-
PDF
- Split View
-
Views
-
Cite
Cite
E K Osman, T Wang, Y Zhan, C R Juneau, S J Morin, E Seli, R T Scott, J M Franasiak, Varying levels of serum estradiol do not alter the timing of the early endometrial secretory transformation, Human Reproduction, Volume 35, Issue 7, July 2020, Pages 1637–1647, https://doi.org/10.1093/humrep/deaa135
Close - Share Icon Share
Abstract
Do supraphysiologic estradiol (E2) levels in the ranges attained during normal and high response superovulation cycles modify the onset of endometrial secretory transformation?
Highly supraphysiologic levels of E2 do not alter the ability of physiologic levels of progesterone (P4) to induce secretory transformation.
Previous studies have demonstrated that premature P4 elevations during IVF cycles are associated with a decrement in clinical pregnancy rates after fresh embryo transfer due to shifts in the window of implantation (WOI). However, alterations in the onset of secretory transformation may not apply uniformly to all patients. High responders with supraphysiologic E2 levels accompanied by similar subtle increases in P4 have not been shown to have decreased sustained implantation rates. This prospective investigation in which whole-genome transcriptomic and methylomic analysis of the endometrium is performed for individual patients under a range of E2 concentrations brings clarity to a long-debated issue.
A randomized, prospective and paired trial was conducted in which 10 participants were enrolled and randomized to the order in which they completed three distinct uterine stimulation cycles, each at a specific E2 concentration: physiologic (∼180 pg/ml), moderately supraphysiologic (600–800 pg/ml) or supraphysiologic (2000 pg/ml). Target E2 ranges were selected to mimic those seen in natural, controlled ovarian stimulation and IVF cycles. E2 valerate was administered in order to maintain stable E2 levels for 12 days followed by intramuscular P4 in oil 10 mg/day for two doses, after which an endometrial biopsy was performed. A total of 30 endometrial biopsies were included in a whole-genome transcriptomic and methylomic analysis.
Healthy volunteers without a history of infertility were included in this study at a single large infertility center. DNA was isolated from the endometrial biopsy specimens and bisulfite sequencing was performed to construct a methylation array. Differential methylation analysis was conducted based on differences in M-values of individuals across treatment groups for each probe as well as carrying out t-tests. RNA was isolated for RNA-Seq analysis and gene expression values were compared using DESeq2. All analyses were performed in a pairwise fashion to compare among the three stimulation cycles within individuals and secondarily to compare all participants in each of the cycles.
The mean peak E2 and P4 levels were 275 pg/ml and 4.17 ng/ml in the physiologic group, 910 pg/ml and 2.69 ng/ml in the moderate group was, and 2043 pg/ml and 2.64 ng/ml in the supraphysiologic group, respectively. Principal component analysis of 834 913 CpG sites was performed on M-values of individuals within the low, moderate and supraphysiologic conditions in a paired approach. There were no differences in genome-wide methylation within participants across E2 groups. A paired analysis revealed that gene expression profiles did not differ within the same individual at each of the three E2 levels. No significant alterations in gene expression as related to endometrial physiology were identified between the low, moderate and supraphysiologic groups in an inter-participant analysis.
Although each participant completed a physiologic cycle in which E2 levels were maintained in a range that would simulate a natural cycle, our findings are limited by lack of an unmedicated control to assess if there was a potential effect from E2V. Additionally, our results were obtained in fertile individuals, who may have a different endometrial response compared to an infertile population. Despite the whole genomic endometrial assessment and rigorous, paired study design, the sample size was limited.
Given that the endometrial response to P4 is unaffected by E2 levels in the supraphysiologic range, diminutions in implantation seen in stimulated cycles may result from embryonic-endometrial dyssynchrony following early P4 elevations or slowly blastulating embryos, which occur independently of the magnitude of the E2 rise.
This study was funded by the Foundation for Embryonic Competence, Basking Ridge, NJ, USA. Dr E.S. reports consultancy work for The Foundation for Embryonic Competence, Basking Ridge, NJ, USA. The other authors declare no conflict of interests related to this topic.
NCT02458404.
Introduction
It has long been recognized that the endometrium is not uniformly receptive throughout the cycle. The relatively narrow interval where successful implantation is supported is termed the window of implantation (WOI) (Navot et al., 1991; Bergh and Navot, 1992; Lessey, 2000). Implantations established near the margins of the WOI have increased risk for loss and overall diminished obstetrical outcomes (Wilcox et al., 1999). Early studies quickly established that the timing of the WOI was controlled by the duration of progesterone (P4) exposure following estrogen driven endometrial proliferation (Navot et al., 1986; Prapas et al., 1998). When P4 levels cross a critical threshold, the onset of secretory transformation in the endometrium begins.
The challenge for clinicians is how best to assure that embryos generated through ART, where natural synchrony is not guaranteed, are ready to implant during the optimal period of receptivity. During stimulated cycles, late follicular rises in P4 lead to shifts in the onset of secretory transformation by 16–24 h, leading to dyssynchrony between the embryo and endometrium (Franasiak et al., 2016). This critical factor—synchrony between the embryo and endometrium—is easily achieved in synthetic hormone replacement cycles. Clinicians determine both the timing of the onset of P4 exposure and the subsequent transfer of expanded blastocysts. However, this is not under direct clinical control in stimulated cycles where P4 levels are commonly elevated in the late follicular phase (Ubaldi et al., 1996; Fanchin et al., 1997; Bosch et al., 2003). The endometrium may begin secretory transformation even though the oocyte has not matured and is one or more days remote from becoming fertilized and initiating development.
