-
PDF
- Split View
-
Views
-
Cite
Cite
Albert Salas-Huetos, Mònica Bulló, Jordi Salas-Salvadó, Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies, Human Reproduction Update, Volume 23, Issue 4, July-August 2017, Pages 371–389, https://doi.org/10.1093/humupd/dmx006
Close - Share Icon Share
Abstract
Infertility is a global public health issue, affecting 15% of all couples of reproductive age. Male factors, including decreased semen quality, are responsible for ~25% of these cases. The dietary pattern, the components of the diet and nutrients have been studied as possible determinants of sperm function and/or fertility.
Previous systematic reviews have been made of the few heterogeneous low-quality randomized clinical trials (RCTs) conducted in small samples of participants and investigating the effect of specific nutrients and nutritional supplements on male infertility. However, as yet there has been no systematic review of observational studies.
A comprehensive systematic review was made of the published literature, from the earliest available online indexing year to November 2016, in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We have included cross-sectional, case-control and prospective and retrospective studies in which fertile/infertile men were well defined (men with sperm disorders, sperm DNA damage, varicocele or idiopathic infertility). The primary outcomes were semen quality or fecundability. With the data extracted, we evaluated and scored the quality of the studies selected. We excluded RCTs, animal studies, review articles and low-quality studies.
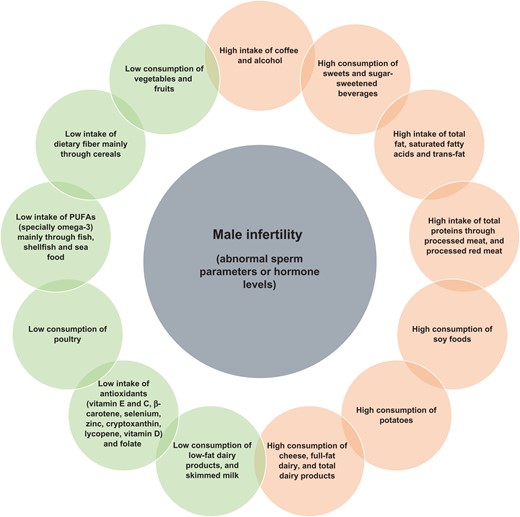
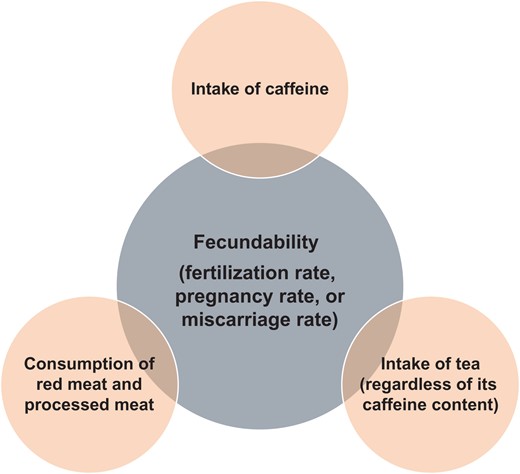
A total of 1944 articles were identified, of which 35 were selected for qualitative analysis. Generally, the results indicated that healthy diets rich in some nutrients such as omega-3 fatty acids, some antioxidants (vitamin E, vitamin C, β-carotene, selenium, zinc, cryptoxanthin and lycopene), other vitamins (vitamin D and folate) and low in saturated fatty acids and trans-fatty acids were inversely associated with low semen quality parameters. Fish, shellfish and seafood, poultry, cereals, vegetables and fruits, low-fat dairy and skimmed milk were positively associated with several sperm quality parameters. However, diets rich in processed meat, soy foods, potatoes, full-fat dairy and total dairy products, cheese, coffee, alcohol, sugar-sweetened beverages and sweets have been detrimentally associated with the quality of semen in some studies. As far as fecundability is concerned, a high intake of alcohol, caffeine and red meat and processed meat by males has a negative influence on the chance of pregnancy or fertilization rates in their partners.
Male adherence to a healthy diet could improve semen quality and fecundability rates. Since observational studies may prove associations but not causation, the associations summarized in the present review need to be confirmed with large prospective cohort studies and especially with well-designed RCTs.
Introduction
Infertility is defined as the failure to achieve a successful pregnancy after 12 months or more of regular unprotected intercourse. In recent decades infertility has become a global public health issue and a major clinical concern, affecting 15% of all reproductive age couples. It has been estimated that 70 million couples worldwide experience subfertility or infertility (Boivin et al., 2007). Male factors, including decreased semen quality, are responsible for ~25% of cases of infertility (Evers, 2002; Sharlip et al., 2002) and, in the USA, the prevalence of men seeking help for fertility is estimated at 3.3–4.7 million (Anderson et al., 2009).
Some studies suggest that human semen quality has declined in certain geographic regions of the world (e.g. Europe and USA) (Merzenich et al., 2010; Mendiola et al., 2013). Currently, the etiology of suboptimal semen quality is poorly understood, and many physiological, environmental and genetic factors, including oxidative stress, have been implicated (WHO, 2010; Jungwirth et al., 2012).
Environmental factors such as air pollution, smoking, stress, chemicals and other toxic agents in the diet have all been considered as possibly responsible for the decrease in semen quality observed in developed countries (Carlsen et al., 1992; Merzenich et al., 2010). In terms of the diet, since the 1980s several components and nutrients have been considered as possible determinants of sperm function, fertility or normal function of the reproductive system (Abbasi et al., 1979).
Accumulating evidence from human in vitro and animal studies indicates that male obesity and some components of the diet may play a pivotal role in modulating spermatogenesis, sperm maturation and fertilizing ability. For example, male obesity has been related to impaired reproductivity because of its effect on the molecular and physical structure of sperm (Mitchell et al., 2011; Palmer et al., 2012a,b). In addition, several foods and some components of the diet that have been associated with an increased risk of obesity, insulin resistance and diabetes have also been related to low sperm quality or function in animal models. For example, diets rich in calories (Rato et al., 2014), trans-fatty acids (TFAs), saturated fats (Ng et al., 2010) or cholesterol (Morgan et al., 2014) have been associated to testicular disruption, involving impairments in spermatogenesis potentially affecting male fertility and the offspring.
Likewise, several cross-sectional, case-control, retrospective and prospective observational studies, some of which were conducted in large samples of individuals, have assessed the associations between diet and semen quality and/or fecundability, with controversial results.
In spite of this, there are many assisted reproductive clinics that recommend simple lifestyle changes such as increases in physical activity, cognitive behavioral therapy and yoga to reduce stress, give advice on how to reduce alcohol and caffeine intake or provide lists of dietary recommendations (Collins and Rossi, 2015) in order to improve semen quality and fertility chances. Nonetheless, reality shows how important it is to have a better understanding of the effect of lifestyle and diet on male fertility before useful recommendations can be made.
Recently, a review was published of randomized clinical trials (RCTs) investigating the effect of specific nutrients and nutritional supplements on male infertility (Giahi et al., 2016). In total, 12 heterogeneous and low-quality RCTs, conducted in small samples of participants, investigating the effect of specific nutrients and nutritional supplements on male infertility were systematically reviewed. Oral complexes of selenium; selenium plus vitamin A; vitamin C; vitamin E; L-carnitine plus L-acetylcarnitine; beta-carotene, alpha-tocopherol and arachidonic acid; coenzyme Q10; clomiphene citrate plus vitamin E; eicoseptanoic plus docohexanoid acid; and ubiquinol were used in an attempt to improve such classical sperm quality parameters as sperm concentration, motility and morphology or sperm DNA fragmentation (SDF). Only a few studies using supplements of carnitine, coenzyme Q10 and selenium have demonstrated some beneficial effects on sperm parameters although they have been unable to give clear explanations about the potential underlying mechanisms. Therefore, Giahi et al. (2016) concluded that studies have reported contradictory evidence on the role that dietary compounds play in male infertility and that large, well-designed RCTs are warranted in the future to better establish recommendations.
In spite of the lack of evidence about the role diet plays in sperm parameters and the effectiveness of supplements to combat male infertility, there has been an invasion of integrative dietary products in the last two decades in some ART clinics. Unfortunately, the safety of these dietary supplements has not been tested, and the dangers for the user population are unknown.
In an attempt to provide a wide-ranging vision of the field and extend the conclusions of Giahi et al. (2016) review, the aim of the present analysis was to systematically review all those observational studies investigating the relationships of diet, food and nutrient consumption to sperm quality and male fecundability.
Methods
Protocol and registration
The protocol of the present study has been registered (PROSPERO 2016: CRD42016039410) in the PROSPERO registry (http://www.crd.york.ac.uk/PROSPERO), an international database for the prospective registration of systematic reviews in health and social care.
Information sources
We conducted a systematic search of the literature published in the MEDLINE-Pubmed database (http://www.ncbi.nlm.nih.gov/pubmed) and a hand searched reference list, from the earliest available online indexing year until November 2016, in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Liberati et al., 2009).
The search used a combination of terms as both Medical Subject Headings and keywords. The search strategy used male infertility-related keywords and words related to nutrition and diet: fertility OR infertility OR male fertility OR male infertility OR sperm dysfunction OR sperm dysfunctions OR sperm DNA damage OR varicocele OR asthenozoospermia OR oligozoospermia OR oligoasthenozoospermia OR oligoasthenoteratozoospermia OR teratozoospermia AND mediterranean diet OR diet OR nutrients OR food OR nuts OR vitamin C OR vitamin E OR zinc OR antioxidants OR vitamins OR cereals OR meat OR vegetables OR fruit OR fish OR legumes OR milk OR yogurt OR cheese OR seeds OR eggs OR dairy product OR micronutrient OR macronutrient OR alcohol OR alcohol consumption OR selenium OR fatty acids OR sugar. We applied the following inclusion filters: Classical Article, Clinical Study, Comparative Study, Congresses Dataset, English Abstract, Evaluation Studies, Introductory Journal Article, Journal Article, Letter, Meta-Analysis, Multicenter Study, Observational Study, Abstract, Humans, Male and English.
Eligibility criteria, search and study selection
The titles and abstracts of all the articles were screened for eligibility by the three authors, who were specialists in male (in)fertility and nutrition. We included case-control, cross-sectional and observational prospective and retrospective studies in which fertile/infertile men were well defined (men with sperm disorders, sperm DNA damage, varicocele or idiopathic infertility). In addition, the primary outcomes of the studies were semen quality (volume, motility, morphology, sperm count or concentration, sperm DNA damage or chromatin integrity, sperm aneuploidies and hormonal level) or fecundability (fertilization rate, pregnancy rate or miscarriage rate). We excluded RCTs, animal studies, review articles and low-quality studies (see quality assessment section). After the primary screening (evaluation of the scope of the study), and once quality and compliance with all the inclusion/exclusion criteria had been evaluated, the full text of the selected articles was obtained.
