-
PDF
- Split View
-
Views
-
Cite
Cite
Alessio Gasperetti, Mauro Biffi, Firat Duru, Marco Schiavone, Matteo Ziacchi, Gianfranco Mitacchione, Carlo Lavalle, Ardan Saguner, Antonio Lanfranchi, Giacomo Casalini, Marco Tocci, Davide Fabbricatore, Francesca Salghetti, Marco Valerio Mariani, Mattia Busana, Alfonso Bellia, Chiara Beatrice Cogliati, Pierluigi Viale, Spinello Antinori, Massimo Galli, Nazzareno Galiè, Claudio Tondo, Giovanni Battista Forleo, Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings, EP Europace, Volume 22, Issue 12, December 2020, Pages 1855–1863, https://doi.org/10.1093/europace/euaa216
Close - Share Icon Share
Abstract
The aim of the study was to describe ECG modifications and arrhythmic events in COVID-19 patients undergoing hydroxychloroquine (HCQ) therapy in different clinical settings.
COVID-19 patients at seven institutions receiving HCQ therapy from whom a baseline and at least one ECG at 48+ h were available were enrolled in the study. QT/QTc prolongation, QT-associated and QT-independent arrhythmic events, arrhythmic mortality, and overall mortality during HCQ therapy were assessed. A total of 649 COVID-19 patients (61.9 ± 18.7 years, 46.1% males) were enrolled. HCQ therapy was administrated as a home therapy regimen in 126 (19.4%) patients, and as an in-hospital-treatment to 495 (76.3%) hospitalized and 28 (4.3%) intensive care unit (ICU) patients. At 36–72 and at 96+ h after the first HCQ dose, 358 and 404 ECGs were obtained, respectively. A significant QT/QTc interval prolongation was observed (P < 0.001), but the magnitude of the increase was modest [+13 (9–16) ms]. Baseline QT/QTc length and presence of fever (P = 0.001) at admission represented the most important determinants of QT/QTc prolongation. No arrhythmic-related deaths were reported. The overall major ventricular arrhythmia rate was low (1.1%), with all events found not to be related to QT or HCQ therapy at a centralized event evaluation. No differences in QT/QTc prolongation and QT-related arrhythmias were observed across different clinical settings, with non-QT-related arrhythmias being more common in the intensive care setting.
HCQ administration is safe for a short-term treatment for patients with COVID-19 infection regardless of the clinical setting of delivery, causing only modest QTc prolongation and no directly attributable arrhythmic deaths.
Arrhythmic safety data from a large cohort of patients with COVID-19 infection treated with hydroxychloroquine (HCQ) alone or in combination with other QT-prolonging drugs were reported.
The use of HCQ was associated with a significant QT and QTc interval prolongation, but the magnitude of the increase was modest [median +13 (9–16) ms]
Over a median follow-up of 16 days, no arrhythmia-related deaths were reported. The overall major ventricular arrhythmia rate was low, with all events being reported in critical patients, and these were found not to be QT or HCQ therapy related.
Baseline QT/QTc length and presence of fever at admission represent the most important determinants for QT/QTc prolongation.
Introduction
In late December 2019, an outbreak of an emerging disease (COVID-19) due to a novel coronavirus (named SARS-CoV-2 later) started in Wuhan. It quickly spread all around the world and was declared a pandemic by the Word Health Organization (WHO) on 12 March 2020.1
In the quest for an effective therapy, antimalarial drugs have been suggested to be effective in treating COVID-19.2 Given its better arrhythmic safety profile compared with chloroquine3 and a recent proof of concept study from Gautret et al.,4 hydroxychloroquine (HCQ) has been proposed as a potential treatment for COVID-19 patients, both as a stand-alone treatment and combined with azithromycin (AM). Large-scale data regarding the effectiveness of HCQ from randomized control trials are currently lacking, with recent observational data showing either no improvement or no increases in adverse patient outcomes.5
QT prolongation is associated with an increase in both arrhythmic and non-arrhythmic mortality, and it is often used as a metric of drug safety.6 One of the main concerns regarding the use of HCQ is its potential impact on the QT interval, particularly when prescribed in association with other QT-prolonging drugs such as AM or the combination of lopinavir/ritonavir (LPV/RTV).7
HCQ has been extensively used in the treatment of malaria, lupus, and rheumatoid arthritis;8,9 however, this is the first time in history that a similar widespread use of HCQ has been advocated. The safety of its large-scale use in acutely ill patients with multiple comorbidities, possibly receiving other QT-prolonging drugs and potentially at risk of electrolyte disbalance, still needs to be assessed.
The aim of this study is to report arrhythmic safety HCQ data from a multicentre cohort of real-world COVID-19 patients undergoing treatment with HCQ.
Methods
Cohort definition
The study was designed as a multicentre cohort study. All consecutive confirmed cases of COVID-19 undergoing HCQ treatment at seven Italian and international institutions from 10 March to 10 April were screened. A confirmed case of COVID-19 was defined by a positive result on a reverse transcription–polymerase chain reaction (RT–PCR) assay of a specimen retrieved from a nasopharyngeal swab as assessed by the local diagnostic lab of each institution.
Patients for which: (i) a pre-HCQ therapy 12-lead ECG; and (ii) either an ‘early’ or ‘late’ ECG control, as per definition below, were available were deemed eligible for enrolment.
