-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroyuki Miyamoto, Atsushi Shimohata, Manabu Abe, Teruo Abe, Emi Mazaki, Kenji Amano, Toshimitsu Suzuki, Tetsuya Tatsukawa, Shigeyoshi Itohara, Kenji Sakimura, Kazuhiro Yamakawa, Potentiation of excitatory synaptic transmission ameliorates aggression in mice with Stxbp1 haploinsufficiency, Human Molecular Genetics, Volume 26, Issue 24, 15 December 2017, Pages 4961–4974, https://doi.org/10.1093/hmg/ddx379
Close - Share Icon Share
Abstract
Genetic studies point to a major role of de novo mutations in neurodevelopmental disorders of intellectual disability, autism spectrum disorders, and epileptic encephalopathy. The STXBP1 gene encodes the syntaxin-binding protein 1 (Munc18–1) that critically controls synaptic vesicle exocytosis and synaptic transmission. This gene harbors a high frequency of de novo mutations, which may play roles in these neurodevelopmental disorders. However, the system and behavioral-level pathophysiological changes caused by these genetic defects remain poorly understood. Constitutional (Stxbp1+/−), dorsal-telencephalic excitatory (Stxbp1fl/+/Emx), or global inhibitory neuron-specific (Stxbp1fl/+/Vgat) mice were subjected to a behavioral test battery examining locomotor activity, anxiety, fear learning, and social interactions including aggression. Furthermore, measurements of local field potentials in multiple regions of the brain were performed. Stxbp1+/− male mice exhibited enhanced aggressiveness and impaired fear learning associated with elevated gamma activity in several regions of the brain including the prefrontal cortex. Stxbp1fl/+/Emx mice showed fear-learning deficits, but neither Stxbp1fl/+/Emx nor Stxbp1fl/+/Vgat mice showed increased aggressiveness. Pharmacological potentiation of the excitatory transmission at active synapses via the systemic administration of ampakine CX516, which enhances the excitatory postsynaptic function, ameliorated the aggressive phenotype of Stxbp1+/− mice. These findings suggest that synaptic impairments of the dorsal telencephalic and subcortical excitatory neurons cause learning deficits and enhanced aggression in Stxbp1+/− mice, respectively. Additionally, normalizing the excitatory synaptic transmission is a potential therapeutic option for managing aggressiveness in patients with STXBP1 mutations.
Introduction
Regulated synaptic transmission underlies normal brain functioning, while its dysregulation causes various pathological states (1,2). The Stxbp1 gene encodes syntaxin-binding protein 1 (Munc18–1), which is a synaptic protein indispensable for neurotransmitter release (3,4). Munc18–1 binds to syntaxin and interacts with the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, regulating docking and fusion of synaptic vesicles to presynaptic membranes (5). Homozygous deletions of Stxbp1 resulted in complete loss of glutamatergic and gamma-aminobutyric acid (GABA)ergic neurotransmitter release (3). In heterozygous Stxbp1 knockout mice, the synaptic transmission rapidly drops during repetitive stimulations due to the limited size of readily releasable pools (4).
Human genetic studies have linked STXBP1 mutations with a broad spectrum of neurodevelopmental diseases. STXBP1 mutations have been described in patients with epileptic encephalopathies including Ohtahara syndrome that is associated with severe intellectual disabilities (6–9). These mutations have also been reported in cases with intellectual disabilities (10,11) and autism spectrum disorders (ASD) (10,12–14). According to a comprehensive review of patients with STXBP1 mutations, almost all patients exhibited epilepsy and intellectual disability, 20% were autistic, and some exhibited increased aggressive behaviors (13). Consistently, neurodevelopmental, psychiatric, and neurological disorders have been occasionally associated with aggressiveness (15–17). Animal models with Stxbp1 mutation may therefore provide mechanistic insights into the neurodevelopmental disorders associated with intellectual disabilities and aggression. Heterozygous Stxbp1 knockout (Stxbp1+/−) mice are outwardly healthy and fertile, though homozygous Stxbp1 knockout mice are lethal (3). Enhanced anxiety-like emotional response assessed based on heart rate was reported in Stxbp1+/− mice in novel open field and fear-conditioning tests (18). Transgenic mice with Stxbp1 overexpression displayed schizophrenic behavior (19). Thus, deviations from the normal level of Munc18–1 protein and the resultant synaptic deficits may trigger neurodevelopmental disorders such as autism and neuropsychiatric symptoms; however, it remains undescribed how dysfunction of synaptic transmission causes behavioral abnormalities from cognition to social interaction related to neurodevelopmental disorders. Here, we directly addressed this question by characterizing behavioral phenotype of constitutive or conditional Stxbp1 knockout mice, and found impaired excitatory neurotransmission-dependent cognitive deficits and increased aggressiveness in Stxbp1+/− mice and amelioration of the aggressive phenotype by potentiating excitatory transmission.
Results
Generation of floxed Stxbp1 and knockout mice
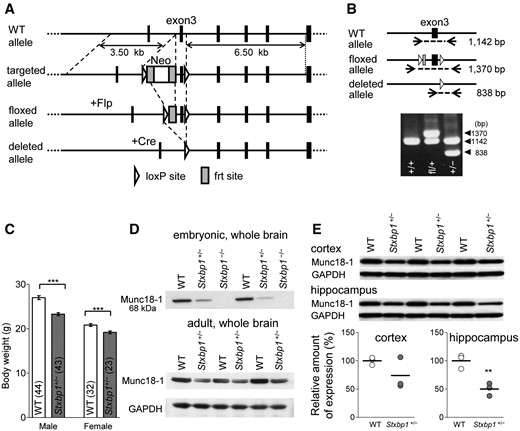
We generated mice with a floxed exon 3 of Stxbp1 gene and subsequently knockout mice by mating with EIIA-Cre driver mice (Fig. 1A and B). Stxbp1 homozygous knockout mice (Stxbp1−/−) were lethal as previously reported (3), while heterozygous Stxbp1 (Stxbp1+/−) mice survived similarly to WT mice (189 Stxbp1+/− and 193 WT mice were obtained from the litters at 4 weeks after birth). The Stxbp1+/− mice had a normal appearance indistinguishable from WT mice except for a lower body weight (Fig. 1C), which is in line with previous findings (18). Our results confirmed the reduced expression of Munc18–1 protein in Stxbp1+/− mice and its absence in embryonic Stxbp1−/− mice (Fig. 1D and E).
Generation of Stxbp1 floxed and knockout mice. (A) Stxbp1 WT, targeted, floxed, and deleted alleles. Stxbp1 knockout mice were obtained by crossing the floxed mice with EIIA-Cre mice. Flp, Flp recombinase; frt, Flp recognition target site. (B) PCR analyses of genomic DNAs of WT (+/+), heterozygous floxed (fl/+), and heterozygous knockout (+/−) mice. (C) Smaller body weight of Stxbp1+/− mice. WT male mice (n = 44) vs. Stxbp1+/− male mice (n = 43), t-test, t85 = 6.544, ***P < 0.0001; WT female mice (n = 32) vs. Stxbp1+/− female mice (n = 23), t-test, t54 = 3.679, ***P = 0.0005. All groups were assessed at 9 weeks of age. (D) Western blots of embryonic (2 WT, 2 Stxbp1+/−, 2 Stxbp1−/−) and young adult (3 WT, 3 Stxbp1+/−; 4 weeks) whole brain samples probed with anti-Munc18-1 or anti-glyceraldehyde-3-phosphatase dehydrogenase (GAPDH) antibodies. GAPDH protein was used for Munc18-1 protein normalization. (E) Quantification of Munc18-1 protein expression in the neocortex and the hippocampus of WT and Stxbp1+/− mice (n = 3, each group, 4 weeks). WT vs. Stxbp1+/− mice, t-test, cortex: t4 = 1.565, P = 0.1926; hippocampus: t4 = 5.190, **P = 0.0066.
