-
PDF
- Split View
-
Views
-
Cite
Cite
Swati Sharma, Joachim Wistuba, Tim Pock, Stefan Schlatt, Nina Neuhaus, Spermatogonial stem cells: updates from specification to clinical relevance, Human Reproduction Update, Volume 25, Issue 3, May-June 2019, Pages 275–297, https://doi.org/10.1093/humupd/dmz006
Close - Share Icon Share
Abstract
Human spermatogonia are target for exploration of adult stem cell characteristics and potential source for the development of therapeutic applications. Almost 50 years ago, Yves Clermont stated with regard to the nature of the true stem cells: ‘there is the possibility that other classes of spermatogonia exist beside the three classes (Adark, Apale and type B)…; …we still know too little about the human spermatogonial stem cells’… This review seeks to provide current knowledge, focusing on different aspects of human spermatogonia, and novel information based on species comparisons with regard to the adaptation of their proliferative potential. Moreover, the objective is to provide an update on the state of the art concerning the potential use of human spermatogonia for clinical applications. Germ cell specification mechanisms and epigenetic as well as transcriptional features of primordial germ cells (PGC) and adult spermatogonia at the single-cell level are reviewed. Studies on single-cell analyses have been included as they provide hitherto unequaled resolution of the transcriptional profiles of unselected human testicular cells and, thereby, new insights into the molecular aspects of germ cell differentiation. Datasets on models of spermatogonial expansion were identified and spermatogonial turnover and lifetime sperm production rates in various species were calculated, based exclusively on studies employing the optical dissector approach. Finally, the state of the art concerning causes of impaired spermatogonial function and fertility preservation were comprehensively reviewed. RNA sequencing data from PGC and spermatogonia indicate that transcriptional heterogeneity is a feature of germ cells prior to differentiation. Based on these data as well as lineage-tracing studies it is now debated whether spermatogonia are a rather plastic population of undifferentiated germ cells with the stem cell niche being the regulatory unit for cell fate decisions. Based on our novel calculations we suggest that spermatogonia are adapted to the individual reproductive lifespan and that the life-long sperm output from a spermatogonium is balanced against the duration of a generation. Thereby, the risk of jeopardizing genome integrity is balanced against a maximized sperm output. With reference to Yves Clermont’s statement, and based on recent datasets, we suggest that the question that needs to be answered is: ‘Is there a true stem cell?’ or better ‘Is there a population of various cells with distinct features serving as a stem cell pool?’. This review provides an update including novel views on various aspects of spermatogonial biology (from embryonic to adult stages). We consider this review relevant for all research scientists and clinicians dealing with fertility, spermatogenesis and fertility preservation.
Introduction
When stem cells came into focus in biological research—several decades ago—the stem cells of the testis were identified as an experimental target for the exploration of adult stem cell features and a potential source for the development of stem cell based therapies. The state of the art—in other words the starting point for research on human spermatogonial stem cells (SSCs)—was nicely stated by Emil Steinberger and Yves Clermont when discussing the nature of the true testicular stem cell almost 50 years ago (Clermont, 1970). Clermont stated: ‘here is the possibility that other classes of spermatogonia exist beside the three classes (Adark, Apale and type B)…; it is not impossible that other spermatogonia with type Apale would be present along the limiting membrane and could serve as stem cells; we still know too little about the human spermatogonial stem cells’.
In the adult testis, SSCs (the definitions of all abbreviations used in the review are shown in Table I) are the least differentiated germ cells and are located at the basement membrane of seminiferous tubules. These SSCs are a subpopulation of the entity of diploid spermatogonia and are defined by their ability to self-renew and to give rise to daughter cells undergoing differentiating divisions, which finally result in the formation of spermatozoa. SSCs are therefore defined by their functional properties and we will use this term throughout the review provided that this functional evidence is available.
Glossary of terms used in the review of spermatogonia, from specification to clinical relevance.
| A: counting area |
| ACTA2: actin alpha 2, smooth muscle |
| AMH: anti-Müllerian hormone |
| ART: assisted reproduction techniques |
| BLIMP1: B lymphocyte-induced maturation protein 1 |
| Bmp4: bone morphogenetic protein 4 |
| Bmp8b: bone morphogenetic protein 8b |
| BRACHYURY: brachyury, T-box transcription factor T |
| CARHSP1: calcium-regulated heat-stable protein 1 |
| CD38: cluster of differentiation 38 |
| cKIT: KIT proto-oncogene receptor tyrosine kinase |
| Csf1: colony stimulating factor |
| CXCR4: CXC chemokine receptor 4 |
| CXCR7: CXC chemokine receptor 7 |
| CXCL12: C-X-C motif chemokine ligand 12 |
| Dnmt1: DNA methyltransferase 1 |
| DSP: daily sperm production |
| E: embryonic |
| EGF: epithelial growth factor |
| ESCs: embryonic stem cells |
| FACS: fluorescence-activated cell sorting |
| Fgf2: fibroblast growth factor 2 |
| GAD1: glutamate decarboxylase 1 |
| Gata4: GATA binding protein 4 |
| Gdnf: Glial cell-line derived neurotrophic factor |
| GFP: Green fluorescent protein |
| GFRɑ1: GDNF family receptor alpha-1 |
| Gy: Gray is the international (SI) unit of ionizing radiation expressed in terms of absorbed energy per unit mass of tissue |
| H: height of plane above the counting frame |
| HMGN3: high mobility group nucleosomal binding domain 3 |
| hPGCLC: human primordial germ cell-like cells |
| hPSC: human pluripotent stem cells |
| ICSI: intra-cytoplasmic sperm injection |
| Id4: DNA-binding protein inhibitor ID-4 |
| IGF1: insulin-like growth factor 1 |
| iPS cells: induced pluripotent stem cells |
| KLF2: krüppel-like factor 2 |
| KLF4: krüppel-like factor 2 |
| KLF6: krüppel-like factor 6 |
| LEF1: lymphoid enhancer binding factor 1 |
| LIF: leukemia inhibitory factor |
| LSP: lifetime sperm production |
| MCS: methylcellulose system |
| N: numerical cell density |
| NANOG: DNA binding homeobox transcription factor involved in embryonic stem (ES) cell proliferation, renewal, and pluripotency. |
| Nanos2: nanos C2HC-type zinc finger 2 |
| Nanos3: nanos C2HC-type zinc finger 3 |
| Ngn3: neurogenin 3 |
| NHPs: non-human primates |
| OCT4: octamer-binding transcription factor 4 |
| P: pluripotency |
| PGC: primordial germ cells |
| PLZ: pre-leptotene–zygotene |
| PLZF: promyelocytic leukemia zinc finger |
| PND: postnatal day |
| PRDM1: PR domain containing zinc finger protein 1 |
| PRDM14: PR domain containing zinc finger protein 14 |
| PS: pachytene spermatocytes |
| Q: number of nuclei |
| SSC: spermatogonial stem cells |
| Smad1: Smad (mothers against DPP homolog) family member 1 |
| Smad4: Smad (mothers against DPP homolog) family member 4 |
| SOX2: SRY (sex determining region Y)-box 2 |
| SOX12: SRY (sex determining region Y)-box 12 |
| SOX17: SRY (sex determining region Y)-box 17 |
| SPG: spermatogonia |
| SRY: sex-determining region on the Y chromosome |
| SSC: spermatogonial stem cells |
| SSEA1: stage-specific embryonic antigen 1 |
| SSEA4: stage-specific embryonic antigen 4 |
| STRA8: stimulated by retinoic acid 8 |
| SYCP1: synaptonemal complex protein 1 |
| TEAD4: TEA domain transcription factor 4 |
| Tet1: Tet methylcytosine dioxygenase 1 |
| Tet2: Tet methylcytosine dioxygenase 1 |
| Tet3: Tet methylcytosine dioxygenase 3 |
| TEX11: testis-expressed gene 11 |
| Tfap2c: transcription factor AP-2 gamma |
| TFCP2L1: transcription factor CP2 like 1 |
| Thy1: thymus cell antigen 1 |
| TPH1: tryptophan hydroxylase 1 |
| TXNIP: thioredoxin interacting protein |
| VASA: member of the DEAD (Asp-Glu-Ala-Asp) box family of ATP-dependent RNA helicases |
| A: counting area |
| ACTA2: actin alpha 2, smooth muscle |
| AMH: anti-Müllerian hormone |
| ART: assisted reproduction techniques |
| BLIMP1: B lymphocyte-induced maturation protein 1 |
| Bmp4: bone morphogenetic protein 4 |
| Bmp8b: bone morphogenetic protein 8b |
| BRACHYURY: brachyury, T-box transcription factor T |
| CARHSP1: calcium-regulated heat-stable protein 1 |
| CD38: cluster of differentiation 38 |
| cKIT: KIT proto-oncogene receptor tyrosine kinase |
| Csf1: colony stimulating factor |
| CXCR4: CXC chemokine receptor 4 |
| CXCR7: CXC chemokine receptor 7 |
| CXCL12: C-X-C motif chemokine ligand 12 |
| Dnmt1: DNA methyltransferase 1 |
| DSP: daily sperm production |
| E: embryonic |
| EGF: epithelial growth factor |
| ESCs: embryonic stem cells |
| FACS: fluorescence-activated cell sorting |
| Fgf2: fibroblast growth factor 2 |
| GAD1: glutamate decarboxylase 1 |
| Gata4: GATA binding protein 4 |
| Gdnf: Glial cell-line derived neurotrophic factor |
| GFP: Green fluorescent protein |
| GFRɑ1: GDNF family receptor alpha-1 |
| Gy: Gray is the international (SI) unit of ionizing radiation expressed in terms of absorbed energy per unit mass of tissue |
| H: height of plane above the counting frame |
| HMGN3: high mobility group nucleosomal binding domain 3 |
| hPGCLC: human primordial germ cell-like cells |
| hPSC: human pluripotent stem cells |
| ICSI: intra-cytoplasmic sperm injection |
| Id4: DNA-binding protein inhibitor ID-4 |
| IGF1: insulin-like growth factor 1 |
| iPS cells: induced pluripotent stem cells |
| KLF2: krüppel-like factor 2 |
| KLF4: krüppel-like factor 2 |
| KLF6: krüppel-like factor 6 |
| LEF1: lymphoid enhancer binding factor 1 |
| LIF: leukemia inhibitory factor |
| LSP: lifetime sperm production |
| MCS: methylcellulose system |
| N: numerical cell density |
| NANOG: DNA binding homeobox transcription factor involved in embryonic stem (ES) cell proliferation, renewal, and pluripotency. |
| Nanos2: nanos C2HC-type zinc finger 2 |
| Nanos3: nanos C2HC-type zinc finger 3 |
| Ngn3: neurogenin 3 |
| NHPs: non-human primates |
| OCT4: octamer-binding transcription factor 4 |
| P: pluripotency |
| PGC: primordial germ cells |
| PLZ: pre-leptotene–zygotene |
| PLZF: promyelocytic leukemia zinc finger |
| PND: postnatal day |
| PRDM1: PR domain containing zinc finger protein 1 |
| PRDM14: PR domain containing zinc finger protein 14 |
| PS: pachytene spermatocytes |
| Q: number of nuclei |
| SSC: spermatogonial stem cells |
| Smad1: Smad (mothers against DPP homolog) family member 1 |
| Smad4: Smad (mothers against DPP homolog) family member 4 |
| SOX2: SRY (sex determining region Y)-box 2 |
| SOX12: SRY (sex determining region Y)-box 12 |
| SOX17: SRY (sex determining region Y)-box 17 |
| SPG: spermatogonia |
| SRY: sex-determining region on the Y chromosome |
| SSC: spermatogonial stem cells |
| SSEA1: stage-specific embryonic antigen 1 |
| SSEA4: stage-specific embryonic antigen 4 |
| STRA8: stimulated by retinoic acid 8 |
| SYCP1: synaptonemal complex protein 1 |
| TEAD4: TEA domain transcription factor 4 |
| Tet1: Tet methylcytosine dioxygenase 1 |
| Tet2: Tet methylcytosine dioxygenase 1 |
| Tet3: Tet methylcytosine dioxygenase 3 |
| TEX11: testis-expressed gene 11 |
| Tfap2c: transcription factor AP-2 gamma |
| TFCP2L1: transcription factor CP2 like 1 |
| Thy1: thymus cell antigen 1 |
| TPH1: tryptophan hydroxylase 1 |
| TXNIP: thioredoxin interacting protein |
| VASA: member of the DEAD (Asp-Glu-Ala-Asp) box family of ATP-dependent RNA helicases |
Glossary of terms used in the review of spermatogonia, from specification to clinical relevance.
