-
PDF
- Split View
-
Views
-
Cite
Cite
Giselle Crawford, Arpita Ray, Anil Gudi, Amit Shah, Roy Homburg, The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta-analysis, Human Reproduction Update, Volume 21, Issue 2, March/April 2015, Pages 275–284, https://doi.org/10.1093/humupd/dmu052
Close - Share Icon Share
There is a growing body of evidence surrounding the role played by seminal plasma in human implantation. Seminal fluid contains several proteins that interact with cervical and uterine epithelial cells inducing active immune tolerance. We sought to answer the study question: Does exposure to seminal plasma improve pregnancy outcomes in women undergoing IVF?
Randomized controlled trials (RCTs) were searched for via MEDLINE, EMBASE, the Cochrane Library, National Research Register, ISI conference proceedings, ISRCTN register and Meta-register, from 1966 to December 2013. Search terms included: ‘seminal plasma’, ‘seminal fluid’, ‘sexual intercourse’, ‘IVF’, ‘ICSI’, ‘ART’, ‘pregnancy rate’, ‘implantation’, ‘embryo transfer’ and ‘live birth’. This analysis included all RCTs comparing the outcome of IVF treatments in patients exposed to seminal plasma near the time of oocyte pickup (OPU) or embryo transfer (ET) with that of placebo controls or controls with no exposure to seminal plasma. The main intervention was exposure to seminal plasma around the time of OPU or embryo transfer during an IVF cycle. The main outcomes were clinical pregnancy and live birth/ongoing pregnancy rates. Data were collected by two independent authors and statistically pooled via meta-analysis following intention to treat and per protocol principles using RevMan (v5.2.10). I2 statistic, forest plots and chi-squared heterogeneity tests were used.
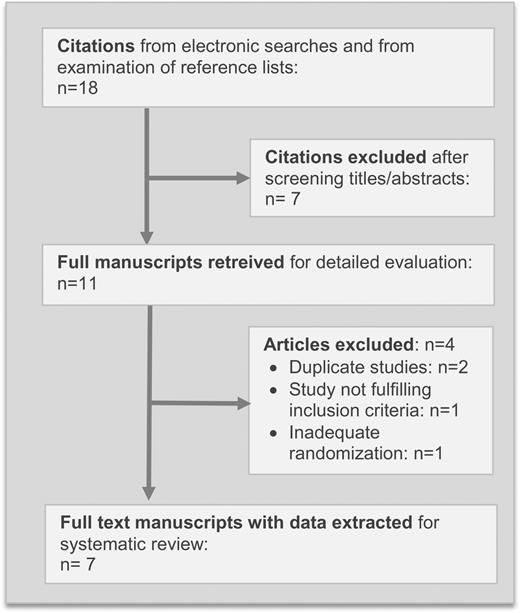
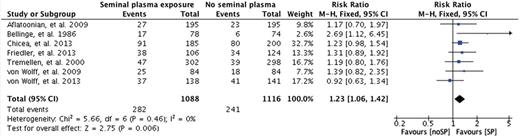
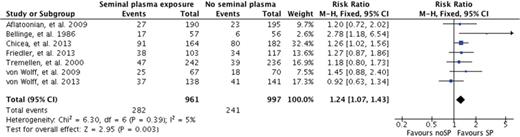
In total 2204 patients were included in seven RCTs. Meta-analysis revealed a statistically significant improvement in clinical pregnancy rate (RR 1.23, 95% CI 1.06–1.42, P = 0.006) by intention to treat. Per protocol analysis also revealed a statistically significant improvement in clinical pregnancy rate (RR 1.24, 95% CI 1.07–1.43, P = 0.003). There was no statistically significant improvement seen for the outcome of ongoing pregnancy/live birth rate, but the available data were very limited. The methodology and quality of the studies were variable.
There are significantly improved outcomes when women are exposed to seminal plasma around the time of ovum pick-up or embryo transfer, with statistical significance for clinical pregnancy but not for ongoing pregnancy/live birth rates being achieved. This meta-analysis is limited by the small number of studies of variable methodology. Further research is required to determine the effect on live birth rate; however, this meta-analysis indicates a significantly improved clinical pregnancy rate and a potential method for improving IVF outcomes.
Introduction
Despite the many advances in in vitro fertilization (IVF) in recent years the success, namely clinical pregnancy and ultimately live birth rates, remains somewhat disappointingly low. One of the many factors contributing to the outcome of IVF is the implantation rate, which remains limited, despite the transfer of high-quality embryos. Inadequate uterine receptivity is considered to be one of the important causes for implantation failure.
Reproduction imposes several intricate immunological challenges: the semi-allogenic developing conceptus induces maternal immune responses, which need to be modified to allow for successful implantation and for the pregnancy to progress (Trowsdale and Betz, 2006). For a number of years, data have been available from both human and animal studies (Alexander and Anderson, 1987; Thaler, 1989; Robertson et al., 2000) to suggest that seminal plasma interacts with the endometrium inducing immunoregulation to promote immune tolerance and thereby facilitating implantation. Animal models have shown that these processes of active immune tolerance in reproductive end organs are very similar to processes in other peripheral and mucosal tissues (O'Leary et al., 2004; Rhodes et al., 2006). There is much research being undertaken by reproductive biologists, immunologists and endocrinologists alike in the attempt to identify various measures in order to reach a fine balance between regulation and dysregulation of endometrial action. The ultimate goal of such measures is to increase the implantation rate, which should consequently result in a greater likelihood of success for each IVF cycle.
