-
PDF
- Split View
-
Views
-
Cite
Cite
Susan Benoff, Grace M. Centola, Colleen Millan, Barbara Napolitano, Joel L. Marmar, Ian R. Hurley, Increased seminal plasma lead levels adversely affect the fertility potential of sperm in IVF, Human Reproduction, Volume 18, Issue 2, February 2003, Pages 374–383, https://doi.org/10.1093/humrep/deg020
Close - Share Icon Share
Abstract
BACKGROUND: Lead remains in high levels in the environment and is known to reduce fertility in animal models, but a direct link between lead exposures and human infertility has not yet been established. METHODS: In a prospective, double‐blind study of the metal ion levels and sperm function, semen was obtained from partners of 140 consecutive women undergoing their first IVF cycle. Lead in seminal plasma was determined by atomic absorption spectroscopy. Motile sperm populations were assessed for surface receptors for mannose binding, and the ability to undergo premature (‘spontaneous’), and free mannose‐induced acrosome reactions. Fertile donor (n = 9) sperm were exposed to exogenous lead during capacitating incubations and then assessed for mannose receptor expression and acrosome loss. RESULTS: Lead levels were negatively correlated with IVF rates. Lead levels were negatively correlated to two of the three sperm function biomarkers (mannose receptors, mannose‐induced acrosome reactions). Lead levels positively correlated with the spontaneous acrosome reaction. These findings were mimicked by in‐vitro exposure of fertile donor sperm to lead. CONCLUSIONS: Multiple sperm parameters are affected as lead levels rise. Increased lead levels may contribute to the production of unexplained male infertility.
Introduction
Reports that human sperm concentration and male fertility are declining have renewed interest in the role of environmental exposures in the aetiology of human male infertility (reviewed in Benoff et al., 2000b). The role of lead in male factor subfertility is of particular current interest (Benoff et al., 2000b; Wong et al., 2000).
Lead is ubiquitous (Lockitch, 1993; Telisman et al., 2000). It is a major metal in the number of tons mined and refined each year. Lead appears in homes in many forms: as lead piping, lead‐containing solders, paints, ceramic glazes, pewter and base metal utensils and fixtures. Common alloys not commonly associated with lead, such as many brasses, contribute to overall lead exposures in homes. For example, brass kitchen and bathroom fixtures are a principal source of the lead found in ‘first draw’ drinking water samples. Lead can leach into foods and beverages stored in lead‐glazed ceramics, lead‐containing‐glass decanters, and metal alloy containers in which lead is a minor component, and pass by ingestion into the body. Past use of lead‐containing ‘self‐cleaning’ exterior house paint, lead chromate metal primer, fungicides and lead flashing on roofs and chimneys has contaminated soils near human dwellings. Children playing in and eating such soil may exhibit significantly elevated lead levels. Agricultural soil contamination may be responsible for lead found in many herbal medicines and cigarettes. Inhalation of lead particulates from combustion of petroleum products, vehicles and homes, and lead‐contaminated coal‐fired power plant fly ash also contribute to environmental lead exposures.
The mean concentrations of lead in bone and soft tissues of men markedly exceed those of women (Barry, 1975; Christensen, 1995). In animal models, lead exposure consistently decreases male reproductive function at the level of the hypothalamic–pituitary–testicular axis (Sokol, 1987). Endocrine dysfunction has also been reported in some human males chronically exposed to lead in the workplace (e.g. Ng et al., 1991). However, in contrast to animals, deleterious effects of lead on human reproduction can occur in the absence of endocrine dysfunction (e.g. Lancranjan et al., 1975; Assennato et al., 1986; Telisman et al., 2000).
Lead exposures also increase testicular lead levels in animal models (review: Winder, 1993). However, even though elevated lead levels have been reported to be spermatotoxic in both animals and man (e.g. Winder, 1989), prior attempts to correlate lead levels in seminal plasma with human male subfertility produced equivocal results (reviewed in Benoff et al., 2000b). Similar attempts in occupationally exposed cohorts have also been inconclusive (Benoff et al., 2000b). In fact, some investigators are not convinced that human lead exposures do affect semen parameters (e.g. Aribarg and Sukchareon, 1996; Hovatta et al., 1998). However, a recent study contains compelling evidence that even moderate exposures to lead have a negative impact on human semen quality (Telisman et al., 2000). This study is supported by our preliminary data, which suggest that a significant fraction of male partners from couples with ‘unexplained’ infertility exhibit high levels of lead in seminal plasma and may in fact represent cases of ‘environmental’ infertility (Benoff, 1999; Benoff et al., 2000b).
In animal studies, lead exposure has been associated with aberrant sperm function, e.g. premature acrosome breakdown (Johansson, 1989; Hsu et al., 1998). Our preliminary in‐vitro data indicate that the same is true in man (Benoff et al., 2000b). Therefore, to study the effects of lead upon the human male reproductive tract, we have undertaken a systematic analysis of lead levels in relation to other trace metals, to semen parameters, to sperm fertilization potential in conventional IVF and to biomarkers of human sperm function in men without occupational exposure to lead.
Materials and methods
Media and chemicals
Modified Ham’s F‐10 medium without hypoxanthine (Catalogue No. 9461) was obtained from Irvine Scientific (Santa Ana, CA, USA). Optima grade (trace metal ion‐free) concentrated hydrochloric and nitric acids were obtained from Fisher Scientific Co. (Pittsburgh, PA, USA). Unless otherwise specified, all chemicals were reagent grade or higher, and were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Human subjects protections
All protocols employing human subjects were reviewed and approved by the Institutional Review Boards of North Shore University Hospital, the University of Rochester Medical Center and Cooper Hospital.
Human semen specimens
Donors of proven fertility (n = 9) participated after giving written informed consent. These subjects were recruited from among semen donors in an artificial insemination programme at the University of Rochester Medical Center. All semen parameters of these donors were within the normal range based on World Health Organization (World Health Organization, 1992) criteria.
Portions of coded seminal plasma and semen specimens produced for diagnostic purposes by consecutive patients enrolled in an IVF programme at North Shore University Hospital were obtained at the point of discard. All subjects (n = 140) were planning to undergo their first cycle of IVF during the period February 1995 to August 1996. The presence of a varicocele and/or anti‐sperm antibodies as well as occupational exposure to metal ions served as study exclusion criteria. Hormonal profiles (FSH, LH and testosterone) were assessed in 55 of the subjects. Informed consent was not required for these unidentified specimens.
Testicular and prostate biopsies
These are control samples used to help validate our lead analytical protocol. Testicular biopsies (n = 5) from men with obstructive azoospermia and prostate biopsies (n = 6) were obtained at the time of clinically dictated procedures and were obtained as pathological specimens from anonymous sources at point of discard. No patient underwent a biopsy solely for research purposes. Testicular tissue was obtained by the percutaneous needle aspiration biopsy technique developed by Marmar (1998) and prostate tissue cores were obtained using spring‐loaded needle biopsy guns (Djavan et al., 2001). All biopsy material was immediately fixed in formalin.
Study design
Analysis of lead in seminal plasma as a biomarker to predict IVF success was performed as part of a larger prospective study examining parameters that could affect IVF outcome. Semen was collected, clinical analyses were performed, and the remainder of the semen specimen was used for experimental studies. The experimental studies were conducted as follows. An aliquot was removed for the Acrobeads test (Hershlag et al., 1997) and then motile sperm populations were isolated and assessed for sperm function in order of priority, based on yield: mannose receptor expression (Hershlag et al., 1998) > spontaneous acrosome reaction (Hershlag et al., 1997) > mannose‐stimulated acrosome loss (Benoff et al., 1997b) > measurements of sperm cholesterol and phospholipids content > non‐nuclear progesterone receptor expression (Jacob et al., 1998) > progesterone‐stimulated acrosome loss (Jacob et al., 1998). Seminal plasma was assessed for metal ion content in order of priority: zinc (Hurley et al., 1997) > cadmium (Benoff et al., 1997a) > lead > nickel. Note that not all experimental analyses were performed on all specimens because of limitations in specimen size.
Only specimens from patients ultimately assigned to conventional IVF insemination (n = 78) or to both insemination and ICSI (n = 18) are considered herein. All specimens from patients exclusively undergoing ICSI (n = 20) based on clinical criteria or who did not proceed to IVF (n = 24) have been excluded from this study.
