-
PDF
- Split View
-
Views
-
Cite
Cite
Evan C Adelstein, David Schwartzman, Sandeep Jain, Raveen Bazaz, Norman C Wang, Samir Saba, Left ventricular dimensions predict risk of appropriate shocks but not mortality in cardiac resynchronization therapy-defibrillator recipients with left bundle-branch block and non-ischemic cardiomyopathy, EP Europace, Volume 19, Issue 10, October 2017, Pages 1689–1694, https://doi.org/10.1093/europace/euw323
Close - Share Icon Share
Abstract
Patients with non-ischaemic cardiomyopathy (NICM) and left bundle-branch block (LBBB) often benefit markedly from cardiac resynchronization therapy (CRT). Cardiac resynchronization therapy responders have a lower risk of appropriate device shocks from CRT-defibrillators (CRT-D) than do non-responders. Larger baseline left ventricular (LV) dimensions may be associated with less CRT response and thus greater risk of appropriate shocks.
We analysed all (n = 249; 55% female) primary prevention CRT-D recipients at our institution with LBBB, NICM, and measured LV dimensions prior to device implant for the outcomes of (i) appropriate shocks, (ii) any appropriate tachyarrhythmia therapies, and (iii) risk of death, transplant, or left ventricular assist device (LVAD). During 59 months (interquartile range 21.5–91.5) follow-up, 19 (8%) patients received ≥1 appropriate shock, and 67 (27%) patients died, received a transplant, or required LVAD. Receiver-operating characteristic analysis of LV end-diastolic diameter (LVEDD) per meter height vs. appropriate shock(s) revealed an area under the curve of 0.75 (95% CI 0.65–0.85; P < 0.001). No patient with indexed LVEDD <3.36 cm/m (n = 76) received a shock. There was no statistically significant difference in risk of death, transplant, or LVAD (corrected HR 1.67, 95% CI 0.90–3.03; P = 0.103) in patients with indexed LVEDD above this cut-off compared to those with smaller dimension. Among 102 patients with paired quantitative echocardiograms, there was no difference in LVEF change between patients with indexed LVEDD <3.36 cm/m (n = 27; median 11%) and larger (n = 75; median 14%).
Patients with LVEDD <3.36 cm/m height prior to CRT-D implant in the setting of NICM and LBBB have minimal risk of appropriate shocks but similar risk of death, transplant- and LVAD and similar extent of LV functional improvement as patients with larger LVEDD. CRT-pacemakers may be appropriate in such patients.
What’s new?
Patients with left bundle-branch block and non-ischemic cardiomyopathy who have a baseline left ventricular end-diastolic dimension <3.36 cm/m height have minimal risk of appropriate device shocks after receiving primary prevention cardiac-resynchronization therapy-defibrillators.
The low risk of ventricular arrhythmias in patients with left ventricular end-diastolic dimension <3.36 cm/m height compared to patients with larger ventricles is not related to baseline comorbid conditions or differences in reverse remodelling after receiving cardiac resynchronization therapy, and both groups have similar risk of death, transplant, or left ventricular assist device.
