-
PDF
- Split View
-
Views
-
Cite
Cite
Nathalie Sermondade, Stéphanie Huberlant, Vanessa Bourhis-Lefebvre, Elisangela Arbo, Vanessa Gallot, Marina Colombani, Thomas Fréour, Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis, Human Reproduction Update, Volume 25, Issue 4, July-August 2019, Pages 439–451, https://doi.org/10.1093/humupd/dmz011
Close - Share Icon Share
Abstract
A worldwide increase in the prevalence of obesity has been observed in the past three decades, particularly in women of reproductive age. Female obesity has been clearly associated with impaired spontaneous fertility, as well as adverse pregnancy outcomes. Increasing evidence in the literature shows that obesity also contributes to adverse clinical outcomes following in vitro fertilization (IVF) procedures. However, the heterogeneity of the available studies in terms of populations, group definition and outcomes prevents drawing firm conclusions. A previous meta-analysis published in 2011 identified a marginal but significant negative effect of increased female body mass index (BMI) on IVF results, but numerous studies have been published since then, including large cohort studies from national registries, highlighting the need for an updated review and meta-analysis.
Our systematic review and meta-analysis of the available literature aims to evaluate the association of female obesity with the probability of live birth following IVF. Subgroup analyses according to ovulatory status, oocyte origin, fresh or frozen-embryo transfer and cycle rank were performed.
A systematic review was performed using the following key words: (‘obesity’, ‘body mass index’, ‘live birth’, ‘IVF’, ‘ICSI’). Searches were conducted in MEDLINE, EMBASE, Cochrane Library, Eudract and clinicaltrial.gov from 01 January 2007 to 30 November 2017. Study selection was based on title and abstract. Full texts of potentially relevant articles were retrieved and assessed for inclusion by two reviewers. Subsequently, quality was assessed using the Newcastle-Ottawa Quality Assessment Scales for patient selection, comparability and assessment of outcomes. Two independent reviewers carried out study selection and data extraction according to Cochrane methods. Random-effect meta-analysis was performed using Review Manager software on all data (overall analysis), followed by subgroup analyses.
A total of 21 studies were included in the meta-analysis. A decreased probability of live birth following IVF was observed in obese (BMI ≥ 30 kg/m2) women when compared with normal weight (BMI 18.5–24.9 kg/m2) women: risk ratio (RR) (95% CI) 0.85 (0.82–0.87). Subgroups analyses demonstrated that prognosis was poorer when obesity was associated with polycystic ovary syndrome, while the oocyte origin (donor or non-donor) did not modify the overall interpretation.
Our meta-analysis clearly demonstrates that female obesity negatively and significantly impacts live birth rates following IVF. Whether weight loss can reverse this deleterious effect through lifestyle modifications or bariatric surgery should be further evaluated.
Introduction
A worldwide increase in the prevalence of obesity has been observed in the past three decades (Finucane et al., 2011, Ng et al., 2014), with 13% of the general population being obese in 2016 (WHO, 2018). This increase concerns both sexes and all age groups, with more than half of women of reproductive age being overweight (defined by BMI ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2) (Flegal et al., 2012; Gallus et al., 2015).
Female obesity has been clearly associated with several adverse pregnancy outcomes, such as miscarriage, hypertension, pre-eclampsia and diabetes (Jungheim et al., 2011; Hawkins Bressler et al., 2015). Obesity also leads to impaired fertility, mainly due to anovulation (Rich-Edwards et al., 2002). Yet other factors may also be involved, since time to conception for obese women is longer than in normal weight women, even in the case of regular menstrual cycles (Gesink Law et al., 2007).
The obese infertile female population is characterized by poorer in vitro fertilization (IVF) outcomes, such as use of higher doses of gonadotrophins, increased cycle cancellation rate, lower oocyte recovery and increased miscarriage rate (Maheshwari et al., 2007; Koning et al., 2012). Growing evidence in the literature shows that obesity impacts clinical outcomes following IVF procedures. However, the heterogeneity of available studies in terms of populations, group definitions and outcomes described prevents drawing firm conclusions. A previous meta-analysis published in 2011 concluded that increased female BMI was associated significantly with adverse pregnancy outcomes following IVF (Rittenberg et al., 2011). Numerous studies have been published since then, including large cohort studies from national registries, highlighting the need for an updated review and meta-analysis.
This systematic review and meta-analysis aims to evaluate the association between female obesity and live birth rates (LBRs) following IVF. Subgroups analyses were performed according to ovulatory status, oocyte origin, fresh or frozen-embryo transfer and cycle rank. The association between female overweight and LBR was also studied secondarily.
Methods
Literature search strategy and eligibility criteria
The search strategy, selection criteria, data extraction, quality assessment and statistical analyses described below were defined a priori. The conduct and reporting of this review was guided by PRISMA guidelines and prospectively registered (PROSPERO: CRD42018090645). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used while writing this review. Cohort studies comparing IVF patients identified as obese according to the World Health Organization (WHO, 2000) (BMI ≥ 30 kg/m2) versus normal weight (BMI in 18.5–24.9 kg/m2) were included. As live birth was the outcome of interest, studies were required to report values of live birth for obese and normal weight females. Studies describing only overweight, underweight and/or obese with another cut-off point than BMI ≥ 30 kg/m2 were excluded.
PubMed, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews were searched for relevant literature. The search strategy was limited to articles published in English or French between 01 January 2007 and 21 June 2017 for all electronic databases, except for Pubmed (end date: 30 November 2017). Further efforts were made to identify all available studies, including searching clinical-trial registries (ClinicalTrials.gov and EU Clinical-trial register) and conference abstracts from ESHRE, and the American Society for Reproductive Medicine, both in 2015 and 2016. The search strategy for electronic databases used the following combined search terms: (‘obesity’[MeSH Terms] OR ‘obesity’[Title/Abstract] OR ‘obese’[Title/Abstract] OR ‘body mass index’[MeSH Terms] OR ‘body mass index’[Title/Abstract] OR BMI[Title/Abstract]) AND (‘live birth’[MeSH Terms] OR ‘live birth’[Title/Abstract]) AND (‘in vitro fertilization’[Title/Abstract] OR ‘fertilization in vitro’[Title/Abstract] OR ‘fertilization in vitro’[MeSH Terms] OR ‘fertilization in vitro’[Title/Abstract] OR ‘in vitro fertilization’[Title/Abstract] OR ‘ivf’ [Title/Abstract] OR ‘sperm injections, intracytoplasmic’[MeSH Terms] OR ‘intracytoplasmic sperm injections’[Title/Abstract] OR ‘sperm injections, intracytoplasmic’[Title/Abstract] OR ‘icsi’[Title/Abstract]) AND (English[lang] OR French[lang]) AND (‘humans’[MeSH Terms] OR ‘Hum Reprod’[Journal] OR (‘women’[MeSH Terms] OR ‘women’[All Fields] OR ‘woman’[All Fields])).