ECG tracings were defined according to the time of retrieval as follows:
T0—baseline ECG: recorded within 5 days before the first dose of HCQ;
T1—first (‘early’) ECG control: recorded at least 36–72 h after the first HCQ dose;
T2—second (‘late’) ECG control: recorded at least 96 h after the first HCQ dose.
Patients were prospectively enrolled and data were retrospectively analysed. This study was approved by the local ethical boards and was conducted in accordance with the Declaration of Helsinki; informed consent was retrieved or a waiver was obtained at centre level, in accordance with single-centre and national regulations regarding experimental treatment protocols.
Clinical settings
Patients were enrolled from three different clinical settings, defined as follows.
Home management (HM): the patient’s first assessment was performed at the treating hospital, then the patient was discharged home on HCQ, with a scheduled follow-up appointment for active surveillance. Only patients with mild respiratory symptoms, a PaO2/FiO2 ratio >300 mmHg, and a low or moderate Tisdale score (TS; see below) were deemed eligible for home management.
Medical ward (MW) management: the patient was hospitalized into a mid-intensity medical ward.
Intensive care unit (ICU) management: the patient was hospitalized into an ICU facility.
For MW and ICU patients, ECGs were obtained in hospital. A team consisting of Cardiologists and Infective Diseases specialists with dedicated nurses (both hospital nurses and territorial care nurses) took care of patients allocated to HM. In the case of patients incapable of leaving home (e.g. feverish or markedly weakened), early and late ECG controls were recorded at home and transmitted using the nurse’s smartphone as the internet access point (Supplementary material online, Figure S1). The ECG was instead recorded in a dedicated area of a COVID-19 outpatient clinic for patients fit to be independently managed. All patients in the HM group received education to report to the managing physicians symptoms potentially suggestive of Torsades de Pointes (TdP) or of situations possibly leading to hypokalaemia (diarrhoea, vomiting, insufficient intake). In the case of hypokalaemia or of situations possibly leading to hypokalaemia, potassium supplementation was suggested: in the HM group, an oral supplementation was set in place, while in the MW and ICU groups either oral or i.v. supplementation, in accordance with clinical judgement, was performed. The Cardiology team was responsible for night-time consultation. Advice was also given to seek consultation with the general practitioner or the hospital team before the intake of any new drug, to exclude potential interactions.
Data collection
For every enrolled patient, baseline demographics, cardiovascular comorbidities, clinical COVID-19 presentation, baseline blood tests, pre-HCQ therapy baseline 12-lead ECG, pharmacological history, 12-lead ECG on HCQ therapy, and arrhythmic events were collected into a centralized, de-identified database. QT values were extracted from all ECGs as non-corrected, with QT intervals being determined using the tangent method. QT corrections were performed using the Bazett’s formula and the linear functions according to Fridericia and Framingham methods.
As suggested by a recent consensus on the use of drugs prolonging the QT interval in COVID-19 patients, a TS value, assessing the risk of QT prolongation, was determined for every patient at the beginning of HCQ therapy.10,11 Additionally, the TS was also used at patient triage to determine the most appropriate setting in which to deliver HCQ therapy, patients with a high TS not being eligible for HM allocation. A QTc prolongation >60 ms was considered abnormal and called for re-assessment of the patient’s medical status and of drug administration/interactions. HCQ suspension was mandatory for a QTc >550 ms, but suspension for shorter values based on clinical judgement was also performed.
Outcomes
PQ, QRS, QT, and QTc durations before and after HCQ administration were collected. Data regarding acute sustained ventricular arrhythmic events [namely sustained ventricular tachycardias (VTs), ventricular fibrillation (VF), and TdP] were collected from available healthcare documentation. Overall cardiovascular, and arrhythmic mortality rates were also assessed. Arrhythmic safety was assessed with regard to malignant QT-prolonging arrhythmias. Data from all patients reported to have died underwent a centralized collegial allocation assessment to determine the cause of death. A committee of three members blinded to the treatment at the time of occurrence of the arrhythmic event adjudicated the possible relationship with HCQ administration based on all available medical information.
Statistical analysis
Numerical values were reported as mean ± standard deviation (SD) or as median and interquartile range (IQR) for normally and non-normally distributed variables, respectively. Categorical variables were reported as counts (percentage). Comparisons among different risk groups were performed using a one-way analysis of variance (ANOVA) or its non-parametrical equivalent; post-hoc analyses were performed with a Tukey correction. Contingency tables were used to compare categorical variables among groups. Univariate or multivariate linear regression were used to assess correlation between variables. A two-tailed P-value of significance was set at 0.05. All statistical analysis were performed using Python 3.7 and the related packages (pandas, numpy, matplotlib, seaborn, and pingouing).
Results
Baseline characteristics
A total of 649 consecutive COVID-19 patients treated with HCQ meeting the inclusion criteria were prospectively enrolled as the study cohort. The mean age was 61.9 ± 18.7 years, and 299 (46.1%) were males. The most common presentation symptoms were fever, dry cough, and dyspnoea, that were present in 590 (90.9%), 529 (81.5%), and 449 (69.2%) of the enrolled patients, respectively. Patients accessed the treating institution after a median of 2 (1–7) days from the appearance of the index symptoms. A comprehensive list of patients’ baseline characteristics, arterial blood gas analysis, and routine blood tests is presented in Table 1.
