-
PDF
- Split View
-
Views
-
Cite
Cite
Penny M. Hopkins, Limb Regeneration in the Fiddler Crab, Uca pugilator: Hormonal and Growth Factor Control, American Zoologist, Volume 41, Issue 3, June 2001, Pages 389–398, https://doi.org/10.1093/icb/41.3.389
Close - Share Icon Share
Abstract
This paper summarizes recent work on various aspects of hormonal control of regeneration in the crustacean, Uca pugilator. Hormonal control in this crab is effected by means of the crustactean steroid hormones, the ecdysteroids. New evidence is presented supporting a role for the retinoid hormones, all-trans retinoic acid and 9-cis retinoic acid, in the control of regeneration in these animals. The possible role of fibroblast growth factors in organization of the limb blastema is explored and the similarities between vertebrate and invertebrate control of regeneration are discussed.
REGENERATION IN CRUSTACEANS
The hard exoskeleton of crustaceans makes growth in these animals quite remarkable. In order to increase in size, these animals must periodically shed (called molt or ecdysis) their old exoskeleton, then expand a new, soft, pliant exoskeleton that has been formed beneath the old one. The length of time from one molt to the next is called the intermolt cycle. The length of the cycle is variable and determined by time of year, nutritional status, and other factors including whether or not the animal is regenerating a limb.
Regeneration is a special type of growth found in many crustaceans. In the fiddler crab Uca pugilator, limbs that are lost due to injury or predation and as a result of the reflexive autotomy response, can be regenerated completely during a single intermolt cycle (for reviews see Skinner, 1985; Hopkins, 1993; Hopkins et al., 1999). Autotomy occurs at the base of all walking legs. Regardless of where along the length of the limb an injury occurs, the autotomy response insures that the limb is cast off at a predetermined point at the base of the leg between the coxa and the basiischium. Loss at the predetermined point insures the most efficient and fastest regeneration. Regeneration of limbs following autotomy occurs in two phases. The first phase immediately follows the loss of the limb and is called basal growth. Basal growth can take place at any time during the molt cycle. The second phase is called proecdysial growth and occurs only as the animal is preparing for ecdysis.
Basal growth
When a limb is cast off the opening left by the discarded limb is sealed by an autotomy membrane (AM). The AM in Uca consists of a complex double membrane that spans the entire coxa (Hopkins, 1993). It is traversed by the large pedal nerve and a few blood sinuses. No other tissues cross the AM, therefore, there is limited tissue damage at autotomy. The nerve, however, is severed at the preformed autotomy breakage plane and the nerve stub retracts into the coxal stump. At autotomy, the AM splits and the most distal portion of the membrane balloons outward to seal the breach. The more proximal portion of the AM remains attached to the sheath of the severed nerve (Hopkins, 1993). This tether prevents the nerve from retracting too far into the stump and keeps it near the center of the developing blastema. In the absence of the pedal nerve, an empty limb shell will regenerate (see Needham, 1965). The pedal nerve appears necessary for muscle development during arthropod limb regeneration.
Vertebrates that regenerate limbs also require an intact nerve for induction of a new regenerate (Brockes, 1984). Limbs will not regenerate in the axolotl if the limb blastema is denervated. The severed nerve in vertebrates releases a fibroblast growth factor (FGF 2) that has a role in organizing the apical wound epidermis in chick embryo limbs and in the axolotl regenerating limb (Taylor et al., 1994; Mullen et al., 1996). FGFs are a family of peptide growth factors initially identified in vertebrates. There are at least nine different mammalian FGF genes and four FGF receptor genes (Coulier et al., 1997). These growth-promoting peptides function as mitogens, motogens, and differentiation factors. FGFs are the predominant outgrowth signal for normal developing vertebrate limb buds (Johnson and Tabin, 1997).
FGF gene homologues have been identified in invertebrates (Coulier et al., 1997). An FGF homologue, called branchless, has been identified in Drosophila melanogaster (Klambt et al., 1992). This gene functions to control tracheal branching in the fruit fly by acting as a motogen in attracting migrating cells in early development and as an inducer of later branching programs (Sutherland et al., 1996).
