-
PDF
- Split View
-
Views
-
Cite
Cite
Siobhan Hogan, Emmanouil Kasotakis, Sean Maher, Brenton Cavanagh, James P O'Gara, Abhay Pandit, Tia E Keyes, Marc Devocelle, Eoghan O'Neill, A novel medical device coating prevents Staphylococcus aureus biofilm formation on medical device surfaces, FEMS Microbiology Letters, Volume 366, Issue 9, May 2019, fnz107, https://doi.org/10.1093/femsle/fnz107
Close - Share Icon Share
ABSTRACT
Prevention of device related infections due to Staphylococcus aureus biofilms on devices represents a significant challenge. Such infections have recently been shown to be dependent on the coagulation pathway via activation of pro-thrombin and fibrin production. Three direct-thrombin inhibitors, argatroban, hirudin and dabigatran, were examined to determine their effect on preventing S. aureus biofilm on plastic biochip surfaces under shear stress using an in vivo relevant model of infection. Surface functionalization of polyurethane discs via dityrosine covalent crosslinking with hirudin was performed and changes in bacterial density and microscopic appearances determined. The three direct-thrombin inhibitors prevented S. aureus biofilm formation on plasma-coated surfaces treated with these agents. Coating of polyurethane with one of these agents, hirudin, significantly inhibited biofilm formation on the modified surface. These findings reveal the exciting potential for coating biomaterial surfaces with direct thrombin inhibitors to prevent staphylococcal binding and subsequent device-related infections.
INTRODUCTION
The use of invasive medical devices is increasingly common in modern healthcare systems. Among the most commonly used medical devices are intravascular catheters (IVCs), such as arterial lines, central venous and peripheral venous catheters. Medical device related infections are a recognised complication of use of these, with staphylococci representing the most common causative organisms associated with these infections. In recent years, several anti-bacterial coatings have been suggested for use on these medical devices in an effort to reduce the incidence of these often difficult to treat infections by preventing bacterial attachment and subsequent biofilm formation (Hanna et al. 2006; Zhao et al. 2011; Monzillo et al. 2012). However, the use of these catheters within clinical practice is varied and surface modification has been limited by concerns relating to safety, expense and the potential emergence of resistance to the antimicrobial coating. Hence, novel coating of IVCs and other medical devices in prevention of infection is an area of significant research.
Based on emerging evidence of the role of S. aureus in the coagulation pathway, direct thrombin inhibitors have been recently suggested to have the potential to reduce S. aureus infection by blocking the interaction of these bacteria with human pro-thrombin (Van Ryn et al. 2013; Vanassche et al. 2010). Pro-thrombin is an important component of human blood and is involved in the coagulation cascade and subsequent clot development. Staphylococcus aureus has been shown to hijack this coagulation cascade by binding and activating pro-thrombin, leading to fibrin production (Vanassche et al. 2013). Fibrin production has recently been shown to be essential for S. aureus biofilm development in vivo (Zapotoczna et al. 2015) and S. aureus binds pro-thrombin at exo-site I via a coagulase/von Willebrand factor binding protein mediated interaction. Therefore, applying coatings to medical devices that are in contact with blood, in order to interfere with the attachment of staphylococci, is a rationale route to biofilm formation prevention. Three anti-coagulant drugs, which act as direct-thrombin inhibitors, were examined in this study; namely, argatroban, hirudin and dabigatran. Argatroban is a synthetic derivative of L-arginine, hirudin is a peptide of 65 amino acids with three disulfide bonds and dabigatran is a benzimidazole-based low molecular weight inhibitor of serine proteases. As these agents bind directly to thrombin, do not require a cofactor such as antithrombin to exert their effect and can inhibit both soluble thrombin and fibrin-bound thrombin (Lee and Ansell 2011), we therefore hypothesized that they have the potential to inhibit S. aureus binding to a foreign body in a competitive manner.
MATERIALS AND METHODS
Bacterial strains and growth conditions
The bacterial strains used in this study were MSSA strain SH1000 and MRSA strain USA300 JE2 (Horsburgh et al. 2002; Fey et al. 2013). Staphylococcus aureus strains were grown with aeration at 37°C in RPMI-1640 media (Gibco, Thermo fisher, MA, USA).
