-
PDF
- Split View
-
Views
-
Cite
Cite
Albana Cumashi, Natalia A. Ushakova, Marina E. Preobrazhenskaya, Armida D'Incecco, Antonio Piccoli, Licia Totani, Nicola Tinari, Galina E. Morozevich, Albert E. Berman, Maria I. Bilan, Anatolii I. Usov, Nadezhda E. Ustyuzhanina, Alexey A. Grachev, Craig J. Sanderson, Maeve Kelly, Gabriel A. Rabinovich, Stefano Iacobelli, Nikolay E. Nifantiev, and on behalf of the Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO), Italy, A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds, Glycobiology, Volume 17, Issue 5, May 2007, Pages 541–552, https://doi.org/10.1093/glycob/cwm014
Close - Share Icon Share
Abstract
The anti-inflammatory, antiangiogenic, anticoagulant, and antiadhesive properties of fucoidans obtained from nine species of brown algae were studied in order to examine the influence of fucoidan origin and composition on their biological activities. All fucoidans inhibited leucocyte recruitment in an inflammation model in rats, and neither the content of fucose and sulfate nor other structural features of their polysaccharide backbones significantly affected the efficacy of fucoidans in this model. In vitro evaluation of P-selectin-mediated neutrophil adhesion to platelets under flow conditions revealed that only polysaccharides from Laminaria saccharina, L. digitata, Fucus evanescens, F. serratus, F. distichus, F. spiralis, and Ascophyllum nodosum could serve as P-selectin inhibitors. All fucoidans, except that from Cladosiphon okamuranus carrying substantial levels of 2-O-α-d-glucuronopyranosyl branches in the linear (1 → 3)-linked poly-α-fucopyranoside chain, exhibited anticoagulant activity as measured by activated partial thromboplastin time whereas only fucoidans from L. saccharina, L. digitata, F. serratus, F. distichus, and F. evanescens displayed strong antithrombin activity in a platelet aggregation test. The last fucoidans potently inhibited human umbilical vein endothelial cell (HUVEC) tubulogenesis in vitro and this property correlated with decreased levels of plasminogen-activator inhibitor-1 in HUVEC supernatants, suggesting a possible mechanism of fucoidan-induced inhibition of tubulogenesis. Finally, fucoidans from L. saccharina, L. digitata, F. serratus, F. distichus, and F. vesiculosus strongly blocked MDA-MB-231 breast carcinoma cell adhesion to platelets, an effect which might have critical implications in tumor metastasis. The data presented herein provide a new rationale for the development of potential drugs for thrombosis, inflammation, and tumor progression.
Introduction
Fucoidans represent a class of fucose-enriched sulfated polysaccharides found in the extracellular matrix of brown algae. Similar fucan sulfates were isolated also from marine invertebrates (the jelly coat of sea urchin eggs and the body wall of sea cucumbers) (Berteau and Mulloy 2003). Several biological properties of fucoidans have been investigated in different experimental models. Fucoidans, like heparin, may inhibit thrombin activity by directly acting on the enzyme (Grauffel et al. 1989) or through the activation of thrombin inhibitors, including antithrombin III and heparin cofactor II. Some fucoidans activate antithrombin only, whereas others interact with both inhibitors (Mauray et al. 1995; Pereira et al. 1999; Kuznetsova et al. 2003). Based on these observations and other previous findings (Nardella et al. 1996; Pomin et al. 2005), it has been proposed that fucoidans may represent promising candidates as anticoagulant agents. In addition, fucoidans have also been shown to possess antiproliferative and antiadhesive activities (McCaffrey et al. 1992) and can also protect cells from infection by viruses (Boisson-Vidal et al. 1995; Damonte et al. 2004).
A growing body of experimental evidence indicates that fucoidans may function as anti-inflammatory agents in several experimental murine models. In this regard, perfusion of fucoidans into the myocardium can suppress the infiltration of neutrophils and injury after ischemia-reperfusion of this organ (Kubes et al. 1995; Omata et al. 1997). Also, fucoidans can decrease the extravasation of leucocytes to the cerebrospinal fluid during meningitis (Granert et al. 1999). Possible mechanisms have been postulated by which fucoidans may affect leukocyte recruitment. The ability of these agents to prevent selectin-mediated cell–cell interactions is supported by in vitro experiments showing that fucoidans may indeed bind to purified and membrane-exposed P- and L-selectins (Foxall et al. 1992) but not to E-selectin (Game et al. 1998).
A role of fucoidans as antineoplastic agents has also been suggested. Different studies have reported the antitumor and antimetastatic activity of fucoidans in xenograft mouse models (Yamamoto et al. 1984; Coombe et al. 1987; Riou et al. 1996). Moreover, fucoidans have been shown to induce apoptosis in cancer cell lines and promote macrophage-induced tumor cell death. In addition, these compounds have been shown to block the interactions between cancer cells and the basement membrane. Finally, some fucoidans, particularly those extracted from Fucus vesiculosus, have been found to inhibit angiogenesis by interfering with the binding of vascular endothelial growth factor (Koyanagi et al. 2003) and basic fibroblast growth factor (bFGF) (Soeda et al. 2000) to their respective receptors.
Due to the difficulties in identifying the chemical structure of algal fucoidans, the relation between their structure and biological activities remain largely unknown. For this reason, these compounds have become commercially available mainly as noncharacterized structurally crude preparations, for example, of the fucoidan from F. vesiculosus. This preparation may contain the impurities of other types of polysaccharides and non-carbohydrate concomitants. Therefore some important correlations between structure and biological activity came out by investigating the anticoagulant activity of fucan sulfates isolated from invertebrates, which are available in very limited amounts, but possess, unlike brown algal fucoidans, a regular structure. The fucan chains of animal origin are made of repeating oligosaccharide units differing in the number and arrangement of sulfate groups (Pereira et al. 1999). Their anticoagulant activity has been shown to depend not only on their molecular weight and sulfation degree, but also on the distribution of sulfate groups in the repeating units and on the structure of the polymeric backbone (Pereira et al. 1999).
Due to the wide variety of biological effects elicited by fucoidans, a current challenge is to investigate whether there could be any difference or similarity in the structural features of fucoidans which may account for certain biological effects of these compounds, including their ability to regulate selectin-mediated inflammation, blood coagulation, angiogenesis, and cell adhesion. Our results show several noteworthy differences in the activities of fucoidans from different species of algae, which are likely connected to differences in the chemical structure of these compounds. This work was directed to the selection of the most active fucoidan samples to be studied further as potential novel drugs for thrombosis, inflammation, and cancer therapy.
Results
Structural motifs of the brown seaweed fucoidans
The chemical composition of the fucoidans under investigation is summarized in Table I. Contrary to regular animal fucan sulfates, the seaweed fucoidans are heterogenic and represent the mixtures of structurally related polysaccharides with certain variations of the content of carbohydrate units (l-fucopyranose and non-fucose ones) and non-carbohydrate substituents (mainly sulfate and acetyl groups). Due to this circumstance, the precise assessment of their structures and determination of the exact location of minor structural elements is not practically possible in all cases. Therefore, the investigation of their backbones and branches which form essential structural motifs are the main targets of structural analysis of fucoidans.