Patients were assigned consecutive numbers by the IVF nurse co‐ordinating specimen and clinical information collection. Specimens were identified for experimental analysis and in the database solely by these numbers. All links to the patients were destroyed by the nurse co‐ordinator at the conclusion of clinical treatment.
Preparation of patient semen for experimental analysis
Preliminary evaluation of patients’ semen was conducted 2–4 weeks prior to an IVF cycle. All semen was collected between 06:30 and 09:00 and brought to the laboratory ≤1 h after collection. Fresh semen specimens were collected by masturbation after 2–3 days of abstinence and allowed to liquefy for ≥20 min before analysis. Semen analysis was performed to determine concentration, motility (World Health Organization, 1992) and levels of antisperm antibodies (Bronson et al., 1982). Sperm were then selected for motility by swim‐up (Bronson et al., 1982; Benoff et al., 1993a) and morphological assessments (Benoff et al., 1999) were performed. Seminal plasma was stored for metal ion analysis (see below).
Portions of one or two motile sperm populations were obtained from each IVF patient and were subjected to sperm function testing as previously described (see below). Untreated (‘uncapacitated’) sperm were prepared for analysis by centrifugation of the motile fraction (500 g for 8 min) to concentrate sperm. To induce capacitation, motile sperm were pelleted, resuspended in Ham’s F‐10 containing 30 mg/ml human serum albumin, delipidated with charcoal to remove any bound progesterone (Benoff et al., 1995), at a concentration of 12×106/ml and incubated for 16–20 h at 37°C in 5% CO2 in air. At the end of incubation, sperm were collected by centrifugation (500 g for 8 min) and their motility was assessed by phase‐contrast microscopy and viability by eosin Y dye exclusion.
Determination of lead in storage vessels and experimental plastic ware
Stringent efforts were made to exclude exogenous lead during sampling, sample processing and analysis, because we have found that chance contamination significantly degrades data quality (Hurley et al., 1997; I.R.Hurley and S.Benoff, unpublished observations). All sample storage, processing and analysis took place in a closed, dedicated room, with access restricted to persons actively involved in this work. All solutions were prepared using 18 MΩ reagent water from a Millipore MillRO system, which was produced as near the time of its use as possible. This protocol was based on recommendations for analysis of trace metals in environmental samples (Eaton et al., 1995). Only polypropylene and fluorocarbon labware was used in these assays to avoid lead contamination of our samples through diffusion and ion‐exchange of lead impurities from glass containers. All plasticware (sample collection tubes, fluorocarbon digestion vessels, sample storage vessels, autosampler vials, etc.) used in these steps were soaked sequentially for 24 h each in 1:4 concentrated hydrochloric acid:reagent water followed by 1:4 concentrated nitric acid:reagent water (both Optima grade; Fisher Scientific Co.) to dissolve and leach out traces of lead. The polypropylene leaching containers, including their tight‐fitting covers, were themselves equilibrated with several volumes of the same acids before being used to clean this plastic ware. Every container was kept closed except during plasticware transfers. Only fluorocarbon tools (tongs and stirring rods) touched plastic ware during these transfers to minimize contamination of the leaching solutions. Traces of leaching acids were removed by exhaustive washing (∼20 container‐volumes) with reagent water. Plastic ware was stored under reagent water in similarly leached and tightly capped 500 ml wide‐mouthed polypropylene bottles until they were used. Fresh samples and samples in all stages of processing and analysis were kept covered or sealed at all times that they were not being actively manipulated.
Analysis of lead levels leached from randomly selected vessels was performed to document the absence of significant contamination (Table IB). Portions (400 µl) of reagent water were processed through the same steps as a sample (see below, and baseline column of Table IB), starting from the step in which the indicated vessel was first used in our seminal plasma sample preparation and analysis scheme. Multiple starting points in the process were tested to identify steps particularly susceptible to contamination. No detectable lead was introduced during the analysis itself, while the mean lead level in reagent water subjected to all steps of sample processing and analysis was 0.6 µg/l of lead, much lower than found in semen samples.
Determination of trace metals in seminal plasma and biopsies
The subject of this report is lead in seminal plasma. However, we also determined lead levels in testis and prostate biopsies. These tissues were used as controls for our analytical protocol.
Semen was collected by masturbation into sterile lead‐free plastic containers. After isolation of motile sperm populations by swim‐up (see above), the residual sperm were removed from seminal plasma by centrifugation at 27 000 g for 10 min at room temperature, and the supernatant was passed through a 0.22 µm syringe filter (Millipore Corp., Bedford, MA, USA) and stored at –70°C. The protein matrix from each sample was removed by acid digestion. Samples (400 µl) of filtered seminal plasma were added to 400 µl portions of Optima grade (Fisher Scientific Co.) concentrated nitric acid in a 2 ml perfluoroalkoxy microwave digestion vessel. The effective power of the commercial microwave oven (Model SNAC‐70D; Menumaster, St Louis, MO, USA) employed to effect digestion of biological matrices was measured to be 130 W at 50% duty cycle based on the rate of heat absorption of a 1000 ml water ballast. Each aliquot of acid/seminal plasma mix was digested separately for 1 min 18.6 s in the presence of the water ballast in this calibrated system. The additional volume of acidified sample was too small to alter microwave power levels significantly. The resulting aqueous digests were diluted 5‐fold and 50‐fold with 2% nitric acid to provide sufficient volume of each sample to determine several metals by graphite furnace atomic absorption spectroscopy.
The diluted seminal plasma digests were assayed on a SpectrAA 250 Plus atomic absorption spectrometer equipped with a GTA 97 graphite furnace (Varian Instruments, Walnut Creek, CA, USA) at 282.3 nm wavelength in the presence of NH4H2PO4 matrix modifier. AA furnace conditions were: 40 s at 95°C (drying), 15 s at 480°C (ashing) and 2.1 s at 2600°C with interrupted gas flow (atomization). The calibration curve for lead was prepared from a serially diluted (2–32 µg/l) lead standard (Inorganic Ventures, Inc., Lakewood, NJ, USA). The calibration curves for zinc and cadmium were similarly prepared. Measurements of zinc and cadmium levels followed previously published protocols (Benoff et al., 1997a; Hurley et al., 1997). Mean metal ion concentrations in seminal plasma were then compared with reference values (Rosecrans et al., 1987; Xuezhi et al., 1992; Keck et al., 1995; Aribarg and Sukcharoen, 1996; El‐Zohairy et al., 1996; e.g. see Table IB).
A portion of each testis or prostate biopsy was weighed and lyophilized until a constant reduced weight was obtained. The dry sample was then dispersed in 800 µl 50% Optima grade nitric acid and was microwave‐digested as described for seminal plasma. Lead and cadmium levels were quantified by graphite furnace atomic absorption spectroscopy as described above for seminal plasma.
We compared our measured lead and cadmium levels in normal human prostate and testis with reference values (Barry, 1975; Oldereid et al., 1993; Brys et al., 1998; Table IB) and found good agreement. This validates in other tissues the calibration curves that we used to measure lead levels in seminal plasma.
We ‘spiked’ 400 µl portions of reagent water with lead to final concentrations of 4 µg/l and 16 µg/l and analysed them as samples, to demonstrate the accuracy and precision of our analytical protocol (see lead ‘spike’ columns of Table IB). We started processing these spikes at various stages to validate individual steps. These spike values were chosen to be typical of concentrations found in our diluted seminal plasma samples. Results are presented as percentages of the spiking concentration (to give a measure of accuracy) ± 1 SD (to give a measure of precision).
We also ‘spiked’ some of our seminal plasma, prostate and testis samples with lead at the point of microwave digestion and determined the percentage recovery of the added lead by comparison with paired unspiked tissue sections (Table IC). The accuracy and precision of lead spike recoveries parallels that seen in spiked reagent water (Table IC), showing that the following sample processing steps did not systematically add lead to, or lose lead from, these biological samples.
Examination of biological markers
Measurements of mannose receptor expression (Benoff et al., 1993a) were performed on selected IVF subjects using fluorescein isothiocyanate‐conjugated mannosylated bovine serum albumin. Premature (‘spontaneous’) acrosome breakdown and the ability to undergo acrosome loss induced by exposure of sperm to zona ligands containing mannose were quantified by staining of acrosome content with rhodamine‐labelled Pisum sativum agglutinin (Benoff et al., 1997b).
All analyses of sperm function were completed prior to the respective IVF cycles. The physicians and embryologists involved in the clinical procedures were blinded to the outcome of these research findings. No relationship was detected between expression of the biomarkers analysed and the standard parameters of semen analysis (Benoff, 1999).