| A: counting area |
| ACTA2: actin alpha 2, smooth muscle |
| AMH: anti-Müllerian hormone |
| ART: assisted reproduction techniques |
| BLIMP1: B lymphocyte-induced maturation protein 1 |
| Bmp4: bone morphogenetic protein 4 |
| Bmp8b: bone morphogenetic protein 8b |
| BRACHYURY: brachyury, T-box transcription factor T |
| CARHSP1: calcium-regulated heat-stable protein 1 |
| CD38: cluster of differentiation 38 |
| cKIT: KIT proto-oncogene receptor tyrosine kinase |
| Csf1: colony stimulating factor |
| CXCR4: CXC chemokine receptor 4 |
| CXCR7: CXC chemokine receptor 7 |
| CXCL12: C-X-C motif chemokine ligand 12 |
| Dnmt1: DNA methyltransferase 1 |
| DSP: daily sperm production |
| E: embryonic |
| EGF: epithelial growth factor |
| ESCs: embryonic stem cells |
| FACS: fluorescence-activated cell sorting |
| Fgf2: fibroblast growth factor 2 |
| GAD1: glutamate decarboxylase 1 |
| Gata4: GATA binding protein 4 |
| Gdnf: Glial cell-line derived neurotrophic factor |
| GFP: Green fluorescent protein |
| GFRɑ1: GDNF family receptor alpha-1 |
| Gy: Gray is the international (SI) unit of ionizing radiation expressed in terms of absorbed energy per unit mass of tissue |
| H: height of plane above the counting frame |
| HMGN3: high mobility group nucleosomal binding domain 3 |
| hPGCLC: human primordial germ cell-like cells |
| hPSC: human pluripotent stem cells |
| ICSI: intra-cytoplasmic sperm injection |
| Id4: DNA-binding protein inhibitor ID-4 |
| IGF1: insulin-like growth factor 1 |
| iPS cells: induced pluripotent stem cells |
| KLF2: krüppel-like factor 2 |
| KLF4: krüppel-like factor 2 |
| KLF6: krüppel-like factor 6 |
| LEF1: lymphoid enhancer binding factor 1 |
| LIF: leukemia inhibitory factor |
| LSP: lifetime sperm production |
| MCS: methylcellulose system |
| N: numerical cell density |
| NANOG: DNA binding homeobox transcription factor involved in embryonic stem (ES) cell proliferation, renewal, and pluripotency. |
| Nanos2: nanos C2HC-type zinc finger 2 |
| Nanos3: nanos C2HC-type zinc finger 3 |
| Ngn3: neurogenin 3 |
| NHPs: non-human primates |
| OCT4: octamer-binding transcription factor 4 |
| P: pluripotency |
| PGC: primordial germ cells |
| PLZ: pre-leptotene–zygotene |
| PLZF: promyelocytic leukemia zinc finger |
| PND: postnatal day |
| PRDM1: PR domain containing zinc finger protein 1 |
| PRDM14: PR domain containing zinc finger protein 14 |
| PS: pachytene spermatocytes |
| Q: number of nuclei |
| SSC: spermatogonial stem cells |
| Smad1: Smad (mothers against DPP homolog) family member 1 |
| Smad4: Smad (mothers against DPP homolog) family member 4 |
| SOX2: SRY (sex determining region Y)-box 2 |
| SOX12: SRY (sex determining region Y)-box 12 |
| SOX17: SRY (sex determining region Y)-box 17 |
| SPG: spermatogonia |
| SRY: sex-determining region on the Y chromosome |
| SSC: spermatogonial stem cells |
| SSEA1: stage-specific embryonic antigen 1 |
| SSEA4: stage-specific embryonic antigen 4 |
| STRA8: stimulated by retinoic acid 8 |
| SYCP1: synaptonemal complex protein 1 |
| TEAD4: TEA domain transcription factor 4 |
| Tet1: Tet methylcytosine dioxygenase 1 |
| Tet2: Tet methylcytosine dioxygenase 1 |
| Tet3: Tet methylcytosine dioxygenase 3 |
| TEX11: testis-expressed gene 11 |
| Tfap2c: transcription factor AP-2 gamma |
| TFCP2L1: transcription factor CP2 like 1 |
| Thy1: thymus cell antigen 1 |
| TPH1: tryptophan hydroxylase 1 |
| TXNIP: thioredoxin interacting protein |
| VASA: member of the DEAD (Asp-Glu-Ala-Asp) box family of ATP-dependent RNA helicases |
| A: counting area |
| ACTA2: actin alpha 2, smooth muscle |
| AMH: anti-Müllerian hormone |
| ART: assisted reproduction techniques |
| BLIMP1: B lymphocyte-induced maturation protein 1 |
| Bmp4: bone morphogenetic protein 4 |
| Bmp8b: bone morphogenetic protein 8b |
| BRACHYURY: brachyury, T-box transcription factor T |
| CARHSP1: calcium-regulated heat-stable protein 1 |
| CD38: cluster of differentiation 38 |
| cKIT: KIT proto-oncogene receptor tyrosine kinase |
| Csf1: colony stimulating factor |
| CXCR4: CXC chemokine receptor 4 |
| CXCR7: CXC chemokine receptor 7 |
| CXCL12: C-X-C motif chemokine ligand 12 |
| Dnmt1: DNA methyltransferase 1 |
| DSP: daily sperm production |
| E: embryonic |
| EGF: epithelial growth factor |
| ESCs: embryonic stem cells |
| FACS: fluorescence-activated cell sorting |
| Fgf2: fibroblast growth factor 2 |
| GAD1: glutamate decarboxylase 1 |
| Gata4: GATA binding protein 4 |
| Gdnf: Glial cell-line derived neurotrophic factor |
| GFP: Green fluorescent protein |
| GFRɑ1: GDNF family receptor alpha-1 |
| Gy: Gray is the international (SI) unit of ionizing radiation expressed in terms of absorbed energy per unit mass of tissue |
| H: height of plane above the counting frame |
| HMGN3: high mobility group nucleosomal binding domain 3 |
| hPGCLC: human primordial germ cell-like cells |
| hPSC: human pluripotent stem cells |
| ICSI: intra-cytoplasmic sperm injection |
| Id4: DNA-binding protein inhibitor ID-4 |
| IGF1: insulin-like growth factor 1 |
| iPS cells: induced pluripotent stem cells |
| KLF2: krüppel-like factor 2 |
| KLF4: krüppel-like factor 2 |
| KLF6: krüppel-like factor 6 |
| LEF1: lymphoid enhancer binding factor 1 |
| LIF: leukemia inhibitory factor |
| LSP: lifetime sperm production |
| MCS: methylcellulose system |
| N: numerical cell density |
| NANOG: DNA binding homeobox transcription factor involved in embryonic stem (ES) cell proliferation, renewal, and pluripotency. |
| Nanos2: nanos C2HC-type zinc finger 2 |
| Nanos3: nanos C2HC-type zinc finger 3 |
| Ngn3: neurogenin 3 |
| NHPs: non-human primates |
| OCT4: octamer-binding transcription factor 4 |
| P: pluripotency |
| PGC: primordial germ cells |
| PLZ: pre-leptotene–zygotene |
| PLZF: promyelocytic leukemia zinc finger |
| PND: postnatal day |
| PRDM1: PR domain containing zinc finger protein 1 |
| PRDM14: PR domain containing zinc finger protein 14 |
| PS: pachytene spermatocytes |
| Q: number of nuclei |
| SSC: spermatogonial stem cells |
| Smad1: Smad (mothers against DPP homolog) family member 1 |
| Smad4: Smad (mothers against DPP homolog) family member 4 |
| SOX2: SRY (sex determining region Y)-box 2 |
| SOX12: SRY (sex determining region Y)-box 12 |
| SOX17: SRY (sex determining region Y)-box 17 |
| SPG: spermatogonia |
| SRY: sex-determining region on the Y chromosome |
| SSC: spermatogonial stem cells |
| SSEA1: stage-specific embryonic antigen 1 |
| SSEA4: stage-specific embryonic antigen 4 |
| STRA8: stimulated by retinoic acid 8 |
| SYCP1: synaptonemal complex protein 1 |
| TEAD4: TEA domain transcription factor 4 |
| Tet1: Tet methylcytosine dioxygenase 1 |
| Tet2: Tet methylcytosine dioxygenase 1 |
| Tet3: Tet methylcytosine dioxygenase 3 |
| TEX11: testis-expressed gene 11 |
| Tfap2c: transcription factor AP-2 gamma |
| TFCP2L1: transcription factor CP2 like 1 |
| Thy1: thymus cell antigen 1 |
| TPH1: tryptophan hydroxylase 1 |
| TXNIP: thioredoxin interacting protein |
| VASA: member of the DEAD (Asp-Glu-Ala-Asp) box family of ATP-dependent RNA helicases |
During the last few decades we had to learn that even the unipotent SSC have a more complex physiology than previously assumed and that we do not have sufficient markers to identify them properly. In addition, our understanding of fate decisions made in this unique cell population is rather limited and we are still not able to preserve these stem cells from fading in case of diseases which require chemotherapy or radiation therapy. Here, following an introduction of the germline and male specification, we review the molecular aspects, including transcriptional and epigenetic properties, of primordial germ cells (PGC) and spermatogonia. Embedded into these considerations we describe models of SSC systems in different species as well as the diversity of testicular organization including the spermatogonial niches. It is the prerequisite for normal spermatogenesis that this intricate system of spermatogonia and their respective niches is intact. We therefore next present a new model for spermatogonial turnover, which applies under healthy conditions. In the final sections we discuss the dysfunction of the seminiferous epithelium, focusing on the disturbance caused by chemotherapy, and provide an update on the spermatogonia-based therapeutic options for male fertility preservation.
Characteristics of the germline from specification to male sex differentiation
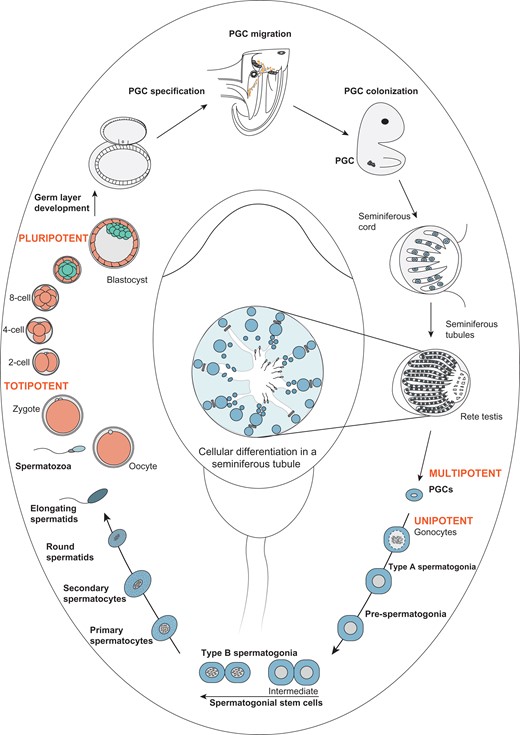
In general, sexual reproduction is essential for efficient recombination of the genome. In mammalian species, initiation of the life cycle of an individual organism begins with the formation of a zygote, generated by the fusion of two individual cells (spermatozoa and oocyte) produced by the two sexes (male and female, respectively) (Fig. 1). During preimplantation development, totipotency is gradually lost during the blastomere to blastocyst transition (Seydoux and Braun, 2006; Reik and Surani, 2015). The first identifiable tissues are trophectoderm, responsible for implantation and the inner cell mass, giving rise to primitive endoderm and epiblast cells (Thomson et al., 1998). The epiblast brings forth the following cellular lineages—ectoderm, mesoderm and endoderm—which differentiate into all somatic tissues (Seydoux and Braun, 2006; Murry and Keller, 2008; Reik and Surani, 2015). Human PGC arise from extraembryonic sites, and they are specified at the onset of gastrulation in the extraembryonic endoderm of the yolk sac from mesoderm cells (Leitch et al., 2013; Tang et al., 2015; von Meyenn and Reik, 2015; Harrison et al., 2017). Most of the existing knowledge on human PGC specification is based on recent studies demonstrating in vitro specification of human PGC-like cells (hPGCLC) from human pluripotent stem cells (hPSC), both embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) (Irie et al., 2015; Kojima et al., 2017; Yamashiro et al., 2018). The factors identified to be involved in human PGC specification highlight distinct genetic regulatory networks, as compared to rodents, and sex determining region Y-box 17 (SOX17) as well as B lymphocyte-induced maturation protein 1 (BLIMP1) as crucial players for human PGC specification (Irie et al., 2015; Tang et al., 2016; Kojima et al., 2017; Yamashiro et al., 2018). It is of note however, that the in vitro models may not reflect the true in vivo situation for germ cell specification in man. Apart from this, novel insights regarding the molecular properties of human PGC were recently gained by single-cell analyses and are outlined later in the review.
Developmental pathway illustrating male germline stem cell development and maturation from primordial germ cells to spermatozoa. Fusion of oocyte and spermatozoa leads to the formation of a totipotent zygote, which undergoes multi-step cleavage and gives rise to a blastocyst. From the inner cell mass of the blastocyst, the epiblast arises, which differentiates into the three germ cell layers ectoderm, mesoderm and endoderm. The formation and specification of primordial germ cells (PGC) in the endoderm initiates male and female-specific germ cell development. In male-specific germ cell developmental pathways, multipotent PGC migrate and colonize the gonadal ridges and further differentiate into unipotent gonocytes in seminiferous tubules. Gonocytes undergo sequential cell divisions differentiating into spermatogonia (including the spermatogonial stem cell (SCC) population), spermatocytes, spermatids and spermatozoa, thereby completing the cycle of spermatogenesis.
Following organogenesis, starting at week 3 of intrauterine development in human, germ cells migrate and colonize the indifferent gonadal ridges. This process is accompanied by active germ cell proliferation. During weeks 7–10 gestation, when human gonadal development occurs, PGCs undergo sex-specification by entering either male or female sex-specific pathways (Tang et al., 2016). With regard to the somatic environment, the expression of sex-determining region on the Y chromosome (SRY) protein initiates a cascade leading to male-specific gonadal differentiation (Capel, 1998). The appearance of Sertoli cells, their aggregation and formation of testicular cords are initial cellular features of male sex differentiation. Testicular hormones (e.g. anti-Mullerian hormone (AMH), testosterone) released from Sertoli and Leydig cells evoke subsequent sex-specific differentiation of the organism (Schlatt and Ehmcke, 2014b).
Once the early germ cells are located within the seminiferous tubules of the male gonad, they are termed gonocytes, which later home into their niches at the basal membrane to become spermatogonia. At the molecular level, it has been demonstrated in mice that the transition of mitotically rather quiescent gonocytes to active spermatogonia is not accompanied by a general increase in mRNA abundance but by a more efficient translation of available mRNAs (Chappell et al., 2013). The process of migration of gonocytes towards the basement membrane and the associated differentiation of gonocytes into spermatogonia continues postnatally in marmosets (4–6 months) as well as in humans (6–9 months) (Sharpe et al., 2003; Honecker et al., 2004; Mitchell et al., 2008). The neonatal period is followed by a phase termed ‘testicular quiescence’. Contrary to this term, the quantification of cells immunopositive for proliferation and germ cell marker proteins, has demonstrated that spermatogonia in the marmoset continue to proliferate (Kelnar et al., 2002; Albert et al., 2010). In line with this, a meta-analysis on spermatogonial numbers in prepubertal human testes revealed increasing numbers from the age of 4–7 years, suggesting that proliferation of germ cells is also ongoing in prepubertal human testes (Masliukaite et al., 2016).
Taken together, these processes during early development of the male germ line are species-specific and highly co-ordinated: somatic cells arranging for later niche formation, endocrine secretion providing the necessary signaling set up and the PGCs making their way into this environment to ensure the ability for life-long gamete production. These actions require a fine-tuned sequence of gene expression and cell-to-cell interaction. Keeping this in mind, it is also quite clear that this system is sensitive and at risk of being disturbed—which in consequence can be causative for a number of infertility and disease related phenotypes that we will report on below in more detail.
