-
PDF
- Split View
-
Views
-
Cite
Cite
Yahya Tamimi, Jonathan M. Skarie, Tim Footz, Fred B. Berry, Brian A. Link, Michael A. Walter, FGF19 is a target for FOXC1 regulation in ciliary body-derived cells, Human Molecular Genetics, Volume 15, Issue 21, 1 November 2006, Pages 3229–3240, https://doi.org/10.1093/hmg/ddl400
Close - Share Icon Share
Abstract
The forkhead C1 (FOXC1) transcription factor is involved in the development and regulation of several organs, including the eye, where FOXC1 alterations cause iris, trabecular meshwork and corneal anomalies. Using nickel agarose chromatin enrichment with human anterior segment cells, we previously identified the fibroblast growth factor 19 (FGF19) locus as a gene potentially regulated by FOXC1. Here, we demonstrate that FGF19 is a direct target of FOXC1 in the eye. FOXC1 positively regulates FGF19 expression in corneal and periocular mesenchymal cells in cell culture and in zebrafish embryos. Through the FGFR4 tyrosine kinase, FGF19 promotes MAPK phosphorylation in the developing and mature cornea. During development, loss of either FOXC1 or FGF19 results in complementary, but distinct, anterior segment dysgeneses. This study reveals an important role for FOXC1 in the direct regulation of the FGF19–FGFR4-MAPK pathway to promote both the development and maintenance of anterior segment structures within the eye.
INTRODUCTION
Glaucoma is a complex eye disorder characterized by degeneration of the optic nerve, leading first to the loss of peripheral and then to central vision and ultimately to total blindness. Significantly, glaucoma ranks as one of the leading cause of irreversible blindness with seventy million people affected worldwide (1–3). Because of the subtly progressive nature of glaucoma, patients often ignore the beginning signs of the disease, which typically lead to late detection and irreversible visual impairment. Glaucoma has been shown to be linked to inherited malformations of the eye, with 20–60% penetrance in patients (4). Axenfeld–Rieger (AR) syndrome is a disorder affecting the development of the anterior segment of the eye and is strongly associated with glaucoma. Two genes encoding transcription factors, PITX2 and FOXC1, are involved in the AR malformation phenotype (5–7). Other AR genes have been mapped to chromosomes 13q14, and 16q24, but remain unidentified (8,9).
The FOXC1 gene (formerly FKHL7), is a member of the forkhead box (FOX) family of transcription factors and maps to chromosome 6p25 (6,7,10). FOXC1 is a single exon gene of 1.6 kb encoding a 553 amino acid protein (11). A well-conserved 110 amino acid DNA-binding domain characterizes FOX family members. The FOX DNA-binding motif is a variant of the helix–turn–helix motif, composed of three α-helices and two large loops that form the ‘wing’ structure (12). This domain is indispensable for DNA recognition and transcription initiation/repression. Analysis of FOXC1 forkhead domain mutations revealed that missense mutations reduce FOXC1 transactivation ability, protein stability and DNA binding (13–15). In mice, the homozygous null (Foxc1−/−) mutant is lethal in the perinatal period with severely abnormal development of the anterior segment of the eye, congenital hydrocephalus and skeletal defects (16). Moreover, targeted deletions of Foxc1 in mice have shown the important role of the protein in vertebrate mesoderm-derived tissues, including the formation of the kidney, eye, heart, bone and cartilage (16–18). Therefore, investigating genes under the control of FOXC1 is a crucial step to learn more about development and diseases that result when the function of FOXC1 is impaired.
We have recently developed the nickel agarose chromatin enrichment (NACE) technique, a modified form of chromatin immunoprecipitation (ChIP) that allows for the enrichment of chromatin fragments bound by epitope-tagged transcription factors (19). To identify potential FOXC1 target genes, we used non-pigmented ciliary epithelial (NPCE) cells, which express endogenous FOXC1, and are derived from tissues responsible for aqueous humor secretion (19). In this report, we describe a functional role of an NACE-enriched candidate FOXC1 target gene, fibroblast growth factor 19 (FGF19). FGF19 is a secreted 24 kDa heparin-dependant signaling molecule that exclusively binds to the FGFR4 receptor and signals through the MAPK pathway (20). We identified a FOXC1 regulatory element upstream of FGF19 and show that FOXC1 binding increases the transcriptional activity of this promoter. Importantly, we validated FGF19 as a FOXC1 target in the zebrafish model system by demonstrating that expression of fgf19 is down-regulated in zebrafish subjected to morpholino antisense oligonucleotide knockdown of the zebrafish FOXC1 homologs, foxC1.1 and foxC1.2. Investigation into the function of FGF19 in developing and mature corneal endothelial (CE) cells points to a new pathway where FOXC1 regulates development and maintenance of the anterior segment through FGF19/FGFR4-directed MAPK activation.
RESULTS
NACE identification of FGF19 as a possible target of FOXC1
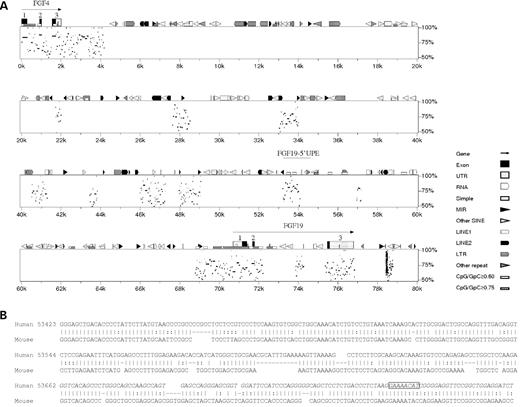
To study FOXC1's regulation of gene expression within the anterior chamber of the eye, we chose the human ciliary body-derived NPCE cells that endogenously express FOXC1 (19). Chromatin fragments less than 2 kb in size were generated by sonication, enriched by NACE and then sequenced. Among potential candidate genes obtained, a 1.3 kb fragment was identified that is located ∼17 kb upstream from the start codon of the fibroblast growth factor 19 gene, FGF19 (Fig. 1A). A percent identity plot (PIP) (21) was generated comparing 80 kb of human genomic sequence (hChr11; GenBank accession no. NT_078088) to 40 kb of the homologous region in mouse (mChr7; GenBank accession no. AC161763). As shown in Figure 1A, there are nine distinct regions of homologous sequence, among the interspersed repeat elements, between FGF19 and the nearest upstream neighboring gene, FGF4. None of these regions match known genes or ESTs and are therefore assumed to be non-coding regulatory sequences. The NACE-enriched 1.3 kb fragment overlaps one of these homologous regions that is ∼68% identical to sequence upstream of mouse fgf15 (Fig. 1B), the mouse homolog of human FGF19 (22). This finding is consistent with the suggestion that the function of the FOXC1 NACE-selected upstream region of FGF19 is conserved in humans and mice.
