-
PDF
- Split View
-
Views
-
Cite
Cite
Yefim Sheynkin, Michael Jung, Peter Yoo, David Schulsinger, Eugene Komaroff, Increase in scrotal temperature in laptop computer users, Human Reproduction, Volume 20, Issue 2, February 2005, Pages 452–455, https://doi.org/10.1093/humrep/deh616
Close - Share Icon Share
Abstract
BACKGROUND: Scrotal hyperthermia has been identified as a risk factor for male infertility. Laptop computers (LC) have become part of a contemporary lifestyle and have gained popularity among the younger population of reproductive age. LC are known to reach high internal operating temperatures. We evaluated the thermal effect of LC on the scrotum. METHODS: Right and left scrotal temperature (ScT) was measured in 29 healthy volunteers in two separate 60 min sessions. ScT was recorded from thermocouples on a digital datalogger every 3 min with the working LC in a laptop position and in the same sitting position with approximated thighs without LC. RESULTS: ScT increased significantly on the right and left side in the group with working LC (2.8°C and 2.6°C, respectively; P<0001) and without LC (2.1°C, P<0.0001). However, ScT elevation with working LC was significantly higher (P<0.0001). CONCLUSIONS: Working LC in a laptop position causes significant ScT elevation as a result of heat exposure and posture-related effects. Long-term exposure to LC-related repetitive transient scrotal hyperthermia is a modern lifestyle feature that may have a negative impact upon spermatogenesis, specifically in teenage boys and young men. Further studies of such thermal effects on male reproductive health are warranted.
Introduction
It has been estimated that 15–20% of couples attempting to achieve pregnancy are unable to conceive. A male factor is the main single diagnostic category in more than half of them (Gilbaugh and Lipshultz, 1994). Gradual decline in sperm production in men has become a growing concern and subject of widespread debates in the last decades (Carlsen et al., 1992; Olsen et al., 1995; Fisch et al., 1996; Lerchl and Nieschlag, 1996; Swan et al., 1997; Jegou et al., 1999). Several factors have been implicated as possible causes of the deterioration of the male reproductive function, including endocrine disrupters, changes in lifestyle and exposure to heat (Figa-Talamanca et al., 1992; Mieusset and Bujan, 1995; Toppari et al., 1996; Thonneau et al., 1998; Bujan et al., 2000; Rozati et al., 2002).
Testicular function is temperature dependent and requires a temperature 2–4°C below body temperature (Thonneau et al., 1998). Elevated testicular temperature is a well-documented mechanism of abnormal spermatogenesis in common diseases associated with male infertility, e.g. varicocele, undescended testis (Mieusset et al., 1985; Goldstein and Eid, 1989; Lerchl et al., 1993; Wright et al., 1997).
Numerous factors can elevate scrotal temperature either by whole body or local scrotal heating. Elevated scrotal temperature was found in men with febrile illness, retractile testes, occupations associated with high temperature exposure, hot bath and sauna users, men wearing tight jockey shorts and suspensories, car drivers (Mills, 1919; MacLeod, 1951; Kapadia and Phadke, 1955; Robinson and Rock, 1967; Brindley, 1982; Brown-Woodman et al., 1984; Nistal and Paniagua, 1984; Rubben et al., 1986; Figa-Talamanca et al., 1992; Saikhun et al., 1999; Bujan et al., 2000). Multiple human studies have confirmed deleterious effects of scrotal hyperthermia on spermatogenesis (Rock and Robinson, 1965; Robinson et al., 1968; Mieusset and Bujan, 1995; Kandeel and Swerdloff, 1988).
Local scrotal hyperthermia can be achieved by direct heat exposure or effect of body temperature and blunted physiological testicular cooling mechanism (Kapadia and Phadke, 1955; Robinson and Rock, 1967; Brindley, 1982).These factors have been experimentally studied by various methods including local scrotal hot water bath, direct heating with a 150 Wt electric light bulb, sitting position with thighs approximated to the scrotum, and scrotal insulation (Rock and Robinson, 1965; Robinson et al., 1968; Brindley, 1982). Recently potential exposure of male reproductive function to certain lifestyle factors (sedentary work position, prolonged car driving, wearing plastic lined diapers by children) has been linked to increased scrotal temperature and delayed conception (Bujan et al., 2000; Hjollund et al., 2000, 2002a; Partsch et al., 2000).
Continued improvements in power, size and price of LC have favored their increased use in a younger population of reproductive age. However, LC actively generate heat and can reach internal operating temperature >70°C. Frequently positioned close to the scrotum, this device is capable of producing direct local heat exposure. In addition, the use of a LC requires a special body position in order to balance the computer on a lap when the scrotum is trapped between closely approximated thighs.
