-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer Gadkari, Tobias Goris, Christian L Schiffmann, Raffael Rubick, Lorenz Adrian, Torsten Schubert, Gabriele Diekert, Reductive tetrachloroethene dehalogenation in the presence of oxygen by Sulfurospirillum multivorans: physiological studies and proteome analysis, FEMS Microbiology Ecology, Volume 94, Issue 1, January 2018, fix176, https://doi.org/10.1093/femsec/fix176
Close - Share Icon Share
Abstract
Reductive dehalogenation of organohalides is carried out by organohalide-respiring bacteria (OHRB) in anoxic environments. The tetrachloroethene (PCE)-respiring Epsilonproteobacterium Sulfurospirillum multivorans is one of few OHRB able to respire oxygen. Therefore, we investigated the organism's capacity to dehalogenate PCE in the presence of oxygen, which would broaden the applicability to use S. multivorans, unlike other commonly oxygen-sensitive OHRB, for bioremediation, e.g. at oxic/anoxic interphases. Additionally, this has an impact on our understanding of the global halogen cycle. Sulfurospirillum multivorans performs dehalogenation of PCE to cis-1,2-dichloroethene at oxygen concentrations below 0.19 mg/L. The redox potential of the medium electrochemically adjusted up to +400 mV had no influence on reductive dehalogenation by S. multivorans in our experiments, suggesting that higher levels of oxygen impair PCE dechlorination by inhibiting or inactivating involved enzymes. The PCE reductive dehalogenase remained active in cell extracts of S. multivorans exposed to 0.37 mg/L oxygen for more than 96 h. Analysis of the proteome revealed that superoxide reductase and cytochrome peroxidase amounts increased with 5% oxygen in the gas phase, while the response to atmospheric oxygen concentrations involved catalase and hydrogen peroxide reductase. Taken together, our results demonstrate that reductive dehalogenation by OHRB is not limited to anoxic conditions.
INTRODUCTION
Halogenated organic groundwater contaminants (e.g. chlorinated ethenes) are dehalogenated by various aerobic and anaerobic microorganisms (Fetzner 1998). Aerobic bacteria dehalogenate organohalides via oxidation reactions using different enzymes (van Pée and Unversucht 2003), whereas anaerobic bacteria often use halogenated compounds as terminal electron acceptors in an anaerobic respiration, in which they couple reductive dehalogenation to energy conservation. This process, termed organohalide respiration (OHR), involves corrinoid-containing reductive dehalogenases as terminal reductase (Leys, Adrian and Smidt 2013; Schubert and Diekert 2016). Organohalide-respiring bacteria (OHRB) are mainly found within the phyla Chloroflexi (Dehalococcoides and Dehalogenimonas), Firmicutes (Dehalobacter and Desulfitobacterium), Deltaproteobacteria (e.g. Geobacter, Anaeromyxobacter, Desulfomonile) and Epsilonproteobacteria (Sulfurospirillum) (Atashgahi, Lu and Smidt 2016). Chlorinated ethenes are widely occurring as groundwater contaminants (Moran, Zogorski and Squillace 2007). While chlorinated ethenes with one or two chlorine substituents (vinyl chloride (VC) and dichloroethenes (DCE)) can be dechlorinated oxidatively under oxic conditions (Coleman et al.2002; Mattes, Alexander and Coleman 2010), tetrachloroethene (PCE) is persistent to microbial degradation in the presence of oxygen but is transformed via reductive dechlorination catalyzed by OHRB (Atashgahi, Lu and Smidt 2016). Although formerly described as an obligate anaerobic bacterium (Scholz-Muramatsu et al.1995), Sulfurospirillum multivorans was recently shown to be able to respire oxygen at low O2 concentrations (Goris et al.2014). This is an exception among OHRB. Most of these bacteria are sensitive to even very low amounts of oxygen, especially obligate organohalide respirers such as Dehalococcoides mccartyi (Amos et al.2008). Besides S. multivorans also Anaeromyxobacter dehalogenans, a Deltaproteobacterium dechlorinating 2-chlorophenol, but not PCE, is an OHRB respiring oxygen (Sanford, Cole and Tiedje 2002; Thomas et al.2008). Additionally, Desulfitobacterium dehalogenans and D. hafniense DCB-2 were described to survive in the presence of air (Madsen and Licht 1992; Utkin, Woese and Wiegel 1994) and to encode proteins responsible for reactive oxygen species (ROS) detoxification (Kim et al.2012). A few other aerobic bacteria mainly belonging to the Betaproteobacteria (e.g. Delftia sp. EOB-17 and Comamonas sp. 7D-2) carry out reductive dehalogenation (but not OHR) under oxic conditions; however, they seem to exclusively dehalogenate brominated aromatic compounds (Chen et al.2013, 2015). Microbial reductive dechlorination of PCE has been shown to be performed only under anoxic conditions at environmental redox potentials below –180 mV (Kästner 1991). The oxygen sensitivity of this process can be partly explained by the low stability of reductive dehalogenases in the presence of oxygen, the enzymatic half-life ranging from 1 to 4 h under atmospheric oxygen concentrations (Holliger, Wohlfarth and Diekert 1998). This oxygen sensitivity is most likely due to the instability of [4Fe-4S] clusters (Imlay 2006) also present in the PCE reductive dehalogenase of S. multivorans (Bommer et al.2014).
Soil and groundwater, which are often contaminated with chlorinated ethenes, are heterogeneous with respect to their oxygen concentration and include microoxic zones. We use the term microoxic to refer to dissolved oxygen concentrations of less than 2 mg/L (corresponding to ∼5% oxygen in the gas phase). In this study, we addressed the question if reductive dechlorination could be catalyzed by S. multivorans in the presence of oxygen, since this would enhance the practicability of bioremediation in microoxic contaminated environments (e.g. at oxic/anoxic interphases or in microoxic aquifers). As a comparison, the PCE-dechlorinating, oxygen-tolerating D. hafniense Y51 was tested for PCE dechlorination in the presence of oxygen. Since little is known about the physiological reaction of microaerobic OHRB or Epsilonproteobacteria in general to oxygen, we also investigated the cellular response of S. multivorans to oxygen by analyzing the organism's proteome.
