-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer Brawn, Matteo Morotti, Krina T. Zondervan, Christian M. Becker, Katy Vincent, Central changes associated with chronic pelvic pain and endometriosis, Human Reproduction Update, Volume 20, Issue 5, September/October 2014, Pages 737–747, https://doi.org/10.1093/humupd/dmu025
Close - Share Icon Share
Chronic pelvic pain (CPP) is a significant public health problem with 1 million affected women in the UK. Although many pathologies are associated with CPP, the pain experienced is often disproportionate to the extent of disease identified and frequently no pathology is found (chronic pelvic pain syndrome). The central nervous system (CNS) is central to the experience of pain and chronic pain conditions in general are associated with alterations in both the structure and function of the CNS. This review describes the available evidence for central changes in association with conditions presenting with CPP.
A detailed literature search was performed to identify relevant papers, however, this is not a systematic review.
CPP is associated with central changes similar to those identified in other pain conditions. Specifically these include, alterations in the behavioural and central response to noxious stimulation, changes in brain structure (both increases and decreases in the volume of specific brain regions), altered activity of both the hypothalamic–pituitary–adrenal axis and the autonomic nervous system (ANS) and psychological distress.
The evidence reviewed in this paper demonstrates that CPP is associated with significant central changes when compared with healthy pain-free women. Moreover, the presence of these changes has the potential to both exacerbate symptoms and to predispose these women to the development of additional chronic conditions. These findings support the use of adjunctive medication targeting the CNS in these women.
Introduction
Chronic pelvic pain (CPP) is a major public health problem throughout the developed world (Mathias et al., 1996; Zondervan et al., 2001; Grace and Zondervan, 2004; Garcia-Perez et al., 2010). In the UK alone it affects >1 million women and has been recently highlighted as a key area of unmet need (Donaldson, 2009). CPP can present at all ages, with studies suggesting that the prevalence increases with age (18.2/1000 in 15–20 year olds; 27.6/1000 in women over 60) (Zondervan et al., 1999a, b). Yet despite being as common as asthma and back pain (Zondervan et al., 1999a, b), with a significant financial cost to both the individual and society (Mathias et al., 1996), there is frequently a significant delay in diagnosis and treatment. In fact, studies suggest that up to 50% of women have not received a diagnosis after many years follow-up (Grace, 1995; Zondervan et al., 1999a, b; Grace and Zondervan, 2004). A variety of pelvic pathologies are associated with CPP in women, including endometriosis, adenomyosis or chronic infection and it can be associated with functional disorders of the bowel (irritable bowel syndrome (IBS)) and the bladder (interstitial cystitis/painful bladder syndrome (IC/PBS)). In many cases, however, detailed investigation of the pelvis/pelvic viscera via both imaging and surgery reveals no abnormality (chronic pelvic pain syndrome (CPPS)) (Baranowski et al., 2012). Furthermore, even when pathology is identified and treated the pain may still persist, suggesting that the pathology was either an incidental finding or that other mechanisms continue to generate pain without the need for a peripheral input (Baranowski, 2009). CPP has recently been defined as ‘chronic or persistent pain perceived in structures related to the pelvis of either men or women. It is often associated with negative cognitive, behavioural, sexual and emotional consequences as well as with symptoms suggestive of lower urinary tract, sexual, bowel, pelvic floor or gynaecological dysfunction’ (Baranowski et al., 2012). Despite this definition acknowledging that the patient and clinician localize the pain as being perceived in the specified anatomical area, the conscious experience of pain is the result of co-ordinated activity within the central nervous system (CNS) (Tracey and Mantyh, 2007). If the focus is shifted away from the pelvis and onto the CNS, it can be seen that women with CPP do exhibit central changes analogous to those observed in other chronic pain conditions (Apkarian et al., 2005; Tracey and Bushnell, 2009; Kaya et al., 2013).
This review will describe the available evidence for central changes in association with conditions presenting with CPP, focussing on both functional (specifically the response during experimentally-delivered painful stimulation, the activity of the hypothalamic–pituitary–adrenal (HPA) axis and the ANS) and structural alterations. As endometriosis is such a common finding in women with CPP presenting to gynaecologists, we will highlight areas where there is evidence relating specifically to endometriosis. We will then discuss the implications of these changes in CPP, before considering whether endometriosis is a ‘special case’ that is particularly vulnerable to central changes.