Homology between human FGF19 and mouse fgf15. (A) PIP detailing the organization of conserved sequences and interspersed repeat elements near the FGF19 locus. Sequences conserved between human and mice were discovered in exons (coding) and also in introns and the intergenic space (both non-coding) between FGF4/fgf4 and FGF19/fgf15. The NACE-enriched 1.3 kb FGF19-5′-UPE fragment is shown at the ∼53–54k position, overlapping a large conserved non-coding region. The cluster of hits at ∼78.5k is due to a microsatellite repeat (CTTT)n with multiple coincidental occurrences in the mouse genomic sequence analyzed. (B) Comparison of the 354 bp FGF19-RE to the mouse fgf15 upstream sequence shows ∼68% sequence identity. The alignment was extracted from the PIPMaker raw data. The position of the 100 bp ChIP-PCR fragment (Fig. 3B) is in italics. The putative FOXC1-binding site is boxed.
FOXC1 activates transcription from the FGF19 5′-upstream element
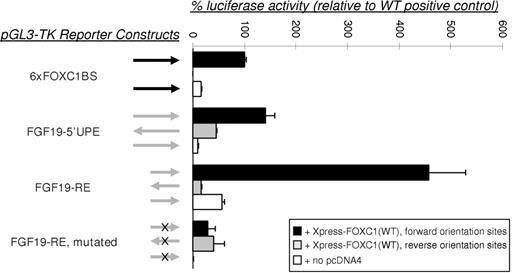
To determine whether the NACE-enriched FGF19 fragment possessed FOXC1 responsive elements, we cloned the 1.3 kb upstream element (FGF19-5′-UPE) into the pGL3-TK-luciferase vector (13) and monitored luciferase activity, after co-transfecting COS-7 cells with pcDNA4-FOXC1 and FGF19-5′-UPE-pGL3-TK expression vectors. A reporter construct containing six consensus FOXC1-binding sites (6×FOXC1BS) (13) was used as a positive control. Xpress-tagged FOXC1 significantly enhanced the expression from the FGF19-5′-UPE luciferase reporter (Fig. 2). The FGF19-5′-UPE cloned in the reverse orientation resulted in markedly reduced reporter activation (∼50% of control), indicating that the 5′-UPE contains a uni-directional FOXC1-responsive element (FGF19-RE) (Fig. 2).
Transactivation assays of the FGF19 promoter region. The FGF19-5′-UPE (1.3 kb) and FGF19-RE (354 bp) (Fig. 1) were cloned into the pGL3-TK-luciferase vector, upstream of the TK promoter, and were co-transfected with pcDNA4-FOXC1 expression vector and pRL-CMV control plasmid into COS-7 cells. Arrows indicate the orientation of the subcloned binding site fragments. Dual-luciferase activity ratios were calculated relative to Xpress-tagged FOXC1 activation of the 6×FOXC1BS reporter. The error bars represent the relative standard error of the mean. The FGF19-5′-UPE promotes reporter activation at a level similar to the 6×FOXC1BS control and activation from the FGF19-RE is more than 4-fold higher, whereas activation from the mutated FGF19-RE approaches background levels of the system.
Validation of a putative FOXC1-RE within the FGF19-5′-UPE
On the basis of the core DNA-binding sequence for FOX transcription factors (AAAYA) (23), we selected a closely related sequence (GAAAACAT) within a 354 bp amplicon in the FGF19-5′-UPE as a putative responsive element (FGF19-RE) able to bind FOXC1 protein (Fig. 1B). To confirm that this site was mediating the FGF19 response to FOXC1, mutations of the selected sequence were introduced within the FGF19-RE. Changing the sequence (GAAAACAT) to (AGGGGTGC) completely abolished the putative responsive site. This mutated version of the FGF19-RE was cloned into the pGL3-TK-luciferase reporter (13) and examined by transactivation assays. In COS-7 cells, FOXC1 was able to significantly activate luciferase expression from the reporter containing the FGF19-RE (Fig. 2). Interestingly, this reporter was activated by FOXC1 to a level of ∼460% of that from the reporter containing the positive control 6×FOXC1BS (Fig. 2). In contrast, mutation of the putative FOXC1-binding site in the FGF19-RE completely abolished the FOXC1 responsiveness of the construct, demonstrating that the 5′-GAAAACAT-3′ site within the FGF19-5′-UPE mediated the FOXC1 response (Fig. 2). These results indicate that FGF19 contains a functional FOXC1 responsive element within the NACE-selected upstream region of FGF19.
FOXC1 binds to the FOXC1-RE within the FGF19 promoter
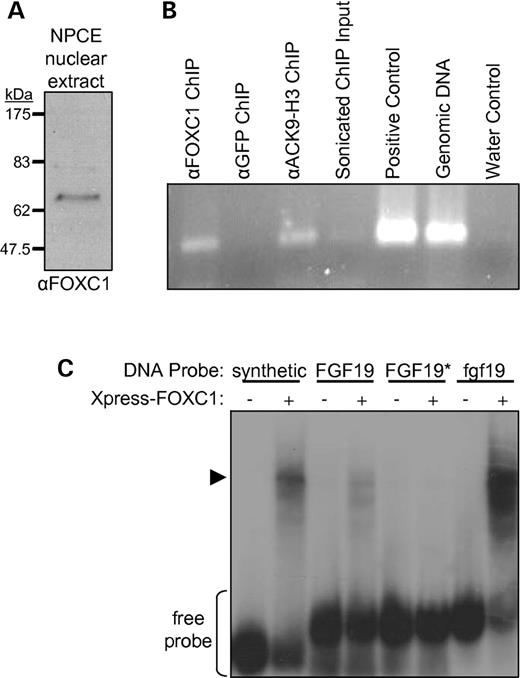
To determine whether endogenous FOXC1 could bind to the FGF19 promoter in vivo, we performed ChIP assays in NPCE cells using antibodies recognizing endogenous FOXC1. Immunoblot analysis of NPCE nuclear extract was performed to confirm the specificity of the anti-FOXC1 antibody to be used in ChIP experiments. As illustrated in Figure 3A, this antibody detected a single band at an apparent molecular weight of 62 kDa, consistent with the predicted molecular weight of FOXC1. As indicated in Figure 3B, a 100 bp region of the FGF19-RE containing the putative FOXC1-binding site (Fig. 1B) was amplified from FOXC1 and histone-H3-acetylated-lysine-9 immunoprecipitates, indicating that endogenous FOXC1 binds to the FGF19 promoter region.
FOXC1 specifically binds to sequences in the FGF19/fgf19 promoters. (A) Western analysis of FOXC1 protein expression in NPCE nuclear extract. (B) The 100 bp fragment of the FGF19-5′-UPE (Fig. 1) was PCR-amplified from sonicated chromatin purified by antibodies to FOXC1 or to histone H3 acetylated at position lysine 9 (ACK9-H3), but not from a control sample using antibodies to GFP. The positive control template was the 1.3 kb FGF19-5′-UPE cloned in pGL3-basic. Sonicated ChIP input is a 1:80 000 dilution of cross-linked chromatin used for immunoprecipitations. (C) EMSA to test direct binding of recombinant FOXC1 to potential target sequences: a ‘synthetic’ in vitro-selected control binding site, the FOXC1-binding site from the ‘FGF19’-RE, the same element containing alterations (asterisk) to the FOX core DNA-binding sequence and a computer-predicted putative binding site upstream of the zebrafish ‘fgf19’ ORF. −, untransfected COS-7 lysate; +, lysate from COS-7 cells transfected with plasmid expressing Xpress-tagged FOXC1.