In‐vitro exposure of motile sperm populations to lead
To determine the in‐vitro effect of lead on the biological sperm function markers, motile sperm populations were isolated from frozen–thawed semen from known fertile donors using three‐step Percoll density centrifugation as described previously (Benoff et al., 1993a). Our prior studies have established that mannose receptor expression and the acrosome reaction are unaffected by sperm freezing (Benoff et al., 1993b). Since mannose receptor expression reaches plateau levels in specimens from fertile donors after 16–18 h of incubation in albumin‐supplemented media (Benoff et al., 1993a), motile sperm populations were divided and incubated for 18–24 h in capacitating media supplemented with varying concentrations of lead acetate (Benoff et al., 2000a). Control aliquots were not exposed to lead.
IVF and embryo transfer
All female factors were resolved before proceeding to IVF. Ovarian stimulation was achieved with a combination of pure FSH (Metrodin; Serono Laboratories, Randolph, MA, USA) and hMG (Pergonal; Serono Laboratories; or Humegon; Organon Inc., W.Orange, NJ, USA) after leuprolide acetate (Lupron; Tap Pharmaceuticals, Chicago, IL, USA) suppression starting in the luteal phase. hCG (Pregnyl; Serono Laboratories) was administered when at least two follicles reached an average diameter of 17 mm. Transvaginal oocyte retrieval was performed 34 h post‐hCG administration.
On the day of oocyte retrieval, sperm characteristics were recorded. Motile sperm were isolated by swim‐up (n = 84) or mini‐Percoll gradients (n = 12; Ord et al., 1990), depending on total sperm number. Percoll gradient centrifugation improved sperm recoveries from specimens with abnormally low count or motility and/or high viscosity. Irrespective of isolation protocol, morphologies of the motile sperm fractions were assessed as wet mounts following phenol fixation (Benoff et al., 1999).
Insemination policy was based solely on semen analysis parameters [as defined by World Health Organization (1992) criteria and by acrosome morphology (Benoff et al., 1999)]. Conventional dose‐compensated IVF inseminations were used in most cases. The number of sperm to be inseminated was adjusted based on sperm motility and acrosome morphology, such that each oocyte was exposed to ≥25 000 sperm with normal acrosomes per ml (Benoff et al., 1999). Retrieved oocytes were split equally between conventional insemination and ICSI if one or more of the following conditions applied: (i) the sperm count of whole semen was >5×106 but <10×106 sperm/ml, (ii) >30% tapering head forms were present, and/or (iii) the couple presented with unexplained infertility of >1 year’s duration (an ongoing policy at NSUH; Hershlag et al., 2002). Conditions for assignment to ICSI only (n = 20) included: (i) ≤5% normal oval head forms, (ii) <5×106 sperm/ml, and/or (iii) poor motility.
Fertilization rate was defined as the ratio between the number of oocytes that developed into normal, two‐pronuclear embryos 16–18 h after insemination and the total number of mature metaphase II oocytes collected from that patient and inseminated by a specific protocol. The fertilization rates in the 96 cases assigned to conventional numerical dose‐compensated IVF inseminations or split between insemination and ICSI were not normally distributed. Therefore, we used the median value (= 0.8) as the measure of central tendency. We employed the interquartile range (Q1 to Q3 = 0.63 to 0.9) as the confidence interval of these observations and defined Q1 as the lower boundary for normal fertilization. Based on these criteria, fertilization rates of ≤63% were defined as ‘reduced’ fertilization.
The normal fertilization and reduced fertilization groups did not differ with respect to female partner age, the number of mature oocytes retrieved or the number of embryos transferred (Hershlag et al., 1998). The distribution of diagnosed male factor differed slightly relative to the normal and reduced fertilization groups (Fisher’s exact test, P < 0.044). The normal fertilization group was comprised of 64.8% unexplained infertility (n = 46), 22.5% asthenozoospermia (n = 16), and 12.7% multiple semen defects (n = 9). The reduced fertilization group had 40% unexplained infertility (n = 10), 28% asthenozoospermia (n = 7), 4% oligozoospermia (n = 1), and 28% multiple semen defects (n = 7).
Confounding variables
The IVF patients’ occupations and whether or not they smoked cigarettes, drank alcoholic beverages, had a history of urogenital tract infection or testicular injury, and/or were taking prescription medications or vitamins were addressed by questionnaire administered by the nurse who assigned each subject a code number.
Statistical methods
All statistical analyses were performed using the SAS/PC software package (SAS Institute Inc., Cary, NC, USA). All statistical methods employed are fully described in Draper and Smith (1966), Zar (1984), Ingelfinger et al. (1987) and Zweig and Campbell (1993). Statistical significance was defined as P < 0.05.
Results
Metal ion levels in seminal plasma
Each seminal plasma specimen was assayed in triplicate for lead, zinc and cadmium (Table IIB and Table IIB). There was <7% intra‐specimen variation between lead measurements, <5% intra‐specimen variation between zinc measurements and <5% intra‐specimen variation between cadmium measurements.
The measured zinc and cadmium in the seminal plasma samples tested were tightly clustered (Table IIB), and were consistent with reference values for adults not occupationally exposed to these metals (Rosecrans et al., 1987; Benoff et al., 2000b).
Seminal plasma lead levels varied over a wide range (Table IIB). The mean lead value was significantly higher than reference values for control, fertile populations not occupationally exposed to lead (Table IB). This range was similar to ranges reported for men occupationally exposed to lead (Xuezhi et al., 1992; Robins et al., 1997).
Seminal plasma lead values displayed a significant negative correlation with seminal plasma zinc levels (Table IIB). In contrast, seminal plasma cadmium concentrations varied independently of seminal plasma lead and zinc levels (Table IIB).
IVF outcome and lead levels
A significant negative relationship was detected between seminal plasma lead levels and the fertilization rate in IVF (Spearman correlation, n = 74, r = –0.447, P < 0.0001). The percentage variation explained by the regression model was determined using the square of the Pearson correlation coefficient (r2 = 0.201) (Draper and Smith, 1966). Changes in seminal lead levels accounted for 20% of the variance in fertilization rates. As lead levels were randomly distributed across subjects with unexplained infertility (normal semen analyses) and those with a defined male factor (oligozoospermia, asthenozoospermia or multiple semen parameter defects) (Kruskal–Wallis test, P = 0.171, not significant), this suggested that a seminal plasma lead test might predict human male fertility.
To obtain a threshold value for the level of lead in seminal plasma that was predictive of IVF fertilization outcome, cases were assigned to the normal fertilization group (>63% of oocytes fertilized) or a low fertilization group (≤63% of oocytes fertilized) (Benoff et al., 1999) and a receiver operating characteristic (ROC) curve analysis (Zweig and Campbell, 1993) was performed. This analysis identified 422.9 µg/l lead as the best threshold value (Table III). This value is very similar to that considered normal for lead in blood of men not occupationally exposed to lead (World Health Organization, 1980; Staudinger and Roth, 1998).
To make comparisons with other diagnostic tests, the likelihood ratio of a positive test and the likelihood ratio of a negative test were calculated (Table III). Muller (2000) has championed the use of these measures for this purpose and has proposed values of a ‘good’ test. Comparing our results (Table III) with target values that Muller (2000) proposed for these measures suggests that a simple measurement of seminal plasma lead levels might be a useful addition in the evaluation of the male partner prior to an IVF attempt.
Seminal plasma lead and other parameters
In animal models, increased lead levels are associated with endocrine dysfunction (Sokol, 1987). However, the concentration of lead in seminal plasma was not related to circulating levels of FSH, LH or testosterone in blood (Table IV), indicating that the hypothalamic–pituitary–testicular axis was unaffected in the subjects exhibiting relatively high seminal plasma lead levels.
To address the potential mechanism by which seminal plasma lead levels may reduce sperm fertility potential, the relationship between concentration of lead in seminal plasma, standard semen parameters and measures of human sperm function were examined (Table IV).
Only weak inverse correlations (e.g. r values of ≤–0.3) were found in this study between seminal plasma lead levels and the standard parameters of semen analysis, i.e. sperm count, motility and morphology. This is consistent with our inability to differentiate between men with unexplained infertility and those with a defined male factor on the basis of seminal plasma lead levels (see above). Stronger negative relationships existed between seminal plasma lead values and mannose receptor expression (r = –0.383), and mannose‐stimulated acrosome loss (r = –0.423). In contrast, percentages of sperm undergoing premature acrosome breakdown during capacitating incubations exhibited a low level increase with increasing seminal plasma lead levels.