Introduction
Cardiac resynchronization therapy (CRT) reduces heart failure (HF) morbidity and mortality in patients with severe left ventricular (LV) dysfunction and abnormal LV intraventricular conduction.1,2 Patients with left bundle-branch block (LBBB)3 and non-ischaemic cardiomyopathy4,5 derive particular benefit from CRT, manifesting profound improvement in clinical status and LV function. However, some studies have shown that marked baseline LV dilatation portends less favourable outcomes after CRT,5 whereas randomized trials have paradoxically excluded patients without significant LV dilatation.1,2 Patients who exhibit marked reverse remodelling with CRT are known to have lower ongoing risk of ventricular arrhythmias,6 yet a priori identification of very low-risk patients has been difficult. Identifying CRT candidates who are at low risk for ventricular arrhythmias may lead to better outcomes if implanted with CRT-P instead of CRT-D, given the risk of inappropriate shocks, lead-related mechanical complications,7 and increased cost with the latter.8
We examined whether LV dimensions prior to CRT are associated with appropriate shocks subsequent to CRT-D implant, hypothesizing that smaller LV dimensions may be associated with less risk. We chose a relatively homogeneous population comprising patients with strictly defined LBBB9 and non-ischaemic cardiomyopathy to minimize potential confounders that may impact CRT response, including ischemic scar burden4 and QRS pattern.3
Methods
Patient selection
We included all patients from a prospectively maintained CRT-D database at the University of Pittsburgh who fulfilled the following criteria: (i) LVEF ≤35%, (ii) strictly defined LBBB, (iii) primary prevention indication for a defibrillator, (iv) non-ischaemic cardiomyopathy, and (v) measured LV dimensions prior to implant. The database includes 1742 CRT-D recipients, of whom 1016 were excluded because of ischemic cardiomyopathy. An additional 328 non-ischaemic cardiomyopathy patients were excluded because of baseline right bundle-branch block, non-specific intraventricular conduction delay, or complete heart block, 35 were excluded because of prior sustained ventricular arrhythmias, and 82 were excluded because there were no available baseline LV dimensions at the University of Pittsburgh Medical Center.
left bundle-branch block was defined as a QRS duration ≥130 ms in women and ≥140 ms in men, a QS or rS complex in lead V1, and notching of the QRS complex in at least two contiguous leads involving I and aVL, V1 and V2, or V5, and V6.9 Non-ischaemic cardiomyopathy was defined as LVEF ≤35% in the absence of (i) prior coronary revascularization, (ii) stenosis ≥80% in a major epicardial coronary artery, and (iii) definitive history of myocardial infarction with corroborating non-invasive imaging.6 Patients had neither a history of aborted cardiac arrest nor spontaneous ventricular arrhythmias lasting ≥30 s or causing hemodynamic collapse. As the primary exposure for the study was LV dimension, we included only patients who underwent transthoracic echocardiography at the hospitals of the University of Pittsburgh Medical Center.
Echocardiography
Echocardiograms were performed at baseline (median 1 month prior to CRT) and ≥3 months (median 17 months) after CRT implant. Echocardiograms were performed by sonographers and interpreted by cardiologists board-certified in echocardiography. LV dimensions were measured in the parasternal long axis view according to standard techniques.10 Patients with echocardiograms available in digital format also underwent offline analysis, using Simpson’s biplane technique in the apical 4- and 2-chamber views to measure LV end-diastolic and end-systolic volumes; these values were used to calculate LVEF.
Device and heart failure therapy
Patients were implanted with CRT-D between April 2002 May 2016 at one of the hospitals of the University of Pittsburgh Medical Center, including a high-voltage lead in the right ventricular apex, a standard pacing lead in the right atrial free wall, and a LV lead preferentially placed in a posterolateral or lateral coronary vein. Devices were programmed at the discretion of the implanting physician. Anti-tachycardia pacing during charging was universally activated in the VF zone when this feature became available, and tachycardia detection and therapy was programmed and reprogrammed according to published guidelines.11
Patients received maximally tolerated guideline-directed medical therapy with β-adrenergic antagonists and inhibitors of the renin-angiotensin-aldosterone system. Medications were recorded at the time of device implant.
Study outcomes and statistical analysis
The outcome measure of primary interest in this exploratory analysis was the incidence and timing of first appropriate device shock. Additional measures of interest were time to any appropriate tachyarrhythmia therapy and survival free from transplant or left ventricular assist device (LVAD). Electrograms from all device shocks were examined by an electrophysiologist and confirmed to be appropriate or inappropriate. We also assessed absolute change in LVEF and relative change in LV end-systolic volume in the subset of patients with paired echocardiograms before and ≥3 months after CRT implant.10
We initially performed receiver-operating characteristic analysis to examine the relationship between LV end-diastolic dimension (LVEDD) indexed to height in meters and any appropriate shocks. Because no patient with indexed LVEDD <3.36 cm/m received an appropriate shock, and our primary goal was to identify a group of patients at particularly low risk of ventricular arrhythmias, we divided the cohort into two groups: (i) indexed LVEDD <3.36 cm/m and (ii) LVEDD ≥3.36 cm/m. Continuous variables were assessed for normal distribution and were compared using Student’s t-test if normally distributed and the Mann–Whitney U test if otherwise. Discrete variables were compared using Fisher’s exact test or χ2 test, as appropriate. Kaplan–Meier curves were constructed for time-dependent outcomes and were compared using the log-rank test. Baseline intrinsic variables differing between the groups with a P-value <0.1 were entered into a multivariate Cox regression model, in addition to variables identified a priori that were deemed to be important predictors of post-CRT outcomes. P-values ≤0.05 were considered statistically significant. SPSS version 23.0 (IBM, Inc., Armonk, NY) was used for statistical analyses.