Investigations into potentially altered receptivity in fresh ART cycles focused on variations in mid-luteal histology (Mirkin et al., 2004; Saadat et al., 2004), but were largely uninformative. Silverberg et al. (1991) shifted the focus away from direct measurements of the endometrium to focusing on the timing of the stimulus, demonstrating that delivery rates declined as late follicular P4 surpassed certain thresholds. Interestingly, these levels were well below those typically associated with premature LH surges and the onset of luteinization. A subsequent landmark study by Hofmann utilized an oocyte donation model and demonstrated that the oocytes from cycles with subtle P4 rises had normal reproductive potential, thus isolating the adverse effect to changes in the endometrium (Hofmann et al., 1993).
The adverse consequences of slow-cryopreservation at the cleavage stage meant that most clinicians elected to transfer the embryos in a fresh cycle and accept diminished outcomes. The increasing use of extended culture to the blastocyst stage and the integration of vitrification with survival rates of >95% provided an alternative therapeutic path. Clinicians could now cryopreserve the embryos for subsequent synchronous transfer in cryopreserved embryo transfer cycles, leading to superior outcomes (Shapiro et al., 2010; Roque et al., 2015; Healy et al., 2016; Coates et al., 2017; Wei et al., 2019).
Armed with a therapeutic alternative when managing patients at risk for embryonic-endometrial dyssynchrony, the focus shifted to defining which patients were at risk for diminished outcomes. A powerful retrospective review of over 4000 patients from Bosch et al. (2010) demonstrated that women whose P4 levels were over 1.5 ng/ml were at clear risk for a meaningful decrement in pregnancy rates. While the exact threshold may vary among laboratories based on the dynamics of the assays being used, subsequent investigators confirmed that subtle P4 levels were consistently associated with diminished outcomes (Xu et al., 2012; Venetis et al., 2015).
Alterations in the onset of secretory transformation due to late follicular elevations in P4 may not apply uniformly to all patients. In a review of the literature to date, Griesinger et al. (2013) found that low and normal responders demonstrated the expected decline in clinical outcomes when subtle P4 elevations were observed, but most interestingly found no such diminution in high responders. They speculate that higher estradiol (E2) levels may alter the intrinsic threshold for the onset of secretory transformation. If the circulating level of P4 required to initiate endometrial secretory transformation was altered in the presence of markedly different E2 levels, then the clinical management of those cycles might continue to fresh transfer thereby eliminating the need to deter to a subsequent cryopreserved embryo transfer cycle.
Modest elevations in circulating levels of P4 are both necessary and sufficient to induce secretory transformation. Following a proliferative phase stimulated by physiologic E2 levels, it appears that the transcription of the endometrium begins to change once circulating progesterone levels reach ∼2.5 ng/ml (Usadi et al., 2008). Consistent with the findings from Bosch and others, a P4 level of 1.5 ng/ml on the day of trigger would continue to increase and be additive to the changes induced by the mid-cycle surge injection. To bring clarity to a complex matter, the endometrial transcriptome of women with P4 levels lower than 2.5 ng/ml did not transition to the pattern seen in the normal secretory phase while those above the threshold did (Usadi et al., 2008; Young et al., 2017).
More contemporary functional genomic studies examining the effect of elevated P4 levels on the endometrium do so in the mid-secretory phase, rather than at the onset of secretory transformation (Labarta et al., 2011). It has been demonstrated that epigenetic alterations within the endometrium in the peri-implantation period occur as a result of premature P4 elevations (Xiong et al., 2017); however, there has been no current genome-wide methylation assessment in the early secretory phase. Large inter-patient variation in endometrial gene expression and CpG methylation exists, further challenging the reliability of such studies and highlighting the need for a study design in which each person serves as their own control.
These data provide a powerful baseline from which to study the impact of varying circulating levels of estradiol on the sensitivity of the endometrium to progesterone. This study seeks to determine if the progesterone threshold for endometrial secretory transformation is impacted by varying levels of estradiol during the proliferative phase which mimic low, normal and high responders during superovulation.
Materials and methods
Patient population
Healthy, reproductive-aged volunteers with regular menstrual cycles were deemed eligible to participate in this study. Participants were recruited through telephone and e-mail communication. Women with a history of infertility, known gynecological problems, intrauterine procedures within the previous 90 days, or with any contraindication to treatment with E2 were excluded. This project was approved by the Copernicus Group Institutional Review Board. The clinicaltrials.gov registration number is NCT02458404. Written informed consent was obtained after enrollment and prior to randomization. All participants were compensated for their participation after each uterine stimulation cycle and received a total of $3000 after completion of all three cycles.
