-
PDF
- Split View
-
Views
-
Cite
Cite
Giovanni Coticchio, Cristina Lagalla, Roger Sturmey, Francesca Pennetta, Andrea Borini, The enigmatic morula: mechanisms of development, cell fate determination, self-correction and implications for ART, Human Reproduction Update, Volume 25, Issue 4, July-August 2019, Pages 422–438, https://doi.org/10.1093/humupd/dmz008
Close - Share Icon Share
Abstract
Assisted reproduction technology offers the opportunity to observe the very early stages of human development. However, due to practical constraints, for decades morphological examination of embryo development has been undertaken at a few isolated time points at the stages of fertilisation (Day 1), cleavage (Day 2–3) and blastocyst (Day 5–6). Rather surprisingly, the morula stage (Day 3–4) has been so far neglected, despite its involvement in crucial cellular processes and developmental decisions.
The objective of this review is to collate novel and unsuspected insights into developmental processes occurring during formation of the morula, highlighting the key importance of this stage for a better understanding of preimplantation development and an improvement of ART.
PubMed was used to search the MEDLINE database for peer-reviewed English-language original articles and reviews concerning the morula stage in mammals. Searches were performed by adopting ‘embryo’, ‘morula’, ‘compaction’, ‘cell fate’ and ‘IVF/assisted reproduction’ as main terms, in association with other keywords expressing concepts relevant to the subject (e.g. cell polarity). The most relevant publications, i.e. those concerning major phenomena occurring during formation of the morula in established experimental models and the human species, were assessed and discussed critically.
Novel live cell imaging technologies and cell biology studies have extended our understanding of morula formation as a key stage for the development of the blastocyst and determination of the inner cell mass (ICM) and the trophectoderm (TE). Cellular processes, such as dynamic formation of filopodia and cytoskeleton-mediated zippering cell-to-cell interactions, intervene to allow cell compaction (a geometrical requisite essential for development) and formation of the blastocoel, respectively. At the same time, differential orientation of cleavage planes, cell polarity and cortical tensile forces interact and cooperate to position blastomeres either internally or externally, thereby influencing their cellular fate. Recent time lapse microscopy (TLM) observations also suggest that in the human the process of compaction may represent an important checkpoint for embryo viability, through which chromosomally abnormal blastomeres are sensed and eliminated by the embryo.
In clinical embryology, the morula stage has been always perceived as a ‘black box’ in the continuum of preimplantation development. This has dictated its virtual exclusion from mainstream ART procedures. Recent findings described in this review indicate that the morula, and the associated process of compaction, as a crucial stage not only for the formation of the blastocyst, but also for the health of the conceptus. This understanding may open new avenues for innovative approaches to embryo manipulation, assessment and treatment.
Introduction
In assisted reproduction technology (ART), embryos are routinely assessed and selected for transfer at isolated time points on Day 2, 3 and, in case of culture to the blastocyst stage, Day 5/6. Embryos may also be observed, but are rarely selected, on Day 4, since embryo transfer (ET) at this stage does not imply the practical advantages of transfer on Days 2–3 (reduced costs and management) or Day 5/6 (higher self-selection and increased cycle efficiency). Day 4 ET is also hindered by difficulties in assessing accurately the morphology of embryos at this stage of development.
In normal undisturbed development, Day 4 embryos have already started, and have often completed, the transition from the cleavage stages, in which blastomeres appear as well-distinct approximately spherical units, to the compacted morula stage, characterised instead by tightly interconnected cells with ill-defined margins, localised either internally or externally in the embryo (http://atlas.eshre.eu). At the sub-cellular level, this phase of development is also when the blastomeres undergo cell polarisation. Cells localised internally are characterised by a nucleus positioned approximately centrally and organelles and other specialised structures distributed in the cell environment without an apparent pattern. Cells distributed externally respond instead to a more defined pattern: the nucleus is positioned basally, cell adhesion structures and stable acetylated microtubules are localised baso-laterally, while more dynamic microtubules, actin filaments and microvilli are found in the apical region. The regulation of the process of polarisation remains unclear, with significant efforts on unpicking the origin of cell polarity in mammalian embryos. Furthermore, the chromosomal status of the Day 4 embryo has received little attention, despite the hypothesis that during development from the cleavage to the blastocyst stage, mosaic embryos can implement mechanisms of self-correction to reduce the aneuploidy load.
Being a developmental phase less amenable to observation and morphological classification, knowledge of the human embryo on Day 4 is limited compared to other stages of development, especially concerning the relationship between morphology and developmental ability. Consequently, this review will highlight the relevance of the morula relative to developmental processes that occur during preimplantation development and discuss downstream implications of such processes for practices used in human ART.
Methods
PubMed was systematically searched for peer-reviewed original articles and reviews identified by relevant keywords, such as ‘embryo’, ‘blastomere’, ‘morula’, ‘compaction’, ‘blastocyst’, ‘blastocoel’, ‘cell fate’, ‘cell lineage’, ‘cell determination’, ‘cell polarity’, ‘cell junctions’, ‘IVF’, ‘assisted reproduction’, ‘inner cell mass’ and ‘trophectoderm’. Keywords were used in multiple and overlapping combinations in order to identify those publications strictly relevant to the morula. Additional studies were identified by thorough analysis of reference lists from relevant publications. The ‘English language’ limit was applied. The most relevant publications, i.e. those concerning major phenomena occurring during the developmental morula stage were assessed and discussed critically to offer a comprehensive description of the process bridging early segmentation and the blastocyst. Concerning animal studies, priority was given to publications relevant to species more consistently used as experimental models for the human.
The morula stage in the continuum of preimplantation development
The crucial phases of preimplantation development have been described in meticulous detail; Day 1 (fertilisation and first cleavage), Days 2–3 (second and third rounds of cleavage) and Days 5–7 (blastocyst stage) of human development have been assessed morphologically in thousands of studies. In contrast, the morula stage has received astonishingly little attention, as illustrated by an authoritative source describing the morula simply as ‘an indistinguishable mass of cells on Day 4 of development’ (ESHRE Atlas of Human Embryology). In the human, compaction typically occurs usually between the 8- and 10-cell stage, although sporadically can be observed as early as the 4-cell stage (Iwata et al., 2014). Qualitative assessment of the morula also appears rather vague; a ‘good quality morula’ is defined as ‘composed of 16–32 blastomeres and all the blastomeres should be included in the compaction process’ (ESHRE Atlas of Human Embryology). Exclusion of one or more cells from the compacted morula is thought to be associated with reduced developmental competence (Ebner et al., 2009). However, the morula remains poorly described. This is due in part to the lack of well-defined morphological markers that can be considered typical of this stage. In addition, compaction and formation of the morula are dynamic processes, difficult to assess in IVF routine practice if observation is carried out at isolated time points. Nevertheless, overlooking this ‘Cinderella’ stage of development is naïve, since recent data indicate that the morula is pivotal for blastocyst formation, establishment of the first cell lineages and, therefore, the entire developmental process. This role is underpinned by molecular, metabolic and cellular changes, which are temporally and spatially regulated, and a transition from a group of individual cells to a coordinated, responsive structure (Brison et al., 2014). Stage-specific modulations of gene expression and metabolism are indicative of such changes.
The early stages of embryo development are largely under the molecular control of maternally-inherited mRNAs and regulatory proteins stockpiled throughout oogenesis. The function of genes, referred to as ‘maternal effect genes’, encoding for such regulators was initially ascertained in invertebrates and lower vertebrates, but knowledge of their action in mammals, including the human, is emerging (Condic, 2016). This initial maternal control of development is gradually superseded as the embryo expresses its own genes. The first studies in the human reported that embryonic genome activation (EGA) began at 4 to 8-cell stage (Braude et al., 1988), but details of the overall dynamics of gene expression during preimplantation development remained unclear for several years. More recently, sophisticated DNA technologies have revealed a more detailed and complex picture of EGA, which has relevance for the morula stage. Briefly, we now know that the first signs of transcription in the human embryonic genome are detectable at the 2-cell stage, much earlier than previously thought. Notably, analysis of the whole preimplantation period showed that EGA occurs in a multiphasic fashion at designated stages: a pattern seen mirrored in a range of mammalians embryos (Svoboda et al., 2015). A major wave of transcription activation occurs at the 4–6 cell stage, involving mainly genes with a role in the translation machinery. This sets the stage for the two following and more extensive transcriptional bursts, which take place towards the end of Day 3 of development (from the 8–10 cell stage interval), i.e. shortly before compaction of the morula (Vassena et al., 2011). Many genes transcribed in these phases encode for proteins related to the metabolism of lipids, proteins, amino acids and carbohydrates. Embryo compaction is therefore preceded by changes in gene expression that are suggestive of major shifts in embryo metabolism.