Methods
Literature searches were conducted using CPP OR CPPS OR known individual causes of CPP (including IBS, endometriosis, IC) AND (i) brain/CNS/nervous system/central or (ii) MRI/fMRI/PET/imaging. Reference lists from other publications were also examined for any missed studies from the initial literature search. We included studies published up until 2013. However, it should be stated that this is not a systematic review.
Brain Function in Women with CPP
The use of imaging techniques to investigate organ structure is central to clinical practice in gynaecology. However, over recent decades the use of functional imaging techniques, particularly neuroimaging, has become widespread in many fields of research and has now entered some areas of clinical practice, such as pre-surgical planning in neurosurgery to ensure key functional areas are not damaged during removal of a lesion (Voets and Matthews, 2005). The main technologies used in pain research are functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). Both techniques identify metabolically active areas (fMRI by harnessing the difference in magnetism between oxygenated and deoxygenated haemoglobin and PET by detecting gamma rays emitted by a radioactive tracer) to give information about activity of regions at rest or during a task (including pain processing in the brain). There is now a significant body of literature demonstrating that chronic pain, regardless of cause, is associated with alterations in brain function, particularly with regard to the processing of pain and other sensory information (Apkarian et al., 2005; Tracey and Bushnell, 2009). Here we will review the evidence that such changes also occur in women with CPP.
Investigating the response to experimental pain
One way to investigate the processes involved in pain perception is to evaluate the response to an experimental noxious stimulus. There are many methods available for generating a painful sensation including temperature change, application of pressure or electrical energy or distension of a hollow viscus. Painful stimuli can be applied at the location where the clinical pain is experienced/referred to or an unaffected site. A variety of different responses to the stimulus can be measured depending on the aims/design of the study. For example, in psychophysical (behavioural) studies, it is the patient's report of pain (usually on a visual analogue scale or a numerical rating scale) that is assessed, whilst neuroimaging studies combine such data with an objective measurement of brain activity in response to the stimulus. Whilst noxious experimental stimuli are not directly comparable to the experience of chronic pain, such a strategy allows insights into the central processes involved in pain perception and allows comparison between patients and healthy pain-free controls.
Behavioural responses to experimental pain in women with CPP
Numerous studies have evaluated differences in the behavioural response to both somatic and visceral noxious stimuli between women with CPP and healthy pain-free women (Supplementary Data). Compared with healthy controls, differences have been identified in response to stimulation of the lower abdomen (Giamberardino et al., 1997; Bajaj et al., 2002; Vincent et al., 2011) and vulva (Bohm-Starke et al., 2001; Pukall et al., 2002, 2005; Giesecke et al., 2004; Lowenstein et al., 2004; Schweinhardt et al., 2008; Sutton et al., 2009; Hampson et al., 2013) but not always the back (Bajaj et al., 2002, 2003). Similarly, women with CPP have shown increased behavioural response to stimulation (both noxious and innocuous) of their bladder (Ness et al., 2005; Powell-Boone et al., 2005) and bowel (Song et al., 2006; Brinkert et al., 2007; Wilder-Smith and Robert-Yap, 2007; Elsenbruch et al., 2010; Piche et al., 2010). However, these findings are not consistent, as not all studies observed an increase in all measures of the pain experience (Giamberardino et al., 1997; Bohm-Starke et al., 2001; Bajaj et al., 2002, 2003; Pukall et al., 2002; Song et al., 2006; Brinkert et al., 2007; Wilder-Smith and Robert-Yap, 2007; Sutton et al., 2009; Elsenbruch et al., 2010), whilst some studies did not observe any heightened response in women with CPP (Wilder-Smith et al., 2004; Ringel et al., 2008). It is possible that methodological differences in these studies (inclusion criteria, differing experimental pain techniques, methods for determining pain thresholds, pain rating protocols, number of study visits, and sample sizes) contributed to this inconsistency (see Supplementary Data).
