-
PDF
- Split View
-
Views
-
Cite
Cite
Geerling E. J. Langenbach, Theo M. G. J. van Eijden, Mammalian Feeding Motor Patterns, American Zoologist, Volume 41, Issue 6, December 2001, Pages 1338–1351, https://doi.org/10.1093/icb/41.6.1338
Close - Share Icon Share
Abstract
Both the anatomy and function of the mammalian masticatory system have attracted substantial interest. This review will discuss the general mammalian feeding patterns. An overview will be given of the evolutionary development and ontogeny of these patterns, the influence of occlusal forces, and recent developments in computer modeling. In mammals, basic symmetrical food transport cycles have been described for lapping and soft food ingestion. To increase chewing efficiency, a unilateral occlusal motion has been evolved replacing the slow closing phase in the basic cycle. The relative uniformity of the mammalian jaw-closer motor patterns during this chewing behavior, as characterized by electromyography (EMG), is striking. Nevertheless, several adaptations, clearly different from the primitive mammalian asymmetric masticatory motor pattern, can be distinguished. In contrast to the relative uniformity in motor patterns, the anatomical diversity of jaw systems is impressive and probably reflects the adaptation to diet. Detailed studies on the influence of occlusal force have been performed in the last decade. Data suggest that the masticatory cycles are largely shaped by sensory feedback. Also, the suckling food intake preceding mastication has been a point of interest. The suckling motor pattern resembles that of mastication, suggesting that the transition could be gradual during postnatal development. Recently, dynamic computer 3D-modeling has emerged as an analytical tool. The approach has the potential to help explain how structure and function interact.
INTRODUCTION
The masticatory system of mammals has attracted significant attention throughout the 20th century. The first published studies described the macroscopic morphology of the masticatory muscles and skull, often comparing different species (e.g.,Fiedler, 1953; Starck, 1933, 1935; Storch, 1968; Gaspard et al., 1976; Schumacher, 1961; Turnbull, 1970). Both the attachment sites and the internal architecture of the jaw elevators (masseter, temporalis and medial pterygoid muscles) were analyzed. To a lesser extent, the jaw depressors (mainly digastric, geniohyoid and lateral pterygoid muscles) were examined. Also in the first half the 20th century, the postnatal development of the masticatory muscles and the skull was studied (Bluntschli, 1929; Müller, 1933; Zey, 1939). More recently, detailed examination of the muscle fiber composition has become possible with the introduction of methods like enzyme- and immuno-histochemistry (Ericksson and Thornell, 1983; Thornell et al., 1984; Bredman et al., 1992; Korfage et al., 2000). Large differences in fiber types can be found between species (d'Albis et al., 1986), between individual muscles and even between muscle parts (Korfage et al., 2000). By extracellular stimulation of trigeminal motoneurons, and simultaneous registration of force, the physiological properties of these fibers, grouped in motor units, have been studied (see for review: van Eijden and Turkawski, 2001).
The actual process of feeding was studied as soon as techniques to record jaw motion and muscle activity were accessible. Hildebrand (1931) described a method to record the jaw motions in human subjects by using three-dimensional röntgen cinematography. Surface electromyographic recordings of the jaw muscles followed in 1949 (Moyers). Techniques for both motion and muscle activity recordings were refined over the years, and since the early 1970s (Kallen and Gans, 1972) have been used in animals, occasionally as models for the human masticatory system. Biomechanical methods, including bite force registration (van Eijden et al., 1990), the use of strain-gauges (Hylander, 1979) and static (Koolstra et al., 1988) and dynamic computer modeling (Koolstra and van Eijden, 1995; Langenbach and Hannam, 1999; Peck et al., 2000) enabled the examination of the loading within the masticatory system.
Although enormous differences in motor behavior can be detected between different species, Hiiemae (1978) suggested a basic uniformity in their activity pattern. The uniformity was refined by Weijs (1994) classifying five masticatory motor patterns based on the different function of muscles. Jaw motion is mainly brought about by the action of the masticatory muscles, and is greatly influenced by the anatomy of the temporomandibular joint and occlusal pattern. Motor patterns are flexible and adjust to the mechanical demands of food. These food demands are different during the development of the system, as the main feeding system changes from suckling to chewing. Motor patterns are greatly determined by central programs that can be modified by peripheral feedback (e.g., periodontal receptors, muscle spindles and temporomandibular joint receptors) (Lund and Enomoto, 1988; Rossignol et al., 1988; Taylor, 1990; Lund, 1991; Abbink, 1999). The fact that, within the order of mammals, a tooth replacement system has been developed in combination with the change in feeding method makes the ontogeny of the masticatory system an intriguing area.