Results
Patient characteristics
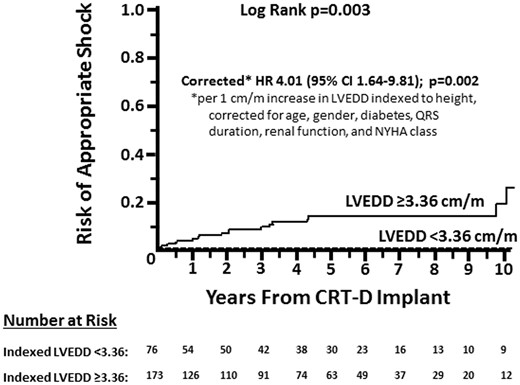
The study cohort included 249 patients, almost evenly split between males and females. Additional baseline characteristics are listed in Table 1. Baseline LVEDD was normally distributed between 3.7 and 10.2 cm, with a mean of 6.1 ± 1.1. Indexing LVEDD to height in meters, patients’ dimensions were non-parametrically distributed between 2.06–5.90 cm/m, with a median of 3.58 and interquartile range of 3.28–3.96. During a median of 59 months (interquartile range 21.5–91.5) follow-up, 19 patients (8%) received an appropriate shock. Initial receiver operating characteristic analysis of LVEDD/m vs. any appropriate shock yielded an area under the curve of 0.75 (95% CI 0.65–0.85; P < 0.001). None of the 76 patients with a baseline indexed LVEDD <3.36 cm/m (or 96 patients with LVEDD <5.8 cm) received an appropriate shock (Figure 1). This LVEDD also corresponded to the lowest tertile cut-off.
Baseline characteristics
| Clinical Characteristic . | Overall Cohort (n = 249) . | LVEDD <3.36 cm/m (n = 76) . | LVEDD ≥3.36 cm/m (n = 173) . | P-value* . |
|---|---|---|---|---|
| Age, y | 64 (54, 74) | 69 (59, 75) | 61 (54, 73) | 0.02 |
| Male | 112 (45%) | 36 (47%) | 76 (44%) | 0.68 |
| NYHA class | 0.14 | |||
| 2 | 33 (13%) | 15 (20%) | 18 (10%) | |
| 3 | 205 (82%) | 58 (76%) | 147 (85%) | |
| 4 | 11 (4%) | 3 (4%) | 8 (5%) | |
| Diabetes | 62 (25%) | 23 (30%) | 39 (23%) | 0.21 |
| Paroxysmal AF | 46 (19%) | 17 (22%) | 29 (17%) | 0.29 |
| Permanent AF | 14 (6%) | 7 (9%) | 7 (4%) | 0.13 |
| Glomerular filtration rate, mL/min | 69 ± 25 | 68 ± 25 | 69 ± 26 | 0.76 |
| QRS duration, ms | 170 (156, 184) | 164 (152, 172) | 176 (161, 188) | <0.001 |
| β-blocker | 221 (89%) | 69 (91%) | 152 (88%) | 0.66 |
| ACE-I or ARB | 214 (86%) | 69 (91%) | 145 (84%) | 0.17 |
| Loop diuretic | 177 (71%) | 47 (62%) | 130 (76%) | 0.03 |
| Statin | 112 (45%) | 42 (55%) | 70 (41%) | 0.04 |
| Digoxin | 80 (32%) | 28 (24%) | 62 (36%) | 0.08 |
| Aldosterone antagonist | 66 (27%) | 16 (21%) | 50 (29%) | 0.22 |
| LVEF, % | 22 (17, 27) | 27 (22, 32) | 20 (16, 22) | <0.001 |
| LVEDD, cm | 6.1 ± 1.1 | 5.2 ± 0.5 | 6.6 ± 0.9 | <0.001 |