Data extraction
We extracted the following information from each study: author/s, year of publication, journal, title of the article, location of the study, cohort name (if appropriate), age, infertility problem, number of patients or participants (sample size), study design, exposure (nutrient, food, food group or dietary pattern), primary outcomes and major findings or principal conclusion. After the data had been extracted, they were checked by the authors for discrepancies in order to minimize the possibility of errors.
Quality assessment
Using all the data extracted, we evaluated and scored the quality of the studies selected on a six-point scale (Hayden et al., 2006). The quality scores were assessed in parallel by the three authors, and discrepancies were re-evaluated together. With this system, we assessed the quality of individual studies using the following criteria (one point per criterion): (i) study participation (the study sample represents the key characteristics of the population of interest sufficiently well to limit potential bias to the results); (ii) study attrition (loss to follow-up is not associated with key characteristics); (iii) prognostic factor measurement (the prognostic factor of interest is measured in study participants in such a way that potential bias is limited); (iv) confounding measurement and account (the outcome of interest is measured in study participants in such a way that potential bias is limited); (v) outcome measurement (important potential confounders are appropriately accounted for, limiting potential bias with respect to the prognostic factor of interest); and (vi) analysis (the statistical analysis is appropriate for the design of the study, and limits the potential for invalid results). Studies with a score between 0 and 3 points were considered to be of low quality, while >3 to 6 were considered to be of high quality.
Results
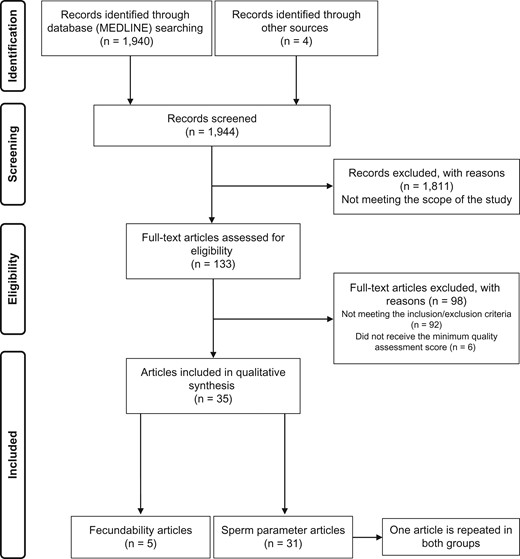
We identified 1940 articles after a primary search by MEDLINE-Pubmed and four from other sources (Google Scholar and reviews references) (Fig. 1). By analyzing the abstracts (n = 1944), we screened and excluded 1811 for reasons of the scope of the study. A total of 133 articles were collected as full texts so that the inclusion/exclusion criteria and quality could be assessed: 92 articles were excluded because they did not meet the inclusion/exclusion criteria and six articles because they were not given the minimum quality assessment score. After applying all the eligibility parameters, 35 articles were included for qualitative analysis.
Summary of selected studies and design
The articles included subjects from 18 countries: Argentine, Brazil, Canada, Denmark, Estonia, Finland, France, Germany, Greece, Iran, Italy, Lithuania, the Netherlands, Norway, Poland, Spain, Sweden and USA. The age of the participants ranged between 18 and 80 years old. There were 11 cross-sectional studies (n = 21 articles), six case-control studies (n = 8 articles), three prospective studies and three retrospective studies.
Primary outcomes of interest
Of the 35 articles included, 31 (n = 12 672 participants) evaluated the effect of dietary patterns and food intake on sperm parameters and quality (Table I) (Goverde et al., 1995; Serra-Majem et al., 2003; Stutz et al., 2004; Eskenazi et al., 2005; Silver et al., 2005; Chavarro et al., 2008, 2014; Young et al., 2008; Mendiola et al., 2009, 2010; Vujkovic et al., 2009; Attaman et al., 2012; Braga et al., 2012; Eslamian et al., 2012, 2015, 2016; Gaskins et al., 2012; Mínguez-Alarcón et al., 2012; Schmid et al., 2012; Afeiche et al., 2013, 2014a,b,c; Jensen et al., 2013, 2014; Zareba et al., 2013; Anifandis et al., 2014; Chiu et al., 2014; de Jong et al., 2014; Cutillas-Tolin et al., 2015; Karayiannis et al., 2016), and five (n = 13 125 participants) on fecundability (Table II) (Florack et al., 1994; Curtis et al., 1997; Olsen et al., 1997; Braga et al., 2012; Xia et al., 2015). It should be pointed out that the Braga et al. (2012) study is included in the two primary outcome groups: sperm parameters and fecundability of partners.
Summary of the 31 studies that investigated associations between nutrition and sperm parameters.
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality score* . |
|---|---|---|---|---|---|---|---|---|
| Stutz et al. (2004) | Argentine | 20–30 | 34 healthy participants | Retrospective | Alcohol frequency intake questionnaire | Plasma testosterone and sperm quality (volume, vitality, motility, morphology and concentration) | Non-significant associations between alcohol consumption and routine semen parameters were found. | 4.5 |
| Goverde et al. (1995) | The Netherlands | Controls (32.5 ± 3.8) Cases (33.2 ± 3.5) | 68 controls and 47 cases with poor semen quality attending an infertility clinic | Case-control | Alcohol and caffeine frequency intake questionnaire | Sperm quality (volume, motility, morphology and concentration) | A lower percentage of normal sperm morphology was observed in the daily alcohol drinkers. No differences with respect to alcohol consumption between male patients with poor semen quality and controls. | 4 |
| Serra-Majem et al. (2003) | Spain | 30–50 | 379 fertile controls and 405 cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | The ingestion of cyclamate, aspartame and saccharin has no association with sperm quality. | 5.5 |
| Mendiola et al. (2009) | Spain | Cases (34.2 ± 3.7) Controls (32.8 ± 3.9) | 31 fertile controls and 30 infertile cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology and concentration) and hormonal levels (FSH, LH, T) | Controls had a higher intake of skimmed milk, shellfish, tomatoes, and lettuce, and cases consumed more yogurt, meat products and potatoes. In the logistic regression model, cases had a lower intake of lettuce and tomatoes, fruits (apricots and peaches), and a significantly higher intake of dairy and processed meat products. | 5 |
| Mendiola et al. (2010) | Compared to cases, control subjects presented significantly higher intakes of carbohydrates, fiber, folate, vitamin C and lycopene and lower intakes of proteins and total fat. | 5 | ||||||
| de Jong et al. (2014) | The Netherlands | Cases (41 ± 5) Controls (38 ± 7) | 121 fertile male controls and 42 infertile cases with asthenozoospermia | Case-control | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Alcohol consumption was not associated with such sperm parameters as volume, sperm count, motility and morphology or pregnancy outcome. | 4.5 |
| Eslamian et al. (2012) | Iran | Cases (32.2 ± 3.5) Controls (33.5 ± 3.7) | 169 normozoospermic controls and 72 asthenozoospermic cases | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T and PRL) | An increased intake of fruits and vegetables (especially oranges, tomatoes and dark green vegetables), poultry, skimmed milk and seafood was associated with a significantly lower risk of asthenozoospermia. An increased intake of processed meats, dairy products and sweets was associated with a significantly higher risk of asthenozoospermia. | 5.5 |
| Eslamian et al. (2015) | Iran | Cases (32.8 ± 3.6) Controls (33.4 ± 3.5) | 235 normospermic controls and 107 asthenozoospermic cases | Case-control | FFQ | Sperm quality and endocrine parameters (FSH, LH, T and PRL) | A high intake of saturated fatty acids, TFAs, palmitic acid and stearic acid was positively related to the odds of having asthenozoospermia. Inverse and dose-dependent associations were found between asthenozoospermia and intake of omega-3 PUFAs and docosahexaenoic acid. | 5.5 |
| Eslamian et al. (2016) | A high intake of vitamin E, vitamin D, vitamin C, zinc, selenium and PUFAs was significantly associated with a lower risk of asthenozoospermia. | 5.5 | ||||||
| Eskenazi et al. (2005) | USA | 20–80 | 87–97 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) | Positive associations were observed between vitamin C intake and sperm number as reflected in the higher mean count, concentration and total progressively motile sperm count; between vitamin E intake and progressive motility or total progressively motile sperm count; and between b-carotene intake and sperm concentration or progressive motility. Folate and zinc intake were not associated with improved semen quality. | 4.5 |
| Silver et al. (2005) | Sperm chromatin integrity | Non-significant associations between high antioxidant intake and sperm chromatin integrity in men with fertility problems. | 5 | |||||
| Young et al. (2008) | Sperm aneuploidy | Men with high folate intake had lower overall frequencies of several types of sperm aneuploidy. | 4.5 | |||||
| Vujkovic et al. (2009) | The Netherlands | 28.6–53.9 | 161 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) and sperm DNA damage | The ‘Health Conscious’ diet was inversely associated with SDF. The ‘Traditional Dutch’ diet was positively correlated with sperm concentration. | 5 |
| Mínguez-Alarcón et al. (2012) | Spain | 18–23 | 209–215 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A positive association was observed between the dietary intake of cryptoxanthin, vitamin C, lycopene and b-carotene and the total motile sperm count. The semen volume is increased in those individuals with a high intake of vitamin C, lycopene and b-carotene. | 5 |
| Chavarro et al. (2014) | Cholesterol intake was inversely related to the ejaculate volume. Intake of TFAs was inversely related to the total sperm count. | 5.5 | ||||||
| Cutillas-Tolin et al. (2015) | The Mediterranean pattern was positively associated with total sperm count. The Western pattern was positively related to the percentage of morphologically normal sperm. | 5.5 | ||||||
| Chavarro et al. (2008) | USA | 18–55 (36.4 ± 5) | 99 male partners of couples attending an infertility clinic | Cross-sectional | FFQ evaluating soy product consumption | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A higher intake of soy foods was associated with a lower sperm concentration. | 4 |
| Attaman et al. (2012) | FFQ | Total fat intake was negatively related to total sperm count and sperm concentration. The intake of omega-3 PUFAs was positively related to normal sperm morphology. | 5.5 | |||||
| Schmid et al. (2012) | USA | 22–80 | 80 healthy participants | Cross-sectional | FFQ | Sperm DNA damage | Men with the highest intake of vitamin C had less SDF than men with the lowest intake. Findings were similar for vitamin E, folate and zinc (but not b-carotene). Older men (>44 years) with the highest vitamin C intake had less SDF than older men with the lowest intake. Findings were similar for vitamin E and zinc. Men (especially older men) with higher dietary and supplement intake of certain micronutrients may produce sperm with less SDF. | 4.5 |
| Jensen et al. (2013) | Denmark | 18.4–22.9 | 701 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Individuals with a higher intake of saturated fatty acids had lower sperm concentrations and lower total sperm count. The percentage of spermatozoa with normal morphology was lower among men with a high percentage of energy from monounsaturated fatty acids, and semen volume was higher among men with a high intake of omega-3 fatty acids. | 4.5 |
| Gaskins et al. (2012) | USA | 18.9–20.5 | 188–189 healthy participants | Cross-sectional | FFQ | Sperm quality (motility, morphology and sperm concentration) | A Prudent dietary pattern was associated with higher progressive sperm motility and unrelated to sperm concentration and morphology. The consumption of a Western dietary pattern was unrelated to rutine semen parameters. | 5 |
| Zareba et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Lycopene intake was associated with better sperm morphology. High dietary intake of vitamin C was associated with lower sperm concentration and sperm count. | 5.5 | |||||
| Afeiche et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Intake of full-fat dairy products was inversely related to sperm motility and morphology. | 5 | |||||
| Chiu et al. (2014) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Higher consumption of sugar-sweetened beverages was associated with lower sperm motility. Sugar-sweetened beverage intake was unrelated to other semen quality parameters or reproductive hormone levels. | 5.5 | |||||