Recent papers have focussed on the jaw motion during the entire feeding process or during feeding methods other than mastication. Studies including the motor patterns of the masticatory system often deal with the ontogeny of the apparatus or the influence of food consistency. In 1994, Weijs published an excellent review of the evolutionary development of the mammalian feeding patterns. In the present review, a synopsis will be given of mammalian feeding. The review is divided into five parts. Part 1 will provide a brief introduction to the general mammalian feeding pattern. In part 2, evolutionary development will be summarized. Part 3 concentrates on the area of ontogeny of the mammalian feeding patterns, and in part 4, the influence of occlusal forces on the masticatory motor pattern is discussed. Finally, in part 5, recent developments in computer modeling of the masticatory system will be summarized.
MAMMALIAN FEEDING PATTERNS
Feeding sequence
Different stages can be distinguished within the feeding behavior. Successively, ingestion, food transport to the processing teeth, chewing and bolus formation, and swallowing can be recognized. Ingestion has been little investigated and is extremely species- and food-dependent. Moreover, no muscle activity studies have been performed. Comparing the rapid and repetitive gnawing motions of the rat with the tear-off behavior shown by carnivores explains the difficulty that would be encountered to generalize the existing patterns of ingestion. After ingestion, the food is transported from the incisal area to the molar region of the oral cavity (transport I cycles, Hiiemae and Crompton, 1985) by pro- and retraction of the tongue combined with jaw closure and opening. Recent studies (Hiiemae et al., 1995; Palmer et al., 1997; Hiiemae and Palmer, 1999) confirm these motions in macaque and human, but do not involve muscle activities. The next stage of food ingestion, chewing, has been studied the most. Both the masticatory motor patterns and the accompanying jaw motion have been studied in many different species. Because the literature on this stage of feeding is so extensive, it will be summarized below. Swallowing occurs, in most mammals, with almost no interruption of the masticatory rhythm, but can sometimes be recognized from the prolonged duration of a chewing cycle. Although differences in jaw motion are evident, the different stages of a feeding sequence seem to have a lot of features in common. Most illustrative for this is the predictable pattern of the tongue cycle relative to the jaw motion (Palmer et al., 1997).
Mastication
Chewing is a cyclic motion of the mandible and tongue apparatus, whereby food is reduced between the maxillary and mandibular teeth. Opening of the jaw is mainly brought about by activity of the digastric, geniohyoid and lateral pterygoid muscles. Jaw opening starts slowly while the tongue apparatus moves forward. The transition to the fast opening phase is accompanied by a reversal of the tongue motion, and can be very distinct (insectivores), or gradual (rodents, ungulates). Thus, jaw opening can be subdivided into a slow and fast opening phase, sometimes even separated by a pause in the jaw movement (Lund and Enomoto, 1988). The infrahyoideal muscles facilitate jaw opening by stabilizing the hyoid bone, and its digastric and geniohyoid muscle attachments. During jaw opening, the jaw often deviates from the midline, a motion that is continued during jaw closure.
Jaw closing is the result of the action of the jaw elevators, i.e., masseter, temporalis and medial pterygoid muscles. Food is usually chewed only or mainly on one side, the working side. The other side is defined as the balancing side. From maximal opening, the jaw closes at high velocity (fast closing), until food contact begins. This transition is sometimes arbitrary. During the power stroke the working side lower teeth move medially and often across the upper teeth. Large differences in the power stroke motion can be found between species and even animals. True bilateral chewing with little transverse motion is mostly found in rodents, but also occurs as the first preparatory chewing cycles. Often the muscle activity during the power stroke is larger than during the initial closing phase. Both muscle activity levels, needed to close the jaw and crush the food, increase sharply with jaw motion speed (Abbink et al., 1999).
