 years of age with CVD or at high risk for CVD.330 Patients were treated with pravastatin 40 mg daily or placebo. The relative risk reduction for a combined CAD endpoint was reduced by 15%, whereas no reduction was shown for stroke. In the Studies Assessing Goals in the Elderly (SAGE) trial, 893 patients 65–83 years of age with stable CAD were recruited to treatment with atorvastatin 80 mg or pravastatin 40 mg.331 The atorvastatin group had a lower all-cause mortality (HR 0.33) and a non-significant trend towards reduction in major CAD events.
Subgroup analyses in randomized trials have been performed for several studies. In the Scandinavian Simvastatin Survival Study (4S) trial, patients >65 years of age had a similar relative risk reduction as younger patients.332 In the Heart Protection Study (HPS), 20 536 individuals were allocated to simvastatin or placebo. After 5 years the relative risk reduction was 18% for coronary death and 25% for coronary events. The reduction was similar in age groups <65, 65–70 and >70 years.333 Similar results were found in subgroup analyses of the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID),334 Cholesterol and Recurrent Events (CARE)335 and TNT trials.336 From data in the LIPID trial the authors calculated that per 1000 persons treated, 45 deaths and 47 major coronary events would be prevented in the older group over 6 years compared with 22 deaths and 32 major coronary events in younger patients over the same time period.
In a CTT meta-analysis, rate ratios for the effects of statins on major vascular events were 0.78, 0.78 and 0.84 in age groups <65, 65–75 and >75 years, respectively.64 Results from an MI registry study in Sweden demonstrate that statin treatment is associated with lower CV mortality in very old post-MI patients without (which is important to stress) increasing the risk of cancer.337
9.4.3 Adverse effects, interactions and adherence
The safety and adverse effects of statins are a matter of special concern in older adults because they often have co-morbidities, take multiple medications and have altered pharmacokinetics and pharmacodynamics. Statin–drug interactions are a concern primarily because of their potential to increase muscle-related statin-associated adverse effects such as myalgia without CK elevation, myopathy with CK elevation and the rare but serious rhabdomyolysis. Statin treatment should be started at a low dose to avoid adverse events and then titrated to achieve optimal LDL-C levels with an appropriate dose.
Older adults are less likely to be prescribed lipid-lowering medications, or adhere to statin therapy, than younger or middle-age subjects. Cost, adverse effects, coronary events occurring despite being on lipid-lowering agents and the wrong perception that the drug is not beneficial are among the reasons for non-adherence. Improving patient understanding of CV risk, the medication regimen and potential benefits of persistence with statin therapy may enhance adherence.
Table 24 lists the recommendations for treatment of dyslipidaemia in older adults.
Recommendations for the treatment of dyslipidaemia in older adults
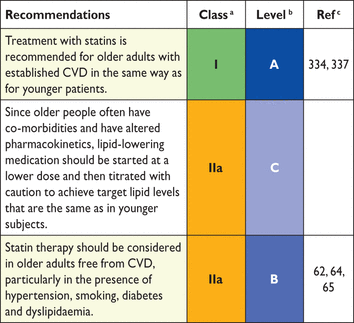 |
 |
CVD = cardiovascular disease.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for the treatment of dyslipidaemia in older adults
 |
 |
CVD = cardiovascular disease.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.5 Diabetes and metabolic syndrome
Diabetes seems to be the fastest increasing disease in the world and it is estimated that the number of people with diabetes will increase from ∼350 million today up to 550 million by 2030.338 Despite significant advantages in the management strategies that lessen CVD risk factors, CVD has remained the leading cause of morbidity and mortality in patients with type 2 diabetes mellitus (T2DM). It has been estimated that the years of life lost for a 50-year-old person with diabetes averages at 6 years and ∼58% of this difference is due to excess vascular disease.339 Diabetes itself is an independent risk factor for CVD and is associated with a higher risk of CVD, even more so in women. Although the difference in CVD risk between individuals with and without diabetes has narrowed substantially over the last decades, there are strong associations between diabetes and vascular outcomes.340,341 Recent data indicate that diabetes per se increases CVD risk about two-fold on average, but the risk is subject to wide variation depending on the population.342 Importantly, those with diabetes and CAD are at substantially higher CVD risk for future events. Hypertension, dyslipidaemia and abdominal obesity commonly co-exist with T2DM and further aggravate the risk, which is highest in people with T2DM and features of MetS.343,344 Importantly, diabetes confers excess mortality risk following ACS despite modern therapies, highlighting the poor prognosis of coronary patients with T2DM and the need for intensive therapy.345
Even more frequent are conditions predisposing to diabetes, such as the so-called metabolic syndrome. The term MetS refers to the clustering of different cardiometabolic risk factors: central obesity, raised serum TGs, reduced HDL-C, glucose intolerance and high blood pressure.346,347 Scoring systems that dichotomize these variables may miss some of the associated risk; a practical approach is that if one component is identified, a systematic search should be made for others.
MetS identifies people at a higher risk of CVD than the general population. Data from recent meta-analyses indicate that people with MetS have a two-fold increase in CV outcomes and a 1.5-fold increase in all-cause mortality.348 How to capture the extra risk beyond the traditional risk factors in clinical practice is a debated issue. A combination of large waist circumference and elevation of TGs is a simple and inexpensive screening tool to discriminate people with MetS at high CVD risk for global risk evaluation.180
9.5.1 Specific features of dyslipidaemia in insulin resistance and type 2 diabetes (Table 25)
Diabetic dyslipidaemia is a cluster of plasma lipid and lipoprotein abnormalities that are metabolically interrelated. The increase in large VLDL particles in T2DM initiates a sequence of events that generates atherogenic remnants, small dense LDL and small TG-rich dense HDL particles.349 These components are not isolated abnormalities but are closely linked to each other. Both LDL and HDL particles show variable compositional changes that are reflected in their functions. Notably apoCIII levels are increased in subjects with T2DM.350 Together, TRL remnants, small dense LDL and small dense HDL comprise the atherogenic lipid profile, which is also characterized by an increase in apoB concentration due to an increased number of apoB-containing particles. Importantly, TRLs, including chylomicrons, VLDL and their remnants, carry a single apoB molecule, also like LDL particles. Therefore the malignant nature of diabetic dyslipidaemia is not always revealed by the lipid measures used in clinical practice, as LDL-C may remain within the normal range. It may be better revealed by non-HDL-C. Elevation of TGs or low HDL-C is seen in about half of subjects with T2DM.351 The abnormal features of the lipid profile precede the onset of T2DM by several years and are common in subjects with central obesity, MetS and T2DM.
Summary of dyslipidaemia in metabolic syndrome and in type 2 diabetes
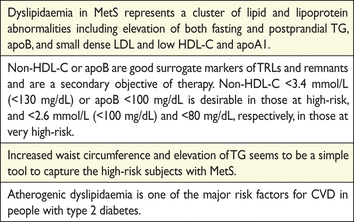 |
 |
apoB = apolipoprotein B; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; MetS = metabolic syndrome; TG = triglycerides; TRLs = triglyceride-rich lipoproteins.
Summary of dyslipidaemia in metabolic syndrome and in type 2 diabetes
 |
 |
apoB = apolipoprotein B; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; MetS = metabolic syndrome; TG = triglycerides; TRLs = triglyceride-rich lipoproteins.
9.5.2 Evidence for lipid-lowering therapy
9.5.2.1 Low-density lipoprotein cholesterol
LDL-C is stipulated to be the primary target of lipid-lowering therapy in diabetes. Trials specifically performed in subjects with T2DM as well as subsets of individuals with diabetes in major statin trials have consistently demonstrated significant benefits of statin therapy on CVD events in people with T2DM.64 Statin therapy reduces the 5-year incidence of major CVD events by 23% per 1 mmol/L reduction in LDL-C, regardless of the initial LDL-C or other baseline characteristics based on meta-analysis.64 The CTT meta-analysis further indicates that subjects with T2DM will have a relative risk reduction that is comparable to that seen in non-diabetic patients, but being at higher absolute risk, the absolute benefit will be greater, resulting in a lower number needed to treat (NNT). Recent studies have suggested an increased incidence of diabetes in patients treated with statins.225 This effect must not lessen our attention to the treatment of patients, as the overall benefit in CV events reduction remains.
9.5.2.2 Triglycerides and high-density lipoprotein cholesterol
Clinical benefits achieved by the treatment of atherogenic dyslipidaemia (high TGs and low HDL-C) are still a matter of discussion. Although the Helsinki Heart Study reported a significant reduction in CVD outcomes with gemfibrozil, neither the FIELD nor the ACCORD study showed a reduction in total CVD outcomes.261,262,265 The FIELD trial failed to reduce significantly the primary endpoint of CAD events (CAD death or non-fatal MI). CVD events were reduced significantly by 11%. In a post hoc analysis of the FIELD study, fenofibrate reduced CVD events by 27% in those with elevated TGs [>2.3 mmol/L (∼204 mg/dL)] and reduced HDL-C (NNT = 23).351 The ACCORD trial confirmed this: patients who had both TG levels in the higher third [≥2.3 mmol/L (204 mg/dL)] and an HDL-C level in the lower third [≤0.88 mmol/L (34 mg/dL)], representing 17% of all participants, appeared to benefit from adding fenofibrate to simvastatin.262
A post hoc analysis of patients with low HDL-C [<1 mmol/L (∼40 mg/dL)] and elevated TGs [>1.80 mmol/L (∼160 mg/dL)] in the 4S trial demonstrated a relative risk for major coronary events of 0.48 with simvastatin. The respective relative risk for overall mortality was 0.44.352 Consistent with these findings, a meta-analysis of fibrates in the prevention of CVD in 11 590 people with T2DM showed that fibrates significantly reduced the risk of non-fatal MI by 21%, but had no effect on the risk of overall mortality or coronary mortality.353
The concept of raising HDL-C seems attractive based on the strength of the relationship between low HDL-C and increased CVD risk in observational studies. Evidence for a beneficial clinical effect of raising HDL-C is lacking, with lifestyle modification providing the first option due to its multifaceted effects.
9.5.3 Treatment strategies for subjects with type 2 diabetes and metabolic syndrome
Lifestyle therapy to improve the atherogenic lipid profile should be recommended to all subjects with T2DM and MetS (see section 5). Dietary advice should be tailored according to the individual's needs. If LDL-C goals are not achieved on maximally tolerated doses of statins, drug combinations may offer additional lowering of LDL-C, but the evidence from outcome studies is limited.354 Patients with T2DM <40 years of age, with a short duration of therapy, without other risk factors, without complications and with an LDL-C level <2.6 mmol/L (100 mg/ dL) may not need lipid-lowering drugs.