| Afeiche et al. (2014c) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Processed red meat intake was inversely related to total sperm count and total progressive motile sperm count. | 5.5 | |||||
| Afeiche et al. (2014a) | USA | 18–55 | 155 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Low-fat dairy intake, particularly low-fat milk, was related to a higher sperm concentration and progressive motility, whereas cheese intake is related to a lower sperm concentration. | 5.5 |
| Afeiche et al. (2014b) | Processed meat intake was negatively associated with sperm morphology, whereas fish intake was positively associated with total sperm count and sperm morphology. | 5.5 | ||||||
| Anifandis et al. (2014) | Greece | 37.4 ± 0.3 | 207 potentially infertile | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, number of spermatozoa and concentration) and SDF | Alcohol consumption was associated with a lower sperm volume and higher SDF. Cigarrete plus alcohol consumption was associated with higher SDF. | 3.5 |
| Jensen et al. (2014) | Europe and USA | 18–45 | 8344 healthy participants | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T, Inhibin B, SHBG) | Moderate alcohol intake is not adversely associated with semen quality, but is associated with higher levels of serum testosterone. | 5.5 |
| Karayiannis et al. (2016) | Greece | 26–55 | 225 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Greater adherence to the MedDiet, was significantly associated with higher sperm concentration, total sperm count and sperm motility. | 5.5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Sperm quality (motility, morphology, and concentration) | Sperm concentration was negatively related to alcohol consumption and positively associated to cereal consumption. Sperm motility was positively associated with the consumption of fruits and cereals, and negatively associated with alcohol and coffee consumption. | 4.5 |
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality score* . |
|---|---|---|---|---|---|---|---|---|
| Stutz et al. (2004) | Argentine | 20–30 | 34 healthy participants | Retrospective | Alcohol frequency intake questionnaire | Plasma testosterone and sperm quality (volume, vitality, motility, morphology and concentration) | Non-significant associations between alcohol consumption and routine semen parameters were found. | 4.5 |
| Goverde et al. (1995) | The Netherlands | Controls (32.5 ± 3.8) Cases (33.2 ± 3.5) | 68 controls and 47 cases with poor semen quality attending an infertility clinic | Case-control | Alcohol and caffeine frequency intake questionnaire | Sperm quality (volume, motility, morphology and concentration) | A lower percentage of normal sperm morphology was observed in the daily alcohol drinkers. No differences with respect to alcohol consumption between male patients with poor semen quality and controls. | 4 |
| Serra-Majem et al. (2003) | Spain | 30–50 | 379 fertile controls and 405 cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | The ingestion of cyclamate, aspartame and saccharin has no association with sperm quality. | 5.5 |
| Mendiola et al. (2009) | Spain | Cases (34.2 ± 3.7) Controls (32.8 ± 3.9) | 31 fertile controls and 30 infertile cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology and concentration) and hormonal levels (FSH, LH, T) | Controls had a higher intake of skimmed milk, shellfish, tomatoes, and lettuce, and cases consumed more yogurt, meat products and potatoes. In the logistic regression model, cases had a lower intake of lettuce and tomatoes, fruits (apricots and peaches), and a significantly higher intake of dairy and processed meat products. | 5 |
| Mendiola et al. (2010) | Compared to cases, control subjects presented significantly higher intakes of carbohydrates, fiber, folate, vitamin C and lycopene and lower intakes of proteins and total fat. | 5 | ||||||
| de Jong et al. (2014) | The Netherlands | Cases (41 ± 5) Controls (38 ± 7) | 121 fertile male controls and 42 infertile cases with asthenozoospermia | Case-control | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Alcohol consumption was not associated with such sperm parameters as volume, sperm count, motility and morphology or pregnancy outcome. | 4.5 |
| Eslamian et al. (2012) | Iran | Cases (32.2 ± 3.5) Controls (33.5 ± 3.7) | 169 normozoospermic controls and 72 asthenozoospermic cases | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T and PRL) | An increased intake of fruits and vegetables (especially oranges, tomatoes and dark green vegetables), poultry, skimmed milk and seafood was associated with a significantly lower risk of asthenozoospermia. An increased intake of processed meats, dairy products and sweets was associated with a significantly higher risk of asthenozoospermia. | 5.5 |
| Eslamian et al. (2015) | Iran | Cases (32.8 ± 3.6) Controls (33.4 ± 3.5) | 235 normospermic controls and 107 asthenozoospermic cases | Case-control | FFQ | Sperm quality and endocrine parameters (FSH, LH, T and PRL) | A high intake of saturated fatty acids, TFAs, palmitic acid and stearic acid was positively related to the odds of having asthenozoospermia. Inverse and dose-dependent associations were found between asthenozoospermia and intake of omega-3 PUFAs and docosahexaenoic acid. | 5.5 |
| Eslamian et al. (2016) | A high intake of vitamin E, vitamin D, vitamin C, zinc, selenium and PUFAs was significantly associated with a lower risk of asthenozoospermia. | 5.5 | ||||||
| Eskenazi et al. (2005) | USA | 20–80 | 87–97 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) | Positive associations were observed between vitamin C intake and sperm number as reflected in the higher mean count, concentration and total progressively motile sperm count; between vitamin E intake and progressive motility or total progressively motile sperm count; and between b-carotene intake and sperm concentration or progressive motility. Folate and zinc intake were not associated with improved semen quality. | 4.5 |
| Silver et al. (2005) | Sperm chromatin integrity | Non-significant associations between high antioxidant intake and sperm chromatin integrity in men with fertility problems. | 5 | |||||
| Young et al. (2008) | Sperm aneuploidy | Men with high folate intake had lower overall frequencies of several types of sperm aneuploidy. | 4.5 | |||||
| Vujkovic et al. (2009) | The Netherlands | 28.6–53.9 | 161 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) and sperm DNA damage | The ‘Health Conscious’ diet was inversely associated with SDF. The ‘Traditional Dutch’ diet was positively correlated with sperm concentration. | 5 |
| Mínguez-Alarcón et al. (2012) | Spain | 18–23 | 209–215 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A positive association was observed between the dietary intake of cryptoxanthin, vitamin C, lycopene and b-carotene and the total motile sperm count. The semen volume is increased in those individuals with a high intake of vitamin C, lycopene and b-carotene. | 5 |
| Chavarro et al. (2014) | Cholesterol intake was inversely related to the ejaculate volume. Intake of TFAs was inversely related to the total sperm count. | 5.5 | ||||||
| Cutillas-Tolin et al. (2015) | The Mediterranean pattern was positively associated with total sperm count. The Western pattern was positively related to the percentage of morphologically normal sperm. | 5.5 | ||||||
| Chavarro et al. (2008) | USA | 18–55 (36.4 ± 5) | 99 male partners of couples attending an infertility clinic | Cross-sectional | FFQ evaluating soy product consumption | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A higher intake of soy foods was associated with a lower sperm concentration. | 4 |
| Attaman et al. (2012) | FFQ | Total fat intake was negatively related to total sperm count and sperm concentration. The intake of omega-3 PUFAs was positively related to normal sperm morphology. | 5.5 | |||||
| Schmid et al. (2012) | USA | 22–80 | 80 healthy participants | Cross-sectional | FFQ | Sperm DNA damage | Men with the highest intake of vitamin C had less SDF than men with the lowest intake. Findings were similar for vitamin E, folate and zinc (but not b-carotene). Older men (>44 years) with the highest vitamin C intake had less SDF than older men with the lowest intake. Findings were similar for vitamin E and zinc. Men (especially older men) with higher dietary and supplement intake of certain micronutrients may produce sperm with less SDF. | 4.5 |
| Jensen et al. (2013) | Denmark | 18.4–22.9 | 701 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Individuals with a higher intake of saturated fatty acids had lower sperm concentrations and lower total sperm count. The percentage of spermatozoa with normal morphology was lower among men with a high percentage of energy from monounsaturated fatty acids, and semen volume was higher among men with a high intake of omega-3 fatty acids. | 4.5 |
| Gaskins et al. (2012) | USA | 18.9–20.5 | 188–189 healthy participants | Cross-sectional | FFQ | Sperm quality (motility, morphology and sperm concentration) | A Prudent dietary pattern was associated with higher progressive sperm motility and unrelated to sperm concentration and morphology. The consumption of a Western dietary pattern was unrelated to rutine semen parameters. | 5 |
| Zareba et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Lycopene intake was associated with better sperm morphology. High dietary intake of vitamin C was associated with lower sperm concentration and sperm count. | 5.5 | |||||
| Afeiche et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Intake of full-fat dairy products was inversely related to sperm motility and morphology. | 5 | |||||
| Chiu et al. (2014) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Higher consumption of sugar-sweetened beverages was associated with lower sperm motility. Sugar-sweetened beverage intake was unrelated to other semen quality parameters or reproductive hormone levels. | 5.5 | |||||
| Afeiche et al. (2014c) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Processed red meat intake was inversely related to total sperm count and total progressive motile sperm count. | 5.5 | |||||
| Afeiche et al. (2014a) | USA | 18–55 | 155 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Low-fat dairy intake, particularly low-fat milk, was related to a higher sperm concentration and progressive motility, whereas cheese intake is related to a lower sperm concentration. | 5.5 |
| Afeiche et al. (2014b) | Processed meat intake was negatively associated with sperm morphology, whereas fish intake was positively associated with total sperm count and sperm morphology. | 5.5 | ||||||
| Anifandis et al. (2014) | Greece | 37.4 ± 0.3 | 207 potentially infertile | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, number of spermatozoa and concentration) and SDF | Alcohol consumption was associated with a lower sperm volume and higher SDF. Cigarrete plus alcohol consumption was associated with higher SDF. | 3.5 |
| Jensen et al. (2014) | Europe and USA | 18–45 | 8344 healthy participants | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T, Inhibin B, SHBG) | Moderate alcohol intake is not adversely associated with semen quality, but is associated with higher levels of serum testosterone. | 5.5 |
| Karayiannis et al. (2016) | Greece | 26–55 | 225 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Greater adherence to the MedDiet, was significantly associated with higher sperm concentration, total sperm count and sperm motility. | 5.5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Sperm quality (motility, morphology, and concentration) | Sperm concentration was negatively related to alcohol consumption and positively associated to cereal consumption. Sperm motility was positively associated with the consumption of fruits and cereals, and negatively associated with alcohol and coffee consumption. | 4.5 |
E2, estradiol; FFQ, food frequency questionnaire; MedDiet, mediterranean diet; PRL, prolactin; PUFAs, polyunsaturated fatty acid; SDF, sperm DNA fragmentation; SHBG, sex hormone binding globulin; T, testosterone; TFAs, trans-fatty acids.