Environmental stimuli-dependent increase of anxiety in Stxbp1+/− mice
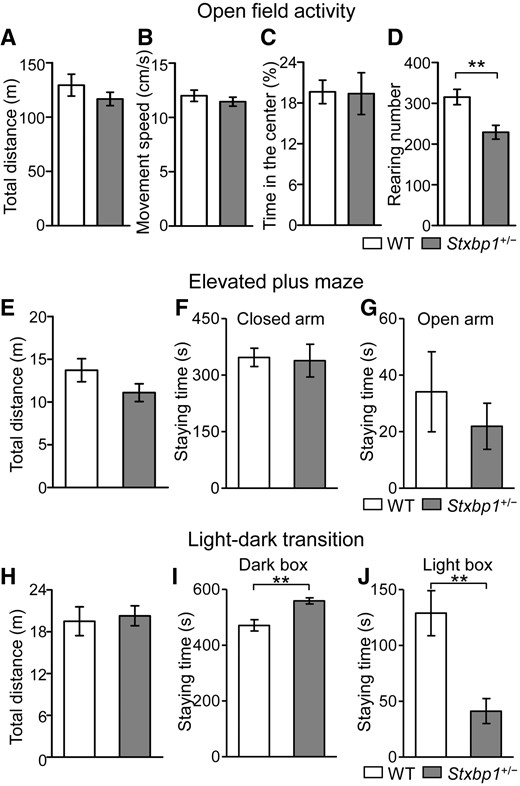
In the novel open field activity test, the total distance moved and movement speed of Stxbp1+/− mice were equivalent to those of WT mice (Fig. 2A and B). Time spent in the center of the arena, reflecting the anxiety level, was also similar to WT mice (Fig. 2C), but the rearing number, an index of repetitive behavior, was significantly lower in Stxbp1+/− mice than in WT mice (Fig. 2D).
Increased anxiety in Stxbp1+/− mice. (A–D) Novel open field test in Stxbp1+/− (n = 10) and WT (n = 10) male mice (2.5 months of age). No motor disturbance was observed in Stxbp1+/- mice. Total distance (A) (t-test, t36 = 1.311, P = 0.1982) and movement speed (B) (t-test, t36 = 0.8448, P = 0.4038) of Stxbp1+/- mice were comparable with those of WT mice (measured for 30 min). The amount of time spent in the center of the open field (C) did not significantly differ between WT and Stxbp1+/- mice (t-test, t36 = 1.680, P = 0.10). Rearing number (D) was significantly decreased in Stxbp1+/- mice (t-test, t36 = 4.034, ***P = 0.0003). (E–G) Elevated plus maze test in Stxbp1+/− (n = 10) and WT (n = 10) male mice (2 months of age). Total distances traveled (E) were similar (t-test, t18 = 1.541, P = 0.1407). The differences of time spent in the closed arms (F) (t-test, t18 = 0.1766, P = 0.8618) and the open arms (G) (t-test, t18 = 0.7479, P = 0.4642) were not statistically different between genotypes. (H–J) Light-dark transition test in Stxbp1+/− (n = 10) and WT (n = 10) male mice (2 months of age). Total distances traveled (H) were similar (t-test, t18 = 1.541, P = 0.1407), however, Stxbp1+/− mice spent a significantly longer time in the dark box (I) (t-test, t18 = 3.810, **P = 0.0013) and shorter time in the light box (J) (t-test, t18 = 3.810, **P = 0.0013) than those of WT mice.
We noticed that Stxbp1+/− mice often displayed hyperactive responses to the experimenter during handling. To examine further the emotional response, we investigated the anxiety-related behavior in Stxbp1+/− mice using the elevated plus maze and the light-dark transition test. The total distance traveled, time spent in the open or closed arms, and the numbers of entry to the open or closed arms did not differ significantly between WT and Stxbp1+/− mice on the elevated plus maze (Fig. 2E–G). In contrast, the time spent in the dark box in the light-dark transition test was significantly longer in the Stxbp1+/− mice than the WT mice (Fig. 2H–J). These results suggest an elevation of anxiety in Stxbp1+/− mice depending on the environmental stimuli/conditions.
Impaired fear learning and changes in gamma activity after fear conditioning in Stxbp1+/− mice
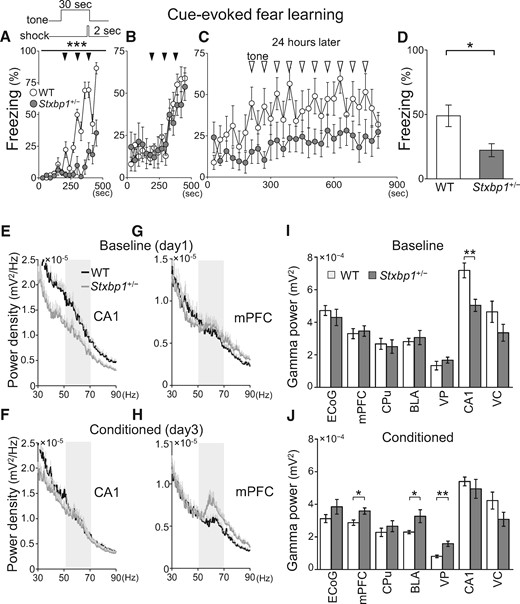
Since patients with Ohtahara, West, or Lennox-Gastaut syndrome suffer from severe intellectual disability, we further investigated the cognitive ability of Stxbp1+/− mice using the fear conditioning memory test (Fig. 3A–D). Although naive Stxbp1+/− mice, which had not experienced any previous behavioral test, failed to form a conditioned freezing response (Fig. 3A), Stxbp1+/− mice that had previously undergone behavioral tests (open field, open field interaction, resident-intruder test) could form fear conditioning similarly to WT mice (Fig. 3B). We further tested the sensory cue-evoked fear memory of these mice in the same chamber 24 h after fear conditioning. The results indicated that the number of freezing responses to the auditory cues was significantly lower in Stxbp1+/− mice than in WT mice (Fig. 3C and D). These results suggest that cognitive functions such as the encoding and retention of fear memory are vulnerable and impaired in Stxbp1+/− mice. In contrast, both Stxbp1+/− and WT mice performed similarly on the Y-maze test, suggesting that short-term or working memory (20) is retained in Stxbp1+/− mice (Supplementary Material, Fig. S1A and B). The reduced freezing in Stxbp1+/− mice is unlikely to be caused by a low sensitivity to the fear stimulus, because the estimated anxiety levels of Stxbp1+/− mice were comparable or even slightly increased compared with WT mice in the elevated plus maze, light–dark box test, and novel open field activity test (Fig. 2). These results are consistent with a recent report that demonstrated increased fear and anxiety in Stxbp1+/− mice using heart rate measurements (18). In the same report, however, auditory delay and trace fear conditioning were significantly enhanced in Stxbp1+/− mice. This discrepancy with our observations can be attributed to the methods used for quantifying the fear responses; freezing response was used in our study, while heart rate was used in the previous study (18).
