-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth Carmel Murphy, Inga-Maria Frick, Gram-positive anaerobic cocci – commensals and opportunistic pathogens, FEMS Microbiology Reviews, Volume 37, Issue 4, July 2013, Pages 520–553, https://doi.org/10.1111/1574-6976.12005
Close - Share Icon Share
Abstract
Among the Gram-positive anaerobic bacteria associated with clinical infections, the Gram-positive anaerobic cocci (GPAC) are the most prominent and account for approximately 25–30% of all isolated anaerobic bacteria from clinical specimens. Still, routine culture and identification of these slowly growing anaerobes to the species level has been limited in the diagnostic laboratory, mainly due to the requirement of prolonged incubation times and time-consuming phenotypic identification. In addition, GPAC are mostly isolated from polymicrobial infections with known pathogens and therefore their relevance has often been overlooked. However, through improvements in diagnostic and in particular molecular techniques, the isolation and identification of individual genera and species of GPAC associated with specific infections have been enhanced. Furthermore, the taxonomy of GPAC has undergone considerable changes over the years, mainly due to the development of molecular identification methods. Existing species have been renamed and novel species have been added, resulting in changes of the nomenclature. As the abundance and significance of GPAC in clinical infections grow, knowledge of virulence factors and antibiotic resistance patterns of different species becomes more important. The present review describes recent advances of GPAC and what is known of the biology and pathogenic effects of Anaerococcus , Finegoldia , Parvimonas , Peptoniphilus and Peptostreptococcus , the most important GPAC genera isolated from human infections.
Introduction
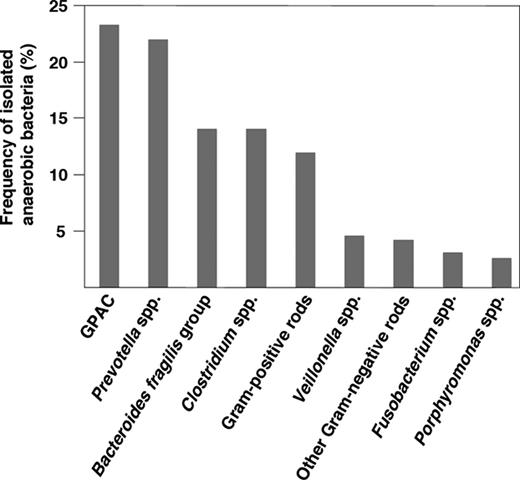
The normal microbiota that colonizes the skin and mucosal surfaces of the human body consists of a plethora of bacterial species of which anaerobic bacteria constitute a large group. Usually, commensals do not breach these protective barriers, but in case of a wound or when the host becomes immuno-compromised, commensals and pathogenic microorganisms can cause infection and disease. Among the Gram-positive anaerobic bacteria associated with clinical infections, the Gram-positive anaerobic cocci (GPAC) are the most prominent. Of all isolated anaerobic bacteria from clinical specimens, GPAC account for approximately 25–30% (Murdoch, 1998 ; Boyanova et al ., 2004 ; Wildeboer-Veloo et al ., 2007 ; Brazier et al ., 2008 ; Mikamo et al ., 2011 ), see Fig. 1 . In the literature, GPAC in general have been described by various synonyms, such as ‘anaerobic coccus’, ‘anaerobic streptococcus’, anaerobic Gram-positive coccus, and ‘ Peptococcus and Peptostreptococcus ’. The term GPAC will be used in this review. Constituting a major part of the normal microbiota, this heterogeneous group of bacteria colonise the skin and mucosal surfaces of the mouth and upper respiratory tract, the gastrointestinal tract and the female genitourinary tract (for references, see Murdoch, 1998 ). Clinically, GPAC are often present in deep-seated anaerobic soft-tissue infections, infections of bones and joints and infections of the female genital tracts (Murdoch, 1998 ; Brazier et al ., 2003 ). Several previous studies have also reported on the isolation of GPAC from wounds, both acute and chronic wounds such as chronic ulcers (for references, see Wall et al ., 2002 ). For an overview of reported infections associated with GPAC see Table 1 .
Overview of taxonomic changes in GPAC from 1997–2012
| Genus | 1997 (Murdoch, 1998 ) | 2012 | References | Clinical infections |
| Peptococcus | P. niger | P. niger | Chronic wounds (Dowd et al ., 2008a ) | |
| Peptostreptococcus | P. anaerobius | P. anaerobius | Abscesses, infections of abdominal cavity and female urogenitary tract, pleural empyema chronic wounds (for references see Peptostreptococcus section) | |
| P. stomatis | New species proposed by Downes and Wade ( 2006 ) | Infections of oral cavity (Downes & Wade, 2006 ; Rocas & Siqueira, 2008 ) | ||
| P. russellii | New species proposed by Whitehead et al . ( 2011 ) | |||
| P. asaccharolyticus | ||||
| P. barnesae | ||||
| P. harei | ||||
| P. heliotrinreducens | Reclassified to Slackia heliotrinreducens in the family Coriobacteriaceae by Wade et al . ( 1999 ) | |||
| P. hydrogenalis | ||||
| P. indolicus | ||||
| P. ivorii | ||||
| P. lacrimalis | ||||
| P. lactolyticus | ||||
| P. magnus | ||||
| P. micros | ||||
| P. octavius | ||||
| P. prevotii | ||||
| P. tetradius | ||||
| P. vaginalis | ||||
| Finegoldia | F. magna | P. magnus reclassified by Murdoch and Shah ( 1999 ) | Soft tissue and wound infections, bone and joint infections, vaginoses, chronic wounds, septic arthritis, prosthetic valve endocarditis, osteoarticular, pleural empyema (for references see Finegoldia section) | |
| MicromonasParvimonas | P. micra | P. micros reclassified by Murdoch & Shah, 1999 ; Renamed to Parvimonas by Tindall and Euzeby ( 2006 ) | Oral infections, skin infections, chronic wounds, joint infections, abscesses, plueral empyema (for references see Parvimonas section) | |
| Anaerococcus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Vaginal discharges and ovarian abscesses, skin and soft tissue infections chronic wounds (for references see Anaerococcus section) | ||
| A. hydrogenalis | ||||
| A. lactolyticus | Urinary tract infections (Domann et al ., 2003 ), chronic ulcers (Dowd et al ., 2008a ; Han et al ., 2011 ) | |||
| A. octavius | ||||
| A. prevotii | Abscesses and vaginal infections (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. tetradius | (Ezaki et al ., 2001 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| A. vaginalis | Chronic ulcers (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. murdochii | New species proposed by Song et al . ( 2007b ) | Infected foot ulcers, soft tissue infections, chronic wound infection (Song et al ., 2007a,b ) | ||
| A. senegalensis | New species proposed by Lagier et al . ( 2012 ) | |||
| Peptoniphilus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Chronic wounds, skin and soft tissue, bone and genitourinary tract, chronic rhinosinusitis (for references see Peptoniphilus section) | ||
| P. asaccharolyticus | Osteoarticular samples (La Scola et al ., 2011 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| P. harei | Skin and soft tissue (Brazier et al ., 2008 ), pressure ulcers (Dowd et al ., 2008a ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. lacrimalis | Vaginal infections (Murdoch, 1998 ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. indolicus | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. ivorii | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. gorbachii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. olsenii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. methioninivorax | New species proposed by Rooney et al . ( 2011 ) | |||
| P. tyrrelliae | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. coxii | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. duerdenii | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| P. koenoeneniae | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| Gallicola | G. barnesae | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | ||
| Murdochiella | M. asaccharolytica | Genus proposed by Ulger-Toprak et al . ( 2010 ) | A case of abdominal wall abscess and a case of sacral pilonidal cyst (Ulger-Toprak et al ., 2010 ) | |
| Atopobium | A. parvulum | A. parvulum | Dental abscesses and abdominal wounds (Olsen et al ., 1991 ), odontogenic infections (Downes et al ., 2001 ) | |
| Anaerosphaera | A. aminiphila | New genus proposed by Ueki et al . ( 2009 ) | ||
| Coprococcus | C. eutactus (T) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ) | ||
| Sarcina | S. ventriculi | A case of emphysematous gastritis (Laass et al ., 2010 ) | ||
| S. maxima | ||||
| RuminococcusBlautia | R. productus | B. producta | Proposed transfer of P. productus to genus Ruminococcus by Ezaki et al . ( 1994 ). Reclassified to a novel genus Blautia by Liu et al . ( 2008 ) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ), a case of necrotising fasciitis (Livaoglu et al ., 2008 ) |
| Genus | 1997 (Murdoch, 1998 ) | 2012 | References | Clinical infections |
| Peptococcus | P. niger | P. niger | Chronic wounds (Dowd et al ., 2008a ) | |
| Peptostreptococcus | P. anaerobius | P. anaerobius | Abscesses, infections of abdominal cavity and female urogenitary tract, pleural empyema chronic wounds (for references see Peptostreptococcus section) | |
| P. stomatis | New species proposed by Downes and Wade ( 2006 ) | Infections of oral cavity (Downes & Wade, 2006 ; Rocas & Siqueira, 2008 ) | ||
| P. russellii | New species proposed by Whitehead et al . ( 2011 ) | |||
| P. asaccharolyticus | ||||
| P. barnesae | ||||
| P. harei | ||||
| P. heliotrinreducens | Reclassified to Slackia heliotrinreducens in the family Coriobacteriaceae by Wade et al . ( 1999 ) | |||
| P. hydrogenalis | ||||
| P. indolicus | ||||
| P. ivorii | ||||
| P. lacrimalis | ||||
| P. lactolyticus | ||||
| P. magnus | ||||
| P. micros | ||||
| P. octavius | ||||
| P. prevotii | ||||
| P. tetradius | ||||
| P. vaginalis | ||||
| Finegoldia | F. magna | P. magnus reclassified by Murdoch and Shah ( 1999 ) | Soft tissue and wound infections, bone and joint infections, vaginoses, chronic wounds, septic arthritis, prosthetic valve endocarditis, osteoarticular, pleural empyema (for references see Finegoldia section) | |
| MicromonasParvimonas | P. micra | P. micros reclassified by Murdoch & Shah, 1999 ; Renamed to Parvimonas by Tindall and Euzeby ( 2006 ) | Oral infections, skin infections, chronic wounds, joint infections, abscesses, plueral empyema (for references see Parvimonas section) | |
| Anaerococcus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Vaginal discharges and ovarian abscesses, skin and soft tissue infections chronic wounds (for references see Anaerococcus section) | ||
| A. hydrogenalis | ||||
| A. lactolyticus | Urinary tract infections (Domann et al ., 2003 ), chronic ulcers (Dowd et al ., 2008a ; Han et al ., 2011 ) | |||
| A. octavius | ||||
| A. prevotii | Abscesses and vaginal infections (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. tetradius | (Ezaki et al ., 2001 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| A. vaginalis | Chronic ulcers (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. murdochii | New species proposed by Song et al . ( 2007b ) | Infected foot ulcers, soft tissue infections, chronic wound infection (Song et al ., 2007a,b ) | ||
| A. senegalensis | New species proposed by Lagier et al . ( 2012 ) | |||
| Peptoniphilus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Chronic wounds, skin and soft tissue, bone and genitourinary tract, chronic rhinosinusitis (for references see Peptoniphilus section) | ||
| P. asaccharolyticus | Osteoarticular samples (La Scola et al ., 2011 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| P. harei | Skin and soft tissue (Brazier et al ., 2008 ), pressure ulcers (Dowd et al ., 2008a ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. lacrimalis | Vaginal infections (Murdoch, 1998 ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. indolicus | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. ivorii | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. gorbachii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. olsenii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. methioninivorax | New species proposed by Rooney et al . ( 2011 ) | |||
| P. tyrrelliae | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. coxii | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. duerdenii | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| P. koenoeneniae | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| Gallicola | G. barnesae | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | ||
| Murdochiella | M. asaccharolytica | Genus proposed by Ulger-Toprak et al . ( 2010 ) | A case of abdominal wall abscess and a case of sacral pilonidal cyst (Ulger-Toprak et al ., 2010 ) | |
| Atopobium | A. parvulum | A. parvulum | Dental abscesses and abdominal wounds (Olsen et al ., 1991 ), odontogenic infections (Downes et al ., 2001 ) | |
| Anaerosphaera | A. aminiphila | New genus proposed by Ueki et al . ( 2009 ) | ||
| Coprococcus | C. eutactus (T) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ) | ||
| Sarcina | S. ventriculi | A case of emphysematous gastritis (Laass et al ., 2010 ) | ||
| S. maxima | ||||
| RuminococcusBlautia | R. productus | B. producta | Proposed transfer of P. productus to genus Ruminococcus by Ezaki et al . ( 1994 ). Reclassified to a novel genus Blautia by Liu et al . ( 2008 ) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ), a case of necrotising fasciitis (Livaoglu et al ., 2008 ) |
Overview of taxonomic changes in GPAC from 1997–2012
| Genus | 1997 (Murdoch, 1998 ) | 2012 | References | Clinical infections |
| Peptococcus | P. niger | P. niger | Chronic wounds (Dowd et al ., 2008a ) | |
| Peptostreptococcus | P. anaerobius | P. anaerobius | Abscesses, infections of abdominal cavity and female urogenitary tract, pleural empyema chronic wounds (for references see Peptostreptococcus section) | |
| P. stomatis | New species proposed by Downes and Wade ( 2006 ) | Infections of oral cavity (Downes & Wade, 2006 ; Rocas & Siqueira, 2008 ) | ||
| P. russellii | New species proposed by Whitehead et al . ( 2011 ) | |||
| P. asaccharolyticus | ||||
| P. barnesae | ||||
| P. harei | ||||
| P. heliotrinreducens | Reclassified to Slackia heliotrinreducens in the family Coriobacteriaceae by Wade et al . ( 1999 ) | |||
| P. hydrogenalis | ||||
| P. indolicus | ||||
| P. ivorii | ||||
| P. lacrimalis | ||||
| P. lactolyticus | ||||
| P. magnus | ||||
| P. micros | ||||
| P. octavius | ||||
| P. prevotii | ||||
| P. tetradius | ||||
| P. vaginalis | ||||
| Finegoldia | F. magna | P. magnus reclassified by Murdoch and Shah ( 1999 ) | Soft tissue and wound infections, bone and joint infections, vaginoses, chronic wounds, septic arthritis, prosthetic valve endocarditis, osteoarticular, pleural empyema (for references see Finegoldia section) | |
| MicromonasParvimonas | P. micra | P. micros reclassified by Murdoch & Shah, 1999 ; Renamed to Parvimonas by Tindall and Euzeby ( 2006 ) | Oral infections, skin infections, chronic wounds, joint infections, abscesses, plueral empyema (for references see Parvimonas section) | |
| Anaerococcus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Vaginal discharges and ovarian abscesses, skin and soft tissue infections chronic wounds (for references see Anaerococcus section) | ||
| A. hydrogenalis | ||||
| A. lactolyticus | Urinary tract infections (Domann et al ., 2003 ), chronic ulcers (Dowd et al ., 2008a ; Han et al ., 2011 ) | |||
| A. octavius | ||||
| A. prevotii | Abscesses and vaginal infections (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. tetradius | (Ezaki et al ., 2001 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| A. vaginalis | Chronic ulcers (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. murdochii | New species proposed by Song et al . ( 2007b ) | Infected foot ulcers, soft tissue infections, chronic wound infection (Song et al ., 2007a,b ) | ||
| A. senegalensis | New species proposed by Lagier et al . ( 2012 ) | |||
| Peptoniphilus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Chronic wounds, skin and soft tissue, bone and genitourinary tract, chronic rhinosinusitis (for references see Peptoniphilus section) | ||