*We evaluated and scored the quality of the studies selected on a six-point scale (Hayden et al., 2006): (i) study participation; (ii) study attrition; (iii) prognostic factor measurement; (iv) confounding measurement and account; (v) outcome measurement; and (vi) analysis. Studies with a score between 0 and 3 points were considered to be of low quality, while >3 to 6 were considered to be of high quality.
Summary of the 31 studies that investigated associations between nutrition and sperm parameters.
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality score* . |
|---|---|---|---|---|---|---|---|---|
| Stutz et al. (2004) | Argentine | 20–30 | 34 healthy participants | Retrospective | Alcohol frequency intake questionnaire | Plasma testosterone and sperm quality (volume, vitality, motility, morphology and concentration) | Non-significant associations between alcohol consumption and routine semen parameters were found. | 4.5 |
| Goverde et al. (1995) | The Netherlands | Controls (32.5 ± 3.8) Cases (33.2 ± 3.5) | 68 controls and 47 cases with poor semen quality attending an infertility clinic | Case-control | Alcohol and caffeine frequency intake questionnaire | Sperm quality (volume, motility, morphology and concentration) | A lower percentage of normal sperm morphology was observed in the daily alcohol drinkers. No differences with respect to alcohol consumption between male patients with poor semen quality and controls. | 4 |
| Serra-Majem et al. (2003) | Spain | 30–50 | 379 fertile controls and 405 cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | The ingestion of cyclamate, aspartame and saccharin has no association with sperm quality. | 5.5 |
| Mendiola et al. (2009) | Spain | Cases (34.2 ± 3.7) Controls (32.8 ± 3.9) | 31 fertile controls and 30 infertile cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology and concentration) and hormonal levels (FSH, LH, T) | Controls had a higher intake of skimmed milk, shellfish, tomatoes, and lettuce, and cases consumed more yogurt, meat products and potatoes. In the logistic regression model, cases had a lower intake of lettuce and tomatoes, fruits (apricots and peaches), and a significantly higher intake of dairy and processed meat products. | 5 |
| Mendiola et al. (2010) | Compared to cases, control subjects presented significantly higher intakes of carbohydrates, fiber, folate, vitamin C and lycopene and lower intakes of proteins and total fat. | 5 | ||||||
| de Jong et al. (2014) | The Netherlands | Cases (41 ± 5) Controls (38 ± 7) | 121 fertile male controls and 42 infertile cases with asthenozoospermia | Case-control | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Alcohol consumption was not associated with such sperm parameters as volume, sperm count, motility and morphology or pregnancy outcome. | 4.5 |
| Eslamian et al. (2012) | Iran | Cases (32.2 ± 3.5) Controls (33.5 ± 3.7) | 169 normozoospermic controls and 72 asthenozoospermic cases | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T and PRL) | An increased intake of fruits and vegetables (especially oranges, tomatoes and dark green vegetables), poultry, skimmed milk and seafood was associated with a significantly lower risk of asthenozoospermia. An increased intake of processed meats, dairy products and sweets was associated with a significantly higher risk of asthenozoospermia. | 5.5 |
| Eslamian et al. (2015) | Iran | Cases (32.8 ± 3.6) Controls (33.4 ± 3.5) | 235 normospermic controls and 107 asthenozoospermic cases | Case-control | FFQ | Sperm quality and endocrine parameters (FSH, LH, T and PRL) | A high intake of saturated fatty acids, TFAs, palmitic acid and stearic acid was positively related to the odds of having asthenozoospermia. Inverse and dose-dependent associations were found between asthenozoospermia and intake of omega-3 PUFAs and docosahexaenoic acid. | 5.5 |
| Eslamian et al. (2016) | A high intake of vitamin E, vitamin D, vitamin C, zinc, selenium and PUFAs was significantly associated with a lower risk of asthenozoospermia. | 5.5 | ||||||
| Eskenazi et al. (2005) | USA | 20–80 | 87–97 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) | Positive associations were observed between vitamin C intake and sperm number as reflected in the higher mean count, concentration and total progressively motile sperm count; between vitamin E intake and progressive motility or total progressively motile sperm count; and between b-carotene intake and sperm concentration or progressive motility. Folate and zinc intake were not associated with improved semen quality. | 4.5 |
| Silver et al. (2005) | Sperm chromatin integrity | Non-significant associations between high antioxidant intake and sperm chromatin integrity in men with fertility problems. | 5 | |||||
| Young et al. (2008) | Sperm aneuploidy | Men with high folate intake had lower overall frequencies of several types of sperm aneuploidy. | 4.5 | |||||
| Vujkovic et al. (2009) | The Netherlands | 28.6–53.9 | 161 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) and sperm DNA damage | The ‘Health Conscious’ diet was inversely associated with SDF. The ‘Traditional Dutch’ diet was positively correlated with sperm concentration. | 5 |
| Mínguez-Alarcón et al. (2012) | Spain | 18–23 | 209–215 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A positive association was observed between the dietary intake of cryptoxanthin, vitamin C, lycopene and b-carotene and the total motile sperm count. The semen volume is increased in those individuals with a high intake of vitamin C, lycopene and b-carotene. | 5 |
| Chavarro et al. (2014) | Cholesterol intake was inversely related to the ejaculate volume. Intake of TFAs was inversely related to the total sperm count. | 5.5 | ||||||
| Cutillas-Tolin et al. (2015) | The Mediterranean pattern was positively associated with total sperm count. The Western pattern was positively related to the percentage of morphologically normal sperm. | 5.5 | ||||||
| Chavarro et al. (2008) | USA | 18–55 (36.4 ± 5) | 99 male partners of couples attending an infertility clinic | Cross-sectional | FFQ evaluating soy product consumption | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A higher intake of soy foods was associated with a lower sperm concentration. | 4 |
| Attaman et al. (2012) | FFQ | Total fat intake was negatively related to total sperm count and sperm concentration. The intake of omega-3 PUFAs was positively related to normal sperm morphology. | 5.5 | |||||
| Schmid et al. (2012) | USA | 22–80 | 80 healthy participants | Cross-sectional | FFQ | Sperm DNA damage | Men with the highest intake of vitamin C had less SDF than men with the lowest intake. Findings were similar for vitamin E, folate and zinc (but not b-carotene). Older men (>44 years) with the highest vitamin C intake had less SDF than older men with the lowest intake. Findings were similar for vitamin E and zinc. Men (especially older men) with higher dietary and supplement intake of certain micronutrients may produce sperm with less SDF. | 4.5 |
| Jensen et al. (2013) | Denmark | 18.4–22.9 | 701 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Individuals with a higher intake of saturated fatty acids had lower sperm concentrations and lower total sperm count. The percentage of spermatozoa with normal morphology was lower among men with a high percentage of energy from monounsaturated fatty acids, and semen volume was higher among men with a high intake of omega-3 fatty acids. | 4.5 |
| Gaskins et al. (2012) | USA | 18.9–20.5 | 188–189 healthy participants | Cross-sectional | FFQ | Sperm quality (motility, morphology and sperm concentration) | A Prudent dietary pattern was associated with higher progressive sperm motility and unrelated to sperm concentration and morphology. The consumption of a Western dietary pattern was unrelated to rutine semen parameters. | 5 |
| Zareba et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Lycopene intake was associated with better sperm morphology. High dietary intake of vitamin C was associated with lower sperm concentration and sperm count. | 5.5 | |||||
| Afeiche et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Intake of full-fat dairy products was inversely related to sperm motility and morphology. | 5 | |||||
| Chiu et al. (2014) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Higher consumption of sugar-sweetened beverages was associated with lower sperm motility. Sugar-sweetened beverage intake was unrelated to other semen quality parameters or reproductive hormone levels. | 5.5 | |||||
| Afeiche et al. (2014c) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Processed red meat intake was inversely related to total sperm count and total progressive motile sperm count. | 5.5 | |||||
| Afeiche et al. (2014a) | USA | 18–55 | 155 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Low-fat dairy intake, particularly low-fat milk, was related to a higher sperm concentration and progressive motility, whereas cheese intake is related to a lower sperm concentration. | 5.5 |
| Afeiche et al. (2014b) | Processed meat intake was negatively associated with sperm morphology, whereas fish intake was positively associated with total sperm count and sperm morphology. | 5.5 | ||||||
| Anifandis et al. (2014) | Greece | 37.4 ± 0.3 | 207 potentially infertile | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, number of spermatozoa and concentration) and SDF | Alcohol consumption was associated with a lower sperm volume and higher SDF. Cigarrete plus alcohol consumption was associated with higher SDF. | 3.5 |
| Jensen et al. (2014) | Europe and USA | 18–45 | 8344 healthy participants | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T, Inhibin B, SHBG) | Moderate alcohol intake is not adversely associated with semen quality, but is associated with higher levels of serum testosterone. | 5.5 |
| Karayiannis et al. (2016) | Greece | 26–55 | 225 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Greater adherence to the MedDiet, was significantly associated with higher sperm concentration, total sperm count and sperm motility. | 5.5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Sperm quality (motility, morphology, and concentration) | Sperm concentration was negatively related to alcohol consumption and positively associated to cereal consumption. Sperm motility was positively associated with the consumption of fruits and cereals, and negatively associated with alcohol and coffee consumption. | 4.5 |
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality score* . |
|---|---|---|---|---|---|---|---|---|
| Stutz et al. (2004) | Argentine | 20–30 | 34 healthy participants | Retrospective | Alcohol frequency intake questionnaire | Plasma testosterone and sperm quality (volume, vitality, motility, morphology and concentration) | Non-significant associations between alcohol consumption and routine semen parameters were found. | 4.5 |