Overall population data (n = 649)
| Age (years), mean ± SD | 61.9 ± 18.7 |
| Male, n (%) | 299 (46.1) |
| BMI, mean ± SD | 25.3 ± 3.7 |
| Hypertension, n (%) | 188 (29.1) |
| Diabetes, n (%) | 73 (11.3) |
| Coronary artery disease, n (%) | 56 (8.3) |
|
|
| Time since symptoms onset (days), median (IQR) | 2 (1–7) |
|
|
| SaO2 (%), median (IQR) | 97 (94–98) |
|
|
| Time between symptoms and HCQ beginning (days), median (IQR) | 2 (0–7) |
|
|
| Loading dose at HCQ therapy start, n (%) | 380 (58.6) |
|
|
|
|
|
|
|
|
| Age (years), mean ± SD | 61.9 ± 18.7 |
| Male, n (%) | 299 (46.1) |
| BMI, mean ± SD | 25.3 ± 3.7 |
| Hypertension, n (%) | 188 (29.1) |
| Diabetes, n (%) | 73 (11.3) |
| Coronary artery disease, n (%) | 56 (8.3) |
|
|
| Time since symptoms onset (days), median (IQR) | 2 (1–7) |
|
|
| SaO2 (%), median (IQR) | 97 (94–98) |
|
|
| Time between symptoms and HCQ beginning (days), median (IQR) | 2 (0–7) |
|
|
| Loading dose at HCQ therapy start, n (%) | 380 (58.6) |
|
|
|
|
|
|
|
|
Baseline and pharmacological characteristics of the enrolled population.
CRP, C-reactive protein; FiO2, fraction of inspired oxygen; Hb, haemoglobin; HCQ, hydroxychloroquine; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; SaO2, saturation of O2.
Overall population data (n = 649)
| Age (years), mean ± SD | 61.9 ± 18.7 |
| Male, n (%) | 299 (46.1) |
| BMI, mean ± SD | 25.3 ± 3.7 |
| Hypertension, n (%) | 188 (29.1) |
| Diabetes, n (%) | 73 (11.3) |
| Coronary artery disease, n (%) | 56 (8.3) |
|
|
| Time since symptoms onset (days), median (IQR) | 2 (1–7) |
|
|
| SaO2 (%), median (IQR) | 97 (94–98) |
|
|
| Time between symptoms and HCQ beginning (days), median (IQR) | 2 (0–7) |
|
|
| Loading dose at HCQ therapy start, n (%) | 380 (58.6) |
|
|
|
|
|
|
|
|
| Age (years), mean ± SD | 61.9 ± 18.7 |
| Male, n (%) | 299 (46.1) |
| BMI, mean ± SD | 25.3 ± 3.7 |
| Hypertension, n (%) | 188 (29.1) |
| Diabetes, n (%) | 73 (11.3) |
| Coronary artery disease, n (%) | 56 (8.3) |
|
|
| Time since symptoms onset (days), median (IQR) | 2 (1–7) |
|
|
| SaO2 (%), median (IQR) | 97 (94–98) |
|
|
| Time between symptoms and HCQ beginning (days), median (IQR) | 2 (0–7) |
|
|
| Loading dose at HCQ therapy start, n (%) | 380 (58.6) |
|
|
|
|
|
|
|
|
Baseline and pharmacological characteristics of the enrolled population.
CRP, C-reactive protein; FiO2, fraction of inspired oxygen; Hb, haemoglobin; HCQ, hydroxychloroquine; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; SaO2, saturation of O2.
Management of HCQ therapy
HCQ was administered early after symptom onset [days from symptoms onset to first dose: 2 (0–7)] in three different care settings: 126 (19.4%) patients received HCQ in a HM setting, 495 (76.3%) patients were hospitalized into a MW and received HCQ during hospital stay, while 28 (4.3%) patients received HCQ in an ICU setting.
HCQ therapeutic protocols varied slightly across centres and are reported in Table 1. Among other co-administered, potentially QT-prolonging drugs, AM and the combination of LPV/RTV were given alongside HCQ in 130 (20.0%) and 125 (19.3%) patients, respectively, with 42 (6.5%) patients receiving HCQ, AM, and LPN/RTV. All data regarding pharmacological therapy are summarized in Table 1.
Baseline Tisdale score, ECG modifications, and QT modification
A TS assessment was performed in the entire cohort at baseline, showing a predominance of patients at low (55.5%) and moderate (38.5%) risk. Patients selected for home treatment had only mild respiratory symptoms and frequently (88.1%) a low arrhythmic risk, with no patients presenting a high arrhythmic risk; the majority of moderate risk patient and the totality of high risk patients receiving HCQ were instead managed as in-patients. The risk stratification for the entire cohort is reported in Table 2.
QT risk score characteristics, as assessed by the Tisdale risk score from Tisdale et al.11
| . | Overall (n = 649) . | HM (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Tisdale score (points), median (IQR) | 6 (4–8) | 4 (4–5) | 7 (5–8) | 8 (6–9) |
|
|
|
|
|
|
|
|
|
|
| . | Overall (n = 649) . | HM (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Tisdale score (points), median (IQR) | 6 (4–8) | 4 (4–5) | 7 (5–8) | 8 (6–9) |
|
|
|
|
|
|
|
|
|
|
HM, home management patient; ICU, intensive care unit patient; MI, myocardial infarction; MW, medical ward patient.