FGF 2 is able to rescue denervated regenerates in newts by replacing the function of the wound epidermis and restoring the expression of an important developmental gene (Dxl3) in the epidermis (Mullen et al., 1996). FGF 2 peptide has no signal sequence, and therefore, is not a secreted protein. The only way such a protein can be released from a cell or tissue is after tissue damage (Poulin et al., 1993).
Following autotomy, the area distal to the severed pedal nerve of Uca shows distinct immunostaining with heterologous vertebrate antibodies to vertebrate growth factor, FGF 2 (Hopkins et al., 1999). The staining at the nerve ending is diffuse—as would be expected if the antigen was released into the hemocoelic space. More proximal portions of the pedal nerve deeper in the coxa show FGF 2-like staining of specific nerve tracts (Hopkins et al., 1999).
The apical epidermis of vertebrate regenerating limb buds releases other fibroblast growth factors (FGFs 4, 8, and 10) that serve to precipitate a cascade of organizational and positional signals that are necessary for successful regeneration to occur (Boilly et al., 1991; Christen and Slack, 1997). The cytoplasm of the apical epidermis cells of the regenerating limb bud in Uca stains with a heterologous antibody to vertebrate FGF 4 (Hopkins et al., 1999). The expression of FGF 2- and FGF 4-like immunoreactivity in the pedal nerve and in the cytoplasm of epidermal cells in Uca limb buds supports a role for multiple FGF-like growth factors in early blastema development in this crustacean.
Proecdysial growth
During basal growth, the limb bud becomes differentiated but not completely grown. All of the leg segments are formed during basal growth and epidermal chromatophores are evident (Hopkins, 1993). The small segments of the limb bud are folded upon one another and encased in a flexible cuticular sac. Further proecdysial growth of these basal limb buds is due to hypertrophic growth of the myotubules formed during basal growth and is due primarily to muscle protein synthesis and water uptake (Hopkins, 1989). The cuticular sac surrounding the regenerating limb bud is flexible and can expand with the rapidly growing bud during proecdysial growth. At this time the limb bud can undergo as much as a three-fold increase in size (Hopkins, 1993).
CONTROL OF REGENERATION BY ECDYSTEROIDS
The interdependence of regeneration and molting in arthropods has long been known. Arthropods that continue to molt and grow as adults (primitive insects and crustaceans), continue to regenerate as adults. Needham (1965) noted that regeneration was dependent upon the presence of an active molt hormone-secreting organ, and Bliss (1956) noted that the regenerating limb bud was an excellent criterion for the crustacean molt cycle stages. Multiple regeneration (the loss of more than one limb) interrupts a normal molting cycle in the crabs Gecarcinus lateralis and Uca pugilator and the shrimp Palaemonetes kadiakensis (Skinner and Graham, 1972; Fingerman and Fingerman, 1974; Stoffel and Hubschman, 1974). Removal of regenerating limb buds formed in response to multiple autotomies lowers levels of circulating molt hormone and slows the growth of remaining limb buds (see Hopkins, 1989). Thus the same hormones that control molting and growth in crustaceans are also implicated in the control of limb regeneration.
The arthropod molting and growth hormones are called ecdysteroids. These steroid hormones are produced by the crustacean Y-organs and the insect prothoracic glands. In the crabs Uca pugilator and Carcinus maenas, the Y-organs produce at least two steroids—25-deoxyecdysone (25DE) and ecdysone (E) that are released and converted peripherally to ponasterone A (PA) and 20-hydroxyecdysone (20HE) respectively (Lachaise et al., 1986; Hopkins, 1989).
Ecdysteroids, like vertebrate steroid hormones, affect their target tissues by interacting with nuclear receptors. The ecdysone receptor gene (EcR) has been identified and sequenced in a number of insects and in one crustacean. The ecdysone receptor homologue from Uca pugilator regenerating limbs was isolated using RT-PCR generated clones (Durica and Hopkins, 1996). The ecdysone receptor in Uca has similar nucleic acid and deduced amino acid sequences to those of ecdysone receptors identified in insects. The ecdysone receptor from Uca (UpEcR) is a member of the large family of nuclear receptors (Chung et al., 1998a).