Composition of seaweed fucoidans studied (in %, w/w)
| Seaweed source . | Short depiction name . | Fuc . | Xyl . | Man . | Glc . | Gal . | Uronic acids . | SO3Na . |
|---|---|---|---|---|---|---|---|---|
| Laminaria saccharina | L.s. | 36.7 | 1.2 | 1.0 | 2.2 | 4.6 | 4.8 | 29.6 |
| Laminaria digitata | L.d. | 30.1 | 1.9 | 1.7 | 1.4 | 6.3 | 7.0 | 27.5 |
| Cladosiphon okamuranus | C.o. | 30.9 | 0.7 | — | 2.2 | — | 23.4 | 15.1 |
| Fucus evanescens | F.e. | 58.7 | 1.6 | — | — | 1.6 | <1 | 36.3 |
| Fucus vesiculosus | F.v. | 26.1 | 2.4 | 3.1 | 2.2 | 5.0 | 10.3 | 23.6 |
| Fucus serratus | F.se. | 24.8 | 2.4 | 2.1 | 2.0 | 4.8 | 8.2 | 29.2 |
| Fucus distichus | F.d. | 40.8 | 0.8 | — | — | 0.8 | <1 | 34.8 |
| Fucus spiralis | F.s. | 33.0 | 2.8 | 1.4 | 1.2 | 3.0 | 8.2 | 25.9 |
| Ascophyllum nodosum | A.n. | 26.6 | 4.4 | 2.6 | 1.1 | 4.7 | 9.4 | 24.4 |
| Seaweed source . | Short depiction name . | Fuc . | Xyl . | Man . | Glc . | Gal . | Uronic acids . | SO3Na . |
|---|---|---|---|---|---|---|---|---|
| Laminaria saccharina | L.s. | 36.7 | 1.2 | 1.0 | 2.2 | 4.6 | 4.8 | 29.6 |
| Laminaria digitata | L.d. | 30.1 | 1.9 | 1.7 | 1.4 | 6.3 | 7.0 | 27.5 |
| Cladosiphon okamuranus | C.o. | 30.9 | 0.7 | — | 2.2 | — | 23.4 | 15.1 |
| Fucus evanescens | F.e. | 58.7 | 1.6 | — | — | 1.6 | <1 | 36.3 |
| Fucus vesiculosus | F.v. | 26.1 | 2.4 | 3.1 | 2.2 | 5.0 | 10.3 | 23.6 |
| Fucus serratus | F.se. | 24.8 | 2.4 | 2.1 | 2.0 | 4.8 | 8.2 | 29.2 |
| Fucus distichus | F.d. | 40.8 | 0.8 | — | — | 0.8 | <1 | 34.8 |
| Fucus spiralis | F.s. | 33.0 | 2.8 | 1.4 | 1.2 | 3.0 | 8.2 | 25.9 |
| Ascophyllum nodosum | A.n. | 26.6 | 4.4 | 2.6 | 1.1 | 4.7 | 9.4 | 24.4 |
Composition of seaweed fucoidans studied (in %, w/w)
| Seaweed source . | Short depiction name . | Fuc . | Xyl . | Man . | Glc . | Gal . | Uronic acids . | SO3Na . |
|---|---|---|---|---|---|---|---|---|
| Laminaria saccharina | L.s. | 36.7 | 1.2 | 1.0 | 2.2 | 4.6 | 4.8 | 29.6 |
| Laminaria digitata | L.d. | 30.1 | 1.9 | 1.7 | 1.4 | 6.3 | 7.0 | 27.5 |
| Cladosiphon okamuranus | C.o. | 30.9 | 0.7 | — | 2.2 | — | 23.4 | 15.1 |
| Fucus evanescens | F.e. | 58.7 | 1.6 | — | — | 1.6 | <1 | 36.3 |
| Fucus vesiculosus | F.v. | 26.1 | 2.4 | 3.1 | 2.2 | 5.0 | 10.3 | 23.6 |
| Fucus serratus | F.se. | 24.8 | 2.4 | 2.1 | 2.0 | 4.8 | 8.2 | 29.2 |
| Fucus distichus | F.d. | 40.8 | 0.8 | — | — | 0.8 | <1 | 34.8 |
| Fucus spiralis | F.s. | 33.0 | 2.8 | 1.4 | 1.2 | 3.0 | 8.2 | 25.9 |
| Ascophyllum nodosum | A.n. | 26.6 | 4.4 | 2.6 | 1.1 | 4.7 | 9.4 | 24.4 |
| Seaweed source . | Short depiction name . | Fuc . | Xyl . | Man . | Glc . | Gal . | Uronic acids . | SO3Na . |
|---|---|---|---|---|---|---|---|---|
| Laminaria saccharina | L.s. | 36.7 | 1.2 | 1.0 | 2.2 | 4.6 | 4.8 | 29.6 |
| Laminaria digitata | L.d. | 30.1 | 1.9 | 1.7 | 1.4 | 6.3 | 7.0 | 27.5 |
| Cladosiphon okamuranus | C.o. | 30.9 | 0.7 | — | 2.2 | — | 23.4 | 15.1 |
| Fucus evanescens | F.e. | 58.7 | 1.6 | — | — | 1.6 | <1 | 36.3 |
| Fucus vesiculosus | F.v. | 26.1 | 2.4 | 3.1 | 2.2 | 5.0 | 10.3 | 23.6 |
| Fucus serratus | F.se. | 24.8 | 2.4 | 2.1 | 2.0 | 4.8 | 8.2 | 29.2 |
| Fucus distichus | F.d. | 40.8 | 0.8 | — | — | 0.8 | <1 | 34.8 |
| Fucus spiralis | F.s. | 33.0 | 2.8 | 1.4 | 1.2 | 3.0 | 8.2 | 25.9 |
| Ascophyllum nodosum | A.n. | 26.6 | 4.4 | 2.6 | 1.1 | 4.7 | 9.4 | 24.4 |
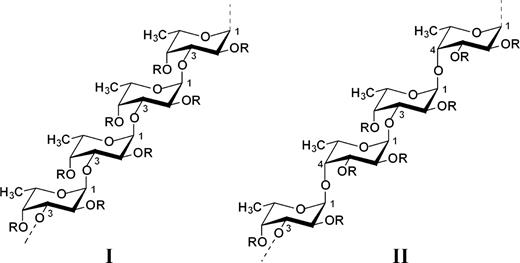
According to our data, the polysaccharide backbones in studied fucoidans are similar to that of type (I) or (II) chains (Figure 1). The type (I) chains are organized in repeating (1 → 3)-linked α-l-fucopyranose residues, whereas type (II) chains contain alternating (1 → 3)- and (1 → 4)-linked α-l-fucopyranose residues. These two backbones could carry carbohydrate [first of all l-fucopyranose (Fuc) and d-glucuronic acid (GlcA)] and non-carbohydrate (sulfate and acetyl groups) substituents R as depicted in Figure 1. Location of minor monosaccharide constituents, also found in known seaweed fucoidan samples [galactose (Gal), mannose (Man), xylose (Xyl), glucose (Glc)], remains unknown.
Two types of homofucose backbone chains in brown seaweed fucoidans. Chains (I) are constructed only of repeating (1 → 3)-linked α-l-fucopyranose residues whereas chains (II) contain alternating (1 → 3)- and (1 → 4)-linked α-l-fucopyranose residues. R depicts the places of potential attachment of carbohydrate (α-l-fucopyranose, α-d-glucuronic acid) and non-carbohydrate (sulfate and acetyl groups) substituents.