Preparation of test agents
Treatment agents were prepared to the following final concentrations in sterile water, argatroban 0.15–10 µM (Sigma-Aldrich), hirudin 8.3–34 µM (Sigma-Aldrich) and dabigatran 0.1–1.5 µM (Sigma-Aldrich).
Biofilm development under shear stress using a microfluidic flow system
Staphylococcus aureus biofilm was formed as previously described (Zapotoczna et al. 2015; Hogan et al. 2016). Platelet-poor plasma was obtained from healthy volunteers. A flow cell and pump was used to create a microfluidic model (Cellix Ltd., Ireland). Vena8 Fluoro+ flow chambers were coated with 100% plasma for 1 h at 37°C. Surfaces of the flow chambers were treated with PBS, argatroban 0.15–10 µM, hirudin 8.3–34 µM or dabigatran 0.1–1.5 µM for a further 1 h. Exponentially growing S. aureus cultures were adjusted to an OD600 0.7 in RPMI-1640 and then injected into each chamber and allowed to attach for 1 h at 37°C on an inverted microscope with a heated platform. The pump was activated and RPMI-1640 at a shear rate of 6.25 dynes (200 µl/min) was infused through chambers of the chip for 24 h. Biofilm density was examined in each chamber using brightfield, confocal and SEM image microscopy, experiments were performed in triplicate and representative images are shown.
Biofilm development under shear stress on polyurethane discs
Polyurethane (PU) discs (Direct plastics, UK) with a diameter of 2cm and a thickness of 0.6cm were placed in a 30mm petri dish (Thermo fisher, UK). Plasma was diluted to 20% (v/v) in carbonate buffer (pH 9.6) and used to precondition the surfaces of PU discs at 37°C for 1 h (Zapotoczna et al. 2015). Discs were then washed once with sterile water and treated with PBS, argatroban 0.15–10 µM, hirudin 8.3–34 µM or dabigatran 0.1–1.5 µM for a further 1 h. An overnight culture of the test organism, grown in RPMI-1640, that was diluted 1:1 to an OD600 of 0.7, was used to coat discs for 1 h statically before they were placed in fresh media under shear stress (4 dynes (highest available shear)) for 24 h at 37°C. Biofilm density and viability was determined by harvesting bacteria from discs to calculate CFU/ml and by confocal and SEM imaging, experiments were performed in triplicate and representative images are shown.
Surface functionalization of PU discs
Plasma etching
PU discs of 1 cm diameter and 0.2 cm thickness were deposited in an air plasma chamber (Harrick PDC-002 plasma cleaner) at 1000 mT with room air. Two time points were selected: 5 and 10 min.
Surface functionalization
Surface functionalization was performed as described elsewhere (Fancy and Kodadek 1999; Truong et al. 2011; Loebel et al. 2015). Briefly, the samples were individually immersed in 10 mL of 0.1 M NaCl and placed in an ice bath. Then 10 ml of 6 M NaOH aqueous solution was slowly added to each of the previous solutions while shaking, until the temperature reached 15°C. All the samples were then removed from ice bath and crystalline Bromoacetic acid (Sigma-Aldrich) (400mg, 2.8 mmol) was added and incubated for 60 min at 25°C, with constant shaking. The discs were finally washed with 0.1 M NaCl four times to remove excess reagent. After the washing step, the carboxyl coated discs were added in 10 ml of MES buffer (100 mM, pH 6.7), where 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride (DMTMM) (0.4 g, 1.4 mmol) and an equimolar concentration of Tyrosine amide (Bachem) (0.26 g, 1.4 mmol) were added at the same time and the solutions incubated for 1 h.
Hirudin functionalization
Hirudin (H0393-100UN) was dissolved in water at a 24μg/ml final concentration. 30 s prior to photo-crosslinking 35 μl of Hirudin solution were mixed with 6 μl of tris(bipyridine) ruthenium(Sugimoto et al. 2007) chloride (Ru(II)) 3 mM and 6 μl of ammonium persulfate 30 mM in an eppendorf tube in dark environment. Then the solution was deposited on the surface of the functionalized PU and was illuminated with a source of light with custom mesh of LEDs, 425 nm (Kingbright) from a distance of approximately 2 cm (luminous intensity of 500 mW/cm2). Finally, the samples were washed with PBS 1X.
