-
PDF
- Split View
-
Views
-
Cite
Cite
A Broome, D Ray, R Mitchell, R Harmer, Responding to ash dieback (Hymenoscyphus fraxineus) in the UK: woodland composition and replacement tree species, Forestry: An International Journal of Forest Research, Volume 92, Issue 1, January 2019, Pages 108–119, https://doi.org/10.1093/forestry/cpy040
Close - Share Icon Share
Abstract
Common ash (Fraxinus excelsior L.) is an important timber species that is widespread in broadleaved woodlands across Europe, where it is currently declining due to the fungal pathogen (Hymenoscyphus fraxineus (T. Kowal) Baral et al., 2014) causing ash dieback. Using the UK as our case study, we assess: (1) likely woodland composition following ash dieback and (2) choice of replacement species for production planting. The greatest impacts on woodland composition will occur where ash forms a larger proportion of the canopy. In such woodlands, larger gaps formed from the loss of ash, are likely to be filled by sycamore (Acer pseudoplatanus L.) and beech (Fagus sylvatica L.) under current climatic conditions and where there is little management intervention. Native woodland policy regarding sycamore and beech may need to be reviewed in UK-designated woodlands where these species are considered non-native. For actively managed production woodlands, 27 replacement tree species for ash are considered, some of these are non-native and present options for continuing production forestry objectives on former ash sites. An assessment of replacement species shows there is no single species that can substitute for the wide range of site conditions associated with the good growth of ash. In deciding to replace ash with another tree species, the decision on selection should be made based on particular site conditions and woodland objectives.
Introduction
Broadleaved woodland communities and ecosystems are changing due to increases in tree pests and pathogens (Santini et al., 2013). A number of historic (and current) examples include: the loss of elm (Ulmus glabra Huds.) in Europe, as a result of Dutch elm disease (Brunet et al., 2014; Bartnik et al., 2015); beech (Fagus sylvatica L.) decline (Jung, 2009) resulting from Phytophthora spp. in Central Europe; in North America the near complete loss of chestnut (Castanea dentata (Marshall) Borkh.) as a result of chestnut blight canker (Cryphonectria parasitica) (Ellison et al., 2005), a decline in American beech (Fagus grandifolia Ehrh.) (Lovett et al., 2010) as a result of beech bark fungal disease; and declines in a variety of ash species (Fraxinus spp) in European Russia and North America due to Emerald ash borer, Agrilus planipennis, (Orlova-Bienkowskaja & Volkovitsh, 2015). The spread of tree pests and diseases has been shown to result from socio-ecological factors such as the increased global trade of timber and wood products (Guo et al., 2012; Boyd et al., 2013), the nursery trade (Santini et al., 2013) and also to climatic change – particularly warmer and wetter winters (Jung, 2009; Tubby & Webber, 2010). Globally, as a result of these factors, invasive forest pathogen introductions have increased exponentially in the last 200 years (Santini et al., 2013). For these reasons, it is important that foresters adapt and manage woodlands affected by pests and pathogens such that, where possible, woodland objectives continue to be met.
Ash dieback is a serious tree disease caused by an invasive fungus (Hymenoscyphus fraxineus (T. Kowal.) Baral et al., 2014) from East Asia that has spread quickly through eastern, central and northern European continental countries and Russia (e.g. Kjær et al., 2012; Davydenko et al., 2013). Ash dieback was first confirmed in the UK in February 2012, and by December 2017 the disease was confirmed in 44 per cent of UK 10 km squares (Forestry Commission, 2017). The disease causes crown dieback and root collar necroses, and in a high forest situation usually leads to tree death either directly (Kowalski, 2006; Halmschlager and Kirisits, 2008; Ogris et al., 2009; Enderle et al., 2013) or indirectly due to attack by bark beetles or infection by Armillaria species, the latter is particularly common in oceanic, humid sites (Lenz et al., 2016;). Between 1 and 5 per cent of common ash genotypes in a population (hereafter referred to as ash) are considered to show some level of tolerance (Pliûra et al., 2011; Kjær et al., 2012; Stener, 2013; McKinney et al., 2011, 2014). The identification of resistance or tolerant genotypes is a current research objective (McKinney et al., 2014; Sollars et al., 2017) to select and maintain ash populations in European woodlands in the future.
Knowledge in continental Europe on management of high forest stands of ash under the threat of ash dieback has led to the recommended general strategy of retaining healthy or slightly damaged ash trees and harvesting commercial timber where trees are severely affected (Skovsgaard et al., 2017). Current UK policy on managing ash woods affected by ash dieback accords with these recommendations (Forestry Commission, 2017). Using this approach, the chance of identifying tolerant individuals is maximized, thereby supporting the overall strategy of developing resistant material to allow the continued use of ash in forestry in the future (Kjær et al., 2012; Boshier and Buggs, 2015). Forest managers also need to consider which species to use to replace ash in productive broadleaved forestry, where ash has been widely planted (Fraxigen, 2005), and the choice will vary with site types across the UK and Europe.
In continental Europe, where ash comprises less than 1 per cent of forest land (Skovsgaard et al., 2017), forest managers are responding to ash dieback with changes in thinning practice, the early felling of ash prior to the normal commercial felling age and replacement planting with other species (Dobrowolska et al., 2011; Skovsgaard et al., 2017). These actions are driving changes in the composition of woodlands (Lygis et al., 2014; Pušpure et al., 2017). Whilst strategies for ash silviculture have been developed based on the experience of ash dieback in continental Europe (Skovsgaard et al., 2017), the effects of applying these strategies have yet to be fully understood, especially in areas where the ash dieback epidemic is still advancing, and where ash occupies a greater proportion of the forest area, such as the UK (5 per cent of the UK forest area – Forestry Commission, 2012). In the UK, ~142 000 ha of broadleaved woodlands have a component of ash (average canopy area 11 per cent). An analysis of the National Forest Inventory (NFI) sample square data (Forestry Commission, 2012) estimated that of the squares in which ash occurred in woodlands, lots had an ash canopy dominance of less than 10 per cent of the total canopy area. On neutral and calcareous lithologies, ash trees form a sizeable component in broadleaved woodland stands across a variety of site types in the UK (Rodwell, 1991; Kerr and Cahalan, 2004). Ash accounts for ~34 million m3 of the timber volume in UK woodlands (Defra, 2013; Broome et al., 2014).
Using the UK as a case study, we attempt to fill the knowledge gaps on how woodland composition could change, and consider the planting choices available to support timber production. Specifically, we:
identify the likely changes in woodland composition due to natural succession over 100 years following loss of ash
suggest tree species which could be introduced as alternatives to ash for timber production in the UK.
Methods
Identifying the distribution of broadleaved woodland communities with different classes of ash cover in the canopy
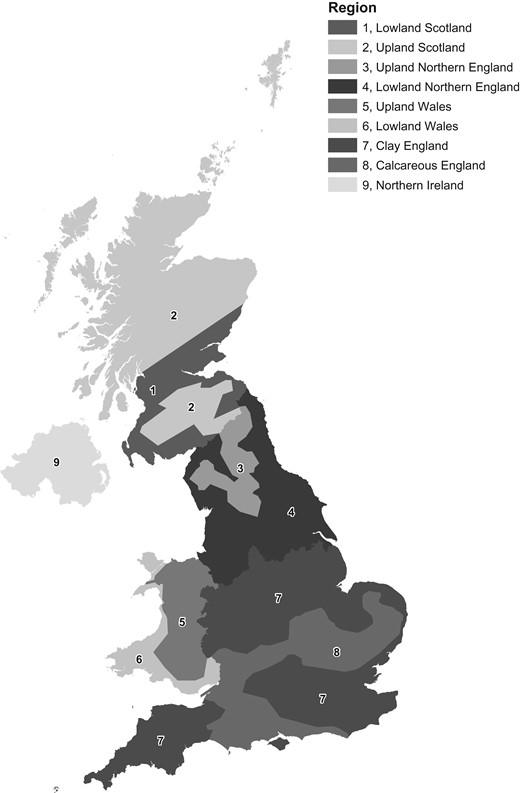
Ash is not uniformly distributed across the UK, the amount present being influenced by management, location, climate and soil type. We used the nine ash regions developed by Mitchell et al. (2014a) to delineate broad regions of variation in ash woodland types and communities (Figure 1). In Scotland, Northern England and Wales, the separation of regions was according to lowland and upland areas; lowland being defined by the accumulated temperature (degree-days above 5.6°C – [DD]) threshold of more than 1200 DD for Northern Britain and more than 1400 DD for Wales (Pyatt et al., 2001). In southern England, a separation was made between two distinct regions with different site lithologies; base-rich clay soils (Region 7) and mainly calcareous soils on chalk and limestone (Region 8) (British Geological Survey, 2017). Northern Ireland was considered as a single climo-geographic region.
Ash regions of the UK. Contains public sector information licenced under the Open Government Licence v3.0 UK.
The NFI sampled woodlands across 15 000 one-hectare sample squares representing ~0.6 per cent of the total woodland area of Britain. Woodland summaries from the NFI sample squares have been made for aggregated NFI regions, and the Forestry Commission (2012) technical report describes the broadleaved woodland cover in Britain with a special emphasis on ash. Using the NFI data, Mitchell et al. (2014b) made four canopy cover classes to describe the distribution of ash canopy cover in UK broadleaved woodlands (<10 per cent, 10–20 per cent, 20–60 per cent and >60 per cent). We used their classification, but combined the last two classes – as few woodlands have >60 per cent ash canopy cover (Forestry Commission, 2012). This provided three ash canopy classes: low (<10 per cent), medium (10–20 per cent) and high (>20 per cent). The British National Vegetation Classification (NVC) provides tables of frequency and abundance for species present in woodland communities and sub-communities (Rodwell, 1991) based on an extensive field survey sample of native woodlands. Although published nearly 30 years ago, and accepting that some changes in composition will have resulted from woodland management, increased deer browsing and nitrogen deposition (Hopkins and Kirby, 2007; Corney, et al., 2008), we made an assumption that the NVC should still provide a reasonably robust classification of woodland communities in Britain. Using data on the occurrence of ash in the NVC communities, we grouped communities and sub-communities (Table 1) into three ash canopy classes of low (<10 per cent), medium (10–20 per cent) and high (>20 per cent). Table 1 shows how these classes fall broadly within the range of Domin score values from sampled ash communities described by Rodwell (1991).