EVOLUTIONARY DEVELOPMENT OF MASTICATORY PATTERNS
Evolution of the mammalian jaw apparatus consists of several important anatomical changes (Barghusen, 1968; Allin, 1975; Crompton and Hylander, 1986). First, the collection of bones forming the lower jaw in primitive forms converts such that the dentary bone develops a new articulation with the temporal bone of the skull. Moreover, the articulation becomes much smaller, requiring a rearrangement of the jaw muscles to reduce the loads imposed on the temporomandibular joint. This can be achieved when good peripheral feedback (e.g., temporomandibular joint receptors) is in place, enabling the masticatory system to evolve, while maintaining the condylar loads below a maximum, degenerative level. Second, the mass of the external adductor increases its attachment site downward to the lateral side of the lower jaw. Both the temporalis and masseter muscles (respectively, the original and newly formed external adductor) attain a larger insertion site on the lower jaw because of the development of a coronoid process and mandibular angle.
Hiiemae and Crompton (1985) suggested that mammalian mastication is derived from a mechanism seen during lapping. In lapping, a slow jaw closing phase in combination with tongue retraction is followed by a slow opening phase combined with tongue protraction. During chewing, the cycles are modified by (1) an increased maximum gape, result of an addition of a fast opening and closing phase, and by (2) a replacement of the slow closing movement by the power stroke. In many species the first chewing cycles are different from the rest because the jaw fails to reach occlusion as the food mass intervenes between the teeth.
Crompton et al. (1977) first described the tongue and hyoid cycles for the opossum. Since then they have been found in many species (shrew: Fish and Mendel, 1982; fruit-eating bat: deGueldre and de Vree, 1984; macaque: Franks et al., 1984; hyrax: German and Franks, 1991; cat: Hiiemae et al., 1981; rabbit: Anapol, 1988; armadillo: Smith and Redford, 1990; sloth: Naples, 1985; human: Hiiemae and Palmer, 1999). The forward motion of the tongue always stops at the start of fast jaw opening, but the rest of the cycle is more variable and perhaps less stereotyped than originally suggested (Mendel et al., 1985). Bramble and Wake (1985) have expanded the idea of a food transport cycle basic to all feeding modes for terrestrially feeding tetrapods. A motor pattern involving adductor, neck, tongue and hyoid musculature would have been conserved in tetrapod evolution.
It is suggested that, during evolution, the bilateral contraction of jaw closers was retained when the first mammals developed a unilateral power stroke, but the firing level at the balancing side was reduced to avoid overloading of the symphysis. In many groups, such as some rodents, carnivores, ungulates and primates, the symphysis was strengthened and the participation of the balancing side musculature in the power stroke was increased. The cooperation pattern of the jaw elevators and depressors has been studied in many different mammalian species. Although differences can be detected, Hiiemae (1978) suggested a basic uniformity in their activity pattern.
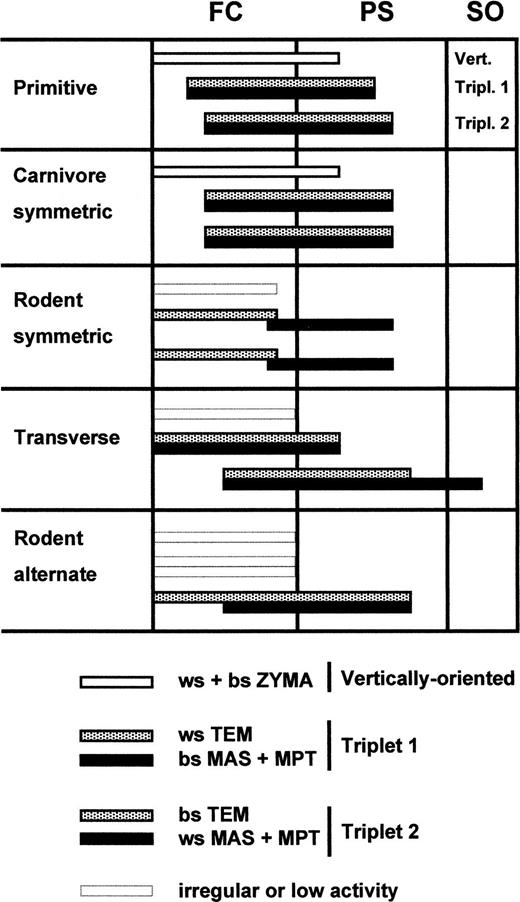
Because skull and tooth forms differ greatly among species (cutting, crushing and grinding types), the accompanying masticatory patterns differ equally. Weijs (1994) suggested separating the closing musculature according to their function, related to their direction of pull. Three different functional muscle groups (Fig. 1) can be distinguished: (1) the symmetrical vertical closers (i.e., the zygomatico-mandibularis muscles or the deep parts of the masseter muscles, and the vertical temporalis parts), active at the start of jaw closing; (2) a triplet of muscles (i.e., working side temporalis, balancing side masseter and medial pterygoid muscles) that moves the jaw to the working side (Triplet I), active during late fast closing; (3) a triplet of muscles (i.e., working side masseter and medial pterygoid, and balancing side temporalis muscles) that move the jaw to the balancing side (Triplet II), active during the power stroke. Such a separation of the jaw