-
PDF
- Split View
-
Views
-
Cite
Cite
J Haas, R Bassil, N Samara, E Zilberberg, C Mehta, R Orvieto, R F Casper, GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study, Human Reproduction, Volume 35, Issue 7, July 2020, Pages 1648–1654, https://doi.org/10.1093/humrep/deaa107
Close - Share Icon Share
Abstract
Does co-administration of GnRH agonist and Human chorionic gonadotropin (hCG; dual trigger) in IVF cycles improve the number of mature oocytes and pregnancy outcome compared to hCG alone?
Using the dual trigger for final follicular maturation increases the number of oocytes, mature oocytes and number of blastocysts (total and top-quality) compared to triggering with hCG alone.
hCG is used at the end of controlled ovarian hyperstimulation as a surrogate LH surge to induce final oocyte maturation. Recently, based on retrospective studies, the co-administration of GnRH agonist and hCG for final oocyte maturation (dual trigger) has been suggested to improve IVF outcome and pregnancy rates
A single center, randomized controlled, double-blinded clinical trial between May 2016 and June 2018 analyzed by intention to treat (ITT).
One hundred and fifty-five normal responder patients were randomized either to receive hCG or dual trigger for final oocyte maturation. Data on patients age, BMI, AMH, number of oocytes retrieved, number of metaphase 2 (MII) oocytes, zygotes and blastocysts, clinical pregnancy rate and live birth rate were assessed and compared between the dual trigger group and the hCG group. We performed a planned interim analysis after the recruitment of 50% of the patients. Based on the totality of outcomes at the interim analysis we decided to discontinue further recruitment.
One hundred and fifty-five patients were included in the study. The age (36 years versus 35.3 years P = NS), BMI (24 kg/m2 versus 23.7 kg/m2) and the AMH (20.1 pmol/l versus 22.4 pmol/l) were comparable between the two groups. Based on ITT analysis, the number of eggs retrieved (11.1 versus 13.4, P = 0.002), the MII oocytes (8.6 versus 10.3, P = 0.009), total number of blastocysts (2.9 versus 3.9, P = 0.01) and top-quality blastocysts transferred (44.7% versus 64.9%; P = 0.003) were significantly higher in the dual trigger group compared to the hCG group. The clinical pregnancy rate (24.3% versus 46.1%, OR 2.65 (1.43–1.93), P = 0.009) and the live birth rate per transfer (22% versus 36.2%, OR= 1.98 (1.05–3.75), P = 0.03) were significantly higher in the dual trigger group compared to the hCG group.
None.
The enhanced response observed with the dual trigger might lead to better IVF outcomes were it used more widely.
The study was funded by TRIO Fertility. There are no conflicts of interest to declare.
ClinicalTrials.gov identifier: NCT02703584
March 2016
May 2016
Introduction
In most mammalian species, spontaneous ovulation is preceded by a surge of both FSH and LH, which is thought to induce final follicular maturation with a loss of gap junctions between the oocyte and cumulus cells, cumulus expansion, germinal vesicle breakdown, resumption of meiosis and luteinization of the granulosa cells (GCs).
In contrast, in the standard/conventional controlled ovarian hyperstimulation (COH) regimen, final follicular maturation has traditionally been triggered by one bolus of human chorionic gonadotropin (hCG) (5000–10 000 units) or more recently, by a bolus of GnRH agonist or both hCG and GnRH agonist (Orvieto, 2015). hCG, a surrogate to the naturally occurring LH surge, is administered as close as possible to the time of ovulation, i.e. 36 h before oocyte recovery and its activity lasts for several days throughout the luteal phase.
In contrast to hCG, the GnRH agonist-induced surge more closely resembles the natural mid-cycle surge of gonadotropins and exposes follicles to both LH and FSH. However, after GnRH agonist triggering, the surge of gonadotropins is shorter in duration (12–36 h versus 48 h) (Gonen et al., 1990; Itskovitz et al., 1991) and there is often poor LH support for the corpora lutea, or actual luteolysis, after ovulation so that intensive luteal support is required to ensure implantation and ongoing pregnancy (Casper, 2015, Humaidan et al., 2015).
Studies comparing the GnRH agonist versus hCG trigger have revealed that the number of oocytes retrieved, percentage of mature oocytes and number of top-quality embryos (TQE) were either comparable or in favor of the GnRH agonist trigger (Humaidan et al., 2005; Orvieto, 2015). Moreover, when analyzing the downstream effects of LH receptor activation, hCG was shown to generate higher intracellular cAMP accumulation, which stimulates steroidogenesis, while LH had a greater impact on extracellular signal-regulated protein kinase (ERK1/2) phosphorylation and AKT, responsible for GCs proliferation, differentiation and survival (Casarini et al., 2012).