Impaired fear memory and abnormal gamma activity in Stxbp1+/− mice. (A) Fear conditioning of naive mice. Tones and concomitant electrical foot shocks (inset) were presented three times (arrow heads) during conditioning. Freezing is expressed as percent of recording time (30-s bin). Note that the naive Stxbp1+/− mice mostly failed to form conditioned fear. Two-way repeated measures ANOVA, WT vs. Stxbp1+/− conditioning: F1, 196 = 63.28, ***P < 0.0001. WT (n = 8) and Stxbp1+/− (n = 8) adult male mice (2–2.5 months of age). (B–D) Cue-evoked fear-learning test in experienced mice. (B) Tones and concomitant electrical foot shocks (three times, arrow head) formed conditioned fear response. (C) Twenty-four hours later, only tones (open triangle, 10 times) were presented to the mice in another chamber. WT (n = 10) and Stxbp1+/− (n = 10) adult male mice (9 months of age) were used. Two-way repeated measures ANOVA, WT vs. Stxbp1+/− conditioning: F1, 252 = 0.11, P = 0.7458; testing: F1, 468 = 3.81, P = 0.0666. (D) Group analysis of cue-evoked freezing, t-test, t18 = 2.726, *P = 0.0138. (E–J) Changes in regional brain activity after fear learning of naive mice. (E, F) Power spectrum of averaged CA1 local field potential (LFP) (mean + SEM) of WT and Stxbp1+/− mice before (baseline, day1) and after fear conditioning (day 3). (G, H) Power spectrum of averaged mPFC LFP (mean + SEM) of WT and Stxbp1+/− mice before (baseline, day1) and after fear conditioning (day 3). Stxbp1+/− mice with simultaneously recorded LFPs showed impairments of fear learning (Supplementary Material, Fig. S2). Note the specific increase of gamma band activity (50–70 Hz, shaded area) in awake Stxbp1+/− mice after conditioning (H) (5 WT and 5 Stxbp1+/− mice; 5 months of age). (I) Regional gamma band activity (50–70 Hz) during 10 tone presentations (30-s duration) prior to fear conditioning. LFP recordings (1 amygdala and 1 CPu from 1 Stxbp1+/− mouse) were excluded from the analysis due to recording problems. Baseline, WT vs. Stxbp1+/− mice, t-test, S1 ECoG: t8 = 0.7169, P = 0.4939; medial prefrontal cortex (mPFC): t8 = 0.3576, P = 0.7299; caudate putamen (CPu): t7 = 0.3117, P = 0.7644; basolateral amygdala (BLA): t7 = 0.5476, P = 0.601; Ventroposterior thalamus (VP): t8 = 1.038, P = 0.3297; hippocampal CA1 region (CA1): t8 = 3.653, **P = 0.0065; visual cortex (VC): t8 = 1.990, P = 0.0818. Two-way repeated measures ANOVA, genotype: F1, 42 = 0.92, P = 0.3684; region: F6, 42 = 44.42, P < 0.0001; interaction: F6, 42 = 2.81, P = 0.0217; Bonferroni post-hoc test, not significant. (J) Regional brain power spectrum of mice after fear conditioning, WT vs. Stxbp1+/− mice, t-test, S1 ECoG: t8 = 1.415, P = 0.1949; mPFC: t8 = 2.825, *P = 0.0223; CPu: t7 = 0.9092, P = 0.3935; BLA: t7 = 2.656, *P = 0.0326; VP: t8 = 4.235, **P = 0.0029; CA1: t8 = 0.7089, P = 0.4985; VC: t8 = 1.699, P = 0.4985. Two-way repeated measures ANOVA, genotype: F1, 42 = 2.53, P = 0.1560; region: F6, 42 = 37.61, P < 0.0001; interaction: F6, 42 = 2.61, P = 0.0305; Bonferroni post-hoc test, not significant.
Gamma activity has been assumed to underlie cognitive processing (21,22), and its abnormalities were reported in models of psychiatric disorders including schizophrenia and autism (23,24). Therefore, we measured electrocorticogram (ECoG) and local field potentials (LFPs) in WT and Stxbp1+/− mice before and after fear conditioning (Fig. 3E–J and Supplementary Material, Fig. S2). In this experiment, we used naive mice for the fear-conditioning test, which again failed to form fear memory (Supplementary Material, Fig. S2A). Prior to the fear conditioning, the gamma (50–70 Hz) activity of awake mice during baseline recordings was significantly decreased in the hippocampal CA1 region of Stxbp1+/− mice (Fig. 3E and I, Supplementary Material, Fig. S2B). Twenty-four hours after fear conditioning, we observed a significantly higher gamma activity in the medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and ventroposterior thalamus (VP) of Stxbp1+/− mice compared with WT mice (Fig. 3H and J, Supplementary Material, Fig. S2C) during the tone presentation. However, there was no statistical difference in the time freezing in the neutral chamber before the tone presentation (Supplementary Material, Fig. S2D), and the beta band activity (10–30 Hz, an index of arousal) or gamma band activity (50–70 Hz, also related to attentive states) of most brain regions were not increased in Stxbp1+/− mice (Supplementary Material, Fig. S2E and F), implying that Stxbp1+/− mice after fear conditioning exhibited similar arousal and fearful states as WT mice. These data may suggest that changes in the gamma activity in multiple brain regions and regional interactions are involved in impairments related to learning or innate information processing in Stxbp1+/− mice.
Enhanced aggression in Stxbp1+/− mice
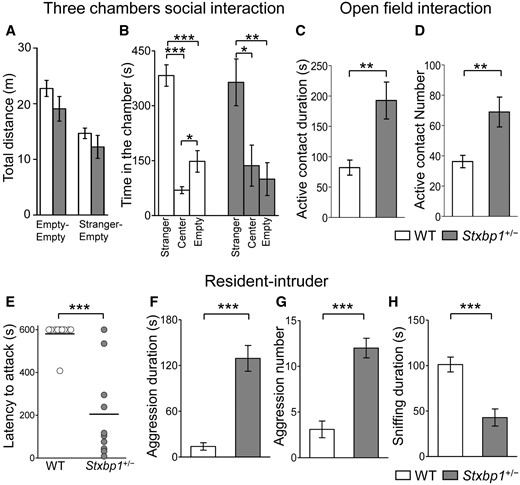
Because autistic features have been reported in patients with STXBP1 mutations (13), we investigated social interaction in male Stxbp1+/− mice using the three-chambered social interaction test. Stxbp1+/− mice and their WT littermates similarly exhibited a strong preference for a cage with a stranger mouse compared with an empty cage (Fig. 4A and B).