Lysates containing recombinant FOXC1 were prepared to evaluate direct binding of FOXC1 to potential target sequences. Radioactive 32P-labeled 30 bp oligomers were prepared that correspond to the putative FOXC1-binding site in the FGF19-RE, the altered binding site used in the transactivation study (discussed earlier and Fig. 2) and a potential binding site upstream of the zebrafish fgf19 open-reading frame (Materials and Methods). In an electrophoretic mobility shift assay (EMSA) (Fig. 3C), Xpress-tagged FOXC1-containing lysates were able to specifically shift a portion of the synthetic in vitro-preferred binding site probe (13), as well as the probes for the FGF19-RE and fgf19. However, FOXC1 lysates were unable to detectably shift the probe containing the alterations of the FGF19-RE that greatly reduce transactivation responsiveness (Fig. 2).
Mutation screening of FGF19 in patients with ar/anterior segment dysgenesis
Approximately 60% of AR patients do not have mutations of any of the known AR genes (24). To investigate whether FGF19 was mutated in AR patients, all three exons of FGF19 were directly sequenced in a cohort of 45 AR/anterior segment dysgenesis patients. A single base substitution (CTG>TTG) was observed in 16 individuals, all heterozygous for the change. Hardy–Weinberg equilibrium analysis indicated that the observed allele frequency did not deviate from the expected results. This substitution is a silent change (L18L), unlikely to be pathogenic. Therefore, mutation of FGF19 does not appear to be a significant cause of human anterior segment disorders.
Validation of the role of FOXC1 in regulating FGF19 expression in zebrafish
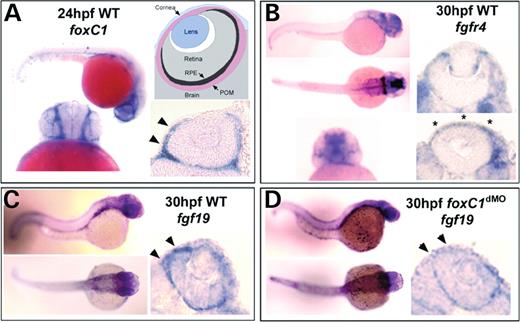
Zebrafish is emerging as a powerful model organism to study the fundamental mechanisms that underlie human diseases including glaucoma (25). The accessibility to large numbers of embryos and relative ease in which gene expression can be extinguished by morpholino oligonucleotide (MO) knockdown makes this system an attractive candidate to validate expression of FOXC1 target genes in vivo. In zebrafish, foxC1 is duplicated and both foxC1.1 and foxC1.2 transcripts are expressed in periocular mesenchyme (POM) and developing anterior segment structures (25). Previous studies in zebrafish have investigated the consequences of loss of foxC1.1 and foxC1.2 during somitogenesis (26). In that study, two non-overlapping MOs specific for each foxC1 transcript were found to efficiently block foxC1 translation. We have used these same MOs to investigate ocular phenotypes associated with loss of foxC1 in the zebrafish and to investigate the in vivo regulation of fgf19 by FoxC1. To confirm in vitro experiments showing fgf19 as a target of FoxC1, levels of fgf19 transcripts were assessed by qRT-PCR in 48 h post-fertilization (hpf) foxC1.1/foxC1.2 (foxC1dMO) morphant zebrafish and compared with control MO-injected sibling fish. To enrich for ocular-expressed transcripts, tissue from embryonic eyes and surrounding POM was collected separately for analysis. Total RNA from three pools of 100 dissected eyes was isolated for both foxC1dMO and control morphants. Analysis revealed a significant 2.5-fold decrease in fgf19 levels in foxC1dMO morphant eyes, when compared with controls (P=0.01). Expression levels of fgf19 were also assessed by whole-mount in situ hybridization analysis. Groups of 10–15 embryos were assessed which, upon sectioning, revealed consistent, robust expression within the retinal pigmented epithelium and the anterior POM, along with lower expression levels throughout the retina (Fig. 4). The only area of foxC1 and fgf19 co-expression in ocular structures was within the anterior POM (Fig. 4). Consistent with cell culture and qRT-PCR studies, a reduction in fgf19 expression was observed in the anterior POM by in situ analysis of all foxC1dMO morphants analyzed (Fig. 4). Levels of fgf19 in other ocular structures were not affected.
Expression of foxC1, fgf19 and fgfr4 (the receptor for Fgf19). (A) Expression of foxC1 at 24 hpf. Note the strong POM expression adjacent to the eyes (arrowheads). The model shows an ocular section with sub-components labeled. (B) Expression of fgfr4 in a 30 hpf wild-type embryo and eyes. Expression is found in areas of the POM, retina ciliary margin, equatorial regions of the lens and the cornea. Asterisks in (B) denote corneal expression of fgfr4. (C) Whole embryo and ocular expression of fgf19 in 30 hpf zebrafish embryos. Note the enriched expression in the RPE, throughout retina, and in anterior POM (arrowheads). (D) Expression of fgf19 in 30 hpf foxC1.1/foxC1.2 (16 ng/embryo) morphant embryos and eyes. Note the reduced expression in the POM (arrowheads). Pigmentation was inhibited by PTU in all embryos analyzed by in situ hybridization.