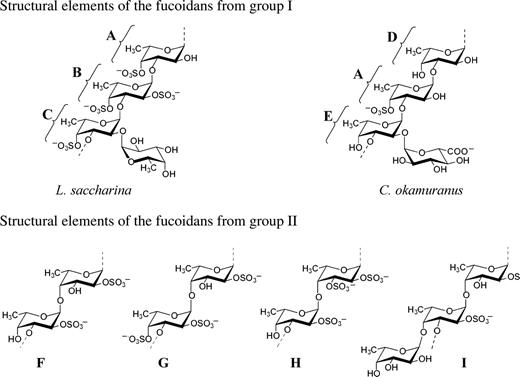
The polysaccharide chain of the fucoidan from Laminaria saccharina (Usov et al. 1998) belongs to type (I) and is built up mainly of 4-sulfated (1 → 3)-linked α-l-fucopyranose residues A (Figure 2), some of which are additionally 2-sulfated (B) or carry 2-O-α-l-fucopyranosyl substituent (C). The ratio of units A–C is approximately 5:1:1 as evaluated by methylation analysis.
Reported structural motifs for fucoidans isolated from the brown seaweeds L. saccharina (A–C) (Usov et al. 1998), C. okamuranus (A, D, E) (Nagaoka et al. 1999; Sakai et al. 2003), F. evanescens (F, G) (Bilan et al. 2002), F. distichus (G) (Bilan et al. 2004), F. vesiculosus (F, H) (Chevolot et al. 2001), A. nodosum (F major, H, G minor) (Chevolot et al. 1999; 2001), and F. serratus (F, G, I) (Bilan et al. 2006).
The same (1 → 3)-linked backbone of type (I) was discovered for Cladosiphon okamuranus fucoidan and found to contain non-sulfated (A), 4-sulfated (D) units and also a branch (E) carrying α-d-glucuronyl residues linked through the (1 → 2) bond (Figure 2). The ratio Fuc:GlcA:sulfate in this polysaccharide was reported as 6.1:1:2.9 (Nagaoka et al. 1999) or 4:1:2 (Sakai et al. 2003). The main chain of a similar type (I) is in the fucoidan derived from L. digitata (Usov et al. unpublished results), but the location of the substituents along the backbone chain remains to be investigated.
Other preparations isolated from algae of the genus Fucus and Ascophyllum nodosum contain the backbone of type (II) with alternating (1 → 3) and (1 → 4) linkages. In particular, the fucoidan from F. evanescens is built up of di-(F) and trisulfated (G) disaccharide repeating units in the ratio of about 3:1 (Bilan et al. 2002), whereas units G form the main fragment of the fucoidan from F. distichus (Bilan et al. 2004), which is comparatively much more regular than other brown seaweed fucoidans.
The chain of the fucoidan from F. vesiculosus is built up mainly of units H (Chevolot et al. 2001), which are also present in the fucoidans from F. spiralis (Usov et al. unpublished results) and from A. nodosum as the major repeating units, together with minor units G and probably some units containing other substituents (xylose, fucose or sulfated fucose) at O-4 (Chevolot et al. 1999; 2001). The backbone of the fucoidan from F. serratus is built up mainly of the repeating units F (Bilan et al. 2006), whereas about half of the 3-linked residues are substituted at O-4 by trifucoside side chains I.
Gel-permeation chromatography investigation of fucoidan samples demonstrated their comparable elution profile and domination of the polysaccharide fractions with molecular weight of 200–500 kDa.
Anti-inflammatory activity of the brown seaweed fucoidans
We have previously shown (Preobrazhenskaya et al. 1997; Ushakova et al. 1999) that a fucoidan from L. saccharina reacts with L- and P-selectins and decreases the escape of neutrophils [polymorphonuclear leucocytes (PMNs)] to the abdominal cavity and the induction of acute peritonitis. The present study showed that all fucoidans at a dose of about 4 mg/kg inhibited at different extents neutrophil extravasation into peritoneal cavity in an acute peritonitis rat model (Table II). The most active inhibitors were fucoidans from L. saccharina and F. evanescens, which inhibited neutrophil extravasation by more than 90% as compared to controls. The least active compounds were fucoidans from F. distichus and F. spiralis, which inhibited the neutrophil transmigration by approximately 60%. In all cases, the differences between the groups of control and treated animals were statistically significant (P < 0.05). It is noticeable that in this assay the fucoidan from C. okamuranus demonstrated a very potent inhibitory activity, whereas in other assays it was remarkably less active when compared with other fucoidans.
Anti-inflammatory and anticoagulant activities of brown seaweed fucoidans
| Seaweed source . | Inflammationa . | APTT (U/mg)b . | ||
|---|---|---|---|---|
| n . | Number of neutrophils per rat (×106) . | Inhibition (% to control) . | ||
| Controlc | 16 | 42.2 ± 3.7 | ||
| Laminaria saccharina | 13 | 2.5 ± 0.4 | 94.1 | 33.0 ± 2.0 |
| Laminaria digitata | 4 | 3.8 ± 1.8 | 91.0 | 24.2 ± 1.2 |
| Cladosiphon okamuranus | 7 | 4.8 ± 1.5 | 88.6 | 0.5 ± 0.1 |
| Fucus evanescens | 4 | 3.0 ± 0.2 | 92.9 | 15.1 ± 0.9 |
| Fucus vesiculosus | 4 | 5.4 ± 3.6 | 87.2 | 9.4 ± 1.2 |
| Fucus serratus | 5 | 6.9 ± 1.8 | 83.6 | 19.1 ± 1.6 |
| Fucus distichus | 6 | 14.1 ± 3.2 | 66.6 | 26.9 ± 1.7 |
| Fucus spiralis | 6 | 14.2 ± 3.1 | 66.4 | 13.6 ± 1.4 |
| Ascophyllum nodosum | 4 | 8.9 ± 4.6 | 78.9 | 13.4 ± 1.1 |
| Seaweed source . | Inflammationa . | APTT (U/mg)b . | ||
|---|---|---|---|---|
| n . | Number of neutrophils per rat (×106) . | Inhibition (% to control) . | ||
| Controlc | 16 | 42.2 ± 3.7 | ||
| Laminaria saccharina | 13 | 2.5 ± 0.4 | 94.1 | 33.0 ± 2.0 |
| Laminaria digitata | 4 | 3.8 ± 1.8 | 91.0 | 24.2 ± 1.2 |
| Cladosiphon okamuranus | 7 | 4.8 ± 1.5 | 88.6 | 0.5 ± 0.1 |
| Fucus evanescens | 4 | 3.0 ± 0.2 | 92.9 | 15.1 ± 0.9 |
| Fucus vesiculosus | 4 | 5.4 ± 3.6 | 87.2 | 9.4 ± 1.2 |
| Fucus serratus | 5 | 6.9 ± 1.8 | 83.6 | 19.1 ± 1.6 |
| Fucus distichus | 6 | 14.1 ± 3.2 | 66.6 | 26.9 ± 1.7 |
| Fucus spiralis | 6 | 14.2 ± 3.1 | 66.4 | 13.6 ± 1.4 |
| Ascophyllum nodosum | 4 | 8.9 ± 4.6 | 78.9 | 13.4 ± 1.1 |
aThe anti-inflammatory activity was determined as the effect on neutrophil extravasation to the peritoneal cavity of rats (for details see the Materials and methods section). Fucoidans were injected intravenously in a dose of 4.0 mg/kg of rat weight. Data are presented as mean ± SEM; n, number of rats in a group.
bAnticoagulant activity was measured as the activated partial thromboplastin time (APTT) related to the heparin standard (Fluka) with an activity of 140 U/mg. Data are shown as mean ± SEM; n = 4.
cAnimals received 0.9% NaCl instead of fucoidan.