| P. asaccharolyticus | Osteoarticular samples (La Scola et al ., 2011 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| P. harei | Skin and soft tissue (Brazier et al ., 2008 ), pressure ulcers (Dowd et al ., 2008a ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. lacrimalis | Vaginal infections (Murdoch, 1998 ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. indolicus | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. ivorii | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. gorbachii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. olsenii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. methioninivorax | New species proposed by Rooney et al . ( 2011 ) | |||
| P. tyrrelliae | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. coxii | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. duerdenii | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| P. koenoeneniae | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| Gallicola | G. barnesae | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | ||
| Murdochiella | M. asaccharolytica | Genus proposed by Ulger-Toprak et al . ( 2010 ) | A case of abdominal wall abscess and a case of sacral pilonidal cyst (Ulger-Toprak et al ., 2010 ) | |
| Atopobium | A. parvulum | A. parvulum | Dental abscesses and abdominal wounds (Olsen et al ., 1991 ), odontogenic infections (Downes et al ., 2001 ) | |
| Anaerosphaera | A. aminiphila | New genus proposed by Ueki et al . ( 2009 ) | ||
| Coprococcus | C. eutactus (T) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ) | ||
| Sarcina | S. ventriculi | A case of emphysematous gastritis (Laass et al ., 2010 ) | ||
| S. maxima | ||||
| RuminococcusBlautia | R. productus | B. producta | Proposed transfer of P. productus to genus Ruminococcus by Ezaki et al . ( 1994 ). Reclassified to a novel genus Blautia by Liu et al . ( 2008 ) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ), a case of necrotising fasciitis (Livaoglu et al ., 2008 ) |
| Genus | 1997 (Murdoch, 1998 ) | 2012 | References | Clinical infections |
| Peptococcus | P. niger | P. niger | Chronic wounds (Dowd et al ., 2008a ) | |
| Peptostreptococcus | P. anaerobius | P. anaerobius | Abscesses, infections of abdominal cavity and female urogenitary tract, pleural empyema chronic wounds (for references see Peptostreptococcus section) | |
| P. stomatis | New species proposed by Downes and Wade ( 2006 ) | Infections of oral cavity (Downes & Wade, 2006 ; Rocas & Siqueira, 2008 ) | ||
| P. russellii | New species proposed by Whitehead et al . ( 2011 ) | |||
| P. asaccharolyticus | ||||
| P. barnesae | ||||
| P. harei | ||||
| P. heliotrinreducens | Reclassified to Slackia heliotrinreducens in the family Coriobacteriaceae by Wade et al . ( 1999 ) | |||
| P. hydrogenalis | ||||
| P. indolicus | ||||
| P. ivorii | ||||
| P. lacrimalis | ||||
| P. lactolyticus | ||||
| P. magnus | ||||
| P. micros | ||||
| P. octavius | ||||
| P. prevotii | ||||
| P. tetradius | ||||
| P. vaginalis | ||||
| Finegoldia | F. magna | P. magnus reclassified by Murdoch and Shah ( 1999 ) | Soft tissue and wound infections, bone and joint infections, vaginoses, chronic wounds, septic arthritis, prosthetic valve endocarditis, osteoarticular, pleural empyema (for references see Finegoldia section) | |
| MicromonasParvimonas | P. micra | P. micros reclassified by Murdoch & Shah, 1999 ; Renamed to Parvimonas by Tindall and Euzeby ( 2006 ) | Oral infections, skin infections, chronic wounds, joint infections, abscesses, plueral empyema (for references see Parvimonas section) | |
| Anaerococcus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Vaginal discharges and ovarian abscesses, skin and soft tissue infections chronic wounds (for references see Anaerococcus section) | ||
| A. hydrogenalis | ||||
| A. lactolyticus | Urinary tract infections (Domann et al ., 2003 ), chronic ulcers (Dowd et al ., 2008a ; Han et al ., 2011 ) | |||
| A. octavius | ||||
| A. prevotii | Abscesses and vaginal infections (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. tetradius | (Ezaki et al ., 2001 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| A. vaginalis | Chronic ulcers (Labutti et al ., 2009 ), blood infections (La Scola et al ., 2011 ) | |||
| A. murdochii | New species proposed by Song et al . ( 2007b ) | Infected foot ulcers, soft tissue infections, chronic wound infection (Song et al ., 2007a,b ) | ||
| A. senegalensis | New species proposed by Lagier et al . ( 2012 ) | |||
| Peptoniphilus | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | Chronic wounds, skin and soft tissue, bone and genitourinary tract, chronic rhinosinusitis (for references see Peptoniphilus section) | ||
| P. asaccharolyticus | Osteoarticular samples (La Scola et al ., 2011 ), pleural empyema (Boyanova et al ., 2004 ) | |||
| P. harei | Skin and soft tissue (Brazier et al ., 2008 ), pressure ulcers (Dowd et al ., 2008a ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. lacrimalis | Vaginal infections (Murdoch, 1998 ), osteoarticular samples (La Scola et al ., 2011 ) | |||
| P. indolicus | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. ivorii | Pressure ulcers (Dowd et al ., 2008a ) | |||
| P. gorbachii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. olsenii | New species proposed by Song et al . ( 2007b ) | Low grade infections of lower extremities (Song et al ., 2007a,b ) | ||
| P. methioninivorax | New species proposed by Rooney et al . ( 2011 ) | |||
| P. tyrrelliae | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. coxii | New species proposed by Citron et al . ( 2012 ) | Leg infection, back cyst and abscesses (Citron et al ., 2012 ) | ||
| P. duerdenii | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| P. koenoeneniae | New species proposed by Ulger-Toprak et al . ( 2012 ) | Wound infection (Ulger-Toprak et al ., 2012 ) | ||
| Gallicola | G. barnesae | Reclassification to novel genus from Peptostreptococcus by Ezaki et al . ( 2001 ) | ||
| Murdochiella | M. asaccharolytica | Genus proposed by Ulger-Toprak et al . ( 2010 ) | A case of abdominal wall abscess and a case of sacral pilonidal cyst (Ulger-Toprak et al ., 2010 ) | |
| Atopobium | A. parvulum | A. parvulum | Dental abscesses and abdominal wounds (Olsen et al ., 1991 ), odontogenic infections (Downes et al ., 2001 ) | |
| Anaerosphaera | A. aminiphila | New genus proposed by Ueki et al . ( 2009 ) | ||
| Coprococcus | C. eutactus (T) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ) | ||
| Sarcina | S. ventriculi | A case of emphysematous gastritis (Laass et al ., 2010 ) | ||
| S. maxima | ||||
| RuminococcusBlautia | R. productus | B. producta | Proposed transfer of P. productus to genus Ruminococcus by Ezaki et al . ( 1994 ). Reclassified to a novel genus Blautia by Liu et al . ( 2008 ) | Intestinal GPAC rarely seen in clinical infections (Maukonen et al ., 2012 ), a case of necrotising fasciitis (Livaoglu et al ., 2008 ) |
Frequency of isolated anaerobic bacteria found in clinical infection (1994–2004). Figure adapted from (Mikamo et al. , 2011 ).
Despite the fact that GPAC are frequently isolated from infections involving anaerobic bacteria, the significance of different isolates have not been well studied. The culture and identification of many GPAC strains in diagnostic laboratories remains difficult. The limitation of their isolation is mainly due to their sensitivity to oxygen, which requires appropriate methods of collection, transportation and strictly anaerobic cultivation of specimens. Also, the slow growth of these organisms combined with time-consuming phenotypic identification methods have often resulted in inconclusive identification of the GPAC species. In addition, GPAC are often isolated from polymicrobial infections with known pathogens and therefore, their relevance has been largely overlooked. Furthermore, in clinical specimens, they have often been reported as anaerobic streptococci (Murdoch, 1998 ). However, the development and application of molecular methods in clinical microbiology, such as PCR (Lisby, 1998 ), multiplex PCR (Song et al ., 2003b ), sequencing of the 16S rRNA gene (Li et al ., 1994 ; Conrads et al ., 1997 ; Clarridge, 2004 ) and pyrosequencing (Dowd et al ., 2008a , b ) have led to improved identification of GPAC in clinical specimens, including chronic wounds (Wolcott et al ., 2009 ). It is thus evident that these bacteria have been largely understudied and as the clinical significance of GPAC grows, it becomes essential to precisely identify the isolated bacteria from clinical infections. Moreover, various genera and species of GPAC can express different virulence factors and they can also exhibit variations in antimicrobial susceptibility emphasising the importance of rapid and correct species identification in clinical samples. Thus, the introduction of newer diagnostic methods may lead to improved treatment of GPAC infections. A renewing interest in clinical microbiology to study anaerobes combined with the frequent isolation of GPAC from clinical materials (Fig. 1 and Table 1 ) further emphasize the need to study this important group of bacteria.
Data from molecular methods have led to extensive taxonomic changes during the last decades and also to the occurrence of new genera and species. Currently, important genera of GPAC that may be isolated from humans are Peptostreptococcus , Finegoldia (Murdoch & Shah, 1999 ), Parvimonas (Tindall & Euzeby, 2006 ), Anaerococcus and Peptoniphilus (Ezaki et al ., 2001 ). Other related GPAC genera are Gallicola (Ezaki et al ., 2001 ), Murdochiella (Ulger-Toprak et al ., 2010 ), Atopobium (Collins & Wallbanks, 1992 ) and Anaerosphaera (Ueki et al ., 2009 ). In addition, genera such as Sarcina , Coprococcus and Blautia (previously Ruminococcus ) (Ezaki et al ., 1994 ; Liu et al ., 2008 ) are phylogenetically more distantly related GPAC. This review describes what is known of the classification of GPAC, clinical relevance of individual genera and species, antibiotic resistance and a more in-depth description of known virulence factors for some species that are more commonly associated with clinical infections.
Classification
GPAC belongs to the Firmicutes phylum and are classified in the order of Clostridiales having low DNA GC contents. Historically, the GPAC have undergone a considerable taxonomic revision. The genera Peptococcus and Peptostreptococcus were originally classified on the basis of morphological characteristics (Kluyver & van Niel, 1936 ); peptococci were arranged in clusters and considered the anaerobic equivalent of staphylococci, whereas peptostreptococci were arranged in long chains and thus considered the anaerobic equivalent to streptococci. Peptococcus and Peptostreptococcus were for many years separated from each other by cellular arrangement, metabolic end products, and utilisation of peptides and carbohydrates (Kluyver & van Niel, 1936 ; Rogosa, 1971 , 1974 ; Holdeman & Moore, 1974 ). Furthermore, they were together with the genera Ruminococcus , Sarcina and Coprococcus , assigned in the Peptococcaceae family of strict anaerobic Gram-positive cocci or coccobacilli (Rogosa, 1971 , 1974 ; Holdeman & Moore, 1974 ).
In 1983 , Ezaki et al . reclassified the GPAC species on the basis of DNA base composition, DNA–DNA hybridization data, the cellular fatty acid profiles and other biochemical characteristics. As a result, four species of Peptococcus ( Peptococcus asaccharolyticus , Peptococcus indolicus , Peptococcus prevotii and Peptococcus magnus ) were transferred to the genus Peptostreptococcus leaving Peptococcus niger as the only remaining species in the genus Peptococcus . However, a later study using similar hybridization techniques did not support this revision (Huss et al ., 1984 ), and in 1994, 16S rRNA sequence analysis confirmed that the genus Peptostreptococcus was phylogenetically disordered (Ezaki et al ., 1994 ; Li et al ., 1994 ), thereby emphasising the need for a radical taxonomic revision of the genus. In a comprehensive review of GPAC by Murdoch in 1998, the taxonomic changes until 1997 were summerised and a possible revised classification was discussed (Murdoch, 1998 ). The 1997 existing classification of the genera Peptococcus and Peptostreptococcus (Murdoch, 1998 ) is shown in Table 1 together with an overview of the taxonomic changes in GPAC up to 2012.
Since 1998, the genus Peptostreptococcus has been divided into several novel genera. The type species of the genus, Peptostreptococcus anaerobius , was found to be distantly related to other members of the genus, and thus a division into six new groups was proposed (Murdoch & Shah, 1999 ; Ezaki et al ., 2001 ) (Table 1 ). Peptostreptococcus magnus and Peptostreptococcus micros were transferred to two new genera, Finegoldia and Micromonas , respectively, where each strain is the only species in its respective genus (Murdoch & Shah, 1999 ). The genus name Micromonas was found to be illegitimate, as Micromonas are green algae, and has therefore, more recently been replaced by Parvimonas (Tindall & Euzeby, 2006 ), with Parvimonas micra being the only species present in this genus. For the remaining peptostreptococci, three new genera were proposed; Peptoniphilus , Anaerococcus and Gallicola , which contains only one species, Gallicola barnesae (Ezaki et al ., 2001 ). Rajendram et al . ( 2001 ) proposed an alternative reclassification of the peptostreptococci, but in the current literature, the classification according to Ezaki et al . (Ezaki et al ., 2001 ) is used.
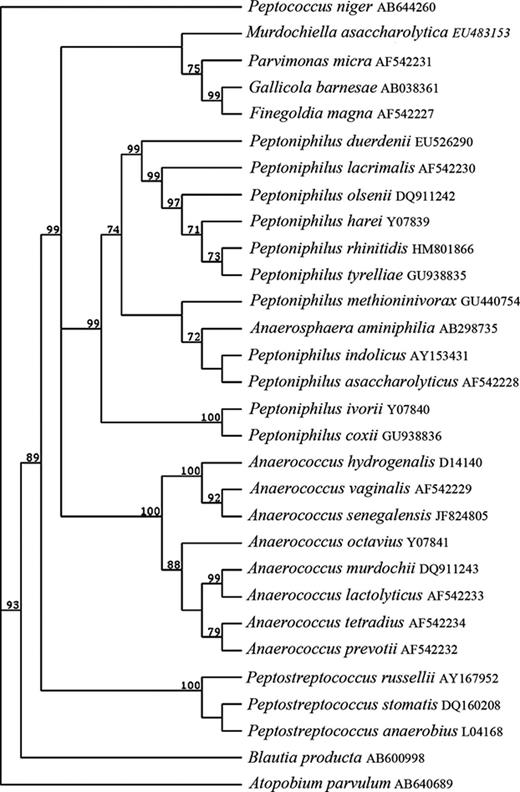
Other changes within the GPAC, as shown in Table 1 , include the reclassification of Ruminococcus productus to Blautia producta (Liu et al ., 2008 ) and the distantly related Peptostreptococcus heliotrinreducans to the family Coriobacteriaceae (Wade et al ., 1999 ). Moreover, two novel genera have been proposed; Anaerosphaera , with the type species A. aminiphila most closely related to species of the genus Peptoniphilus (Ueki et al ., 2009 ) and Murdochiella with the type species M. asaccharolytica most closely related to P. micra and Finegoldia magna (Ulger-Toprak et al ., 2010 ). For an overview of the phylogenetic organisation within GPAC, see Fig. 2 .
Phylogenetic tree showing phylogenetic relationships within GPAC. This tree was constructed by the neighbour-joining method, by inputting 16S rRNA gene sequences into MacVector. Significant bootstrap values, expressed as a percentage of 32 000 replications, are indicated at branching points.
Isolation and identification
Isolation of GPAC is usually performed on fastidious anaerobe agar or blood agar anaerobically incubated for 48 h or up to 7 days (Heginbothom et al ., 1990 ; Health-Protection-Agency, 2009 ). In most laboratories, identification is phenotypically based on morphological appearance, Gram's stain reaction and sensitivity to metronidazole. However, resistance to metronidazole has been reported for GPAC (Hecht, 2006 ) and such organisms may be overlooked by that approach. For classification to species or even genus level, further biochemical identification tests, for instance inhibition by sodium polyanethol sulphonate (SPS disc) (Graves et al ., 1974 ), pigment production, nitrate reduction, urease production, indole test and analysis of proteolytic enzyme profiles are required. Carbohydrate fermentation and detection of volatile fatty acids by gas-liquid chromatography are other methods used for classification, but today many laboratories do not have facilities to perform these tests. In addition, identification and differentiation between species by these conventional protocols are both problematic and time-consuming. See Table 2 for an overview of the biochemical characteristics of GPAC. In the 1980s, a number of commercial biochemical assays, such as RapID ANA (Innovative Diagnostic Systems, Atlanta, GA), API 20A and AN-Ident (Analytlab Products, Plainview, NY), and Rapid ID 32A (bioMérieux, Marcy l'Etoile, France), for detection of anaerobes, were developed. These systems are based on the evaluation of the action of a range of bacterial enzymes and other test reactions.