Relationship between percentage of ash in the canopy and frequency and abundance (Domin score) for ash in NVC woodland communities and sub-communities (Rodwell, 1991).
| Ash canopy cover class . | % Ash in canopy . | NVC frequency . | NVC communities and sub-communities containing ash . | Max median Domin score1 (and % cover2) . |
|---|---|---|---|---|
| Low | <10% | I | W10a, d; W14; W2b; W12b | 3.5 (<10%) |
| Medium | ≥10%–≤20% | II–III | W10b, c, e; W7a, b, c; W12c; W9b; W2a; W6a | 4.5 (4–25%) |
| High | >20% | IV–V | W12a; W9a; W8 a, b, c, d, e, g | 6.0 (25–33%) |
| Ash canopy cover class . | % Ash in canopy . | NVC frequency . | NVC communities and sub-communities containing ash . | Max median Domin score1 (and % cover2) . |
|---|---|---|---|---|
| Low | <10% | I | W10a, d; W14; W2b; W12b | 3.5 (<10%) |
| Medium | ≥10%–≤20% | II–III | W10b, c, e; W7a, b, c; W12c; W9b; W2a; W6a | 4.5 (4–25%) |
| High | >20% | IV–V | W12a; W9a; W8 a, b, c, d, e, g | 6.0 (25–33%) |
1A range of Domin scores are provided for ash within each NVC community. For each community, the median Domin score was calculated. The maximum median Domin score for the communities listed is presented.
2The Domin score as a percentage cover as given by Rodwell (1991).
Relationship between percentage of ash in the canopy and frequency and abundance (Domin score) for ash in NVC woodland communities and sub-communities (Rodwell, 1991).
| Ash canopy cover class . | % Ash in canopy . | NVC frequency . | NVC communities and sub-communities containing ash . | Max median Domin score1 (and % cover2) . |
|---|---|---|---|---|
| Low | <10% | I | W10a, d; W14; W2b; W12b | 3.5 (<10%) |
| Medium | ≥10%–≤20% | II–III | W10b, c, e; W7a, b, c; W12c; W9b; W2a; W6a | 4.5 (4–25%) |
| High | >20% | IV–V | W12a; W9a; W8 a, b, c, d, e, g | 6.0 (25–33%) |
| Ash canopy cover class . | % Ash in canopy . | NVC frequency . | NVC communities and sub-communities containing ash . | Max median Domin score1 (and % cover2) . |
|---|---|---|---|---|
| Low | <10% | I | W10a, d; W14; W2b; W12b | 3.5 (<10%) |
| Medium | ≥10%–≤20% | II–III | W10b, c, e; W7a, b, c; W12c; W9b; W2a; W6a | 4.5 (4–25%) |
| High | >20% | IV–V | W12a; W9a; W8 a, b, c, d, e, g | 6.0 (25–33%) |
1A range of Domin scores are provided for ash within each NVC community. For each community, the median Domin score was calculated. The maximum median Domin score for the communities listed is presented.
2The Domin score as a percentage cover as given by Rodwell (1991).
For this study, we wanted to predict spatially, the proportion of different NVC woodland communities containing ash in the UK. Our method aims to use the floristic lists of the NVC ash woodland communities to identify the species of trees and shrubs which could respond to loss of ash, and the proportion of woodlands in which this response is likely. Rodwell (1991) does provide a coarse spatial summary of NVC communities, but no measure of the proportion or amount of these in the woodland landscape. The NFI sample square ‘Map 3’ (Forestry Commission, 2012 p. 21) provides an approximate location of broadleaved woodland, as well as the proportion of broadleaved woodland with an ash canopy component in our three classes (Table 1) but does not specify the NVC woodland community. We used the ash regions (Figure 1) as the spatial units for our study. We intersected the ash region map with the NFI sample square data to summarize the proportion of broadleaved woodlands in a region which contained ash in each of our three canopy cover classes (Table 1). We used the link between ash canopy class and NVC communities (Table 1), and the regional distribution of NVC communities to predict the proportion of different NVC communities in each of the ash regions. To further refine and qualify our ash NVC woodland community prediction, we used expert knowledge of site factors relating to woodland community: topography, climate, lithology and soil – and referring to Rodwell (1991), Pyatt et al. (2001), the Soilscapes Viewer (2017) and Lilly et al. (2010) (Table 2). The NVC was not available for Northern Ireland and so we used data from the Northern Ireland Habitat Action Plan for Mixed Ash Woodlands (http://www.doeni.gov.uk/niea/mixedashwoods_pdf-2.pdf (accessed on 1 March 2018)) and the Northern Ireland Forest Service Woodland Register (2013) (http://www.dardni.gov.uk/ (accessed on 1 March 2018)).
Ecological site factors and NVC types associated with broadleaved woodland sites containing ash for each of the ash-relevant regions described by Mitchell et al. (2014c), showing proportion of total broadleaved woodland in each of the three categories of percentage ash in canopy (<10%, ≥10%, ≤20% or >20%) for each region.
| Region . | Typical climate zone . | Ash in canopy . | Proportion of broadleaved woods (%) . | Typical lthology (BGS, 2017) . | Typical soil types and soil pH 2,3 or 4 . | Main associated NVC sub-community (Hall, 1997; Rodwell, 1991) . |
|---|---|---|---|---|---|---|
| 1 Lowland Scotland | Warm Moist | <10% | 75 | Silurian | Brown gley2 | W10a |
| Warm Moist | ≥10%–≤20% | 10 | Andesite | Loamy surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Warm Moist | >20% | 15 | Carboniferous limestone | Calcareaous brown earth4 | W9a; W8b, e | |
| 2 Upland Scotland | Cool Wet | <10% | 90 | Quartz mica-schist | Upland brown earth2 | W11b; W17a, d |
| Cool Moist | ≥10%–≤20% | 6 | Lower Old Red Sandstone | Loamy gleyed brown earth3 | W9b; W7b; W10e | |
| Cool Wet | >20% | 4 | Limestone | Calcareous upland brown earth4 | W9a | |
| 3 Upland Northern England | Cool Moist | <10% | 75 | Andesitic tuff | Upland brown earth2 | W10a; W11b; W17b, c |
| Warm Moist | ≥10%–≤20% | 12 | Permian sandstones | Surface water gley3 | W7b; W9b; W10e | |
| Warm Moist | >20% | 13 | Carboniferous limestone | Loamy surface water gley4 | W9a; W8e | |
| 4 Lowland Northern England | Warm Dry | <10% | 71 | Triassic mudstones | Alluvium, loamy brown earths and gleys2 | W2a, b; W6b; W10a, d |
| Warm Dry | ≥10%–≤20% | 10 | Permian sandstone and Keuper marl | Alluvium, loamy surface water gley3 | W10b, c, e; W7a, b, c; W6a | |
| Warm Dry | >20% | 19 | Magnesian limestone | Calcareous brown earth4 | W8b; e, g | |
| 5 Upland Wales | Cool Wet | <10% | 76 | Carboniferous Pennant measures | Gleyed upland brown earth, Surface water gley2 | W6b; W10a; W17b, c |
| Cool Wet | ≥10%–≤20% | 6 | Silurian Llandovery | Surface water gley3 | W10c, e; W8f; W7a, b, c; W6a; W11a | |
| Cool Wet | >20% | 18 | Lower Old Red Sandstone | Brown earth4 | W8b, e, f; W9a | |
| 6 Lowland Wales | Warm Moist | <10% | 42 | Lower Cambrian | Brown gley2 | W6b; W10a |
| Warm Moist | ≥10%–≤20% | 10 | Ordovician | Surface watery gley3 | W10b, c, e; W8f | |
| Warm Dry | >20% | 48 | Carboniferous limestone | Calcareous surface water gley4 | W8b, d, e; W9a | |
| 7 Clay South England | Warm Dry | <10% | 58 | Bagshot beds | Loamy surface water gley2 | W2a, b; W6b; W10a, d; W12b; W13a, b |
| Warm Dry | ≥10%–≤20% | 12 | London clay | Surface water gley3 | W10b, c; W12c W8f; W6a | |
| Warm Dry | >20% | 30 | Weald clay | Calcareous surface water gley4 | W12a; W8a, b, c, d, e | |
| 8 Calcareous South England | Warm Dry | <10% | 47 | Oxford clay | Surface water gley (clay with flints)2 | W6b; W10d; W13a, b; W14 |
| Warm Dry | ≥10%–≤20% | 12 | Great oolite | Calcareous brown gley3 | W8f; W6a; W10b, c | |
| Warm Dry | >20% | 41 | Chalk | Rendzina, Calcareous brown earth4 | W8a, b, c, d, e | |
| 9 Northern Ireland | Cool Wet1 | <10% | 75 | Dalradian quartz mica-schist | Brown gley2 | W10a |
| Cool Moist1 | ≥10%–≤20% | 10 | Silurian | Surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Cool Moist1 | >20% | 15 | Carboniferous limestone | Gleyed brown earth4 | W9a; W8b, e |
| Region . | Typical climate zone . | Ash in canopy . | Proportion of broadleaved woods (%) . | Typical lthology (BGS, 2017) . | Typical soil types and soil pH 2,3 or 4 . | Main associated NVC sub-community (Hall, 1997; Rodwell, 1991) . |
|---|---|---|---|---|---|---|
| 1 Lowland Scotland | Warm Moist | <10% | 75 | Silurian | Brown gley2 | W10a |
| Warm Moist | ≥10%–≤20% | 10 | Andesite | Loamy surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Warm Moist | >20% | 15 | Carboniferous limestone | Calcareaous brown earth4 | W9a; W8b, e | |
| 2 Upland Scotland | Cool Wet | <10% | 90 | Quartz mica-schist | Upland brown earth2 | W11b; W17a, d |
| Cool Moist | ≥10%–≤20% | 6 | Lower Old Red Sandstone | Loamy gleyed brown earth3 | W9b; W7b; W10e | |
| Cool Wet | >20% | 4 | Limestone | Calcareous upland brown earth4 | W9a | |
| 3 Upland Northern England | Cool Moist | <10% | 75 | Andesitic tuff | Upland brown earth2 | W10a; W11b; W17b, c |
| Warm Moist | ≥10%–≤20% | 12 | Permian sandstones | Surface water gley3 | W7b; W9b; W10e | |
| Warm Moist | >20% | 13 | Carboniferous limestone | Loamy surface water gley4 | W9a; W8e | |
| 4 Lowland Northern England | Warm Dry | <10% | 71 | Triassic mudstones | Alluvium, loamy brown earths and gleys2 | W2a, b; W6b; W10a, d |
| Warm Dry | ≥10%–≤20% | 10 | Permian sandstone and Keuper marl | Alluvium, loamy surface water gley3 | W10b, c, e; W7a, b, c; W6a | |
| Warm Dry | >20% | 19 | Magnesian limestone | Calcareous brown earth4 | W8b; e, g | |
| 5 Upland Wales | Cool Wet | <10% | 76 | Carboniferous Pennant measures | Gleyed upland brown earth, Surface water gley2 | W6b; W10a; W17b, c |
| Cool Wet | ≥10%–≤20% | 6 | Silurian Llandovery | Surface water gley3 | W10c, e; W8f; W7a, b, c; W6a; W11a | |
| Cool Wet | >20% | 18 | Lower Old Red Sandstone | Brown earth4 | W8b, e, f; W9a | |
| 6 Lowland Wales | Warm Moist | <10% | 42 | Lower Cambrian | Brown gley2 | W6b; W10a |
| Warm Moist | ≥10%–≤20% | 10 | Ordovician | Surface watery gley3 | W10b, c, e; W8f | |
| Warm Dry | >20% | 48 | Carboniferous limestone | Calcareous surface water gley4 | W8b, d, e; W9a | |
| 7 Clay South England | Warm Dry | <10% | 58 | Bagshot beds | Loamy surface water gley2 | W2a, b; W6b; W10a, d; W12b; W13a, b |
| Warm Dry | ≥10%–≤20% | 12 | London clay | Surface water gley3 | W10b, c; W12c W8f; W6a | |
| Warm Dry | >20% | 30 | Weald clay | Calcareous surface water gley4 | W12a; W8a, b, c, d, e | |
| 8 Calcareous South England | Warm Dry | <10% | 47 | Oxford clay | Surface water gley (clay with flints)2 | W6b; W10d; W13a, b; W14 |
| Warm Dry | ≥10%–≤20% | 12 | Great oolite | Calcareous brown gley3 | W8f; W6a; W10b, c | |
| Warm Dry | >20% | 41 | Chalk | Rendzina, Calcareous brown earth4 | W8a, b, c, d, e | |
| 9 Northern Ireland | Cool Wet1 | <10% | 75 | Dalradian quartz mica-schist | Brown gley2 | W10a |
| Cool Moist1 | ≥10%–≤20% | 10 | Silurian | Surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Cool Moist1 | >20% | 15 | Carboniferous limestone | Gleyed brown earth4 | W9a; W8b, e |
Typical climate zone = 1not included in Pyatt et al., 2001.