MATERIALS AND METHODS
Cultivation
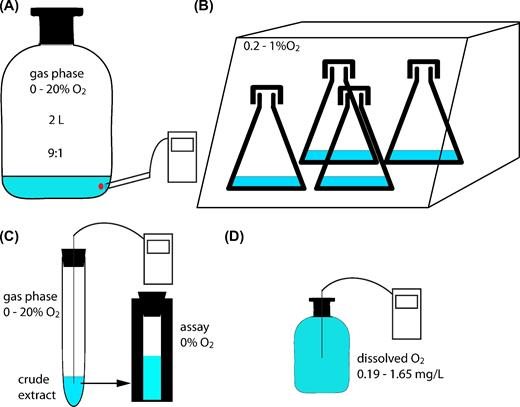
Sulfurospirillum multivorans (DSMZ 12446) was cultivated under anoxic conditions at 28°C in a defined mineral medium (Scholz-Muramatsu et al.1995) without vitamin B12 (cyanocobalamin). Desulfitobacterium hafniense Y51 was cultivated in the same medium but with 0.2% (w/v) yeast extract. Pyruvate (40 mM) was used as electron donor, oxygen and/or PCE as electron acceptor. PCE was added to the medium (10 mM nominal concentration, corresponding to ∼200 μM in the aqueous phase) from a hexadecane stock solution (0.5 M, resulting in a hexadecane to medium ratio of 2% (v/v)). The initial content of oxygen was measured with a manometer by adding the required amount of pure oxygen to bottles containing a gas phase of 1 bar nitrogen. The starting culture for all experiments contained S. multivorans cultivated for 60 transfers on pyruvate (40 mM) and fumarate (40 mM) plus 0.2% yeast extract, resulting in a downregulated PCE respiration gene expression (John et al.2009; Goris et al.2015). For adaptation and to avoid transfer of remaining substrates, three sequential pre-cultures were cultivated in rubber-stoppered 200-mL glass serum bottles with a ratio of gas to liquid phase of 1:1 and with the same electron acceptor used in the experiment (i.e. PCE or oxygen). For the experiments, the medium was inoculated with the pre-cultures (10%) and the cultures were cultivated in rubber-stoppered glass bottles (Fig. 1A). Gas to liquid phase ratio was 9:1 in 2-L bottles containing cultures with oxygen (Linde AG, Leuna, Germany) or 1:1 with PCE as terminal electron acceptor. The cultures were shaken at 150 rpm at 28°C.
Schematic overview of cultivation conditions and methods applied. (A) Cells cultivated in 2-L bottles for proteomics and PCE dechlorination in the presence of oxygen measured with optode spots. (B) Cells cultivated in Erlenmayer flasks closed with aluminum caps in hypoxic station. (C) Photometric PCE dechlorination assay to measure oxygen stability of crude extracts. (D) Cell suspension experiment to elucidate the effect of dissolved oxygen on PCE dechlorination.
When investigating the oxygen stress response of S. multivorans, oxygen was added to the gas phase of exponentially growing cells with PCE as electron acceptor to a final amount of 5% or 20% oxygen. Cultivation was then continued for 6.5 h until harvesting. To analyze the proteome after induction with PCE, S. multivorans cells cultivated for at least 60 transfers with fumarate as sole electron acceptor were transferred to medium with pyruvate/PCE (three replicates harvested in the exponential growth phase) or pyruvate/PCE plus 1.86 mg/L oxygen (5% oxygen in the gas phase, six bottles). Three of the latter bottles were harvested when the oxygen concentration in the medium was consumed to <0.17 mg/L. The remaining three cultures were harvested after PCE dechlorination had started, as measured by cDCE production. The cultivation of S. multivorans with PCE in the presence of constant oxygen concentrations was performed as follows. First, a culture of S. multivorans (100 ml) cultivated with PCE was harvested under anoxic conditions. The resulting cell pellet was washed twice with anoxic buffer (100 mM Tris-HCl pH 7.5) to get rid of residual chlorinated ethenes. The cells were then resuspended in 100 ml medium containing pyruvate (40 mM) without PCE. This cell suspension served as inoculum for the cultures (10 ml pyruvate/PCE medium in 50 ml Erlenmeyer flasks closed with loose aluminum caps to allow gas exchange) to achieve an initial OD578 of 0.05 to 0.08. The cultures were incubated at 28°C under rigorous shaking (360 rpm) in a Whitley H35 hypoxystation (Don Whitley Scientific, UK) with a nitrogen atmosphere containing 0.2%, 0.5% or 1% oxygen (Fig. 1B). When the experiment was performed with D. hafniense Y51, the medium contained yeast extract (0.2%). Over a period of 6 h, samples were taken hourly for the quantification of chlorinated ethenes. Uninoculated medium-containing flasks treated the same way served as control for PCE evaporation during incubation.
Bacterial growth was monitored photometrically by measuring the optical density at 578 nm or by the determination of the protein concentration with the Bio-Rad reagent (Bio-Rad Laboratories, München, Germany) using the Bradford assay (Bradford 1976). Bovine serum albumin was used as standard. All cultivations were performed in triplicates, except where stated otherwise. Growth phases were determined by plotting the growth curves to semilogarithmic scales.
