-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Mueller, Theodoros Maltaris, Joern Siemer, Helge Binder, Inge Hoffmann, Matthias W. Beckmann, Ralf Dittrich, Uterine contractility in response to different prostaglandins: results from extracorporeally perfused non-pregnant swine uteri, Human Reproduction, Volume 21, Issue 8, 1 August 2006, Pages 2000–2005, https://doi.org/10.1093/humrep/del118
Close - Share Icon Share
Abstract
BACKGROUND: Prostaglandins (PGs) are important stimulators of uterine contractility. Limited data are available at present on the effects of different PGs on uterine contractility, measured using intraluminal pressure changes in the complete uterus. The goal of this study was to assess dynamic changes in uterine contractility and peristalsis in response to PGs in comparison with the effects of oxytocin administration. METHODS: An extracorporeal perfusion model of swine uteri was used, which keeps the uterus in a functional condition, and is appropriate for the study of physiological questions. Oxytocin- and PG-induced uterine contractility and peristalsis were assessed using an intrauterine double-chip microcatheter. RESULTS: A dose-dependent increase in intrauterine pressure (IUP) in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) was observed after the administration of PGF2α and oxytocin, which reached a plateau after further stimulation. A dose-dependent increase in IUP in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) was also observed after the administration of PGE1 and PGE2, with a plateau in IUP in the middle-concentration range and a decrease in the course of further stimulation. PGE2 caused significantly more contractions starting in the corpus uteri and moving to the isthmus uteri (P = 0.008). The direction of most contractions caused by PGE1, PGE2 and oxytocin differed from that of PGF2α. CONCLUSIONS: This study demonstrates that the PGs tested modulate contractility in non-pregnant swine uteri in a characteristic way, resulting in different contractility patterns.
Introduction
Adequate uterine contractility and intact uterotubal transport function are required for the transport of semen and gametes and for successful embryo implantation, whereas inadequate uterine contractility may lead to ectopic pregnancies, miscarriages, retrograde bleeding and endometriosis (Bulletti et al., 2001, 2002; Kissler et al., 2004a; Leyendecker et al., 2004; Bulletti and de Ziegler, 2005).
Oxytocin levels fluctuate throughout the menstrual cycle and correlate with genital lubrication and sexual arousal in women (Carmichael et al., 1987; Murphy et al., 1990; Carter, 1992; Gimpl and Fahrenholz, 2001; Argiolas and Melis, 2003); they are therefore believed to play a role in the peripheral activation of sexual function (Evans et al., 2003; Benoussaidh et al., 2004; Salonia et al., 2005). Oxytocin is used clinically to induce labour at term, as one of the most potent uterotonic agents, and exerts a wide spectrum of central and peripheral physiological effects (McNeilly et al., 1983; Carmichael et al., 1987; Murphy et al., 1990; Carter, 1992; Russell and Leng, 1998; Gimpl and Fahrenholz, 2001; Thornton et al., 2001; Argiolas and Melis, 2003; Caba et al., 2003; Woodcock et al., 2004). On the contrary, prostaglandins (PGs) are known to play a key role in the maintenance of human pregnancy and the onset of labour, whereas their possible role in regulation of uterine contractility in non-pregnant uteri is still enigmatic. PGE1, in particular, is secreted by the seminal vesicle and prostate gland and is present in human seminal plasma (Bygdeman and Samuelsson, 1967; Robertson, 2005). In addition, the endometrium itself is able to generate different PGs, which may play a significant role in placentation and in regulating the lifespan of the corpus luteum (Herath et al., 2006; Waclawik et al., 2006). In addition to steroids and oxytocin, PGs are also believed to modulate myometrial contractility in a characteristic way not only at the time of labour but also at the time of human reproduction (Hertelendy and Zakar, 2004). In a study of myometrial PG receptor expression, a region-dependent, heterogeneous distribution of contractile and relaxant prostanoid receptors and cyclooxygenase was reported in the non-pregnant porcine myometrium (Cao et al., 2005). In pregnancy, it is known that the regulation of uterine contractility involves PG interactions (Hurd et al., 2005; Ziganshin et al., 2005). Hirst et al. (2005) have reported that it is possible to suppress uterine contractility and delay preterm labour using a novel PGF2α receptor antagonist in sheep. To date, however, only cell-culture experiments using isolated muscle strips have been conducted to record uterine contractility (Palliser et al., 2005). Differences have also been found in the localization and expression of PG receptors in human myometrium during pregnancy (Astle et al., 2005). To our knowledge, however, uterine contractility caused by different PGs has not previously been examined in an experimental model using the complete uterus.
