-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel M. Campagne, Should fertilization treatment start with reducing stress?, Human Reproduction, Volume 21, Issue 7, 1 July 2006, Pages 1651–1658, https://doi.org/10.1093/humrep/del078
Close - Share Icon Share
Abstract
In the past few decades, new and more efficient techniques to help solve fertility problems have become widely available throughout the developed world. These techniques include hormonal stimulation, ICSI, gamete intra-Fallopian transfer (GIFT) and IVF, and their cost is, on average, considerable. There is substantial initial evidence that the psychological disposition of the parents-to-be influences their fertility and thus the outcome of fertilization techniques. Many fertility treatments include consultation with a psychologist and do try to keep the stress produced by the treatment itself to a minimum, using concurrent therapy. However, the accumulating evidence points to the need to program medical fertility treatment, bearing in mind both chronic and acute stress levels, and to treat for their reduction before commencing the (actual) fertility treatment. There is ample evidence that lower stress levels mean better female and male natural fertility, though there is as yet no conclusive experimental evidence that lower stress levels result in better fertility treatment outcome. However, first reducing stress may diminish the number of treatment cycles needed before pregnancy is obtained, may prepare the couple for an initial failure of treatment or even make the more invasive techniques unnecessary. Primary psychological treatment for trait and state stress, being a less invasive method than IVF, ICSI or GIFT, is to be applied whenever indicated. Also, treatment and therapy to reduce stress, and in so doing enhance fertility, do not provoke the ethical and religious objections raised by infertility treatments.
Introduction
Evidence-based medicine has become the objective. The efficacy of treatments and therapies needs to be established and publicized to inform the professional world but also—and importantly so—the persons who seek solutions for their problems. Professionals will of course prefer evidence that is conclusive, but this does not mean that partial or inconclusive evidence, if that is the best available, may be disregarded. This is especially the case in preventive medicine, and in general in those cases where, for a particular condition, probably or possibly effective treatments without negative side effects are available. From an ethical point of view, such treatments cannot be disregarded. Most psychological treatments have no known negative side effects. However, high-tech medical interventions do not usually consider psychological factors relevant and only sparingly co-apply psychological treatments or therapies. Time and evidence is proving that to be a mistake, possibly perpetuated because of the different criteria as to when a procedure may be rated ‘evidence-based’.
Medical science wants hard-core experimental evidence that a particular procedure remedies the condition earlier or better than another, and earlier and better than no procedure or a sham one (placebo). Differently, psychological science looks for experimental evidence that a particular procedure improves the chances that a person can herself prevent or correct factors that, in the medium or long term, would affect quality of life and/or result in pathology. The medical and the psychological objectives differ not only in that the first looks for certainties and the second works with probabilities, they also differ as to the source of change, being either external—through drugs or intervention—or internal—through cognitive, behavioural or psychodynamic changes.
The professional person or team involved in our subject—fertility—can and should combine these objectives and coordinate to increase success rates and improve cost-effectiveness. Preventive and supportive psychological treatments should be applied even when their efficacy has only provisionally been established; thus, they should be rated as ‘evidence-based’ at an earlier level of evidence than would apply to a medical treatment.
I shall proceed with a brief outline of some of the neurobiological pathways through which stress may influence fertility and review the experimental evidence as to that influence in healthy persons. Then follows a short review of the available evidence as to the relevance of some of the ‘markers’ for stress for IVF outcome. Finally, some technical and ethical reasons as to why fertilization techniques should include at least one serious prior attempt to reduce existing stress levels will be discussed.
Part 1—Ways of interaction between stress and fertility
Over the past 30 years, the majority of investigations into the interaction between emotional stress and infertility have shown that infertility causes stress, but stress does not necessarily cause infertility. Several factors play a part in this difference, a decisive one being the chronicity of stress. Preclinical studies indicate that stress can promote long-term changes in multiple neurochemical systems (Kaufman et al. 2000).