elevators enabled a classification of the different masticatory patterns among species by defining the timing differences of these three muscle groups. The three muscle groups cooperate variously in five different masticatory patterns, as defined by EMG. (1) In the initial mammalian pattern, these groups can be discerned and discharge in an overlapping but usually distinct sequence (opossum). (2) Both triplets can contract simultaneously resulting in an almost purely vertical jaw closing movement (carnivores). (3) All muscle groups can contract symmetrically, while a separation in time is present in forward and backward pulling muscles, resulting in a retraction and protraction of the jaw added to the hinge movement (rodents). (4) The activity of both triplets can be clearly separated in time, resulting in a smaller or larger transversal component in the jaw movement (ungulates, lagomorphs, primates). (5) Only triplet II activity can be prominent, resulting in an extreme transversal movement, alternating from the left to the right side (some rodents). It can be concluded that, although differences within the group of mammals exists, the general pattern is consistent with the generalized feeding cycle as suggested by Bramble and Wake (1985).
Although this classification facilitates comparison of the masticatory function in different animal groups and unifies more or less all mammalian masticatory systems, the diversity in the morphology of jaw systems is remarkable. Adaptation to diet may play an important role in this variation in morphology. But many different functions converge in the head and neck area, including vision, hearing, olfaction, locomotion (head posture), and brain volume. These factors all constrain the possibilities of the masticatory system development. Except for the diet influence, these factors have not been thoroughly examined.
ONTOGENY OF THE MASTICATORY MOTOR PATTERN
Although the main function of the masticatory system continues to be food uptake, the change in behavior (from suckling into mastication) during growth is dramatic. Liquid food is replaced by solid foods, the mode of uptake changes from suction to mechanical breakdown, while a conversion in main muscle activity occurs from jaw opening and tongue muscles to jaw closing muscles. At the same time, mammals share the trend of early neurocranial and late facial expansion (DuBrul, 1950; Moore and Spence, 1969). As a result, young mammals are relatively short-snouted (Schmidt-Nielsen, 1984). As an effect of this differential growth, large changes in the muscle orientations occur (sheep: Zey, 1939; pig: Wineski and Herring, 1983; rat: Hurov et al., 1988; rabbit: Langenbach and Weijs, 1990). Moreover, a differential growth of jaw closers and openers takes place resulting in relatively strong jaw closers, often in combination with a decrease in motion range (Hurov et al., 1988; Langenbach and Weijs, 1990). This morphological development is critical to the transition from suckling to chewing and the maturation of the masticatory behavior. An attempt to relate the anatomical growth and changes in the motor patterns has been made in the rabbit (see below).
The mechanism of food intake during suckling has been a point of controversy (Herring, 1985a). Opening of the jaw may produce negative pressure and draw the milk into the oral cavity (Colley and Creamer, 1958). Tongue retraction combined with jaw closure can expel the milk from the nipple (Ardran et al., 1958). The true mechanism is probably a combination of both functions. Jaw and tongue motions during suckling have been investigated in the macaque (German et al., 1992), opossum (German and Crompton, 1996) and pig (German et al., 1997). Muscle activity during suckling has been examined by Wineski and Herring (1983) and Herring (1985b) for pig, Langenbach et al. (1992) for rabbit, Iinuma et al. (1991) for dog, Westneat and Hall (1992) for rat, and Sakashita et al. (1996) for human. The transition to mastication has attracted little attention (rabbit: Yardin, 1974; Langenbach et al., 1992, 2001; hamster: Lakars and Herring, 1980; pig: Wineski and Herring, 1983; Huang et al., 1994). This is, in view of the large morphological and functional changes, surprising.