Anti-inflammatory and anticoagulant activities of brown seaweed fucoidans
| Seaweed source . | Inflammationa . | APTT (U/mg)b . | ||
|---|---|---|---|---|
| n . | Number of neutrophils per rat (×106) . | Inhibition (% to control) . | ||
| Controlc | 16 | 42.2 ± 3.7 | ||
| Laminaria saccharina | 13 | 2.5 ± 0.4 | 94.1 | 33.0 ± 2.0 |
| Laminaria digitata | 4 | 3.8 ± 1.8 | 91.0 | 24.2 ± 1.2 |
| Cladosiphon okamuranus | 7 | 4.8 ± 1.5 | 88.6 | 0.5 ± 0.1 |
| Fucus evanescens | 4 | 3.0 ± 0.2 | 92.9 | 15.1 ± 0.9 |
| Fucus vesiculosus | 4 | 5.4 ± 3.6 | 87.2 | 9.4 ± 1.2 |
| Fucus serratus | 5 | 6.9 ± 1.8 | 83.6 | 19.1 ± 1.6 |
| Fucus distichus | 6 | 14.1 ± 3.2 | 66.6 | 26.9 ± 1.7 |
| Fucus spiralis | 6 | 14.2 ± 3.1 | 66.4 | 13.6 ± 1.4 |
| Ascophyllum nodosum | 4 | 8.9 ± 4.6 | 78.9 | 13.4 ± 1.1 |
| Seaweed source . | Inflammationa . | APTT (U/mg)b . | ||
|---|---|---|---|---|
| n . | Number of neutrophils per rat (×106) . | Inhibition (% to control) . | ||
| Controlc | 16 | 42.2 ± 3.7 | ||
| Laminaria saccharina | 13 | 2.5 ± 0.4 | 94.1 | 33.0 ± 2.0 |
| Laminaria digitata | 4 | 3.8 ± 1.8 | 91.0 | 24.2 ± 1.2 |
| Cladosiphon okamuranus | 7 | 4.8 ± 1.5 | 88.6 | 0.5 ± 0.1 |
| Fucus evanescens | 4 | 3.0 ± 0.2 | 92.9 | 15.1 ± 0.9 |
| Fucus vesiculosus | 4 | 5.4 ± 3.6 | 87.2 | 9.4 ± 1.2 |
| Fucus serratus | 5 | 6.9 ± 1.8 | 83.6 | 19.1 ± 1.6 |
| Fucus distichus | 6 | 14.1 ± 3.2 | 66.6 | 26.9 ± 1.7 |
| Fucus spiralis | 6 | 14.2 ± 3.1 | 66.4 | 13.6 ± 1.4 |
| Ascophyllum nodosum | 4 | 8.9 ± 4.6 | 78.9 | 13.4 ± 1.1 |
aThe anti-inflammatory activity was determined as the effect on neutrophil extravasation to the peritoneal cavity of rats (for details see the Materials and methods section). Fucoidans were injected intravenously in a dose of 4.0 mg/kg of rat weight. Data are presented as mean ± SEM; n, number of rats in a group.
bAnticoagulant activity was measured as the activated partial thromboplastin time (APTT) related to the heparin standard (Fluka) with an activity of 140 U/mg. Data are shown as mean ± SEM; n = 4.
cAnimals received 0.9% NaCl instead of fucoidan.
The comparison of the data presented in Tables I and II and the available information regarding the structure of fucoidans did not show any direct relation between the anti-inflammatory activity of fucoidans and the content of monosaccharides and sulfate, as well as other parameters of their main chains, such as the presence of branching points. Nevertheless, taking into account that the interaction of P- and L-selectins with their receptors could be inhibited by relatively small natural ligand, namely the tetrasaccharide sialyl Lewis X (SLeX), we might expect that SLeX could be mimicked by some structural motifs presented on the fucoidans belonging both to groups (I) and (II). This conclusion could be confirmed in the future by studying the activity of the fragments of the fucoidans of both types, whose systematic synthesis and conformational analysis are currently in progress (recent communications from the series: Grachev et al. 2006; Ustuzhanina et al. 2006).
Effect of fucoidans on PMNs adhesion to platelet-coated glass surface under flow conditions
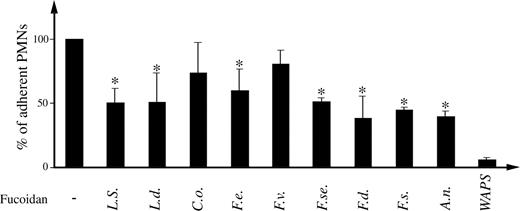
In order to elucidate the mechanisms underlying the efficacy of fucoidans in reducing PMNs extravasation into the peritoneum in the rat inflammatory model, we examined the effects of fucoidans on P-selectin-dependent adhesion of PMNs to adherent platelets under flow. In this model, P-selectin blockage by a specific anti-P-selectin antibody (WAPS) almost completely prevented PMNs adhesion (Figure 3). These results demonstrated that PMNs recruitment in this model involves a P-selectin-dependent mechanism. In the presence of fucoidans from L. saccharina, L. digitata, F. evanescens, F. serratus, F. distichus, F. spiralis, and A. nodosum, (100 µg/mL), PMNs adhesion to platelets monolayers was reduced by 50–60% (P < 0.05) as compared to controls. In contrast, the same concentration of other fucoidans did not significantly affect this interaction.
Effect of fucoidans in polymorphonuclear leucocyte (PMN) adhesion to P-selectin expressed on platelet-coated surface under flow conditions. Fucoidans at 100 µg/mL final concentration were added to platelet-coated surface and incubated for 10 min at room temperature. The same concentration of fucoidans was also added to PMN suspensions before PMN addition to platelets. Under flow conditions, the migration of PMNs was followed and photographs were taken using a camera. The number of attached PMNs per field were counted. The mean percentage ± SEM with respect to control of at least three independent experiments are represented. *P < 0.05.
Anticoagulant activities of fucoidans
The study of anticoagulant activities in activated partial thromboplastin time (APTT) model showed considerable differences among fucoidans obtained from different seaweeds (Table II). The fucoidans tested can be conventionally divided into three main groups according to their anticoagulant activity. The most active anticoagulants were fucoidans from L. saccharina, L. digitata, F. distichus, and F. serratus, whose activities exceeded 19 arbitrary heparin U/mg. Fucoidans of the second group, such as fucoidan from F. evanescens, F. spiralis, A. nodosum, and F. vesiculosus, exhibited an approximately halved activity, being 9–15 U/mg. It is remarkable that the fucoidan from C. okamuranus was the least active among all the fucoidans and had virtually no anticoagulant effect (Table II). The absence of its anticoagulant activity could be explained by the fact that this preparation contains the lowest amount of sulfate in its polysaccharide backbone. Another important structural feature distinguishing this polysaccharide from the others is the presence of vicinal 2,3-branching point formed by 2-O-α-d-glucuronyl substituents (Nagaoka et al. 1999; Sakai et al. 2003).