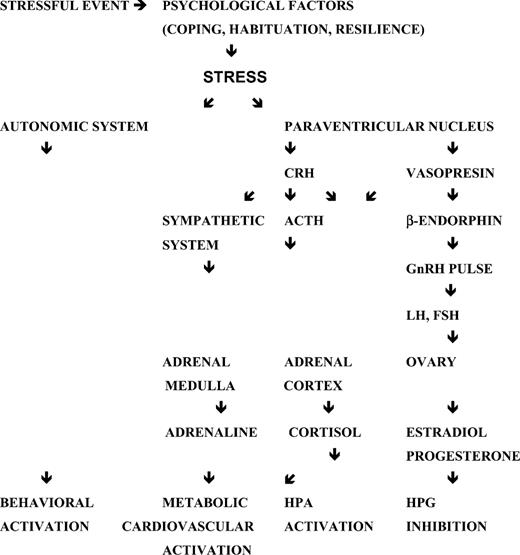
Stress involves the reciprocal and differential reactions of the hypothalamic–pituitary–adrenal (HPA) axis and the noradrenergic and adrenergic nerves to different types of ‘stressors’ and also the physiological differences between male and female response. Recent models contemplate many more interactions with other hormonal and neurobiological systems, such as the hypothalamic–pituitary–gonadal (HPG) axis or the sympathetic–adrenal–medullar system (Figure 1).
Schematic central response to stress and inhibition of the hypothalamic–pituitary–gonadal (HPG) axis (adapted from Ferin, 1999). ACTH, adrenocorticotrophic hormone; CRH, corticotrophin-releasing hormone; HPA, hypothalamic–pituitary–adrenal axis.
Stress mediators can have both protective and damaging effects, depending on the time course of their secretion. In the long run, they produce what has been called ‘allostatic overload’, meaning a change in the stability of important physiological systems with negative consequences, affecting fertility (McEwen, 2005).
Stress differs from anxiety, and biological markers are not conclusive. Elevated stress is not necessarily psychologically perceived as anxiety, and vice versa. Sanders and Bruce (1999) established a relationship between psychosocial stress and fertility in women, independent of stress hormone levels.
The early work of Selye (1950) that observed ovarian atrophy in rats exposed to stress has been followed by a number of studies that confirm the potential of stress to inhibit the HPG axis and to affect fertility (Berga, 1996). However, attempts to isolate single causal links between stress and infertility have been less successful. This partial failure is not surprising, considering the complex nature of that relationship. Stress hormones and the HPA axis interact with hormones which influence fertility directly, such as GnRH, prolactin, LH and FSH, as well as with hormones that may interfere with fertility such as cortisol, endogenous opioids and melatonin.
The fact that similar neurotransmitters and nuclei within the hypothalamus control both stress and reproduction augments the possibilities for reciprocal interference. Other substances apparently unrelated to HPA/adrenergic interaction have proven to exert a significant influence on fertility, as in the case of activated T cells in the peripheral blood, associated with a reduced implantation rate in women undergoing IVF (Gallinelli et al., 2001; Palter et al., 2001; Dobson et al., 2003).
Recent studies report that although serum levels of glucocorticoids often but not always differ between women who become pregnant and those that do not, the follicular levels of glucocorticoid hormones, especially lower follicular cortisone and a higher cortisol/cortisone ratio have shown to have a significant effect on pregnancy rates in IVF. A link was found between fertility and the activity of the ovarian 11β-hydroxysteroid dehydrogenase (11β-HSD) enzyme that catalyses the interconversion of cortisol and the biologically inactive cortisone (Arcuri et al., 1996; Edwards et al., 1996; Smith et al., 1997). Other studies found significant differences in the estradiol (E2) and progesterone areas under the curve (AUC) in the luteal phase between those women who became pregnant after IVF and those who failed (Czemiczky et al., 2000). Thus, stress may appear only in other substances or only in certain sites, or at very low levels, and cannot be determined by glucocorticoid measurement only (Irvine et al., 1994; Lewicka et al., 2003).
Stress has also been shown to have a negative impact on various parameters associated with semen quality, which similarly declines in patients undergoing IVF/ICSI (Boivin et al., 1998; Clarke et al., 1999). Recent studies have offered data as to why and how stress may reduce sperm quality and motility, for instance through loss of glutathione and free sulphydryl content of seminal plasma on account of stress (Eskiocak et al., 2005) or through the inhibition of the conversion of androstenedione into testosterone in Leydig cells on account of higher adrenocorticotrophic hormone (ACTH) and cortisol levels (Klimek et al., 2005).
Part 2—Are markers for stress and anxiety relevant for IVF outcome?