Biochemical characteristics of the majority of GPAC species
| Species (no. of strains examined) | Terminal major VFA | Production of * | Saccharolytic enzymes * | Proteolytic enzymes * | Carbohydrate fermentation reactions † | |||||||||||||||||||||||||||||||||||
| ALP | ADH | Indole | Urease | αGAL | βGAL | αGLU | βGUR | ArgA | ProA | PheA | LeuA | PyrA | TyrA | HisA | Glu-cose | Lac-tose | Man-nose | Raffi-nose | Ri-bose | |||||||||||||||||||||
| P. anaerobius ( n = 63) | IC(IV) | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | + | − | w | − | − | |||||||||||||||||||
| P. stomatis ( n = 2) | ND | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | w | − | − | − | − | |||||||||||||||||||
| P. russellii ( n = 1) | A | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | ND | − | − | ND | |||||||||||||||||||
| Parvimonas micros ( n = 31) | A | + | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | − | − | |||||||||||||||||||
| F. magna ( n = 116) | A | v | v | − | − | − | − | − | − | + | − | − | + | + | −/w | −/w | −/w | − | − | − | − | |||||||||||||||||||
| A. prevotii ( n = 1) | B | − | − | − | + | + | − | + | + | + | − | − | − | + | w | + | − | − | + | + | + | |||||||||||||||||||
| A. tetradius ( n = 1) | B | − | − | − | + | − | − | + | + | + | − | w | + | w | w | w | + | − | + | − | − | |||||||||||||||||||
| A. lactolyticus ( n = 1) | B | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − | + | + | + | − | − | |||||||||||||||||||
| A. hydrogenalis ( n = 14) | B | −/w | − | + | v | − | − | v | − | − | − | − | − | − | − | − | + | + | + | + | − | |||||||||||||||||||
| A. vaginalis ( n = 29) | B | −/w | + | v | − | − | − | − | − | + | − | − | + | − | − | + | + | − | v | − | − | |||||||||||||||||||
| A. octavius ( n = 6) | C | − | − | − | − | − | − | − | − | − | + | − | − | w | − | − | + | − | + | − | + | |||||||||||||||||||
| A. murdochii ( n = 6) | A/B | + | + | − | − | − | v | − | − | + | − | − | + | + | − | +/w | + | ND | + | − | ND | |||||||||||||||||||
| Peptoniphilus ivorii ( n = 4) | IV | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus harei ( n = 13) | B | − | − | v | − | − | − | − | − | + | − | − | −/w | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus niger ( n = 1) | C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| P. olsenii ( n = 4) | A | + | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| P. gorbachii ( n = 6) | A | − | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| Peptoniphilus indolicus ( n = 6) | B | + | − | + | − | − | − | − | − | + | − | + | + | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus asaccharolyticus ( n = 52) | B | − | − | v | − | − | − | − | − | + | − | − | v | − | v | w | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus lacrimalis ( n = 1) | B | − | − | − | − | − | − | − | − | + | − | + | + | − | v | + | − | − | − | − | − | |||||||||||||||||||
| P. coxii (new) ( n = 7) | ND | −/w | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | ND | − | − | ND | |||||||||||||||||||
| P. tyrrelliae ( n = 4) | ND | − | − | + | − | − | − | − | − | + | − | − | +/w | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| B. producta ( n = 1) | A | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | + | + | v | + | v | |||||||||||||||||||
| G. barnesae ( n = 1) | A(B) | − | − | w | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| Species (no. of strains examined) | Terminal major VFA | Production of * | Saccharolytic enzymes * | Proteolytic enzymes * | Carbohydrate fermentation reactions † | |||||||||||||||||||||||||||||||||||
| ALP | ADH | Indole | Urease | αGAL | βGAL | αGLU | βGUR | ArgA | ProA | PheA | LeuA | PyrA | TyrA | HisA | Glu-cose | Lac-tose | Man-nose | Raffi-nose | Ri-bose | |||||||||||||||||||||
| P. anaerobius ( n = 63) | IC(IV) | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | + | − | w | − | − | |||||||||||||||||||
| P. stomatis ( n = 2) | ND | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | w | − | − | − | − | |||||||||||||||||||
| P. russellii ( n = 1) | A | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | ND | − | − | ND | |||||||||||||||||||
| Parvimonas micros ( n = 31) | A | + | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | − | − | |||||||||||||||||||
| F. magna ( n = 116) | A | v | v | − | − | − | − | − | − | + | − | − | + | + | −/w | −/w | −/w | − | − | − | − | |||||||||||||||||||
| A. prevotii ( n = 1) | B | − | − | − | + | + | − | + | + | + | − | − | − | + | w | + | − | − | + | + | + | |||||||||||||||||||
| A. tetradius ( n = 1) | B | − | − | − | + | − | − | + | + | + | − | w | + | w | w | w | + | − | + | − | − | |||||||||||||||||||
| A. lactolyticus ( n = 1) | B | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − | + | + | + | − | − | |||||||||||||||||||
| A. hydrogenalis ( n = 14) | B | −/w | − | + | v | − | − | v | − | − | − | − | − | − | − | − | + | + | + | + | − | |||||||||||||||||||
| A. vaginalis ( n = 29) | B | −/w | + | v | − | − | − | − | − | + | − | − | + | − | − | + | + | − | v | − | − | |||||||||||||||||||
| A. octavius ( n = 6) | C | − | − | − | − | − | − | − | − | − | + | − | − | w | − | − | + | − | + | − | + | |||||||||||||||||||
| A. murdochii ( n = 6) | A/B | + | + | − | − | − | v | − | − | + | − | − | + | + | − | +/w | + | ND | + | − | ND | |||||||||||||||||||
| Peptoniphilus ivorii ( n = 4) | IV | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus harei ( n = 13) | B | − | − | v | − | − | − | − | − | + | − | − | −/w | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus niger ( n = 1) | C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| P. olsenii ( n = 4) | A | + | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| P. gorbachii ( n = 6) | A | − | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| Peptoniphilus indolicus ( n = 6) | B | + | − | + | − | − | − | − | − | + | − | + | + | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus asaccharolyticus ( n = 52) | B | − | − | v | − | − | − | − | − | + | − | − | v | − | v | w | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus lacrimalis ( n = 1) | B | − | − | − | − | − | − | − | − | + | − | + | + | − | v | + | − | − | − | − | − | |||||||||||||||||||
| P. coxii (new) ( n = 7) | ND | −/w | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | ND | − | − | ND | |||||||||||||||||||
| P. tyrrelliae ( n = 4) | ND | − | − | + | − | − | − | − | − | + | − | − | +/w | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| B. producta ( n = 1) | A | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | + | + | v | + | v | |||||||||||||||||||
| G. barnesae ( n = 1) | A(B) | − | − | w | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
A, acetate; B, butyrate; IV, isovalerate; C, n -caproate; ALP, alkaline phosphatase; ADH, argininge dihydrolase; αGAL, α-galactosidase; βGAL, β-galactosidase; αGLU, α-glucosidase; βGUR, β-glucuronidase; ArgA, arginine arylamidase (AMD); ProA, proline AMD; PheA, phenylalanine AMD; LeuA, leucine AMD; PyrA, pyroglutamyl AMD; TyrA, tyrosine AMD; HisA, histidine AMD; −, > 90% negative; w, weakly positive; +, > 90% positive; v, varied reactions; ND, not determined.
Biochemical characteristics of the majority of GPAC species
| Species (no. of strains examined) | Terminal major VFA | Production of * | Saccharolytic enzymes * | Proteolytic enzymes * | Carbohydrate fermentation reactions † | |||||||||||||||||||||||||||||||||||
| ALP | ADH | Indole | Urease | αGAL | βGAL | αGLU | βGUR | ArgA | ProA | PheA | LeuA | PyrA | TyrA | HisA | Glu-cose | Lac-tose | Man-nose | Raffi-nose | Ri-bose | |||||||||||||||||||||
| P. anaerobius ( n = 63) | IC(IV) | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | + | − | w | − | − | |||||||||||||||||||
| P. stomatis ( n = 2) | ND | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | w | − | − | − | − | |||||||||||||||||||
| P. russellii ( n = 1) | A | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | ND | − | − | ND | |||||||||||||||||||
| Parvimonas micros ( n = 31) | A | + | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | − | − | |||||||||||||||||||
| F. magna ( n = 116) | A | v | v | − | − | − | − | − | − | + | − | − | + | + | −/w | −/w | −/w | − | − | − | − | |||||||||||||||||||
| A. prevotii ( n = 1) | B | − | − | − | + | + | − | + | + | + | − | − | − | + | w | + | − | − | + | + | + | |||||||||||||||||||
| A. tetradius ( n = 1) | B | − | − | − | + | − | − | + | + | + | − | w | + | w | w | w | + | − | + | − | − | |||||||||||||||||||
| A. lactolyticus ( n = 1) | B | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − | + | + | + | − | − | |||||||||||||||||||
| A. hydrogenalis ( n = 14) | B | −/w | − | + | v | − | − | v | − | − | − | − | − | − | − | − | + | + | + | + | − | |||||||||||||||||||
| A. vaginalis ( n = 29) | B | −/w | + | v | − | − | − | − | − | + | − | − | + | − | − | + | + | − | v | − | − | |||||||||||||||||||
| A. octavius ( n = 6) | C | − | − | − | − | − | − | − | − | − | + | − | − | w | − | − | + | − | + | − | + | |||||||||||||||||||
| A. murdochii ( n = 6) | A/B | + | + | − | − | − | v | − | − | + | − | − | + | + | − | +/w | + | ND | + | − | ND | |||||||||||||||||||
| Peptoniphilus ivorii ( n = 4) | IV | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus harei ( n = 13) | B | − | − | v | − | − | − | − | − | + | − | − | −/w | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus niger ( n = 1) | C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| P. olsenii ( n = 4) | A | + | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| P. gorbachii ( n = 6) | A | − | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| Peptoniphilus indolicus ( n = 6) | B | + | − | + | − | − | − | − | − | + | − | + | + | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus asaccharolyticus ( n = 52) | B | − | − | v | − | − | − | − | − | + | − | − | v | − | v | w | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus lacrimalis ( n = 1) | B | − | − | − | − | − | − | − | − | + | − | + | + | − | v | + | − | − | − | − | − | |||||||||||||||||||
| P. coxii (new) ( n = 7) | ND | −/w | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | ND | − | − | ND | |||||||||||||||||||
| P. tyrrelliae ( n = 4) | ND | − | − | + | − | − | − | − | − | + | − | − | +/w | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| B. producta ( n = 1) | A | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | + | + | v | + | v | |||||||||||||||||||
| G. barnesae ( n = 1) | A(B) | − | − | w | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| Species (no. of strains examined) | Terminal major VFA | Production of * | Saccharolytic enzymes * | Proteolytic enzymes * | Carbohydrate fermentation reactions † | |||||||||||||||||||||||||||||||||||
| ALP | ADH | Indole | Urease | αGAL | βGAL | αGLU | βGUR | ArgA | ProA | PheA | LeuA | PyrA | TyrA | HisA | Glu-cose | Lac-tose | Man-nose | Raffi-nose | Ri-bose | |||||||||||||||||||||
| P. anaerobius ( n = 63) | IC(IV) | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | + | − | w | − | − | |||||||||||||||||||
| P. stomatis ( n = 2) | ND | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | w | − | − | − | − | |||||||||||||||||||
| P. russellii ( n = 1) | A | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | ND | − | − | ND | |||||||||||||||||||
| Parvimonas micros ( n = 31) | A | + | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | − | − | |||||||||||||||||||
| F. magna ( n = 116) | A | v | v | − | − | − | − | − | − | + | − | − | + | + | −/w | −/w | −/w | − | − | − | − | |||||||||||||||||||
| A. prevotii ( n = 1) | B | − | − | − | + | + | − | + | + | + | − | − | − | + | w | + | − | − | + | + | + | |||||||||||||||||||
| A. tetradius ( n = 1) | B | − | − | − | + | − | − | + | + | + | − | w | + | w | w | w | + | − | + | − | − | |||||||||||||||||||
| A. lactolyticus ( n = 1) | B | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − | + | + | + | − | − | |||||||||||||||||||
| A. hydrogenalis ( n = 14) | B | −/w | − | + | v | − | − | v | − | − | − | − | − | − | − | − | + | + | + | + | − | |||||||||||||||||||
| A. vaginalis ( n = 29) | B | −/w | + | v | − | − | − | − | − | + | − | − | + | − | − | + | + | − | v | − | − | |||||||||||||||||||
| A. octavius ( n = 6) | C | − | − | − | − | − | − | − | − | − | + | − | − | w | − | − | + | − | + | − | + | |||||||||||||||||||
| A. murdochii ( n = 6) | A/B | + | + | − | − | − | v | − | − | + | − | − | + | + | − | +/w | + | ND | + | − | ND | |||||||||||||||||||
| Peptoniphilus ivorii ( n = 4) | IV | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus harei ( n = 13) | B | − | − | v | − | − | − | − | − | + | − | − | −/w | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus niger ( n = 1) | C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
| P. olsenii ( n = 4) | A | + | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| P. gorbachii ( n = 6) | A | − | − | v | − | − | − | − | − | + | − | +/w | + | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| Peptoniphilus indolicus ( n = 6) | B | + | − | + | − | − | − | − | − | + | − | + | + | − | w | + | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus asaccharolyticus ( n = 52) | B | − | − | v | − | − | − | − | − | + | − | − | v | − | v | w | − | − | − | − | − | |||||||||||||||||||
| Peptoniphilus lacrimalis ( n = 1) | B | − | − | − | − | − | − | − | − | + | − | + | + | − | v | + | − | − | − | − | − | |||||||||||||||||||
| P. coxii (new) ( n = 7) | ND | −/w | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | ND | − | − | ND | |||||||||||||||||||
| P. tyrrelliae ( n = 4) | ND | − | − | + | − | − | − | − | − | + | − | − | +/w | − | + | + | − | ND | − | − | ND | |||||||||||||||||||
| B. producta ( n = 1) | A | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | + | + | v | + | v | |||||||||||||||||||
| G. barnesae ( n = 1) | A(B) | − | − | w | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||||||||
A, acetate; B, butyrate; IV, isovalerate; C, n -caproate; ALP, alkaline phosphatase; ADH, argininge dihydrolase; αGAL, α-galactosidase; βGAL, β-galactosidase; αGLU, α-glucosidase; βGUR, β-glucuronidase; ArgA, arginine arylamidase (AMD); ProA, proline AMD; PheA, phenylalanine AMD; LeuA, leucine AMD; PyrA, pyroglutamyl AMD; TyrA, tyrosine AMD; HisA, histidine AMD; −, > 90% negative; w, weakly positive; +, > 90% positive; v, varied reactions; ND, not determined.
With the introduction of new molecular approaches, such as 16S rRNA gene sequencing and pyrosequencing, analysis of bacterial composition in clinical samples without the need for culturing is allowed. 16S rRNA gene sequencing represents an accurate method for both bacterial classification and identification, and through available genotypic data, molecular techniques can be developed for identification of GPAC. In a study by Song et al . ( 2003a ), sequence data for 13 type strains of GPAC from established species, including F. magna , P. micra , Peptoniphilus harei and P. anaerobius , were determined. Based on these data, a collection of clinical isolates previously identified by phenotypic tests were reidentified by the use of 16S rRNA gene sequencing. By this method, 84% of the clinical GPAC isolates were accurately identified to species level (Song et al ., 2003a ). With assays such as 16S ribosomal PCR (Riggio & Lennon, 2003 ), multiplex PCR (Song et al ., 2003a ) and 16S rRNA gene-based fluorescent probes (Wildeboer-Veloo et al ., 2007 ), rapid and reliable identification of GPAC species is possible. Recently, a short biochemical scheme was developed by Song et al . for simple identification of GPAC in the clinical laboratory (Song et al ., 2007a ). This scheme was based on the solid identification of strains obtained from 16S rRNA gene sequencing (Song et al ., 2003a ), and included both reference strains and clinical isolates. Lin et al . ( 2010 ) developed an oligonucleotide array based on the 16S-23S rRNA intergenic spacer region of clinically important anaerobes, including Anaerococcus prevotii , Anaerococcus tetradius , F. magna , Peptoniphilus asaccharolyticus , P. anaerobius and P. micra . Reference strains and clinical isolates were identified by the array and the sensitivity for identification of pure cultures was 99.7%, whereas the specificity was 97.1%. Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) can also be utilised for detection of proteins in bacteria isolated from clinical specimens (Veloo et al ., 2011a ). By constructing a database, using commonly isolated strains of GPAC as reference, Veloo et al . could identify clinical isolates by MALDI-TOF MS and results were then compared with other identification methods. Of 107 unknown GPAC isolates, 96 could be identified with reliability. The other strains (11/107) showed < 98% sequence similarities to their closest reference strain, and therefore, it was concluded that these isolates probably represented a new species (Veloo et al ., 2011a ). With these methods, the reliability of identification of slow-growing anaerobes will most likely increase.
Clinical relevance
Apart from being major constituents of the normal anaerobic microbiota, GPAC are also considered as opportunistic pathogens. The introduction of foreign materials (such as joint replacement, catheters, etc.), a growing elderly population and number of immuno-compromised individuals has contributed to a situation where the clinical significance of infections caused by opportunistic pathogens has increased. Mostly, GPAC are isolated from polymicrobial infections, but in many cases, the organisms are isolated in pure culture and these involve mainly F. magna , although other species like P. micra , P. harei and P. anaerobius also occur (Murdoch, 1998 ; Wildeboer-Veloo et al ., 2007 ). The clinical importance of individual GPAC species has not been extensively studied, probably due to the mixed nature of infections and difficulties in identifying many strains in the diagnostic laboratory. Special media and prolonged cultivation times are required for isolation of anaerobes, thus culture results are delayed and usually, treatment of the infection has already been started. The polymicrobial nature of GPAC infections, in addition to inadequate classification, has most likely contributed to the neglect of the clinical significance of individual species of GPAC.
A number of surveys have looked at the frequency of anaerobes in clinical specimens and found that GPAC constitute 24–31% of all anaerobic isolates (Holland et al ., 1977 ; Wren et al ., 1977 ; Brook, 1988a ; Murdoch et al ., 1994 ; Murdoch, 1998 ; Boyanova et al ., 2004 ; Wildeboer-Veloo et al ., 2007 ; Brazier et al ., 2008 ; Mikamo et al ., 2011 ). They can be isolated from a wide variety of sites, of which the dominating are abscesses and infections of skin and soft tissue, mouth, bone and joint, upper respiratory and female genital tracts. In cases of pleural empyema, a life-threatening pleuropulmonary infection with high mortality rate, P. micra has been the predominant species, but also F. magna , P. anaerobius and Anaerococcus vaginalis have been reported (Murdoch, 1998 ). When the incidence of anaerobic bacteria in patients with pleural empyema were investigated, GPAC were among the predominant anaerobic bacteria isolated from the infections and found in 26.3% of all cases (Boyanova et al ., 2004 ). Among these, the incidences of P. micra , F. magna , and P. anaerobius were similar, but P. asaccharolyticus , A. tetradius and A. prevotii were detected as well (Boyanova et al ., 2004 ). Other recent retrospective studies have also reported on high incidences of GPAC isolated from various clinical samples, such as infections of the abdominal cavity, skin, soft tissues, and bone (Mikamo et al ., 2011 ; Rodriguez-Cavallini et al ., 2011 ). The frequency of GPAC in relation to other anaerobic bacteria isolated from clinical specimens is shown in Fig. 1 .