In‐vitro modelling of lead effects
To determine whether the association between increased seminal plasma lead levels and altered motility and/or biomarker expression in IVF patients corresponded to a causal relationship, motile sperm populations from fertile donors were exposed to increasing doses of exogenous lead (51.5–5180 µg/l) during overnight incubation under sperm capacitating conditions. This range of lead levels spans and exceeds those observed in IVF patient seminal plasma.
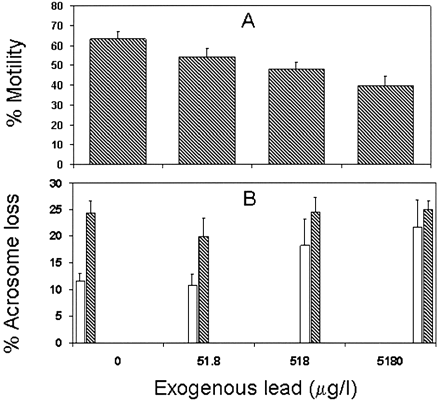
Lead in the incubation media decreased mannose receptor expression (Benoff et al., 2000a) and motility of incubated sperm (Figure 1A) in a dose‐dependent manner. Lead in the incubation media also increased spontaneous acrosome reactions (Figure 1B) in a dose‐dependent manner. The mannose‐induced acrosome reaction was apparently suppressed in a dose‐dependent manner in lead‐exposed sperm compared with no‐lead controls (Figure 1B). These findings mimic those of the IVF subjects under study.
Lead levels and potential confounding variables
Testicular cadmium concentrations have been reported to increase with increasing age (Oldereid et al., 1993). Consistent with this, a weak relationship was detected between subject age and seminal plasma cadmium levels (Spearman correlation, n = 114, r = 0.244, P < 0.006). In contrast, no relationship has been detected between the concentration of lead in testis and male age (Barry, 1975; Oldereid et al., 1993). Similarly, in the IVF patient population under study, male age was not correlated with seminal plasma lead levels (Table V).
Some vitamin and mineral supplements are contaminated by lead (e.g. Rogan et al., 1999; Scelfo and Flegal, 2000). Although their usage could potentially confound the outcome of these studies, blood lead levels have been reported to be unaffected by such contamination (Rogan et al., 1999; Gulson et al., 2001). Consistent with this, comparison of seminal plasma lead levels in subjects taking vitamin and mineral supplements (n = 17) with those not taking such supplements found no difference (Mann–Whitney test, P = 0.759, not significant).
Alcohol consumption is a source of lead exposure and alcohol has been reported to increase the amount of biologically active lead (review, Telisman et al., 2000). Of the 96 IVF patients, 18 reported drinking between 0.25 and 4 glasses of an alcoholic beverage per day. However, no relationship was detected between seminal plasma lead levels and alcohol consumption (Table V).
Cigarette smoking was considered potentially to be an ‘confounding variable’ in this analysis that might not prove to be really independent of lead measures (Benoff et al., 2000b), as between 0.6 and 2.0 µg of lead can be found within a single cigarette (Chiba and Masironi, 1991). Seventeen of those subjects who used alcoholic beverages also smoked cigarettes. Lead levels in seminal plasma of the men who smoked cigarettes (n = 11) and those who did not smoke (n = 86) were similar (Mann–Whitney U‐test, P = 0.941, not significant). Within the small subgroup of patients who used tobacco products, no relationship was detected between cigarette smoking, defined as packs per day, and the concentration of lead in seminal plasma (Table V). In contrast, when the relationship between lead levels and the number of years the patient smoked cigarettes was examined, a Spearman correlation coefficient was obtained, which, although not significant, was sufficiently large (r = 0.454; Table V) to suggest that a relationship might be detected if the sample size were to be increased. Given the long clearance rate for lead, we made a lifetime estimate for cigarette smoking (i.e. number of packs per day×20 cigarettes per pack×365.25 days per year×number of years smoking). Lead levels in seminal plasma were highly correlated (r = 0.964) with this lifetime estimate for smoking (Table V).
Discussion
Blood lead levels [determined by the National Institute for Occupational Safety and Health (1994) protocol] of ≥400 µg/l require medical intervention (Staudinger and Roth, 1998). However, studies by several groups now suggest that lead intake levels below this threshold are associated with a variety of adverse health effects in somatic tissues (Davis and Svendsgaard, 1987; Lippmann, 1990; Miller et al., 1990; Mushak, 1992; Xuezhi et al., 1992). Blood plasma lead levels have also been reported in some studies to be inversely related to semen quality (Lancranjan et al., 1975; Assennato et al., 1986; Hu et al., 1992; Lerda, 1992; Xuezhi et al., 1992; Robins et al., 1997) and, as in somatic tissues, negative effects may be observed at lead levels below permissible exposure limits (Viskum et al., 1999; Telisman et al., 2000).
Our preliminary work indicated that blood plasma lead levels were elevated in some IVF patients (Benoff et al., 2000b) and were inversely correlated with the rate of fertilization (Benoff, 1999). The partitioning of lead in whole blood between the erythrocyte and plasma fractions varies considerably (Smith et al., 1998; Chuang et al., 2001) and appears dependent upon plasma purification protocol (e.g. compare Zorbas et al., 1994 and Smith et al., 1998). Since the fraction of whole blood lead in plasma is more readily available for distribution to other organs, it therefore should be considered the biologically relevant measure (Bergdahl et al., 1999; Chuang et al., 2001). However, use of blood plasma lead levels in the study of human infertility is limited by the current uncertainty as to baseline blood plasma lead levels in normal individuals.
Positive relationships between blood lead levels and seminal plasma lead levels have been reported after both occupational exposures (Aribarg and Sukchareon, 1996) and environmental exposures (Telisman et al., 2000) to lead. This suggested that a mechanism must exist in which lead exposures were readily transferred to the male reproductive tract. Therefore, in the current study, we probed the relationship between seminal plasma lead levels and male infertility. Our findings of high seminal lead levels were unexpected as, based on questionnaire responses, none of the subjects was engaged in an occupation likely to produce exposure to metal ions.
We also report that the level of lead in seminal plasma is negatively correlated with male fertility potential, as measured by IVF outcome. Our data confirm prior findings that increased seminal plasma lead levels can occur without detectable effects on male reproductive endocrine function, and that increased lead intake may be weakly associated with decreased sperm concentration, decreased normal morphology and decreased sperm motility.
By employing dose‐compensated IVF inseminations (Benoff et al., 1999), all oocytes are exposed to the same number of motile sperm with normal morphology. We thus removed the effect of count and motility from the equation and are studying deficits in sperm function that are independent of standard semen analysis parameters. We therefore find it significant that increased seminal plasma lead levels are associated with decreased human sperm function: one measure predictive of zona binding (reduced mannose receptor expression) and another measure mimicking physiological acrosome reaction agonists (mannose). These biomarkers provide relatively unambiguous endpoints for lead‐induced reproductive toxicity compared with those previously employed, since they monitor specific steps in the process of fertilization, and expression of these two markers are independent of each other (Benoff et al., 1997b). These data suggest that lead is acting at multiple levels in testis and sperm to decrease human male fertility. The current study has used these biomarkers to demonstrate that lead exposures play a significant role in ‘unexplained’ infertility.
The stimulatory effect of lead on premature acrosome breakdown of human sperm may also be important, as it is observed both in lead‐exposed animal models (Johansson, 1989; Hsu et al., 1998) and after in‐vitro exposure of fertile human donor sperm to lead (Benoff et al., 2000b). As only a small subpopulation within motile human sperm is apparently capable of acrosome exocytosis (Benoff et al., 1995), lead inhibition of the mannose‐stimulated acrosome reaction is probably a consequence of lead‐induced increase in premature acrosome breakdown. The lead‐induced reaction selectively depletes the sperm subpopulation capable of expressing mannose‐binding lectins at a concentration required to induce a mannose‐stimulated acrosome reaction, or a zona pellucida‐induced reaction after zona pellucida binding (Liu and Baker, 1990, 1994).
High seminal plasma lead levels in up to 29 of the IVF patients was associated with cigarette smoking and/or consumption of alcoholic beverages. However, the high lead values in the remaining patients who did not smoke cigarettes and/or did not use alcoholic beverages is currently unexplained as none were occupationally exposed to lead. One possibility to be explored is the role of physical activity (Zorbas et al., 1994), as blood lead levels increase with decreased physical activity. Another possibility is diet, since low calcium diets and those high in lactose or fat apparently enhance lead accumulation (Goyer, 1995).