The original extracorporeal perfusion model of an isolated uterus was first described by Bulletti et al. (1986). Previous investigations have validated the feasibility of this perfusion model of isolated uteri for various purposes (Bulletti et al., 1987, 1988a, 1988b; Richter et al., 2000, 2003, 2004, 2006). This perfusion model is able to keep the uterus in a functional condition and is suitable for the study of physiological questions (Bulletti et al., 1993, 2001, 2002). The development of an extracorporeal perfusion model of the swine uterus enabled our group to carry out general assessments of uterine contractility and peristalsis (Dittrich et al., 2003; Maltaris et al., 2005a, 2005b; Mueller et al., 2005, 2006).
The aim of the present study was to monitor uterine contractility in the corpus uteri and the isthmus uteri continuously, to determine intrauterine pressure (IUP) changes and to evaluate the role of PGE1, PGE2 and PGF2α in the regulation of uterine contractility in comparison with oxytocin. The advantage of the animal model used is that it makes it possible to test a large number of uteri, similar in size and condition, from young and healthy animals within the reproductive lifespan.
Materials and methods
Swine uterus
Swine (Sus scrofa domestica) are widely used in research. The swine uterus is a long bicornuate uterus with a single corpus and a single cervix. The uterine wall has a similar architecture to that in humans and in other domestic animals, with the three classic histological elements of the uterine wall—the endometrium; the myometrium, which consists of clearly oriented smooth-muscle cells; and the perimetrium. The endometrium contains many hundreds of glands in a cross-section of the uterine wall, and the myometrium is clearly differentiated into inner circular and outer longitudinal layers. The Fallopian tubes in the adult female have the same diameter as those in humans. However, they are much longer, and the uterine corpus is also longer in comparison with human uteri. The sow has an estrus cycle of 20–21 days.
Swine uteri were obtained from the local slaughterhouse. They all came from healthy animals aged 5–18 months. Sixty uteri were selected on the basis of their size and overall condition, as well as the condition of the uterine arterial stumps, and randomly assigned to four groups (n = 15 in each group) that were comparable with regard to the size, weight, condition and age of the animals and unrelated to phase of the estrus cycle. The mean weight of all the swine uteri was 128 g (range 85.5–162.8 g). Swine uteri are very easily separated from the rest of the body within approximately 2 min, shortly after the animal is killed by electric shock (1.5 A, 400 V, 4 s).
Perfusion system
After catheter placement in the uterine vessels with 16–24-gauge needles (Abbocath-T; Abbott Ireland, Sligo, Ireland), depending on the uterus size, the organ was placed in a controlled temperature perfusion chamber (Karl Lettenbauer, Erlangen, Germany) filled with the perfusion medium. The uterus was then connected bilaterally with two reservoirs containing the perfusion medium (Krebs–Ringer bicarbonate glucose buffer, Sigma, Deisenhofen, Germany). The perfusion medium was oxygenated with carbogen gas (a mixture of 95% oxygen and 5% carbon dioxide) and then forced into the uterine arterial catheters with two roller pumps. The flow rate of the perfusion medium was constantly monitored and kept at 15 ml/min and 100 mmHg.