Enhanced aggression in Stxbp1+/− mice. (A, B) Three-chamber social interaction test in Stxbp1+/− (n = 10) and WT (n = 10) male mice (3 months of age). (A) Total distance traveled did not differ significantly between genotypes. Empty-Empty: t18 = 1.383, P = 0.1836; Stranger-Empty: t18 = 1.070, P = 0.2987. (B) Time spent in the chamber of a stranger mouse (C57BL/6N, male, 6 weeks), the center chamber, or the chamber of empty cage during a 10-min test period. Both genotypes spent a significantly longer time in the chambers with stranger mice. WT mice, stranger vs. center: t18 = 10.16, ***P < 0.0001, stranger vs. empty: t18 = 5.644, ***P < 0.0001, center vs. empty: t18 = 2.539, P = 0.0206, t-test. Stxbp1+/− mice, stranger vs. center: *P = 0.0155, stranger vs. empty: t18 = 3.387, **P = 0.0033, center vs. empty: t18 = 0.5123, P = 0.6147, t-test. (C, D) Open field social interaction test in Stxbp1+/− (n = 10) and WT (n = 10) male mice (5–6 months of age). Stxbp1+/− mice showed a significantly longer duration (t-test, t18 = 3.369, **P = 0.0034) and higher number (t-test, t18 = 3.060, **P = 0.0067) of active contacts with stranger mice (C57BL/6N, male, 6 weeks) than WT mice. (E–H) Resident-intruder test in Stxbp1+/− (n = 10) and WT (n = 10) male mice (7 months of age). Stxbp1+/- mice showed enhanced aggressiveness (10-min test period) against an intruder mouse (C57BL/6N, male, 6 weeks) with a significantly shorter latency to attack (E) (t-test, t = 5.396, ***P < 0.0001; 600 s were assigned to mice that did not attack), longer aggression duration (F) (t18 = 6.574, **P < 0.0001), and higher aggression number (G) (t18 = 6.313, ***P < 0.0001). Sniffing duration (H) was shorter in Stxbp1+/− mice (t18 = 4.665, ***P = 0.0002).
Subsequently, we tested social interaction between freely moving male mice in the open field (Fig. 4C and D). We quantified active contacts by WT or Stxbp1+/− mice to unfamiliar mice (C57B6), including sniffing, touching, and following. The Stxbp1+/− mice exhibited a significantly larger number and longer duration of active contacts than the WT mice. Because attacking behaviors were occasionally observed in Stxbp1+/− mice in the open field interaction test, we further quantified the aggressiveness of Stxbp1+/− mice using the resident-intruder test (Fig. 4E–H). The latency to the first aggressive behavior (attacking, biting) against the intruder (C57B6 young male mouse) was significantly shorter (Fig. 4E), and the duration and number of aggressive responses against the intruder were also significantly higher in resident Stxbp1+/− mice (Fig. 4F and G, Supplementary Material, Movie S1). In contrast, the sniffing behaviors were significantly decreased in Stxbp1+/− mice (Fig. 4H).
Consistent with the increased aggressiveness of Stxbp1+/− mice, there was a high incidence of injury in mice in their home cages. Although severe injury rarely occurred in our WT mouse colonies, we frequently observed fighting and injuries among male Stxbp1+/− littermates (injuries found in 26 cages out of 67 cages; 7/126 injured WT mice and 33/120 Stxbp1+/− mice; 1 month to 1 year of age; mixed housing of both genotypes). Most injuries were found on the backs or at the bases of the tails. A high incidence of injuries in Stxbp1+/− mice is possibly due to their smaller body size (Fig. 1C) and fight-backs from larger WT littermates. A few female Stxbp1+/− mice with tail injuries were also noted.
No increase in aggression after conditional deletions of Stxbp1 in the dorsal-telencephalic excitatory neurons or global inhibitory neurons
To evaluate comparatively the impact of Stxbp1 deletion in excitatory and inhibitory neurons on cognition and aggressiveness, we generated two conditional knockout mice: [1] Emx1-Cre (25,26)-mediated dorsal-telencephalic excitatory neurons-specific deletion, namely in the cerebral cortex, hippocampus, amygdala, and olfactory bulb, but not the basal ganglia and thalamus; [2] Vgat-Cre (26)-mediated global inhibitory neurons-specific deletion. At 4 weeks of age, we obtained 42 heterozygous floxed mice (Stxbp1fl/+) and 44 heterozygous floxed mice with Emx1-Cre-mediated deletion (Stxbp1fl/+/Emx) from mating of Stxbp1fl/+ and Emx1-Cre driver mice. Furthermore, 26 Stxbp1fl/+ and 24 Stxbp1fl/+/Vgat mice were obtained from mating the Stxbp1fl/+ and Vgat-Cre driver mice; no homozygous Stxbp1fl/fl/Emx or Stxbp1fl/fl/Vgat mice were obtained. Based on this, we suggest that, while the homozygous deletion of Stxbp1 in excitatory or inhibitory neurons results in premature death, both heterozygous lines survive well postnatally. The body weights of Stxbp1fl/+/Emx and Stxbp1fl/+/Vgat mice were similar to those of the control littermates (Supplementary Material, Fig. S3A and B). Western blot analysis indicated a significant reduction of Munc18–1 expression in the cerebral cortex and hippocampus of Stxbp1fl/+/Emx mice (Supplementary Material, Fig. S3C–E), but not in Stxbp1fl/+/Vgat mice (Supplementary Material, Fig. S3F–H). In the novel open field test, Stxbp1fl/+/Emx and Stxbp1fl/+/Vgat mice displayed mostly normal behaviors including locomotor activity, center stay, and rearing (Fig. 5A–H). The memory retention deficits were partially replicated in contextual fear conditioning in Stxbp1fl/+/Emx mice (Fig. 5I–K). This tendency was also seen in Stxbp1fl/+/Vgat mice, but was not statistically significant (Fig. 5L–N). Both Stxbp1fl/+/Emx and Stxbp1fl/+/Vgat mice, however, did not exhibit enhanced aggressiveness, but rather displayed a lower tendency (Fig. 5O–R). Consistently, we did not observe inter-male aggression in their home cages. These results suggest that non-dorsal telencephalic subcortical brain structures are responsible for the enhanced aggressiveness in Stxbp1+/− mice, while reduced excitatory synaptic transmission in the dorsal telencephalon primarily contributes to the impaired fear-learning.