Typical soil pH = 2slightly acid, 3neutral, 4neutral/calcareous (Table 8, p. 14 Pyatt et al., 2001).
Ecological site factors and NVC types associated with broadleaved woodland sites containing ash for each of the ash-relevant regions described by Mitchell et al. (2014c), showing proportion of total broadleaved woodland in each of the three categories of percentage ash in canopy (<10%, ≥10%, ≤20% or >20%) for each region.
| Region . | Typical climate zone . | Ash in canopy . | Proportion of broadleaved woods (%) . | Typical lthology (BGS, 2017) . | Typical soil types and soil pH 2,3 or 4 . | Main associated NVC sub-community (Hall, 1997; Rodwell, 1991) . |
|---|---|---|---|---|---|---|
| 1 Lowland Scotland | Warm Moist | <10% | 75 | Silurian | Brown gley2 | W10a |
| Warm Moist | ≥10%–≤20% | 10 | Andesite | Loamy surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Warm Moist | >20% | 15 | Carboniferous limestone | Calcareaous brown earth4 | W9a; W8b, e | |
| 2 Upland Scotland | Cool Wet | <10% | 90 | Quartz mica-schist | Upland brown earth2 | W11b; W17a, d |
| Cool Moist | ≥10%–≤20% | 6 | Lower Old Red Sandstone | Loamy gleyed brown earth3 | W9b; W7b; W10e | |
| Cool Wet | >20% | 4 | Limestone | Calcareous upland brown earth4 | W9a | |
| 3 Upland Northern England | Cool Moist | <10% | 75 | Andesitic tuff | Upland brown earth2 | W10a; W11b; W17b, c |
| Warm Moist | ≥10%–≤20% | 12 | Permian sandstones | Surface water gley3 | W7b; W9b; W10e | |
| Warm Moist | >20% | 13 | Carboniferous limestone | Loamy surface water gley4 | W9a; W8e | |
| 4 Lowland Northern England | Warm Dry | <10% | 71 | Triassic mudstones | Alluvium, loamy brown earths and gleys2 | W2a, b; W6b; W10a, d |
| Warm Dry | ≥10%–≤20% | 10 | Permian sandstone and Keuper marl | Alluvium, loamy surface water gley3 | W10b, c, e; W7a, b, c; W6a | |
| Warm Dry | >20% | 19 | Magnesian limestone | Calcareous brown earth4 | W8b; e, g | |
| 5 Upland Wales | Cool Wet | <10% | 76 | Carboniferous Pennant measures | Gleyed upland brown earth, Surface water gley2 | W6b; W10a; W17b, c |
| Cool Wet | ≥10%–≤20% | 6 | Silurian Llandovery | Surface water gley3 | W10c, e; W8f; W7a, b, c; W6a; W11a | |
| Cool Wet | >20% | 18 | Lower Old Red Sandstone | Brown earth4 | W8b, e, f; W9a | |
| 6 Lowland Wales | Warm Moist | <10% | 42 | Lower Cambrian | Brown gley2 | W6b; W10a |
| Warm Moist | ≥10%–≤20% | 10 | Ordovician | Surface watery gley3 | W10b, c, e; W8f | |
| Warm Dry | >20% | 48 | Carboniferous limestone | Calcareous surface water gley4 | W8b, d, e; W9a | |
| 7 Clay South England | Warm Dry | <10% | 58 | Bagshot beds | Loamy surface water gley2 | W2a, b; W6b; W10a, d; W12b; W13a, b |
| Warm Dry | ≥10%–≤20% | 12 | London clay | Surface water gley3 | W10b, c; W12c W8f; W6a | |
| Warm Dry | >20% | 30 | Weald clay | Calcareous surface water gley4 | W12a; W8a, b, c, d, e | |
| 8 Calcareous South England | Warm Dry | <10% | 47 | Oxford clay | Surface water gley (clay with flints)2 | W6b; W10d; W13a, b; W14 |
| Warm Dry | ≥10%–≤20% | 12 | Great oolite | Calcareous brown gley3 | W8f; W6a; W10b, c | |
| Warm Dry | >20% | 41 | Chalk | Rendzina, Calcareous brown earth4 | W8a, b, c, d, e | |
| 9 Northern Ireland | Cool Wet1 | <10% | 75 | Dalradian quartz mica-schist | Brown gley2 | W10a |
| Cool Moist1 | ≥10%–≤20% | 10 | Silurian | Surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Cool Moist1 | >20% | 15 | Carboniferous limestone | Gleyed brown earth4 | W9a; W8b, e |
| Region . | Typical climate zone . | Ash in canopy . | Proportion of broadleaved woods (%) . | Typical lthology (BGS, 2017) . | Typical soil types and soil pH 2,3 or 4 . | Main associated NVC sub-community (Hall, 1997; Rodwell, 1991) . |
|---|---|---|---|---|---|---|
| 1 Lowland Scotland | Warm Moist | <10% | 75 | Silurian | Brown gley2 | W10a |
| Warm Moist | ≥10%–≤20% | 10 | Andesite | Loamy surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Warm Moist | >20% | 15 | Carboniferous limestone | Calcareaous brown earth4 | W9a; W8b, e | |
| 2 Upland Scotland | Cool Wet | <10% | 90 | Quartz mica-schist | Upland brown earth2 | W11b; W17a, d |
| Cool Moist | ≥10%–≤20% | 6 | Lower Old Red Sandstone | Loamy gleyed brown earth3 | W9b; W7b; W10e | |
| Cool Wet | >20% | 4 | Limestone | Calcareous upland brown earth4 | W9a | |
| 3 Upland Northern England | Cool Moist | <10% | 75 | Andesitic tuff | Upland brown earth2 | W10a; W11b; W17b, c |
| Warm Moist | ≥10%–≤20% | 12 | Permian sandstones | Surface water gley3 | W7b; W9b; W10e | |
| Warm Moist | >20% | 13 | Carboniferous limestone | Loamy surface water gley4 | W9a; W8e | |
| 4 Lowland Northern England | Warm Dry | <10% | 71 | Triassic mudstones | Alluvium, loamy brown earths and gleys2 | W2a, b; W6b; W10a, d |
| Warm Dry | ≥10%–≤20% | 10 | Permian sandstone and Keuper marl | Alluvium, loamy surface water gley3 | W10b, c, e; W7a, b, c; W6a | |
| Warm Dry | >20% | 19 | Magnesian limestone | Calcareous brown earth4 | W8b; e, g | |
| 5 Upland Wales | Cool Wet | <10% | 76 | Carboniferous Pennant measures | Gleyed upland brown earth, Surface water gley2 | W6b; W10a; W17b, c |
| Cool Wet | ≥10%–≤20% | 6 | Silurian Llandovery | Surface water gley3 | W10c, e; W8f; W7a, b, c; W6a; W11a | |
| Cool Wet | >20% | 18 | Lower Old Red Sandstone | Brown earth4 | W8b, e, f; W9a | |
| 6 Lowland Wales | Warm Moist | <10% | 42 | Lower Cambrian | Brown gley2 | W6b; W10a |
| Warm Moist | ≥10%–≤20% | 10 | Ordovician | Surface watery gley3 | W10b, c, e; W8f | |
| Warm Dry | >20% | 48 | Carboniferous limestone | Calcareous surface water gley4 | W8b, d, e; W9a | |
| 7 Clay South England | Warm Dry | <10% | 58 | Bagshot beds | Loamy surface water gley2 | W2a, b; W6b; W10a, d; W12b; W13a, b |
| Warm Dry | ≥10%–≤20% | 12 | London clay | Surface water gley3 | W10b, c; W12c W8f; W6a | |
| Warm Dry | >20% | 30 | Weald clay | Calcareous surface water gley4 | W12a; W8a, b, c, d, e | |
| 8 Calcareous South England | Warm Dry | <10% | 47 | Oxford clay | Surface water gley (clay with flints)2 | W6b; W10d; W13a, b; W14 |
| Warm Dry | ≥10%–≤20% | 12 | Great oolite | Calcareous brown gley3 | W8f; W6a; W10b, c | |
| Warm Dry | >20% | 41 | Chalk | Rendzina, Calcareous brown earth4 | W8a, b, c, d, e | |
| 9 Northern Ireland | Cool Wet1 | <10% | 75 | Dalradian quartz mica-schist | Brown gley2 | W10a |
| Cool Moist1 | ≥10%–≤20% | 10 | Silurian | Surface water gley3 | W9b; W7a, b, c; W10e; W8f | |
| Cool Moist1 | >20% | 15 | Carboniferous limestone | Gleyed brown earth4 | W9a; W8b, e |
Typical climate zone = 1not included in Pyatt et al., 2001.
Typical soil pH = 2slightly acid, 3neutral, 4neutral/calcareous (Table 8, p. 14 Pyatt et al., 2001).
Estimating the changes in woodland composition due to natural succession following loss of ash
For woodlands where ash occupies a Low proportion of the canopy, the loss of all the ash from such woodlands would amount to less than ‘crown thinning’ and, although there could be seedling regeneration, the canopy of remaining tree species would be expected to grow and fill openings created (Kerr and Haufe, 2011). Thus the following procedure was only carried out for woodlands with medium and high-ash canopy cover (Table 1). To identify the most likely tree and shrub species to fill gaps created as a result of ash dieback, within each NVC sub-community shown in Table 1, we used the following five-step procedure:
The species of tree seedlings, saplings and shrubs (ash was excluded) listed as being present in the understorey in the different NVC communities were identified from the NVC floristic lists. Definitions of trees and shrubs follow Rodwell and Patterson (1994).