| Goverde et al. (1995) | The Netherlands | Controls (32.5 ± 3.8) Cases (33.2 ± 3.5) | 68 controls and 47 cases with poor semen quality attending an infertility clinic | Case-control | Alcohol and caffeine frequency intake questionnaire | Sperm quality (volume, motility, morphology and concentration) | A lower percentage of normal sperm morphology was observed in the daily alcohol drinkers. No differences with respect to alcohol consumption between male patients with poor semen quality and controls. | 4 |
| Serra-Majem et al. (2003) | Spain | 30–50 | 379 fertile controls and 405 cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | The ingestion of cyclamate, aspartame and saccharin has no association with sperm quality. | 5.5 |
| Mendiola et al. (2009) | Spain | Cases (34.2 ± 3.7) Controls (32.8 ± 3.9) | 31 fertile controls and 30 infertile cases with poor semen quality | Case-control | FFQ | Sperm quality (volume, motility, morphology and concentration) and hormonal levels (FSH, LH, T) | Controls had a higher intake of skimmed milk, shellfish, tomatoes, and lettuce, and cases consumed more yogurt, meat products and potatoes. In the logistic regression model, cases had a lower intake of lettuce and tomatoes, fruits (apricots and peaches), and a significantly higher intake of dairy and processed meat products. | 5 |
| Mendiola et al. (2010) | Compared to cases, control subjects presented significantly higher intakes of carbohydrates, fiber, folate, vitamin C and lycopene and lower intakes of proteins and total fat. | 5 | ||||||
| de Jong et al. (2014) | The Netherlands | Cases (41 ± 5) Controls (38 ± 7) | 121 fertile male controls and 42 infertile cases with asthenozoospermia | Case-control | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Alcohol consumption was not associated with such sperm parameters as volume, sperm count, motility and morphology or pregnancy outcome. | 4.5 |
| Eslamian et al. (2012) | Iran | Cases (32.2 ± 3.5) Controls (33.5 ± 3.7) | 169 normozoospermic controls and 72 asthenozoospermic cases | Case-control | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T and PRL) | An increased intake of fruits and vegetables (especially oranges, tomatoes and dark green vegetables), poultry, skimmed milk and seafood was associated with a significantly lower risk of asthenozoospermia. An increased intake of processed meats, dairy products and sweets was associated with a significantly higher risk of asthenozoospermia. | 5.5 |
| Eslamian et al. (2015) | Iran | Cases (32.8 ± 3.6) Controls (33.4 ± 3.5) | 235 normospermic controls and 107 asthenozoospermic cases | Case-control | FFQ | Sperm quality and endocrine parameters (FSH, LH, T and PRL) | A high intake of saturated fatty acids, TFAs, palmitic acid and stearic acid was positively related to the odds of having asthenozoospermia. Inverse and dose-dependent associations were found between asthenozoospermia and intake of omega-3 PUFAs and docosahexaenoic acid. | 5.5 |
| Eslamian et al. (2016) | A high intake of vitamin E, vitamin D, vitamin C, zinc, selenium and PUFAs was significantly associated with a lower risk of asthenozoospermia. | 5.5 | ||||||
| Eskenazi et al. (2005) | USA | 20–80 | 87–97 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) | Positive associations were observed between vitamin C intake and sperm number as reflected in the higher mean count, concentration and total progressively motile sperm count; between vitamin E intake and progressive motility or total progressively motile sperm count; and between b-carotene intake and sperm concentration or progressive motility. Folate and zinc intake were not associated with improved semen quality. | 4.5 |
| Silver et al. (2005) | Sperm chromatin integrity | Non-significant associations between high antioxidant intake and sperm chromatin integrity in men with fertility problems. | 5 | |||||
| Young et al. (2008) | Sperm aneuploidy | Men with high folate intake had lower overall frequencies of several types of sperm aneuploidy. | 4.5 | |||||
| Vujkovic et al. (2009) | The Netherlands | 28.6–53.9 | 161 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, number of spermatozoa and concentration) and sperm DNA damage | The ‘Health Conscious’ diet was inversely associated with SDF. The ‘Traditional Dutch’ diet was positively correlated with sperm concentration. | 5 |
| Mínguez-Alarcón et al. (2012) | Spain | 18–23 | 209–215 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A positive association was observed between the dietary intake of cryptoxanthin, vitamin C, lycopene and b-carotene and the total motile sperm count. The semen volume is increased in those individuals with a high intake of vitamin C, lycopene and b-carotene. | 5 |
| Chavarro et al. (2014) | Cholesterol intake was inversely related to the ejaculate volume. Intake of TFAs was inversely related to the total sperm count. | 5.5 | ||||||
| Cutillas-Tolin et al. (2015) | The Mediterranean pattern was positively associated with total sperm count. The Western pattern was positively related to the percentage of morphologically normal sperm. | 5.5 | ||||||
| Chavarro et al. (2008) | USA | 18–55 (36.4 ± 5) | 99 male partners of couples attending an infertility clinic | Cross-sectional | FFQ evaluating soy product consumption | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | A higher intake of soy foods was associated with a lower sperm concentration. | 4 |
| Attaman et al. (2012) | FFQ | Total fat intake was negatively related to total sperm count and sperm concentration. The intake of omega-3 PUFAs was positively related to normal sperm morphology. | 5.5 | |||||
| Schmid et al. (2012) | USA | 22–80 | 80 healthy participants | Cross-sectional | FFQ | Sperm DNA damage | Men with the highest intake of vitamin C had less SDF than men with the lowest intake. Findings were similar for vitamin E, folate and zinc (but not b-carotene). Older men (>44 years) with the highest vitamin C intake had less SDF than older men with the lowest intake. Findings were similar for vitamin E and zinc. Men (especially older men) with higher dietary and supplement intake of certain micronutrients may produce sperm with less SDF. | 4.5 |
| Jensen et al. (2013) | Denmark | 18.4–22.9 | 701 healthy participants | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Individuals with a higher intake of saturated fatty acids had lower sperm concentrations and lower total sperm count. The percentage of spermatozoa with normal morphology was lower among men with a high percentage of energy from monounsaturated fatty acids, and semen volume was higher among men with a high intake of omega-3 fatty acids. | 4.5 |
| Gaskins et al. (2012) | USA | 18.9–20.5 | 188–189 healthy participants | Cross-sectional | FFQ | Sperm quality (motility, morphology and sperm concentration) | A Prudent dietary pattern was associated with higher progressive sperm motility and unrelated to sperm concentration and morphology. The consumption of a Western dietary pattern was unrelated to rutine semen parameters. | 5 |
| Zareba et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Lycopene intake was associated with better sperm morphology. High dietary intake of vitamin C was associated with lower sperm concentration and sperm count. | 5.5 | |||||
| Afeiche et al. (2013) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Intake of full-fat dairy products was inversely related to sperm motility and morphology. | 5 | |||||
| Chiu et al. (2014) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Higher consumption of sugar-sweetened beverages was associated with lower sperm motility. Sugar-sweetened beverage intake was unrelated to other semen quality parameters or reproductive hormone levels. | 5.5 | |||||
| Afeiche et al. (2014c) | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, E2, T, free T, Inhibin B, SHBG) | Processed red meat intake was inversely related to total sperm count and total progressive motile sperm count. | 5.5 | |||||
| Afeiche et al. (2014a) | USA | 18–55 | 155 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Low-fat dairy intake, particularly low-fat milk, was related to a higher sperm concentration and progressive motility, whereas cheese intake is related to a lower sperm concentration. | 5.5 |
| Afeiche et al. (2014b) | Processed meat intake was negatively associated with sperm morphology, whereas fish intake was positively associated with total sperm count and sperm morphology. | 5.5 | ||||||
| Anifandis et al. (2014) | Greece | 37.4 ± 0.3 | 207 potentially infertile | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, number of spermatozoa and concentration) and SDF | Alcohol consumption was associated with a lower sperm volume and higher SDF. Cigarrete plus alcohol consumption was associated with higher SDF. | 3.5 |
| Jensen et al. (2014) | Europe and USA | 18–45 | 8344 healthy participants | Cross-sectional | Alcohol frequency intake questionnaire | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) and hormonal levels (FSH, LH, T, Inhibin B, SHBG) | Moderate alcohol intake is not adversely associated with semen quality, but is associated with higher levels of serum testosterone. | 5.5 |
| Karayiannis et al. (2016) | Greece | 26–55 | 225 partners of couples attending an infertility clinic | Cross-sectional | FFQ | Sperm quality (volume, motility, morphology, number of spermatozoa and concentration) | Greater adherence to the MedDiet, was significantly associated with higher sperm concentration, total sperm count and sperm motility. | 5.5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Sperm quality (motility, morphology, and concentration) | Sperm concentration was negatively related to alcohol consumption and positively associated to cereal consumption. Sperm motility was positively associated with the consumption of fruits and cereals, and negatively associated with alcohol and coffee consumption. | 4.5 |
E2, estradiol; FFQ, food frequency questionnaire; MedDiet, mediterranean diet; PRL, prolactin; PUFAs, polyunsaturated fatty acid; SDF, sperm DNA fragmentation; SHBG, sex hormone binding globulin; T, testosterone; TFAs, trans-fatty acids.
*We evaluated and scored the quality of the studies selected on a six-point scale (Hayden et al., 2006): (i) study participation; (ii) study attrition; (iii) prognostic factor measurement; (iv) confounding measurement and account; (v) outcome measurement; and (vi) analysis. Studies with a score between 0 and 3 points were considered to be of low quality, while >3 to 6 were considered to be of high quality.
Summary of the studies that investigated associations between nutrition and fecundability.