No aggression in mice with Stxbp1 heterozygous deletion in dorsal-telencephalic excitatory neurons or global inhibitory neurons. (A–D) Open field activity test in Stxbp1fl/+/Emx (n = 6) and control (5 Stxbp1fl/+ and 1 Stxbp1+/+/Emx, n = 6) mice (3 months of age). There was no significant difference between genotypes in the total distance traveled (A) (t10 = 1.022, P = 0.3308), moving speed (B) (t10 = 1.558, P = 0.1504), time spent in the center (C) (t10 = 1.467, P = 0.1732), and rearing number (D) (t10 = 0.9046, P = 0.3870). (E–H) Open field activity test in Stxbp1fl/+/Vgat (n = 8) and control (Stxbp1fl/+, n = 8) mice (2 months of age). There is no significant difference between genotypes in the total distance traveled (E) (t14 = 0.9809, P = 0.3433), moving speed (F) (t14 = 0.6945, P = 0.4987), time in the center (G) (t14 = 0.1888, P = 0.8530), and number of rearing (H) (t14 = 1.429, P = 0.1750). (I–K) Contextual fear memory test in Stxbp1fl/+/Emx (n = 8) and control (1 WT, 3 Stxbp1fl/+, 4 Stxbp1+/+/Emx, n = 8) mice (5 months of age). Stxbp1fl/+/Emx mice acquired conditioned fear responses (I), but attenuated fear expression 24 h after fear conditioning (J, K). Two-way repeated measures ANOVA, control vs. Stxbp1fl/+/Emx, conditioning: F1, 196 = 2.46, P = 0.1393; testing: F1, 70 = 7.42, *P = 0.0164. Averaged freezing time expressed as percent of recording time (30-s bin). (K) Averaged freezing time (%) during the test, controls vs. Stxbp1fl/+/Emx1-Cre mice, t-test, t14 = 2.725, *P = 0.0164. (L–N) Contextual fear memory test on Stxbp1fl/+/Vgat (n = 8) and control (Stxbp1fl/+, n = 8) mice (2 months of age). Stxbp1fl/+/Vgat mice acquired conditioned fear responses (L), but tended to lose the fear memory 24 h after fear conditioning (M, N). Two-way repeated measures ANOVA, control vs. Stxbp1fl/+/Vgat, conditioning: F1, 196 = 0.01, P = 0.9352; testing: F1, 70 = 3.21, P = 0.0950. (N) Averaged freezing time (%) during the test, controls vs. Stxbp1fl/+/Vgat mice, t-test, t14 = 1.791, P = 0.0950. (O, P) Resident-intruder test in Stxbp1fl/+/Emx (n = 6) and control (5 Stxbp1fl/+, 1 Stxbp1+/+/Emx, n = 6) male mice (4 months of age). Stxbp1fl/+/Emx mice did not differ from control mice in the duration of aggression (t10 = 0.9196, P = 0.3794) and sniffing (t10 = 0.0000, P = 1.0000) (O). Numbers of aggression (t10 = 0.3552, P = 0.7298) and sniffing (t10 = 0.2717, P = 0.7194) did not differ between genotypes (P). (Q, R) Resident-intruder test in Stxbp1fl/+/Vgat (n = 8) and control (Stxbp1fl/+, n = 8) male mice (2 months of age). Stxbp1fl/+/Vgat mice did not differ from control mice in the duration of aggression (t14 = 1.556, P = 0.1420), sniffing (t14 = 1.038, P = 0.3168) (Q), and number of aggression (t14 = 1.963, P = 0.0698) and sniffing (t14 = 0.8011, P = 0.4364) (R).
Mitigation of aggressiveness in Stxbp1+/− mice by potentiating excitatory transmission
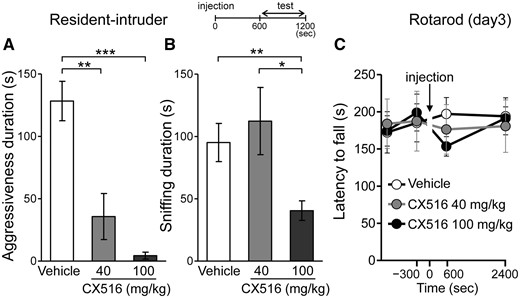
Haploinsufficiency of Stxbp1 results in faster rundown of excitatory synaptic transmission (4). Therefore, we hypothesized that recovering the excitatory synaptic transmission may mitigate aggressiveness in Stxbp1+/− mice. Ampakines potentiate the function glutamatergic alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor by slowing the deactivation and desensitization of ion channels; they are only effective in the presence of glutamate at synapses (27). Ampakines have been widely used as cognitive enhancers in normal animals or animal models with cognitive impairments (27). At comparable doses, which are reported to improve various types of memory performance, CX516, a first-generation ampakine with a short half-life (28), or vehicle was intraperitoneally administered to Stxbp1+/− mice 10 min prior to the resident-intruder test. While vehicle-treated Stxbp1+/− mice still showed robust aggressiveness against the intruders, CX516-treated mice significantly decreased (40 mg/kg) and totally suppressed (100 mg/kg) their aggressive responses (Fig. 6A). Sniffing was unchanged at 40 mg/kg, but significantly decreased at 100 mg/kg compared with vehicle-treated mice (Fig. 6B). CX516-treated Stxbp1+/− mice remained occasionally at a distance from the intruder mouse, a behavior that was also observed in CX516-treated WT mice (n = 6). No sluggish or dyskinetic movements were observed (Supplementary Material, Movie S2). Furthermore, no significant impairments in motor coordination in the CX516-treated Stxbp1+/− mice were observed on the rotarod test (Fig. 6C). We additionally performed the light-dark transition and contextual fear-learning tests in male Stxbp1+/− mice. CX516 (40 mg/kg) was administrated 10 min before the light-dark transition test or foot shock conditioning. There was no significant difference between the vehicle or CX516-treated mice in both the light-dark transition test (Supplementary Material, Fig. S4A–C) and contextual fear learning (Supplementary Material, Fig. S4D–F). The effect of CX516 lasted for approximately 30 min, after which aggressiveness re-emerged in the mice (Supplementary Material, Movie S3). These results indicate that the potentiation of glutamatergic neurotransmission by ampakine efficiently suppresses aggressiveness in Stxbp1+/− mice without major side effects. Adult WT mice at 7 months of age exhibited significantly lower aggressiveness compared with adult Stxbp1+/− mice (Fig. 4E–G). Therefore, we tested the effects of CX516 on WT mice at a younger age (3–4 months), who are prone to aggressiveness. Our results indicated that the aggressiveness of WT mice tended to be mitigated by CX516 (100 mg/kg), but not at a statistically significant level (vehicle: 66.0 ± 34.2 s, n = 4; CX: 9.75 ± 9.8 s, n = 4; t-test, p = 0.164). This result suggested that CX516 is generally effective on aggressiveness, thus providing evidence that enhanced glutamatergic transmission may suppress aggressiveness.
Ampakine ameliorated the enhanced aggression in Stxbp1+/− mice. (A, B) Resident-intruder test in Stxbp1+/− mice treated with ampakine CX516. Male Stxbp1+/− mice (3–7 months of age) were intraperitoneally injected with 40 mg/kg CX516 (n = 8), 100 mg/kg CX516 (n = 10), or saline vehicle (n = 9), and the tests started at 10 min and ended at 20 min after the injections (inset). (A) Duration of the aggression (sec) of Stxbp1+/− mice was drastically suppressed by CX516. Mann-Whitney test, vehicle vs. CX516 (40 mg/kg), U = 9, **P = 0.0079; vehicle vs. CX516 (100 mg/kg), U = 0, ***P < 0.0001. (B) Duration of sniffing in Stxbp1+/− mice was unchanged after 40 mg/kg of CX516, but was suppressed after 100 mg/kg of CX516. t-test, vehicle vs. CX516 (40 mg/kg), t15 = 0.5690, P = 0.5777; vehicle vs. CX516 (100 mg/kg), t17 = 3.274, **P = 0.0045; CX516 (40 mg/kg) vs. CX516 (100 mg/kg), t16 = 2.815, *P = 0.0124. (C) Rotarod test in Stxbp1+/− mice treated with CX516. Trials on an accelerating rotarod were performed for 3 consecutive days (4 trials per day, total 12 trials). The latency when Stxbp1+/− mice (male, 4–7 months) fell off the rod was recorded. Vehicle (n = 6), low CX516 (40 mg/kg, n = 6), or high CX516 (100 mg/kg, n = 6) were injected 10 min before the 11th trial (time = 0 s, arrow). The latency to fall off the rod at the 11th trial (time = 600 s) was compared; t-test, vehicle vs. CX516 (40 mg/kg): t10 = 0.5211, P = 0.6136; vehicle vs. CX516 (100 mg/kg): t10 = 1.704, P = 0.1192.