Molecular insights into the transcriptional and epigenetic processes associated with human male PGC development
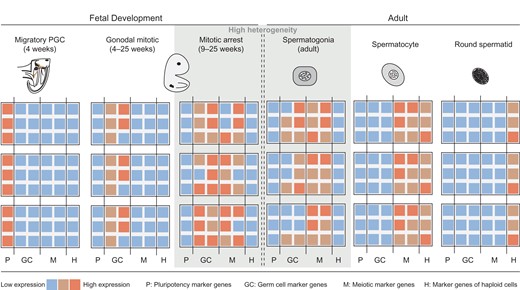
Single-cell technologies have taken the research on male germ cells a major step forward. Four landmark studies have isolated human PGC and unveiled their epigenetic and transcriptional changes using single-cell approaches (Gkountela et al., 2015; Guo et al., 2015; Tang et al., 2015; Li et al., 2017). One hallmark of germ cell development is genome-wide DNA demethylation. While some authors consider that the depletion of methylation prevents the transmission of sex-specific methylation patterns in sperm and eggs to the offspring (Heard and Martienssen, 2014) others assume that the demethylation occurs in specific regions of the genome and thereby signals information from one generation to the next (Seisenberger et al., 2012; Radford et al., 2014). The process of genome-wide DNA demethylation appears to be conserved among mammals, as demonstrated by the decreasing methylation levels in early PGC from mouse, pig and human assessed by semi-quantitative immunohistochemistry (Seki et al., 2005; Hyldig et al., 2011b; Eguizabal et al., 2016). Evaluating global DNA methylation levels in human male PGC obtained from 4 to 19 week-old fetuses revealed the lowest methylation levels, of only 7–8%, at week 11. This is compared to over 80% global methylation levels in the post-implantation embryo (Guo et al., 2015). Following this epigenetic ground state, de novo methylation is initiated following week 19 of development as indicated by increasing global methylation levels (Gkountela et al., 2015; Guo et al., 2015). While it has been demonstrated that this process of de novo methylation continues until well after birth in primates (Langenstroth-Röwer et al., 2017), data on early human postnatal germ cells are not yet available. Integrative analysis of methylation levels and transcriptional data showed that the transcriptional properties of PGC remain rather constant irrespective of the global methylation changes (Guo et al., 2015). Nonetheless, global expression profiles of human PGC from gestational weeks 4–26 enabled the distinction of three germ cell subtypes: migrating PGC (week 4), gonadal and mitotically active PGC (weeks 4–25) and gonadal and mitotically arrested PGC (weeks 9–25; Li et al., 2017). The overlapping time periods highlight that PGC development occurs via transcriptionally distinct subpopulations, which may be present at the same time. During migration and expansion, PGC express pluripotency marker genes [octamer-binding transcription factor 4 (OCT4) and Nanog homeobox (NANOG)], however, these transcripts are downregulated when cells arrest. In contrast, genes associated with meiosis (stimulated by retinoic acid 8 (STRA8), synaptonemal complex protein 1 (SYCP1)) become upregulated in the mitotically arrested subpopulation of human PGC (Fig. 2). Therefore, distinct transcriptional profiles can be associated with distinct functional properties (Li et al., 2017), i.e. it can be observed that mitotic arrest as the final stage of PGC differentiation incurs higher transcriptional heterogeneity compared to the other two subpopulations (Fig. 2; Guo et al., 2015; Li et al., 2017). During subsequent germ cell development, PGC gradually differentiate into various subtypes. These fine-tuned steps are mirrored by the number of terms used to categorize them (including gonocytes, pre- and pro-spermatogonia as well as further subtypes of spermatogonia), which are mainly defined on morphological criteria (Fig. 1).
Heat map showing the general gene expression profiles of human germ cells during different developmental stages. On the basis of gene expression analysis using RNA sequencing at single cell level, three PGC subpopulations can be identified during human fetal development: migratory, gonadal mitotic and mitotically arrested PGC. Based on single-cell analysis of human adult spermatogonia, transcriptionally distinct subpopulations can be distinguished. Note that a high degree of transcriptional heterogeneity can be observed in germ cell populations prior to differentiation, specifically in mitotically arrested PGC and undifferentiated spermatogonia (grey shaded area). In contrast, more mature germ cells, including spermatocytes and round spermatids, appear to be more homogenous cell populations. As information on transcriptional properties of germ cells from birth until puberty is lacking, this period is indicated by two dashed lines. P = expression of pluripotency marker genes; GC = germ cell marker genes; M = meiotic marker genes; H = marker genes of haploid cells.
Comprehensive analyses of human PGC have long been hampered by the limited access to human fetal testes and the low number of early germ cells. The advent of single-cell transcriptome analyses has therefore enabled a hitherto unequaled resolution of the transcriptional properties of early germ cells, unveiling the existence of at least three distinct subpopulations of human PGC. This better understanding of the early germ cell subpopulations can now be taken into account in studies assessing, for instance, the impact of gonadotoxic substances or underlying causes of early germ cell loss.
The molecular processes associated with male germ cells following week 29 of human fetal development and throughout puberty remain largely unknown. However, based on current progress in the field of high throughput single-cell transcriptome analyses, we expect that the datasets, which will close the knowledge gap between embryonic and adult germ cells, will become available in the near future.
Models of SSC systems in rodents and primates
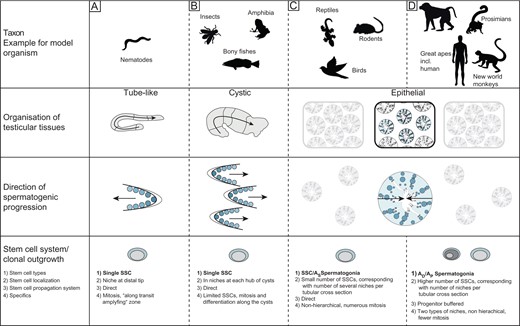
In the adult testis, spermatogonia are the least differentiated germ cell type present. Differentiation of these cells involves incomplete mitotic divisions resulting in interconnected cell clones. These syncytial clones are physically connected by intercellular cytoplasmic bridges, thus giving rise to a cytoplasmic continuum, i.e. a clone. In organisms like Drosophila, spermatogonial syncytia are enveloped by somatic cells, and the integrated structure is known as a cyst (Fig. 3; Matunis et al., 2012). The epithelial arrangements in mammals can be also regarded as arrangement of cystic germ cell clones. Instead of cuboidal cysts the germ cell clones are now arranged as several layers of fully flattened cysts (Schlatt and Ehmcke, 2014b). Hence, the clonal arrangement is considered a highly conserved feature of spermatogonia.
Comparison of various types of testicular organization in model organisms. The figure shows three types of testes, representative of most model organisms (A–C). Representative tube shaped Caenorhabditis elegans (A) testis containing the stem cell niche at the distal tip with SSC differentiating linearly. The cystic testis (B) type composed of a number of synchronously differentiating cysts; with SSC accompanied by supporting somatic cells forming the testicular niches (one per cyst) at the hub region. Clonal outgrowth from spermatogonia to spermatids occurs longitudinally along the testicular axis resulting in homogenous progress. The lobular or epithelial testis (C, D) present in reptiles, birds and mammals is divided into two compartments (the interstitium and the seminiferous tubules) with germ cells differentiating radially from the basal lamina to the lumen. In mammals, two stem cell systems were described: a ‘direct’ (C) and a ‘progenitor-buffered’ system (in primates) (D). In the figure, AD represents Adark spermatogonia, AP represents Apale spermatogonia and AS represents Asingle spermatogonia.
As described in Fig. 3C and D, two stem cell systems have been classified in mammals: a direct (non-progenitor buffered) system in rodents and a progenitor-buffered system in primates (Ehmcke et al., 2006). Mouse spermatogenic differentiation is driven by a chain of mitotic divisions based on the Asingle spermatogonia, of which seven types of A spermatogonia are derived (Asingle, Apair, Aaligned, A1, A2, A3 and A4) (De Rooij, 1998; De Rooij and Russell, 2000; Dettin et al., 2003). The Asingle spermatogonia are considered to be the SSC, which self-renew and give rise to the Apair and Aaligned spermatogonia. Those are expanded to larger cohorts. Continued mitotic expansions result in the A1–A4 spermatogonia, spermatogonial clones that are synchronized with the seminiferous epithelial cycle. Of those, B and Intermediate spermatogonia are formed, leading to large interconnected cohorts of spermatogonia (Ehmcke et al., 2006). As in rodents, primate spermatogonia expand in clonogenic patterns forming syncytial chains connected by intercellular bridges (Ehmcke et al., 2005; Yoshida, 2010). However, in contrast to rodents, two types of distinct A spermatogonia exist in primates, based on nuclear morphology. As originally proposed by Clermont, these represent reserve stem cells (Adark) and self-renewing progenitors (Apale). Several studies also indicate the presence of a transiting spermatogonial population (constituting 25–50% of the spermatogonial population), which are morphologically intermediate and distinct from Adark and Apale and known as Atransition (Ehmcke et al., 2005; Ehmcke and Schlatt, 2006). This purely morphological characterization was challenged when using molecular and histological markers as well as functional tests (Hermann et al., 2010). Based on the characterization of spermatogonia in rodents, further characterization of colonizing primate stem cell subpopulations was performed employing the fluorescence-activated cell sorting (FACS) approach. Different phenotypic subpopulations of primate spermatogonia representing Adark and Apale were distinguished based on molecular marker profiles. The most undifferentiated phenotypic profile [glial cell-line derived neurotrophic factor (GDNF) family receptor alpha-1+/promyelocytic leukemia zinc finger+/KIT proto-oncogene receptor tyrosine kinase- (GFRα1+/PLZF+/cKIT−)] was demonstrated by the Adark subpopulation and a small proportion of Apale cells, which was a striking observation. However, the majority of the Apale population expressed the more differentiated phenotype (GFRα1+/PLZF+/cKIT+). These observations in primates were proposed to be in line with the ‘Asingle’ spermatogenesis model in rodents, indicating the existence of a comparable spermatogonial system in primates (Hermann et al., 2010). This further raises the question of whether a population of Apale cells showing identical phenotypic expression as Adark cells is the Atransition cell type (transiting or intermediate cell populations).
Models for SSC self-renewal and expansion
Different models have been proposed describing distinct stem cell expansion modes (including hierarchical expansion or stochastic differentiation), proliferative hierarchies and patterns of stem cell renewal (Klein et al., 2010; Hara et al., 2014). In rodents, the most intensely discussed model is the ‘clonal fragmentation model’ proposed by Yoshida and Klein (Klein et al., 2010). In this study, real-time lineage tracing and pulse chase studies of GFRA1-GFP (expressed in Asingle, Apaired) and neurogenin 3 (NGN3)-GFP (expressed in Aaligned) expressing spermatogonial populations were performed. Interestingly, most of the NGN3+ cells of the type A spermatogonial population differentiated whereas few NGN3+ cells retained the ability to self-renew (Nakagawa et al., 2007, 2010). In contrast, long-term tracking of GFRA1+ cells indicates that the majority of the GFRA1+ population represents a single stem cell pool (including individual Asingle to syncytial states i.e. Apaired, Aal-3, Aal-4, Aal-5, Aal-6, Aal-7, Aal-8), yet only 5% of GFRA1+ cells undergo complete cell divisions. Based on this it was proposed by the authors that the GFRA1+ cells remain undifferentiated single stem cells (Hara et al., 2014), a hypothesis which needs to be tested further. In addition, it was reported that most clonally expanding spermatogonia died after 3 months whereas others expand (Klein et al., 2010). The pattern of loss and expansion was stochastic and fate decisions to undergo self-renewal or differentiation were dominated by competition between neighboring clones (Klein et al., 2010). Neutral drift dynamics was the common hallmark inherent in stem cell populations under a normal physiological state. Neutral drift dynamics refers to the phenomenon that cell populations are renewing to maintain tissue homeostasis. Applying a model of population dynamics, homeostasis can be achieved by random events of mitotic expansion and clonal splitting. These population dynamics are therefore non directed (neutral drift) and stochastic. In case of tissue injury or insult, stem cells changed splitting and expansion patterns and thereby replenished the reserve pool (Klein et al., 2010; Krieger and Simons, 2015).
Taking data from different adult stem cell systems into account, a stem cell model has been proposed in which a heterogeneous stem cell pool enables individual cells to respond differentially, depending on their momentary marker profile. The induction by stimuli from the microenvironment and the actual status of the individual cell leads to diverse fate decisions, such as undergoing self-renewal and entry into apoptosis or differentiation (Lee et al., 2014; Krieger and Simons, 2015). This new stem cell model assumes that the cells are not undergoing a unidirectional process of differentiation. Applying it to germ cells, spermatogonia may not develop unidirectionally and stepwise from stem cells to differentiating B spermatogonia but may go back and forth between different spermatogonial subtypes (Krieger and Simons, 2015), similar to the model (reported previously in Drosophila) (Stine and Matunis, 2013). Evidence from the lineage-tracing studies in mouse (mentioned above) substantiates the stochastic behavior of germ cells (Klein et al., 2010; Hara et al., 2014) indicating that molecular expression may specify the momentary hierarchical stage of a cell, but whether it has any influence on stem cell fate decisions needs to be determined by evaluating the potential of transcriptionally distinct subpopulations through functional assays.
In contrast to the stochastic turnover concept, which was based on experimental data and proposed by the Yoshida group, De Rooij et al. proposed an alternate model. This model is based on a computational approach and suggests that a steady state is maintained by migration of self-renewing stem cells to the areas with depleting spermatogonial clones (De Rooij and Beek, 2013; De Rooij, 2017). The various models for spermatogonial expansion were recently revisited in two reviews (De Rooij, 2017; Lord and Oatley, 2017). Additional experimental data is still required to identify the model which reflects the in vivo situation best.
However, studies investigating SSC kinetics (epithelial stages, proliferation patterns, division and clonal size) proposed different expansion models in rodents and primates (Ehmcke and Schlatt, 2006). Therefore, the recently proposed models for clonal dynamics of spermatogonia in rodents point to the need to also study clonal proliferation and differentiation mechanisms in primates, including the human, to understand the clonal dynamics of a progenitor-buffered SSC system in depth. These dynamics would have many further implications, for example concerning (reproductive) ageing or mutation frequency in the germ line.
Based on available data, we propose the presence of a heterogeneous stem cell pool, with ‘no linear developmental hierarchy’. Another aspect not taken into account in traditional SSC models is that spermatogonia remain mobile (Yoshida et al., 2007; De Rooij, 2009; Heckmann et al., 2018a) and can thereby enter different microenvironments along the basement membrane (e.g. close/away from blood vessels or interstitial cells). This migratory behavior in addition to the presence of various subtypes may generate a highly complex scenario for their fate decisions. In addition, clonal expansion and random clonal splitting may also play a relevant role. The evidence for the existence of a heterogeneous spermatogonial population could be increased when performing single-cell analyses of spermatogonial (stem) cells. If heterogeneity can be demonstrated, such results would support the idea of a cell pool consisting of various ‘types’ and forming the basis of spermatogenic progress. In the following sections we summarize data obtained with regard to the individual profiles of spermatogonia reflecting potential plastic fate decision processes.