Cell harvesting and sample preparation
Sulfurospirillum multivorans cells were harvested by centrifugation (12 000 × g, 10 min at 10°C). The cell pellets were washed once in 50 mM Tris-HCl (pH 7.5) and resuspended (2 ml per g wet weight) in the same buffer containing ∼20 mg DNase I (AppliChem, Darmstadt, Germany) and ∼40 mg of protease inhibitor (cOmplete Mini, EDTA-free; Roche, Mannheim, Germany). The cells were disrupted using a French Press (6.9 MPa = 1000 psi). Cell debris was removed by centrifugation (6000 × g, 10 min at 4°C). Protein was precipitated using acetone and 50 μg was used for further analysis. Protein pellets were resuspended in 5× sample buffer (0.31 M Tris-HCl pH 6.8; 10% sodium dodecylsulphate (SDS) (w/v); 30% glycerol (v/v); 25% mercaptoethanol (v/v), 0.05% bromophenol blue (w/v)) and denatured for 10 min at 66°C. SDS polyacrylamide gel electrophoresis was carried out in a 12.5% acrylamide gel over a running distance of 2 cm. Lanes were cut, destained, and proteins reduced and alkylated as described before (Goris et al.2016) prior to digest with trypsin (Promega, Madison, WI, USA) overnight at 37°C. Extracted peptides were desalted using ZipTip-μC18 material (Merck Millipore, Darmstadt, Germany). After evaporation of solvents under vacuum, samples were resuspended in 0.1% formic acid containing 2% acetonitrile before liquid chromatography coupled mass spectrometry (LC–MS/MS) analysis.
Mass spectrometry and proteome data analysis
Mass spectrometric analysis was performed as described previously (Goris et al.2015, 2016). In short, an Ultimate 3000 nanoRSLC system (Thermo Scientific, Germering, Germany) was used to separate peptides with a 90-min gradient. After electron spray ionization, peptide masses were analyzed in an Orbitrap Fusion mass spectrometer (Thermo Scientific, San Jose, CA, USA) operated in data-dependent mode. Further details of the applied method can be found in the supplementary materials section (Supporting Information).
LC–MS/MS data were analyzed using Proteome Discoverer (v1.4.1.14, Thermo Scientific). MS/MS spectra were searched against the S. multivorans database containing 3191 non-redundant protein-coding sequence entries (NCBI Genbank, accession number CP007201.1) using the SEQUEST HT and MS Amanda search engines with the following settings: trypsin as cleaving enzyme, oxidation of methionine as dynamic and carbamidomethylation of cysteine as static modification, up to two missed cleavages, MS mass tolerance set to 10 ppm and MS/MS mass tolerance to 0.05 Da. False discovery rate for peptides was set to <0.01 (Table S1, Supporting Information). Quantification of proteins was performed using the average of top 3 peptide area (Bondarenko, Chelius and Shaler 2002) as implemented in ProteomDiscoverer 1.4. Protein quantification was considered successful for proteins detected in >50% of biological replicates, otherwise they were classified as identified but not quantified proteins. After log10 transformation, the protein abundance values were normalized to the median and bioinformatic analysis was applied by principal component analysis and t-test statistics (R). Significance threshold P < 0.05 in a two-tailed test were considered as significantly altered.
Tetrachloroethene reductive dehalogenase (PceA) activity measurement in crude cell extracts
The cultures were harvested in the exponential growth phase (as determined by growth curve recording) by centrifugation (12 000 × g, 10 min at 10°C). The cells were resuspended in 2 volumes (ml/g biomass) of anoxic buffer (100 mM Tris-HCl pH 7.5, made anoxic via 30 vacuum/nitrogen flush cycles) and disrupted using a French Press (6.9 MPa = 1000 psi). Defined amounts of the cell extracts were transferred to anoxic vials (nitrogen atmosphere) closed with rubber stoppers (Fig. 1C). In the vials, different oxygen concentrations (0%, 1%, 2.5% and 5%) were adjusted by transferring defined amounts of air into the gas phase with a syringe. For 20% oxygen in the gas phase, the nitrogen atmosphere in the vial was replaced with air. The oxygen concentration in the medium and gas phase was monitored with a Microx4 oxygen meter (PreSense-Precision Sensing GmbH, Regensburg, Germany). Vials were incubated at 28°C under stirring. At defined time points, the activity of PceA was measured as described earlier using a photometric assay with Tris-HCl buffer pH 7.5, containing methyl viologen reduced by titanium(III) citrate, as artificial electron donor (Neumann, Wohlfarth and Diekert 1996). Specific activity values are given as nkat/mg or U/mg (1 U = 1 μmol per min).
PCE dechlorination in cell suspensions (resting cells)
A defined mineral medium (Scholz-Muramatsu et al.1995) with pyruvate (40 mM) was used, but without resazurin, cysteine, iron and trace element solution to prevent bacterial growth. The experiment was conducted in 200-mL rubber-stoppered glass serum bottles. To adjust specific oxygen concentrations in the reaction medium (0%, 2.5%, 5% and 7.5% oxygen), air was injected with a syringe to a 100-ml medium. To equilibrate the oxygen concentrations of the gas and liquid phases, the bottles were shaken rigorously (300 rpm) for 6 h. The medium of two bottles (1:1 gas:liquid phase) was then combined in one bottle for the experiment to ensure a gas phase as small as possible (Fig. 1D). PCE (200 μM) was dissolved in the medium by stirring overnight. To ensure a constant reaction temperature, the bottles were pre-incubated for 1 h at 28°C. Afterwards, 5% of an exponentially growing S. multivorans or D. hafniense Y51 culture was added. The inoculum was resuspended in buffer beforehand to minimize the transfer of nutrients. The bottles were incubated at 28°C while stirring. Samples were taken every 20 min and analyzed for chlorinated ethenes; growth was determined at the beginning and at the end of the experiment.
Immunoblot analysis
From all biological replicates used in the proteome analysis, 10 μg protein was subjected to denaturing SDS-PAGE (12.5%) and afterwards blotted onto a polyvinylidene difluoride membrane (Roche) using a semidry transfer cell (Bio-Rad) according to the protocol described by John et al. (2009). The PceA antiserum (primary antibody) was diluted 500 000-fold. The primary antibody was detected via a secondary antibody (diluted 1:20 000) coupled to alkaline phosphatase (Sigma-Aldrich, Munich, Germany).
Analysis of metabolites
Dechlorination of PCE was monitored by gas phase GC-FID analysis of culture samples using a Clarus 500 gas chromatograph as described in Mac Nelly et al. (2014). Detection limit of chlorinated ethenes was 0.5 μM. The oxygen concentration in the medium and in the gas phase during oxygen respiration in 2-L bottles was measured via fluorescence intensity changes of oxygen-sensitive optode spots (POF-PfSt3, Presens, Regensburg, Germany) and a corresponding detection device from the same company (Fibox 3 LCD trace v7, Fig. 1A).