| LVESD, cm | 5.3 ± 1.2 | 4.3 ± 0.6 | 5.7 ± 1.1 | <0.001 |
| LVEDV, mLa | 177 (135, 223) | 127 (103, 166) | 198 (158, 235) | <0.001 |
| LVESV, mLa | 129 (96, 170) | 94 (74, 119) | 150 (114, 194) | <0.001 |
| VF rate cut-off, bpm | 210 (200, 220) | 210 (200, 220) | 210 (200, 220) | 0.80 |
| VT rate, cut-off, bpm | 182 (176, 187) | 182 (176, 188) | 182 (176, 187) | 0.68 |
| Clinical Characteristic . | Overall Cohort (n = 249) . | LVEDD <3.36 cm/m (n = 76) . | LVEDD ≥3.36 cm/m (n = 173) . | P-value* . |
|---|---|---|---|---|
| Age, y | 64 (54, 74) | 69 (59, 75) | 61 (54, 73) | 0.02 |
| Male | 112 (45%) | 36 (47%) | 76 (44%) | 0.68 |
| NYHA class | 0.14 | |||
| 2 | 33 (13%) | 15 (20%) | 18 (10%) | |
| 3 | 205 (82%) | 58 (76%) | 147 (85%) | |
| 4 | 11 (4%) | 3 (4%) | 8 (5%) | |
| Diabetes | 62 (25%) | 23 (30%) | 39 (23%) | 0.21 |
| Paroxysmal AF | 46 (19%) | 17 (22%) | 29 (17%) | 0.29 |
| Permanent AF | 14 (6%) | 7 (9%) | 7 (4%) | 0.13 |
| Glomerular filtration rate, mL/min | 69 ± 25 | 68 ± 25 | 69 ± 26 | 0.76 |
| QRS duration, ms | 170 (156, 184) | 164 (152, 172) | 176 (161, 188) | <0.001 |
| β-blocker | 221 (89%) | 69 (91%) | 152 (88%) | 0.66 |
| ACE-I or ARB | 214 (86%) | 69 (91%) | 145 (84%) | 0.17 |
| Loop diuretic | 177 (71%) | 47 (62%) | 130 (76%) | 0.03 |
| Statin | 112 (45%) | 42 (55%) | 70 (41%) | 0.04 |
| Digoxin | 80 (32%) | 28 (24%) | 62 (36%) | 0.08 |
| Aldosterone antagonist | 66 (27%) | 16 (21%) | 50 (29%) | 0.22 |
| LVEF, % | 22 (17, 27) | 27 (22, 32) | 20 (16, 22) | <0.001 |
| LVEDD, cm | 6.1 ± 1.1 | 5.2 ± 0.5 | 6.6 ± 0.9 | <0.001 |
| LVESD, cm | 5.3 ± 1.2 | 4.3 ± 0.6 | 5.7 ± 1.1 | <0.001 |
| LVEDV, mLa | 177 (135, 223) | 127 (103, 166) | 198 (158, 235) | <0.001 |
| LVESV, mLa | 129 (96, 170) | 94 (74, 119) | 150 (114, 194) | <0.001 |
| VF rate cut-off, bpm | 210 (200, 220) | 210 (200, 220) | 210 (200, 220) | 0.80 |
| VT rate, cut-off, bpm | 182 (176, 187) | 182 (176, 188) | 182 (176, 187) | 0.68 |
120 patients had volumetric analysis of baseline echocardiogram, 40 in the LVEDD <3.36 cm/m group and 80 in the LVEDD ≥3.36 cm/m group.
P-value reflects comparison between patients with indexed LVEDD <3.36 cm/m and ≥3.36 cm/m.