Discussion
In this study, we demonstrated that impaired synaptic transmission in Stxbp1+/− mice results in cognitive deficits and enhanced aggressiveness, which was pharmacologically ameliorated by potentiating excitatory postsynaptic function. We also observed mild epileptic phenotypes in the mice (Miyamoto et al., in preparation). Despite the high homology of Munc18–1 between human and mouse, the phenotypic severity in mice with Munc18–1 haploinsufficiency is moderate compared with that in humans, and the reasons for these differences are currently unknown. Indeed, the mouse models carrying human disease mutations do not always fully recapitulate the disease phenotype of the patients. For instance, we have previously shown that Scn1a knockout mice well reproduced the severe phenotype of Dravet syndrome (26,29), while the phenotype of laforin knockout mouse phenotype was considerably milder than that reported in patients with Lafora disease (30).
Impulsive aggression is often associated with various types of psychiatric and neurodevelopmental disorders, including schizophrenia, clinical depression, epilepsy, ASD, and attention-deficit/hyperactivity disorder (15,17,31–33). Patients with intellectual disabilities or neurodegenerative diseases such as Alzheimer‘s disease are also at a high-risk of aggressive behaviors (34,35). Stxbp1+/− mice recapitulate clinical features of these disorders. Although Ohtahara syndrome in many newborn patients (typically within 3 months) is caused by de novo STXBP1 mutations (6), enhanced aggressiveness has not been described so far, likely due to the severe mental retardation and motor deficits. However, seventy-five percent of patients with Ohtahara syndrome (within 3 months of age) transit to West syndrome (mostly within 1 year), and 59% of those with West syndrome further evolve to Lennox-Gastaut syndrome (1 year or later) (9), which display aggressive behaviors at high rates (36,37). Therefore, Stxbp1+/− mice should serve as a model for aggression in neurodevelopmental disorders.
To our knowledge, the present study is the first to report on the mitigation of aggression through the activation of glutamatergic AMPA receptors. Memory improvements by ampakines such as CX516 have been repeatedly documented in the literature (27,28,38,39). The administration of CX516 has been previously reported to reduce the spontaneous locomotor activity in a dose dependent manner (40,41). Therefore, CX516 might reduce aggressive behaviors due to possible adverse effects on brain and behavior. However, our results indicated that the aggressive behavior was significantly (40 m/kg) and drastically (100 mg/kg) suppressed, while putative non-aggressive behaviors such as sniffing was either not affected (40 m/kg) or reduced by 50% following the 100 mg/kg dosage. Even at a higher CX516 dose (100 mg/kg), the motor performance on the rotarod test was only modestly affected. Previous studies have reported significant improvements in learning and memory after the administration of CX516 at 35 mg/kg in fear conditioning (38), spatial delayed non-match-to-sample task (28), and olfactory learning (40), and at 80 mg/kg in the novel object recognition task (42). It is worth noting that these tasks require high cognitive abilities and motivation, thus indicating minimal to absent adverse effects of CX516. Moreover, CX516 at 40 mg/kg relieved depressive symptoms of pain (43). Thus, the effect of CX516 on aggressiveness in our model is unlikely due to non-specific sedative or adverse effects.
Several studies reported that glutamate stimulation facilitates aggressiveness, suggesting that glutamatergic inputs to the hypothalamic attack area or the raphe nucleus promote aggression, while blockade of glutamate receptors reduces aggression [reviewed in (44)]. The basis of the contradictory glutamatergic actions on aggression in the current and previous studies is so far unknown. However, it could stem from different contributions of glutamate receptor subtypes (e.g. AMPA, NMDA, kainite, and metabotropic glutamate receptors) (44), brain regions (e.g. prefrontal cortex, amygdala, hypothalamus, nucleus accumbens, habenula, and raphe nucleus) (44,45), or use-dependent actions of the ampakine (27). A recently introduced anti-epileptic drug ‘perampanel,’ which is a non-competitive antagonist of AMPA receptors, was reported to induce aggressive behaviors frequently in patients with epilepsy (46). Thus, it may be of interest to explore whether STXBP1 mutations are also a marker for patients with epilepsy who are at risk of developing aggression in response to perampanel treatment.
Our present results showed that, unlike Stxbp1+/− mice, Stxbp1fl/+/Emx mice and Stxbp1fl/+/Vgat mice did not exhibit increased aggressiveness. These findings suggest that impaired excitatory neurotransmission in non-dorsal telencephalic subcortical brain structures are responsible for inducing increased aggressiveness in Stxbp1+/− mice. There are several mouse models with enhanced aggression (47). Particularly, hypofunction of the brain serotonergic system has been suggested to accelerate aggressiveness (48,49). Therefore, we propose that reduced glutamatergic inputs to the raphe nucleus (50) or reduced serotonergic transmission caused by Stxbp1 haploinsufficiency may contribute to the enhanced aggressiveness in both mice and patients.
Urigüen and colleagues (19) reported an elevated level of anxiety in mice overexpressing Munc18–1, while in the present study Stxbp1+/− mice also showed elevated anxiety. This may derive from the varying behavioral paradigms used. Their study performed the open field and elevated plus maze tests, while our present study used the light-dark transition test. Alternatively, deviations from the optimal level of neurotransmission may similarly affect the level of anxiety.
Contextual and cue-evoked fear conditioning require proper functioning of the amygdala, while contextual fear conditioning is hippocampus dependent (51). Since Stxbp1+/− mice displayed deficits in cue-evoked fear conditioning, impaired functions of the amygdala and hippocampus are suggested. The partial impairment of fear memory in Stxbp1fl/+/Emx mice is consistent with this idea. Possibly different or opposed learning effects can be expected from reduced glutamate release in Stxbp1fl/+/Emx mice compared with reduced GABA release Stxbp1fl/+/Vgat mice. However, these mice have similar memory deficits. Since the brain glutamatergic and GABAergic neurons are both involved in fear learning (52) in ways of excitatory, inhibitory, or disinhibitory manners, it is possible that the dysfunction of either neurotransmitter system may consequently hamper fear learning. Alternatively, fear-learning paradigm which focuses on freezing response might not be sufficient to distinguish differences between them in fear-memory processing. In addition, dissociations of fear-learning and cognitive abilities have been reported (53,54).
Endocannabinoids suppress the synaptic transmission through the phosphorylation of Munc18–1 (55,56), thus serving the homeostatic control of synaptic strength through negative feedback. In particular, endocannabinoids are implicated in fear, anxiety, synaptic plasticity, and learning (57). These findings are indeed consistent with our present results in Stxbp1+/− mice. We also observed differences between WT and Stxbp1+/− mice in the gamma activity elicited by fear conditioning in multiple brain regions. Gamma activity is believed to be associated with various cognitive functions such as attention, sensory perception, learning/memory, and social activity (21,22), and may also underlie neural computations and inter-regional communications (58). Therefore, the abnormal gamma activity in Stxbp1+/− mice may affect neuronal processing and coordination among brain regions. Significantly higher LFP gamma activities in the mPFC, amygdala, thalamus, and hippocampus of Stxbp1+/− mice suggest that these brain regions are responsible for the impaired fear memory formation. Indeed, these brain regions have been previously implicated in fear memory (52,59). Aberrant gamma oscillations have also been implicated in neuropsychiatric diseases such as schizophrenia and ASD (24). Excessive gamma activity was reported in young patients with ASD (23) and in an animal of schizophrenia (60). Furthermore, impairments of social behavior and conditional learning together with increased gamma power have been associated with the selective activation of PFC pyramidal neurons (61). Thus, neuronal dysfunction caused by excessive gamma activity could explain the deficits in learning/memory and emotional processing in Stxbp1+/− mice. Thus, the aberrant regional gamma activity could be a biomarker of these disorders.