The observed sex‐related differences in lead accumulation in non‐occupationally exposed individuals (Barry, 1975; Christensen, 1995) may arise from differences in lifestyle variables, i.e. that men consume a greater bulk of food, alcohol and tobacco and have lower calcium intake than females (Barry, 1975; Grandjean et al., 1992). However, our data indicate that there may also be inter‐individual differences in response to lead exposure: 9/53 cases with normal fertilization rates had seminal plasma lead levels >400 µg/l and 6/21 cases with reduced fertilization had seminal plasma lead levels <400 µg/l. Thus, a question remains as to whether genetic variation (as postulated in somatic tissues; Bjorkman et al., 2000; Onalaja and Claudio, 2000; Schwartz et al., 2000; also see Benoff, 1999; Benoff et al., 2000b) or some unstudied toxicant contributes to susceptibility to lead (Todd et al., 1996).
In summary, there appears to be a direct negative correlation between seminal plasma lead levels and IVF rates. Lead levels also negatively correlated with standard semen parameters and sperm function biomarkers, and positively correlated with premature acrosome breakdown. Given the need for sperm function tests to predict fertilization success, and to assist clinicians and patients in determining the appropriate course of infertility therapy and treatment, the clinician should consider lead measurements when evaluating male partners from couples with unexplained infertility, together with measurements of some combination of mannose receptor expression and mannose‐induced acrosome reaction monitoring. Finally, environmental exposure limits for lead might be re‐evaluated in light of these results.
Acknowledgements
This paper is dedicated to Professor Wolf‐Bernhard Schill on the occasion of his 60th birthday. The authors thank Jerrold J.Heindel, PhD for insightful discussion and encouragement, Robert G.Pergolizzi, PhD and Raul Wapnir, PhD for critical reading of the manuscript, David L.Rosenfeld, MD, Gerald M.Scholl, MD, Avner Hershlag, MD, George W.Cooper, PhD and Terry Paine, BS for contribution of clinical findings, Francine S.Mandel, PhD and Cristina Sison, PhD for additional statistical input, and Zhu Jing Zhang, MD, Patricia Guhring, RN, Stephanie Canaras, MA, Michele Barcia, MA, Georgina Kvapil, BS, Deborah Liotta, MLT, Rita Herko, AAS and Evelyn Andolina, BS for their technical assistance. This work was supported by Grant No. ES 06100 to S.B. from the National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, Maryland.
Figure 1. Motile sperm populations from known fertile donors were divided and incubated overnight in media supplemented with increasing doses of lead. Data were analysed using a repeated measures analysis of variance model. (A) When compared with lead unexposed control aliquots, lead treatment results in a dose‐dependent inhibition of sperm motility (n = 7, P < 0.003). (B) Lead also affected the acrosome reaction (n = 9); the open bars represent values for spontaneous acrosome loss while the closed bars represent those following mannose treatment. In the absence of lead, mannose treatment increased the percentages of sperm undergoing acrosome breakdown (P < 0.02). In contrast, lead exposure caused a dose‐dependent increase in the spontaneous acrosome reaction (P < 0.04) with a concomitant dose‐dependent inhibition of the induced acrosome reaction (P < 0.0008).
| Table IA. Results from graphite furnace atomic absorption (AA) spectroscopy quality control analyses: plasticware | ||||||
| Vessel | Baseline | Lead ‘spike’ | ||||
| n | Lead level(µg/l) | 4 µg/l | 16 µg/l | |||
| n | % recovery | n | % recovery | |||
| AA sample cup | 23 | 0.00 ± 0.19 | 15 | 95.00 ± 14.02 | 9 | 86.81 ± 12.28 |
| Microwave digestion vessel | 11 | 0.55 ± 0.41 | 8 | 84.38 + 8.10 | 8 | 94.54 ± 7.13 |
| Semen collection cup | 11 | 0.64 ± 0.47 | 8 | 84.38 ± 16.96 | 8 | 95.33 ± 5.50 |
| Table IA. Results from graphite furnace atomic absorption (AA) spectroscopy quality control analyses: plasticware | ||||||
| Vessel | Baseline | Lead ‘spike’ | ||||
| n | Lead level(µg/l) | 4 µg/l | 16 µg/l | |||
| n | % recovery | n | % recovery | |||
| AA sample cup | 23 | 0.00 ± 0.19 | 15 | 95.00 ± 14.02 | 9 | 86.81 ± 12.28 |
| Microwave digestion vessel | 11 | 0.55 ± 0.41 | 8 | 84.38 + 8.10 | 8 | 94.54 ± 7.13 |
| Semen collection cup | 11 | 0.64 ± 0.47 | 8 | 84.38 ± 16.96 | 8 | 95.33 ± 5.50 |
| Table IB.Results from graphite furnace atomic absorption spectroscopy quality control analyses: comparison with previously reported values for lead in reproductive tissues | ||||
| Tissue | Observed valuea | Reference valueb | P (t‐test) | |
| n | Mean | |||
| Prostatea | 6 | 0.177 ± 0.035 | 0.210 ± 0.085 | 0.418 (NS) |
| Testisa,c | 5 | 0.063 ± 0.016 | 0.120 ± 0.057 | 0.108 (NS) |
| Seminal plasmaa | 94 | 382.3 ± 340.6 | 153.7 ± 39.2 | 0.0001 |
| NS = not significant. | ||||
| Table IB.Results from graphite furnace atomic absorption spectroscopy quality control analyses: comparison with previously reported values for lead in reproductive tissues | ||||
| Tissue | Observed valuea | Reference valueb | P (t‐test) | |
| n | Mean | |||
| Prostatea | 6 | 0.177 ± 0.035 | 0.210 ± 0.085 | 0.418 (NS) |
| Testisa,c | 5 | 0.063 ± 0.016 | 0.120 ± 0.057 | 0.108 (NS) |
| Seminal plasmaa | 94 | 382.3 ± 340.6 | 153.7 ± 39.2 | 0.0001 |
| NS = not significant. | ||||
| Table IC. Results from graphite furnace atomic absorption spectroscopy quality control analyses: percentage lead spike recoveries from human tissues | ||||
| Tissue | Lead ‘spike’ | |||
| + 4 µg/l | + 16 µg/l | |||
| n | % recovery | n | % recovery | |
| Prostate | 7 | 112.5 ± 12.5 | 7 | 97.6 ± 11.51 |
| Testis | 3 | 91.67 ± 16.67 | 3 | 95.83 ± 13.66 |
| Seminal plasma | 5 | 85.0 ± 16.96 | 7 | 87.50 ± 12.20 |
| Table IC. Results from graphite furnace atomic absorption spectroscopy quality control analyses: percentage lead spike recoveries from human tissues | ||||
| Tissue | Lead ‘spike’ | |||
| + 4 µg/l | + 16 µg/l | |||
| n | % recovery | n | % recovery | |
| Prostate | 7 | 112.5 ± 12.5 | 7 | 97.6 ± 11.51 |
| Testis | 3 | 91.67 ± 16.67 | 3 | 95.83 ± 13.66 |
| Seminal plasma | 5 | 85.0 ± 16.96 | 7 | 87.50 ± 12.20 |
% recovery = (lead concentration in sample aliquot to which lead has been added – lead concentration in paired sample aliquot unspiked with lead) / [expected increase in lead (input) concentration due to added lead] ×100.
aMetal ion concentrations for solid tissues are reported as ng/mg dry weight while those for seminal plasma are in µg/l.
bValues from Barry (1975), Xuezhi et al. (1992), Oldereid et al. (1993), Aribarg and Sukcharoen (1996) and El‐Zohairy et al. (1996) for occupationally unexposed males.
cTestis biopsies taken from men with obstructive azoospermia and normal spermatogenesis [i.e. Johnsen (1970) score ≥8.0].