The species were ranked first by frequency and then by abundance in the NVC community and the five most frequent and abundant species were selected. Definitions of frequent and abundant follow Rodwell (1991).
Species identified in step 2 were assigned a tolerance to shading (good, moderate, poor) taken from Harmer et al. (2010) (p. 112) and where good = ‘Shade tolerant’, moderate = ‘Intermediate’ and poor = ‘Shade intolerant’.
For NVC communities within the medium ash canopy class, in which a small canopy gap size was anticipated with loss of ash (and therefore resulting light levels were anticipated to be low), the list from step 2 was re-ordered to give the species with higher tolerance to shading a higher ranking.
The distribution of each species was checked against data in the ‘New Atlas of the British and Irish Flora’ (Preston et al., 2002) to ensure an allocation to ash regions within their main range.
The procedure produced a list of trees and shrubs henceforth termed ‘responder species’ for each of the medium and high-ash canopy classes which were likely to dominate the gaps in woodlands in three periods following dieback of ash in each of the nine ash regions. To predict the relative dominance of responder species in the first 10 years, between 10–50 years and for more than 50 years after the loss of ash, a further analysis was carried out. For every group of responder species by ash region and NVC ash woodland-type sub-community, we considered the competitive ability (Grime et al., 2007) and the expected longevity of each responder species. For example, if beech seedlings and hazel (Corylus avellana L.) occurred together in a gap as the initial responder species, beech would be expected to replace hazel as it is a large, long-lived tree regarded as a competitor and hazel is a comparatively small, short-lived shrub regarded as a stress-competitor. For the purposes of this analysis, it was assumed that the woodland remained unmanaged, under the same influences (e.g. levels of deer pressure and eutrophication) as when the NVC field data were collected. Additionally, we assumed no interacting effect of major management interventions or large natural disturbance events that might substantially change the tree species composition of woodlands.
Identification of species that could be planted as replacements for productive ash woodland
Productive ash woodlands are generally associated with warm climates (greater than 1200 DD >5°C – Pyatt et al., 2001), and so the rationale for identifying possible replacement species for use in productive woodlands was based on the climatic suitability of the nine regions. Mitchell et al. (2014c) assessed the suitability of 58 alternative tree species as replacements for ash with conservation objectives in mind (support of ash-associated species and replication of ash ecological function). This list was reviewed, focussing on the suitability of species that could occupy some ash woodland site types and fulfil production objectives in different parts of the UK. The potential of alternative species to produce high-quality timber will depend on a variety of site quality criteria, including moisture availability, fertility and soil depth. The tolerance of the alternative species to conditions of shade, alkaline soil, soil moisture, thin soils and spring frosts and their preference for deep and fertile soil was compared with that of ash using information from the Ecological Site Classification Decision Support Tool (http://www.forestdss.org.uk/geoforestdss/), the Tree Species Guide (https://www.forestresearch.gov.uk/tools-and-resources/tree-species-database/), and work by Niinemets and Valladares (2006), Moffat (2014) and Pyatt et al. (2001) and were used in the selection of alternative species. Based on all the information, a score was ascribed to each criterion of suitability for each of the alternative tree species assessed for replacing ash in the nine ash regions.
Results
Expected changes in tree and shrub species following loss of ash using the five-step procedure
The number of seedling and sapling species recorded in the floristic tables of the NVC woodland sub-communities (Rodwell, 1991), and offering potential as responder species, varied between 6 and 21 (Table 3). Hazel, sycamore and hawthorn (Crataegus monogyna Jacq.) were present in many sub-communities with differing percentages of ash in the canopy. In general, based on their frequency and then abundance in the floristic lists, we considered that these species would show the greatest overall response following loss of ash. Species such as downy birch (Betula pubescens Ehrh.) and silver birch (B. pendula Roth) were ranked as being intolerant to shade (based on Harmer et al., 2010, Table 3). Shade-intolerant species were predicted to occur as responder species only in woods with a high percentage of ash in the canopy (>20 per cent) where large gaps would be created following ash dieback. Shade-tolerant species (Table 3) were predicted to fill smaller gaps created in woods with medium levels of ash in the canopy (10–20 per cent).
Responder saplings, seedlings and shrubs present in different broadleaved woodland communities containing ash.
| % Ash canopy cover . | NVC community . | Overstorey . | Saplings, seedlings and shrubs . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | No . | 1 . | 2 . | 3 . | 4 . | 5 . |
| high | W12 a | Fagus sylvatica | 17 | Corylus avellana | Acer pseudoplatanus | Crataegus monogyna | Fagus sylvatica | Acer campestre |
| high | W9 a | Fraxinus excelsior | 8 | Corylus avellana | Crataegus monogyna | Ulmus glabra | Acer pseudoplatanus | Betula pubescens |
| high | W8 all | Fraxinus excelsior | 21 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8a | Fraxinus excelsior | 16 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8b | Fraxinus excelsior | 14 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8c | Fraxinus excelsior | 11 | Corylus avellana | Crataegus monogyna | Acer pseudoplatanus | Cornus sanguinea | Prunus spinosa |
| high | W8d | Fraxinus excelsior | 15 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Prunus spinosa |
| high | W8e | Fraxinus excelsior | 13 | Crataegus monogyna | Corylus avellana | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8f | Fraxinus excelsior | 10 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8g | Fraxinus excelsior | 13 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Rhamnus cathartica | Viburnum opulus |
| med | W11 a | Quercus petraea | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Betula pendula | Quercus robur |
| med | W10 b, c, e | Quercus robur | 20 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Betula pubescens |
| med | W7 all | Alnus glutinosa | 16 | Corylus avellana | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Betula pubescens |
| med | W12 c | Fagus sylvatica | 10 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Taxus baccata | Ligustrum vulgare |
| med | W9 b | Sorbus aucuparia/ Fraxinus excelsior | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Sorbus aucuparia | Salix cinerea |
| med | W6 a | Alnus glutinosa | 8 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Alnus glutinosa | Salix cinerea |
| low | W10 a, d | Quercus robur | 18 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Carpinus betulus |
| low | W14 all | Fagus sylvatica | 7 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Sambucus nigra | Betula pendula |
| low | W2 all | Betula pubescens/ Salix cinerea | 9 | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Salix fragilis | Betula pendula |
| low | W12 b | Fagus sylvatica | 12 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Acer campestre |
| low | W17 all | Quercus petraea | 12 | Corylus avellana | Fagus sylvatica | Crataegus monogyna | Quercus petraea | Betula pubescens |
| low | W11 b | Betula pubescens | 5 | Corylus avellana | Crataegus monogyna | Betula pubescens | Quercus robur | |
| low | W6 d, e | Alnus glutinosa/Betula pubescens | 11 | Acer pseudoplatanus | Sambucus nigra | Prunus spinosa | Crataegus monogyna | Salix cinerea |
| low | W13 a, b | Taxus baccata | 8 | Buxus sempervirens | Taxus baccata | Acer pseudoplatanus | Sambucus nigra | Euonymus europaeus |
| % Ash canopy cover . | NVC community . | Overstorey . | Saplings, seedlings and shrubs . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | No . | 1 . | 2 . | 3 . | 4 . | 5 . |
| high | W12 a | Fagus sylvatica | 17 | Corylus avellana | Acer pseudoplatanus | Crataegus monogyna | Fagus sylvatica | Acer campestre |
| high | W9 a | Fraxinus excelsior | 8 | Corylus avellana | Crataegus monogyna | Ulmus glabra | Acer pseudoplatanus | Betula pubescens |
| high | W8 all | Fraxinus excelsior | 21 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8a | Fraxinus excelsior | 16 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8b | Fraxinus excelsior | 14 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8c | Fraxinus excelsior | 11 | Corylus avellana | Crataegus monogyna | Acer pseudoplatanus | Cornus sanguinea | Prunus spinosa |
| high | W8d | Fraxinus excelsior | 15 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Prunus spinosa |
| high | W8e | Fraxinus excelsior | 13 | Crataegus monogyna | Corylus avellana | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8f | Fraxinus excelsior | 10 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8g | Fraxinus excelsior | 13 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Rhamnus cathartica | Viburnum opulus |
| med | W11 a | Quercus petraea | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Betula pendula | Quercus robur |
| med | W10 b, c, e | Quercus robur | 20 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Betula pubescens |
| med | W7 all | Alnus glutinosa | 16 | Corylus avellana | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Betula pubescens |
| med | W12 c | Fagus sylvatica | 10 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Taxus baccata | Ligustrum vulgare |
| med | W9 b | Sorbus aucuparia/ Fraxinus excelsior | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Sorbus aucuparia | Salix cinerea |
| med | W6 a | Alnus glutinosa | 8 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Alnus glutinosa | Salix cinerea |
| low | W10 a, d | Quercus robur | 18 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Carpinus betulus |
| low | W14 all | Fagus sylvatica | 7 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Sambucus nigra | Betula pendula |
| low | W2 all | Betula pubescens/ Salix cinerea | 9 | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Salix fragilis | Betula pendula |
| low | W12 b | Fagus sylvatica | 12 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Acer campestre |
| low | W17 all | Quercus petraea | 12 | Corylus avellana | Fagus sylvatica | Crataegus monogyna | Quercus petraea | Betula pubescens |
| low | W11 b | Betula pubescens | 5 | Corylus avellana | Crataegus monogyna | Betula pubescens | Quercus robur | |
| low | W6 d, e | Alnus glutinosa/Betula pubescens | 11 | Acer pseudoplatanus | Sambucus nigra | Prunus spinosa | Crataegus monogyna | Salix cinerea |
| low | W13 a, b | Taxus baccata | 8 | Buxus sempervirens | Taxus baccata | Acer pseudoplatanus | Sambucus nigra | Euonymus europaeus |
%Ash canopy cover = percentage of ash in the canopy, see Methods for further details; community = NVC woodland community and sub-communities (Rodwell, 1991); overstorey = predominant species in the overstorey; No. = total number of species recorded as saplings, seedlings and woody shrubs; 1–5 = species ranked by order of expected initial response to loss of ash due to their abundance, tolerance to light levels (tolerant, intermediate, intolerant) and expected light availability (more shade-tolerant species favoured where light levels are lower but excessive light assumed to suppress growth of F. sylvatica and Acer campestre)
Responder saplings, seedlings and shrubs present in different broadleaved woodland communities containing ash.