The female reproductive tract contains catecholamine receptors (Moran, 1975); thus, catecholamines—which are related to stress, see Table I—may affect fertility, for example, by interfering with the transport of gametes through the Fallopian tube or by altering uterine blood flow (Schenker et al., 1992). A substantial number of studies found that anticipatory anxiety and high anticipatory cortisol levels prior to oocyte retrieval (OR) and embryo transfer (ET) result in lower pregnancy rates, as do depression, high active coping, high avoidance and high expression of emotion (Demyttenaere et al., 1992; Smeenk et al., 2001, 2005).
Markers for stress
| Substance/method . | Relevance for acute/chronic stress . | Relevance as stress marker for IVF outcome . |
|---|---|---|
| Adrenaline | High | High (at OR, ET) |
| Noradrenaline | High | High (only at ET) |
| ACTH | High | High |
| Amylase | Variable | Questioned |
| Dehydroepiandrosterone | High | ? |
| Cortisol | Variable | Site-dependent |
| Estrogen | Variable | ? |
| Prolactin | Probable | Probable |
| Progesterone/allopregnanolone | Variable | Questioned |
| LH | Probable | Probable |
| Vasopressin | High | ? |
| NK cells | High | High |
| Cardiovascular reaction to provoked stress | High | High |
| Depression (even subclinical) | High | High |
| High active coping | High | Probable |
| High avoidance | High | Probable |
| High expression of emotion | High | High |
| State anxiety | High | High |
| State-anxiety self-report | Questioned | Questioned |
| Trait anxiety | Some | Some |
| Trait-anxiety self-report | Questioned | Questioned |
| Substance/method . | Relevance for acute/chronic stress . | Relevance as stress marker for IVF outcome . |
|---|---|---|
| Adrenaline | High | High (at OR, ET) |
| Noradrenaline | High | High (only at ET) |
| ACTH | High | High |
| Amylase | Variable | Questioned |
| Dehydroepiandrosterone | High | ? |
| Cortisol | Variable | Site-dependent |
| Estrogen | Variable | ? |
| Prolactin | Probable | Probable |
| Progesterone/allopregnanolone | Variable | Questioned |
| LH | Probable | Probable |
| Vasopressin | High | ? |
| NK cells | High | High |
| Cardiovascular reaction to provoked stress | High | High |
| Depression (even subclinical) | High | High |
| High active coping | High | Probable |
| High avoidance | High | Probable |
| High expression of emotion | High | High |
| State anxiety | High | High |
| State-anxiety self-report | Questioned | Questioned |
| Trait anxiety | Some | Some |
| Trait-anxiety self-report | Questioned | Questioned |
ACTH, adrenocorticotrophic hormone; ET, embryo transfer; NK, natural killer; OR, oocyte retrieval. See text for references. Important differences between female and male factors have been found.
Markers for stress
| Substance/method . | Relevance for acute/chronic stress . | Relevance as stress marker for IVF outcome . |
|---|---|---|
| Adrenaline | High | High (at OR, ET) |
| Noradrenaline | High | High (only at ET) |
| ACTH | High | High |
| Amylase | Variable | Questioned |
| Dehydroepiandrosterone | High | ? |
| Cortisol | Variable | Site-dependent |
| Estrogen | Variable | ? |
| Prolactin | Probable | Probable |
| Progesterone/allopregnanolone | Variable | Questioned |
| LH | Probable | Probable |
| Vasopressin | High | ? |
| NK cells | High | High |
| Cardiovascular reaction to provoked stress | High | High |
| Depression (even subclinical) | High | High |
| High active coping | High | Probable |
| High avoidance | High | Probable |
| High expression of emotion | High | High |
| State anxiety | High | High |
| State-anxiety self-report | Questioned | Questioned |
| Trait anxiety | Some | Some |
| Trait-anxiety self-report | Questioned | Questioned |
| Substance/method . | Relevance for acute/chronic stress . | Relevance as stress marker for IVF outcome . |
|---|---|---|
| Adrenaline | High | High (at OR, ET) |
| Noradrenaline | High | High (only at ET) |
| ACTH | High | High |
| Amylase | Variable | Questioned |
| Dehydroepiandrosterone | High | ? |
| Cortisol | Variable | Site-dependent |
| Estrogen | Variable | ? |
| Prolactin | Probable | Probable |
| Progesterone/allopregnanolone | Variable | Questioned |
| LH | Probable | Probable |
| Vasopressin | High | ? |
| NK cells | High | High |
| Cardiovascular reaction to provoked stress | High | High |
| Depression (even subclinical) | High | High |
| High active coping | High | Probable |
| High avoidance | High | Probable |
| High expression of emotion | High | High |
| State anxiety | High | High |
| State-anxiety self-report | Questioned | Questioned |
| Trait anxiety | Some | Some |
| Trait-anxiety self-report | Questioned | Questioned |
ACTH, adrenocorticotrophic hormone; ET, embryo transfer; NK, natural killer; OR, oocyte retrieval. See text for references. Important differences between female and male factors have been found.