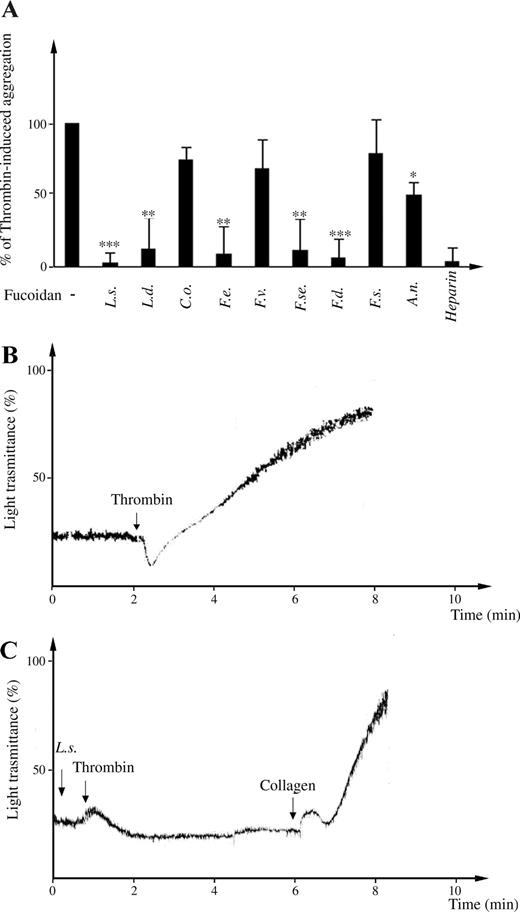
Since thrombin is a primary inducer of platelet activation and coagulation, we also examined the effects of 100 µg/mL fucoidans on thrombin-induced platelet aggregation. After exposure to fucoidans from L. saccharina (Figure 4C), or others such as L. digitata, F. evanescens, F. serratus, and F. distichus (Figure 4A), the platelets showed no or little response after additional exposure to 0.5 U/mL thrombin, but aggregated in response to thrombin receptor activating peptide (TRAP) or collagen (Figure 4C). Lower concentrations (10 µg/mL) of the same fucoidans did not prevent thrombin-induced aggregation of platelets (data not shown). Under the same conditions, fucoidan from A. nodosum induced a lower effect on the inhibition of aggregation, reducing by 50% the effects of thrombin, whereas others from C. okamuranus, F. vesiculosus, and F. spiralis could not prevent thrombin-induced platelet aggregation (Figure 4A).
Effect of fucoidans on human platelet aggregation. (A) Washed platelets were preincubated in the absence or presence of 100 µg/mL of fucoidans and the ability to prevent thrombin-induced platelet aggregation was evaluated. Percentages of thrombin-induced activation of platelets, in absence or in presence of different fucoidans, were reported. Data are collected from at least three independent experiments. ***P < 0.001; **P < 0.01; and *P < 0.05. (B, C) Representative aggregation curves are shown. (B) Aggregation in response of 0.5 U/mL thrombin was recorded. (C) The addition of L. saccharina caused no aggregation of human washed platelets. Subsequently, thrombin was added and light transmittance was measured for at least 3 min.
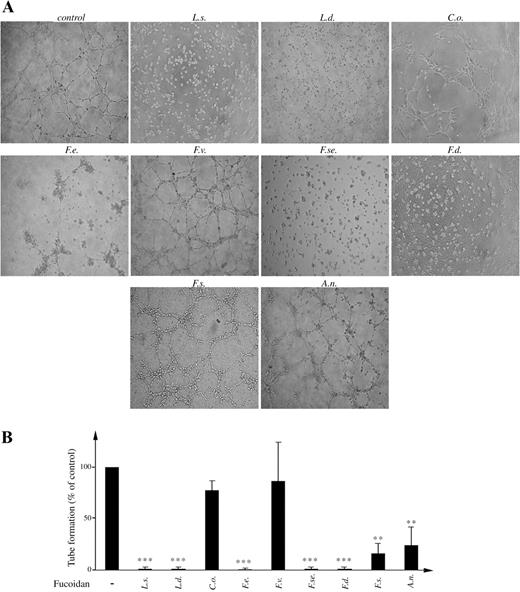
Fucoidan effects on the formation of human umbilical vein endothelial cell (HUVEC) capillary-like structures in vitro (tubulogenesis)
To determine whether the difference in fucoidan structures might have an impact on their ability to modulate angiogenesis, we analyzed their properties in an in vitro assay of HUVEC tubulogenesis. HUVECs have been shown to form capillary-like structures (tubes) when plated on matrigel, and this phenomenon is known as in vitro tubulogenesis. As seen in Figure 5, in the presence of serum, HUVECs reorganize into tube-like structures, and this effect is blocked (99% of inhibition, P < 0.0001) by the addition of 100 µg/mL of fucoidans from L. saccharina, L. digitata, F. evanescens, F. serratus, and F. distichus. To ensure that the suppression of in vitro HUVEC tubulogenesis was not due to toxic effects, cells were analyzed by trypan-blue exclusion test after 18 h of culture in the presence of fucoidans and then compared to controls. None of fucoidans caused significant cell death (data not shown).
Differential inhibitory effects of fucoidans on human umbilical vein endothelial cell (HUVEC) tubulogenesis. (A) Representative pictures of HUVEC on matrigel in presence of fetal bovine serum (FBS) along with 100 µg/mL of each of the indicated fucoidans. (B) Quantitative analysis of tube formation was performed by counting of closed areas (tubes) in four different fields. Data are collected from at least three independent experiments. ***P < 0.001 and **P < 0.01.
The fucoidans from F. spiralis and A. nodosum were less active (Figure 5B), since tubulogenesis was only partially inhibited when they were used at a concentration of 100 µg/mL. Under the same conditions, addition of 100 µg/mL of fucoidans from C. okamuranus and F. vesiculosus was not able to impair the formation of tubes. These results confirmed the specificity and the selectivity of each polysaccharide in the regulation of angiogenesis. Particularly, from the comparison of the structures of the fucoidans from L. saccharina and C. okamuranus, one might speculate that the lack of antiangiogenic activity in the latter could be connected with the lower content of sulfates and/or the presence of 2-O-α-d-glucuronyl substituents along the linear polysaccharide backbone (Table I, Figure 2).
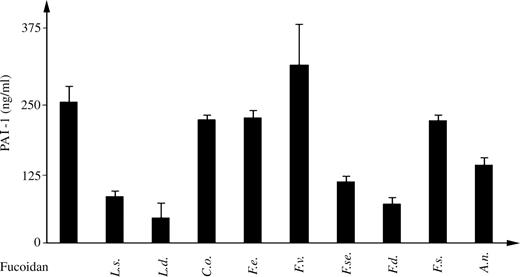
Effects of fucoidan on plasminogen-activator inhibitor-1 (PAI-1) release from HUVEC
In order to identify a potential mechanism responsible for the antiangiogenic activity described above, we measured the levels of PAI-1 in the conditioned medium (CM) of HUVEC cultured in the absence or presence of fucoidans. As shown in Figure 6, the levels of PAI-1 were markedly reduced when cells were exposed to fucoidans from L. saccharina, L. digitata, F. serratus, and F. distichus. Other fucoidans did not affect the release of PAI-1. The addition of fetal bovine serum (FBS) significantly enhanced the levels of PAI-1 present in CM of HUVEC (174 ng/mL in CM versus 258.7 ng/mL in the presence of 10% of FBS, P < 0.01).
Effect of fucoidans in plasminogen-activator inhibitor-1 (PAI-1) (ng/mL) released from HUVECs. HUVECs were plated onto matrigel in the presence or absence of fucoidans. After 18 h, HUVEC supernatants were collected and PAI-1 levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit.
