-
PDF
- Split View
-
Views
-
Cite
Cite
Claus Yding Andersen, Anne Cathrine Bollerup, Stine Gry Kristensen, Defining quality assurance and quality control measures in connection with ovarian tissue cryopreservation and transplantation: a call to action, Human Reproduction, Volume 33, Issue 7, July 2018, Pages 1201–1204, https://doi.org/10.1093/humrep/dey105
Close - Share Icon Share
Abstract
Freezing of ovarian tissue for fertility preservation has been gaining ground as a valid method in recent years. More than 100 children have been born from this procedure worldwide. As a result, many fertility clinics are now implementing this method. However, the practical procedures that need to be mastered to successfully implement the freezing of ovarian tissue are different in many aspects from those normally used in fertility clinics and are not well defined. Furthermore, success is difficult to measure since patients usually do not return for transplantation until several years after freezing, which puts extra emphasis of good quality control and quality assurance measures to secure a transplantation of tissue with surviving follicles that can sustain fertility. The present paper describes the procedures and a checklist implemented in Denmark in order to secure a successful clinical service. To standardize and implement uniform measures for this new method, we suggest a consensus conference to collectively agree on the best technical and clinical practice.
Introduction
Ovarian tissue cryopreservation (OTC) is currently gaining ground as a valid method for fertility preservation in young women with various cancer diseases and fertility clinics worldwide are starting to implement the technique. To date, more than 100 children have been conceived based on transplantation of frozen/thawed ovarian tissue (Donnez and Dolmans, 2017; Jensen et al., 2017a). Moreover, transplanted ovarian tissue reinstates the ovarian endocrine function, often with the development of more or less regular menstrual cycles with sex hormone production at magnitudes similar to the natural situation (Donnez et al., 2013; Jensen et al., 2015). The longevity of the transplanted tissue is variable depending mainly on the age of the woman at OTC (Donnez and Dolmans, 2017), but a number of other conditions are most likely involved, including the speed at which revascularization occurs after transplantation (Xia et al., 2015). Nonetheless, transplanted tissue has been active for several years and examples of more than 10 years of function resulting from one transplantation procedure have been reported (Andersen et al., 2012). This surprisingly long period of duration has prompted the use of OTC in a number of other clinical situations such as severe genetic conditions with a risk of primary ovarian insufficiency (POI), including thalassemia, sickle cell anemia, Turner syndrome and galactosemia (Jensen et al., 2017b). It has also been suggested that autologous ovarian tissue stored at a young age may be used as a method to postpone menopause if transplanted after exhausting the existing follicle pool present in vivo (Andersen & Kristensen, 2015). Thus, there is no universal successful endpoint for the OTC procedure in contrast to ART in which a positive hCG followed by the birth of a healthy child is the definitive endpoint.
Although OTC is currently being performed mainly in fertility clinics, the procurement and the whole set-up of OTC differ profoundly in many aspects from conventional ART such as IVF, ICSI and IUI. Whereas the success of conventional ART procedures is demonstrated within a relatively short time period (e.g. a positive pregnancy test obtained after 14 days), the period between OTC and transplantation may take years and, in case of OTC in children, sometimes decades. Furthermore, once transplanted the tissue may be active for many years and potentially provide fertility a number of years after transplantation. In Denmark for instance, two patients conceived more than 5 years following transplantation (Jensen et al., 2015). Therefore, the period from procurement of the tissue until a potential conception based on transplanted frozen/thawed tissue may last several years, and decades in case of tissue frozen in childhood. The success rate in connection with OTC and transplantation is different from conventional IVF/ART in many aspects and currently it is difficult to predict the final success rate in terms of birth rate (Yding Andersen, 2015). Further, in addition to restoration of fertility, OTC and transplantation also reconstitutes the endocrine function of the ovary and basically reinstalls the organ function, which in some patients may be the desired effect rather than fertility restoration. However, it is important to understand that fertility and endocrine function go hand in hand and that both are a result of follicular activity.
