-
PDF
- Split View
-
Views
-
Cite
Cite
Luda Diatchenko, Gary D. Slade, Andrea G. Nackley, Konakporn Bhalang, Asgeir Sigurdsson, Inna Belfer, David Goldman, Ke Xu, Svetlana A. Shabalina, Dmitry Shagin, Mitchell B. Max, Sergei S. Makarov, William Maixner, Genetic basis for individual variations in pain perception and the development of a chronic pain condition, Human Molecular Genetics, Volume 14, Issue 1, 1 January 2005, Pages 135–143, https://doi.org/10.1093/hmg/ddi013
Close - Share Icon Share
Abstract
Pain sensitivity varies substantially among humans. A significant part of the human population develops chronic pain conditions that are characterized by heightened pain sensitivity. We identified three genetic variants (haplotypes) of the gene encoding catecholamine- O -methyltransferase (COMT) that we designated as low pain sensitivity (LPS), average pain sensitivity (APS) and high pain sensitivity (HPS). We show that these haplotypes encompass 96% of the human population, and five combinations of these haplotypes are strongly associated ( P =0.0004) with variation in the sensitivity to experimental pain. The presence of even a single LPS haplotype diminishes, by as much as 2.3 times, the risk of developing myogenous temporomandibular joint disorder (TMD), a common musculoskeletal pain condition. The LPS haplotype produces much higher levels of COMT enzymatic activity when compared with the APS or HPS haplotypes. Inhibition of COMT in the rat results in a profound increase in pain sensitivity. Thus, COMT activity substantially influences pain sensitivity, and the three major haplotypes determine COMT activity in humans that inversely correlates with pain sensitivity and the risk of developing TMD.
INTRODUCTION
Pain perception is a complex process that is influenced by a variety of environmental and genetic factors ( 1 ). Although the relative importance of genetic versus environmental factors in human pain perception remains unclear, reported heritability for nociceptive and analgesic sensitivities in mice is estimated to range from 28 to 76% ( 1 ). Even though animal studies have provided a list of candidate ‘pain genes’, only a few genes have been identified that are associated with the perception of pain in humans. Single nucleotide polymorphisms (SNPs) in genes that code for melanocortin-1 receptor and neuronal cytochrome P4502D6 are associated with alterations in opioid analgesia in humans ( 1 , 2 ). Congenital insensitivity to pain (CIP) type I has been linked to the gene encoding a subunit of serine palmitoyltransferase, and CIP type IV has been linked to the gene encoding for a nerve growth factor-specific tyrosine kinase receptor ( 1 , 3 ). Furthermore, a common SNP in codon 158 ( val158met ) of the gene that codes for catecholamine- O -methyltransferase (COMT) has been proposed to contribute to differences in the human experience of pain ( 4 ). This enzyme has broad biological functions including the regulation of the levels of catecholamines and enkephalins ( 5 ). Thus, the examination of the association of COMT polymorphism with human pain perception and persistent pain conditions is of considerable importance.
A pathological pain condition that appears to be associated with COMT activity is myogenous temporomandibular joint disorder (TMD). This condition is characterized by persistent facial pain, impaired oral function ( 6 – 8 ) and heightened sensitivity to pain-evoking stimuli (e.g. mechanical, thermal and ischemic) at numerous body sites ( 9 , 10 ). TMD impacts 5–15% of the adult population and incurs billions of dollars in health care costs ( 6 ). In 1976, Marbach and Levitt ( 11 ) reported that patients with facial pain conditions comparable to TMD show increased urinary levels of catecholamine metabolites and express diminished erythrocytic COMT activity, suggesting a role for COMT in this persistent pain condition. In the present study, we examined the relationship between COMT polymorphism, pain sensitivity and the risk of TMD development.
RESULTS
Pain sensitivity varies significantly in the human population
Data for this study were collected from 202 healthy female volunteers. The subjects participated in a 3 year prospective cohort study that was designed to identify risk factors for TMD ( 9 , 10 , 12 ). Only females were included in this study as they exhibit a higher prevalence for the condition relative to males ( 13 ). During an initial screening exam, we assessed the sensitivity of subjects to experimental noxious stimuli and collected peripheral blood samples for genetic analyses. Subjects were subsequently followed for up to 3 years, by both trimonthly interviews and annual physical examinations, to identify newly developed cases of TMD.
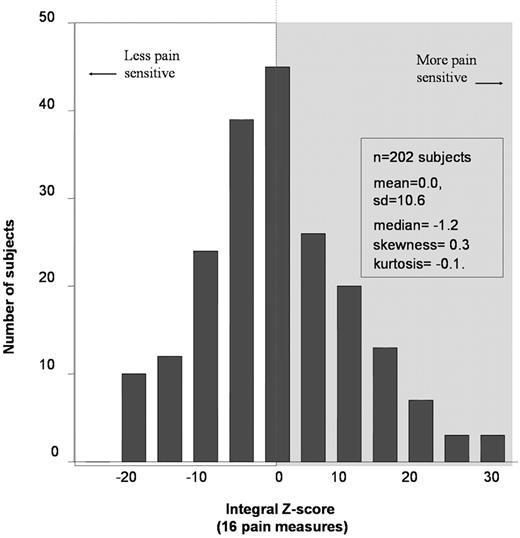
In order to evaluate each participant's pain sensitivity, a unique approach was used to derive a unitary measure of pain sensitivity for both cutaneous and deep muscle pain, which are transmitted and modulated by different neural mechanisms ( 14 , 15 ). To accomplish this, each of 16 measures of pain sensitivity was normalized to a mean of 0 and standard deviation of 1, producing a unit normal deviate ( z -score) for each test procedure. A sum of these 16 scores produced a normalized single score of pain sensitivity (integral z -score) for each individual (see Supplemental Material for details). As shown in Figure 1 , measures of individual pain sensitivity (integral z -scores) were distributed approximately normally (skewness=0.3 and kurtosis=−0.1), ranging from −22.4 (least responsive to painful stimuli) to 28.0 (most responsive to painful stimuli); although the majority of individuals display average pain sensitivity, the individual variability in pain sensitivity between people is substantial.