Because of these observations, hCG combined with GnRH agonist trigger for final follicular maturation has been implemented in clinical practice aiming to improve IVF cycle outcome. Lin et al. (2013) examined whether dual triggering of final oocyte maturation with a combination of GnRH agonist and hCG (both administered 36 h prior to oocyte retrieval) could improve the live birth rate of normal responders undergoing the GnRH antagonist COH protocol. In their retrospective cohort study, including almost 400 cycles, the mean numbers of oocytes retrieved and mature metaphase II (MII) oocytes were both statistically significantly greater in the dual-trigger group compared to an hCG trigger alone. Moreover, while the mean number of embryos and TQE, as well as the rate of blastocyst progression, were similar between the two groups, the dual trigger group demonstrated statistically significantly higher implantation, clinical pregnancy and live birth rates compared with the hCG trigger group.
In 2017, a meta-analysis was published comparing hCG with dual triggering and concluded that the number of oocytes, embryos and TQE were comparable, but the pregnancy rate was significantly higher in the dual trigger group (Ding et al., 2017).
Prompted by the aforementioned observations, we sought to perform a prospective, randomized controlled double-blinded study in normal responders, comparing cycles triggered with hCG versus dual trigger (hCG + GnRH agonist) 36 h prior to oocytes retrieval.
Materials and methods
A prospective, randomized, double-blinded clinical trial (ClinicalTrials.gov identifier: NCT02703584), enrolled patients attending our university-affiliated Infertility and IVF center between May 2016 and June 2018. The study was approved by our institutional review board (IRB) and all participants provided a written informed consent before entry.
The inclusion criteria for participating in the study were: women age 18–41 years, with BMI (body mass index) of 18–35 kg/m2, AMH (anti-Müllerian hormone) >1 ng/ml, AFC (antral follicular count) 6–20 and FSH ˂20 IU/l undergoing one of their first three IVF cycle attempts.
We excluded patients with poor ovarian reserve (AMH <1 ng/ml), patients at high risk of developing ovarian hyperstimulation syndrome (OHSS; E2 levels >15 000 pmol/l or AFC >20), patients with BMI >35 and patients with moderate–severe endometriosis.
Stimulation protocol
Gonadotropin treatment was initiated on the third day of menses with the use of recombinant FSH (Gonal-F, EMD Serono, Mississauga, Ontario, Canada). Once the leading follicle reached a size of 13 mm, and/or E2 levels exceeded 1200 pmol/l, co-treatment with GnRH antagonist 0.25 mg/day (Cetrotide, Serono or Orgalutran, Merck, Kirkland, Quebec, Canada) and recombinant LH (Luveris, Serono) or highly purified human menopausal gonadotropin (Menopur, Ferring, Toronto, Ontario, Canada) were commenced. Follicle growth and hormone levels were serially monitored by ultrasound and blood tests. When the dominant follicles reached an average diameter of 18–20 mm (the morning of the trigger day) the investigators recruited the patients for the study. After the participants provided a written informed consent, the investigators provided the patients a sealed envelope with a syringe (and a number on the syringe). The nurses, physicians, embryologists and the patients were all blinded. The study coordinator prepared the syringes with GnRH agonist/placebo once a week. Only the study coordinator had access to the coding of the syringes.
The patients were prospectively randomized into two double-blinded groups: (i) hCG group: Patients were triggered for final follicular maturation with hCG (Pregnyl 10 000 IU; Merck) and placebo (normal saline)—36 h prior to oocyte aspiration. (ii) The dual trigger group: Patients were triggered with GnRH agonist (Suprefact 0.5 mg; Sanofi-Aventis, Lval, Quebec, Canada) and hCG (Pregnyl 10 000 IU) 36 h prior to the oocyte aspiration.
The GnRH agonist or placebo was provided by the clinic without any additional cost to the patient.
All the patients were supplemented with 200 mg micronized progesterone in vaginal ovules three times daily, that is, in the morning, at mid-day, and before bed (Prometrium, Merck).
Outcomes
The primary outcome was number of mature oocytes (MII) analyzed by ITT. The secondary outcomes included: total number of oocytes, zygotes, blastocysts, top-quality blastocysts, clinical pregnancy rate, implantation rate, live birth rate per transfer and per patient, also analyzed by ITT.
Each blastocyst was also given a grade according to the simplified SART (Society for Assisted Reproductive Technology) grading system which was proposed by Heitmann et al. (2013). SART grade good was assigned for inner cell mass (ICM) grade A and trophectoderm (TE) grade A or B (AA or AB blastocysts).