Biofilm formation on hirudin coated PU surfaces
PU discs were coated as described above. Plasma was diluted to 20% (v/v) in carbonate buffer (pH 9.6) and used to precondition the surfaces of discs at 37°C for 1 h (Zapotoczna et al. 2015). An overnight culture of the test organism, grown in RPMI-1640, that was diluted 1:1 to an OD600 of 0.7, was used to coat discs for 1 h statically before they were placed in fresh media under shear stress (4 dynes) for 24 h at 37°C. Biofilm density and viability was determined by harvesting bacteria from discs and by confocal and SEM imaging.
Determining bacterial cell density
After treatment, biofilms were harvested from the surface of PU discs using TrypLETM (1X) (Gibco), a dissociation reagent, as described previously (Zapotoczna et al. 2015). Bacterial numbers were determined using a broth dilution and spread plate method on muller hinton agar (MHA) (Fannin, LIP, Ireland). Log10 transformation of CFU data were used to ensure normal distribution of the data.
Microscopy
Biofilm structure and treatment efficacy was analysed using a light microscope (Zeiss) and a confocal microscope (Zeiss) and image capture software LSM510. Brightfield images were captured using 100 X magnification. Bacterial cells within the biofilm were visualized using propidium iodine (20 mM). Optimized laser (HeNe (632.8 nm)) were used to excite the dyes and capture the fluorescence emitted from the cells, under a 400 X magnification. Laser strength and detector gain were selected before capturing the first image and not adjusted for the duration of the experiment. ImageJ software was used to calculate fluorescent intensity for each chamber.
Scanning electron microscopy
Bacteria were heat fixed onto the surfaces of each disc at 56°C for 1 h. Discs were submerged in liquid nitrogen, transferred to a quorum preparation chamber, sublimated for 40 min at −100°C, coated in platinum and imaged using a Zeiss Ultra Plus SEM and imaged at 5Kv using a SE2 detector. Discs were maintained at a temperature of −190°C throughout imaging.
Contact angle
The effect of surface modification on surface hydrophilicity was assessed by contact angle goniometry. Sessile drop profile was obtained using deionized water as contacting liquid at treated PU substrates under air at room temperature using a Dataphysics OCA 35 Series goniometer, with an electronic syringe unit, dropping a volume of 5 μL with a speed of 1 μL s−1.
Statistical analysis
For the CFU data generated from the assessment of biofilm prevention on PU discs, descriptive statistics (including mean and standard deviation) values were calculated for each test agent.
Ethical approvals
Blood donations were obtained from healthy adult donors. Written, informed consent was obtained from participants at the time of collection. Ethics approval for collection and use of blood was granted by the Ethics Committee of the Royal College of Surgeons in Ireland (REC820).
RESULTS
Pro-thrombin binding agents for the prevention of S. aureus biofilm formation
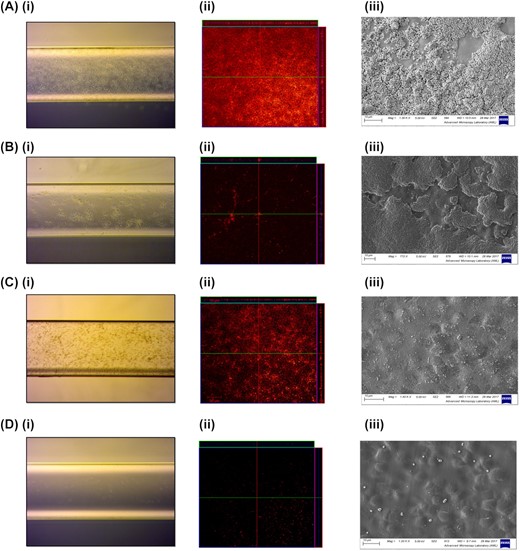
Three exosite I targeting agents, argatroban, hirudin and dabigatran, were examined at a range of concentrations to determine their effect on preventing S. aureus biofilm development on plastic biochip surfaces using an in vivo relevant model of infection. This involved exposing surfaces to human plasma (20% v/v) for 1 h prior to formation of the bacterial biofilm. Plasma coated surfaces treated with PBS were shown to have high levels of S. aureus SH1000 biofilm development. Biofilm was visualized using brightfield, confocal and SEM images after 24 h of growth and the most effective treatment concentration of each test agent is shown in Fig. 1 (Fig. 1 A i, ii, iii). Dabigatran treatment (1.5 µM) resulted in the largest reduction in biofilm development when compared to the untreated (PBS) control, with only single cells visible on treated surfaces (Fig. 1 D i, ii, iii). Argatroban treatment (10 µM) was shown to reduce the amount of visible biofilm development with SH1000 when examined using brightfield and confocal microscopy (Fig. 1 B i, ii); however, high levels of SH1000 biofilm growth were evident in images taken using SEM (Fig. 1 B iii), which is likely due to the higher sensitivity of this method. Hirudin treatment (34 µM) was also shown to have strong inhibitory properties, confirmed by SEM imaging (Fig. 1 C i, ii, iii). When SH1000 bacterial cells were harvested from the surfaces of discs, average bacterial density from the untreated discs was 1 × 106 CFU/ml (n = 3) whilst markedly reduced in the treatment groups with a significant reduction in bacterial density via quantitative measurement (Table 1). Microscopy experiments were repeated with MRSA strain USA300 JE2 and very similar results were observed for this strain (Fig. S1, Supporting Information).