Molecular insights into the transcriptional and epigenetic properties of murine and human spermatogonial subpopulations
For 50 years, morphological criteria have been used as main determinants of spermatogonial subtypes. Clermont’s distinction of Apale and Adark became the most accepted model in man and monkeys but has always been disputed (Clermont, 1970; Ehmcke and Schlatt, 2006). The question discussed in this conversation ‘What is the true stem cell?’ still holds true significance. The fact that the question could not be answered after 50 years of investigation leads us to speculate if it is the right question, or if we should revisit the question itself. Perhaps addressing the question ‘Is there a true stem cell?’ or better ‘Is there a heterogeneous population of cells with distinct features serving as a stem cell pool?’ might be more precise and important.
Generally, the presence of heterogeneous stem cell populations is in line with datasets from other adult stem cell systems (hematopoiesis: Dykstra et al., 2007; Benz et al., 2012; intestine: Lopez-Garcia et al., 2010). Using mice as a model organism, a number of studies have reported on the heterogeneity of the spermatogonial population. Applying DNA-binding protein inhibitor (Id4) as a marker for isolation of murine spermatogonia from postnatal Day 6 (PND6) testes, insights into their transcriptional profiles were gained. Initially unexpected, substantial transcriptional heterogeneity was found even among this population of highly selected spermatogonia, which was confirmed at protein level (Hermann et al., 2015). Comparative analyses of the protein marker profile of murine spermatogonia collected at different developmental time points suggests the existence of a phase characterized by a high degree of spermatogonial heterogeneity comprising the expression of early and late germ cell markers, specifically from P4 to P10. After this time however, selected phase markers were expressed by distinct populations of undifferentiated and differentiating spermatogonia (Niedenberger et al., 2015). Heterogeneous expression of selected markers proteins (nanos C2HC-type zinc finger 2 and 3 (NANOS2 and NANOS3)) was observed however, also in spermatogonia of adult mice (Suzuki et al., 2009). More recently comprehensive datasets have been generated employing single-cell RNA sequencing (RNA-Seq) analyses of testicular cell suspensions from adult mice. These analyses showed the continuous nature of germ cell differentiation (Chen et al., 2018; Green et al., 2018; Lukassen et al., 2018). Transcriptome data obtained from 1136 individual testicular cells unveiled stage and cell type-specific transcription profiles. Furthermore these datasets suggest that the spermatogonia only gradually commit to meiosis (Chen et al., 2018). Focused analyses of c. 2500 individual spermatogonia by Green et al. (2018) resulted in the identification of four spermatogonial subtypes. Attempts to assign the most undifferentiated spermatogonial population into a hierarchical organization or to spermatogonial states were not feasible, indicating that available datasets are not sufficient yet for this analysis or that these undifferentiated spermatogonia are indeed characterized by transcriptional plasticity (Green et al., 2018).
Recent studies have unveiled novel information on the epigenetic and transcriptional properties of spermatogonia of the adult human testis. It is still a matter of debate whether DNA methylation plays a role during establishment of various stages of post-pubertal human germ cell development. A comparison of bulk samples of stage-specific embryonic antigen 4 (SSEA4)-positive spermatogonia with sperm showed highly comparable methylation profiles among germ cell subtypes, indicating no major role for DNA methylation (Guo et al., 2017). An alternative interpretation of this finding was provided by the same group in a subsequent publication, suggesting, that the lack of ‘epigenetic boundaries’ may be a prerequisite for the plastic nature of the spermatogonial population (Guo et al., 2018). Overall, comparative analyses of self-renewing thymus cell antigen 1 (THY1+) positive to differentiating KIT+ spermatogonia isolated from adult mouse testes also yielded highly similar results. In-depth analyses revealed that more than a 30% change in methylation level was observed in seven promoter regions of genes involved in meiosis or encoding potassium channels (Hammoud et al., 2014). These data suggest that methylation changes of a certain gene set may indeed be associated with germ cell differentiation. Corresponding studies in the human assessing the methylation profile of different spermatogonial subpopulations remain to be performed. Transcriptional analyses of pure populations of human spermatogonia have long been hampered by the lack of spermatogonia-specific cell surface markers required for isolation of defined spermatogonial subpopulations among large numbers of differentiating and more mature germ cells. Morphologically selected human spermatogonia from in vitro cultures and subsequent single-cell gene expression analyses of selected marker genes have demonstrated heterogeneous transcriptional profiles (Neuhaus et al., 2017). Moreover, using morphological parameters, Jan et al. (2017) captured cell pools of spermatogonial subtypes (Adark and Apale), spermatocytes (leptotene/zygotene, early and late pachytene) and round spermatids from testicular tissues of adult men with qualitatively normal spermatogenesis using laser capture microdissection and subsequently performed RNA-Seq. However, B spermatogonia as well as pre-leptotene spermatocytes were not included in these analyses as the required number of 500 cells per germ cell subtype could not be reached. Nonetheless analyses of remaining cell types provided exciting new insights into the molecular properties of human spermatogonia. RNA-Seq data unveiled that spermatogonia have the highest degree of transcriptional complexity among the analyzed germ cell types, which then declines in the course of spermatogenesis. Also, Adark and Apale spermatogonia displayed a higher level of transcriptional heterogeneity compared to the more differentiated cell types and could not be assigned distinct transcriptional profiles, questioning whether the transition from a mitotically inactive to an active state is regulated by transcriptional changes. However, two genes, glutamate decarboxylase 1 (GAD1) and tryptophan hydroxylase 1 (TPH1), were differentially expressed in Adark (high) and Apale spermatogonia (low) (Jan et al., 2017). As these genes repress proliferation of mouse SSCs (Du et al., 2013) and erythroid precursors (Amireault et al., 2011) they might put and maintain Adark spermatogonia in a quiescent state. In line with published datasets, Jan et al. also found that spermatogonia already express a high number of genes, which are only required during later stages of spermatogenesis (Jan et al., 2017). Mechanistically, recent data indicate that this uncoupling of transcription and translation may be regulated by an intron retention programme, keeping those transcripts required during later stages of germ cell differentiation in an un-spliced state (Naro et al., 2017). This may be causative for the pools of Adark and Apale spermatogonia displaying a comparably high degree of transcriptional heterogeneity when compared to meiotic and post-meiotic germ cells as well as overlapping transcriptional patterns.
Moreover, Guo et al. performed single cell-transcriptome analysis of SSEA4-sorted cells comparing expression of pluripotency marker genes OCT4, NANOG and sex determining region Y-box 2 (SOX2), revealing that expression was restricted to ESCs and PGCs but not detectable in adult human spermatogonia. In contrast, meiosis-related genes were upregulated in adult human spermatogonia compared to ESCs and PGCs. Focusing on further analysis of adult human spermatogonia isolated by surface markers SSEA4 (n = 60 cells) and KIT (n = 32 cells) showed the existence of four distinct cellular subpopulations based on their transcriptional profiles (Guo et al., 2017). It is of note, that the data were obtained from limited cell numbers, which were isolated from individual testicular tissues that were not further characterized. Yet, these findings supported the transcriptional heterogeneity even among sorted cell populations implying a high transcriptional variability in the entire population of spermatogonia (Fig. 2). Key transcripts varied among the subtypes: undifferentiated spermatogonia were characterized by increased levels of stem cell-specific transcripts and genes known to inhibit uptake of glucose (thioredoxin interacting protein, TXNIP), which is in line with their low metabolic and quiescent state. Most differentiated spermatogonia were characterized by an upregulation of transcripts associated with DNA replication/repair, mitochondrial activities and spermatogonial differentiation (Guo et al., 2017).
More recent publications provided single cell RNA-Seq data from a total of 2854, 6490 and 7134 unselected human testicular cells, respectively (Guo et al., 2018; Hermann et al., 2018; Wang et al., 2018). Importantly, Guo et al. (2018) used three testicular tissues as starting material and performed two technical replicates. In line with data from the mouse, human germ cells also presented as a continuum based on transcriptional profiles (Guo et al., 2018; Hermann et al., 2018; Wang et al., 2018). Focusing on the spermatogonial population and on the extended dataset, Guo et al. (2018) identified an additional state yielding in total five spermatogonial clusters, which were independent of the cell cycle phase. These recent datasets, with a stronger focus also on the protein marker profile of spermatogonial subpopulations expand the information on the heterogeneous nature of undifferentiated spermatogonia and corroborate the suggestion that heterogeneous profiles also at the protein level may facilitate the bi-directional transition between spermatogonial states (Guo et al., 2018).
Based on the recent datasets, the first part of Clermont’s statement ‘there is the possibility that other classes of spermatogonia exist beside the three classes (Adark, Apale and type B)’ appears quite visionary (Clermont, 1970). Indeed, based on single-cell data there seem to be more classes of spermatogonia than just the three suggested ones, which need to be further analyzed in terms of function. What is more, there is no entirely distinct transcriptional profile associated with the nuclear morphology, which is the basis for classification of Adark, Apale and type B spermatogonia. The existence of five spermatogonial subpopulations suggests that the spermatogonial differentiation is not a binary but rather a more gradual process than suggested by the classification based on nuclear morphology. This may be of functional relevance, as gradual differentiation potentially enables more cells to revert back to a more undifferentiated state, if necessary.
The characteristics of male germ cells from specification to differentiation in the adult have been discussed above, the key aspects of which are summarized in Table II. Thus, summarizing the state of the art provocatively, one might even argue that there is no distinct ‘stem cell class’ as such but rather a population of undifferentiated cells of individual ‘stemness’ associated with high plasticity.
Developmental and epigenetic processes occurring at specific stages in perinatal life, and the key regulators and factors influencing these processes, in male mouse (Mus musculus), pig (Sus scrofa scrofa), marmoset (Callithrix jacchus) and human (Homo sapiens).
| Developmental and epigenetic processes . | Species . | Age . | Key regulators and factors involved . | References . |
|---|---|---|---|---|
| PGC specification | Mouse | E6.25 | Blimp1, Prdm14, Tfap2c, Prdm1, Klf2, Sox2, Nanog, Oct4, Bmp4, Bmp8b, Smad1, Smad4 | Magnúsdóttir et al. (2012, 2013); Nakaki et al. (2013); Irie et al. (2015); Gillich et al. (2012); Kurimoto et al. (2008); Ying et al. (2000); Lawson et al. (1999); Hayashi et al. (2002); Chang and Matzuk (2001), Tang et al. (2015); Hargan-Calvopina et al. (2016); Saitou and Miyauchi (2016) |
| PGC specification | Pig | E9.5–16 | NANOG, OCT4, SOX2, BRACHYURY | Kobayashi et al. (2017) |
| PGC specification | Human | E10.5–13.5 | SOX17, PRDM1, PRDM14, BLIMP1, BRACHYURY, NANOS3, SOX12, KLF6, LEF1, TFCP2L1, KLF4 | Irie et al. (2015); Chen and Clark (2015); Sugawa et al. (2015); Gkountela et al. (2015); Surani (2015); Tang et al. (2015); Kojima et al. (2017); Yamashiro et al. (2018) |
| PGC migration—loss of DNA methylation | Mouse | E8–10.5 | Gata4, Tead4, Ssea4 | Guibert et al. (2012); Irie et al. (2015); Surani (2015); Sugawa et al. (2015); Tang et al. (2015) |
| PGC migration | Pig | E17–20 | OCT4, SSEA1 | Hyldig et al. (2011a,b) |
| PGC migration | Human | E29–35 | SOX17, HMGN3, CARHSP1, GATA4, TEAD4, CKIT, VASA, SSEA1 | Tang et al. (2015); Li et al. (2017); Surani et al. (2007) |
| PGC colonization | Mouse | E10.5–12 | Tet1, Tet2, Dnmt1 | Hill et al. (2018); Hargan-Calvopina et al. (2016); Seisenberger et al. (2012); Guibert et al. (2012) |
| -Genome-wide loss of methylcytosine | ||||
| PGC colonization | Pig | E23–24 | OCT4 | Hyldig et al. (2011a,b) |
| PGC colonization | Human | E36–42 | CKIT, VASA | Gkountela et al. (2013); Tang et al. (2015) |
| Male sex differentiation | Mouse | E12–13.5 | Dnmt1 | Hargan-Calvopina et al. (2016); Tang et al. (2015) |
| -Hypomethylated epigenetic ground state, X reactivation, chromatin reorganization, decrease and increase in H3K27me3 histone methylation | ||||
| Male sex differentiation | Pig | E25–31 | OCT4 | Hyldig et al. (2011a,b) |
| Male sex differentiation | Human | E43–63 | NANOS2, NANOG, CD38, NANOS3, PRDM1 | Gkountela et al. (2015) |
| Methylation erasure | Mouse | E13.5 | – | Gkountela et al. (2015) |
| -Two-step process necessary for normal spermatogenesis in adults | ||||
| Methylation erasure | Pig | E22–42 | Hyldig et al. (2011a,b) | |
| Methylation erasure | Human | E70–77 | – | Gkountela et al. (2015); Guo et al. (2015) |
| -Lowest global methylation levels | ||||
| De novo DNA methylation | Mouse | PND4–5 | Tet3 | Williams et al. (2011) |
| De novo DNA methylation | Pig | E31–E42 | – | Hyldig et al. (2011a,b) |
| De novo DNA methylation | Human/Marmoset | E59–137 Continues postnatally in primate animal-model even 4–8 months after birth (Cj) | – | Gkountela et al. (2015); Langenstroth-Röwer et al. (2017) |
| Developmental and epigenetic processes . | Species . | Age . | Key regulators and factors involved . | References . |
|---|---|---|---|---|
| PGC specification | Mouse | E6.25 | Blimp1, Prdm14, Tfap2c, Prdm1, Klf2, Sox2, Nanog, Oct4, Bmp4, Bmp8b, Smad1, Smad4 | Magnúsdóttir et al. (2012, 2013); Nakaki et al. (2013); Irie et al. (2015); Gillich et al. (2012); Kurimoto et al. (2008); Ying et al. (2000); Lawson et al. (1999); Hayashi et al. (2002); Chang and Matzuk (2001), Tang et al. (2015); Hargan-Calvopina et al. (2016); Saitou and Miyauchi (2016) |
| PGC specification | Pig | E9.5–16 | NANOG, OCT4, SOX2, BRACHYURY | Kobayashi et al. (2017) |
| PGC specification | Human | E10.5–13.5 | SOX17, PRDM1, PRDM14, BLIMP1, BRACHYURY, NANOS3, SOX12, KLF6, LEF1, TFCP2L1, KLF4 | Irie et al. (2015); Chen and Clark (2015); Sugawa et al. (2015); Gkountela et al. (2015); Surani (2015); Tang et al. (2015); Kojima et al. (2017); Yamashiro et al. (2018) |
| PGC migration—loss of DNA methylation | Mouse | E8–10.5 | Gata4, Tead4, Ssea4 | Guibert et al. (2012); Irie et al. (2015); Surani (2015); Sugawa et al. (2015); Tang et al. (2015) |
| PGC migration | Pig | E17–20 | OCT4, SSEA1 | Hyldig et al. (2011a,b) |
| PGC migration | Human | E29–35 | SOX17, HMGN3, CARHSP1, GATA4, TEAD4, CKIT, VASA, SSEA1 | Tang et al. (2015); Li et al. (2017); Surani et al. (2007) |
| PGC colonization | Mouse | E10.5–12 | Tet1, Tet2, Dnmt1 | Hill et al. (2018); Hargan-Calvopina et al. (2016); Seisenberger et al. (2012); Guibert et al. (2012) |
| -Genome-wide loss of methylcytosine | ||||
| PGC colonization | Pig | E23–24 | OCT4 | Hyldig et al. (2011a,b) |
| PGC colonization | Human | E36–42 | CKIT, VASA | Gkountela et al. (2013); Tang et al. (2015) |
| Male sex differentiation | Mouse | E12–13.5 | Dnmt1 | Hargan-Calvopina et al. (2016); Tang et al. (2015) |
| -Hypomethylated epigenetic ground state, X reactivation, chromatin reorganization, decrease and increase in H3K27me3 histone methylation | ||||
| Male sex differentiation | Pig | E25–31 | OCT4 | Hyldig et al. (2011a,b) |
| Male sex differentiation | Human | E43–63 | NANOS2, NANOG, CD38, NANOS3, PRDM1 | Gkountela et al. (2015) |
| Methylation erasure | Mouse | E13.5 | – | Gkountela et al. (2015) |
| -Two-step process necessary for normal spermatogenesis in adults | ||||
| Methylation erasure | Pig | E22–42 | Hyldig et al. (2011a,b) | |
| Methylation erasure | Human | E70–77 | – | Gkountela et al. (2015); Guo et al. (2015) |
| -Lowest global methylation levels | ||||
| De novo DNA methylation | Mouse | PND4–5 | Tet3 | Williams et al. (2011) |
| De novo DNA methylation | Pig | E31–E42 | – | Hyldig et al. (2011a,b) |
| De novo DNA methylation | Human/Marmoset | E59–137 Continues postnatally in primate animal-model even 4–8 months after birth (Cj) | – | Gkountela et al. (2015); Langenstroth-Röwer et al. (2017) |
E, Embryonic; PND, postnatal day; PGC, primordial germ cell.