9.5.4 Type 1 diabetes
Type 1 diabetes is associated with high CVD risk, in particular in patients with microalbuminuria and renal disease.355 Conclusive evidence supports the proposition that hyperglycaemia accelerates atherosclerosis. Emerging evidence highlights the frequent coexistence of MetS with type 1 diabetes, resulting in this so-called double diabetes increasing CVD risk.356
The lipid profile in type 1 diabetic subjects with good glycaemic control is ‘supernormal’ and characterized by subnormal TGs and LDL-C, whereas HDL-C is usually within the upper normal range or slightly elevated. This is explained by subcutaneous administration of insulin that increases LPL activity in adipose tissue and skeletal muscle and consequently the turnover rate of VLDL particles. However, there are potentially atherogenic changes in the composition of both HDL and LDL particles. In all patients with type 1 diabetes and in the presence of microalbuminuria and renal disease, LDL-C lowering (at least 30%) with statins as the first choice (drug combination may be considered if needed) is recommended irrespective of the basal LDL-C concentration.
Recommendations for the treatment of dyslipidaemia in diabetes are shown in Table 26.
Recommendations for the treatment of dyslipidaemia in diabetes
 |
 |
apoB = apolipoprotein B; CKD = chronic kidney disease; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoproteincholesterol; MetS = metabolic syndrome; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for the treatment of dyslipidaemia in diabetes
 |
 |
apoB = apolipoprotein B; CKD = chronic kidney disease; CVD = cardiovascular disease; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoproteincholesterol; MetS = metabolic syndrome; TG = triglycerides.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.6 Patients with acute coronary syndrome and patients undergoing percutaneous coronary intervention
Patients who have presented recently with ACS are at high risk of experiencing further CV events. In these patients, lipid management should be undertaken in the context of a comprehensive global risk management strategy that includes lifestyle adaptations, management of risk factors and the use of cardioprotective drugs in certain subgroups. Ideally, this can be well coordinated through participation in a multidisciplinary cardiac rehabilitation programme.
9.6.1 Specific lipid management issues in acute coronary syndrome
Data from specific trials358–360 and meta-analysis support routine early use of prompt, intensive and prolonged statin therapy. Thus we recommend that high-intensity statin therapy be initiated during the first 1–4 days of hospitalization for the index ACS; the dose should aim at reaching the LDL-C goal of <1.8 mmol/L (∼70 mg/dL) or a 50% reduction of LDL-C as indicated in Table 11 on treatment goals. The use of lower-intensity statin therapy should be considered in patients at increased risk of adverse effects with high-intensity statins (e.g. the elderly, hepatic impairment, renal impairment or potential for interaction with essential concomitant therapy). Ezetimibe further reduced LDL-C and offered additional benefit (6.4% relative risk reduction in composite clinical endpoint) in association with simvastatin in post-ACS patients.63 Results in studies with PCSK9 inhibitors that included post-ACS/very high-risk patients were promising115,116 and definitive outcome studies are awaited.
Lipids should be re-evaluated 4–6 weeks after ACS to determine whether lipid goals have been reached and whether there are any safety issues; the therapeutic regimen can then be adapted accordingly.
Supplementation with highly purified n-3 PUFAs reduced mortality in survivors of MI in one study (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)], but failed to affect clinical outcomes in two more recent trials using modern evidence-based prevention therapies (most patients were on statins), and therefore cannot be recommended in routine practice.361 Furthermore, in patients who had experienced a recent ACS, the CETP inhibitor dalcetrapib did not reduce the risk of recurrent CV events.362
9.6.2 Lipid management issues in patients undergoing percutaneous coronary intervention
In an individual patient meta-analysis of 13 randomized studies that included 3341 patients, it was shown that either pretreatment with high-dose statin (ranging from >2 weeks to a single dose) in statin-naïve patients (11 studies) or loading of a high-dose statin in patients receiving chronic statin therapy decreased periprocedural MI and 30-day adverse events in patients undergoing percutaneous coronary intervention (PCI).363–365 In all but one study, PCI was performed in the setting of stable angina, or in a non-emergent fashion in non-ST elevation ACS (NSTE-ACS). In one study in the setting of primary PCI that was included in the meta-analysis, coronary flow was improved.366 Thus a strategy of routine short pretreatment or loading (on the background of chronic therapy) with high-dose statin before PCI should be considered in elective PCI or in NSTE-ACS (class IIa, level of evidence A).363–365 High-dose statin pretreatment or loading before primary or delayed PCI for ST elevation MI (STEMI) requires further study. Statin pretreatment is also effective in reducing the risk of contrast-induced acute kidney injury after coronary angiography or intervention.367
Recommendations for lipid-lowering therapy in patients with ACS and patients undergoing PCI are shown in Table 27.
Recommendations for lipid-lowering therapy in patients with acute coronary syndrome and patients undergoing percutaneous coronary intervention
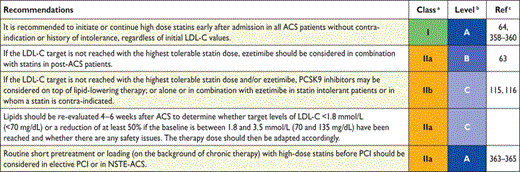 |
 |
ACS = acute coronary syndrome; LDL-C = low-density lipoprotein-cholesterol; NSTE-ACS = non-ST elevation acute coronary syndrome; PCI = percutaneous coronary intervention; PCSK9 = proprotein convertase subtilisin/kexin type 9.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for lipid-lowering therapy in patients with acute coronary syndrome and patients undergoing percutaneous coronary intervention
 |
 |
ACS = acute coronary syndrome; LDL-C = low-density lipoprotein-cholesterol; NSTE-ACS = non-ST elevation acute coronary syndrome; PCI = percutaneous coronary intervention; PCSK9 = proprotein convertase subtilisin/kexin type 9.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.7 Heart failure and valvular diseases
9.7.1 Prevention of incident heart failure in coronary artery disease patients
The onset of heart failure (HF) increases the risk of mortality and morbidity three to four times compared with patients without HF. Pooling of results from RCTs suggested that cholesterol lowering with statin treatment reduced incident HF by 9–45% in patients with CAD.368,369 Four key prospective RCTs compared more intensive vs. less intensive drug regimens. The more intense approach reduced the incidence of hospitalization due to HF by an average of 27% in patients with acute and stable CAD without previous HF.358,370–372 However, there is no evidence that statins can prevent HF of non-ischaemic origin.
9.7.2 Chronic heart failure
HF patients have lower TC and LDL-C than patients without HF. In contrast to patients without HF, low TC portends a poor prognosis in HF. Routine administration of statins in patients with HF is not advised. In two large RCTs373,374 there was no benefit on hard endpoints, such as CV mortality and non-fatal MI and stroke, in spite of a decrease in hospitalizations373,375 and a marked reduction of LDL-C and hs-CRP in patients with mainly systolic heart failure. In any case, there is no evidence for harm in patients on statin treatment after the occurrence of HF, and therefore there is no need for discontinuation if patients are already on this medication. n-3 PUFAs may have a small benefit. In the GISSI-HF RCT, a significant effect on primary endpoints (all-cause death and hospitalization for HF) was found in HF patients with New York Heart Association (NYHA) classifications II–IV.376
9.7.3 Valvular disease
Aortic stenosis increases the risk of CV events and mortality. Suggestions for an association between aortic stenosis, LDL-C and Lp(a), as well as between cholesterol and increased risk for calcification of bioprosthetic valves, were matched with early observational non-controlled trials that showed beneficial effects of aggressive lipid lowering in slowing the progression of aortic stenosis. However, this was not confirmed in RCTs.243,377,378 The Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE; 155 patients, 80 mg atorvastatin or placebo), the SEAS (1873 patients, simvastatin 40 mg plus ezetimibe 10 mg or placebo) and the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER; 269 patients, rosuvastatin 40 mg or placebo) trials failed to show a reduction in the progression of aortic stenosis or related events in patients with mild to moderate aortic stenosis. Notably, ischaemic events were reduced by 21% in the SEAS trial. Furthermore, in a post hoc analysis of the RCTs Incremental Decrease In End-points Through Aggressive Lipid-lowering Trial (IDEAL) and Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL), high-dose versus usual-dose statin therapy or placebo did not impact the incidence of aortic valve stenosis among patients without known aortic valve stenosis.379 Aortic valve sclerosis (calcification of the aortic leaflets without impairment in leaflet excursion or a significant transvalvular pressure gradient) is associated with an increased risk of CAD even in the absence of increased risk profiles. In such patients, statins, acting at an earlier disease stage, may be useful both for aortic valve disease and CAD progression; however, this warrants further investigation.380 Regarding rheumatic mitral stenosis and bioprosthetic valves, small observational studies suggest a benefit of statin treatment.381,382
Recommendations for lipid-lowering therapy in patients with HF and valvular diseases are shown in Table 28.
Recommendations for the treatment of dyslipidaemia in heart failure or valvular disease
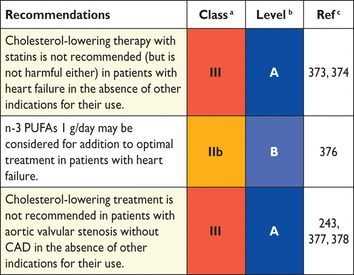 |
 |
CAD = coronary artery disease; PUFAs = polyunsaturated fatty acids.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for the treatment of dyslipidaemia in heart failure or valvular disease
 |
 |
CAD = coronary artery disease; PUFAs = polyunsaturated fatty acids.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.8 Autoimmune diseases
Autoimmune diseases, including rheumatoid arthritis, SLE, psoriasis and anti-phospholipid syndrome, are characterized by enhanced atherosclerosis and consequently higher CV morbidity and mortality rates compared with the general population.383–385 The immune system is believed to be involved in the pathogenesis of atherosclerosis. Inflammatory components of the immune response as well as autoimmune elements (e.g. autoantibodies, autoantigens and autoreactive lymphocytes) are involved in these processes. These diseases are characterized by inflammatory vasculitis and endothelial dysfunction. Therefore particular attention should be paid to conventional CVD risk factor treatment, including dyslipidaemia, in these patients. Statins are effective in reducing disease activity, CV events and mortality (particularly in primary prevention) in this setting, while their discontinuation increases MI and mortality.386 However, there is no firm indication to use lipid-lowering therapy only on the basis of the presence of the disease (Table 29). Furthermore, no specific LDL-C goal beyond that indicated by individual total risk has been set for such patients.