In other experiments, the oxygen concentration was measured with the Microx4 oxygen meter and a needle-type oxygen microsensor NTH-PSt7 (PreSense-Precision Sensing GmbH, Fig. 1C and D). Temperature was measured in separate bottles to calculate the oxygen concentration in the medium. The measurement range of the oxygen sensor was from 0% to 100% (0–45 mg/L) oxygen. The optode spots had a detection limit of 15 μg/L (0.03%) dissolved oxygen, the microsensor NTH-PSt7 of 10 μg/L (0.02%).
Redox measurements and redox potential adjustment
The redox potential of the medium in growing cultures (40 mM formate as electron donor, 5 mM acetate as carbon source and 10 mM PCE in a hexadecane phase as electron acceptor) was adjusted using a 3-electrode-potentiostat (Wenking LB 81 M, Bank Elektronik, Göttingen, Germany) with a graphite working electrode. For the detailed experimental set-up see Fig. S1 (Supporting Information). The potential was measured against a Ag+/AgCl reference electrode (Ref100, Unisense A/S, Århus, Denmark) with a standard potential of +207 mV. Redox potentials in the medium were measured with a redox electrode (Pt4800-M5-S7, Mettler Toledo, Gießen, Germany). Before inoculation, the medium was made anoxic (30 vacuum/flush cycles with nitrogen) and adjusted to a defined redox potential of either –600, –200, +200 or +400 mV. Incubation temperature was 28°C. During cultivation, the redox potential of the medium was adjusted and kept constant at the given value by the potentiostat under anoxic conditions.
RESULTS
Proteome response to oxygen respiration compared to PCE respiration
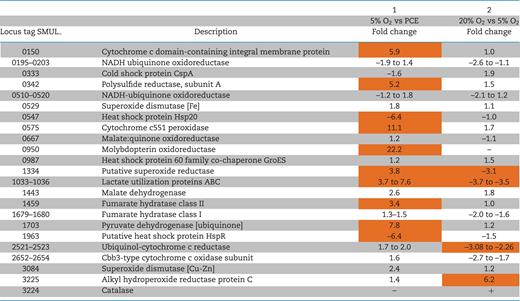
In a previous study, Sulfurospirillum multivorans was shown to grow with O2 as terminal electron acceptor and an O2 concentration of 5% proved optimal for growth (Goris et al.2014). To identify proteins of the oxygen respiratory chain or involved in stress response to ROS, the proteomes of S. multivorans cells cultivated with either PCE or O2 (5% in the gas phase) were analyzed and compared. The protein abundance value (mean of top 3 peptide area) of 35 quantified proteins differed significantly between the two different growth conditions (Table S1, data sheet S3 and Fig. S2A, Supporting Information), while the variance of the datasets from three replicates was low (Fig. S3A, Supporting Information). The proteins encoded by genes in the OHR region were found to be present exclusively in PCE-cultivated cells. The catalytic subunit of a molybdopterin oxidoreductase of the uncharacterized MopB3 family (SMUL_950) was the protein with the highest increase in the presence of oxygen compared to PCE (22-fold, Table 1). Two proteins putatively involved in oxygen-related stress response were also found to be present in higher amounts with oxygen: a cytochrome c551 peroxidase belongs to one of the most differentially quantified proteins (SMUL_0575, 11-fold), while the amount of a putative superoxide reductase was 4-fold increased (SMUL_1334, Table 1). An uncharacterized cytochrome c-like membrane protein (encoded by SMUL_150) with 445 amino acid residues and 8 transmembrane helices showed higher abundance in oxygen-cultivated cells than in PCE-cultivated cells (6-fold higher, P-value 0.002). The main enzyme complexes involved in oxygen respiration, cbb3-type cytochrome c oxidase (encoded by SMUL_2652–2655) and cytochrome c reductase (bc1 complex, encoded by SMUL_2521–2525) were found in similar amounts in the two compared proteomes (see Table 1 and Table S1, data sheet S2, Supporting Information), indicating a constitutive production.
Level changes of selected proteins involved in oxygen respiration, redox reactions, stress response to 5% oxygen or stress response to atmospheric oxygen concentration.
 |
 |
Proteins marked in orange are significantly up- or downregulated (fold change of ±2.82, P-value ≤ 0.05).
–: not detected. + : quantified only with 20% O2.
Level changes of selected proteins involved in oxygen respiration, redox reactions, stress response to 5% oxygen or stress response to atmospheric oxygen concentration.
 |
 |
Proteins marked in orange are significantly up- or downregulated (fold change of ±2.82, P-value ≤ 0.05).
–: not detected. + : quantified only with 20% O2.
Proteome response of cells exposed to atmospheric oxygen concentrations
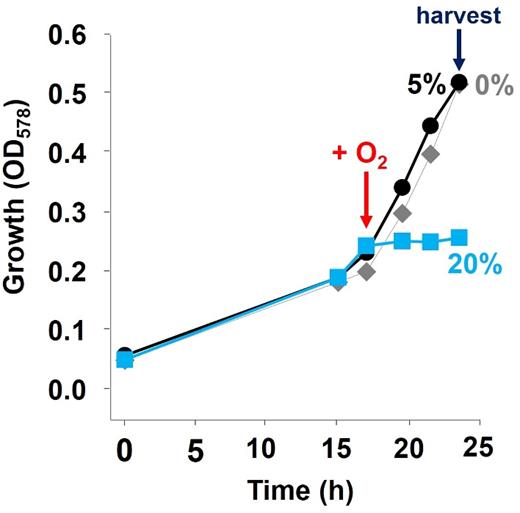
Next, we tested the impact of 6.5 hours exposure to 20% oxygen compared to exposure to 5% oxygen on the proteome of PCE-cultivated S. multivorans cells. The growth of cultures exposed to 5% oxygen was similar to that of control cultures (pyruvate/PCE without oxygen), while the culture exposed to 20% oxygen stopped growing (Fig. 2). The dechlorination ceased after O2 addition (data not shown). Eighteen proteins were significantly regulated when comparing both conditions. Four of these were found to be more abundant when cells were exposed to 20% oxygen (Table S1, data sheet S4, Supporting Information). Of these four proteins, one is predicted to be functionally related to oxygen stress response, an alkyl hydroperoxide reductase AhpC (SMUL_3225) with 6.2-fold higher amounts than in cells exposed to 5% oxygen (Table 1). A catalase, not detected in any other proteome investigated in this study, was detected at low levels (abundance of 6.8, in the lowest quartile of quantified proteins) in cells incubated with 20% oxygen.