| % Ash canopy cover . | NVC community . | Overstorey . | Saplings, seedlings and shrubs . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | No . | 1 . | 2 . | 3 . | 4 . | 5 . |
| high | W12 a | Fagus sylvatica | 17 | Corylus avellana | Acer pseudoplatanus | Crataegus monogyna | Fagus sylvatica | Acer campestre |
| high | W9 a | Fraxinus excelsior | 8 | Corylus avellana | Crataegus monogyna | Ulmus glabra | Acer pseudoplatanus | Betula pubescens |
| high | W8 all | Fraxinus excelsior | 21 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8a | Fraxinus excelsior | 16 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8b | Fraxinus excelsior | 14 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8c | Fraxinus excelsior | 11 | Corylus avellana | Crataegus monogyna | Acer pseudoplatanus | Cornus sanguinea | Prunus spinosa |
| high | W8d | Fraxinus excelsior | 15 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Prunus spinosa |
| high | W8e | Fraxinus excelsior | 13 | Crataegus monogyna | Corylus avellana | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8f | Fraxinus excelsior | 10 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8g | Fraxinus excelsior | 13 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Rhamnus cathartica | Viburnum opulus |
| med | W11 a | Quercus petraea | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Betula pendula | Quercus robur |
| med | W10 b, c, e | Quercus robur | 20 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Betula pubescens |
| med | W7 all | Alnus glutinosa | 16 | Corylus avellana | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Betula pubescens |
| med | W12 c | Fagus sylvatica | 10 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Taxus baccata | Ligustrum vulgare |
| med | W9 b | Sorbus aucuparia/ Fraxinus excelsior | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Sorbus aucuparia | Salix cinerea |
| med | W6 a | Alnus glutinosa | 8 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Alnus glutinosa | Salix cinerea |
| low | W10 a, d | Quercus robur | 18 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Carpinus betulus |
| low | W14 all | Fagus sylvatica | 7 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Sambucus nigra | Betula pendula |
| low | W2 all | Betula pubescens/ Salix cinerea | 9 | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Salix fragilis | Betula pendula |
| low | W12 b | Fagus sylvatica | 12 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Acer campestre |
| low | W17 all | Quercus petraea | 12 | Corylus avellana | Fagus sylvatica | Crataegus monogyna | Quercus petraea | Betula pubescens |
| low | W11 b | Betula pubescens | 5 | Corylus avellana | Crataegus monogyna | Betula pubescens | Quercus robur | |
| low | W6 d, e | Alnus glutinosa/Betula pubescens | 11 | Acer pseudoplatanus | Sambucus nigra | Prunus spinosa | Crataegus monogyna | Salix cinerea |
| low | W13 a, b | Taxus baccata | 8 | Buxus sempervirens | Taxus baccata | Acer pseudoplatanus | Sambucus nigra | Euonymus europaeus |
| % Ash canopy cover . | NVC community . | Overstorey . | Saplings, seedlings and shrubs . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | No . | 1 . | 2 . | 3 . | 4 . | 5 . |
| high | W12 a | Fagus sylvatica | 17 | Corylus avellana | Acer pseudoplatanus | Crataegus monogyna | Fagus sylvatica | Acer campestre |
| high | W9 a | Fraxinus excelsior | 8 | Corylus avellana | Crataegus monogyna | Ulmus glabra | Acer pseudoplatanus | Betula pubescens |
| high | W8 all | Fraxinus excelsior | 21 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8a | Fraxinus excelsior | 16 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Sambucus nigra | Acer pseudoplatanus |
| high | W8b | Fraxinus excelsior | 14 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8c | Fraxinus excelsior | 11 | Corylus avellana | Crataegus monogyna | Acer pseudoplatanus | Cornus sanguinea | Prunus spinosa |
| high | W8d | Fraxinus excelsior | 15 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Prunus spinosa |
| high | W8e | Fraxinus excelsior | 13 | Crataegus monogyna | Corylus avellana | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8f | Fraxinus excelsior | 10 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Acer pseudoplatanus | Ulmus glabra |
| high | W8g | Fraxinus excelsior | 13 | Corylus avellana | Crataegus monogyna | Cornus sanguinea | Rhamnus cathartica | Viburnum opulus |
| med | W11 a | Quercus petraea | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Betula pendula | Quercus robur |
| med | W10 b, c, e | Quercus robur | 20 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Betula pubescens |
| med | W7 all | Alnus glutinosa | 16 | Corylus avellana | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Betula pubescens |
| med | W12 c | Fagus sylvatica | 10 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Taxus baccata | Ligustrum vulgare |
| med | W9 b | Sorbus aucuparia/ Fraxinus excelsior | 6 | Corylus avellana | Crataegus monogyna | Betula pubescens | Sorbus aucuparia | Salix cinerea |
| med | W6 a | Alnus glutinosa | 8 | Corylus avellana | Crataegus monogyna | Sambucus nigra | Alnus glutinosa | Salix cinerea |
| low | W10 a, d | Quercus robur | 18 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Carpinus betulus |
| low | W14 all | Fagus sylvatica | 7 | Fagus sylvatica | Acer pseudoplatanus | Corylus avellana | Sambucus nigra | Betula pendula |
| low | W2 all | Betula pubescens/ Salix cinerea | 9 | Alnus glutinosa | Crataegus monogyna | Salix cinerea | Salix fragilis | Betula pendula |
| low | W12 b | Fagus sylvatica | 12 | Corylus avellana | Acer pseudoplatanus | Fagus sylvatica | Crataegus monogyna | Acer campestre |
| low | W17 all | Quercus petraea | 12 | Corylus avellana | Fagus sylvatica | Crataegus monogyna | Quercus petraea | Betula pubescens |
| low | W11 b | Betula pubescens | 5 | Corylus avellana | Crataegus monogyna | Betula pubescens | Quercus robur | |
| low | W6 d, e | Alnus glutinosa/Betula pubescens | 11 | Acer pseudoplatanus | Sambucus nigra | Prunus spinosa | Crataegus monogyna | Salix cinerea |
| low | W13 a, b | Taxus baccata | 8 | Buxus sempervirens | Taxus baccata | Acer pseudoplatanus | Sambucus nigra | Euonymus europaeus |
%Ash canopy cover = percentage of ash in the canopy, see Methods for further details; community = NVC woodland community and sub-communities (Rodwell, 1991); overstorey = predominant species in the overstorey; No. = total number of species recorded as saplings, seedlings and woody shrubs; 1–5 = species ranked by order of expected initial response to loss of ash due to their abundance, tolerance to light levels (tolerant, intermediate, intolerant) and expected light availability (more shade-tolerant species favoured where light levels are lower but excessive light assumed to suppress growth of F. sylvatica and Acer campestre)
Expected changes in dominant responder species years 1–10
Three shrubs, hazel, elder (Sambucus nigra, L.) and hawthorn were assessed as the dominant responder shrub species in gaps during the first 10 years following the loss of ash (Table 4). Hazel appeared in all regions; elder is confined to the Regions 4, 5, 6, 7 and 8 (Southern England, lowland Northern England and Wales); and hawthorn occurs in all the regions except the upland regions of Scotland and England. Five responder tree species were predicted. Sycamore is the most widespread and was considered likely to become dominant in all regions except 2 and 3. Silver birch and downy birch and alder (Alnus glutinosa (L.) Gaertn.) are less widespread and were considered as responder trees in Regions 2 and 3. Elm was identified as a potential responder tree only in north Scotland (Region 2) but this may be short-lived as Dutch elm disease is currently advancing north in Scotland (Brasier, pers. comm.). Beech was also identified as a responder tree in Regions 4, 5, 6, 7 and 8, and field maple (Acer campestre L.) was identified as a responder tree in south England on clay soils (Region 7).
From ash woodland community and sub-community floristic lists of Rodwell (1991), responder shrub and tree species are predicted to develop in gaps created by loss of ash in different regions of the UK at three time periods. Dominant species are listed first. Ca Corylus avellana, Ap Acer pseudoplatanus, Cm Crataegus monogyna, Ag Alnus glutinosa, Bp Betula pubescens/pendula, Ug Ulmus glabra, Fs Fagus sylvatica, Sn Sambucus nigra, Ac Acer campestre.
| Region . | Ash canopy percentage . | Years . | ||
|---|---|---|---|---|
| 1–10 . | 11–50 . | 51–100 . | ||
| 1 Lowland Scotland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
| 2 Upland Scotland | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Ca, Bp, Ug | Ca, Bp, Ug | Ca, Ug | |
| 3 Upland Northern England | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Bp, Ca | Bp, Ca | Ca | |
| 4 Lowland Northern England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ap, Ca, Sn, Cm | Ap | Ap | |
| 5 Upland Wales | 10–20 | Ca, Fs,Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ca, Ap | Ca, Ap | |
| 6 Lowland Wales | 10–20 | Ca, Fs, Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ap | Ap | |
| 7 Clay South England | 10–20 | Ca, Ap, Fs | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn, Ac | Ap, Ac | Ap, Ac | |
| 8 Calcareous South England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ca, Cm, Sn, Ap | Ap | Ap | |
| 9 Northern Ireland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
| Region . | Ash canopy percentage . | Years . | ||
|---|---|---|---|---|
| 1–10 . | 11–50 . | 51–100 . | ||
| 1 Lowland Scotland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
| 2 Upland Scotland | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Ca, Bp, Ug | Ca, Bp, Ug | Ca, Ug | |
| 3 Upland Northern England | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Bp, Ca | Bp, Ca | Ca | |
| 4 Lowland Northern England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ap, Ca, Sn, Cm | Ap | Ap | |
| 5 Upland Wales | 10–20 | Ca, Fs,Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ca, Ap | Ca, Ap | |
| 6 Lowland Wales | 10–20 | Ca, Fs, Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ap | Ap | |
| 7 Clay South England | 10–20 | Ca, Ap, Fs | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn, Ac | Ap, Ac | Ap, Ac | |
| 8 Calcareous South England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ca, Cm, Sn, Ap | Ap | Ap | |
| 9 Northern Ireland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
From ash woodland community and sub-community floristic lists of Rodwell (1991), responder shrub and tree species are predicted to develop in gaps created by loss of ash in different regions of the UK at three time periods. Dominant species are listed first. Ca Corylus avellana, Ap Acer pseudoplatanus, Cm Crataegus monogyna, Ag Alnus glutinosa, Bp Betula pubescens/pendula, Ug Ulmus glabra, Fs Fagus sylvatica, Sn Sambucus nigra, Ac Acer campestre.