Recommendation for the treatment of dyslipidaemia in autoimmune diseases
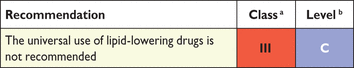 |
 |
aClass of recommendation.
bLevel of evidence.
Recommendation for the treatment of dyslipidaemia in autoimmune diseases
 |
 |
aClass of recommendation.
bLevel of evidence.
9.9 Chronic kidney disease
CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health. CKD can be classified on the basis of the GFR into five categories.387 In the adult population, a decreasing GFR is associated with an increased CVD risk, independent of other CV risk factors.388–391 The CV mortality in patients with stage 3 and stage 4 CKD is two-fold and three-fold higher, respectively, when compared with patients with normal renal function.391 Patients with CKD and established CVD have a much higher mortality rate compared with patients with CVD and normal renal function.392 Therefore patients with CKD are considered high risk (stage 3 CKD) or very high risk (stage 4–5 CKD or on dialysis) and there is no need to use risk estimation models in these patients.
9.9.1 Lipoprotein profile in chronic kidney disease
The lipid profile shows both quantitative and qualitative abnormalities that worsen with declining GFR, being most pronounced in subjects with end-stage renal disease (ESRD). In the initial stages of CKD, TGs are specifically elevated and HDL-C lowered; the elevation of TGs is caused by both increased production and impaired removal of TRLs due to changes in regulatory enzymes and proteins. Consequently, non-HDL-C and apoB levels are clearly increased. LDL subclasses display a shift to an excess of small dense LDL particles. In patients with ESRD, the catabolic rate of LDL is markedly prolonged, resulting in clear elevation of both TC and LDL-C levels. Plasma Lp(a) levels also start to increase early due to the prolonged residence times of these particles in the circulation. Altogether, most patients with stage 3–5 CKD have mixed dyslipidaemia and the lipid profile is highly atherogenic, with adverse changes in all lipoproteins.
9.9.2 Evidence for lipid management in patients with chronic kidney disease
In a systematic review of 50 studies including 45 285 participants, the benefits and harms of statins were evaluated in comparison with placebo or no treatment (47 studies) or another statin (3 studies) in adults with CKD and no CVD at baseline.393 Statins consistently lowered the death rate and major coronary events by 20%; statin-related effects on stroke and kidney function were found to be uncertain. These results are in line with those from a meta-analysis of 11 RCTs including 21 293 patients with CKD, of whom 6857 were receiving dialysis.394 In patients with CKD not on dialysis, treatment with statins reduced all-cause mortality by 34%, CV mortality by 31%, CV events by 45% and stroke by 34%. In patients receiving dialysis, treatment with statins had no effect on all-cause mortality and stroke, but reduced CV mortality by 21% and CV events by 19%. Stage 5 CKD (or on dialysis) is indeed a very high-risk condition in which different factors influence outcome; results from RCTs of lipid-altering therapies have not provided convincing evidence of reduced CVD events in such patients.
In the 4D trial (Die Deutsche Diabetes Dialyse studie) involving a cohort of 1200 patients with diabetes on haemodialysis, atorvastatin had no positive effect on the primary composite endpoint of CVD.395 The results from AURORA (A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events), involving 2776 patients on haemodialysis, show that rosuvastatin lowered LDL-C as expected but had no significant effect on the composite CVD endpoint.396 The neutral results question the benefits of statins in these very high-risk patients with poor outcomes.
In the SHARP study397 simvastatin and ezetimibe combination therapy was associated with lower risk for major atherosclerotic events (coronary death, MI, non-haemorrhagic stroke or any revascularization) compared with placebo in persons with CKD stage 3A–5. The trial did not have sufficient power to assess the effects in the primary outcome separately in dialysis and non-dialysis patients, but there was no good statistical evidence that the proportional effects in dialysis patients differed from those seen in patients not on dialysis; in general, CV risk was much lower in the patients in the SHARP trial compared with those of the AURORA and 4D trials, reflected in the lower event and mortality rates.
A cost-effectiveness analysis of statins for primary CVD prevention in CKD398 showed that statins reduced absolute CVD risk in patients with CKD but that the increased risk for rhabdomyolysis, and competing risks associated with progressive CKD, partly offset these gains. A systematic review of the benefits and harms of statins in patients with a functioning kidney transplant included 3465 patients, free of CHD, from 22 studies. The authors concluded that statins may reduce CV events, although treatment effects were imprecise; due to heterogeneity, different statin regimens could not be compared and additional studies are recommended.228
9.9.3 Safety of lipid management in patients with chronic kidney disease
Safety issues and dose adjustment are important in advanced stages of CKD (stages 3–5), as adverse events are commonly dose related and due to increased blood concentration of the compound. Preference should be given to regimens and doses that have been shown to be beneficial in RCTs conducted specifically in such patients.399 Prevention of coronary events has been documented with fluvastatin 80 mg, atorvastatin 20 mg, rosuvastatin 10 mg, simvastatin/ezetimibe 20/10 mg, pravastatin 40 mg and simvastatin 40 mg. Lower doses than those used in the trials may be appropriate in Asian countries and in patients on polypharmacy and with co-morbidities. Furthermore, statins that are eliminated mainly by the hepatic route may be preferred (fluvastatin, atorvastatin, pitavastatin). Statins metabolized via CYP3A4 may result in adverse effects due to drug–drug interactions, and special caution is required.
9.9.4 Recommendations of lipid management for patients with chronic kidney disease
Based on the evidence for lipid management in patients with CKD, the Kidney Disease: Improving Global Outcomes (KDIGO) organization developed an updated clinical practice guideline for lipid management in CKD.399 In line with this, but with a focus on those patients at high or very high risk for developing CVD, recommendations are summarized in Table 30.
Recommendations for lipid management in patients with moderate to severe chronic kidney disease
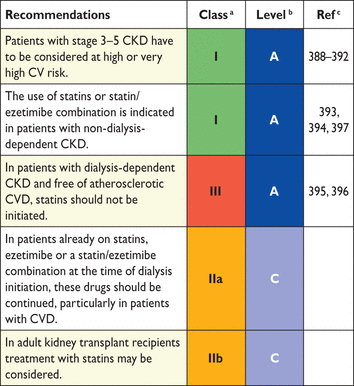 |
 |
CKD = chronic kidney disease; CV = cardiovascular.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for lipid management in patients with moderate to severe chronic kidney disease
 |
 |
CKD = chronic kidney disease; CV = cardiovascular.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.10 Transplantation (Table 31)
Lipid abnormalities are common in patients who have undergone solid organ transplantation and predispose patients to the development of both atherosclerotic disease and transplant arterial vasculopathy, resulting in major vascular events. Common general causes of dyslipidaemia in these patients are diabetes, obesity, MetS and CKD.
Immunosuppressive drug regimens also have important adverse effects on lipid metabolism. Glucocorticoid therapy causes weight gain and exacerbates insulin resistance, leading to increases in TC, VLDL and TGs and in the size and density of LDL particles. Calcineurin inhibitors increase the activity of hepatic lipase, decrease LPL and bind LDLR, resulting in reduced clearance of atherogenic lipoproteins. A greater adverse impact on lipid profiles is seen with ciclosporin than with tacrolimus. Sirolimus, a structural analogue of tacrolimus, causes dyslipidaemia in almost half of the patients receiving it. Patients should receive healthy lifestyle advice as recommended for patients at increased risk of CVD.
Statins have a similar effect on lipids in transplant recipients as in the general population. Although randomized trial data have shown that statins have the potential to improve outcomes in heart transplant patients400–402 and renal transplant patients,403 the amounts of outcome data are not extensive. A recent systematic review demonstrated a strong trend towards reduced CVD events and mortality with statins in renal transplant patients.403 Several potential drug interactions must also be considered, especially with ciclosporin which is metabolized through CYP3A4 and may increase systemic statin exposure and the risk of myopathy. Fluvastatin, pravastatin, pitavastatin and rosuvastatin have less potential for interaction.402 Tacrolimus is also metabolized by CYP3A4, but appears to have less potential for harmful interaction with statins than ciclosporin. Other drugs that influence CYP3A4 activity should be avoided if possible and used with extreme caution in patients receiving both calcineurin inhibitors and statins. Statins are recommended as the first-line agents for lipid lowering in transplant patients. Initiation should be at low doses with careful up-titration and caution regarding potential drug–drug interactions. Initiation of therapy with low-dose pravastatin or fluvastatin is recommended for those on ciclosporin. For patients with dyslipidaemia who are unable to take statins, ezetimibe could be considered as an alternative in those with high LDL-C.404 No outcome data are available for this drug, which should generally be reserved for second-line use. Care is required with the use of fibrates, as they can decrease ciclosporin levels and have the potential to cause myopathy. Extreme caution is required if fibrate therapy is planned in combination with a statin. Cholestyramine is not effective as monotherapy in heart transplant patients and has the potential to reduce absorption of immunosuppressants, minimized by separate administration.