Stress response of S. multivorans to atmospheric oxygen concentrations after cultivation with PCE. Cells were supplied with either 5% or 20% oxygen in the gas phase in the exponential phase. After further 6.5 h, both cultures were harvested for proteomics. Gray diamonds represent the growth curve of the control culture without oxygen supplement.
The two superoxide dismutases encoded by S. multivorans SMUL_0529 (Fe-type) and SMUL_3084 (Cu-Zn-type) were found in similar amounts. The first one is among the top 25% of proteins from cells cultivated with 5% oxygen (value of 8.5), and the latter among the bottom 30% (7.0). Several proteins involved in oxygen respiration, e.g. the bc1 complex and the cytochrome c oxidase, were found to be downregulated in cells exposed to 20% oxygen.
PCE dechlorination by cell extracts and resting cells in the presence of oxygen
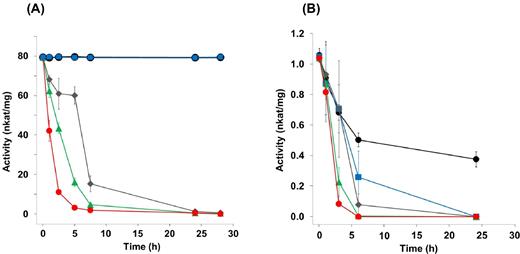
Since the proteome data showed that the oxygen respiratory chain proteins are also expressed under PCE-respiring conditions, oxygen might be consumed at the membrane of S. multivorans. This rapid oxygen reduction could protect oxygen-labile proteins involved in PCE respiration, which might allow the latter to proceed even when cells are cultivated in the presence of oxygen. Therefore, we examined the oxygen sensitivity of PCE dechlorination in cell extracts and resting cells obtained from cultures cultivated with PCE. Since the activity assays of reductive dehalogenases are dependent on artificial electron donors, which would be immediately oxidized by oxygen, we incubated crude extracts of S. multivorans in reaction buffer without electron donor, but containing different amounts of oxygen. PceA activities from samples taken at defined time points after starting the incubation were measured using reduced methyl viologen as artificial electron donor (redox potential E°' = –446 mV). The initial PceA activity for all experiments was ∼80 nkat mg−1 (4.8 U/mg) (Fig. 3A). At atmospheric oxygen concentrations, the enzymatic activity half-life was about 2 h, comparable to that reported for the purified enzyme (Neumann et al.1996). However, when lowering the oxygen concentration to 1% in the gas phase, the activity was stable and comparable to that with 0% oxygen (Fig. 3A) and the PCE dechlorination remained constant for about 96 h (data not shown).
Activity of the reductive dehalogenase PceA of S. multivorans and D. hafniense Y51 in the presence of different oxygen concentrations applied to crude extracts before measurement. (A) PceA activity of crude extracts of S. multivorans. (B) PceA activity of crude extracts of D. hafniense Y51. The crude extracts were incubated at 28°C under stirring and were exposed to the oxygen concentrations indicated. Black dots, without O2; blue squares, 0.37 mg/L O2; gray diamonds, 0.93 mg/L O2; green triangles, 1.86 mg/L O2; red dots, 7.44 mg/L O2.
Since it was reported that Desulfitobacterium spp. can tolerate low amounts of oxygen (Madsen and Licht 1992; Utkin, Woese and Wiegel 1994), the same experiment was repeated with cell extracts of the PCE-dechlorinating Desulfitobacterium hafniense Y51. Even in the absence of oxygen, the enzyme was not stable in crude extracts, losing 50% of its dehalogenation activity after 6 h (Fig. 3B). In cell extracts incubated with 1% oxygen in the gas phase (dissolved oxygen 0.37 mg/L), the enzymatic half-life was 3 h, indicating a much lower PceA stability in D. hafniense Y51 compared to PceA in cell extracts of S. multivorans.
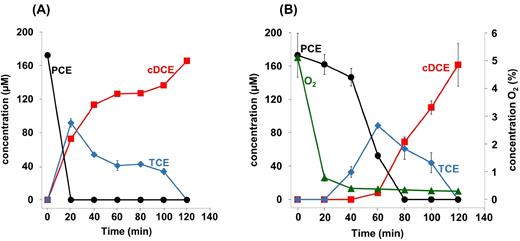
To investigate PCE dechlorination under oxic conditions in cell suspensions (resting cells), S. multivorans cells were transferred into a pyruvate/PCE/oxygen-containing solution similar to the growth medium, but lacking essential supplements to prevent growth. Cell suspensions without oxygen were used as positive control. Initial oxygen concentrations 0.932, 1.864 or 2.796 mg/l in the medium were applied. The dechlorination of PCE started in cell suspensions (∼20 μg/ml cell protein) without oxygen directly after the inoculation of the reaction mixture with S. multivorans (Fig. 4A). In contrast, in cell suspensions with oxygen the dechlorination of PCE did not start before oxygen was depleted to concentrations below 0.19 mg/l (0.5%) (Fig. 4B). The oxygen concentration in the medium decreased to a constant level of ∼0.1 mg/ml as measured with a needle-type oxygen microsensor (see Materials and Methods section). The oxygen concentration in the control with anoxic medium was below the detection limit (0.01 mg/ml).
Dechlorination of PCE by cell suspensions of S. multivorans in the presence of different oxygen concentrations. (A) Dechlorination of PCE and formation of cDCE and TCE in the absence of oxygen. (B) Dechlorination of PCE and formation of cDCE and TCE as well as the consumption of oxygen. The initial oxygen concentration was 5% in the gas phase (as measured to be 1.86 mg/L in the medium); the protein concentration was 12–20 μg/ml.