Some studies found no effects of psychological stress on the IVF success rate (Harlow et al., 1996; Milad et al., 1998), probably because they relied on ‘traditional’ biological stress markers that have shown to be not necessarily relevant, whilst recent studies identify, as referred earlier (Czemiczky et al., 2000), significant links with other, more specific, markers.
Ferin (1999) rightly warns not to generalize data on the basis of responses to stress challenges, because each particular one may well activate HPA through a different central pathway with its own particular sensitivity to the ovarian steroids. Until further investigations can establish generally valid stress markers or, more probably, a combination that has been validated as such, each professional must select his own combination of those markers that have proven relevance for fertility treatment outcome.
Stress or its negative associated mood state, anxiety, represents a threat to the outcome of IVF/ICSI fertilization, but it is not the only mood disorder that does. Depression also has been shown to have negative impact and is significantly correlated with anxiety. A fair-sized (n = 291), recent Dutch study established that a significant relationship exists between both psychological variables and the probability of becoming pregnant after IVF/ICSI treatment, after controlling for other factors. State anxiety had a slightly stronger correlation (P = 0.01 versus P = 0.03) with treatment outcome than depression, but both mood states relate to persistent or chronic ‘trait’ stress (Smeenk et al., 2001). Other studies arrive at (partially) different conclusions, but inadequate methodology, small sample sizes and emphasis on stable factors instead of on variable factors (such as in the now questioned Templeton model) make these results hard to evaluate (Anderheim et al., 2005).
A 2004 study indicates that depressive symptoms in the non-clinical range may heighten the adrenaline but not the noradrenaline response to acute stress and significantly slow recovery. This result is in line with the accumulating evidence that subclinical levels of depressive symptoms can have major health implications (Bush et al., 2001; Gold et al., 2004).
Different types of response to stress, or ‘resilience’, determine its ultimate effect. Psychobiological characteristics may heighten or reduce the responsiveness to a stressor. Women with a high chronic ineffectiveness of coping show higher anticipatory stress that, in 34–59% of cases, affects prolactin and cortisol release. Some studies propose that these personality-dependent stress responses affect conception rates in spontaneous cycles as well as in stimulated cycles (Demyttenaere et al., 1991, 1992). Twenty-five years of animal and human research provide evidence that prior exposure to a chronic stressor significantly elevates neuroendocrine reactivity to a novel acute stressor. Consistent with literature, acute endocrine response in cortisol and adrenaline was significantly predicted by change in acute high stress but not in self-report (Gold et al., 2003); hence, chronic stress is not necessarily reflected in the state anxiety inventory questionnaires widely used in investigation. As said earlier, neither do ‘traditional’ biological stress markers necessarily reflect perceived stress. For instance, anxiogenic norepinephrine impact is counteracted by neuropeptide Y, and cortisol impact is counteracted by dehydroepiandrosterone (DHEA). Studies show several more attenuating interactions, relevant for fertility (Rosenbaum and Covino, 2005).
These findings make it mandatory to measure stress, before and during fertilization treatment, by a combination of biological and psychological means.
Evidence is accumulating that stress responses and the return to normality not only differ notably between subjects, but also that some biological mediators, specifically adrenaline and noradrenaline, need much more time to return to pre-stressor levels, indicating differences in habituation that underlie the chronicity of stress impact (Schommer et al., 2003) and thus long-term influence on fertility.