Recommendations for the treatment of dyslipidaemia in transplant patients
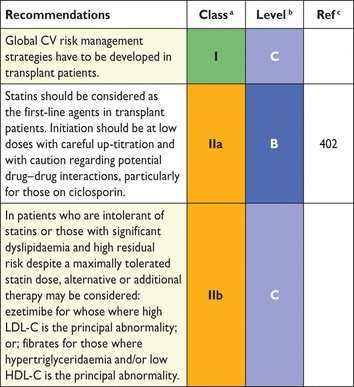 |
 |
CV = cardiovascular; HDL = high-density lipoprotein-cholesterol; LDL = low-density lipoprotein-cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for the treatment of dyslipidaemia in transplant patients
 |
 |
CV = cardiovascular; HDL = high-density lipoprotein-cholesterol; LDL = low-density lipoprotein-cholesterol.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.11 Peripheral arterial disease
The term PAD encompasses all vascular sites, including carotid, vertebral, upper extremity, mesenteric, renal and lower extremity arteries. The aorta is often included in the term.405 PAD is a common manifestation of atherosclerosis, and such patients are at elevated risk of coronary events, with PAD representing an independent risk factor for MI and CV death.405,406 Elevated CV risk has led to inclusion of PAD among the list of ‘risk equivalent’ conditions, and therapeutic strategies of secondary prevention should be implemented. Yet, despite the high CV morbidity and mortality risk, PAD patients are usually inadequately managed compared with CAD patients.406
9.11.1 Lower extremities arterial disease
A low ABI (<0.90) is diagnostic for lower extremities arterial disease (LEAD). Either a low (<0.90) or a high (>1.40, related to stiffened arteries) ABI is predictive of CV morbidity and mortality. Cholesterol-lowering therapy reduces the risk of ischaemic CV events and worsening of claudication, while it also improves walking performance. As for cardiac events, a systematic review of 18 trials including >10 000 patients, with cholesterol levels ranging from normal to elevated, reported that lipid-lowering therapy in subjects affected by atherosclerosis of the lower limbs is associated with a 20% reduction in total CV events, together with a non-significant 14% reduction of all-cause mortality.407 Regarding limb prognosis, in the Reduction of Atherothrombosis for Continued Health (REACH) registry, statin use was associated with an ∼18% lower rate of adverse limb outcomes.408 Even in the most advanced stages of the disease (critical limb ischaemia), statin therapy improved rates of 1-year mortality and major adverse CV events and increased amputation-free survival.409
9.11.2 Carotid artery disease
While there are currently no randomized studies that have assessed whether lipid-lowering treatments reduce the incidence of CV events in patients enrolled on the basis of carotid atherosclerotic disease and without previous CV events, lipid-lowering therapy reduced stroke in numerous studies. In a meta-analysis of RCTs enrolling >90 000 patients, Amarenco et al.410 reported that statin therapy leads to a 21% reduction in the incidence of all strokes in different populations and that this effect is mainly driven by the extent of LDL-C reduction. CIMT is reduced with statins in RCTs,410,411 yet the predictive role of this biomarker (but not of carotid plaque) is somewhat compromised in light of recent data.60 Modest regression of carotid atherosclerosis with niacin in most (but not all) imaging studies was not supported by clinical benefit in the AIM-HIGH and HPS2-THRIVE trials.251,252
9.11.3 Retinal vascular disease
Atherosclerotic changes of retinal arteries correlate with TC, LDL-C, TG and apoB levels and also with CAD.412 Fenofibrate reduces the progression of diabetic retinopathy.413,414
9.11.4 Secondary prevention in patients with aortic abdominal aneurysm
The presence of an abdominal aortic aneurysm represents a risk-equivalent condition and is associated with age, male gender, personal history of atherosclerotic CVD, smoking, hypertension and dyslipidaemia,415 while, in contrast, diabetic patients are at decreased risk.
There are currently no available clinical trials on the reduction of CV risk with lipid-lowering therapy in patients affected by this condition. Systematic reviews,416 mostly based on retrospective non-randomized studies, have reported that there is still inconclusive evidence that statin therapy reduces perioperative CV morbidity and mortality. In an RCT comparing atorvastatin 20 mg with placebo, the composite endpoint of cardiac death, MI, stroke and unstable angina was significantly reduced in 100 patients undergoing vascular non-cardiac surgery, including abdominal aortic aneurysm repair.417 In another double-blind placebo-controlled trial in 497 patients undergoing vascular surgery, perioperative fluvastatin therapy (80 mg/day) was associated with an improvement in postoperative cardiac outcome.418
Based on a recent meta-analysis, statin therapy is likely effective in the prevention of growth of small (<55 mm in diameter) abdominal aortic aneurysms.419
9.11.5 Renovascular atherosclerosis
Although lipid-lowering therapy has never been tested in an RCT in patients affected by renovascular atherosclerosis, a recent population-based study showed that in patients >65 years of age with atherosclerotic renovascular disease, the risk of a major cardiorenal composite endpoint (MI, stroke, HF, acute renal failure, dialysis and death) was significantly lower in statin users than in non-users.420
The recommendations for lipid-lowering drugs in patients with PAD are shown in Table 32.
Recommendations for lipid-lowering drugs in patients with peripheral arterial disease (including carotid artery disease)
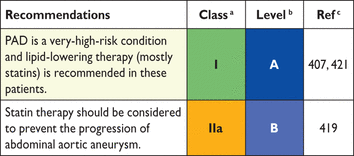 |
 |
PAD = peripheral arterial disease.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for lipid-lowering drugs in patients with peripheral arterial disease (including carotid artery disease)
 |
 |
PAD = peripheral arterial disease.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.12 Stroke
Stroke has a heterogeneous aetiology, including cardiac thromboembolism (often associated with atrial fibrillation), carotid artery and proximal aortic atherosclerosis and thromboembolism, small vessel cerebrovascular disease and intracranial haemorrhage (including intracerebral and subarachnoid haemorrhage). Dyslipidaemia may play a variable role in the pathogenesis of stroke according to the particular aetiology. The relationship between dyslipidaemia and atherothrombotic events, including ischaemic stroke and TIA, is well recognized, while the association of dyslipidaemia with other types of stroke is uncertain. Notwithstanding, concomitant control of other aetiologic factors, such as hypertension, is of paramount importance.
9.12.1 Primary prevention of stroke
The use of statin therapy in adults at high risk of CVD due to LDL-C or other CV risk factors, including arterial hypertension, as well as in the setting of established CVD, reduces the risk of ischaemic stroke or TIA.64,69,128,330,422–426. Risk reduction for a first ischaemic stroke is 21% per 1.0 mmol/L LDL-C reduction,64 and it is similar between men and women.65 Beneficial effects are retained over long-term follow-up.427 A recent meta-analysis of RCTs in subjects >65 years of age at high CV risk without established CV disease showed that statins significantly reduce the incidence of MI and stroke, but do not significantly prolong survival in the short-term.327 More intensive lipid lowering with statins is associated with a lower risk of stroke compared with less intensive regimens.64,65,128,422 Concerns for increased risk of haemorrhagic stroke with statin treatment do not appear to be justified.423 The addition of ezetimibe to simvastatin in post-ACS patients had an incremental beneficial effect for ischaemic stroke or all strokes (marginal statistical significance for the latter).63 Niacin did not reduce stroke over long-term follow-up in patients with CVD in the AIM-HIGH and HPS2-THRIVE trials.251,252 In fact, the increased rate of ischaemic stroke in the AIM-HIGH trial and the trend (P = 0.08) for increased haemorrhagic stroke in the HPS2-THRIVE trial raised concerns and contributed to halting of the AIM-HIGH trial before its planned conclusion. Primary prevention of stroke contributes to the overall indication for starting treatment with statins in all patients with established atherosclerotic disease and in patients at high risk for developing CVD, in accordance with the recommendations given in Table 33.
Recommendations for lipid-lowering drugs for primary and secondary prevention of stroke
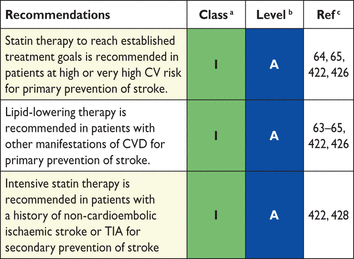 |
 |
CVD = cardiovascular disease; TIA = transient ischaemic attack.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for lipid-lowering drugs for primary and secondary prevention of stroke
 |
 |
CVD = cardiovascular disease; TIA = transient ischaemic attack.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
9.12.2 Secondary prevention of stroke
Following stroke or TIA, patients are at risk not only of recurrent cerebrovascular events, but also of other major CV events, including MI. Secondary prevention therapy with statins reduces the risk of recurrent stroke (by 12%), MI and vascular death.422,428 Statin pretreatment at TIA onset was associated with reduced recurrent early stroke risk in patients with carotid stenosis in a pooled data analysis, supporting an as-early-as-possible initiation of statins after stroke.429 However, the aetiology of stroke may influence the response to statins, and those patients with evidence of atherothrombosis underlying their cerebrovascular events appear to benefit most, while those with haemorrhagic stroke may not benefit.422
9.13 Human immunodeficiency virus patients
HIV-infected patients typically have low TC and LDL-C, as well as low HDL-C and increased TGs.430,431 Antiretroviral treatment (ART) or highly active antiretroviral treatment (HAART; when drugs are used in combination) causes marked increases in TC, LDL-C and TGs and a predominance of small dense LDL particles, while HDL-C remains low. The extent of lipid changes differs between and within antiretroviral drug classes. Newer protease inhibitors, non-nucleoside reverse transcriptase inhibitors (NNRTIs) or integrase inhibitors affect lipoprotein metabolism to a lesser extent. ART also reduces insulin sensitivity and promotes hypertension and body fat redistribution (lipodystrophy that involves lipoatrophy, which refers to a loss of fat in the face, buttocks and limbs, and/or lipohypertrophy, in which there is an accumulation of fat in the breasts, neck, back or stomach region) that additionally contribute to CVD risk. HIV-infected patients have a higher risk for CVD when compared with HIV-uninfected individuals [RR 1.61 (95% CI 1.43, 1.83)], while ART (and especially older protease inhibitors) further increases this risk, up to two-fold [RR 2.00 (95% CI 1.70, 2.37)].431–433 CVD risk remains high even after adjusting for traditional risk factors.434 ART may particularly accelerate the onset of CAD-related events in young male heavy smokers with dyslipidaemia. Nevertheless, absolute CVD risk increase with ART is moderate and should be put into perspective with the benefits of HIV treatment.
Dietary changes and regular physical activity, as well as switching to another ART regimen, may act favourably on dyslipidaemia, but most patients still need pharmacological therapy to reach lipid goals. Statins are effective, but drug interactions with ART need to be considered. Statins metabolized in the liver via the CYP3A4 or CYP2C9 are susceptible to drug interactions with protease inhibitors and the NNRTI efavirenz. Pravastatin is not significantly metabolized via the CYP isoenzyme system and is therefore a preferred statin in HIV-infected individuals. Preferred statins include atorvastatin, fluvastatin, pitavastatin and rosuvastatin, although caution should be exercised. Combination of simvastatin or lovastatin with any protease inhibitor or efavirenz is not recommended. The HIV drug interaction database of the University of Liverpool (http://www.hiv-druginteractions.org) is a very helpful tool for checking drug interactions (Supplementary Figure B). For patients who cannot tolerate statin treatment, ezetimibe could be an option.435 Fibrates and fish oil may be prescribed when HTG is predominant.436 Use of bile acid sequestrants is not recommended because they increase TGs and their effects on the absorption of antiretroviral drugs have not been studied.
There are no data on the effects of statins, ezetimibe or fibrates on CV events in dyslipidaemic HIV-infected patients.
HIV-drug interaction database of the University of Liverpool.
The recommendations for lipid-lowering drugs in HIV patients are shown in Table 34.