Developmental and epigenetic processes occurring at specific stages in perinatal life, and the key regulators and factors influencing these processes, in male mouse (Mus musculus), pig (Sus scrofa scrofa), marmoset (Callithrix jacchus) and human (Homo sapiens).
| Developmental and epigenetic processes . | Species . | Age . | Key regulators and factors involved . | References . |
|---|---|---|---|---|
| PGC specification | Mouse | E6.25 | Blimp1, Prdm14, Tfap2c, Prdm1, Klf2, Sox2, Nanog, Oct4, Bmp4, Bmp8b, Smad1, Smad4 | Magnúsdóttir et al. (2012, 2013); Nakaki et al. (2013); Irie et al. (2015); Gillich et al. (2012); Kurimoto et al. (2008); Ying et al. (2000); Lawson et al. (1999); Hayashi et al. (2002); Chang and Matzuk (2001), Tang et al. (2015); Hargan-Calvopina et al. (2016); Saitou and Miyauchi (2016) |
| PGC specification | Pig | E9.5–16 | NANOG, OCT4, SOX2, BRACHYURY | Kobayashi et al. (2017) |
| PGC specification | Human | E10.5–13.5 | SOX17, PRDM1, PRDM14, BLIMP1, BRACHYURY, NANOS3, SOX12, KLF6, LEF1, TFCP2L1, KLF4 | Irie et al. (2015); Chen and Clark (2015); Sugawa et al. (2015); Gkountela et al. (2015); Surani (2015); Tang et al. (2015); Kojima et al. (2017); Yamashiro et al. (2018) |
| PGC migration—loss of DNA methylation | Mouse | E8–10.5 | Gata4, Tead4, Ssea4 | Guibert et al. (2012); Irie et al. (2015); Surani (2015); Sugawa et al. (2015); Tang et al. (2015) |
| PGC migration | Pig | E17–20 | OCT4, SSEA1 | Hyldig et al. (2011a,b) |
| PGC migration | Human | E29–35 | SOX17, HMGN3, CARHSP1, GATA4, TEAD4, CKIT, VASA, SSEA1 | Tang et al. (2015); Li et al. (2017); Surani et al. (2007) |
| PGC colonization | Mouse | E10.5–12 | Tet1, Tet2, Dnmt1 | Hill et al. (2018); Hargan-Calvopina et al. (2016); Seisenberger et al. (2012); Guibert et al. (2012) |
| -Genome-wide loss of methylcytosine | ||||
| PGC colonization | Pig | E23–24 | OCT4 | Hyldig et al. (2011a,b) |
| PGC colonization | Human | E36–42 | CKIT, VASA | Gkountela et al. (2013); Tang et al. (2015) |
| Male sex differentiation | Mouse | E12–13.5 | Dnmt1 | Hargan-Calvopina et al. (2016); Tang et al. (2015) |
| -Hypomethylated epigenetic ground state, X reactivation, chromatin reorganization, decrease and increase in H3K27me3 histone methylation | ||||
| Male sex differentiation | Pig | E25–31 | OCT4 | Hyldig et al. (2011a,b) |
| Male sex differentiation | Human | E43–63 | NANOS2, NANOG, CD38, NANOS3, PRDM1 | Gkountela et al. (2015) |
| Methylation erasure | Mouse | E13.5 | – | Gkountela et al. (2015) |
| -Two-step process necessary for normal spermatogenesis in adults | ||||
| Methylation erasure | Pig | E22–42 | Hyldig et al. (2011a,b) | |
| Methylation erasure | Human | E70–77 | – | Gkountela et al. (2015); Guo et al. (2015) |
| -Lowest global methylation levels | ||||
| De novo DNA methylation | Mouse | PND4–5 | Tet3 | Williams et al. (2011) |
| De novo DNA methylation | Pig | E31–E42 | – | Hyldig et al. (2011a,b) |
| De novo DNA methylation | Human/Marmoset | E59–137 Continues postnatally in primate animal-model even 4–8 months after birth (Cj) | – | Gkountela et al. (2015); Langenstroth-Röwer et al. (2017) |
| Developmental and epigenetic processes . | Species . | Age . | Key regulators and factors involved . | References . |
|---|---|---|---|---|
| PGC specification | Mouse | E6.25 | Blimp1, Prdm14, Tfap2c, Prdm1, Klf2, Sox2, Nanog, Oct4, Bmp4, Bmp8b, Smad1, Smad4 | Magnúsdóttir et al. (2012, 2013); Nakaki et al. (2013); Irie et al. (2015); Gillich et al. (2012); Kurimoto et al. (2008); Ying et al. (2000); Lawson et al. (1999); Hayashi et al. (2002); Chang and Matzuk (2001), Tang et al. (2015); Hargan-Calvopina et al. (2016); Saitou and Miyauchi (2016) |
| PGC specification | Pig | E9.5–16 | NANOG, OCT4, SOX2, BRACHYURY | Kobayashi et al. (2017) |
| PGC specification | Human | E10.5–13.5 | SOX17, PRDM1, PRDM14, BLIMP1, BRACHYURY, NANOS3, SOX12, KLF6, LEF1, TFCP2L1, KLF4 | Irie et al. (2015); Chen and Clark (2015); Sugawa et al. (2015); Gkountela et al. (2015); Surani (2015); Tang et al. (2015); Kojima et al. (2017); Yamashiro et al. (2018) |
| PGC migration—loss of DNA methylation | Mouse | E8–10.5 | Gata4, Tead4, Ssea4 | Guibert et al. (2012); Irie et al. (2015); Surani (2015); Sugawa et al. (2015); Tang et al. (2015) |
| PGC migration | Pig | E17–20 | OCT4, SSEA1 | Hyldig et al. (2011a,b) |
| PGC migration | Human | E29–35 | SOX17, HMGN3, CARHSP1, GATA4, TEAD4, CKIT, VASA, SSEA1 | Tang et al. (2015); Li et al. (2017); Surani et al. (2007) |
| PGC colonization | Mouse | E10.5–12 | Tet1, Tet2, Dnmt1 | Hill et al. (2018); Hargan-Calvopina et al. (2016); Seisenberger et al. (2012); Guibert et al. (2012) |
| -Genome-wide loss of methylcytosine | ||||
| PGC colonization | Pig | E23–24 | OCT4 | Hyldig et al. (2011a,b) |
| PGC colonization | Human | E36–42 | CKIT, VASA | Gkountela et al. (2013); Tang et al. (2015) |
| Male sex differentiation | Mouse | E12–13.5 | Dnmt1 | Hargan-Calvopina et al. (2016); Tang et al. (2015) |
| -Hypomethylated epigenetic ground state, X reactivation, chromatin reorganization, decrease and increase in H3K27me3 histone methylation | ||||
| Male sex differentiation | Pig | E25–31 | OCT4 | Hyldig et al. (2011a,b) |
| Male sex differentiation | Human | E43–63 | NANOS2, NANOG, CD38, NANOS3, PRDM1 | Gkountela et al. (2015) |
| Methylation erasure | Mouse | E13.5 | – | Gkountela et al. (2015) |
| -Two-step process necessary for normal spermatogenesis in adults | ||||
| Methylation erasure | Pig | E22–42 | Hyldig et al. (2011a,b) | |
| Methylation erasure | Human | E70–77 | – | Gkountela et al. (2015); Guo et al. (2015) |
| -Lowest global methylation levels | ||||
| De novo DNA methylation | Mouse | PND4–5 | Tet3 | Williams et al. (2011) |
| De novo DNA methylation | Pig | E31–E42 | – | Hyldig et al. (2011a,b) |
| De novo DNA methylation | Human/Marmoset | E59–137 Continues postnatally in primate animal-model even 4–8 months after birth (Cj) | – | Gkountela et al. (2015); Langenstroth-Röwer et al. (2017) |
E, Embryonic; PND, postnatal day; PGC, primordial germ cell.
Regulatory aspects of spermatogonial niches
Clermont was the first to report the typical clonal arrangements of active and inactive spermatogonia. Based on this arrangement of undifferentiated germ cells, he postulated the necessity of somatic cells to form a surrounding environment along the adjoining tubular walls (nowadays termed niches) (Clermont, 1963, 1966). Interestingly, the characteristics of these niches are as undefined as the stem cell itself. So far, lineage tracing studies in mice suggest that stem cell niches are specific areas in close proximity to the blood vessels and vasculature (Chiarini-Garcia et al., 2001; Yoshida et al., 2007), and it appears likely that the spermatogonial niche provides factors promoting cell proliferation (for review: Kanatsu-Shinohara and Shinohara, 2013) but also factors inhibiting cell division (Kanatsu-Shinohara et al., 2010, 2014). There are different models to explain a correlation of niches with the blood vessel system. However, the exact mechanisms for how the niches may function and which spermatogonial subtypes are located at specific sites have to be further elucidated. The testicular stem cell niche plays a significant role in maintaining a pool of undifferentiated precursors and thereby the regenerative potential of the testis. More specifically, niches harbor the stem cells and render them quiescent. This is of crucial importance, as only spermatogonia colonizing these niches can act as stem cells. Furthermore, niches protect such spermatogonia from undergoing many divisions and this ‘calming down’ of proliferating activity is an important contribution in order to sustain genetic germ line integrity at the stem cell level by minimizing mutations. Thus, as in other stem cell systems, these niches play a significant role in providing a steady state, i.e. maintaining a persisting pool of undifferentiated precursors and, as a result, also a stable production rate of differentiating germ cells once meiotic progression is initiated.
We consider the inhibition of expansion in the niche an active process that is regulated by the testicular microenvironment. In the past few years, several in vivo and in vitro studies have investigated the role of specific transcriptional regulators and testicular factors in maintaining homeostasis and modulating stem cell fate decisions (such as self-renewal or differentiation) (Chan et al., 2014; Kimura et al., 2014; Morimoto et al., 2015; Takashima et al., 2013, 2015; Kanatsu-Shinohara et al., 2013, 2014, 2016a,b). Quite a few specific factors have been described to act on SSCs (Schlatt and Sharma, 2019), for instance: Kit ligand produced by Sertoli cells influences the expansion of type A spermatogonia (Sorrentino et al., 1991; Rossi et al., 1993). Colony stimulating factor (CSF1) and GDNF are involved in regulation of SSC self-renewal and spermatogonial proliferation, respectively (Yomogida et al., 2003; Oatley et al., 2009). Other growth factors, including fibroblast growth factor (FGF2; Mullaney and Skinner, 1992), epithelial growth factor (EGF), leukemia inhibitory factor (LIF) and insulin-like growth factor 1 (IGF1), may be complimenting GDNF in regulating SSC numbers (Oatley and Brinster, 2012). Also, the chemokine C-X-C motif chemokine ligand 12 (CXCL12) with its receptors C-X-C motif chemokine receptor 4 (CXCR4) and C-X-C motif chemokine receptor 7 (CXCR7) is known to be involved in regulating germ cell migration, the homing of germ cells into their niches in testis and various aspects of germ cell development in different species (Heckmann et al., 2018a). However, despite the functional influence of various factors we propose that the crucial regulatory aspect is the release of the strong inhibition to generate an adequate number of precursors which will start a species-specific cascade of mitotic divisions prior to meiosis. Since many species, such as rodents, generate large clones from one stem cell, only a few precursors should be released at defined distances on the basement membrane to maintain a full load of the seminiferous epithelium with several expanding large clones of differentiating germ cells (Yomogida et al., 2003; Ogawa et al., 2005). Species with fewer mitotic expansions prior to meiosis (as in human) require a more frequent generation of differentiating spermatogonia per unit area of testis as many more small clones are colonizing the seminiferous epithelium (Schlatt and Ehmcke, 2014). The nature of the inhibitory actions controlling the kinetics of stem cell turnover are not yet understood in mammals and it has to be explored how the niche regulates the stem cell pool and which specific factors are functionally involved in maintenance of homeostasis, self-renewal and differentiation. Insight into these processes has so far been hampered by the lack of platforms providing information on single cell expression and, in particular, the cell-to-cell interactome level. The advent of high throughput single cell-expression analyses has provided novel information on germ cells and somatic cells from the same testicular samples. Importantly, this information is also now available on the first samples from an infant, as well as adult tissues (Guo et al., 2018). Comparative analysis of normal samples from different developmental stages will provide information on the properties of the spermatogonial niche in the infant, prepubertal, pubertal and adult testes allowing insight into the cross-talk between different somatic cells as well as germ cells throughout development. Comparative approaches with datasets from the mouse have already shown species-specific differences: for example, the colony stimulating factor 1 (CSF1) receptor in humans at the transcriptional level is not expressed in spermatogonia but rather in macrophages, indicating distinct regulatory mechanisms (Guo et al., 2018). It is to be expected that future analyses will focus on comparative analyses of normal testicular tissues and those with impaired spermatogenesis. These studies will help to decipher the transcriptional pathways associated with infertility and will provide insight into the underlying causes of impaired germ cell development. Moreover, recent advances in the field of nanostructures put microscopical, as well a single-cell secretome analyses of selectively placed interacting cells into reach.