Basal growth
Using anti-sense 32P-UTP-labeled RNA probes and in situ hybridization, Uca ecdysone receptor mRNA has been measured in limb buds during basal growth. During the first four days of basal growth the limb bud epidermis becomes organized and begins to secrete a thin cuticle. At this time, the levels of UpEcR mRNA in the limb bud rise slowly (Chung et al., 1998a).
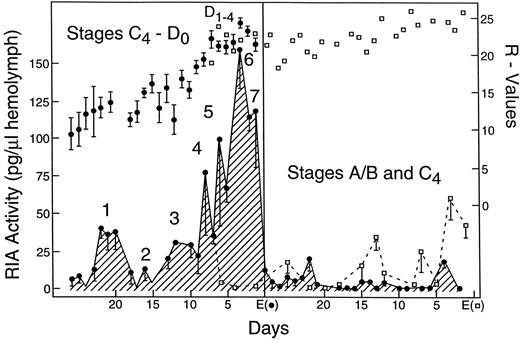
Levels of circulating ecdysteroids in Uca, however, remain low (<8.3 × 10−8M) during basal growth (molt stages A/B and C4; Fig. 1; Hopkins, 1983). If high concentrations of exogenous ecdysteroids are injected or perfused into the crab, Gecarcinus lateralis, during this time basal growth is inhibited (Hopkins et al., 1979). Thus, while ecdysteroids may be required for basal growth and ecdysone receptor mRNA is present in the basal limb bud throughout this growth period, apparently the circulating levels of ecdysteroids must remain low in order for basal growth to occur.
Proecdysial growth
Proecdysis in Uca is distinguished by a series of pulses (or peaks) of circulating ecsysteroids (Figs. 1 and 2a). The transition from anecdysis (C4) to proecdysis (D0) is dependent upon a transient pulse of ecdysteroids (Fig. 1—Peak 1 = 1.6 × 10−7M; see Hopkins, 1983) that occurs two to three weeks prior to ecdysis. Rapid limb regeneration occurs during the proecdysial substage of D0. The first pulse of ecdysteroids must be followed by a drop in circulating levels to anecdysial levels (Fig. 1—Peak 2 = 8.3 × 10−8M) in order for proecdysial growth of limbs to begin. This drop is obligatory in crabs that are regenerating limbs (Hopkins, 1989). The signal(s) that control the ecdysteroid levels during this period are not understood, but it seems likely that it is generated by the limb buds themselves: Increasing numbers of missing limbs cause increasing levels of inhibition (Fingerman and Fingerman, 1974; Hopkins, 1982; Skinner, 1985).
If ecdysteroid levels fail to drop after peak 1, proecdysial regeneration fails to occur (Hopkins, 1989). In the crayfish, Astacus leptodactylus,in vitro control of the C4–D transition of integument protein synthesis is induced by a two day exposure to 10−8 M of 20HE, followed by an essential one day withdrawal of the hormone (Traub et al., 1987). Regeneration of imaginal leg discs from the flesh fly, Sarcophaga peregrina, also needs low levels of 20HE (2.5 × 10−8M) in vitro to initiate disc regeneration and wound healing. The effective concentration of 20HE was forty times lower than levels needed to induce differentiation in vitro (Kunieda et al., 1997). Thus it is clear that both basal growth and early proecdysial growth in Uca limb buds can only occur when there are low levels of circulating ecdysteroids.