A large part of human infectious diseases are comprised of chronic infections, including chronic wounds. The colonisation of wounds often involves polymicrobial biofilm communities and populating bacteria often become resistant to many antibiotics. Numerous studies have reported the recovery and isolation of GPAC from both acute and chronic wounds (for references see Wall et al ., 2002 ). For instance, Bowler and Davies found that more than 50% of anaerobes isolated from leg ulcers were strains of GPAC belonging to the genus previously known as Peptostreptococcus (Bowler & Davies, 1999a ). More recently, several reports have shed light about the bacterial profile associated with wound infections, and these studies demonstrate that besides aerobic species, like Staphylococcus spp., Streptococcus spp., Enterococcus spp. and Pseudomonas aerugionosa , anaerobes, including Peptoniphilus , Finegoldia and Anaerococcus are prominent colonisers (Dowd et al ., 2008a , b ; Wolcott et al ., 2009 ; Gontcharova et al ., 2010 ; Han et al ., 2011 ). In these studies, molecular methods such as bacterial Tag-encoded FLX amplicon pyrosequencing, 16S-based amplification followed by pyrosequencing and shotgun Sanger sequencing were utilised.
A large part of human infectious diseases are comprised of chronic infections, including chronic wounds. The colonisation of wounds often involves polymicrobial biofilm communities and populating bacteria often become resistant to many antibiotics. Numerous studies have reported the recovery and isolation of GPAC from both acute and chronic wounds (for references see Wall et al ., 2002 ). For instance, Bowler and Davies found that more than 50% of anaerobes isolated from leg ulcers were strains of GPAC belonging to the genus previously known as Peptostreptococcus (Bowler & Davies, 1999a ). More recently, several reports have shed light about the bacterial profile associated with wound infections, and these studies demonstrate that besides aerobic species, like Staphylococcus spp., Streptococcus spp., Enterococcus spp. and Pseudomonas aerugionosa , anaerobes, including Peptoniphilus , Finegoldia and Anaerococcus are prominent colonisers (Dowd et al ., 2008a , b ; Wolcott et al ., 2009 ; Gontcharova et al ., 2010 ; Han et al ., 2011 ). In these studies, molecular methods such as bacterial Tag-encoded FLX amplicon pyrosequencing, 16S-based amplification followed by pyrosequencing and shotgun Sanger sequencing were utilised.
When the bacterial diversity in chronic wound tissue samples was examined using both standard culturing and pyrosequencing, culturing revealed an average of three common bacterial species in each wound. In contrast, pyrosequencing revealed an average of 17 genera and most of these were anaerobes, including Anaerococcus , Finegoldia and Peptoniphilus (Han et al ., 2011 ). Thus, it can be concluded that the development of molecular methods, such as bacterial Tag-encoded FLX amplicon pyrosequencing, has lead to improved identification of GPAC within various wound types. By symbiotically existing together with aerobic colonisers, which use up the oxygen, obligate anaerobes like GPAC may gain advantages and also act synergistically in causing disease. These bacteria and their metabolites may significantly impair normal wound-healing processes, such as inflammation, remodelling of extracellular matrix and re-epithelialisation, and it is now evident that polymicrobial biofilm communities constitute important barriers to the healing of chronic wounds (Bjarnsholt et al ., 2008 ). Considering that different virulence factors can be expressed by various genera and species of GPAC (for instance Finegoldia and Parvimonas , see below), a correct identification to the species level in clinical samples is important. For clinicians, a better understanding of the bacterial composition in a wound will naturally benefit the management of the particular wound.
A relative increase of GPAC in cases of anaerobic bacteraemia has also been observed (Lassmann et al ., 2007 ), and by using 16S rRNA gene sequencing, it was found that GPAC accounted for 7% of all anaerobic bacteria isolated from bloodstream infections over a 5-year period at Duke University Medical Center (Simmon et al ., 2008 ). Furthermore, in a recent study, anaerobes isolated by routine culture of samples or biopsies obtained from normally sterile sites, were identified by the use of MALDI-TOF MS and 16S rRNA gene sequencing (La Scola et al ., 2011 ). By this method, GPAC were identified in blood cultures and osteoarticular samples (La Scola et al ., 2011 ). A case of bacteraemia caused by Finegoldia was also recently reported (Rosenthal et al ., 2012 ). Oral infections caused by GPAC genera, like Peptostreptococcus and Parvimonas have been reported as well (described below). Also, Atopobium spp., including Atopobium parvulum , occur in human gingival crevices and may be isolated from dental abscesses and abdominal wounds (Olsen et al ., 1991 ). Atopobium parvulum has not only been associated with the saliva of healthy persons, but also with odontogenic infections, such as dental implants (Downes et al ., 2001 ). In addition, members of A. parvulum are associated with patients suffering from halitosis (oral malodour) (Riggio et al ., 2008 ).
An overview of infections associated with various GPAC genera and species are shown in Table 1 and the clinical relevance of the genera Anaerococcus , Peptoniphilus , Finegoldia , Peptostreptococcus and Parvimonas are described in more detail below.
Antibiotic resistance
In general, GPAC have variable resistance to penicillins (7–10%), clindamycin (7–20%), and metronidazole (5–10%), whereas these bacteria are more susceptible to β-lactam/β-lactamase inhibitors, cephalosporins, carbapenems, and chloramphenicol (Hecht, 2006 ). Also, resistance to tetracycline and erythromycin has been reported (Brazier et al ., 2003 ; Boyanova et al ., 2004 ). Data describing differences in antimicrobial susceptibility between various species of GPAC are increasing (Bowker et al ., 1996 ; Brazier et al ., 2003 , 2008 ; Koeth et al ., 2004 ; Roberts et al ., 2006 ; Könönen et al ., 2007 ) and are described in more detail for the major groups below.
Regarding the continuous rise in antibiotic resistance amongst GPAC and anaerobes in general, more surveillance testing will be needed. Moreover, due to differences in antibiotic susceptibility between GPAC species, it is important to identify isolates in clinical specimens for susceptibility testing to adapt the correct antibacterial therapy.
Anaerococcus
Description and overview
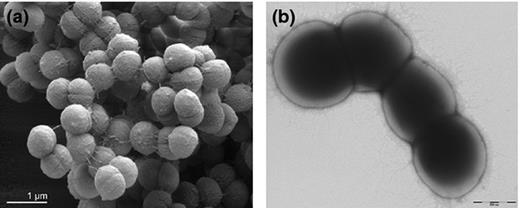
The type species of the genus Anaerococcus is A. prevotii (Ezaki et al ., 2001 ) (see Fig. 3a ). This strain was originally designated as Micrococcus prevotii , then placed in the genus Peptococcus (Foubert & Douglas, 1948 ), transferred to the genus Peptostreptococcus in 1983 (Ezaki et al ., 1983 ) and finally to the genus Anaerococcus (Ezaki et al ., 2001 ). Anaerococcus prevotii and several other species have been described, namely A. tetradius , Anaerococcus lactolyticus , Anaerococcus hydrogenalis , A. vaginalis , Anaerococcus murdochii , Anaerococcus octavius and Anaerococcus senegalensis (see Fig. 3b ) (Ezaki et al ., 2001 ; Song et al ., 2007a ; Lagier et al ., 2012 ). Cells occur in pairs, tetrads, short chains or clumps and individual cells vary in size from 0.6–0.9 μm in diameter, and on enriched blood agar, colonies also vary in size (0.5–2 mm) (Labutti et al ., 2009 ). Peptones and amino acids are used as major energy sources and butyrate is the major metabolite (Ezaki et al ., 2001 ). Most species are able to weakly ferment carbohydrates and are also indole-negative and coagulase-negative (Ezaki et al ., 2001 ).
The completed gene sequence of the type strain A. prevotii PC1 T , originally isolated from human plasma, was recently published (Labutti et al ., 2009 ). The genome is 1 797 577 bp long (chromosome and one plasmid), has an average G + C content of 35.6% and a total of 1913 open reading frames (ORFs). Of these, 1852 are protein-coding genes and 1399 of the genes have been assigned a predicted function.
Clinical importance
Anaerococcus prevotii is frequently recovered from clinical specimens, such as vaginal discharges and ovarian, peritoneal, sacral or lung abscesses (Labutti et al ., 2009 ). The species is also a common member of the normal flora of skin, oral cavity and the gut (Ezaki et al ., 1983 ). In an rRNA gene-based study of the armpit microbiota of healthy males, Anaerococcus spp. were abundant (Egert et al ., 2011 ). By using multiplex PCR, 16 out of 190 clinical isolates were identified as Anaerococcus spp., mainly A. vaginalis (Song et al ., 2003b ) and in a European study on antimicrobial susceptibility amongst 299 GPAC isolates, mainly from skin and soft-tissue infections, 26 were identified as Anaerococcus (Brazier et al ., 2008 ). Using a culture-independent molecular approach, A. lactolyticus was identified in urinary tract specimens in coinfection with both known and unknown uropathogens (Domann et al ., 2003 ). In a recent survey of the bacterial diversity in biofilms of various wound types, A. lactolyticus and A. vaginalis were identified among the predominant species in grouped samples of diabetic foot ulcers and pressure ulcers using 16S rRNA gene-based molecular amplification followed by shotgun Sanger sequencing (Dowd et al ., 2008a ). When the bacterial diversity in individual chronic diabetic foot ulcers was investigated using a pyrosequencing approach, Anaerococcus spp. were highly prevalent and found in 22 of 40 samples (Dowd et al ., 2008a ). Recently, A. vaginalis and A. prevotii were also identified in blood cultures by mass spectrometry and 16S rRNA gene sequencing, (La Scola et al ., 2011 ). In addition, five isolates of Anaerococcus were identified of which at least two, based on sequence similarity with known species, most likely belong to new species (La Scola et al ., 2011 ). Two of these isolates were obtained from osteoarticular samples, one from cervical abscess and the others from blood.
Antibiotic resistance
Anaerococcus prevotii is susceptible to penicillins (Murdoch, 1998 ) but resistant to SPS (Song et al ., 2007a ). Brazier et al . ( 2003 ) also suggests that A. prevotii is resistant to tetracycline, erythromycin and clindamycin, although the number of isolates in this study was very low. Other studies have shown resistance of A. prevotii , isolated from diabetic foot infections, to clindamycin, levofloxacin and ceftazidine (Goldstein et al ., 2006 a; Goldstein et al ., 2006b ). Clinical isolates of A. murdochii (six strains) were reported to be resistant to colistin sulphate, two strains to kanamycin, one to clindamycin and three showed intermediate resistance to penicillin (Song et al ., 2007a ).
Peptoniphilus
Description and overview
The genus Peptoniphilus uses peptone as a major energy source, butyrate is the major metabolic end-product and carbohydrates are not fermented (Ezaki et al ., 2001 ). Cells vary in size depending upon species (from 0.5–1.5 μm in diameter for P. harei ), colonies are 1–2 mm, circular, entire and opaque, the G+C content of DNA of members of this genus is 30–34 mol% (Ezaki et al ., 1983 ). The type species is P. assacharolyticus , originally classified in the genus Peptococcus , transferred to the genus Peptostreptococcus in 1986 (Holdeman et al ., 1986 ) and finally reclassified to the genus Peptoniphilus (Ezaki et al ., 2001 ). However, the type strain of P. asaccharolyticus (ATCC 14963) is not representative of the species and a low DNA–DNA homology between clinical isolates and the type strain was described. Thus, a number of type strains were reidentified using 16S rRNA gene sequencing and the closest relative for the strains was P. harei (Veloo et al ., 2011a ). Peptoniphilus harei and P. asaccharolyticus share the same biochemical features and it was concluded that the isolates of P. asaccharolyticus were misidentified. Veloo et al . ( 2011a ) therefore suggested the incidence of P. asaccharolyticus in clinical material to be highly overestimated. In a recent rRNA-based study of the armpit microbiota of healthy males, Peptoniphilus spp. were also found to be abundant (Egert et al ., 2011 ).
Clinical relevance
With new molecular techniques like pyrosequencing, the clinical importance of the genus Peptoniphilus has been acknowledged. Several recent studies have found high frequency of Peptoniphilus spp. DNA within chronic wound samples. For instance, Dowd et al . ( 2008a ) found that Peptoniphilus DNA comprises 38.4% of total sequences within pressure ulcer samples, 7% in diabetic wounds, but only 0.2% within venous leg ulcers. Dominating bacteria in pressure ulcers were Peptoniphilus ivorii , but also high frequencies of P. harei and Peptoniphilus indolicus were found (Dowd et al ., 2008a ). Other studies using pyrosequencing also report high prevalence of Peptoniphilus spp. in diabetic ulcers (Dowd et al ., 2008b ; Gontcharova et al ., 2010 ). Recently, species of Peptonophilus , such as P. harei , P. assacharolyticus and Peptoniphilus lacrimalis , were also identified by mass spectrometry and 16S rRNA gene sequencing in clinical material from osteoarticular samples (La Scola et al ., 2011 ). Other reported sites of isolation of P. lacrimalis are from vaginal specimens and discharge of the eye (Murdoch, 1998 ). Recently, two novel species isolated from clinical specimens, including leg infection, back cyst, and abscesses, were proposed, Peptoniphilus coxii and Peptoniphilus tyrrelliae (Citron et al ., 2012 ). Also, two strains from human wound specimens were recently isolated and proposed to belong to two novel species, Peptoniphilus duerdenii and Peptoniphilus koenoeneniae (Ulger-Toprak et al ., 2012 ). Moreover, a novel food-borne Peptoniphilus spp. was identified in a study investigating microorganisms from retail ground beef and was named Peptoniphilus methioninivorax (Rooney et al ., 2011 ).
Interestingly, when the microbial flora was identified with pyrosequencing in patients with chronic rhinosinusitis, anaerobic genera like Peptoniphilus predominated, in contrast to conventional culturing methods, where mainly Staphylococcus aureus and coagulase-negative Staphylococcus were detected (Stephenson et al ., 2010 ). In a European study on antimicrobial susceptibility among 299 GPAC isolates, mainly from skin and soft-tissue infections, 70 isolates were identified as Peptoniphilus with P. harei being the dominating species (Brazier et al ., 2008 ). The clinical importance of P. harei is further emphasised by Song et al . ( 2003b ) who identified 48 of 190 clinical isolates as P. harei . A retrospective report on anaerobic isolates collected in Costa Rica between 1999 and 2008 revealed approximately 60% Gram-positive bacteria and of these, 25% were cocci (Rodriguez-Cavallini et al ., 2011 ). Species were identified by the use of two commercial phenotypic systems (RapID 32A and API 20A) and 12% were identified as Peptoniphilus spp. dominating in skin, soft tissue, bone and genitourinary tract samples.
Antibiotic resistance
A recent study reported that P. coxii strains were resistant to doxycycline and 29% were resistant to moxifloxacin and clindamycin, whereas all strains of P. tyrrelliae were susceptible to doxycycline but resistant to moxifloxacin and 25% to clindamycin (Citron et al ., 2012 ). All strains were susceptible to linezolid, metronidazole and penicillin (Citron et al ., 2012 ). In another study, the in vitro activity of the broad-spectrum cephalosporin ceftobiprole was compared with other antibiotics against 20 strains of P. asaccharolyticus , isolated from diabetic foot infections (Goldstein et al ., 2006 a). They were highly resistant to levofloxacin and ceftazidine but sensitive to ceftobiprole. Peptoniphilus harei has been reported as resistant to tetracycline, in contrast to P. lacrimalis and P. ivorii (Brazier et al ., 2003 ). Clindamycin resistance by strains of P. asaccharolyticus has also been reported (Citron et al ., 2005 ; Goldstein et al ., 2006b ). Song et al . ( 2007a ) reported that Peptoniphilus gorbacchi showed resistance to clindamycin (2 of 6 strains) and one strain showed intermediate resistance to penicillin.
Finegoldia
Description and overview
The genus Finegoldia is named after the American microbiologist S. M. Finegold and the type species is F. magna (Murdoch & Shah, 1999 ). The original classification of F. magna remains unclear, but it might have been described first as Diplococcus magnus in 1933 (Prevot, 1933 ), and in 1974, given the name Peptococcus magnus (Rogosa, 1974 ). The taxonomic revision by Ezaki and coworkers transferred the species to the genus Peptostreptococcus (Ezaki et al ., 1983 ). In 1999, Peptostreptococcus magnus was reclassified in the current genus Finegoldia as F. magna (Murdoch & Shah, 1999 ).