When interpreting the results of these studies to date it is not possible to determine the extent to which any increase in response to noxious stimuli reflects local changes (skin/organ or peripheral nerves) as opposed to truly reflecting central changes. Therefore, many studies have also investigated the response to noxious stimulation at a site distant to where the pain is experienced or referred (Supplementary Data). In the majority of studies it is the hand or arm that is used as a distant site, however, the leg has also been used. Again, the findings are not consistent, but increased behavioural responses to noxious stimulation at a distant site have been demonstrated in women with CPP including in association with dysmenorrhoea (Giamberardino et al., 1997; Granot et al., 2001; Bajaj et al., 2002; Vincent et al., 2011), endometriosis (Bajaj et al., 2003; Laursen et al., 2005; He et al., 2010; Neziri et al., 2010; As-Sanie et al., 2012), IBS (Wilder-Smith and Robert-Yap, 2007; Piche et al., 2010), IC (Clauw et al., 1997; Ness et al., 2005; Powell-Boone et al., 2005) and vulvodynia/provoked vestibulodynia (Granot et al., 2002; Pukall et al., 2002, 2006; Giesecke et al., 2004; Foster et al., 2005; Granot, 2005; Granot and Lavee, 2005; Johannesson et al., 2007; Sutton et al., 2012; Hampson et al., 2013). Such observations are likely to reflect plastic changes in the CNS, leading to increased sensitivity to noxious stimuli (central sensitization) (for review, see Latremoliere and Woolf (2009)). Whilst there is some evidence that this increased sensitivity may in part be reversible after successful treatment of the underlying pathology (Bajaj et al., 2003; He et al., 2010), it does not appear to be purely a reflection of the presence of ongoing pain, as women with dysmenorrhoea demonstrated enhanced behavioural responses to noxious stimulation of both the abdomen and arm throughout the menstrual cycle and not just at the time they were experiencing pain (Vincent et al., 2011).
Identifying changes in central processing using neuroimaging
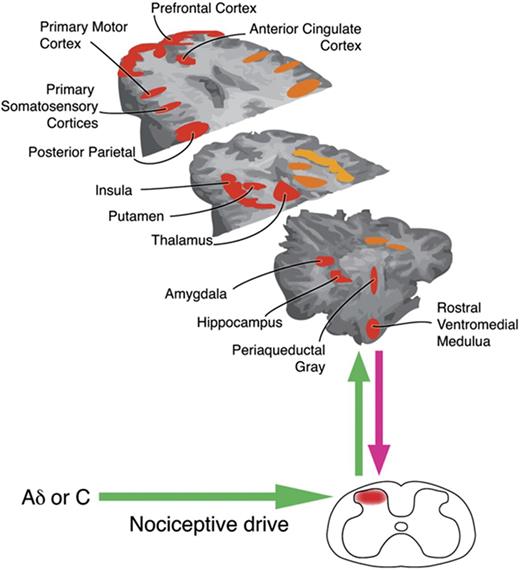
Reprinted from Neuron, Tracey and Mantyh (2007) with permission from Elsevier. Nociceptive input is processed and modulated by ascending and descending pathways in the central nervous system. Many factors influence how the brain processes pain so the pain experience varies both within and between individuals.However, fMRI studies have shown that certain regions are frequently active in response to acute pain. Alterations in activity in some of these regions have been demonstrated in women with CPP (fMRI, functional magnetic resonance imaging).
The concept of a ‘brain-gut axis’ has been widely accepted for many years (Tillisch and Labus, 2011), initially focussing on the conscious processes controlling defecation, but subsequently extended to the functional bowel syndromes. It is therefore not surprising that IBS is the pelvic pain condition most investigated with neuroimaging (see Supplementary Data). A recent meta-analysis of the studies investigating differences in the brain response to rectal distension between individuals with IBS and controls found differences in activation of the amygdala, thalamus, prefrontal, anterior cingulate, and insular cortices (Tillisch et al., 2011), all of which play an important role in pain processing (Tracey and Mantyh, 2007). When studies are limited to those only including female subjects, the findings are regionally similar, with rectal distension eliciting decreased activation in the amygdala and hippocampus (Wilder-Smith et al., 2004) and increased activation in the insular and prefrontal cortices (Elsenbruch et al., 2010) in women with IBS compared with healthy women. However, in the latter study, significance was lost when anxiety and depression measures were included. As before, however, these findings are not consistent in all reported studies (see Supplementary Data) including one study that found no differences; however, it only investigated regions within the cingulate cortex (Ringel et al., 2008).