| Table IA. Results from graphite furnace atomic absorption (AA) spectroscopy quality control analyses: plasticware | ||||||
| Vessel | Baseline | Lead ‘spike’ | ||||
| n | Lead level(µg/l) | 4 µg/l | 16 µg/l | |||
| n | % recovery | n | % recovery | |||
| AA sample cup | 23 | 0.00 ± 0.19 | 15 | 95.00 ± 14.02 | 9 | 86.81 ± 12.28 |
| Microwave digestion vessel | 11 | 0.55 ± 0.41 | 8 | 84.38 + 8.10 | 8 | 94.54 ± 7.13 |
| Semen collection cup | 11 | 0.64 ± 0.47 | 8 | 84.38 ± 16.96 | 8 | 95.33 ± 5.50 |
| Table IA. Results from graphite furnace atomic absorption (AA) spectroscopy quality control analyses: plasticware | ||||||
| Vessel | Baseline | Lead ‘spike’ | ||||
| n | Lead level(µg/l) | 4 µg/l | 16 µg/l | |||
| n | % recovery | n | % recovery | |||
| AA sample cup | 23 | 0.00 ± 0.19 | 15 | 95.00 ± 14.02 | 9 | 86.81 ± 12.28 |
| Microwave digestion vessel | 11 | 0.55 ± 0.41 | 8 | 84.38 + 8.10 | 8 | 94.54 ± 7.13 |
| Semen collection cup | 11 | 0.64 ± 0.47 | 8 | 84.38 ± 16.96 | 8 | 95.33 ± 5.50 |
| Table IB.Results from graphite furnace atomic absorption spectroscopy quality control analyses: comparison with previously reported values for lead in reproductive tissues | ||||
| Tissue | Observed valuea | Reference valueb | P (t‐test) | |
| n | Mean | |||
| Prostatea | 6 | 0.177 ± 0.035 | 0.210 ± 0.085 | 0.418 (NS) |
| Testisa,c | 5 | 0.063 ± 0.016 | 0.120 ± 0.057 | 0.108 (NS) |
| Seminal plasmaa | 94 | 382.3 ± 340.6 | 153.7 ± 39.2 | 0.0001 |
| NS = not significant. | ||||
| Table IB.Results from graphite furnace atomic absorption spectroscopy quality control analyses: comparison with previously reported values for lead in reproductive tissues | ||||
| Tissue | Observed valuea | Reference valueb | P (t‐test) | |
| n | Mean | |||
| Prostatea | 6 | 0.177 ± 0.035 | 0.210 ± 0.085 | 0.418 (NS) |
| Testisa,c | 5 | 0.063 ± 0.016 | 0.120 ± 0.057 | 0.108 (NS) |
| Seminal plasmaa | 94 | 382.3 ± 340.6 | 153.7 ± 39.2 | 0.0001 |
| NS = not significant. | ||||
| Table IC. Results from graphite furnace atomic absorption spectroscopy quality control analyses: percentage lead spike recoveries from human tissues | ||||
| Tissue | Lead ‘spike’ | |||
| + 4 µg/l | + 16 µg/l | |||
| n | % recovery | n | % recovery | |
| Prostate | 7 | 112.5 ± 12.5 | 7 | 97.6 ± 11.51 |
| Testis | 3 | 91.67 ± 16.67 | 3 | 95.83 ± 13.66 |
| Seminal plasma | 5 | 85.0 ± 16.96 | 7 | 87.50 ± 12.20 |
| Table IC. Results from graphite furnace atomic absorption spectroscopy quality control analyses: percentage lead spike recoveries from human tissues | ||||
| Tissue | Lead ‘spike’ | |||
| + 4 µg/l | + 16 µg/l | |||
| n | % recovery | n | % recovery | |
| Prostate | 7 | 112.5 ± 12.5 | 7 | 97.6 ± 11.51 |
| Testis | 3 | 91.67 ± 16.67 | 3 | 95.83 ± 13.66 |
| Seminal plasma | 5 | 85.0 ± 16.96 | 7 | 87.50 ± 12.20 |
% recovery = (lead concentration in sample aliquot to which lead has been added – lead concentration in paired sample aliquot unspiked with lead) / [expected increase in lead (input) concentration due to added lead] ×100.
aMetal ion concentrations for solid tissues are reported as ng/mg dry weight while those for seminal plasma are in µg/l.
bValues from Barry (1975), Xuezhi et al. (1992), Oldereid et al. (1993), Aribarg and Sukcharoen (1996) and El‐Zohairy et al. (1996) for occupationally unexposed males.
cTestis biopsies taken from men with obstructive azoospermia and normal spermatogenesis [i.e. Johnsen (1970) score ≥8.0].
| Table IIA. Seminal plasma metal ion levels in subjects who proceeded to IVF: seminal plasma metal ion ranges in µg/l as determined by graphite furnace atomic absorption spectroscopy | ||||||
| Metal ion | n | Mean | SD | Median | Minimum | Maximum |
| Lead | 74 | 395.0 | 359.7 | 282.8 | <10 | 1650 |
| Cadmium | 91 | 0.294 | 0.091 | 0.280 | 0.091 | 0.692 |
| Zinc | 83 | 50 800 | 24 485 | 43 739 | 10 526 | 102 974 |
| Table IIA. Seminal plasma metal ion levels in subjects who proceeded to IVF: seminal plasma metal ion ranges in µg/l as determined by graphite furnace atomic absorption spectroscopy | ||||||
| Metal ion | n | Mean | SD | Median | Minimum | Maximum |
| Lead | 74 | 395.0 | 359.7 | 282.8 | <10 | 1650 |
| Cadmium | 91 | 0.294 | 0.091 | 0.280 | 0.091 | 0.692 |
| Zinc | 83 | 50 800 | 24 485 | 43 739 | 10 526 | 102 974 |
| Table IIB.Seminal plasma metal ion levels in subjects who proceeded to IVF: examination of the relationship between seminal plasma metal levels: results of Spearman correlational analyses | ||||
| Variable 1 | Variable 2 | n | r | P value |
| Seminal plasma lead | Seminal plasma cadmium | 74 | –0.043 | 0.715 (NS) |
| Seminal plasma zinc | 67 | –0.384 | < 0.001 | |
| Seminal plasma cadmium | Seminal plasma zinc | 83 | 0.190 | 0.085 (NS) |
| Table IIB.Seminal plasma metal ion levels in subjects who proceeded to IVF: examination of the relationship between seminal plasma metal levels: results of Spearman correlational analyses | ||||
| Variable 1 | Variable 2 | n | r | P value |
| Seminal plasma lead | Seminal plasma cadmium | 74 | –0.043 | 0.715 (NS) |
| Seminal plasma zinc | 67 | –0.384 | < 0.001 | |
| Seminal plasma cadmium | Seminal plasma zinc | 83 | 0.190 | 0.085 (NS) |
n = number of subjects; NS = not significant.
NS = not significant.
| Table IIA. Seminal plasma metal ion levels in subjects who proceeded to IVF: seminal plasma metal ion ranges in µg/l as determined by graphite furnace atomic absorption spectroscopy | ||||||
| Metal ion | n | Mean | SD | Median | Minimum | Maximum |
| Lead | 74 | 395.0 | 359.7 | 282.8 | <10 | 1650 |
| Cadmium | 91 | 0.294 | 0.091 | 0.280 | 0.091 | 0.692 |
| Zinc | 83 | 50 800 | 24 485 | 43 739 | 10 526 | 102 974 |
| Table IIA. Seminal plasma metal ion levels in subjects who proceeded to IVF: seminal plasma metal ion ranges in µg/l as determined by graphite furnace atomic absorption spectroscopy | ||||||
| Metal ion | n | Mean | SD | Median | Minimum | Maximum |
| Lead | 74 | 395.0 | 359.7 | 282.8 | <10 | 1650 |
| Cadmium | 91 | 0.294 | 0.091 | 0.280 | 0.091 | 0.692 |
| Zinc | 83 | 50 800 | 24 485 | 43 739 | 10 526 | 102 974 |
| Table IIB.Seminal plasma metal ion levels in subjects who proceeded to IVF: examination of the relationship between seminal plasma metal levels: results of Spearman correlational analyses | ||||
| Variable 1 | Variable 2 | n | r | P value |
| Seminal plasma lead | Seminal plasma cadmium | 74 | –0.043 | 0.715 (NS) |
| Seminal plasma zinc | 67 | –0.384 | < 0.001 | |
| Seminal plasma cadmium | Seminal plasma zinc | 83 | 0.190 | 0.085 (NS) |
| Table IIB.Seminal plasma metal ion levels in subjects who proceeded to IVF: examination of the relationship between seminal plasma metal levels: results of Spearman correlational analyses | ||||
| Variable 1 | Variable 2 | n | r | P value |
| Seminal plasma lead | Seminal plasma cadmium | 74 | –0.043 | 0.715 (NS) |
| Seminal plasma zinc | 67 | –0.384 | < 0.001 | |
| Seminal plasma cadmium | Seminal plasma zinc | 83 | 0.190 | 0.085 (NS) |
n = number of subjects; NS = not significant.