Study design and uterine stimulation protocol
This was a single-center, prospective, randomized study, designed to provide a paired comparison of methylomic and transcriptomic profiles of the endometrium of individual participants under three distinct E2 concentrations. Target E2 ranges were 180–300 pg/ml for the physiologic, 600–800 pg/ml for moderately supraphysiologic and >2000 pg/ml for the supraphysiologic cycles. Individuals were randomized to the order in which they each completed three sequential and distinct uterine stimulation cycles between June 2015 and December 2016. Randomization was performed utilizing a computer-generated random number sequence to assign subjects to the physiologic, moderately supraphysiologic and supraphysiologic groups.
Participants administered leuprolide acetate 10 mg subcutaneous (SQ) injection daily (qD) for ∼2 weeks during the mid-luteal phase for ovarian suppression. On cycle Day 3, study participants presented for a baseline ultrasound as well as a blood draw for E2, P4 and hCG levels. Leuprolide acetate dose was then decreased to 5 mg qD and continued until cycle Day 12, 2 days prior to the start of P4 in oil (PIO). Estradiol Valerate (E2V) was injected intramuscularly every 48 h in a physician specified dose of 2–15 mg to attain E2 levels in the specified ranges. This formulation of synthetic E2 was chosen for its constant steady-state levels that more accurately simulate conditions during the proliferative phase (Schug et al., 2012).
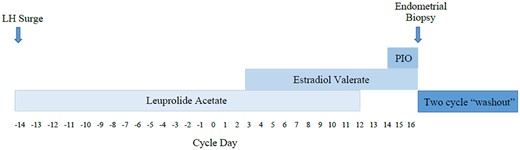
Transvaginal ultrasound (TVUS) along with serum measurement of E2 and P4 was performed every 48 h and E2V dosing was adjusted accordingly to attain E2 measurements in the desired ranges depending on cycle allocation. On cycle Day 14, PIO 10 mg, a dose known to mimic the P4 rise seen prior to the onset of secretory transformation (Young et al., 2017), was administered intramuscularly every 24 h for two doses and an endometrial biopsy was performed on the morning of cycle Day 16 after two full days of P4 exposure. A 10-day course of medroxyprogesterone acetate was given to provoke a withdrawal bleed after the biopsy was completed and the patient underwent a ‘washout’ period lasting ∼6 weeks without any stimulation in which their E2 levels returned to baseline. Two weeks prior to the subsequent uterine stimulation cycle, participants initiated leuprolide acetate treatment again in preparation for the following cycle to which they were allocated. E2V and PIO were then sequentially administered until cycle Day 14 according to study protocol in order to attain E2 levels in the target ranges for the specific cycle to which participants were randomized. Participation was concluded after completion of all three stimulation cycles and collection of endometrial biopsies at the end of each. Stimulation cycle protocol is summarized in Fig. 1.
Cycle design. Enrolled participants initiated treatment with leuprolide acetate 10 mg qD after they were confirmed to be in the luteal phase based on transvaginal ultrasound (TVUS) as well as estradiol (E2) and progesterone (P4) serum testing results. On cycle Day 3, estradiol valerate (E2V) was administered intramuscularly every 48 h and dose-adjustment was performed in order to maintain E2 levels in the desired range for the cycle that the participant was completing. Progesterone in oil (PIO) was started on cycle Day 14 and an endometrial biopsy was performed after two completed doses. A two cycle washout period was required between each consecutive stimulation cycle.
Biopsy collection
After two completed days of P4 exposure, an endometrial biopsy was performed in the outpatient setting using the Pipelle® endometrial suction curette (Cooper Surgical, Trumbull, CT, USA). The sample was obtained after passing the curette into the endometrial cavity and removing tissue from the functional portion of the endometrium for analysis. Three physicians performed all endometrial biopsies in a standardized fashion. One pass was performed for each procedure utilizing a four-quadrant technique. If blood contamination was present within a portion of the biopsy specimen, those portions were excluded from the cryopreserved specimen. All specimens were washed with phosphate-buffered saline prior to RNA and DNA isolation.
RNA isolation and sequencing
Samples were divided into two aliquots, snap frozen in liquid nitrogen immediately after the endometrial biopsy and stored at −80°C until time of analysis. RNA was extracted from one aliquot of the same tissue sample using RNeasy™ mini kit (Qiagen, Redwood City, CA, USA) according to the manufacturer’s protocol. The quality and integrity of the isolated RNA were confirmed by running a portion of each RNA sample on the NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA) and Agilent 2200 Tape Station (Agilent Technologies, Santa Clara, CA, USA). Purified total RNA was suspended in nuclease-free water. RNA was qualified on the NanoDrop with an OD260/280 ratio of 1.8–2.0 and an OD 260/230 ratio of 1.8–2.0. The RNA-Seq library was prepared using TruSeq™ RNA Library Prep Kit v2 (Illumina, San Diego, CA, USA) as directed by standard protocol. Libraries were sequenced on Illumina’s HiSeq 2500 with paired-end 75 base pair reads.