Part 3—Efficacy of the reduction of stress before fertility treatment commences
There is substantial evidence that personality dimensions, coping modes, stress susceptibility and resilience correlate with IVF outcome (Sanders and Bruce, 1999; Czemiczky et al., 2000; Smeenk et al., 2001; Hjelmstedt et al., 2003; Klonoff-Cohen, 2005). These influences may contribute to infertility from well before the problem manifests itself. Thus, the acute stress caused by the fertility problem needs to be distinguished from the chronic stress levels the persons involved may be experiencing, which are not causally related to infertility (Chan et al., 1989; Newton et al., 1990; Demyttenaere et al., 1991; Collins et al., 1992; Facchinetti et al., 1997; Eugster et al., 2004).
Those studies on the influence of stress on fertility treatment outcome that measured both chronic (or ‘trait’) stress/anxiety and procedural (or ‘state’) stress/anxiety did so, starting measurement at the first consultation at the fertility clinic. However, the chronic stress score obtained then necessarily includes the accumulated anticipatory stress provoked by the previous stages of the ‘infertility experience’ that commenced the day the couple came to suspect that something could be amiss. Studies show that baseline acute and chronic stress affects biological end-points, i.e. the number of oocytes retrieved and fertilized, but also affects pregnancy, live birth delivery, birthweight and multiple gestations, whereas procedural stress only influenced biological end-points (Klonoff-Cohen et al., 2001); hence, managing baseline stress is of even greater importance than managing the stress inherent to fertility treatment itself.
Recent studies indicate that (acute) stress increases the dropout rates from treatment (Olivius et al., 2004; Schroder et al., 2004; Rajkhowa et al., 2006).
To protect the early embryo, the acute stress produced by or during the fertility treatment must be treated only by psychological techniques or treatments, possibly supported by dietary adjustments and changes in procedure. Chronic stress, however, would benefit more from treatment before fertility treatment (Cwikel et al., 2004). In both cases, the approach should be differentially adjusted to males and females. Female response to marital stress, for instance, differs significantly from male response and causes greater and more persistent hormonal and immunological change (Kiecolt-Glaser et al., 1997, 1998).
Psychological interventions, such as behavioural treatment, aimed at reducing stress in IVF, have shown to be effective in a number of studies (Sarrel and DeCherney, 1985; Domar et al., 1990, 2000; Terzioglu, 2001; Facchinetti et al., 2004). A recent meta-analysis showed that psychotherapy accompanying IVF also is effective (de Liz and Strauss, 2005). It is difficult to calculate how much improvement of pregnancy rates we can expect from psychological interventions. The influence of psychological interventions on pregnancy rates varies considerably from one study to another, depending on size, selection criteria, female/male factor and, especially, study design. More research is needed to further specify and validate efficacy.
Experimental studies confirm that ‘intuitive remedies’ not only do not always work for acute stress reduction but may actually increase distress and significantly reduce fertility. With intuitive remedies, I refer to those that ‘logically’ should reduce stress because they have shown to be effective in other stress-related problems. They include getting in control of the situation, being more involved as a couple with the procedure or talking about one’s emotional reactions to the fertility problem with other people.
These are the remedies without sustaining evidence of their efficacy in the particular situation of infertility treatment that nevertheless are being recommended by a number of well-meaning professionals. Several studies disclose that cognitive-behavioural training also is effective in improving sperm concentration and increases live birth rate (Pook et al., 1999; Tuschen-Caffier et al., 1999). However, another study in 1999 revealed that efficacy is male/female factor dependent. First, the study found that in males, contrary to females, the less prominent the overall coping efforts were, the better. Second, the more distance the subject could take from the problem, the better, although this may not be understood well by the person if becoming pregnant is a priority and may increase marital distress because of the effect this attitude would have on the woman. Third, it appeared that the less cognitive involvement in the infertility problem, the better (Takefman et al., 1990).
Other differences in response were found. The wife often requires more engagement in their infertility from the husband (Wright et al., 1991), whereas this engagement may result in more distress and lower fertility-related adjustment for the man (Forsythe and Compas, 1987). These findings apparently contradict others that found high commitment or social support effective for reducing stress (Pengilly and Dowd, 2000) but should be interpreted considering the special kind of ‘existential stress’ that is generated by infertility. This stress needs specific measurements, a specific treatment environment and specific control conditions.