With the exception of an anecdotal report of penile and scrotal burns after LC use (Ostenson, 2002), the effect of portable computers in a laptop position on scrotal temperature is not known. We performed the first study to investigate scrotal temperature changes in LC users.
Materials and methods
Twenty-nine healthy male volunteers, 21 to 35 years old (median age 24) were recruited. All subjects completed the study. The study was approved by the Institutional Review Board and conducted at the General Clinical Research Center. All men signed an informed consent form, completed a health questionnaire and underwent a physical exam. Exclusion criteria included history or presence of varicocele, cryptorchidism, scrotal surgery, skin disease, infertility, testicular size discrepancy, recent febrile illness and prolonged or occupational exposure to heat (e.g. sauna or hot bath users, professional drivers, workers exposed to high temperature). Two 1 h sessions of scrotal temperature measurements were performed on different days in the same room with median room temperature of 22.28°C (range 21.89–22.61°C). Men were dressed in the same casual attire for each session. Sessions with and without LC were conducted at the same time of the day between 8.00 and 16.00 (median time 11.37).
Body temperature was taken orally prior to each session. Each participant spent 15 min standing in the room to adjust to the room temperature before being seated in the chair. Two cutaneous thermocouples (5SRTC-TT J type Teflon insulated wire, Omega Engineering Inc., Stamford, CT; maximum service temperature of 260°C) were attached to the unshaved scrotal skin anteriorly corresponding to the right and left testis using thin transparent tape to cover the sensor end of the thermocouple. Nonworking LC was positioned on the lap. After the participant adopted the position with approximated thighs necessary to comfortably balance the LC on the lap, the LC was removed. This position was maintained throughout the complete session. The thermocouples were connected to digital thermometer/datalogger HH84 (Omega Engineering Inc., Stamford, CT). Separate measurements of scrotal temperature on the right and left side were recorded in 3 min intervals. The datalogger was calibrated daily.
Two different brand name Pentium 4 LC were used randomly. The LC was turned on for 15 min before being positioned on the lap. Thermocouples were attached the same way and at the same place on the right and left side of the scrotum. Then the LC was comfortably positioned on the lap and the participant adopted and maintained the same sitting posture as at the previous session without the LC. Temperature measurements were performed at 3 min intervals. Measurements of the external bottom surface temperature of the working LC were taken randomly at the same time intervals.
Statistical analysis
Statistical analysis was carried out in SAS version 8.2 (SAS Institute Inc, Cary, NC). Data are summarized with medians and ranges (min and max) in centigrade units. Wilcoxon signed-ranks tests for two related samples were used to test for differences between scrotal temperatures for each side separately with P<0.05 considered statistically significant.
Results
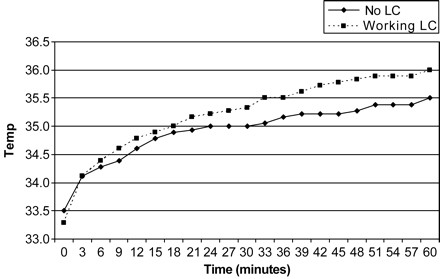
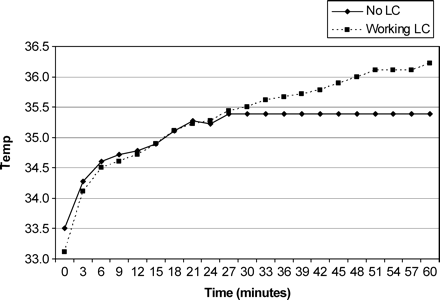
Median body temperature for both sessions was 37°C (range 36.78–37.01°C). There was no significant difference in median baseline scrotal temperatures of the right and left side between groups with LC and without LC (P=0.075 and P=0.083, respectively). As shown in Figure 1, median left scrotal temperature increased in men with working LC and without LC. Median right scrotal temperature also increased in both groups, although it remained constant in men without LC after 30 min (Figure 2). Scrotal temperature elevation on the right and left side in 60 min was significant in both groups (P<0.0001). However, this temperature elevation (60 min gradient) on the right and left side was significantly higher in the group with working LC compared to the group without LC (P<0.0001; Table I).
The median external bottom surface temperature of the two working LCs increased from 30.91°C (29.11–32.56°C) at the beginning of the experiment to 39.92°C (39.50–40.28°C) at 60 min. There were no significant differences in the initial (P=0.329) and final external bottom surface temperature (P=0.999) between two LC brands based on Wilcoxon rank sums tests.
Discussion
The negative effect of exogenous scrotal heat exposure on spermatogenesis has been demonstrated by numerous experimental human and animal studies. Recently, scrotal hyperthermia has been linked to certain lifestyle factors including use of disposable plastic lined diapers in children, prolonged car driving and sedentary work (Bujan et al., 2000; Hjollund et al., 2000, 2002a; Partsch, 2000). Laptop computers have become an integral part of a modern lifestyle. By 2005, LC use in USA will grow to 60 million units, while worldwide usage is predicted to be at 150 million units. Use of LC is growing among teenagers and young men of reproductive age.