Study selection and data extraction
Two reviewers (MC and TF) independently performed a screening of titles and abstracts of all articles, clinical studies and abstracts of congresses to exclude citations deemed irrelevant by both observers. Based on the pre-established inclusion criteria, full texts of potentially relevant articles were retrieved and assessed for inclusion by two reviewers (NS and VG, SH and TF, VBL and MC). Any disagreement or uncertainty was resolved by discussion with a third reviewer. The methodological quality of the selected studies was assessed using the Cochrane Handbook methods: patient selection, baseline comparability and outcomes selection and measurement with a total of nine stars were judged using the Newcastle-Ottawa Quality Assessment Scale for cohort studies (Stang, 2010). Data were extracted from included articles by two independent reviewers (NS and VG, SH and TF, VBL and MC) using a data extraction form developed for the present review. All qualifying articles with quantitative data for LBR, with documented numbers for obese females (BMI ≥ 30 kg/m2) and for normal weight females (BMI in [18.5 kg/m2; 24.9 kg/m2]) were included in the meta-analysis. No replacement of missing data has been done. When only percentages were available, and when possible, the number of events was derived from total number of cycles/transfer in each population group. Data were not extracted for clinical studies and conference abstracts.
Data synthesis and meta-analysis
The following study details were extracted to characterize the included studies: study authors, publication year, study time frame, country, study design, eligibility criteria, cycle rank, oocyte donation, women with polycystic ovary syndrome (PCOS), fertilization method, type of embryo transfer. For each group (normal weight and obese), the sample size, age, percentage and/or number of live births were noted. When data were dispatched by subgroups in the article (e.g. PCOS and non-PCOS), extracted data were pooled for overall meta-analysis. Dichotomous variables were expressed as risk ratios (RR) and the precision of the estimates was evaluated by the 95% CI. The clinical relevance of all comparisons was assessed based on the precision of the estimates. A random effects model was used to address the differences in true effect size across studies, since it is unlikely that observed differences among study results are due solely to the role of chance.
The software Review Manager 5.3.5 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to combine and analyse the results for the meta-analysis. Meta-analysis was performed using a random-effect model with the Mantel–Haenszel (M-H) method. Pooled effect sizes were deemed statistically significant at P < 0.05. In addition to computed estimates of between-study variance (Tau2), the statistic heterogeneity across the studies was calculated by chi-square statistic, and inconsistency was judged by the value of I2 statistic. I2 ≥ 50% indicates substantial heterogeneity (Higgins et al., 2003). For each study included in the meta-analysis, risk of bias was assessed by two independent reviewers (NS and VG, SH and TF, VBL and MC) using ROBIN-1 tools (Sterne et al., 2016): confounding, selection of participants, intervention classification, intervention deviations, missing data, outcome measurement and selection of reported results. Each study was assigned a ‘low’, ‘high’ or ‘unclear’ risk of bias. A funnel plot was used to assess the presence of small-study effects suggestive of publication bias.
Sensitivity analysis
Several pre-specified sensitivity analyses were performed. To explore statistical heterogeneity, meta-analysis using a fixed-effect model was also performed to compare the estimates of the intervention effect of fixed- and random-effect models. Furthermore, sensitivity analysis was performed by excluding the outliers identified in the Funnel plot. To assess the possible impact of study weights, sensitivity analyses were performed. First, a forest plot was displayed in ascending order of study weight and checked visually. Second, studies with a high number of patients were analysed, after lowering their weight under 20%. To verify whether the conclusion would have been different if eligibility was restricted to studies with low risk of bias, another sensitivity analysis was performed though omitting all studies with at least one high risk of bias. Subgroup analyses were performed to separate the distinct kinds of embryo transfer, cycle rank of the IVF, oocyte source and patients diagnosed with PCOS or not.
Results
Study selection
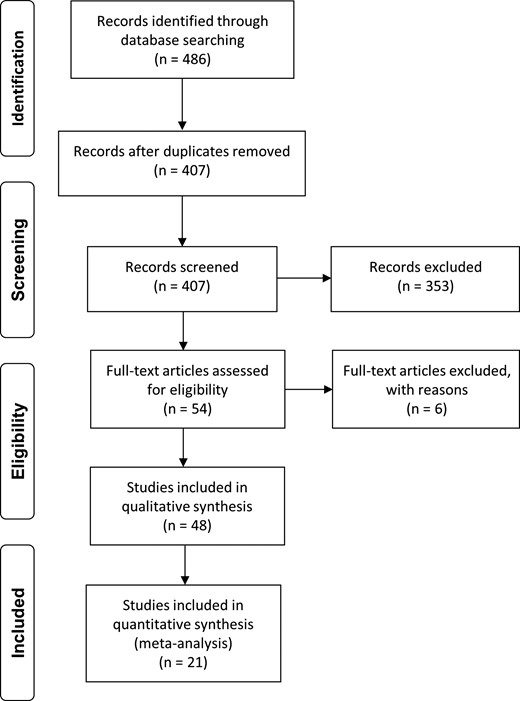
The search strategy identified a total of 486 articles, including duplicates and articles irrelevant to the primary research questions. After removing duplicates, 407 abstracts were reviewed, and 54 full-text articles were assessed for eligibility for quantitative analysis. Among them, 21 articles seemed potentially appropriate to be included in the meta-analysis (Fig. 1).
Flow chart of study selection for systematic review and meta-analysis.
