-
PDF
- Split View
-
Views
-
Cite
Cite
Karen Sliwa, Mark C Petrie, Peter van der Meer, Alexandre Mebazaa, Denise Hilfiker-Kleiner, Alice M Jackson, Aldo P Maggioni, Cecile Laroche, Vera Regitz-Zagrosek, Maria Schaufelberger, Luigi Tavazzi, Jolien W Roos-Hesselink, Petar Seferovic, Karin van Spaendonck-Zwarts, Amam Mbakwem, Michael Böhm, Frederic Mouquet, Burkert Pieske, Mark R Johnson, Righab Hamdan, Piotr Ponikowski, Dirk J Van Veldhuisen, John J V McMurray, Johann Bauersachs, on behalf of the EurObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy, Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry, European Heart Journal, Volume 41, Issue 39, 14 October 2020, Pages 3787–3797, https://doi.org/10.1093/eurheartj/ehaa455
Close - Share Icon Share
Abstract
We sought to describe the clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy (PPCM) globally.
In 2011, >100 national and affiliated member cardiac societies of the European Society of Cardiology (ESC) were contacted to contribute to a global registry on PPCM, under the auspices of the ESC EURObservational Research Programme. These societies were tasked with identifying centres who could participate in this registry. In low-income countries, e.g. Mozambique or Burkina Faso, where there are no national societies due to a shortage of cardiologists, we identified potential participants through abstracts and publications and encouraged participation into the study. Seven hundred and thirty-nine women were enrolled in 49 countries in Europe (33%), Africa (29%), Asia-Pacific (15%), and the Middle East (22%). Mean age was 31 ± 6 years, mean left ventricular ejection fraction (LVEF) was 31 ± 10%, and 10% had a previous pregnancy complicated by PPCM. Symptom-onset occurred most often within 1 month of delivery (44%). At diagnosis, 67% of patients had severe (NYHA III/IV) symptoms and 67% had a LVEF ≤35%. Fifteen percent received bromocriptine with significant regional variation (Europe 15%, Africa 26%, Asia-Pacific 8%, the Middle East 4%, P < 0.001). Follow-up was available for 598 (81%) women. Six-month mortality was 6% overall, lowest in Europe (4%), and highest in the Middle East (10%). Most deaths were due to heart failure (42%) or sudden (30%). Re-admission for any reason occurred in 10% (with just over half of these for heart failure) and thromboembolic events in 7%. Myocardial recovery (LVEF > 50%) occurred only in 46%, most commonly in Asia-Pacific (62%), and least commonly in the Middle East (25%). Neonatal death occurred in 5% with marked regional variation (Europe 2%, the Middle East 9%).
Peripartum cardiomyopathy is a global disease, but clinical presentation and outcomes vary by region. Just under half of women experience myocardial recovery. Peripartum cardiomyopathy is a disease with substantial maternal and neonatal morbidity and mortality.
See page 3798 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa564)
Introduction
Peripartum cardiomyopathy (PPCM) is a life-threatening condition which presents with heart failure towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is identified.1 , 2 Estimates of the incidence of PPCM vary markedly from 1 to 100 in 10 000 live births depending on region (even though these studies used similar definitions).1 In cohort studies from single countries, persistent reduction in left ventricular ejection fraction (LVEF) is common and death can occur, although cardiac function may also recover.3–5 Internationally, many unknowns remain: clinical presentation across different regions, worldwide mortality and morbidity (including hospitalizations, cardiovascular events, and thromboembolic events), and neonatal outcomes. Therefore, we conducted a prospective, global, observational registry to investigate PPCM around the world.
Methods
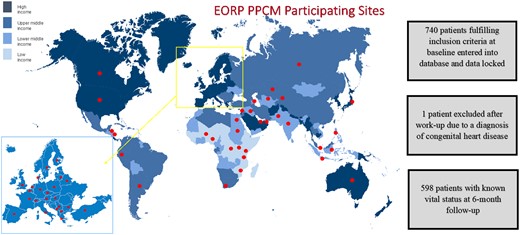
In 2011, >100 national and affiliated member cardiac societies of the European Society of Cardiology (ESC) were invited to contribute to a global registry on PPCM, under the auspices of the ESC EURObservational Research Programme (EORP). Activities of the ESC reach far beyond the boundaries of Europe, and nearly 90 000 members come from all over the world. These societies were tasked with identifying centres who could participate in this registry. In low-income countries, e.g. Mozambique or Burkina Faso, where there are no national societies due to a shortage of cardiologists, we identified potential participants through abstracts and publications and encouraged participation into the study.
Women with PPCM were enrolled prospectively between 2012 and 2018. Only newly diagnosed cases (those consented within 6 months of the diagnosis of PPCM) were eligible. Study design, patient selection, and data collection have been published previously.6 , 7 Eligibility criteria for participating centres were (i) availability of echocardiography; (ii) clinical expertise to make the diagnosis; and (iii) the ability to follow-up patients for 6 months. A dedicated central study management team coordinating the EORP at the ESC Heart House assisted physicians with regulatory and ethical approval and with data entry. Mandatory inclusion criteria were (i) peripartum state (no precise time windows were mandated as per the 2010 ESC Position Statement1); (ii) signs and/or symptoms of heart failure; (iii) LVEF ≤45%; and (iv) exclusion of other causes of heart failure. This was an observational study with no specific protocols or recommendations for diagnosis or management. Regions were defined as Europe, Africa, Asia-Pacific, and the Middle East as per the World Health Organization definitions (Supplementary Material, Appendix Methods Section).8 Ethnicity was self-reported.
The baseline visit was the first visit to the specialist making the diagnosis of PPCM. Data collected included baseline characteristics (demographics, comorbidities, obstetric history, signs, and symptoms), blood tests and findings from electrocardiography, chest radiography, and echocardiography. Ventricular assist device implantation was recorded at baseline. Echocardiographic parameters included LVEF, left atrial and left ventricular dimensions, right ventricular function, and valvular abnormalities. Echocardiography was performed at baseline and at 6 months. Information on pharmacological therapy initiated up to 6 months was collected and included guideline-recommended cardiovascular drugs, anticoagulation, and bromocriptine. Outcomes reported during the index PPCM hospitalization were death, stroke, and thromboembolism. Outcomes reported at 6 months following the diagnosis of PPCM were death, re-hospitalization, stroke, and thromboembolic events. Left ventricular function at 6 months was categorized as: (i) recovery (defined as LVEF ≥50%); (ii) persisting moderate left ventricular dysfunction (defined as LVEF 36–49%); and (iii) persisting severe left ventricular dysfunction (defined as LVEF ≤35%). A composite outcome of persisting severe left ventricular dysfunction or death was also pre-specified.
Core clinical data, such as data from the electrocardiogram and echocardiogram and information on the neonate, were centrally validated by a data monitor who contacted the sites. Comorbid conditions, deaths, re-hospitalizations, and thromboembolic events were reported by investigators using a standard case report form (see Supplementary Material, Appendix Methods section). Laboratory measurements (including N-terminal Pro-B-Type natriuretic peptide) were processed locally. All data are held by the EORP department of the ESC.
Statistical analysis
Continuous variables are reported as means and standard deviations or medians and interquartile ranges. Between-group comparisons for continuous variables were made by using non-parametric (Kruskal–Wallis) testing. Categorical variables are reported as percentages. Between-group comparisons for categorical variables were made using the χ2 test. For categorical variables with more than two categories, exact P-values were estimated according to the Monte Carlo method. A two-sided P-value <0.05 was considered statistically significant. Kaplan–Meier survival curves were generated for outcomes where time-to-event data were available. All analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Enrolment
Between 2012 and 2018, 740 women with suspected PPCM were recruited in 49 countries. One patient was excluded due to a subsequent diagnosis of congenital heart disease, resulting in the inclusion of 739 women. Patients were enrolled from Europe (33%), Africa (29%), Asia-Pacific (15%), and the Middle East (22%), respectively (Figure 1, Table 1). Race was reported as Caucasian in 33%, Black in 28%, Asian in 21%, Middle Eastern in 13%, Hispanic in 1%, and ‘other’ in 4% (Table 1).