DNA isolation and methylation sequencing
Genomic DNA was isolated from 20 mg of snap-frozen endometrial tissue using DNeasy™ 96 blood and tissue kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. The purity and concentration of the DNA were estimated using a NanoDrop 2000 and Agilent 2200 Tape Station. Bisulfite conversion was then performed using the EZ DNA Methylation Gold Kit (Zymo Research, Irvine, CA, USA) according to protocol as directed by the manufacturer. Genome-wide DNA methylation was assessed using the Infinium MethylationEPIC BeadChip (Illumina Inc., USA).
Statistical analyses
Comparison of cycle characteristics.
Differences between groups in mean endometrial thickness, P4 level on the day of PIO administration and P4 level on the day of endometrial biopsy were calculated using a one-way analysis of variance (ANOVA) utilizing the web-based program OpenEpi (www.openepi.com).
RNA-Seq analysis.
After sequencing, reads were trimmed for quality, and aligned with the reference human genome hg38 with gencode annotation (Frankish et al., 2019). While the standard annotation has ∼50K entries, the gencode annotation has over 100K annotated regions on the genome. HiSAT2 was used for alignment to a reference genome, and both StringTie and BallGown for transcript abundance estimation (Pertea et al., 2016). DESEq2 (Love et al., 2014) was utilized for differential gene expression. In total, 58 288 gene transcripts were analyzed and compared to the annotated reference genome. Gene expression values were calculated as transcripts per million mapped reads (TPM) values. For downstream processing and visualization of the data, R was utilized. Genes were deemed differentially expressed between different conditions if they showed an FDR (adjusted P-value) of <0.05. Intra-subject analysis was conducted in an age-based approach utilizing a paired t-test while controlling for multiple comparisons in order to detect differences between conditions within individuals.
DNA methylation analysis.
The minfi package (v1.26.2) (Aryee et al., 2014) in R (v3.5.0) was used for EPIC microarray data quality control (QC) and calculations of beta values, which are estimates of the fractions of methylation at CpG loci. All samples passed QC based on methylated/unmethylated signal intensities and control single nucleotide polymorphism (SNP) genotypes. Background corrections and data normalization were carried out using the ssNoob method (Triche et al., 2013) within the minfi package. Probes with an SNP at the marker location or at the single nucleotide extension location with an SNP minor allele frequency >0.01 based on dbSNP137 and probes with at least three samples with detection P-values >0.01 were removed from further analysis, leaving 834 913 markers in the EPIC microarray.
Downstream data analysis was based on M-values, the logit transformation of beta values, for their more desirable statistical properties (Du et al., 2010). Principal component analysis (PCA) was carried out on centered and scaled M-values of 834 913 markers for all 30 samples. To compare a pair of stimulation treatment groups, a delta M-value (the difference in M-values of two samples) was calculated for every marker for the same individual between two groups. The delta M-values for each of 10 individuals were then quantile normalized using the profile of the individual with the median of delta M-values of all markers closer to zero between the two individuals with the standard deviation of delta M-values closest to the median standard deviation of the 10 individuals. A one sample t-test was carried out for each marker with the normalized delta M-values under the null hypothesis that the expected delta M-value is zero. Assuming that the number of independent tests is around 500 000 genome-wide, a P-value of <1e−7 would be considered significant after Bonferroni correction.
Results
A total of 34 endometrial samples from 14 participants were collected. Nineteen individuals initially were consented and enrolled. Five subjects dropped out of the study prior to initiation of any uterine stimulation cycle while four individuals elected to drop out of the study after the first cycle. The remaining 10 participants completed all three arms of the study. Of those who withdrew, three did so due to personal reasons and one patient choose to electively cryopreserve her oocytes. There were no adverse reactions in any enrolled study participants. Average patient age was 30.5 ± 6.45 years and average BMI was 28.3 ± 3.85 kg/m2.The endometrial thickness, mean peak E2, average P4 on cycle Day 12 prior to PIO treatment, average P4 on the day of endometrial biopsy (cycle Day 16) and total mean dosage of E2V are demonstrated in Table I.
Average endometrial thickness, estradiol (E2), progesterone (P4) levels and E2 Valerate (E2V) consumption in each cycle.
| . | Physiologic . | Moderately Supraphysiologic . | Supraphysiologic . |
|---|---|---|---|
| Mean endometrial thickness (mm) on day of biopsy | 9.71 ± 2.45* | 9.70 ± 2.43* | 8.75 ± 1.38* |
| Mean peak E2 (pg/ml) | 275 ± 86.3 | 909.7 ± 280.0 | 2043.4 ± 355.8 |
| Mean peak P4 (ng/ml) prior to progesterone in oil treatment | 0.59 ± 0.66‡ | 0.45 ± 0.13‡ | 0.54 ± 0.32‡ |
| Mean P4 on day of endometrial biopsy (ng/ml) | 4.17 ± 3.78¤ | 2.69 ± 1.05¤ | 2.63 ± 0.59¤ |
| Total mean dosage (mg) of E2valerate consumed | 13.5 ± 6.92 | 35.2 ± 9.1 | 89.5 ± 20.1 |
| . | Physiologic . | Moderately Supraphysiologic . | Supraphysiologic . |
|---|---|---|---|
| Mean endometrial thickness (mm) on day of biopsy | 9.71 ± 2.45* | 9.70 ± 2.43* | 8.75 ± 1.38* |
| Mean peak E2 (pg/ml) | 275 ± 86.3 | 909.7 ± 280.0 | 2043.4 ± 355.8 |
| Mean peak P4 (ng/ml) prior to progesterone in oil treatment | 0.59 ± 0.66‡ | 0.45 ± 0.13‡ | 0.54 ± 0.32‡ |
| Mean P4 on day of endometrial biopsy (ng/ml) | 4.17 ± 3.78¤ | 2.69 ± 1.05¤ | 2.63 ± 0.59¤ |
| Total mean dosage (mg) of E2valerate consumed | 13.5 ± 6.92 | 35.2 ± 9.1 | 89.5 ± 20.1 |
Despite the wide range of E2 levels, there were no significant differences between groups in regard to the mean endometrial thickness (*P = 0.52). Mean P4 levels prior to initiation of progesterone in oil (PIO) treatment (‡P = 0.76) as well as on the day of endometrial biopsy (¤P = 0.25) did not vary between conditions. The mean peak E2 and total mean dosage of E2V were highest amongst participants in the supraphysiologic group as compared to moderately supraphysiologic or physiologic groups.