All 21 eligible studies were cohort studies. The study sample sizes ranged from 117 (Hill et al., 2011) to 494 097 (Kawwass et al., 2016) cycles, for a total of 682 532 cycles (Table I). Study participants were mainly from the USA (McCormick et al., 2008; Sneed et al., 2008; Hill et al., 2011; Luke et al., 2011; Chavarro et al., 2012; Bailey et al., 2014; Schliep et al., 2015; Zhang et al., 2015; Kawwass et al., 2016; Provost et al., 2016a, 2016b; Insogna et al., 2017; Russo et al., 2017), but also Australia (Zander-Fox et al., 2012), China (Zhang et al., 2010), Denmark (Pinborg et al., 2011), France (Sifer et al., 2014), India (Sharma, 2014), Macedonia (Petanovski et al., 2011) and Spain (Bellver et al., 2010, 2013). Included women were recruited during their first cycle (Sneed et al., 2008; Petanovski et al., 2011; Bellver et al., 2013; Schliep et al., 2015; Zhang et al., 2010, 2015; Insogna et al., 2017; Russo et al., 2017), all of them (McCormick et al., 2008; Bellver et al., 2010; Pinborg et al., 2011; Zander-Fox et al., 2012; Bailey et al., 2014; Sifer et al., 2014; Kawwass et al., 2016; Provost et al., 2016a, 2016b) or not specified (Hill et al., 2011; Luke et al., 2011; Chavarro et al., 2012; Sharma, 2014). Studies concerned either autologous cycles (McCormick et al., 2008; Sneed et al., 2008; Petanovski et al., 2011; Chavarro et al., 2012; Zander-Fox et al., 2012; Bailey et al., 2014; Sharma, 2014; Zhang et al., 2015; Kawwass et al., 2016; Provost et al., 2016a; Russo et al., 2017), oocyte donation cycles (Bellver et al., 2013; Provost et al., 2016b), both (Luke et al., 2011; Insogna et al., 2017) or not specified (Bellver et al., 2010; Zhang et al., 2010; Hill et al., 2011; Pinborg et al., 2011; Sifer et al., 2014; Schliep et al., 2015). Studies included only fresh embryo transfers (McCormick et al., 2008; Sneed et al., 2008; Bellver et al., 2010; Zhang et al., 2010; Hill et al., 2011; Petanovski et al., 2011; Chavarro et al., 2012; Zander-Fox et al., 2012; Bailey et al., 2014; Sharma, 2014; Sifer et al., 2014; Schliep et al., 2015; Kawwass et al., 2016; Provost et al., 2016a, 2016b), frozen-embryo transfers (Insogna et al., 2017) or both (Luke et al., 2011; Pinborg et al., 2011; Bellver et al., 2013; Zhang et al., 2015; Russo et al., 2017) (Table I).
Characteristics of studies included in the meta-analysis.
| Author, year country . | BMI groupsa and number of cycles . | Inclusion criteria . | Exclusion criteria . | Cycle rank . | Autologous or donor oocytes cycle . | Ovarian status of the patients . | Type of embryo transfer . | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal 18.5–24.9 kg/m2 . | Overweight 25.0–29.9 kg/m2 . | Obese ≥ 30.0 kg/m2 . | |||||||
| Bailey et al. (2014), USA | 51 | 19 | 31 | Age <40 years, PCOS, IVF | IVM cycles, FSH >10 IU/L, uncontrolled thyroid disease, previous chemotherapy or radiotherapy, recurrent pregnancy loss, uterine factor, translocation, endometriosis or pelvic inflammatory disease, adenomyosis, myoma | All | Autologous | Only PCOS | Fresh |
| Bellver et al. (2010), Spain | 3930* | 1081 | 419 | All cycles performed | Not specified | All | Not specified | Not specified | Fresh |
| Bellver et al. (2013), Spain | 5706* | 1770 | 653 | First donor cycle, donor being of normal BMI and 18–35 years | Not specified | First | Donor | Not specified | Both |
| Chavarro et al. (2012), USA | 103* | 46 | 37 | Not specified | Not specified | Not specified | Autologous | Both | Fresh |
| Hill et al. (2011), USA | 58 | 38 | 21 | Age 19–42 years, FSH <12 IU/L | FSH ≥12 IU/L, age >42 years | Not specified | Not specified | Not specified | Fresh |
| Insogna et al. (2017), USA | 288 | 106 | 59 | Initial frozen-embryo transfer cycles | Not specified | First | Both | Both | Frozen-thawed |
| Kawwass et al. (2016), USA | 271 985 | 116 788 | 91 646 | All fresh cycles from this time period for which BMI data are available | Cancellation, missing BMI data, cryopreservation with no fresh transfer | All | Autologous | Not specified | Fresh |
| Luke et al. (2011), USA | 25 860 | 10 581 | 7467 | Cycles resulting in transfer of one or more embryos, heigh and weight recorded | Not specified | Not specified | Both | Not specified | Both |
| McCormick et al. (2008), USA | 64 | 30 | Age <42 years, fresh non-donor IVF | Not specified | All | Autologous | Both | Fresh | |
| Petanovski et al. (2011), Macedonia | 533 | 255 | 99 | First cycle, IVF or ICSI, no donor | Not specified | First | Autologous | Both | Fresh |
| Pinborg et al. (2011), Denmark | 702 | 257 | 178 | IVF, ICSI or frozen-embryo transfer | Not specified | All | Not specified | Not specified | Both |
| Provost et al. (2016a), USA | 134 588 | 54 822 | 42 508 | All fresh cycles from this time period for which physiologically reasonable data had been entered for height and weight | BMI >48; BMI <16 | All | Autologous | Both | Fresh |
| Provost et al. (2016b), USA | 13 058 | 5394 | 3228 | Height <48 inches and weight <70 pounds | All | Donor | Not specified | Fresh | |
| Russo et al. (2017), USA | 294 | 64 | 112 | First embryo transfer, age <40 years, single top-quality autologous blastocyst transfer | Congenital uterine anomalies, endometrial polyps, intrauterine synechiae, adenomyosis, intra-cavitary fibroids, hydrosalpinges, donor embryo transfer, age >40 years | First | Autologous | Not specified | Both |
| Schliep et al. (2015), USA | 407 | 147 | 135 | Patients undergoing first fresh IVF cycle | Men with non-obstructive azoospermia | First | Not specified | Not specified | Fresh |
| Sharma (2014), India | 208 | 213 | 69 | Fresh IVF/ICSI cycles, non-donor, age 25–35 years | Age >35 years, frozen-embryo transfer, donor and gestational surrogacy cycles, accompanying medical problem which may lead to abnormal BMI | Not specified | Autologous | Not specified | Fresh |