Recommendation for lipid-lowering drugs in human immunodeficiency virus patients
 |
 |
HIV = human immunodeficiency virus; LDL-C = low-density lipoproteincholesterol.
aClass of recommendation.
bLevel of evidence.
Recommendation for lipid-lowering drugs in human immunodeficiency virus patients
 |
 |
HIV = human immunodeficiency virus; LDL-C = low-density lipoproteincholesterol.
aClass of recommendation.
bLevel of evidence.
9.14 Mental disorders
The presence of a major mental illness such as schizophrenia or bipolar disorder has deleterious effects on the risk of developing CVD. This is related to an unhealthy lifestyle in the majority of these patients (sedentary behaviour, unbalanced diet, smoking), but also to drug treatment. Some antipsychotics, antidepressants, anxiolytics and mood stabilizers are associated with weight gain and cardiometabolic disturbances, including dyslipidaemia and dysglycaemia.
In patients with first-episode schizophrenia spectrum disorders, cardiometabolic risk factors were present early in the illness; this was related to the underlying disorder, unhealthy lifestyle and antipsychotic medications, which interact with each other.437 All this explains the higher prevalence of obesity, MetS, diabetes and dyslipidaemia in patients with these psychiatric diseases.438 It also results in a greater incidence of CVD and in more CV deaths in psychiatric patients suffering from these disorders.
In a Finnish cohort of patients with schizophrenia, life expectancy was about 2 decades less than in persons of similar age from the general population.439 In patients with bipolar disorder there was a reduction in life expectancy of 12–14 years.440 Among 654 patients with bipolar disorder in the Fondamental Advanced Centers of Expertise in Bipolar Disorders (FACE-BD) cohort, 18.5% met the criteria for MetS; only 11% and 28% of the patients with hypercholesterolaemia and high fasting glycaemia, respectively, were treated for these conditions.441 Patients with the aforementioned psychiatric diseases are in general less compliant with chronic drug treatments and therefore have their CV risk factors less well controlled.
CVD accounts for much of the excess mortality in psychiatric patients.442 CVD develops more than a decade earlier in patients with bipolar disorders than in controls.443 Therefore it could be recommended to start primary prevention earlier rather than later in these patients. This is well summarized in a position paper of the European Psychiatric Association supported by the European Association for the Study of Diabetes and by the ESC.444
Statins are equally effective in lowering LDL-C in psychiatric patients treated with second-generation antipsychotics445; however, in only a limited number of these patients are preventive actions taken both in regard to lifestyle and to the use of cardioprotective drugs. The odds of the use of statins was approximately halved in patients with schizophrenia compared with controls.446
Unfortunately, no RCTs with ‘hard’ CV outcome measures have so far been conducted in patients with these major mental diseases. It is reasonable to expect that the favourable metabolic effects of treatment that have been demonstrated result in the prevention of CV events in the long term. However, several questions remain that need to be addressed in further studies in patients with these major mental disorders, regarding long-term safety of statins in association with antipsychotics that also predispose to diabetes and prevention of premature CV mortality and incidence.
Table 35 lists the recommendations for the lipid-lowering drug treatment of patients with mental disorders.
Recommendations for lipid-lowering pharmacological treatment in patients with mental disorders
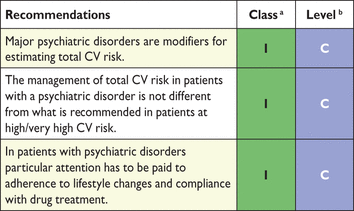 |
 |
CV = cardiovascular.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations for lipid-lowering pharmacological treatment in patients with mental disorders
 |
 |
CV = cardiovascular.
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
10. Monitoring of lipids and enzymes in patients on lipid-lowering therapy (Table 36)
Evidence from trials for what tests should be carried out to monitor lipids in patients on treatment is limited. Similar limited evidence applies to tests of possible toxicity, such as ALT and CK. Recommendations stem from consensus rather than evidence-based medicine.
Summary of recommendations for monitoring lipids and enzymes in patients on lipid-lowering therapy
 |
 |
ACS = acute coronary syndrome; ALT = alanine aminotransferase; CK = creatine kinase; ULN = upper limit of normal.
Summary of recommendations for monitoring lipids and enzymes in patients on lipid-lowering therapy
 |
 |
ACS = acute coronary syndrome; ALT = alanine aminotransferase; CK = creatine kinase; ULN = upper limit of normal.
Algorithm for treatment of muscular symptoms during statin treatment.211.
Response to therapy can be assessed at 6–8 weeks from initiation of therapy, but response to lifestyle may take longer. Standard practice for subsequent follow-up monitoring is 6–12 months, but such monitoring intervals are arbitrary. As a minimum, LDL-C should be assessed whenever available, but better management decisions will probably occur if a full lipid profile is performed, including HDL-C and TGs. Non-HDL-C or apoB should also be analysed, and used as a secondary treatment target. A separate issue is the impact of regular lipid monitoring in promoting patient adherence to lifestyle changes or drug regimens that impact positively on their health, as found in a range of studies. It is unclear whether only the process of monitoring is critical in achieving this or whether a combination of education, regular contact and adherence assessment is required.
Where lipid-lowering therapy is implemented, safety blood tests are advised, including ALT and CK at baseline, to identify the limited number of patients where treatment is contraindicated. CK should be checked in patients with high risk for myopathy, such as the very elderly with co-morbidities, patients with earlier muscle symptoms or patients on interacting drugs. A systematic review found that the incidence of drug-induced hepatotoxicity in patients taking statins is unknown, with very few cases occurring in large-scale RCTs.212,214 Recent reviews are encouraging about the safety of long-term statin therapy.221,222 ALT is recommended 8–12 weeks after the start of lipid-lowering therapy or dose change, but routine control of ALT during treatment is not recommended and should be performed, if indicated, based on clinical observations. In patients whose liver function tests rise above three times the ULN, explanations such as alcohol ingestion or non-alcoholic fatty liver disease should be sought and the levels monitored. If levels remain elevated then lipid-lowering therapy should be stopped, but may be cautiously reintroduced under monitoring after levels have returned to normal.
There is no predictive value of routine repeat CK testing for rhabdomyolysis since the level can increase for many reasons, including muscle injury or excess muscular exercise. However, CK must be assessed immediately in patients, especially the elderly, presenting with muscle pain and weakness, and treatment stopped if >10 times the ULN. Strategies to handle CK elevations are given in Table 35 and in the supplementary material. Due to the increased frequency of diabetes during statin treatment, regular checks of HbA1C should be considered in patients at high risk of developing diabetes, such as the elderly or those with MetS, obesity or signs of insulin resistance.
11. Strategies to encourage adoption of healthy lifestyle changes and adherence to lipid-modifying therapies
Terminology to describe the way patients follow their medication regimens and sustain behavioural changes has evolved over the years to include terms such as compliance, adherence and concordance. Compliance (http://medical-dictionary.thefreedictionary.com/Compliance) is defined as a ‘willingness to follow a prescribed course of treatment’, but also has connotations of obeying orders in a subservient way. Adherence (http://apps.who.int/medicinedocs/en/d/Js4883e/6.html) is defined as ‘the extent to which a person's behaviour—taking medication, following a diet, and/or executing lifestyle changes—corresponds with agreed recommendations from a healthcare provider’ and is literally defined as sticking to something. Finally, concordance (http://www.drugs.com/dict/concordance.html) is defined as ‘a negotiated, shared agreement between clinician and patient concerning treatment regimen(s), outcomes, and behaviours; a more cooperative relationship than those based on issues of compliance and noncompliance’.
While the terms adherence and concordance are today considered more acceptable than compliance, for the purposes of this guideline, the term adherence will be used, as it is most commonly used in practice and research today.
11.1 Achieving and adhering to healthy lifestyle changes
Behavioural strategies to promote the adoption of healthy lifestyle habits are covered in brief in this section; however, more detail is available in the ESC Guidelines on cardiovascular disease prevention in clinical practice.6
No smoking, healthy eating and being physically active are the foundations of preventive cardiology because of their favourable impact on CV risk, including modification of the lipid profile. Healthy lifestyle habits will also enhance effectiveness and reduce the need for drug therapy.
Helping patients to change to healthier lifestyle habits is most effectively achieved through formal programmes of preventive care, possibly because of the intensive follow-up and multidisciplinary expertise they provide.447 However, in everyday care, adherence to both healthy lifestyle changes and medication regimens is a challenge to both professionals and patients.
A comprehensive patient- and family-centred approach located in one healthcare setting is recommended rather than addressing single risk factors with more than one intervention in different locations. Drawing on expertise from different disciplines in smoking cessation, dietetics, physical activity, exercise and health psychology is also essential, regardless of whether these experts have direct involvement with patients as dedicated members of a team or whether this expertise is achieved by the provision of training to doctors and nurses delivering care.447
The adoption of effective strategies to help patients to change behaviours is now made easier with the development of a hierarchical taxonomy of behaviour change strategies.448 The taxonomy has developed a system of standardized labelling of behavioural strategies, which allows a clear description of complex interventions449 in research reports and their subsequent translation into clinical practice initiatives.
Box 11 includes some useful techniques when counselling patients for behavioural change.
In addition, it is important to be aware of the following barriers to change:
Healthy choices are not always easy choices.
Socio-economic status and cultural and environmental factors influence behaviour change.
Your agenda for change as a health professional may conflict with that of the person you are trying to help.
Helping people to change requires dedicated time from the health professional to provide support and follow-up.
People may have feelings of ambivalence towards changing behaviour that need exploring.
11.2 Adhering to medications
Despite a wealth of evidence on the efficacy and effectiveness of statins in both primary and secondary prevention, adherence remains a consistent barrier, with rates of <50% demonstrated in several studies. Adherence declines over the duration of treatment450–454; however, this is truer in patients treated for primary compared with secondary prevention of CVD, with reported rates of up to 77% discontinuing their statins within 2 years. Adherence is better in patients recruited to clinical trials compared with those treated in the real world.455,456 Not surprisingly, this non-adherence has an impact on healthcare costs, morbidity, hospital readmissions and mortality.457–461 Poor adherence rates are not only limited to statins but are also true of other lipid-lowering drugs and all medications used to prevent CVD, as demonstrated in a systematic review and meta-analysis.462
The reasons for non-adherence are complex and include misconceptions about tolerability on the part of both patients and professionals alike. These barriers prevent patients from gaining the maximum benefit from their treatment.