Genotyping of the COMT locus
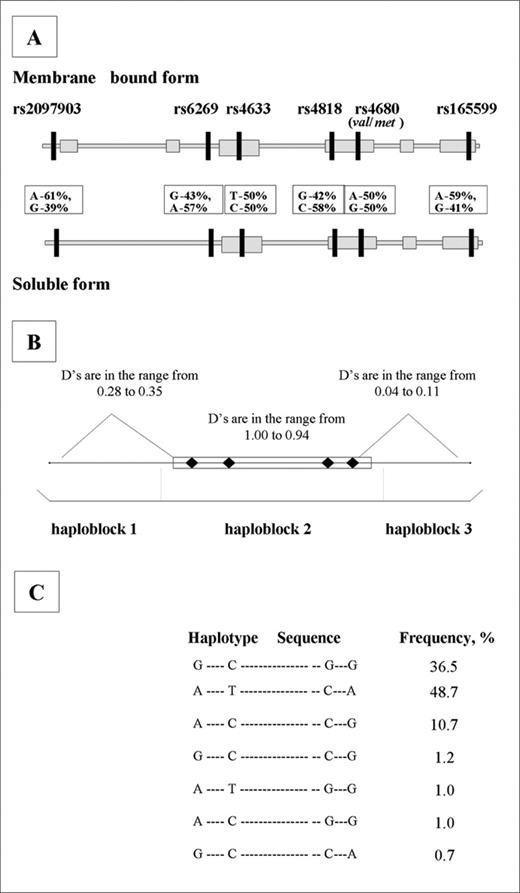
Genomic DNA from peripheral blood samples was genotyped for SNPs within the COMT gene locus. Six SNPs were chosen that display high polymorphism frequency in the human population (>40% prevalence). Figure 2 A shows the positions of the SNPs within the COMT locus that code for two major forms of COMT enzyme: membrane bound (MB-COMT) and soluble (S-COMT). The first SNP (rs2097903) is located at position −1217 in the estrogen sensitive portion of the MB-COMT promoter region ( 16 , 17 ), whereas the second SNP (rs6269) is located in the promoter region of S-COMT ( 16 , 18 ). The next three SNPs (rs4633, rs4818 and rs4680) are located within the coding region for both S - and MB-COMT ( 19 ) (NCBI genome database). Variations in SNPs rs4633 and rs4818 are synonymous (i.e. do not produce a change in amino acid composition). In contrast, SNP rs4680 is non-synonymous and codes for a substitution of valine ( val ) to methionine ( met ) at codon 158. The last SNP (rs165599) is located in the very end of the 3′-UTR of the gene and its G allele has been reported to be associated with schizophrenia ( 18 ). Importantly, the COMT SNP map has been thoroughly constructed (NCBI database, http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=1312 ). In the coding region of the gene, there are no other SNPs with frequencies greater than 0.15. We further confirmed this by comparing more than 300 COMT EST sequences from NCBI database using the CLUSTALW program for multiple sequence alignments. As it has been discussed by Risch ( 20 ), alleles with polymorphic frequencies <0.15% are very unlikely to have significant impact on common population-based diseases/disorders such as TMD.
Statistically significant associations were found between the integral z -score and two SNPs (Table 1 ; Supplementary Material, Fig. S1). The SNP rs6269 accounted for 6% of the variation in pain sensitivity as determined by analysis of variance (ANOVA, P <0.01), whereas SNP rs4818 accounted for 7% of the variation (ANOVA, P <0.01). For both SNPs, the homozygous genotypes were associated with significant differences in mean pain sensitivity ( t -test, P <0.01). Consistent with previously reported results ( 4 ), the val158met SNP (rs4680) showed a marginal, but not statistically significant, relationship with pain sensitivity, accounting for 2% of the variation in the summary pain measure (ANOVA, P =0.18). Individuals homozygous for met/met tended to be more pain responsive than those homozygous for val/val , but again the association was marginal ( t -test, P =0.06). SNPs A- rs2097903, rs4633 and rs16559 were not significantly associated with pain sensitivity (Table 1 ; Supplementary Material, Fig. S1). Multivariate analysis revealed that all possible combinations of the four SNPs in the coding region accounted for 10.6% of the variation in the summary measure of pain sensitivity. In this model, we first controlled for rs4680 (accounting for 2% of variance), then for rs4818 (7% of variance); the remaining SNPs did not individually contribute significantly ( P >0.10) to the variance in pain sensitivity (Supplementary Material, Table S3).
COMT haplotypes determine sensitivity to pain
We next determined which combinations of alleles (haplotypes) were formed by the six SNPs. It has been shown that alleles form associations (haploblocks) of variable length with the average span of 18 kb in populations of European descent and only a few common haplotypes are observed ( 21 ). Three haploblocks were determined in our study sample by LD analysis (Supplementary Material, Table S4). Because the association with pain sensitivity was observed only for SNPs rs6269 and rs4818, located in the central COMT locus haploblock (Table 1 , Fig. 2 B; Supplementary Material, Fig. S1), we focused our analyses on this haploblock.
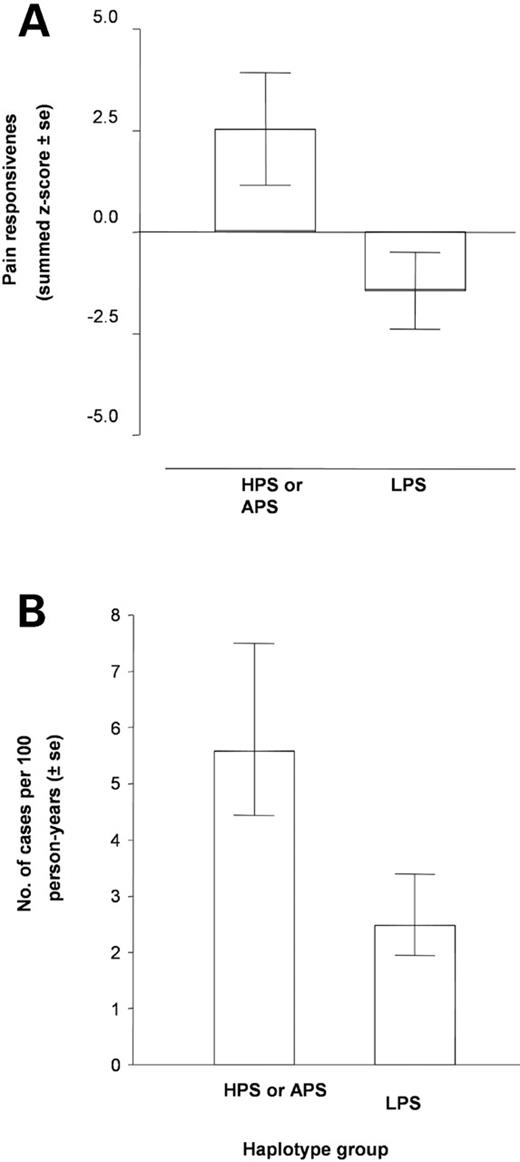
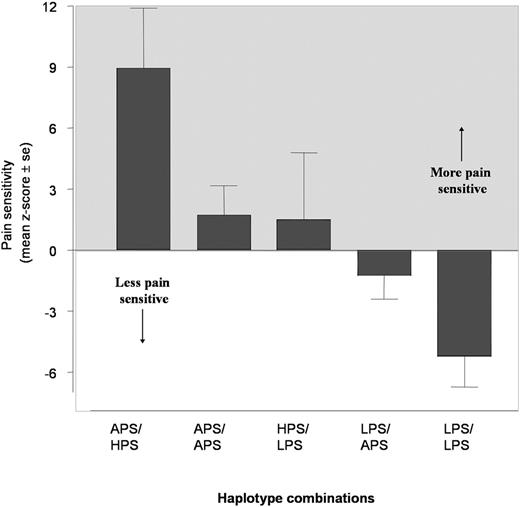
Seven haplotypes with a frequency >0.5% were detected, three of them representing 95.9% of all haplotypes observed in this study (Fig. 2 C). Five combinations of these three haplotypes were present in 92% of subjects and were associated with marked gradients in pain responsiveness (Fig. 3 ). Subjects homozygous for the G_C_G_G haplotype had the lowest pain responsiveness (mean summed z -score=−5.23±1.5; Fig. 3 , Table 1 ); thus, G_C_C_G is designated as the ‘low pain sensitivity’ (LPS) haplotype. Intermediate pain responsiveness was observed in individuals homozygous for A_T_C_A, which we refer to as the APS haplotype (mean summed z -score=1.75±1.47; Fig. 3 , Table 1 ). The greatest pain responsiveness was observed in individuals heterozygous for A_T_C_A (APS) and A_C_C_G haplotypes (mean summed z -score=8.9±2.9; Fig. 3 , Table 1 ). We refer to the A_C_C_G haplotype as the ‘high pain sensitivity’ (HPS) haplotype. Differences among the five combinations of haplotypes were significant (Table 1 , overall ANOVA, P =0.0004) and factorial analysis demonstrated that each haplotype had independent effects on pain sensitivity (Table 2 , factorial ANOVA model, P ≤0.01). These haplotypes accounted for 10.4% of the variation ( P <0.01) in pain sensitivity, representing virtually all of the variation (10.6%) explained by combinations of the four individual SNPs in the central haploblock.