| Region . | Ash canopy percentage . | Years . | ||
|---|---|---|---|---|
| 1–10 . | 11–50 . | 51–100 . | ||
| 1 Lowland Scotland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
| 2 Upland Scotland | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Ca, Bp, Ug | Ca, Bp, Ug | Ca, Ug | |
| 3 Upland Northern England | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Bp, Ca | Bp, Ca | Ca | |
| 4 Lowland Northern England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ap, Ca, Sn, Cm | Ap | Ap | |
| 5 Upland Wales | 10–20 | Ca, Fs,Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ca, Ap | Ca, Ap | |
| 6 Lowland Wales | 10–20 | Ca, Fs, Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ap | Ap | |
| 7 Clay South England | 10–20 | Ca, Ap, Fs | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn, Ac | Ap, Ac | Ap, Ac | |
| 8 Calcareous South England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ca, Cm, Sn, Ap | Ap | Ap | |
| 9 Northern Ireland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
| Region . | Ash canopy percentage . | Years . | ||
|---|---|---|---|---|
| 1–10 . | 11–50 . | 51–100 . | ||
| 1 Lowland Scotland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
| 2 Upland Scotland | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Ca, Bp, Ug | Ca, Bp, Ug | Ca, Ug | |
| 3 Upland Northern England | 10–20 | Ca, Ag | Ca, Ag | Ag |
| >20 | Bp, Ca | Bp, Ca | Ca | |
| 4 Lowland Northern England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ap, Ca, Sn, Cm | Ap | Ap | |
| 5 Upland Wales | 10–20 | Ca, Fs,Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ca, Ap | Ca, Ap | |
| 6 Lowland Wales | 10–20 | Ca, Fs, Ap | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn | Ap | Ap | |
| 7 Clay South England | 10–20 | Ca, Ap, Fs | Fs | Fs |
| >20 | Ap, Cm, Ca, Sn, Ac | Ap, Ac | Ap, Ac | |
| 8 Calcareous South England | 10–20 | Ca, Ap, Fs | Ap, Fs | Fs, Ap |
| >20 | Ca, Cm, Sn, Ap | Ap | Ap | |
| 9 Northern Ireland | 10–20 | Ca, Ap | Ap | Ap |
| >20 | Ca, Cm, Ap | Ap | Ap | |
Expected changes in dominant responder species years 10–50
During this period, responder species were expected to be trees: shrub responders remaining only in the upland regions of Scotland, Wales and northern England (Table 4). Within these three regions, hazel, was predicted to fill gaps previously occupied by ash trees. In Regions 1 and 9, sycamore was expected to dominate the gaps, and we predicted that sycamore would remain a responder tree in a large proportion of ash woods in Regions 4, 5, 6, 7 and 8, although in these regions beech would also be a responder species. With sycamore, field maple would continue to be a responder species where ash formed more than 20 per cent of the canopy in woodlands of Region 7. Regions 2 and 3 differed from others in that alder and downy birch/silver birch were expected to be responder trees dominating the gaps previously occupied by ash.
Expected changes in dominant responder species years 50–100
Little change was predicted in responder species in the 50–100-year time period, with gaps being dominated by larger trees of the same species. The exceptions were Regions 2 and 3 where alder was predicted to replace hazel and Region 5 where sycamore was predicted to replace hazel; (Table 4).
Selection of replacement species
Of the 58 species listed by Mitchell et al. (2014c), assessments were made for 27 of these as replacement species. We removed non-native Fraxinus species following new evidence (Forest Research, 2018) of ash dieback in the UK on F. americana, F. caroliniana, F. latifolia, F. mandshurica and F. ornus. Other species in the Mitchell et al. (2014c) list being shrubs for which an assessment of production, using yield class (m3 ha−1 yr−1) predicted from Ecological Site Classification (Pyatt et al., 2001), is not applicable. The 27 productive replacement species include 17 that are non-native to the UK, including four non-native conifers (Table 5). All 27 species were assessed as potential replacements for ash, particularly for broadleaved woodland production. Their biodiversity value is compared with ash (Table 5), and this metric is based on the proportion of ash-associated species described by Broome et al. (2014), for the tree species shown in Table 5.
Site-related constraints, tolerances and preferences for potential replacement species (native, non-native, broadleaf and conifer) for ash based on production potential and biodiversity value based on the percentage of use by ash-associated species (after Broome et al., 2014). The list has been ranked by decreasing production value.
| Species . | Native (Na) Non-native (Nn) Broadleaf (Bl) Conifer (Co) . | Region . | Potential . | Tolerance to site constraints1 . | Soil type tolerance/preference1 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prod . | Biod (%) . | Shade . | Calc . | Wet2 . | Fresh2 . | Dry2 . | Spring frost . | Thin . | Deep . | Fertile . | |||
| Fraxinus excelsior L. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 100 | M | M | M | G | M | M/P | M | G | G |
| Pseudotsuga menziesii (Mirb.) Franco | NnCo | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 29 | M | P | P | G | M | P | P | M | M |
| Quercus petraea (Matt.) Liebl. | NaBl | 1, 2, 3, 4, 5, 6, 9 | G | 94 | M | P | M | G | G | M | M | M | G |
| Fagus sylvatica L. | NaBl | 1, 3, 4, 6, 7, 8, 9 | G | 92 | G | M | P | G | M | M | M | G | M |
| Prunus avium L. | NaBl | 1, 4, 5, 6, 7, 8, 9 | G | 88 | M | M | P | G | M | G | P | G | G |
| Acer pseudoplatanus L. | NnBl | 1, 3, 4, 5, 6, 7, 9 | G | 88 | M | M | M | G | P | G | M | M/G | M |
| Quercus rubra L. | NnBl | 1, 2, 3, 4, 5, 6, 9 | G | 29 | M | P | M | G | M | G | M | M | M |
| Thuja plicata Donn ex D. Don | NnCo | 1, 2, 3, 4, 5, 6, 9 | G | 22 | G | M | M | G | M | G | P | M | M/G |
| Quercus robur L. | NaBl | 1, 2, 3, 4, 7, 8 | G | 94 | P/M | P | M | G | M | M | P | G | G |
| Acer platanoides L. | NnBl | 1, 4, 6, 7, 8, 9 | G | 60 | G | M | P | G | M | G | P | M/G | M/G |
| Larix decidua Mill. | NnCo | 1, 2, 3, 4, 9 | G | 79 | P | M | P | G | G | M | P | M | M |
| Tilia cordata Mill. | NaBl | 4, 6, 7, 8 | G | 31 | G | M | P | G | M | G | M | G | G |
| Castanea sativa Mill. | NnBl | 4, 6, 7, 8 | G | 88 | M | P | P | G | M | M | M | M | L/M |
| Juglans regia L. | NnBl | 4, 6, 7, 8 | G | 81 | P | M | P | G | P | P | P | G | G |
| Juglans nigra L. | NnBl | 4, 6, 7, 8 | G | 80 | P | M | P | G | P | P | P | G | G |
| Quercus cerris L. | NnBl | 6, 7, 8 | G | 32 | M | M | M | G | G | P | M | M | M |
| Betula pendula Roth | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 90 | P | P | M | G | M | G | M | M | M |
| Alnus glutinosa (L.) Gaertn. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 89 | P | P | G | M | P | G | P | M | M |
| Populus tremula L. | NaBl | 1, 2, 3, 4, 5, 6, 9 | M | 89 | M | P | M | G | M | G | M | M | M |
| Acer campestre L. | NaBl | 1, 4, 6, 7, 8, 9 | M | 88 | M | G | P | G | M | M | M | M/G | G |
| Carya cordiformis (Wangenh.) C. Koch | NnBl | 1, 4, 6, 7, 8, 9 | M | 19 | P/M | M | M | G | G | G | P | M | M |
| Abies alba Mill. | NnCo | 1, 2, 3, 4, 5, 9 | M | 30 | G | M | P | G | P | P | P | M/G | M |
| Carpinus betulus L. | NaBl | 1, 3, 4, 7, 8 | M | 88 | G | P | M | G | G | M | M | M | M |
| Pterocarya fraxinifolia (Poir.) Spach | NnBl | 4, 5, 6, 7, 8 | M | 19 | M | M | M | G | P | M | P | M | M |
| Alnus cordata (Loisel.) Duby | NnBl | 6, 7, 8 | M | 23 | P | M | P | G | G | P/M | M | M | M/G |
| Ostrya carpinifolia Scop. | NnBl | 6, 7, 8 | M | 20 | M/G | G | P | G | G | P | M | M | M |
| Platanus hybrida Brot. | NnBl | 7, 8 | M | 76 | M/G | G | M | G | P/M | G | P/M | M | M |
| Betula pubescens Ehrh. | NaBl | 1, 2, 3, 4, 5, 6, 7, 9 | L | 90 | P | P | G | M | M | G | M | M | M |
| Species . | Native (Na) Non-native (Nn) Broadleaf (Bl) Conifer (Co) . | Region . | Potential . | Tolerance to site constraints1 . | Soil type tolerance/preference1 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prod . | Biod (%) . | Shade . | Calc . | Wet2 . | Fresh2 . | Dry2 . | Spring frost . | Thin . | Deep . | Fertile . | |||
| Fraxinus excelsior L. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 100 | M | M | M | G | M | M/P | M | G | G |
| Pseudotsuga menziesii (Mirb.) Franco | NnCo | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 29 | M | P | P | G | M | P | P | M | M |
| Quercus petraea (Matt.) Liebl. | NaBl | 1, 2, 3, 4, 5, 6, 9 | G | 94 | M | P | M | G | G | M | M | M | G |
| Fagus sylvatica L. | NaBl | 1, 3, 4, 6, 7, 8, 9 | G | 92 | G | M | P | G | M | M | M | G | M |
| Prunus avium L. | NaBl | 1, 4, 5, 6, 7, 8, 9 | G | 88 | M | M | P | G | M | G | P | G | G |
| Acer pseudoplatanus L. | NnBl | 1, 3, 4, 5, 6, 7, 9 | G | 88 | M | M | M | G | P | G | M | M/G | M |
| Quercus rubra L. | NnBl | 1, 2, 3, 4, 5, 6, 9 | G | 29 | M | P | M | G | M | G | M | M | M |
| Thuja plicata Donn ex D. Don | NnCo | 1, 2, 3, 4, 5, 6, 9 | G | 22 | G | M | M | G | M | G | P | M | M/G |
| Quercus robur L. | NaBl | 1, 2, 3, 4, 7, 8 | G | 94 | P/M | P | M | G | M | M | P | G | G |
| Acer platanoides L. | NnBl | 1, 4, 6, 7, 8, 9 | G | 60 | G | M | P | G | M | G | P | M/G | M/G |
| Larix decidua Mill. | NnCo | 1, 2, 3, 4, 9 | G | 79 | P | M | P | G | G | M | P | M | M |
| Tilia cordata Mill. | NaBl | 4, 6, 7, 8 | G | 31 | G | M | P | G | M | G | M | G | G |
| Castanea sativa Mill. | NnBl | 4, 6, 7, 8 | G | 88 | M | P | P | G | M | M | M | M | L/M |
| Juglans regia L. | NnBl | 4, 6, 7, 8 | G | 81 | P | M | P | G | P | P | P | G | G |
| Juglans nigra L. | NnBl | 4, 6, 7, 8 | G | 80 | P | M | P | G | P | P | P | G | G |
| Quercus cerris L. | NnBl | 6, 7, 8 | G | 32 | M | M | M | G | G | P | M | M | M |
| Betula pendula Roth | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 90 | P | P | M | G | M | G | M | M | M |
| Alnus glutinosa (L.) Gaertn. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 89 | P | P | G | M | P | G | P | M | M |
| Populus tremula L. | NaBl | 1, 2, 3, 4, 5, 6, 9 | M | 89 | M | P | M | G | M | G | M | M | M |
| Acer campestre L. | NaBl | 1, 4, 6, 7, 8, 9 | M | 88 | M | G | P | G | M | M | M | M/G | G |
| Carya cordiformis (Wangenh.) C. Koch | NnBl | 1, 4, 6, 7, 8, 9 | M | 19 | P/M | M | M | G | G | G | P | M | M |
| Abies alba Mill. | NnCo | 1, 2, 3, 4, 5, 9 | M | 30 | G | M | P | G | P | P | P | M/G | M |
| Carpinus betulus L. | NaBl | 1, 3, 4, 7, 8 | M | 88 | G | P | M | G | G | M | M | M | M |
| Pterocarya fraxinifolia (Poir.) Spach | NnBl | 4, 5, 6, 7, 8 | M | 19 | M | M | M | G | P | M | P | M | M |
| Alnus cordata (Loisel.) Duby | NnBl | 6, 7, 8 | M | 23 | P | M | P | G | G | P/M | M | M | M/G |
| Ostrya carpinifolia Scop. | NnBl | 6, 7, 8 | M | 20 | M/G | G | P | G | G | P | M | M | M |
| Platanus hybrida Brot. | NnBl | 7, 8 | M | 76 | M/G | G | M | G | P/M | G | P/M | M | M |
| Betula pubescens Ehrh. | NaBl | 1, 2, 3, 4, 5, 6, 7, 9 | L | 90 | P | P | G | M | M | G | M | M | M |
1Using Forest Research species and provenance notes.