Vitality parameters
Perfusate samples were taken at 1-h intervals for measurement of pH, PO2, PCO2, HCO3, lactate and oxygen saturation. The perfusate samples were analysed using an i-STAT portable clinical analyzer (Abbott Diagnostics, Abbott Park, IL, USA).
Intrauterine pressure measurement
IUP was recorded using an intrauterine double-chip microcatheter (Urobar 8 DS-F, Raumedic, Muenchberg, Germany) with a distance of 8 cm between the two pressure sensors. One sensor was placed in the isthmus uteri, and the other was placed in the corpus uteri in the swine uterus. The Fallopian tubes and cervix uteri were not closed. The double-chip microcatheter was connected to a Datalogger (MPR1, Raumedic) for continuous monitoring of IUP at both the locations, with the data being transferred to a personal computer.
Induction of uterine contractions
Oxytocin (Syntocinon; Novartis Germany Ltd., Nuremberg, Germany), at increasing dosages of 0.5–10 IU, and PGE1 (alprostadil, Caverject; Pharmacia, Erlangen, Germany), PGE2 (dinoprostone, Minprostin E2; Pharmacia, Erlangen, Germany) and PGF2α (sulprostone, Nalador; Schering, Berlin, Germany), at increasing dosages of 0.5–10 µg, were used to induce contractions of the uterus and were administered every 30 min. All medications were administered as bolus injections through the uterine arterial catheters.
Statistical analysis
A paired t-test was used for statistical evaluation of IUP increases during uterine contractions in the isthmus uteri in comparison with that in the corpus uteri for each tested concentration of the test substances, whereas an unpaired t-test was used for statistical evaluation of IUP caused by the different test substances. Pressure differences between the different concentrations of test substances were evaluated using analysis of variance for the repeated measurements. In addition, the chi-squared test of independence was used to assess whether uterine contractions started in the isthmus uteri or the corpus uteri. All calculations were performed using the Statistical Program for the Social Sciences (SPSS, version 10.1 for Windows; SPSS, Inc., Chicago, IL, USA). P values of less than 0.05 were considered statistically significant.
Results
The experiments were only carried out when it was possible to maintain constant flow rates of the perfusion medium of 15 ml/min through each artery, with an ideal pressure of 100 mmHg, throughout the duration of the experiments. The vitality parameters remained physiological during the first 8 h of perfusion (data not shown; for details, see Dittrich et al., 2003; Maltaris et al., 2005a, 2005b; Mueller et al., 2005). Regularly recurring peristaltic waves, with IUP increases in both the corpus uteri and the isthmus uteri, were achieved in all of the perfused swine uteri and were continuously measured using the intrauterine double-chip catheter described (Mueller et al., 2006). For all groups, IUP changes showed similar values for the amplitude and duration of the uterine contractions. Data for increases in IUP are demonstrated as pooled data (each group contained 15 uteri) with mean values ± SD.
Oxytocin
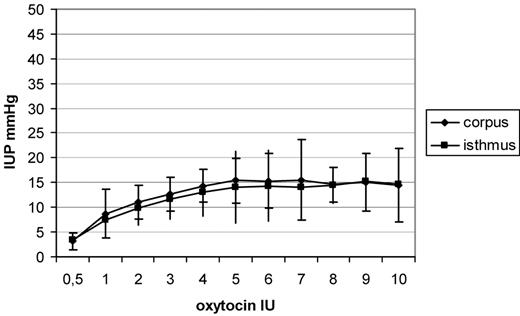
In general, a dose-dependent increase in IUP in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) was observed following oxytocin administration. A plateau in IUP was observable after administration of 5 IU of oxytocin, while no increase of IUP was measured using more than 5 IU of oxytocin. The differences between IUP in the isthmus and that in the corpus uteri were not significant (Figure 1).
Increases in intrauterine pressure (IUP), shown as means and SD after administration of increasing dosages of oxytocin. A dose-dependent increase in IUP was observed in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001), following oxytocin administration.