COMT haplotypes determine enzyme activity
Functional polymorphism in the COMT gene has been described only for SNP rs4680 ( val158met ) ( 4 , 5 , 22 ), which codes for a substitution of val to met . The met substitution produces a COMT enzyme with lower thermostability, resulting in decreased enzyme activity ( 22 ). It is generally accepted that genetic variability in codon 158 is the primary source of individual variation in COMT activity in humans ( 5 ); however, in many cases, the observed association between several human diseases and disorders and the met allele (low enzyme activity) is relatively modest and occasionally opposite, such as for obsessive-compulsive disorder (reviewed in 23 ) and schizophrenia (reviewed in 24 ).
Although it appears that the val158met amino acid substitution in COMT can explain the greater pain responsiveness observed for individuals with the APS haplotype when compared with the LPS haplotype, because the APS haplotype codes for the less stable met variant, it cannot explain the greater pain responsiveness that we found for subjects with the HPS haplotype when compared with the LPS haplotype. This is because both the HPS and LPS haplotypes possess the G allele that codes for the more stable val variant (Fig. 2 C). Thus, the val158met SNP alone cannot account for the observed variations in pain perception. Furthermore, even though polymorphism in SNPs rs6269 and rs4818 is significantly associated with pain z -scores, both pain sensitive haplotypes HPS and APS contain the A allele of rs6269 and the C allele of rs4818. Consequently, variations in these SNPs cannot explain why the HPS and APS haplotypes are associated with different levels of pain sensitivity. Instead, the interaction of the val158met SNP with other SNPs determines the functional outcomes. The other SNPs are either synonymous (i.e. code for the same amino acid) or are located in the promoter region of S-COMT . A haplotype-dependent regulation of mRNA expression is also unlikely because SNP rs6269, which is located in the promoter region of S-COMT , does not independently contribute to pain sensitivity (Supplementary Material, Table S3). Therefore, we hypothesized that haplotype-specific secondary structures of mRNA affect COMT mRNA stability and/or efficiency of protein translation ( 25 ).
To test these possibilities, we transiently transfected HEK 293 cells with full-length COMT cDNA clones that corresponded to the three major haplotypes. The expression of COMT protein was assessed by measuring COMT enzymatic activity in the lysate of transfected cells. Figure 4 A shows that the LPS haplotype provides 4.8 times higher COMT activity when compared with the APS haplotype ( P <0.01), which is in agreement with a previous report of reduced thermal stability of COMT protein containing met (APS haplotype) in comparison with COMT protein containing val (LPS haplotype) ( 22 ). However, our finding that the HPS haplotype provides 11.4 times lower COMT activity compared with the LPS haptotype ( P <0.01) can only be attributed to the lower amount of protein produced by the HPS haplotype as these two haplotypes code for COMT protein with exactly the same amino acid composition. No differences in COMT RNA abundance were detected in the transfected cells as measured by real-time PCR (Fig. 4 B); therefore, the three major haplotypes affect the efficiency of protein synthesis, but not RNA stability.
Inhibition of COMT enhances sensitivity to noxious stimuli
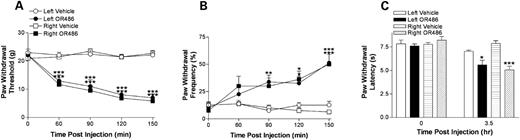
Our association and in vitro studies strongly suggest that reductions in COMT enzymatic activity enhances pain sensitivity. To directly test whether decreased COMT activity enhances pain sensitivity, the COMT inhibitor OR486 was administered to naive rats. OR486 decreased paw withdrawal thresholds to mechanical and thermal stimuli ( P <0.0001 and P <0.0007, respectively) and increased paw withdrawal frequency to noxious punctate mechanical stimuli ( P <0.0001) (Fig. 5 ). The degree of mechanical and thermal hyperalgesia produced by OR486 was comparable to that produced by carrageenan-induced inflammation in the hindpaw (data not shown).
The high activity COMT haplotype protects from developing TMD
To determine the clinical relevance of these findings, we examined whether COMT polymorphism is related to the incidence of TMD onset among 170 subjects with the five most common haplotype combinations who completed one or more follow-up visits. Fifty-eight of the participants had only ‘low COMT activity’ haplotypes (HPS and/or APS) and the remaining 112 subjects had at least one ‘high activity’ haplotype (LPS). We first confirmed that HPS and/or APS subjects were more sensitive to experimental pain at their baseline assessment when compared with LPS subjects ( P =0.02; Fig. 6 A). During the 3 year observational period, 15 new cases of TMD were diagnosed ( 12 ) at varying time periods ranging from 9 months to 3 years after recruitment, yielding an average incidence rate of 3.5 cases per 100 person-years of follow-up. The incidence rate was more than twice as high among individuals having only HPS and/or APS haplotypes (5.6 cases per 100 person-years) when compared with individuals with at least one LPS haplotype (2.5 cases per 100 person-years, Fig. 6 B). The derived incidence density ratio of 2.3 was significant (95% confidence interval=1.1–4.8), suggesting that the HPS and/or APS haplotypes represent significant risk factors for TMD onset.
DISCUSSION
Several important conclusions can be drawn. First, COMT genotype is highly associated with human pain perception. There are three major COMT haplotypes (LPS, APS and HPS) that determine COMT enzymatic activity, encompassing ∼96% of the examined genotypes. The LPS haplotype is associated with low pain sensitivity, APS is associated with higher pain sensitivity and HPS with the highest pain sensitivity. Collectively, these three haplotypes account for ∼11% of the variability in pain perception. Given the inevitably polygenic nature of pain perception, the magnitude of the effect of COMT haplotypes on pain perception is substantial. Indeed, quantitative trait locus (QTL) mapping studies for related traits in mice have shown that each single QTL usually accounts for 5–25% of the overall variance in nociceptive sensitivity ( 2 , 26 ).