Two studies have investigated central pain processing in women with vulvodynia/provoked vestibulodynia (Pukall et al., 2005; Hampson et al., 2013). In the first, pressure stimulation of the vulva elicited activation of similar pain regions between women with provoked vestibulodynia and healthy controls; however, the women with provoked vestibulodynia demonstrated significantly stronger activation in several of these regions (Pukall et al., 2005). As outlined by the researchers, these results emphasize potential overactivity of the CNS leading to increased sensitivity in the patient cohort, as seen in other chronic pain conditions. The amplification within these regions might be due to compromised spinal and/or brainstem pain modulatory circuits in the patient cohort, since neural activity often differed by magnitude, not just brain region. Similar findings were reported by the second study (Hampson et al., 2013), with patients showing increased activation of known pain processing regions in response to both vulval and thumb stimulation compared with controls. Of note, this study also identified subtle differences in the central response to stimulation between vulvodynia patients with different clinical presentations (primary versus secondary and provoked versus unprovoked pain). This observation suggests that the pain mechanisms underlying the symptoms in vulvodynia may differ depending on the clinical phenotype and warrants further investigation.
There has recently been an increased interest in dysmenorrhoea within the pain field (Berkley, 2013), including the publication of two neuroimaging studies investigating pain processing in women with dysmenorrhoea (Tu et al., 2009; Vincent et al., 2011). Perhaps not surprisingly, the first of these (Tu et al., 2009), showed that brain metabolism during menstruation was different in women experiencing dysmenorrhoea compared with those whose periods were not painful. More strikingly, the second study showed that even when women with dysmenorrhoea were not experiencing pain (Days 10–12 and 20–22 of the menstrual cycle), their brain response to noxious stimulation was different from that of control women (Vincent et al., 2011). Thus despite requiring a lower temperature for stimulation to produce the same perception of pain intensity, there was increased activity in two important pain-processing regions, the inferior/middle temporal gyrus and left entorhinal cortex (ERC), when compared with controls. Moreover, the strength of activation in the ERC during these non-menstrual phases was positively correlated with reported pain severity at menstruation.
Whilst it is important to remember that these studies use a variety of noxious stimuli and utilize relatively small subject cohorts (although with sophisticated statistical methods), it is noteworthy that the mechanisms amplifying the experience appear to vary between pelvic pain conditions (see Supplementary Data).
Changes in endogenous pain inhibition
It is well established that endogenous central pain inhibitory mechanisms exist, mediated through a variety of spinal and cortical mechanisms (for review, see McMahon (2013)). The extent to which an individual is able to engage these mechanisms depends on a variety of factors including genetics (Mogil, 1999; Zubieta et al., 2003), psychological state and cognitive factors (Tracey and Mantyh, 2007). Dysfunctional pain inhibition has been demonstrated in a variety of chronic pain states (for reviews, see Tracey and Mantyh (2007) and Tracey and Bushnell (2009)) and it has even been suggested that the inability to engage these systems may predispose to the development of chronic pain after an acute insult (De Felice et al., 2011). Some of the findings described above and in Supplementary Data may represent abnormal pain inhibition, as many of the studies identify changes in the prefrontal cortices, amygdala and insula, which are key regions of the descending pain inhibitory system.
Experimentally, there are techniques available to specifically probe the ability of an individual to engage these systems. One such strategy that has been used to investigate women with CPP is to investigate the phenomenon of diffuse noxious inhibitory control (DNIC). DNIC is a mechanism by which painful stimuli to one location decreases perceived pain elicited by noxious stimuli at a distant part of the body (for review, see McMahon (2013)). DNIC has been investigated in women with IBS and provoked vestibulodynia. In women with IBS, four studies found that dual painful stimulation decreased pain in healthy controls, while not significantly decreasing pain in patients (Wilder-Smith et al., 2004; Song et al., 2006; Wilder-Smith and Robert-Yap, 2007; Piche et al., 2010). The increased activity in the midbrain of individuals with IBS identified in the recent fMRI meta-analysis may well be a reflection of increased activity/dysfunction of the endogenous modulatory systems (Tillisch et al., 2011). However, women with provoked vestibulodynia were shown to have similar endogenous pain modulation to healthy controls (Johannesson et al., 2007; Sutton et al., 2012). Thus, while studies are conflicting, the literature suggests that women with CPP may have altered endogenous pain modulation. Further research is required to understand how these changes develop and whether their presence may predispose to the development of chronic pain.