Prostaglandins
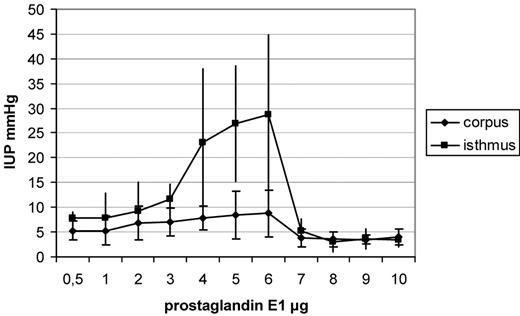
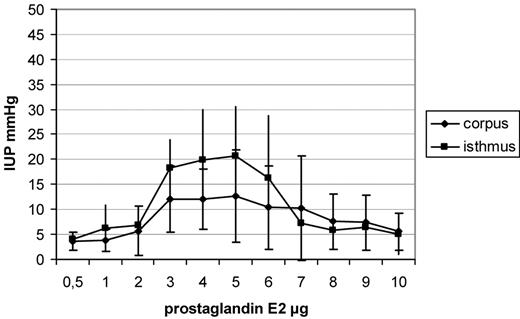
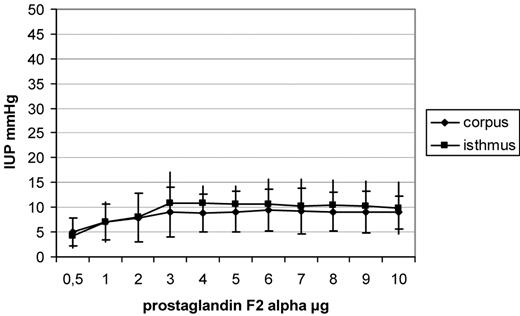
A dose-dependent increase in IUP in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) was observed following PGE1 administration. A plateau in IUP was observable after administration of 4–6 µg, whereas when higher dosages were used, a decrease in IUP was observed in both the locations. Dosages of 3–6 µg caused a significantly higher IUP in the isthmus uteri (for 3 µg, P = 0.001; 4 µg, P = 0.002; 5 µg, P < 0.001 and 6 µg, P < 0.001) in comparison with the corpus uteri (Figure 2). After administration of PGE2, a dose-dependent increase in IUP in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) was observed. A plateau in IUP was observable after administration of 3–5 µg, whereas a decrease in IUP in both the locations was seen using higher dosages (Figure 3). Dosages of 3–5 µg PGE2 caused a significantly higher increase in IUP in the isthmus uteri (3 µg, P = 0.04; 4 µg, P = 0.03 and 5 µg, P = 0.04) in comparison with that in the corpus uteri, whereas the other concentrations tested caused no significant differences between the two locations. After administration of PGF2α, a similar contractility pattern was seen in comparison with oxytocin administration, with a dose-dependent increase in IUP in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) and with a plateau reached after administration of 5 µg PGF2α. No differences were observed in the IUP increase measured in the isthmus uteri in comparison with that in the corpus uteri (Figure 4). The greatest increase in IUP was seen in the isthmus uteri after administration of PGE1 (P < 0.001 versus PGE2, P < 0.0001 versus PGF2α and P < 0.0001 versus oxytocin) followed by PGE2 (P < 0.001 versus PGF2α and P < 0.001 versus oxytocin) at the dosages tested. Both PGE1 and PGE2 caused a statistically significantly higher increase in IUP in the isthmus uteri in comparison with that in the corpus uteri, resulting in a cervicofundic pressure gradient.
Increases in intrauterine pressure (IUP), shown as means and SD after administration of increasing dosages of prostaglandin E1. Dosages of 3–6 µg caused a significantly higher IUP in the isthmus uteri (for 3 µg, P = 0.001; 4 µg, P = 0.002; 5 µg, P < 0.001 and 6 µg, P < 0.001) in comparison with that in the corpus uteri.