| Sneed et al. (2008), USA | 613 | 325 | 307 | First fresh non-donor cycle, age 21–44 years | Frozen-embryo transfer, donor oocyte and gestational surrogacy cycles | First | Autologous | Both | Fresh |
| Sifer et al. (2014), France | 260 | 90 | 59 | Age <37 years, first or second IVF cycle, at least two good quality embryos, including at least one top-quality embryo | Single Embryo Transfer for obstetrical cause | All | Not specified | Not specified | Fresh |
| Zander-Fox et al. (2012), Australia | 1065 | 486 | 506 | Gonadotropin-stimulated cycles involving oocyte retrieval and insemination with either partner or donor sperm, age <38 years | Donor cycle, natural cycle | All | Autologous | Not specified | Fresh |
| Zhang et al. (2010), China | 2222 | 379 | 27 | First cycle, receiving aGnRH and r-FSH stimulation protocol | Severe endometriosis, PGD, frozen-embryo transfer | First | Not specified | Both | Fresh |
| Zhang et al. (2015), USA | 243 | 142 | 52 | Male, unexplained, tubal factors | Hypothyroidism, hyperprolactinemia | First | Autologous | Not specified | Both |
| Author, year country . | BMI groupsa and number of cycles . | Inclusion criteria . | Exclusion criteria . | Cycle rank . | Autologous or donor oocytes cycle . | Ovarian status of the patients . | Type of embryo transfer . | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal 18.5–24.9 kg/m2 . | Overweight 25.0–29.9 kg/m2 . | Obese ≥ 30.0 kg/m2 . | |||||||
| Bailey et al. (2014), USA | 51 | 19 | 31 | Age <40 years, PCOS, IVF | IVM cycles, FSH >10 IU/L, uncontrolled thyroid disease, previous chemotherapy or radiotherapy, recurrent pregnancy loss, uterine factor, translocation, endometriosis or pelvic inflammatory disease, adenomyosis, myoma | All | Autologous | Only PCOS | Fresh |
| Bellver et al. (2010), Spain | 3930* | 1081 | 419 | All cycles performed | Not specified | All | Not specified | Not specified | Fresh |
| Bellver et al. (2013), Spain | 5706* | 1770 | 653 | First donor cycle, donor being of normal BMI and 18–35 years | Not specified | First | Donor | Not specified | Both |
| Chavarro et al. (2012), USA | 103* | 46 | 37 | Not specified | Not specified | Not specified | Autologous | Both | Fresh |
| Hill et al. (2011), USA | 58 | 38 | 21 | Age 19–42 years, FSH <12 IU/L | FSH ≥12 IU/L, age >42 years | Not specified | Not specified | Not specified | Fresh |
| Insogna et al. (2017), USA | 288 | 106 | 59 | Initial frozen-embryo transfer cycles | Not specified | First | Both | Both | Frozen-thawed |
| Kawwass et al. (2016), USA | 271 985 | 116 788 | 91 646 | All fresh cycles from this time period for which BMI data are available | Cancellation, missing BMI data, cryopreservation with no fresh transfer | All | Autologous | Not specified | Fresh |
| Luke et al. (2011), USA | 25 860 | 10 581 | 7467 | Cycles resulting in transfer of one or more embryos, heigh and weight recorded | Not specified | Not specified | Both | Not specified | Both |
| McCormick et al. (2008), USA | 64 | 30 | Age <42 years, fresh non-donor IVF | Not specified | All | Autologous | Both | Fresh | |
| Petanovski et al. (2011), Macedonia | 533 | 255 | 99 | First cycle, IVF or ICSI, no donor | Not specified | First | Autologous | Both | Fresh |
| Pinborg et al. (2011), Denmark | 702 | 257 | 178 | IVF, ICSI or frozen-embryo transfer | Not specified | All | Not specified | Not specified | Both |
| Provost et al. (2016a), USA | 134 588 | 54 822 | 42 508 | All fresh cycles from this time period for which physiologically reasonable data had been entered for height and weight | BMI >48; BMI <16 | All | Autologous | Both | Fresh |
| Provost et al. (2016b), USA | 13 058 | 5394 | 3228 | Height <48 inches and weight <70 pounds | All | Donor | Not specified | Fresh | |
| Russo et al. (2017), USA | 294 | 64 | 112 | First embryo transfer, age <40 years, single top-quality autologous blastocyst transfer | Congenital uterine anomalies, endometrial polyps, intrauterine synechiae, adenomyosis, intra-cavitary fibroids, hydrosalpinges, donor embryo transfer, age >40 years | First | Autologous | Not specified | Both |
| Schliep et al. (2015), USA | 407 | 147 | 135 | Patients undergoing first fresh IVF cycle | Men with non-obstructive azoospermia | First | Not specified | Not specified | Fresh |
| Sharma (2014), India | 208 | 213 | 69 | Fresh IVF/ICSI cycles, non-donor, age 25–35 years | Age >35 years, frozen-embryo transfer, donor and gestational surrogacy cycles, accompanying medical problem which may lead to abnormal BMI | Not specified | Autologous | Not specified | Fresh |
| Sneed et al. (2008), USA | 613 | 325 | 307 | First fresh non-donor cycle, age 21–44 years | Frozen-embryo transfer, donor oocyte and gestational surrogacy cycles | First | Autologous | Both | Fresh |
| Sifer et al. (2014), France | 260 | 90 | 59 | Age <37 years, first or second IVF cycle, at least two good quality embryos, including at least one top-quality embryo | Single Embryo Transfer for obstetrical cause | All | Not specified | Not specified | Fresh |
| Zander-Fox et al. (2012), Australia | 1065 | 486 | 506 | Gonadotropin-stimulated cycles involving oocyte retrieval and insemination with either partner or donor sperm, age <38 years | Donor cycle, natural cycle | All | Autologous | Not specified | Fresh |
| Zhang et al. (2010), China | 2222 | 379 | 27 | First cycle, receiving aGnRH and r-FSH stimulation protocol | Severe endometriosis, PGD, frozen-embryo transfer | First | Not specified | Both | Fresh |
| Zhang et al. (2015), USA | 243 | 142 | 52 | Male, unexplained, tubal factors | Hypothyroidism, hyperprolactinemia | First | Autologous | Not specified | Both |
aNormal weight was defined by BMI 20–24.9 kg/m2. PCOS, polycystic ovary syndrome.
Characteristics of studies included in the meta-analysis.