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality Score . |
|---|---|---|---|---|---|---|---|---|
| Florack et al. (1994) | The Netherlands | ND | 259 partners of couples attending an infertility clinic | Prospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Male partners with ≥10 alcoholic drinks/week had a higher probability of fecundability. Consumers of ≥7 cups/day of coffee had a lower probability of fecundity. | 4.5 |
| Curtis et al. (1997) | Canada | Couples of women <44 | 2607 healthy partners of farmers (2593 men) | Retrospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Heavy drinkers of tea had decreased fecundability. | 4.5 |
| Olsen et al. (1997) | Denmark, Germany, Italy, France, Sweden, Poland and Spain | Couples of women 25–44 | 6630 theoretically healthy couples (6279 men) from a general population and 4035 couples (3603 men) from a pregnancy register population | Retrospective | Alcohol frequency intake questionnaire | Fecundability | Non-significant associations between alcohol consumption and fecundability were reported. | 5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, pregnancy rate and miscarriage rate) | Alcohol consumption was negatively associated with the fertilization rate. The consumption of red meat had a negative impact on the implantation rate. The consumption of red meat was negatively associated to the chance of pregnancy. | 4.5 |
| Xia et al. (2015) | USA | 18–55 | 141 partners from couples attending an infertility clinic | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, clinical pregnancy and live-birth rates per initiated cycle) | Poultry intake was positively associated with fertilization rates. Processed meat intake was negatively associated with fertilization rates. | 5 |
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality Score . |
|---|---|---|---|---|---|---|---|---|
| Florack et al. (1994) | The Netherlands | ND | 259 partners of couples attending an infertility clinic | Prospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Male partners with ≥10 alcoholic drinks/week had a higher probability of fecundability. Consumers of ≥7 cups/day of coffee had a lower probability of fecundity. | 4.5 |
| Curtis et al. (1997) | Canada | Couples of women <44 | 2607 healthy partners of farmers (2593 men) | Retrospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Heavy drinkers of tea had decreased fecundability. | 4.5 |
| Olsen et al. (1997) | Denmark, Germany, Italy, France, Sweden, Poland and Spain | Couples of women 25–44 | 6630 theoretically healthy couples (6279 men) from a general population and 4035 couples (3603 men) from a pregnancy register population | Retrospective | Alcohol frequency intake questionnaire | Fecundability | Non-significant associations between alcohol consumption and fecundability were reported. | 5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, pregnancy rate and miscarriage rate) | Alcohol consumption was negatively associated with the fertilization rate. The consumption of red meat had a negative impact on the implantation rate. The consumption of red meat was negatively associated to the chance of pregnancy. | 4.5 |
| Xia et al. (2015) | USA | 18–55 | 141 partners from couples attending an infertility clinic | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, clinical pregnancy and live-birth rates per initiated cycle) | Poultry intake was positively associated with fertilization rates. Processed meat intake was negatively associated with fertilization rates. | 5 |
ND, no-data.
Summary of the studies that investigated associations between nutrition and fecundability.
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality Score . |
|---|---|---|---|---|---|---|---|---|
| Florack et al. (1994) | The Netherlands | ND | 259 partners of couples attending an infertility clinic | Prospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Male partners with ≥10 alcoholic drinks/week had a higher probability of fecundability. Consumers of ≥7 cups/day of coffee had a lower probability of fecundity. | 4.5 |
| Curtis et al. (1997) | Canada | Couples of women <44 | 2607 healthy partners of farmers (2593 men) | Retrospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Heavy drinkers of tea had decreased fecundability. | 4.5 |
| Olsen et al. (1997) | Denmark, Germany, Italy, France, Sweden, Poland and Spain | Couples of women 25–44 | 6630 theoretically healthy couples (6279 men) from a general population and 4035 couples (3603 men) from a pregnancy register population | Retrospective | Alcohol frequency intake questionnaire | Fecundability | Non-significant associations between alcohol consumption and fecundability were reported. | 5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, pregnancy rate and miscarriage rate) | Alcohol consumption was negatively associated with the fertilization rate. The consumption of red meat had a negative impact on the implantation rate. The consumption of red meat was negatively associated to the chance of pregnancy. | 4.5 |
| Xia et al. (2015) | USA | 18–55 | 141 partners from couples attending an infertility clinic | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, clinical pregnancy and live-birth rates per initiated cycle) | Poultry intake was positively associated with fertilization rates. Processed meat intake was negatively associated with fertilization rates. | 5 |
| Reference . | Location . | Age (years) . | Population studied . | Study design . | Exposure . | Outcome . | Principal conclusion . | Quality Score . |
|---|---|---|---|---|---|---|---|---|
| Florack et al. (1994) | The Netherlands | ND | 259 partners of couples attending an infertility clinic | Prospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Male partners with ≥10 alcoholic drinks/week had a higher probability of fecundability. Consumers of ≥7 cups/day of coffee had a lower probability of fecundity. | 4.5 |
| Curtis et al. (1997) | Canada | Couples of women <44 | 2607 healthy partners of farmers (2593 men) | Retrospective | Alcohol and caffeine frequency intake questionnaire | Fecundability | Heavy drinkers of tea had decreased fecundability. | 4.5 |
| Olsen et al. (1997) | Denmark, Germany, Italy, France, Sweden, Poland and Spain | Couples of women 25–44 | 6630 theoretically healthy couples (6279 men) from a general population and 4035 couples (3603 men) from a pregnancy register population | Retrospective | Alcohol frequency intake questionnaire | Fecundability | Non-significant associations between alcohol consumption and fecundability were reported. | 5 |
| Braga et al. (2012) | Brazil | 38.4 ± 9.3 | 250 male patients undergoing ICSI cycles | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, pregnancy rate and miscarriage rate) | Alcohol consumption was negatively associated with the fertilization rate. The consumption of red meat had a negative impact on the implantation rate. The consumption of red meat was negatively associated to the chance of pregnancy. | 4.5 |
| Xia et al. (2015) | USA | 18–55 | 141 partners from couples attending an infertility clinic | Prospective | FFQ | Fecundability (fertilization rate, implantation rate, clinical pregnancy and live-birth rates per initiated cycle) | Poultry intake was positively associated with fertilization rates. Processed meat intake was negatively associated with fertilization rates. | 5 |
ND, no-data.
Sperm parameters
Only one retrospective study was included in this systematic review (Table I) (Stutz et al., 2004). It found no significant associations between alcohol consumption and plasma testosterone concentrations or seminal parameters in 34 healthy participants from Argentina.
Case-control studies are shown in Table I (Goverde et al., 1995; Serra-Majem et al., 2003; Mendiola et al., 2009, 2010; Eslamian et al., 2012, 2015, 2016; de Jong et al., 2014).
Two of them focused on analyzing the relationship between alcohol consumption and semen quality and their conclusions are controversial. Goverde et al. (1995) studied 47 cases and 68 controls with poor semen quality attending an infertility clinic, and showed that men who drank alcohol every day had a lower percentage of normal sperm morphology than men who did not drink alcohol. In contrast, de Jong et al. (2014) did not find an association between alcohol consumption and such sperm parameters as volume, sperm count, motility and morphology in 42 infertile cases with asthenozoospermia and 121 fertile male controls.
A large case-control study based on 405 males with poor semen quality and 379 fertile controls investigated the possible association of cyclamate, cyclohexylamine and other artificial sweeteners with male infertility. The conclusion was that the ingestion of these sweeteners was not related to sperm quality (Serra-Majem et al., 2003).
Several studies have evaluated the relationship between food groups and fertility. One of these is Mendiola et al. (2009) who analyzed infertile cases with poor semen quality and fertile controls (30 cases and 31 controls). They showed that, compared to the controls, infertile cases presented a lower consumption of skimmed milk, shellfish, tomatoes and lettuce; and they consumed more yogurt, meat products and potatoes (Mendiola et al., 2009). In another article using the same population, the authors also show that infertile cases presented significantly lower intakes of carbohydrates, fiber, folate, vitamin C and lycopene; and higher intakes of proteins and total fat (Mendiola et al., 2010).
The associations between the consumption of different food groups and the risk of having different idiopathic asthenozoospermia infertility were also evaluated in 72 asthenozoospermic cases and 169 normozoospermic controls from Iran. Individuals with asthenozoospermia were observed to consume less fruit and vegetables (i.e. oranges, tomatoes and dark green vegetables), poultry, skimmed milk and seafood than controls. Nonetheless, an increased intake of processed meats, dairy products and sweets was associated with a significantly higher risk of asthenozoospermia (Eslamian et al., 2012).
More recent studies by the same group, but with 235 normozoospermic controls and 107 asthenozoospermic cases, concluded that a high intake of saturated fatty acids (SFAs), TFAs, and palmitic and stearic fatty acids was positively related to the probability of having asthenozoospermia. They also found inverse and dose-dependent associations between the intake of omega-3 polyunsaturated fatty acids (PUFAs) and docosahexaenoic acid (DHA) and the risk of having asthenozoospermia (Eslamian et al., 2015). In another published report, a high intake of some vitamins (vitamin E, vitamin D and vitamin C), zinc, folate, total fiber, selenium and PUFAs was significantly associated with a lower risk of asthenozoospermia (Eslamian et al., 2016).
Table I summarizes the cross-sectional studies (Eskenazi et al., 2005; Silver et al., 2005; Chavarro et al., 2008, 2014; Young et al., 2008; Vujkovic et al., 2009; Attaman et al., 2012; Gaskins et al., 2012; Mínguez-Alarcón et al., 2012; Schmid et al., 2012; Afeiche et al., 2013, 2014a,b,c; Jensen et al., 2013, 2014; Zareba et al., 2013; Anifandis et al., 2014; Chiu et al., 2014; Cutillas-Tolin et al., 2015; Karayiannis et al., 2016).
Two studies focused on alcohol consumption and semen quality, the SDF index and serum reproductive hormones (Anifandis et al., 2014; Jensen et al., 2014). While one of these studies, conducted in 8344 healthy participants, found that a moderate alcohol intake was associated with higher levels of serum testosterone, but was not harmfully associated with semen quality (Jensen et al., 2014), the other (n = 207 potentially infertile participants) associated total alcohol consumption with lower sperm volume and higher SDF. This same study found that the combination of cigarette and alcohol consumption was associated with higher SDF (Anifandis et al., 2014).
The Age and Genetic Effects in Sperm study, conducted in healthy participants, focused on three different factors, which were reported in three different articles (Eskenazi et al., 2005; Silver et al., 2005; Young et al., 2008): (i) sperm quality (volume, motility, number of spermatozoa and concentration), (ii) sperm chromatin integrity, and (iii) sperm aneuploidy. A positive relationship was demonstrated between vitamin C intake and total sperm count, concentration and total progressive motility; vitamin E intake, and progressive motility or total progressively motile sperm count; and β-carotene intake, and sperm concentration and progressive motility. However, folate and zinc intake were not associated with several parameters of semen quality (Eskenazi et al., 2005). Furthermore, a high antioxidant intake was not related to improved sperm chromatin structure and, therefore, to fertility problems in 87 healthy men (Silver et al., 2005). Finally, compared to those with low intake, men with high folate intake had lower overall frequencies of several types of sperm aneuploidy (for chromosomes X, Y and 21) (Young et al., 2008).
Only one article, conducted in 80 healthy participants, primarily focused on sperm DNA damage. Compared to those in the reference quartile, men in the top quartile of vitamin C intake had less SDF, with similar findings for vitamin E, folate and zinc (but not β-carotene). Also, older men (>44 years) in the top quartile of vitamin C intake had less SDF than older men in the reference quartile of intake, with similar findings for vitamin E and zinc. However, younger men (<44 years) did not benefit from higher intakes of the micronutrients surveyed (Schmid et al., 2012).
Two articles conducted in the same population (99 male partners of subfertile couples from the Massachusetts General Hospital Fertility Center (USA) who presented for semen analyses) focused on the association between soy rich isoflavone food, fat intake and semen quality parameters. A higher intake of soy foods was associated with lower sperm concentration (Chavarro et al., 2008), and total fat intake was negatively related to total sperm count and sperm concentration (Chavarro et al., 2008). These associations appeared to be driven primarily by intake of saturated fat. Conversely, intake of omega-3 PUFAs was positively related to normal sperm morphology (Attaman et al., 2012).