In the UK in 2014, controversy was sparked among general practitioners (GPs) and other physicians463 in relation to the recent updated recommendation from NICE to offer atorvastatin 20 mg for the primary prevention of CVD to people who have an estimated ≥10% absolute 10-year risk of developing CVD using the QRISK2 assessment tool (http://www.nice.org.uk/guidance/cg181). Together with the additional controversy generated by Abramson et al.464,465 regarding their analysis of the adverse effects of statins, which was subsequently corrected, it is not surprising that GPs, in particular, have a certain degree of reluctance to proceed with the NICE strategy. If there is a lack of local consensus on the prescription of statins in primary prevention, then GPs may be less inclined to prescribe them, let alone encourage patients to adhere to their statin therapy, even in the face of minor adverse effects.
Various empirical models of health behaviour and behaviour change theory have been shown to predict adherence, including the Theory of Planned Behaviour466 and the Health Belief Model.467 Studies that investigated adherence to medications in long-term conditions identified factors such as high susceptibility, severity of the condition, strong intentions and high self-efficacy as being associated with good adherence, while poor lifestyle habits and low perceived behavioural control were associated with poor adherence.468,469 However, these theoretical models are limited in that they do not take into account important social, economic, health system and therapy-related factors. Most recently, the COM-B (Capability, Opportunity and Motivation) theoretical model,470 developed by Michie et al.,471 which takes a broader look at factors influencing adherence, proposed a framework for assessing and addressing adherence, taking the interaction between capability (defined as both the psychological and physical capacity of an individual to engage in a behaviour), opportunities (defined as factors outside the control of an individual) and their motivation to do so.
Predictors of non-adherence with statins have been identified450,472–474 and include their use in individuals for primary prevention as compared with their use in patients with disease or with multiple risk factors, lower income, being elderly, complex polypharmacy, cost and forgetfulness due to a lack of symptoms and psychological co-morbidities. In addition, reasons for reluctance to collect a first prescription of statin medication were investigated in a cross-sectional telephone survey conducted in California from recruits to an RCT.475 The most commonly reported reasons included general concerns about the medication, wanting to try lifestyle measures first and fear of adverse effects; however, a significant proportion reported financial hardship, a lack of understanding of why they needed to take the medication and what the medication was for (indicating a need to address the patient–professional relationship and poor health literacy). Health literacy is defined as ‘the degree to which individuals have the capacity to obtain, process and understand basic health info and services needed to make appropriate health decisions’ (http://nnlm.gov/outreach/consumer/hlthlit.html).
Poor health literacy is of particular concern in regard to medication adherence.475 Elderly patients and those with low socio-economic status and chronic health conditions may be especially vulnerable. These patients may get confused, especially when their regimens are complex and include many drugs (polypharmacy) that need to be taken on more than one occasion per day. Important steps to empower patients to get more benefit from health interventions include the following476:
1. Use good interpersonal skills (good eye contact, warm manner) and an empathetic, non-judgmental attitude.
2. Provide clear and simple instructions on a drug regimen backed up with written instructions, which can also be seen by a spouse or caregiver.
3. Speak slowly in plain language and avoid medical jargon when giving instructions.
4. Limit the number of instructions to no more than three key points—principle of ‘need to know’ (Figure 8).
5. Use ‘teachback’ to confirm understanding; e.g. ‘I want to make sure that I explained things clearly. Let's review what we discussed. What are the three strategies that will help keep your cholesterol down?’
6. Use supplemental materials, e.g. images, videos and audio sources, to improve recall (Figure 9).
7. Encourage questions and discussion—enlist the family or others important to the individual.
8. Motivational interviewing skills may be helpful in communicating with patients who are ambivalent or seem against starting or continuing with medications:37,477
Counsel patients using the OARS method (Box 11).
Use the ‘elicit–provide–elicit’ model to tailor the information you give (elicit what the patient wants to know, provide that information, elicit from the patient how they can use this new knowledge to their benefit).
Acknowledge and reflect your patient's resistance.
Support your patient's autonomy to make their own decisions about their health and treatment.
Explore your patient's ambivalence to adhere to their treatment.
Develop a plan of action together and share decision-making.
9. Build self-efficacy and confidence, drawing on social learning theory.478
Being able to identify patients with low health literacy is important. Indicators may include seeking help when an illness is already advanced, inarticulacy in explaining concerns, making excuses like ‘I forgot my glasses’ to cover for the shame associated with illiteracy, being passive or aggressive and missing appointments.
Interventions to improve adherence have been reviewed in a Cochrane review from 2010,479 which looked at interventions to improve adherence to all forms of lipid-lowering therapy, including reminders, simplification of drug regimens and provision of information and education. Most effective was reminders, such as setting alarms, connecting medication-taking to other tasks to trigger memory and phone reminders from nurses. Reminder systems have the potential to be developed with the help of innovations in technology, like the use of text messaging, the Internet and applications for mobile phones or tablets to assist in self-monitoring and management. Adherence research is weak in this area, mainly because it has not kept abreast of the rapid developments in technology;480 however, these methods may come into their own in the future with a stronger knowledge base.
Prescription of a statin should include a shared decision-making approach481 that engages the patient in a discussion before initiating treatment, especially when it is being considered for primary prevention of CVD. This discussion should be based on risk estimation and adequate communication of this risk to patients. Involving the patient in such a way is likely to be empowering and motivate adherence. This discussion is not exclusively about the prescription of a statin to manage lipids; a comprehensive approach includes addressing all lifestyle and other biomedical factors that contribute to CV risk.
Once treatment has been prescribed, communication should focus on conveying achievements in reaching goals, assessment of adherence and possible reasons for non-adherence, such as adverse effects. In relation to lipid-lowering medications, and statins in particular, misconceptions and misleading media reports are in abundance. Many patients report adverse effects of statins to their GPs and this may be because of an increased likelihood to anticipate them. However, a recent large review of RCTs213 found that in 83 880 patients receiving blinded placebo-controlled statin therapy, few reported adverse effects were actually due to the drug. This study calculated the PSN, defined as the proportion of symptoms not attributable to its pharmacological action, in order to provide GPs with a clear metric to use in advising their patients on whether reported symptoms are genuinely likely to be pharmacologically caused by the statin or not.
Recently, promising results for improving adherence have been demonstrated in the use of a fixed-dose combination (FDC) drug or ‘polypill’ in both primary and secondary prevention. The Use of a Multidrug Pill In Reducing cardiovascular Events (UMPIRE) RCT482 compared an FDC containing aspirin, statin and two blood pressure–lowering agents with usual care in both primary and secondary prevention in 2004 randomized patients in India and Europe. At 15 months, statistically significant differences between intervention and usual care were seen in self-reported adherence and changes in systolic blood pressure and LDL-C. The Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention (FOCUS) study483 had a cross-sectional first phase, which identified factors contributing to non-adherence after MI in 2118 patients from five countries in South America and Europe. In the second phase, 695 patients from the first phase were randomized to receive either a polypill containing aspirin, statin and ramipril in varying doses or were given the three drugs separately. Adherence was measured with the self-reported Morisky–Green questionnaire and pill counts and was statistically significantly superior in the intervention group compared with usual care at 9 months. In phase 1, factors associated with non-adherence were younger age, depression, complex regimen, poorer health insurance coverage and low social support.
Given the benefits for adherence demonstrated with simplified dosing reported in a Cochrane overview of interventions to improve safe and effective medicines use by consumers,484 it makes sense that a pill that contains multiple medications in one tablet will enhance adherence. This overview also found that the use of self-management or self-monitoring programmes, as well as a regular pharmacy review of prescribed medications with a view to taking out unnecessary medications, was helpful.
Many of the studies included in the Cochrane review of interventions to improve medication adherence480 drew on the support of allied professionals such as nurses and pharmacists to deliver complex interventions, which may include telephone follow-up, interim appointments and monitoring of repeat prescriptions. The reviewed interventions may be difficult to replicate in everyday clinical care due to the cost and the availability of personnel. Drawing on the support of non-professional people within the social context of the patient, such as spouses, other family members, caregivers or other key figures, as well as lay groups in the community, may prove to be a cost-effective way to improve adherence.
Box 12 lists a number of tips to use when prescribing multiple medications to patients in order to help them adhere.
12. To do and not to do messages from the Guidelines
13. Appendix
ESC Committee for Practice Guidelines (CPG): Jose Luis Zamorano (Chairperson) (Spain), Victor Aboyans (France), Stephan Achenbach (Germany), Stefan Agewall (Norway), Lina Badimon (Spain), Gonzalo Barón-Esquivias (Spain), Helmut Baumgartner (Germany), Jeroen J. Bax (The Netherlands), Héctor Bueno (Spain), Scipione Carerj (Italy), Veronica Dean (France), Çetin Erol (Turkey), Donna Fitzsimons (UK), Oliver Gaemperli (Switzerland), Paulus Kirchhof (UK/Germany), Philippe Kolh (Belgium), Patrizio Lancellotti (Belgium), Gregory Y. H. Lip (UK), Petros Nihoyannopoulos (UK), Massimo F. Piepoli (Italy), Piotr Ponikowski (Poland), Marco Roffi (Switzerland), Adam Torbicki (Poland), António Vaz Carneiro (Portugal), Stephan Windecker (Switzerland).