The end of proecdysial substage D0 (and the end of proecdysial regeneration) is signaled by a second transitional pulse of ecdysteroids (Fig. 1—Peak 4 = 2.5 × 10−7M). There is a marked and sustained increase in circulating ecdysteroids (Fig. 1—Peaks 5,6,7 = up to 5.0 × 10−7M) following Peak 4 that corresponds to a period of no regenerative limb growth (called terminal plateau—D1–4; see Bliss, 1956). Terminal plateau is the time when the major influence of ecdysteroids switches from the control of regeneration (Hopkins, 1992) to control of apolysis (Freeman and Costello, 1979), and ecdysis (Krishnakumaran and Schneiderman, 1970).
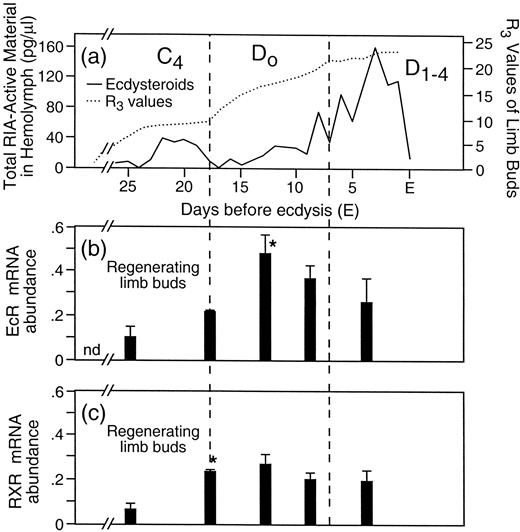
There is a significant increase in UpEcR mRNA abundance in limb buds (expressed as pg of cRNA per ug total RNA) during D0 (Fig. 2b; Chung et al., 1998b). Abundance stays high until ecdysis, but responsiveness of limb buds to ecdysteroids varies during this period. Proecdysial limb buds are able to respond in vitro to E or 20HE by increased incorporation of labeled leucine into protein (Hopkins, 1989). This effect was observed in limb buds that were removed immediately following peak 1 (Early D0, Fig. 1) but not in limb buds that were removed later (late D0 and D1–4). E had a greater effect on protein synthesis than did 20HE. The steroid concentrations used in vitro (1.7 × 10−7M) were similar to levels measured during the transition peak (Fig. 1, Peak 1). These steroids had no effect on muscle protein synthesis in limb buds removed during late D0 through D1–4, when circulating levels of steroids are highest. Thus, the ecdysone receptor mRNA abundances seen during stages D0 and D1–4 (Fig. 2b) may represent more than one isoform of the receptor with differing affinities for ligand(s). Multiple isoforms of the ecdysone receptor have been reported for Drosophila tissues (Talbot et al., 1993).
In summary, basal growth of limb buds occurs during a time when levels of circulating ecdysteroids and UpEcR mRNA levels are low. The transition from anecdysial (C4) to proecdysial (D0) growth requires a transitory pulse of ecdysteroids (1.6 × 10−7M). Following this pulse, levels of UpEcR rise. Circulating levels of ecdysteroids, however, return to anecdysial levels (8.3 × 10−8M), because early proecdysial growth of the regenerating limb also requires low levels of circulating ecdysteroids. A second transitory pulse coincides with the end of proecdysial regeneration and the beginning of terminal plateau. During terminal pleateau there are a series of large (up to 5.0 × 10−7M) pulses of circulating ecdysteroids which appear to be necessary for successful ecdysis of the entire exoskeleton.
CONTROL OF REGENERATION BY RETINOIDS
In the fruit fly, Drosophila melanogaster and the tobacco hornworm, Manduca sexta, the ecdysteroid receptor does not function by itself. It has an obligate dimer partner—Ultraspiracle (USP). USP is a member of the retinoid receptor family (Oro et al., 1990; Yao et al., 1993). The crab homologue of USP in Uca has been isolated, cloned, and sequenced (Chung et al., 1998a). It is a molecule with high identity to USP in the DNA-binding domain, but is more similar to vertebrate retinoid-X-receptors (RXRs) in the ligand-binding domain, so it is named Uca RXR (UpRXR). An RXR-like receptor has also been identified in the ixodid tick, Amblyomma americanum (Palmer et al., 1999).