The development of oral behavior in mammals is suggested to follow a fixed order of phases: (1) active jaw depression; (2) active jaw closure, tongue, and lip movements; (3) incisive movements; and (4) grinding movements (Lakars and Herring, 1980). The speed at which oral function develops differs greatly between mammals, and is, apparently to a large extent, defined by the eruption of the teeth. Precocious animals, like the guinea pig, have fully erupted cheek teeth at birth and perform masticatory movements in utero (Ainamo, 1971; Teaford and Walker, 1983). Three weeks after birth, pigs have their first deciduous molars in occlusion and show large and often clumsy jaw movements (Wineski and Herring, 1983; Huang et al., 1994). Altricial mammals on the other hand, are characterized by almost complete absence of large jaw movements in the new-born, followed by an extremely rapid development of oral behavior (hamster: Lakars and Herring, 1980; rabbit: Yardin, 1974; Langenbach et al., 1992). In the rabbit, incisal food manipulation can be seen at day 11, a couple of days later followed by the first masticatory movements, coinciding with the newly formed molar occlusion.
The pig, which is a clearly precocial animal, shows soon after birth a wide variety of jaw motions, but they do not take any solid foods until the age of three weeks as the molars erupt. During suckling in the pig, simultaneous activity bursts in the temporalis and masseter muscles alternate with longer activity bursts in the anterior suprahyoidal muscles (Herring, 1985b). Chew-like motions have been recorded in these first postnatal weeks, but the motor patterns involve left-right contraction asymmetry, whereas in the adult pattern the transverse jaw motions are the result of differential contractions within mainly the masseter muscle (Herring et al., 1979). This maturation of the masticatory motor pattern takes several months. The first functional masticatory jaw motions involve irregular contractions of both the masseter and temporalis muscles (Huang et al., 1994), while the still present suckling behavior involves very regular contractions. The irregular chewing contractions are significantly changed in the first period of the maturation by a decrease of the activity duration of the jaw closers and the development of a clear predominance of the working side muscles' activity. The masticatory behavior and the motor patterns change until the age of 21–26 wk when they are indistinguishable from that of adults (Huang et al., 1994).
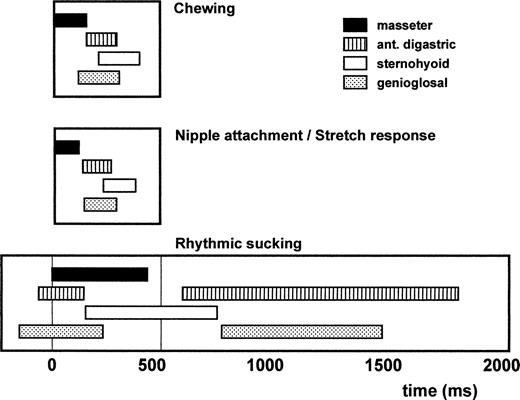
Compared to the pig, a faster development of the adult masticatory motor pattern can be seen in the other three examined species, all altricial animals. In two of three examined animals (rat and rabbit), a detailed description of the suckling and chewing motor patterns has been given. From this, it becomes clear that the first chewing motor patterns resemble the suckling activity. In the rat (Westneat and Hall, 1992), three different stages within the suckling behavior have been identified. During nipple attachment, the infant rat develops a good nipple contact, while during rhythmic sucking, the milk flow is initiated with powerful suction cycles. The actual feeding cycles occur in the last stage, the stretch response. Of these, the first and last stages display motor patterns similar to that of chewing (Fig. 2). The preparatory rhythmic sucking cycles show a clearly different motor pattern, in that the digastric and genioglossus muscles undergo two activity bursts. Chewing EMGs are present at the age of 12 days, at the time the teeth erupt. In just one week's time, this irregular and immature pattern changes into the adult masticatory pattern. Frequencies of chewing, nipple attachment, and stretch response have a similar range of 3–5 Hz, while the rhythmic sucking is much slower (0.5–1 Hz).
In contrast to work performed in the rat, Langenbach et al. (1992) examined the activity behavior of all jaw closing muscles during suckling and the transition to mastication. During suckling, all muscles fire symmetrically both with respect to timing and to activity level. No changes in the suckling motor pattern in the course of the examined two weeks were found. Jaw opening shows a biphasic muscle activity. A burst of the suprahyoidal muscles is followed by an activity burst of the lateral pterygoid and digastric muscles. Jaw closing is generated by activity of all jaw closers. Except for the posterior portions of the masseter, these muscles are simultaneously active. The posterior superficial masseter initiates jaw closing, and is compared to the other jaw closers, more active. It is thought that it may counteract, in addition to the lateral pterygoid, the strongly retrusive digastric action. Irregular horizontal jaw motions at maximum opening may be attributed to variation in this antagonistic action.