Finegoldia magna cells vary from 0.8 to 1.6 μm in diameter and occur predominantly in masses but occasionally in pairs or short chains (see Fig. * ). The growth rate in vitro is relatively slow. In liquid Todd-Hewitt medium (supplemented with 0.5% Tween-80), the bacteria reach stationary phase after incubation for 70–90 h (Karlsson et al ., 2007 ). Following growth on enriched blood agar for 2–5 days, colonies range 1–2 mm in diameter. The colour of the colonies is most frequently translucent, but can vary from white to grey and even yellow (Murdoch & Mitchelmore, 1991 ; Murdoch, 1998 ). Finegoldia magna is an anaerobic bacterium requiring an oxygen-free environment for growth. However, F. magna isolates on enriched blood agar plates that were exposed to air still had some viable cells after 48 h, indicating that resting cells may be relatively aerotolerant (Murdoch, 1998 ). Acetic acid is the major fermentation product and most strains produce weak acid from fructose and only a few strains from glucose (Ezaki et al ., 1983 ; Murdoch, 1998 ). Instead peptones and amino acids can be used as major energy sources. All strains produce ammonia from glycine and most strains produce ammonia from threonine and serine (Ezaki et al ., 1983 ). Aminopeptidase activities have been reported (Ng et al ., 1998 ) and also catalase activity (Murdoch & Mitchelmore, 1991 ; Krepel et al ., 1992 ). Coagulase, indole and urease are not formed, and no strain reduces nitrate (Ezaki et al ., 1983 ; Murdoch, 1998 ). For a summary of the biochemical features and major characteristics of F. magna , see Table 2 .
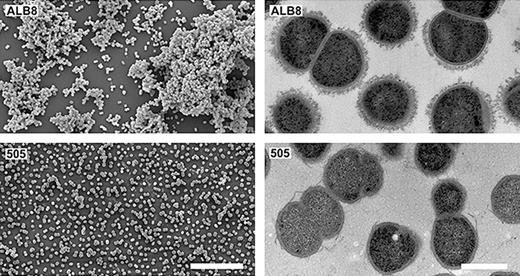
Electron microscopy images of Finegoldia magna ALB8. Left: Scanning electron micrographs of F. magna strains ALB8 (top row) and 505 (non-FAF expressing strain) (bottom row) Bar represents 10 μm. Right: Transmission electron micrographs of same. Bar represents 5 μm. Reproduced with premission from (Frick et al ., 2008 ).
Genome description
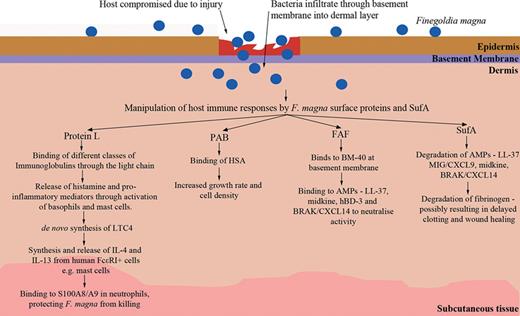
The first complete genome sequence of F. magna strain ATCC 29328, originally isolated from an abdominal wound, was published in 2008 (Goto et al ., 2008 ). The genome consists of a circular chromosome (1.8 Mb) and a plasmid pPEP1 (0.2 Mb) with a GC content of 32.3% for the chromosome and 29.7% for the plasmid. A total of 1813 ORFs were found, 1631 in the chromosome and 182 in the plasmid. Genomic analysis revealed that only F. magna has a complete glycolysis pathway for fructose in accordance with previous reports that most strains produce weak acid from fructose and only few strains from glucose (Ezaki et al ., 1983 ; Murdoch, 1998 ). The genome has many aminopeptidases and amino acid/oligopeptide transporters that may facilitate the uptake of amino acids from the environment, thereby, amino acids can be used as major energy sources. As compared to other GPAC species, F. magna was reported to possess more aminopeptidase activities (Ng et al ., 1998 ), implicating a higher pathogenicity for this species. Other types of transporters found include electron, ion, multidrug-efflux and ATP-binding cassette transporters. Genes for superoxide reductase, NADH oxidase and a putative NADH dehydrogenase are also detected and these genes may help F. magna to survive in aerobic conditions.
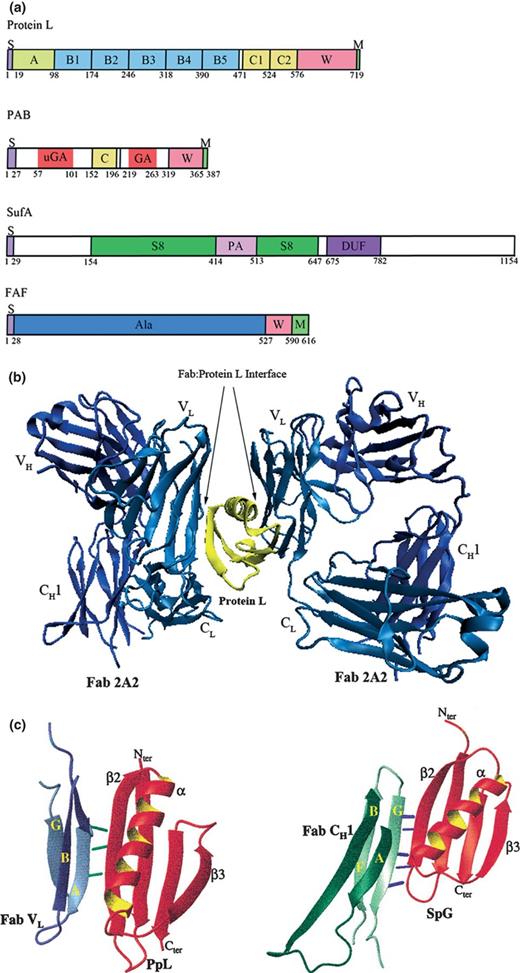
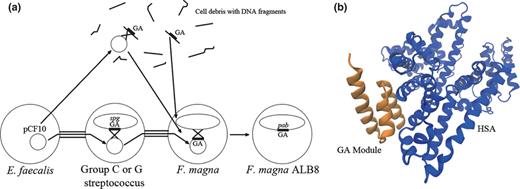
In the F. magna genome, four genes encoding the functional albumin-binding domain (GA module) are identified. The GA module is found in the Peptostreptococcal Albumin Binding (PAB) protein, originally isolated from F. magna in 1994 (de Château & Björck, 1994 ). The isolation and characterisation of protein PAB is described in more detail in the following section. Also, collagen adhesion homologues, amidase homologues, a serine proteinase precursor and a putative biofilm-associated surface protein were identified. Moreover, in silico analysis of the genome identified eleven genes encoding sortases; four on the chromosome and seven on the plasmid. Sortases are extracellular transpeptidases, that catalyse the covalent anchoring of proteins with LPXTG-like motifs to the bacterial cell wall by cleaving the threonine and glycine residues (Navarre & Schneewind, 1999 ; Novick, 2000 ; Mazmanian et al ., 2001 ). The presence of seven sortase genes on the plasmid seems to be a unique feature of F. magna , as searching of sortases in 29 plasmids of 14 Gram-positive bacterial species revealed only one sortase gene present on a Clostridium perfringens plasmid (Goto et al ., 2008 ). Thus, the plasmid-encoded sortases in F. magna might be of importance in terms of pathogenicity through enrichment of the variety of surface proteins leading to enhancement of the bacterial interaction with host tissues (Goto et al ., 2008 ).
Clinical importance
Amongst the GPAC, F. magna is probably the most pathogenic organism and is the species most frequently isolated in pure culture from various clinical infection sites (Bourgault et al ., 1980 ; Murdoch, 1998 ). Typical infections connected with F. magna are soft-tissue abscesses, wound infections, bone and prosthetic joint infections (Fitzgerald et al ., 1982 ; Davies et al ., 1988 ; Murdoch, 1998 ; Brook & Frazier, 2000 ; Brazier et al ., 2008 ; Brook, 2008 ; Holst et al ., 2008 ; Levy et al ., 2009 ; Martin et al ., 2009 ). The bacterium has also been described in septic arthritis (Fitzgerald et al ., 1982 ; Hunter & Chow, 1988 ), nonpuerperal breast abscesses (Edmiston et al ., 1990 ; Krepel et al ., 1992 ; Castello et al ., 2007 ) and in vaginoses (Kastern et al ., 1990 ; Ricci et al ., 2001 ; Aggarwal et al ., 2003 ). In addition, rare cases of infectious endocarditis on prosthetic valves (Cofsky & Seligman, 1985 ; Pouëdras et al ., 1992 ; van der Vorm et al ., 2000 ; Bassetti et al ., 2003 ; Fournier et al ., 2008 ) and postoperative mediastinitis (Kernéis et al ., 2009 ) caused by F. magna have been described. In a clinical study, the incidence of anaerobic bacteria in patients with pleural empyema were analysed (Boyanova et al ., 2004 ). GPAC were found in 35.4% of the patients that were positive for anaerobic bacteria (147 of 198 patients), and among those F. magna accounted for 7.5%. Also a case of necrotising pneumonia caused by F. magna was recently described (Sedano Gómez et al ., 2011 ).
Most likely, the incidence of F. magna is highly underestimated due to problems of obtaining good quality anaerobic clinical specimens. For instance, detection of F. magna in blood cultures was found to be dependent on the blood culture system used (Bassetti et al ., 2003 ). In this case of prosthetic valve endocarditis caused by F. magna , several blood cultures incubated in BacT/ALERT (BioMérieux) and BACTEC 9240 (Becton Dickinson) systems were negative despite growth of F. magna from biopsies of the aortic wall of the patient (Bassetti et al ., 2003 ). Additional tests demonstrated that the isolated strain did grow in other blood culture systems like SEPTI-CHEK BHI-S (Becton-Dickinson) and ISOLATOR (Du Pont Co.) or in thioglycolate medium and on blood agar (Bassetti et al ., 2003 ). This is consistent with other reports on prosthetic valve endocarditis caused by F. magna , where the bacterium could only be detected in cultures from the infected valve (Pouëdras et al ., 1992 ; van der Vorm et al ., 2000 ). Thus, the relevance of F. magna as the infectious agent in patients with apparent culture-negative endocarditis has to be considered. Interestingly, by the use of molecular amplification in combination with traditional culturing of samples from prosthetic joint infections, correct diagnoses was made by PCR in cases where culturing was negative (Holst et al ., 2008 ; Levy et al ., 2009 ). A more accurate detection and identification of the bacteria might also lead to a changed view of the frequency of F. magna in relation to certain clinical conditions. The prospect of giving the correct antibiotic therapy will also be improved.
Finegoldia magna has been shown to be one of the most common anaerobes isolated from skin specimens (Higaki & Morohashi, 2003 ), and several surveys have reported F. magna as highly prevalent in chronic wounds, including diabetic ulcers and pressure ulcers (Hansson et al ., 1995 ; Stephens et al ., 2003 ; Dowd et al ., 2008a , b ; Gontcharova et al ., 2010 ). In a study by Hansson et al . ( 1995 ), F. magna was present in 29% of patients suffering from venous leg ulcers (58 patients) without any clinical sign of infection. In another study, the bacterial flora was characterised in venous leg ulcers of 178 patients during 12 weeks and 153 individual bacterial species were identified. Finegoldia magna was among the most frequently isolated species and was found in 21.4% of the isolates (Moore et al ., 2010 ). Furthermore, in samples from 40 diabetic foot ulcers, F. magna was present in 23 of the samples (Dowd et al ., 2008a ). Recently, anaerobes isolated by routine culture from deep samples, were identified by the use of MALDI-TOF MS and 16S rRNA gene sequencing (La Scola et al ., 2011 ). From a total of 544 isolates, from various sampling sites, 332 isolates (61%) were identified by MALDI-TOF MS whereas the remaining 212 isolates (39%) could be identified by 16S rRNA gene sequencing. Finegoldia magna was amongst the most common anaerobes and mainly isolated from osteoarticular samples (La Scola et al ., 2011 ).
Finegoldia magna has recently been implicated in a case of toxic shock syndrome (Rosenthal et al ., 2012 ). The case involved a fatal monomicrobial F. magna bacteraemia and it was believed to be caused by the superantigen activity of protein L, binding to the variable domain of the κ light chains of IgG. This report suggests that the overall significance of F. magna as a pathogen is underestimated and that more sensitive detection methods will see it being identified more frequently in clinical infections in the future (Rosenthal et al ., 2012 ).
Antibiotic resistance
In general, F. magna is susceptible to the antibiotics that are used for treatment of anaerobic infections, but lower antibiotic resistance rates (10–20%) to clindamycin, metronidazole, penicillin and higher resistance rates (> 20%) to erythromycin and tetracycline have been reported (Aldridge et al ., 2001 ; Brazier et al ., 2003 , 2008 ; Martin et al ., 2009 ; Hawser, 2010 ). Various rates of resistance to some fluoroquinolones (levofloxacin and moxifloxacin) and cephalosporins (cefotaxime, cefepime and ceftazidime) have also been described (Goldstein et al ., 2006 a; Veloo et al ., 2011b ). In the study by Veloo et al . ( 2011a ), F. magna showed the highest MIC50 (Minimum Inhibitory Concentration required to inhibit the growth of 50% of the organisms) and MIC90 values for penicillin G, amoxicillin–clavulanic acid, clindamycin, and tigecycline and it also had the highest MIC90 values for levofloxacin and moxifloxacin as compared to other GPAC, like P. micra and P. harei , also tested. Furthermore, F. magna isolates displaying various rates of resistance to erythromycin, azithromycin, ampicillin and levofloxacin were found susceptible to telithromycin, a ketolide structurally related to clarithromycin (Mikamo et al ., 2003 ). Telithromycin was shown to exhibit a rapid and prolonged inhibitory activity against F. magna and other common anaerobic and aerobic pathogens, suggesting a clinical use in mixed respiratory infections (Stein et al ., 2006 ).
Metronidazole, a 5-nitroimidazole, has been one of the preferred antimicrobials for serious anaerobic infections; however, resistance has been noted for some anaerobic species, for instance Bacteroides fragilis , Clostridium spp. and Peptostreptococcus spp. (Pankuch et al ., 1993 ). Resistance mechanisms have not been conclusively identified, but in Bacteroides the presence of nim genes encoding nitroimidazole reductases has been implicated as a possible cause of resistance (Trinh & Reysset, 1996 ). Out of nine tested F. magna strains, two were found highly resistant to metronidazole (MIC > 128 mg L −1 ), and also possessed a nimB gene (Theron et al ., 2004 ). The nimB gene was however, also found in three of the seven susceptible F. magna strains, implying the possibility of a silent nimB gene (Theron et al ., 2004 ).
Virulence factors
The most well-described virulence factors of F. magna are protein L (Björck, 1988 ), PAB (de Château & Björck, 1994 ), SufA (Karlsson et al ., 2007 ) and FAF (Frick et al ., 2008 ) (Fig. 5 ). These four proteins are described in more detail in the following section. Other virulent properties have been described in F. magna . However, the proteins responsible for these actions have not yet been identified. Finegoldia magna strains with enhanced collagenase, gelatinase and hippurate hydrolase activity have been isolated from nonpuerperal abscesses and diabetic foot infections (Krepel et al ., 1991 , 1992 ). Furthermore, supernatant from strains isolated from chronic leg ulcers inhibited keratinocyte wound repopulation and endothelial tubule formation in vitro (Stephens et al ., 2003 ).
Overview of Finegoldia magna surface proteins and SufA and their effects on the host during infection.
Finegoldia magna has recently been described to have the ability to form biofilm (Donelli et al ., 2012 ). It was found to be strongly adherent and could develop as a dual-species biofilm with both B. fragilis and Clostridium difficile . The ability of F. magna to form a biofilm could protect it from host immune defences, as well as targeted antibiotic therapies (Donelli et al ., 2012 ).
Protein L
Protein L – an F. magna surface protein with affinity for Ig L chains
Protein L (76–106 kDa depending on the strain from which it is isolated) was initially isolated from F. magna strain 312 and could not be identified in other investigated strains (Björck, 1988 ). In binding experiments, 125 I-radiolabelled protein L was able to bind IgG, F(ab')2 fragments and Fab fragments of IgG, κ and λ L chains, IgM and IgA. In binding assays with human plasma, it binds exclusively and highly specifically with human Ig L chains (Björck, 1988 ). Protein L binds to IgM, IgA and IgG with an equilibrium constant of approximately 10 10 M −1 for each, indicating a similar binding to all three immunoglobulin classes (Åkerstrom & Björck, 1989 ).
Protein L interacts with the light chain of Ig through the κ variable domain (Nilson et al ., 1992 ). The ability of protein L to bind V κ domains makes it of potential value in the isolation of antigen-binding V L domains and Fv fragments prepared from monoclonal antibodies (Nilson et al ., 1992 ).
Structure of protein L and interaction with domains of Ig
Protein L was the first gene in F. magna to be sequenced. Its basic structure bears both similarities and differences with cell-wall proteins of Gram-positive bacteria. Its signal sequence is considerably shorter, consisting of 18 amino acid residues compared to at least 33 in most other Gram-positive cell-wall proteins (see schematic representation Fig. 6a ). Following the signal sequence, there is region A which consists of 79 residues. The κ-binding property was mapped to the B repeats, which consist of 72–76 amino acid residues each (Kastern et al ., 1992 ). This is analogous to protein A and protein G whose IgG Fc-binding activity is also located in repeat units (Sjödahl, 1977 ; Fahnestock et al ., 1986 ; Guss et al ., 1986 ). The κ-binding B repeats of protein L shared no homology with Ig-binding domains of any other bacterial protein, which is expected, due to its unique specificity for Ig light chains. Protein L also contains two twin-C repeats (52 amino acid residues each) with unknown function and shares no homology with protein or gene sequences in major databanks. Following on from the C repeats comes the cell wall-spanning region (W), an LPXTG-type motif and a hydrophobic membrane-spanning region (Fischetti et al ., 1990 ; Kastern et al ., 1992 ) (Fig. 6a ).