Disruption of Foxc1 or Fgf19 expression results in ocular anterior segment dysgenesis in zebrafish
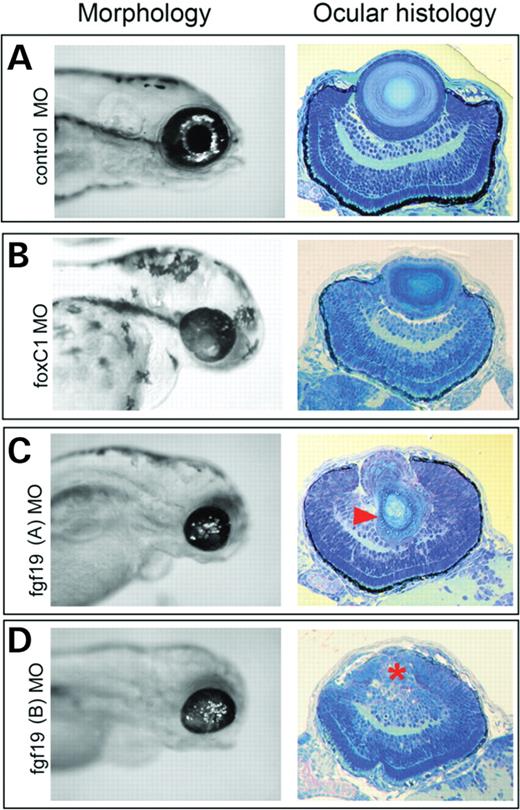
The foxC1 MOs described earlier and another against fgf19 (27) were used to investigate the roles of these genes in ocular morphogenesis. Whole-mount analysis of foxC1 morphants showed a small eye phenotype (Fig. 5B). Histological inspection showed reduced cellular differentiation in the POM and delayed retinal lamination (Fig. 5B). Non-ocular phenotypes included hydrocephaly and defects in neural crest derived structures (28). As previously described, fgf19 knockdown results in severe forebrain defects by 24 hpf, which prevents the eyes from forming (27). To characterize the role of Fgf19 during ocular development, we performed injections with various concentrations of fgf19 MO. Concentrations of 3.2 ng fgf19 MO per embryo resulted in either anophthalmia or severe microphthalmia, consistent with the observations of Miyake et al. (27) (data not shown). However, fgf19 MO injections at 2.1 ng per embryo, which mimics the reduced expression of fgf19 in foxC1dMO morphants, permitted specification and initiation of ocular development (Fig. 5C and D). However, the eyes of fgf19 morphants were still smaller in size, showed delayed retinal lamination and exhibited dysmorphic anterior segment structures similar to foxC1dMO morphants (Fig. 5C). However, in contrast to foxC1dMO morphants, the lenses of fgf19 morphants were often absent or showed severe polarization with posterior morphogenesis defects (Fig. 5D).
Morphology and ocular histology of control, foxC1 and fgf19 morphants. Whole-view morphology (left panels) and ocular histology (right panels) of 72 hpf zebrafish embryos. (A) Control MO-injected embryos (16 ng/embryo), (B) foxC1.1+foxC1.2 MO-injected (16 ng/embryo) and (C and D) fgf19 MO-injected embryos (2.1 ng/embryo) with (C) dysmorphic lens (arrowhead) and (D) aphakic (lack of lens) phenotype (asterisk). Both foxC1.1+foxC1.2 MO-injected and fgf19 MO-injected embryos showed 100% penetrance for anterior segment dysgenesis. For fgf19 morphants, although anterior segment dysgenesis was completely penetrant, the expressivity of the lens dysmorphogenesis was variable. For the fgf19 morphant embryos sectioned, 8/12 were aphakic or showed severely dysmorphic lenses. Survival after 24 hpf for each condition was: control MO, 117/130; foxC1.1+foxC1.2 MO, 57/86; fgf19 MO, 148/175 (combined n from three independent experiments).
FGFR4 expression in the anterior chamber of the eye
FGF19 acts uniquely through the transmembrane protein-tyrosine kinase fibroblast growth factor receptor 4 (FGFR4) (20). To investigate functional roles of secreted ciliary body-derived FGF19, we assessed FGFR4 expression in the anterior chamber of the developing zebrafish eye using in situ hybridization. High levels of fgfr4 mRNA were found within the cornea, the proliferative marginal zones of the lens, throughout the retina, and in the POM (Fig. 4). The expression of FGFR4 within a human corneal cell line (data not shown) and within the developing zebrafish cornea suggests a possible FOXC1-mediated FGF19/FGFR4 signaling pathway within the cornea.
The FGF19–FGFR4 pathway mediates the ERK1/2 signaling cascade in the cornea
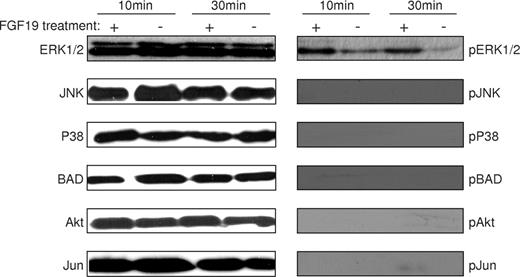
Binding of FGF19 to FGFR4 has been shown to result in signaling through MAPK activation in the liver (29). To determine whether FGF19 can activate the FGFR4 receptor, and consequently, the downstream MAPK cascade in the anterior segment of the eye, human corneal endothelium (CE) cells that endogenously express FGFR4 protein (data not shown) were treated with recombinant FGF19 protein. Antibodies that specifically recognize Thr180/Tyr182 phospho-p38, Thr202/Tyr204 phospho-p44/42 (ERK1/2), Ser473 phospho-Akt, Ser136 phospho-BAD, Thr183/Tyr185 phospho-SAPK/JNK and Ser63 phospho-Jun were used to test for protein kinase activation. Non-phosphorylated forms of these proteins were used as references. FGF19 treatment increased the relative abundance of phosphorylated ERK1/2 in CE cells, as shown in Figure 6. Phosphorylated forms of the other protein kinases were not detected. These results suggest that ERK1/2 is a key element in the FOXC1-FGF19–FGFR4 signaling pathway.
Activation of ERK1/2 by FGF19. Human recombinant FGF19 was used to stimulate human CE cells to determine MAPK response. Cells were either treated with (+) or without (−) human recombinant FGF19 and were harvested after 10 or 30 min of incubation. Antibodies recognizing phospho-p38, phospho-ERK1/2, phospho-Akt, phospho-BAD, phospho- SAPK/JNK and phospho-Jun were used to detect kinase activity. Non-phosphorylated forms of these proteins were used as references.
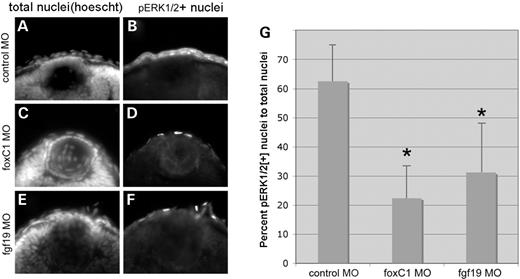
To study the relevance of the FOXC1 signaling pathway in vivo, activation of ERK1/2 was studied in the cornea of the developing zebrafish. At 50 hpf, the corneas of wild-type zebrafish showed high levels of ERK1/2 activation, as measured by the percentage of corneal nuclei with phosphoERK1/2 immunoreactivity (Fig. 7). In comparison to control MO-injected embryos, the proportion of corneal cells with nuclear phosphoERK1/2 was reduced to 38% in foxC1dMO morphants and 60% in fgf19 morphants expressed as a fraction of total corneal Hoechst stained nuclei (Fig. 7).
MAPK activity in the developing cornea. Total nuclei (Hoechst, left panels) and immunohistochemistry for MAPK activation (phosphoERK1/2, right panels) in 50 hpf zebrafish embryos. (A and B) Control morphant (16 ng), (C and D) foxC1.1+foxC1.2 MO morphant (16 ng) and (E and F) fgf19 morphant (2.1 ng) embryos. (G) Graph of proportion of total cornea nuclei positive for pERK1/2 in each condition. Note the reduction in proportion of pERK1/2-positive nuclei in foxC1 and fgf19 morphants when compared with control morphants. *P<0.01 versus control MO (Student's t-test). For each condition, n=8 embryos.