Average endometrial thickness, estradiol (E2), progesterone (P4) levels and E2 Valerate (E2V) consumption in each cycle.
| . | Physiologic . | Moderately Supraphysiologic . | Supraphysiologic . |
|---|---|---|---|
| Mean endometrial thickness (mm) on day of biopsy | 9.71 ± 2.45* | 9.70 ± 2.43* | 8.75 ± 1.38* |
| Mean peak E2 (pg/ml) | 275 ± 86.3 | 909.7 ± 280.0 | 2043.4 ± 355.8 |
| Mean peak P4 (ng/ml) prior to progesterone in oil treatment | 0.59 ± 0.66‡ | 0.45 ± 0.13‡ | 0.54 ± 0.32‡ |
| Mean P4 on day of endometrial biopsy (ng/ml) | 4.17 ± 3.78¤ | 2.69 ± 1.05¤ | 2.63 ± 0.59¤ |
| Total mean dosage (mg) of E2valerate consumed | 13.5 ± 6.92 | 35.2 ± 9.1 | 89.5 ± 20.1 |
| . | Physiologic . | Moderately Supraphysiologic . | Supraphysiologic . |
|---|---|---|---|
| Mean endometrial thickness (mm) on day of biopsy | 9.71 ± 2.45* | 9.70 ± 2.43* | 8.75 ± 1.38* |
| Mean peak E2 (pg/ml) | 275 ± 86.3 | 909.7 ± 280.0 | 2043.4 ± 355.8 |
| Mean peak P4 (ng/ml) prior to progesterone in oil treatment | 0.59 ± 0.66‡ | 0.45 ± 0.13‡ | 0.54 ± 0.32‡ |
| Mean P4 on day of endometrial biopsy (ng/ml) | 4.17 ± 3.78¤ | 2.69 ± 1.05¤ | 2.63 ± 0.59¤ |
| Total mean dosage (mg) of E2valerate consumed | 13.5 ± 6.92 | 35.2 ± 9.1 | 89.5 ± 20.1 |
Despite the wide range of E2 levels, there were no significant differences between groups in regard to the mean endometrial thickness (*P = 0.52). Mean P4 levels prior to initiation of progesterone in oil (PIO) treatment (‡P = 0.76) as well as on the day of endometrial biopsy (¤P = 0.25) did not vary between conditions. The mean peak E2 and total mean dosage of E2V were highest amongst participants in the supraphysiologic group as compared to moderately supraphysiologic or physiologic groups.
Endometrial transcriptome
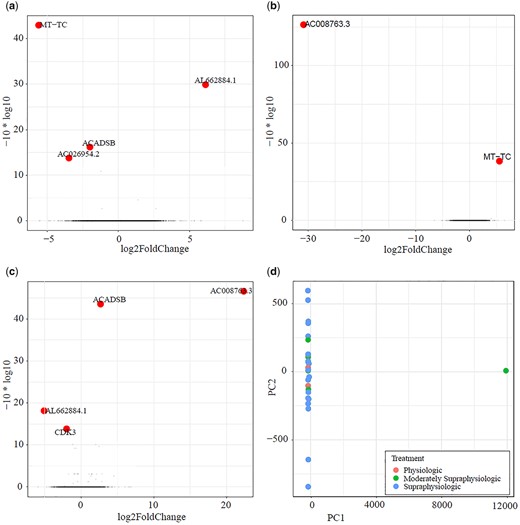
An inter-participant grouped analysis was conducted by carrying out a PCA on 58 288 gene expression levels between the physiologic, moderately supraphysiologic and supraphysiologic E2 groups. Three genes were noted to be significantly downregulated (ACADSB, P = 0.024; MT-TC, P = 5.15e−4; AC026954.2, P = 0.042) while AL662884.1 was upregulated (P = 0.001) in a comparison of the physiologic to moderately supraphysiologic conditions. While two of these genes have not been categorized and have no protein product, modulation of MT-TC and ACADSB is unrelated to varying conditions within the endometrium. In comparing the moderately supraphysiologic to the supraphysiologic group, MT-TC was noted to be upregulated (P = 1.56e−4) and AC008763.3, a gene without a known protein product, was noted to be downregulated (P = 0.00e−6). Two genes were noted to have increased expression (AC008763.3, P = 2.2e−5; ACADSB, P = 4.5e−5) while two were downregulated (CDK3, P = 0.04; AL662884.1, P = 0.02) in a comparison of the physiologic to supraphysiologic group. No genes were noted to be significantly modulated across all three conditions when comparing individuals in each group. These results are summarized in Fig. 2.