Statistical analysis
Sample size calculation was performed for the primary outcome mature oocytes (MII). In order to increase the rate of MII from to 65% to 80% with an alpha error of 5% and beta error of 80% the sample size needed is 276 patients in both groups. We performed a planned interim analysis after the recruitment of 50% of the patients. Based on the totality of outcomes at the interim analysis we decided to discontinue further recruitment. All randomized subjects were included in an intention to treat (ITT) analysis. Statistical analysis was performed with Student’s independent t-test, and Fisher’s exact test, as appropriate. Results are presented as mean + 95% confidence intervals (CIs); P-values <0.05 were considered significant.
Logistic regression analysis was employed for multivariate analysis. Variables used in the regression model included maternal age, number of top-quality blastocysts and method of triggering.
Results
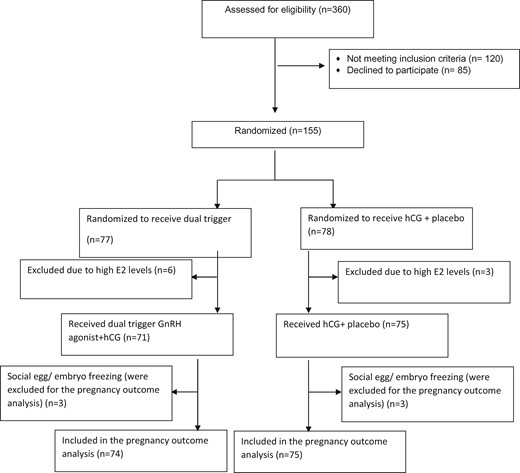
One hundred and fifty-five patients were recruited in the study. This included 75 of 78 patients in the hCG group and 71 of 77 patients in the dual trigger group. Nine patients did not meet inclusion criteria a few hours after the randomization (six patients in the dual trigger arm and three patients in the hCG arm) due to E2 levels >15 000 pmol/l or AFC >20 (exclusion criteria) and those patients were triggered with GnRH agonist only (Fig. 1). We included the data from all the patients in the study for ITT analysis.
No between-group differences were observed in patient demographic characteristics (Table I) nor COH variables such as: the length of stimulation, total dose of gonadotropins, peak E2 levels at the trigger day and number of follicles >10 and >15 mm at the trigger day (Table II).
Patients’ characteristics.
| . | hCG . | Dual trigger . |
|---|---|---|
| N | 78 | 77 |
| Age, year | 36, (35.2–36.8) | 35.4, (34.6–36.2) |
| BMI, kg/m2 | 24.1, (22.9–25.1) | 23.6, (22.9–24.5) |
| Gravidity | 0.8, (0.50–1.09) | 0.7, (0.39–1 |
| Parity | 0.2, (0.04–0.35 | 0.2, (0.10–0.29) |
| Etiology | ||
| Unexplained | 34 | 36 |
| Male factor | 17 | 17 |
| Mechanical | 4 | 1 |
| Other | 23 | 23 |
| AMH (pmol/l) | 20, (16–24.2) | 22.41, (19.1–25.7) |
| . | hCG . | Dual trigger . |
|---|---|---|
| N | 78 | 77 |
| Age, year | 36, (35.2–36.8) | 35.4, (34.6–36.2) |
| BMI, kg/m2 | 24.1, (22.9–25.1) | 23.6, (22.9–24.5) |
| Gravidity | 0.8, (0.50–1.09) | 0.7, (0.39–1 |
| Parity | 0.2, (0.04–0.35 | 0.2, (0.10–0.29) |
| Etiology | ||
| Unexplained | 34 | 36 |
| Male factor | 17 | 17 |
| Mechanical | 4 | 1 |
| Other | 23 | 23 |
| AMH (pmol/l) | 20, (16–24.2) | 22.41, (19.1–25.7) |
Data are means (95%CI) or n.
AMH, anti-Müllerian hormone.
Patients’ characteristics.
| . | hCG . | Dual trigger . |
|---|---|---|
| N | 78 | 77 |
| Age, year | 36, (35.2–36.8) | 35.4, (34.6–36.2) |
| BMI, kg/m2 | 24.1, (22.9–25.1) | 23.6, (22.9–24.5) |
| Gravidity | 0.8, (0.50–1.09) | 0.7, (0.39–1 |
| Parity | 0.2, (0.04–0.35 | 0.2, (0.10–0.29) |
| Etiology | ||
| Unexplained | 34 | 36 |
| Male factor | 17 | 17 |
| Mechanical | 4 | 1 |
| Other | 23 | 23 |
| AMH (pmol/l) | 20, (16–24.2) | 22.41, (19.1–25.7) |
| . | hCG . | Dual trigger . |
|---|---|---|
| N | 78 | 77 |
| Age, year | 36, (35.2–36.8) | 35.4, (34.6–36.2) |
| BMI, kg/m2 | 24.1, (22.9–25.1) | 23.6, (22.9–24.5) |
| Gravidity | 0.8, (0.50–1.09) | 0.7, (0.39–1 |
| Parity | 0.2, (0.04–0.35 | 0.2, (0.10–0.29) |
| Etiology | ||
| Unexplained | 34 | 36 |
| Male factor | 17 | 17 |
| Mechanical | 4 | 1 |
| Other | 23 | 23 |
| AMH (pmol/l) | 20, (16–24.2) | 22.41, (19.1–25.7) |
Data are means (95%CI) or n.