The association between food consumption and sperm parameters was also studied by the same group of researchers in another sample of partners of couples presenting for semen analyses (n = 155). Low-fat dairy intake, particularly low-fat milk, was related to higher sperm concentration and progressive motility, whereas cheese consumption was related to lower sperm concentrations, but only among past or current smokers (Afeiche et al., 2014a). In addition, fish consumption was positively related to total sperm count and morphology, whereas processed meat consumption was negatively associated with sperm morphology (Afeiche et al., 2014b).
Using data from the Rochester Young Men's Study (RYMS), a cross-sectional study conducted in 189 healthy young men in 2009–2010, five articles were published (Gaskins et al., 2012; Afeiche et al., 2013, 2014c; Zareba et al., 2013; Chiu et al., 2014). When participants were categorized by factor analysis, the ‘Prudent’ diet (characterized by a high intake of fish, chicken, fruit, vegetables, legumes and whole grains) was significantly associated with higher progressive sperm motility (but not with sperm concentration and morphology) while the ‘Western’ diet (characterized by high intake of red and processed meat, refined grains, pizza, snacks, high-energy drinks and sweets) was not (Gaskins et al., 2012). As far as dietary antioxidants are concerned, lycopene intake was related to better sperm morphology, whereas a high intake of vitamin C from food sources alone was associated with a lower sperm concentration and sperm count (Zareba et al., 2013). Three articles from the RYMS analyzed which foods were related to sperm parameters and reproductive hormone levels. The intake of full-fat dairy products was inversely related to sperm motility and morphology, and these associations were driven primarily by the intake of cheese and were independent of overall dietary patterns. However, non-significant associations were reported between dairy food consumption and hormone levels (Afeiche et al., 2013). As far as the consumption of beverages is concerned, men in the highest quartile of sugar-sweetened beverages showed lower sperm motility than those in the reference quartile. However, no association between sugar-sweetened beverages and other semen quality parameters or reproductive hormone levels were described (Chiu et al., 2014). Finally, intake of processed red meat was inversely related to total sperm count and total progressive motile sperm count (Afeiche et al., 2014c).
In the Murcia Young Men's cross-sectional Study carried out on ~200 healthy Spanish university students (18–23 years old), a positive association was observed between the dietary intake of several antioxidant nutrients (cryptoxanthin, vitamin C, lycopene and β-carotene) and total motile sperm count. Moreover, semen volume increased in those individuals with a high intake of vitamin C, lycopene and β-carotene (Mínguez-Alarcón et al., 2012). Cutillas-Tolin et al. (2015) and collaborators show that traditional Mediterranean diets (characterized by high intakes of vegetables, fruits and seafood) may have a positive impact on male reproductive potential because this pattern was positively associated with total sperm count, although a ‘Western’ pattern was positively related to the percentage of morphologically normal sperm. The same authors reported that dietary cholesterol was inversely related to ejaculate volume after adjusting for potential confounders, whereas the intake of TFAs was inversely related to total sperm count (Chavarro et al., 2014).
One of the largest cross-sectional studies examining the association between dietary fat intake and semen quality was conducted in 701 healthy young Danish men. Compared to those in the bottom quartile, men in the top quartile of energy intake as SFAs showed lower sperm concentrations and lower total sperm counts. In the same study, the percentage of normal morphology spermatozoa was lower among men consuming a high percentage of energy from monounsaturated fatty acids, whereas semen volume was higher among men with a high intake of n-3 fatty acids (Jensen et al., 2013).
In the FOod, Lifestyle and Fertility Outcome study (FOLFO-study) conducted in 161 healthy Dutch men, Vujkovic et al. (2009) compared two dietary patterns. When participants were categorized by factor analysis, the ‘Health Conscious’ diet (high intakes of fruits, vegetables, fish and whole grains) was significantly associated with less SDF but the ‘Traditional Dutch’ diet (high intakes of meat, potatoes and whole grains and low intakes of beverages and sweets), was positively correlated with sperm concentration.
Recently, adherence to the Mediterranean diet assessed by a validated score, was positively associated with higher sperm concentration, total sperm count and sperm motility in a cross-sectional study in 225 men (26–55 years old) from couples attending a fertility clinic (Karayiannis et al., 2016).
In the only prospective study conducted to date (Table I), 250 male participants whose partners had undergone ICSI cycles were analyzed. In this study, the sperm concentration was negatively associated with the frequency of cereal consumption and the number of meals per day. In addition, sperm motility was negatively associated with alcohol and coffee consumption, and positively associated with the consumption of cereals and fruits (Braga et al., 2012).
The quality scores of the articles related to the sperm parameters included in this review are modest (case-control studies, mean = 5.06/6, cross-sectional studies, mean = 5.27/6 and prospective and retrospective studies = 4.5/6).
Fecundability
Table II shows retrospective and prospective studies analyzing the association between male food consumption and fecundability. The two retrospective studies included in this review (Curtis et al., 1997; Olsen et al., 1997) are both large and focus on the frequency of alcohol consumption. In the study by Curtis et al. (1997) of 2607 healthy partners of farmers (2593 men), individuals who were heavy tea drinkers (regardless of caffeine content) had decreased fecundability. However, no association was reported between alcohol consumption and fecundability (Curtis et al., 1997). The largest multicentric study conducted by Olsen et al. (1997) in 6630 theoretically healthy couples (6279 men) from the general population and 4035 couples (3603 men) from a pregnancy register population, non-significant associations were detected between alcohol consumption and fecundability.
The three prospective studies (Florack et al., 1994; Braga et al., 2012; Xia et al., 2015) (Table II) are small studies that analyze between 141 and 259 male partners of women attending an infertility clinic. Florack et al. show that male partners with a consumption of ≥10 alcoholic drinks per week had a higher probability of fecundity, whereas heavy consumers of caffeine (≥7 cups/day) had a lower probability (Florack et al., 1994). The other two prospective studies aimed to assess the influence on fecundability of food consumption by men (250 and 141 male partners whose couples had undergone ART cycles, respectively). In the Braga et al. study, alcohol consumption had a negative influence on the fertilization rate, whereas the consumption of red meat had a negative impact on the implantation rate and on the chance of pregnancy (Braga et al., 2012). In Xia's study, poultry intake was positively associated with fertilization rates, whereas processed meat intake was negatively associated with fertilization rates among couples undergoing conventional IVF (Xia et al., 2015).
The quality scores of the articles related to the fecundability studies included in this review are also modest (mean = 4.7/6).
Discussion
The present review of epidemiological/observational studies provides the most comprehensive analysis to date of the associations between diet or nutrient intake and the risk of infertility. It suggests that diet modifications may be useful in modulating male fertility and fecundability.
As far as sperm quality is concerned, the results of this systematic review indicated that healthy diets (i.e. the Mediterranean diet) rich in such nutrients as omega-3 fatty acids, some antioxidants and vitamins, and low in SFAs and TFAs are inversely associated with low semen quality parameters. In terms of food groups, fish, shellfish and seafood, poultry, cereals, vegetables and fruits, and low-fat dairy products have been positively related to sperm quality. However, diets rich in processed meat, soy foods, potatoes, full-fat dairy products, coffee, alcohol and sugar-sweetened beverages and sweets have been inversely associated with the quality of semen in some studies (Fig. 2).
Nutrition-related factors reported in this review that were associated with male infertility. Different colors denote the type of association with male infertility: positive association in green and negative association in orange.
The few studies relating male nutrient or food intake and fecundability also suggest that diets rich in red meat, processed meat, tea and caffeine are associated with a lower rate of fecundability. This association is only controversial in the case of alcohol (Fig. 3).
Diet-related factors reported in this review that were associated with fecundability. Negative associations are shown in orange.
The potential biological mechanisms linking diet with sperm function and fertility are largely unknown and require further study. Below we show some of the mechanisms deserving attention in relation to the consumption of some foods.
Fruits, vegetables and cereals
Fruits and vegetables are rich in water, antioxidant vitamins (especially vitamin C, vitamin A, β-carotene and polyphenols, but also other phytochemicals), some minerals with antioxidant properties (potassium and magnesium), folate and fiber.
There is a direct association between antioxidant status and the production of reactive oxygen species (ROS) in spermatozoa (Ross et al., 2010). In addition, high concentrations of ROS negatively affect sperm DNA and, consequently, sperm motility, vitality and concentration, but also miscarriage and developmental abnormalities in the offspring (Saleh et al., 2003; Tremellen, 2008; Aitken et al., 2016; Aitken, 2016). Antioxidants are considered to be ‘scavengers’ of ROS and their use has been studied as a treatment to reverse the adverse impact of high ROS concentrations on semen parameters (Ross et al., 2010). In fact most of the clinical trials conducted in humans, based on relatively little scientific evidence, have demonstrated some possible benefits of several types of antioxidants on sperm quality (Scott et al., 1998; Ghanem et al., 2010). Antioxidants have also shown some promise in treating idiopathic oxidative stress in spermatozoa (Gharagozloo and Aitken, 2011; Showell et al., 2014). However, these results should be replicated in the future before solid recommendations can be made.
As has been reported, men with a high folate intake had lower frequencies of several types of sperm aneuploidy (Young et al., 2008), which suggests that this vitamin could be important in spermatogenesis. Folate, which is mainly present in green leafy vegetables, is essential for DNA maintenance, and transfer RNA and protein synthesis (Molloy, 2012). Because DNA synthesis is an essential part of spermatogenesis, folate is probably important to the process. In fact, in an RCT, the total normal sperm count increased after combined sulfate and folic acid treatment in both subfertile and fertile men (Wong et al., 2002).
In addition, fruits, vegetables, legumes and whole cereals are the principal sources of fiber. Some studies have demonstrated that fiber consumption reduced plasma estrogen levels by binding directly to unconjugated estrogens (Goldin et al., 1982), and low plasma estrogen levels in males are essential for normal fertility (Amarnath et al., 2016).
Soy foods
The principal hypothesis for the negative effect of soy foods on male fertility was the phytoestrogen concentration. Phytoestrogens have known deleterious effects on the male endocrine system (Santti et al., 1998) with potential effects on fertility. Nevertheless, the question of whether phytoestrogens are beneficial or harmful to human health remains unresolved. Animal studies have suggested that exposure to phytoestrogens in the developmental period is not advisable because they may disrupt the endocrine system (McMichael-Phillips et al., 1998). However, the only RCT study testing the effect of consuming soy foods in humans has demonstrated no effect on serum gonadotrophin and sex hormone levels, or on semen quality (Mitchell et al., 2001).