Volcano plots comparing participants: (a) in the physiologic versus moderately supraphysiologic (b) in the moderately supraphysiologic versus supraphysiologic and (c) in the physiologic versus supraphysiologic groups as well as a (d) principal component analysis (PCA) plot of differentially expressed genes between groups. No differences in expression of endometrial related genes were noted between groups. In the physiologic versus moderately supraphysiologic group comparison (a), ACADSB, AC026954.2 and MT-TC were downregulated, while AL662884.1 was upregulated. In a comparison of the moderately supraphysiologic to the supraphysiologic group (b), MT-TC was upregulated, while AC008763.3 was downregulated. When comparing the physiologic to supraphysiologic groups (c), AC008763.3 and ACADSB were upregulated while CDK3 and AL662884.1 were downregulated. The PCA plot (d) further emphasizes that there are no significant changes in gene expression between groups. AC026954.2, AL662884.1 and AC008763.3 have no protein product, while MT-TC, ACADSB and CDK3 are genes unrelated to endometrial function.
A paired intra-participant analysis was performed as described previously to compare conditions within individuals in each of the three study conditions. In each of the comparisons, there were genes that were differentially expressed (P < 0.05) within individual conditions in some participants, but none were noted to be related to endometrial receptivity or the onset of the secretory transformation. A heat map of the differentially expressed genes is included in Supplementary Fig. S1. One exception was the gene progestin association endometrial protein (PAEP), also known as glycodelin, normally upregulated in the mid-secretory phase, was downregulated in two participants between both the physiologic and supraphysiologic groups (P = 2.6e−6) as well as when comparing the moderately physiologic and supraphysiologic groups (P = 6.8e−6) for two individuals within one group only. Other genes noted to be significantly different were not endometrial related, nor were they noted to be hormonally responsive.
Endometrial methylome
With respect to treatment group, a PCA was carried out on M-values of 834 913 markers. There were no statistically significant differences between gene methylation profiles when comparing physiologic and moderately supraphysiologic groups. The smallest P-value was 2.80e−6, which was far from genome-wide significance. The markers with the lowest P-values were noted to have nearby markers with very small P-values as well. To inspect if there was local enrichment of small P-values in genome regions, groups of 10 markers across the genome were tested for the number of P-values that were <0.01. Four CpG markers met such criteria, and this count was not determined to be significant (P = 0.276).
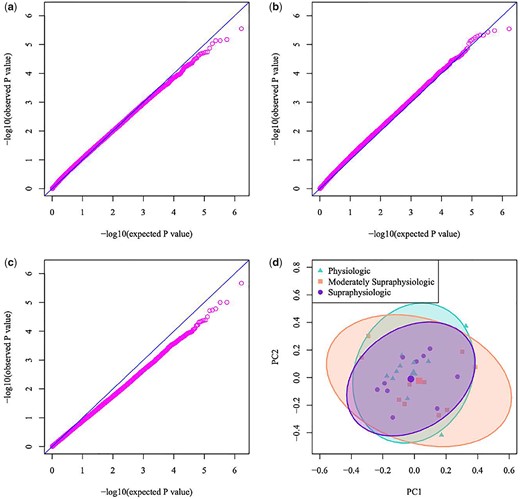
The methylation profiles of the endometrium in the moderately supraphysiologic and supraphysiologic groups did not differ (P = 2.84e−6). Local enrichment testing was performed as described above and no markers were determined to be significant in any genomic region (P = 0.580). Similarly, there were no significant differences between the endometrial methylome under physiologic as compared to supraphysiologic conditions (P = 2.14e−6), summarized in Fig. 3. The P-value was 0.916 for testing local enrichment markers with association to treatment group when comparing the physiologic and supraphysiologic conditions. The above results do not support a systematic difference between treatment groups either between or within an individual participant.
Q/Q plot comparing participants: (a) in the physiologic versus moderately supraphysiologic (b) in the moderately supraphysiologic versus supraphysiologic (c) in the physiologic versus supraphysiologic groups, (d) principal component analysis (PCA) plot of differentially expressed genes within each comparison. A total of 834 913 CpG markers were evaluated and no differences in genome-wide methylation were noted between groups in each of three distinct conditions.
A total of 834 913 single CpG sites, similarly to the inter-subject analysis, were tested for association within each cycle in individual participants. There were noted to be 11 429 markers which were statistically different within each subject; however, all beta values associated with these differences were consistent with the presence of genome variations including SNPs and indels. Manual inspection of the genome annotation data confirmed such findings. No statistically significant differences in the methylomic profile of the endometrium were found in individuals within each distinct treatment group.