These circumstances demand a completely different approach to quality control and quality assurance of OTC and transplantation as compared to traditional work in fertility clinics. A number of additional and different measures to assure quality assurance and quality control are necessary as compared to conventional ART procedures. Thus, as many fertility clinics have been starting to perform OTC, the aim of this article is to provide recommendations and a checklist on how to structure quality control and quality assurance given the invariably long time between procurement and utilization. Moreover, the manuscript highlights some aspects that are important to consider when starting a new OTC service, which differs compared to most other activities of fertility clinics. The article describes our experience with quality control and quality assurance as they have developed in Denmark, but standardized and uniform methods of quality control do not currently exist, including monitoring, for example, clinical outcome. We therefore propose that an international consensus conference is needed to define appropriate quality control and quality assurance measures for the OTC procedure.
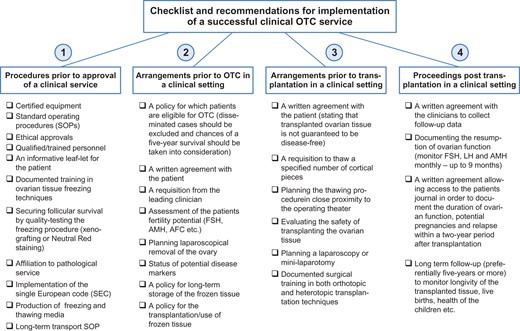
The following guidelines and recommendations comprise four consecutive steps which may be used as a checklist (Fig. 1) and facilitate the initiation of a successful clinical service.
A checklist of guidelines and recommendations that can be used to secure proper quality assurance and quality control measures in connection with ovarian tissue cryopreservation and subsequent transplantation. OTC, ovarian tissue cryopreservation; AMH, anti-Müllerian hormone; AFC, antral follicle count.
Step 1: Recommended procedures prior to approval of a clinical service
Appropriate equipment, standard operating procedures (SOPs), potential ethical approvals, suitable facilities and qualified/trained personnel.
An information leaflet with easy understandable information about the procedure that the patient can take home to read.
Documented supervised training in the preparation of ovarian cortical tissue from large domestic animal species, preferably cow or sheep.
Documented education in ovarian tissue freezing techniques.
Ability to secure successful survival of early stage human follicles following freezing and thawing. This may be performed following different protocols; either in vivo or in vitro methods are available. The golden standard is demonstrating the presence of healthy follicles in histological sections prepared from human tissue transplanted to immunodeficient mice for a period exceeding 3 weeks (in order to secure reabsorption of non-viable follicles) (Rosendahl et al., 2011) or by an in vitro test that determines the follicular survival rate by estimating the number of viable and the number of total follicles (for instance Neutral Red staining) in small pieces of ovarian cortical tissue.
Affiliation to a qualified pathological service that will be able to diagnose potential unexpected macroscopic findings in the ovarian tissue in connection with a clinical service.
Implementation of donation identification sequence and of production identification sequence, Single European Code (SEC), a 40-digit code, which individual ampoules will be requested to be labeled with in countries attached to the European Union (EU).
Ability to locally produce, or have available, validated freezing and thawing media that comply with basic standards to be used in a clinical setting as no commercial media are currently available.
In case of long-term transport (1–20 h) of the ovarian tissue prior to procurement, a SOP describing transport conditions must be in place including procedures to secure a documented sending and receipt of the tissue by the responsible currier and by the cryopreservation facility.
Step 2: Arrangements prior to OTC in a clinical setting
A written policy on patient conditions in which OTC will be offered including potential upper age limit, disease progression, general health conditions, etc.
A written formal agreement in which the patient states that she wants, and agrees to, the procedure and is informed about the potential side effects and other relevant information.
A written formal statement requesting freezing from the clinician in charge with information about the patient including diagnosis, status of disease markers and consent.
In connection with cancer diseases, OTC should currently only be performed in non-disseminated cases unless special conditions argue in favor of OTC.
The chances of a successful 5-year survival period post cancer treatment should be taken into consideration.
The fertility potential should be documented by several hormone parameters, including anti-Müllerian hormone (AMH) and FSH measurements combined with other medically relevant measures, such as antral follicle count, prior to ovarian excision.
A plan for excising ovarian tissue (usually performed laparoscopically) and sufficient qualified personnel.
A document with the status of potential disease markers for HIV, hepatitis, etc. must be present before the handling and procurement of the ovarian tissue for cryopreservation.