No ligand has been identified for USP, but the vertebrate RXRs are ligand-activated transcription regulators that affect gene transcription by interacting with a variety of cognate DNA response elements (HREs) either as homodimers or as heterodimers. The ligand for vertebrate RXR is 9-cis Retinoic Acid (9-cis RA—Manglesdorf et al., 1994). In the presence of 9-cis RA, the formation of RXR/RXR homodimers is favored in vertebrate systems (Pfahl et al., 1994). Heterodimeric partners of RXR include the thyroid hormone receptor, the Vitamin D receptor, and the ecdysteroid receptor (Manglesdorf et al., 1994).
Basal growth
During the first four days of basal growth and throughout proecdysis, UpRXR mRNA is present in the blastema of the regenerating limb bud of Uca pugilator (Fig. 2c; Chung et al., 1998b). Moreover, the activated epidermis (=wound epidermis) at one and four days after autotomy shows strong nuclear UpRXR immunoreactivity using Uca-specific antibodies (Hopkins et al., 1999). These are the same cells that show strong FGF 4-like immunoreactivity in the cytoplasm during this time. As mentioned above, both retinoids and FGFs act together in developing and regenerating vertebrate limbs to stimulate important down-stream gene responses (Niswander et al., 1994; Cohn et al., 1995).
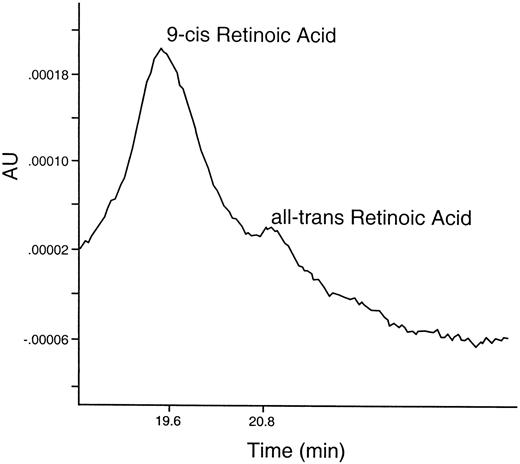
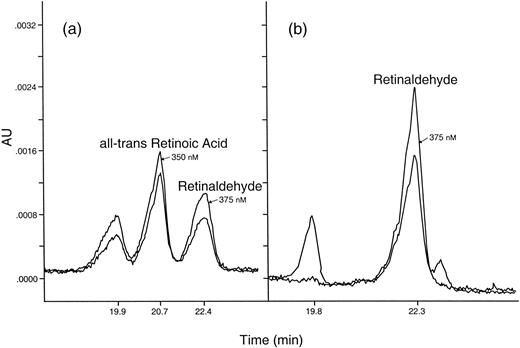
Endogenous retinoids have been isolated from crab limb blastemas (Fig. 3). Blastemas removed from crabs four days after autotomy (A + 4) contain very small amounts of both all-trans retinoic acid (19 pg/ug protein/blastema) and 9-cisRA (83 pg/ug protein/blastema). The source of these retinoids within the blastema has not been identified at this time in Uca, however, 9-cis RA is produced by wound epidermis in the regenerating newt limb during early limb organization (Viviano et al., 1995). Uca blastemas also contain an aldehyde dehydrogenase enzyme necessary to convert retinaldehyde (RH) to retinoic acid (RA) in vitro (Fig. 4a). This conversion was totally blocked by addition of the aldehyde dehydrogenase-specific enzyme inhibitor, Citral (Fig. 4b). Moreover, only about 50% of the RH recovered in the controls was accounted for in the experimentals by RA or RH. Thus, there may be other enzymes in addition to the aldehyde dehydrogenase in these blastemas that utilize retinoids as substrates. Some RA may be photoisomerized to 9-cisRA (see the peak at 19.8 min in Fig. 4). The presence of Citral in the incubation, however, eliminated only the 350 nm absorbing peak. The nature of the other retinoid-metabolizing enzymes and their products is unknown at this time.