Indeed, it is well documented both in model species and the human that during preimplantation development, metabolism undergoes important phase-dependent modifications in response to specific requirements of the embryo (Leese, 2012). During the early cleavage stages, the embryo is comparatively quiescent (Smith and Sturmey, 2013). Cell divisions occur at a moderate pace, net mass does not increase and most of the energy requirements are focused on general homoeostatic functions, with limited need for biosynthetic processes. Consequently, metabolic needs, in terms of ATP during the cleavage stages, are satisfied largely from low levels of oxidation of pyruvate and lactate, which are abundantly present in the surrounding environment (Smith and Sturmey, 2013). In addition, a high ATP:ADP ratio negatively regulates the flux of glucose through the glycolytic pathway prior to compaction (Rozell et al., 1992). This picture changes in the second half of preimplantation development, corresponding to a period when gene transcription is more sustained, the rate of cell division increases and embryo mass increases. Importantly, considerable energy is invested in Na+/K+ ATPase, an enzyme needed for an active transport of those ions from the surrounding environment to intercellular spaces (Martin and Leese, 1999; Houghton et al., 2003). A high ion concentration in the intercellular space facilitates movement of water down a concentration gradient from the extra-embryonic environment, leading ultimately to the formation of the blastocoel (Watson and Barcroft, 2001). The energy demands for such activities are satisfied by a marked increase in metabolic function. The rate of oxygen consumption rises significantly, as a range of metabolic fuels including pyruvate, glutamine and fatty acids are oxidised (Houghton et al., 1996; ,Thompson et al., 1996; Houghton et al., 2003; Sturmey and Leese, 2003; Lopes et al., 2005). In parallel, there is a marked, significant rise in glucose consumption via aerobic glycolysis (Gardner et al., 2001; Krisher and Prather, 2012). Importantly, aerobic utilisation of glucose can support multiple biosynthetic pathways, as well as providing ATP (Krisher and Prather, 2012). In addition to aerobic glycolysis, the increased uptake of glucose is important for sustaining the Pentose Phosphate Pathway, essential for the provision of ribose sugars necessary for nucleotide synthesis (Smith and Sturmey, 2013). Crucially, genes whose products, such as the pyruvate dehydrogenase complex, functionally link glycolysis to the activity of the oxidative TCA cycle, may be regulated at the transcriptional level and expressed in a timely fashion. Therefore, as the embryo evolves from a low-energy, low activity, almost automatic system to one that is highly energetic, highly proliferative and developmentally active, the morula stage represents a key regulatory landmark during which a pyruvate-based metabolism characterised by low oxidative levels gives way to a more active aerobic utilisation of glucose, combined with other metabolic fuels, in response to modified developmental needs. Unfortunately, detailed knowledge of metabolism of the morula per se is sparse, compared to the wealth of data on cleavage and blastocyst stage embryos, mostly due to the challenges described above.
Inner/outer cells and morula geometry
During preimplantation development, the cells of the embryo make their first developmental decision, i.e. whether to contribute to the formation of the inner cell mass (ICM) or the trophectoderm (TE) of the blastocyst. After implantation, the former will develop mainly into the soma of the future organism, while the latter will give raise to extra-embryonic tissues supporting the embryo proper. Classical studies carried out in the mouse have suggested the concept (referred to as inside-outside theory) that, as cleavage progresses, blastomeres that become totally internalised within the structure of the embryo will exclusively form the ICM, while blastomeres that remain external and exposed to the surrounding environment will contribute mainly to the TE. These conclusions were drawn by simple, but ingenious, experiments in which outer cells of 16-cell mouse embryos were labelled with fluorescent microparticles and labelling patterns of ICM and TE were observed following development of morulae into blastocysts (Fleming, 1987). However, the precise timing and mechanism of the fate decision remains unclear and answers to the questions of how blastomeres acquire an inner or outer position, how inner and outer cells become different and how positional cues are translated into developmental decisions still elude us. In a fascinating recent study, Biase et al. (2018) used an RNASeq approach, combined with cellular barcoding, to indicate that lineage differences were apparent as early as the two-cell stage in mouse embryos, indicating that the fate of the morula may be determined after the first cleavage. The morula and the process of compaction are particularly relevant to such questions, because they represent the functional manifestation of repositioning the inner and outer cells (as discussed below).
The creation of the morula presents a geometrical challenge, in which cells are required to distinctly occupy internal or external positions. Up until the third round of divisions, all cells (up to eight) are approximately spherical and are all exposed to the surrounding environment, in addition to being in contact with each other. During the fourth round of cleavage, cell number increases from 8 to 16, and, on average, five blastomeres become positioned in the interior of the mouse embryo (Morris et al., 2010). If blastomeres were to retain a spherical shape and remain in mutual contact, for mere geometrical rules (the Newton’s kissing number), only a single cell would find allocation internally in the embryo at the 13-cell stage (White et al., 2017). Beyond such a stage, the addition of further cells in the interior of the embryo would be impossible while maintaining blastomere and embryonic sphericity and cell-to-cell contact. In the mouse, compaction occurs normally between the 8- and 16-cell stage, concomitantly with the formation of inner and outer cells. Cell compaction is therefore the geometrical, functionally-essential, solution that permits the development of populations of inner and outer cells in a definite proportion and to preserve its overall shape of a homogeneous sphere within the constraints of the zona pellucida. In the majority of cases in the human, compaction also occurs during the same developmental interval (Iwata et al., 2014), although the overall process of commitment of inner and outer cells in the formation of ICM and TE, respectively, is less certain (see below).
The forces that shape the morula in the process of compaction
Geometry is however only one of the several obstacles that the developing embryo has to overcome to ensure the generation of two cell lines. One of the most topical questions that concern the morula stage and indeed preimplantation development revolves around the forces that make compaction possible. Until the 8-cell stage, cleavage unfolds apparently uneventfully. Compaction marks a dramatic change in cell behaviour and shape, so profound that it can be observed by a simple transmitted light microscope. At compaction, blastomeres lose their sphericity to acquire a rather flattened epithelial-like shape in which cell contour is difficult to discriminate. This has prompted the hypothesis that cellular specialisations that ensure cell-to-cell contact and adhesion play an essential role in compaction. Early investigations on the Ca2+-dependent cell adhesion molecule E-cadherin were consistent with this hypothesis. In the mouse, E-cadherin is uniformly distributed in the cell membrane during the first cleavages but from the 8-cell stage, its localisation becomes restricted to intercellular adherens junctions, also referred to as zonulae adherens (Vestweber et al., 1987), which ensure mutual lateral adhesion between epithelial cells and contribute to the maintenance of epithelial cell polarity (Table I). In the human, E-cadherin follows a similar pattern of redistribution, with a preferential localisation in adherens junctions from Day 3.5 to 4.0 of development (Campbell et al., 1995). The initial finding that mouse compacted morulae exposed to functionally interfering anti-E-cadherin antibodies undergo decompaction (Vestweber and Kemler, 1985) inspired subsequent gene targeting studies. Indeed, mouse morulae homozygous for a null mutation of the E-cadherin-encoding gene were unable to persist in a compacted state, progressively losing cohesiveness and reverting to a decompacted morphology, with cells still able to cleave but not mutually adhesive and unable to organise themselves into blastocyst (Riethmacher et al., 1995). One explanation for this is that the early embryo is endowed with E-cadherin molecules of maternal origin that can initiate compaction, but new, zygotic-derived E-cadherin is necessary to complete the process. Interestingly, loss of E-cadherin function and compaction is accompanied by intracellular alterations, particularly the loss of cell polarity (Riethmacher et al., 1995). However, compaction and cell polarity are not strictly mutually dependent because under specific experimental conditions, one process can occur in the absence of the other (Stephenson et al., 2010).
Schematic description of function of proteins in key developmental processes that characterise the morula stage.
| Protein . | Developmental role . | References . |
|---|---|---|
| Contribute to the formation of adherens junctions (zonulae adherens), which ensure mutual lateral adhesion between epithelial cells, thus contributing to the maintenance of epithelial cell polarity | Vestweber et al. (1987); Haegel et al. (1995); Riethmacher et al. (1995); Campbell et al. (1995); Torres et al. (1997); Perez-Moreno et al. (2003). |
| Contribute to the organisation and function of filopodia, which extend between adjacent blastomeres and determine cell flattening during compaction | Fierro-González et al. (2013). |
| E-cadherin | Intervenes in the formation of tight junctions (zonulae occludens), which create belt-like impermeable structures located at the upper-lateral sides of adjacent outer cells | Eckert and Fleming (2008). |
| Cooperate to lead to the formation of an actomyosin ring localised at the margins of adjacent cells. This ring produces the tension forces required for the zippering mechanism that acts in coordination with cell junctions to seal the intercellular contact between outer cells | Zenker et al. (2018). |
| Myosin II | Generates tensile forces at cell borders and constriction of the apical domain, by which blastomeres are internalised | Samarage et al. (2015). |
| Cooperate in the formation of a regulatory mechanism involving signalling of Hippo for the phosphorylation and consequent cytoplasmic localisation of Yap. Absence of nuclear localisation of Yap prevents the activation of the transcription regulator TEAD. Inactivation of Hippo leads to localisation of dephosphorylated Yap in the nucleus and activation of TEAD | Ota and Sasaki (2008); Zhao et al. (2008); reviewed in Harvey et al. (2013); Nishioka et al. (2009); Hirate et al. (2012). |
| TEAD | Upon activation by unphosphorylated Yap, TEAD initiates the transcription of several effector genes, among which those that determine the trophectoderm cell fate (e.g. Cdx2 and Gata3) | Yagi et al. (2007); Nishioka et al. (2008); Nishioka et al. (2009); Ralston et al. (2010). |
| Interact mutually to establish apical (Par-aPCK) and baso-lateral domains in outer cells | Suzuki and Ohno (2006). |
| Par3-Par6-aPKC (Par-aPCK) | Inhibit the Hippo pathway, which acts against the specification of trophectoderm characteristics | Hirate et al. (2013). |
| Cooperate to activate Hippo | Hirate et al. (2013); Leung and Zernicka-Goetz (2013) |
| Once expressed as an effect of positive regulation by TEAD, induce the expression of trophectoderm characteristics. | Jedrusik et al. (2008); Ralston and Rossant (2008); Ralston et al. (2010) |
| Act as apical regulators for the maintenance of cell pluripotency | Chambers et al. (2003); Takahashi and Yamanaka (2006); Okita et al. (2007) |
| Are regulated by Oct4 and Sox2, contributing to the preservation of pluripotency | Ambrosetti et al. (1997); Okuda et al. (1998); Cauffman et al. (2009). |
| Protein . | Developmental role . | References . |
|---|---|---|
| Contribute to the formation of adherens junctions (zonulae adherens), which ensure mutual lateral adhesion between epithelial cells, thus contributing to the maintenance of epithelial cell polarity | Vestweber et al. (1987); Haegel et al. (1995); Riethmacher et al. (1995); Campbell et al. (1995); Torres et al. (1997); Perez-Moreno et al. (2003). |
| Contribute to the organisation and function of filopodia, which extend between adjacent blastomeres and determine cell flattening during compaction | Fierro-González et al. (2013). |
| E-cadherin | Intervenes in the formation of tight junctions (zonulae occludens), which create belt-like impermeable structures located at the upper-lateral sides of adjacent outer cells | Eckert and Fleming (2008). |
| Cooperate to lead to the formation of an actomyosin ring localised at the margins of adjacent cells. This ring produces the tension forces required for the zippering mechanism that acts in coordination with cell junctions to seal the intercellular contact between outer cells | Zenker et al. (2018). |
| Myosin II | Generates tensile forces at cell borders and constriction of the apical domain, by which blastomeres are internalised | Samarage et al. (2015). |
| Cooperate in the formation of a regulatory mechanism involving signalling of Hippo for the phosphorylation and consequent cytoplasmic localisation of Yap. Absence of nuclear localisation of Yap prevents the activation of the transcription regulator TEAD. Inactivation of Hippo leads to localisation of dephosphorylated Yap in the nucleus and activation of TEAD | Ota and Sasaki (2008); Zhao et al. (2008); reviewed in Harvey et al. (2013); Nishioka et al. (2009); Hirate et al. (2012). |
| TEAD | Upon activation by unphosphorylated Yap, TEAD initiates the transcription of several effector genes, among which those that determine the trophectoderm cell fate (e.g. Cdx2 and Gata3) | Yagi et al. (2007); Nishioka et al. (2008); Nishioka et al. (2009); Ralston et al. (2010). |
| Interact mutually to establish apical (Par-aPCK) and baso-lateral domains in outer cells | Suzuki and Ohno (2006). |
| Par3-Par6-aPKC (Par-aPCK) | Inhibit the Hippo pathway, which acts against the specification of trophectoderm characteristics | Hirate et al. (2013). |
| Cooperate to activate Hippo | Hirate et al. (2013); Leung and Zernicka-Goetz (2013) |
| Once expressed as an effect of positive regulation by TEAD, induce the expression of trophectoderm characteristics. | Jedrusik et al. (2008); Ralston and Rossant (2008); Ralston et al. (2010) |
| Act as apical regulators for the maintenance of cell pluripotency | Chambers et al. (2003); Takahashi and Yamanaka (2006); Okita et al. (2007) |
| Are regulated by Oct4 and Sox2, contributing to the preservation of pluripotency | Ambrosetti et al. (1997); Okuda et al. (1998); Cauffman et al. (2009). |
These include compaction, intercellular sealing to allow blastocoel formation, positioning of inner and outer cells, establishment of cell polarity and cell fate determination.