QT risk score characteristics, as assessed by the Tisdale risk score from Tisdale et al.11
| . | Overall (n = 649) . | HM (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Tisdale score (points), median (IQR) | 6 (4–8) | 4 (4–5) | 7 (5–8) | 8 (6–9) |
|
|
|
|
|
|
|
|
|
|
| . | Overall (n = 649) . | HM (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Tisdale score (points), median (IQR) | 6 (4–8) | 4 (4–5) | 7 (5–8) | 8 (6–9) |
|
|
|
|
|
|
|
|
|
|
HM, home management patient; ICU, intensive care unit patient; MI, myocardial infarction; MW, medical ward patient.
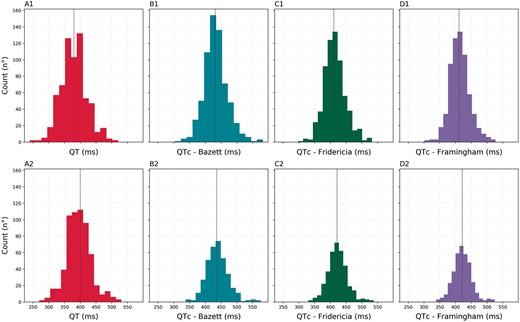
Serial ECG recordings showed a significant reduction in heart rate (HR) between T0 and T1 [mean difference HR01: –3.3 (–4.9 to –1.70) b.p.m; P < 0.001] and between T0 and T2 [mean difference HR02: –5.0 (–6.8 to –3.2) b.p.m.; P < 0.001] in the overall cohort, while no differences in atrial rhythm, PR interval, and QRS width were observed (Table 3). A significant QT/QTc interval prolongation was observed at different time points, regardless of the rate correction formula used (Table 3). QT/QTc values before HCQ therapy onset and at last available ECG follow-up of the entire cohort are reported in Figure 1. The QT/QTc modification across different clinical setting groups did not differ, as reported in Supplementary material online, Table S1 and Figure S2.
QT/QTc distribution at T0 (upper panels) and at the last available ECG (lower panels) on hydroxychloroquine for the cohort. (A1/A2) QT interval (ms); (B1/B2) QTc Bazett (ms); (C1/C2) QTc Fridericia (ms); (D1/D2) QTc Framingham (ms).
Overall ECG data analysis
| . | . | . | . | Per group comparisons . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline (n = 649) . | ECG at T1 (n = 358) . | ECG at T2 (n = 404) . | T0–T1P . | Mean Δ . | T0–T2P . | Mean Δ . | T1–T2P . | Mean Δ . | |
| Heart rate (b.p.m.), median (IQR) | 78(68–89) | 75(65–82) | 74(65-82) | <0.001 | –3.3(–4.9 to –1.7) | <0.001 | –5.0(–6.8 to –3.2) | 0.287 | ||
| Rhythm Sinus, n (%) Atrial fibrillation/flutter, n (%) | 610 (94.0) 39 (6.0) | 337 (94.1) 21 (5.9) | 372 (92.1) 32 (7.9) | 0.401 | ||||||
| PQ interval (ms), median (IQR) | 160(140–176) | 160(140–180) | 160(150–180) | 0.287 | ||||||
| QRS width (ms), median (IQR) | 90(81–100) | 90(82–100) | 90(84–100) | 0.259 | ||||||
| QT interval (ms), median (IQR) | 380(356–400) | 395(370–415) | 400(370–420) | <0.001 | +13(+10 to +17) | <0.001 | +20(+16 to +23) | <0.001 | +9(+4 to +13) | |
| QTc Bazett (ms), median (IQR) | 431(410–452) | 436(416–459) | 438(417–462) | 0.478 | <0.001 | +9(+4 to +12) | <0.001 | +8(+3 to +12) | ||
| QTc Fridericia (ms), median (IQR) | 411(392–432) | 421(404–440) | 423(406–442) | 0.004 | +8(+5 to +12) | <0.001 | +13(+9 to +16) | <0.001 | +8(+4 to +12) | |
| QTc Framingham (ms), median (IQR) | 411 (393–431) | 421(405–440) | 422(407–441) | 0.003 | +8(+5 to +11) | < 0.001 | +12(+9 to +15) | < 0.001 | +7(+3 to+10) | |
| . | . | . | . | Per group comparisons . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline (n = 649) . | ECG at T1 (n = 358) . | ECG at T2 (n = 404) . | T0–T1P . | Mean Δ . | T0–T2P . | Mean Δ . | T1–T2P . | Mean Δ . | |
| Heart rate (b.p.m.), median (IQR) | 78(68–89) | 75(65–82) | 74(65-82) | <0.001 | –3.3(–4.9 to –1.7) | <0.001 | –5.0(–6.8 to –3.2) | 0.287 | ||
| Rhythm Sinus, n (%) Atrial fibrillation/flutter, n (%) | 610 (94.0) 39 (6.0) | 337 (94.1) 21 (5.9) | 372 (92.1) 32 (7.9) | 0.401 | ||||||
| PQ interval (ms), median (IQR) | 160(140–176) | 160(140–180) | 160(150–180) | 0.287 | ||||||
| QRS width (ms), median (IQR) | 90(81–100) | 90(82–100) | 90(84–100) | 0.259 | ||||||
| QT interval (ms), median (IQR) | 380(356–400) | 395(370–415) | 400(370–420) | <0.001 | +13(+10 to +17) | <0.001 | +20(+16 to +23) | <0.001 | +9(+4 to +13) | |
| QTc Bazett (ms), median (IQR) | 431(410–452) | 436(416–459) | 438(417–462) | 0.478 | <0.001 | +9(+4 to +12) | <0.001 | +8(+3 to +12) | ||
| QTc Fridericia (ms), median (IQR) | 411(392–432) | 421(404–440) | 423(406–442) | 0.004 | +8(+5 to +12) | <0.001 | +13(+9 to +16) | <0.001 | +8(+4 to +12) | |
| QTc Framingham (ms), median (IQR) | 411 (393–431) | 421(405–440) | 422(407–441) | 0.003 | +8(+5 to +11) | < 0.001 | +12(+9 to +15) | < 0.001 | +7(+3 to+10) | |
Δ, difference.