Our findings would be applicable to understand a broad spectrum of neurodevelopmental and psychiatric diseases with synaptic deficits, and provide insights into the role of excitatory transmission in aggression. Furthermore, while atypical antipsychotics are often used to treat clinical aggression in schizophrenia, autism, or dementia (33,62–64), our present study offers new possibilities of therapeutic alternatives to manage aggression by normalizing excitatory transmission.
Materials and Methods
All animal experimental protocols were approved by the Animal Experiment Committee of RIKEN Brain Science Institute. Mice were handled in accordance with the guidelines of the Animal Experiment Committee. Food and water were available ad libitum, and cages were kept at 23 °C and 55% humidity on a 12-h light/dark cycle with the lights off at 20: 00. All mice were housed in groups of two to five mice per cage until behavioral tests.
Stxbp1 constitutional and conditional knockout mice
Stxbp1 floxed mice were produced by homologous recombination using the C57BL/6N-derived ES cell line RENKA (65). A genomic fragment carrying exon 2–6 of the Stxbp1 gene was isolated by PCR from C57BL/6N genome. A DNA fragment carrying the loxP sequence and neomycin-resistance cassette flanked by two frt sites was inserted into the site 162 bp upstream of the exon 3, while the other loxP sequence was inserted into the site 159 bp downstream of the exon 3. The targeting vector contained the floxed exon, 3.5 kb upstream and 6.5 kb downstream genomic sequences, and 4.3 kb pMC1DTpA. Establishment of targeted ES clones and generation of chimeras were performed as described previously (65). Stxbp1 floxed mice were maintained on a C57BL/6N background. To generate Stxbp1 knockout mice, Stxbp1fl/+ mice were cross-mated with EIIA-Cre mice (66). Heterozygous (Stxbp1fl/+/EIIA-Cre) offspring were subsequently backcrossed with C57BL/6N mice to obtain Stxbp1+/− mice lacking the EIIA-Cre transgene. Empty spiracles homolog 1 (Emx1)-Cre knock-in line (25,67) and Vesicular GABA transporter (Vgat)-Cre BAC transgenic line (26) was described previously, and maintained on a C57BL/6J background.
Western blot analysis
Brains were removed from mice and cortex and hippocampus were dissected, frozen in liquid nitrogen, and stored at −80 °C until analyzed. The brain tissues were homogenized for 12 strokes in homogenization buffer [1× PBS (−) and 1× complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA), pH 7.4] using Potter-type homogenizer. The homogenates were sonicated and centrifuged for 5 min at 3300g, with resulting supernatants centrifuged for 10 min at 20 000g, and the supernatants were recovered as protein extracts. The protein concentration was determined by the Bradford method. Protein (8.4 µg) was resolved by SDS–PAGE using 5–20% polyacrylamide SuperSep™ Ace gels (Wako Pure Chemical Industries, Osaka, Japan). Western blot analysis was performed using a mouse monoclonal antibody against whole Munc18–1 (epitope undefined) (catalog number 116 011, 1: 1000, Synaptic Systems GmbH, Goettingen, Germany), or rabbit polyclonal antibody (catalog number116 002, 1: 1000, Synaptic Systems GmbH, Goettingen, Germany) raised against Munc18–1 C-terminal sequence (amino acids 580–594). GAPDH (catalog number: 1D4-ADI-CSA-335-E, 1: 2000, Enzo Life Sciences, Farmingdale, NY, USA) was used for normalization of Western blot analyses. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (catalog number: W4021, 1: 5000; Promega, Madison, WI, USA) antibodies. Bound antibodies were detected using enhanced chemiluminescence reagent (PerkinElmer, Boston, MA, USA). Semi-quantitation of proteins was performed using the NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA). Mean expression levels were estimated by comparison with serial dilutions of homogenates from age-matched control mice and represented as percentages relative to control mice.
Behavioral tests
Adult (2–7 months of age) Stxbp1+/− and wild-type (WT) littermate male mice from age-matched litters were used. During behavioral tests, all mice were housed individually to avoid intermale aggression. Time intervals between the tests were separated by over a week. The order of behavioral tests was basically as follows: novel open field activity test, elevated-plus maze test, light-dark transition test, Y-maze test, three-chambered social interaction test, open field interaction test, resident-intruder test and fear memory test.
Novel open field activity test
Exploratory and locomotor activities of mice in a novel open field arena (60 × 60 × 40 cm, 70 lux) were automatically analyzed every 10 min during a 30-min test period (O'Hara & Co., Tokyo, Japan). Total distance traveled, speed, rearing number, and time spent in the center of the open field (area >12.5 cm from the walls) were quantified. Images were captured at 2 frames per second. The Stxbp1+/− mice were subsequently used for the open field interaction test, resident-intruder test and cue-evoked fear-learning test. The Stxbp1fl/+/Emx and Stxbp1fl/+/Vgat mice were subsequently used for contextual fear-learning test.
Elevated-plus maze test
The elevated-plus maze test was performed as previously described (68). The maze was consisted of two orthogonal closed and open arms (25 cm long) with a quadrangular area located at the intersection (O’Hara & Co.). The arms were set 50 cm above the floor. The walls of the closed arms were made from transparent, clear plates. Light intensity was 70 lux at the center field of the maze. Each mouse was placed in the center of the maze facing the closed arms, and allowed to explore for 10 min. The time spent in the open arms, closed arms and center field and the number of entries into the open and closed arms were measured. These Stxbp1+/− mice were subsequently used for the light-dark transition test and three-chambered social interaction test.
Light–dark transition test
The light–dark transition test was performed as previously described (68). The light-dark box consisted of two equal-sized plastic boxes (20 × 20 × 20 cm) connected by a shuttle door (3 × 5 cm) located in the center of the partition (O'Hara & Co.). Each mouse was placed on the dark side of the apparatus and allowed to explore the boxes for 10 min. Measurements of the number of transitions between the light (250 lux) and dark (2 lux) sides and time spent in each side were made.
Y-maze test
In Y-maze test (69), size of each arm is 40 cm long, 12 cm high, 3 cm wide at the bottom and 10 cm wide at the top. After placing a mouse at the end of an arm, the mouse was allowed to move freely through the maze during a 5 min test session. A sequence of arm entries was used for calculation of an alternation which is defined as the number of entries into all three arms sequentially. The percent alternation is calculated as (actual alternations/maximum alternations) ×100.
Three-chambered social interaction test
The apparatus was a rectangular, three chambered box made from non-transparent, gray plates, which was placed in a soundproof outer box (68) (O’Hara & Co.). Two transparent partitions divide the box into three identical 20-cm-long chambers. Square openings in the bottom of the partitions allowed mice to move among the three chambers. A stranger moue (C57BL/6J males, 8 weeks) was placed in a cage with vertical grid, and the stranger mouse was habituated in the cage for 10 min before the test. Initially, a test mouse was placed in the center chamber of the three-chambered box, where empty cages with vertical grid were located on the corner of the both side chambers, and allowed to explore the entire box for 10 min (‘empty-empty’). Next, the empty cage was replaced with the wire cage containing the stranger mouse (‘stranger-empty’), then the test mouse was allowed to explore the test box for 10 min (test for sociability). Time spent around each cage, time spent in each chamber and total distance traveled were automatically quantified.