Effect of surface coating on biofilm development under shear stress. Surfaces were coated with 20% (v/v) human plasma for 1 h, before being treated with PBS (control) (A), Argatroban 10 µM (B), Hirudin 34 µM (C) or Dabigatran 1.5 µM (D). Biofilms of S. aureus SH1000 were grown on surfaces for 24 h at 37°C under shear stress. Images were captured with (i) a light microscope for unstained bacterial cells, (ii) a Zeiss confocal microscope for bacteria stained with propidium iodide for 1 h and (iii) a Zeiss Ultra Plus scanning electron microscope of adherent cells on PU discs. Experiments were performed in triplicate and representative images are shown.
Effect of treatment with argatroban, hirudin and dabigatran on bacterial density of discs (experiments performed in triplicate, standard deviation (SD) indicated).
| Treatment group . | S. aureus SH1000 . |
|---|---|
| Untreated control (PBS) | 1 × 106 CFU/ml (mean, n = 3, SD = 1 × 104 CFU/ml) |
| Argatroban (10 µM) | 2100 CFU/ml (mean, n = 3, SD = 150 CFU/ml) |
| Hirudin (34 µM) | 160 CFU/ml (mean, n = 3, SD = 41 CFU/ml) |
| Dabigatran (1.5 µM) | 32 CFU/ml (mean, n = 3, SD = 6 CFU/ml) |
| Treatment group . | S. aureus SH1000 . |
|---|---|
| Untreated control (PBS) | 1 × 106 CFU/ml (mean, n = 3, SD = 1 × 104 CFU/ml) |
| Argatroban (10 µM) | 2100 CFU/ml (mean, n = 3, SD = 150 CFU/ml) |
| Hirudin (34 µM) | 160 CFU/ml (mean, n = 3, SD = 41 CFU/ml) |
| Dabigatran (1.5 µM) | 32 CFU/ml (mean, n = 3, SD = 6 CFU/ml) |
Effect of treatment with argatroban, hirudin and dabigatran on bacterial density of discs (experiments performed in triplicate, standard deviation (SD) indicated).
| Treatment group . | S. aureus SH1000 . |
|---|---|
| Untreated control (PBS) | 1 × 106 CFU/ml (mean, n = 3, SD = 1 × 104 CFU/ml) |
| Argatroban (10 µM) | 2100 CFU/ml (mean, n = 3, SD = 150 CFU/ml) |
| Hirudin (34 µM) | 160 CFU/ml (mean, n = 3, SD = 41 CFU/ml) |
| Dabigatran (1.5 µM) | 32 CFU/ml (mean, n = 3, SD = 6 CFU/ml) |
| Treatment group . | S. aureus SH1000 . |
|---|---|
| Untreated control (PBS) | 1 × 106 CFU/ml (mean, n = 3, SD = 1 × 104 CFU/ml) |
| Argatroban (10 µM) | 2100 CFU/ml (mean, n = 3, SD = 150 CFU/ml) |
| Hirudin (34 µM) | 160 CFU/ml (mean, n = 3, SD = 41 CFU/ml) |
| Dabigatran (1.5 µM) | 32 CFU/ml (mean, n = 3, SD = 6 CFU/ml) |
Novel coating with hirudin prevents S. aureus biofilm
Testing was expanded to determine if applying the exosite I targeting agents directly to a PU surface, a commonly used material in catheters, would reduce the development of S. aureus biofilm in the presence of human plasma. The challenge of immobilizing inhibitor molecules, covalently, onto the surface of PU is to retain availability of the active site to the exosite I of prothrombin post-immobilization. Hirudin forms a biomolecular complex with thrombin via its acidic C-terminus and therefore, due to the biological activity of hirudin as a direct thrombin inhibitor and its functional groups suitable for covalent attachment to PU, it was chosen for inclusion in this testing.