The cell suspension experiments were also performed with D. hafniense Y51 cells (∼15 μg/ml cell protein). With initial oxygen concentrations of 5% and 7.5%, neither the oxygen nor the PCE concentration decreased. When using lower oxygen concentrations of 1% or 2.5%, a decrease of oxygen to <0.03% or 0.7%, respectively, was measured. In the experiment with an initial oxygen concentration of 1%, PCE dechlorination was only observed after oxygen was depleted (Fig. S4, Supporting Information). An increase in biomass, as determined at the beginning and at the end point of the experiment, was not observed in both D. hafniense and S. multivorans.
PCE dechlorination by growing cells in the presence of oxygen
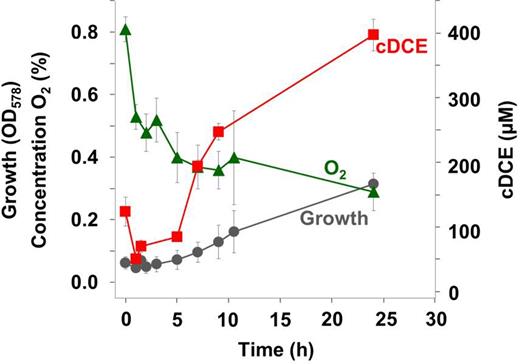
To assess if PCE can be dehalogenated under oxic conditions by growing S. multivorans cells, the organism was cultivated with O2 concentrations of 5%, 2% and 1% in the gas phase corresponding to measured aqueous concentrations of 1.86, 0.746 and 0.373 mg/L, respectively (Fig. 1A). PCE dechlorination was not detected at oxygen concentrations of 5% or 2%, even after oxygen consumption due to respiration (data not shown). With 1% initial oxygen in the gas phase, the cells started to dechlorinate PCE as soon as the oxygen concentration dropped to less than about 0.5% within about 2 h (Fig. 5). Obviously, it was not possible to maintain constant oxygen concentrations in these bottles, despite the large gas phase. Moreover, the used optode spots did not allow for accurate measurement of oxygen concentrations in the medium below 0.2 mg/L. Therefore, cultures were incubated in flasks in the hypoxystation at defined constant oxygen concentrations of 0.2%, 0.5% or 1% in a second approach (Fig. 1B). The formation of the PCE dechlorination products trichloroethene (TCE) and cDCE was observed up to an oxygen concentration of 0.5% but not at 1%. Product formation started approximately 1 h after starting the experiment. The quantification of PCE dechlorination was not possible, since a major portion of the chlorinated ethenes evaporated under the experimental conditions applied. All four replicates showed formation of cDCE and TCE at an oxygen concentration of 0.2%, while TCE was the only dechlorination product detected in two of the four samples at an oxygen concentration of 0.5%. From these observations, it can be concluded that S. multivorans is able to dechlorinate PCE at oxygen concentrations of up to ∼0.5% in the gas phase, corresponding to 0.19 mg/L dissolved oxygen in the liquid phase. Desulfitobacterium hafniense Y51 did not dechlorinate PCE in the presence of any of the tested oxygen concentrations in both experimental set-ups.
Dechlorination of PCE by cultivating S. multivorans cells in the presence of oxygen. Product (cDCE) formation and growth of S. multivorans with pyruvate as electron donor and PCE as electron acceptor in the presence of oxygen (initial concentration in the gas phase 1%, 0.373 mg/L O2 in the medium). The experiment was performed in duplicates. The higher initial cDCE concentration is caused by carrying over cDCE from the pre-culture which subsequently dissolved in the medium.
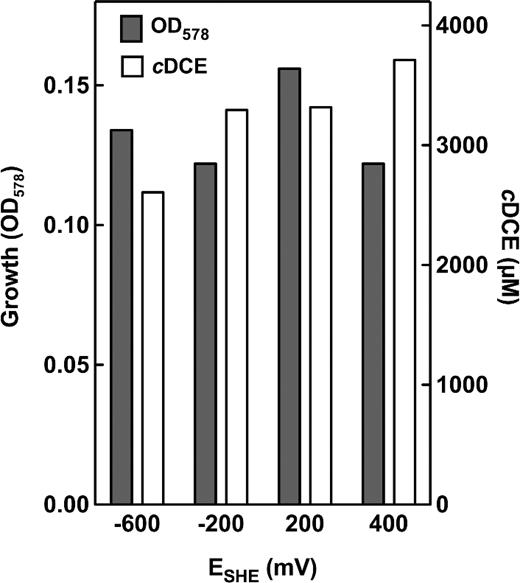
The redox potential of the growth medium with 5% oxygen was measured to be ∼ +230 mV (E°' vs the hydrogen electrode; data not shown). Since it was not known whether oxygen or the redox potential or both affect the organism's ability to respire PCE, the influence of the redox potential on PCE respiration was tested in the absence of oxygen. The redox potential in the range of –600 to +400 mV as applied by potentiostat had no significant effect on growth or PCE dechlorination as detected by cDCE formation (Fig. 6).
Redox potential-dependent dechlorination of PCE to cDCE in S. multivorans cultures. During cultivation (formate/acetate/PCE), the redox potential of the medium was kept constant at the desired value. After 20 h of cultivation, OD and cDCE were determined.