Baseline characteristics
| Clinical Characteristic . | Overall Cohort (n = 249) . | LVEDD <3.36 cm/m (n = 76) . | LVEDD ≥3.36 cm/m (n = 173) . | P-value* . |
|---|---|---|---|---|
| Age, y | 64 (54, 74) | 69 (59, 75) | 61 (54, 73) | 0.02 |
| Male | 112 (45%) | 36 (47%) | 76 (44%) | 0.68 |
| NYHA class | 0.14 | |||
| 2 | 33 (13%) | 15 (20%) | 18 (10%) | |
| 3 | 205 (82%) | 58 (76%) | 147 (85%) | |
| 4 | 11 (4%) | 3 (4%) | 8 (5%) | |
| Diabetes | 62 (25%) | 23 (30%) | 39 (23%) | 0.21 |
| Paroxysmal AF | 46 (19%) | 17 (22%) | 29 (17%) | 0.29 |
| Permanent AF | 14 (6%) | 7 (9%) | 7 (4%) | 0.13 |
| Glomerular filtration rate, mL/min | 69 ± 25 | 68 ± 25 | 69 ± 26 | 0.76 |
| QRS duration, ms | 170 (156, 184) | 164 (152, 172) | 176 (161, 188) | <0.001 |
| β-blocker | 221 (89%) | 69 (91%) | 152 (88%) | 0.66 |
| ACE-I or ARB | 214 (86%) | 69 (91%) | 145 (84%) | 0.17 |
| Loop diuretic | 177 (71%) | 47 (62%) | 130 (76%) | 0.03 |
| Statin | 112 (45%) | 42 (55%) | 70 (41%) | 0.04 |
| Digoxin | 80 (32%) | 28 (24%) | 62 (36%) | 0.08 |
| Aldosterone antagonist | 66 (27%) | 16 (21%) | 50 (29%) | 0.22 |
| LVEF, % | 22 (17, 27) | 27 (22, 32) | 20 (16, 22) | <0.001 |
| LVEDD, cm | 6.1 ± 1.1 | 5.2 ± 0.5 | 6.6 ± 0.9 | <0.001 |
| LVESD, cm | 5.3 ± 1.2 | 4.3 ± 0.6 | 5.7 ± 1.1 | <0.001 |
| LVEDV, mLa | 177 (135, 223) | 127 (103, 166) | 198 (158, 235) | <0.001 |
| LVESV, mLa | 129 (96, 170) | 94 (74, 119) | 150 (114, 194) | <0.001 |
| VF rate cut-off, bpm | 210 (200, 220) | 210 (200, 220) | 210 (200, 220) | 0.80 |
| VT rate, cut-off, bpm | 182 (176, 187) | 182 (176, 188) | 182 (176, 187) | 0.68 |
| Clinical Characteristic . | Overall Cohort (n = 249) . | LVEDD <3.36 cm/m (n = 76) . | LVEDD ≥3.36 cm/m (n = 173) . | P-value* . |
|---|---|---|---|---|
| Age, y | 64 (54, 74) | 69 (59, 75) | 61 (54, 73) | 0.02 |
| Male | 112 (45%) | 36 (47%) | 76 (44%) | 0.68 |
| NYHA class | 0.14 | |||
| 2 | 33 (13%) | 15 (20%) | 18 (10%) | |
| 3 | 205 (82%) | 58 (76%) | 147 (85%) | |
| 4 | 11 (4%) | 3 (4%) | 8 (5%) | |
| Diabetes | 62 (25%) | 23 (30%) | 39 (23%) | 0.21 |
| Paroxysmal AF | 46 (19%) | 17 (22%) | 29 (17%) | 0.29 |
| Permanent AF | 14 (6%) | 7 (9%) | 7 (4%) | 0.13 |
| Glomerular filtration rate, mL/min | 69 ± 25 | 68 ± 25 | 69 ± 26 | 0.76 |
| QRS duration, ms | 170 (156, 184) | 164 (152, 172) | 176 (161, 188) | <0.001 |
| β-blocker | 221 (89%) | 69 (91%) | 152 (88%) | 0.66 |
| ACE-I or ARB | 214 (86%) | 69 (91%) | 145 (84%) | 0.17 |
| Loop diuretic | 177 (71%) | 47 (62%) | 130 (76%) | 0.03 |
| Statin | 112 (45%) | 42 (55%) | 70 (41%) | 0.04 |
| Digoxin | 80 (32%) | 28 (24%) | 62 (36%) | 0.08 |
| Aldosterone antagonist | 66 (27%) | 16 (21%) | 50 (29%) | 0.22 |
| LVEF, % | 22 (17, 27) | 27 (22, 32) | 20 (16, 22) | <0.001 |
| LVEDD, cm | 6.1 ± 1.1 | 5.2 ± 0.5 | 6.6 ± 0.9 | <0.001 |
| LVESD, cm | 5.3 ± 1.2 | 4.3 ± 0.6 | 5.7 ± 1.1 | <0.001 |