NS = not significant.
Identification of a threshold seminal plasma lead level predicting ‘normal’ fertilization rates
| Results from ROC curve analysisa | Value | Estimated 95% CI |
| Threshold | 422.9 µg/l | |
| Sensitivityb (%) | 75.9 | 62.4–86.5 |
| Specificityc (%) | 70.0 | 45.7–88.1 |
| Positive predictive valued (%) | 87.2 | 74.3–95.2 |
| Negative predictive valuee (%) | 51.9 | 32.0–71.3 |
| Likelihood ratio of a positive testf | 2.53 | |
| Likelihood ratio of a negative testg | 0.33 |
| Results from ROC curve analysisa | Value | Estimated 95% CI |
| Threshold | 422.9 µg/l | |
| Sensitivityb (%) | 75.9 | 62.4–86.5 |
| Specificityc (%) | 70.0 | 45.7–88.1 |
| Positive predictive valued (%) | 87.2 | 74.3–95.2 |
| Negative predictive valuee (%) | 51.9 | 32.0–71.3 |
| Likelihood ratio of a positive testf | 2.53 | |
| Likelihood ratio of a negative testg | 0.33 |
aReceiver operating characteristic (ROC) curve analysis was performed as described by Zweig and Campbell (1993).
bSensitivity = the probability that a seminal plasma lead level ≥422.9 µg/l will correctly predict fertilization rates of ≤63%.
cSpecificity = the probability that a seminal plasma lead level <422.9 µg/l will correctly predict fertilization rates of ≥63%.
dPositive predictive value = the probability that seminal plasma lead level <422.9 µg/l will correspond to a fertilization rate >63%.
eNegative predictive value = the probability that seminal plasma lead level ≥422.9 µg/l will correspond to a fertilization rate ≤63%.
fLikelihood ratio of a positive test = sensitivity/(1 – specificity); the likelihood ratio for a positive test can range from 1.0 to infinity, with higher ratios being better (Muller, 2000).
gLikelihood ratio of a negative test = (1 – sensitivity)/specificity; the likelihood ratio of a negative test can range from 1.0 to 0.0, with lower being better (Muller, 2000).
CI = confidence interval.
Identification of a threshold seminal plasma lead level predicting ‘normal’ fertilization rates
| Results from ROC curve analysisa | Value | Estimated 95% CI |
| Threshold | 422.9 µg/l | |
| Sensitivityb (%) | 75.9 | 62.4–86.5 |
| Specificityc (%) | 70.0 | 45.7–88.1 |
| Positive predictive valued (%) | 87.2 | 74.3–95.2 |
| Negative predictive valuee (%) | 51.9 | 32.0–71.3 |
| Likelihood ratio of a positive testf | 2.53 | |
| Likelihood ratio of a negative testg | 0.33 |
| Results from ROC curve analysisa | Value | Estimated 95% CI |
| Threshold | 422.9 µg/l | |
| Sensitivityb (%) | 75.9 | 62.4–86.5 |
| Specificityc (%) | 70.0 | 45.7–88.1 |
| Positive predictive valued (%) | 87.2 | 74.3–95.2 |
| Negative predictive valuee (%) | 51.9 | 32.0–71.3 |
| Likelihood ratio of a positive testf | 2.53 | |
| Likelihood ratio of a negative testg | 0.33 |
aReceiver operating characteristic (ROC) curve analysis was performed as described by Zweig and Campbell (1993).
bSensitivity = the probability that a seminal plasma lead level ≥422.9 µg/l will correctly predict fertilization rates of ≤63%.
cSpecificity = the probability that a seminal plasma lead level <422.9 µg/l will correctly predict fertilization rates of ≥63%.
dPositive predictive value = the probability that seminal plasma lead level <422.9 µg/l will correspond to a fertilization rate >63%.
eNegative predictive value = the probability that seminal plasma lead level ≥422.9 µg/l will correspond to a fertilization rate ≤63%.
fLikelihood ratio of a positive test = sensitivity/(1 – specificity); the likelihood ratio for a positive test can range from 1.0 to infinity, with higher ratios being better (Muller, 2000).
gLikelihood ratio of a negative test = (1 – sensitivity)/specificity; the likelihood ratio of a negative test can range from 1.0 to 0.0, with lower being better (Muller, 2000).
CI = confidence interval.
Seminal plasma lead levels and other parameters in subjects who proceeded to IVF: results of Spearman correlational analyses
| Variable | n | r | P value |
| FSH (mIU/ml) | 28 | 0.148 | 0.452 (NS) |
| LH (mIU/ml) | 28 | 0.103 | 0.603 (NS) |
| Testosterone (ng/dl) | 28 | –0.083 | 0.674 (NS) |
| Sperm concentration (×106/ml) | 74 | –0.277 | < 0.017 |
| % normal oval morphology | 74 | –0.306 | < 0.008 |
| % sperm motility | 74 | –0.282 | < 0.015 |
| % increase in mannose receptor expression on incubation | 67 | –0.383 | < 0.001 |
| % premature acrosome loss (incubated minus freshly isolated) | 56 | 0.265 | < 0.05 |
| % increase in acrosome loss induced by mannose treatment after incubation | 46 | –0.423 | < 0.003 |
| Variable | n | r | P value |
| FSH (mIU/ml) | 28 | 0.148 | 0.452 (NS) |
| LH (mIU/ml) | 28 | 0.103 | 0.603 (NS) |
| Testosterone (ng/dl) | 28 | –0.083 | 0.674 (NS) |
| Sperm concentration (×106/ml) | 74 | –0.277 | < 0.017 |
| % normal oval morphology | 74 | –0.306 | < 0.008 |
| % sperm motility | 74 | –0.282 | < 0.015 |
| % increase in mannose receptor expression on incubation | 67 | –0.383 | < 0.001 |
| % premature acrosome loss (incubated minus freshly isolated) | 56 | 0.265 | < 0.05 |
| % increase in acrosome loss induced by mannose treatment after incubation | 46 | –0.423 | < 0.003 |
n = number of subjects; NS = not significant.
Seminal plasma lead levels and other parameters in subjects who proceeded to IVF: results of Spearman correlational analyses
| Variable | n | r | P value |
| FSH (mIU/ml) | 28 | 0.148 | 0.452 (NS) |
| LH (mIU/ml) | 28 | 0.103 | 0.603 (NS) |
| Testosterone (ng/dl) | 28 | –0.083 | 0.674 (NS) |
| Sperm concentration (×106/ml) | 74 | –0.277 | < 0.017 |
| % normal oval morphology | 74 | –0.306 | < 0.008 |
| % sperm motility | 74 | –0.282 | < 0.015 |
| % increase in mannose receptor expression on incubation | 67 | –0.383 | < 0.001 |
| % premature acrosome loss (incubated minus freshly isolated) | 56 | 0.265 | < 0.05 |
| % increase in acrosome loss induced by mannose treatment after incubation | 46 | –0.423 | < 0.003 |
| Variable | n | r | P value |
| FSH (mIU/ml) | 28 | 0.148 | 0.452 (NS) |
| LH (mIU/ml) | 28 | 0.103 | 0.603 (NS) |
| Testosterone (ng/dl) | 28 | –0.083 | 0.674 (NS) |
| Sperm concentration (×106/ml) | 74 | –0.277 | < 0.017 |
| % normal oval morphology | 74 | –0.306 | < 0.008 |
| % sperm motility | 74 | –0.282 | < 0.015 |
| % increase in mannose receptor expression on incubation | 67 | –0.383 | < 0.001 |
| % premature acrosome loss (incubated minus freshly isolated) | 56 | 0.265 | < 0.05 |
| % increase in acrosome loss induced by mannose treatment after incubation | 46 | –0.423 | < 0.003 |
n = number of subjects; NS = not significant.
Examination of the relationship between seminal plasma lead levels and lifestyle variables: results of Spearman correlational analyses
| Variable | n | r | P value |
| Male age | 88 | –0.181 | 0.090 (NS) |
| Alcohol consumption | 18 | –0.132 | 0.602 (NS) |
| Cigarette smokinga | 11 | 0.227 | 0.396 (NS) |
| Years smoking | 8 | 0.454 | 0.258 (NS) |
| Lifetime smoking estimateb | 7 | 0.964 | < 0.0005 |
| Variable | n | r | P value |
| Male age | 88 | –0.181 | 0.090 (NS) |
| Alcohol consumption | 18 | –0.132 | 0.602 (NS) |
| Cigarette smokinga | 11 | 0.227 | 0.396 (NS) |
| Years smoking | 8 | 0.454 | 0.258 (NS) |
| Lifetime smoking estimateb | 7 | 0.964 | < 0.0005 |
aPacks per day.
bNumber of packs per day×20 cigarettes per pack×365.25 days per year×number of years smoking.
n = number of subjects; NS = not significant.