ESC National Cardiac Societies actively involved in the review process of the 2016 ESC/EAS Guidelines for the management of dyslipidaemias:
Armenia: Armenian Cardiologists Association, Parounak H. Zelveian; Austria: Austrian Society of Cardiology, Peter Siostrzonek; Azerbaijan: Azerbaijan Society of Cardiology, Firdovsi Ibrahimov; Belarus: Belorussian Scientific Society of Cardiologists, Volha Sujayeva; Belgium: Belgian Society of Cardiology, Marc J. Claeys; Bosnia and Herzegovina: Association of Cardiologists of Bosnia and Herzegovina, Belma Pojskić; Bulgaria: Bulgarian Society of Cardiology, Arman Postadzhiyan; Croatia: Croatian Cardiac Society, Davor Miličić; Cyprus: Cyprus Society of Cardiology, George C. Georgiou; Czech Republic: Czech Society of Cardiology, Hana Rosolova; Denmark: Danish Society of Cardiology, Christian Klausen; Estonia: Estonian Society of Cardiology, Margus Viigimaa; Finland: Finnish Cardiac Society, Kari Kervinen; Former Yugoslav Republic of Macedonia: Macedonian FYR Society of Cardiology, Sasko Kedev; France: French Society of Cardiology, Jean Ferrières; Georgia: Georgian Society of Cardiology, Shalva Petriashvili; Germany: German Cardiac Society, Ulrich Kintscher; Greece: Hellenic Cardiological Society, Loukianos Rallidis; Hungary: Hungarian Society of Cardiology, Róbert Gábor Kiss; Iceland: Icelandic Society of Cardiology, Thorarinn Guðnason; Ireland: Irish Cardiac Society, Vincent Maher; Israel: Israel Heart Society, Yaakov Henkin; Italy: Italian Federation of Cardiology, Gian Francesco Mureddu; Kazakhstan: Association of Cardiologists of Kazakhstan, Aisulu Mussagaliyeva; Kosovo: Kosovo Society of Cardiology, Pranvera Ibrahimi; Kyrgyzstan: Kyrgyz Society of Cardiology, Erkin Mirrakhimov; Latvia: Latvian Society of Cardiology, Gustavs Latkovskis; Libya: Libyan Cardiac Society, Hisham Ben Lamin; Lithuania: Lithuanian Society of Cardiology, Rimvydas Slapikas; Luxembourg: Luxembourg Society of Cardiology, Laurent Visser; Malta: Maltese Cardiac Society, Philip Dingli; Moldova: Moldavian Society of Cardiology, Victoria Ivanov; The Netherlands: Netherlands Society of Cardiology, Janneke Wittekoek; Norway: Norwegian Society of Cardiology, Anders Hovland; Poland: Polish Cardiac Society, Andrzej Rynkiewicz; Portugal: Portuguese Society of Cardiology, Quiteria Rato; Russian Federation: Russian Society of Cardiology, Marat Ezhov; San Marino: San Marino Society of Cardiology, Marco Zavatta; Serbia: Cardiology Society of Serbia, Milan A. Nedeljkovic; Slovakia: Slovak Society of Cardiology, Daniel Pella; Slovenia: Slovenian Society of Cardiology, Zlatko Fras; Spain: Spanish Society of Cardiology, Domingo Marzal; Sweden: Swedish Society of Cardiology, Lennart Nilsson; Switzerland: Swiss Society of Cardiology, Francois Mach; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Faouzi Addad; Turkey: Turkish Society of Cardiology, Meral Kayıkcıoglu; Ukraine: Ukrainian Association of Cardiology, Olena Mitchenko; United Kingdom: British Cardiovascular Society, David Wald.
14. References
Author notes
ESC Committee for Practice Guidelines (CPG) and National Cardiac Society Reviewers can be found in the Appendix.
ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), European Association for Cardiovascular Prevention & Rehabilitation (EACPR), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI), Heart Failure Association (HFA)
Councils: Council on Cardiovascular Nursing and Allied Professions, Council for Cardiology Practice, Council on Cardiovascular Primary Care, Council on Hypertension
Working Groups: Atherosclerosis & Vascular Biology, Cardiovascular Pharmacotherapy, Coronary Pathophysiology & Microcirculation, E-cardiology, Myocardial and Pericardial Diseases, Peripheral Circulation, Thrombosis.
The content of these European Society of Cardiology (ESC) and European Atherosclerosis Society Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC (journals.permissions@oup.com).
Disclaimer. The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their publication. The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and, where appropriate and/or necessary, the patient's caregiver. Nor do the ESC Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities in order to manage each patient's case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
The disclosure forms of the authors and reviewers are available on the ESC website www.escardio.org/guidelines.
- dyslipidemias
- ldl cholesterol lipoproteins
- high density lipoprotein cholesterol
- triglycerides
- statins
- lipoproteins
- cardiovascular diseases
- heart disease risk factors
- kidney failure, chronic
- diabetes mellitus, type 2
- cholesterol
- apolipoproteins b
- life style
- plasma
- guidelines
- lipids
- mortality
- pharmacokinetics
- lipid-lowering therapy
- european society of cardiology



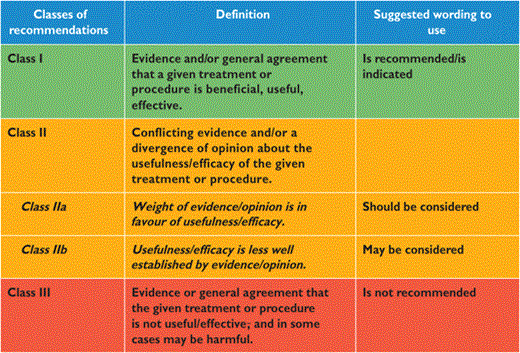
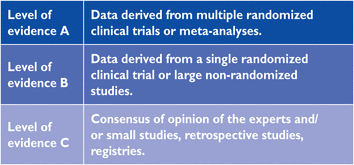

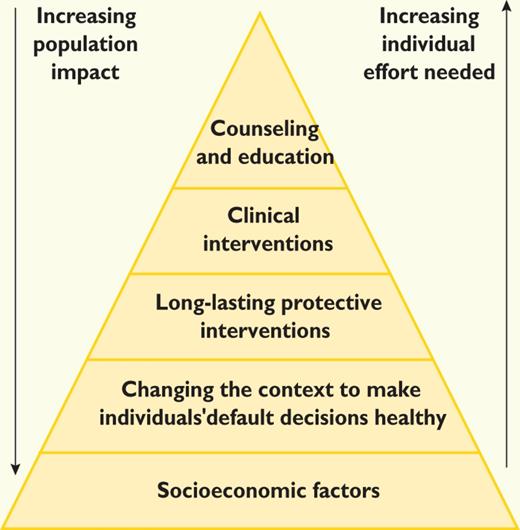

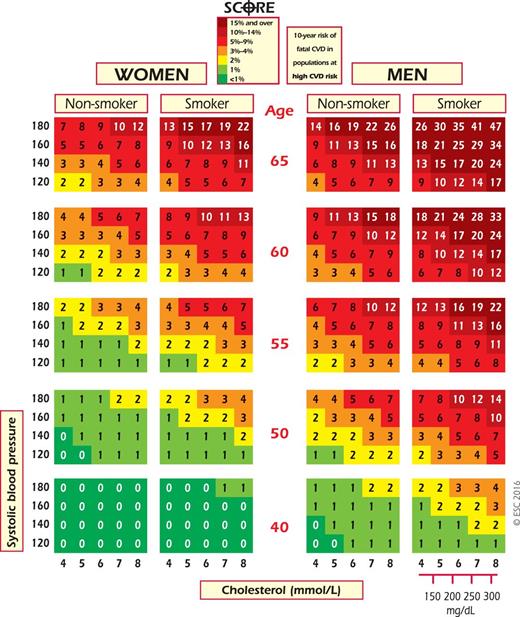
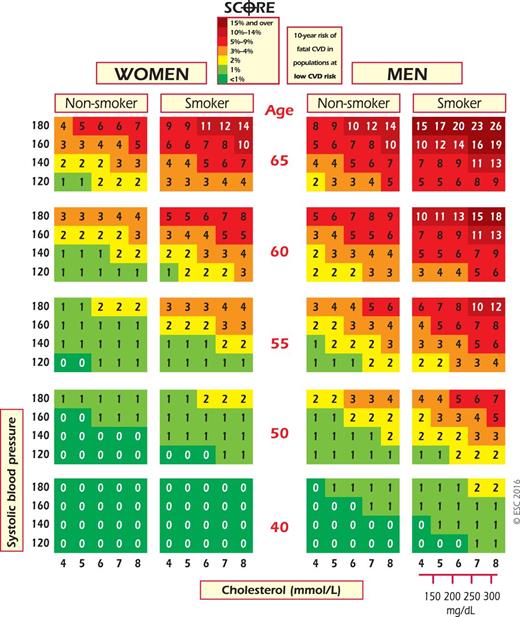
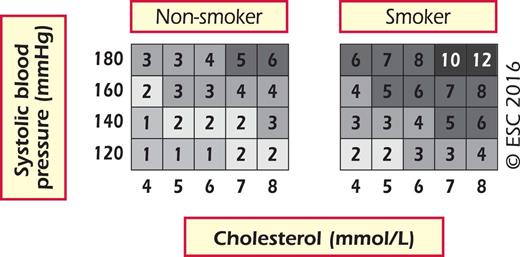
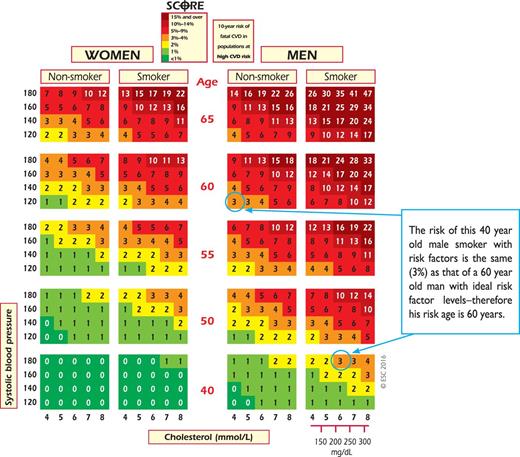
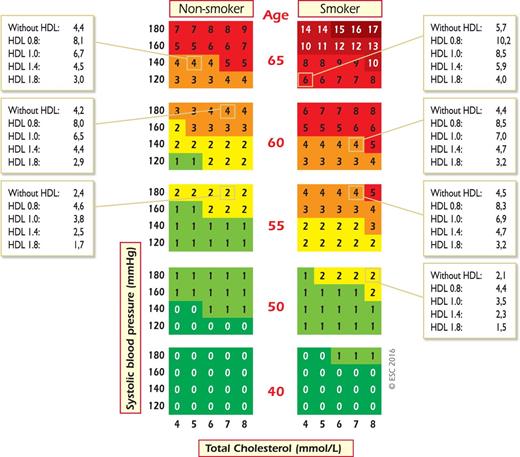
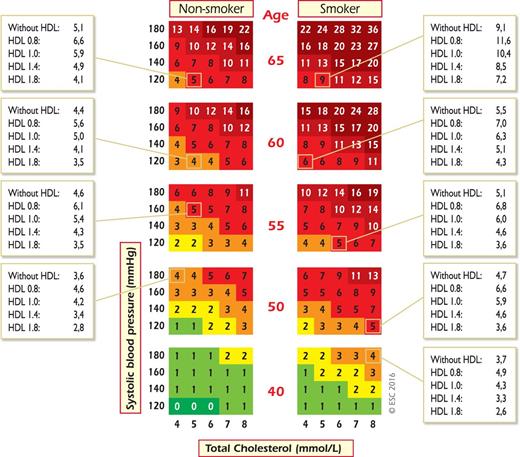

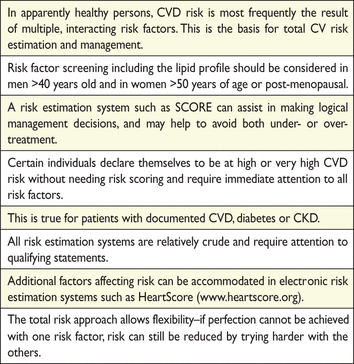


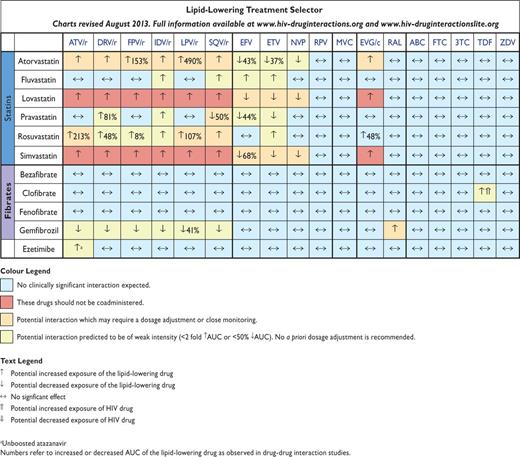
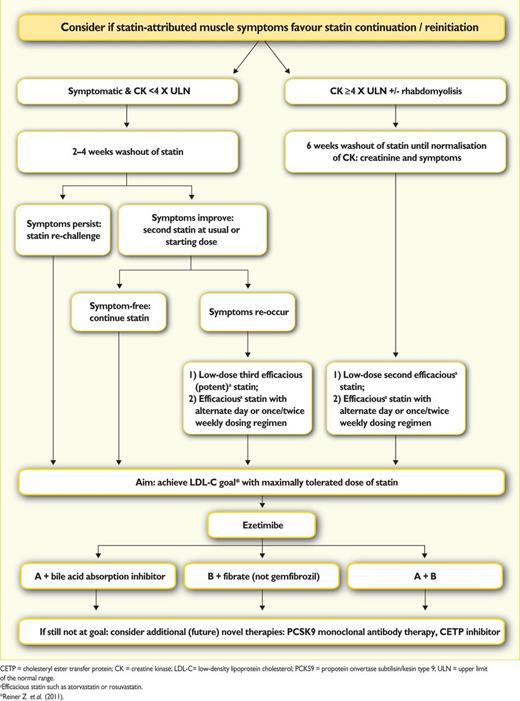


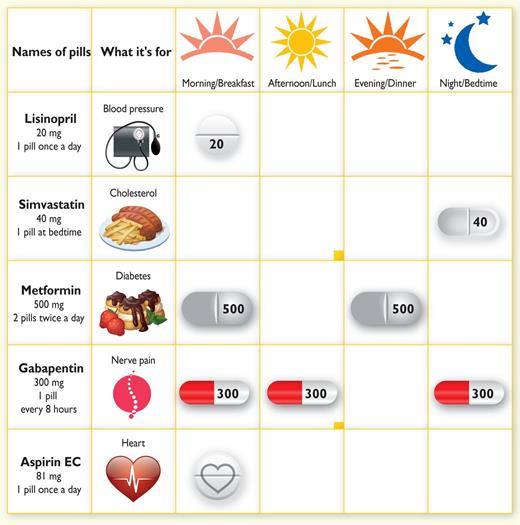
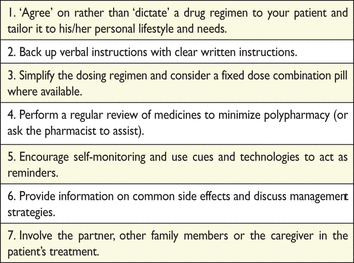
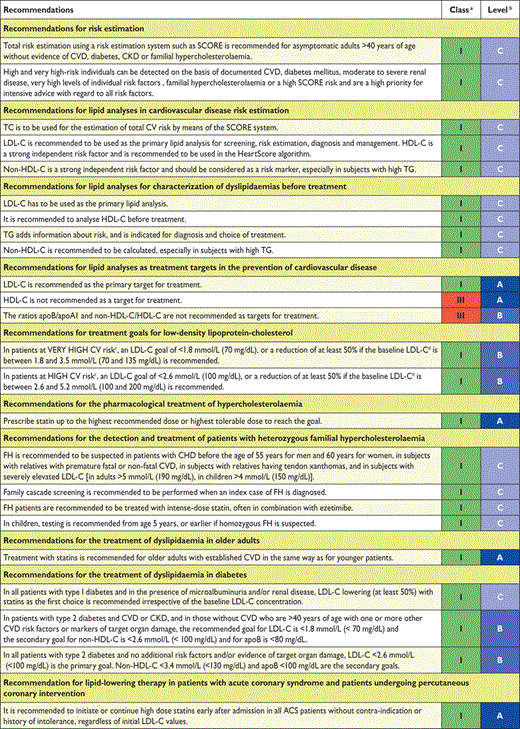
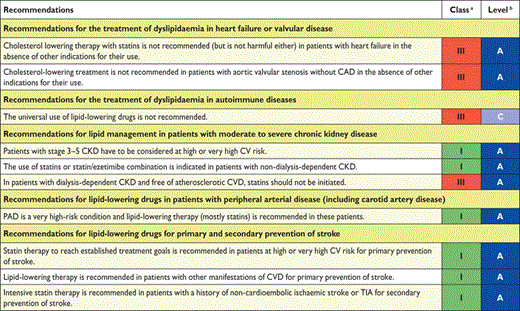

Comments