Schematic description of function of proteins in key developmental processes that characterise the morula stage.
| Protein . | Developmental role . | References . |
|---|---|---|
| Contribute to the formation of adherens junctions (zonulae adherens), which ensure mutual lateral adhesion between epithelial cells, thus contributing to the maintenance of epithelial cell polarity | Vestweber et al. (1987); Haegel et al. (1995); Riethmacher et al. (1995); Campbell et al. (1995); Torres et al. (1997); Perez-Moreno et al. (2003). |
| Contribute to the organisation and function of filopodia, which extend between adjacent blastomeres and determine cell flattening during compaction | Fierro-González et al. (2013). |
| E-cadherin | Intervenes in the formation of tight junctions (zonulae occludens), which create belt-like impermeable structures located at the upper-lateral sides of adjacent outer cells | Eckert and Fleming (2008). |
| Cooperate to lead to the formation of an actomyosin ring localised at the margins of adjacent cells. This ring produces the tension forces required for the zippering mechanism that acts in coordination with cell junctions to seal the intercellular contact between outer cells | Zenker et al. (2018). |
| Myosin II | Generates tensile forces at cell borders and constriction of the apical domain, by which blastomeres are internalised | Samarage et al. (2015). |
| Cooperate in the formation of a regulatory mechanism involving signalling of Hippo for the phosphorylation and consequent cytoplasmic localisation of Yap. Absence of nuclear localisation of Yap prevents the activation of the transcription regulator TEAD. Inactivation of Hippo leads to localisation of dephosphorylated Yap in the nucleus and activation of TEAD | Ota and Sasaki (2008); Zhao et al. (2008); reviewed in Harvey et al. (2013); Nishioka et al. (2009); Hirate et al. (2012). |
| TEAD | Upon activation by unphosphorylated Yap, TEAD initiates the transcription of several effector genes, among which those that determine the trophectoderm cell fate (e.g. Cdx2 and Gata3) | Yagi et al. (2007); Nishioka et al. (2008); Nishioka et al. (2009); Ralston et al. (2010). |
| Interact mutually to establish apical (Par-aPCK) and baso-lateral domains in outer cells | Suzuki and Ohno (2006). |
| Par3-Par6-aPKC (Par-aPCK) | Inhibit the Hippo pathway, which acts against the specification of trophectoderm characteristics | Hirate et al. (2013). |
| Cooperate to activate Hippo | Hirate et al. (2013); Leung and Zernicka-Goetz (2013) |
| Once expressed as an effect of positive regulation by TEAD, induce the expression of trophectoderm characteristics. | Jedrusik et al. (2008); Ralston and Rossant (2008); Ralston et al. (2010) |
| Act as apical regulators for the maintenance of cell pluripotency | Chambers et al. (2003); Takahashi and Yamanaka (2006); Okita et al. (2007) |
| Are regulated by Oct4 and Sox2, contributing to the preservation of pluripotency | Ambrosetti et al. (1997); Okuda et al. (1998); Cauffman et al. (2009). |
| Protein . | Developmental role . | References . |
|---|---|---|
| Contribute to the formation of adherens junctions (zonulae adherens), which ensure mutual lateral adhesion between epithelial cells, thus contributing to the maintenance of epithelial cell polarity | Vestweber et al. (1987); Haegel et al. (1995); Riethmacher et al. (1995); Campbell et al. (1995); Torres et al. (1997); Perez-Moreno et al. (2003). |
| Contribute to the organisation and function of filopodia, which extend between adjacent blastomeres and determine cell flattening during compaction | Fierro-González et al. (2013). |
| E-cadherin | Intervenes in the formation of tight junctions (zonulae occludens), which create belt-like impermeable structures located at the upper-lateral sides of adjacent outer cells | Eckert and Fleming (2008). |
| Cooperate to lead to the formation of an actomyosin ring localised at the margins of adjacent cells. This ring produces the tension forces required for the zippering mechanism that acts in coordination with cell junctions to seal the intercellular contact between outer cells | Zenker et al. (2018). |
| Myosin II | Generates tensile forces at cell borders and constriction of the apical domain, by which blastomeres are internalised | Samarage et al. (2015). |
| Cooperate in the formation of a regulatory mechanism involving signalling of Hippo for the phosphorylation and consequent cytoplasmic localisation of Yap. Absence of nuclear localisation of Yap prevents the activation of the transcription regulator TEAD. Inactivation of Hippo leads to localisation of dephosphorylated Yap in the nucleus and activation of TEAD | Ota and Sasaki (2008); Zhao et al. (2008); reviewed in Harvey et al. (2013); Nishioka et al. (2009); Hirate et al. (2012). |
| TEAD | Upon activation by unphosphorylated Yap, TEAD initiates the transcription of several effector genes, among which those that determine the trophectoderm cell fate (e.g. Cdx2 and Gata3) | Yagi et al. (2007); Nishioka et al. (2008); Nishioka et al. (2009); Ralston et al. (2010). |
| Interact mutually to establish apical (Par-aPCK) and baso-lateral domains in outer cells | Suzuki and Ohno (2006). |
| Par3-Par6-aPKC (Par-aPCK) | Inhibit the Hippo pathway, which acts against the specification of trophectoderm characteristics | Hirate et al. (2013). |
| Cooperate to activate Hippo | Hirate et al. (2013); Leung and Zernicka-Goetz (2013) |
| Once expressed as an effect of positive regulation by TEAD, induce the expression of trophectoderm characteristics. | Jedrusik et al. (2008); Ralston and Rossant (2008); Ralston et al. (2010) |
| Act as apical regulators for the maintenance of cell pluripotency | Chambers et al. (2003); Takahashi and Yamanaka (2006); Okita et al. (2007) |
| Are regulated by Oct4 and Sox2, contributing to the preservation of pluripotency | Ambrosetti et al. (1997); Okuda et al. (1998); Cauffman et al. (2009). |
These include compaction, intercellular sealing to allow blastocoel formation, positioning of inner and outer cells, establishment of cell polarity and cell fate determination.
In the mouse, other molecules interacting with E-cadherin in the formation of adherens junctions have been implicated in the process of compaction, further supporting a role for cell adhesion in this developmental phase. The intracellular domain of E-cadherin is connected with β- or γ-catenin. This complex is in contact with α-catenin, through which it is anchored to the actin cytoskeleton (Perez-Moreno et al., 2003). In mice, experimental ablation of α-catenin produces a phenotype similar to that of the previously described E-cadherin null mutants, with embryos able to initiate but not complete compaction (Torres et al., 1997). By contrast, in β-catenin knockout embryos, compaction occurs unperturbed, with developmental anomalies emerging only at gastrulation, perhaps as an effect of redundancy due to the presence of other catenins or protracted action of maternal molecules (Haegel et al., 1995). Ephitin, a transmembrane serine protease, is another example of a cell adhesion molecule that appears to be involved in compaction. It co-localises with E-cadherin at areas of the cell membrane of contact between blastomeres of compacted mouse 8-cell embryos. When its expression is ablated in RNAi experiments, cell adhesion is lost and embryo death occurs shortly afterwards (Khang et al., 2005). Importantly, and unlike the mouse, the culture conditions may also affect the stability and function of junctional complexes occurring between blastomeres in human embryos, with implications for the process of compaction and ultimately embryo viability in vitro (Eckert et al., 2007).