Examination of the relationship between seminal plasma lead levels and lifestyle variables: results of Spearman correlational analyses
| Variable | n | r | P value |
| Male age | 88 | –0.181 | 0.090 (NS) |
| Alcohol consumption | 18 | –0.132 | 0.602 (NS) |
| Cigarette smokinga | 11 | 0.227 | 0.396 (NS) |
| Years smoking | 8 | 0.454 | 0.258 (NS) |
| Lifetime smoking estimateb | 7 | 0.964 | < 0.0005 |
| Variable | n | r | P value |
| Male age | 88 | –0.181 | 0.090 (NS) |
| Alcohol consumption | 18 | –0.132 | 0.602 (NS) |
| Cigarette smokinga | 11 | 0.227 | 0.396 (NS) |
| Years smoking | 8 | 0.454 | 0.258 (NS) |
| Lifetime smoking estimateb | 7 | 0.964 | < 0.0005 |
aPacks per day.
bNumber of packs per day×20 cigarettes per pack×365.25 days per year×number of years smoking.
n = number of subjects; NS = not significant.
References
Aribarg, A. and Sukchareon, N. (
Assennato, G., Paci, G., Baser, M.E., Molinini, R., Candela, R.G., Altamura, B.M. and Gioginor, R. (
Barry, P.S.I. (
Benoff, S., Cooper, G.W., Hurley, I. Napolitano, B., Rosenfeld, D.L., Scholl, G.M. and Hershlag, A. (
Benoff, S., Hurley, I., Cooper, G.W. Mandel, F.S., Hershlag, A., Scholl, G.M. and Rosenfeld, D.L. (
Benoff, S., Rushbrook, J.I., Hurley, I.R., Mandel, F.S., Barcia, M., Cooper, G.W. and Hershlag, A.(
Benoff, S., Hurley, I.R., Barcia, M., Mandel, F.S., Cooper, G.W. and Hershlag, A. (
Benoff, S., Hurley, I.R., Mandel, F.S., Paine, T., Jacob, A., Cooper, G.W. and Hershlag, A. (
Benoff, S., Cooper, G.W., Paine, T., Hurley, I.R., Napolitano, B., Jacob, A., Scholl, G.M. and Hershlag, A. (
Benoff, S., Cooper, G.W, Centola, G.M., Jacob, A., Hershlag, A. and Hurley, I.R. (
Benoff, S., Jacob, A. and Hurley, I.R. (
Bergdahl, I.A., Vahter, M., Counter, A., Schutz, A., Buchanan, L.H., Ortega, F., Laurell, G. and Skerfving, S. (
Bjorkman, L., Vahter, M. and Pedersen, N.L. (
Bronson, R.A., Cooper, G.W. and Rosenfeld, D.L. (
Brys, M., Nawrocka, A.D, Miekos, E. Zydek, C., Foksinski, M., Barecki, A. and Krajewska, W.M. (
Chiba, M. and Masironi, R. (
Christensen, J.M. (
Chuang, H.Y, Schwartz, J., Gonzales‐Cossio, T., Lugo, M.C., Palazuelos, E., Aro, A., Hu, H. and Hernandez‐Avila, M. (
Djavan, B., Waldert, M., Zlotta, A., Dobronski, P., Seitz, C., Remzi, M., Borkowski, A., Shculman, C. and Marberger, M. (
Eaton, A.D., Clescerio, L.S. and Greenburg, A.E. (eds) Standard Methods for the Examination of Water and Waste Water, 19th edn. American Public Health Association, Washington, DC.
El‐Zohairy, E.A., Youseff, A.F., Abul‐Nasr, S.M., Fahmy, I.M., Salem, D., Kahil, A.K. and Madkour, M.K. (
Grandjean, P., Nielsen, G.D., Jorgensen, P.J. and Horder, M. (
Gulson, B.L., Mizon, K.J., Palmer, J.M., Korsch, M.J. and Taylor, A.J. (
Hershlag, A., Paine, T., Scholl, G.M., Rosenfeld, D.L., Mandel, F.S., Zhu, J.Z., Guhring, P, Mercerod, D. and Benoff, S. (
Hershlag, A., Scholl, G.M., Jacob, A., Mandel, F.S., Guhring, P., Paine, T., Cooper, G.W. and Benoff, S. (
Hershlag, A., Paine, T., Kvapil, G., Feng, H. and Napolitano, B. (
Hovatta, O., Venalainen, E.‐R., Kuusimaki, L., Heikkila, J., Hirvi, T. and Reima, I. (
Hsu, P.C., Hsu, C.C., Liu, M.Y., Chen, L.Y. and Guo, Y.L (
Hu, W.Y., Wu, S.H., Wang, L.L., Wang, G.I., Fan, H. and Liu, Z.M. (
Hurley, I.R., Hershlag, A. and Benoff, S. (
Ingelfinger, J.A., Mosteller, F., Thibodeau, L.A. and Ware, L.A. (
Jacob, A., Hurley, I., Mandel, F.S., Hershlag, A., Cooper, G.W. and Benoff, S. (
Johansson, L. (
Johnsen, S.G. (
Keck, C., Bramkamp, G., Behre, H.M., Muller, C., Jockenhovel, F. and Nieschlag, E. (
Lancranjan, I., Popescu, H.I., Gavanescu, O., Klepsch, I. and Serbanescu, M. (
Lerda, D. (
Lippmann, M. (
Liu, D.Y. and Baker, H.W.G. (
Liu, D.Y. and Baker, H.W.G. (
Marmar, J.L. (
Miller, G.D., Massaro, T.F. and Massaro, E.J. (
Muller, C.H. (
Mushak, P. (
National Institute for Occupational Safety and Health (1994) Method 8003. In Lead in Blood and Urine. NIOSH Manual of Analytical Methods, 4th edn.
Ng, T.P., Goh, H.H., Ng, Y.L., Ong, H.Y., Ong, C.N., Chia, K.S., Chia, S.E. and Jeyaratnam, J. (
Oldereid, N.B., Thomassen, Y., Attramadal, A., Olaisen, B. and Purvis, K. (
Onalaja, A.O. and Claudio, L. (
Ord, T., Patrizio, P., Marello, E., Balmaceda. J.P. and Asch, R.H. (
Robins, T.G., Bornman, M.S., Ehrlich, R.I., Cantrell, A.C., Pienaar, E., Vallabh, J. and Miller, S. (
Rogan, W.J., Ragan, N.B., Damokosh, A.I., Davoli, C., Schaffer, T.R. and Jones, R.L. (
Rosecrans, R.R., Jeyendran, R.S., Perez‐Pelaez, M. and Kennedy, W.P. (
Scelfo, G.M. and Flegal, A.R. (
Schwartz, B.S., Lee, B.‐K., Lee, G.‐S., Stewart, W.F., Simon, D., Kelsey, K. and Todd, A.C. (
Smith, D.R., Ilustre, R.P. and Osterloh, J.D. (
Sokol, R.A. (
Telisman, S., Cvitkovic, P., Juraasovic, J., Pizent, A., Gavella, M. and Rocic, B. (
Todd, A.C., Wetmur, J.G., Moline, J.M., Gobold, J.H., Levin, S.M. and Landrigan, P.J. (
Viskum, S., Rabjerg, L., Jorgensen, P.J. and Grandjean, P. (
Winder, C. (
Wong, W.Y., Thomas, C.M.G., Merkus, J.M.W.M., Zielhuis, G.A. and Steegers‐Theunissen, R.P. (
World Health Organization (1980) Report of a study group: recommended health‐based limits in occupational exposure to heavy metals. Technical Report 647. WHO, Geneva.
World Health Organization (1992) Laboratory Manual for the Examination of Human Semen and Human Semen–Cervical Mucus Interaction, 3rd edn. Cambridge University Press, New York, pp.
Xuezhi, J., Youxin, L. and Yilan, W. (
Zorbas, Y.G., Federenko, Y.F. and Naexu, K.A. (