Not only do female patients react differently from male patients to infertility and fertility treatment, also, male factor differ from female factor male patients in their stress responses and attitudes towards infertility (Clarke et al., 1999). Although acute stress should not affect sperm production because of the spermatogenic cycle of 70 days, its negative influence probably channels through the hormonal component of spermatogenesis. In female patients, the distinction between female factor and male factor female patients also must be taken into account and treatment and stress control adjusted in consequence.
For both females and males, the short-term goal should be to reduce the patient’s feeling of helplessness, by means of including advice on coping with infertility, changes in sexual behaviour, modification of negative cognitions related to infertility, overcoming deficiencies in knowledge about fertility and improving marital communication skills (Table II).
Short-term goals for male and female fertility patients (Pook et al., 1999)
| Reduction of feelings of helplessness, through coping with infertility |
| Changes in sexual behaviour |
| Modification of negative cognitions as to infertility |
| Overcoming deficiencies in knowledge about fertility |
| Improving marital communication skills |
| Reduction of feelings of helplessness, through coping with infertility |
| Changes in sexual behaviour |
| Modification of negative cognitions as to infertility |
| Overcoming deficiencies in knowledge about fertility |
| Improving marital communication skills |
Short-term goals for male and female fertility patients (Pook et al., 1999)
| Reduction of feelings of helplessness, through coping with infertility |
| Changes in sexual behaviour |
| Modification of negative cognitions as to infertility |
| Overcoming deficiencies in knowledge about fertility |
| Improving marital communication skills |
| Reduction of feelings of helplessness, through coping with infertility |
| Changes in sexual behaviour |
| Modification of negative cognitions as to infertility |
| Overcoming deficiencies in knowledge about fertility |
| Improving marital communication skills |
Two main factors appear in chronic stress that determine what influence the psychological condition and mood state of a woman or a man may have on her or his fertility. The first factor is the way in which the person handles stressors in general. If that ‘coping’ way is less than adequate, stress may become chronic, and chronic stress affects fertility (McEwen, 2005). The second factor refers to how the person handles the threat to her or his self-esteem, or her or his biological and social value, which results from the knowledge that something is amiss with her and/or his fertility. As a result, anxiety and altered mood states in general are common from the moment doubts as to a couple’s fertility arise and increase sharply on account of the fertility treatment itself. The clinical relevance of this second factor is well established (Czemiczky et al., 2000) and is, up to now, the one factor that fertility treatments (sometimes) take into account and will try to control with psychological approaches. However, more and earlier attention should be paid to the first factor, as chronic stress is an important influence on treatment outcome. Religious and social beliefs could be considered markers for fertility-related stress in their own right (Schenker, 2005).
As we have seen earlier, present evidence indicates that levels of psychosocial stress do not necessarily induce measurable changes in the presence of stress hormones that would affect fertility, nor does self-reported stress levels always correlate significantly with existing biological stress levels. This complication asks for generally agreed upon parameters on how and when mood factors should be measured and at what levels they should be considered relevant for fertility treatment outcome, as well as what evidence-based options are available to obtain a reduction of stress to levels that may be considered acceptable.
The algorithm of Figure 2 is based upon the following assertions:
A physician other than the fertility clinic specialist, often the gynaecologist but sometimes the family doctor, usually makes a preliminary diagnosis of infertility.
This physician should establish stress levels with the help of validated questionnaires or refer to a psychologist for this purpose, to see if there is reason to suspect that chronic stress plays a significant role. His findings will not yet be affected by the additional stress produced by visits to a fertility clinic.
If indicated, stress-reduction techniques should be applied, and after 3 months stress levels should again be measured with the same questionnaires. The couple is then referred to the fertility clinic.
The fertility clinic again establishes stress levels, this time both psychological and biological (see Table I for a selection), and compares findings with the previous uncontaminated measurements to determine the susceptibility to stress treatment.
If levels are still over established individual thresholds, another 3 months of stress reduction is attempted.
When stress reduction is obtained, fertility treatment is initiated with concurrent stress management for both.
If stress levels remain over established thresholds, the couple should be advised on the increased probability of a negative result of the fertility treatment and should be recommended to continue with therapy until lower stress levels are attained. However, if they insist, an informed consent should be signed.