(a) Overview of domain layout in Finegoldia magna proteins. S, signal peptide; W, wall spanning region; M, membrane spanning region; S8, peptidase S8 domain; PA, protease associated domain; DUF, domain of unknown function; Ala, alanine rich region. (b) Structure showing interaction of protein L C* domain (same three dimensional structure as domain B1) with two human IgM Fab 2A2 domains. The protein L domain (yellow) is sandwiched between and interacting with the light chains of both Fab domains (light blue). Structure was built using the 1HEZ pdb file (Graille et al ., 2001 ). This image was made with VMD. VMD is developed with NIH support by the Theoretical and Computational Biophysics group at the Beckman Institute, University of Illinois at Urbana-Champaign. (c) Comparison of protein L (PpL) with protein G (SpG) fold. The β sheets are orientated to show the structural similarities in both domains. The β2 sheet of both domains interacts with an Fab VL (protein L) or an Fab CH1 (protein G) β sheet, through a β zipper interaction. Coloured lines represent hydrogen bonds between the main-chain atoms. Figure reproduced with permission from (Graille et al ., 2001 )
The three dimensional structure of the 76 amino acid residue domain B1 of protein L was resolved using NMR spectroscopy and distance geometry-restrained stimulated annealing. It comprises a 15-amino acid residue disordered NH 2 -terminus followed by an α-helix packed against a four-stranded β-sheet. It shares very limited sequence homology with the protein G domains interacting with the heavy chains of IgG (Wikström et al ., 1994 ). However, despite their different binding properties, the B1 domain and the IgG-binding protein G domain have similar folds (see Fig. 6c ) (Wikström et al ., 1993 ). The B1 domain of protein L is thought to interact with the human Ig κ chain through most of the residues in β-sheet 2, the COOH-terminal residues of the α-helix and the loop connecting the α-helix with the third β-strand (Wikström et al ., 1995 ).
The crystal structure of the complex between a human Ig Fab fragment and a single domain of protein L was refined to 2.7 Å (Graille et al ., 2001 ). The protein L domain used in the study, C* (61 residues), has the same three-dimensional structure as domain B1 as determined by NMR (Wikström et al ., 1994 ) and the two structures superimpose with an rmsd of 1.31 Å over 59 residues (Graille et al ., 2001 ). The crystal structure revealed that C* has two separate regions that can interact with κ-light chains (see Fig. 6b ). Therefore, it can bind two Fab fragments simultaneously, involving similar sites on the V L domains of the κ-chain, but with markedly different affinities. The possibility of a single protein L domain interacting with two V L regions suggests that protein L could bridge two Ig molecules anchored at the membrane of B cells (Graille et al ., 2001 ).
Protein L as a tool for antibody detection
Two proteins widely used for binding and detection of IgG antibodies are protein A from staphylococcal strains (Forsgren & Sjöquist, 1966 ) and protein G from group C and G streptococci (Björck & Kronvall, 1984 ; Reis et al ., 1984 ). These proteins that have been well-characterised have affinity for the Fc part of mammalian IgG. They display similar physiochemical properties and are fibrous, elongated, monomeric proteins with several binding sites for IgG (Åkerstrom & Björck, 1986 ). Protein G binds to all human IgG subclasses and has a higher affinity for human IgG than protein A (Langone, 1982 ; Åkerstrom & Björck, 1986 ). However, the ability of protein L to bind to the light chain of Ig means that it can bind all classes of Ig with a κ-light chain. Therefore, protein L represents a new tool for the binding and detection of antibodies due to its broader Ig-binding spectrum (Åkerstrom & Björck, 1989 ). Furthermore, as protein L does not bind bovine immunoglobulins (Château et al ., 1996 ), the protein provides a convenient way to purify κ-light chain-containing monoclonal antibodies produced from culture supernatant or ascites where foetal calf serum is used.
Biological implications for protein L – Ig light chain interactions
By expressing protein L on the surface of some strains, F. magna is able to bind different classes of immunoglobulin on its cell surface with high affinity during infection. As a result, the bacterium could influence cellular and molecular events (Kastern et al ., 1990 ). Although most of protein L is bound at the bacterial surface, some of it is also released into the medium during growth (Björck, 1988 ; Kastern et al ., 1990 ). In a study, Kastern et al . found a significant correlation between expression of protein L and F. magna isolated from bacterial vaginosis (Kastern et al ., 1990 ). Protein L and Protein L-expressing bacteria were found to induce an in vitro mediator release from human basophils and mast cells from lung and skin tissues (Patella et al ., 1990 ), most likely through an interaction with IgE on the surface of the cells. Mediator release was dose- dependent with histamine secretion gradually increasing with increase in bacterial concentration (Patella et al ., 1990 ).
Protein L had a further effect on human basophils through inducing the de novo synthesis of leukotriene C 4 (LTC4) (Patella et al ., 1990 ). LTC4 is a proinflammatory chemical mediator that possesses many biological and vasoactive properties in humans (Samuelsson et al ., 1987 ; Marone et al ., 1988 ). In human skin mast cells (HSMC), protein L induced a greater histamine release than anti-IgE, whereas protein A and protein G failed to have any effect. In addition, protein L also induced the de novo synthesis of the chemical mediator, prostaglandin D 2 (PGD 2 ), from HSMC. Both LTC4 and PGD 2 have significant biological importance in inflammation, suggesting that protein L-expressing F. magna could have increased virulence during infection. In the same study by Patella et al . ( 1990 ), intradermal injection of protein L induced a wheal-and-flare reaction in nonallergic subjects. These results abide by previous findings that expression of protein L is correlated with virulence (Kastern et al ., 1990 ).
To investigate the virulent effects of protein L in infection, the human oral commensal, Streptococcus gordonii GP1291, was used to construct a recombinant bacterium expressing four of the Ig-binding domains of protein L (B1–B4) (Ricci et al ., 2001 ). As a control, a GP1292 strain was used which expressed another M6-based fusion protein. After 6 weeks, 33.3% of the mice inoculated with GP1291 were still colonised, as compared to 5.5% of GP1292 inoculated mice. Furthermore, in the murine vagina, S. gordonii GP1291 persisted for 14 weeks, whereas GP1292 persisted for 8 weeks. These results suggest that Ig-binding domains of protein L on the surface of S. gordonii enhance the duration of vaginal colonisation (Ricci et al ., 2001 ). Ricci et al . hypothesise that the effect seen could be due to protein L facilitating the adherence of F. magna to the vaginal mucosa through interaction with surface associated IgA during oestrus, as S. gordonii colonisation occurs during this time. However, during proestrus, colonisation is at its lowest and IgG and IgA reach their highest concentrations in vaginal fluid. During this time, protein L could mainly interfere with the defence functions of soluble Ig and thus, prevent bacterial clearance (Ricci et al ., 2001 ).
Another biological function for protein L is that it is a potent inducer of the synthesis and release of IL-4 and IL-13 from human FcεRI + cells (human basophils and mast cells expressing the FcεRI high affinity receptor for IgE) (Genovese et al ., 2003 ). This activity classifies protein L as a bacterial superantigen due to its ability to induce the release of two cytokines critical for Th2 polarisation from human FcεRI + cells. This action is mediated by binding to the κ-chains of IgE present on human basophils (Genovese et al ., 2003 ). A recent study by Nunomura et al . ( 2012 ) suggests that cell-surface FcεRI expression is an important participant in protein L-mediated full activation of mast cells. Engagement of FcεRI with the κ-chains of IgE and protein L induces tyrosine phosphorylation of ITAM in the FcRβ- and γ-signalling subunits leading to intracellular signalling and the release of proinflammatory mediators such as TNF-α and LTC4. Both TNF-α and LTC4 play important roles in adaptive immunity (Nunomura et al ., 2012 ). The ability of protein L to induce de novo synthesis of immunoregulatory cytokines has an important relevance as to the virulence of protein L expressing F. magna strains. Furthermore, protein L appears to be the first bacterial protein capable of inducing de novo synthesis and release of IL-4 and IL-13 from basophils (Genovese et al ., 2003 ). During infection, this could be both beneficial and harmful to the bacterium. Inflammation leads to an increase in vascular permeability causing an influx of nutrient-rich plasma. However, inflammation also induces the production of many antimicrobial peptides and chemokines. Nevertheless, if the bacterium is capable of counteracting these mechanisms more effectively than other bacterial species in the same locality, this will create a selective advantage (Åkerstrom & Björck, 1989 ).
To investigate in vivo trafficking of protein L, Smith et al . ( 2004a ) used whole body autoradiography in a murine model system. Analysis showed that protein L primarily targets secondary lymphoid organs, the spleen and lymph nodes. The main target of protein L was the white pulp of the spleen, which is composed of highly organised lymphoid tissue containing T and B lymphocytes (Picker & Siegelman, 1999 ). Therefore, protein L preferentially targets cells expressing surface Ig or cells that can interact with Ig, which agrees with previous studies. The major cell population targeted by protein L was B lymphocyte B220 + cells, whose interaction was rapid and transient (Smith et al ., 2004a ). This targeting is most likely mediated through direct interaction of protein L to surface Ig on B cells. In addition, this interaction in vivo resulted in B-cell activation as splenic B cells showed upregulation of MHC-II and CD86 after administration of protein L (Smith et al ., 2004a ). The ability of protein L to target a specific cell population in vivo could lead to the development of new therapeutic disease treatments.
Further investigations in the effects of protein L on B-cell subpopulations discovered that in a mature pool of B cells, addition of protein L caused a reduction of splenic marginal zone B cells and peritoneal B-1 cells (Viau et al ., 2004 ). These two subsets of B cells are important in innate B-cell immunity and induce the rapid clearance of pathogens. Therefore, F. magna may use protein L as a tool to subvert the first line of the host's immune defence (Viau et al ., 2004 ).
Apart from the B1–B5 repeats which have been shown to bind to human Ig L chains, a function has also been determined for the A domain. The A domain is the outermost domain of protein L and a short segment (< 10 aa) most proximal to the B1 domain was found to have high affinity for the neutrophil cytosolic proteins S100A8 and S100A9 (Åkerstrom & Björck, 2009 ). S100A8 and S100A9 make up almost 40% of the total protein cytosolic content of neutrophils and monocytes (Odink et al ., 1987 ). At certain Ca levels, the two proteins form the tetramer, (S100A8/A9) 2 , known as calprotectin, which has the ability to inhibit the growth of various bacterial and fungal species (Steinbakk et al ., 1990 ). S100A8/A9 was found to kill F. magna 505 (a strain not expressing protein L), but not F. magna strain 312, which further suggests that resistance to killing is due to surface protein L (Åkerstrom & Björck, 2009 ). The resistance is thought to be due to protein L forming a barrier by binding the neutrophil proteins at a distance from the cell surface (Åkerstrom & Björck, 2009 ). S100A8/A9 action on F. magna 505 was seen at pH 5.5 and not pH 7.5 which is relevant to where F. magna are most commonly isolated – the skin, mucosal surfaces in the vagina and in mouth and tissue abscesses (Rentzsch & Wilke, 1970 ; Bryant et al ., 1980 ; Kastern et al ., 1990 ; Grinstein et al ., 1991 ; Ohman & Vahlquist, 1994 ; Robinson et al ., 2002 ). In addition, an acidic pH is found in tissues and wound fluids during inflammation and infection (Johne et al ., 1997 ).
PAB – peptostreptococcal albumin binding protein
Protein PAB: mosaic organisation and a product of intergenic interspecies recombination of a functional domain
Protein PAB is a human serum albumin (HSA)-binding protein isolated from a HSA-binding strain of F. magna (de Château & Björck, 1994 ) (see schematic representation Fig. 6a ). It could be purified from F. magna ALB8 from mutanolysin-released cell-surface proteins and from the growth medium. Protein PAB is composed of 387 amino acids and harbours an amino acid stretch comprised of 45 amino acids sharing 60% homology with the repeated albumin-binding sequence of protein G of group C and G streptococci. This section is known as the GA module in protein PAB and it corresponds to the HSA-binding domain of the protein (protein G-related albumin-binding module) (de Château & Björck, 1994 ). PAB contains no repeated domains, in contrast to proteins L and G and most other surface proteins of Gram-positive bacteria. When the amino acid sequence of the GA module is lined up with two HSA-binding domains from protein DG12 from a bovine group G streptococcal strain, three HSA-binding protein G domains of the G148 strain and protein PAB from F. magna ALB1, there is a high degree of homology and 14 conserved residues. Hence, this module appears to have been transferred across species borders from one prokaryotic gene to another, possibly originating in group C or G streptococcus and transferred to F. magna through the pCF10 conjugated plasmid from Enterococcus faecalis (de Château & Björck, 1994 ). See Fig. 7a for a model of GA module shuffling between different bacteria.
(a) Possible modes of shuffling of the GA module to form the pab gene in Finegoldia magna . Putative methods of uptake of the GA module include: (1) direct uptake of GA module fragment from cell debris and subsequent homologous/nonhomologous recombination with F. magna chromosome. (2) Release of pCF10 from E. faecalis into cell debris from group G streptococcus, recombination with the GA module and transformation into F. magna . (3) Conjugational plasmid pCF10 is transferred by conjugation from E. faecalis to group C or G streptococcus, homologous recombination with the protein G gene ( spg ) on the chromosome and finally, conjugation and homologous recombination with F. magna . Figure adapted from (de Château & Björck, 1994 ) (b) Structure of the HSA-GA complex, showing the GA module binding to a novel site on albumin. Structure was built using the 1TF0 pdb file (Lejon et al ., 2004 ). This image was made with VMD. VMD is developed with NIH support by the Theoretical and Computational Biophysics group at the Beckman Institute, University of Illinois at Urbana-Champaign.
Structure of the GA module
The GA module consists of 45 amino acid residues and was the first example of module shuffling in prokaryotes. Prokaryotes lack introns, making exon or module shuffling less probable. However, the gene sequences of bacterial HSA-binding domains were analysed with regard to their global fold and this led to the identification of ‘recer’ sequences (de Château & Björck, 1996 ). Recer sequences act as structureless spacer sequences of 15 nucleotides in length flanking the different modules, that promote flexibility and interdomain in-frame recombination in the corresponding protein (de Château & Björck, 1996 ). The presence of recer sequences in HSA-binding domains of bacterial proteins could help explain how the GA module was incorporated into protein PAB. These recer sequences are quite rare and after their identification, they have not been found on a wide scale in other genomes.
Protein PAB contains two GA modules – GA and uGA – which share 38% identity and provide two binding sites for HSA (de Château & Björck, 1996 ). The uGA domain has a much weaker binding affinity for HSA, if the same binding site for HSA on different GA modules is assumed (de Château et al ., 1996 ). uGA is present on protein PAB's predecessor – protein urPAB, which contains uGA, but not the GA module (de Château & Björck, 1996 ).
Initial studies on the structure of the GA module in F. magna ALB8 using NMR spectroscopy showed that it consisted of a three-helix-bundle with a left-handed folding topology (Johansson et al ., 1995 ). The presence of similar folds in different proteins with different protein-binding capabilities suggests that this is an energetically favourable fold that is conserved during evolution (Johansson et al ., 1995 ). Further NMR studies on the structure of the same GA module using NMR spectroscopy agreed with the structural prediction produced in 1995 (Johansson et al ., 1997 ). The region responsible for binding to HSA was predicted to be the NH 2 -terminal well-conserved region of the GA module (Johansson et al ., 1997 ).
The crystal structure of the GA module in complex with HSA was resolved at 2.7 Å (pdb code 1tf0) (Lejon et al ., 2004 ). The GA module binds to HSA at a site in domain II of the albumin molecule (see Fig. 7b ). In the GA module, the region involved in binding was discovered to be the residues from the second helix and the two loops surrounding it (Lejon et al ., 2004 ). The interaction between Phe-27 in the GA module and Met-329 in HSA was found to be crucial for the hydrophobic interaction. Binding of HSA by protein PAB in F. magna appears to enhance it's pathogenicity as protein PAB-expressing strains were mostly isolated from patients with localised suppurative infections. Binding of HSA could provide the bacteria with fatty acids and other nutrients carried by HSA, causing faster growth rates at an infection site (Lejon et al ., 2004 ). The biological implications of HSA binding will be discussed in more detail in the next section.
Biological functions and clinical implications of protein PAB
Protein PAB and PAB-expressing F. magna were found to bind HSA with high specificity (de Château et al ., 1996 ). In a study of 30 isolates from localised suppurative infections (abscesses, soft-tissue and wound infections), 16 were found to bind significant amounts of HSA. Vaginosis and commensal isolates showed no affinity for HSA (de Château et al ., 1996 ). These results suggest a link between the albumin binding phenotype and suppurative infections. Therefore, protein PAB plays an important role in enhancing bacterial virulence during infection. Furthermore, strains found to express protein PAB did not express Ig light-chain binding protein L (de Château et al ., 1996 ).