L = low; M = moderate; G = good; P = poor; ND = no data; Region = regions which have suitable climate; Potential-Prod = productive; Biod = biodiversity.
https://www.forestresearch.gov.uk/tools-and-resources/tree-species-database/ (Niinemets and Valladares, 2006;,Moffat, 2014; Pyatt et al., 2001)
2See Pyatt et al. (2001) for soil moisture regime definitions of wet, fresh and dry.
Site-related constraints, tolerances and preferences for potential replacement species (native, non-native, broadleaf and conifer) for ash based on production potential and biodiversity value based on the percentage of use by ash-associated species (after Broome et al., 2014). The list has been ranked by decreasing production value.
| Species . | Native (Na) Non-native (Nn) Broadleaf (Bl) Conifer (Co) . | Region . | Potential . | Tolerance to site constraints1 . | Soil type tolerance/preference1 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prod . | Biod (%) . | Shade . | Calc . | Wet2 . | Fresh2 . | Dry2 . | Spring frost . | Thin . | Deep . | Fertile . | |||
| Fraxinus excelsior L. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 100 | M | M | M | G | M | M/P | M | G | G |
| Pseudotsuga menziesii (Mirb.) Franco | NnCo | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 29 | M | P | P | G | M | P | P | M | M |
| Quercus petraea (Matt.) Liebl. | NaBl | 1, 2, 3, 4, 5, 6, 9 | G | 94 | M | P | M | G | G | M | M | M | G |
| Fagus sylvatica L. | NaBl | 1, 3, 4, 6, 7, 8, 9 | G | 92 | G | M | P | G | M | M | M | G | M |
| Prunus avium L. | NaBl | 1, 4, 5, 6, 7, 8, 9 | G | 88 | M | M | P | G | M | G | P | G | G |
| Acer pseudoplatanus L. | NnBl | 1, 3, 4, 5, 6, 7, 9 | G | 88 | M | M | M | G | P | G | M | M/G | M |
| Quercus rubra L. | NnBl | 1, 2, 3, 4, 5, 6, 9 | G | 29 | M | P | M | G | M | G | M | M | M |
| Thuja plicata Donn ex D. Don | NnCo | 1, 2, 3, 4, 5, 6, 9 | G | 22 | G | M | M | G | M | G | P | M | M/G |
| Quercus robur L. | NaBl | 1, 2, 3, 4, 7, 8 | G | 94 | P/M | P | M | G | M | M | P | G | G |
| Acer platanoides L. | NnBl | 1, 4, 6, 7, 8, 9 | G | 60 | G | M | P | G | M | G | P | M/G | M/G |
| Larix decidua Mill. | NnCo | 1, 2, 3, 4, 9 | G | 79 | P | M | P | G | G | M | P | M | M |
| Tilia cordata Mill. | NaBl | 4, 6, 7, 8 | G | 31 | G | M | P | G | M | G | M | G | G |
| Castanea sativa Mill. | NnBl | 4, 6, 7, 8 | G | 88 | M | P | P | G | M | M | M | M | L/M |
| Juglans regia L. | NnBl | 4, 6, 7, 8 | G | 81 | P | M | P | G | P | P | P | G | G |
| Juglans nigra L. | NnBl | 4, 6, 7, 8 | G | 80 | P | M | P | G | P | P | P | G | G |
| Quercus cerris L. | NnBl | 6, 7, 8 | G | 32 | M | M | M | G | G | P | M | M | M |
| Betula pendula Roth | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 90 | P | P | M | G | M | G | M | M | M |
| Alnus glutinosa (L.) Gaertn. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 89 | P | P | G | M | P | G | P | M | M |
| Populus tremula L. | NaBl | 1, 2, 3, 4, 5, 6, 9 | M | 89 | M | P | M | G | M | G | M | M | M |
| Acer campestre L. | NaBl | 1, 4, 6, 7, 8, 9 | M | 88 | M | G | P | G | M | M | M | M/G | G |
| Carya cordiformis (Wangenh.) C. Koch | NnBl | 1, 4, 6, 7, 8, 9 | M | 19 | P/M | M | M | G | G | G | P | M | M |
| Abies alba Mill. | NnCo | 1, 2, 3, 4, 5, 9 | M | 30 | G | M | P | G | P | P | P | M/G | M |
| Carpinus betulus L. | NaBl | 1, 3, 4, 7, 8 | M | 88 | G | P | M | G | G | M | M | M | M |
| Pterocarya fraxinifolia (Poir.) Spach | NnBl | 4, 5, 6, 7, 8 | M | 19 | M | M | M | G | P | M | P | M | M |
| Alnus cordata (Loisel.) Duby | NnBl | 6, 7, 8 | M | 23 | P | M | P | G | G | P/M | M | M | M/G |
| Ostrya carpinifolia Scop. | NnBl | 6, 7, 8 | M | 20 | M/G | G | P | G | G | P | M | M | M |
| Platanus hybrida Brot. | NnBl | 7, 8 | M | 76 | M/G | G | M | G | P/M | G | P/M | M | M |
| Betula pubescens Ehrh. | NaBl | 1, 2, 3, 4, 5, 6, 7, 9 | L | 90 | P | P | G | M | M | G | M | M | M |
| Species . | Native (Na) Non-native (Nn) Broadleaf (Bl) Conifer (Co) . | Region . | Potential . | Tolerance to site constraints1 . | Soil type tolerance/preference1 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prod . | Biod (%) . | Shade . | Calc . | Wet2 . | Fresh2 . | Dry2 . | Spring frost . | Thin . | Deep . | Fertile . | |||
| Fraxinus excelsior L. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 100 | M | M | M | G | M | M/P | M | G | G |
| Pseudotsuga menziesii (Mirb.) Franco | NnCo | 1, 2, 3, 4, 5, 6, 7, 8, 9 | G | 29 | M | P | P | G | M | P | P | M | M |
| Quercus petraea (Matt.) Liebl. | NaBl | 1, 2, 3, 4, 5, 6, 9 | G | 94 | M | P | M | G | G | M | M | M | G |
| Fagus sylvatica L. | NaBl | 1, 3, 4, 6, 7, 8, 9 | G | 92 | G | M | P | G | M | M | M | G | M |
| Prunus avium L. | NaBl | 1, 4, 5, 6, 7, 8, 9 | G | 88 | M | M | P | G | M | G | P | G | G |
| Acer pseudoplatanus L. | NnBl | 1, 3, 4, 5, 6, 7, 9 | G | 88 | M | M | M | G | P | G | M | M/G | M |
| Quercus rubra L. | NnBl | 1, 2, 3, 4, 5, 6, 9 | G | 29 | M | P | M | G | M | G | M | M | M |
| Thuja plicata Donn ex D. Don | NnCo | 1, 2, 3, 4, 5, 6, 9 | G | 22 | G | M | M | G | M | G | P | M | M/G |
| Quercus robur L. | NaBl | 1, 2, 3, 4, 7, 8 | G | 94 | P/M | P | M | G | M | M | P | G | G |
| Acer platanoides L. | NnBl | 1, 4, 6, 7, 8, 9 | G | 60 | G | M | P | G | M | G | P | M/G | M/G |
| Larix decidua Mill. | NnCo | 1, 2, 3, 4, 9 | G | 79 | P | M | P | G | G | M | P | M | M |
| Tilia cordata Mill. | NaBl | 4, 6, 7, 8 | G | 31 | G | M | P | G | M | G | M | G | G |
| Castanea sativa Mill. | NnBl | 4, 6, 7, 8 | G | 88 | M | P | P | G | M | M | M | M | L/M |
| Juglans regia L. | NnBl | 4, 6, 7, 8 | G | 81 | P | M | P | G | P | P | P | G | G |
| Juglans nigra L. | NnBl | 4, 6, 7, 8 | G | 80 | P | M | P | G | P | P | P | G | G |
| Quercus cerris L. | NnBl | 6, 7, 8 | G | 32 | M | M | M | G | G | P | M | M | M |
| Betula pendula Roth | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 90 | P | P | M | G | M | G | M | M | M |
| Alnus glutinosa (L.) Gaertn. | NaBl | 1, 2, 3, 4, 5, 6, 7, 8, 9 | M | 89 | P | P | G | M | P | G | P | M | M |
| Populus tremula L. | NaBl | 1, 2, 3, 4, 5, 6, 9 | M | 89 | M | P | M | G | M | G | M | M | M |
| Acer campestre L. | NaBl | 1, 4, 6, 7, 8, 9 | M | 88 | M | G | P | G | M | M | M | M/G | G |
| Carya cordiformis (Wangenh.) C. Koch | NnBl | 1, 4, 6, 7, 8, 9 | M | 19 | P/M | M | M | G | G | G | P | M | M |
| Abies alba Mill. | NnCo | 1, 2, 3, 4, 5, 9 | M | 30 | G | M | P | G | P | P | P | M/G | M |
| Carpinus betulus L. | NaBl | 1, 3, 4, 7, 8 | M | 88 | G | P | M | G | G | M | M | M | M |
| Pterocarya fraxinifolia (Poir.) Spach | NnBl | 4, 5, 6, 7, 8 | M | 19 | M | M | M | G | P | M | P | M | M |
| Alnus cordata (Loisel.) Duby | NnBl | 6, 7, 8 | M | 23 | P | M | P | G | G | P/M | M | M | M/G |
| Ostrya carpinifolia Scop. | NnBl | 6, 7, 8 | M | 20 | M/G | G | P | G | G | P | M | M | M |
| Platanus hybrida Brot. | NnBl | 7, 8 | M | 76 | M/G | G | M | G | P/M | G | P/M | M | M |
| Betula pubescens Ehrh. | NaBl | 1, 2, 3, 4, 5, 6, 7, 9 | L | 90 | P | P | G | M | M | G | M | M | M |
1Using Forest Research species and provenance notes.