Several studies present promising results of psychological interventions leading to higher pregnancy rates (Domar et al., 2000). As said, however, there is uncertainty as to which markers should be measured to assess stress levels. State anxiety has considerable validity as an independent marker for better pregnancy rates, on account of the effect of anxiety on the implantation period of the fertility cycle. Nevertheless, to improve overall pregnancy rates is not simply a matter of reducing acute stress, but rather of finding ways to improve the efficiency of the adaptive response to stressors whilst minimizing over-activity of the systems involved, since such over-activity lies at the heart of the problem (McEwen, 2005). Thus, to determine whether an infertile woman or man suffers from stress levels that would exert a negative influence on fertility treatment success rates, we cannot exclusively rely on objective thresholds of a common biological stress marker such as adrenaline or cortisol, as there is considerable overlap of its levels between successfully and unsuccessfully treated women (Smeenk et al., 2005).
To initially establish stress levels, a prospective state and trait anxiety questionnaire could produce reasonably acceptable data to decide whether to initiate stress-reduction therapy and to measure its efficacy, on the condition that the questionnaire is applied as early as possible and preferably well before the first consultation at the fertility clinic. As the level of stress is always subjective and personal, initial thresholds are indicative only and serve as a screening method for further selection. Treatment objectives should aim at a proportional reduction of baseline levels of the markers that did show relevancy in each particular case.
Research is needed to further establish the efficacy of additional or alternative interventions to minimize stress-induced negative changes in fertility as well as infertility-induced stress. Meanwhile, closer monitoring for stress and stress-induced negative changes, together with early interventions aimed at reducing the influence of stress on fertility and fertility treatment success rates, should be part of treatment protocol. These interventions would benefit from physician–psychologist co-operation (see Table III).
Interventions for stress control and fertility treatment
| Procedural means | Protocols to include patient selection according to existing chronic stress levels and response to acute stressors |
| Preliminary treatment to reduce anxiety and depression before fertilization cycles are initiated | |
| Psychological means | Cognitive-behavioural therapy |
| Relaxation training | |
| Differential orientation as to infertility | |
| Fertility sabbatical permit | |
| Technical means | Frozen back-up semen samples taken at low-stress moments outside the fertilization cycle |
| Further refinement of fertilization techniques, such as removal of the acrosome before ICSI (Morozumi and Yanagimachi, 2005) | |
| Neurobiological means | Establishing individual baselines and specific stress markers |
| Establishing thresholds for referring | |
| Monitoring for stress before and during fertility treatment |
| Procedural means | Protocols to include patient selection according to existing chronic stress levels and response to acute stressors |
| Preliminary treatment to reduce anxiety and depression before fertilization cycles are initiated | |
| Psychological means | Cognitive-behavioural therapy |
| Relaxation training | |
| Differential orientation as to infertility | |
| Fertility sabbatical permit | |
| Technical means | Frozen back-up semen samples taken at low-stress moments outside the fertilization cycle |
| Further refinement of fertilization techniques, such as removal of the acrosome before ICSI (Morozumi and Yanagimachi, 2005) | |
| Neurobiological means | Establishing individual baselines and specific stress markers |
| Establishing thresholds for referring | |
| Monitoring for stress before and during fertility treatment |
Interventions for stress control and fertility treatment
| Procedural means | Protocols to include patient selection according to existing chronic stress levels and response to acute stressors |
| Preliminary treatment to reduce anxiety and depression before fertilization cycles are initiated | |
| Psychological means | Cognitive-behavioural therapy |
| Relaxation training | |
| Differential orientation as to infertility | |
| Fertility sabbatical permit | |
| Technical means | Frozen back-up semen samples taken at low-stress moments outside the fertilization cycle |
| Further refinement of fertilization techniques, such as removal of the acrosome before ICSI (Morozumi and Yanagimachi, 2005) | |
| Neurobiological means | Establishing individual baselines and specific stress markers |
| Establishing thresholds for referring | |
| Monitoring for stress before and during fertility treatment |
| Procedural means | Protocols to include patient selection according to existing chronic stress levels and response to acute stressors |
| Preliminary treatment to reduce anxiety and depression before fertilization cycles are initiated | |
| Psychological means | Cognitive-behavioural therapy |
| Relaxation training | |
| Differential orientation as to infertility | |
| Fertility sabbatical permit | |
| Technical means | Frozen back-up semen samples taken at low-stress moments outside the fertilization cycle |
| Further refinement of fertilization techniques, such as removal of the acrosome before ICSI (Morozumi and Yanagimachi, 2005) | |
| Neurobiological means | Establishing individual baselines and specific stress markers |
| Establishing thresholds for referring | |
| Monitoring for stress before and during fertility treatment |
Part 4—Ethical and technical reasons for stress management before fertility treatments
Presently available evidence support the following three assertions:
Infertility causes stress in the couple involved.