Studies carried out by de Château et al . ( 1996 ) showed that by the addition of HSA to the growth medium, the growth rates and maximum cell densities of HSA-binding strains of F. magna were significantly increased. This was not the case for nonbinding strains of F. magna . The reason for this growth increase is thought to be the access of the bacteria to diverse ligands carried by HSA. HSA is a major transporter of long and short-chain fatty acids, tryptophan, thyroxine, calcium ions, etc. By binding HSA to the bacterial surface through protein PAB, these ligands are made available to the bacteria. Moore et al . ( 1977 ) have shown that F. magna grows better in the presence of Tween 80, a detergent containing an oleic acid tail. Free fatty acids (FFAs) are present at a concentration of 0.5 mM in human plasma and 99% of it is tightly bound to the FFA-binding sites of HSA. This is due to FFAs having a rapid turnover, t 1/2 , of 2 min in human plasma. HSA is present extravascularly in most tissues and in inflammatory secretions at mucosal surfaces. Therefore, HSA-binding F. magna will have ample access to HSA and its ligands. This will give the bacteria selective advantages and faster growth rates when establishing infection.
In a recent study by Egesten et al . ( 2011 ), binding of HSA by the GA module was found to have a function in addition to nutrient access and fast growth. Group G streptococci, carrying the HSA-binding protein G, bound HSA from both saliva and plasma, and the bound HSA inactivated the antibacterial peptide, MIG/CXCL9. This protects the bacterium from harmful antibacterial peptides that are released during bacterial invasion, through a protein G-dependent HSA coating (Egesten et al ., 2011 ). This could also be a survival strategy used by F. magna to survive on an activated epithelial surface, via a protein PAB-dependent HSA coating.
The GA module of protein PAB was found to bind HSA with much higher affinity than its predecessor, G148-GA3, reflecting the power of bacterial evolution (Johansson et al ., 2002 ). Worryingly, PAB-expressing F. magna strains were found to be tetracycline- resistant, suggesting that antibiotics are providing the selective pressure behind module shuffling (Johansson et al ., 2002 ). If this trend continues, F. magna could turn from being a member of the normal bacterial flora into a potential pathogen. Finegoldia magna 's current role as a commensal and opportunistic pathogen means it utilises every opportunity to establish infection. Continued selective pressure through the use of antibiotics could mean that F. magna acquires more virulent traits, making infections more serious and difficult to treat.
SufA – subtilase of F. magna
SufA is a subtilisin-like proteinase of F. magna that was found to have a wide range of functions, which could result in enhanced pathogenicity of F. magna during infection. SufA was initially identified as a papain-released bacterial surface protein with a putative molecular mass of 127 kDa (Karlsson et al ., 2007 ). The putative locations of the catalytic triad are Asp181, His247 and Ser578. The consensus pattern and order of this catalytic triad is consistent with peptidases of the S8 family – subtilisin family (Siezen et al ., 1991 ; Siezen & Leunissen, 1997 ). See Fig. 6a for an illustrated domain layout of SufA.
Investigations were carried out to determine the capability of SufA to degrade antibacterial peptides, as this appears to be an important characteristic of proteinases of major human pathogens (Schmidtchen et al ., 2002 ; Sieprawska-Lupa et al ., 2004 ). SufA was shown to completely degrade the antibacterial peptide, LL-37, during an incubation of 1–3 h (Karlsson et al ., 2007 ). LL-37 is a human cathelicidin, which kills target microorganisms by disrupting membrane integrity (Turner et al ., 1998 ). SufA was also capable of degrading the chemokine MIG/CXCL9 ( M onokine I nduced by G amma-interferon) (Cole et al ., 2001 ; Egesten et al ., 2007 ) into small fragments. SufA cleavage of LL-37 and MIG/CXCL9 resulted in enhanced survival of F. magna during antibacterial assays with these peptides (Karlsson et al ., 2007 ).
SufA was found to be unable to cleave antibacterial peptides of the defensin family, possibly due the presence of cysteines in the defensin family, which protect the peptides from SufA proteolysis (Karlsson et al ., 2007 ). LL-37, on the other hand, is a linear α-helical peptide without any cysteines (Bals & Wilson, 2003 ) and can be completely degraded by SufA. In addition, the COOH-terminal end of MIG/CXCL9 where SufA is predicted to cleave has been reported to have an α-helical structure (Egesten et al ., 2007 ). The ability of SufA to cleave and inactivate certain antimicrobial peptides could lead to enhanced survival and proliferation during infection. This theory was further investigated by a study carried out by Frick et al . ( 2011 ) using the antibacterial proteins Midkine (MK) and BRAK/CXCL14, which are both expressed constitutively in the epidermal layer of skin. Here, SufA-generated fragments of MK and BRAK/CXCL14, after a 1 h digestion, were found to still efficiently kill the virulent human pathogen, Streptococcus pyogenes . Finegoldia magna was also killed, but to a much lesser extent (Frick et al ., 2011 ). This could provide selective advantages to F. magna during the early stages of infection.
Further studies on SufA interaction with MIG/CXCL9 showed that SufA was able to modulate the chemokine's activities to promote bacterial survival during epithelial inflammation. MIG/CXCL9 is an ELR-negative CXC- chemokine and is produced by human keratinocytes in response to inflammatory stimuli, e.g. the cytokine IFN-γ (Liao et al ., 1995 ). In IFN-γ-stimulated keratinocytes, F. magna failed to increase production of MIG/CXCL9, in sharp contrast to S. pyogenes (Karlsson et al ., 2009a ). This may be due to PAMPs on the surface of F. magna being less exposed, resulting in a decreased inflammatory response and allowing F. magna to survive as a commensal.
MIG/CXCL9 exerts its bactericidal activity through membrane perturbation and translocation through the bacterial membrane into the cytoplasm resulting in possible inhibition of essential enzymatic activities (Karlsson et al ., 2009a ). SufA-degraded fragments of MIG/CXCL9 still retained the ability to enter the cytoplasm of S. pyogenes , but not that of F. magna . This may be explained, in part, by the fact that SufA-processed MIG/CXCL9 loses its ability to form dimers, which is essential for the activity of many chemokines (Proudfoot et al ., 2003 ; Campanella et al ., 2006 ). In addition, differences in cell-wall architecture and membrane composition between the two bacterial species, may explain their stark difference in susceptibility to the processed chemokine (Karlsson et al ., 2009a ). As shall be discussed in the following section, FAF, the adhesion protein of F. magna , when released from the bacterial surface by SufA, is capable of binding and neutralising the effect of both MIG/CXCL9 and LL-37 in the growth medium (Frick et al ., 2008 ). Therefore, SufA and FAF appear to play a dual role in protecting F. magna from antibacterial peptides present in the environment.
The role that SufA plays in the interaction with the host coagulation system was also explored. Many pathogenic bacteria manipulate the host coagulation system through proteolytic degradation or binding of its components (Sun, 2006 ). SufA was found to specifically and rapidly cleave fibrinogen, by removing the COOH-terminal part of the fibrinogen Aα chains (αC) (Karlsson et al ., 2009a ). Further processing by SufA results in an attack on the NH 2 -terminal part of the Bβ chains and further processing of the Aα chains. The αC chains are important for lateral fibril associations and clot formation and stabilisation. SufA-treated plasma showed an increased thrombin clotting time (TCT assay) and this could be explained by the removal of the αC chains (Karlsson et al ., 2009a ). The cleavage of fibrinogen by SufA could not only affect fibrin polymerisation and clotting but also some of the other important functions of fibrinogen, such as wound healing. During wound healing, the fibrin network provides a temporary matrix into which cells can proliferate (Drew et al ., 2001 ; Laurens et al ., 2006 ). SufA inhibition of this network could significantly delay wound repair.
SufA was shown to prevent the formation of a fibrin network around F. magna which had adhered to human keratinocytes (Karlsson et al ., 2009a ). On the other hand, an intact fibrin network was formed around F. magna bacteria, from which the SufA gene had been knocked out. These results suggest that cleavage of fibrinogen by SufA prevents the formation of a fibrin network around F. magna that have adhered to human keratinocytes. This could prevent the bacteria from becoming trapped and facilitate the establishment of infection, promoting virulence (Karlsson et al ., 2009a ). In addition, it could add selective advantages to the bacteria as a member of the normal flora.
FAF – F. magna adhesion factor
FAF is an alpha helical coiled coil protein that mediates bacterial aggregation by interacting with FAF molecules on neighbouring F. magna bacteria (see Fig. 6a for a domain layout). The structure and location of FAF and the virulent M proteins of S. pyogenes are highly similar, suggesting that both of these proteins share comparable functions. In addition, both proteins are released from the bacterial surface. FAF is released in the form of a 53 kDa fragment by the subtilisin-like protease, SufA, which was described in the previous section (Frick et al ., 2008 ). FAF is present at the surface of most F. magna strains; however, the gene is not identical. There was found to be strain-to-strain sequence variation in FAF, mainly in the NH 2 -terminal part.
Studies on the biological function of FAF are still at a preliminary stage. However, initial studies have identified ligands for FAF. FAF was found to bind to BM-40, a noncollagenous glycoprotein, which is a linking molecule in the basement membrane of skin. The binding site region for BM-40 was localised to the COOH-terminal end of the molecule, which is conserved amongst FAF homologues (Frick et al ., 2008 ). This sequence conservation suggests an evolutionary pressure to maintain this region of the FAF molecule, signifying the importance for a FAF-BM40 interaction in infection. The biological significance of this interaction was further illustrated by the coincubation of F. magna bacteria with human skin biopsies, which showed bacteria present at the basement membrane through the colocalisation of gold-labelled FAF and BM-40 (Frick et al ., 2008 ). Interestingly, most of the FAF-expressing strains in this study (20 of 28) were also found to express PAB, the albumin-binding surface protein. As previously described, binding of albumin promotes growth of F. magna and BM-40 has been reported to increase albumin transport across the epithelium (Goldblum et al ., 1994 ). Therefore, BM-40 bound by FAF could influence transport and as a result, increase bacterial multiplication. BM-40 has also been found in soluble form in wound fluid and has been shown to stimulate wound healing and cell proliferation (Brekken & Sage, 2000 ). Therefore, through its interaction with BM-40, both soluble FAF and FAF-expressing F. magna could impair wound healing in chronic wounds, which would explain why these bacteria are so effective at causing infection in this niche.
LL-37 was identified as another ligand for FAF and both the NH 2 - and COOH-terminal regions of FAF were required for full binding. LL-37 was shown to be able to effectively kill a non-FAF expressing strain, but, addition of exogenous FAF significantly lowered killing (Frick et al ., 2008 ). FAF-expressing F. manga were found to be much more resistant to killing from LL-37 than non-FAF expressing bacteria. FAF was also found to have a neutralising capacity on other bactericidal proteins – MK, BRAK/CXCL14 and hBD-3 – in a dose-dependent manner (Frick et al ., 2011 ). Interestingly, hBD-3, which is resistant to SufA cleavage, was the peptide most efficiently neutralised by FAF. The ability of F. magna to effectively block the activity of antimicrobial peptides could help it to survive as a commensal at the skin epidermis (Frick et al ., 2011 ).
Large amounts of FAF were found to be released from F. magna and this exogenous FAF could act as a protective barrier around the bacteria during infection (Frick et al ., 2008 ). FAF appears to significantly affect the interaction between the bacteria and the host and helps in understanding how F. magna is able to colonise and survive in the human host.
Peptostreptococcus
Description and overview
The genus Peptostreptococcus used to be genetically and phenotypically heterogeneous, but has undergone extensive taxonomic change in the last decade. A number of new genera have been proposed to account for the many differences in fundamental characteristics between the species, including Finegoldia , Parvimonas , Gallicola , Peptoniphilus and Anaerococcus (Murdoch & Shah, 1999 ; Ezaki et al ., 2001 ; Tindall & Euzeby, 2006 ). The genus now consists of just three species – the type species P. anaerobius and the recently identified Peptostreptococcus stomatis and Peptostreptococcus russellii (Downes & Wade, 2006 ; Whitehead et al ., 2011 ). Peptostreptococcus anaerobius have a cell morphology, which is usually coccobacillary with a diameter of 0.5–0.7 μm and occurs in short chains (Holdeman et al ., 1986 ; Murdoch & Mitchelmore, 1991 ). Colonies are grey with a slightly raised off-white centre and grow on enriched blood agar more rapidly than other GPAC species, giving off a distinctive, sickly sweet odour (Murdoch & Mitchelmore, 1991 ). It is found in the gastrointestinal and vaginal flora (Neut et al ., 1985 ; Holdeman et al ., 1986 ; Hillier et al ., 1993 ).
Peptostreptococcus stomatis cells (0.8 × 0.8–0.9 μm) are arranged in pairs or short chains. Their colonies have a diameter of 0.8–1.8 mm and are circular, high convex to pyramidal in the centre, opaque, shiny and cream to off-white with a narrow, grey peripheral outer ring in colour (Downes & Wade, 2006 ). Peptostreptococcus stomatis grow moderately in broth media and growth can be enhanced through the addition of fermentable carbohydrates. Cells ferment fructose, glucose and maltose weakly and are mildly saccharolytic. End products of metabolism include major amounts of acetic and isocaproic acids, minor amounts of isobutyric and isovaleric acids and trace to minor amounts of butyric acid. It has a G + C content of 36 mol% (Downes & Wade, 2006 ). It is a member of the oral commensal flora and probably accounts for isolates that were previously thought to be P. anaerobius , as it is now hypothesised that P. anaerobius is not, in fact, an oral commensal (Downes & Wade, 2006 ).
Peptostreptococcus russellii was isolated from a swine manure storage pit and cells (0.8–1 μm) occur in pairs and chains of 3–10 cells. Colonies are 2–3 mm in diameter, convex, opaque, smooth and whitish in colour (Whitehead et al ., 2011 ). Cells are weakly saccharolytic and the major end-product of glucose metabolism is acetate. Copious amounts of ammonia are produced (> 40 mM) from various nitrogen sources. The type strain is RT-10B T and has a G + C content of 35.6 mol% (Whitehead et al ., 2011 ).
Clinical importance
Peptostreptococcus anaerobius is one of the most common GPAC associated with infections of the abdominal cavity and female urogenitary tract (Brook, 1988a , 1989a ; Murdoch et al ., 1994 ). Infections of the female urogenitary tract include bacterial vaginosis, which is a polymicrobial syndrome, where Lactobacillus populations are replaced by a mixture of bacteria including P. anaerobius , Gram-negative anaerobic rods such as Prevotella spp., the facultative Gardnerella vaginalis and the genital mycoplasmas Mycoplasma hominis and Ureaplasma urealyticum (Hill, 1993 ). Pybus et al . discovered that P. anaerobius was unable to grow in vaginal defined medium that had not been conditioned by peptone-supplementation through prior incubation with Prevotella bivia . Prevotella bivia culture supernatants have a net accumulation of amino acids which supported the growth of P. anaerobius . This commensal symbiosis is thought to exist in bacterial vaginoses whereby Prevotella bivia supports the growth of P. anaerobius (Pybus & Onderdonk, 1998 ).
It has also been isolated from abscesses from a wide range of human clinical specimens including the brain, ear, jaw, pleural cavity, blood, spinal and joint fluid and pelvic, urogenital and abdominal regions (Holdeman et al ., 1986 ). It is mostly associated with mixed infection sites but there have been some reports of isolation from pure culture (Brook & Frazier, 1993 ; Montejo et al ., 1995 ).
It was also isolated in pure culture from a case of orbital cellulitis, suggesting it has the potential to cause severe orbital disease (Malik et al ., 2004 ). Peptostreptococcus anaerobius has recently been associated with some cases of infective endocarditis, however, the occurrence is rare and there is a more favourable prognosis than with patients with infective endocarditis from other anaerobic bacteria (Minces et al ., 2010 ; Wu et al ., 2011 ). It has been isolated from some cases of skin infection, in association with aerobic bacteria, such as S. aureus (Higaki et al ., 2000 ). It has also been reported to be isolated from a range of acute and chronic wounds (Sanderson et al ., 1979 ; Bowler & Davies, 1999a ) including dermal ulcers (Alper et al ., 1983 ), leg ulcers (Bowler & Davies, 1999a ), post-thoractomy sternal wound (Brook, 1989a ), chronic venous leg ulcers (Gilchrist & Reed, 1989 ; Hansson et al ., 1995 ; Brook & Frazier, 1998 ), nonpuerperal breast infection (Edmiston et al ., 1990 ), diabetic foot ulcers (Johnson et al ., 1995 ), burn wound infections (Mousa, 1997 ), surgical wounds (Steffen & Hentges, 1981 ) and diabetic foot infections (Wheat et al ., 1986 ).
Peptostreptococcus stomatis is associated with infections of the human oral cavity, such as dentoalveolar abscesses and endodontic infections (Downes & Wade, 2006 ). However, it is only a recently described species, so there are limited clinical data available. As mentioned in the previous section, oral infections reported to be caused by P. anaerobius were probably incorrectly identified and were in fact caused by P. stomatis . It was detected in one-quarter of the cases from a large number of necrotic root canal samples and was one of the most dominant taxa in some of the communities (Rocas & Siqueira, 2008 ).