Induction of PCE respiration in the presence of oxygen
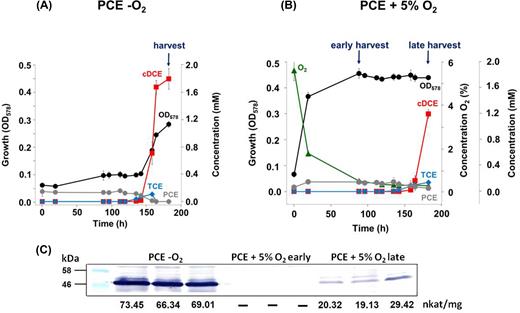
The influence of oxygen exposure on induction of PCE respiration in OHR-downregulated cells was investigated by inoculating OHR-downregulated cells (cultivated without PCE for more than 60 transfers) in medium containing 10 mM PCE only or PCE plus oxygen (5%) as electron acceptor. The latter cultures reached the stationary phase within 88 h and reached an optical density of about 0.47. At this time point, three of six cultures fed with oxygen and PCE were harvested for proteomic and biochemical studies (designated O2 early) and the other three cultures were further incubated and harvested later (O2 late) (Fig. 7B). PCE dechlorination started approximately at the same time in both cultures (160 h for PCE/5% oxygen and 135 h for PCE only) (Fig. 7A and B). After another 48 h, when PCE was completely depleted in the PCE only culture (=exponential growth phase) (Fig. 7A) and 72% of the PCE was depleted in the O2 late culture (Fig. 7B), both cultures were harvested. In all samples, PceA was monitored by western blot analysis and enzymatic PCE dechlorination activity. Active reductive dehalogenase PceA was detected in all cultures harvested after 183 h of cultivation, although at a much lower level in the PCE/oxygen-cultivated cells as shown in a western blot of the respective cultures (Fig. 7).
Induction of PCE respiration in the presence of oxygen. For the induction of organohalide respiration in S. multivorans in the presence of oxygen, cells with downregulated PCE respiration were used (see Materials and Methods section). Pyruvate served as electron donor for all cultures. (A) As control, three cultures were amended with PCE as sole electron donor. (B) Six cultures were supplied with PCE + 5% O2. Three of these cultures were harvested earlier in the stationary phase (early), while the other three cultures were harvested in the late stationary phase (late) when the cells had started PCE dechlorination. PCE dechlorination start is indicated by a red arrow, harvesting time points are indicated by dark-blue arrows. Black dots, growth curve; gray dots, PCE; blue diamonds, TCE; red squares, cDCE; green triangles, oxygen. There is no apparent stoichometry between PCE and cDCE concentration due to the different solubility of the two chlorinated ethenes. (C) Western blot and PCE-dechlorinating activities of crude cell extracts.
In the proteome, the samples of cells harvested early (O2 early) showed a wider distribution of the replicates in the principle component analysis compared to the samples harvested at the second time point (Fig. S5, Supporting Information). This can most likely be attributed to the heterogeneity of the cultures harvested early in the stationary phase of the oxygen-amended cultures.
In cultures harvested directly after oxygen consumption (O2 early), only PceA, the two-component response regulator (SMUL_1539) and a putative FMN-binding protein (SMUL_1575) were quantified from the OHR core region, in which the PCE respiration proteins are clustered (Fig. 8, Table S1, data sheet S5 and S7, Supporting Information). While the response regulator (SMUL_1539) was detected in similar amounts as in cells cultivated with PCE as sole electron acceptor, the reductive dehalogenase PceA and the FMN-binding protein were quantified in more than 100-fold lower amounts. In the O2 late cells, nine proteins of the OHR region (PceA, the regulator, an IscU-like protein and seven proteins inside or downstream of the corrinoid biosynthesis gene cluster) were quantified in contrast to 28 proteins encoded by this region in cells cultivated with PCE only (Fig. 8, Table S1, data sheet S7, Supporting Information). Additionally, seven corrinoid biosynthesis proteins were identified in one replicate. Seven of the nine quantified OHR proteins were upregulated (5 to 16-fold) in cells cultivated with PCE only compared to the O2 late cells (Fig. 8, Table S1, data sheets S5 and S6).
Identified gene products of the OHR core region as detected by proteomic analysis. Each square correlates to a given gene product identified or quantified under given cultivation/experimental condition (at the left). For quantified proteins, the protein intensity is provided (color code at the far right, normalized and logarithmized average of top 3 peptide area as described in the Materials and methods section), proteins identified but not quantified are represented by shaded squares.
DISCUSSION
Response of Sulfurospirillum multivorans to oxygen
The terminal oxidase used by S. multivorans for oxygen respiration is a cbb3-type cytochrome oxidase, known for its high affinity to oxygen (Pitcher, Brittain and Watmugh 2002). This enzyme is most likely constitutively expressed, as suggested by its high protein abundance levels not only with oxygen, but also in the presence of PCE (this study), fumarate or nitrate (Goris et al.2015) as electron acceptors. Anaeromyxobacter dehalogenans encodes for a similar cbb3-type cytochrome oxidase (Thomas et al.2008), putatively used by this OHRB for its microaerobic lifestyle. Desulfitobacterium hafniense Y51 instead contains a cluster encoding a putative cytochrome bd oxidase (DSY4054– 4056) known for their low oxygen affinity (Borisov et al.2011). The presence of this enzyme might be responsible for the observed oxygen consumption in D. hafniense Y51 cell extracts. The two other putative terminal oxidases (bd- and bo3-type) encoded by S. multivorans (Goris et al.2014) were not detected in any proteome data and might serve other functions not related to the presence of oxygen. The oxygen-dependent upregulation of another cytochrome c-like protein and a molybdopterin oxidoreductase with unknown function might hint to their role in oxygen respiration or oxygen stress response in S. multivorans.