| Author, year country . | BMI groupsa and number of cycles . | Inclusion criteria . | Exclusion criteria . | Cycle rank . | Autologous or donor oocytes cycle . | Ovarian status of the patients . | Type of embryo transfer . | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal 18.5–24.9 kg/m2 . | Overweight 25.0–29.9 kg/m2 . | Obese ≥ 30.0 kg/m2 . | |||||||
| Bailey et al. (2014), USA | 51 | 19 | 31 | Age <40 years, PCOS, IVF | IVM cycles, FSH >10 IU/L, uncontrolled thyroid disease, previous chemotherapy or radiotherapy, recurrent pregnancy loss, uterine factor, translocation, endometriosis or pelvic inflammatory disease, adenomyosis, myoma | All | Autologous | Only PCOS | Fresh |
| Bellver et al. (2010), Spain | 3930* | 1081 | 419 | All cycles performed | Not specified | All | Not specified | Not specified | Fresh |
| Bellver et al. (2013), Spain | 5706* | 1770 | 653 | First donor cycle, donor being of normal BMI and 18–35 years | Not specified | First | Donor | Not specified | Both |
| Chavarro et al. (2012), USA | 103* | 46 | 37 | Not specified | Not specified | Not specified | Autologous | Both | Fresh |
| Hill et al. (2011), USA | 58 | 38 | 21 | Age 19–42 years, FSH <12 IU/L | FSH ≥12 IU/L, age >42 years | Not specified | Not specified | Not specified | Fresh |
| Insogna et al. (2017), USA | 288 | 106 | 59 | Initial frozen-embryo transfer cycles | Not specified | First | Both | Both | Frozen-thawed |
| Kawwass et al. (2016), USA | 271 985 | 116 788 | 91 646 | All fresh cycles from this time period for which BMI data are available | Cancellation, missing BMI data, cryopreservation with no fresh transfer | All | Autologous | Not specified | Fresh |
| Luke et al. (2011), USA | 25 860 | 10 581 | 7467 | Cycles resulting in transfer of one or more embryos, heigh and weight recorded | Not specified | Not specified | Both | Not specified | Both |
| McCormick et al. (2008), USA | 64 | 30 | Age <42 years, fresh non-donor IVF | Not specified | All | Autologous | Both | Fresh | |
| Petanovski et al. (2011), Macedonia | 533 | 255 | 99 | First cycle, IVF or ICSI, no donor | Not specified | First | Autologous | Both | Fresh |
| Pinborg et al. (2011), Denmark | 702 | 257 | 178 | IVF, ICSI or frozen-embryo transfer | Not specified | All | Not specified | Not specified | Both |
| Provost et al. (2016a), USA | 134 588 | 54 822 | 42 508 | All fresh cycles from this time period for which physiologically reasonable data had been entered for height and weight | BMI >48; BMI <16 | All | Autologous | Both | Fresh |
| Provost et al. (2016b), USA | 13 058 | 5394 | 3228 | Height <48 inches and weight <70 pounds | All | Donor | Not specified | Fresh | |
| Russo et al. (2017), USA | 294 | 64 | 112 | First embryo transfer, age <40 years, single top-quality autologous blastocyst transfer | Congenital uterine anomalies, endometrial polyps, intrauterine synechiae, adenomyosis, intra-cavitary fibroids, hydrosalpinges, donor embryo transfer, age >40 years | First | Autologous | Not specified | Both |
| Schliep et al. (2015), USA | 407 | 147 | 135 | Patients undergoing first fresh IVF cycle | Men with non-obstructive azoospermia | First | Not specified | Not specified | Fresh |
| Sharma (2014), India | 208 | 213 | 69 | Fresh IVF/ICSI cycles, non-donor, age 25–35 years | Age >35 years, frozen-embryo transfer, donor and gestational surrogacy cycles, accompanying medical problem which may lead to abnormal BMI | Not specified | Autologous | Not specified | Fresh |
| Sneed et al. (2008), USA | 613 | 325 | 307 | First fresh non-donor cycle, age 21–44 years | Frozen-embryo transfer, donor oocyte and gestational surrogacy cycles | First | Autologous | Both | Fresh |
| Sifer et al. (2014), France | 260 | 90 | 59 | Age <37 years, first or second IVF cycle, at least two good quality embryos, including at least one top-quality embryo | Single Embryo Transfer for obstetrical cause | All | Not specified | Not specified | Fresh |
| Zander-Fox et al. (2012), Australia | 1065 | 486 | 506 | Gonadotropin-stimulated cycles involving oocyte retrieval and insemination with either partner or donor sperm, age <38 years | Donor cycle, natural cycle | All | Autologous | Not specified | Fresh |
| Zhang et al. (2010), China | 2222 | 379 | 27 | First cycle, receiving aGnRH and r-FSH stimulation protocol | Severe endometriosis, PGD, frozen-embryo transfer | First | Not specified | Both | Fresh |
| Zhang et al. (2015), USA | 243 | 142 | 52 | Male, unexplained, tubal factors | Hypothyroidism, hyperprolactinemia | First | Autologous | Not specified | Both |
| Author, year country . | BMI groupsa and number of cycles . | Inclusion criteria . | Exclusion criteria . | Cycle rank . | Autologous or donor oocytes cycle . | Ovarian status of the patients . | Type of embryo transfer . | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal 18.5–24.9 kg/m2 . | Overweight 25.0–29.9 kg/m2 . | Obese ≥ 30.0 kg/m2 . | |||||||
| Bailey et al. (2014), USA | 51 | 19 | 31 | Age <40 years, PCOS, IVF | IVM cycles, FSH >10 IU/L, uncontrolled thyroid disease, previous chemotherapy or radiotherapy, recurrent pregnancy loss, uterine factor, translocation, endometriosis or pelvic inflammatory disease, adenomyosis, myoma | All | Autologous | Only PCOS | Fresh |
| Bellver et al. (2010), Spain | 3930* | 1081 | 419 | All cycles performed | Not specified | All | Not specified | Not specified | Fresh |