Various systemic therapies, such as the use of immunoglobulin, aspirin, heparin or cytokines, have not improved the success rate of IVF treatment (Stern et al., 2003). These treatments may have failed due to a lack of clear pathophysiological concepts. An enhanced understanding of the physiological changes during implantation has stimulated interest in the role of seminal plasma in human implantation.
Background
Immune factors involved in implantation
At implantation, a state of maternal immune tolerance is required in order to avoid an immune attack on the developing conceptus (Guerin et al., 2011). The conceptus has reduced antigenicity, yet despite this maternal T cells still recognize and respond to the fetal antigens (Erlebacher et al., 2007; Moldenhauer et al., 2009).
The embryo produces a number of pro-inflammatory cytokines, suggesting that the embryo directly induces inflammatory pathways in the endometrium during the process of implantation (Jabbour et al., 2009). Numerous pro- and anti-inflammatory cytokines, chemokines, leucocyte adhesion molecules and angiogenic factors are locally expressed in a carefully regulated manner allowing successful implantation to occur (Hutchinson et al., 2011). These mediators in epithelial, stromal and immune cells support blastocyst attachment, endometrial decidualisation and placentation (van Mourik et al., 2009). There are various inflammatory resolution pathway targets involved in these processes, including IL1, IL6, IL10, VEGF, TGFβ, TNF-α, LIF and others (Simon et al., 1993; Charnock-Jones et al., 1994; von Wolff et al., 2000; Jasper et al., 2007; Hutchinson et al., 2011). Many of these factors are up-regulated during the preimplantation period. However, evidence suggests that they are suppressed in infertile patients and in patients with recurrent miscarriage (Lim et al., 2000; von Wolff et al., 2000).
The active immune system, specifically, the T cell subset of regulatory T cells (Treg), is essential in the peri-implantation period for the establishment of the allogeneic pregnancy (Aluvihare et al., 2006; Zhao et al., 2007; Guerin et al., 2009). Treg cells act by suppressing both the proliferation and function of leucocyte subsets, such as CD4 and CD8 T cells, B cells and natural killer (NK) cells. Treg cells also affect the function of dendritic cells (DCs) and macrophages (Shevach, 2002; Guerin et al., 2009). Evidence from murine studies suggests that deficiency in Treg cells leads to implantation failure or miscarriage (Aluvihare et al., 2006; Shima et al., 2010).
The role of seminal plasma
Seminal plasma (SP) has conventionally only been thought of as a transport and nutrient medium for spermatozoa. It is released from the seminal vesicles and the prostate gland and is rich in estrogen, testosterone and prostaglandins and has high concentrations of signalling agents, such as transforming growth factor-beta (TGFβ), prostaglandins, interleukin-8 (IL-8), and interferon-gamma (IFN-γ), along with bacterial polysaccharide (Robertson, 2005). Exposure of seminal fluid at coitus is the earliest point at which the female immune system is exposed to paternal antigens that will be again be encountered in the implanting conceptus. This delivery of the male seminal fluid at coitus induces an inflammation-like response activating immune changes in both humans (Pandya and Cohen, 1985; Sharkey et al., 2007) and rodents (Robertson et al., 1996; Johansson et al., 2004; Guerin et al., 2011).
It is probable that seminal fluid interacts with both cervical and endometrial components. Sharkey et al. (2012) examined the human cervix via biopsy following unprotected vaginal coitus, vaginal coitus with use of a condom, or no coitus. They demonstrated that SP introduced at intercourse (without the use of a condom) elicits expression of pro-inflammatory cytokines and chemokines, and a robust recruitment of macrophages, dendritic cells, and memory T cells. The leucocyte and cytokine environment induced in the cervix by seminal fluid appears competent to initiate adaptions in the female immune response that promote fertility.
It is speculated that SP moves up through the cervix by subendometrial and myometrium peristaltic waves (Leyendecker et al., 1996; Kunz et al., 1997), where it alters endometrial receptivity. The human cervix produces cervical mucus, which is thought to prevent large concentrations of spermatozoa and seminal plasma from reaching the uterine cavity during intercourse. Despite this studies performed by Kunz et al. (1997) and Leyendecker et al. (1996) have demonstrated that labelled albumin macrospheres, placed at the external cervical os, reach the fallopian tubes within 1 min due to subendometrial and peristaltic waves. A recent study by Chen et al. (2014) looked at the in vitro effect of SP on endometrial epithelial cells and stromal fibroblasts. This demonstrated that SP exposure increases expression of genes and secreted proteins associated with cellular migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts.
These experiments further support the hypothesis that SP may interact with both cervical and endometrial components.
It has been demonstrated in mice that high molecular weight seminal proteins generate an inflammatory response in post-mating mice (Robertson et al., 1996) and also stimulate infiltration of subepithelial stromal tissues by macrophages, dendritic cells and granulocytes (Tremellen et al., 1998). In humans, semen stimulates recruitment of macrophages and lymphocytes into the layers of the cervix (epithelial and deep stromal tissues) (Robertson et al., 2001). Pro-inflammatory markers such as IL-6, IL-11 and LIF, amongst others (Stewart et al., 1992; Laird et al., 1997; von Wolff et al., 2002; Sharkey et al., 2004) are activated and are responsible for regulation of cervical leucocyte infiltration. Gene knockout murine models have shown that both IL-11 and LIF play important roles in implantation while the cytokine IL-6 influences fertility and implantation efficiency (Stewart et al., 1992; Robb et al., 1998; Jasper et al., 2007).