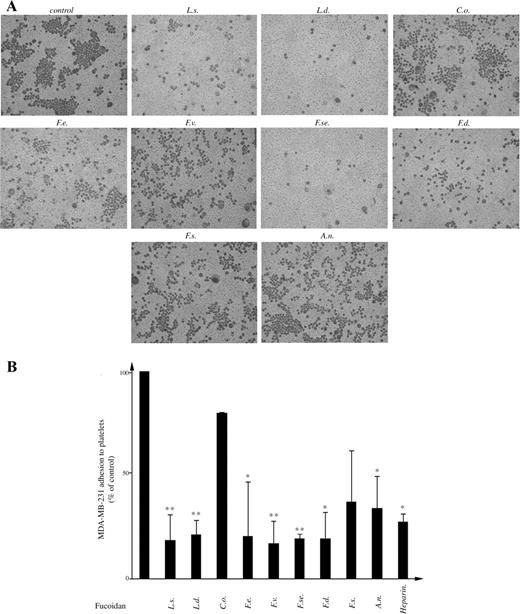
Effect of fucoidans on the adhesion of breast cancer cells to immobilized platelets
Several studies have established a key role of tumor cell–platelet interaction as one of the earliest processes favoring tumor metastasis (Hejna et al. 1999). Therefore, we aimed to determine the effect of fucoidans on the adhesion of a highly metastatic breast cancer cell line MDA-MB-231 to a platelet-coated surface under static conditions. Adherent platelets are known to have partially released their α-granule content (Lahav and Hynes 1981), becoming activated and expressing different glycoproteins (GPs) or adhesion molecules, such as P-selectin and integrins. Breast cancer cells were added on a platelet-covered surface, under static condition. Fucoidans (added at a final concentration of 100 µg/mL) were preincubated for 10 min at room temperature with tumor cells (1 × 105 cells). Cells were left to adhere to platelet-coated plates for 1 h. After washing, adherent cells were fixed and stained with hematoxylin/eosin. The number of cells present in different fields was counted. As shown in Figure 7, fucoidans from L. saccharina, L. digitata, F. vesiculosus, F. serratus, and F. distichus significantly reduced by approximately 80% (P < 0.01) the tumor cell adhesion to human platelets. On the other hand, fucoidans from F. evanescens and A. nodosum were less active and showed 78% and 66% inhibition, respectively (P < 0.05), whereas the fucoidans from C. okamuranus and F. spiralis did not significantly inhibit heterotypic cell adhesion of tumor cells to human platelets.
Effect of fucoidans on breast cancer cell adhesion to platelets. (A) The MDA-MB-231 cells were preincubated with fucoidans prior to exposure to platelet-coated plates. Photographs are representative of at least three independent experiments. (B) Quantification of cell adhesion was performed by counting cells adhered to at least three different fields. The results were expressed as % of treated sample in respect to control **P < 0.01 and *P < 0.05.
Discussion
Fucoidans represent an intriguing group of naturally occurring polysaccharides that might have promising therapeutic applications in various clinical settings. Because algal fucoidans are characterized by a wide variety of biological activities and by a highly complex and heterogeneous structure, which obviously vary with algal species, we currently aimed to determine whether fucoidans from various sources might differentially affect inflammation, coagulation, and some cancer-associated processes.
Our finding show that the i.v. administration of each fucoidan results in remarkable decrease of leucocytes recruitment in an experimental model of peritonitis in rat. One of the possible mechanisms by which fucoidans could successfully prevent PMNs accumulation is by interfering with P-selectin binding activity (Ushakova et al. 1999). In fact, by analyzing the activities of fucoidans in a flow model of P-selectin-mediated PMNs adhesion to platelets, we found that only fucoidans from L. saccharina, L. digitata, F. evanescens, F. serratus, F. distichus, F. spiralis, and A. nodosum could serve as more efficient P-selectin inhibitors than fucoidan from C. okamuranus. The high content of 2-O-α-d-glucuronyl substituent in the polysaccharide chain of fucoidan from C. okamuranus suggests that these lateral branches may impair the antiadhesive effect of this polysaccharide.
The observed inhibitory effect of fucoidans on PMNs adhesion on platelets is consistent with the hypothesis that fucoidans may inhibit PMNs recruitment in the peritonitis inflammatory model, at least in part, by interfering with P-selectin adhesive function. However, the interaction of fucoidans with other leukocyte adhesion receptors cannot be excluded; in fact it has been reported that fucoidans can also bind L-selectin (Ley et al. 1993). Moreover, other putative selectin-independent mechanisms that can mediate fucoidans activity in vivo might also be taken into consideration. For instance, studies in selectin-deficient mice suggest that selectins are not required for the observed effects of sulfated polysaccharides in mobilization of stem cells in vivo (Sweeney et al. 2000).
Many studies have long reported that fucoidans are active modulators of the coagulation, and represent potential therapeutic compounds as an alternative to heparin (Mourão 2004). In this regard, we confirmed here that, like heparin, all fucoidans were able to prolong the clotting time of human plasma, with the exception of C. okamuranus. Interestingly, a higher specificity regarding fucoidan's activity was observed in a thrombin-induced platelet aggregation test. We documented here that only five fucoidans derived from L. saccharina, L. digitata, F. distichus, F. serratus, and F. evanescens could strongly inhibit thrombin activity on human platelet aggregation. An antithrombin effect for some fucoidans was previously described in a study of rabbit platelet aggregation (Trento et al. 2001). The most plausible hypothesis to explain the specific inhibitory effect of fucoidans on thrombin-induced platelet activation could be their ability to inhibit the catalytic activity of thrombin at the concentrations used in the present study. Supporting this theory, chromatographic studies have demonstrated that thrombin can indeed bind to fucoidans (Minix and Doctor 1997). In addition, other authors have shown that some highly branched sulfated fucoidans from brown algae directly inhibit thrombin (Pereira et al. 1999). However the possibility that, like heparin, fucoidans may inhibit thrombin-induced platelet aggregation by blocking the interaction between thrombin and its two main receptors on human platelets, protease-activated receptor-1 and GP-1b (De Candia et al. 1999; 2001) should not be completely excluded. Additionally, we suggest that the specific antithrombin effects of fucoidans might be determined by specific features of their chemical structure. In particular, we hypothesize that the high presence of glucuronic acid branches is the most probable feature responsible for the lack of anticoagulant activity, as seen by C. okamuranus fucoidan-induced effect on APPT assay (Figure 2; Tables I and II). Nevertheless, the absence of this chemical group was thought to be important but might not represent the only factor determining the antithrombin properties of fucoidans (Figure 4).
Anticoagulants have historically been proposed as a complementary treatment in cancer, especially for their ability to negatively affect hemostasis and angiogenesis (Carmeliet 2003). Therefore, we have further explored the antiangiogenic properties of fucoidans in vitro. Interestingly, our data show that fucoidans displaying strong antithrombin properties, such as those from L. saccharina, L. digitata, F. evanescens, F. serratus, and F. distichus, are also potent inhibitors of tubulogenesis. On the contrary, fucoidans from C. okamuranus and F. vesiculosus (Table I) lack any inhibitory activity on tubulogenesis. In our opinion, these variable effects on tube formation might be related to differences in chemical composition of the different fucoidans. Particularly, the less active compounds are characterized by a low degree of sulfation and a high presence of 2-O-α-d-glucuronyl substituents along the linear polysaccharide backbone (see for example, C. okamuranus) . On the other hand, we also observed that the inhibition of tubulogenesis correlated well with a reduction of PAI-1 levels found in the HUVEC supernatants, suggesting a potential mechanism responsible for inhibition of angiogenesis. Indeed, our results concur with previous studies that reported several pathways by which PAI-1 could exert proangiogenic activity (Bajou et al. 2001; Kim 2003). Reduction of PAI-1 levels may be explained by the ability of fucoidan to hijack PAI-1 antigen, which might generate the formation of a fucoidan-PAI-1 complex, thus reducing PAI-1 availability as reported previously (Minix and Doctor 1997). However, other PAI-1-independent mechanisms responsible for the inhibitory activity observed on tubulogenesis should not be ruled out, since a strong inhibitor of tubulogenesis in vitro, F. evanescens did not cause a significant reduction on PAI-1 antigen levels found in HUVEC supernatants (Figures 5 and 6). Moreover, preliminary data from our group documented that fucoidan from L. saccharina could completely abolish bFGF-induced tubulogenesis (data not shown). Collectively these data indicate that several fucoidans may serve as potent antiangiogenic agents in vitro.