Diversity of testicular organization
Although the general processes of spermatogenic initiation by SSCs are grossly identical across species (SSCs self-renew and also produce differentiating daughter cells undergoing haploidization), the adaptations of testicular morphology are rather variable. Distinct anatomical testicular features exist in different species, which form the microenvironment for germ cells in the male. We illustrate this by describing three types of testes using representative model organisms (Fig. 3). Caenorhabditis elegans is a protandrous hermaphrodite nematode producing first sperm and later eggs in the same gonad, interestingly from the same germline stem cell population (Ramm et al., 2014). The male gonad, a tube (shaped like a ‘U’ with a truncated arm) contains the stem cell niche at the distal tip. Some features of this rather simple organization resemble those in a cystic testis; present in a variety of taxa reaching from insects to fishes and amphibians (Ramm et al., 2014). At the testis tip, a somatic hub region homes rarely dividing SSCs and also contains somatic cyst stem cells. Cyst cells engulf individual or groups of spermatogonia forming small cysts. These are growing by rapidly dividing germ cells. In each cyst, germ cells differentiate synchronously while they are pushed towards the distal end. In the distal region germ cells in each cyst develop into spermatocytes and spermatids (Fig. 3). A third morphologically distinct testis type is present in reptiles, birds and mammals, namely the epithelial type testis. Here, the testis is divided into two compartments, the interstitium and the seminiferous tubules (Wistuba et al., 2007). At the basement membrane of the seminiferous tubules, niches are located to home the SSCs. They give rise to differentiating germ cells, which are arranged in concentric layers. Sertoli cells are the structural constituents of the seminiferous epithelium, which form a blood–testis barrier by intense cell–cell contact. The germ cells differentiate radially, i.e. from the basal lamina to the adluminal part of the epithelium where the testicular spermatozoa are released into the lumen and transported via the rete testis into the epididymis. Contractile movements are generated by the myoid peritubular cells lining the outside of the tubular wall (Wistuba et al., 2007). The different testicular types are also relevant for the male germline cells and their physiological features. Stem cell niches and the mode of expansion need to be adapted to the anatomical arrangements.
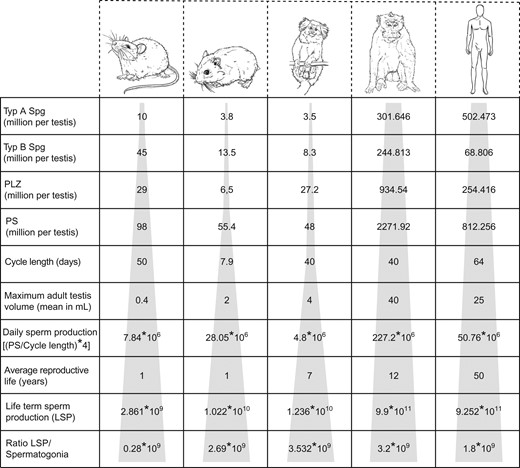
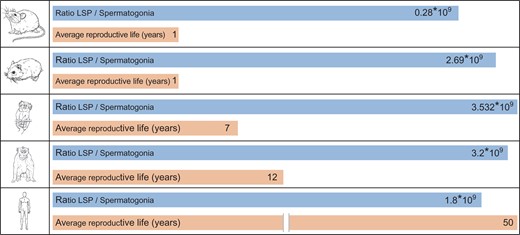
Inter-species comparison of spermatogonial turnover and sperm production rate
Sophisticated stereological microscopy was one of the first modern approaches employed for estimation of cell/nuclear numbers. Cellular and nuclear volume was estimated by employing either model-based or serial section reconstitution methods. The ‘optical dissector’ method was developed 2 years later (Sterio, 1984; Gundersen, 1986). This method involved cutting thick tissue sections (20–30 μm) and employing an oil-immersion objective with a high numerical aperture for observations. Very shallow focal depth (using a high numerical aperture lens) enables optical sectioning of thick sections. Sequential sections at a uniform distance can be observed and in-focus cells per nucleus are quantified. This technique was applied to testicular tissues for the first time in 1995 (Wreford, 1995). The major advantage of the optical dissector approach is that it avoids the aligning of physically distinct sections, which is very advantageous for quantification of more densely associated germ cells present in the seminiferous epithelium of a tubule (Wreford, 1995). Fortunately, optical dissector data are now available for a couple of mammalian species, enabling comparative reanalysis of relationships between the different germ cell types. This morphometric approach creates the most reliable dataset as it is based on a systematic random sampling using 3D stereological analyses in tissues retaining their structural integrity. These papers, which we reanalyzed, are highly comparable, fully valid in terms of the recognition of germ cell subtypes, replicates and counting accuracy using almost identical evaluation strategies. In order to introduce a new perspective, we used the existing data to alternatively calculate new and thus far neglected aspects of testis biology. To do this, we introduce the concept of a balanced stem cell system to cope with the needs of species-specific sperm production and a fixed maximal turnover of A-spermatogonia.
A stem cell driven process, such as the production of gametes, has to fulfill certain tasks, i.e. it should provide a maximum of fertile gametes but also protect the genetic information transmitted to the next generation by these. Therefore, the potential to produce gametes over the entire reproductive lifespan should have resulted in adaptations balancing the optimal sperm output versus a minimized loss of genetic integrity. In order to check whether this assumption holds true, we analyzed published data on sperm production in various species of different generational time course, assuming that a short-lived species should produce less sperm per spermatogonium during its reproductive life than species that reproduce for decades. We performed a systematic literature review to identify all valid evidence for calculating species-specific spermatogenic turnover rates per generation in five different species, representing two distinct SSC-systems: rat (Rattus rattus) (Meachem et al., 1998), Djungarian hamster (Phodopus sungorus) (Meachem et al., 2005) versus marmoset (Callithrix jacchus) (Weinbauer et al., 2001), macaque (Macaca fascicularis) (Zhengwei et al., 1997, 1998; Donnell et al., 2001) and man (Homo sapiens) (Zhengwei et al., 1998; Raleigh et al., 2004; Matthiesson et al., 2006) (Fig. 4). As we intended the most valid and unbiased information on absolute cell numbers to be incorporated, only studies on absolute germ cell numbers (type A and type B spermatogonia, spermatocytes) using the optical dissector approach were included in the analysis. Numerical cell density is estimated by using the following formula (Berndtson, 2011): N = Q−/(a * h) (‘Q−’ represents number of nuclei, ‘a’ represents counting area; ‘h’ represents height of plane above the counting frame). We have re-calculated the raw data, and transformed and analyzed them uniformly to allow for a species comparison of these data (Supplementary Table SI).
Proposed models of SSC kinetics in five different mammalian species. The calculated sperm turnover rate/generation in five different species (rats (Rattus rattus), Djungarian hamsters (Phodopus sungorus), marmosets (Callithrix jacchus), macaques (Macaca fascicularis), humans (Homo sapiens)), representing the two stem cell systems (‘direct’ and ‘progenitor-buffered’) is shown. Average numbers of type A spermatogonia (SPG), type B spermatogonia, pre-leptotene–zygotene (PLZ) and pachytene spermatocytes (PS) per testis, respectively, were used to calculate daily sperm production, lifetime sperm production (LSP) and the ratio of LSP/type A spermatogonium. The tip of the grey-shaded pyramid represents the population size of type A spermatogonia. The base of the pyramid shows that the LSP ratio is in the range of 109–1011 for all the species. The ratio of LSP/type A spermatogonium is in the similar order of 109 for the respective species, possibly pointing to a general adaptation process in terms of testicular productivity.
The data reveal comparable daily sperm production (DSP) rates (in the order of 106) in all the five species (Fig. 4) representing different stem cell systems. The average number of sperm produced in the entire reproductive lifespan in one generation was calculated, to estimate the lifetime sperm production (LSP) rates for all species. LSP rates for all species were in the range 109–1011. We revealed that all five species show a comparable sperm output per generation (Fig. 4). Irrespective of the physiological differences, including testicular size, testicular volume, number of undifferentiated spermatogonia per testis, progenitor-buffered or non-progenitor buffered stem cell systems and the distinct reproductive lifespans, the ratio of LSP per type A spermatogonium in different species during one generation was found to be comparable (in the order of 109) (Figs. 4 and 5). Comparable DSP rates in different species have been reported in the literature (Senger, 2004), however, a species comparison of LSP rates has never been investigated or reported. Our findings provide evidence in support of the presence of a conserved mechanism across species, which uniformly regulates reproductive function and capacity to produce comparable numbers of sperm in a single generation (Fig. 5). Despite the reproductive lifespans stretching from 1 year to 50 years in various species, the total lifetime sperm production and the turnover rates of spermatogonia are similar, indicating a strict regulation of these assets. Taken together, these data point to an adaptation of spermatogonia to achieve maximal production of sperm during their life—likely balanced against a minimum of mutation errors. Whether total sperm production during life is limited by mechanisms intrinsic to the germ cell or to the exhaustion limits of the niche, or to both, remains to be revealed.
Ratio of LSP rates/type A spermatogonium in five different mammalian species. For rats (Rattus rattus), Djungarian hamsters (Phodopus sungorus), marmosets (Callithrix jacchus), macaques (Macaca fascicularis), and humans (Homo sapiens), the ratio of LSP/type A spermatogonium was found to be in the order of 109 in all species. Blue bars represent the ratios and orange bars represent the average reproductive lifespan (numbered in years).
We suggest that the different spermatogonial systems might be an adaptation to the varying reproductive lifespans of the species in order to achieve this goal.
Options for spermatogonial therapies
While the previous sections focused on regulatory aspects and transcriptional as well as epigenetic features of spermatogonia under normal conditions, we now focus on dysfunction of the seminiferous epithelium affecting either the niche or the spermatogonia. To date, genetic causes affecting germ cell and/or niche function have been identified in ~4% of infertile men (Tüttelmann et al., 2018). Few single-gene defects causative of male infertility have been identified. Testis-expressed gene 11 (TEX11), however, represents such a gene and mutations have been shown to cause meiotic arrest and azoospermia (Yatsenko et al., 2015). Apart from that, 47,XXY Klinefelter syndrome presents a chromosomal aberration, in which the additional X-chromosome may lead to aberrant transcriptional profiles owing to incomplete X-inactivation (Aksglaede et al., 2006; Zitzmann et al., 2015) resulting not only in impaired spermatogonial function but also potentially niche cell function. As a consequence of this, testicular tissues of affected patients show a reduction in spermatogonial numbers throughout testicular development (Aksglaede et al., 2006; Heckmann et al., 2018b). Klinefelter syndrome is the most common chromosomal aberration with a prevalence of 1:500–1:600 in newborn boys (Nieschlag et al., 2016). Apart from these natural causes leading to malfunction of germ cells and/or their niches, malfunction may also be induced, for example, by exposure to radiation or chemotherapy. Stem cell-related dysfunctions might be as yet an underestimated major contributor to male infertility.
Effect of chemotherapy and irradiation therapy on male germ cells
Histo-morphometric analysis has been used as one of the major approaches to analyse the effect of chemotherapy and irradiation on testicular germ cell survival. Spermatogonial quantitative data from clinical samples (healthy testicular biopsies) of prepubertal boys assessed to establish clinical reference values indicate an age-dependent variation in spermatogonial quantity during testicular development: a decrease (1–3 years), an increase (until 6–7 years), a constant level (until 11 years) and then a sharp increase (13–14 years) in spermatogonial numbers in different age groups were recorded (Masliukaite et al., 2016). Based on morphology, an accurate classification into Adark, Apale and B spermatogonia is not possible in the immature testicular tissues (Ehmcke et al., 2005; Ehmcke and Schlatt, 2006), therefore, we expect that an unbiased analysis of the transcriptional profiles of the populations of spermatogonia that naturally occur during development may help to establish a meaningful classification of germ cell development from birth until puberty.
Spermatogonial quantification in immature human testicular samples cryopreserved for fertility preservation showed a high degree of heterogeneity and low spermatogonial numbers in patients exposed to irradiation or chemotherapeutic treatment (Heckmann et al., 2018b). The finding that spermatogonial numbers are significantly different at an inter-individual level depending on disease or pre-treatment led us to speculate that the composition of spermatogonial subpopulations will also change, with hitherto unknown consequences for chances of recovery of spermatogenesis. Employing the established spermatogonial reference values (Masliukaite et al., 2016), the effects of therapeutic treatment on spermatogonial numbers was evaluated by spermatogonial quantification in a cohort of prepubertal patients suffering from acute leukemia (Poganitsch-Korhonen et al., 2017). A reduced spermatogonial pool was observed in patients who had undergone therapy with alkylating agents. Similar findings (reduced spermatogonial quantity in testes) were reported in another patient cohort of prepubertal boys treated with alkylating agents (Stukenborg et al., 2018). This finding substantiates the long-term adverse impact of therapy with alkylating agents.