| LVEDV, mLa | 177 (135, 223) | 127 (103, 166) | 198 (158, 235) | <0.001 |
| LVESV, mLa | 129 (96, 170) | 94 (74, 119) | 150 (114, 194) | <0.001 |
| VF rate cut-off, bpm | 210 (200, 220) | 210 (200, 220) | 210 (200, 220) | 0.80 |
| VT rate, cut-off, bpm | 182 (176, 187) | 182 (176, 188) | 182 (176, 187) | 0.68 |
120 patients had volumetric analysis of baseline echocardiogram, 40 in the LVEDD <3.36 cm/m group and 80 in the LVEDD ≥3.36 cm/m group.
P-value reflects comparison between patients with indexed LVEDD <3.36 cm/m and ≥3.36 cm/m.
Time to first appropriate CRT-D shock in patients with baseline indexed LVEDD <3.36 cm/m versus ≥3.36 cm/m.
The 76 patients with indexed LVEDD <3.36 cm/m and 173 patients with LVEDD ≥3.36 cm/m are compared in Table 1. Patients with indexed LVEDD <3.36 cm/m were older, had a narrower QRS complex, were less likely to be prescribed loop diuretics, and had a higher baseline LVEF. The groups were otherwise similar, including programmed rate cut-offs for VT and VF zones, in particular.
Device tachyarrhythmia therapies
The unadjusted risk of at least 1 appropriate shock was significantly greater as indexed LVEDD increased (HR 3.62 per 1 cm/m increase, 95% CI 1.78–7.37; P < 0.001). The hazard ratio per 1 cm/m increase in indexed LVEDD was 4.01 (95% CI 1.64–9.81; P = 0.002) when corrected for age, gender, diabetes, QRS duration, renal function, and NYHA class.
The unadjusted risk of anti-tachycardia pacing in patients with indexed LVEDD <3.36 cm/m compared to ≥3.36 cm/m was 1.29 (95% CI 0.72–2.33; P = 0.39). Adjusting for age, gender, diabetes, QRS duration, renal function, and NYHA class did not appreciably change this finding (hazard ratio 1.82, 95% CI 0.87–3.82; P = 0.11). The corresponding unadjusted risk of any appropriate tachyarrhythmia therapy was significantly greater in the group with indexed LVEDD <3.36 cm/m (hazard ratio 1.75, 95% CI 1.07–2.88; P = 0.026), which corrected to 1.94 (95% CI 1.05–3.59; P = 0.034) on multivariate analysis.
Survival free from cardiac transplant or ventricular assist device
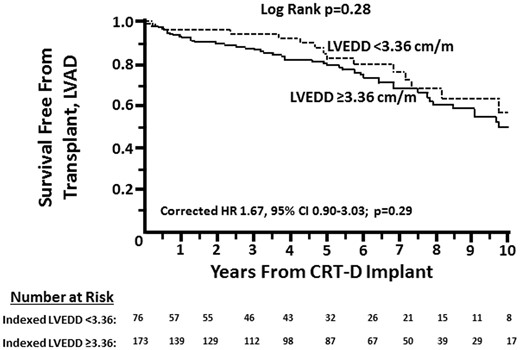
A total of 67 patients (27%) died (n = 52), received a transplant (n = 7), or required LVAD (n = 8), including 15 (20%) patients with indexed LVEDD <3.36 cm/m and 52 (30%) patients with indexed LVEDD ≥3.36 cm/m. Overall survival free from transplant or LVAD was similar (Figure 2; HR 0.73, 95% CI 0.41–1.30; P = 0.29). A multivariate Cox model incorporating age, gender, renal function, diabetes, NYHA class, and QRS duration yielded similar findings (HR 1.67, 95% CI 0.90–3.03; P = 0.103). Using a continuous variable for indexed LVEDD rather than a dichotomous cut-off value in this model also demonstrated no statistically significant difference in survival free from transplant or VAD per 1 cm/m increase (corrected HR 1.57, 95% CI 0.98–2.53; P = 0.063).