Brain Structure in Women with CPP
Perhaps surprisingly, many chronic pain conditions have been associated with a reduction in brain volume. Of particular note, these changes are not global across the brain but occur in specific regions which are functionally involved in the processing of pain (May, 2011). The mechanisms behind these alterations in brain volume are not completely understood but may include a direct neurotoxic effect of repeated episodes of pain leading to neuronal atrophy or death, an effect of altered metabolic activity or neurotransmitter concentration, neurotoxic effects of drugs, neurodegeneration secondary to pain-related inactivity, and an effect related to psychological correlates such as anxiety and depression (May, 2008, 2011). Morphological studies exist for a variety of non-pelvic chronic pain conditions including chronic low back pain, fibromyalgia and headache. The volume of the grey matter in the anterior cingulate cortex is reduced in almost all these conditions; however, reductions are also commonly seen in the volume of other regions particularly the thalamus, insula and prefrontal cortices (May, 2011). In many cases the volume of specific brain regions correlated with the duration of the symptom suggesting that these volumetric changes are a consequence rather than a cause of the pain.
Volumetric studies have also been undertaken in women with dysmenorrhoea, provoked vestibulodynia and CPP, both without an obvious cause, and related to endometriosis and IBS (Supplementary Data). Interestingly, although decreases in grey matter were observed in many pain processing regions, areas with an increased volume were also observed (Schweinhardt et al., 2008; Blankstein et al., 2010; Seminowicz et al., 2010; Tu et al., 2010, 2013; As-Sanie et al., 2012). Although these increases were observed in different brain regions from those where reductions in volume have been observed in other conditions, these regions are also known to be involved in the processing of noxious stimuli. A number of reasons have been proposed to explain why increases in brain volume are seen with these specific pain conditions in contrast to the other conditions investigated, including the fact that the patients are generally younger, pain is intermittent/cyclical/provoked as opposed to continuous and that most women with these conditions do not use large doses of pain modifying drugs for a prolonged time period (Schweinhardt et al., 2008).
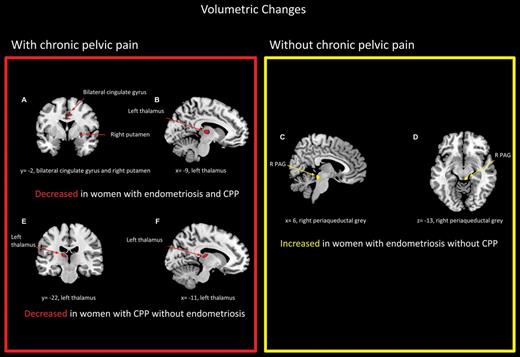
Adapted from As-Sanie et al. (2012) with permission. The figure has been reproduced with permission of the International Association for the Study of Pain® (IASP). The figure may not be reproduced for any other purpose without permission. Regional grey matter changes in women with and without endometriosis and chronic pelvic pain compared with healthy pain-free controls. Red regions represent areas in which grey matter decreased, while yellow regions represent where grey matter increased.
Changes in Activity of the Hypothalamic–Pituitary–Adrenal Axis
Activation of the HPA axis is central to the stress response (Frodl and O'Keane, 2013). Given that chronic pain can be considered as a repeated stressor, both physiologically and psychologically, it is probably not surprising that dysfunction in the activity of the HPA axis is an almost universal finding in chronic pain conditions (Griep et al., 1998; Gaab et al., 2005; Galli et al., 2009). Moreover, cortisol, as one of the principal players in the HPA axis, appears to be central to the phenomenon of stress-induced analgesia (Filaretov et al., 1996) and therefore a reduction in cortisol levels secondary to dysfunction of the HPA axis may exacerbate pain.