DISCUSSION
Techniques monitoring global changes in gene expression, such as microarray expression analysis, PCR differential display and serial analysis of gene expression, are unable to identify transcription factor-targeted promoters (30). Thus, ChIP technology has emerged as a robust option to isolate specific gene regulatory sequences from the complexes formed by the interaction of transcription factors and chromatin (31). We have previously demonstrated that the ChIP-derived technique NACE is useful to enrich chromatin in the absence of a specific antibody to a transcription factor of interest and have specifically used NACE to enrich for candidate genes regulated by Xpress-tagged FOXC1 (19). Our NACE data indicated that FOXC1 target genes are involved in a broad range of activities, including cell signaling, regulation, development, differentiation and malignancy (19).
Among the potential FOXC1 targets obtained by NACE, a 1.3 kb FGF19 5′-upstream element (FGF19-5′-UPE) was particularly interesting. FOXC1 and FGF19 are co-expressed in a variety of human tissues, including the eye (19). The NACE-enriched fragment located upstream of FGF19 was conserved between human and mice (Fig. 1) and represents one of the most proximal upstream non-coding putative regulatory regions. Our data here demonstrate that the FGF19-5′-UPE is sufficient to allow FOXC1-dependant activation of gene expression in transactivation assays and led to the identification of a bona fide FOXC1 responsive element within a 354 bp fragment of the FGF19-5′-UPE (Fig. 2) which contains a DNA sequence to which FOXC1 can directly bind (Fig. 3C). Endogenous FOXC1 binds directly to a sequence in the FGF19 upstream element (Fig. 3B) and mutagenesis of the FOXC1 responsive element drastically reduced FOXC1-dependent reporter activation (Fig. 2). Potential FOXC1-binding cis-elements upstream of zebrafish fgf19 were computationally detected using the Possum (32) website. Recombinant FOXC1 was shown to directly bind one of these fgf19 upstream elements (Fig. 3C), suggesting that FoxC1-dependent regulation of fgf19 is conserved in zebrafish.
Human FGF19 is located on chromosome 11, in a region associated with skeletal and retinal defects (20). FGF19 is expressed in several tissues, including the fetal brain, cartilage, skin and adult gall bladder, and is over-expressed in colon cancer cell lines (20,33). Interestingly, both FGF19 and its receptor FGFR4 are expressed in the developing and adult retina (20,34). Since FGF19 is a direct target of FOXC1, a known cause of the human ocular disease AR syndrome (6,7), we tested whether mutations of FGF19 were also associated with human anterior segment defects. Our analyses of a panel of 45 patients revealed a silent L18L alteration, but no disease-associated mutations, indicating that mutation of FGF19 is not likely to be a significant cause of human AR and/or anterior segment dysgenesis.
Zebrafish embryos were used as an in vivo model to assess the regulation of FGF19 by FOXC1. Upon morpholino knockdown of FoxC1 activity, a 2.5-fold reduction in fgf19 transcript expression was found in 48 hpf dissected ocular tissue by qRT-PCR analysis, when compared with controls. This reduction is consistent with predictions from the in vitro experiments and is further supported by whole-mount in situ hybridization analysis. In foxC1dMO embryos, a decrease in fgf19 transcript was found in the anterior POM; the only ocular area where both genes are co-expressed (Fig. 4). Along with the promoter analysis, these data provide compelling evidence that FOXC1 is a functional regulator of FGF19.
Ocular dysgenesis in foxC1dMO and fgf19 morphants is consistent with a role of FoxC1 in regulating fgf19 within the POM. Both morphants displayed a small eye phenotype and delayed retinal lamination, along with a lack of cellular differentiation within the POM. Interestingly, fgf19 morphants also showed severe defects in lens development. The lens was often absent or displayed a severe posterior dismorphogenesis. In contrast, no lens defects were found in foxC1 morphants. These findings correlate well with the observed expression patterns of the foxC1 transcripts, fgf19, and its receptor fgfr4 (Fig. 4). Defects in POM and corneal differentiation were observed in both foxC1 and fgf19 morphants, where all genes were co-expressed. These shared phenotypes, our qRT-PCR analyses and in situ hybridization results, coupled with in vitro experiments suggest that FoxC1 regulates the transcription of fgf19 that activates Fgfr4 and facilitates normal anterior segment development.
Our comparison of foxC1 and fgf19 morphants, however, also revealed POM- and FoxC1-independent functions of Fgf19. In foxC1 morphants, fgf19 was down regulated in the POM, but not in the neural retina. No lens defects were induced in foxC1 morphants. We therefore attribute the lens defects in fgf19 morphants to the loss of fgf19 expression in the neural retina that activates Fgfr4 present on the lateral margins of the lens epithelium.
Following activation by FGF ligands, FGF tyrosine kinase receptors activate downstream components and initiate signal transduction (35,36). MAPK is a prominent component of many FGF mediated signal transduction events (29). The formation of an FGF19/FGFR4 complex induces receptor dimerization and subsequent phosphorylation of tyrosine kinases, leading to a variety of different signal transduction pathways. Induction assays on CE cells using recombinant FGF19 were performed to investigate the effect of FGF19 on different MAPK signaling pathways in the cornea. Among the MAPKs tested, only ERK1/2 was phosphorylated in CE cells in response to exposure to FGF19 (Fig. 6), suggesting that the specific FGF19/FGFR4 interaction is recruiting ERK1/2 for signaling. These results were confirmed in zebrafish, where reduced ERK1/2 activation was found in both foxC1dMO and fgf19 morphants (Fig. 7). Interestingly, ERK1/2 activation in foxC1dMO morphants was reduced further than that in the fgf19 morphants. This may reflect synergistic effects of multiple FoxC1 targets in addition to Fgf19, which affect MAPK signaling.
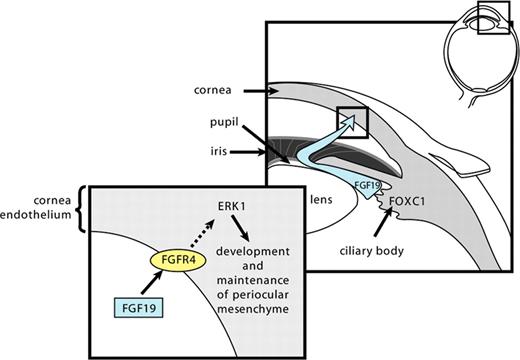
We have shown that FOXC1-regulated FGF19–FGFR4-MAPK signaling is important for proper development and maintenance of anterior segment structures. These pathways have been previously shown to be involved in cell survival, proliferation, differentiation and the inflammatory response (29,37,38). In the cornea, the ERK1/2 pathway is involved in tight junction formation and maintenance and proper FOXC1 gene dosage is required for proper development (39). Mouse embryos homozygous for null mutations in Foxc1 displayed severe abnormalities of the anterior segment, particularly in the cornea, due to abnormal development where a failure of separation occurs between the cornea and the lens (17). Moreover, corneal stroma in these mutants is disorganized with a thicker epithelium when compared with normal (17). Importantly, the increased dosage of FOXC1 also results in increased central corneal thickness and cellular hyperplasia in humans (40). Our results reveal that FOXC1 expression in ocular structures can alter expression of genes in the cornea through a novel FOXC1-FGF19–FGFR4-MAPK pathway (Fig. 8). In addition to discovering a key aspect of FOXC1 function in the anterior chamber of adult eye, our findings illustrate a possible mechanism that could underlie FOXC1-associated corneal phenotypes.