L = low; M = moderate; G = good; P = poor; ND = no data; Region = regions which have suitable climate; Potential-Prod = productive; Biod = biodiversity.
https://www.forestresearch.gov.uk/tools-and-resources/tree-species-database/ (Niinemets and Valladares, 2006;,Moffat, 2014; Pyatt et al., 2001)
2See Pyatt et al. (2001) for soil moisture regime definitions of wet, fresh and dry.
Regional climatic constraints
Between 11 and 26 species were found to be suitable for each region; fewer in upland Scotland (Region 2), and more in lowland Wales (Region 6) (Table 5). Eight of the suggested replacements have a more restricted southerly range in the UK than ash, these are: Italian alder (Alnus cordata (Loisel.) Duby), black walnut (Juglans nigra L.) and common walnut (Juglans regia L.), hop-hornbeam (Ostrya carpinifolia Scop.), London plane (Platanus hybrida Brot.), Turkey oak (Quercus cerris L.), sweet chestnut (Castania sativa Mill.) and small-leaved lime (Tilia cordata Mill.). In contrast, species such as alder, silver birch and Douglas fir (Pseudotsuga menziessii (Mirb.) Franco) have more widespread potential and suitable for northerly and upland ash regions, assuming that a local or suitable provenance is selected (Hubert and Cundall, 2006). Other species listed in Table 5 would be more suited to northerly areas, such as silver fir (Abies alba Mill.), European larch (Larix decidua Mill.), aspen (Populus tremula L.), sessile oak (Quercus petraea (Matt.) Liebl.) and red oak (Quercus rubra L.). Continentality is an important criterion for silver fir, hornbeam (Carpinus betulus L.), pedunculate oak (Quercus robur L.), sycamore, small-leaved lime and Norway maple (Acer platanoides L.). These species should grow well in sheltered central and easterly sites and might exhibit poor performance in the more exposed west of the UK.
Soil type constraints and preferences
The comparison of site constraints and preferences (Table 5) shows that ash grows well on a wide range of soil types. No one alternative species grows as well as ash on all site types and different alternative species will have to be selected for different site conditions. If replacing ash on calcareous soils, then a species with either moderate or good tolerance of neutral to high pH should be selected (e.g. field maple, hop-hornbeam, London plane, Table 5). On neutral to slightly acid soils a greater range of alternative species may be considered.
Discussion
Study limitations
We have interpreted vegetation dynamics in UK broadleaved woodlands containing ash, from descriptions of vegetation composition, rather than measuring changes in woodland composition over time. Woodland seedling, sapling and shrub composition was estimated from surveys in the 1980s, although ash canopy composition data were sampled in the 2010s. Our study may therefore under represent some of the responder species. We assume a direction of change in woodland composition based on the competitive trait and longevity of woody species (Grime et al., 2007), and acknowledge that longer term ecological changes have occurred (Hopkins and Kirby, 2007; Corney, et al., 2008; Hermy, 2015). In particular, a shift from ruderal to competitive species (sensuGrime et al., 2007) was recognized by Hermy (2015) in broadleaved woodlands in Belgium over a similar period. Hermy (2015) showed how changes in acidification and the humus quality of woodland soils had altered the field layer, leading also to regeneration of more common, shade-tolerant tree species (e.g. sycamore, beech and western hemlock) at the expense of more light demanding species (e.g. oak). Whilst we have identified sycamore and beech as main responders, we may have underestimated the contribution conifers e.g. Douglas fir and Scots pine might make to UK woodlands where ash is lost. Other influential factors such as deer browsing (Gill and Beardall, 2001), eutrophication from atmospheric deposition (Bobbink et al., 2010), and the application of different silvicultural systems on vegetation dynamics have not been directly assessed.
Broadleaved woodland composition changes with loss of ash
A shift in tree species composition in broadleaved woodlands in the UK is likely as a result of ash dieback (Needham et al., 2016), and this will occur in broadleaved woodlands with a high percentage of ash in the canopy (e.g. southern England (Harmer et al., 1997)). Harmer et al. (1997) studied regeneration in woodlands across Regions 7 and 8, and they surveyed and noted all the species we predict as responder species. The most frequently occurring species predicted in this study were among the top nine most frequently encountered shrubs, saplings or seedlings in the Harmer et al.'s (1997) study. Differences in the size of gaps explains some of the differences. Their gaps were bigger than we predicted would occur, and 45 of their 78 sites had less canopy cover than we predict if all the ash died in our high-ash category. Our results concur with Mabbett (2014) and Needham et al. (2016) in suggesting that sycamore and beech are the main species likely to replace ash. This leads to implications for woodland policy in the UK. The native range of F. sylvatica has been considered restricted to southern England, although a recent study shows this may not be the case (Sjölund et al., 2017). The regeneration of beech in broadleaved woods, particularly with a conservation status (SSSI or SAC, NNRs), has not always been tolerated in central and northern England, or in Scotland and much of Wales (e.g. Walker and Philip, 2010). Conservation policy has also sought to remove sycamore wherever it colonized ancient woodlands throughout the UK, but on many sites the species has been recognized as valuable for production and biodiversity (Leslie, 2005), however, both beech and sycamore exhibit a greater susceptibility to grey squirrel damage (Mayle and Broome, 2013).
Woodland species composition has already changed in countries where ash dieback has been present for more than a decade. For example in Latvia (Pušpure et al., 2017), ash loss has favoured grey alder (Alnus incana (L.) Moench), elm and Norway maple; in Lithuania a shift in species composition has favoured grey alder and birch (Betula spp.) (Lygis et al., 2014). Our expectation for non-intervention is that shrub species would dominate in the early years, being gradually replaced by predominantly shade-tolerant trees after a decade. Experience of woodland dynamics in the UK (Harmer et al., 2010 – Box 4.2) shows that many suppressed shrubs, such as common dogwood (Cornus sanguinea L.), spindle (Euonymus europaeus L.), blackthorn (Prunus spinosa L.), elder, hazel and hawthorn, will grow in response as occurred following Dutch elm disease. These shrubs might also form locally dominant vegetation patches that suppress the growth of regenerating high-canopy seedlings and saplings. In which case the seedlings and saplings more likely to regenerate may be from shade-bearing species, and light demanders such as birch and willow (Salix spp.) may respond less vigorously in smaller gaps with an established understorey. Where birch and alder occur in ash dieback-affected woodlands, they are likely to dominate if gap sizes are larger; and there is a high likelihood that felling (more so than non-intervention) will provide an opportunity for high density regeneration of these species (Harmer et al., 1997; Lygis et al., 2014), and this may or may not fit with management objectives.
In this study, we have ruled out any ash survival because there is uncertainty of the percentage of resistant ash genotypes to the disease in the UK, although 1–3 per cent has been suggested from trials (Lee, pers. comm.). This accords with current estimates in Europe which report 1–5 per cent of ash genotypes may be less affected by the disease (McKinney et al., 2011; Pliûra et al., 2011; Enderle et al., 2013).
Species options for replacing ash on productive sites
The most productive ash typically occurs on deep fertile moist soils, but ash is tolerant of a wide range of site conditions. Prior to ash dieback this had allowed managers the option to grow ash as a productive broadleaved species, in many situations (Kerr, 1995; Dobrowolska et al., 2011). Our results show many potential replacement species are generally less tolerant of one or more specific characters associated with ash sites. Consequently, although there are several potential replacement species suitable for the prevailing climate in each ash region, the number actually suitable for each location may be fewer due to other site constraints. Therefore, managers must match carefully the replacement species requirements to former ash sites.
Many of the species assessed in this study to replace ash in the UK are currently being used in Europe (Enderle et al., 2017). In Germany, species choice for planting following sanitary felling includes: pedunculate oak (on wetter sites); and black walnut (on freely draining deep soils); sometimes in mixture with small-leaved lime and hornbeam (both on drier sites); Norway maple and Norway spruce (Picea abies (L.) H. Karst.) (riparian freely draining sites, with occasional flooding) and beech (Enderle et al., 2017).
The silviculture for some of the replacement non-native species is less well understood in UK conditions, and the value of non-native species to support the ash-associated biodiversity is either low or not known (Broome et al., 2014; Mitchell et al., 2014b, c). Thus where conservation of biodiversity is a priority objective, we recommend site native species for selection.
Management and policy implications
Our findings generally support the UK forest policy on managing ash dieback-affected woodlands. Ash makes up a minor component of most UK broadleaved woodlands (Forestry Commission, 2012) and changes in woodland composition may be minimal. Where ash forms a larger component of broadleaved woodlands (>10 per cent), the impacts will be more severe and loss of ash will cause a change in species composition. In unmanaged woodlands, or where managed with minimal disturbance for biodiversity, species such as beech and sycamore will be favoured. Consequently, existing views on the native status of tree species in the UK, or locally native within the UK, may need to be challenged.
In woodland managed for timber production or for biodiversity, our suggested replacements may be considered for planting by managers. Although these species have been identified suitable for the UK, there are constraints in their use such as lack of silvicultural knowledge, the need for careful site matching and a supply of suitable material from nurseries all need to be considered. Interpreting the results of this study more widely in Europe will require a careful assessment of site type, relevant responder species and a consideration of winter cold, frost and light (Ellenberg, 1988), particularly where production is the primary objective.
A comparison of our study with experiences from other countries (Pliûra et al., 2011; Kjær et al., 2012; Stener, 2013; Enderle et al. 2017) impacted by ash dieback suggests that we may be too pessimistic about the lack of ash regeneration survival, as we have assumed virtually none. The future for ash as a component in broadleaved woodlands may be subjected to the selection of resistant or less affected genotypes, bred and translocated to suitable broadleaved woodland sites.
Funding
Initial funding from Defra, Natural England, Scottish Natural Heritage, Natural Resources Wales, Northern Ireland Environment Agency and the Forestry Commission. The data were then further analysed in research funded by: Forestry Commission Science and Innovation Strategy research programme 3 (to A.B., D.R., R.H.) and Scottish Government’s Rural and Environment Science and Analytical Services (RESAS) research programme (to R.M.). We are grateful for the comments received from two anonymous reviewers and the editor.
Conflict of interest statement
None declared.