The findings in CPP are consistent with other chronic pain conditions (Griep et al., 1998; Gaab et al., 2005; Galli et al., 2009). Thus, abnormally low cortisol levels have been observed in women with endometriosis and CPP (Petrelluzzi et al., 2008), dysmenorrhoea (Vincent et al., 2011) and provoked vulvodynia (Ehrstrom et al., 2009) and replicated over repeated measurements (Petrelluzzi et al., 2008; Vincent et al., 2011). In two of these studies, levels of reported stress were also assessed and found to be higher in patients than controls, despite their lower cortisol levels (Petrelluzzi et al., 2008; Ehrstrom et al., 2009). The mechanism(s) by which suppression of the HPA axis is produced are as yet unclear. Burnout of the system has been proposed as one possibility (Hellhammer and Wade, 1993; Fries et al., 2005) and the recent finding that cortisol levels were negatively correlated with the duration of dysmenorrhoea (Vincent et al., 2011) would be consistent with this, suggesting that HPA axis suppression is a consequence not a cause of their symptoms. In women with endometriosis, the circadian pattern of cortisol secretion was maintained but suppression of cortisol secretion occurred throughout the day (Petrelluzzi et al., 2008).
In view of the negative impact of chronic stress on important physiological processes including the immune system and memory function (McEwen, 2005), it has been proposed that suppression of HPA axis activity might in fact be a protective mechanism (Fries et al., 2005). However, as we will discuss below, a reduction in serum cortisol levels would be expected to exacerbate symptoms from any underlying infective or inflammatory pathology.
Autonomic Nervous System Changes
It has been suggested that abnormal functioning of the ANS might responsible for some of the symptoms associated with IBS (Bockus et al., 1928). In healthy controls, experimentally increasing sympathetic activity has been shown to heighten visceral organ sensitivity to distension (Iovino et al., 1995). In women with IBS, altered ANS functioning (increased sympathetic and/or decreased parasympathetic activity) has been observed during sleep (Thompson et al., 2002), over a 24 h period (Heitkemper et al., 1998), and after stimulation of the sympathetic nervous system (Waring et al., 2004) when compared with healthy women. In response to painful distension of the sigmoid colon and rectum, individuals with IBS have demonstrated decreases in parasympathetic and increases in sympathetic nervous system activity when compared with healthy controls (although this finding was only significant in men with IBS) (Tillisch et al., 2005).
Whether these changes in ANS activity are unique to IBS or, as with the other changes described here, are common to all patients with CPP is as yet unclear. A recent questionnaire study, assessed for the presence of autonomic symptoms (such as vasomotor symptoms, orthostatic intolerance and sleep disorder) in a small cohort of women with CPP (n = 16) and pain-free controls (n = 15) (Janicki et al., 2013). They found a significantly higher incidence of autonomic symptoms in the women with CPP, however, there are a number of difficulties in interpreting these results. Firstly, the autonomic symptom profile included symptoms relating to bowel and bladder function yet ten of the fifteen CPP patients had pain with a full bladder and/or with bowel movement. Secondly, there was no assessment of drug use in the CPP cohort and, as the authors describe, many of the autonomic symptoms may be secondary to the drugs prescribed to treat their pain. Furthermore, the sample size was small and the symptoms not validated by a medical practitioner. However, this is certainly an area that deserves further investigation in the future.
Psychological Changes
Many chronic pain conditions are comorbid with depression and anxiety (for review, see Blackburn-Munro and Blackburn-Munro (2001)). Negative psychological states potentially put individuals at increased risk for developing or exacerbating chronic pain. However, it is important to remember that psychological dysfunction can also be a consequence of the pain (for reviews, see Blackburn-Munro and Blackburn-Munro (2001) and Blackburn-Munro (2004)).