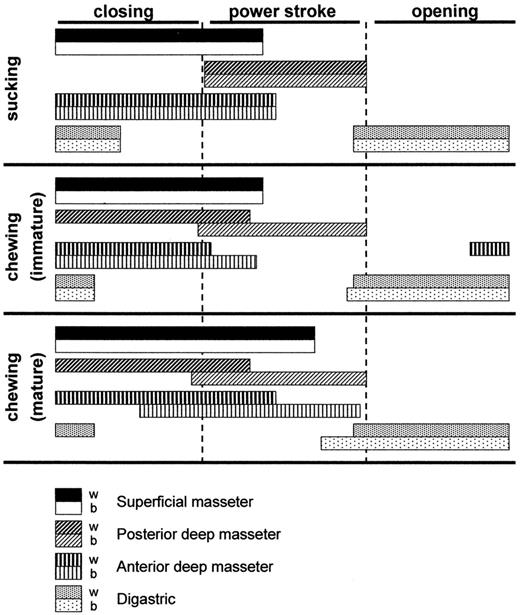
In contrast to rats, rabbits chew their food with a clear laterodeviation of the jaw. Chewing starts at the age of 13 days, immediately after their molars erupt. It is surprising that, during the first recognizable chewing cycles (performed with a similar frequency as suckling) the motor pattern of the balancing side muscles resembles the suckling motor pattern (Fig. 3). The newly attained working side activity pattern shows a higher amplitude compared to the balancing side. Apart from the amplitude difference, some of the working side jaw closers show timing differences compared to the balancing side muscles. During the course of development the balancing side muscles become more involved, presumably to increase the maximal chewing force that can be exerted at occlusion, comparable to the findings in the pig (Huang et al., 1994). Except for the increasing activity level of the balancing side muscles, two other changes in the chewing motor pattern can be recognized. First, most jaw closers shift or prolong their activity towards the power stroke, and second, the digastric muscle attains a clear asymmetric contraction pattern (Fig. 3).
During growth, the masticatory system, as a static-lever apparatus, retains its initial characteristics of force transfer, despite the considerable changes in skull shape (Langenbach and Weijs, 1990). Differential growth of the muscle cross-sectional areas results in increasingly larger bite forces which can be exerted in a decreasing range of directions. Especially in the pro- and retractive directions, the rabbit masticatory system loses its ability to generate large forces (Langenbach et al., 1991). These anatomical changes are instrumental for the change to chewing in which large occlusal forces in a mainly vertical direction have to be generated. However, in spite of the enormous anatomical changes, the functional jaw motions stay well within the capabilities of the system. Therefore, it can be concluded that, in the rabbit, the necessary change in oral behavior from suckling to chewing is engendered by the development of a different motor pattern, not by an overall change in morphology.
In the dog, Iinuma et al. (1991) described the oral behavior and the corresponding activity patterns of the temporal and masseter muscles during the first seven weeks. At the time the molars erupt, a chewing-like oral behavior is developed. While the temporal muscle is the dominant muscle during suckling, the masseter attains a higher activity level at the time the behavior changes into the chewing-like behavior, similar to that found during the adult masticatory pattern.
The similarity of frequency and timing of suckling and chewing muscle activity found for the rat, rabbit, and dog suggests a close relationship between the control mechanisms of both feeding methods. Collections of neurons that produce rhythmical changes in excitability of motoneurons are called central pattern generators (Lund, 1991). These generators, located in the brain stem, are involved in rhythmic motions (Rossignol et al., 1988), and sufficient proof has been provided for their role in mastication. The central pattern generator for mastication can be activated by electrical stimulation of the cortical masticatory area, resulting in rhythmic jaw motions, resembling mastication. It is interesting to notice that in newborn guinea pigs, suckling can be generated by stimulating a cortical area adjacent to the cortical masticatory area (Iriki et al., 1988). Possibly both cortical areas project onto the same central pattern generator. It is not clear, however, how the change in use of cortical area is induced. In all likelihood, this can be the result of a change in peripheral stimulus. In decerebrated animals, rhythmic masticatory motions can also be induced by stimuli in and around the oral area. A study by Bremer (1923), in rabbits, showed that the position of the stimulus within the oral cavity resulted in a change from gnawing to mastication when the site of stimulation was moved from the front to the back of the mouth. This is in coherence with the development of masticatory behavior at the time of molar eruption in the still sucking young.