| Bellver et al. (2013), Spain | 5706* | 1770 | 653 | First donor cycle, donor being of normal BMI and 18–35 years | Not specified | First | Donor | Not specified | Both |
| Chavarro et al. (2012), USA | 103* | 46 | 37 | Not specified | Not specified | Not specified | Autologous | Both | Fresh |
| Hill et al. (2011), USA | 58 | 38 | 21 | Age 19–42 years, FSH <12 IU/L | FSH ≥12 IU/L, age >42 years | Not specified | Not specified | Not specified | Fresh |
| Insogna et al. (2017), USA | 288 | 106 | 59 | Initial frozen-embryo transfer cycles | Not specified | First | Both | Both | Frozen-thawed |
| Kawwass et al. (2016), USA | 271 985 | 116 788 | 91 646 | All fresh cycles from this time period for which BMI data are available | Cancellation, missing BMI data, cryopreservation with no fresh transfer | All | Autologous | Not specified | Fresh |
| Luke et al. (2011), USA | 25 860 | 10 581 | 7467 | Cycles resulting in transfer of one or more embryos, heigh and weight recorded | Not specified | Not specified | Both | Not specified | Both |
| McCormick et al. (2008), USA | 64 | 30 | Age <42 years, fresh non-donor IVF | Not specified | All | Autologous | Both | Fresh | |
| Petanovski et al. (2011), Macedonia | 533 | 255 | 99 | First cycle, IVF or ICSI, no donor | Not specified | First | Autologous | Both | Fresh |
| Pinborg et al. (2011), Denmark | 702 | 257 | 178 | IVF, ICSI or frozen-embryo transfer | Not specified | All | Not specified | Not specified | Both |
| Provost et al. (2016a), USA | 134 588 | 54 822 | 42 508 | All fresh cycles from this time period for which physiologically reasonable data had been entered for height and weight | BMI >48; BMI <16 | All | Autologous | Both | Fresh |
| Provost et al. (2016b), USA | 13 058 | 5394 | 3228 | Height <48 inches and weight <70 pounds | All | Donor | Not specified | Fresh | |
| Russo et al. (2017), USA | 294 | 64 | 112 | First embryo transfer, age <40 years, single top-quality autologous blastocyst transfer | Congenital uterine anomalies, endometrial polyps, intrauterine synechiae, adenomyosis, intra-cavitary fibroids, hydrosalpinges, donor embryo transfer, age >40 years | First | Autologous | Not specified | Both |
| Schliep et al. (2015), USA | 407 | 147 | 135 | Patients undergoing first fresh IVF cycle | Men with non-obstructive azoospermia | First | Not specified | Not specified | Fresh |
| Sharma (2014), India | 208 | 213 | 69 | Fresh IVF/ICSI cycles, non-donor, age 25–35 years | Age >35 years, frozen-embryo transfer, donor and gestational surrogacy cycles, accompanying medical problem which may lead to abnormal BMI | Not specified | Autologous | Not specified | Fresh |
| Sneed et al. (2008), USA | 613 | 325 | 307 | First fresh non-donor cycle, age 21–44 years | Frozen-embryo transfer, donor oocyte and gestational surrogacy cycles | First | Autologous | Both | Fresh |
| Sifer et al. (2014), France | 260 | 90 | 59 | Age <37 years, first or second IVF cycle, at least two good quality embryos, including at least one top-quality embryo | Single Embryo Transfer for obstetrical cause | All | Not specified | Not specified | Fresh |
| Zander-Fox et al. (2012), Australia | 1065 | 486 | 506 | Gonadotropin-stimulated cycles involving oocyte retrieval and insemination with either partner or donor sperm, age <38 years | Donor cycle, natural cycle | All | Autologous | Not specified | Fresh |
| Zhang et al. (2010), China | 2222 | 379 | 27 | First cycle, receiving aGnRH and r-FSH stimulation protocol | Severe endometriosis, PGD, frozen-embryo transfer | First | Not specified | Both | Fresh |
| Zhang et al. (2015), USA | 243 | 142 | 52 | Male, unexplained, tubal factors | Hypothyroidism, hyperprolactinemia | First | Autologous | Not specified | Both |
aNormal weight was defined by BMI 20–24.9 kg/m2. PCOS, polycystic ovary syndrome.
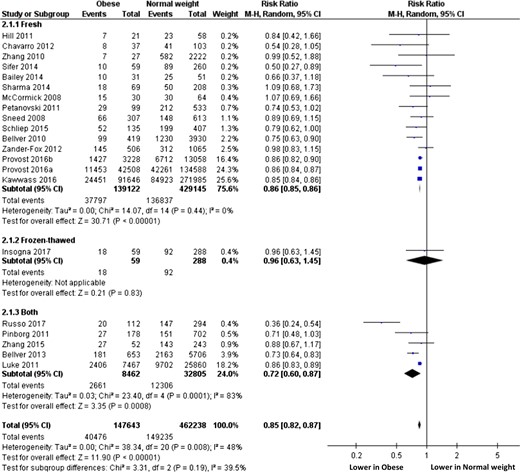
Live birth rates following IVF in obese versus normal weight women
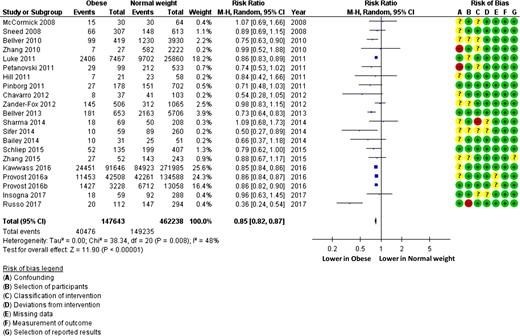
When comparing obese to normal weight women, the RR (95% CI) for live birth following IVF was 0.85 (0.82–0.87) confirming a significant negative association between obesity and live birth (Fig. 2) (n = 609 881 cycles analysed). Heterogeneity was moderate at 48%.
Live birth rate following IVF in obese and normal weight women (random effects model). ‘Events’ relates to IVF cycles leading to live birth, ‘Total’ relates to the total number of IVF cycles included in the study. Obesity was considered BMI ≥ 30 kg/m2, normal weight BMI 18.5–24.9 kg/m2.
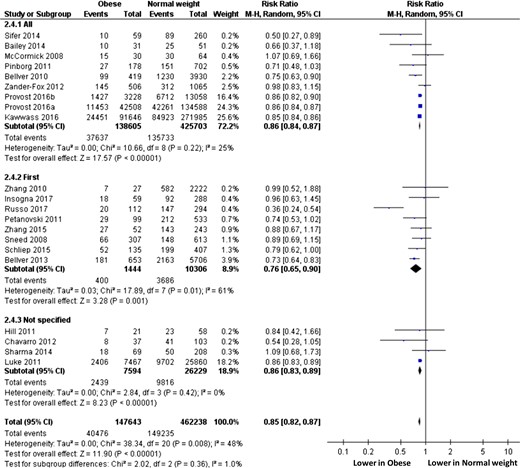
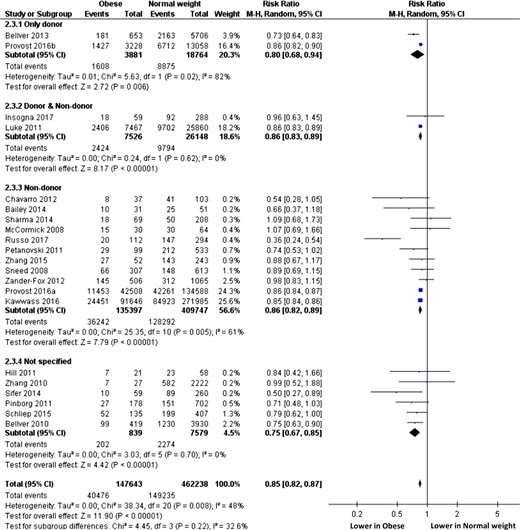
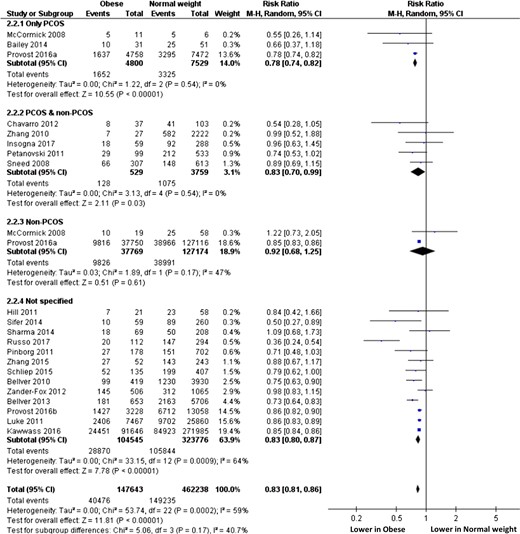
Subgroup analyses performed according to cycle rank (only first cycle, all cycles, unspecified, Fig. 3) or oocyte origin (autologous, oocyte donation, both, unspecified, Fig. 4) did not modify the overall interpretation. Subgroup analyses performed according to embryo transfer type (fresh, frozen, both, Fig. 5) cannot be interpreted due to the selection of only one study evaluating only frozen-embryo transfers. Subgroup analyses performed according to ovarian status (PCOS only, non-PCOS, both, undetermined, Fig. 6) showed significantly lower RR (95% IC) of live birth in obese than in normal weight women when only PCOS patients were selected (0.78, 0.74–0.82), whereas LBR was comparable between obese and normal weight women when only non-PCOS women were considered (RR, 95% IC: 0.92, 0.68–1.25).