AMH, anti-Müllerian hormone.
Stimulation cycle characteristics.
| . | hCG . | Dual trigger . |
|---|---|---|
| Length of stimulation (days) | 11 (10.6–11.4) | 10.9 (10.5–11.3) |
| Total dose of gonadotropins (IU) | 3184 (2880–3490) | 3209 (2950–3570) |
| E2 levels at the trigger day (pmol/l) | 6818 (5610–7240) | 8120 (6980–8920 |
| Number of follicles >10 mm at the trigger day | 14.3 (13.2–15.4) | 14.8 (13.6–16) |
| Number of follicles >15 mm at the trigger day | 9.2 ( 8.4–10) | 9.7 (8.8–10.5) |
| . | hCG . | Dual trigger . |
|---|---|---|
| Length of stimulation (days) | 11 (10.6–11.4) | 10.9 (10.5–11.3) |
| Total dose of gonadotropins (IU) | 3184 (2880–3490) | 3209 (2950–3570) |
| E2 levels at the trigger day (pmol/l) | 6818 (5610–7240) | 8120 (6980–8920 |
| Number of follicles >10 mm at the trigger day | 14.3 (13.2–15.4) | 14.8 (13.6–16) |
| Number of follicles >15 mm at the trigger day | 9.2 ( 8.4–10) | 9.7 (8.8–10.5) |
Data are mean (95% CI).
E2, estradiol.
Stimulation cycle characteristics.
| . | hCG . | Dual trigger . |
|---|---|---|
| Length of stimulation (days) | 11 (10.6–11.4) | 10.9 (10.5–11.3) |
| Total dose of gonadotropins (IU) | 3184 (2880–3490) | 3209 (2950–3570) |
| E2 levels at the trigger day (pmol/l) | 6818 (5610–7240) | 8120 (6980–8920 |
| Number of follicles >10 mm at the trigger day | 14.3 (13.2–15.4) | 14.8 (13.6–16) |
| Number of follicles >15 mm at the trigger day | 9.2 ( 8.4–10) | 9.7 (8.8–10.5) |
| . | hCG . | Dual trigger . |
|---|---|---|
| Length of stimulation (days) | 11 (10.6–11.4) | 10.9 (10.5–11.3) |
| Total dose of gonadotropins (IU) | 3184 (2880–3490) | 3209 (2950–3570) |
| E2 levels at the trigger day (pmol/l) | 6818 (5610–7240) | 8120 (6980–8920 |
| Number of follicles >10 mm at the trigger day | 14.3 (13.2–15.4) | 14.8 (13.6–16) |
| Number of follicles >15 mm at the trigger day | 9.2 ( 8.4–10) | 9.7 (8.8–10.5) |
Data are mean (95% CI).
E2, estradiol.
The number of oocytes (11.1 versus 13.4, P = 0.002), number of MII (8.6 versus 10.3, P = 0.009), number of blastocysts (2.9 versus 3.9, P = 0.01) and number of top-quality blastocysts (1.4 versus 2.2, P = 0.001) were significantly higher in the dual trigger group (Table III), as compared to the hCG group. In the hCG group, 14/78 patients decided to perform preimplantation genetic testing for aneuploidies (PGT-A) on their embryos, and in the dual trigger group 20/77 of the patients performed PGT-A.
Embryology outcome, calculated by intention to treat.