A proposed model for FOXC1 regulation of gene expression in the cornea through a novel FOXC1-FGF19–FGFR4-ERK1/2 signaling pathway. FOXC1 is expressed in the ciliary body, where, by activating the expression of FGF19, allows FGF19 to circulate within the aqueous humor to tissues of the anterior chamber of the eye. FGF19 binds to the FGFR4 in the CE and triggers the ERK1/2 signaling pathway.
MATERIALS AND METHODS
Nomenclature
FOXC1 has been previously named FKHL7 and FREAC3 in humans and Mf1 in mice. In zebrafish, FoxC1 is duplicated, with both foxC1.1 (FoxC1a) and foxC1.2 (FoxC1b) present. The double foxC1.1/foxC1.2 morpholino knockdown zebrafish embryos are signified by foxC1dMO throughout the text. The mouse homolog of human FGF19 is Fgf15, and this gene (fgf19) is not duplicated in zebrafish.
Cell culture and transient transfection
Cell lines were cultured in media containing 50 U/ml penicillin and 50 µg/ml streptomycin, at 37°C in a 5% CO2, humidified incubator. Human NPCE cells were grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Burlington, ON, USA) supplemented with 10% heat inactivated fetal calf serum (Gemini Bioproducts, Calabasas, CA, USA). Human Cornea Endothelial (CE) cells were propagated in a 1:1 mixture of F-12 Nutrient Mixture (Ham) and Media 199 (Invitrogen) containing 5% fetal calf serum (Medicorp Inc., Montreal, QB) and incubated in plates coated with 0.01 mg/ml fibronectin (GE Healthcare, Baie d'Urfe, QB), 0.03 mg/ml Vitrogen 100 (Cohesion Technologies, Palo Alto, CA, USA) and 0.01 mg/ml BSA (Sigma-Aldrich, Oakville, ON, USA). COS-7 and HeLa cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were propagated in DMEM. Transient cell transfections were performed as described (13) using FuGENE6™ reagent (Roche, Mississauga, ON, USA) according to the manufacturer recommendations. Four micrograms of plasmid DNA (pcDNA4-His/Max-FOXC1) was used to transfect 100 mm plates of cells at 70–80% confluence. Cells were incubated for another 48 h before being subjected to NACE analysis.
Nickel agarose chromatin enrichment
We have previously described the development of NACE as a method using affinity between nickel agarose beads and histidine residues to enrich for downstream genes regulated by FOXC1 (19). FGF19 was selected as a possible FOXC1 target gene in NPCE cells, using NACE, as described previously (19).
In silico analyses
The 80/40 kb of genomic sequence from hChr11/mChr7 was extracted from the GenBank contigs NT_078088 (the reverse complement of nucleotides 370 000–450 000) and AC61763 (the reverse complement of nucleotides 113 000–153 000), respectively. The human sequence was first submitted to RepeatMasker (A.F.A. Smit, R. Hubley and P. Green, unpublished data) (http://www.repeatmasker.org) to generate the annotation file needed for the PIPMaker submission (21) (http://pipmaker.bx.psu.edu/pipmaker). The gene structures of FGF4 and FGF19 were derived from annotation in the human genomic contig file. The PIPMaker dot plot (data not shown) confirmed the co-linearity of the homologous regions in the two genomes.
Constructs and mutagenesis
The FOXC1 cDNA encoding the entire open-reading frame was subcloned into EcoRI–XbaI sites in the pcDNA4-His/Max plasmid (Invitrogen) as previously reported (13). A SmaI–KpnI fragment of 1340 bp representing the entire NACE-enriched FGF19-5′-UPE fragment and the PCR-amplified 354 bp FGF19-RE fragment were cloned in both orientations upstream of the luciferase gene in pGL3 vector (Promega, Nepean, ON) on which the cytomegalovirus promoter was replaced by the herpes simplex-virus thymidine kinase (TK) promoter (13). A plasmid containing six copies of the FOXC1-binding site (6×FOXC1BS) subcloned upstream of the TK promoter within the pGL3 vector (13) was used as a positive control for transactivation assays.
In vitro mutagenesis was carried out on the 354 bp fragment cloned into pGEM-T Easy vector (Invitrogen), using the Quickchange mutagenesis kit (Stratagene, La Jolla, CA, USA), following the guidelines provided by the manufacturer. The primers used to mutate the potential FOXC1-binding site (in italics) of the FGF19 upstream region within the 354 bp fragment were: forward (5′-CCT CTG ACC CTC TAA GAG GGG TGC CGG GGA GGT TCC GGC-3′); reverse (5′-GCC GGA ACC TCC CCG GCA CCC CTC TTA GAG GGT CAG AGG-3′). Mutagenesis was confirmed by sequencing.
Transactivation assays
COS-7 cells were cultured in six-well plates at a density of 1×105 cells/ml 24 hours prior to transfection with 500 ng of pcDNA4-FOXC1 and 200 ng of either the 6×FOXC1BS luciferase reporter (13) or the FGF19-5′-UPE or FGF19-RE luciferase reporters in pGL3-TK. One nanogram of pRL-CMV (Promega) was added as an internal control. Cells were harvested after 24 h of incubation and luciferase activity was monitored, following the manufacturer's protocol (Promega). Transfections were performed in triplicates and repeated at least twice for confirmation.