Survival free from transplant or LVAD in patients with baseline indexed LVEDD <3.36 cm/m versus ≥3.36 cm/m.
Echocardiography
A follow-up echocardiogram with paired volumetric data was available in 102 patients (41%), including 27 (36%) with indexed LVEDD <3.36 cm/m and 75 (43%) with indexed LVEDD ≥3.36 cm/m. Overall, LVEF increased by 14% and LVESV decreased 38% from baseline, with post-CRT measurements significantly different from pre-CRT measurements (P < 0.001). The absolute LVEF increase was identical between patients with indexed LVEDD <3.36 and ≥3.36 (median 11% vs. 14%, respectively; P = 0.32) and the relative LVESV decrease was also similar (median −40% vs. −37%, respectively; P = 0.38).
Discussion
In this large, single centre registry study, smaller LV dimensions prior to CRT-D implant were associated with lower risk of appropriate device shocks in patients with non-ischaemic cardiomyopathy, LBBB, and no prior sustained ventricular arrhythmias. In particular, patients with indexed LVEDD <3.36 cm/m height had no appropriate device shocks. Despite the significant difference in risk of appropriate shocks based upon baseline LVEDD, there was no difference in survival free from transplant or LVAD, and among patients with paired echocardiograms before and after CRT, there was no quantitative difference in reverse remodelling or LV functional improvement based upon baseline LVEDD.
Cardiomyopathy patients with LBBB rather than non-specific intraventricular conduction delay or right bundle-branch block derive the greatest benefit from CRT,3 a finding reflected in current practice guidelines.12 Non-ischaemic cardiomyopathy is also associated with more reverse remodelling, greater LV functional improvement, and better survival outcomes compared to patients with ischaemic heart disease.4 Furthermore, patients who experience significant reverse remodelling with CRT are at lower risk of ventricular arrhythmias,6 and LV reverse remodelling is associated with long-term survival in CRT recipients.13 It is in this context that we examined outcomes in patients with non-ischaemic cardiomyopathy, strictly defined LBBB,9 and a primary prevention indication for a CRT-D, postulating that LV dilatation may be related to risk of appropriate device shocks. We examined patients with non-ischaemic cardiomyopathy in order to remove the element of ischaemic scar burden from ongoing risk of ventricular arrhythmias. We also sought to study a population of ‘ideal’ CRT candidates and therefore excluded patients with non-LBBB conduction abnormalities or atrioventricular block requiring pacing. As such, our study population is fairly homogenous compared to the majority of CRT datasets described in the literature.
Patients without significant LV dilatation have largely been excluded from larger CRT clinical trials; CARE-HF2 excluded patients with LVEDD <3.0 cm/m height, and COMPANION1 excluded patients with LVEDD <6.0 cm. COMPANION included both CRT-D and CRT-pacemakers, and by excluding patients with less severe LV dilatation who were possibly less likely to benefit from a defibrillator, it may have overestimated the survival benefit of CRT-D vs. CRT-pacemakers.
Baseline LV dilatation has been associated with worse outcomes after CRT, although our study is the first to our knowledge to examine specifically the relationship between LV dimensions and outcomes in patients with NICM and LBBB. Rickard et al.14 found an inverse relationship between baseline LVEDD and LVEF improvement after CRT, although there was significant LVEF improvement across the spectrum of LV dimensions. Furthermore, mortality increased as LV dimensions increased. However, no tachyarrhythmia therapy data were included, 58% of patients had ischaemic cardiomyopathy, and only 39% had LBBB. Carluccio et al. found an inverse relationship between baseline LV end-systolic volume index and extent of LVEF improvement after CRT and a direct relationship between baseline LV volume and HF events.15 Among pacemaker-dependent patients without coronary artery disease, LVEF normalization after CRT upgrade has been observed more commonly in those with less baseline LV dilatation.16 A post-hoc analysis of MADIT-CRT also showed that patients who received appropriate shocks demonstrated greater baseline LV dilatation.17 The disconnect between shocks and survival free from transplant or LVAD may be explained by prevention of sudden death in patients with larger LV dimensions, shifting the cause of death towards progressive HF, which may be similar regardless of LV dimensions, as evidenced by similar degrees of LV reverse remodelling and LV functional improvement.