| . | hCG . | Dual trigger . | Mean difference, (95% CI) . | P-value . |
|---|---|---|---|---|
| Number of patients | 78 | 77 | ||
| Oocytes (n) | 11.1 | 13.4 | 2.4 (0.8–3.9) | 0.002 |
| Oocytes/n of follicles >10 | 77.8% | 93.4% | 16% (5.2–26.7%) | 0.005 |
| MII (n) | 8.6 | 10.3 | 1.7 (0.4–3) | 0.009 |
| 2PN (n) | 6.3 | 7.8 | 1.6 (0.4–2.7) | 0.007 |
| Cleavage stage embryos (n) | 5.6 | 7.0 | 1.3 (0.2–2.4) | 0.02 |
| Blastocyst (n) | 2.9 | 3.9 | 1 (0.2–1.9) | 0.01 |
| Top-quality blastocyst (n) | 1.4 | 2.4 | 0.9 (0.4–1.5) | 0.001 |
| . | hCG . | Dual trigger . | Mean difference, (95% CI) . | P-value . |
|---|---|---|---|---|
| Number of patients | 78 | 77 | ||
| Oocytes (n) | 11.1 | 13.4 | 2.4 (0.8–3.9) | 0.002 |
| Oocytes/n of follicles >10 | 77.8% | 93.4% | 16% (5.2–26.7%) | 0.005 |
| MII (n) | 8.6 | 10.3 | 1.7 (0.4–3) | 0.009 |
| 2PN (n) | 6.3 | 7.8 | 1.6 (0.4–2.7) | 0.007 |
| Cleavage stage embryos (n) | 5.6 | 7.0 | 1.3 (0.2–2.4) | 0.02 |
| Blastocyst (n) | 2.9 | 3.9 | 1 (0.2–1.9) | 0.01 |
| Top-quality blastocyst (n) | 1.4 | 2.4 | 0.9 (0.4–1.5) | 0.001 |
MII, metaphase 2; 2PN, 2-pronucleate.
Embryology outcome, calculated by intention to treat.
| . | hCG . | Dual trigger . | Mean difference, (95% CI) . | P-value . |
|---|---|---|---|---|
| Number of patients | 78 | 77 | ||
| Oocytes (n) | 11.1 | 13.4 | 2.4 (0.8–3.9) | 0.002 |
| Oocytes/n of follicles >10 | 77.8% | 93.4% | 16% (5.2–26.7%) | 0.005 |
| MII (n) | 8.6 | 10.3 | 1.7 (0.4–3) | 0.009 |
| 2PN (n) | 6.3 | 7.8 | 1.6 (0.4–2.7) | 0.007 |
| Cleavage stage embryos (n) | 5.6 | 7.0 | 1.3 (0.2–2.4) | 0.02 |
| Blastocyst (n) | 2.9 | 3.9 | 1 (0.2–1.9) | 0.01 |
| Top-quality blastocyst (n) | 1.4 | 2.4 | 0.9 (0.4–1.5) | 0.001 |
| . | hCG . | Dual trigger . | Mean difference, (95% CI) . | P-value . |
|---|---|---|---|---|
| Number of patients | 78 | 77 | ||
| Oocytes (n) | 11.1 | 13.4 | 2.4 (0.8–3.9) | 0.002 |
| Oocytes/n of follicles >10 | 77.8% | 93.4% | 16% (5.2–26.7%) | 0.005 |
| MII (n) | 8.6 | 10.3 | 1.7 (0.4–3) | 0.009 |
| 2PN (n) | 6.3 | 7.8 | 1.6 (0.4–2.7) | 0.007 |
| Cleavage stage embryos (n) | 5.6 | 7.0 | 1.3 (0.2–2.4) | 0.02 |
| Blastocyst (n) | 2.9 | 3.9 | 1 (0.2–1.9) | 0.01 |
| Top-quality blastocyst (n) | 1.4 | 2.4 | 0.9 (0.4–1.5) | 0.001 |
MII, metaphase 2; 2PN, 2-pronucleate.
More fresh transfers were performed in the hCG group compared to the dual trigger group (33 versus 23, respectively) and the clinical pregnancy rate was comparable between the two groups in those cycles (51.5% versus 52.1%, P = 1). In the hCG group, there were 1.07 embryos per transfer and in the dual trigger group 1.03 embryos per transfer. When selecting embryos for transfer, 64.9% (63/97) of the embryos transferred in the dual trigger group were TQE and 44.7% (51/114) of the embryos transferred in the hCG group were TQE (P = 0.003).
The implantation rate (22.8% versus 43.7% P = 0.003), the live birth rate per transfer (22% versus 36.2%, P = 0.03) and the clinical pregnancy rate per patient (37.3% versus 56.8% P = 0.02) were significantly higher in the dual trigger group. The cumulative live birth rate per patient was not significantly different (32% versus 44.6%, P = 0.11) (Table IV). There were no cases of OHSS in either group.