Baseline characteristics
| . | All (n = 739) . | Europe (n = 247) . | Africa (n = 213) . | Asia-Pacific (n = 113) . | Middle East (n = 166) . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 31 ± 6 | 32 ± 6 | 28 ± 6 | 32 ± 6 | 31 ± 7 | <0.001 |
| Ethnicity, n (%) | <0.001 | |||||
| Caucasian | 233 (33) | 183 (80) | 5 (2) | 13 (12) | 32 (20) | |
| Black | 203 (28) | 14 (6) | 186 (88) | 1 (0.9) | 2 (1) | |
| Asian | 147 (21) | 17 (7) | 1 (0.5) | 87 (78) | 42 (26) | |
| Middle Eastern | 96 (13) | 8 (4) | 0 (0) | 0 (0) | 88 (54) | |
| Hispanic | 9 (1) | 0 (0) | 0 (0) | 9 (8) | 0 (0) | |
| Other | 28 (4) | 6 (3) | 20 (9) | 2 (2) | 0 (0) | |
| Comorbidities, n (%) | ||||||
| Diabetes | 22 (3) | 9 (4) | 1 (0.5) | 1 (0.9) | 11 (7) | 0.001 |
| Smoking | 94 (13) | 67 (30) | 11 (5) | 14 (13) | 2 (1) | <0.001 |
| Human immunodeficiency virus | 29 (6) | 0 (0) | 29 (18) | 0 (0) | 0 (0) | <0.001 |
| Hypertension in current pregnancy | 281 (39) | 91 (38) | 70 (33) | 67 (60) | 53 (33) | <0.001 |
| Pre-eclampsia in current pregnancy | 183 (25) | 56 (24) | 45 (21) | 52 (46) | 30 (19) | <0.001 |
| Parity, n (%) | 0.013 | |||||
| 0 | 13 (3) | 8 (6) | 3 (2) | 2 (3) | 0 (0) | |
| 1 | 110 (23) | 37 (28) | 28 (20) | 22 (28) | 23 (18) | |
| ≥2 | 360 (75) | 89 (66) | 112 (78) | 56 (70) | 103 (82) | |
| Previous peripartum cardiomyopathy, n (%) | 46 (10) | 6 (4) | 20 (14) | 2 (3) | 18 (14) | 0.002 |
| Timing of symptom-onset, n (%) | ||||||
| Antepartum | <0.001 | |||||
| Prior to final month | 81 (13) | 21 (10) | 18 (10) | 7 (7) | 35 (25) | |
| Within final month | 132 (21) | 43 (21) | 26 (15) | 37 (36) | 26 (19) | |
| Postpartum | 0.004 | |||||
| Within 1 month | 275 (44) | 99 (47) | 83 (47) | 36 (35) | 57 (41) | |
| Months 2–3 | 90 (14) | 33 (16) | 30 (17) | 15 (15) | 12 (9) | |
| Months 4–5 | 34 (5) | 6 (3) | 17 (10) | 4 (4) | 7 (5) | |
| Beyond 5 months | 15 (2) | 7 (3) | 3 (2) | 3 (3) | 2 (1) | |
| Time between symptom-onset and diagnosis (days) | 10 (3–34) | 6 (1–20) | 23 (5–61) | 7 (2–29) | 14 (5–47) | <0.001 |
| NYHA class, n (%) | <0.001 | |||||
| I/II | 241 (33) | 76 (32) | 90 (43) | 22 (20) | 53 (32) | |
| III | 243 (34) | 83 (34) | 80 (38) | 26 (24) | 54 (33) | |
| IV | 241 (33) | 82 (34) | 40 (19) | 62 (56) | 57 (35) | |
| Signs and symptoms, n (%) | ||||||
| Chest pain | 140 (19) | 38 (16) | 60 (29) | 7 (6) | 35 (21) | <0.001 |
| Palpitations | 363 (50) | 108 (45) | 100 (48) | 47 (42) | 108 (65) | <0.001 |
| Dizziness | 186 (26) | 71 (29) | 45 (21) | 20 (18) | 50 (30) | 0.032 |
| Third heart sound | 327 (46) | 56 (24) | 138 (66) | 54 (50) | 79 (52) | <0.001 |
| Elevated jugular venous pressure | 291 (42) | 41 (18) | 108 (51) | 66 (62) | 76 (50) | <0.001 |
| Peripheral oedema | 428 (58) | 130 (53) | 130 (62) | 76 (68) | 92 (56) | 0.038 |
| Pulmonary crepitations | 429 (59) | 128 (53) | 134 (64) | 77 (69) | 90 (57) | 0.012 |
| Blood tests | ||||||
| N-terminal pro-B-type natriuretic peptide (pg/mL) | 3308 (933– 8905) | 3521 (1123– 8612) | 2337 (473– 12 344) | 1861 (915– 2661) | 1307 (271– 12 973) | 0.760 |
| Electrocardiography | ||||||
| Heart rate (b.p.m.) | 100 ± 22 | 97 ± 23 | 101 ± 20 | 105 ± 22 | 100 ± 22 | 0.005 |
| Atrial fibrillation or flutter, n (%) | 16 (2) | 6 (3) | 1 (0.5) | 0 (0) | 9 (6) | 0.003 |
| Echocardiography | ||||||
| Left ventricular ejection fraction (%) | 31 ± 10 | 31 ± 11 | 32 ± 10 | 32 ± 11 | 31 ± 9 | 0.828 |
| Left ventricular ejection fraction, n (%) | 0.429 | |||||
| ≤25% | 224 (31) | 81 (35) | 62 (29) | 29 (26) | 52 (32) | |
| 26–35% | 258 (36) | 70 (30) | 82 (39) | 45 (40) | 61 (37) | |
| ≥36% | 237 (33) | 79 (34) | 68 (32) | 39 (35) | 51 (31) | |
| Left ventricular measurements, n (%) | ||||||
| Interventricular septum diastole (mm) | 9.0 (8.0–11.0) | 9.8 (8.9–11.0) | 9.0 (8.0–10.0) | 9.0 (8.0–11.0) | 10.0 (8.0–10.0) | 0.017 |
| Left ventricular end-diastolic diameter (mm) | 59 ± 8 | 59 ± 8 | 60 ± 8 | 55 ± 6 | 62 ± 8 | <0.001 |
| Corrected for body surface area (mm/m2) | 35 ± 6 | 33 ± 5 | 36 ± 6 | 34 ± 5 | 35 ± 6 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 49 ± 9 | 49 ± 10 | 50 ± 8 | 46 ± 7 | 51 ± 9 | <0.001 |
| Corrected for body surface area (mm/m2) | 29 ± 6 | 27 ± 6 | 31 ± 6 | 28 ±5 | 29 ± 6 | <0.001 |
| Mitral regurgitation, n (%) | <0.001 | |||||
| Moderate | 178 (32) | 79 (41) | 60 (32) | 19 (18) | 20 (29) | |
| Severe | 84 (15) | 31 (16) | 23 (12) | 5 (5) | 25 (36) | |
| Right ventricular impairment, n (%) | 0.002 | |||||
| Mild | 209 (32) | 67 (30) | 79 (43) | 20 (19) | 43 (31) | |
| Severe | 61 (9) | 19 (9) | 15 (8) | 10 (9) | 17 (12) | |
| Chest radiography, n (%) | ||||||
| Cardiomegaly | 385 (80) | 103 (63) | 114 (97) | 77 (83) | 91 (83) | <0.001 |
| Congestion | 364 (76) | 118 (73) | 86 (74) | 74 (79) | 86 (79) | 0.596 |
| . | All (n = 739) . | Europe (n = 247) . | Africa (n = 213) . | Asia-Pacific (n = 113) . | Middle East (n = 166) . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 31 ± 6 | 32 ± 6 | 28 ± 6 | 32 ± 6 | 31 ± 7 | <0.001 |
| Ethnicity, n (%) | <0.001 | |||||
| Caucasian | 233 (33) | 183 (80) | 5 (2) | 13 (12) | 32 (20) | |
| Black | 203 (28) | 14 (6) | 186 (88) | 1 (0.9) | 2 (1) | |
| Asian | 147 (21) | 17 (7) | 1 (0.5) | 87 (78) | 42 (26) | |
| Middle Eastern | 96 (13) | 8 (4) | 0 (0) | 0 (0) | 88 (54) | |
| Hispanic | 9 (1) | 0 (0) | 0 (0) | 9 (8) | 0 (0) | |
| Other | 28 (4) | 6 (3) | 20 (9) | 2 (2) | 0 (0) | |
| Comorbidities, n (%) | ||||||
| Diabetes | 22 (3) | 9 (4) | 1 (0.5) | 1 (0.9) | 11 (7) | 0.001 |
| Smoking | 94 (13) | 67 (30) | 11 (5) | 14 (13) | 2 (1) | <0.001 |
| Human immunodeficiency virus | 29 (6) | 0 (0) | 29 (18) | 0 (0) | 0 (0) | <0.001 |
| Hypertension in current pregnancy | 281 (39) | 91 (38) | 70 (33) | 67 (60) | 53 (33) | <0.001 |
| Pre-eclampsia in current pregnancy | 183 (25) | 56 (24) | 45 (21) | 52 (46) | 30 (19) | <0.001 |
| Parity, n (%) | 0.013 | |||||
| 0 | 13 (3) | 8 (6) | 3 (2) | 2 (3) | 0 (0) | |
| 1 | 110 (23) | 37 (28) | 28 (20) | 22 (28) | 23 (18) | |
| ≥2 | 360 (75) | 89 (66) | 112 (78) | 56 (70) | 103 (82) | |
| Previous peripartum cardiomyopathy, n (%) | 46 (10) | 6 (4) | 20 (14) | 2 (3) | 18 (14) | 0.002 |
| Timing of symptom-onset, n (%) | ||||||
| Antepartum | <0.001 | |||||
| Prior to final month | 81 (13) | 21 (10) | 18 (10) | 7 (7) | 35 (25) | |
| Within final month | 132 (21) | 43 (21) | 26 (15) | 37 (36) | 26 (19) | |
| Postpartum | 0.004 | |||||
| Within 1 month | 275 (44) | 99 (47) | 83 (47) | 36 (35) | 57 (41) | |
| Months 2–3 | 90 (14) | 33 (16) | 30 (17) | 15 (15) | 12 (9) | |
| Months 4–5 | 34 (5) | 6 (3) | 17 (10) | 4 (4) | 7 (5) | |
| Beyond 5 months | 15 (2) | 7 (3) | 3 (2) | 3 (3) | 2 (1) | |
| Time between symptom-onset and diagnosis (days) | 10 (3–34) | 6 (1–20) | 23 (5–61) | 7 (2–29) | 14 (5–47) | <0.001 |
| NYHA class, n (%) | <0.001 | |||||
| I/II | 241 (33) | 76 (32) | 90 (43) | 22 (20) | 53 (32) | |
| III | 243 (34) | 83 (34) | 80 (38) | 26 (24) | 54 (33) | |
| IV | 241 (33) | 82 (34) | 40 (19) | 62 (56) | 57 (35) | |
| Signs and symptoms, n (%) | ||||||
| Chest pain | 140 (19) | 38 (16) | 60 (29) | 7 (6) | 35 (21) | <0.001 |
| Palpitations | 363 (50) | 108 (45) | 100 (48) | 47 (42) | 108 (65) | <0.001 |
| Dizziness | 186 (26) | 71 (29) | 45 (21) | 20 (18) | 50 (30) | 0.032 |
| Third heart sound | 327 (46) | 56 (24) | 138 (66) | 54 (50) | 79 (52) | <0.001 |
| Elevated jugular venous pressure | 291 (42) | 41 (18) | 108 (51) | 66 (62) | 76 (50) | <0.001 |
| Peripheral oedema | 428 (58) | 130 (53) | 130 (62) | 76 (68) | 92 (56) | 0.038 |
| Pulmonary crepitations | 429 (59) | 128 (53) | 134 (64) | 77 (69) | 90 (57) | 0.012 |