Cellular responses to different doses of irradiation have also been systematically evaluated in irradiation studies with non-human primates (NHPs) (Van Alphen et al., 1988; De Rooij et al., 2002; Jahnukainen et al., 2007, 2011; Tröndle et al., 2017). Spermatogonia from different age groups of monkeys were observed to be susceptible to different doses of irradiation. A dose dependent decline in spermatogonial numbers (Type Adark and Apale) and number of tubules with type A and B spermatogonia has been reported in irradiated (0, 0.5, 1.0 and 4 Gy) testicular fragments of immature juvenile rhesus monkeys subjected to short-term (organ culture) and long-term evaluation (xenografting) (Jahnukainen et al., 2007). The highest dose of irradiation resulted in a sharp decline in A spermatogonial numbers and a rise in apoptotic germ and Sertoli cells in cultured fragments. Number of tubules with a Sertoli cell only phenotype increased with time, and tubule elongation in the grafts was influenced by the highest dose of irradiation (4 Gy) (Jahnukainen et al., 2007). In contrast, low irradiation doses influence differentiating germ cells, whereas more advanced germ cell types (spermatocytes and spermatids) remained unaffected (Jahnukainen et al., 2007). The rates of testicular germ cell and somatic cell recovery after irradiation varied in both prepubertal and pubertal NHPs. Irradiation was observed to have a greater adverse effect on the tubular outgrowth of prepubertal compared to the pubertal NHP testis. In prepubertal testis, somatic cells, specifically Sertoli cells, show a more adverse response to irradiation compared to a pubertal testis, indicating a significant influence on the increased mitotic expansion and germ cell differentiation in a pubertal testis (Jahnukainen et al., 2011). These findings are in line with datasets showing adverse effects of irradiation (4 Gy) on marker expression especially actin alpha 2, smooth muscle (ACTA2), marker of the peritubular cells in prepubertal macaque testes (Tröndle et al., 2017). An age-dependent effect of irradiation of testicular stem cells or their niches has not been yet assessed and detected in NHPs. Besides spermatogonial quantification data based on morphological evaluation and scoring, our knowledge on germ cell recovery and functional potential of the testis post irradiation is still limited (Jahnukainen et al., 2012) and should be further explored using transplantation studies in NHPs. The use of primate models is an unequivocal prerequisite for this research as access to human tissues is rather limited but the need for fertility preservation strategies is increasing (Heckmann et al., 2018b). Thus, understanding the defects and the damage done by curing life-threatening diseases is crucial in order to develop strategies to counter the loss of fertility, in particular in prepubertal patients for whom no other strategy is available. In the following section we summarize the efforts undertaken and the experimental progress in the development of approaches to preserve fertility beyond gonadotoxic treatment.
Spermatogonia-based approaches to male fertility preservation
Stem cell-based approaches have a long history and were originally of general academic interest in terms of germ cell maturation and later for transgenesis (Brinster, 2007; Reuter et al., 2012). However, it soon became obvious that these methods could serve as the basis for fertility preservation or restoration in infertile patients or those at risk of losing their fertility as a result of gonadotoxic treatment, for example, prepubertal boys who are excluded from the possibility of freezing semen for later ART. Stem cell approaches can generally be classified into the two categories, using testicular cell suspensions or intact testicular tissues as the starting material. We will review those methods briefly, describing their experimental proof of principle and their current state with regard to preclinical and clinical application (Table III and Fig. 6).
In vitro and in vivo approaches to fertility preservation.
| Method . | First used in: species/model . | Current state of success . | Therapeutic state and potential . | References . |
|---|---|---|---|---|
| Xenotransplantation of testicular tissue (ectopic and orthotopic) | Pig to mouse | Complete donor-derived spermatogenesis | NA* | Honaramooz et al. (2002); Hou et al. (2007), Kaneko et al. (2017) |
| Man to mouse | Premeiotic cells | Experimental/ex situ maturation of spermatozoa for ART, risk of retrovirus transmission and potential cancer relapse | Schlatt et al. (2003); Yu et al. (2006) | |
| Autotransplantation of testicular tissue | Macaque | Complete spermatogenesis in orthotopic transplantation | Experimental, no human data | Jahnukainen et al. (2012) |
| Marmoset | Complete spermatogenesis in orthotopic transplantation, meiotic arrest in ectopic transplantation | NA* | Luetjens et al. (2008) | |
| Germ cell transplantation | Rat to mouse, also leukemic rat model, | Complete donor-derived spermatogenesis, transition of blood born cancer | NA* | Brinster (2007); Jahnukainen et al. (2001) |
| Marmoset to mouse, | Colonization, no differentiation | NA* | Langenstroth et al. (2014) | |
| Macaque to Macaque, | Enhanced re-initiation of spermatogenesis | NA* | Schlatt et al. (2002a,b); Hermann et al. (2012) | |
| Man to mouse | Colonization, no differentiation | Experimental ex situ maturation of spermatozoa for ART, risk of retrovirus transmission | Reis et al. (2000); Schlatt et al. (1999a) | |
| In vitro culture of spermatogonial stem cells, spermatogonia, germ cells | Mouse | Isolation, characterization, enrichment and long-term survival | NA* | Kanatsu Shinohara and Shinohara (2007) |
| Pig | Isolation, characterization, enrichment and long-term survival | NA* | Zhang et al. (2017) | |
| Marmoset | Isolation, characterization, enrichment and short-term survival | NA* | Langenstroth et al. (2014) | |
| Human | Isolation, characterization, enrichment and short-term survival | Experimental, long-term culture and reliable cell lines not yet established | Kossack et al. (2013), Schneider et al. (2015); Sadri-Ardekani et al. (2009, 2011) | |
| Organ culture | Mouse | Full spermatogenesis, ART possible with sperm harvested from culture, offspring achieved | NA* | Sato et al. (2011) |
| Reconstruction of testicular tissue in vitro, conventional culture | Rat | Reassembly of somatic cells into cord like structures | NA* | Schlatt et al. (1999b); Pan et al. (2013) |
| Human | Reassembly of somatic cells into cord like structures | Experimental, ex situ maturation of spermatozoa for ART or autologous re-transplantation, no risks foreseeable | Mincheva et al. (2018); von Kopylow et al. (2018) | |
| Reconstruction of testicular tissue in vitro 3D culture systems, ‘organoids’ | Mouse, Rat | Reassembly of somatic cells into tubule-like arrangement in matrigel and collagen sponges, differentiation into morphologically mature sperm in SACS and MCS | NA* | Gassei et al. (2008); Stukenborg et al. (2008, 2009); Reuter et al. (2014), Alves-Lopes et al. (2017, 2018) |
| Human | Colonization of isolated extracellular matrices by human testicular cells | Experimental, ex situ maturation of spermatozoa for ART, no risks foreseeable | Baerts et al. (2017) |
| Method . | First used in: species/model . | Current state of success . | Therapeutic state and potential . | References . |
|---|---|---|---|---|
| Xenotransplantation of testicular tissue (ectopic and orthotopic) | Pig to mouse | Complete donor-derived spermatogenesis | NA* | Honaramooz et al. (2002); Hou et al. (2007), Kaneko et al. (2017) |
| Man to mouse | Premeiotic cells | Experimental/ex situ maturation of spermatozoa for ART, risk of retrovirus transmission and potential cancer relapse | Schlatt et al. (2003); Yu et al. (2006) | |
| Autotransplantation of testicular tissue | Macaque | Complete spermatogenesis in orthotopic transplantation | Experimental, no human data | Jahnukainen et al. (2012) |
| Marmoset | Complete spermatogenesis in orthotopic transplantation, meiotic arrest in ectopic transplantation | NA* | Luetjens et al. (2008) | |
| Germ cell transplantation | Rat to mouse, also leukemic rat model, | Complete donor-derived spermatogenesis, transition of blood born cancer | NA* | Brinster (2007); Jahnukainen et al. (2001) |
| Marmoset to mouse, | Colonization, no differentiation | NA* | Langenstroth et al. (2014) | |
| Macaque to Macaque, | Enhanced re-initiation of spermatogenesis | NA* | Schlatt et al. (2002a,b); Hermann et al. (2012) | |
| Man to mouse | Colonization, no differentiation | Experimental ex situ maturation of spermatozoa for ART, risk of retrovirus transmission | Reis et al. (2000); Schlatt et al. (1999a) | |
| In vitro culture of spermatogonial stem cells, spermatogonia, germ cells | Mouse | Isolation, characterization, enrichment and long-term survival | NA* | Kanatsu Shinohara and Shinohara (2007) |
| Pig | Isolation, characterization, enrichment and long-term survival | NA* | Zhang et al. (2017) | |
| Marmoset | Isolation, characterization, enrichment and short-term survival | NA* | Langenstroth et al. (2014) | |
| Human | Isolation, characterization, enrichment and short-term survival | Experimental, long-term culture and reliable cell lines not yet established | Kossack et al. (2013), Schneider et al. (2015); Sadri-Ardekani et al. (2009, 2011) | |
| Organ culture | Mouse | Full spermatogenesis, ART possible with sperm harvested from culture, offspring achieved | NA* | Sato et al. (2011) |
| Reconstruction of testicular tissue in vitro, conventional culture | Rat | Reassembly of somatic cells into cord like structures | NA* | Schlatt et al. (1999b); Pan et al. (2013) |
| Human | Reassembly of somatic cells into cord like structures | Experimental, ex situ maturation of spermatozoa for ART or autologous re-transplantation, no risks foreseeable | Mincheva et al. (2018); von Kopylow et al. (2018) | |
| Reconstruction of testicular tissue in vitro 3D culture systems, ‘organoids’ | Mouse, Rat | Reassembly of somatic cells into tubule-like arrangement in matrigel and collagen sponges, differentiation into morphologically mature sperm in SACS and MCS | NA* | Gassei et al. (2008); Stukenborg et al. (2008, 2009); Reuter et al. (2014), Alves-Lopes et al. (2017, 2018) |
| Human | Colonization of isolated extracellular matrices by human testicular cells | Experimental, ex situ maturation of spermatozoa for ART, no risks foreseeable | Baerts et al. (2017) |
SACS, soft agar culture system; MCS, methylcellulose system.
NA* – no therapeutic potential has been established or experimentally proven yet.
In vitro and in vivo approaches to fertility preservation.
| Method . | First used in: species/model . | Current state of success . | Therapeutic state and potential . | References . |
|---|---|---|---|---|
| Xenotransplantation of testicular tissue (ectopic and orthotopic) | Pig to mouse | Complete donor-derived spermatogenesis | NA* | Honaramooz et al. (2002); Hou et al. (2007), Kaneko et al. (2017) |
| Man to mouse | Premeiotic cells | Experimental/ex situ maturation of spermatozoa for ART, risk of retrovirus transmission and potential cancer relapse | Schlatt et al. (2003); Yu et al. (2006) | |
| Autotransplantation of testicular tissue | Macaque | Complete spermatogenesis in orthotopic transplantation | Experimental, no human data | Jahnukainen et al. (2012) |
| Marmoset | Complete spermatogenesis in orthotopic transplantation, meiotic arrest in ectopic transplantation | NA* | Luetjens et al. (2008) | |
| Germ cell transplantation | Rat to mouse, also leukemic rat model, | Complete donor-derived spermatogenesis, transition of blood born cancer | NA* | Brinster (2007); Jahnukainen et al. (2001) |
| Marmoset to mouse, | Colonization, no differentiation | NA* | Langenstroth et al. (2014) | |
| Macaque to Macaque, | Enhanced re-initiation of spermatogenesis | NA* | Schlatt et al. (2002a,b); Hermann et al. (2012) | |
| Man to mouse | Colonization, no differentiation | Experimental ex situ maturation of spermatozoa for ART, risk of retrovirus transmission | Reis et al. (2000); Schlatt et al. (1999a) | |
| In vitro culture of spermatogonial stem cells, spermatogonia, germ cells | Mouse | Isolation, characterization, enrichment and long-term survival | NA* | Kanatsu Shinohara and Shinohara (2007) |
| Pig | Isolation, characterization, enrichment and long-term survival | NA* | Zhang et al. (2017) | |
| Marmoset | Isolation, characterization, enrichment and short-term survival | NA* | Langenstroth et al. (2014) | |
| Human | Isolation, characterization, enrichment and short-term survival | Experimental, long-term culture and reliable cell lines not yet established | Kossack et al. (2013), Schneider et al. (2015); Sadri-Ardekani et al. (2009, 2011) | |
| Organ culture | Mouse | Full spermatogenesis, ART possible with sperm harvested from culture, offspring achieved | NA* | Sato et al. (2011) |
| Reconstruction of testicular tissue in vitro, conventional culture | Rat | Reassembly of somatic cells into cord like structures | NA* | Schlatt et al. (1999b); Pan et al. (2013) |
| Human | Reassembly of somatic cells into cord like structures | Experimental, ex situ maturation of spermatozoa for ART or autologous re-transplantation, no risks foreseeable | Mincheva et al. (2018); von Kopylow et al. (2018) | |
| Reconstruction of testicular tissue in vitro 3D culture systems, ‘organoids’ | Mouse, Rat | Reassembly of somatic cells into tubule-like arrangement in matrigel and collagen sponges, differentiation into morphologically mature sperm in SACS and MCS | NA* | Gassei et al. (2008); Stukenborg et al. (2008, 2009); Reuter et al. (2014), Alves-Lopes et al. (2017, 2018) |
| Human | Colonization of isolated extracellular matrices by human testicular cells | Experimental, ex situ maturation of spermatozoa for ART, no risks foreseeable | Baerts et al. (2017) |
| Method . | First used in: species/model . | Current state of success . | Therapeutic state and potential . | References . |
|---|---|---|---|---|
| Xenotransplantation of testicular tissue (ectopic and orthotopic) | Pig to mouse | Complete donor-derived spermatogenesis | NA* | Honaramooz et al. (2002); Hou et al. (2007), Kaneko et al. (2017) |
| Man to mouse | Premeiotic cells | Experimental/ex situ maturation of spermatozoa for ART, risk of retrovirus transmission and potential cancer relapse | Schlatt et al. (2003); Yu et al. (2006) | |
| Autotransplantation of testicular tissue | Macaque | Complete spermatogenesis in orthotopic transplantation | Experimental, no human data | Jahnukainen et al. (2012) |
| Marmoset | Complete spermatogenesis in orthotopic transplantation, meiotic arrest in ectopic transplantation | NA* | Luetjens et al. (2008) | |
| Germ cell transplantation | Rat to mouse, also leukemic rat model, | Complete donor-derived spermatogenesis, transition of blood born cancer | NA* | Brinster (2007); Jahnukainen et al. (2001) |
| Marmoset to mouse, | Colonization, no differentiation | NA* | Langenstroth et al. (2014) | |
| Macaque to Macaque, | Enhanced re-initiation of spermatogenesis | NA* | Schlatt et al. (2002a,b); Hermann et al. (2012) | |
| Man to mouse | Colonization, no differentiation | Experimental ex situ maturation of spermatozoa for ART, risk of retrovirus transmission | Reis et al. (2000); Schlatt et al. (1999a) | |
| In vitro culture of spermatogonial stem cells, spermatogonia, germ cells | Mouse | Isolation, characterization, enrichment and long-term survival | NA* | Kanatsu Shinohara and Shinohara (2007) |
| Pig | Isolation, characterization, enrichment and long-term survival | NA* | Zhang et al. (2017) | |
| Marmoset | Isolation, characterization, enrichment and short-term survival | NA* | Langenstroth et al. (2014) | |
| Human | Isolation, characterization, enrichment and short-term survival | Experimental, long-term culture and reliable cell lines not yet established | Kossack et al. (2013), Schneider et al. (2015); Sadri-Ardekani et al. (2009, 2011) | |
| Organ culture | Mouse | Full spermatogenesis, ART possible with sperm harvested from culture, offspring achieved | NA* | Sato et al. (2011) |
| Reconstruction of testicular tissue in vitro, conventional culture | Rat | Reassembly of somatic cells into cord like structures | NA* | Schlatt et al. (1999b); Pan et al. (2013) |
| Human | Reassembly of somatic cells into cord like structures | Experimental, ex situ maturation of spermatozoa for ART or autologous re-transplantation, no risks foreseeable | Mincheva et al. (2018); von Kopylow et al. (2018) | |
| Reconstruction of testicular tissue in vitro 3D culture systems, ‘organoids’ | Mouse, Rat | Reassembly of somatic cells into tubule-like arrangement in matrigel and collagen sponges, differentiation into morphologically mature sperm in SACS and MCS | NA* | Gassei et al. (2008); Stukenborg et al. (2008, 2009); Reuter et al. (2014), Alves-Lopes et al. (2017, 2018) |
| Human | Colonization of isolated extracellular matrices by human testicular cells | Experimental, ex situ maturation of spermatozoa for ART, no risks foreseeable | Baerts et al. (2017) |
SACS, soft agar culture system; MCS, methylcellulose system.