Psychological distress has been observed in women with CPP. Thus even women who only suffer with dysmenorrhoea (as opposed to pain throughout the month) have increased tendencies for depression and anxiety (Dorn et al., 2009), although this is not a universal finding (Vincent et al., 2011). Similarly, women with endometriosis (Sepulcri Rde and do Amaral, 2009; Smorgick et al., 2013) and provoked vestibulodynia (Nunns and Mandal, 1997) are more likely to have anxiety or depression, but may otherwise be psychologically healthy (Eriksen et al., 2008). Neuroimaging studies have shown that psychological factors can augment the pain experience by specific mechanisms that are different from those observed when the noxious input is increased (for review, see Tracey and Mantyh (2007)). Thus anxiety amplifies pain by increasing activity within the hippocampus and entorhinal cortex (Ploghaus et al., 2001), whilst depression enhances the affective component of pain, increasing unpleasantness, and appears to disrupt activity within a network of brain regions including the amygdala and inferior frontal gyrus (Berna et al., 2010). In the context of dysmenorrhoea, Tu and colleagues observed regionally specific (prefrontal and orbitofrontal cortices) metabolic increases in the brain during dysmenorrhoea, which they suggest may be related to negative psychological symptoms (Tu et al., 2009).
Implications of Central Changes
A major criticism of many of the studies cited in this review is that they are ‘snapshot’ studies and therefore they can only inform us about central factors that occur in association with chronic pain, rather than giving us any information about cause and effect. Unfortunately, we currently know very little about why some people develop chronic pain. It is extremely difficult to undertake longitudinal studies, evaluating the factors that predispose to the development of chronic pain and assessing the central changes that occur after such pain has developed. Whilst these are clearly questions that future large collaborative studies need to answer, there are many implications of the central features of chronic pain that have been described above which justify their identification and treatment where possible, no matter what their aetiology might be.
Exacerbation of symptoms
Many of the pathologies associated with CPP in women have an infective or inflammatory component (endometriosis, adenomyosis, potentially interstitial cystitis/painful bladder syndrome (IC/PBS), chronic pelvic inflammatory disease (PID), etc.), therefore a reduction in circulating glucocorticoids would be expected to exacerbate these pathologies (Silverman and Sternberg, 2012). Such an increase in disease burden alone could be expected to intensify symptoms. Additionally, many of the changes described above can aggravate pain even if the peripheral nociceptive input stays constant. These include a loss of a variety of endogenous analgesic mechanisms including that mediated by cortisol and an amplification of pain by psychological factors such as anxiety and depression (Tracey and Mantyh, 2007). Furthermore, the development of central sensitization, as has been demonstrated in a number of conditions associated with CPP (Kaya et al., 2013), can lead to the generation of pain without a peripheral noxious input potentially at least partly explaining the pain that persists despite optimum treatment of identified pathology.
Predisposition to other chronic conditions
It is well known that chronic pain conditions cluster and that having one pain condition can increase the likelihood of developing another. This is true for conditions within the pelvis (e.g. IBS and IC/PBS (Rodriguez et al., 2009)) and at distant sites (e.g. CPP and migraine (Karp et al., 2011; Yang et al., 2012)). There are a number of reasons as to why this might occur including common predisposing factors, such as genetics (Diatchenko et al., 2007) or psychological/cognitive state (for review, see Blackburn-Munro (2004)), and similar neural mechanisms (Giamberardino et al., 2010). However, it is also plausible that the central changes secondary to one chronic pain condition could then predispose to the development of another (Woolf, 2011). For example, a reduction in circulating cortisol could increase the chances of repeated urinary tract infections, or central sensitization could predispose to the development of chronic whiplash after a minor car accident. In this context, the central changes observed in women with dysmenorrhoea (Tu et al., 2009, 2010, 2013; Vincent et al., 2011) are of particular concern given the high prevalence of dysmenorrhoea in adolescents and young women.
The correlation between CPP and other clinical conditions is not exclusive to those in the realm of pain. Chronic fatigue syndrome (CFS) has been correlated with vulvodynia (Arnold et al., 2007) and IBS (Whitehead et al., 2002), whilst endometriosis is associated with a variety of autoimmune and endocrine conditions including hypothyroidism, systemic lupus erythematosus and multiple sclerosis (Sinaii et al., 2002). Other chronic conditions have also been associated with HPA axis dysfunction, including CFS and major depression, as has post-traumatic stress disorder (Yehuda et al., 1991; Pariante and Lightman, 2008; Papadopoulos and Cleare, 2012). Thus, activity of the HPA axis may conceivably be a biological mechanism relating these conditions. Moreover, it could underlie the frequently discussed, though not well proven (Raphael et al., 2004), link between abuse and chronic pain.