Heat remains one of the most critical and unresolved issues in computer design. Frequent use of LC in a laptop position directly exposes the scrotum to the dissipated high internal operating temperature of the machine.
Maintenance of a proper testicular temperature is essential for normal spermatogenesis. Our study demonstrates statistically significant elevation of scrotal temperature in LC users. Since scrotal skin temperature strongly correlates with testicular temperature, such elevation corresponds to a testicular hyperthermia (Kitayama, 1965; Kurz and Goldstein, 1986; Hjollund et al., 2002a). Portable computers in a laptop position produce scrotal hyperthermia by both the direct heating effect of the computer and the sitting position necessary to balance computer on the lap with the scrotum trapped tightly between the thighs. Increased scrotal temperature in a sitting position with thighs together has been reported in previous studies (Rock and Robinson, 1965; Brindley, 1982; Bujan et al., 2000). However, the thermal impact of working LC in a laptop position is significantly higher than positional scrotal hyperthermia itself.
In our study, median left and right side scrotal temperature increase in the group with working LC was 2.6°C and 2.8°C, respectively. The magnitude of scrotal hyperthermia associated with abnormal spermatogenesis is unclear. While an increase in scrotal temperature of 1°C was sufficient to suppress spermatogenesis in some studies, others did not confirm those findings when scrotal temperature rose by 0.8–1°C (Rock and Robinson, 1965; Wang et al., 1997). Higher testicular or scrotal temperature elevation between 1 and 2.9°C was more consistently associated with a sustained and considerable negative effect on spermatogenesis and fertility (Robinson et al., 1968; Zorgniotti and MacLeod, 1973; Mieusset et al., 1985; Shafik, 1991). Therefore, a scrotal temperature increase of more than 1°C above baseline has been suggested as a possible minimal thermal gradient capable of inhibiting spermatogenesis (Wang et al., 1997; Partsch, 2000). A strong negative association was found between high scrotal temperature and sperm count as well as Inhibin B, which is considered a biochemical marker of spermatogenesis. Sperm concentration decreased by 40% per 1°C increment of median daytime scrotal temperature (Hjollund et al., 2002b). In one animal study, an increase in scrotal temperature resulted in impaired fertility even without any detectable changes in semen analysis (Mieusset et al., 1992).
The frequency and time of heat exposure capable of producing reversible or irreversible changes in human spermatogenesis are not known. Studies of frequency of heat exposure and durability of inhibition of spermatogenesis revealed significant but reversible (within weeks or months) changes after single or multiple short-term scrotal heating (Robinson et al., 1968; Kandeel and Swerdloff, 1988) and total body heating (Procope, 1965; Brown-Woodman et al., 1984). However, LC may produce significant repetitive transient scrotal hyperthermia for years. Insufficient recovery time between heat exposures may cause irreversible or partially reversible changes in male reproductive function. In one study, men exposed to high temperature for 5–7 years were found to have oligoasthenoteratozoospermia, while those exposed for 12–15 years had azoospermia (Dada et al., 2003). Another study of 449 male partners of infertile couples revealed that patients with ‘idiopathic’ oligoasthenoteratozoospermia are more exposed to genital heat stress than normozoospermic men (Jung et al., 2002).
Our study demonstrates that working with a LC produces significant elevation of scrotal temperature. While scientific background suggests a negative impact of scrotal hyperthermia upon spermatogenesis, further studies of this particular type of thermal exposure with LC are warranted. Meanwhile, limited use of LC in a laptop position by teenage boys and young men in reproductive age may be considered, to avoid intermittent scrotal heat exposure.
Median left scrotal temperature (°C) in men with working LC and without LC.
Median right scrotal temperature (°C) in men with working LC and without LC.