| Blood tests | ||||||
| N-terminal pro-B-type natriuretic peptide (pg/mL) | 3308 (933– 8905) | 3521 (1123– 8612) | 2337 (473– 12 344) | 1861 (915– 2661) | 1307 (271– 12 973) | 0.760 |
| Electrocardiography | ||||||
| Heart rate (b.p.m.) | 100 ± 22 | 97 ± 23 | 101 ± 20 | 105 ± 22 | 100 ± 22 | 0.005 |
| Atrial fibrillation or flutter, n (%) | 16 (2) | 6 (3) | 1 (0.5) | 0 (0) | 9 (6) | 0.003 |
| Echocardiography | ||||||
| Left ventricular ejection fraction (%) | 31 ± 10 | 31 ± 11 | 32 ± 10 | 32 ± 11 | 31 ± 9 | 0.828 |
| Left ventricular ejection fraction, n (%) | 0.429 | |||||
| ≤25% | 224 (31) | 81 (35) | 62 (29) | 29 (26) | 52 (32) | |
| 26–35% | 258 (36) | 70 (30) | 82 (39) | 45 (40) | 61 (37) | |
| ≥36% | 237 (33) | 79 (34) | 68 (32) | 39 (35) | 51 (31) | |
| Left ventricular measurements, n (%) | ||||||
| Interventricular septum diastole (mm) | 9.0 (8.0–11.0) | 9.8 (8.9–11.0) | 9.0 (8.0–10.0) | 9.0 (8.0–11.0) | 10.0 (8.0–10.0) | 0.017 |
| Left ventricular end-diastolic diameter (mm) | 59 ± 8 | 59 ± 8 | 60 ± 8 | 55 ± 6 | 62 ± 8 | <0.001 |
| Corrected for body surface area (mm/m2) | 35 ± 6 | 33 ± 5 | 36 ± 6 | 34 ± 5 | 35 ± 6 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 49 ± 9 | 49 ± 10 | 50 ± 8 | 46 ± 7 | 51 ± 9 | <0.001 |
| Corrected for body surface area (mm/m2) | 29 ± 6 | 27 ± 6 | 31 ± 6 | 28 ±5 | 29 ± 6 | <0.001 |
| Mitral regurgitation, n (%) | <0.001 | |||||
| Moderate | 178 (32) | 79 (41) | 60 (32) | 19 (18) | 20 (29) | |
| Severe | 84 (15) | 31 (16) | 23 (12) | 5 (5) | 25 (36) | |
| Right ventricular impairment, n (%) | 0.002 | |||||
| Mild | 209 (32) | 67 (30) | 79 (43) | 20 (19) | 43 (31) | |
| Severe | 61 (9) | 19 (9) | 15 (8) | 10 (9) | 17 (12) | |
| Chest radiography, n (%) | ||||||
| Cardiomegaly | 385 (80) | 103 (63) | 114 (97) | 77 (83) | 91 (83) | <0.001 |
| Congestion | 364 (76) | 118 (73) | 86 (74) | 74 (79) | 86 (79) | 0.596 |
Baseline characteristics
| . | All (n = 739) . | Europe (n = 247) . | Africa (n = 213) . | Asia-Pacific (n = 113) . | Middle East (n = 166) . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 31 ± 6 | 32 ± 6 | 28 ± 6 | 32 ± 6 | 31 ± 7 | <0.001 |
| Ethnicity, n (%) | <0.001 | |||||
| Caucasian | 233 (33) | 183 (80) | 5 (2) | 13 (12) | 32 (20) | |
| Black | 203 (28) | 14 (6) | 186 (88) | 1 (0.9) | 2 (1) | |
| Asian | 147 (21) | 17 (7) | 1 (0.5) | 87 (78) | 42 (26) | |
| Middle Eastern | 96 (13) | 8 (4) | 0 (0) | 0 (0) | 88 (54) | |
| Hispanic | 9 (1) | 0 (0) | 0 (0) | 9 (8) | 0 (0) | |
| Other | 28 (4) | 6 (3) | 20 (9) | 2 (2) | 0 (0) | |
| Comorbidities, n (%) | ||||||
| Diabetes | 22 (3) | 9 (4) | 1 (0.5) | 1 (0.9) | 11 (7) | 0.001 |
| Smoking | 94 (13) | 67 (30) | 11 (5) | 14 (13) | 2 (1) | <0.001 |
| Human immunodeficiency virus | 29 (6) | 0 (0) | 29 (18) | 0 (0) | 0 (0) | <0.001 |
| Hypertension in current pregnancy | 281 (39) | 91 (38) | 70 (33) | 67 (60) | 53 (33) | <0.001 |
| Pre-eclampsia in current pregnancy | 183 (25) | 56 (24) | 45 (21) | 52 (46) | 30 (19) | <0.001 |
| Parity, n (%) | 0.013 | |||||
| 0 | 13 (3) | 8 (6) | 3 (2) | 2 (3) | 0 (0) | |
| 1 | 110 (23) | 37 (28) | 28 (20) | 22 (28) | 23 (18) | |
| ≥2 | 360 (75) | 89 (66) | 112 (78) | 56 (70) | 103 (82) | |
| Previous peripartum cardiomyopathy, n (%) | 46 (10) | 6 (4) | 20 (14) | 2 (3) | 18 (14) | 0.002 |
| Timing of symptom-onset, n (%) | ||||||
| Antepartum | <0.001 | |||||
| Prior to final month | 81 (13) | 21 (10) | 18 (10) | 7 (7) | 35 (25) | |
| Within final month | 132 (21) | 43 (21) | 26 (15) | 37 (36) | 26 (19) | |
| Postpartum | 0.004 | |||||
| Within 1 month | 275 (44) | 99 (47) | 83 (47) | 36 (35) | 57 (41) | |
| Months 2–3 | 90 (14) | 33 (16) | 30 (17) | 15 (15) | 12 (9) | |
| Months 4–5 | 34 (5) | 6 (3) | 17 (10) | 4 (4) | 7 (5) | |
| Beyond 5 months | 15 (2) | 7 (3) | 3 (2) | 3 (3) | 2 (1) | |
| Time between symptom-onset and diagnosis (days) | 10 (3–34) | 6 (1–20) | 23 (5–61) | 7 (2–29) | 14 (5–47) | <0.001 |
| NYHA class, n (%) | <0.001 | |||||
| I/II | 241 (33) | 76 (32) | 90 (43) | 22 (20) | 53 (32) | |
| III | 243 (34) | 83 (34) | 80 (38) | 26 (24) | 54 (33) | |
| IV | 241 (33) | 82 (34) | 40 (19) | 62 (56) | 57 (35) | |
| Signs and symptoms, n (%) | ||||||
| Chest pain | 140 (19) | 38 (16) | 60 (29) | 7 (6) | 35 (21) | <0.001 |
| Palpitations | 363 (50) | 108 (45) | 100 (48) | 47 (42) | 108 (65) | <0.001 |
| Dizziness | 186 (26) | 71 (29) | 45 (21) | 20 (18) | 50 (30) | 0.032 |
| Third heart sound | 327 (46) | 56 (24) | 138 (66) | 54 (50) | 79 (52) | <0.001 |
| Elevated jugular venous pressure | 291 (42) | 41 (18) | 108 (51) | 66 (62) | 76 (50) | <0.001 |
| Peripheral oedema | 428 (58) | 130 (53) | 130 (62) | 76 (68) | 92 (56) | 0.038 |
| Pulmonary crepitations | 429 (59) | 128 (53) | 134 (64) | 77 (69) | 90 (57) | 0.012 |
| Blood tests | ||||||
| N-terminal pro-B-type natriuretic peptide (pg/mL) | 3308 (933– 8905) | 3521 (1123– 8612) | 2337 (473– 12 344) | 1861 (915– 2661) | 1307 (271– 12 973) | 0.760 |
| Electrocardiography | ||||||
| Heart rate (b.p.m.) | 100 ± 22 | 97 ± 23 | 101 ± 20 | 105 ± 22 | 100 ± 22 | 0.005 |
| Atrial fibrillation or flutter, n (%) | 16 (2) | 6 (3) | 1 (0.5) | 0 (0) | 9 (6) | 0.003 |
| Echocardiography | ||||||
| Left ventricular ejection fraction (%) | 31 ± 10 | 31 ± 11 | 32 ± 10 | 32 ± 11 | 31 ± 9 | 0.828 |
| Left ventricular ejection fraction, n (%) | 0.429 | |||||
| ≤25% | 224 (31) | 81 (35) | 62 (29) | 29 (26) | 52 (32) | |
| 26–35% | 258 (36) | 70 (30) | 82 (39) | 45 (40) | 61 (37) | |
| ≥36% | 237 (33) | 79 (34) | 68 (32) | 39 (35) | 51 (31) | |
| Left ventricular measurements, n (%) | ||||||
| Interventricular septum diastole (mm) | 9.0 (8.0–11.0) | 9.8 (8.9–11.0) | 9.0 (8.0–10.0) | 9.0 (8.0–11.0) | 10.0 (8.0–10.0) | 0.017 |
| Left ventricular end-diastolic diameter (mm) | 59 ± 8 | 59 ± 8 | 60 ± 8 | 55 ± 6 | 62 ± 8 | <0.001 |
| Corrected for body surface area (mm/m2) | 35 ± 6 | 33 ± 5 | 36 ± 6 | 34 ± 5 | 35 ± 6 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 49 ± 9 | 49 ± 10 | 50 ± 8 | 46 ± 7 | 51 ± 9 | <0.001 |
| Corrected for body surface area (mm/m2) | 29 ± 6 | 27 ± 6 | 31 ± 6 | 28 ±5 | 29 ± 6 | <0.001 |
| Mitral regurgitation, n (%) | <0.001 | |||||
| Moderate | 178 (32) | 79 (41) | 60 (32) | 19 (18) | 20 (29) | |
| Severe | 84 (15) | 31 (16) | 23 (12) | 5 (5) | 25 (36) | |
| Right ventricular impairment, n (%) | 0.002 | |||||
| Mild | 209 (32) | 67 (30) | 79 (43) | 20 (19) | 43 (31) | |
| Severe | 61 (9) | 19 (9) | 15 (8) | 10 (9) | 17 (12) | |
| Chest radiography, n (%) | ||||||
| Cardiomegaly | 385 (80) | 103 (63) | 114 (97) | 77 (83) | 91 (83) | <0.001 |
| Congestion | 364 (76) | 118 (73) | 86 (74) | 74 (79) | 86 (79) | 0.596 |
| . | All (n = 739) . | Europe (n = 247) . | Africa (n = 213) . | Asia-Pacific (n = 113) . | Middle East (n = 166) . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 31 ± 6 | 32 ± 6 | 28 ± 6 | 32 ± 6 | 31 ± 7 | <0.001 |
| Ethnicity, n (%) | <0.001 | |||||
| Caucasian | 233 (33) | 183 (80) | 5 (2) | 13 (12) | 32 (20) | |
| Black | 203 (28) | 14 (6) | 186 (88) | 1 (0.9) | 2 (1) | |
| Asian | 147 (21) | 17 (7) | 1 (0.5) | 87 (78) | 42 (26) | |
| Middle Eastern | 96 (13) | 8 (4) | 0 (0) | 0 (0) | 88 (54) | |
| Hispanic | 9 (1) | 0 (0) | 0 (0) | 9 (8) | 0 (0) | |
| Other | 28 (4) | 6 (3) | 20 (9) | 2 (2) | 0 (0) | |
| Comorbidities, n (%) | ||||||
| Diabetes | 22 (3) | 9 (4) | 1 (0.5) | 1 (0.9) | 11 (7) | 0.001 |
| Smoking | 94 (13) | 67 (30) | 11 (5) | 14 (13) | 2 (1) | <0.001 |
| Human immunodeficiency virus | 29 (6) | 0 (0) | 29 (18) | 0 (0) | 0 (0) | <0.001 |
| Hypertension in current pregnancy | 281 (39) | 91 (38) | 70 (33) | 67 (60) | 53 (33) | <0.001 |