Secondly, synonymous SNPs within COMT haplotypes can have effects on protein function that exceed the effects of individual SNPs. Several recently published studies support the theory that the collective grouping of SNPs in haplotypes has a stronger association with the assessed phenotype than individual SNPs ( 18 , 25 , 27 – 29 ). However, almost all previously published haplotype association studies demonstrate the importance of haplotype reconstruction because combinations of SNPs result in synergistic effects on protein function. Recently, Duan et al. ( 25 ) showed that synonymous SNPs within haplotypes can have functional consequences drastically different from those of each isolated mutation. This effect was attributed to alternations in the secondary structure of mRNA, which results in alterations in mRNA stability. In contrast, our studies show that genomic variations of the COMT gene do not alter the amount of COMT mRNA, suggesting that the differences in enzymatic activity result from differences in protein translation. The fact that expressed cDNA constructs, which differed in only three SNPs rs4633, rs4818 and rs4680 ( val158met ), showed >11-fold difference in expressed enzyme activity, confirms that the observed association between haplotypes and pain sensitivity is largely caused by combinations of these three SNPs and not by other SNPs in the haploblock located in the 5′ or intronic region of the COMT gene that can affect RNA transcription. Although the precise molecular mechanisms underlying this phenomenon are unknown, our ongoing studies suggest that interactions between SNPs have profound effects on the secondary mRNA structure, which controls the efficacy of protein translation. The identification of new functional haplotypes in the present study reinforces the findings presented by Shifman et al. ( 18 ) and Bray et al. ( 28 ), suggesting that haplotype reconstruction, rather than polymorphism at codon 158, needs to be considered in future studies that examine the relationship between COMT polymorphism, human pain and pain-related or affective disorders.
Thirdly, COMT inhibition results in a robust increase in pain sensitivity. These data provide evidence that COMT activity regulates pain sensitivity and strongly suggests that the observed association between COMT genotype and pain perception is unlikely to be epiphenomenal or caused by other functional polymorphisms in neighboring genes that possess high LDs with the tested COMT SNPs.
Finally, our results are of considerable clinical significance and represent the first study of its kind to demonstrate an association between a genetic polymorphism that impacts pain sensitivity and the risk for a clinical chronic pain condition. The presence of even a single high COMT activity (LPS) haplotype diminishes, by as much as 2.3 times, the risk of developing TMD, a common musculoskeletal pain condition. The risk ratio of 2.3 is of a magnitude comparable to genetic risk factors for other multifactorial conditions, such as schizophrenia ( 18 ), and is similar to other predictors of TMD, such as a history of chronic pain at other body sites ( 7 , 8 ). The clinical relevance of this finding is best quantified by the measure of population attributable risk for having HPS and/or APS, which was 29% in this cohort of women, indicating that nearly one-third of new TMD cases can be attributed to this COMT genotype.
The mechanism by which diminished COMT activity influences pain perception and the development of TMD is not known. It has been suggested that reduction in COMT activity associated with the met allele at codon 158 leads to a reduction in the content of enkephalins (endogenous opioid-like peptides) in certain regions of the CNS associated with pain and mood ( 4 ). Another possible mechanism is that reduced COMT activity results in elevated levels of catecholamimes, such as epinephrine, which promote the production of persistent pain states via the stimulation of β 2 -adrenergic receptors in the peripheral and central nervous system ( 30 ). Future studies are required to establish the precise mechanisms. However, the clinical, animal and molecular data presented in this study are in complete agreement with the conclusion that COMT activity substantially influences pain sensitivity and that the three major haplotypes determine COMT activity in humans in a fashion that inversely correlates with pain sensitivity and the risk of developing TMD and possibly other chronic pain conditions.
MATERIALS AND METHODS
Detailed descriptions for each procedure are provided in Supplementary Material.
Clinical assessments and procedures
Study participants consisted of 202 healthy pain-free females aged 18–34 years who provided a blood sample and consent for genotyping from among a larger cohort of 244 females. All subjects underwent pain perception assessments and head/neck examinations. Pressure pain thresholds were assessed over the right and left temporalis muscles, masseter muscles, temporomandibular joints and ventral surfaces of the wrists with a hand-held pressure algometer ( 31 ). Thermal pain thresholds and tolerances (in °C) were determined with a computer-controlled thermal stimulator on the skin overlying the right masseter muscle, the skin overlying the right hairy forearm and the skin overlying the dorsal surface of the right foot ( 32 ). The temporal summation of heat pain was determined by delivering 15 heat pulses of 53°C to the skin overlying the thenar region of the right hand ( 33 ). Subjects verbally rated the intensity of each thermal pulse using a numerical scale ( 34 ). Ischemic pain thresholds and tolerances (in min) were assessed via the submaximal effort tourniquet procedure ( 9 ).
Genotyping
Genomic DNA was purified from 202 subjects using QIAamp™ 96 DNA Blood Kit (Qiagen, Valencia, CA, USA) and used for either 5′exonuclease TaqMan™ PCR for the first five COMT SNPs (rs2097903, rs6269, rs4633, rs4818, rs4680) ( 35 ) or duplex-specific nuclease assays ( 36 ) for SNP rs165599. The NCBI SNP databases were used to assign SNP numbers. SAS proc haplotype was used for haplotype reconstruction.
Assessment of COMT activity corresponding to different haplotypes
Full-length S-COMT cDNA clones corresponding to LPS, APS or HPS haplotypes were obtained from the IMAGE clone collection (Open Biosystems, Huntsville, AL, USA). Clones BG290167 and BG818517 represented LPS haplotype, clones BI821094 and F037202 represented APS haplotype and clones BI759217 and BF035214 represented HPS haplotype. Human embryonic kidney cells ( HEK 293 ) were transiently transfected using SuperFect Reagent (Qiagen). To control the efficiency of transfection, pSV-βGalactosidase vector (Promega, Madison, WI, USA) was cotransfected with the COMT-containing clones. Cells lysates were collected ∼24 h post-transfection. The COMT enzymatic assay was based on the method described by Masuda et al. ( 37 ). Normetanephrine (NMN) amount was measured with a NMN ELISA kit [Immuno-Biological Laboratories, Inc. (IBL-America), Minneapolis, MN, USA]. COMT activity was then normalized for transfection efficiency by measuring the β-galactosidase activity from each lysate using a β-galactosidase enzyme system (Promega). RNA abundance was measured using a Opticon-2 Real Time Fluorescence Detection System (MJ Research, Reno, NV, USA) with a DyNAmo-SYBRGreen qPCR kit (MJ Research).
Statistical analysis of clinical data
Sixteen measures of pain sensitivity were standardized to unit normal deviates ( z -scores) and summed to produce one summary measure of pain responsiveness for each subject. Associations with each of the six SNPs were evaluated for 202 genotyped subjects using ANOVA and Student's t -test (Table 1 ; Supplementary Material, Fig. S1). Independent effects of SNPs were evaluated in a multivariable ANOVA model. Associations between pain responsiveness and haplotypes were assessed in a factorial ANOVA model for 186 subjects carried one of five combinations of the three most prevalent haplotypes. Incidence of newly diagnosed TMD was computed for 170 of those subjects who had one or more follow-up evaluations over the 3 year study period. Incidence rates for two haplotype groups were computed as the number of TMD cases divided by the person-years of follow-up and compared using Poisson regression with the log of person-years as the offset.