Increases in intrauterine pressure (IUP), shown as means and SD after administration of increasing dosages of prostaglandin E2 (PGE2). Dosages of 3–5 µg PGE2 caused a significantly higher IUP increase in the isthmus uteri (3 µg, P = 0.04; 4 µg, P = 0.03 and 5 µg, P = 0.04) in comparison with that in the corpus uteri.
Increases in intrauterine pressure (IUP), shown as means and SD after administration of increasing dosages of prostaglandin F2α (PGF2α). After administration of PGF2α, a dose-dependent increase in IUP in the isthmus uteri (P < 0.001) and the corpus uteri (P < 0.001) was observed, reaching a plateau after administration of 5 µg PGF2α.
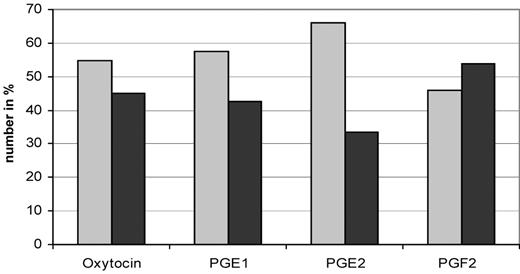
After administration of oxytocin and PGE1, most contraction waves were observed to start in the corpus uteri with peristalsis directed towards the isthmus uteri, whereas after administration of PGF2α, most contraction waves were found to start in the isthmus uteri with peristalsis directed towards the corpus uteri, although these differences were not statistically significant. However, administration of PGE2 resulted in a significant increase in peristalsis starting in the corpus uteri and moving in the direction of the isthmus uteri (P = 0.008) (Figure 5).
Number in % of peristaltic waves starting in the corpus uteri (grey bars) and the isthmus uteri (black bars). Each group contains 15 perfused uteri. Uterine contractions starting in the isthmus uteri or the corpus uteri were tested using the chi-squared test of independence. For prostaglandin E2 (PGE2), P = 0.008 for corpus versus isthmus uteri.
Discussion
This study investigated the effects of different PGs on the regulation of uterine contractility in extracorporeally perfused non-pregnant swine uteri in comparison with oxytocin. To our knowledge, uterine contractility in response to different PGs is evaluated here for the first time by measuring IUP in different uterine compartments in an experimental model using the complete organ.
During PGF2α administration, the perfused uteri showed a similar contractility pattern in comparison with oxytocin, with a dose-dependent increase in IUP being measured at both locations and a plateau in IUP being reached at higher dosages. In contrast, PGE1 and PGE2 caused significantly higher increases in IUP in the isthmus uteri in comparison with that in the corpus uteri, resulting in a cervicofundic pressure gradient in middle-concentration ranges at the dosages tested, whereas the administration of higher dosages caused relative quiescence in the uterine myometrium, with a decrease in IUP being measured during the further course of stimulation at both the locations. These results suggest that small amounts of PGE1 and PGE2 may play an important role in the regulation of contractility in non-pregnant uteri by generating a cervicofundic pressure gradient. PGF2α was not able to generate a pressure gradient between different compartments in non-pregnant uteri.
The periovulatory cervicofundic peristalsis has been described as ‘rapid sperm transport’ to the side bearing the dominant follicle, which is a precondition for successful reproduction in humans (Kissler et al., 1995, 2004b; Kunz et al., 1996; Wildt et al., 1998). However, during menstruation, contraction waves appear to be directed towards the cervix (Lyons et al., 1991; Fukuda and Fukuda, 1994). The investigation of uterine contractility and its regulation by PGs is of clinical interest not only in obstetrics—for example, to induce labour or to delay birth and prolong pregnancy—but also in gynaecology and reproductive medicine, especially concerning embryo implantation (Bulletti et al., 2005).