P-values of significance are reported for direct comparisons among groups only in the case of an overall all-comparison significant P. The reported level of significance is assessed after adequate post-hoc comparison corrections.
Overall ECG data analysis
| . | . | . | . | Per group comparisons . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline (n = 649) . | ECG at T1 (n = 358) . | ECG at T2 (n = 404) . | T0–T1P . | Mean Δ . | T0–T2P . | Mean Δ . | T1–T2P . | Mean Δ . | |
| Heart rate (b.p.m.), median (IQR) | 78(68–89) | 75(65–82) | 74(65-82) | <0.001 | –3.3(–4.9 to –1.7) | <0.001 | –5.0(–6.8 to –3.2) | 0.287 | ||
| Rhythm Sinus, n (%) Atrial fibrillation/flutter, n (%) | 610 (94.0) 39 (6.0) | 337 (94.1) 21 (5.9) | 372 (92.1) 32 (7.9) | 0.401 | ||||||
| PQ interval (ms), median (IQR) | 160(140–176) | 160(140–180) | 160(150–180) | 0.287 | ||||||
| QRS width (ms), median (IQR) | 90(81–100) | 90(82–100) | 90(84–100) | 0.259 | ||||||
| QT interval (ms), median (IQR) | 380(356–400) | 395(370–415) | 400(370–420) | <0.001 | +13(+10 to +17) | <0.001 | +20(+16 to +23) | <0.001 | +9(+4 to +13) | |
| QTc Bazett (ms), median (IQR) | 431(410–452) | 436(416–459) | 438(417–462) | 0.478 | <0.001 | +9(+4 to +12) | <0.001 | +8(+3 to +12) | ||
| QTc Fridericia (ms), median (IQR) | 411(392–432) | 421(404–440) | 423(406–442) | 0.004 | +8(+5 to +12) | <0.001 | +13(+9 to +16) | <0.001 | +8(+4 to +12) | |
| QTc Framingham (ms), median (IQR) | 411 (393–431) | 421(405–440) | 422(407–441) | 0.003 | +8(+5 to +11) | < 0.001 | +12(+9 to +15) | < 0.001 | +7(+3 to+10) | |
| . | . | . | . | Per group comparisons . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline (n = 649) . | ECG at T1 (n = 358) . | ECG at T2 (n = 404) . | T0–T1P . | Mean Δ . | T0–T2P . | Mean Δ . | T1–T2P . | Mean Δ . | |
| Heart rate (b.p.m.), median (IQR) | 78(68–89) | 75(65–82) | 74(65-82) | <0.001 | –3.3(–4.9 to –1.7) | <0.001 | –5.0(–6.8 to –3.2) | 0.287 | ||
| Rhythm Sinus, n (%) Atrial fibrillation/flutter, n (%) | 610 (94.0) 39 (6.0) | 337 (94.1) 21 (5.9) | 372 (92.1) 32 (7.9) | 0.401 | ||||||
| PQ interval (ms), median (IQR) | 160(140–176) | 160(140–180) | 160(150–180) | 0.287 | ||||||
| QRS width (ms), median (IQR) | 90(81–100) | 90(82–100) | 90(84–100) | 0.259 | ||||||
| QT interval (ms), median (IQR) | 380(356–400) | 395(370–415) | 400(370–420) | <0.001 | +13(+10 to +17) | <0.001 | +20(+16 to +23) | <0.001 | +9(+4 to +13) | |
| QTc Bazett (ms), median (IQR) | 431(410–452) | 436(416–459) | 438(417–462) | 0.478 | <0.001 | +9(+4 to +12) | <0.001 | +8(+3 to +12) | ||
| QTc Fridericia (ms), median (IQR) | 411(392–432) | 421(404–440) | 423(406–442) | 0.004 | +8(+5 to +12) | <0.001 | +13(+9 to +16) | <0.001 | +8(+4 to +12) | |
| QTc Framingham (ms), median (IQR) | 411 (393–431) | 421(405–440) | 422(407–441) | 0.003 | +8(+5 to +11) | < 0.001 | +12(+9 to +15) | < 0.001 | +7(+3 to+10) | |
Δ, difference.