Open field interaction test
Mice were housed individually for over 7 days before open field interaction test. An Stxbp1+/− or WT test mouse was introduced together with a stranger male mouse (6 weeks, C57BL/6N, Japan SLC) into the open field arena (60 × 60 × 40 cm, 70 lux) and allowed to explore freely for 10 min. Social interaction was monitored by a color charge-coupled device camera (2 frames/s) and analysis was manually performed. The number of active contacts initiated by the test mice (WT or Stxbp1+/−), mean duration per contact and total duration of contact were quantified by the investigator blinded to genotypes of the mice. Active contact includes attacking, sniffing, touching, following, and chasing by the test mice.
Resident-intruder test
Stxbp1+/− or WT resident test mice were housed in home cages individually for over 7 days before the test (70). After introducing a male intruder mouse (C57BL/6N, 6 weeks) to the home cage (28 × 17 × 13 cm, 70 lux) of the resident mouse (WT or Stxbp1+/−), the duration and number of attack, pursuit/chasing and sniffing by the resident mice for 10 min were scored by the investigator blinded to genotypes of the mice.
Contextual fear and cue-evoked fear memory test
Fear conditioning (71) was performed in a translucent plastic chamber with a stainless-steel grid floor (10 × 10 × 10 cm, 210 lux) in a soundproof box with white noise (50 dB). Initially, a mouse was allowed to explore the chamber for 3 min, and received three pairs of conditioned stimulus (CS: 60 dB white noise for 30 s) and unconditioned stimulus (US: 0.3 mA electrical foot shock for 2 s) delivered at the last 2 s of the CS period. The interval between the CS-US pairings was set to 1 min. After the end of the CS-US presentation, the mouse remained in the chamber for 1 min and was returned to the home cage. Twenty-four hours after the conditioning, contextual fear memory was tested in the same conditioning chamber for 3 min without the CS and US.
Auditory cue-evoked fear memory test was performed in a test chamber distinct from the conditioning chamber in shape (triangular prism), luminance (50 lux), floor (paper chips bedding) and background white noise (60 dB). Twenty-four hours after the conditioning, the mouse was put in the test chamber for 3 min exploration, and then 10 pairs of CS alone were presented (65 dB white noise for 30 s). The interval between the CS was set to 30 s. Following the CS presentation, the mouse remained in the chamber for another 1 min. Freezing response (immobility excluding respiration) of the mice was automatically quantified by ImageJ software (O’Hara). Once mice were used for fear memory test, behavioral experiments of the mice were terminated.
Rotarod test
The tests using an accelerating-rotarod (72) were conducted for consecutive 3 days. On the first day, animals were habituated to the rotarod apparatus (Muromachi Kikai Co., Ltd., Tokyo, Japan) at constant 4 rpm for 30 s. Four trials on the rotarod linearly accelerating from 4 to 40 rpm in 300 s were performed in a day with a 600 s interval. The latency when the mouse fell off was recorded.
Drug administration
In the resident-intruder and rotarod tests, mice were injected vehicle (saline, 5 µl/g of body weight), low CX516 (40 mg/kg, 8 mg/ml in saline) or high CX516 (100 mg/kg, 20 mg/ml in saline) intraperitoneally 10 min before the tests.
Cue-evoked fear conditioning combined with somatosensory electrocorticogram (S1 ECoG) and local field potential (LFP) recordings
Stainless steel screws (1.1 mm diameter) served as S1 ECoG electrode was placed over the right somatosensory cortex (1.0 mm posterior to bregma, 1.5 mm to the midline) (73). A stainless screw as a reference and ground electrode was placed on the cerebellum. Stainless wire monopolar electrode was inserted in the cervical region of the trapezius muscle for electromyogram (EMG). For monopolar local field potential (LFP) recordings of brain regions, stainless wires (200 μm diameter) were stereotaxically implanted contralateral to the ECoG electrode according to the following coordinates (anterior-posterior, medial-lateral, depth from the cortical surface, mm): medial prefrontal cortex (1.9, 0.3, 1.4), caudate-putamen (0.0, 2.4, 2.5), basolateral amygdala (−1.4, 2.9, 3.7), ventroposterior thalamus (−1.8, 1.5, 3.2), hippocampus CA1 region (−2.5, 2.2, 1.1), visual cortex binocular zone (−3.4, 3.0, 0.4). Contacts between the electrode and brain surface were covered with a small amount of Vaseline and secured with dental acrylic. An antibiotic (ampicillin) was used in surgery. After at least 1 week recovery from implant surgery, the animals were tethered to a 16-channel commutator (Plexon, Dallas, TX) and allowed to move freely during recording in an electrically shielded cage with food and water available ad libitum. ECoG, EMG and LFPs (filtered 0.7–170 Hz, 1 KHz sampling) were recorded using the MAP data acquisition system (Plexon, Dallas, TX) and analyzed off-line using a software (NeuroExplorer, Nex Technology, Madison, AL).
The mice implanted electrodes were subjected to the cue-evoked fear conditioning test. Baseline brain activity with and without 30 s buzzer sound as a CS was recorded in the recording chamber (LE100290, PanLab, Spain). Twenty-four hours later, fear conditioning was conducted using a chamber with metal grid (20 × 20 × 25 cm, Harvard Apparatus), simultaneously animals’ behavior was recorded using an infrared camera. Three pairs of auditory CS and US (2 s, 0.3 mA electrical foot shock) delivered at the last 2 s of the CS period was controlled by a software (PACKWIN, Panlab, Spain). Again, 24 h after conditioning, brain activity of the conditioned mouse was recorded in the same chamber for a baseline recording. Freezing behavior was scored by the investigator blinded to genotypes of the mice. Power spectrum analysis of ECoG and LFPs were calculated (0–100 Hz, 0.5 Hz bin) by NeuroExplorer. Power densities corresponding to defined oscillations were summed for absolute LFP power (e.g. gamma: 50–70 Hz). Upon completion of the experiment using ECoG and LFP recordings, positions of electrodes and cannulas were verified histologically using hematoxylin and eosin staining (30 μm brain sections).
Statistics
Statistical analyses were performed using Prism 5 (GraphPad Software, La Jolla, CA, USA). Comparisons between two genotype groups were performed using Student‘s t-test, two-sided. When the variance of data set was significantly different, we used nonparametric statistical analysis, Mann-Whitney U test. P value smaller than 0.05 was considered significant. For comparison between genotypes in fear conditioning test, two-way repeated measures analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons was applied. Data are represented as mean ± standard error of mean (SEM). *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Supplementary Material is available at HMG online.
Acknowledgements
We are grateful to all members of Laboratory for Neurogenetics at RIKEN Brain Science Institute (RIKEN-BSI) for helpful discussion, Ms. Miyuki Murayama for biochemical analysis, Ms. Nancy Malintan and the Support Unit for Animal Resources Development in RIKEN-BSI Research Resources Center for mouse maintenance.
Conflict of Interest statement. None declared.
Funding
Strategic Research Program for Brain Sciences (SRPBS) of Japanese Ministry of Education, Culture, Sports, Sciences and Technology (MEXT) and Japan Agency for Medical Research and Development (AMED), and RIKEN-BSI (K.Y.); MEXT Grants-in-Aid for Scientific Research (C) (15K09848), Kawano Masanori Memorial Foundation for Promotion of Pediatrics and Japan Epilepsy Research Foundation (H.M.).