Endogenous retinoids have long been considered to supply constituitive signals that play important roles in the normal development of the vertebrate limb (Tabin, 1991). Endogenous retinoids have been detected in regenerating tissues from axolotls, frogs and chicks (Scadding and Maden, 1994; Viviano et al., 1995).
Exogenously applied retinoids have been shown to respecify pattern formation during normal development and regeneration in a number of vertebrates (for review see Johnson and Tabin, 1997). Important developmental genes involved in both intra- and extracellular signal transduction are affected by exogenous retinoid exposure during normal development or during regeneration (Niswander et al., 1994; Akimenko and Ekka, 1995; Mullen et al., 1996; Helms et al., 1997).
Exogenous retinoids disrupt normal regeneration in Uca (Hopkins and Durica, 1995). Crabs treated with RA have poorly differentiated limb buds and an extraordinary abundance of apparently undifferentiated blastemal cells. There is strong immunoreactivity for UpRXR in blastemal epidermal cells and we have shown that immersion of regenerating crabs in RA significantly elevates the UpRXR mRNA levels in early blastemas (Chung et al., 1998a). In vertebrates, the limb apical epidermis promotes proliferation and inhibits differentiation of underlying mesenchymal cells (Paulson, 1994). In Uca, exogenously applied RA appears to inhibit differentiation and promote proliferation of blastemal cells (Hopkins and Durica, 1995).
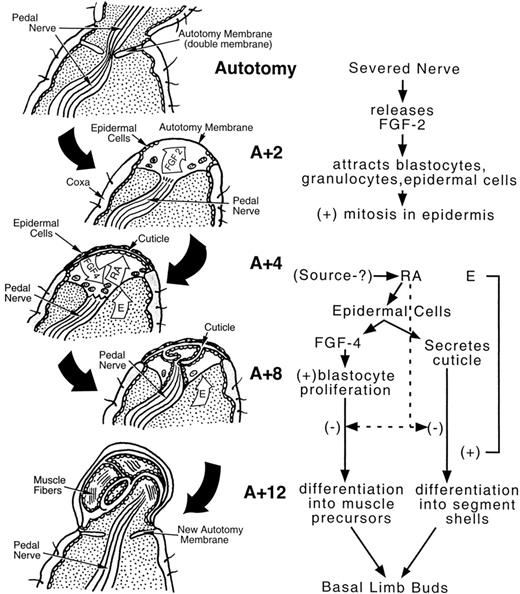
I propose that the epidermis in the regenerating blastema of the crab, Uca pugilator (Fig. 5), is analogous to the wound epidermis of vertebrates. UpRXR is expressed in the epidermis soon after autotomy during early wound healing and exogenous RA applied during this period disrupts the subsequent development of limb buds. FGF-like immunoreactivity occurs during wound healing in Uca in both the severed pedal nerve and the wound epidermis. I suggest that the release of growth factor(s) from the severed nerve and the production of FGF-like compounds by the wound epidermis are very early events in crustacean limb regeneration and that the effects of these growth factors may be modulated by endogenous retinoids (Fig. 5).
Ecdysteroids may also play a role during blastema formation. Ecdysteroid receptor mRNA is present in the blastema as early as the day after autotomy (Chung et al., 1998b) and ecdysteroids have a role in arthropod epidermal events such as mitosis and cuticle secretion.
Fibroblast-like cells migrate into the empty segments of the developing blastema to establish cell lines that later become myoblasts. Proecdysial growth of the limb bud consists of rapid growth of the muscle cells that were differentiated during the basal growth period. During early proecdysis, the limb bud responds to ecdysteroids by elevated levels of muscle protein synthesis in the fully differentiated limb cells. Ecdysteroids may help mobilize putative pre-myoblast cells during basal growth and stimulate hypertrophic muscle protein synthesis in those same cells during early proecdysial growth.