Antibiotic resistance
Antibiotic susceptibility data of Peptostreptococcus are not widely available. However, a recent study looked at the antibiotic susceptibilities of 61 isolates of P. anaerobius and P. stomatis against amoxicillin, amoxicillin-clavulanic acid, cefoxitin, ertapenem, azithromycin, clindamycin, metronidazole and moxifloxacin (Könönen et al ., 2007 ). The isolates originated from various clinical specimens from anatomical sites such as ulcer and skin specimens, pus specimens of the genitourinary tract and oropharyngeal and gastrointestinal specimens. Peptostreptococcus stomatis was found to be susceptible to all antibiotics with a slightly increased resistance to clindamycin. Thirteen per cent of P. anaerobius isolates displayed intermediate-to-resistant MICs to one or more drugs including amoxicillin, amoxicillin-clavulanic acid, cefoxitin and moxifloxacin (Könönen et al ., 2007 ). This study also found most P. anaerobius isolates to be from infection sites of the lower extremities and genitourinary tract, whereas, most P. stomatis isolates were from oral, pharyngeal and gastrointestinal specimens. These findings are in line with the studies of Downes and Wade and Riggio and Lennon, suggesting that P. anaerobius is not involved in colonisation/infection of the oral and pharyngeal areas (Riggio & Lennon, 2003 ; Downes & Wade, 2006 ). Metronidazole proved to be one of the most effective antibiotics against both Peptostreptococcus spp. . However, the nitroimidazole resistance gene – nimB – has been found in 31% of P. anaerobius strains in another study (Theron et al ., 2004 ). Therefore, metronidazole resistance in Peptostreptococcus will have to be monitored in the future.
Peptostreptococcus anaerobius has also been shown to be extremely susceptible to a new antibiotic, oritavancin, which is being developed for infections caused by vancomycin-susceptible and -resistant organisms (Citron et al ., 2005 ). It is a glycopeptide antibiotic that inhibits bacterial cell-wall formation through blocking the transglycosylation step in peptidoglycan hydrolysis. It was found to be twofold more active than vancomycin against eleven strains of P. anaerobius (Citron et al ., 2005 ).
Parvimonas
Description and overview
The Parvimonas genus contains just one species – P. micra – which has undergone much reclassification in recent times. Originally classified as Peptostreptococcus micros , it was transferred to the Micromonas genus in 1999 by Murdoch and Shah and known as Micromonas micros (Murdoch & Shah, 1999 ). The Micromonas genus was replaced by Parvimonas by Tindall and Euzeby in 2006 , with P. micra as it's sole species (Tindall & Euzeby, 2006 ).
Parvimonas micra cells (0.3 –0.7 μm) are usually arranged in pairs and chains (Holdeman et al ., 1986 ). Their colonies have a diameter of 1 mm and are usually white in colour, domed, glistening and typically surrounded by a yellow-brown halo of discoloured agar up to 2 mm wide on enriched blood agar plates (Murdoch et al ., 1988 ; Murdoch & Mitchelmore, 1991 ; van Dalen et al ., 1993 ). There is both a rough and a smooth morphotype of P. micra , which differ with regard to the presence of fibrillar structures on the cell wall, hydrophobic activity, the ability to lyse erythrocytes and the composition of cell-wall proteins (van Dalen et al ., 1993 ). The smooth morphotype appear as small, domed, bright white, nonhaemolytic colonies and the rough morphotype appear as white, dry, haemolytic colonies with wrinkled edges and long, thin fibrillar structures outside the cell envelope. Repeated subculturing in broth results in the rough morphotype changing into the smooth colony morphology with no fibrillar structures (van Dalen et al ., 1993 ).
Clinical importance
Parvimonas micra is part of the normal commensal flora of the gastrointestinal tract (Finegold et al ., 1974 ; Bartlett, 1990 ) and the gingival crevice (Holdeman et al ., 1986 ; Rams et al ., 1992 ). With regard to its role in clinical infections, it is mainly recognised as an oral pathogen and is particularly isolated from polymicrobial infections such as periodontitis. Periodontitis is caused by a group of bacteria and results in destruction of the periodontal tissues (Socransky et al ., 1999 ). Recent advances in bacterial identification, such as microarray and 16S rRNA gene sequencing have confirmed the association of P. micra with periodontal infections. Nonnenmacher et al . targeted the 16S rRNA gene sequences of a range of periodontal pathogens using real-time PCR from 50 subgingival plaque samples from periodontitis patients and 33 from periodontally healthy subjects. They found higher counts of P. micra in samples from periodontitis patients (Nonnenmacher et al ., 2004 ). A microarray study carried out by Vianna et al . ( 2005 ) looked at the microbial composition of necrotic root canals from 20 patients and compared it with methods of culture identification. From the culture-based identification, P. micra was present in 10% of samples. However, using microarray, P. micra was found in 50% of samples and was the most prevalent organism detected (Vianna et al ., 2005 ). This study shows that the prevalence of P. micra in oral infections could have previously been underestimated due to the use of culture-based techniques for identification. A nucleic acid-based technique will provide a more comprehensive picture of bacteria associated with necrotic teeth.
Parvimonas micra has been identified as a prominent oral pathogen in endoperiodontal lesions, apical abscesses and periodontitis in many recent studies (Lee et al ., 2003 ; Fritschi et al ., 2008 ; Siqueira et al ., 2009 ; Rocas et al ., 2011 ; Didilescu et al ., 2012 ). Rocas and Siqueira ( 2008 ) identified P. micra in 28% of samples from infected root canals of 43 teeth with chronic apical periodontitis. Another study found a positive association between P. micra and severe gingival overgrowth in patients taking cyclosporin A following organ transplantation, where P. micra was found in 66% of subgingival samples (Romito et al ., 2004 ). Parvimonas micra was found to be present in 5.9% of isolates in a study looking at the profiling of the microbiota in infected root canals (Sato et al ., 2012 ). Sato et al . used restriction fragment length polymorphism analysis of PCR-amplified 16S ribosomal RNA genes and sequencing, for identification of live bacterial cells.
It has also been implicated in infection in other parts of the body. It has been reported to have been isolated from a range of skin infections including chronic wounds (Bowler & Davies, 1999a ), leg ulcers (Bowler & Davies, 1999a ), nonpuerperal breast infection (Edmiston et al ., 1990 ), burn wound infections (Mousa, 1997 ), acute (surgical) wounds (Sanderson et al ., 1979 ) and diabetic foot infections (Wheat et al ., 1986 ). Riesbeck and Sanzen ( 1999 ) identified P. micra as the causative microorganism in a case of destructive knee joint infection with rapid progress of cartilage destruction. Bartz et al . ( 2005 ) found P. micra to be the causative microorganism in a prosthetic joint infection of the hip, which was linked to a tooth extraction. A study by Lafaurie et al . ( 2007 ) looked at the frequency of periodontic and subgingival anaerobic and facultative bacteria in the bloodstream following scaling and root planing as a result of preventative dental procedures and periodontal therapy. They found that P. micra was one of the most frequently identified periodontopathogens in peripheric blood. It was still present in the bloodstream, in some cases, 30 min after the dental procedure. As the capability of neutralising a bacterial threat in the bloodstream varies between patients, this may represent a risk factor for developing remote infections (Lafaurie et al ., 2007 ). Studies by Murdoch et al . ( 1988 , 1994 ) isolated P. micra from abscesses from numerous infection sites, including soft-tissue abscesses, plueral empyemas, anorectal abscess and from an intrauterine contraceptive device and patients with chronic sinusitis. They found that it usually occurred in a particular flora at the infection site which consisted of microaerophilic streptococci, Fusobacterium spp. and Bacteroides spp. (Murdoch et al ., 1988 , 1994 ).
Antibiotic resistance
Overall, P. micra is highly susceptible to antibiotics (Aldridge et al ., 1983 , 2001 ; Mitchelmore et al ., 1995 ; Bowker et al ., 1996 ). A recent study looked at the antimicrobial susceptibility of GPAC of clinical strains isolated in 10 European countries, found P. micra to be present in 17.7% of isolates (53 strains) (Brazier et al ., 2008 ). All P. micra strains were found to be susceptible to imipenem, metroniazole, vancomycin, linezolid and metronidazole. Two strains were found to be resistant to either penicillin or clindamycin. The penicillin-resistant strain did not produce β-lactamase, so penicillin resistance could be due to modifications in the penicillin-binding proteins (Reig & Baquero, 1994 ). Clindamycin resistance is caused by an RNA methylase that modifies the site of action of the antibiotic (Garcia-Rodriguez et al ., 1995 ).
A study by Veloo et al . ( 2011a ) looked at the antimicrobial susceptibilities of 115 isolates of clinically relevant GPAC over a 3-year period in the Netherlands. One strain of P. micra was identified that was resistant to metronidazole, but no significant resistances to any other antibiotics were found (Veloo et al ., 2011a ). Rams et al . looked at spiramycin, amoxicillin and metronidazole resistance in human periodontitis microbiota from 37 patients with untreated severe periodontitis (Rams et al ., 2011 ). A 10.8% of P. micra isolates were found to be resistant to spiramycin, 2.7% to amoxicillin and none to metronidazole. Whereas antibiotic resistance in P. micra is not yet a significant issue when treating infection, monitoring through continuous antimicrobial susceptibility testing still seems highly justified.
Virulence factors
Parvimonas micra is one of the best studied of the GPAC in terms of characterisation of its virulence factors. The demonstration of synergy with facultative and anaerobic bacteria during the growth of abscesses, capsule formation and the ability to form hydrogen sulphide from glutathione have been described as important virulence factors (Brook & Walker, 1985 ; Brook, 1987 , 1988a ; Carlsson et al ., 1993 ). Furthermore, it has been described to adhere to gingival epithelial cells (Dzink et al ., 1989 ) and to express immunoglobulin Fc-binding proteins (Grenier & Michaud, 1994 ). As described earlier, P. micra cells exist in either a rough or a smooth morphotype, with the rough morphotype having fibrillar material on their surface (van Dalen et al ., 1993 ). A study by van Dalen et al . ( 1998 ) looking at the pathogenicity of the two different morphotypes, found that abscesses induced by the rough morphotype were slightly, but significantly larger. The same study also looked at virulence of both morphotypes in a mixed infection with two Prevotella spp. and found that the most effective combination in terms of transmissibility of infection, bacterial counts from pus and larger abscesses produced, was the rough morphotype of P. micra with Prevotella intermedia . They also discovered that the rough morphotype had an increased interaction with polymorphonuclear leukocytes in the absence of serum and that this could be due to the increased amount of fibrillar material on the surface of the bacteria (van Dalen et al ., 1998 ).
Kremer et al . ( 1999a ) looked at the function of the fibrillar surface appendages in adherence to epithelial cells. They discovered that adherence of the rough morphotype was significantly lower than the smooth and the smooth variant of the rough type. Thus, the fibril-like surface structures appear to have an obstructive effect on adherence. This difference was abolished by protease removal of the fibrillar appendages on the rough morphotype. The adherence was pH-independent and is probably mediated by periodate-sensitive extracellular polysaccharides (Kremer et al ., 1999a ). Kremer et al . cloned a component of the fibril-like structure and designated it fibA (Kremer et al ., 1999a ). It encodes a 45 kDa protein that is recognised by fibril-specific antibodies and has a 38-residue leader peptide, which directs exportation of the protein. Its COOH-terminus comprises of a large number of aromatic amino acids, which may be involved in anchoring the protein to cell-surface carbohydrate structures (Kremer et al ., 1999a ).
Data on proteolytic activity in the virulence of P. micra have started to emerge. Ng et al . ( 1998 ) found that 15 of 19 strains of P. micra tested possessed cell-associated gelatinase activity. Mikamo et al . ( 1999 ) reported that nine out of 18 strains of P. micra that were isolated from amniotic fluid with preterm premature rupture of membranes demonstrated elastase activity. A study by Grenier and Bouclin ( 2006 ) investigated the contribution of proteases and plasmin-acquired activity to P. micra virulence. They discovered the production of a chymotrypsin-like enzyme from P. micra that is both cell-bound and secreted. Furthermore, they verified the gelatinase activity previously reported by Ng et al . and found that it was restricted to the rough morphotype only, which expresses three gelatinase bands. An in vitro model of bacterial virulence using a reconstituted basement membrane showed that the chymotrypsin-like protease and gelatinases directly contributed to bacterial penetration of the rough morphotype. Both the rough and smooth morphotype were found to bind human plasminogen to the cell surface and once bound, plasminogen activators of bacterial (streptokinase) and human (urokinase) origin activated plasminogen to plasmin. Activated plasmin on the bacterial surface may interact with extracellular matrix and P. micra cells coated with plasmin were found to have a significantly greater tissue penetration activity through an in vitro basement membrane model (Grenier & Bouclin, 2006 ). A more recent study by Ota-Tsuzuki and Alves Mayer ( 2010 ) investigated collagenase production and haemolytic activity in P. micra oral isolates. All of the studied isolates demonstrated collagenolytic activity to some extent. Only two of the 38 isolates tested demonstrated elastase activity, which is in stark contrast to the study on elastase activity in P. micra isolates from amniotic fluids in uterine infections (Mikamo et al ., 1999 ). This finding suggests a difference in the proteolytic profile of strains isolated from different sites of infection. Furthermore, oral P. micra isolates demonstrated a high haemolytic activity against chicken blood erythrocytes and to a less extent, rabbit, but none towards sheep blood (Ota-Tsuzuki & Alves Mayer, 2010 ).
Some studies have also been carried out looking at the ability of P. micra to stimulate an inflammatory response in cells. Nonnenmacher et al . ( 2003 ) found that a DNA preparation containing unmethylated CpG motifs from P. micra stimulated macrophages and gingival fibroblasts to produce TNF-α and IL-6. A study by Yoshioka et al . ( 2005 ) reported that P. micra cells bound Actinobacillus actinomycetemcomitans lipopolysaccharide. Binding of Gram-negative lipopolysaccharide significantly increased its capacity to induce TNF-α production by human macrophages.
Further studies into P. micra virulence were carried out by Tanabe et al . ( 2007 ), who investigated the response of human macrophages to a cell-wall preparation of P. micra . They reported that the cell-wall preparation stimulated the production of TNF-α and IL-1β, both of which are important determinants of the progression of periodontitis (Graves & Cochran, 2003 ). In addition, the cell-wall preparation induced significant amounts of IL-6, IL-8 and RANTES secretion by macrophages (Tanabe et al ., 2007 ). In vivo effects of IL-6 secretion during periodontitis may include promotion of bone resorption (Ishimi et al ., 1990 ). IL-8 and RANTES are both potent chemokines which favour the accumulation of chemokines during inflammation (Luster, 1998 ). This could help contribute to periodontal tissue destruction during infection. Furthermore, RANTES has been reported to be important in the initiation and progression of periodontitis (Gamonal et al ., 2000 ; Johnson et al ., 2004 ). The induction of proinflammatory cytokines and chemokines by the P. micra cell wall activate host-mediated destructive processes that are seen during periodontitis (Tanabe et al ., 2007 ). Significantly, P. micra cell-wall extracts were also found to induce increased expression of active MMP-9. MMP-9 has been linked to periodontal tissue destruction and is highly expressed in inflamed gingival tissues (Teng et al ., 1992 ; Smith et al ., 2004a ). The same study also found that various intracellular signalling pathways are induced by the P. micra cell wall, including PKA, ERK2, JNK and p38, leading to an increased production of the proinflammatory cytokines seen above and possibly contributing to periodontal tissue destruction (Tanabe et al ., 2007 ).
Conclusions and future work
GPAC are part of the commensal microbiota of humans in multiple sites of the body and account for approximately one-third of isolated anaerobic bacteria from clinical specimens (Murdoch et al ., 1994 ). GPAC have undergone extensive taxonomic reclassification in the past 15 years with the Peptostreptococcus genus being subdivided into six new groups, along with the identification of newly described species. Although mainly isolated from polymicrobial infections, some species have been isolated in pure culture. The most common GPAC isolated from clinical infections are F. magna , P. anaerobius , P. micra and P. asaccharolyticus (Wren, 1996 ). Previously, the lack of efficient and specific identification techniques for the detection of GPAC in infection meant that some species were misidentified or even overlooked. The advent of the multiplex PCR assay, 16S rRNA gene-based probes and MALDI-TOF mass spectrometry through the availability of genotypic data mean that, in the future, GPAC will be more correctly and comprehensively identified from clinical samples. This will ensure correct treatment of infection and provide more extensive data on the clinical importance of some species. Recent data on the antibiotic resistance of GPAC have shown that variable resistance exists towards penicillins, clindamycin and metronidazole. Reports on differences in antimicrobial susceptibility between various species of GPAC is increasing and should continued to be studied, to monitor a potential increasing resistance of GPAC to antibiotics. Whereas there are some data on the virulence factors and pathogenic mechanisms of F. magna and P. micra , these studies should be extended to the other clinically relevant GPAC species.
Acknowledgements
This work was supported by the Swedish Research Council (project 7480), the Foundations of Crafoord, Bergvall and Österlund, the Royal Physiographic Society, and Hansa Medical AB.
References
Editor: Wilbert Bitter