P-values of significance are reported for direct comparisons among groups only in the case of an overall all-comparison significant P. The reported level of significance is assessed after adequate post-hoc comparison corrections.
At univariate analysis, QT/QTc changes from baseline were found to be significantly associated with HR changes, presence of fever at admission, and baseline QT/QTc value at first ECG (Supplementary material online, Table S2). At multivariate analysis, QT/QTc changes were associated with baseline QT/QTc values at T0 [coefficient –0.29 (–0.34 to –0.23), P < 0.001] and HR changes [coefficient –1.14 (–1.27 to –1.0), P < 0.001]. The entire multivariate analysis is reported in Supplementary material online, Table S3.
A subanalysis assessing the impact of fever reduction on QT/QTc modification has been performed for 144 (22.2%) patients, for which the body temperature both at baseline ECG and at the 96 h ECG were available. The impact of the variation of body temperature on QT/QTc was not significant. The impact of body temperature variation on HR variation [coefficient +4.6 (+1.8 to +7.4); P < 0.001] is reported in Supplementary material online, Figure S3.
Arrhythmic outcomes
Over a median time of 16 (9–20) days, no TdP events were reported. Five (0.7%) patients (n = 3 in the ICU; n = 2 in the MW setting) suspended HCQ as a medical decision based on a QTc prolongation between 500 and 540 ms, although no arrhythmic events were observed in these patients.
The overall major ventricular arrhythmia rate was very low (Table 4), with 7 (1.1%) acutely ill patients presenting a major ventricular event (n = 3 VF; n = 4 sustained monomorphic VT), 5 of which occurred in ICU patients. All episodes were reported in elderly patients [median age 71 (67–84)], with multiple comorbidities (n = 6 ischaemic cardiomyopathy; n = 7 hypertension; n = 3 diabetes). In three patients, an acute myocardial infarction was the underlying cause of VF, while acute de-compensation led to VT in three patients with pre-existing heart failure, and respiratory failure was the clinical grounds in the seventh patient. HCQ was suspended in all 7 patients. The centralized adjudication committee deemed that none of these episodes was directly related to HCQ treatment. A total of 12 (1.8%) and 3 (0.5%) new-onset atrial fibrillation/flutter episodes were observed, alongside 9 (1.3%) symptomatic bradycardias requiring medical de-escalation of non-HCQ medications. No cardiac or major ventricular arrhythmic events occurred in the HM group. The all-cause mortality rate was 6.5%: a total of 42 patients died, of which only 3 were in association with one of the aforementioned major ventricular arrhythmic events, as reported in Table 4.
Outcome during HCQ therapy
| . | Overall (n = 649) . | HT (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Follow up time (days), median [IQR] | 16 (9–20] | 15 [8–20] | 16 (9–210 | 19 (6–23) |
| HCQ suspension due to QT prolongation, n (%) | 5 (0.8) | 0 | 2 (0.4) | 3 (10.7) |
| Sustained VT during HCQ, n (%) | 4 (0.6) | 0 | 2 (0.4) | 2 (7.1) |
| VF during HCQ, n (%) | 3 (0.5) | 0 | 0 | 3 (10.7) |
| TdP during HCQ, n (%) | 0 | 0 | 0 | 0 |
| AF/AFl episodes, n (%) | 15 (2.3) | 2 (1.6) | 6 (1.2) | 7 (25.0) |
| Bradycardia episodes, n (%) | 9 (1.4) | 0 | 4 (0.8) | 5 (17.8) |
| Overall mortality, n (%) | 42 (6.5) | 2 (1.6) | 30 (6.1) | 10 (35.7) |
| . | Overall (n = 649) . | HT (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Follow up time (days), median [IQR] | 16 (9–20] | 15 [8–20] | 16 (9–210 | 19 (6–23) |
| HCQ suspension due to QT prolongation, n (%) | 5 (0.8) | 0 | 2 (0.4) | 3 (10.7) |
| Sustained VT during HCQ, n (%) | 4 (0.6) | 0 | 2 (0.4) | 2 (7.1) |
| VF during HCQ, n (%) | 3 (0.5) | 0 | 0 | 3 (10.7) |
| TdP during HCQ, n (%) | 0 | 0 | 0 | 0 |
| AF/AFl episodes, n (%) | 15 (2.3) | 2 (1.6) | 6 (1.2) | 7 (25.0) |
| Bradycardia episodes, n (%) | 9 (1.4) | 0 | 4 (0.8) | 5 (17.8) |
| Overall mortality, n (%) | 42 (6.5) | 2 (1.6) | 30 (6.1) | 10 (35.7) |
AF, atrial fibrillation; AFl, atrial flutter; HCQ, hydroxychloroquine; VT, ventricular tachycardia.