Recent advances in in-vivo labelling and time lapse imagining techniques have extended our understanding of the mechanisms of compaction and the role of E-cadherin and associated molecules. It is now possible to view E-cadherin distribution in live cells, by microinjecting RNA molecules encoding E-cadherin with a GFP fluorescent tag. This approach has revealed a complex and regulated cell-to-cell interaction occurring at the time of compaction (Fierro-González et al., 2013). Before the 8-cell stage, E-cadherin is found throughout the cell membrane, although particularly abundant at adherens junctions. During the 8- to 16-cell stage interval, concomitant with compaction, E-cadherin-rich filopodia become visible. Originating from an area situated between the adherens junctions and the cell apical domain, these filopodia have a length of 10–12 μm and extend over the apical membrane of adjacent cells. These blastomere protrusions are positive for F-actin and Myo10, but do not appear to contain α-tubulin, consistent with typical filopodia structure (Table I). Detailed 4D analysis during the 8- to 16-cell interval unveiled an astonishing regulation of filopodia (Fierro-González et al., 2013). They are detected only in 55–60% of all blastomeres. Each blastomere projects 5–6 filopodia over the apical membrane of 2–3 adjacent cells; however, adjacent blastomeres never project filopodia reciprocally. Filopodia are dynamic, appearing to extend and retract in coordination with cell division. Remarkably, they are retracted before a cell division is initiated, while cells receiving filopodia do not divide as long as filopodia extend over their membranes. In addition, approximately two-thirds of blastomeres with filopodia undergo symmetric division and remain to the outer of the embryo organisation, while the remaining one-third divide asymmetrically giving rise to one inner and one outer cell. Notably, such a 7:3 ratio of symmetric/asymmetric divisions is believed to occur during compaction (Morris et al., 2010). The observation that, before division, retraction of filopodia is followed by a change in cell shape from elongated to rounded, has inspired further experiments confirming a causal role of filopodia in the process of compaction. When filopodia were ablated by laser micromanipulation, the juxtaposed membranes of two adjacent cells retracted immediately, indicating that filopodia impose mechanical forces between cells that can influence membrane tension and therefore cell shape. The same effect was not observed when adherens junctions were ablated (Fierro-González et al., 2013).
Molecular manipulations have confirmed a role for filopodia in determining cell elongation behaviours required by compaction. In effect, mouse blastomeres microinjected with E-cadherin short interfering RNA (siRNA) form few filopodia, remain rounded and fail to integrate in a compacted structure formed by non-injected cells. Knock down of α-catenin and β-catenin, which co-localise with E-cadherin in filopodia, also cause a drastic reduction in filopodia number and inability of the knocked-down blastomeres to elongate and compact (Fierro-González et al., 2013). Together, these findings confirm an instrumental role for E-cadherin and associated proteins in embryo compaction, while indicating filopodia, but not adherens junctions, as the prominent cell specialisation required for a change in cell shape from rounded to elongated.
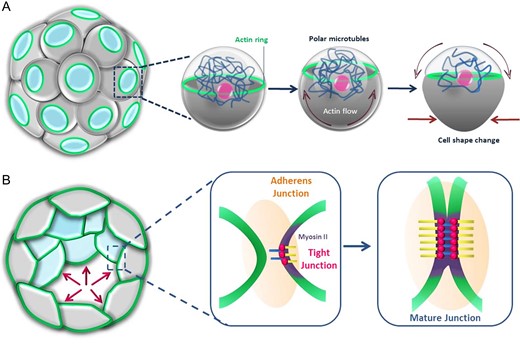
Collectively, this evidence indicates a critical role for cell adhesion in the process of compaction. However, cell adhesion appears to have other significant implications for the function of the morula and initiation of blastocyst formation. In particular, by preventing the free diffusion of membrane proteins, adherens junctions maintain the integrity of the baso-lateral and apical membrane domains. Such a difference represents a prominent aspect of cell polarity. Differentiations in the composition and function of cell membrane domains are central for the mechanism of accumulation of intercellular fluid which leads to formation of the blastocoel. As a further consequence of the localisation and function of adherens junctions, the Na+/K+ ATPase system is localised only at the baso-lateral membrane domain of outer cells of mouse embryos (Watson and Kidder, 1988). By hydrolysation of one molecule of ATP, the enzyme transports three Na+ ions out of the cell, in exchange for two K+ ions. This allows the establishment of an ion concentration gradient between the intercellular spaces of the morula, which become enriched in Na+ ions, and the surrounding environment. As a result, and assisted by increased and coordinated expression of aquaporins (Barcroft et al., 2003), water flows unidirectionally from the exterior through the epithelial-like outer cells, accumulating extracellularly and thereby forming the blastocoel (Watson and Barcroft, 2001). Concomitantly at this stage, E-cadherin-dependent formation of tight junctions (zonulae occludens) creates a belt-like impermeable structure located at the upper-lateral sides of adjacent outer cells (Table I). This specialisation of cell-to-cell contact contributes to the retention of water in the intercellular spaces of the embryo, as shown in the mouse, the human and other species (Eckert and Fleming, 2008). Live cell imaging used to study the development of mouse embryos has revealed that the creation of a barrier that seals the interior of the mouse embryo from the outside is assisted by an actin-zippering mechanism. Before formation of the blastocoel, actin-rich rings form at the apical side of outer cells. These rings do not have contractile ability but instead expand in size and diameter until they reach cell-to-cell areas of contact. At this level, they interact with and stabilise cell junctions by recruiting the relevant components. This is followed by focused association with myosin II (Table I). In this fashion, the newly formed actomyosin ring localised at the margins of adjacent cells produces the tension forces required for the zippering mechanism that acts in coordination with cell junctions to seal the intercellular contact between outer cells (Zenker et al., 2018).
Cellular arrangements in the nascent morula
If indeed compaction, encompassing cell elongation and flattening, represents a cellular ‘trickery’ to accommodate inner and outer cells in the structure of the morula beyond the 8-cell stage, it poses the question of how these two cell populations are generated in the first place. Collective evidence suggests that the modalities by which inner and outer cell are generated are multiple and probably not mutually exclusive. One of such modalities, the first to be identified in studies on mouse embryo development, is based on two major elements, i.e. cell polarity and symmetry of cell division (Mihajlović and Bruce, 2017). As mentioned above, between the 8-cell and the 16-cell stage, blastomeres acquire a well-defined polarised organisation, with nuclear (Reeve and Kelly, 1983), cytoskeletal (Johnson and Maro, 1984; Houliston and Maro, 1989) and cell membrane components (Fleming and Pickering, 1985; Korotkevich et al., 2017) distributed differently in the baso-lateral (internal) and apical (external) domains. Blastomere polarisation was also observed in human embryos from the 8-cell stage (Nikas et al., 1996). Different orientations of cleavage planes will therefore determine alternative destinies of daughter cells (Johnson and Ziomek, 1981a). Planes oriented parallel to the cell baso-apical axis will produce two symmetric daughter cells, both remaining in an external position and inheriting approximately the same polarised organisation of their progenitor cell. On the contrary, planes oriented orthogonally to the cell baso-apical axis will generate two asymmetric cells, one external cell inheriting the organisation and molecules of the apical domain and one internal cell characterised by baso-lateral attributes of the mother cell. These two types of cell division are also referred to as conservative and differentiative, respectively, depending on whether they preserve and reproduce a pre-existing cellular status or generate diversity in the position (external or internal) and inheritance of cellular organisation (apical or baso-lateral) between daughter cells (Sutherland et al., 1990). Recent studies (Korotkevich et al., 2017) indicate that orientation of the cleavage plane at the 8-cell stage is not a random event, but rather seems regulated by innate cell contact-independent factors. For example, in isolated blastomeres of mouse embryos which already show signs of cell polarisation, the mitotic spindle aligns preferentially to the basal–apical axis producing asymmetric cells in more than 80% of cases (Korotkevich et al., 2017). In addition, experimental disruption of the apical domain in isolated blastomeres of 8-cell embryos is associated with a random spindle (and cleavage plan) orientation, suggesting that the cortical domain can regulate spindle positioning and ultimately symmetric or asymmetric division. This hypothesis is in line with micro-transplantation experiments in which the cortical domain of polarised cells integrated in non-polarised cells was able to induce asymmetric cell division (Korotkevich et al., 2017). The polarised cortical domain seems therefore not only necessary, but also sufficient to control the orientation of the cleavage plan and the occurrence of asymmetric division. Consequently, the geometry of cell division at the 8–16-cell stage introduces a new structural dimension (inner–outer) in the multicellular organisation of the mouse embryo and creates diversity among blastomeres (Johnson, 2009). Notably, asymmetric division unequally redistributes cell fate determining factors (localised especially in the apical domain) in inner and outer cells, making them not only positionally, but also functionally, different (see below). Importantly, the frequency of symmetric and asymmetric divisions has implications for the relative proportion of inner and outer cells and, as a consequence, abundance of ICM and TE cells in the ensuing blastocyst (Bischoff et al., 2008). Studies on isolated blastomeres of 8-cell mouse embryos suggest that the probability by which symmetric and asymmetric divisions can occur may be an intrinsic cell characteristic at this stage (Johnson and Ziomek, 1981b), In particular, symmetric divisions, which preserve polarity in both daughter cells, are more likely to occur in blastomeres with larger apical domains (Pickering et al., 1988) and higher levels of expression of apical-specific determinants.