Another effect investigated in the present study is related to the activity of fucoidans on platelet–tumor cell interactions. A growing body of experimental evidence has indicated that the formation of platelet–tumor aggregates in the bloodstream is important in facilitating the metastasis process (Hejna et al. 1999), and the blockade of platelet adhesion molecules, such as P-selectin (Kim et al. 1998), or the combined inhibition of GPIIb/IIIa and alpha V beta 3 integrin could provide significant tools for the inhibition of tumor metastasis (Trikha et al. 2002; Gomes et al. 2004). In this regard, we investigated the ability of fucoidans to affect the adhesion of MDA-MB-231 (breast cancer cell) to platelets. Our results demonstrate that specific fucoidans may significantly reduce the number of breast cancer cells adhered to immobilized platelets under static conditions, whereas the polysaccharides derived from C. okamuranus, enriched with glucuronic acid substituents, and also those from F. spiralis, failed to prevent this type of interaction. Although we have not identified specific cell adhesion molecules involved in cell–cell interactions, we hypothesize that, similarly to heparin (Borsig et al. 2001), fucoidans could initially block P-selectin-mediated cell adhesion. Furthermore, the contribution of other adhesion molecules is assured, since the use of only WAPS (anti-P-selectin antibody) was not sufficient to completely block cell–cell interactions (data not shown). One of the possible candidate molecules involved in this process might be thrombospondin, a heparin-binding GP present in the platelet granules (Lawler et al. 1978). It has been shown that once released, thrombospondin is capable of binding to the surface of resting and activated platelets (Nelson et al. 1993). On the other hand, Incardona et al. (1996) indicated that similarly to heparin, a natural fucoidan was capable of blocking the binding of thrombospondin to MDA-MB-231 breast cancer cells. However, the possibility that fucoidans might also block other important candidate molecules involved in tumor cell adhesion to platelets, such as integrins (Haroun-Bouhedja et al. 2002; Liu et al. 2005), should not be ruled out.
Materials and methods
Extraction and purification of fucoidans
The fucoidans used in the present work are listed in Table I. The procedure for isolating fucoidans from L. saccharina has been described earlier (Usov et al. 1998). The procedure includes the extraction of the dry defatted algal biomass with a dilute solution of calcium chloride, precipitation of acidic polysaccharides with Cetavlon, transformation of Cetavlonic salts into calcium salts, and an alkaline treatment to remove acetyl groups (if any in the native polysaccharide) and to transform the fucoidan into the sodium salt. The same procedure was used to obtain preparations L. digitata, F. vesiculosus, F. spiralis, and A. nodosum from the corresponding algae (Table I). Three preparations were isolated by similar extraction procedures, but the alkaline treatment was omitted and an ion-exchange chromatography of the native polysaccharide was performed to obtain the most sulfated fraction. This method produces polysaccharides from F. evanescens (Bilan et al. 2002), F. serratus (Bilan et al. 2006), and F. distichus (Bilan et al. 2004) (for detailed isolation procedures, see cited works). Fucoidan from C. okamuranus was a gift from Dr M. Iha (South Product Co. Ltd, Suzaki, Japan). The structure of the polysaccharide from C. okamuranus has been investigated previously (Nagaoka et al. 1999; Sakai et al. 2003), and this preparation is currently under study as a cell adhesion inhibitor of Helicobacter pylori (Shibata et al. 2003) but also exhibits other biological properties (Shibata et al. 2000; Matsumoto et al. 2004). The monosaccharide and sulfate contents of fucoidans were determined as previously described (Usov et al. 1998; Bilan et al. 2002; 2004).
Gel-permeation chromatography characterization of fucoidans
The molecular-weight distribution of fucoidan samples were characterized by gel-permeation chromatography on a column with TSK-HW-65(S) gel (2.7 × 60 cm, separation range of 10–1000 kDa) by elution with water (2 mL/min) and detection with differential refractometer (Knauer, Berlin, Germany). Standard dextran samples (50, 75, 150, 250, and 500 kDa) were used for column calibration.
Rat peritoneal inflammation model
A rat model of acute peritonitis was used as described earlier (Ushakova et al. 1999) with some modifications. A 9.0% solution of peptone (7 mL) in 0.9% NaCl was injected intraperitoneally into female Wistar rats (about 250 g) under ethereal anesthesia. Fucoidans were injected into the femoral vein of rats in sterile 0.9% NaCl (0.3 mL) 15 min after peptone injection. The same volume of 0.9% NaCl was injected to control animals. After 3 h, the animals were anesthetized, sacrificed, and their peritoneal cavities were washed with phosphate buffered saline (PBS) (30 mL) containing heparin (60 U/mL), 0.02% ethylenediaminetetraacetic acid, and 0.03% bovine serum with vigorous peritoneum massage for 1 min. The cell number in the lavage fluid was counted and the cell suspension was concentrated by centrifugation at 400g for 10 min. The cell pellet was then diluted 1:1 with bovine serum; the smears were prepared and stained according to the Pappenheim method. The number of PMNs was determined in two parallel smears, each containing 600 cells.
Isolation of platelets and PMNs from human blood
Blood was collected from healthy volunteers who had not received any medication for at least 2 weeks. Nine parts of blood were anticoagulated with one part of 3.8% trisodium citrate. Human platelets were prepared by differential centrifugations as described (Cumashi et al. 2001). After removing the platelet-rich plasma, PMNs were isolated by dextran sedimentation followed by Ficoll–Hypaque gradient and hypotonic lysis of erythrocytes. PMNs were washed and resuspended in HEPES–Tyrode's buffer (pH 7.4) containing 129 mmol/L NaCl, 9.9 mmol/L NaHCO3, 2.8 mmol/L KCl, 0.8 mmol/L KH2PO4, 0.8 mmol/L MgCl2 · 6H2O, 5.6 mmol/L glucose, 10 mmol/L HEPES, and 1 mmol/L CaCl2.
Platelets monolayers
Glass coverslips were coated with 4% 3-aminopropyl-triethoxysilane (APES) in acetone. Platelet suspension of 0.5 mL in 1 mol/L Ca2 + containing 3.5 × 107 PLT/mL was stratified on APES-coated glass-slide, and platelets allowed to adhere for 3 h at room temperature. Density and confluence of platelet layers were examined by light microscopy.
Flow adhesion assay
PMNs adhesion under physiologic flow was investigated in a parallel plate flow chamber. Platelet-coated slides were mounted in a flow chamber and placed in a thermoregulated plexiglass box maintained at 37° by an electric heating element. Platelet surface was perfused with 5 mL of PMNs suspension [106 mL−1 in 0.1% bovine serum albumin-Dulbecco's modified eagle (BSA-DME) medium], at a wall shear stress of 2 dynes/cm2 for 2 min, followed by perfusion with medium without cells at wall shear stress of 10 dyne/cm2 for 2 min, in order to remove nonadherent PMNs. The interaction of PMNs with platelets was observed by phase contrast video microscopy with a 10 × objective (Olympus, Hamburg, Germany) and images were continuously recorded for playback analysis (Pro-Series video camera, High Performance CCD camera, Media Cybernetics, Silver Spring, MD). Adherent PMNs were counted at the end of the perfusion, in four randomized fields, by using an ad hoc software for image analysis (Image Pro-Plus for Windows, Media Cybernetics, Silver Spring, MD), and reported as mean ± SEM. P-selectin was immunologically blocked by incubating platelets with the monoclonal antibody WAPS 12.2 (20 µg/mL) for 10 min at room temperature. Fucoidans were added to platelet surfaces for 15 min, at a concentration of 100 µg/mL, and then exposed to PMNs suspension.