Outcome during HCQ therapy
| . | Overall (n = 649) . | HT (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Follow up time (days), median [IQR] | 16 (9–20] | 15 [8–20] | 16 (9–210 | 19 (6–23) |
| HCQ suspension due to QT prolongation, n (%) | 5 (0.8) | 0 | 2 (0.4) | 3 (10.7) |
| Sustained VT during HCQ, n (%) | 4 (0.6) | 0 | 2 (0.4) | 2 (7.1) |
| VF during HCQ, n (%) | 3 (0.5) | 0 | 0 | 3 (10.7) |
| TdP during HCQ, n (%) | 0 | 0 | 0 | 0 |
| AF/AFl episodes, n (%) | 15 (2.3) | 2 (1.6) | 6 (1.2) | 7 (25.0) |
| Bradycardia episodes, n (%) | 9 (1.4) | 0 | 4 (0.8) | 5 (17.8) |
| Overall mortality, n (%) | 42 (6.5) | 2 (1.6) | 30 (6.1) | 10 (35.7) |
| . | Overall (n = 649) . | HT (n = 126) . | MW (n = 495) . | ICU (n = 28) . |
|---|---|---|---|---|
| Follow up time (days), median [IQR] | 16 (9–20] | 15 [8–20] | 16 (9–210 | 19 (6–23) |
| HCQ suspension due to QT prolongation, n (%) | 5 (0.8) | 0 | 2 (0.4) | 3 (10.7) |
| Sustained VT during HCQ, n (%) | 4 (0.6) | 0 | 2 (0.4) | 2 (7.1) |
| VF during HCQ, n (%) | 3 (0.5) | 0 | 0 | 3 (10.7) |
| TdP during HCQ, n (%) | 0 | 0 | 0 | 0 |
| AF/AFl episodes, n (%) | 15 (2.3) | 2 (1.6) | 6 (1.2) | 7 (25.0) |
| Bradycardia episodes, n (%) | 9 (1.4) | 0 | 4 (0.8) | 5 (17.8) |
| Overall mortality, n (%) | 42 (6.5) | 2 (1.6) | 30 (6.1) | 10 (35.7) |
AF, atrial fibrillation; AFl, atrial flutter; HCQ, hydroxychloroquine; VT, ventricular tachycardia.
Discussion
Our study represents the largest experience available assessing the arrhythmic impact of HCQ therapy in patients with COVID-19 treated in all the different clinical scenarios: ICU (continuous monitoring), medical wards (serial ECG recordings), and home management (serial planned ECG recordings).
In our analysis, HCQ’s effect on electrophysiological parameters was found to be only a modest prolongation of the QTc value (11 ms) in the absence of treatment-related sustained arrythmias and sudden deaths, therefore demonstrating the arrhythmic safety of this treatment in consecutive unselected recipients. Of note, HCQ therapy was found to be associated with a reduction of the heart rate, the magnitude of which may also have been the result of the concomitant fever control and body temperature reduction. A bradycardia dictating modulation of concomitant rate-lowering agents occurred in 1.3% of patients.
Provided that an adequate ECG monitoring strategy is implemented, the short-term HCQ treatment used in COVID-19 patients seems to be safe, regardless of the clinical setting in which it is started.
HCQ in the COVID-19 pandemics
The call for immediate action arising from political entities and the general population has placed massive mediatic pressure on healthcare professionals and has led to several experimental treatments being introduced into clinical practice with only moderate evidence backing them.2,4,7 HCQ in particular has been used as first-line agent in the treatment of COVID-19 after preliminary reports of clinical improvement in mildly ill patients.4
At first used as an antimalarial agent as well as an antiarrhythmic agent,12 HCQ typically results in a milder prolongation of the PR interval, QRS, and QTc compared with other antimalarial drugs such as as quinidine and halofrantine.13 Apart from sporadic cases of TdP occurring in patients with severe ventricular dysfunction or after excessive self-administration for the purpose of suicide, the use of HCQ has been reported as safe in patients chronically treated for immunological diseases.14 The incidence of TdP on HCQ remained elusive in large series, despite a mean 25 ms QT prolongation during long-term treatment. In rheumatic patients, conduction disorders due to HCQ have been reported to be more common side effects than rhythm disorders,14 and can be mitigated by the favourable drug effect on the underlying disease, as observed for chloroquine in patients with systemic lupus erythematosus.15
Data regarding HCQ effectiveness for COVID-19 treatment are currently controversial: this study was neither powered nor planned to assess the clinical efficacy of HCQ, and will therefore be interpreted accordingly. In order to draw definitive conclusions regarding the impact of HCQ on COVID-19 patients, results from one of the several ongoing trials are needed. On the other hand, this study aims to present the available data regarding the HCQ arrhythmic safety profile in COVID-19 patients, to provide physicians with real-world evidence coming from three different clinical settings, upon which to build a more evidence-based decision-making process.
QT/QTc modification under HCQ administration
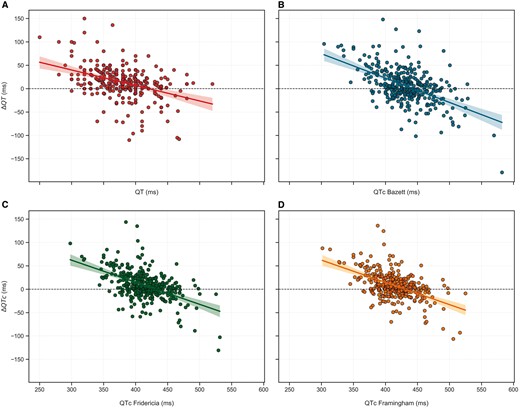
Following HCQ administration, we observed only modest QT/QTc changes from baseline, regardless of the linear rate correction formula used (Figure 1). Greater prolongation of QT/QT on HCQ treatment was not associated with a longer baseline value (Figure 2), mostly due to the changing conditions of patients with an acute illness and due to a lower QT/QTc reserve.