More recent studies on cell allocation in the mouse embryo have shown that the geometry of cell division (symmetric/asymmetric) is not the exclusive, and perhaps not even the most important, modality by which the two populations of inner and outer cells are formed. Such studies have been made possible by an advanced and highly sophisticated live imaging technique. This approach involves embryos that are microinjected with RNA encoding for a fluorescent protein (mCherry) targeting the cell membrane. The emitted fluorescence can be then detected by two-photon confocal microscopy and used to track cell positioning in 4D at a high resolution, following analysis by a methodology referred to as computational membrane segmentation (Fierro-González et al., 2013). Mouse embryos at the 8 to 16-cell stage studied with this approach showed unexpected properties (Samarage et al., 2015). In the first place, it was observed that more than 80% of inner cells derive from symmetric divisions and that 60% of embryos produce inner cells without undergoing asymmetric divisions. Therefore, asymmetric division, although important in the process of cell allocation as demonstrated by early studies, is not a frequent or ‘sine qua non’ condition to determine the position of the first inner cells to be formed. Rather, computational membrane segmentation has revealed a different morphogenetic mechanism causing cell internalisation. Starting from the 12-cell stage, some cells undergo a well-defined change in shape that involves constriction of the apical portion and expansion of the baso-lateral domain, with a consequent gradual repositioning towards the geometrical centre of the embryo. Once positioned centrally, after the 16-cell stage these cells divide to increase their number, although more inner cells can be generated through the classical mechanism of asymmetric division (Samarage et al., 2015).
Further observations clarified how apical constriction can occur in cells destined to internalisation (Samarage et al., 2015). In principle, cell constriction can result from different morphogenetic forces acting between adjacent cells, such as adhesion, involving E-cadherin, and cortical tension, derived by contractility of actomyosin structures. In 8- to 16-cell mouse embryos, E-cadherin is localised preferentially in the baso-lateral region of all cells, while no differences in its distribution are seen in the apical domain of constricting and non-constricting cells (Fierro-González et al., 2013). Modulation in cell-to-cell adhesion is therefore unlikely to generate apical constriction. On the contrary, myosin II, but not actin, distribution can differ between adjacent cells (Fig. 1). In particular, Myosin II is more abundant at the borders between constricting and non-constricting cells, tending to accumulate as apical constriction progresses (Samarage et al., 2015). Micromanipulation experiments involving laser ablation of areas of the actomyosin organisation situated at the margins between constricting and non-constricting cells have produced data informing on directionality and magnitude of tensile interactions. Such information is consistent with a biomechanical model, according to which forces acting at the border of constricting cells promote their internalisation, while forces surrounding the same cells act against apical constriction and cell internalisation (Table I). Myosin II is essential in this mechanism. This is shown by the evidence that, when Myosin II is experimentally downregulated in some, but not all, cells of an 8- to 16-cell embryo, control cells with normal levels of Myosin II fail to constrict and undergo internalisation if they are delimited by three or more knockdown cells. Therefore, tensile forces acting in non-constricting cells are also important for internalisation (Samarage et al., 2015).
Forces that shape blastomeres and secure sealing of intercellular spaces at the morula stage. (A) Flows of cortical cytoplasm and a network of polar microtubules cooperate to generate a ring of actin in the apical domain of outer cells. This ring expands reaching the cell boundaries, recruits myosin II, becomes contractile and allows cell shape remodelling. (B) At cell boundaries, expanded actin rings cooperate with tight and adherens junctions to achieve intercellular zippering and sealing to finally assist accumulation of intercellular fluid and formation of the blastocoel.
The overall choreography by which cell shape changes to allow the accommodation of an adequate number and positioning of inner and outer cells in the growing mouse morula is therefore complex. Adherens junctions and filopodia drive modification of the cell shape from approximately spherical, until the 8-cell stage, to elongated and flattened at later stages to overcome the geometrical limitations imposed by the Newton’s kissing number. At the beginning of compaction, opposite tensile forces produced by actomyosin accumulation at the cell borders induce some cells to undergo apical constriction coupled to basal broadening. As a result of these changes, such cells reposition internally. At later stages of compaction, inner and outer cells are also generated by differential orientation of the cleavage planes by which outer cells divide, with planes oriented parallel or orthogonal to the cell basal–apical axis giving rise to only outer or both inner and outer cell, respectively. Finally, actomyosin rings localised at the areas of contact between outer cells generate tensile forces required to seal the barrier formed from adherens junctions, making it impermeable. This ultimately allows accumulation and retention of fluid in the blastocoel.
Roles of cell positioning and polarity in cell fate determination
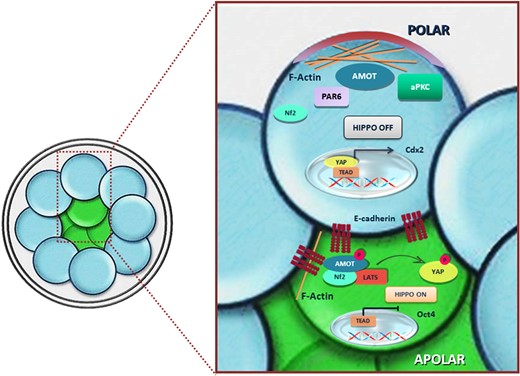
Experimental removal and spatial rearrangements of blastomeres have shown that early mouse embryos are highly plastic. When one blastomere is removed from a 2-cell embryo, the remaining half embryo can develop to term without significant consequences. Also, blastomeres of 4- to 8-cell embryos, still morphologically and positionally identical, can be disassembled and reassembled to form chimaeric associations able to support normal pre- and postimplantation development (Morris et al., 2012). During the 8- to 16-cell interval, inner and outer cells are formed, developing preferentially into the ICM and TE compartments of the blastocyst, respectively. This could suggest a change in developmental rules by which cell potency is progressively restricted. While in general this concept is correct (Suwińska et al., 2008), the reality is more complex. Elegant cell disassociation–reassociation experiments have revealed that all blastomeres of mouse 16-cell embryos maintain the ability to contribute to the formation of both the ICM and the TE (Ziomek and Johnson, 1982; Suwińska et al., 2008). Blastomeres of 32-cell embryos appear to have lost this plasticity, developing only into ICM or TE depending on whether they have an inner or outer origin, respectively (Suwińska et al., 2008). Therefore, under undisturbed conditions during the 8–16-cell transition, blastomeres appear to have made ‘decisions’ about position and ultimate fate, but these seem not to be irreversible commitments to a specific lineage at this stage. What makes blastomeres different during the 8–16-cell interval is their inner/outer position and apolar/polar symmetry. Historically, both these conditions have been proposed to cause cell determination at this stage (reviewed in Mihajlović and Bruce, 2017). According to one model, by definition, inner cells are totally engaged in cell-to-cell contacts throughout their surface, while outer cells have also cell-to-cell contact-free surfaces exposed to the surrounding environment. These two statuses are believed to coincide with different micro-environmental cues that determine the ICM and TE cell fate, respectively. In the polarity model, at the 8-cell stage blastomeres become polarised acquiring a baso-lateral domain oriented towards the interior and an apical domain facing the exterior. Depending on whether cleavage occurs parallel or orthogonal to the baso-apical axis, daughter cells lose or preserve their polarity, respectively, and inherit differently distributed regulatory molecules that commit them into the ICM or TE pathway. The two models are not mutually exclusive, though, because predominantly inner cells are apolar and outer cells are polar. These concepts were already described in this review, but notably several more recent studies point toward an interplay between polarity, position and a third major regulator of cell fate, the Hippo signalling pathway. Hippo is a tumour-suppressor pathway described initially in Drosophila and conserved across species (Mihajlović and Bruce, 2017) (reviewed in Harvey et al., 2013). Its activation depends on several stimuli among which, importantly in the case of embryos, cell contact. When Hippo is active, transcriptional co-activator proteins Yap (Yap1 and Wwtr1) are phosphorylated by Lats protein kinase. In its phosphorylated form, Yap is retained in the cytoplasmic compartment and degraded. By contrast, inactivity of Hippo allows nuclear localisation of unphosphorylated Yap. In the nucleus, Yap can bind several transcription factors, among which TEA domain transcription factors (TEAD). Then, the binary complex TEAD-Yap targets several effector genes (Ota and Sasaki, 2008; Zhao et al., 2008) (Table I). Clues that the TEAD and Hippo systems are involved in embryonic cell fate determination derives from experiments showing that in mutated TEAD4 embryos, caudal-type homeoboxprotein-2 (Cdx2), which commit cells into the TE fate, is largely downregulated and all blastomeres acquire ICM characteristics (Yagi et al., 2007; Nishioka et al., 2008) (Table I). However, because TEAD4 is ubiquitously expressed in the embryo, in normal development a modality must exist that prevents its action in blastomeres that develop into ICM. The key regulatory factor of this network in indeed Hippo (Fig. 2) (Nishioka et al., 2009). Its signalling pathway is activated only in the inner cells, where Yap becomes phosphorylated, and so remains in the cytoplasm meaning that TEAD4 cannot trigger the TE pathway. At the same time, in the outer cells, unphosphorylated Yap can be transported into the nucleus and activate TEAD4 (Hirate et al., 2012). Finally, TEAD4 directly promotes the transcription of TE-determining genes, such as the previously mentioned Cdx2, and GATA-binding protein 3 (Gata3) (Nishioka et al., 2009; Ralston et al., 2010) (Table I).
Example of integration of positional, polarity and molecular cues to achieve differential gene expression and determination of alternative cell fates. In inner cells (in green), Amot is associated with the adherens junctions throughout the cell membrane and has reduced activity for F-actin. This condition promotes interaction with and phosphorylation by Lats and ultimately activation of the Hippo regulatory pathway, which prevents intranuclear localisation of Yap and expression of TE determining genes. In outer cells (in blue), polarisation generated by Par-aPKC sequesters Amot in the apical domain bound to F-actin and prevents the interaction of the same protein with E-cadherin at the level of adherens junctions. In this fashion, Amot is not phosphorylated by Lats and Hippo cannot be activated (Hirate et al., 2013).