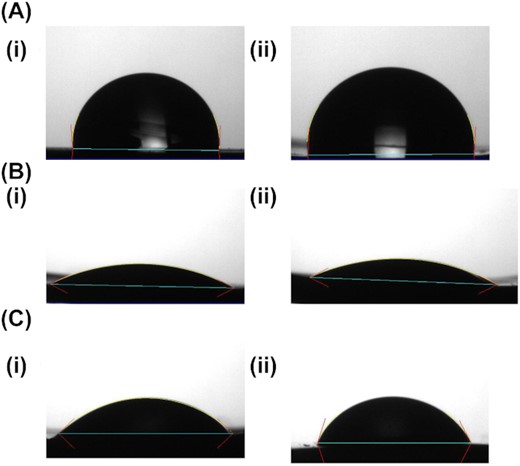
Hirudin was anchored on PU surface and the surfaces were first subjected to air plasma treatment, followed by chemical treatment with bromoacetic acid, to functionalise them with carboxylate groups as described elsewhere (Alves et al. 2011). Surface modification was confirmed by measuring contact angle goniometry as an indicator of surface hydrophilicity (Fig. 2). Untreated PU is hydrophobic, reflected in a static water contact angle of 92 ± 2°. Plasma treatment rendered the surfaces hydrophilic with a contact angle of 23 ± 3°. Within experimental error, there was no difference between contact angle at 5 or 10 min of plasma treatment. Following chemical treatment, the surfaces remained hydrophilic, with contact angle less than 60°, but the hydrophilicity was significantly reduced compared to the pristine plasma treated surfaces. The carboxyl terminated surfaces were then conjugated to hirudin. In order to avoid interference with the N- or C-terminus of the peptide, domains that are crucial for the binding to the thrombin and exosite I respectively, we identified tyrosine (Y, Tyr) as a suitable amino acid to provide the covalent linkage to the PU, whilst maintaining hirudin activity (Liu et al. 2014). Dityrosine crosslinks were identified approximately 50 yr ago and are located in proteins connected with age and oxidative stress (DiMarco and Giulivi 2007; Partlow et al. 2016). Furthermore, the dityrosine bond formation is not reversible, compared to the disulphide bond. Indeed, one of the strategies that nature uses for the reinforcement of natural fibrous proteins is such covalent crosslinking. In order to generate the dityrosine crosslinking, the PU had to be functionalized first with tyrosine residues and a photogenerated oxidant method was selected to generate the crosslink with hirudin's tyrosine (Truong et al. 2011).
Contact angle of PU discs. PU discs with untreated surfaces A (i), (ii). Plasma treatment after 5 min B (i), and after 10 min B (ii). Functionalization with carboxylic acid groups at 5 min plasma sample C (i) and 10 min plasma sample C (ii). Experiments were performed in triplicate and representative images are shown.
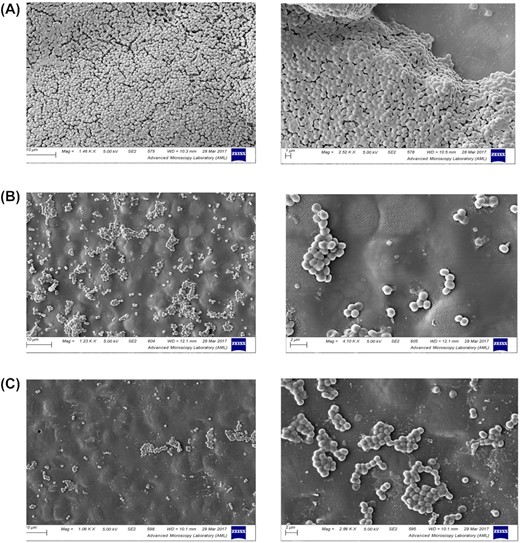
Staphylococcus aureus biofilms were then grown on uncoated (control) and hirudin coated discs under shear stress for 24 h following exposure to plasma, and biofilm density was examined using SEM imaging. High-level biofilm production on untreated disks (Fig. 3 A), was dramatically reduced on discs coated with hirudin (Fig. 3 B and C). Only small isolated clusters of adherent S. aureus cells were evident on the hirudin-coated discs. No significant difference was seen between the discs coated with hirudin by a photogenerated oxidant treatment of 5 or 10 min.