Pregnancy outcome, calculated by intention to treat.
| . | hCG . | Dual trigger . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Number of patients included in the analysis | 75 | 74 | |||
| Clinical pregnancy rate (fresh transfers) | 17/33 (52%) | 12/23 (52%) | 1.02 | 0.35–2.98 | 0.96 |
| Total Implantation rate | 28/114 (23%) | 42/97 (43%) | 2.74 | 1.32–4.29 | 0.003 |
| TQE transferred/all embryos transferred | 51/114 (45%) | 63/97 (65%) | 2.29 | 1.31–3.99 | 0.003 |
| Clinical pregnancy rate per transfer | 28/107 (24%) | 42/91 (46%) | 2.65 | 1.33–4.39 | 0.009 |
| Clinical pregnancy rate per patient | 28/75 (37) | 42/74 (57%) | 2.20 | 1.14–4.24 | 0.01 |
| Live birth per transfer | 24/107 (22%) | 33/91 (36%) | 1.98 | 1.05–3.67 | 0.03 |
| Cumulative live birth per patient | 24/75 (32%) | 33/74 (45%) | 1.67 | 0.87–3.32 | 0.11 |
| . | hCG . | Dual trigger . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Number of patients included in the analysis | 75 | 74 | |||
| Clinical pregnancy rate (fresh transfers) | 17/33 (52%) | 12/23 (52%) | 1.02 | 0.35–2.98 | 0.96 |
| Total Implantation rate | 28/114 (23%) | 42/97 (43%) | 2.74 | 1.32–4.29 | 0.003 |
| TQE transferred/all embryos transferred | 51/114 (45%) | 63/97 (65%) | 2.29 | 1.31–3.99 | 0.003 |
| Clinical pregnancy rate per transfer | 28/107 (24%) | 42/91 (46%) | 2.65 | 1.33–4.39 | 0.009 |
| Clinical pregnancy rate per patient | 28/75 (37) | 42/74 (57%) | 2.20 | 1.14–4.24 | 0.01 |
| Live birth per transfer | 24/107 (22%) | 33/91 (36%) | 1.98 | 1.05–3.67 | 0.03 |
| Cumulative live birth per patient | 24/75 (32%) | 33/74 (45%) | 1.67 | 0.87–3.32 | 0.11 |
Pregnancy outcome, calculated by intention to treat.
| . | hCG . | Dual trigger . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Number of patients included in the analysis | 75 | 74 | |||
| Clinical pregnancy rate (fresh transfers) | 17/33 (52%) | 12/23 (52%) | 1.02 | 0.35–2.98 | 0.96 |
| Total Implantation rate | 28/114 (23%) | 42/97 (43%) | 2.74 | 1.32–4.29 | 0.003 |
| TQE transferred/all embryos transferred | 51/114 (45%) | 63/97 (65%) | 2.29 | 1.31–3.99 | 0.003 |
| Clinical pregnancy rate per transfer | 28/107 (24%) | 42/91 (46%) | 2.65 | 1.33–4.39 | 0.009 |
| Clinical pregnancy rate per patient | 28/75 (37) | 42/74 (57%) | 2.20 | 1.14–4.24 | 0.01 |
| Live birth per transfer | 24/107 (22%) | 33/91 (36%) | 1.98 | 1.05–3.67 | 0.03 |
| Cumulative live birth per patient | 24/75 (32%) | 33/74 (45%) | 1.67 | 0.87–3.32 | 0.11 |
| . | hCG . | Dual trigger . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|---|---|
| Number of patients included in the analysis | 75 | 74 | |||
| Clinical pregnancy rate (fresh transfers) | 17/33 (52%) | 12/23 (52%) | 1.02 | 0.35–2.98 | 0.96 |
| Total Implantation rate | 28/114 (23%) | 42/97 (43%) | 2.74 | 1.32–4.29 | 0.003 |
| TQE transferred/all embryos transferred | 51/114 (45%) | 63/97 (65%) | 2.29 | 1.31–3.99 | 0.003 |
| Clinical pregnancy rate per transfer | 28/107 (24%) | 42/91 (46%) | 2.65 | 1.33–4.39 | 0.009 |
| Clinical pregnancy rate per patient | 28/75 (37) | 42/74 (57%) | 2.20 | 1.14–4.24 | 0.01 |
| Live birth per transfer | 24/107 (22%) | 33/91 (36%) | 1.98 | 1.05–3.67 | 0.03 |
| Cumulative live birth per patient | 24/75 (32%) | 33/74 (45%) | 1.67 | 0.87–3.32 | 0.11 |
We performed a logistic regression (Table V) and included the age, number of TQE and the method of triggering. We found the age and the number of TQE to be correlated with increased cumulative live birth. The method of triggering was not found to be correlated with the live birth per patient. In the dual trigger group, the number of oocytes, total number of blastocysts and top-quality blastocysts were increased, and due to the increased number of blastocysts, the live birth rate was increased as well. We looked at the average number of remaining blastocysts for patients that conceived in both groups. In the dual trigger group, the number of remaining embryos was increased compared with the control group (4 versus 1.8, P = 0.002).