Chromatin immunoprecipitations
ChIP assays were performed as described in Weinmann and Farnham (41,42) with modifications. NPCE cells (∼108) were cross-linked with 1% formaldehyde solution added directly to the cell culture media for 10 min at room temperature. Glycine was added to a final concentration of 0.125 m to stop cross-linking and cells were incubated for 5 min. The cell culture media was removed and cells were washed two times with phosphate-buffered saline (PBS). Cells were collected into 50 ml conical tubes by scraping with PBS and centrifuging at 650×g for 5 min at 4°C. The cell pellet was resuspended in 5 ml of ChIP Cell Lysis Buffer [5 mm PIPES, pH 8.0; 85 mm KCl; 0.5% IGEPAL CA-630; 1 mm PMSF; and 1/200 dilution of mammalian protease inhibitor cocktail (Sigma)] and incubated on ice for 10 min. The cell lysate was homogenized with six strokes of a Dounce homogenizer (pestle B) on ice and centrifuged at 2500×g for 5 min at 4°C. The cell pellet was then resuspended in 1 ml of ChIP Nuclear Lysis Buffer (50 mm Tris–Cl, pH 8.0; 10 mm EDTA; 1% SDS; 1 mm PMSF; and 1/200 dilution of mammalian protease inhibitor cocktail). The lysate was sonicated for six, 15 s bursts on a Fisher Scientific Sonic Dismembrator 60 to shear chromatin to an approximate average length of 500 bp. The lysate was centrifuged at 15 000×g for 10 min at 4°C. The chromatin lysate was then pre-cleared with 50 µl of Protein G (Sigma-Aldrich) beads (blocked with sheared salmon sperm DNA) for 1 h with rocking at 4°C. The lysate was then centrifuged for 1 min at 6000×g at 4°C and the supernatant was dispensed into 250 µl aliquots. Each aliquot was diluted five times with ChIP Dilution Buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mm EDTA, 16.7 mm Tris–Cl, pH 8.0; 167 mm NaCl) and incubated with antibodies against green fluorescent protein (GFP) (Abcam, Cambridge, MA, USA); FOXC1 (Abcam) or acetylated lysine 9 of histone H3 (Cell Signaling) overnight at 4°C. Two micrograms of each antibody was used. Protein G-ssDNA beads (25 µl) were added to each tube and incubated a further 2 h at 4°C. The beads were collected by centrifugation at 6000×g for 1 min at 4°C. The beads were then washed three times in ChIP Wash Buffer (20 mm Tris–Cl, pH 8.0; 150 mm NaCl; 2 mm EDTA; 0.1% SDS and 1% Triton X-100), followed by a single wash in ChIP Final Wash Buffer (20 mm Tris–Cl, pH 8.0; 500 mm NaCl; 2 mm EDTA; 0.1% SDS; and 1% Triton X-100). The DNA was eluted from the beads by adding 300 ml of Elution Buffer (100 mm NaHCO3 and 1% SDS) and rotated for 15 min at room temperature. The tubes were centrifuged at 6000×g for 1 min and the supernatant was transferred to a fresh tube. Five micrograms of 20 mg/ml proteinase K was added to each tube and incubated overnight at 65°C. The DNA was collected by QIAGEN PCR cleanup protocols (QIAGEN Inc., Mississauga, ON, USA) and eluted in 80 µl of distilled, deionized water. Five microliters of each ChIP product was used for PCR analyses.
A 100 bp fragment in the FGF19-RE was amplified with the following primers, using standard PCR conditions: 5′-GCA GCC AAG CCA GTT AGC-3′ and 5′-AGA TCC TCC AGC CGG AAC-3′.
Electrophoretic mobility shift assay
A potential FOXC1-binding site within the zebrafish fgf19 upstream region was determined by computer prediction of FOXC1-binding sites upstream of the fgf19 ORF in zebrafish genomic sequence. The TRANSFAC (release 4.0) binding site distribution matrix for FOXC1 (Freac-3) was retrieved from http://www.genome.jp/dbget-bin/www_bget?tfmatrix+M00291 and entered as ‘cis-elements’ for querying the 18 kb intergenic interval between zebrafish fgf19 and fgf4 from clone CH211-188I17 (GenBank accession no. AL929586, from position 142 904–124 716) with the online program Possum (32) (http://zlab.bu.edu/~mfrith/possum). Of the several sites identified on the (+) strand (relative to fgf19), the highest scoring hit (score of 7.90) was located 1.8 kb upstream of the start codon. EMSA procedures were performed as described (13) with the zebrafish potential FOXC1-binding site, the site within the FGF19-RE and controls. DNA oligos for newly described probes are as follows, with the putative FOXC1-binding sites italicized on the forward strand: FGF19, forward: 5′-GAT CGA CCC TCT AAG GAA AAC ATC GGG GAG GTT C-3′; reverse: 5′-GAT CGA ACC TCC CCG ATG TTT TCC TTA GAG GGT C-3′; FGF19*, forward: 5′-GAT CGA CCC TCT AAG AGG GGT GCC GGG GAG GGT C-3′ reverse: 5′-GAT CGA ACC TCC CCG GCA CCC CTC TTA GAG GGT C-3′; fgf19, forward: 5′-GAT CAA AAT TGT TAA GTA AAC AAA CTG TGT GCA T-3′; reverse, 5′-GAT CAT GCA CAC AGT TTG TTT ACT TAA GAA TTT T-3′.
Fgf19 stimulation of human CE cells
CE cells were starved for 48 h in serum-free medium (1:1 volume of F12+Medium 199) containing 5 µg/ml of each of insulin and transferrin (Sigma-Aldrich) before treatment with 50 ng/ml recombinant FGF19 (PeproTech EC Ltd, London, UK) and 10 U/ml heparin sulfate (Sigma-Aldrich). Cells were subsequently incubated for 5 min, 15 min, 30 min, 60 min and 24 h. Proteins were extracted and processed for western blot analyses.
Western analysis
For the detection of endogenous FOXC1, 125 µg of NPCE nuclear extract was resolved on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Immunoblot analysis for FOXC1 was performed with goat polyclonal anti-FOXC1 antibody (Abcam; diluted 1:100), incubated overnight at 4°C and followed by a 2 h incubation at room temperature with an HRP-conjugated donkey anti-goat IgG secondary antibody (Jackson Immunolabs; 1:10 000).
For studying the potential stimulation of protein kinase signaling upon FGF19 treatment, the following antibodies (all from Cell Signaling Technology Inc., Danvers, MA, USA) were used: α-p38, α-phospho-p38 (Thr180/Tyr182), α-p44/42 (ERK1/2), α-phospho-p44/42 (ERK1/2) (Thr202/Tyr204), α-Akt, α-phospho-Akt (Ser473), α-Bad, α-phospho-Bad (Ser136), α-SAPK/JNK, α-phospho-SAPK/JNK (Thr183/Tyr185), α-c-Jun and α-phospho-c-Jun (Ser63) II. All antibodies were used at a dilution of 1/500 and were polyclonal antibodies raised in rabbit. Forty-eight hours after cell transfection, or after FGF19 treatment, cells were washed in ice-cold PBS and harvested by gentle centrifugation. Cells were lysed in 20 mm Tris buffer (pH 7.4), 50 mm NaCl, 1 mm EGTA, 1% Nonidet P-40, 2.5 mm sodium pyrophosphate, 1 mm sodium orthovanadate and protease inhibitor cocktail tablets (Roche). Samples were sonicated and centrifuged to remove cellular debris prior to protein concentration determination, according to Bradford assay (Bio-Rad, Mississauga, ON, USA). Samples of 10 µg were resolved on a 10% SDS–polyacrylamide gel and transferred to a nitrocellulose membrane (Invitrogen). Blots were subsequently probed with primary antibody. Finally, a suitable HRP-conjugated secondary antibody was applied and immunoreactions revealed by using a Pierce chemiluminescence kit (Fisher Scientific Company, Ottawa, ON, USA).