At this stage, cell polarity and position assume importance. In outer cells, polarity is imposed by mutual regulatory influences by which the Par3-Par6-aPKC (Par-aPCK) system promotes the organisation of the apical domain (Table I), while Par1 induces the configuration of the baso-lateral domain (Suzuki and Ohno, 2006). Par-aPCK appears to have the ability to inhibit the Hippo pathway, which acts against the specification of TE characteristics. This is indicated by the finding that at the 32-cell stage in Par-aPCK-mutated embryos showing loss of polarity in outer cells, Hippo is activated and Yap remains localised in the cytoplasm in all blastomeres, not only in inner cells (Hirate et al., 2013). On another hand, Hippo signalling requires strong cell–cell adhesion, which is particularly enhanced in inner cells; this is confirmed by the fact that Hippo is not activated in dissociated blastomeres from polarity-disrupted embryos, irrespective of the original inner or outer cell position (Hirate et al., 2013).
In the human, studies on the role of Yap in cell determination are lacking. However, cell programming experiments showed that Yap is activated when embryonic fibroblasts are induced to become pluripotent stem cells, suggesting that the hippo regulatory pathway is involved in the expression of pluripotency (Lian et al., 2010).
Some details on how, at the molecular level, polarity and cell adhesion interact to regulate Hippo activation are known (Fig. 2). Hippo activation depends on angiomotin (Amot) proteins (Table I). These regulatory proteins are also crucial for cell fate determination because their loss is sufficient to commit blastomeres into the TE fate irrespective of their position or polarity (Hirate et al., 2013; Leung and Zernicka-Goetz, 2013). Amot has a different intracellular distribution in inner and outer cells. In the former it is found associated with the adherens junctions throughout the membrane, while in the latter it is selectively localised at the apical domain (Hirate et al., 2013; Leung and Zernicka-Goetz, 2013). Amot also has binding activities which are differentially regulated in inner and outer cells. At the adherens junctions of inner cell, one region of the protein binds the cytoplasmic segment of E-cadherin and the junction-associated protein Nf2. Another subdomain of Amot has F-actin binding activity. In the same inner cells when the Amot-E-cadherin-Nf2 complex is formed, phosphorylation of this site by Lats reduces the affinity for F-actin, promotes interaction with Lats itself and ultimately stimulates the ability of Lats to activate Hippo (Hirate et al., 2013). In outer cells, polarisation generated by Par-aPKC sequesters Amot in the apical domain bound to F-actin and prevents the interaction of the same protein with E-cadherin at the level of adherens junctions. In this fashion, Amot is not phosphorylated by Lats and Hippo cannot be activated (Hirate et al., 2013). These conditions are permissive for a nuclear localisation of Yap and expression of TE-determining genes. Taken together, regulation of the Hippo signalling exemplifies how cell–cell adhesion, whose intensity depends on cell position and cell polarity, can influence call fate. This scenario, however, does not rule out that other factors can affect the Hippo-Yap pathway and ultimately cell fate. For example, in compacted mouse morulae, when biomechanical forces of outer blastomeres are experimentally weakened by knocking down the maternal myosin gene (Myh9), loss of cell contractility alone can lead to increased Yap phosphorylation and cytoplasmic localisation. As a consequence of reduced intranuclear YAP presence, although such blastomeres remain external, they fail to express the TEAD-Yap-controlled genes that are responsible for the acquisition of TE characteristics (Maître et al., 2016). These experiments demonstrate an extreme integration of mechanical, positional and molecular cues in a single regulative network of cell fate determination.
Molecular control of cell fate determination
As described, Hippo signalling is a major regulatory mechanism which ultimately prevents expression of transcription factors that elicit the TE phenotype. However, several other questions concern the pathways that promote the specification of the TE and ICM lineages and establish mutual regulatory loops. Notably, decisions on cell fate are not necessarily made at the same time by the cells of the same compartment, making the understanding of regulatory networks particularly arduous. In addition, the large majority of data have been generated in the mouse model (see below), while information concerning the human embryos remains scant.
TE specification
As discussed above, Cdx2 is a crucial component for specification of TE characteristics. In the mouse 8-cell stage embryo, this regulator is expressed only in some blastomeres but as the morula forms, it is found in a higher proportion of cells, to become expressed in outer cells of the morula and finally in elements of the TE (Jedrusik et al., 2008; Ralston and Rossant, 2008). Cdx2 –/– is embryonic lethal, due to the inability to form a TE. Embryos begin to cavitate but the blastocoel do not form properly because outer cells do not acquire their typical epithelial phenotype and fail to form a selective barrier which is instrumental for inward transport of water and expansion of the embryo (Strumpf et al., 2005). In the absence of Cdx2 expression, a lack of epithelial characteristics is associated with specific molecular features, such as repression of Eomesodermin (Eomes) and Hand1, while ICM-determining genes are expressed ectopically (Strumpf et al., 2005). Eomes is a T-box-specific factor (Ciruna and Rossant, 1999). In null mutants, its absence coincides with the inability of embryos to develop a functional TE. This occurs in association with normal levels of Cdx2 while Hand1 and P11, typical of epithelial differentiation, are undetectable (Strumpf et al., 2005). Combined, these data suggest that the relative position of Eomes in the pathway that specifies the TE fate is downstream of Cdx2 and upstream of more terminal markers of TE differentiation. Another key regulator of TE formation is the zinc-finger transcription factor Gata3. Loss of function of this factor, through RNAi action, leads to a phenotype very similar to Cdx2 null mutants, in which blastocyst formation is impeded and development arrests at the blastocyst stage (Ralston et al., 2010). While there is consensus on the opinion that Gata3 is not governed by the Hippo pathway, the question of the hierarchical position with respect to Cdx2 is more controversial. However, a comparable temporal pattern of expression between Gata3 and Cdx2 suggest that the two regulators act in parallel (Ralston and Rossant, 2008), with no upstream influence of one over the other. Indeed, Gata3 is co-expressed with Cdx2 already at the 8-cell stage and is found in TE, but not ICM cells, at the blastocyst stage (Ralston et al., 2010).
ICM specification
As the inner cells commit to their lineage, they must not only not express TE characteristics as an effect of hippo activation, but also, they must initiate the ICM pathway. In such cells, the master regulatory factor is the octamer-binding transcription factor 3/4 (Oct4, also known as Pou5f1) (Table I). Oct4 positively regulates gene transcription by recognising the sequence ATGCAAAT situated in the promoters and enhancer regions of several genes (Schöler et al., 1990). While its expression is observed throughout preimplantation development, as the embryo develops its presence becomes restricted to the ICM. Oct4 appears downregulated in embryonic stem cells following differentiation induced by leukaemia inhibitory factor (LIF) (Palmieri et al., 1994), whereas its absence in Oct4 –/– mutant embryos does not prevent decidualisation but causes peri-implantation death (Nichols et al., 1998). This factor is therefore considered instrumental for pluripotency. Other factors contribute to pluripotency of the ICM and are differentially regulated in a cell- and stage-specific fashion. For example, in the mouse, Nanog (Chambers et al., 2003) is expressed in the inner cells of the morula and becomes restricted to the epiblast of the ICM in the blastocyst, from which the embryo proper derives (Chambers et al., 2003). Indeed, reprogramming experiments of somatic cells induced into pluripotent stem cells illustrate the critical importance of both Oct4 and Nanog in maintaining pluripotency (Takahashi and Yamanaka, 2006; Okita et al., 2007).
The binding domain-containing family of transcription factors, Sox2, is a member of the High Mobility Group (HMG) protein family that is associated with maintenance of pluripotency in the embryo (Table I). It is detectable throughout mouse preimplantation development, from the zygote until the morula stage, when it disappears in the cells that will form the TE (Avilion et al., 2003). The observations that Sox2 –/– embryos have an unchanged pattern of protein expression and that Sox2 mRNA starts to be produced from the morula stage indicate that this factor is accumulated in the oocyte during oogenesis (Avilion et al., 2003). Sox2 appears crucial for ICM and in particular the epiblast at the time of implantation (Avilion et al., 2003). Furthermore, and interaction between Sox2 and Oct4 appears essential for the expression of downstream pluripotency genes, such as Fgf4 (Ambrosetti et al., 1997), Utf1 (Okuda et al., 1998) and Nanog (Rodda et al., 2005) (Table I). Again, in the human, the timing of expression may differ; indeed, nuclear localisation of Sox2 was observed only from the compacted morula stage, i.e. much later than in the mouse embryo (Cauffman et al., 2009).
Mutual negative regulation
Several lines of evidence converge towards a model involving mutual interaction at the intracellular level between the ICM and TE regulatory systems. Indeed, Cdx2 knockout mouse embryos are unable to promote positive regulation of TE specific genes such as Eomes and Hand1 in outer cells, but instead express Oct4 and Nanog, which are typically associated with inner cells. At the molecular level, this cross-regulation is explained by the fact that at the blastocyst stage Cdx2 and SWI/SNF chromatin remodelling factor cooperate to bind the promoter region of Oct4 in cells that will develop into TE, thus inhibiting the action of this pluripotency factor. This replicates a scheme observed in other types of epithelial cells (Wang et al., 2010).