| Pre-eclampsia in current pregnancy | 183 (25) | 56 (24) | 45 (21) | 52 (46) | 30 (19) | <0.001 |
| Parity, n (%) | 0.013 | |||||
| 0 | 13 (3) | 8 (6) | 3 (2) | 2 (3) | 0 (0) | |
| 1 | 110 (23) | 37 (28) | 28 (20) | 22 (28) | 23 (18) | |
| ≥2 | 360 (75) | 89 (66) | 112 (78) | 56 (70) | 103 (82) | |
| Previous peripartum cardiomyopathy, n (%) | 46 (10) | 6 (4) | 20 (14) | 2 (3) | 18 (14) | 0.002 |
| Timing of symptom-onset, n (%) | ||||||
| Antepartum | <0.001 | |||||
| Prior to final month | 81 (13) | 21 (10) | 18 (10) | 7 (7) | 35 (25) | |
| Within final month | 132 (21) | 43 (21) | 26 (15) | 37 (36) | 26 (19) | |
| Postpartum | 0.004 | |||||
| Within 1 month | 275 (44) | 99 (47) | 83 (47) | 36 (35) | 57 (41) | |
| Months 2–3 | 90 (14) | 33 (16) | 30 (17) | 15 (15) | 12 (9) | |
| Months 4–5 | 34 (5) | 6 (3) | 17 (10) | 4 (4) | 7 (5) | |
| Beyond 5 months | 15 (2) | 7 (3) | 3 (2) | 3 (3) | 2 (1) | |
| Time between symptom-onset and diagnosis (days) | 10 (3–34) | 6 (1–20) | 23 (5–61) | 7 (2–29) | 14 (5–47) | <0.001 |
| NYHA class, n (%) | <0.001 | |||||
| I/II | 241 (33) | 76 (32) | 90 (43) | 22 (20) | 53 (32) | |
| III | 243 (34) | 83 (34) | 80 (38) | 26 (24) | 54 (33) | |
| IV | 241 (33) | 82 (34) | 40 (19) | 62 (56) | 57 (35) | |
| Signs and symptoms, n (%) | ||||||
| Chest pain | 140 (19) | 38 (16) | 60 (29) | 7 (6) | 35 (21) | <0.001 |
| Palpitations | 363 (50) | 108 (45) | 100 (48) | 47 (42) | 108 (65) | <0.001 |
| Dizziness | 186 (26) | 71 (29) | 45 (21) | 20 (18) | 50 (30) | 0.032 |
| Third heart sound | 327 (46) | 56 (24) | 138 (66) | 54 (50) | 79 (52) | <0.001 |
| Elevated jugular venous pressure | 291 (42) | 41 (18) | 108 (51) | 66 (62) | 76 (50) | <0.001 |
| Peripheral oedema | 428 (58) | 130 (53) | 130 (62) | 76 (68) | 92 (56) | 0.038 |
| Pulmonary crepitations | 429 (59) | 128 (53) | 134 (64) | 77 (69) | 90 (57) | 0.012 |
| Blood tests | ||||||
| N-terminal pro-B-type natriuretic peptide (pg/mL) | 3308 (933– 8905) | 3521 (1123– 8612) | 2337 (473– 12 344) | 1861 (915– 2661) | 1307 (271– 12 973) | 0.760 |
| Electrocardiography | ||||||
| Heart rate (b.p.m.) | 100 ± 22 | 97 ± 23 | 101 ± 20 | 105 ± 22 | 100 ± 22 | 0.005 |
| Atrial fibrillation or flutter, n (%) | 16 (2) | 6 (3) | 1 (0.5) | 0 (0) | 9 (6) | 0.003 |
| Echocardiography | ||||||
| Left ventricular ejection fraction (%) | 31 ± 10 | 31 ± 11 | 32 ± 10 | 32 ± 11 | 31 ± 9 | 0.828 |
| Left ventricular ejection fraction, n (%) | 0.429 | |||||
| ≤25% | 224 (31) | 81 (35) | 62 (29) | 29 (26) | 52 (32) | |
| 26–35% | 258 (36) | 70 (30) | 82 (39) | 45 (40) | 61 (37) | |
| ≥36% | 237 (33) | 79 (34) | 68 (32) | 39 (35) | 51 (31) | |
| Left ventricular measurements, n (%) | ||||||
| Interventricular septum diastole (mm) | 9.0 (8.0–11.0) | 9.8 (8.9–11.0) | 9.0 (8.0–10.0) | 9.0 (8.0–11.0) | 10.0 (8.0–10.0) | 0.017 |
| Left ventricular end-diastolic diameter (mm) | 59 ± 8 | 59 ± 8 | 60 ± 8 | 55 ± 6 | 62 ± 8 | <0.001 |
| Corrected for body surface area (mm/m2) | 35 ± 6 | 33 ± 5 | 36 ± 6 | 34 ± 5 | 35 ± 6 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 49 ± 9 | 49 ± 10 | 50 ± 8 | 46 ± 7 | 51 ± 9 | <0.001 |
| Corrected for body surface area (mm/m2) | 29 ± 6 | 27 ± 6 | 31 ± 6 | 28 ±5 | 29 ± 6 | <0.001 |
| Mitral regurgitation, n (%) | <0.001 | |||||
| Moderate | 178 (32) | 79 (41) | 60 (32) | 19 (18) | 20 (29) | |
| Severe | 84 (15) | 31 (16) | 23 (12) | 5 (5) | 25 (36) | |
| Right ventricular impairment, n (%) | 0.002 | |||||
| Mild | 209 (32) | 67 (30) | 79 (43) | 20 (19) | 43 (31) | |
| Severe | 61 (9) | 19 (9) | 15 (8) | 10 (9) | 17 (12) | |
| Chest radiography, n (%) | ||||||
| Cardiomegaly | 385 (80) | 103 (63) | 114 (97) | 77 (83) | 91 (83) | <0.001 |
| Congestion | 364 (76) | 118 (73) | 86 (74) | 74 (79) | 86 (79) | 0.596 |
Baseline characteristics
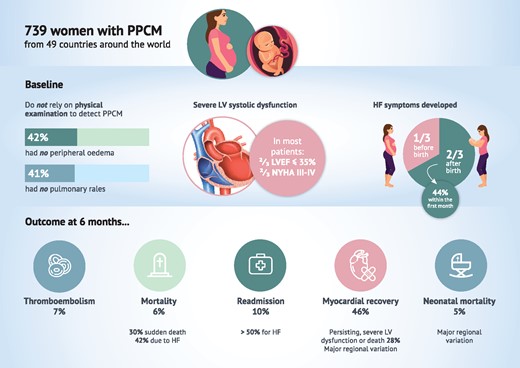
Mean (±SD) age was 31 ± 6 years, mean LVEF was 31 ± 10%, and 67% of women had a LVEF ≤35% (Table 1). At diagnosis, 67% of patients had severe (NYHAIII/IV) symptoms. There was a poor correlation between symptoms and LVEF and a quarter of those with mild (NYHA I/II) symptoms had a LVEF ≤25% (Supplementary material online, Appendix Figure S1). Peripheral oedema and pulmonary rales were present in 58 and 59%, respectively, and 76% had pulmonary congestion on chest radiography. A third heart sound was heard in 46%.
One-third of patients developed symptoms before birth and two-thirds after birth (44% within the first month after delivery, 14% in months 2–3 after delivery and 8% ≥4 months after delivery, Supplementary material online, Appendix Figure S2). The most common week in which women were diagnosed with PPCM antepartum was the final week prior to delivery (Supplementary material online, Appendix Figure S3). A prior episode of PPCM had occurred in 10% (i.e. the pregnancy included in the registry was a subsequent PPCM pregnancy, Table 1). Concomitant hypertension and pre-eclampsia were reported in 39% and 25%, respectively.
Baseline characteristics by region
Women with PPCM in Europe were older and were more likely to be smokers than in other regions (Table 1). Women from the Middle East had the highest prevalence of diabetes and multiparity. African women were the youngest, had the highest prevalence of human immunodeficiency virus (HIV) and the lowest prevalence of diabetes. There were marked geographic differences in concomitant pre-eclampsia and hypertension; pre-eclampsia was much more common in Asia-Pacific (46%) than in Europe (24%), Africa (21%), and the Middle East (19%) (P < 0.001). Hypertension followed a similar pattern. More of the participants recruited in Africa and the Middle East were multiparous (78% and 82%, respectively) compared with Europe (66%) and Asia-Pacific (70%) (P = 0.013). In Europe and Africa, symptom-onset in the first month after delivery was more common (both 47%) than in Asia-Pacific (35%) and the Middle East (41%) (P = 0.004). There was a marked difference in the frequency of antepartum symptom-onset (e.g. Africa 25% and Asia-Pacific 43%) (P < 0.001). The delay between symptom-onset and diagnosis was greatest in Africa [median 23 days (IQR 5–61)] and shortest in Europe [median 6 days (IQR 1–20)]. Women in Asia-Pacific had more severe (NYHA III/IV) symptoms than in other regions (e.g. Asia-Pacific 80% and Africa 57%) (P < 0.001). There was no regional difference in LVEF, but women from the Middle East had higher left ventricular end-systolic and diastolic diameters (e.g. the Middle East 62 ± 8 mm and Asia-Pacific 55 ± 6 mm) (P < 0.001). When corrected for body surface area, women from Africa had the largest left ventricular diameters.
Pharmacological therapy
The use of drugs for heart failure at 6 months was high across all regions; 85% were prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 81% were prescribed beta-blockers, and 45% were prescribed mineralocorticoid receptor antagonists (Supplementary material online, Appendix Figure S4). Digoxin was used in 21% and a diuretic in 74%. During the 6 months after delivery, bromocriptine and anticoagulation were used in 15 and 16%, respectively. There was marked regional variation in bromocriptine-prescribing (Europe 15%, Africa 26%, Asia-Pacific 8%, the Middle East 4%, P < 0.001), but no difference in the use of anticoagulants. There was no difference in the frequency of thromboembolic events in the group who were treated with bromocriptine compared with those who were not [5 events in 84 patients (6%) who received bromocriptine vs. 26 events in 463 patients (6%) not receiving bromocriptine (P = 0.802)].