Clotting time assay
The anticoagulant action of fucoidans was measured within an APTT clotting assay according to Anderson et al. (1976). Normal pooled human plasma (80 µL) was mixed with a solution (20 µL) of fucoidan (0–5 µg) in 0.9% NaCl, and the mixture was incubated for 1 min at 37 °C. Thereafter, a solution (100 µL) containing a mixture of phospholipids and an activator was added, the resulting mixture was incubated for 2 min at 37 °C. Finally, a solution of 0.025 M CaCl2 (100 µL) preheated at 37 °C was added to the mixture. The time of clot formation was detected. The activity of fucoidans was expressed as heparin U/mg, using a parallel curve obtained with the use of heparin standard (Fluka, Buchs, Switzerland; 140 IU/mg activity).
Platelet aggregation test
Platelet aggregation test was performed as described (Cumashi et al. 2001). Briefly, 500 µL of 108/mL washed platelets in 1 mmol/L Ca2 +-containing HEPES-Tyrode's, were incubated with continuous stirring at 37°C in silanized glass tubes placed in the aggregometer. Platelet aggregation was expressed as the increase in light transmission observed after thrombin (0.5 U/mL) addition. Fucoidans, 100 µg/mL, were added to platelets before the thrombin. Five minutes after thrombin, a different stimulus, TRAP (50 µg/mL) or collagen (30 µg/mL) was added to evaluate whether platelets retained the ability to aggregate.
Cell cultures
HUVECs were isolated by collagenase digestion as described (Gimbrone, 1976). The endothelial cells were grown on gelatin-coated dishes in 199 medium containing 10% FBS (Gibco-Invitrogen, Carlsbad, CA), supplemented with 12 U/mL heparin and 50 mg/mL bovine crude endothelial cell growth factor (ECGF) at 37 °C under 5% CO2. HUVECs from passage 1 to 5, were used for experiments. MDA-MB-231 breast cancer cells were grown in DME medium supplemented with 10 % heat-inactivated FBS.
Tubulogenesis assay
The ability of fucoidans to modulate angiogenesis in vitro was evaluated in a capillary tube formation (tubulogenesis assay) as previously described (Rabinovich et al. 2006). Briefly, chamber slides were coated with growth factor-depleted Matrigel (Becton Dickinson, Bedford, MA) for 1 h at 37 °C. HUVECs resuspended in M199 containing 10% FBS were seeded on Matrigel (5 × 104/perwell). Fucoidans were added at the final concentration of 100 µg/mL. After 18–20 h incubation at 37 °C and 5 % CO2, the cultures were photographed. For each individual well, three digitized photographs were taken from different locations. Photographs were analyzed by ImagePro Plussoftware (Media Cybernetics, Silver Spring, MD), and the closed areas (tube-like structures) were counted. The extension of tube formation was expressed as the portion of tubes (%) found on fucoidan-treated samples versus controls. The final results were pooled from at least three independent experiments.
PAI-1 assay
PAI-1 levels were measured in CM from HUVECs plated on Matrigel for the tubulogenesis assay, in the presence or absence of fucoidans. After 20 h incubation, CM were collected, centrifuged, and stored. Five-microliter aliquots of CM were assayed using a specific enzyme-linked immunosorbent assay (ELISA; American Diagnostica GmbH, Pfungstadt, Germany).
In vitro platelet–tumor cell adhesion assay
For tumor cell adhesion assays, platelet-coated surfaces were generated as described (Karpatkin et al. 1988) with modifications. Platelet suspension of 0.1 ml containing 3 × 107 platelets in HEPES Tyrode's buffer was added to flat-bottomed plastic micro-titter wells. Plates were incubated for 1 h at room temperature. Afterwards, the same volume of HEPES–Tyrode's buffer enriched with 2 mM Ca2 + was added to platelets. The plate was then left at 4 °C overnight. The day after, nonadherent platelets were removed by washing with PBS plus 1% of BSA. Two-hundred microliters of this solution was added and incubated for 1 h at 37 °C in order to block “free adherent” sites on the plastic. Tumor cells were detached and resuspended in PBS enriched with Ca2 + and Mg2 +. After counting, 1 × 105 cells were added to each platelet-coated well, in the presence or absence of 100 µg/mL fucoidans and incubated for 1 h at 37 °C. Plates were then washed twice and fixed with methanol. Adherent cells were stained by using a hematoxylin/eosin solution in order to make visible the cancer cell nuclei.
Statistical analysis
Statistical significance among different experimental groups was determined using the Student t test. P values less than 0.05 were considered statistically significant.
Conclusions
Our data demonstrate that fucoidans obtained from brown algal species different from the traditionally studied F. vesiculosus and A. nodosum may act as inhibitors of inflammation, angiogenesis, and heterotypic tumor cell adhesion. The results described herein might suggest the importance of 2-O-α-d-glucuronyl branch in decreasing several biological activities if weaker inhibitory potential of C. okamuranus fucoidan is not due to lower sulfate content. On the contrary, the structure of the polysaccharide backbone (type I versus type II) seems to be less critical. This could be explained by the presence of biological relevant structural elements on both polysaccharide backbones or by nonspecific mechanisms, including their poly-anionic structure. Thus, the decreased inhibitory activity of 2-O-α-d-glucuronylated polysaccharides could be associated with the corresponding conformational changes of the linear backbone influenced by vicinal branching which we could observe by using the corresponding synthetic oligosaccharide models (Gerbst et al., unpublished results).
Among the studied compounds, the fucoidan from L. saccharina seems to be a more powerful inhibitor of angiogenesis and tumor cell adhesion to platelets. Further investigation of different sulfated fractions of this polysaccharide (obtained by ion-exchange chromatography) and of synthetic fragments is in progress to delineate the structural motifs responsible for distinct biological activities. This information could open the perspective for the development of novel low-toxic agents for the treatment of thrombosis, inflammation, and tumor progression.
Acknowledgments
We thank Rachel Miller (Loch Duart Ltd, Badcall Salmon House, Scourie, Lairg, Sutherland, IV27 4TH, UK) and Angus Morrison (Biolitec Pharma Ltd, Breasclete, Isle of Lewis, Scotland, HS2 9ED, UK) for kind help in harvesting and drying of the seaweeds, and Dr Patrizia Di Gregorio and Gabriele Merciaro of “Transfusion Center” SS Annunziata Hospital, Chieti, Italy, for helpful collaboration. We are in debt with Dr Virgilio Evangelista for review of the manuscript. This work was supported in part by GlycoSense AG (Jena, Germany) and the Russian Foundation for Basic Research (grants 04-04-49464a and 06-03-33080).
Conflict of interest statement
None declared.
Abbreviations
- APES
3-aminopropyl-triethoxysilane
- APTT
activated partial thromboplastin time
- bFGF
basic fibroblast growth factor
- BSA
bovine serum albumin
- CM
conditioned medium
- DME
Dulbecco's modified eagle
- ELISA
Enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GP
glycoprotein
- HUVEC
human umbilical vein endothelial cell
- PAI-1
plasminogen-activator inhibitor-1
- PBS
phosphate buffered saline
- PMN
polymorphonuclear leucocytes
- SLeX
sialyl Lewis X
- TRAP
thrombin receptor activating peptide