Hirudin coating of PU discs. PU discs were coated with PBS (control) (A), Hirudin for 5 min (B) or Hirudin for 10 min (C). Discs were coated with 20% (v/v) human plasma for 1 h. Biofilms of S. aureus SH1000 were grown on surfaces for 24 h at 37°C under shear stress. Discs were submerged in liquid nitrogen, transferred to a Quorum preparation chamber, sublimated for 40 min at −100°C, coated in platinum and imaged using a Zeiss Ultra Plus SEM and imaged at 5Kv using a SE2 detector. Discs were maintained at a temperature of −190°C throughout imaging. Experiments were performed in triplicate and representative images are shown.
DISCUSSION
Medical device related infections are notoriously difficult to treat due to the presence of bacterial biofilm on their surfaces. The biofilm matrix and phenotypic characteristics of the bacteria confer resistance to the host immune response and clearance. Staphylococcus aureus, the most common organism causing device related infections, has been shown to be significantly more resistant to treatment with antibiotics and antiseptics once embedded within a biofilm and hence represents a considerable treatment challenge (Hogan et al. 2016; Olson et al. 2002). Therefore, as one of the initial requirements for staphylococcal biofilm infection is adherence to the polymer surface, efforts have been made to develop materials that prevent adherence. Here, we demonstrate the effect of three drugs, argatroban, hirudin and dabigatran to bind prothrombin and prevent the formation of fibrin, a key component of the staphylococcal biofilm matrix. All three drugs, which are currently licensed for use in human medicine as oral anti-coagulants, were shown to reduce the amount of bacterial biofilm after treatment of plasma coated surfaces of biochips and subsequent challenge with S. aureus under conditions of shear stress. Shear stress within a catheter has previously been shown to range from 0.3–48 dynes/cm2 depending on the catheter type (Frumento et al. 2002). One study into atherosclerotic plaques and the vessel wall showed that thrombin binding increased under shear stress of less than 12.6 dynes (Mongrain and Rodes-Cabau 2006); the shear conditions used in this study are both under this value. It would be interesting to assess the impact of a wide range of shear stress on the formation of S. aureus biofilm in a model of catheter infection. Others have investigated the possible therapeutic role of staphylothrombin inhibition (McAdow et al. 2011); with one research group identifying reduced fibrin deposition and S. aureus retention on catheters in the presence of increased plasma concentrations of dabigatran (Vanassche et al. 2010; Vanassche et al. 2013). There have also been reports of catheter coating with other anti-thrombin agents and also of a derivative of hirudin to specifically prevent clot formation (Klement et al. 2002; Yang et al. 2012). However, this study, to the best of our knowledge, has for the first time investigated the anti-staphylococcal biofilm potential of coating catheters with direct thrombin inhibitors and has shown the successful binding of hirudin to a PU surface to be an effective anti-staphylococcal biofilm coating. Although stability and activity of this coating over time needs to be investigated further, based on these promising anti-biofilm results, and the robust safety profile of this drug, this coating has strong potential to be investigated further to prevent catheter related infections due to S. aureus. Furthermore, this approach could also be investigated in other S. aureus device related models of infection such as prosthetic joints, shunt material, etc., in an effort to reduce the impact of staphylococcal device related infections.
ACKNOWLEDGEMENTS
The imaging/analysis for this project (SFI grant number 15/TIDA/2950) was carried out at the Advanced Microscopy Laboratory (AML) at the AMBER center, CRANN Institute (www.crann.tcd.ie/Facilities/Advanced-Microscopy-Laboratory.aspx), Trinity College Dublin, Ireland. AML is an SFI supported imaging and analysis center.
TEK and SM gratefully acknowledge SFI for funding under grant number 14/IA/2488.
FUNDING
This work was supported by funding from Science Foundation Ireland (SFI) to Dr Eoghan O'Neill [Grant Number 15/TIDA/2950] and the European Regional Development Fund [Grant Number 13/RC/2073].
Conflicts of interests. None declared.