Maternal outcomes
Six-month follow-up was available for 598 (81%) patients. A comparison of the baseline characteristics of the 598 patients with 6-month follow-up data and the 141 without 6-month follow-up data showed no major differences, although those without follow-up were more likely to be Middle-Eastern, more likely to have a higher baseline LVEF, more likely to have had a previous PPCM and less likely to have HIV (Supplementary material online, Appendix Table S1).
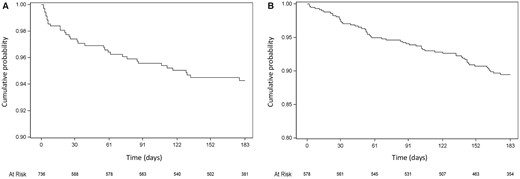
Death in hospital from any cause occurred in 2% of women and a ventricular assist device was implanted in 3% during the index hospitalization (Table 2). Mortality from any cause at 6 months was 6%. Death occurred most frequently in the Middle East (10%) and least frequently in Europe (4%). Just under half of the deaths occurred within 30 days of diagnosis (Figure 2). Over half of deaths in-hospital were due to heart failure (56%) (Table 2). Most deaths through 6 months were also due to heart failure (42%), with the second most common cause of death being sudden (30%). Hospital admission for any reason through 6 months occurred in 10% of women (53% of cases were for heart failure, 32% were for cardiovascular reasons other than heart failure, and 19% of admissions were non-cardiovascular) (Figure 2, Table 2). The incidence of thromboembolic events was 7% by 6 months and was highest in Europe (9%), and lowest in Asia-Pacific region (3%) (Table 2). Stroke occurred in 3% of women. The majority of thromboembolic events and strokes were identified during the index hospitalization. Mean LVEF at 6 months was 46 ± 13%, with a mean increase from baseline to 6 months of 15 ± 13% (signed-rank P < 0.001) (Supplementary material online, Appendix Table S2). Mean reductions in left ventricular end-diastolic and end-systolic diameters from baseline to 6 months were 4 ± 7 and 8 ± 8 mm, respectively. Recovery of left ventricular function (to a LVEF ≥50%) occurred in 46% of women, whereas 23% had persisting and severe left ventricular dysfunction (LVEF ≤35%) at 6 months (Table 2). Persisting, severe left ventricular dysfunction or death occurred in 28%. Recovery occurred most frequently in women in the Asia-Pacific region (62%) and least frequently in those in the Middle East (25%) (P < 0.001).
Kaplan-Meier survival curves for 6-month outcomes: (A) Death from any cause and (B) Re-hospitalization for any cause.
In-hospital and 6-month outcomes
| N (%) . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| In-hospital devices | ||||||
| Assist device | 17 (3) | 12 (7) | 0 (0) | 5 (6) | 0 (0) | <0.001 |
| Implantable cardioverter-defibrillator | 5 (0.9) | 4 (2) | 0 (0) | 0 (0) | 1 (0.7) | 0.101 |
| Cardiac resynchronization therapy | 2 (0.4) | 1 (0.6) | 0 (0) | 0 (0) | 1 (0.7) | 0.833 |
| Death | ||||||
| In-hospital | ||||||
| All-cause | 16 (2) | 7 (3) | 1 (0.5) | 1 (0.9) | 7 (4) | 0.046 |
| Heart failure | 9 (56) | 4 (57) | 0 (0) | 1 (100) | 4 (57) | 1.000 |
| Sudden | 4 (25) | 1 (14) | 1 (100) | 0 (0) | 2 (29) | 0.484 |
| Stroke | 3 (19) | 2 (29) | 0 (0) | 0 (0) | 1 (14) | 1.000 |
| 6 months | ||||||
| All-cause | 35 (6) | 8 (4) | 8 (5) | 8 (8) | 11 (10) | 0.082 |
| Heart failure | 14 (42) | 5 (63) | 0 (0) | 2 (33) | 7 (64) | 0.018 |
| Sudden | 10 (30) | 1 (13) | 2 (25) | 4 (67) | 3 (27) | 0.204 |
| Stroke | 5 (15) | 2 (25) | 2 (25) | 0 (0) | 1 (9) | 0.520 |
| Presumed cardiovascular | 4 (12) | 0 (0) | 4 (50) | 0 (0) | 0 (0) | 0.004 |
| Re-hospitalization at 6 months | ||||||
| All-cause | 58 (10) | 24 (12) | 18 (10) | 7 (7) | 9 (9) | 0.667 |
| Heart failure | 30 (53) | 9 (39) | 11 (61) | 3 (43) | 7 (78) | 0.220 |
| Other cardiac | 13 (23) | 10 (43) | 1 (6) | 1 (14) | 1 (11) | 0.025 |
| Vascular | 5 (9) | 1 (4) | 4 (22) | 0 (0) | 0 (0) | 0.191 |
| Non-cardiovascular | 11 (19) | 4 (17) | 3 (17) | 3 (43) | 1 (11) | 0.441 |
| Thromboembolism | ||||||
| In-hospital | ||||||
| All-cause | 40 (5) | 19 (8) | 7 (3) | 3 (3) | 11 (7) | 0.083 |
| Venous | 30 (4) | 15 (6) | 5 (2) | 3 (3) | 7 (4) | 0.169 |
| Arterial | 13 (2) | 6 (2) | 2 (0.9) | 0 (0) | 5 (3) | 0.170 |
| After discharge to 6 months | ||||||
| All-cause | 4 (0.7) | 0 (0) | 3 (2) | 0 (0) | 1 (1) | — |
| Venous | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | — |
| Arterial | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 44 (7) | 19 (9) | 10 (6) | 3 (3) | 12 (11) | 0.101 |
| Venous | 31 (5) | 15 (7) | 5 (3) | 3 (3) | 8 (7) | 0.127 |
| Arterial | 16 (3) | 6 (3) | 5 (3) | 0 (0) | 5 (5) | 0.216 |
| Stroke | ||||||
| In-hospital | ||||||
| All-cause | 12 (2) | 7 (3) | 1 (0.5) | 0 (0) | 4 (2) | — |
| Ischaemic | 8 (1) | 4 (2) | 1 (0.5) | 0 (0) | 3 (2) | — |
| Haemorrhagic | 5 (0.7) | 3 (1) | 0 (0) | 0 (0) | 2 (1) | — |
| After discharge to 6 months | ||||||
| All-cause | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Ischaemic | 2 (0.4) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | — |
| Haemorrhagic | 1 (0.2) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 15 (3) | 7 (3) | 4 (2) | 0 (0) | 4 (4) | — |
| Ischaemic | 10 (2) | 4 (2) | 3 (2) | 0 (0) | 3 (3) | — |
| Haemorrhagic | 6 (1) | 3 (1) | 1 (0.6) | 0 (0) | 2 (2) | — |
| Left ventricular function at 6 months | ||||||
| Recovered | 219 (46) | 98 (57) | 53 (37) | 48 (62) | 20 (25) | <0.001 |
| Persisting moderate left ventricular dysfunction | 147 (31) | 51 (29) | 52 (36) | 20 (26) | 24 (30) | 0.367 |
| Persisting severe left ventricular dysfunction | 107 (23) | 24 (14) | 38 (27) | 10 (13) | 35 (44) | <0.001 |
| Persisting severe left ventricular dysfunction or death | 142 (28) | 32 (18) | 46 (30) | 18 (21) | 46 (51) | <0.001 |
| N (%) . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| In-hospital devices | ||||||
| Assist device | 17 (3) | 12 (7) | 0 (0) | 5 (6) | 0 (0) | <0.001 |
| Implantable cardioverter-defibrillator | 5 (0.9) | 4 (2) | 0 (0) | 0 (0) | 1 (0.7) | 0.101 |
| Cardiac resynchronization therapy | 2 (0.4) | 1 (0.6) | 0 (0) | 0 (0) | 1 (0.7) | 0.833 |
| Death | ||||||
| In-hospital | ||||||
| All-cause | 16 (2) | 7 (3) | 1 (0.5) | 1 (0.9) | 7 (4) | 0.046 |
| Heart failure | 9 (56) | 4 (57) | 0 (0) | 1 (100) | 4 (57) | 1.000 |
| Sudden | 4 (25) | 1 (14) | 1 (100) | 0 (0) | 2 (29) | 0.484 |
| Stroke | 3 (19) | 2 (29) | 0 (0) | 0 (0) | 1 (14) | 1.000 |
| 6 months | ||||||
| All-cause | 35 (6) | 8 (4) | 8 (5) | 8 (8) | 11 (10) | 0.082 |
| Heart failure | 14 (42) | 5 (63) | 0 (0) | 2 (33) | 7 (64) | 0.018 |
| Sudden | 10 (30) | 1 (13) | 2 (25) | 4 (67) | 3 (27) | 0.204 |
| Stroke | 5 (15) | 2 (25) | 2 (25) | 0 (0) | 1 (9) | 0.520 |
| Presumed cardiovascular | 4 (12) | 0 (0) | 4 (50) | 0 (0) | 0 (0) | 0.004 |
| Re-hospitalization at 6 months | ||||||
| All-cause | 58 (10) | 24 (12) | 18 (10) | 7 (7) | 9 (9) | 0.667 |
| Heart failure | 30 (53) | 9 (39) | 11 (61) | 3 (43) | 7 (78) | 0.220 |
| Other cardiac | 13 (23) | 10 (43) | 1 (6) | 1 (14) | 1 (11) | 0.025 |
| Vascular | 5 (9) | 1 (4) | 4 (22) | 0 (0) | 0 (0) | 0.191 |
| Non-cardiovascular | 11 (19) | 4 (17) | 3 (17) | 3 (43) | 1 (11) | 0.441 |
| Thromboembolism | ||||||
| In-hospital | ||||||
| All-cause | 40 (5) | 19 (8) | 7 (3) | 3 (3) | 11 (7) | 0.083 |
| Venous | 30 (4) | 15 (6) | 5 (2) | 3 (3) | 7 (4) | 0.169 |
| Arterial | 13 (2) | 6 (2) | 2 (0.9) | 0 (0) | 5 (3) | 0.170 |
| After discharge to 6 months | ||||||
| All-cause | 4 (0.7) | 0 (0) | 3 (2) | 0 (0) | 1 (1) | — |
| Venous | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | — |
| Arterial | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 44 (7) | 19 (9) | 10 (6) | 3 (3) | 12 (11) | 0.101 |
| Venous | 31 (5) | 15 (7) | 5 (3) | 3 (3) | 8 (7) | 0.127 |
| Arterial | 16 (3) | 6 (3) | 5 (3) | 0 (0) | 5 (5) | 0.216 |
| Stroke | ||||||
| In-hospital | ||||||
| All-cause | 12 (2) | 7 (3) | 1 (0.5) | 0 (0) | 4 (2) | — |
| Ischaemic | 8 (1) | 4 (2) | 1 (0.5) | 0 (0) | 3 (2) | — |