There are also hints of an inverse, mutual regulation between the Cdx2 and Oct4 pathways. Indeed, in embryonic stem cells, Oct4 was found to interact with Cdk1. The two proteins form a complex that is required to repress the transcription of Cdx2 (Li et al., 2012). Therefore, in the mouse, not only do the Cdx2 and Oct4 regulatory pathways specify the TE and ICM cell fates, respectively, but also are involved in an elegant interplay of mutual regulation in which one represses the other in its own embryonic compartment. Consistent with the mouse embryo, in the human at the 8-cell stage, Oct4 and Nanog are initially expressed in all blastomeres; afterwards their localisation remains limited to the ICM (Kimber et al., 2008; Niakan et al., 2012). By contrast, human embryos show a different temporal pattern of Cdx2 expression. In fact, this regulator is detectable only after formation of the blastocoel and is initially co-expressed with Oct4 (Niakan et al., 2012). It seems, therefore, that specification of the two cell lines occurs significantly later than in the mouse and does not necessarily require the Oct4/Cdx2 antagonism. This opens the possibility that the fate of human blastomeres can remain plastic until relatively advanced stages of development. Indeed, human outer cells from compacted morulae or early blastocysts can contribute to formation of the ICM if experimentally repositioned inside the embryo (De Paepe et al., 2013). Furthermore, after isolation and reaggregation, human outer cells are able to reconstitute an embryo able to cavitate and form an ICM (De Paepe et al., 2013). Therefore, while mouse and human development share some common features of cell fate determination, further studies are warranted to ascertain possible interspecies differences.
The morula stage in clinical embryology
Embryo selection and transfer at the morula stage
Clinical data on the reproductive performance of Day 4 embryos are scarce. In clinical IVF, embryos are usually transferred in the uterus at early cleavage (Days 2/3) or blastocyst (Days 5/6) stages. Much less commonly, they are replaced on Day 4 when they are expected to have reached the morula stage, owing in part to the challenges described above. Furthermore, where such embryos are used, they will often be transferred together with Day 3 or Day 5 embryos, making the association between embryonic stage and ability to implant and develop to term unachievable. However, there is isolated evidence that embryo transfer (ET) can be successfully implemented on Day 4. In the late 1990s, preliminary reports indicated that morula stage embryos could be used for ET following blastomere biopsy on Day 3 in preimplantation genetic testing (PGT) cycles (Grifo et al., 1998; Gianaroli et al., 1999). In one of the few specific studies carried out on the morula stage, Tao et al. (2002) compared retrospectively the relative clinical performance of Day 3 and Day 4 embryos. In all cases, Day 4 ET were associated with higher implantation and pregnancy rates, suggesting that culture to Day 4 could offer better chances for embryo selection compared to Day 3. A prospective randomised study published several years later and including 350 couples (Pantos et al., 2008) showed that Day 4 ET was associated with implantation and clinical pregnancy rates that were comparable to Day 3 ET (22.0 vs. 21.0 and 49.7% vs. 45.3%, respectively). A further retrospective analysis (Skorupski et al., 2007) claimed that the transfer of Day 4 embryos was compatible with high rates of implantation (45–34%) and live birth (55–33%) across a large spectrum of female age (≤34 to 40 years). However, the absence of a control group and scoring criteria, as well as the fact that some of the embryos had undergone assisted hatching, means that these data need careful interpretation. The clinical outcome of Day 4 and Day 5 ET was the object of another retrospective study (Feil et al., 2008). This analysis adopted a single embryo transfer (SET) approach and reported comparable overall ongoing pregnancy rates between Day 4 and Day 5 transfer (38.7% and 32.1%, respectively). In addition, sub-analysis of the Day 4 group revealed that morulae entirely or partially compacted, and otherwise normal, implanted with comparable rates (40.0% and 37.1%, respectively); however, in cases where partial compaction was associated with large vacuoles and extensive fragmentation, the implantation rate dropped dramatically. Thus, Day 4 embryos at the morula stage can be discriminated morphologically and developmentally to some degree and, in the best-case scenario, appear to have implantation rates comparable to that achieved by Day 5 transfers. Such conclusions are supported by a further retrospective study by Kang et al. (2012). For all major clinical outcomes, i.e. pregnancy rate (51.5% vs. 51.8%), implantation rate (52.3% vs. 52.5%) and live birth rate (39.2% vs. 44.7%), results were comparable between morula and Day 5 ET, respectively, although the miscarriage rate tended to be higher in the Day 4 group. Together, these data suggest that ET of morulae on Day 4 can be contemplated as an option in case of intense laboratory workload and necessity to redistribute ETs more uniformly over consecutive days.
A recent report specifically focused on the predictive value of morphology of Day 4 embryos (Fabozzi et al., 2015). Embryos were considered at the morula stage if showing at least 14 cells and scored according to four morphological classes (A–D, best–worst), depending on a combination of the degrees of compaction and integrity. Out of 393 embryos, the proportions of grade A–D morulae were 47.84%, 26.72%, 20.36% and 5.09%, respectively. The degree of compaction and integrity was positively associated with the blastocyst formation rate which was 87.2%, 63.8%, 41.3% and 15.0% for the A–D classes, respectively. Importantly, this classification system was found to be a better predictor of blastocyst formation and quality compared with a conventional grading system performed on Day 3. Overall, these data confirm previous reports suggesting that the scoring systems based on degree of compaction and integrity of Day 4 embryos have the ability to predict the rate and quality of blastocyst formation (Ebner et al., 2009; Ivec et al., 2011).
The use of morula stage embryos for preimplantation genetic testing
Over the course of the last decade, PGT has undergone major changes. Progress in embryo culture and cryopreservation have shifted the preference for the time of embryo biopsy from Day 3 (6–8 cell stage) to Day 5 or 6 (blastocyst stage). This means that more cells are available for analysis (3–10 instead of 1–2) and, in general, embryos appear more resilient to the biopsy procedure. However, Zakharova et al. (2014) reported on embryo biopsies performed on 709 Day 4 morulae from 215 PGT cycles. To achieve loosening of intercellular contacts, compacted morulae were exposed to a Ca2+-free medium. Three to seven blastomeres were biopsied from each embryo and analysed by fluorescent in-situ hybridisation (FISH). After return to standard medium containing Ca2+, more than 90% of biopsied embryos reached the blastocyst stage by Day 6. In an impressive range of endpoints, including postnatal follow up, no differences were apparent between the PGT and non-PGT groups. Therefore, the authors concluded that embryo biopsy is technically feasible and safe to be carried out at the compacted morula stage. Although encouraging, caution must be exercised, since the relatively small numbers of embryos from a single-centre study are unlikely to offer solid conclusions on the safety of the Day 4 approach. Concerns derive not only from the possible impact of removal of 3–7 cells from an embryo usually formed from 12 to 32 blastomeres, but also from potential undefined physiological effects arising from the disruption, as we have illustrated above, of highly complex and important morphogenetic events occurring at the morula stage. Thus, exposure to Ca2+-free medium, functional ablation of intercellular contacts and consequent reversion of cell shape from elongated to spherical, although transient, could potentially perturb embryo physiology with long-term developmental consequences. Nonetheless, embryo biopsy at the morula stage remains an interesting option, implicating advantages such as (i) the recovery of a higher number of cells compared to Day 3 biopsy, (ii) the possibility to have one full day for chromosome analysis and therefore perform fresh ET of unaffected embryos on Day 5, and (iii) the recovery of intact biopsied cells, whose integrity is amenable to FISH, in cases where this analytical method is preferable.
Cryopreservation of human morulae
Cryopreservation at the morula stage has been reported in numerous studies conducted in animal models, including mouse, rat, cow, pig and goat. Therefore, it appears to be technically possible. However, evidence from animal studies cannot be directly applied to human IVF. Membrane permeability to the various cryoprotectants, on which successful cryopreservation depends, occurs by simple diffusion or via specific channels (aquaporins) according to kinetics that vary from stage to stage often in a species-specific fashion. Early experiments suggested that human blastocysts vitrified using fine plastic capillaries as storage devices gave acceptable rates of survival (Cremades, 2004). Recent progress in vitrification has allowed improvement in survival rates of both intact and biopsied morulae (92.0% and 87.5%, respectively) (Zhang et al., 2009). Isolated cases of healthy live births (one twin and one single) were also reported after the transfer of compacted morulae cryopreserved by slow freezing (Tao et al., 2001). In a larger case series, 54 Day 4 morulae were warmed after vitrification; 38 embryos survived to 24 h after thawing, while 30 developed to the blastocyst stage. In 18 ETs, implantation and birth rates for ET were 20.0% (6/30) and 27.8% (5/18), respectively (Vanderzwalmen et al., 2002). Therefore, while cryopreservation at the morula stage can be applied, the limited available data suggest a reduced efficacy compared with cryopreservation at the cleavage or blastocyst stage. Again however, caution should be exercised as this is an under investigated area of treatment.
Emerging data from TLM
Timing of the morula stage
For decades, the morula has been described as characteristic of Day 4, but little data was available on the specific timing of cleavage events beyond the 8-cell stage or the dynamics of the process of compaction (ESHRE Atlas of Human Embryology). Isolated reports indicated that starting compaction ‘early’ was associated with a higher implantation potential (Skiadas et al., 2006), although the precise timing of initiation of this process was not described.
The introduction of TLM has provided new opportunities to observe the morphokinetics of the morula stage. In one of the first TLM studies describing the morula stage, Campbell et al. (2013) reported the median time of start of compaction in euploid embryos was 79.7 h post ICSI, a value similar to that of embryos affected by a single aneuploidy (80.7 h), but significantly different to that of embryos carrying multiple aneuploidies (85.1 h). Median times of full morula formation in the three study groups were not statistically different (83.5, 87.9 and 88.2 h, respectively). Another study investigated the formation of the fully compacted morula in embryos of PGT cases, reporting mean times of 94.4 and 95.3 h in euploid and aneuploid embryos, respectively (Minasi et al., 2016). Times of full compaction appears different in the two studies, although it should be noted that they were reported using different parameters (median and mean, respectively). In a study specifically focussed on stage of compaction, Iwata et al. (2014) found that compaction can occur at any stage between the 4- and the 16-cell stage, although the majority of embryos initiate compaction at the 8-cell stage or later. In such embryos, almost half go on to form good quality blastocysts, while embryos starting compaction before the 8-cell stage form good blastocysts in less than 20% of cases. Interestingly, early compacting embryos appear to be characterised by cell cycle anomalies, like multinucleation caused by cytokinesis failure (Iwata et al., 2014).