Is Endometriosis Particularly Vulnerable to Central Changes?
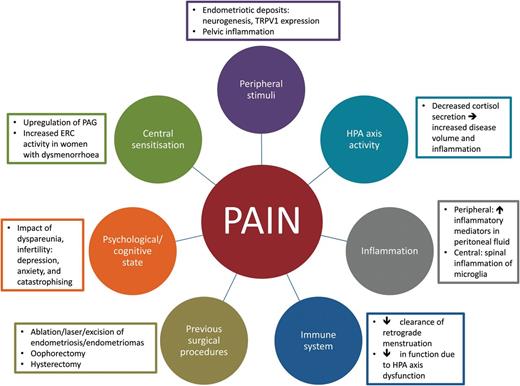
There are many factors that contribute to pain in CPP (circles). These factors might play a role in CPP in women with endometriosis (rectangles). PAG, periaqueductal grey; TRPV1, transient receptor potential cation channel subfamily V member 1; ERC, entorhinal cortex; HPA, hypothalamic pituitary axis.
Moreover, many women with endometriosis are likely to have significant concerns about their fertility. These concerns could be expected to increase both anxiety and pain catastrophising (an exaggerated negative response in anticipation of pain (Quartana et al., 2009)) thereby amplifying the pain experience. Furthermore, women with fertility concerns are more likely to undergo surgical treatment of their disease (Dunselman et al., 2014), thereby increasing the potential for further damage to pelvic and endometriotic nerves. Similarly, the association of endometriosis with dyspareunia could be expected to increase psychological distress in these women (Meana et al., 1999). Furthermore, if this symptom significantly impacts on their relationship, both self-esteem and social support may be reduced, potentially further worsening their pain experience (Culley et al., 2013). It is plausible that these central changes contribute to the well-established disparity between the extent of disease observed at laparoscopy and the pain experienced (Vercellini et al., 2007) and to the persistence of pain despite adequate surgical treatment. However, further work specifically assessing central changes in women with proven endometriosis and their influence on pain symptoms and the response to treatment are needed.
Conclusions
The evidence reviewed in this paper demonstrates that CPP, whilst perceived as a peripheral (pelvic) condition, is in fact associated with significant central changes when compared with healthy pain-free women. These findings support the use of adjunctive medication targeting the CNS (such as amitriptyline, gabapentin, duloxetine, etc.) in women with CPP. This is common practice in other chronic pain conditions (Dworkin et al., 2010; Richards et al., 2012) but is currently poorly investigated in CPP specifically (Sator-Katzenschlager et al., 2005; Horne et al., 2012). Although it remains to be ascertained whether central changes are a cause, effect or both of the painful symptoms, it appears likely that their presence has the potential to not only exacerbate symptoms, but also to predispose these women to the development of additional chronic conditions.
The extent to which these central changes are reversible with treatment remains unknown. However, these findings support the idea that pain should be treated promptly even in the absence of identifiable pathology. Such prompt treatment, as well as being welcomed by the patient, may prevent development or progression of central changes and potentially development of other symptoms/pain conditions, in addition to improving symptoms from any other pain conditions already present (Giamberardino et al., 2010). It is of particular concern that many of the symptoms of CPP start in adolescence or early adult life (Zondervan et al., 1999a, b; Brosens et al., 2013), a time when the CNS is very plastic (Blakemore and Choudhury, 2006; Kalia, 2008) and thus potentially more vulnerable to repeated episodes of pain. Whilst future longitudinal studies are required to assess the impact of pelvic pain on brain development in adolescents and young women, strategies for reducing the amount of pain experienced are likely to be of particular importance in these patients.
Authors' roles
K.V. conceptualized the manuscript. J.B. and K.V. wrote the manuscript. C.M.B, K.T.Z and M.M. were involved in discussions of the content of the review. All authors revised the manuscript and approved the final version.
Funding
Supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health's NIHR Biomedical Research Centres Scheme.
Conflict of interest
K.T.Z. is a member of the scientific advisory boards of AbbVie, Inc., Bayer Healthcare and Roche Diagnostic.
References