| Haemorrhagic | 5 (0.7) | 3 (1) | 0 (0) | 0 (0) | 2 (1) | — |
| After discharge to 6 months | ||||||
| All-cause | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Ischaemic | 2 (0.4) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | — |
| Haemorrhagic | 1 (0.2) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 15 (3) | 7 (3) | 4 (2) | 0 (0) | 4 (4) | — |
| Ischaemic | 10 (2) | 4 (2) | 3 (2) | 0 (0) | 3 (3) | — |
| Haemorrhagic | 6 (1) | 3 (1) | 1 (0.6) | 0 (0) | 2 (2) | — |
| Left ventricular function at 6 months | ||||||
| Recovered | 219 (46) | 98 (57) | 53 (37) | 48 (62) | 20 (25) | <0.001 |
| Persisting moderate left ventricular dysfunction | 147 (31) | 51 (29) | 52 (36) | 20 (26) | 24 (30) | 0.367 |
| Persisting severe left ventricular dysfunction | 107 (23) | 24 (14) | 38 (27) | 10 (13) | 35 (44) | <0.001 |
| Persisting severe left ventricular dysfunction or death | 142 (28) | 32 (18) | 46 (30) | 18 (21) | 46 (51) | <0.001 |
In-hospital and 6-month outcomes
| N (%) . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| In-hospital devices | ||||||
| Assist device | 17 (3) | 12 (7) | 0 (0) | 5 (6) | 0 (0) | <0.001 |
| Implantable cardioverter-defibrillator | 5 (0.9) | 4 (2) | 0 (0) | 0 (0) | 1 (0.7) | 0.101 |
| Cardiac resynchronization therapy | 2 (0.4) | 1 (0.6) | 0 (0) | 0 (0) | 1 (0.7) | 0.833 |
| Death | ||||||
| In-hospital | ||||||
| All-cause | 16 (2) | 7 (3) | 1 (0.5) | 1 (0.9) | 7 (4) | 0.046 |
| Heart failure | 9 (56) | 4 (57) | 0 (0) | 1 (100) | 4 (57) | 1.000 |
| Sudden | 4 (25) | 1 (14) | 1 (100) | 0 (0) | 2 (29) | 0.484 |
| Stroke | 3 (19) | 2 (29) | 0 (0) | 0 (0) | 1 (14) | 1.000 |
| 6 months | ||||||
| All-cause | 35 (6) | 8 (4) | 8 (5) | 8 (8) | 11 (10) | 0.082 |
| Heart failure | 14 (42) | 5 (63) | 0 (0) | 2 (33) | 7 (64) | 0.018 |
| Sudden | 10 (30) | 1 (13) | 2 (25) | 4 (67) | 3 (27) | 0.204 |
| Stroke | 5 (15) | 2 (25) | 2 (25) | 0 (0) | 1 (9) | 0.520 |
| Presumed cardiovascular | 4 (12) | 0 (0) | 4 (50) | 0 (0) | 0 (0) | 0.004 |
| Re-hospitalization at 6 months | ||||||
| All-cause | 58 (10) | 24 (12) | 18 (10) | 7 (7) | 9 (9) | 0.667 |
| Heart failure | 30 (53) | 9 (39) | 11 (61) | 3 (43) | 7 (78) | 0.220 |
| Other cardiac | 13 (23) | 10 (43) | 1 (6) | 1 (14) | 1 (11) | 0.025 |
| Vascular | 5 (9) | 1 (4) | 4 (22) | 0 (0) | 0 (0) | 0.191 |
| Non-cardiovascular | 11 (19) | 4 (17) | 3 (17) | 3 (43) | 1 (11) | 0.441 |
| Thromboembolism | ||||||
| In-hospital | ||||||
| All-cause | 40 (5) | 19 (8) | 7 (3) | 3 (3) | 11 (7) | 0.083 |
| Venous | 30 (4) | 15 (6) | 5 (2) | 3 (3) | 7 (4) | 0.169 |
| Arterial | 13 (2) | 6 (2) | 2 (0.9) | 0 (0) | 5 (3) | 0.170 |
| After discharge to 6 months | ||||||
| All-cause | 4 (0.7) | 0 (0) | 3 (2) | 0 (0) | 1 (1) | — |
| Venous | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | — |
| Arterial | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 44 (7) | 19 (9) | 10 (6) | 3 (3) | 12 (11) | 0.101 |
| Venous | 31 (5) | 15 (7) | 5 (3) | 3 (3) | 8 (7) | 0.127 |
| Arterial | 16 (3) | 6 (3) | 5 (3) | 0 (0) | 5 (5) | 0.216 |
| Stroke | ||||||
| In-hospital | ||||||
| All-cause | 12 (2) | 7 (3) | 1 (0.5) | 0 (0) | 4 (2) | — |
| Ischaemic | 8 (1) | 4 (2) | 1 (0.5) | 0 (0) | 3 (2) | — |
| Haemorrhagic | 5 (0.7) | 3 (1) | 0 (0) | 0 (0) | 2 (1) | — |
| After discharge to 6 months | ||||||
| All-cause | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Ischaemic | 2 (0.4) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | — |
| Haemorrhagic | 1 (0.2) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 15 (3) | 7 (3) | 4 (2) | 0 (0) | 4 (4) | — |
| Ischaemic | 10 (2) | 4 (2) | 3 (2) | 0 (0) | 3 (3) | — |
| Haemorrhagic | 6 (1) | 3 (1) | 1 (0.6) | 0 (0) | 2 (2) | — |
| Left ventricular function at 6 months | ||||||
| Recovered | 219 (46) | 98 (57) | 53 (37) | 48 (62) | 20 (25) | <0.001 |
| Persisting moderate left ventricular dysfunction | 147 (31) | 51 (29) | 52 (36) | 20 (26) | 24 (30) | 0.367 |
| Persisting severe left ventricular dysfunction | 107 (23) | 24 (14) | 38 (27) | 10 (13) | 35 (44) | <0.001 |
| Persisting severe left ventricular dysfunction or death | 142 (28) | 32 (18) | 46 (30) | 18 (21) | 46 (51) | <0.001 |
| N (%) . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| In-hospital devices | ||||||
| Assist device | 17 (3) | 12 (7) | 0 (0) | 5 (6) | 0 (0) | <0.001 |
| Implantable cardioverter-defibrillator | 5 (0.9) | 4 (2) | 0 (0) | 0 (0) | 1 (0.7) | 0.101 |
| Cardiac resynchronization therapy | 2 (0.4) | 1 (0.6) | 0 (0) | 0 (0) | 1 (0.7) | 0.833 |
| Death | ||||||
| In-hospital | ||||||
| All-cause | 16 (2) | 7 (3) | 1 (0.5) | 1 (0.9) | 7 (4) | 0.046 |
| Heart failure | 9 (56) | 4 (57) | 0 (0) | 1 (100) | 4 (57) | 1.000 |
| Sudden | 4 (25) | 1 (14) | 1 (100) | 0 (0) | 2 (29) | 0.484 |
| Stroke | 3 (19) | 2 (29) | 0 (0) | 0 (0) | 1 (14) | 1.000 |
| 6 months | ||||||
| All-cause | 35 (6) | 8 (4) | 8 (5) | 8 (8) | 11 (10) | 0.082 |
| Heart failure | 14 (42) | 5 (63) | 0 (0) | 2 (33) | 7 (64) | 0.018 |
| Sudden | 10 (30) | 1 (13) | 2 (25) | 4 (67) | 3 (27) | 0.204 |
| Stroke | 5 (15) | 2 (25) | 2 (25) | 0 (0) | 1 (9) | 0.520 |
| Presumed cardiovascular | 4 (12) | 0 (0) | 4 (50) | 0 (0) | 0 (0) | 0.004 |
| Re-hospitalization at 6 months | ||||||
| All-cause | 58 (10) | 24 (12) | 18 (10) | 7 (7) | 9 (9) | 0.667 |
| Heart failure | 30 (53) | 9 (39) | 11 (61) | 3 (43) | 7 (78) | 0.220 |
| Other cardiac | 13 (23) | 10 (43) | 1 (6) | 1 (14) | 1 (11) | 0.025 |
| Vascular | 5 (9) | 1 (4) | 4 (22) | 0 (0) | 0 (0) | 0.191 |
| Non-cardiovascular | 11 (19) | 4 (17) | 3 (17) | 3 (43) | 1 (11) | 0.441 |
| Thromboembolism | ||||||
| In-hospital | ||||||
| All-cause | 40 (5) | 19 (8) | 7 (3) | 3 (3) | 11 (7) | 0.083 |
| Venous | 30 (4) | 15 (6) | 5 (2) | 3 (3) | 7 (4) | 0.169 |
| Arterial | 13 (2) | 6 (2) | 2 (0.9) | 0 (0) | 5 (3) | 0.170 |
| After discharge to 6 months | ||||||
| All-cause | 4 (0.7) | 0 (0) | 3 (2) | 0 (0) | 1 (1) | — |
| Venous | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | — |
| Arterial | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 44 (7) | 19 (9) | 10 (6) | 3 (3) | 12 (11) | 0.101 |
| Venous | 31 (5) | 15 (7) | 5 (3) | 3 (3) | 8 (7) | 0.127 |
| Arterial | 16 (3) | 6 (3) | 5 (3) | 0 (0) | 5 (5) | 0.216 |
| Stroke | ||||||
| In-hospital | ||||||
| All-cause | 12 (2) | 7 (3) | 1 (0.5) | 0 (0) | 4 (2) | — |
| Ischaemic | 8 (1) | 4 (2) | 1 (0.5) | 0 (0) | 3 (2) | — |
| Haemorrhagic | 5 (0.7) | 3 (1) | 0 (0) | 0 (0) | 2 (1) | — |
| After discharge to 6 months | ||||||
| All-cause | 3 (0.6) | 0 (0) | 3 (2) | 0 (0) | 0 (0) | — |
| Ischaemic | 2 (0.4) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | — |
| Haemorrhagic | 1 (0.2) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | — |
| Cumulative 6-month | ||||||
| All-cause | 15 (3) | 7 (3) | 4 (2) | 0 (0) | 4 (4) | — |
| Ischaemic | 10 (2) | 4 (2) | 3 (2) | 0 (0) | 3 (3) | — |
| Haemorrhagic | 6 (1) | 3 (1) | 1 (0.6) | 0 (0) | 2 (2) | — |
| Left ventricular function at 6 months | ||||||
| Recovered | 219 (46) | 98 (57) | 53 (37) | 48 (62) | 20 (25) | <0.001 |
| Persisting moderate left ventricular dysfunction | 147 (31) | 51 (29) | 52 (36) | 20 (26) | 24 (30) | 0.367 |
| Persisting severe left ventricular dysfunction | 107 (23) | 24 (14) | 38 (27) | 10 (13) | 35 (44) | <0.001 |
| Persisting severe left ventricular dysfunction or death | 142 (28) | 32 (18) | 46 (30) | 18 (21) | 46 (51) | <0.001 |
Obstetric and neonatal outcomes
Termination of pregnancy took place in 0.5% overall (Table 3). More women diagnosed antepartum had a Caesarean section compared with those diagnosed postpartum (59 vs. 46%). Among those born, the frequency of low birth weight (<2500 g) was 28% (Table 3). Neonatal death occurred in 5%, with regional variation (Europe 2%, Africa 5%, Asia-Pacific 4%, the Middle East 9%, P = 0.012). Half of women in the registry breastfed, most commonly in Asia-Pacific (61%) and least commonly in Europe (34%) (P < 0.001).