Subgroup analysis according to cycle rank. ‘Events’ relates to IVF cycles leading to live birth, ‘Total’ relates to the total number of IVF cycles included in the study.
Subgroup analysis according to oocyte origin. ‘Events’ relates to IVF cycles leading to live birth, ‘Total’ relates to the total number of IVF cycles included in the study.
Subgroup analysis according to embryo transfer type. ‘Events’ relates to IVF cycles leading to live birth, ‘Total’ relates to the total number of IVF cycles included in the study.
Subgroup analysis according to ovarian status. ‘Events’ relates to IVF cycles leading to live birth, ‘Total’ relates to the total number of IVF cycles included in the study.
Sensitivity analyses
The use of the fixed effects model did not modify the result (0.85, 0.85–0.86) (data not shown). Sensitivity analyses revealed an outlier publication (Russo et al., 2017) (Supplementary Fig. S1). Excluding data from Russo et al. did not influence the results: RR (95% CI) for a live birth following IVF was 0.85 (0.84–0.87) for obese women when compared to normal weight women, with very low heterogeneity (Supplementary Fig. S2).
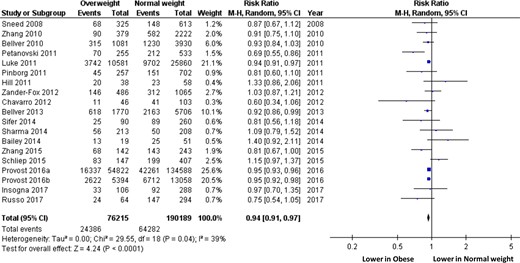
Live birth rates following IVF in overweight versus normal weight women
When compared to normal weight women, the RR (95% CI) for overweight women to obtain a live birth following IVF was 0.94 (0.91–0.97) (Fig. 7) (n = 266 404 cycles analysed). Heterogeneity was moderate (39%).
Live birth rate following IVF in overweight and normal weight women (random effects model). ‘Events’ relates to IVF cycles leading to live birth, ‘Total’ relates to the total number of IVF cycles included in the study.
Risk of bias within studies
Excluding data from articles presenting at least one high risk of bias (Zhang et al., 2010; Petanovski et al., 2011; Sharma, 2014; Russo et al., 2017) did not influence the results: RR (95% IC) for a live birth following IVF was 0.85 (0.84–0.87) for obese women when compared to normal weight women, with moderate heterogeneity (Supplementary Fig. S3 ).
Discussion
This meta-analysis based on 682 532 cycles demonstrates that female obesity has a significant deleterious effect on LBR following IVF. Obese women had a significantly decreased chance of giving birth following IVF when compared with normal weight women (RR, IC95%: 0.85, 0.84–0.87). LBR was also significantly lower in overweight women (RR, IC95%: 0.94, 0.91–0.97). These updated results are in accordance with a previous meta-analysis that showed significantly lower chances of clinical pregnancy and live birth in women who were overweight or obese in 47 967 cycles (Rittenberg et al., 2011).
Impaired ovarian folliculogenesis, oocyte quality, embryonic development and uterine environment might be involved in poorer reproductive outcomes in obese women. However, their respective impacts still remain unclear. Altered function of the hypothalamic-pituitary axis associated with high insulin, androgen and estrogen levels might participate in impairing ovarian folliculogenesis (Jungheim and Moley, 2010). Oocyte quality is the final result of a long intrafollicular process consisting of adequate and simultaneous nuclear and cytoplasmic oocyte maturation. The oocyte is surrounded by granulosa cells and bathes in follicular fluid throughout its maturation. Therefore, any impairment of granulosa cell function and/or follicular fluid composition might impair oocyte maturation, and subsequently oocyte quality. The association between female obesity and alterations of follicular fluid composition has been extensively documented (reviewed in Broughton and Moley, 2017). These metabolic alterations mainly concern lipids, proteins and growth factors, and are associated with increased oxidative stress and disrupted steroidogenesis (Jungheim et al., 2011; Valckx et al., 2012). Higher leptin levels found in obese women have also been shown to affect oocyte and embryo quality (Catteau et al., 2016), as well as granulosa cell function (Lin et al., 2017). Some authors suggested that blastocyst formation may also be impaired in obese women (Comstock et al., 2015). Whether those suspected alterations of oocyte quality might lead to increased risk of embryo aneuploidy in obese women has been recently evaluated. A study of preimplantation genetic testing for aneuploidies did not find any significant association between obesity and aneuploidy rate or the number of euploid embryos (Goldman et al., 2015), suggesting that obesity does not affect oocyte chromosomal competence.
Although this remains controversial, obesity has also been shown to reduce uterine receptivity (Broughton and Moley, 2017). Impaired endometrial stromal cell decidualization has been reported in in vitro studies and animal models (Rhee et al., 2016). Obesity may alter endometrium—embryo cross-talk, leading to impaired implantation (Broughton and Moley, 2017) and leptin could play a key role in this phenomenon (Catteau et al., 2016). Whether this impaired implantation participates in the increased risk of abnormal placentation and early miscarriage found in obese women can be questioned (Metwally et al., 2008).