Animal behavior and analysis
Sixteen adult male Sprague–Dawley rats (285–325 g; Charles River Laboratories, Wilmington, MA, USA) were placed in plexiglass cages positioned over an elevated perforated stainless-steel platform and habituated to the environment for 15–25 min prior to testing. Paw withdrawal threshold to punctate mechanical stimulation was assessed using the up–down method of Chaplan et al. ( 38 ). Immediately following determination of the response threshold, paw withdrawal frequency (%) to the presentations of a noxious von Frey monofilament was calculated. Thermal hyperalgesia was evaluated using the Hargreaves radiant heat method ( 39 ). After establishing stable baseline responsiveness to mechanical and thermal stimuli, separate groups of rats received OR486 (30 mg/kg i.p.; N =8) or vehicle ( N =8) 1 h prior to behavioral testing. Responsiveness to von Frey filaments was reassessed at 30 min intervals for 2 h. Paw withdrawal latencies to radiant heat were subsequently assessed at 2.5 h into the testing procedure. Behavioral data were analyzed by ANOVA for repeated measures and post hoc comparisons were performed using Bonferroni test.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG Online.
ACKNOWLEDGEMENTS
We would like to thank Ollie Monbureau for developing the hardware and software used for pain phenotyping. We also want to thank Julie A. Clarke for performing the genomic DNA purification. Finally, we would also like to express our thanks to Jurgen Westermann from IBL-Hamburg, Germany, for assisting us with optimizing the Normetanephrine ELISA kit for COMT measurements in cell lysates. Supported by DE07509 and DE007333 (W.M.), AR/AI-44564, 5-P60 AR-30701-14 and AR/AI-44030, NIH Intramural Grants DE00366 and AA000301, the Comprehensive Neuroscience Program Grant USUHS G192BR-C4 and by Attagene's R&D funding.
Figure 1. Distribution of a summary measure of pain sensitivity. A summary measure of pain sensitivity was derived from 16 individual pain measures, each standardized to unit normal deviates ( z -scores) with a mean of 0 and standard deviation of 1. The 16 pain measures were thermal pain threshold conveyed by Aδ afferents and both threshold and tolerance conveyed by C-fiber afferents, all measured in °C at each of three anatomical sites (arm, cheek and foot); tolerance to temporal summation of C-fiber mediated pain (as reported on 0–100 visual analog scale); right arm ischemic pain onset and tolerance (s); and mechanical pain thresholds (kg) assessed over the temporalis and masseter muscles, the temporomandibular joint and the ventral surfaces of wrists. Individuals represented at the extreme left-side of the figure are resistant to pain-evoking procedures whereas individuals represented at the extreme right-side of the figure are most sensitive to pain-evoking procedures.
Figure 2. ( A ) Schematic diagram of COMT genomic organization, SNP positions and percentage distribution. COMT gene locus (chromosome 22, band q11.21) spans for 27 221 bases. ( B ) Schematic diagram of linkage disequilibrium between six SNP markers. Four SNPs, rs6269, rs4633, rs4818 and rs4680 ( val158met) , which occur within the central region of the COMT gene, were found to exhibit strong LDs with the strongest associations found between SNPs rs6269 and rs4818 ( D ′=0.94, R2 =0.88) and between SNPs rs4633 and rs4680 ( D ′=0.96, R2 =0.91). In contrast, SNP rs2097903, located in the 5′ promoter region, and SNP rs165599, located in the 3′-UTR, did not show strong LDs (Supplementary Material, Table S4). Thus, LD analysis demonstrated that the COMT locus covers three haploblocks (B). SNP rs2097903 is situated on the first haploblock, SNPs rs6269, rs4633, rs4818 and rs4680 are situated on the second haploblock and SNP rs165599 is situated on the third haploblock. ( C ) Estimated frequencies of the COMT haplotypes. The sequence of alleles in each haplotype for haploblock 2 reflects the order of occurrence from 5′ to 3′ in the COMT gene (SNPs: rs6269, rs4633, rs4818 and rs4680, respectively). Seven haplotypes out of possible 16 were detected for these four SNPs with the most frequent haplotype (48.7%) composed of the most frequent alleles for all four markers (A_T_C_A for SNPs rs6269, rs4633, rs4818 and rs4680, respectively). The second major haplotype (36.5%) was composed of the least frequent alleles for all four markers (G_C_G_G). The third haplotype (10.5%) was composed of a combination of the most frequent alleles for SNPs rs4633 and rs4680 and the least frequent alleles for SNPs rs6269 and rs4818 (A_C_C_G). These three haplotypes accounted for 95.9% of all detected haplotypes. These findings are consistent with the previously reported LD analysis of the COMT SNPs ( 18 , 19 , 28 ) and with the report of Gabriel et al. ( 21 ) that each haploblock within human genomic DNA is usually represented by three to five major haplotypes.
Figure 3. Pain responsiveness categorized by three major COMT haplotype combinations. LPS: haplotype G_C_G_G, APS: haplotype A_T_C_A, HPS: haplotype A_C_C_G. The greater values reflect greater pain sensitivity. Each value represents the mean z -score with associated SEM.
Figure 4. Effect of haplotype on the COMT activity in transfected HEK 293 cells. HEK 293 cells were transiently transfected with six full-length COMT cDNA clones that corresponded to the three major haplotypes (LPS, APS and HPS). The expression of COMT protein was assessed by measurement of COMT enzymatic activity ( A ) in the lysate from transfected cells. COMT activity was assessed from two independent clones for each haplotype. Two independent ELISAs for each of the two independent transfections were performed for each clone. The graph shows the average values for the eight measurements for each haplotype. The COMT activity was calculated in nanograms of NMN, synthesized during 1 h at 37°C under described enzymatic condition per 10 5 transfected cells. The highest COMT activity was observed for LPS haplotypes ([NMN]=34.3±3.0 ng per 10 5 cells; A), the lower activity was observed for APS haplotype ([NMN]=7.2±1.53 ng per 10 5 cells; A) and HPS haplotypes produced the lowest activity ([NMN]=3.0±2.20 ng per 10 5 cells; A). The relative abundance of COMT RNA ( B ) was assessed by real-time PCR. Each value represents the mean value with associated SEM.
Figure 5. The effect of COMT inhibition on rat pain behavior. Baseline sensitivity to mechanical stimuli was estimated by measuring both threshold ( A ) and frequency ( B ) of paw withdrawal. Sensitivity to thermal stimuli was estimated by measuring latency of paw withdrawal ( C ). After establishing baseline sensitivity, animals received the COMT inhibitor OR486 (30 mg/kg i.p.) or vehicle 1 h prior to testing. Data are expressed as mean±SEM. *** P <0.001, ** P <0.01 and * P <0.05 different from the control conditions by ANOVA and Bonferroni post hoc tests. N =8 rats per group.
Figure 6. Pain sensitivity and TMD incidence by haplotype groupings: HPS/APS versus LPS. Because of the limited number of TMD cases in the cohort, all subjects were subdivided into two groups: LPS and HPS/APS. Subjects were assigned to the LPS group if they carried at least one LPS haplotype. Subjects were assigned to the HPS/APS group if they carried only APS and HPS haplotypes. ( A ) Shows that subjects from the LPS group demonstrated significantly lower pain responsiveness than those from the HPS/APS group ( P =0.02, t -test). ( B ) Shows the number of incidence case per 100 person-years as a function of haplotype group.
Variation in pain sensitivity (summed z -score) among tested SNPs and diplotypes of the COMT gene
| SNPs . | Genotype . | Number of subjects . | Genotypes frequencies . | Mean (SD) z -score . | ANOVA aR2 . | P -value . | t -Test bP -value . |
|---|---|---|---|---|---|---|---|
| rs2097903 | A/A | 68 | 0.340 | −1.4 (10.1) | 0.012 | 0.3 | 0.1 |
| A/G | 104 | 0.520 | 0.2 (11.4) | ||||
| G/G | 28 | 0.140 | 2.2 (8.0) | ||||
| rs6269 | G/G | 31 | 0.153 | −4.7 (9.1) | 0.061 | 0.002 | 0.0006 |
| A/G | 97 | 0.480 | −0.7 (10.5) | ||||
| A/A | 74 | 0.366 | 3.0 (10.7) | ||||
| rs4633 | C/C | 52 | 0.257 | −2.0 (10.5) | 0.013 | 0.26 | 0.09 |
| C/T | 98 | 0.485 | 0.4 (10.9) | ||||
| T/T | 52 | 0.257 | 1.3 (10.2) | ||||
| rs4818 | G/G | 28 | 0.139 | −5.2 (8.0) | 0.07 | 0.0007 | 0.0003 |
| G/C | 100 | 0.495 | −0.9 (10.5) | ||||
| C/C | 74 | 0.366 | 3.2 (10.7) | ||||
| rs4680 | G/G | 51 | 0.252 | −2.1 (10.3) | 0.017 | 0.18 | 0.056 |
| A/G | 102 | 0.505 | 0.2 (10.9) | ||||
| A/A | 49 | 0.243 | 1.7 (10.3) | ||||
| rs165599 | G/G | 27 | 0.138 | −2.4 (8.5) | 0.008 | 0.48 | 0.27 |
| A/G | 87 | 0.446 | 0.4 (11.4) | ||||
| A/A | 81 | 0.415 | −0.2 (10.3) | ||||
| Haplotype combination | ATCA_ACCG | 15 | 0.081 | 8.9 (11.4) | 0.107 | 0.0004 | |
| ATCA_ATCA | 49 | 0.263 | 1.7 (10.3) | ||||
| ATCA_GCGG | 80 | 0.430 | −1.3 (10.2) | ||||
| GCGG_ACCG | 14 | 0.075 | 1.5 (12.3) | ||||
| GCGG_GCGG | 28 | 0.151 | −5.2 (8.0) |
| SNPs . | Genotype . | Number of subjects . | Genotypes frequencies . | Mean (SD) z -score . | ANOVA aR2 . | P -value . | t -Test bP -value . |
|---|---|---|---|---|---|---|---|
| rs2097903 | A/A | 68 | 0.340 | −1.4 (10.1) | 0.012 | 0.3 | 0.1 |
| A/G | 104 | 0.520 | 0.2 (11.4) | ||||
| G/G | 28 | 0.140 | 2.2 (8.0) | ||||
| rs6269 | G/G | 31 | 0.153 | −4.7 (9.1) | 0.061 | 0.002 | 0.0006 |
| A/G | 97 | 0.480 | −0.7 (10.5) | ||||
| A/A | 74 | 0.366 | 3.0 (10.7) | ||||
| rs4633 | C/C | 52 | 0.257 | −2.0 (10.5) | 0.013 | 0.26 | 0.09 |
| C/T | 98 | 0.485 | 0.4 (10.9) | ||||
| T/T | 52 | 0.257 | 1.3 (10.2) | ||||
| rs4818 | G/G | 28 | 0.139 | −5.2 (8.0) | 0.07 | 0.0007 | 0.0003 |
| G/C | 100 | 0.495 | −0.9 (10.5) | ||||
| C/C | 74 | 0.366 | 3.2 (10.7) | ||||
| rs4680 | G/G | 51 | 0.252 | −2.1 (10.3) | 0.017 | 0.18 | 0.056 |
| A/G | 102 | 0.505 | 0.2 (10.9) | ||||
| A/A | 49 | 0.243 | 1.7 (10.3) | ||||
| rs165599 | G/G | 27 | 0.138 | −2.4 (8.5) | 0.008 | 0.48 | 0.27 |
| A/G | 87 | 0.446 | 0.4 (11.4) | ||||
| A/A | 81 | 0.415 | −0.2 (10.3) | ||||
| Haplotype combination | ATCA_ACCG | 15 | 0.081 | 8.9 (11.4) | 0.107 | 0.0004 | |
| ATCA_ATCA | 49 | 0.263 | 1.7 (10.3) | ||||
| ATCA_GCGG | 80 | 0.430 | −1.3 (10.2) | ||||
| GCGG_ACCG | 14 | 0.075 | 1.5 (12.3) | ||||
| GCGG_GCGG | 28 | 0.151 | −5.2 (8.0) |
a ANOVA, analysis of variance testing null hypothesis of equality of means among three alleles.
bt -Test for SNPs is Student's t -test testing null hypothesis of equality of means between homozygous.
Variation in pain sensitivity (summed z -score) among tested SNPs and diplotypes of the COMT gene
| SNPs . | Genotype . | Number of subjects . | Genotypes frequencies . | Mean (SD) z -score . | ANOVA aR2 . | P -value . | t -Test bP -value . |
|---|---|---|---|---|---|---|---|
| rs2097903 | A/A | 68 | 0.340 | −1.4 (10.1) | 0.012 | 0.3 | 0.1 |
| A/G | 104 | 0.520 | 0.2 (11.4) | ||||
| G/G | 28 | 0.140 | 2.2 (8.0) | ||||
| rs6269 | G/G | 31 | 0.153 | −4.7 (9.1) | 0.061 | 0.002 | 0.0006 |
| A/G | 97 | 0.480 | −0.7 (10.5) | ||||
| A/A | 74 | 0.366 | 3.0 (10.7) | ||||
| rs4633 | C/C | 52 | 0.257 | −2.0 (10.5) | 0.013 | 0.26 | 0.09 |
| C/T | 98 | 0.485 | 0.4 (10.9) | ||||
| T/T | 52 | 0.257 | 1.3 (10.2) | ||||
| rs4818 | G/G | 28 | 0.139 | −5.2 (8.0) | 0.07 | 0.0007 | 0.0003 |
| G/C | 100 | 0.495 | −0.9 (10.5) | ||||
| C/C | 74 | 0.366 | 3.2 (10.7) | ||||
| rs4680 | G/G | 51 | 0.252 | −2.1 (10.3) | 0.017 | 0.18 | 0.056 |
| A/G | 102 | 0.505 | 0.2 (10.9) | ||||
| A/A | 49 | 0.243 | 1.7 (10.3) | ||||
| rs165599 | G/G | 27 | 0.138 | −2.4 (8.5) | 0.008 | 0.48 | 0.27 |
| A/G | 87 | 0.446 | 0.4 (11.4) | ||||
| A/A | 81 | 0.415 | −0.2 (10.3) | ||||
| Haplotype combination | ATCA_ACCG | 15 | 0.081 | 8.9 (11.4) | 0.107 | 0.0004 | |
| ATCA_ATCA | 49 | 0.263 | 1.7 (10.3) | ||||
| ATCA_GCGG | 80 | 0.430 | −1.3 (10.2) | ||||
| GCGG_ACCG | 14 | 0.075 | 1.5 (12.3) | ||||
| GCGG_GCGG | 28 | 0.151 | −5.2 (8.0) |
| SNPs . | Genotype . | Number of subjects . | Genotypes frequencies . | Mean (SD) z -score . | ANOVA aR2 . | P -value . | t -Test bP -value . |
|---|---|---|---|---|---|---|---|
| rs2097903 | A/A | 68 | 0.340 | −1.4 (10.1) | 0.012 | 0.3 | 0.1 |
| A/G | 104 | 0.520 | 0.2 (11.4) | ||||
| G/G | 28 | 0.140 | 2.2 (8.0) | ||||
| rs6269 | G/G | 31 | 0.153 | −4.7 (9.1) | 0.061 | 0.002 | 0.0006 |
| A/G | 97 | 0.480 | −0.7 (10.5) | ||||
| A/A | 74 | 0.366 | 3.0 (10.7) | ||||
| rs4633 | C/C | 52 | 0.257 | −2.0 (10.5) | 0.013 | 0.26 | 0.09 |
| C/T | 98 | 0.485 | 0.4 (10.9) | ||||
| T/T | 52 | 0.257 | 1.3 (10.2) | ||||
| rs4818 | G/G | 28 | 0.139 | −5.2 (8.0) | 0.07 | 0.0007 | 0.0003 |
| G/C | 100 | 0.495 | −0.9 (10.5) | ||||
| C/C | 74 | 0.366 | 3.2 (10.7) | ||||
| rs4680 | G/G | 51 | 0.252 | −2.1 (10.3) | 0.017 | 0.18 | 0.056 |
| A/G | 102 | 0.505 | 0.2 (10.9) | ||||
| A/A | 49 | 0.243 | 1.7 (10.3) | ||||
| rs165599 | G/G | 27 | 0.138 | −2.4 (8.5) | 0.008 | 0.48 | 0.27 |
| A/G | 87 | 0.446 | 0.4 (11.4) | ||||
| A/A | 81 | 0.415 | −0.2 (10.3) | ||||
| Haplotype combination | ATCA_ACCG | 15 | 0.081 | 8.9 (11.4) | 0.107 | 0.0004 | |
| ATCA_ATCA | 49 | 0.263 | 1.7 (10.3) | ||||
| ATCA_GCGG | 80 | 0.430 | −1.3 (10.2) | ||||
| GCGG_ACCG | 14 | 0.075 | 1.5 (12.3) | ||||
| GCGG_GCGG | 28 | 0.151 | −5.2 (8.0) |
a ANOVA, analysis of variance testing null hypothesis of equality of means among three alleles.
bt -Test for SNPs is Student's t -test testing null hypothesis of equality of means between homozygous.
Factorial ANOVA model of effects of three major haplotypes on pain sensitivity a
| Source . | Degrees of freedom . | Sum of squares . | Sequential R2 . | F -value . | P -value . |
|---|---|---|---|---|---|
| Haplotype 1 (LPS, HPS) | 1 | 466.1 | 0.022 | 4.5 | 0.04 |
| Haplotype 2 (LPS, APS, HPS) | 2 | 1730.5 | 0.082 | 8.3 | <0.01 |
| Error | 182 | 18 900.5 | |||
| Summary z -score least squares means from model | |||||
| Mean | SE | P -value | |||
| Haplotype 1 | |||||
| LPS | −2.1 | 1.7 | 0.01 | ||
| APS | 2.8 | 1.0 | (Reference) | ||
| Haplotype 2 | |||||
| LPS | −3.5 | 1.1 | <0.01 | ||
| APS | −0.7 | 1.7 | 0.04 | ||
| HPS | 5.3 | 1.9 | (Reference) | ||
| Source . | Degrees of freedom . | Sum of squares . | Sequential R2 . | F -value . | P -value . |
|---|---|---|---|---|---|
| Haplotype 1 (LPS, HPS) | 1 | 466.1 | 0.022 | 4.5 | 0.04 |
| Haplotype 2 (LPS, APS, HPS) | 2 | 1730.5 | 0.082 | 8.3 | <0.01 |
| Error | 182 | 18 900.5 | |||
| Summary z -score least squares means from model | |||||
| Mean | SE | P -value | |||
| Haplotype 1 | |||||
| LPS | −2.1 | 1.7 | 0.01 | ||
| APS | 2.8 | 1.0 | (Reference) | ||
| Haplotype 2 | |||||
| LPS | −3.5 | 1.1 | <0.01 | ||
| APS | −0.7 | 1.7 | 0.04 | ||
| HPS | 5.3 | 1.9 | (Reference) | ||
a Model uses data from 186 subjects who were genotyped and had combination of the three major haplotypes. For the full model F3182 =7.05, P <0.01, R2 =0.104. F ‐values for Haplotype 1 and Haplotype 2 test null hypotheses that haplotype variants have no influence on pain sensitivity after adjustment for the other haplotype. P ‐values for means test null hypotheses that mean z -scores for individual haplotypes are equal to reference-group haplotype (marked ‘Reference’).
Factorial ANOVA model of effects of three major haplotypes on pain sensitivity a
| Source . | Degrees of freedom . | Sum of squares . | Sequential R2 . | F -value . | P -value . |
|---|---|---|---|---|---|
| Haplotype 1 (LPS, HPS) | 1 | 466.1 | 0.022 | 4.5 | 0.04 |
| Haplotype 2 (LPS, APS, HPS) | 2 | 1730.5 | 0.082 | 8.3 | <0.01 |
| Error | 182 | 18 900.5 | |||
| Summary z -score least squares means from model | |||||
| Mean | SE | P -value | |||
| Haplotype 1 | |||||
| LPS | −2.1 | 1.7 | 0.01 | ||
| APS | 2.8 | 1.0 | (Reference) | ||
| Haplotype 2 | |||||
| LPS | −3.5 | 1.1 | <0.01 | ||
| APS | −0.7 | 1.7 | 0.04 | ||
| HPS | 5.3 | 1.9 | (Reference) | ||
| Source . | Degrees of freedom . | Sum of squares . | Sequential R2 . | F -value . | P -value . |
|---|---|---|---|---|---|
| Haplotype 1 (LPS, HPS) | 1 | 466.1 | 0.022 | 4.5 | 0.04 |
| Haplotype 2 (LPS, APS, HPS) | 2 | 1730.5 | 0.082 | 8.3 | <0.01 |
| Error | 182 | 18 900.5 | |||
| Summary z -score least squares means from model | |||||
| Mean | SE | P -value | |||
| Haplotype 1 | |||||
| LPS | −2.1 | 1.7 | 0.01 | ||
| APS | 2.8 | 1.0 | (Reference) | ||
| Haplotype 2 | |||||
| LPS | −3.5 | 1.1 | <0.01 | ||
| APS | −0.7 | 1.7 | 0.04 | ||
| HPS | 5.3 | 1.9 | (Reference) | ||
a Model uses data from 186 subjects who were genotyped and had combination of the three major haplotypes. For the full model F3182 =7.05, P <0.01, R2 =0.104. F ‐values for Haplotype 1 and Haplotype 2 test null hypotheses that haplotype variants have no influence on pain sensitivity after adjustment for the other haplotype. P ‐values for means test null hypotheses that mean z -scores for individual haplotypes are equal to reference-group haplotype (marked ‘Reference’).
References
Mogil, J.S. (
Mogil, J.S., Wilson, S.G., Chesler, E.J., Rankin, A.L., Nemmani, K.V., Lariviere, W.R., Groce, M.K., Wallace, M.R., Kaplan, L., Staud, R. et al. (
Nagasako, E.M., Oaklander, A.L. and Dworkin, R.H. (
Zubieta, J.K., Heitzeg, M.M., Smith, Y.R., Bueller, J.A., Xu, K., Xu, Y., Koeppe, R.A., Stohler, C.S. and Goldman, D. (
Mannisto, P.T. and Kaakkola, S. (
National Ambulatory Medical Care Survey, Report of the Panel on Communicative Disorders and Stroke Council. No: 81-1914. 6-1-1979. Public Health Service, NIH, Washington, D.C.
John, M.T., Miglioretti, D.L., LeResche, L., Von Korff, M. and Critchlow, C.W. (
Von Korff, M., Le Resche, L. and Dworkin, S.F. (
Maixner, W., Fillingim, R., Booker, D. and Sigurdsson, A. (
Maixner, W., Fillingim, R., Sigurdsson, A., Kincaid, S. and Silva, S. (
Marbach, J.J. and Levitt, M. (
Dworkin, S.F., Fricton, J.R., Hollender, L., Huggins, K.H., LeResche, L., Lund, J., Mohl, N., Ohrbach, R., Palla, S.F., Sommers, E.E. et al. (
Carlsson, G.E. and Le Resche, L. (
Yu, X.-M., Hua, M. and Mense, S. (
Mense, S. (
Xie, T., Ho, S.L. and Ramsden, D. (
DeMille, M.M., Kidd, J.R., Ruggeri, V., Palmatier, M.A., Goldman, D., Odunsi, A., Okonofua, F., Grigorenko, E., Schulz, L.O., Bonne-Tamir, B. et al. (
Shifman, S., Bronstein, M., Sternfeld, M., Pisante-Shalom, A., Lev-Lehman, E., Weizman, A., Reznik, I., Spivak, B., Grisaru, N., Karp, L. et al. (
Li, T., Ball, D., Zhao, J., Murray, R.M., Liu, X., Sham, P.C. and Collier, D.A. (
Risch, N.J. (
Gabriel, S.B., Schaffner, S.F., Nguyen, H., Moore, J.M., Roy, J., Blumenstiel, B., Higgins, J., DeFelice, M., Lochner, A., Faggart, M. et al. (
Lotta, T., Vidgren, J., Tilgmann, C., Ulmanen, I., Melen, K., Julkunen, I. and Taskinen, J. (
Azzam, A. and Mathews, C.A. (
Glatt, S.J., Faraone, S.V. and Tsuang, M.T. (
Duan, J., Wainwright, M.S., Comeron, J.M., Saitou, N., Sanders, A.R., Gelernter, J. and Gejman, P.V. (
Abiola, O., Angel, J.M., Avner, P., Bachmanov, A.A., Belknap, J.K., Bennett, B., Blankenhorn, E.P., Blizard, D.A., Bolivar, V., Brockmann, G.A. et al. (
Drysdale, C.M., McGraw, D.W., Stack, C.B., Stephens, J.C., Judson, R.S., Nandabalan, K., Arnold, K., Ruano, G. and Liggett, S.B. (
Bray, N.J., Buckland, P.R., Williams, N.M., Williams, H.J., Norton, N., Owen, M.J. and O'Donovan, M.C. (
Davidson, S. (
Khasar, S.G., Green, P.G., Miao, F.J. and Levine, J.D. (
Jaeger, B. and Reeves, J.L. (
Fruhstorfer, H., Lindblom, U. and Schmidt, W.G. (
Price, D.D., Hu, J.W., Dubner, R. and Gracely, R.H. (
Vierck, C.J., Jr, Cannon, R.L., Fry, G., Maixner, W. and Whitsel, B.L. (
Shi, M.M., Bleavins, M.R. and de la Iglesia, F.A. (
Shagin, D.A., Rebrikov, D.V., Kozhemyako, V.B., Altshuler, I.M., Shcheglov, A.S., Zhulidov, P.A., Bogdanova, E.A., Staroverov, D.B., Rasskazov, V.A. and Lukyanov, S. (
Masuda, M., Tsunoda, M., Yusa, Y., Yamada, S. and Imai, K. (
Chaplan, S.R., Pogrel, J.W. and Yaksh, T.L. (


![Figure 4. Effect of haplotype on the COMT activity in transfected HEK 293 cells. HEK 293 cells were transiently transfected with six full-length COMT cDNA clones that corresponded to the three major haplotypes (LPS, APS and HPS). The expression of COMT protein was assessed by measurement of COMT enzymatic activity ( A ) in the lysate from transfected cells. COMT activity was assessed from two independent clones for each haplotype. Two independent ELISAs for each of the two independent transfections were performed for each clone. The graph shows the average values for the eight measurements for each haplotype. The COMT activity was calculated in nanograms of NMN, synthesized during 1 h at 37°C under described enzymatic condition per 10 5 transfected cells. The highest COMT activity was observed for LPS haplotypes ([NMN]=34.3±3.0 ng per 10 5 cells; A), the lower activity was observed for APS haplotype ([NMN]=7.2±1.53 ng per 10 5 cells; A) and HPS haplotypes produced the lowest activity ([NMN]=3.0±2.20 ng per 10 5 cells; A). The relative abundance of COMT RNA ( B ) was assessed by real-time PCR. Each value represents the mean value with associated SEM.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/hmg/14/1/10.1093_hmg_ddi013/2/m_ddi01304.jpeg?Expires=1716360442&Signature=4khflCC1fT2TZn9e6MfBxUlgu36~Mj5Rrt4phh9WejLmclM-lYVHhAeYyEHXc-~Pg8RpFfQcYuVPAE3~BRVI5EpDw8hnB9oT2Z76YjmsCzoLaCqB8I2GnfcZZ0bnJkBTz3QhI9PIXooeigs8ucJDu3zLp9Vq2EO8RmnOeDNmxv8vdyS4RB1wt5WefWHiewLIuIATNPh3UClgLrz01MVecSR1vkb4uR18WIvV0AcZjUf35nHQYZ~KvyCszfm~j4DMzrNJj-HzdnOeMSLHAX3-KxkXyPXO67Qf1dubKPKOCSKMCFlC6rSET0HG~dNd7RbclQVNBfzFqbRxmuVKT9Yapw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)