Obstetric and neonatal outcomes
| . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| Obstetric | ||||||
| Pregnancy outcome, n (%) | ||||||
| Antepartum diagnosis | 0.083 | |||||
| Vaginal delivery | 29 (33) | 7 (28) | 12 (57) | 3 (30) | 7 (22) | |
| Caesarean section | 52 (59) | 18 (72) | 8 (38) | 7 (70) | 19 (59) | |
| Termination | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 3 (9) | |
| Miscarriage | 4 (5) | 0 (0) | 1 (5) | 0 (0) | 3 (9) | |
| Postpartum diagnosis | <0.001 | |||||
| Vaginal delivery | 318 (53) | 77 (39) | 136 (73) | 44 (45) | 61 (52) | |
| Caesarean section | 277 (46) | 119 (60) | 50 (27) | 53 (55) | 55 (47) | |
| Termination | 1 (0.2) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | |
| Miscarriage | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | |
| Peripartum haemorrhage, n (%) | 43 (6) | 11 (5) | 21 (10) | 7 (6) | 4 (2) | 0.016 |
| Twin pregnancy, n (%) | 21 (4) | 6 (3) | 11 (8) | 2 (2) | 2 (1) | 0.019 |
| Breastfeeding, n (%) | 365 (50) | 84 (34) | 123 (58) | 68 (61) | 90 (55) | <0.001 |
| Neonatal | ||||||
| Male sex, n (%) | 285 (51) | 81 (46) | 77 (53) | 55 (52) | 72 (54) | 0.455 |
| Length (cm) | 48 ± 5 | 50 ± 5 | 46 ± 5 | 47 ± 4 | 46 ± 8 | <0.001 |
| Weight (g) | 2861 ± 761 | 2928 ± 759 | 2947 ± 790 | 2778 ± 715 | 2713 ± 749 | 0.043 |
| Birth weight <2500 g, n (%) | 136 (28) | 39 (25) | 35 (26) | 31 (31) | 31 (33) | 0.451 |
| Head circumference (cm) | 32 ± 4 | 33 ± 3 | 32 ± 4 | 34 ± 2 | 30 ± 4 | <0.001 |
| APGAR score | ||||||
| 1 min | 8 ± 2 | 8 ± 2 | 8 ± 2 | 7 ± 2 | 7 ± 2 | <0.001 |
| 5 min | 9 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 2 | 9 ± 1 | 0.062 |
| Death, n (%) | 28 (5) | 3 (2) | 8 (5) | 4 (4) | 13 (9) | 0.012 |
| . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| Obstetric | ||||||
| Pregnancy outcome, n (%) | ||||||
| Antepartum diagnosis | 0.083 | |||||
| Vaginal delivery | 29 (33) | 7 (28) | 12 (57) | 3 (30) | 7 (22) | |
| Caesarean section | 52 (59) | 18 (72) | 8 (38) | 7 (70) | 19 (59) | |
| Termination | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 3 (9) | |
| Miscarriage | 4 (5) | 0 (0) | 1 (5) | 0 (0) | 3 (9) | |
| Postpartum diagnosis | <0.001 | |||||
| Vaginal delivery | 318 (53) | 77 (39) | 136 (73) | 44 (45) | 61 (52) | |
| Caesarean section | 277 (46) | 119 (60) | 50 (27) | 53 (55) | 55 (47) | |
| Termination | 1 (0.2) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | |
| Miscarriage | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | |
| Peripartum haemorrhage, n (%) | 43 (6) | 11 (5) | 21 (10) | 7 (6) | 4 (2) | 0.016 |
| Twin pregnancy, n (%) | 21 (4) | 6 (3) | 11 (8) | 2 (2) | 2 (1) | 0.019 |
| Breastfeeding, n (%) | 365 (50) | 84 (34) | 123 (58) | 68 (61) | 90 (55) | <0.001 |
| Neonatal | ||||||
| Male sex, n (%) | 285 (51) | 81 (46) | 77 (53) | 55 (52) | 72 (54) | 0.455 |
| Length (cm) | 48 ± 5 | 50 ± 5 | 46 ± 5 | 47 ± 4 | 46 ± 8 | <0.001 |
| Weight (g) | 2861 ± 761 | 2928 ± 759 | 2947 ± 790 | 2778 ± 715 | 2713 ± 749 | 0.043 |
| Birth weight <2500 g, n (%) | 136 (28) | 39 (25) | 35 (26) | 31 (31) | 31 (33) | 0.451 |
| Head circumference (cm) | 32 ± 4 | 33 ± 3 | 32 ± 4 | 34 ± 2 | 30 ± 4 | <0.001 |
| APGAR score | ||||||
| 1 min | 8 ± 2 | 8 ± 2 | 8 ± 2 | 7 ± 2 | 7 ± 2 | <0.001 |
| 5 min | 9 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 2 | 9 ± 1 | 0.062 |
| Death, n (%) | 28 (5) | 3 (2) | 8 (5) | 4 (4) | 13 (9) | 0.012 |
Obstetric and neonatal outcomes
| . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| Obstetric | ||||||
| Pregnancy outcome, n (%) | ||||||
| Antepartum diagnosis | 0.083 | |||||
| Vaginal delivery | 29 (33) | 7 (28) | 12 (57) | 3 (30) | 7 (22) | |
| Caesarean section | 52 (59) | 18 (72) | 8 (38) | 7 (70) | 19 (59) | |
| Termination | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 3 (9) | |
| Miscarriage | 4 (5) | 0 (0) | 1 (5) | 0 (0) | 3 (9) | |
| Postpartum diagnosis | <0.001 | |||||
| Vaginal delivery | 318 (53) | 77 (39) | 136 (73) | 44 (45) | 61 (52) | |
| Caesarean section | 277 (46) | 119 (60) | 50 (27) | 53 (55) | 55 (47) | |
| Termination | 1 (0.2) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | |
| Miscarriage | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | |
| Peripartum haemorrhage, n (%) | 43 (6) | 11 (5) | 21 (10) | 7 (6) | 4 (2) | 0.016 |
| Twin pregnancy, n (%) | 21 (4) | 6 (3) | 11 (8) | 2 (2) | 2 (1) | 0.019 |
| Breastfeeding, n (%) | 365 (50) | 84 (34) | 123 (58) | 68 (61) | 90 (55) | <0.001 |
| Neonatal | ||||||
| Male sex, n (%) | 285 (51) | 81 (46) | 77 (53) | 55 (52) | 72 (54) | 0.455 |
| Length (cm) | 48 ± 5 | 50 ± 5 | 46 ± 5 | 47 ± 4 | 46 ± 8 | <0.001 |
| Weight (g) | 2861 ± 761 | 2928 ± 759 | 2947 ± 790 | 2778 ± 715 | 2713 ± 749 | 0.043 |
| Birth weight <2500 g, n (%) | 136 (28) | 39 (25) | 35 (26) | 31 (31) | 31 (33) | 0.451 |
| Head circumference (cm) | 32 ± 4 | 33 ± 3 | 32 ± 4 | 34 ± 2 | 30 ± 4 | <0.001 |
| APGAR score | ||||||
| 1 min | 8 ± 2 | 8 ± 2 | 8 ± 2 | 7 ± 2 | 7 ± 2 | <0.001 |
| 5 min | 9 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 2 | 9 ± 1 | 0.062 |
| Death, n (%) | 28 (5) | 3 (2) | 8 (5) | 4 (4) | 13 (9) | 0.012 |
| . | All . | Europe . | Africa . | Asia-Pacific . | Middle East . | P-value . |
|---|---|---|---|---|---|---|
| Obstetric | ||||||
| Pregnancy outcome, n (%) | ||||||
| Antepartum diagnosis | 0.083 | |||||
| Vaginal delivery | 29 (33) | 7 (28) | 12 (57) | 3 (30) | 7 (22) | |
| Caesarean section | 52 (59) | 18 (72) | 8 (38) | 7 (70) | 19 (59) | |
| Termination | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 3 (9) | |
| Miscarriage | 4 (5) | 0 (0) | 1 (5) | 0 (0) | 3 (9) | |
| Postpartum diagnosis | <0.001 | |||||
| Vaginal delivery | 318 (53) | 77 (39) | 136 (73) | 44 (45) | 61 (52) | |
| Caesarean section | 277 (46) | 119 (60) | 50 (27) | 53 (55) | 55 (47) | |
| Termination | 1 (0.2) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | |
| Miscarriage | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | |
| Peripartum haemorrhage, n (%) | 43 (6) | 11 (5) | 21 (10) | 7 (6) | 4 (2) | 0.016 |
| Twin pregnancy, n (%) | 21 (4) | 6 (3) | 11 (8) | 2 (2) | 2 (1) | 0.019 |
| Breastfeeding, n (%) | 365 (50) | 84 (34) | 123 (58) | 68 (61) | 90 (55) | <0.001 |
| Neonatal | ||||||
| Male sex, n (%) | 285 (51) | 81 (46) | 77 (53) | 55 (52) | 72 (54) | 0.455 |
| Length (cm) | 48 ± 5 | 50 ± 5 | 46 ± 5 | 47 ± 4 | 46 ± 8 | <0.001 |
| Weight (g) | 2861 ± 761 | 2928 ± 759 | 2947 ± 790 | 2778 ± 715 | 2713 ± 749 | 0.043 |
| Birth weight <2500 g, n (%) | 136 (28) | 39 (25) | 35 (26) | 31 (31) | 31 (33) | 0.451 |
| Head circumference (cm) | 32 ± 4 | 33 ± 3 | 32 ± 4 | 34 ± 2 | 30 ± 4 | <0.001 |
| APGAR score | ||||||
| 1 min | 8 ± 2 | 8 ± 2 | 8 ± 2 | 7 ± 2 | 7 ± 2 | <0.001 |
| 5 min | 9 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 2 | 9 ± 1 | 0.062 |
| Death, n (%) | 28 (5) | 3 (2) | 8 (5) | 4 (4) | 13 (9) | 0.012 |
Discussion
Previous studies on PPCM are from individual countries such as South Africa,9 Haiti10 Germany,11 and the USA,12 but international data (especially from Europe and Asia) are lacking. In conjunction with these prior studies, our large multinational registry, including over 700 women with PPCM from 49 countries, shows that this condition affects women regardless of region or ethnicity. Moreover, while recovery of left ventricular function occurred in 46% of women by 6 months, 23% had persisting and severe left ventricular dysfunction, and 6% of women died in this period (Take home figure).