Discussion
This randomized, paired cross-over study demonstrates that supraphysiologic levels of E2 do not meaningfully alter the initiation of P4-induced secretory transformation. Stated simply, the endometrial response to P4 is unaffected by E2 levels in the supraphysiologic range. There was an equivalent endometrial response to P4 regardless of the E2 level. While several genes were noted to be upregulated or downregulated within individuals at varying levels of estradiol, the majority either did not have a protein product, were ‘read through’ segments of DNA adjacent to other unrelated genes, or whose role was unrelated to endometrial function and physiology. The exception to this is PAEP, a gene previously noted to be highly upregulated in the early and mid-secretory phase endometrium (Julkunen et al., 1986; Lindhard et al., 2002), however, was noted to be downregulated in two participants in both the moderately supraphysiologic and supraphysiologic E2 ranges. It has been demonstrated that PAEP is increased dramatically in pathologic conditions such as endometriosis (Focarelli et al., 2018), whereas expression is decreased in recurrent implantation failure (Pathare et al., 2017). Interestingly, it has been shown in a murine model that GnRH agonist treatment during controlled ovarian hyperstimulation (COH) resulted in reduced expression of PAEP in the endometrium (Wu et al., 2018), which may hypothetically account for these findings. Similarly, when comparing all participants in each condition, no genes were significantly modulated related to secretory transformation or endometrial receptivity. There were no genes, regardless of protein product or function, that were noted to be upregulated or downregulated consistently across all E2 conditions either within or between individual subjects.
Echoing the stable transcriptomic profile of the endometrium across a range of E2 levels, the methylation profile of the genome in the early secretory phase remained unaffected by highly supraphysiologic levels of E2. No intra-participant nor inter-participant variations in genome methylation were observed in our study. All CpG markers that were found to be differentially methylated within each patient were attributed to genomic variations within individuals due to beta values that are consistent with the presence of SNPs and indels. This was confirmed by manual inspection of such CpG sites as well. Although characteristic changes in DNA methylation profiles have been defined from the pre-receptive (LH + 2) to the receptive (LH + 8) phase in natural cycles of healthy, fertile women (Kukushkina et al., 2017), as well as in the peri-implantation period of infertile patients with premature rises in progesterone (Xiang et al., 2017), no such observations were noted in our study.
Importantly, there was equivalent endometrial proliferation and progesterone stimulation between each cycle for all patients. There were no differences in mean endometrial thickness, peak P4 levels prior to initiation of PIO, or in peak P4 levels on day of endometrial biopsy regardless of serum E2 level or the dose of E2V consumed. As intended, these parameters were adequately controlled for under a wide range of E2 exposures. We confirmed the onset of secretory transformation on the day of endometrial biopsy (cycle Day 16) by measurement of P4 levels that are concordant with those previously reported to induce the secretory transformation utilizing a PIO dose of 10 mg (Usadi et al., 2008; Young et al., 2017).
Our observations are consistent with previous observations (Groll et al., 2000), which demonstrated that expression of fundamental biomarkers as well as endometrial histology were unchanged despite increasing levels of E2 to the supraphysiologic range. In this study, however, biopsies were performed in the mid- to late-secretory phase after 10 days of P4 exposure. While our current findings concur with these observations, they also expand upon them as we now recognize that the onset of secretory transformation is not altered in the presence of premature P4 elevations.
Given that increasing levels of E2 do not attenuate the ability of subtle rises in P4 to induce secretory transformation, what could account for Greisinger’s findings in which high responders with such elevations did not have diminutions in pregnancy rates? It has been demonstrated that high responding patients with supraphysiologic E2 levels are younger and tend to blastulate earlier in the embryonic window. Despite P4 provoked shifts in the WOI, the blastocyst is capable of implantation at an earlier time, compensating for the premature onset of secretory transformation. In comparison, older patients, often with a poor or normo-ovarian response, tend to blastulate normally or even late within the embryonic window (Forman et al., 2013; Shapiro et al., 2013), detrimentally impacting pregnancy rates through an advanced secretory endometrium and potentially delayed blastocyst. When late blastulating embryos are cryopreserved, pregnancy rates are equivalent, thereby correcting for synchrony issues (Franasiak et al., 2018).
Our findings are useful for clinicians in that the onset of secretory transformation is not affected by the endometrial response to supraphysiologic levels of estradiol. In both fresh and frozen-thawed endometrial transfer cycles, E2 levels often surpass 2000 pg/ml. Our results are reassuring in that there is an equivalent response to P4 despite the level of E2 exposure, making it less likely that variations will be impactful in frozen embryo transfer cycles as well as fresh cycles. As such, clinicians should respond to premature P4 elevations in all patients with the same caution given that patients maintain normal endometrial physiology as evidenced by shifts in the onset of secretory transformation across a range of E2 levels. Clinical pregnancy rates are lower with fresh embryo transfer as compared to frozen-thawed embryo transfer, even in fresh cycles without a premature rise in P4 and regardless of the number of oocytes retrieved (Shapiro et al., 2011a,b; Roque et al., 2015). This has been attributed to deleterious effects of COH on endometrial receptivity, however can be attributed to dyssynchrony between the embryo and endometrium following early P4 elevations or alternatively to slowly blastulating embryos, both of which are independent of the E2 rise.