While S. multivorans is able to grow with 5% oxygen in the gas phase, atmospheric concentrations of oxygen inhibit growth of this microaerobic bacterium. The most likely explanation for the growth inhibition might be the high concentration of ROS in cells confronted with 20% oxygen. This is reflected in the proteome response of S. multivorans cells exposed to atmospheric oxygen concentrations. On the one hand, cells incubated with 20% oxygen stopped growing and exhibited a shutdown of catabolic enzymes that might produce ROS. On the other hand, different enzymes potentially scavenging ROS are found in the proteome of these cells. The major ROS-scavenging enzymes are superoxide dismutase (SOD), superoxide reductases (SOR), catalase and cytochrome c peroxidase (Ccp) (Ezraty et al.2017), all of which are encoded in the genome of S. multivorans. Desulfitobacterium hafniense Y51 and DCB-2 encode three of these four ROS-scavenging enzymes; they lack a gene encoding SOR. However, only catalase gene expression was induced in strain DCB-2 after exposure to oxygen (Kim et al.2012). Two of the ROS-scavenging enzymes seem to be upregulated with 5% oxygen in S. multivorans, Ccp and a putative SOR. The latter reduce superoxide to hydrogen peroxide (Sheng et al.2014), while Ccps are assumed to catalyze the conversion of hydrogen peroxide to water when cells are stressed with oxygen (Atack and Kelly 2007). With 20% oxygen, catalase, another enzyme reducing hydrogen peroxide, was detected. This is in line with observations in Escherichia coli, where catalase is recruited when hydrogen peroxide levels are high (Imlay 2013). Besides the catalase, AhpC is upregulated with 20% oxygen. AhpC is described as the peroxide-reducing protein of the alkyl hydroperoxide reductase system (La Carbona et al.2007), which convert hydrogen peroxide and organic hydroperoxides to water and the corresponding alcohols, respectively (Wood et al.2003). Of the two types of SODs, the Fe-type SOD may be the main superoxide anion radical scavenger, since it was found in larger amounts in all S. multivorans proteomes than the Cu-Zn SOD.
Reductive dechlorination of PCE in the presence of oxygen
Besides Anaeromyxobacter dehalogenans, S. multivorans is the only described microaerobic OHRB. (Sanford, Cole and Tiedje 2002; Goris et al.2014) However, only the latter respires halogenated alkenes and therefore might be also able to reductively dehalogenate PCE and TCE in the presence of oxygen. However, the quantification of reductive dechlorination of PCE in the presence of low amounts of oxygen was difficult to achieve: Besides the technical difficulties in precise oxygen measurements, cultivation of S. multivorans with PCE and oxygen in closed bottles resulted in the rapid consumption of oxygen. The threshold of oxygen concentration, at which PCE dechlorination became possible, could only be roughly determined this way. A constant supply of oxygen in the medium was reached via cultivation of S. multivorans in open flasks, but this made the quantification of the volatile chlorinated ethenes impossible. Therefore, we gathered results of three different experiments involving PCE dechlorination measurement under oxygen. The combined results point toward the reductive dehalogenation being mediated at up to about 0.19 mg/L oxygen. This might be ecologically relevant in the oxic-anoxic interphase of PCE-contaminated environments. Above 0.19 mg/L oxygen, PCE dechlorination was inhibited. A fast downregulation of PceA could be excluded, since the downregulation of this enzyme takes ∼100 generations, when fumarate, nitrate and oxygen are used as sole electron acceptors instead of PCE (John et al.2009; Goris et al.2015, unpublished data). Additionally, the induction of the OHR gene region of PceA downregulated S. multivorans cells was not much slower with additional oxygen as electron acceptor. Cell extracts of S. multivorans containing the key enzyme of the reductive dehalogenation, the corrinoid and Fe-S cluster-containing PceA exhibited a tolerance to 1% oxygen for at least 48 h. Therefore, the reported sensitivity of PceA toward oxygen (Neumann, Wohlfarth and Diekert 1996) might be not the only limiting factor in PCE dechlorination in the presence of oxygen. The oxygen instability of PceA is most likely due to an effect on its two [4Fe-4S] clusters, which often convert to other forms when exposed to oxygen (Imlay 2006). In contrast to S. multivorans, cell extracts of D. hafniense Y51 exhibited low stability at 1% and even without oxygen. Probably due to this, D. hafniense Y51 cells did not mediate PCE dechlorination in the presence of oxygen. Another factor differentiating S. multivorans from D. hafniense Y51 is the presence of different terminal oxidases, which might cause the faster oxygen consumption rate in S. multivorans cell extracts. This possibly contributes to the ability of only S. multivorans to dechlorinate PCE in the presence of oxygen.
Since the PCE respiratory chains of both organisms likely involve other redox-active proteins, it is conceivable that these are even more oxygen-sensitive than PceA. This might apply to the PCE-induced putative quinol dehydrogenase of S. multivorans (Goris et al.2015), since the amino acid sequence of its periplasmic subunit indicates the presence of four [4Fe-4S] cluster-binding motifs (Goris et al.2014). The fact that reductive dehalogenation was independent of the environmental redox potential up to +400 mV came as a surprise, as in general the redox potential suitable for supporting OHR was thought to be lower. Whether reductive dehalogenation is mediated at high redox potentials due to S. multivorans actively lowering the periplasmic potential or whether reductive dehalogenation itself is independent of the redox potential cannot be concluded here.
CONCLUSION
With the current study, we showed that Sulfurospirillum multivorans can dechlorinate PCE in the presence of oxygen concentrations equal to or below 0.19 mg/L (0.5% in the gas phase) in contrast to the anaerobic bacterium Desulfitobacterium hafniense Y51. The proteomic response of S. multivorans to oxygen revealed two main enzymes that are upregulated as a stress response when the organism respires oxygen, an SOR and a hydrogen peroxide reductase. A constitutively expressed terminal oxidase might be one reason for the PCE respiration ability in the presence of oxygen. The findings of this study are important in studies on reductive dehalogenation in oxic-anoxic zones and give first clues into the proteomic response of microaerobic Epsilonproteobacteria to oxygen.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSEC online.
Acknowledgements
Benjamin Scheer is gratefully acknowledged for technical assistance and maintenance of the Orbitrap Fusion mass spectrometer. We thank Bernhard Schink (University of Konstanz) for lending his potentiostat and Manfred Rudolph (FSU Jena) for helpful comments on redox electrodes and the potentiostat. We appreciate the help of Nicole Engert-Ellenberger from the Microbial Immunology group at the Hans Knöll Institute in Jena during the work with their hypoxystation.
FUNDING
This work was supported by the German Research Foundation (DFG), as part of the research unit FOR 1530. The authors used the analytical facilities of the Centre for Chemical Microscopy (ProVIS) at the Helmholtz Centre for Environmental Research, which is supported by European Regional Development Funds (EFRE—Europe funds Saxony) and the Helmholtz Association.
Conflict of interest. None declared.