The pathophysiologic basis for our findings is likely multifactorial. LV dilatation is the final common pathway for multiple processes that cause cardiomyopathy and is known to increase arrhythmia risk. Alterations in the expression of connexins and stretch-mediated changes in ion channel function have been reported in the setting of LV dilatation.18 Smaller baseline dimensions may reflect less interstitial fibrosis19 and therefore less available substrate for malignant re-entrant ventricular arrhythmias. This relationship may apply only to patients without coronary artery disease because denser, less patchy myocardial scar from prior infarction may provide the substrate for re-entry regardless of LV dimensions in patients with ischaemic cardiomyopathy. Among patients with ischaemic cardiomyopathy in our centre’s database, we found no discrete cut-off LVEDD below which the risk of appropriate shocks is zero (data not shown). These findings support a study by Witt et al in which only patients with ischaemic cardiomyopathy derived survival benefit with CRT-D over CRT-pacemakers.20
Our data suggest that CRT-pacemakers may be a viable alternative to CRT-D in non-ischaemic HF patients receiving CRT for primary prevention with LBBB and without significant LV dilatation because of the low or absent risk of malignant ventricular arrhythmias. CRT-pacemakers do have several advantages over CRT-D. There is no risk of inappropriate shocks with CRT-pacemakers, and pacing leads have a better track record of reliability compared to defibrillator leads, some of which have been subjected to class I Food and Drug Administration recalls.7 Furthermore, the risks of infection may be lower with the smaller device size of CRT-pacemakers, and CRT-D devices also cost significantly more than CRT-pacemakers.8
Limitations
As the present study was retrospective and did not prospectively evaluate the hypothesis that smaller LV dimensions are associated with lower risk of appropriate shocks, its conclusions are hypothesis-generating only. Measurement of LV dimensions does not take into account non-homogeneous LV geometry, although difficulty in delineating apical endocardium may render volume measurements more error-prone than measurement of linear dimensions. Paired echocardiograms were only available in less than half our population, representing what may be a biased subset of patients. Only overall mortality was described, although there would be no reason to suspect that non-cardiac mortality would be preferentially segregated to a group of patients with larger or smaller LV dimensions. While tachyarrhythmia therapy was not standardized, a small group of physicians performed all implants and follow-up care, suggesting that there was no bias across patients with differing baseline LV dimensions. The lack of significant difference in survival free from transplant or LVAD may be partly the result of underpowering for this endpoint. Finally, we do not have extensive MRI data regarding evidence of myocardial fibrosis in this patient population. Therefore, we may only speculate as to any possible relationship between LV dimension and myocardial fibrosis.
Conclusions
In primary prevention CRT-D recipients without coronary artery disease and with strictly defined LBBB, smaller baseline LVEDD is associated with lower risk of subsequent appropriate device shocks but no difference in survival free from transplant or VAD. Since no shocks were observed in patients with indexed LVEDD <3.36 cm/m, CRT-pacemakers may be a reasonable alternative in appropriately selected patients.
Conflicts of interest: none declared.
References
- artificial cardiac pacemaker
- primary prevention
- tachycardia
- left ventricular ejection fraction
- echocardiography
- left ventricle
- ventricular assist device
- area under curve
- diastole
- follow-up
- shock
- mortality
- transplantation
- left bundle-branch block
- cardiac resynchronization therapy
- defibrillators
- risk reduction
- medical devices
- cardiomyopathy, non-ischemic
- device implant
- implants
- diameter
- cardiac resynchronization therapy defibrillator systems