Technically, the determination of the time of full compaction is challenging because of its dynamic nature, occurring over several hours. Therefore, significant intra- and inter-operator variability cannot be excluded. Nonetheless, it is realistic that time differences between studies, at the morula and all developmental stages, can reflect intrinsic differences between cohorts of embryos and/or extrinsic influences, such as culture conditions. Indeed, while mean times of formation of fully compacted morulae are very similar in males and females embryos (91.2 and 91.9 h, respectively) (Bronet et al., 2015), embryos from overweight/obese women have an overall faster developmental kinetics. This is also reflected in times of morula formation 17 h shorter than women of normal weight (Leary et al., 2015). Likewise, in another study, it was shown that embryos undergoing repeated contractions and expansions during blastocyst formation, a phenomenon indicated as a negative marker of implantation potential, achieve full compaction ~5 h earlier (80.9 vs. 86.3 h, respectively) compared with those that do not exhibit cyclical contractions/expansions (Marcos et al., 2015). Laboratory manipulations can also impact the morula stage. Indeed, an analysis carried out in embryos produced in PGT cycles described that Day 3 embryos of at least eight cells and subject to removal of 1–2 blastomeres progressed to the morula stage with a 5-h delay and implanted at a lower rate compared with embryos of a control group (Bar-El et al., 2016). These findings are consistent with a previous study reported by Kirkegaard et al. (2011). Notwithstanding such limitations, the time of compaction alone appears to be associated with embryo implantation. This notion derives from a recent study showing that in a selected population of embryos full compaction in accomplished 14 h earlier in those that implant (86.4 vs. 100.3 h, respectively) (Motato et al., 2016).
Morphokinetics and chromosomal status of the morula
Data from a recent investigation (Lagalla et al., 2017) suggest that the morula stage could be an important checkpoint for embryo quality during preimplantation development and that the process of compaction could be involved in mechanisms of self-correction. The study confirmed that diverse cleavage anomalies (e.g. direct cleavage of one cell into three, rapid cleavage, reverse cleavage) were associated with a 50% reduction in the chances of an embryo developing into a good quality blastocyst. Interestingly, the study also showed that blastocysts derived from such abnormally cleaving embryos exhibited a higher rate of euploidy compared with blastocysts developed from embryos without cleavage anomalies (75.0% vs, 49.2% respectively). Even more interestingly, it was found that all euploid blastocysts derived from abnormally cleaving embryos were characterised by the phenomenon of partial compaction. Partial compaction was observed also in embryos with no apparent cleavage anomalies, but at a much lower rate. Taken together, these findings suggest that, on one hand, blastomeres that have become aneuploid as a consequence of cleavage anomalies (e.g. direct cleavage) may in some cases prevent development to blastocyst. On the other hand, it might be possible that such aneuploid blastomeres are excluded at compaction, with a rescue of the original condition of euploidy of the embryo as a whole. The latter hypothesis appears to be confirmed by comparative chromosome analysis of cells excluded from compaction. In the majority of cases, in the same embryo the chromosome constitution of these cells was more affected by segregation errors compared with cells of the trophectoderm, or they could not be analysed due to extensive DNA degradation, a hallmark of apoptosis often triggered in response to complex aneuploidy. Therefore, it is plausible to propose that during compaction, aneuploid cells are excluded from the embryos allowing a restoration of euploidy. This is in line with the well-established notion that mosaicism is more frequent at earlier stages of development than at the blastocyst stage. If true, this suggests a startling critical role for the morula in terms of embryo rescue and elimination of mosaicism, as well as the more widely-described functions relating to morphogenetic events and determination of cell fate. The notion of the existence of mechanisms of self-correction during preimplantation development is also emerging from other observations. For example, a study on embryos displaying direct unequal cleavage (i.e. division of one cell into three or more daughter cells) indicates that extrusion from the compacted morula of blastomeres affected by such a cleavage anomaly is strongly associated with the formation of good quality blastocysts (Zhan et al., 2016). Such information might be of crucial relevance in the application of PGT at the morula stage.
A plea for more research on the morula stage and compaction process
Recent investigations confirm the necessity of intensifying research efforts on the morula stage. Data from Mayer et al. (2018) described the phenomenon of late onset of cell vacuolisation at the morula stage. In particular, they reported that the implantation ability of good quality blastocysts is significantly reduced in case of formation of vacuoles at the morula stage. The morula stage also appears relevant to the management of slow-growing embryos. In fact, Tannus et al. (2018) observed that embryos reaching the morula stage on early Day 5 and achieving the blastocyst stage on Day 6 can implant with higher frequency if cryopreserved and transferred in a frozen embryo replacement, instead of being used fresh, confirming the importance of endometrial receptivity and synchronisation. Even more intriguing information seems to derive from detailed observation of the mechanism of compaction and elimination of presumably abnormal cell from the mass that then organises itself into blastocyst. In a preliminary report, Lagalla and colleagues (2018) proposed the existence of two distinct mechanisms of cell elimination resulting in partial compaction: (a) exclusion of cells from the outset, before the beginning of compaction, or (b) extrusion of cells following their initial involvement in the compaction process. Interestingly, in partially compacted embryos, exclusion was found to occur more frequently in embryos of younger women, while the opposite was observed in case of extrusion. This finding, together with the recently reported differences in chromosome constitution between the embryo and cells eliminated during compaction (Lagalla et al., 2017), is suggestive of the morula stage as an important checkpoint stage during formation of a viable blastocyst. However, research in clinical embryology alone is unlikely to have the capacity to reveal the intimate secrets of the morula stage. Strategic collaboration with other disciplines and approaches will be crucial to ensure progress in this field. Being a dynamic process and extremely difficult to observe even with the aid of TLM, the morula stage could be amenable to investigation by exploiting machine learning approaches, which has already found application to predict embryo implantation ability at the blastocyst stage (Zaninovic et al., 2018). Further progress could derive from the analysis of extruded/excluded cells to reveal their cellular and molecular constitution and gain more in-depth insights on possible mechanisms of self-correction. Live cell imaging, especially if developed in a truly non-invasive fashion, could also give a crucial impulse to research on the morula stage. Three-dimensional modelling approaches have already begun to reveal how cell internalisation, and therefore formation of the two main cell lineages, occurs only when differences in cell surface contractility exceed a predictable threshold (Maître et al., 2016). Molecular, cellular and metabolic analysis of spent media exposed to embryos of different developmental stages could shed new light on the morula and better explain its unique characteristics.
Conclusions
The morula stage and the associated process of compaction have received relatively little attention in ART due to their highly dynamic nature combined with the ambiguous criteria of morphological classification. Morula stage embryos therefore remain scarcely integrated in the mainstream ART procedures, such as embryo quality assessment, ET, cryopreservation and embryo biopsy. This is in striking conflict with the importance that the construction of the morula has for preimplantation development and indeed the entire developmental process. For example, during the temporal span of the morula stage, newly expressed genes of the embryonic genome mediate the transition from a low-activity, low-energy metabolic state characterising the early cleavage stages to a more active glucose-based aerobic capacity required for the formation and expansion of the blastocyst (Leese, 2012). From a developmental standpoint, the morula stage is when the first crucial cell-fate decision is taken, whereby blastomeres commit themselves to contribute to either the ICM or TE compartment (Mihajlović and Bruce, 2017). As early as the 1980s, positional information (internal or external localisation) was recognised as a determining factor for the establishment of an ICM or TE cell fate (Fleming, 1987). Early studies also suggested that, starting from the 8–10 cell stage, differential intracellular distribution of cell determinants cooperates with the geometry of cleavage planes to generate functional diversity between internal and external cells (Johnson and Ziomek, 1981a; 1981b). These notions remain valid today. However, recent studies, aided by extraordinary progress in live cell imaging techniques, have expanded our understanding of the biology of the morula (Table I). We now know that compaction is assisted not only by junctional structures, but also by fine and dynamic regulation of specialised cellular projections, filopodia (Fierro-González et al., 2013). We have also learnt that generation of inner and outer cells occurs not only as a consequence of alternative orientation of cleavage planes, but also as an effect of differences in cortical tension between adjacent outer blastomeres. In addition, new information has revealed the crucial role of an actin-zippering mechanism in ensuring intercellular sealing and establishing a permeability barrier required by the process of blastocoel expansion. New light has also been shed on the mutually regulated pathways of gene expression that commit cells into distinct developmental destinies (Jedrusik, 2015). Although obtained in the mouse model, such a wealth of information has reignited interest in the specific merit of the morula stage for clinical embryology. Information on possible influences of in-vitro culture on developmental decisions taken at the morula stage are lacking in human ART. Indeed, recent morphokinetic data suggest that compaction could represent an opportunity to sense chromosomal anomalies in individual blastomeres and implement self-correction mechanisms aimed at excluding abnormal cells from the developing embryo (Lagalla et al., 2017). Overall, such a knowledge casts new light on this crucial developmental stage. It also indicates new avenues for future research in preimplantation development in the context of clinical embryology.
Authors’ roles
Giovanni Coticchio: manuscript design, literature search, manuscript writing, critical discussion and editing. Cristina Lagalla: manuscript design, literature search, manuscript writing, critical discussion and editing. Roger Sturmey: manuscript writing. Francesca Pennetta: manuscript design, critical reading and editing, design and production of figures. Andrea Borini: manuscript design and critical discussion.
Funding
No external funding was either sought or obtained for this study.
Conflict of interest
None declared.
References
Fleming TP, Pickering SJ.
Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H.
Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A.