Potatoes
Potatoes primarily contain starches with a high glycemic index and glycemic load properties (Atkinson et al., 2008). A high glycemic and insulinemic response to food has been associated with oxidative stress (Hu et al., 2006) which, as mentioned above, has an important effect on semen quality (Ross et al., 2010). In addition, a diet with a high glycemic index and high glycemic load has been associated with an increased risk of inflammation (Kristo et al., 2013) and Type 2 diabetes (Muraki et al., 2016). In fact, the frequency of potato consumption has recently been positively related to an increased risk of diabetes development (Dong et al., 2011), and this has a detrimental effect on semen parameters (Ding et al., 2015). Because glucose metabolism is important to spermatogenesis, an excess of potatoes and other high starch glycemic food can have detrimental effects on sperm parameters through their effect on the glucose metabolism. Indeed, hyperglycemia has been shown to affect sperm motility and fertilization in mature sperm (Miki, 2007).
Fish, shellfish and seafood
The possible benefits of fish, seafood and shellfish on sperm parameters may be the result of their high omega-3 PUFA content. Ecosapentanoic acid (EPA) and DHA are essential fatty acids that play an important role in the anti-inflammatory and antioxidant properties of enzymes such as superoxide dismutase. A significant positive correlation has been reported between DHA sperm concentrations and sperm motility (Gulaya et al., 2001). Because seminal plasma is recognized as one of the most powerful antioxidant fluids, it is not surprising that any defects it may have are often associated with oxidative stress through an increment in ROS, followed by a SDF, which leads to male infertility (Wathes et al., 2007). In fact, the only RCT conducted in infertile men with idiopathic oligoasthenoteratozoospermia and lower levels of EPA and DHA in spermatozoa has demonstrated that omega-3 PUFA supplementation has beneficial effects on some semen quality parameters (Safarinejad, 2011).
Therefore, it is plausible that increased fish intake or fish oil supplementation may result in improved parameters of semen quality.
Dairy products
The possible effect of dairy foods on male fertility is highly controversial. In short, full-fat dairy and total dairy products and cheese have been negatively associated with sperm quality parameters. Total low-fat dairy and skimmed milk, however, have been related to better classical semen indices (Afeiche et al., 2013, 2014a). Commercial milk is a mixture of milk from cows in different stages of pregnancy and non-pregnant cows, with ~75% coming from pregnant cows (Ganmaa et al., 2001, 2004). Naturally occurring estrogens of placental origin are present in the milk of pregnant cows. As mentioned above, estrogens, derived from dairy (or other food sources), could contribute to a decrease in sperm production (Afeiche et al., 2013; Amarnath et al., 2016). In theory all dairy products should have the same effect, although in this case low-fat dairy products do not. On the other hand, low-fat and skimmed milk consumption is associated with higher circulating levels of insulin-like growth factor 1 (IGF-1) and insulin (Afeiche et al., 2014a). Results from animal studies also indicate that insulin has the potential to increase sperm motility and concentration in rats (Huang et al., 2016), and also to rescue spermatogenesis in Type 1 diabetic mice (Schoeller et al., 2012a). The consumption of low-fat and skimmed milk has also been associated with higher peripheral concentrations of IGF-1 in community-dwelling participants and increases in IGF-1 levels in feeding trials (Hoppe et al., 2005; Bonjour et al., 2012). Given that spermatogenesis is a process of active cell division requiring insulin, and that IGF-1 can bind and activate Leydig cell insulin receptors regulating Sertoli cell proliferation, the relations observed between low-fat dairy products and higher sperm concentration and motility may represent a biological effect in humans (Afeiche et al., 2014a). In this case, the IGF-1 levels may have a more important role than hormone homeostasis in humans.
Meat and processed meat
Meat and processed meat are rich in protein, but also in xenobiotics, mainly xenoestrogens (XEs) and in some cases anabolic steroids (Swan et al., 2007). The use of these compounds in the food industry increases the total level of XEs and sex steroids in processed foods, such as meat, the intake of which contributes significantly to daily exposures. XEs are highly lipophilic substances that can accumulate in fat-rich foods like meat, which have estrogenic effects and are suspected to be partially responsible for the decline in semen quality. In an RCT, synthetic estrogens, such as polychlorinated biphenyls and phthalate esters (widely used industrial compounds), showed deleterious effects on some semen parameters in infertile men with unknown etiology (Rozati et al., 2002).
Meat, full-fat dairy products and butter are the principal sources of SFAs. Although improvements in sperm parameters are a response to PUFA omega-3 sources, in human spermatozoa, elevated SFA concentrations and low omega-3 PUFA levels are related to decreased fertility parameters (Esmaeili et al., 2015). In animal studies, some dietary SFAs do not affect sperm quality parameters (Blesbois et al., 1997; Samadian et al., 2010; Esmaeili et al., 2014; Fair et al., 2014). However, several studies in humans have shown higher levels of palmitic acid or stearic acid in spermatozoa in infertile men (Zalata et al., 1998; Aksoy et al., 2006).
Meat and dairy products are also the principal source of natural TFAs. However, in our diet, TFAs mainly come from processed foods such as bakery products, fast foods and snacks, which are made with shortening, margarine or oils that contain partially hydrogenated oils and fats. In rodents, a high intake of TFAs leads to a number of adverse male reproductive outcomes including decreased serum testosterone levels and, in extreme cases, arrest of spermatogenesis and testicular degeneration with consequences such as low sperm count or motility (Hanis et al., 1989; Veaute et al., 2007). However, studies need to be carried out on the effect of TFA intake on humans.
Coffee, tea and alcohol
In the present review, adult caffeine intake did not show a clear association with semen quality, but high caffeine intake was associated with higher plasma levels of testosterone (Ramlau-Hansen et al., 2008). Several studies have found a positive association between the consumption of caffeine (from coffee, tea or caffeinated beverages) and subfecundity in women (Jensen et al., 1998; Hassan and Killick, 2004). In males, the principal hypothesis is that elevated testosterone levels could disrupt the endocrine system and have a detrimental effect on sperm production (Diamanti-Kandarakis et al., 2009).
Some epidemiological studies have examined the relationship between alcohol consumption and reproductive function. Most of them were conducted in small selected populations of infertile men with contradictory results (La Vignera et al., 2013). A recent review of 15 cross-sectional studies has shown a detrimental effect of alcohol consumption on semen volume and morphology, mainly in daily, not occasional, consumers. This suggests that a moderate consumption of alcohol should not adversely affect semen quality parameters (Ricci et al., 2016). A positive association between excess alcohol intake and some semen quality parameters has also been observed in some, but not all, cross-sectional and case-control studies. However, in relation to sperm parameters, the only prospective study included in our review that assesses alcohol has reported an inverse association between alcohol consumption and sperm concentration and motility. In relation to fecundability, prospective studies show contradictory results.
Alcohol has been experimentally shown to have a deleterious effect at all levels of the male reproductive system. It interferes with the regulation of the hypothalamic–pituitary–testicular axis, impairing LH and FSH secretion, decreasing testosterone levels, and disrupting endocrine homeostasis (Muthusami and Chinnaswamy, 2005; Maneesh et al., 2006). Likewise, the ratio between free estradiol and free testosterone has been modified by alcohol intake (Hansen et al., 2012), and spermatogenetic arrest and the Sertoli-cell-only syndrome has been found to be more frequently associated with high alcohol consumption (Pajarinen and Karhunen, 1994).
Sweets and sugar-sweetened beverages
Numerous studies have shown that sugar-sweetened beverages are associated with weight gain and incidence of obesity (Forshee et al., 2008; de Ruyter et al., 2012; Ebbeling et al., 2012; Malik et al., 2013; Te Morenga et al., 2013; Khan and Sievenpiper, 2016), metabolic syndrome (Malik et al., 2010; Ferreira-Pêgo et al., 2016) and Type 2 diabetes (Greenwood et al., 2014; Imamura et al., 2015; Wang et al., 2015). All of these disorders can increase insulin resistance (Stanhope et al., 2009) which could negatively influence semen quality via increased oxidative stress (Park et al., 2009). In addition, sperm cells contain receptors for glucose which are required for sperm motility and post ejaculation maturation, both necessary for successful conception (Williams and Ford, 2001). Moreover, glucose and insulin can also disrupt the hypothalamic–pituitary–testicular axis and, therefore, sperm production (Schoeller et al., 2012a,b). Alternatively, sweets and sugar-sweetened beverages contain many contaminants (for example, bisphenol A and phthalates) that have leached from plastic containers and which may have a negative influence on sperm quality (Jurewicz et al., 2013). The literature on this topic in humans is scarce. However, a recent study in rodents found that sugary drinks negatively impact male fertility (Ruff et al., 2013).
Limitations and strengths
Several limitations of our study should be acknowledged. Although broad search terms were used, and reference lists were hand searched, we may not have identified all publications. In addition, our search strategy was limited to MEDLINE-Pubmed database, and not EMBASE or other databases. However, the main scientific journals are indexed in MEDLINE-Pubmed database. Misclassification of the studies, publication bias and quality score attrition should also be acknowledged as potential limitations and, to avoid this, the three authors independently reviewed all the qualitative studies included. However, the major limitation of the study is that the review was based on epidemiological observational design studies, which limits the ability to determine causality between food and nutrient intake, and parameters of semen quality and fecundability.
Moreover, some intrinsic limitations of the studies included in this review should be mentioned. First, populations were heterogeneous between the studies. For instance, the subfertile populations have different phenotypes (asthenozoospermic participants or individuals with poor semen quality). In addition, the control populations were diverse and were taken from different environments (general population, university students, farmers, etc.), which can potentially influence the findings. Second, other potential confounders, such as health status, weight, age, medication use, energy intake, physical activity and abstinence time, may influence the reported associations. Some of these factors were considered as confounders in some studies, but not all.
Notwithstanding these limitations, this review is the most up to date and exhaustive review of observational studies carried out with a quality validated protocol, a tailored checklist and methodologically rigorous quality control points. Future studies are needed to confirm our conclusions.
Conclusion
The present systematic review of observational studies provides the most comprehensive analysis to date of the associations between diet or nutrient intake and the risk of infertility. It suggests that male adherence to a healthy diet can improve semen quality and fecundability rates. Since observational studies can prove associations but not demonstrate causation, the associations summarized in the present review need to be confirmed with large prospective cohort studies of high quality, and especially with well-designed RCTs.
Acknowledgements
CIBERobn is an initiative of the Instituto de Salud Carlos III.
Authors’ roles
A.S-H. designed the review, collected and selected the data, assessed the articles and wrote the manuscript. M.B. assessed the articles, and critically reviewed the article for important intellectual content. J.S-S. initiated the idea of the review, designed the review, assessed the articles, contributed to the drafting and critically reviewed the article for important intellectual content. The authors approved the final manuscript.
Funding
There was no external source of funding for this work.
Conflict of interest
None declared.