Fig. 1. Profile of daily mean ecdysteroid hemolymph levels (crosshatched areas are pg of RIA active ecdysteroid per ul of hemolymph). Stages of molt cycle are described in Hopkins, 1983. Solid circles are mean R-values (length of regenerate divided by width of crab × 100; see Bliss, 1956). Open squares are R-values and RIA titers from animals that did not molt with cohorts at first ecdysis (Eo). Redrawn from Hopkins, 1983
Fig. 2. Correlation of circulating ecdysteroids and levels of UpEcR and UpRXR receptor mRNA. (a) The same curve as in Figure 1 redrawn on a different scale. (b) Mean abundance of UpEcR mRNA extracted from limb buds removed at the points in the molt cycle corresponding to (a). The vertical bars indicate the standard error of the mean. The star (*) indicates statistically significant increase in levels compared to previous measurement (P = <0.05). (c) Mean abundance of RXR mRNA in limb buds during the molt cycle. Bars and stars indicate same as in (b). Molt cycle stages at top of (a) correspond to stages in Figure 1
Fig. 3. Ultraviolet tracing (330 nm) of extract of 450 Uca limb blastemas removed four days after autotomy. Separated on reversed-phase HPLC. Labels above each peak indicate corresponding elution time for known standards of all-trans Retinoic Acid, and 9-cis Retinoic Acid. (Blastemas were extracted four days after autotomy with ethyl acetate:methyl acetate—8:1—in the presence of 0.5% ascorbic acid and EDTA in Uca saline. The organic phase was evaporated in the dark, under nitrogen, redissolved in methanol and separated on a reversed phase C-18 bondapak column, using a Waters pump system and a gradient of acetonitrile:methanol:1% acetic acid at ratios of 45:15:40 up to 75:25:0 for 30 min. Standard curves of known standards of varying concentrations were run under the same conditions)
Fig. 4. Retinoid metabolism by blastema extracts incubated with retinaldehyde substrate in the presence of or in the absence of the inhibitor, Citral. (800 Uca limb blastemas were extracted four days after autotomy in HEPES (pH 7.35) and centrifuged. To supernatant was added 375 mM KCl, 2 uM retinaldehyde, 2 mM NAD+ and 2 mM dTT—with and without 2 mM Citral (Sigma). Solutions were incubated in glass vials at 4°C for 24 hr in the dark. The resulting retinoids were extracted and analyzed as described in Fig. 3.) Two UV wavelengths were used to simultaneously monitor the HPLC effluent and to help identify the peaks—350 nm for retinoic acid and 375 nm for retinaldehyde
Fig. 5. Drawing of a limb and a limb bud with possible mechanisms of control suggested by data presented in text. Top figure is a longitudinal representation of a limb that includes the preformed plane of autotomy (with double autotomy membrane) prior to autotomy. Two days after autotomy (A + 2) epidermal cells are migrating from sides of coxa under distal edge of autotomy membrane and fibroblast growth factor 2 (FGF 2)-like immunoreactivity is seen in basin formed by proximal edge of autotomy membrane, severed pedal nerve, and distal portion of autotomy membrane. Four days after autotomy (A + 4), a thin cuticle is secreted by the epidermal cells and fibroblast growth factor 4 (FGF 4)-like immunoreactivity is seen in epidermal cells (as well as UpRXR immunoreactivity). Endogenous retinoic acid (RA) is present in the blastemas at this time also. By eight days after autotomy (A + 8), infoldings from the earlier cuticle are seen. These are believed to be the first indication of leg differentiation into segments. Pedal nerve regeneration is evident at this time. At twelve days after autotomy (A + 12), developing muscle fibers are seen in the individual segments of the regenerating limb. A new autotomy membrane begins to form and the regenerating pedal nerve can be seen moving into the newly formed limb segments
From the Symposium Recent Progress in Crustacean Endocrinology: A Symposium in Honor of Milton Fingerman presented at the Annual Meeting of the Society for Integrative and Comparative Biology, 4–8 January 2000, at Atlanta, Georgia.
E-mail: Phopkins@ou.edu
I am very grateful to Susan Jorgenson, Andrea Taylor, and Xiaohui Wu for their technical help in preparing the immunolocalizations, the antibodies and in the isolation of blastemal tissues.