TGF-β is one of the primary components of seminal plasma that has been implicated in regulating the maternal immune response. It is a very strong immune deviating agent driving an active form of immune tolerance. It causes phenotype skewing in antigen presenting cells and lymphocytes and affects the induction and resolution of inflammatory responses. The TGF-β content of seminal plasma is amongst the highest measured in biological fluids, mostly consisting of TGF-β1 and TGF-β3 with TGF-β2 being less abundant (Robertson, 2005). Its synthesis is regulated by testosterone in the seminal vesicles. Unlike TGF-β in serum, which is present exclusively in the latent form, ∼25% of TGF-β in seminal plasma exists in the mature, active form (Robertson et al., 2002). Examination of TBF-β in the uterine fluid of mice after insemination has shown that ∼70% is in the active form, implying that significant activation of seminal vesicle-derived TGF-β occurs at the time of ejaculation or after deposition in the female tract, although the exact mechanism has not yet been determined (Tremellen et al., 1998). The acidic environment of the human vagina (typically pH 5.0) might be sufficient to elicit some partial activation. Other factors that have been implicated in its activation include seminal plasma enzymes, such as plasmin, substilisin-like endoproteases and tissue- and urokinase-type plasminogen activator, as well as female tract derived activating agents such as plasmin (Chu and Kawinski, 1998; Robertson et al., 2002). TGF-β appears to be a principal stimulating agent in the post-coital inflammatory response, likely initiating the process of immune tolerance to seminal antigen, thereby playing an important role in priming of the female immune system for embryo implantation, as several seminal antigens are shared by the fetus. In vitro experiments in rodents suggest that seminal plasma plays a role in the regulation of implantation by regulating endometrial epithelial cells. In conjunction with IL-8, TGF-β stimulates the release of pro-inflammatory cytokines, including GM-CSF (granulocyte macrophage colony-stimulating factor) in vivo and in vitro, along with IL-1β, IL-6 and LIF (Tremellen et al., 1998; Gutsche et al., 2003). Of these, GM-CSF has been the best studied and it is known that regulation of GM-CSF plays an important role in the development of the embryo (Robertson et al., 1999, 2000, 2001).
The female immune response is further stimulated by seminal plasma via the drainage of seminal antigen through the lymphatic system (Leyendecker et al., 1996; Johansson et al., 2004). Guerin et al. (2011) have demonstrated in mice an accumulation of Treg cells in the para-aortic lymph nodes draining the uterus in response to seminal plasma delivered at coitus, resulting in a greater availability of Treg cells for recruitment into the uterus. Further, seminal plasma is thought to play a key regulatory role in the uterine Treg cell population. Migration of Treg cells from the circulation into the endometrium is promoted by the seminal-plasma induced up-regulation of T cell chemokine CCL19 in glandular epithelial cells (Robertson et al., 2009; Shima et al., 2010; Guerin et al., 2011). In summary, seminal fluid induces an elevation in the uterine Treg cell population, which occurs due to an increased availability of Treg cells as well as increased Treg cell migration into the uterine epithelial cells. These Treg cells help to induce a state of maternal immune tolerance thereby avoiding induction of inflammation and recognition of the semi-allogenic conceptus after implantation.
Considerable evidence from both human and animal studies support an immune-regulatory interaction between seminal plasma and the female reproductive tract. But the question remains as to whether such physiological processes can be utilized during in vitro fertilization in an attempt to improve the implantation and cycle outcomes.
Objectives
This study sought to systematically review and summarize the evidence related to the use of seminal plasma (SP) in women undergoing IVF or ICSI for improving cycle outcomes.
Methods
Study eligibility criteria
Only randomized controlled trials (RCTs) in which SP exposure was used as an adjunctive measure in IVF or ICSI in order to improve cycle outcomes were included. The types of participants included in the studies were women of reproductive age, with any cause of infertility, who were undergoing IVF or ICSI cycles. The intervention of SP exposure may be via sexual intercourse or via intra-vaginal, intra-cervical or intrauterine injection, occurring prior to embryo replacement. The main outcome measures considered were the clinical pregnancy rate and the live birth or ongoing pregnancy rate achieved in the index IVF cycle.
Literature search methodology
This study searched electronic databases including MEDLINE (1966–December 2013), EMBASE (1980–December 2013), Cochrane Library (1970–2013) and the National Research Register for any relevant studies. The search was broadened for grey literature and thereby avoiding publication bias by also searching ISI Conference Proceedings (1990–2013), as well as databases for registration of ongoing and archived randomized controlled trials, specifically Meta-register for RCT and ISRCTN Register.
A combination of Medical Subject Headings (MeSH) and text words were used to generate three subsets of citations, one including studies involving seminal plasma (‘seminal plasma’, ‘seminal fluid’, ‘sexual intercourse’ and ‘intercourse’), the second including studies of assisted reproductive technologies (‘in-vitro fertilization’, ‘IVF’, ‘intracytoplasmic sperm injection’, ‘ICSI’, ‘assisted reproductive techniques' or ‘ART’) and the third subset including studies of outcome after IVF and ICSI (‘pregnancy rate’, ‘pregnancy’, ‘implantation’, ‘embryo transfer’, ‘live birth’). The three subsets of citations were then combined with ‘AND’ generating a set of citations relevant to the research question. Limits applied included ‘humans' and ‘randomized control trial’; however, no language restrictions were placed in any of the searches. The reference lists of articles retrieved by the search were hand-searched to identify cited articles not captured by electronic searches.
Study selection
Two review authors scrutinized the titles and abstracts of the identified studies. Those that were clearly irrelevant were removed. The full texts of potentially relevant articles were retrieved and independently assessed for inclusion by A.R. and G.C. Disagreement was resolved by consensus.
Data extraction
The review authors independently assessed the selected studies for methodological quality based upon components of study design related to internal validity (CRD report number 4, 2001). Information on the method of randomization, allocation concealment, blinding, intention-to-treat analysis and follow-up was sought. Study characteristics such as participants' baseline characteristics (female age, aetiology and duration of infertility, mean number of embryos transferred), nature of the intervention (timing of seminal plasma exposure, method of seminal plasma exposure), nature of the control group (abstinence, no treatment or placebo) and features of the IVF treatment cycle were extracted from the individual studies. Where necessary, authors of the original papers were contacted for additional missing information.
Risk of bias in individual studies
The included studies were assessed for risk of bias with consideration given to: sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data and selective outcome reporting. Two review authors assessed the included studies for these aspects and a summary of the findings are presented in Table I.
Quality of the studies included in the systematic review.
| Publication . | Study design . | Comparability of groups at baseline . | Sequence generation . | Allocation concealment . | Blinding of participants and personnel . | ITT analysis . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | RCT | Yes | Low risk | Unclear risk* | Low risk | Number of excluded patients who did not reach ET |
| Bellinge et al. (1986) | RCT | Yes | Low risk | Unclear risk** | Low risk | Number of excluded patients who did not reach ET |
| Chicea et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET or who did not have top quality embryos transferred. |
| Friedler et al. (2013) | RCT | Yes | Low risk | Low risk | Low risk | Number of excluded patients who did not reach ET |
| Tremellen et al. (2000) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2009) | RCT | Yes | Low risk | Unclear risk*** | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Yes |
| Publication . | Study design . | Comparability of groups at baseline . | Sequence generation . | Allocation concealment . | Blinding of participants and personnel . | ITT analysis . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | RCT | Yes | Low risk | Unclear risk* | Low risk | Number of excluded patients who did not reach ET |
| Bellinge et al. (1986) | RCT | Yes | Low risk | Unclear risk** | Low risk | Number of excluded patients who did not reach ET |
| Chicea et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET or who did not have top quality embryos transferred. |
| Friedler et al. (2013) | RCT | Yes | Low risk | Low risk | Low risk | Number of excluded patients who did not reach ET |
| Tremellen et al. (2000) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2009) | RCT | Yes | Low risk | Unclear risk*** | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Yes |
ET, embryo transfer or replacement.
*Unclear risk = ‘random selection by drawing numbered slips from paper bag’, unclear if concealed.
**Unclear risk = ‘table of random numbers’, unclear if open random table of numbers.
***Unclear risk = ‘randomization performed by randomization lists’, unclear if concealed.
Quality of the studies included in the systematic review.
| Publication . | Study design . | Comparability of groups at baseline . | Sequence generation . | Allocation concealment . | Blinding of participants and personnel . | ITT analysis . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | RCT | Yes | Low risk | Unclear risk* | Low risk | Number of excluded patients who did not reach ET |
| Bellinge et al. (1986) | RCT | Yes | Low risk | Unclear risk** | Low risk | Number of excluded patients who did not reach ET |
| Chicea et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET or who did not have top quality embryos transferred. |
| Friedler et al. (2013) | RCT | Yes | Low risk | Low risk | Low risk | Number of excluded patients who did not reach ET |
| Tremellen et al. (2000) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2009) | RCT | Yes | Low risk | Unclear risk*** | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Yes |
| Publication . | Study design . | Comparability of groups at baseline . | Sequence generation . | Allocation concealment . | Blinding of participants and personnel . | ITT analysis . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | RCT | Yes | Low risk | Unclear risk* | Low risk | Number of excluded patients who did not reach ET |
| Bellinge et al. (1986) | RCT | Yes | Low risk | Unclear risk** | Low risk | Number of excluded patients who did not reach ET |
| Chicea et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET or who did not have top quality embryos transferred. |
| Friedler et al. (2013) | RCT | Yes | Low risk | Low risk | Low risk | Number of excluded patients who did not reach ET |
| Tremellen et al. (2000) | RCT | Yes | Low risk | High risk | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2009) | RCT | Yes | Low risk | Unclear risk*** | Low risk | Number of excluded patients who did not reach ET |
| Von Wolff et al. (2013) | RCT | Yes | Low risk | High risk | Low risk | Yes |
ET, embryo transfer or replacement.
*Unclear risk = ‘random selection by drawing numbered slips from paper bag’, unclear if concealed.
**Unclear risk = ‘table of random numbers’, unclear if open random table of numbers.
***Unclear risk = ‘randomization performed by randomization lists’, unclear if concealed.
Statistical analysis
For each included study, binary data were extracted in 2 × 2 tables and results were pooled and expressed as relative risks (RR) with 95% confidence intervals (CI). We performed an intention to treat analysis, despite six of the seven studies not following this principle. We considered any drop outs as failures. Statistical heterogeneity was assessed using the I2 statistic (Higgins, 2008) and considered absent if the I2 statistic was <50%. Heterogeneity of treatment effects was evaluated graphically using forest plots and statistically using the chi-squared heterogeneity test. Exploration of heterogeneity was conducted using variation in features of the population, intervention and study quality. If heterogeneity could not be satisfactorily explained then the data would not be deemed suitable for inclusion in the meta-analysis and would not be pooled. All statistical analyses were performed using the RevMan (version 5.2.10) software (The Cochrane Collaboration, Oxford, UK). Publication bias was assessed by performing a funnel plot analysis using Egger's test (Egger et al., 1997).
Results
All seven studies were randomized control trials and were published in full. Two studies compared sexual intercourse with abstinence (n = 990) (Tremellen et al., 2000; Aflatoonian et al., 2009) and the remaining five studies compared application of SP, whether intra-vaginal, intra-cervical, and/or intrauterine (n = 1215) to no intervention (Bellinge et al., 1986; Chicea et al., 2013) or to placebo (von Wolff et al., 2009, 2013; Friedler et al., 2013). One study was a multi-centre trial performed in Spain and Australia (Tremellen et al., 2000) and the remaining six studies were single-centre studies performed in the Middle East, Europe and Australia. The quality of the seven studies is presented in Table I and the main characteristics of each are presented in Table II.
Characteristics of the studies included in the systematic review.
| Publication . | Number of participants . | Inclusion criteria . | Intervention group . | Control group . | Mean age . | Outcome measures . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | 390 (195 study group, 195 controls) | Couples undergoing IVF or ICSI cycles for any cause of infertility lasting >5 years. | Sexual intercourse at least once 12 h after ET | Abstinence | Study group: 29.40 Controls: 29.59 | CPR, IR |
| Bellinge et al. (1986) | 152 (78 study group, 74 controls) | Couples undergoing IVF with use of partner's fresh semen with any cause of infertility | Insemination of 0.5–6.0 ml of untreated semen 36–48 h prior to ET | No insemination/no placebo | Study group: 32.2 Controls: 31.9 | CPR, IR |
| Chicea et al. (2013) | 385 (185 study group, 200 control group) | Couples undergoing IVF with female age <38 years, 0–3 previous IVF attempts | 0.5 ml of prepared seminal plasma inseminated 1–2 cm in cervical canal and remaining (up to further 0.5 ml) deposited in vagina, after OPU | No insemination/no placebo | Study group: 33.1 Controls: 33.9 | CPR, IR |
| Friedler et al. (2013) | 230 (106 study group, 124 controls) | Couples undergoing IVF or ICSI with female age <41 years who have failed to conceive after at least 1 prior IVF-ET cycle | 0.5 ml of prepared seminal plasma injected into the vaginal vault just after OPU | Placebo controlled – 0.5 ml culture medium injected in the vaginal vault just after OPU | Study group: 32.0 Controls: 31.22 | OPR, CPR, IR |
| Tremellen et al. (2000) | 600 total Centre 1: 200 (102 study group, 98 controls) Centre 2: 400 (200 study group, 200 controls) | Centre 1: Couples undergoing thawed embryo transfer Centre 2: Couples undergoing fresh embryo transfer Both centres: Female age 18–40 years in a stable sexual relationship | Centre 1: Sexual intercourse on at least 1 occasion within 2 days prior to and 2 days after ET Centre 2: Sexual intercourse on at least 2 occasions, once within 12 h prior to OPU and once within 12 h after ET. | Abstinence | Centre 1 Study group: 33.8 Controls: 33.1 Centre 2 Study group: 33.3 Controls: 33.2 | CPR, IR |
| Von Wolff et al. (2009) | 168 (84 study group, 84 controls) | Couples undergoing IVF or ICSI, in stable relationship | 0.5 ml of thawed seminal plasma inseminated 2–3 cm into cervical canal and remaining fluid (up to further 1 ml) was deposited in vagina, after OPU | Placebo controlled – 0.5 ml sodium chloride inseminated into cervical canal and remaining (up to further 1 ml) deposited in vagina, after OPU | Study group: 34.4 Controls: 34.1 | CPR |
| Von Wolff et al. (2013) | 279 (138 study group, 141 controls | Couples undergoing IVF or ICSI | 1.5 ml of thawed diluted seminal plasma (ratio 1:4 with sodium chloride) injected just above the end of the cervix, after OPU | Placebo controlled – 1.5 ml thawed sodium chloride injected just above the end of the cervix, after OPU | Study group: 34.6 Controls: 34.9 | LBR, CPR |
| Publication . | Number of participants . | Inclusion criteria . | Intervention group . | Control group . | Mean age . | Outcome measures . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | 390 (195 study group, 195 controls) | Couples undergoing IVF or ICSI cycles for any cause of infertility lasting >5 years. | Sexual intercourse at least once 12 h after ET | Abstinence | Study group: 29.40 Controls: 29.59 | CPR, IR |
| Bellinge et al. (1986) | 152 (78 study group, 74 controls) | Couples undergoing IVF with use of partner's fresh semen with any cause of infertility | Insemination of 0.5–6.0 ml of untreated semen 36–48 h prior to ET | No insemination/no placebo | Study group: 32.2 Controls: 31.9 | CPR, IR |
| Chicea et al. (2013) | 385 (185 study group, 200 control group) | Couples undergoing IVF with female age <38 years, 0–3 previous IVF attempts | 0.5 ml of prepared seminal plasma inseminated 1–2 cm in cervical canal and remaining (up to further 0.5 ml) deposited in vagina, after OPU | No insemination/no placebo | Study group: 33.1 Controls: 33.9 | CPR, IR |
| Friedler et al. (2013) | 230 (106 study group, 124 controls) | Couples undergoing IVF or ICSI with female age <41 years who have failed to conceive after at least 1 prior IVF-ET cycle | 0.5 ml of prepared seminal plasma injected into the vaginal vault just after OPU | Placebo controlled – 0.5 ml culture medium injected in the vaginal vault just after OPU | Study group: 32.0 Controls: 31.22 | OPR, CPR, IR |
| Tremellen et al. (2000) | 600 total Centre 1: 200 (102 study group, 98 controls) Centre 2: 400 (200 study group, 200 controls) | Centre 1: Couples undergoing thawed embryo transfer Centre 2: Couples undergoing fresh embryo transfer Both centres: Female age 18–40 years in a stable sexual relationship | Centre 1: Sexual intercourse on at least 1 occasion within 2 days prior to and 2 days after ET Centre 2: Sexual intercourse on at least 2 occasions, once within 12 h prior to OPU and once within 12 h after ET. | Abstinence | Centre 1 Study group: 33.8 Controls: 33.1 Centre 2 Study group: 33.3 Controls: 33.2 | CPR, IR |
| Von Wolff et al. (2009) | 168 (84 study group, 84 controls) | Couples undergoing IVF or ICSI, in stable relationship | 0.5 ml of thawed seminal plasma inseminated 2–3 cm into cervical canal and remaining fluid (up to further 1 ml) was deposited in vagina, after OPU | Placebo controlled – 0.5 ml sodium chloride inseminated into cervical canal and remaining (up to further 1 ml) deposited in vagina, after OPU | Study group: 34.4 Controls: 34.1 | CPR |
| Von Wolff et al. (2013) | 279 (138 study group, 141 controls | Couples undergoing IVF or ICSI | 1.5 ml of thawed diluted seminal plasma (ratio 1:4 with sodium chloride) injected just above the end of the cervix, after OPU | Placebo controlled – 1.5 ml thawed sodium chloride injected just above the end of the cervix, after OPU | Study group: 34.6 Controls: 34.9 | LBR, CPR |
CPR, clinical pregnancy rate; OPR, ongoing pregnancy rate; LBR, live birth rate; IR, implantation rate; OPU, oocyte pick-up; ET, embryo transfer.
Characteristics of the studies included in the systematic review.
| Publication . | Number of participants . | Inclusion criteria . | Intervention group . | Control group . | Mean age . | Outcome measures . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | 390 (195 study group, 195 controls) | Couples undergoing IVF or ICSI cycles for any cause of infertility lasting >5 years. | Sexual intercourse at least once 12 h after ET | Abstinence | Study group: 29.40 Controls: 29.59 | CPR, IR |
| Bellinge et al. (1986) | 152 (78 study group, 74 controls) | Couples undergoing IVF with use of partner's fresh semen with any cause of infertility | Insemination of 0.5–6.0 ml of untreated semen 36–48 h prior to ET | No insemination/no placebo | Study group: 32.2 Controls: 31.9 | CPR, IR |
| Chicea et al. (2013) | 385 (185 study group, 200 control group) | Couples undergoing IVF with female age <38 years, 0–3 previous IVF attempts | 0.5 ml of prepared seminal plasma inseminated 1–2 cm in cervical canal and remaining (up to further 0.5 ml) deposited in vagina, after OPU | No insemination/no placebo | Study group: 33.1 Controls: 33.9 | CPR, IR |
| Friedler et al. (2013) | 230 (106 study group, 124 controls) | Couples undergoing IVF or ICSI with female age <41 years who have failed to conceive after at least 1 prior IVF-ET cycle | 0.5 ml of prepared seminal plasma injected into the vaginal vault just after OPU | Placebo controlled – 0.5 ml culture medium injected in the vaginal vault just after OPU | Study group: 32.0 Controls: 31.22 | OPR, CPR, IR |
| Tremellen et al. (2000) | 600 total Centre 1: 200 (102 study group, 98 controls) Centre 2: 400 (200 study group, 200 controls) | Centre 1: Couples undergoing thawed embryo transfer Centre 2: Couples undergoing fresh embryo transfer Both centres: Female age 18–40 years in a stable sexual relationship | Centre 1: Sexual intercourse on at least 1 occasion within 2 days prior to and 2 days after ET Centre 2: Sexual intercourse on at least 2 occasions, once within 12 h prior to OPU and once within 12 h after ET. | Abstinence | Centre 1 Study group: 33.8 Controls: 33.1 Centre 2 Study group: 33.3 Controls: 33.2 | CPR, IR |
| Von Wolff et al. (2009) | 168 (84 study group, 84 controls) | Couples undergoing IVF or ICSI, in stable relationship | 0.5 ml of thawed seminal plasma inseminated 2–3 cm into cervical canal and remaining fluid (up to further 1 ml) was deposited in vagina, after OPU | Placebo controlled – 0.5 ml sodium chloride inseminated into cervical canal and remaining (up to further 1 ml) deposited in vagina, after OPU | Study group: 34.4 Controls: 34.1 | CPR |
| Von Wolff et al. (2013) | 279 (138 study group, 141 controls | Couples undergoing IVF or ICSI | 1.5 ml of thawed diluted seminal plasma (ratio 1:4 with sodium chloride) injected just above the end of the cervix, after OPU | Placebo controlled – 1.5 ml thawed sodium chloride injected just above the end of the cervix, after OPU | Study group: 34.6 Controls: 34.9 | LBR, CPR |
| Publication . | Number of participants . | Inclusion criteria . | Intervention group . | Control group . | Mean age . | Outcome measures . |
|---|---|---|---|---|---|---|
| Aflatoonian et al. (2009) | 390 (195 study group, 195 controls) | Couples undergoing IVF or ICSI cycles for any cause of infertility lasting >5 years. | Sexual intercourse at least once 12 h after ET | Abstinence | Study group: 29.40 Controls: 29.59 | CPR, IR |
| Bellinge et al. (1986) | 152 (78 study group, 74 controls) | Couples undergoing IVF with use of partner's fresh semen with any cause of infertility | Insemination of 0.5–6.0 ml of untreated semen 36–48 h prior to ET | No insemination/no placebo | Study group: 32.2 Controls: 31.9 | CPR, IR |
| Chicea et al. (2013) | 385 (185 study group, 200 control group) | Couples undergoing IVF with female age <38 years, 0–3 previous IVF attempts | 0.5 ml of prepared seminal plasma inseminated 1–2 cm in cervical canal and remaining (up to further 0.5 ml) deposited in vagina, after OPU | No insemination/no placebo | Study group: 33.1 Controls: 33.9 | CPR, IR |
| Friedler et al. (2013) | 230 (106 study group, 124 controls) | Couples undergoing IVF or ICSI with female age <41 years who have failed to conceive after at least 1 prior IVF-ET cycle | 0.5 ml of prepared seminal plasma injected into the vaginal vault just after OPU | Placebo controlled – 0.5 ml culture medium injected in the vaginal vault just after OPU | Study group: 32.0 Controls: 31.22 | OPR, CPR, IR |
| Tremellen et al. (2000) | 600 total Centre 1: 200 (102 study group, 98 controls) Centre 2: 400 (200 study group, 200 controls) | Centre 1: Couples undergoing thawed embryo transfer Centre 2: Couples undergoing fresh embryo transfer Both centres: Female age 18–40 years in a stable sexual relationship | Centre 1: Sexual intercourse on at least 1 occasion within 2 days prior to and 2 days after ET Centre 2: Sexual intercourse on at least 2 occasions, once within 12 h prior to OPU and once within 12 h after ET. | Abstinence | Centre 1 Study group: 33.8 Controls: 33.1 Centre 2 Study group: 33.3 Controls: 33.2 | CPR, IR |
| Von Wolff et al. (2009) | 168 (84 study group, 84 controls) | Couples undergoing IVF or ICSI, in stable relationship | 0.5 ml of thawed seminal plasma inseminated 2–3 cm into cervical canal and remaining fluid (up to further 1 ml) was deposited in vagina, after OPU | Placebo controlled – 0.5 ml sodium chloride inseminated into cervical canal and remaining (up to further 1 ml) deposited in vagina, after OPU | Study group: 34.4 Controls: 34.1 | CPR |
| Von Wolff et al. (2013) | 279 (138 study group, 141 controls | Couples undergoing IVF or ICSI | 1.5 ml of thawed diluted seminal plasma (ratio 1:4 with sodium chloride) injected just above the end of the cervix, after OPU | Placebo controlled – 1.5 ml thawed sodium chloride injected just above the end of the cervix, after OPU | Study group: 34.6 Controls: 34.9 | LBR, CPR |
CPR, clinical pregnancy rate; OPR, ongoing pregnancy rate; LBR, live birth rate; IR, implantation rate; OPU, oocyte pick-up; ET, embryo transfer.
Study participants
The mean age of female study participants across the seven studies was <35 years, with individual study means ranging from 29.5 to 34.8 years. All seven studies included couples with infertility of any aetiology. Four of the seven studies described the duration of infertility, all >2.5 years mean (Aflatoonian et al., 2009; Chicea et al., 2013; Friedler et al., 2013). Five of the seven studies described the inclusion of patients with previous IVF cycle failure (Tremellen et al., 2000; von Wolff et al., 2009, 2013; Chicea et al., 2013; Friedler et al., 2013). Only one study excluded patients with other possible causes of implantation failure (Chicea et al., 2013). All seven studies included patients undergoing fresh IVF cycles, with a varying combination of protocols used. However, Tremellen et al. (2000) enrolled patients undergoing natural cycle frozen embryo replacement (FER) in one of their two centres.
Comparability of the study groups
All the studies selected for this review were confirmed comparable for baseline characteristics between their study and control groups. Specifically, the studies reported comparable female age, aetiology and duration of infertility, and prior IVF cycles. All eight studies also reported similar IVF characteristics, such as the stimulation protocol used, method of fertilization (IVF or ICSI) and the number of embryos transferred. Three studies performed an a priori power calculation (Tremellen et al., 2000; Friedler et al., 2013; von Wolff et al., 2013) but only one of these studies successfully reached the estimated sample size (Friedler et al., 2013). A further two studies performed post hoc power calculations, being described as pilot studies (von Wolff et al., 2009; Chicea et al., 2013).
Intervention
The method of SP exposure was described in all seven studies but was widely variable. In two studies (Tremellen et al., 2000; Aflatoonian et al., 2009) women randomized to the study group were exposed to their partners' SP via timed intercourse around the time of embryo transfer (ET) and, in the case for the Spanish centre in Tremellen et al. (2000), near the time of the ovum pick-up procedure (OPU). In the other five studies, the method of intervention included insemination of untreated semen (Bellinge et al., 1986) 36–48 h prior to embryo transfer, deposition of prepared SP in the vagina just after OPU (Friedler et al., 2013), combinations of intra-cervical and intra-vaginal insemination of prepared SP just after OPU (von Wolff et al., 2009; Chicea et al., 2013) and also intrauterine insemination of diluted SP (von Wolff et al., 2013).
The control groups were also varied. Four studies gave abstinence advice (Bellinge et al., 1986; Tremellen et al., 2000; Aflatoonian et al., 2009; Chicea et al., 2013). The remaining three studies used various placebos including intra-vaginal injection of 0.5 ml of culture medium (Friedler et al., 2013), 0.5–1.5 ml intra-cervical/-vaginal injection of sodium chloride (von Wolff et al., 2009) and 1.5 ml intrauterine insemination of thawed sodium chloride (von Wolff et al., 2013). For further details on the methods of intervention and controls, see Table II.
Outcome measures
Clinical pregnancy rate
Summary of the clinical pregnancy rate (CPR) by ITT for the seven studies included in the review.
Summary of the clinical pregnancy rate (CPR) per protocol for the seven studies included in the review.
Live birth/ongoing pregnancy rate
Summary of the live birth rate/ongoing pregnancy rate for the two studies included in the review.
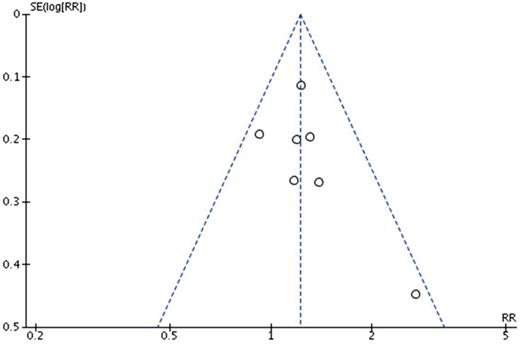
Publication bias
Funnel plot to assess publication bias in the review, outcome: clinical pregnancy rate (CPR).
Discussion
The female reproductive tract's response to seminal fluid is emerging as an interesting and likely important factor in influencing female receptivity to embryo implantation and progression to pregnancy. The role of seminal plasma for improved outcomes in patients undergoing IVF is a not a new concept. As is clear from our systematic review, the concept has been proposed since the late 1980s. However, while there has been substantial work in animals, until recently there has been only sparse information from these earlier studies on the human response. In more recent years, several further human studies have emerged to indicate that similar pathways are at play and this increases the likelihood that seminal fluid has been overlooked as a factor in determining fertility in women and particularly in IVF success. This systematic review is the first, to our knowledge, that has collated the findings of the various relevant human studies that have being undertaken to date.
This systematic review adds valuable benefit to this study topic. Despite the reality that the majority of human studies included did not recruit an adequate sample size and did not reach clinical significance individually, the enhanced precision of the effect estimate provided by this systematic review has shown a significant treatment effect for the use of seminal plasma in patients undergoing IVF for the main outcome, clinical pregnancy rate.
The results of our meta-analysis, from the seven human studies included in the systematic review, reveal a statistically significant improvement in the clinical pregnancy rate following IVF treatment when seminal plasma is used as an adjunct treatment near the time of OPU and/or embryo transfer. The average clinical pregnancy rate in the SP group across the pooled studies is 29 versus 25% in the control groups. This corresponds to a 17% relative increase in clinical pregnancy rate which would be considered desirable for patients undergoing IVF treatments. We did not find a statistically significant improvement in the ongoing pregnancy/live birth rate. The data available were limited for this outcome.
The results of this study lead us to question the traditional abstinence advice given to couples undergoing IVF. There may be a number of reasons as to why couples are advised not to engage in intercourse during their IVF treatment. There is evidence of improved sperm number and semen volume after a 2- to 7-day period of abstinence and this may be an important consideration (Cooper et al., 1993; De Jonge et al., 2004; WHO, 2010). Some clinicians also raise concern regarding the potential increased risk of multiple pregnancy although this is rather unlikely if oocyte retrieval has been sufficiently undertaken in the infertile population undergoing IVF. Consideration might also need to be given to a small increased risk of infection and advice tailored individually. Finally, concern exists regarding the possibility of inducing uterine contractions from the prostaglandins present in seminal plasma, which may make for a more hostile uterine environment for the transferred embryo.
We acknowledge that there are a number of limitations of this systematic review. Notably, there is heterogeneity amongst the studies included with regards to design and study protocols. Some studies randomized couples to intercourse or abstinence while others compared active injection of seminal plasma (with varying preparation and application methods) to either placebo or no intervention. This adds some level of doubt over the pooled results obtained from the meta-analysis. One of the common weaknesses of a systematic review is its reliance on the quality and internal validity of the studies selected for inclusion. The included studies were of variable quality. All individually lacked adequate sample size, thus not allowing their alternate hypotheses to be proven, if there is indeed a true effect.
There remain a number of key gaps in medical knowledge and understanding surrounding this topic. This systematic review has shown a clinically significantly improved CPR in women who are exposed to seminal plasma during their IVF cycle. However, due to the heterogeneity of included studies, firm recommendations cannot be made regarding its clinical application at this current time. Specifically, the exact method of seminal plasma exposure included unprotected normal vaginal intercourse, intra-vaginal, intra-cervical or intrauterine injection but it is not possible for us to differentiate between the clinical and biological validity of the various methodologies. The preparation method, the dose and the timing of SP exposure are all areas that need to be explored further. The best way to answer such questions would be with large double-blinded placebo-control randomized control trials. In order to maximize the physiological and clinical significance of such trials, they should (i) recruit adequately powered sample sizes, (ii) consider various protocols for the application of SP, (iii) include live birth as the primary outcome measure and (iv) follow an intention-to-treat analysis. If further human trials support the findings of this systematic review, the adjunctive use of SP in IVF treatment would be relatively simple to implement, and likely acceptable to patients with no additional expense.
Conclusion
We conclude that the use of seminal plasma may be of benefit for improving the clinical outcomes in patients undergoing IVF treatment. However, there remains much work to be done to ascertain the best mode and timing of delivery as well as its effect on live birth before this adjunctive treatment can be recommended and implemented.
Authors' roles
G.C. and A.R. reviewed the literature, performed the systematic review and prepared the manuscript. R.H. conceived the idea for the review and gave significant contribution in revising the text and preparing the manuscript. A.S. and A.G. contributed in revising the article. All authors read and approved the final manuscript.
Funding
No funding was requested or received.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to acknowledge the excellent and efficient library staff at the Newcomb Library, Homerton University Hospital.