It has been suggested that highly elevated levels of E2 during COH for IVF increases obstetric and neonatal morbidity, namely the risk of pre-eclampsia and small for gestational age infants (Albrecht et al., 2006; Kalra et al., 2011; Imudia et al., 2012). These risks are mitigated by deferral of embryo transfer to a subsequent frozen-thawed transfer cycle. Supraphysiologic levels of E2 as well as an altered ratio of E2 to P4 may alter the normal course of uterine spiral artery invasion that permissibly occurs in the low E2 milieu associated with spontaneous pregnancy. An additional study should be conducted to determine if an altered ratio of E2 to P4 may contribute to the adverse obstetrical outcomes seen in fresh embryo transfer cycles with highly elevated levels of E2.
The principal strength of our study is the randomized and paired cross-over design in which each patient is able to be compared to themselves across a range of E2 levels. This is the first study investigating the onset of secretory transformation in this context, and as such is able to eliminate genomic variability between individuals as a confounder. In addition, our study was the first to look at whole-genome transcriptomics and methylomics under varying exposure to E2, rather than relying on the subjective nature of histologic dating of the endometrium or targeted gene analysis.
Our study also had several limitations. While each participant completed a physiologic cycle in which E2 levels were maintained in a range that would simulate a natural cycle, our findings are restricted by lack of an unmedicated control to assess if there was a potential effect from E2V. However, it was felt to be prudent to include a medicated, physiologic cycle in order to standardize cycle length and accurately capture the onset of secretory transformation given that the exact timing of the initiation of the LH surge and P4 stimulus is difficult to determine in a natural cycle.
Although the number of participants was limited in our study, a total of 30 samples were collected from 10 individuals, allowing for a paired intra-person as well as inter-person comparison. Per biopsy specimen, over 58 000 genes were included in the transcriptomic analysis with >834 000 CpG markers in the methylomic comparison. The breadth of genomic coverage and rigorous design allowed for sufficient power to address the study objective. Previous contemporary studies performing whole-genome molecular phenotyping of the endometrium included fewer than 30 biopsy samples, with one sample from each patient being compared to others under different study conditions (Labarta et al., 2011; Young et al., 2017; Evans et al., 2018; Da Broi et al., 2019).
Our investigation included only fertile participants in order to establish that any variance in gene regulation is due to differing levels of E2 rather than infertility itself, which has been shown to characteristically alter gene expression patterns in the endometrium (Matsuzaki et al., 2009; Altmae et al., 2010; Houshdaran et al., 2016; Lessey and Kim, 2017). Given our findings, further investigation will be necessary to determine if varying levels of E2 differentially affect the endometrial response to P4 in an infertile population.
Our observations help to bring clarity to a long-debated issue and demonstrate that serum E2 levels even in supraphysiologic ranges do not impact the P4 threshold at which secretory transformation occurs.
Acknowledgements
The authors thank Sameet Mehta, PhD, Min Yang, PhD and Shiny Titus, PhD for assistance with study sample processing and statistical analyses for this manuscript. We would also like to thank Dr. Steven Young, MD, PhD for his insight and contributions to the study design.
Authors’ roles
E.K.O., MD: Corresponding author. Development of study idea, design and methodology. Collected and processed study samples, analysis of study results, interpretation of analyzed results and manuscript preparation. T.W., MD, PhD: Assisted with study sample processing, methodology, bioinformatic analysis of transcriptomic results and also assisted with creation of figures for this manuscript. Y.Z., PhD: Assisted with bioinformatic analysis, interpretation of methylomic data and assisted with creation of figures. C.R.J., MD: Assisted with patient recruitment, enrolling, consenting and collection of biopsy specimens. S.J.M., MD: Assisted with patient recruitment, enrolling, consenting and collection of biopsy specimens. E.S., MD: Assisted with methodology, interpretation of results and manuscript preparation. R.T.S. Jr, MD, HCLD: Development of study idea, design and methodology. Assisted with manuscript preparation. Co-principal investigator. J.M.F., MD, HCLD: Development of study idea, design and methodology. Assisted with patient recruitment, enrolling and consenting study participants. Collected and processed study samples, assisted with manuscript preparation. Co-principal investigator.
Funding
Funding for this study was provided by The Foundation for Embryonic Competence, Basking Ridge, NJ, USA.
Conflict of interest
Dr E.S. reports consultancy work for The Foundation for Embryonic Competence, Basking Ridge, NJ, USA. The other authors declare no conflict of interests related to this topic.
References
Du P, Zhang X, Huang C, Jafari N, Kibbe W, Hou L, Lin S. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 2010;11:587.
Forman EJ, Franasiak JM, Hong KH, Scott RT. Late expanding euploid embryos that are cryopreserved with subsequent synchronous transfer have high sustained implantation rates (SIR) similar to fresh normally blastulating euploid embryos. Fertil Steril 2013;100:S99
Franasiak JM, Forman EJ, Patounakis G, Hong KH, Werner MD, Upham KM, Treff NR, Scott Jr. RT. Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open 2018;4:
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Factors related to embryo-endometrium asynchrony in fresh IVF cycles increase in prevalence with maternal age. Fertil Steril 2013;100:S287.