Mutation screening analysis
Mutation screening was performed by PCR amplification of the three exons of the FGF19 gene (primer sequences available upon request), followed by labeled primer fluorescent sequencing as previously described (19). The Hardy–Weinberg equation (p2+2pq+q2=1) was subsequently used to predict allelic and genotypic frequencies within the sequenced cohort of 45 patients. p2 and q2 represent the number of homozygous genotype frequencies, (+/+) and (−/−) respectively, whereas 2pq represents heterozygous individuals (+/−).
Quantitative RT-PCR
Three sets of 100 eyes and associated POM from 48 hpf foxC1dMO (GeneIDs 79374 and 79375) MO and control MO-injected zebrafish embryos were dissected and total RNA was isolated using the QIAGEN RNeasy Plus Mini Kit (QIAGEN Inc.). cDNA was produced using 0.5 µg of RNA with the SuperScript II Reverse Transcriptase enzyme (Invitrogen). qPCR reactions were carried out using the iQ SYBR Green Supermix (Bio-Rad) on the iCycler iQ Real-Time Detection System (Bio-Rad). All independent cDNA samples were assayed in triplicate for each primer set. Primers to amplify a 102 bp fragment of fgf19 (GeneID 368245) were designed (forward: 5′-AAG TAT CGG CGT GGA GAG C-3′; reverse: 5′-TGT CTG GCT GCG TAC AGA TG-3′). Amplification of EF1alpha was used for normalization (5′-TGG GCA CTC TAC TTA AGG AC-3′; 5′-TGT GCC AAC AGG TGC AGT TC-3′). Data were analyzed using the ΔΔCt method (43).
Whole-mount in situ hybridization
A 213 bp fragment of the zebrafish fgf19 gene was amplified by PCR with the following primers: 5′-ATG CAA AGC TCG GAC AGT CT -3′ and 5′-TCA CAA GAG CTC GGT GTT TG-3′. This fragment was TOPO cloned into the pCRII-TOPO vector (Invitrogen) and used as templates for generation of a cRNA probes. For fgfr4 (GeneID 30706), a 738 bp fragment, was PCR amplified using primers: 5′-CGG AGA AGC TCA TCA AAG G-3′ and 5′-ATT GTA ATA CGA CTC ACT ATA GGG CGA GTT TCC GCC TTG CAT ACA T-3′. The 3′-primer includes a T7 RNA polymerase promoter (italics). This PCR product was used as a template for cRNA probe generation. The probe for foxC1.1 was generated as previously described (26). cRNA probes were produced and whole-mount in situ hybridization was conducted as previously described (44) with the addition of a final spin column cRNA probe purification using the ProbeQuant G-50 Micro Column (GE Healthcare). Purple-stained regions are the result of hydrolysis of BCIP/NBT (Roche) by alkaline phosphatase-conjugated anti-digoxigenin. Full details of the procedure are available from the authors. Ten to fifteen phenylthiourea (PTU)-treated embryos were analyzed in each in situ experiment. PTU inhibits melanin synthesis. For post-hybridization sectioning, embryos were fixed in 4% paraformaldehyde/PBS and infiltrated with 15% sucrose, 30% sucrose and then 100% Tissue-Tek OCT (Miles Inc., Elkhart, IN, USA). Embryos were oriented in a freezing mold and 10 µm sections were cut on a cryostat and mounted on gelatin-coated glass slides.
Morpholino knockdown in the zebrafish and histological analysis
MOs previously described were ordered from Gene Tools (Corvallis, OR, USA) and used to knockdown zebrafish foxC1.1, foxC1.2 and fgf19 (26,27).
foxC1.1 MO2: 5′-CCT GCA TGA CTG CTC TCC AAA ACG G-3; foxC1.2 MO1: 5′-GCA ATC GTA CCC CTT TCT TCG GTA C-3; fgf19 MO: 5′-CAG TGA CAA AGA GTA AGA GGA GCAT-3′; control MO: 5′-CCT CTTA CCT CAG TTA CAA TTT ATA-3′. MOs were resuspended in water and injected into one- to two- cell stage embryos using a Nanoject II injector (Drummond Scientific, Broomall, PA, USA). The following amounts per embryo were injected: 8.0 ng foxC1.1 MO2 and 8.0 ng foxC1.2 MO1 were injected to achieve foxC1dMO morphant zebrafish. About 16 ng of control MO was injected for controls. Amounts of fgf19 MO injected ranged from 2.1 to 3.2 ng.
Histological specimens were processed as previously described (45). In brief, embryos were fixed in primary fixative [2% paraformaldehyde, 2.5% glutaraldehyde, 3% sucrose, 0.06% phosphate buffer (pH 7.4)] at 4°C for 24 h and then washed in 0.1 m PBS, dehydrated through an ethanol series and propylene oxide and then infiltrated with EMbed-812/Araldyte resin mixture. Semi-thin plastic sections were cut with a glass knife on a JB4 microtome. Sections were stained with 1% Toluidine blue in 1% Borax buffer. Samples were photographed using a Nikon Coolpix 995 digital color digital camera mounted on a Nikon E800 compound microscope with a 60× oil-emersion objective.
Frozen section immunohistochemistry
Wild-type and morphant embryos were fixed at 50 hpf in 4% paraformaldehyde/PBS and infiltrated with 2 h steps of 15% sucrose, 30% sucrose and 100% Tissue-Tek OCT. Embryos were orientated, sectioned and mounted on gelatin-coated glass slides. Antibodies that recognize pERK1/2 were used at a dilution of 1/200 in 5% goat serum/PBT blocking solution. Immunoreactivity was detected with anti-rabbit fluorescent secondaries and imaged at 40× with a Nikon C1 confocal microscope.
ACKNOWLEDGEMENTS
The authors thank all of the members of Ocular Genetics Laboratory for their help and support. We thank also Mrs Farideh Mirzayans and Dr Michelle Carle for their technical assistance and Ms Penny Snell for creative services. We thank Dr Miguel Coco-Prados for the NPCE cell line. We particularly wish to thank Drs Underhill and Wevrick for their helpful comments on this manuscript. This work was supported by the Alberta Heritage Foundation for Medical Research (AHFMR), the Canadian Institute for Health Research (CIHR Grant MOP MOP64223), the National Institutes of Health (R01EY16060, BAL) and a National Eye Institute Vision Sciences Training Grant (T32-EY014537, J.M.S.).
Conflict of Interest statement. None declared.
REFERENCES
Author notes
The authors wish it to be known that, in their opinion, the first three authors should be regarded as joint First Authors.
- fibroblast growth factors
- congenital abnormality
- cell culture techniques
- mutation
- chromatin
- embryo
- genes
- mitogen-activated protein kinases
- phosphorylation
- protein tyrosine kinase
- sepharose
- trabecular meshwork
- zebrafish
- cornea
- eye
- iris
- nickel
- transcription factor
- mesenchymal stem cells
- immune reconstitution inflammatory syndrome