Regression analysis of different variables correlated with the live birth rate.
| . | Coefficient . | Standard error . | Wald statistic . | P-value . | Odds ratio (95% CI) . |
|---|---|---|---|---|---|
| Maternal age (y) | −0.145 | 0.055 | 6.888 | 0.009 | 0.86 ( 0.78–0.96) |
| TQE blast | 0.283 | 0.110 | 6.572 | 0.010 | 1.33 (1.07–1.65) |
| Dual trigger | 0.288 | 0.386 | 0.557 | 0.456 | 1.33 (0.63–2.84) |
| Constant | 4.025 | 2.014 | 3.992 | 0.046 | 55.97 |
| . | Coefficient . | Standard error . | Wald statistic . | P-value . | Odds ratio (95% CI) . |
|---|---|---|---|---|---|
| Maternal age (y) | −0.145 | 0.055 | 6.888 | 0.009 | 0.86 ( 0.78–0.96) |
| TQE blast | 0.283 | 0.110 | 6.572 | 0.010 | 1.33 (1.07–1.65) |
| Dual trigger | 0.288 | 0.386 | 0.557 | 0.456 | 1.33 (0.63–2.84) |
| Constant | 4.025 | 2.014 | 3.992 | 0.046 | 55.97 |
TQE blast, top-quality embryo blastocyst.
Regression analysis of different variables correlated with the live birth rate.
| . | Coefficient . | Standard error . | Wald statistic . | P-value . | Odds ratio (95% CI) . |
|---|---|---|---|---|---|
| Maternal age (y) | −0.145 | 0.055 | 6.888 | 0.009 | 0.86 ( 0.78–0.96) |
| TQE blast | 0.283 | 0.110 | 6.572 | 0.010 | 1.33 (1.07–1.65) |
| Dual trigger | 0.288 | 0.386 | 0.557 | 0.456 | 1.33 (0.63–2.84) |
| Constant | 4.025 | 2.014 | 3.992 | 0.046 | 55.97 |
| . | Coefficient . | Standard error . | Wald statistic . | P-value . | Odds ratio (95% CI) . |
|---|---|---|---|---|---|
| Maternal age (y) | −0.145 | 0.055 | 6.888 | 0.009 | 0.86 ( 0.78–0.96) |
| TQE blast | 0.283 | 0.110 | 6.572 | 0.010 | 1.33 (1.07–1.65) |
| Dual trigger | 0.288 | 0.386 | 0.557 | 0.456 | 1.33 (0.63–2.84) |
| Constant | 4.025 | 2.014 | 3.992 | 0.046 | 55.97 |
TQE blast, top-quality embryo blastocyst.
Discussion
Triggering of final follicular maturation following COH for IVF has become a field of interest for research during the last few years, and different modes of triggering have been recommended for different patient populations (Orvieto, 2015).
Griffin et al. (2014) published a retrospective cohort study that evaluated the percentage of mature oocytes retrieved in patients with a previous history of more than 25% immature oocytes recovered in an IVF cycle. When these women were triggered with GnRH agonist and hCG (dual trigger) to induce oocyte maturation in a subsequent cycle, they found that the proportion of mature oocytes was increased significantly. They therefore concluded that in patients with a low percentage of mature oocytes retrieved, a combination of GnRH agonist and hCG is able to increase the percentage of mature oocytes retrieved. In vitro fertilization outcomes, however, remained poor, suggesting an underlying oocyte dysfunction.
Concomitant to the previous study, Zilberberg et al. (2014) also evaluated whether the dual trigger would improve the proportion of MII oocytes in patients with a previous low proportion of mature oocytes (66%) per total number of oocytes retrieved. They found a significantly higher number of mature oocytes, a significantly higher proportion of MII oocytes per number of oocytes retrieved, a higher number of embryos transferred and a higher number of TQE.
In another retrospective study by the same group (Haas et al., 2014), patients with low/poor oocyte yield despite an apparently normal follicular development were triggered with hCG and GnRH agonist in the following cycle and demonstrated significantly higher number of oocytes retrieved, an increased number of 2PN, significantly higher proportion of oocytes retrieved to the number of follicles >10 and >14 mm in diameter and increased number of embryos transferred.
Recently, we conducted a pilot RCT (Haas et al., 2019) including patients with poor ovarian response according to Bologna criteria who were prospectively randomized to different modes of triggering final follicular maturation. Despite the small number of patients included in the study, the patients triggered with hCG and GnRH agonist had a significantly higher number of TQE compared with the patients triggered with only hCG.
A prospective randomized controlled study by Decleer et al. (2014) compared normal responder patients triggered with hCG versus patients triggered with hCG and GnRH agonist. While the numbers of oocytes and mature oocytes were comparable between the two groups, the number of TQE was increased in the dual trigger group and so was the number of cryopreserved embryos, with no between-group difference in pregnancy rates. In this study, only ongoing pregnancy rate was described without the live birth rate, or the cumulative pregnancy rate/live birth rate per started stimulation cycle.