Most women in the registry had severe heart failure at the time of presentation, suggesting that the condition is not diagnosed during its initial phase, perhaps because symptoms and signs such as dyspnoea, fatigue, and oedema are attributed to pregnancy or the post-pregnancy state rather than heart failure, or because these characteristic findings can be absent. Of the patients with mild symptoms at diagnosis, a quarter had severe left ventricular dysfunction. Conventional signs are unreliable when attempting to diagnose PPCM (Take home figure); fewer than two-thirds of patients had peripheral oedema or pulmonary rales and fewer than half had a third heart sound. A high index of suspicion, and a low threshold for cardiac investigations, are required.2 Internationally, the median time from symptom onset to diagnosis was 10 days, but this ranged from 6 days in Europe to 23 days in Africa. These data highlight the delay in time from onset of symptoms to diagnosis. Prompt diagnosis should be a priority to allow timely initiation of heart failure therapies. The timing of onset of symptoms also varied by region; for example, 75% in Africa developed symptoms postpartum, compared with 56% in the Middle East. Cohort studies of consecutive peripartum patients would be valuable to determine the true incidence of PPCM and the severity of cardiac abnormalities in pregnancy.
In keeping with prior smaller reports,13 pre-eclampsia occurred in a quarter of patients, with marked regional differences; in Asia-Pacific, the frequency was more than double that of the Middle East and Africa, and nearly double that of Europe. The relationship between PPCM and pre-eclampsia is not well-understood, but shared pathophysiological mechanisms have been postulated.14 , 15
Death occurred in 6% of all patients within 6 months of diagnosis, which is substantially lower than many previous reports of PPCM,16 but 28 times higher than that of all maternal deaths worldwide (0.2% at 42 days).17 The lower frequency of death reported in our registry compared with other PPCM studies could reflect selection bias in the sites who participated in the registry (it is possible that they provided a higher quality of inpatient management and medical therapy post-discharge than in prior studies), but similar death rates have been reported in Germany4 and Japan18 at 6 months. Outcomes for women in Africa3 and the Middle East19 have historically been worse, but our findings show that survival for women in these regions is better than previously reported. It is possible that patients in some countries could have died prior to entering the registry, either due to sensitivities around enrolling very sick patients or patients dying before a diagnosis was made. Prescriptions of guideline-recommended therapies for heart failure in this global registry were similar to those in recent randomized clinical trials for all regions.20
Because an abnormal prolactin fragment (16 kDa prolactin) has been postulated to have a detrimental effect on the heart and vasculature, and play a role in the pathogenesis of PPCM, the use of bromocriptine, which suppresses the production of prolactin, has been advocated in patients with the condition.21 This treatment was used in 15% of patients in our study. Based on current evidence, the use of bromocriptine in patients with PPCM has recently been given a Class II Level B recommendation in the 2018 European Guidelines on Cardiovascular Disease in Pregnancy.22 A large, definitive randomized, placebo-controlled multicentre international trial of bromocriptine in PPCM is yet to be performed.
Cause of death at 6 months was sudden in 30%, suggesting that defibrillators may have a role, in particular subcutaneous defibrillators or wearable cardioverter-defibrillators given the possibility of myocardial recovery. However, only 1% of the patients in this registry received an implantable cardioverter defibrillator by the time of hospital discharge. No prior studies have reported how often patients are hospitalized after a diagnosis of PPCM. In this registry, 10% of patients had a re-hospitalization within 6 months of diagnosis, approximately half of which were due to heart failure. Although re-hospitalizations in women with PPCM occur less frequently than in those with heart failure of other aetiologies,23 efforts should focus on reducing these events including by ensuring optimal guideline-directed therapy for heart failure and by identifying and treating intra-cardiac, venous, or arterial thrombi.
Deep venous thromboses, pulmonary emboli, arterial emboli, and ischaemic strokes were more common than previously appreciated (7% at 6 months). Whether or not anticoagulation should be prescribed in all women, or only those with visible cardiac thrombi, atrial fibrillation, or very low ejection fraction is uncertain. Visible thrombi were evident in approximately 20% of women in two African studies.24 , 25 This is likely due to the pro-coagulant states of pregnancy and heart failure.26 Fewer than a sixth of women in the registry were anticoagulated in the 6 months following discharge. The regional differences in rates of thromboembolic events are striking, with a frequency of 9% in Europe compared with 3% in Asia-Pacific. The higher rates of myocardial recovery seen in Asia-Pacific are likely to, at least partially, explain lower rates of thromboembolism. We believe that anticoagulation should not solely be limited to those with atrial fibrillation and/or visible thrombus, but only randomized clinical trials will definitively identify which patients with PPCM should be anticoagulated. In the absence of data from a randomized clinical trial, and in light of our findings of high rates of thromboembolic events, our recommendation is that the use of therapeutic anticoagulation in women with PPCM during pregnancy and in the postpartum period may be considered. It has been postulated that bromocriptine leads to increased thromboembolic events, but the findings from this registry do not support this. Both warfarin and heparin are considered relatively safe during breast-feeding, but warfarin should be avoided where possible during pregnancy.2
Myocardial recovery is common in PPCM, but reported frequencies vary markedly. Myocardial recovery at 6 months has occurred less frequently in Africa3 , 27 than in other countries such as the USA5 and Germany.4 In this global registry, 46% recovered and 23% had persisting and severely impaired cardiac function. Globally, myocardial recovery appears to be less common than in predominantly Caucasian populations, both in this registry and in North America.12 We found regional differences in myocardial recovery, with a frequency in Asia-Pacific of more than double that of the Middle East. These regional variations in recovery do not appear to reflect differences in the use of heart failure treatments, so may reflect differences in underlying mechanisms, which remain poorly understood.
A striking regional difference was that of neonatal death, which was approximately 4.5 times more frequent in the Middle East than in Europe. In every region, neonatal death far exceeded population-level rates estimates by UNICEF (Western Europe 0.2%, Sub-Saharan Africa 2.8%, South Asia 2.7%, East Asia and Pacific 0.8%, the Middle East and North Africa 1.2%).28 Prior publications have focused on the maternal perspective of PPCM with little attention paid to neonatal outcomes. The higher neonatal death rates do not appear to reflect major regional differences in cardiac function or maternal management. Whether or not there is a need for improved care of neonates should be explored in future studies; these should aim to determine how and why these babies die and identify ways in which to reduce neonatal morbidity and mortality.
Limitations
As with most registries conducted at a global level, there are several limitations. Only core information was validated by monitors. Follow-up was not as complete as would be seen in an industry-funded clinical trial (participation into the registry was voluntarily and unpaid). Many centres lacked extensive resources or did not have universal health care. In certain regions, patients would not be able to afford the time or the expense of travelling long distances to return for review. Data on cardiac transplantation were not captured and data on the use of implantable cardioverter defibrillators and cardiac resynchronization therapy were only captured at hospital discharge. A degree of selection bias for centres interested in this condition is likely and could explain better outcomes than were previously reported.
Conclusion
PPCM is a global disease occurring across continents in patients of all ethnicities. Diverse regional differences in clinical characteristics and outcomes are seen. While myocardial recovery occurs in just under half of patients, thromboembolic events and maternal and neonatal death remain common in these previously healthy young women.
Acknowledgements
EORP Oversight Committee, The Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection were conducted by the EORP department from the ESC by Rachid Mir Hassaine and Souad Mekhaldi as Clinical Project Managers, Emanuela Fiorucci as Project Officer, Marina Andarala as Data Manager. Statistical analyses were performed by Cécile Laroche. Overall activities were coordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator). All investigators are listed in Supplementary material online, Appendix 1. We also acknowledge the support of Mrs Olivia Briton, who spent days and weeks retrieving data from sites, and Sylvia Dennis, from the Hatter Institute for Cardiovascular Research, who helped prepare the manuscript. We also acknowledge all investigators who entered patients into the registry (Supplementary material online, Appendix).
Funding
Since the start of EORP, the following companies have supported the whole research programme: Abbott Vascular Int. (2011–21), Amgen Cardiovascular (2009–18), AstraZeneca (2014–21), Bayer AG (2009–18), Boehringer Ingelheim (2009–19), Boston Scientific (2009–12), The Bristol Myers Squibb and Pfizer Alliance (2011–19), Daiichi Sankyo Europe GmbH (2011–20), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–17), Edwards (2016–19), Gedeon Richter Plc. (2014–16), Menarini Int. Op. (2009–12), MSD-Merck & Co. (2011–14), Novartis Pharma AG (2014–20), ResMed (2014–16), Sanofi (2009–11), SERVIER (2009–21), and Vifor (2019–22). P.v.d.M. is supported by the European Research Council (grant: ERC-2016-StG-715732). M.C.P. is by supported by a British Heart Foundation Centre of Excellence Research Grant (grant number 18/6/34217).
Conflict of interest: none declared.
References
World map region definitions. World Health Organisation member regions.
The World Bank. Maternal mortality ratio (modeled estimate, per 100,000 live births). https://data.worldbank.org/indicator/sh.sta.mmrt (1 October 2019).
Sharieff S, Zaman KS. Prognostic factors at initial presentation in patients with peripartum cardiomyopathy. J Pak Med Assoc 2003;
UNICEF data: monitoring the situation of children and women. Neonatal mortality. https://data.unicef.org/topic/child-survival/neonatal-mortality/ (1 January 2020).
Author notes
Mark C. Petrie shared first authorship.
- myocardium
- left ventricular ejection fraction
- peripartum cardiomyopathy
- heart failure
- bromocriptine
- africa
- asia
- follow-up
- newborn
- middle east
- mothers
- diagnosis
- heart
- morbidity
- mortality
- thromboembolic event
- neonatal death
- new york heart association classification
- geographic difference
- symptom onset
- european society of cardiology