Infertility treatments cause stress in the couple involved.
Stress may be a primary or secondary cause of infertility.
For each, the level of evidence is different, the first two having been repeatedly confirmed by acceptable studies, whereas the third is supported by a number of studies that are acceptable in design and execution and should be taken as probable yet not conclusive evidence.
Professional ethics provide reasons to accept all three assertions as valid and for the physician to act in consequence. Invasive fertility techniques such as IVF, ICSI and gamete intra-Fallopian transfer (GIFT) present a problem for practicing Catholics and persons with the belief that conception is not to be meddled with. Attempts to favour male and female fertility with non-invasive methods do not qualify as meddling with fertility and should, therefore, be acceptable for persons with these beliefs. A more general reason is that if psychology can help to solve infertility, then psychology should be applied as a first option and before more invasive steps are taken. A further reason lies in the ethical obligation that the professional should apply less invasive treatments first. Therefore, considering the ample evidence that stress plays a fundamental or at least supporting role in infertility, treatment protocol should consider stress both as a cause and as a consequence of infertility and should not commence invasive treatment before verifying whether important levels of both types of stress in any particular case exist and whether these can be reduced.
The economic cost of infertility treatment is high. An effort must be made to reduce that financial burden. If the number of treatment cycles or maybe the treatment itself can be limited or deemed unnecessary through better fertility on account of a primary reduction of acute and/or chronic stress, then that should be the guideline for private and public institutions alike.
To the biologist and laboratory technician, being in control of a fertility treatment requires that the number of biological variables be as low as possible. As we have seen, stress results in changes in a number of variables that are difficult to preview or control. It may result in biologically measurable variations, but not necessarily so. The changes may be measurable in one location but show no change in another. Stress may result in psychological indicators that could correlate with biological changes but not necessarily do so. Stress may result in increases of a substance in some cases but in other cases will decrease the levels of that same substance. Thus, from a technical point of view, the treatment cycle and its individual components will be under better control if the exogenous influences caused by chronic or acute stress are reduced or eliminated before the treatment cycle.
Conclusion
Infertility is a relative matter and depends on the changes brought about by man, nature or chance. Acute and chronic stress may cause infertility, or lower the success rate of fertility treatments. Stress acts through different mechanisms, not only by inhibiting the HPA axis but also by altering the concentration of fertility hormones (FSH, GnRH and LH) as well as other substances such as cortisol, opioids and melatonin. It alters the follicular levels of glucocorticoid hormones and of 11β-HSD. It also affects semen quality. Thus, stress levels cannot be determined by glucocorticoid measurements only, and therefore stress in fertility is a subject that requires gynaecology, biology and psychology to co-operate.
Acute stress needs to be differentiated from chronic stress. Acute stress may be caused by the fertility problem or the fertility procedure, whereas chronic stress, or anxiety, would be pre-existing and as such is an important influence on treatment outcome. Both need to be reduced.
The available evidence dictates that fertility treatment protocol should include stress management and stress reduction as factors of major importance. Consensus should be reached as to protocol establishing:
(very) early measurement of stress in the assisted fertility procedure;
which absolute or relative thresholds must be taken to indicate a threat to the success rate of the fertility treatment and thus indicate a necessary pretreatment to reduce acute and chronic stress levels and
which evidence-supported treatments for this purpose can be recommended.
Stress reduction is a non-invasive, less expensive and ethically acceptable way of improving fertility. The professional in reproductive medicine should always test for chronic stress before initiating fertility treatment and adjust selection and treatment protocol accordingly.
Acknowledgements
The author is grateful to Professor R. Bernabeu PhD, MD, Bernabeu Fertility Institute of Alicante, Director Chair Reproductive Medicine, Universidad Miguel Hernández, for his constructive comments.