In our present RCT, we found a higher number of oocytes, mature oocytes, blastocysts, top-quality blastocysts, implantation rate, ongoing pregnancy rate and higher live birth rate in the dual trigger group. To our knowledge, this is the first prospective double-blinded RCT comparing the live birth rate in patients triggered with hCG versus a dual trigger. The live birth per transfer in the hCG group was 22% and in the dual trigger group 36.2% (P = 0.03). The cumulative live birth rate was 32% per started cycle in the hCG group and 44.6% in the dual trigger group. The cumulative live birth rate per started cycle was not significantly different between the two groups but the study was not powered for this outcome. We realize that the overall live birth rate was not high but as demonstrated in the Results section, the average age was 35.3–36. As described in the logistic regression, age had a significant influence on the live birth rate, and because 30% of the patients were aged 39 or above, we find the live birth rate to be acceptable.
As described, when performing the logistic regression, the trigger method was not correlated with higher live birth rate. This finding indicates that when comparing two patients of the same age and with the same number of TQE, the trigger method does not have an impact on the live birth rate, but as demonstrated previously, patients in the dual trigger group had more TQE and therefore a higher cumulative live birth rate.
When we looked at number of embryos remaining cryopreserved for patients that conceived in both groups, we found an increased number of remaining embryos in the dual trigger group compared with the hCG group. The increased number of cryopreserved blastocysts in the dual trigger group suggests that the cumulative live birth rate in this group may be higher still once all blastocysts have been transferred (Smeltzer et al., 2019).
We speculate that the reason for improved results after the dual triggering may be the additional benefit from the endogenous FSH surge following the GnRH agonist trigger and the synergistic effect of both LH and hCG on the LH receptor. The role of the FSH during ovulation that follows the LH mid-cycle surge in the natural menstrual cycle is not clear. In general, FSH is known to promote formation of LH receptors in luteinizing GCs, nuclear maturation and cumulus expansion. Lamb et al. (2011), in their randomized, double-blind, placebo-controlled study, demonstrated that the fertilization rate and the likelihood of obtaining oocytes from mature-sized follicles (oocyte recovery rate) were higher after concomitant FSH (450 IU) administration at the time of the hCG trigger compared with hCG alone.
In our previous study, we investigated GC gene expression from patients triggered with hCG versus those triggered with both GnRH agonist and hCG (Haas et al., 2016). We found decreased expression of conexin43 and increased expression of epiregulin and amphiregulin in the GCs from patients receiving hCG and GnRH agonist compared with hCG alone, which may explain the suggested improved oocyte and embryo quality related to the dual triggering group (Hasegawa et al., 2007, Ben-Ami et al., 2011).
One of the weaknesses of the present study is that we did not include a third arm of patients who were triggered with GnRH agonist alone. If we had added the third arm, we would have been able to test whether it was the administration of GnRH agonist or the co-administration of GnRH agonist and hCG that improved the outcome as demonstrated in the study Although we believe it would have been interesting to add a third study group i.e. patients triggered with GnRH agonist only, we decided to include in this study only two groups—patients triggered with hCG versus patients triggered with hCG + GnRH agonist. Several RCT studies and a meta-analysis (Griesinger et al., 2006) have previously compared patients triggered with GnRH agonist alone versus hCG and did not show any differences in the number of oocytes or blastocysts. In our clinic, the trigger for normal responders is hCG and for hyper-responders is GnRH agonist only in order to reduce the risk of OHSS. The objective of the present study was to evaluate whether the addition of GnRH agonist to hCG would improve the pregnancy outcome in the group of normal responders who would typically not receive GnRH agonist alone.
Another potential weakness of this study was that the decision whether to perform a fresh embryo transfer or to freeze all the embryos was made by the treating physician. The clinical pregnancy rate was higher during the fresh transfers compared with the frozen, but the numbers were too small to draw any conclusion regarding the different mode of embryo transfer.
We think it is important to clarify that this study was performed in normal responders and we do not know whether the positive impact of the dual trigger on pregnancy rate will be the same in older or poor responder patients.
We conclude that using the dual trigger for final follicular maturation in normal responder women undergoing IVF increases the number of oocytes, mature oocytes and number of blastocysts as well as the percentage of top-quality blastocysts compared to triggering with hCG alone. The increase in the number of blastocysts improved the outcome of the IVF cycle.
Authors’ roles
J.H., R.O. and R.C. designed the trial. R.B., N.S., E.Z. and C.M. enrolled patients and completed data collection. J.H. and R.C. conducted the statistical analysis. J.H., R.O. and R.C. completed the article.
Funding
TRIO fertility.
Conflict of interest
None of the authors declare any conflict of interest.