A written formulated policy for keeping the tissue frozen (also long term, which may include decades of storage) both in terms of economic and legal consequences.
A formulated written policy for the transplantation/use of frozen tissue both in terms of autologous and allogenic transplantation in case it is legal.
Written information on the legal and financial conditions for continued storage including the potential death of the patient. It should also be considered under which circumstances the patient may want to donate her tissue for research purposes.
Step 3: Arrangements prior to transplantation in a clinical setting
The patient needs to be disease-free for a sufficient period (this may vary according to diagnosis) prior to transplantation and she needs to be in good health.
A written formal agreement in which the patient agrees to have tissue transplanted, and in which it is stated that currently it cannot be guaranteed that the procedure is completely safe in terms of potentially re-transplanting malignant cells.
A written formal request to the laboratory personnel to thaw out a specified number of pieces of ovarian cortex in which the patient is clearly identified.
A plan for the thawing of the ovarian tissue in appropriate conditions in close proximity of the operating theater in order to minimize the time from thawing to transplantation of the thawed ovarian tissue.
A proper evaluation of the safety for the patient of transplanting ovarian tissue must be conducted by the clinical team and the scientists. This includes current medication, and suitability both in terms of physiological and mental issues.
Documentation of the surgical training and skills required to perform both orthotopic and heterotopic transplantation of the thawed tissue.
A written formal agreement that outlines the willingness of the clinical team to collect follow-up data, as outlined in the point below.
Step 4: Procedures post transplantation in a clinical setting
In order to document resumption of ovarian function it is necessary to monitor FSH, LH, AMH and potentially oestradiol on a monthly basis following transplantation until premenopausal levels of gonadotrophins in blood are reached. Premenopausal levels of gonadotrophins are normally reached within a 3–6-month period following transplantation. If no substantial reduction in gonadotrophins has been achieved with a 9-month period, the transplantations is to be considered a failure.
For quality control and quality assurance, a written formal agreement with the patient allowing access to the patient’s notes in order to keep track of conditions relating to the procedure including not only duration of function and potential pregnancies, but also potential relapse in a 2-year period after transplantation. If this is not possible the patient agrees to be contacted in order to provide this information and make her whereabouts known to the clinic within this period. If relapse occurs subsequent to a 2-year period of transplantation it is currently considered to be unrelated to the transplantation of the tissue. If relapse occurs within a 2-year period following transplantation each individual case requires a medical evaluation to determine whether the transplanted ovarian tissue could be involved in the relapse. Potentially the transplanted ovarian tissue may be recovered and evaluated for the occurrence of disease markers. This is also the case if the patient dies within a 2-year period of transplantation.
A long-term follow-up period is warranted but not a formal requirement—preferentially a 5-year follow-up period.
The consequences of transplantation should be documented as much as possible. Longevity of the tissue in terms of function—length of time with menstrual cycles, pregnancies (positive hCG tests, clinical pregnancies, children born) and the health of the children born.
Conclusion
The above measures are not universally accepted and not all are required, for example, by the EU-tissue directive, but many clinics may benefit from these guidelines by implementing them right from the beginning. In member countries of the EU, the European Tissues and Cells Directive is clearly the legal framework in which OTC should be implemented, but due to the variable end-points for success of OTC, a proper approach to monitor, for example, clinical outcome after transplantation has not yet been agreed upon in the EU and worldwide. The present guidelines attempt to include a more detailed monitoring of clinical outcome after transplantation. In this way, new clinics will be better equipped to monitor the performance of their clinical activity and it will enable them to provide data to the competent authorities and central registers (which hopefully can be established), and further pave the way for a full endorsement of this new technique in a clinical setting worldwide.
The present proposal for guidelines is the result of a collaboration between the competent authorities and the clinic which has performed OTC in Denmark during the past decade. This development has taken place in other countries too, while some countries have not yet started this process. As a consequence, there is no collective agreement on how to perform standardized and uniform quality control and quality assurance including clinical follow-up. As many centers are now embarking on OTC, we suggest to advance OTC and transplantation by conducting a consensus conference to determine in detail the framework for starting this new procedure.
Authors’ roles
C.Y.A., A.C.B. and S.G.K. all contributed to the conception and writing of this article.
Funding
None.
Conflict of interest
None declared.