Endogenous or exogenous PGs are important uterine stimulators (Hirst et al., 2005). PGs play an important role in the onset and maintenance of labour (Astle et al., 2005). Engstrom et al. (2000) have shown that oxytocin treatment down-regulated the uterine contractile response to PGF2α, whereas atosiban (an oxytocin receptor antagonist) did not influence the in vitro response to PGF2α. Interactions between PGs and oxytocin have been described; each is able to influence the receptor status of the other and the corresponding myometrial contractile response, because they act by different mechanisms (Carnahan et al., 1996; Franczak et al., 2005). In addition, oxytocin is involved in the regulation of PGF2α secretion during luteolysis and early pregnancy (Franczak et al., 2005). Hirst et al. (2005) were able to show that suppression of PGF2α induced myometrial contractility by a specific PGF2α receptor antagonist in pregnant sheep. The inhibition of PG production is associated with fewer gap junctions and decreased levels of the oxytocin receptor in sheep myometrium and endometrium (Garfield et al., 1980; Wu et al., 1999). It has been postulated that the use of a PGF2α receptor antagonist might be the best therapeutic approach for new tocolytic drugs. In general, PG receptors may be able to serve as targets for the development of appropriate medications to delay preterm labour (Hannah, 2000). PGE1 and PGE2 are of considerable interest in relation to human reproductive mechanisms and transport functions through the female genital tract, because they are secreted by the seminal vesicle and prostate gland and are present in human seminal plasma (Bygdeman and Samuelsson, 1967; Robertson, 2005). PGs interact with discrete receptors, and different receptors have been identified for each PG on the basis of their different activity profiles and the generation of the second messengers, whereas oxytocin after binding to its receptors activates the intracellular phosphatidylinositol pathway (Whiteaker et al., 1995; Franczak et al., 2005; Hirst et al., 2005). However, other factors are also involved in the regulation of uterine contractility or are able to modulate it—such as epidermal growth factor, vascular endothelial growth factor and metabolites of PGF2α (Ribeiro et al., 2003; Willenburg et al., 2004; Wijayagunawardane et al., 2005). In addition, linoleic acid and lipopolysaccharide are likely to differentially alter the PG metabolism or uterine responsiveness to oxytocin (Ross et al., 2004; Cheng et al., 2005; Herath et al., 2006). On the contrary, estrogen and progesterone have been found to modulate the production of PGE and PGF in response to lipopolysaccharide (Herath et al., 2006), and ovarian steroids also influence oxytocin and vasopressin secretion from the posterior lobe of the pituitary gland (Bossmar et al., 1995b). Oxytocin produced by the posterior lobe of the pituitary gland or locally in the uterus appears to be involved in the initiation of labour, whereas vasopressin is believed to play an important role in the uterine hyperactivity occurring in primary dysmenorrhoea (Ekström et al., 1992; Bossmar et al., 1994; Akerlund et al., 1995; Bossmar et al., 1995a). Although PGE and PGF receptor expression and myometrial sensitivity in different parts of the uterus and myometrium have been described in pregnancy and during labour, there is still a lack of comparable data for the non-pregnant uterus (Astle et al., 2005). Activation of the PGF receptor strongly stimulates the myometrium, whereas stimulation of the PGE receptor may lead to contraction or relaxation, depending on the receptor subtype expression (Palliser et al., 2005). Cao et al. (2005) described a heterogeneous distribution of contractile or relaxant prostanoid receptors in the non-pregnant porcine myometrium. However, results observed in animal models are not of course generally transferable directly to humans.
In summary, administration of PGF2α showed a contractility pattern similar to the effects seen after oxytocin administration. However, administration of PGE1 and PGE2 induced completely different contractility patterns. The role of different PG antagonists or cyclooxygenase inhibitors (e.g. indomethacin) can be investigated also using the experimental model described here. Furthermore, different PG application intervals or a co-treatment with estrogens or progesterone will be a goal of further studies.
Acknowledgement
Research for this study was supported by the ELAN Fund of the University of Erlangen–Nuremberg.