The new 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias have been released recently(1). The 2016 Guidelines focus on a particular problem that relates to young people with high levels of risk factors: a low absolute risk may conceal a high relative risk requiring advice for intensive lifestyle measures. The approach to this problem in 2016 version of the Guidelines is the same than 2012(2) and 2016(3) European Guidelines on cardiovascular disease prevention in clinical practice: calculation of the cardiovascular risk age.
The concept of vascular age or heart age was introduced in 2008 by D’Agostino and cols(4), who established that an individual’s heart age is calculated as the age of a person with the same predicted risk but with all other risk factor levels in normal ranges. They published heart age/vascular age tables derived from the general cardiovascular risk profile obtained from the Framingham Heart Study.
Other vascular age charts were published in 2010 based on the same concept but with SCORE project model(5). These charts are similar to original coloured SCORE charts but with vascular age inside each coloured box. They are the first specific vascular age charts published based on SCORE project. Also, it was demonstrated for the first time that vascular age does not need to be recalibrated: agreement in vascular age between high-risk countries charts and low-risk countries charts was almost total with a intraclass correlation coefficient of 0.997, avoiding the need of recalibration and allowing a broad use of them.
Comparing what it is said about vascular age in both series of guidelines (2012 and 2016 European Guidelines on cardiovascular disease prevention and 2016 guidelines of dyslipidaemias) it is astonishing to see that whole section “2.3.2 Cardiovascular risk age” from the prevention guidelines(3) is included in the dyslipidaemias guidelines(1) word for word except cites. Both guidelines state “Risk age has been shown to be independent of the CV endpoint used”, what was demonstrated in 2012 (with a partial SCORE dataset) as quoted in 2016 prevention guidelines cite 68(6). Because of it, dyslipidaemias guidelines cites 51 and 52 are incorrect (even cite 51 dates from 2009, before SCORE vascular age were published). Both cites are in no way related to SCORE vascular age. Also, both guidelines state “Risk age can be used in any population… and therefore avoids the need for recalibration”. Prevention guidelines refer to the original publication(3) (cite 69), but in dyslipidaemias guidelines it has been ommited. Perhaps, there were manuscript errors that can be amended by changing original cites 51 and 52 for cites 68(6) and 69(5) from 2016 prevention guidelines. A similar error occurred in 2012 prevention guidelines(2) and it was changed after publication.
The new 2016 dyslipidaemia guidelines present a method of estimating vascular risk age with the SCORE charts, not with specific vascular age charts, so that risk age can be estimated visually by looking at the SCORE chart. This method is useful only where there is no high absolute risk and for a maximum absolute risk of 2% in females and 4% in males. It is surprising that the new guidelines suggest this method without mentioning the specific SCORE vascular age charts(5) that are useful for the full range of absolute risk and age that appears in the SCORE charts.
Risk estimation is necessary for management of patients and vascular risk age is a useful tool for communicating risk to them and to get them to comply with lifestyles and drug treatments. The 2010 SCORE vascular age charts(5) can be used with the 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias.
References
1.- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, on behalf of Authors/Task Force Members. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J (2016) DOI: http://dx.doi.org/10.1093/eurheartj/ehw272 ehw272 First published online: 27 August 2016.
2.- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte Op Reimer WJM, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33(13):1635-701.
3.- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-81.
4.- D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care - The Framingham Heart Study. Circulation. 2008;117(6):743-753.
5.- Cuende JI. Cuende N, Calaveras-Lagartos J. How to calculate vascular age with the SCORE project scales: a new method of cardiovascular risk evaluation. Eur Heart J. 2010;31(19):2351-8.
6.- Cooney MT, Vartiainen E, Laatikainen T, De Bacquer D, McGorrian C, Dudina A, Graham I. Cardiovascular risk age: concepts and practicalities. Heart 2012;98:941–946.
Addendum:
Item 1. What “2016 European Guidelines on cardiovascular disease prevention in clinical practice” says:
“Risk age has been shown to be independent of the CV endpoint used (68), which bypasses the dilemma of whether to use a risk estimation system based on CV mortality or on total CV events. Risk age can be used in any population regardless of baseline risk and secular changes in mortality, and therefore avoids the need for recalibration (69). At present, risk age is recommended to help communicate about risk, especially to younger people with a low absolute risk but a high relative risk.”
Item 2. What “2016 ESC/EAS Guidelines for the Management of Dyslipidaemias” says:
“Risk age has been shown to be independent of the CV endpoint used (51-52), which bypasses the dilemma of whether to use a risk estimation system based on CVD mortality or on the more attractive but less reliable endpoint of total CVD events. Risk age can be used in any population regardless of baseline risk or secular changes in mortality, and therefore avoids the need for recalibration. At present, risk age is recommended for helping to communicate about risk, especially to younger people with a low absolute risk but a high relative risk.”
Figures: https://academic.oup.com/DocumentLibrary/EHJ/commentfiles/GLeletter704752figures.pdf