Median scrotal temperature (°C) with working LC in a laptop position and without LC
| Sessions . | Side . | 0 min . | 60 min . | 60 min gradient . | P-value . |
|---|---|---|---|---|---|
| Working LC in a laptop position | Right | 33.1 (32.0–35.2) | 36.2 (35.1–37.5) | 2.8 (1.0–5.1) | <0.0001 |
| Left | 33.3 (32.3–34.9) | 36.0 (35.4–37.4) | 2.6 (1.4–4.3) | <0.0001 | |
| Sitting without LC | Right | 33.5 (31.0–35.3) | 35.4 (33.0–36.9) | 2.1 (–0.1–4.5) | <0.0001 |
| Left | 33.5 (31.7–35.3) | 35.5 (33.9–36.7) | 2.1 (0.0–3.7) | <0.0001 |
| Sessions . | Side . | 0 min . | 60 min . | 60 min gradient . | P-value . |
|---|---|---|---|---|---|
| Working LC in a laptop position | Right | 33.1 (32.0–35.2) | 36.2 (35.1–37.5) | 2.8 (1.0–5.1) | <0.0001 |
| Left | 33.3 (32.3–34.9) | 36.0 (35.4–37.4) | 2.6 (1.4–4.3) | <0.0001 | |
| Sitting without LC | Right | 33.5 (31.0–35.3) | 35.4 (33.0–36.9) | 2.1 (–0.1–4.5) | <0.0001 |
| Left | 33.5 (31.7–35.3) | 35.5 (33.9–36.7) | 2.1 (0.0–3.7) | <0.0001 |
Median scrotal temperature (°C) with working LC in a laptop position and without LC
| Sessions . | Side . | 0 min . | 60 min . | 60 min gradient . | P-value . |
|---|---|---|---|---|---|
| Working LC in a laptop position | Right | 33.1 (32.0–35.2) | 36.2 (35.1–37.5) | 2.8 (1.0–5.1) | <0.0001 |
| Left | 33.3 (32.3–34.9) | 36.0 (35.4–37.4) | 2.6 (1.4–4.3) | <0.0001 | |
| Sitting without LC | Right | 33.5 (31.0–35.3) | 35.4 (33.0–36.9) | 2.1 (–0.1–4.5) | <0.0001 |
| Left | 33.5 (31.7–35.3) | 35.5 (33.9–36.7) | 2.1 (0.0–3.7) | <0.0001 |
| Sessions . | Side . | 0 min . | 60 min . | 60 min gradient . | P-value . |
|---|---|---|---|---|---|
| Working LC in a laptop position | Right | 33.1 (32.0–35.2) | 36.2 (35.1–37.5) | 2.8 (1.0–5.1) | <0.0001 |
| Left | 33.3 (32.3–34.9) | 36.0 (35.4–37.4) | 2.6 (1.4–4.3) | <0.0001 | |
| Sitting without LC | Right | 33.5 (31.0–35.3) | 35.4 (33.0–36.9) | 2.1 (–0.1–4.5) | <0.0001 |
| Left | 33.5 (31.7–35.3) | 35.5 (33.9–36.7) | 2.1 (0.0–3.7) | <0.0001 |
This project has been supported by the Department of Urology, Stony Brook University Hospital and General Clinical research Center grant # 5-MO1-RR-10710.
References
Brindley GS (
Brown-Woodman PD, Post EJ, Glass GC and White IG (
Bujan L, Daudin M, Charlet JP, Thonneau P and Mieusset R (
Carlsen E, Giwercman A, Keiding N and Skakkebæk NE (
Dada R, Gupta NP and Kucheria K (
Figa-Talamanca I, Dell'Orco V, Pupi A et al. (
Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ and Barad DH (
Gilbaugh JH and Lipshultz LI (
Goldstein M and Eid JF (
Hjollund NHI, Bonde JPE, Jensen TK and Olsen J (
Hjollund NHI, Storgaard L, Ernst E, Bonde JP and Olsen J (
Hjollund NHI, Storgaard L, Ernst E, Bonde JP and Olsen J (
Jegou B, Auger J, Multinger L et al. (
Jung A, Schill WB and Schuppe HC (
Kandeel FR and Swerdloff RS (
Kurz KR and Goldstein M (
Lerchl A and Nieschlag E (
Lerchl A, Keck C, Spiteri-Grech J and Nieschlag E (
Mieusset R and Bujan L (
Mieusset R, Grandjean H, Mansat A and Pontonnier F (
Mieusset R, Quintana-Casares P, Sanchez-Partida LG et al. (
Mills RG (
Nistal M and Paniagua R (
Olsen GW, Bodner KM and Ramlow JM (
Partsch C-J, Aukamp M and Sippell WG (
Procope BJ (
Rozati R, Reddy PP, Reddanna P and Mujtaba R (
Robinson D and Rock J (
Robinson D, Rock J and Menkin MF (
Rock J and Robinson D (
Rübben H, Recker F and Lutzeyer W (
Saikhun J, Kitiyanant Y, Vanadurongwan V and Pavasuthipasit K (
Shafik A (
Swan SH, Elkin EP and Fenster L (
Thonneau P, Bujan L, Multingner L and Mieusset R (
Toppari J, Latsen JC, Christiansen P et al. (
Wang C, McDonald V, Leung A et al. (
Wright EJ, Young GPH and Goldstein M (
Author notes
1Department of Urology and 3General Clinical Research Center, State University of New York at Stony Brook, Stony Brook NY 11794-8093, USA