NA* – no therapeutic potential has been established or experimentally proven yet.
SSC-based experimental strategies for male fertility restoration and preservation. The schematic diagram depicts approaches that are based on testicular cell suspensions (top) and testicular tissue fragments (bottom). Cellular approaches involve the following methods: Reprogramming of iPSC to germ cells; SSC isolation, propagation and differentiation by autotransplantation; reconstruction of seminiferous tubules via testicular cells using 2D or 3D organoid structures (with matrix support). Tissue-based approaches preserve the structural integrity of the seminiferous epithelium and include the following methods: Isolation and culture of seminiferous tubules via a perfusion-based non-static microfluidic system; culture of testicular fragments via an agarose or membrane-based static-culture system, and autotransplantation of testicular fragments. Generated spermatozoa could be later used for fertilization by ART or via natural conception.
The isolation and expansion of SSC for subsequent germ cell transplantation is the only approach which could potentially lead to a restoration of fertility enabling a natural pregnancy. The intra-testicular germ cell transplantation method was developed in the 1990s. Here, isolated donor germ cells are transplanted into the host’s seminiferous tubules where they recolonize niches that had been emptied of host germ cells because of mutation or by treatment (Brinster, 2007). Initial experiments were extremely successful. For example, demonstrating donor-derived germ cell maturation to fertile spermatozoa when transplanted between murine strains and species, and the method was developed further into an assay for SSC potential (Brinster, 2007; Table III). However, apart from the imminent risks—retrovirus transmission and potential cancer relapse—the method failed to allow primate gametes (including human) to mature in a mouse testis. There are few publications and all show a colonization by primate spermatogonia only and those were maintained but failed to differentiate (e.g. baboon, marmoset and human) (Reis et al., 2000; Schlatt et al., 1999a; Nagano et al., 2001; Langenstroth et al., 2014; Table III). Conclusively, this method of xenologous intra-testicular germ cell xenotransplantation is still experimental after almost 3 decades and, likely, will never reach the preclinical or clinical stage. However, there have been initial experiments using this transplantation approach autologously in macaques, which show that after irradiation the re-transplantation of the donor’s spermatogonia supported and improved spermatogenic recovery (Schlatt et al., 2002a; Hermann et al., 2012; Table II). In another germ cell transplantation experiment in macaques, the impact of hormone suppression on the effect of cytotoxic therapy was tested. The authors could demonstrate that hormone suppression enhanced spermatogenic recovery from transplanted SSC in primates, a finding with putative clinical meaning once applied to patients (Shetty et al., 2013). Although data on humans are lacking so far, germ cell transplantation might become the basis for a therapeutic route once the risk of any cancer relapse can be excluded by proper sorting methods. Importantly, propagation of human SSC also needs to be achieved to obtain sufficient cell numbers for germ cell transplantation and subsequent recolonization of seminiferous tubules. Isolation and propagation of SSC under various culture conditions is feasible in mice (Kanatsu Shinohara and Shinohara, 2007), pigs (Zhang et al., 2017) and also SSC from marmosets and humans were able to survive in culture. The presence of SSC in these cultures was confirmed by germ cell transplantation assays using mice with germ cell-depleted testes as recipients, demonstrating the ability of cultured SSCs to colonize niches at the basement membrane (Sadri-Ardekani et al., 2009, 2011; Langenstroth et al., 2014). Employing these culture approaches (Kossack et al., 2013; Schneider et al., 2015), the stability of the epigenetic profile in vitro could also be demonstrated (Langenstroth-Röwer et al., 2017) and first insights into the transcriptional properties of adult human spermatogonia were gained (Neuhaus et al., 2017). However, there is still the need to further develop culture conditions that support the propagation of human spermatogonia for possible clinical application (Table III).
Another approach using testicular cell suspensions as the starting material makes use of the ability of testicular somatic cells to reassemble into 3D arrangements. It was shown that these processes work even better when the culture conditions include matrices (e.g. Matrigel, soft agar culture system (SACS) or methylcellulose system (MCS); Gassei et al., 2008; Stukenborg et al., 2008, 2009) or scaffolds (e.g. collagen sponges or decellularized connective tissues; Reuter et al., 2014; Baerts et al., 2017) mimicking the testicular extracellular environment as a surrogate structure. In contrast to conventional cultures, the reassembly is not inverted in such scaffolds. Differentiation of murine cells was demonstrated in 3D matrices up to morphologically normal sperm, however in another experiment using collagen sponges, rat testicular cells formed tubule-like structures but the germ cells did not differentiate (Reuter et al., 2014). Using extracellular matrices derived from connective tissue, somatic cells formed organoids but germ cell differentiation also failed. Recently, aggregation or organoid models for maturation of gametes or male and female germ cells have been reported (Hayashi et al., 2012; Ishikura et al., 2016). In these studies pluripotent precursors were used to derive PGC-like cells in vitro. These PGC-like cells could then be differentiated by transfer into fetal gonadal tissues, which restructure into testes or ovaries. These models reveal the crucial importance of microenvironments to instruct PGC into the correct fate and into somatic cell guided gamete development. This approach holds great promise for unraveling the regulatory aspects of germ cell development and differentiation. Future studies will unveil whether sperm can also be derived from pluripotent human cells, including ESC or iPSC.
Remaining SSC-based approaches employ testicular tissue fragments as the starting material considering that germ cells need the company of their somatic partners, i.e. Sertoli and eventually also peritubular and Leydig cells. These considerations resulted in co-culture approaches, be it as organ culture (testicular fragments) employing static (agarose base) and non-static (microfluidics) culture systems, or as co-culture of germ and somatic cells in the presence of hormones. One of the major early insights of such experiments was that the germ cells not only need the presence of the somatic fraction to colonize and differentiate but also seemingly prefer a spatial environment in which they can reassemble three-dimensionally (Stukenborg et al., 2009; Reuter et al., 2012). Thus, it was not surprising that the most successful outcome was achieved by Sato et al. (2011) when fragments of immature mouse testis tissue were developed in an organ culture where they fully differentiated and produced sperm, which were then used for ICSI and resulted in normal healthy offspring. To date, this approach has only been successful in mice. Reasons for this include that it is relatively easy to obtain sufficient testicular tissues at the appropriate age from mice. To translate this approach to the human however, there is currently still a lack of available tissue, hampering the optimization of the method. Thus, experiments with primate tissues are needed in order to achieve similar results as were obtained in mice. Once this barrier can be passed the (likely quite different) needs of cultured somatic and germ cells will be understood better and these approaches might then open a route for successful human in vitro spermatogenesis in a reasonably short time frame.
Finally, the transplantation of testicular tissue fragments has been considered as an approach for the differentiation of immature germ cells. Inter-species transplantation of immature testis tissues resulting in full spermatogenesis and mature donor-derived spermatozoa being obtained from the ectopic transplants was first reported in a piglet (donor) to mouse (host) experiment (Honaramooz et al., 2002). This approach was then repeated in pig (Kaneko et al., 2017) and various other species including mouse, hamster, bovine and monkeys (Schlatt et al., 2002a,b; Wistuba et al., 2004; Rathi et al., 2005, 2008; Liu et al., 2016), primarily in order to preserve the male germ line in endangered species but finally also in a man to mouse approach. Xenografting of human testicular tissues, which would be an option for fertility preservation, was not fully successful and ended in premeiotic/meiotic arrest of the germ cells (Schlatt et al., 2003; Yu et al., 2006). However, these results are still preliminary as human material is rare and studies still limited. It should be mentioned that such xenotransplantation approaches are not free of risk, for example it cannot yet be excluded that host retroviruses could be transferred into the donors’ genome. More important, there might always be a chance that cancer cells would be retransmitted when the transplants are re-transplanted into the donor, a route originally planned. Using a rat leukemia model it was demonstrated that grafting resulted in a full transmission of cancer into the mouse hosts (Hou et al., 2007). Transmission of cancer was also reported after testicular cell transplantation from leukemic rats (Jahnukainen et al., 2001). As the approach of xenografting failed in achieving complete spermatogenesis in New World primates (Schlatt et al., 2002a,b; Wistuba et al., 2004; Sharma et al. 2018) and humans (Schlatt et al. 2003; Yu et al. 2006) autologous grafting was developed, a route which would anyhow be superior for later clinical use. Autografting was successfully performed in macaques (Jahnukainen et al., 2012) and marmosets (Luetjens et al., 2008); interestingly showing that the site of transplantation seems to be crucial, as ectopic transplants failed, while orthotopic ones produced spermatozoa. Human data are still lacking, rendering the method still experimental, a fact that is (apart from technical issues) also related to the ethical implications, which are expanded upon below.
When summarizing the current barriers to using spermatogonia in a clinical context, it requires mentioning that after almost a century of experimentation it has become evident that for successful in vitro spermatogenesis to occur specific conditions need to be mimicked in a culture dish. Clinically, the efficient maturation of a sufficient number of germ cells would solve the issue of fertility preservation. What we do know so far is that the germ line appears to require a 3D surrounding and the support of co-cultured somatic testicular cells, which provide the niche under natural conditions. The endocrine milieu should also play a role, yet currently available data is inconsistent and this issue remains to be fully resolved. In this context it is of note that somatic cells require relatively poor culture media whilst germ cells need richer ones. This will likely necessitate a staggered, differential culture protocol, starting with the seeding of somatic cells followed by addition of germ cells later on and paralleled by a change of culture conditions (Reuter et al., 2012). Conventional cultures of rodent and human germ and somatic cells resulted in rearrangement processes that resembled cord formation in which Sertoli and peritubular cells interact with each other and form structures which can be seen as inverted seminiferous tubules (Schlatt et al. 1999b; Pan et al., 2013; Mincheva et al., 2018; von Kopylow et al., 2018). Considering the complexity of the interaction between the niche and germ line as well as the need for external regulation of differentiation processes it is not surprising that the differentiation ability of male germ cells in vitro is limited. It is also not surprising that the only proof of principle studies (in rodents) so far were conducted either in 3D matrices (Stukenborg et al., 2008, 2009; Alves-Lopes et al., 2017, 2018) or in organ culture (Sato et al., 2011). As access to immature human testicular tissues is highly limited, hampering systematic studies, experiments with primate tissues are needed to achieve similar results as in mice. Employing these primate tissues may then open a route for successful human in vitro spermatogenesis in a reasonable time.
Ethical reflections on aspects concerning fertility preservation in boys
Fertility preservation in children is a good example of conflicts that have to be balanced in an ethical discourse. A prepubertal individual suffering from a life-threatening disease has to be cured by therapies that jeopardize, if not destroy, his or her chance to build up a family with their own children. Thus, the survival of the patients has to be ensured but their fertility is the price paid, which constitutes a dilemma. Consequently, the development of therapeutic options for infertility is an ethical issue for reproductive medicine, aiming to reverse a severe side effect induced by medical treatment. However, even once protocols for reliable and successful germ line differentiation are established, the ethical and legal questions, so far overlooked or potentially underestimated, need to be addressed. One question may be the following: ‘Who is allowed to decide on the use of cryopreserved tissues in the case where the patient does not survive the disease? Can this decision be made by the parents, the clinician in charge or an independent ethics committee?’ If the former is the case, parents would be confronted with the decision to kill the germ line of their beloved child.
Finally, once germ cells can be differentiated outside the body, technical options for their genetic manipulation will become available at some point in the future. Those techniques will lead to debate over the extent to which such a manipulation is ethically still justifiable (e.g. when a genetic defect in the germ line can be cured) or becomes unethical (e.g. when genes are manipulated for reasons other than disease). In the framework of this review it is impossible to describe and discuss the numerous ethical scenarios that should be considered in terms of ex situ/in vitro germ line maturation for fertility preservation. However, because of the increasing likelihood that solutions for fertility preservation and germ cell maturation will become feasible in the near future, there is an urgent need to start the ethical debate now and to develop appropriate guidelines.
Conclusion
In the introductory remarks of this review we referred to Clermont’s statement proposing that there might be more than the classical Adark and Apale spermatogonia and that knowledge on the human SSC system was too limited. During the past 50 years, we have obtained more insight regarding the features of spermatogonia largely by applying novel experimental approaches and molecular methods. Yet, the SSC as well as its niche remain largely undefined. However, what we know from single-cell expression and lineage-tracing analyses is that spermatogonia are a heterogeneous and plastic population of undifferentiated germ cells, confirming the statement of Clermont. Moreover, we have learned that the niche is the regulatory unit for stem cell fate and that spermatogonia are adapted to the individual reproductive lifespan. Finally, the life-long sperm output of a spermatogonium appears to be balanced against the duration of a generation (i.e. the risk of jeopardizing genome integrity is balanced against a maximized sperm output and this might have led to the direct and progenitor-buffered stem cell systems to function in a non-hierarchical, non-linear way). Concluding these observations, it is not surprising that a system requiring such concerted regulatory action is particularly sensitive to damage caused by therapies for malignant diseases and that preserving male fertility is an enormous challenge. Further research is desperately needed in the field to gain insight into the existence of spermatogonial subpopulations throughout human testicular development and to establish spermatogonia-based approaches for fertility preservation—before another 50 years have passed by.
Authors’ roles
All authors contributed to drafting the article and critical discussion.
Funding
Funding was provided by the FP7 program of the EU as a Marie Curie International Training Program, Growsperm (FP7-PEOPLE-2013-ITN 603568 GROWSPERM) and the German research foundation (Clinical Research Unit CRU326 ‘Male Germ Cells’: DFG CRU SCHL 394/15-1; CRU NE 2190/3-1).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I.