Our results might suggest a dose-effect relationship, even if the design of our study cannot ascertain it. According to subgroups analyses, prognosis seems to be poorer when obesity is associated with PCOS. Although this result is based only on four studies, it suggests that PCOS could be an independent deleterious prognosis factor. Obesity and PCOS are very intricate pathophysiological situations. Whether each one is causative of the other still remains unclear (Alvarez-Blasco et al., 2006; Yildiz et al., 2008), but it is usually considered that the association of PCOS with obesity exacerbates their respective metabolic dysregulations (Moran et al., 2015; Broughton and Moley, 2017) and thus enhances their overall impact on reproductive functions. This is in accordance with a study that showed alterations in endometrial gene expression during the window of implantation in obese women, especially when associated with PCOS (Bellver et al., 2011). In contrast, oocyte origin (donor or non-donor) did not modify the overall interpretation, contrary to the results reported in a previous meta-analysis (Jungheim et al., 2013). This result has to be interpreted carefully, especially because it is based on only two studies, including one that did not analyse donors’ BMI (Provost et al., 2016b). Importantly, the second study was large, including almost 10 000 first egg donation cycles, and showed a detrimental effect of obesity on reproductive outcome, even after controlling for donor BMI (Bellver et al., 2013). These results could suggest that obesity preferentially negatively alters endometrial receptivity rather that oocyte quality, as also underlined by molecular studies evaluating endometrial gene expression in obese patients (Bellver et al., 2011).
Our study has some limitations. First, LBR being the main outcome, details concerning controlled ovarian stimulation parameters, embryo quality, early pregnancy or miscarriage rates are lacking. However, LBR represents the gold standard for the assessment of IVF results, guaranteeing the homogeneity of the results, in particular by avoiding the variations in the definition of ‘pregnancy rate’. On the other hand, it has to be underlined that data allowing evaluation of cumulative LBR were not available in the included studies. Second, we used the definition of obesity according to the WHO standardized classification of BMI. Although unable to distinguish body fat distribution, it remains the most widely used marker of adiposity in clinical and research settings. Third, we chose to compare obese to normal weight women for the main analysis. The comparison with overweight women was also performed, but as a secondary analysis, and our study was not designed to evaluate a potential dose-effect of the BMI on LBR. Last, even if the quality of the studies included in the meta-analysis was carefully evaluated, they still present a relatively important heterogeneity in terms of population or outcome definitions. The numerous confounding factors that potentially influence the chance of live birth following IVF treatment could not be included in our study. More specifically, confounding factors potentially associated with obesity should be considered with care, such as patient age (McLernon et al., 2016), parity and obstetric history, male partner BMI (Mushtaq et al., 2018), smoking status and intensity (Wang et al., 2016; Carreras-Torres et al., 2018), ethnicity (Wang et al., 2016; Yu, 2016; Luke, 2017), socio-demographic status (Merino Ventosa and Urbanos-Garrido, 2016; Wang et al., 2016; Bilger et al., 2017), indication for IVF treatment or embryo transfer strategy (McLernon et al., 2016). Nevertheless, our meta-analysis offers several strengths, including the largest sample size ever published and robust statistical analysis with very short CIs, stable RRs and low heterogeneity after exclusion of an outlier study or studies presenting high risk of bias.
International recommendations state that infertile couples should be informed of the decreased chances of pregnancy and increased obstetrical and fetal risks in case of female obesity (ESHRE, 2010; NICE, 2013; ASRM, 2015). Some of them even do not recommend IVF in case of female morbid obesity (BMI > 35 kg/m2; RANZCOG, 2014). Although the present meta-analysis does not aim at providing clinical recommendations, our results clearly advocate clear information and counselling of obese infertile women before IVF. Indeed, female obesity has an impact on long-term health of not only mothers but also offspring, as stated by the ‘Developmental Origin of Health and Disease’ hypothesis (Barker, 2007), and preconception period may represent the optimal moment to act. It is currently unclear whether weight loss may improve this negative effect of female obesity on IVF results. Although weight loss has been clearly associated with improved natural fertility (Legro et al., 2016; Best et al., 2017) and is advised in clinical international recommendations (ESHRE, 2010; NICE, 2013; RANZCOG, 2014; ASRM, 2015), two recent randomized controlled trials were not able to find any pregnancy or LBR improvement following weight loss and assisted reproductive technology (ART) procedures (Mutsaerts et al., 2016; Einarsson et al., 2017). However, a significantly higher rate of pregnancy was observed during the time prior to the IVF cycle in both studies. Although both studies presented limitations, such as a high degree of heterogeneity, and poor coaching and follow-up, ultimately leading to little weight reduction, their results question current weight loss strategies in obese infertile women and highlight the need for more prospective trials in order to determine the optimal care strategy that will guarantee the highest LBR with minimal complications and burden. Concerning bariatric surgery, current data remains sparse and controversial (Christofolini et al., 2014; Tsur et al., 2014; Milone et al., 2017), calling for further studies. In particular, although weight loss achieved by diet seems to be associated with significantly improved pregnancy outcomes (Bogaerts et al., 2015; Kalliala et al., 2017), results seem more nuanced when obtained by surgery. Bariatric surgery was indeed associated with lower risks of gestational diabetes and large-for-gestational-age infants (Galazis et al., 2014; Johansson et al., 2015), but also with higher risk of small-for-gestational-age infants and shorter gestation (Galazis et al., 2014; Johansson et al., 2015) and possibly increased mortality (Johansson et al., 2015). Altogether, these data suggest that bariatric surgery should be considered as a last option, after appropriate lifestyle therapy combined with tailored follow-up has been actively attempted.
In conclusion, our meta-analysis clearly demonstrates that female obesity negatively and significantly impacts LBRs following IVF. Whether weight loss through lifestyle modifications or bariatric surgery may reverse this deleterious effect should be further evaluated.
Acknowledgements
We thank MONITORING FORCE (Dr Bernadette Darné and Mrs Solène Languille) and the Inter-university Library of Medicine (BIUM) of Paris for methodological support. We also thank Professor Paul Barrière and Dr Astrid Finet for critical review of this work.
Authors’ roles
NS: data collection and analysis, writing and revising of the manuscript. SH: data collection and analysis, co-writing of the manuscript. VBL: data collection and analysis, co-writing of the manuscript. EAJ: study design and supervision. VG: data collection and analysis, co-writing of the manuscript. MC: study design, data collection and analysis, co-writing of the manuscript. TF: study design and supervision, data collection and analysis, writing and revising of the manuscript, validation of the final version of the manuscript. All authors approved the final version of the manuscript.
Funding
This work was sponsored by an unrestricted grant from GEDEON-RICHTER France.
References
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration,