-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher De Jonge, Biological basis for human capacitation—revisited, Human Reproduction Update, Volume 23, Issue 3, May-June 2017, Pages 289–299, https://doi.org/10.1093/humupd/dmw048
Close - Share Icon Share
Abstract
A little more than a decade ago a review entitled ‘Biological basis for human capacitation’ was published. A primary conclusion of the review was that with all the technological advances that have been made since the first experiments demonstrated the in vivo requirement of capacitation for fertilization, very little progress had since been made, most significantly for human.
The present review was carried out to provide an update on the biological basis for human capacitation. It briefly revisits the original schema, presents a review of the literature that urged research interest in human sperm capacitation and puts under the spotlight the original definition of capacitation balanced against the limitations of experiments in vitro to characterize a complex process that necessarily mandates a female component, and very recent findings in the mouse. It also includes proposed considerations for new thinking regarding capacitation, and progress toward understanding the biology of human capacitation.
The PubMed, Google Scholar and Scopus literature databases were reviewed extensively using inclusive, broad and multispecies search terms without publication date limitation.
Comprehensive screening of the literature database showed that no papers regarding human sperm capacitation in vivo have been published in the past 20 years. Recent experiments in the mouse have provided compelling and unanticipated data regarding capacitation and in vivo fertilization. Questions were posed and addressed regarding: stimuli for initiation of capacitation, capacitation relative to the cumulus–oocyte complex, comparison between in vivo and in vitro capacitation, and potential species-specific differences in location and timing of capacitation.
There has been no progress on the in vivo biology of human sperm capacitation since before the turn of the century. Human IVF and its technologies may likely have inhibited, and continue to hold back, any future in vivo experiments that would address one or more questions regarding acquisition of fertilizing capacity in human. The limiting factor for progress in the area is access to funding and human subjects.
Introduction
A little more than a decade ago the review ‘Biological basis for human capacitation’ was published (De Jonge, 2005). The review relied primarily on human in vitro and a paucity of in vivo data to construct a hypothetical framework for the biological factors and processes that possibly contribute to capacitation of human spermatozoa in vivo. The current review briefly revisits the original schema, presents a review of the literature that urged research interest in human sperm capacitation, and puts into spotlight the original definition of capacitation balanced against the limitations of experiments in vitro to characterize a complex process that necessarily mandates a female component, and very recent findings in the mouse. It also includes proposed considerations for new thinking regarding capacitation, and progress toward understanding the biology of human capacitation.
Before embarking on our new journey it is purposeful to revisit the origin and definition of the term ‘capacitation’. ‘Austin (1951, 1952) and Chang (1951) independently described changes that are prerequisite for non-human mammalian spermatozoa to fertilize oocytes in vivo, described as the acquisition of ‘fertilizing capacity’. This acquisition process was termed ‘capacitation’ and is defined as, ‘…the sperm must undergo some form of physiological change or capacitation before it is capable of penetrating the egg’ (Austin, 1952). The definition was later expanded by Austin and Bishop (1958) to include ‘residence of spermatozoa in the female reproductive tract to become capacitated’. To gain understanding of the molecular processes currently purported to play a role in mammalian sperm capacitation, readers are encouraged to investigate recent reviews (e.g. Gervasi and Visconti, 2016; Jin and Yang, 2016).
Methods
The PubMed, Google Scholar and Scopus literature databases were reviewed extensively using inclusive, broad and multispecies search terms without publication date limitation.
Results
A brief review of ‘Biological Basis for Human Capacitation’ (see De Jonge, 2005 for complete details and references).
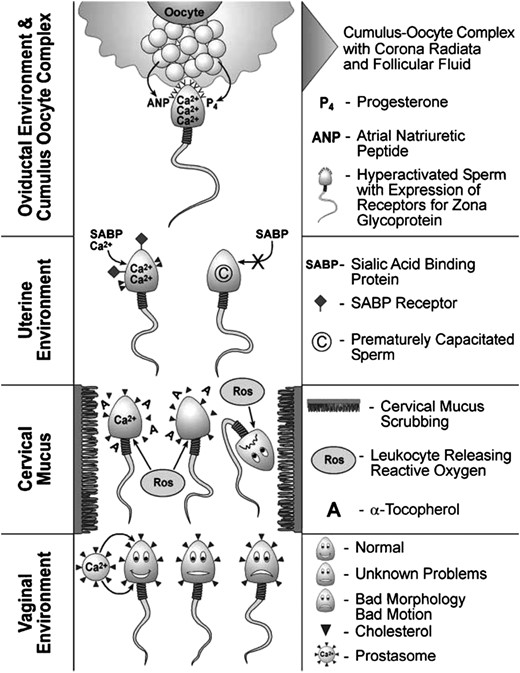
The biological basis for human capacitation. Semen contains secretions from accessory glands, e.g. calcium-containing prostasomes from the prostate, and a heterogeneous sperm population comprised of normally functioning sperm, sperm of questionable function and dysfunctional sperm. Sperm migrate from the membrane-stabilizing, sterol-enriched seminal plasma and acidic vaginal environment into the cervical mucus. Sperm plasma membranes are scrubbed by the ultrastructural elements in the mucus, facilitating the removal from the plasma membrane of adsorbed molecules and sterols. Leukocytes infiltrate the cervical mucus coincident with sperm entry. Leukocytes produce reactive oxygen molecules that have a pro-capacitating influence on normally functioning sperm and a deleterious influence on dysfunctional sperm, facilitating the removal of the latter from the fertilizing sperm population. With the removal of poor quality and dysfunctional sperm, the widely heterogeneous sperm population that entered the cervical mucus has been made somewhat more homogenous upon exit from the mucus and entry into the uterine environment. While the time sperm spend resident in the uterus is likely to be brief, due in part to uterine contractions that propel sperm to the fundus, there is ample opportunity for additional and necessary changes to occur. The sperm plasma membrane is undergoing dynamic changes with the formation of ordered lipid microdomains and sterol removal, facilitated by uterine sterol sulphatase. Consequences of regionalization and removal of sterols are (i) increased permeability to ions such as Ca2+ and (ii) the expression of receptors and binding of stimulatory ligands, such as sialic acid-binding protein. The migrating sperm population becomes more homogenous with the selection out of prematurely capacitating and dysfunctioning sperm. Upon arrival into the oviduct ipsilateral to the ovulatory follicle, sperm are introduced to an environment with a diverse cellular and hormonal composition. With progression to the ampullary region of the oviduct, sperm detect the scent of the oocyte through the action of chemoattractant molecules, e.g. atrial natriuretic peptide, secreted by the COC complex. Progesterone, adjacent and adsorbed to the cumulus, initiates inward Ca2+ transients that bring sperm intracellular Ca2+ concentrations to threshold levels for acrosome reaction stimulation by zona pellucida glycoproteins. The, perhaps, dozen sperm reaching the zona are likely to be very homogeneous with respect to the fully functional signal transduction mechanisms and motility characteristics necessary for fertilization. Thus, it is likely that the sperm that eventually fertilizes the oocyte has one attribute the others did not—luck. Reprinted with permissions from De Jonge (2005).
The influence of the uterus and its secretions on sperm capacitation is largely unstudied due to practical and ethical limitations and this deficit is reflected by the following scant data. Uterine contractions act as part of a facilitative transport mechanism that more rapidly propels sperm to the uterine fundus, utero-tubal junction and, primarily, into the fallopian tube ipsilateral to the ovulatory follicle. In addition to providing facilitative transport, the uterine epithelium is reported to produce sterol sulphatase that may serve to remove cholesterol secondary to a cervical mucus-mediated mechanism. Finally, a 54 kDa sialic acid-binding protein isolated from human endometrial cell secretions is proposed to have two functions: as a candidate for binding and facilitating Ca2+ movement into sperm; for stimulation of sperm plasma membrane remodeling in the form of sialoglycoconjugate removal resulting in a decrease in net negative sperm plasma membrane charge.
The oviductal epithelium and tubal milieu is a region in the female reproductive tract that has received considerable research focus because not only is it the site of fertilization but also early embryogenesis and transport. Based most significantly on non-human animal data, the oviductal epithelium has been proposed to act as a sperm reservoir that seemingly functions to regulate binding and release of sperm. The influence(s) of oviductal epithelium and secretions on human sperm function has relied upon in vitro cell culture experiments, which have yielded conflicting data. The oviductal ampulla is where the ovulatory follicle deposits the cumulus–oocyte complex (COC) and with it some quantity of follicular fluid (FF). The FF and cumulus contain a number of factors, e.g. progesterone (P4), atrial natriuretic peptide, that contribute to regulating sperm function, e.g. capacitation, motility. The oviductal lumen serves as a receptacle in which the spermatozoa are exposed to concentration gradients of factors regulating sperm function.
The presumptive final capacitating factor that spermatozoa encounter prior to the zona-induced acrosome reaction and fertilization is the COC. At ovulation, the cumulus mass that surrounds the oocyte is largely restricted to the innermost layers of cells called the ‘corona radiata’. These corona cells not only produce P4 but also have a proteinaceous matrix consisting, in part, of hyaluronate (hyaluronic acid) that holds the cells together. Hyaluronate has been shown to stimulate sperm function, e.g. motility, capacitation.
In concluding this brief introductory review, Austin's and Chang's observations that came to define ‘capacitation’ can be somewhat modified and adapted to characterize human capacitation in vivo as a transformational process involving functional changes occurring in spermatozoa that begins after the removal and/or redistribution of factors acquired by spermatozoa while resident in seminal plasma; progresses throughout transit in the female reproductive tract; completes when spermatozoa have acquired the ability to undergo the acrosome reaction in response to zona pellucida ligands.
Historical perspective
The original definition of capacitation included the term ‘acquisition’ and, in the classical sense, initiates only after sperm have spent time in the female reproductive tract, suggesting that one or more elements produced within and contributed by the female tract are essential for time-dependent sperm capacitation. As an example, Chang (1951) and Austin (1951) found that very few oocytes were fertilized when semen, washed sperm or sperm from the vas were injected into the oviducts of periovulatory rabbits. This is in spite of the fact that sperm were seen adherent to the zonae of the unfertilized oocytes. However, when the timing of insemination was adjusted to several hours prior to ovulation, a very much larger proportion of oocytes were fertilized. Confirmatory rabbit mating experiments were done in which the number of ampullary sperm, fertilized eggs and total eggs were counted (for review of mating experiments and capacitation, see Noyes, 1959). In the paper by Austin (1952), in which he coined the term ‘capacitation’, he used a rat model for coital experiments using either non-induced or chorionic gonadotrophin-induced ovulation. The results demonstrated and affirmed that a time requirement for spermatozoa to be exposed to the female reproductive tract was required. Furthermore, when capacitated sperm were present in the ampullae just prior to ovulation, fertilization occurred very rapidly after each COC was ovulated. These data suggest that the attraction by sperm to the COC is non-random.
Chang (1957) demonstrated that fertilization was negatively impacted when rabbit sperm, previously capacitated in utero, were treated with a 5–100% dilution of seminal plasma and placed in the oviducts of doe rabbits that had recently ovulated. Seminal plasma had no significant negative impact on zona binding but rather inhibited zona penetration by spermatozoa. When diluted human seminal plasma (5%) was used to treat capacitated rabbit sperm at the same post-ovulatory time, it diminished fertilization by ~50% from controls (Chang, 1957). In addition, bull semen at the lowest dilution (2%) tested was almost completely inhibitory of fertilization, whereas the same dilution using rabbit seminal plasma suppressed fertilization by ~40% relative to controls. When capacitated sperm were treated with 5% or 10% seminal plasma and placed in oviducts 6 h prior to ovulation, oocytes fertilized to the same extent as controls (Chang, 1957). These experiments demonstrated for the first time that seminal plasma could reverse the capacitated state that capacitation could be restored after a typical incubation time in oviducts, and that the inhibitory effects were seemingly non-specific, because first, there was no dose–response for inhibition and second, the inhibition was not species specific. Chang concluded that capacitation is ‘… not … a simple process of elimination by the female reproductive tract of adherent seminal plasma’ and ‘… the metabolism of sperm is much altered by contact with the uterine lumen.’
Chang (1958) published that the endocrine state of the sperm ‘host’ or ‘incubator’ influences the time required for capacitation as opposed to the endocrine state of the ovulatory recipient. Sperm incubated in the uteri of pseudopregnant rabbits at Days 6–14 post ovulation (fully developed corpora lutea at peak progestational activity) were unable to fertilize oocytes of recipient ovulatory rabbits. In contrast, sperm incubated in the uteri of Day 1 or 19 pseudopregnant rabbits (less developed and regressing corpora lutea, respectively, and with lower progestational activity) were able to fertilize recipient rabbit oocytes (at a level 50–60% of control). Further to a hormonal influence, when sperm were incubated in the uteri of P4-treated estrous rabbits capacitation was considered inhibited because when the recovered spermatozoa were placed in the ampullae of recipient rabbits, oocytes failed to fertilize (~2%). However, when spermatozoa were incubated in the oviducts of pseudopregnant rabbits and then subsequently placed into the oviduct of recipient rabbits, capacitation was proven to have occurred as evidenced by high fertilization rates. That capacitation was inhibited by incubation in uteri of P4 pretreated or pseudopregnant ‘host’ rabbits but not in pseudopregnant oviduct-incubated sperm.
Based on the above data, the following conditions can be identified that contribute to capacitation in non-human mammals: sperm must be removed from seminal plasma to initiate capacitation, the time-dependent process of capacitation is modifiable or regulated based on the location and hormonal milieu of the female tract, factors secreted by the oviduct contribute to acquisition of fertilizing capacity, and one or more sperm-attractant messages emanating from the COC are quickly received by the capacitated spermatozoon. As noted, the pioneering experiments that led to the aforementioned ‘conditions’ contributing to the acquisition of sperm fertilizing capacity were conducted using research animals. What research was taking place at the time to understand and characterize the biology of capacitation in the human?
Almost 20 years after the discoveries of Austin and Chang, Bedford (1970) published a comprehensive review on capacitation and fertilization. Interestingly, in the relative plethora of references cited, only three references relate to human reproductive biology. Furthermore, while two of the papers reported on human IVF, none of the three papers addressed human capacitation. Thus, efforts were underway to fertilize human oocytes in vitro but with no fundamental understanding of how human sperm acquire ‘fertilizing capacity’. In fact, almost three decades earlier, Rock and Menkin (1944) published their effort to fertilize human oocytes in vitro. A tantalizing ‘real-time’ observation contained in their report was that the cumulus surrounding the oocyte was being penetrated by active spermatozoa 1 h after incubation. When the oocyte was transferred to another dish, the cumulus was readily dispersed. This observation would much later become reflective of one of the characteristics of (human) sperm capacitation: the enzymatic action of sperm hyaluronidase on the hyaluronic acid glue that holds cumulus cells together.
One of the first publications to discuss human sperm capacitation in vivo was primarily focused on investigating the contraceptive potential of a progestagen (Roland, 1969). Roland speculated that the contraceptive effect seen in norm-ovulatory young women was due not to a hormonal influence on ovulation but rather an effect on the spermatozoa penetrating into and through the cervix. He offered speculation that ‘human sperm capacitation was initiated in the endocervix’ and is essential for human reproduction. He stated further that capacitation was yet to be demonstrated for human spermatozoa and that there was a lack of information regarding where capacitation might initiate. While human sperm capacitation has been demonstrated and characterized in vitro, Roland's comments relative to the in vivo environment still resonate 50 years later.
Beginning in the 1960s, the field of reproductive biology exploded with interest and investigators realized that in vivo experimentation presented obstacles, especially for human. Thus, in vitro cell culture techniques were being rapidly developed (e.g. Norman et al., 1960; Yanagimachi and Chang, 1963; Edwards et al., 1966). Norman et al. (1960) reported the successful long-term culture of washed human spermatozoa in defined media (Medium 199) for up to 5 days. Edwards et al. (1966) attempted to fertilize human oocytes that were matured in vitro (Medium 199). Initially, they co-cultured washed sperm with oocytes for up to 42 h post insemination, but they were disappointed by the very poor and ambiguous fertilization results. They concluded that human spermatozoa required an incubation period to become capacitated, similar to what had previously been shown for other mammalian species. They cultured human sperm with rabbit uterus and fimbria, followed by subsequent insemination of human oocytes and, again, there was no fertilization. The same negative outcome occurred when sperm and oocytes were co-incubated with endosalpinx or when spermatozoa that had migrated out of cervical mucus were used for insemination. The significant conclusion of this investigation was ‘… a supply of capacitated human spermatozoa will be required to achieve fertilization’. Arguably, one cannot discount the possible role of the oocytes in the fertilization outcomes. However, what is certain is that spermatozoa had not acquired the ability for fertilization under the conditions of experiments described in their paper.
Following on from earlier work, Edwards et al. (1969) attempted again to fertilize human oocytes matured in vitro. For this investigation, having previously identified a significant component as missing, i.e. sperm-capacitating medium, they used a new ‘environmental’ medium developed by Bavister that had been shown to be effective for capacitating hamster spermatozoa and fertilization in vitro (Bavister, 1969). The results from using the new ‘capacitating’ medium for the investigation were as follows: of 34 oocytes that were mature (extruded 1st polar body), 16 did not fertilize, 6 had spermatozoa in the zona pellucida, 5 oocytes had sperm in the perivitelline space (PVS) and 7 oocytes were judged to be fertilized based on the presence of 2 pronuclei. Arguably, the most significant observation was that for ‘the first time it was demonstrated that human spermatozoa require an incubation period to become capacitated’ because fertilization did not occur until several hours (~7 h) after insemination of oocytes in vitro. The second notable observation was that more spermatozoa subsequently attached to and penetrated the zona pellucida when the gametes were pre-incubated in FF, resulting in a higher incidence of fertilization. This latter observation contrasted with Bavister (1969) who, in a hamster IVF model, reasoned that tubal or FFs may not capacitate (acrosome react) spermatozoa but merely facilitate it occurring spontaneously (see mouse in vivo/ex vivo experiments below). What might help to explain the apparent difference in response of spermatozoa to FF in these two separate in vitro capacitation experimental models?
Yanagimachi (1969) performed a focused study on the influence of bovine FF on capacitation and acrosome reaction in hamster spermatozoa. He found that bovine FF, when heat-inactivated, had a dialyzable heat stable fraction and a non-dialyzable, heat labile fraction that were stimulatory of different sperm functions. The heat stable fraction induced very active and vigorous motility (while not termed hyperactivation in this paper the characteristic motion would later be named ‘hyperactivation’) that reached a maximum after 4 h of incubation. In contrast, the heat labile fraction induced the acrosome reaction starting 2 h after incubation and peaking at 4 h. Perhaps more significant is that neither fraction alone resulted in fertilization of hamster eggs. It was only when the fractions were combined that the heat labile (non-dialyzable) fraction exerted its full potency in stimulating the acrosome reaction and a dose-dependent increase in fertilization. Yanagimachi concluded by stating that the sperm-capacitating substance in the bovine FF is not chemically identical to secretions of the female reproductive tract but that they do have something in common in their chemical makeup despite their different origins.
In the paper that would forever change childlessness for infertile couples and ultimately lead to a Nobel Prize, Edwards et al. (1970) modified the salts in Bavister's medium to reflect the composition of human FF. The fertilization results were encouraging and the rest, as they say, is history. In their concluding comments, the investigators introduce relatively new knowledge regarding the lysosomal properties of the acrosome. They commented that P4 is known to destabilize lysosomal membranes. They further discussed their unpublished data that P4 is a component of human FF and a product of steroid activity by granulosa cells. These comments would prove to be insightful because in a relatively short time a story would begin to unfold for the role of P4 as being, arguably, a significant modulator of human sperm function.
In conclusion, this ‘Historical Perspective’ section focused on the pivotal investigations that provided early characterization of capacitation in mammals. In contrast, virtually no experiments with similar investigative rigor were published regarding human sperm capacitation in vivo. In fact, it was the advent of investigations into human IVF and associated failures that prompted a requirement of capacitation for human IVF success to be acknowledged. That recognition served as a partial catalyst for the further development of human sperm-capacitating medium but that was primarily driven from the context of optimizing human IVF culture media for oocyte and embryo development.
Bavister (2002) wrote a review article on the ‘early history of in vitro fertilization’. The paper walks beautifully through the sequence of events from the ‘discovery’ of capacitation to development and application of techniques for IVF. Perhaps most notable in the review is the writing devoted to capacitation. Bavister's paper is highlighted herein and, most significantly, for the following concluding questions posed almost 15 years ago and quoted here: ‘What is the stimulus for the initiation of capacitation within the female reproductive tract? What molecular events are involved in capacitation? How exactly does the completion of capacitation lead to the acrosome reaction?’ No human data have been published that definitively answer the first question. The answers to the remaining questions, derived solely from in vitro experimentation, are well investigated and continue to evolve.
Will the real ‘capacitation’ please stand up?
The central topic of this and the earlier paper (De Jonge 2005) regards the biology of human sperm capacitation in vivo, which necessarily and inexorably demands an ovulatory female reproductive tract component, the permanence and fidelity of the original definition balanced against 60 years of intensive in vitro investigation and whether or not distinctions might exist between species. Reports are emerging that stimulate question(s) regarding the use of animal models, specifically the rodent, to serve not only as template for characterizing human biological processes but also, more relevant for this discussion, as de facto surrogate models for characterizing capacitation of human sperm (e.g. Coleman, 2003; Yauger et al., 2011; Avella et al., 2014; Okabe, 2014; Choi et al., 2016; Kaupp and Strunker, 2016). In fact, in a very recent review paper on sperm signaling mechanisms (Kaupp and Strunker, 2016), the authors concluded that ‘there is no unifying model system for sperm signaling or navigation … we caution against the tendency to uncritically apply conclusions drawn for studies on sperm from knockout mouse lines to other species, including humans’. This comment is highly relevant when making conclusions from studies on capacitation between species and in vivo or in vitro studies within a species, and the following supports this assertion.
The original definition of capacitation included the induction of the acrosome reaction and release of acrosomal components. The interrelationship of the two events can be stated more specifically as: ‘the fulfillment in the acquisition of sperm fertilizing capacity is evidenced by the morphologically distinct exocytotic event called the acrosome reaction’. Are there in vivo data that might shed light on where sperm reach fulfillment in capacitation relative to the COC, and does the acrosome reaction represent the finality of capacitation?
Three models were proposed for sperm location and timing of the acrosome reaction relative to interaction with the zona pellucida (Florman and Fissore, 2015). The first and third models adhere to the definition of capacitation as originally conceived (Austin definition): the acrosome reaction occurs en route to making contact with the zona pellucida, exposing the cumulus matrix-penetrating inner acrosomal enzyme, hyaluronidase, followed by subsequent zona binding and penetration. The second model proposes acrosome intact sperm passaging through the cumulus matrix facilitated by plasma membrane-bound hyaluronidase. Acrosome intact sperm upon contact with the zona pellucida are induced to undergo the acrosome reaction by sperm–zona ligand–receptor mechanisms. Models 1 and 3 might be considered as continuum models (perhaps likened to a sensitivity assay or filtration mechanism), in that they are suggestive of one or more agonists distributed at a distance from the zona, such as in the ampulla and/or the cumulus mass, acting to induce the acrosome reaction in capacitated spermatozoa that are in transit and in proximity to the zona pellucida-encased oocyte.
Pioneering experiments published in the late 1970s and early 1980s collectively established that mouse spermatozoa begin capacitation after exposure to the reproductive tract and cumulus mass, and reached fulfillment when sperm were induced by zona protein (ZP3) to undergo the acrosome reaction (Model 2; Saling and Storey, 1979; Florman and Storey, 1982; Bleil and Wassarman, 1983). Recently, several scientifically innovative investigations used the mouse, arguably the most common comparative animal model for human capacitation, to shed new light on the fidelity of the historical data and raise the question regarding which of the sperm–zona interaction models might be most applicable to the mouse.
Jin et al. (2011) explored capacitation by investigating the timing of the acrosome reaction in spermatozoa in relation to cumulus-intact and cumulus-free oocytes cultured in vitro using sperm produced from enhanced green fluorescent protein (EGFP) transgenic mice (the intact sperm acrosome fluoresces green). Acrosomal status was monitored in real time using video microscopy. They found that a significant majority of spermatozoa had completed the acrosome reaction before ever contacting the zona pellucida, and acrosome-reacted sperm spent a very transient time (<1 min) on the zona surface before penetrating it. Furthermore, cumulus-free oocytes co-incubated with cumulus-intact oocytes were found to fertilize equally well (50–60%). In contrast, cumulus-free oocytes incubated in cumulus-conditioned medium fertilized similar to cumulus-free oocytes in non-conditioned medium (~20%). The Jin et al. (2011) report documents for the first time that the majority of oocytes successfully fertilized in vitro resulted from spermatozoa that had reached fulfillment of fertilizing capacity before contact with the zona pellucida (Models 1 and 3). It calls into question the functional role of mouse ZP3 (the presumptive acrosome reaction-inducer). It also draws into question whether other factors, such as the tubal environment and/or cumulus and matrix, have a significant role in mouse fertilization in vitro.
The preceding investigation was performed in vitro and is thus subject to question regarding relevance to what takes place biologically—in vivo. Three similar investigations delved deeper into the location and timing of the acrosome reaction in EGFP mouse sperm relative to the COC using in vivo and ex vivo post-coital female reproductive tracts (Hino et al., 2016; La Spina et al., 2016; Muro et al., 2016). Hino et al. (2016), in contrast to the other two associated reports, discovered that a portion of the sperm population in as early as the lower oviductal isthmus had become fully capacitated based on their acrosomal status. They attribute as explanation for their unique observation that the mouse matings in their investigations were natural and not hormonally induced, as in the other two reports. Each of the three investigations clearly demonstrated that the incidence of fully capacitated spermatozoa (acrosome-reacted) increased during transit through the isthmus, and with the greatest incidence of acrosome reaction (AR) being in the upper isthmus. Furthermore, virtually all fertilizing spermatozoa were acrosome reacted upon reaching the ampulla. And, equally compelling, the number of sperm reaching the ampulla was only a few at any given time and virtually all were acrosome reacted. Lastly, the sperm to egg ratio was almost unity (<2). The following concluding comments bear reflection: (i) ‘the zona pellucida is not the sole or intrinsic acrosome reaction-inducing substance in the mouse’ (Hino et al., 2016), (ii) spermatozoa, when entering the cumulus of an already fertilized oocyte, left quickly, suggesting a ‘chemical recognition’ by the spermatozoon of one or more factors released or produced as a consequence of fertilization (Hino et al., 2016) and lastly (iii) ‘the behavior of mouse spermatozoa in the female reproductive tract was largely beyond our imagination. “Although the in vitro experiments helped clarify aspects of the mechanism of fertilization, we should keep in mind the importance of in vivo experiments in the study of fertilization”’ (Muro et al., 2016).
The preceding in vivo/ex vivo mouse experiments revealed unanticipated and remarkable information regarding the location and timing of capacitation leading to fertilization. These studies used real-time imaging of acrosomal fluorescence to track capacitation status of spermatozoa as they ascended the fallopian tubes, and then visualized fertilization and the intriguing attributes highlighted above. Given the Austin definition of capacitation, some might argue that the sequence to fertilization was predicted. Certainly, the basic sequence is arguably predicted based on Models 1 and 3. However, importantly, a recent study presented findings that clearly fall outside traditional thinking regarding capacitation. Inoue et al. (2011) discovered, using genetically modified mice, that sperm having penetrated the zona pellucida (acrosome reacted) of one oocyte, but unable to fuse with the oolemma, can be harvested from the PVS to successfully inseminate and fertilize fresh cumulus-invested oocytes in vitro. Furthermore, the mouse spermatozoa recovered from the PVS were noted to have vigorous motility, characteristic of hyperactivation, suggestive of a capacitation-linked attribute as persisting past the presumed ‘endpoint’ of capacitation, i.e. the acrosome reaction. Kuzan et al. (1984) found, in rabbit, that acrosome-reacted sperm harvested from the PVS of a first oocyte cohort could fertilize oocytes in a second cohort. These results question the original definition of capacitation because indicators of capacitation status, i.e. acrosome reaction and hyperactivation, persist beyond the endpoint of capacitation, i.e. acrosome reaction.
Tateno et al. (2013) recently published data from mouse that further question ‘traditionally’ held concepts regarding capacitation and associated events. In mouse, molecular changes occurring as part of capacitation are, in part, activation of the cAMP/protein kinase A (PKA) pathway (Krapf et al., 2010), an increase in protein tyrosine phosphorylation (Visconti et al., 1995) and an increase in Cai2+ (Ruknudin and Silver, 1990). Tateno et al. (2013) used a calcium-transporting agent, calcium ionophore A23187, to treat mouse spermatozoa under different incubation conditions and evaluated AR, molecular changes in the aforementioned markers of capacitation and fertilization in vitro. They found that epididymal spermatozoa became motionless after a brief (10 min) incubation with A23187. When A23187 was removed by washing sperm regained motility, became hyperactivated within 30 min and maintained hyperactivated motion for 2 h. Simultaneously, roughly half the sperm population had undergone the acrosome reaction after the brief 10 min incubation with A23187. The addition of A23187 also had an inhibitory effect on PKA activity that was removed upon washing away of the ionophore. And finally, spermatozoa treated (10 min) with and then washed free of A23187 fertilized oocytes in medium containing a PKA inhibitor and with or without bicarbonate (HCO3−, a presumed required component for capacitation). These fertilized oocytes produced live, normal pups. Collectively, the results demonstrated that the cAMP/PKA and tyrosine phosphorylation pathway requirement for capacitation could be bypassed by a transient Cai2+ increase and with fertilization proceeding normally. In human, A23187 and activators of the cAMP/PKA pathway induce the acrosome reaction of capacitated sperm while inhibitors of the pathway prevent the reaction (see, e.g. De Jonge et al., 1989, 1991). A23187 induces the acrosome reaction in human spermatozoa presumptively by bypassing pathways associated with capacitation but with no reported inhibitory effect on human sperm motility (Bielfeld et al., 1994). Comparative human IVF experiments that could probe further are not feasible.
When the aforementioned studies are viewed in total the results compel a revisit to the definition of capacitation, at least as it relates to the mouse (and perhaps the rabbit), and for the following reasons. It is difficult to contest that the terminal event of mouse sperm capacitation, i.e. the acrosome reaction, in vivo is induced by one or more candidate factors that are unrelated to the oocyte both spatially and temporally. And further, the acrosome reaction does not apparently define the fulfillment of capacitation in the mouse model. The belief that sperm viability ends soon after acrosomal loss is not tenable, based on results from Hino et al. (2016), Tateno et al. (2013) and Inoue et al. (2011). None of these observations were expected or predicted based on years of preceding mouse IVF experiments, yet the results and conclusions from those earlier experiments have been made to be bona fide and cross-species applicable. Conclusively, any conceptions regarding translation of historical capacitation observations from mouse model to human are questionable and highlight even more so the essential need for investigation of human sperm capacitation in vivo.
Conundrums and considerations
Based on data in the literature, mouse spermatozoa were ‘proven’ to have fully acquired fertilizing capacity as evidenced by occurrence of the acrosome reaction in response to zona proteins (ZP3). Those data came from experiments conducted in vitro. Based on the results detailed above, a clear difference exists in the process of mouse sperm capacitation depending on whether the system studied is in vivo or in vitro. While mouse sperm capacitation resembles Models 1 and 3 (above) the same is (apparently) not true for human spermatozoa, which currently identifies with Model 2.
In a report on the ultrastructure of human oocytes fertilized in vitro, Soupart and Strong (1974) observed that human spermatozoa (begin to) undergo capacitation before fertilization (zona contact); sperm associated with the zona pellucida were acrosome reacted, demonstrating that they had become fully capacitated and those not in contact with the zona were acrosome intact (see also, e.g. Tesarik, 1989; Liu and Baker, 1990; Liu et al., 2006); in comparison to other mammals studied, the equatorial segment of human spermatozoa changed ultrastructurally as evidenced by its progressive disappearance during penetration of the zona; the oocyte was activated by the time the spermatozoon was embedded deeply within the zona. These observations, and especially the latter, identify significant aspects of capacitation and fertilization in human as being distinct from mouse. Specifically, the timing of the zona reaction and cortical granule release differs between human and mouse/rabbit, as evidenced by the aforementioned mouse and rabbit re-insemination experiments (Kuzan et al., 1984; Inoue et al., 2011). Finally, acrosomal status relative to sperm location in the cumulus and zona suggests that the latter plays an inducing role in human sperm acrosomal loss, signifying that human spermatozoa reach fulfillment in capacitation upon contact with the zona pellucida. Perhaps P4 produced by the cumulus cells and the hyaluronate that contributes to the cumulus matrix have a priming role for capacitation. In human, P4 activates CatSper channels (cation channels of sperm) and Ca2+ influx ensues (Lishko et al., 2011; Strünker et al., 2011). In contrast, P4 does not activate CatSper in mouse sperm (see recent review on sperm signaling, Kaupp and Strunker, 2016). What has and will likely continue to prove a significant and unresolved question is whether or not these observations apply to the in vivo acquisition of fertilizing capacity of human spermatozoa—which then leaves investigations reliant upon in vitro experimentation, and the following calls into question their biological significance.
Human spermatozoa incubated outside the female tract in a balanced salt solution readily undergo capacitation and, as bona fide evidence, can fertilize oocytes in vitro. With limited exception, the vast majority of current knowledge for how human sperm capacitation has come to be defined is overwhelmingly derived from in vitro experimentation using human and, by comparison, non-human spermatozoa. While in vitro experiments have relevance, they arguably do so only for the conditions under which the experiments were conducted. For example, culture media commonly used not only for human IVF but also for basic research studies of human sperm capacitation can differentially influence distinct changes in markers associated with, or that have become a part of, the present-day definition of capacitation, e.g. motility, hyperactivation, protein tyrosine phosphorylation and acrosome reaction (e.g. Calvo et al., 1993; Moseley et al., 2005; Liu et al., 2011). Thus, the culture media used for investigating molecular mechanisms of capacitation have a reasonable potential to contribute unsuspected ‘noise’ to a system presumed to have fidelity. More specifically, one cannot assume that a culture medium being used as the ‘backdrop’ for an experiment to investigate, for example tyrosine phosphorylation, is not unknowingly or unintentionally adding to or subtracting from, or modifying, the outcome measure—thereby leaving a ‘footprint in the sand’. Moseley et al. (2005) found that a ‘complex’ culture medium commonly used in human IVF accelerated human sperm tyrosine phosphorylation-correlated and hyperactivation-associated capacitation in comparison to Earle's medium (a balanced salt solution intended to maintain structural and physiological function of mammalian cells in vitro). They concluded that their ‘…data strongly suggest that factors acting through signaling pathways other than bicarbonate/cAMP/PKA…, may be responsible for this effect’. The latter pathway has been well characterized as having a critical role in capacitation. Given the plethora of research reports on capacitation, a legitimate question then is: might noisy data be contributing to the evolving definition of (human) sperm capacitation? Bona fide evidence for signaling pathways responsible for human sperm capacitation must come from in vivo experiments that incorporate the periovulatory female reproductive tract, until then the caveat of an in vitro mechanism for human sperm capacitation must be applied.
Human sperm do not have to reside in the female tract to fertilize oocytes in vitro. Given current data, is there reasonable evidence to consider that human spermatozoa come with a ‘toolkit’ such that the acquisition of fertilizing capacity has begun prior to ejaculation? In De Jonge (2005), the topic of and putative role for prostasomes in ‘charging’ sperm with Cai2+ at the time of ejaculation was presented. Park et al. (2011) have recently reported a more precise role for prostasomes in regulating human sperm function in vitro. They found that the pH-dependent fusion of prostasomes with, primarily, the sperm mid-piece results in the transfer of a toolkit containing Ca2+ transport-regulating enzymes (cyclic adenosine diphosphoribose–synthesizing enzymes) and receptors (for P4 and ryanodine) that serve to regulate P4-induced sperm motility via CatSper and also the acrosome reaction but via different Ca2+ signaling compartments. See Ronquist (2015) for a comprehensive review of prostasomes.
Seminogelin (SEMG1) is a seminal plasma protein contributed by the seminal vesicles that causes coagulation of the ejaculate. Beyond this seemingly simple function, SEMG1 has been reported to influence capacitation possibly through an ROS inhibitory mechanism (de Lamirande, 2007; de Lamirande and Lamothe, 2010). O'Rand and Widgren (2012) reported that human SEMG1 binds to a receptor to not only inhibit sperm motility but also to cause a loss in intracellular Ca2+. Thus, there are two pre-female reproductive tract factors, prostasomes and SEMG1, that reportedly influence sperm function, and quite possibly capacitation, that are seemingly functioning antagonistically to one another. The point is that it is very difficult to discern whether SEMG1 and/or prostasomes indeed influence the fertilizing sperm population because the in vitro conditions are highly artificial, e.g. ejaculation into a cup followed by a time period for liquefaction. Resolution of factors that possibly influence capacitation by modifying sperm properties, and in this case motility and Cai2+ before exposure to the female tract, demands further investigation and perhaps by starting with a simple post-coital test as described, for example, by Sobrero and MacLeod (1962).
When reviewing the literature on epididymal function, one very frequently reads that sperm acquire both fertilizing and forward motion ability during residence and transit in the epididymis (see e.g. Hinrichsen and Blaquier, 1980; Cooper, 1990; Bedford, 1994; Cornwall, 2009; Sullivan and Mieusset, 2016). Structural differences exist between the human epididymis and other mammals, thus one can question whether functional differences exist. Sperm transit time through the human epididymis is abbreviated (~6 days) relative to other mammals (in some species almost 14 days). It has long been asserted that mammalian sperm, including human sperm, require time for maturation that occurs during transit and storage in the epididymis. Several maturation-associated characteristics that are apparently ubiquitous across mammalian species are acquisition of progressive motility, change in net surface membrane charge, acquisition of epididymis-secreted membrane proteins and increase in disulfide bonds of the sperm nucleus. Given the relative brevity of time that human sperm spend in the epididymis, a requisite role for the human epididymis in sperm maturation beyond the examples provided is less clear due to the very limited number of studies using non-pathological specimens (Cornwall, 2009).
At present, there are no overwhelmingly convincing data that demonstrate an absolute requirement for human sperm passage through the epididymis for subsequent fertilization both in vitro and in vivo. Controversially, several investigations reported that human epididymal sperm are not only capable of progressive motility but also, more significantly, have the capacity and capability to fertilize human oocytes whether in vivo or in vitro (Young, 1970; Silber, 1989a,b; Silber et al., 1990; Asch and Silber, 1991; Mathieu et al., 1992). Results from vasoepididymostomy document natural conception, as evidenced by pregnancy, regardless of the site of the anastomosis, and even if sperm had not transited and experienced regions distal from the caput (Young, 1970; Silber, 1989a,b). With the advent of human IVF it became clear that human epididymal sperm could be used for fertilizing oocytes (Silber et al., 1990; Asch and Silber, 1991; Mathieu et al., 1992). However, while capable of fertilization, fewer epididymal sperm fertilize oocytes as compared to when ejaculated sperm are used. These results suggest that transit through the sequential regions of the epididymis and beyond confers upon spermatozoa attributes that enhance fertilizing capacity. In vivo there is a question concerning a compensatory role of the epididymis that forces maturation, per se, so that sperm from more proximal regions can fertilize. However, IVF models show the same outcome, that human epididymal sperm are motile and can fertilize oocytes. What becomes more apparent from the data is that full in vitro fertilizing potential is not reached until sperm have completely transited the epdidiymis. Since capacitation is, by definition, the acquisition of fertilizing capacity, it is reasonable to speculate that passage through the epididymal environment confers upon spermatozoa factors that, while perhaps not requisite, serve to potentiate or optimize sperm fertilization capacity, in concert with the preexisting capacitation toolkit.
The epididymal epithelium secretes extracellular membrane-bound vesicles called epididymosomes that carry proteins compartmentalized to either the surface or interior for delivery to spermatozoa as they pass through the epididymis (review e.g. Sullivan, 2015; Machtinger et al., 2016). Analysis of human epididymosomes demonstrates that they differ characteristically in protein composition relative to their site of epididiymal origin (Sullivan et al., 2007). An intriguing possibility is that epididymosomes transfer one or more proteins that contribute to the regulation of pre-fertilization events, e.g. mouse SPAM 1 (e.g. Martin-DeLeon, 2015).
Closing comments
The original definition of capacitation (Austin, 1952) is still relevant in so far as spermatozoa must acquire the capacity for fertilization in vivo. However, data from in vitro experimentation have come to characterize the mechanisms of capacitation and especially so for human. Problematic is whether or not in vitro physiological and molecular processes that have contributed to a modern definition of capacitation translate equally to what takes place in vivo.
In vitro investigations on human sperm capacitation begin with an ejaculate produced into a cup, and the coagulum forms instantaneously which then requires a period of time (typically 30–60 min) for liquefaction of seminal fluid to allow sperm to swim freely. The specimen is then manipulated to isolate a population of spermatozoa for experimentation, which adds more time and further exposure to non-biological conditions. In vivo, highly motile spermatozoa migrate into the cervical mucus very quickly after ejaculation, e.g. ~90 s (Sobrero and MacLeod, 1962), thereby limiting the exposure time of putatively fertilizing-capable sperm to the seminal fluid cellular and acellular components. Furthermore, the in vitro incubation time for human sperm capacitation ranges from 3 to 24 h. Capacitation in vivo may likely occur in a much shorter time frame based on the following set of events. First, due to facilitative uterine transport mechanisms, rapid sperm migration occurs such that sperm are present in the oviduct in as early as ~10 min post-coitus (Rubenstein et al., 1951; Settlage et al., 1973; Ahlgren, 1975). These data suggest a minor role of the uterus in capacitation and a more likely significant role for the oviduct. Since the oviductal milieu contains pro-capacitative factors (see e.g. De Jonge, 2005), it is plausible that in vivo capacitation occurs in a shorter time frame than in vitro. Since the in vitro and in vivo conditions preceding to the initiation of capacitation are vastly different, e.g. sperm in vitro are unnaturally bathed in seminal fluid for significantly longer than in vivo, and the time frame for capacitation is likely different, the process of capacitation may then also be consequently different. These points are particularly relevant when considered in light of pro-fertility and contraceptive therapies and research, as well as toxicology research.
There are many outstanding review papers in the literature database that detail the current molecular mechanisms of capacitation, including for the human (see e.g. Baker, 2016; Gervasi and Visconti, 2016; Jin and Yang, 2016). How do the capacitation models to date explain results from the experiments of Inoue et al. (2011) and Hino et al. (2016)? Keeping in mind that spermatozoa have arrested transcription, a number of questions arise, for example, once a signal transduction cascade has been initiated that culminates in the release of a second messenger, such as calcium, can it recycle and continue in perpetuity, and is there a mechanism for receptor recycling and calcium storage replenishment and, if so, what is the time frame and pathway for refueling? The results from these novel ‘back to the future’ types of experiments question the relevance of current molecular models for capacitation. As new data emerge, such as the compelling reports presented herein, it becomes apparent that in vivo experiments are essential to provide biological checks and balances for the in vitro data that have contributed to the complex modern picture of capacitation.
The last paper to investigate sperm transport in the human was published >20 years ago (Williams et al., 1993), near the time that ICSI was introduced to IVF as a tool for ‘treating’ severe male factor infertility. However, ICSI is used more routinely today because it is convenient, there is a (perceived) reduced risk of no fertilization even in the absence of a male factor, and it possibly provides psychological salve to reduce patient anxiety. While ICSI has helped countless couples to have a family, I personally feel it has had a negative impact on basic human reproductive biology research; as evidenced by a 20-year drought in publications. The reasons for this are 2-fold: economical and ethical. Performing human reproduction experiments is scrutinized at all levels from patient, to clinic, institutional review boards, to government funding agencies, e.g. what is the actual ‘need’ (purpose) for such studies since human IVF exists. From a patient perspective, which often drives clinical practice, what is the point of enrolling in a study if it does not help me, the patient, become pregnant faster? None of the above-mentioned stakeholders would likely support studies that could pose a potentially significant risk/benefit imbalance, especially when there is a mindset of ‘in vitro experiments can be done’. Regrettably, the conclusion by Chang (1957), made 60 years ago, that capacitation is ‘… not … a simple process of elimination by the female reproductive tract of adherent seminal plasma’ and ‘… the metabolism of sperm is much altered by contact with the uterine lumen’ may never be resolved for human, the ‘stimulus for initiation of capacitation in the female reproductive tract’ may never be identified, and, finally, how a human spermatozoon navigates the female reproductive tract to find and fertilize an oocyte may never be known.
The intent of this paper was to stimulate thinking about what is known and what has yet to be revealed regarding the biology of human sperm capacitation that culminates in fertilization. The insights from 60 years ago are as relevant today as they were then and going to the past often provides a pathway to the future. Today, we have the benefit of far more advanced technology. We can watch sperm transport real time and fertilization as it happens. The question is, how can technology be exploited so that we can learn more about human reproductive biology? If the desire exists to learn more about the fundamental mechanisms behind human fertilization, then there will be the bright young minds to answer those questions and more.
Authors’ roles
The author is the sole contributor and is responsible for study design, execution, analysis, manuscript drafting and critical discussion.
Funding
Nothing to declare.
Conflict of Interest
Nothing to declare.