Linear correlation between baseline QT/QTc (ms) and QT/QTc variations (ΔQT/ΔQTc) (ms). (A) QT interval; (B) QTc Bazett; (C) QTc Fridericia; (D) QTc Framingham
When stricter formulae for rate adaptation were used, only fever and a longer baseline QTc were associated with QTc modifications towards a smaller value (Supplementary material online, Table S2). Indeed, several factors can change the QT/QTc duration beyond a drug effect: (i) QT/QTc prolongation occurs after defervescence; (ii) glycaemic and electrolyte changes may occur in hospitalized patients following clinical improvement; (iii) heart rate shifts to either of the extremes of the physiological range (where the rate correction formulae prove inadequate for QT/QTc measurement), strongly impacting the QT (Supplementary material online, Figure S4). This latter aspect was particularly important for those patients who developed atrial tachyarrhythmias (marked heart rate increase) or converted to sinus rhythm (marked rate reduction) during HCQ administration, thus exhibiting the most relevant QTc changes. (iv) Lastly, autonomic modulation due to changes in multiple comorbidities such as diabetes or chronic pulmonary disease may heavily impact the QTc interval.16
Outcomes in different clinical settings
Our study captured HCQ effects in a real-life population treated in three different clinical settings: ICU, hospital ward, and home treatment, according to the individual patient’s risk profile.
No significant differences in the HCQ impact on the QT/QTc interval were observed across the different clinical settings. The arrhythmic burden was low overall, but it was skewed towards patients hospitalized in the ICU at a per-group assessment: this was due to their more severe baseline conditions and their more aggressive infective state, alongside the several risk factors for arrhythmias that an ICU patient presents.
Data from our hospitalized and ICU patients were in line with the recent reports from Mercuro et al. and Bessier et al.,17,18 strengthening and further expanding their single-centre experiences with larger multicentre cohorts. Considering both case series, an overall QT interval prolongation was observed, while only a single TdP event was reported.17,18 This low rate of major ventricular arrhythmia events was deemed to be due to termination of HCQ and other QT-prolonging drugs upon detection of QT prolongation at ECG monitoring. These results held true in our cohort as well, even considering the home therapy patient group and the less restrictive QT/QTc prolongation cut-off for HCQ termination suggested in our study. It should also be noted that 30% of our patients received two QT-prolonging drugs, and 13.6% received three, mimicking real-life polypharmacy prescriptions. Nonetheless, the only arrhythmic events observed in our study were typical of acute coronary syndromes, de-compensation in heart failure patients, and respiratory failure. Figure S5 in the Supplementary material online reports two examples of sustained complex arrhythmic events. These events were not deemed to be QT related at the centralized assessment performed, due to their monomorphic morphology and a normal QTc interval preceding the arrhythmic event.
In this light, HCQ administration (alone or in combination) appears safe during the short-term treatment used in COVID-19 patients in all clinical settings, provided that patients’ screening and ECG recordings are available and that an adequate a priori patient-tailored risk assessment has been performed. Of note, particular attention should be given to the identification of patients suffering from congenital arrhythmias syndromes (especially from long QT syndrome), for which HCQ may represent an important arrhythmic trigger.19 Additionally, due to the long half-life of HCQ, we suggest performing serial/continuous ECG analyses in order to monitor these patients over a long period of time.
Limitations
Our study represents the first real-life assessment of the safety of HCQ in all settings in the general population during the COVID-19 pandemic. Some limitations should be noted: 24-h telemetry and loop recorders were not routinely available for all enrolling centres and, therefore, arrhythmic outcome assessment was also achieved by means of symptoms report. However, it appears highly unlikely that major arrhythmic events triggered by QT prolongation (such as TdP) would have gone unnoticed, given their highly symptomatic burden. It is important to remember, however, that regardless of these limitations in data collection, sudden arrhythmic death did not occur and severe ventricular arrhythmias were rare and related to underlying cardiac diseases. Additionally, the HCQ dose used in clinical practice varied across hospitals; although the impact of the loading dose has been taken into account in the multivariate analysis, it should still be mentioned as a potential limitation.
Conclusion
HCQ administration, alone or in combination with other potentially QTc-prolonging drugs, proved safe for a short-term treatment of patients with COVID-19 infection, causing only modest QTc prolongation. Serial ECG recordings at 36–72 h and later than 96 h from treatment onset can detect QTc changes that might suggest therapy modification. This experience provides a framework to enable HCQ therapy implementation in different clinical settings for future efficacy trials.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgments
We thank for their support in this COVID-19 project Professor Massimo Mancone, Professor Francesco Fedele, and Dr Michele Magnocavallo from the University ‘La Sapienza’ of Rome; Dr Carlo Descovich and Dr Maria Luisa De Luca from the Azienda USL di Bologna; Dr Giulia Massaro and D. Andrea Angeletti from the University of Bologna; and Dr Sarah Costa from University Hospital of Zurich.
Conflict of interest: Dr. A.S. own stocks from Gilead Science inc. The rest of authors declare no conflict of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Alessio Gasperetti and Mauro Biffi share first co-authorship.
Claudio Tondo and Giovanni Battista Forleo share senior co-authorship.