The development of occlusion, and thus, sensory feedback from the periodontal receptors, seems to be instrumental in the inception of the chewing motor pattern. Comparison of the EMGs between ages in both the rat and rabbit suggests a degree of continuity of muscular activity across the suckling-to-chewing transition, allowing the young animal to refine its chewing abilities at the time suckling is still its main feeding behavior.
INFLUENCE OF OCCLUSION ON THE MASTICATORY MOTOR PATTERN
It has been propounded that masticatory motor patterns are influenced by occlusal forces (Rossignol et al., 1988). A low level of muscle activity has been observed in the EMG of subjects making chewing movements without food, whereas with food present, the level of observed muscle activity was much higher (Olthoff et al., 1985). Also, during a feeding sequence, the closing muscle activity is low during the early stages of closing, whereas the activity increases after teeth encounter the food and resistance to further closing is felt (opossum: Thexton, 1976). When the food is squashed and softened during chewing, the amplitude of the closing muscle activity decreases. Also, the texture of food influences the total amount of EMG, without affecting the chewing rhythm (human: Horio and Kawamura, 1989), and the food hardness modifies muscle activity levels during the power stroke (Weijs and Dantuma, 1981; Hylander et al., 1987). The larger the food particles, the earlier jaw closing activity starts after the moment of maximal jaw opening (Gorniak and Gans, 1980). It has been shown that decerebrated or anesthetised animals start to chew with no other stimulation than placing some food in the mouth. The centrally induced masticatory activity pattern remains present even after removal of sensory feedback (Dellow and Lund, 1971), indicating that sensory feedback is not essential for the masticatory movements. However, comparison of the stereotyped motions and activity patterns produced by cortical stimulation with the large variability in motion and activity seen in natural chewing, demonstrates the role that sensory feedback plays. It can be concluded that a portion of the closer muscle activity is needed just to move the jaw, and additional muscle activity is needed to overcome the resistance of food.
The relationship between EMG and bite force is mostly estimated in an indirect way, which makes it infeasible to draw concrete conclusions about the influence of occlusal forces on the masticatory motor patterns. A clever method to investigate this relationship in a detailed and direct way has been described by Ottenhoff et al. (1992a). Subjects performed rhythmic open-close motions, while food resistance was simulated by a computer-controlled force, supplied by a magnet-coil system. The coil was attached to the subject's mandible and could freely move up and down. By varying the current through the coil, a force could be generated. With this method the two parts of the closing muscle activity (i.e., activity solely to move the lower jaw and additional muscle activity to overcome the occlusal resistance) could be defined. When an occlusal force was present, an anticipating mechanism was activated for the next cycle which generates muscle activity even before the onset of the occlusal force. From their experiments, it becomes clear that adaptation to a changed occlusal condition occurred within two cycles. It was concluded that the additional muscle activity is mainly generated by peripheral feedback. The central nervous system is able to generate jaw closer muscle activity with a shorter latency if the counteracting force is expected than when it is not expected. The likely origins of the feedback are muscle spindles (Morimoto et al., 1995; Abbink, 1999) and periodontal receptors (Lavigne et al., 1987; Morimoto et al., 1989). The magnitude of the mechanism is scaled by information about the resistance gained in previous cycles (Ottenhoff et al., 1992b). A similar, but much slower, mechanism was found for the digastric muscle when jaw opening was opposed (Abbink et al., 1998).
BIOMECHANICAL MODELING OF THE MASTICATORY SYSTEM
The evolutionary development, ontogeny, morphology, and motor patterns of the mammalian masticatory system are characterized by large variations. These variations complicate comparisons between grouped data, and efforts to relate cause with effect. To explain these relationships in the masticatory system, structure and function have to be linked in a dependent manner, in which biomechanical theories have to play an important role.
Interactions between structure and function in the masticatory system can be simulated with computer models which use established biomechanical principles. The first simulations involved static rigid-beam models, assuming the mandible to be nonflexible and muscles to contract isometrically. This approach has been reviewed by Weijs and van Spronsen (1992), and the most refined example is the model described by Koolstra et al. (1988). These models have been used to examine a broad range of topics, such as joint loading (Faulkner et al., 1987; Korioth and Hannam, 1990), the dependency between the angulation and size of articular and bite forces (van Eijden, 1991), the importance of the size and angulation of the muscle tensions (Throckmorton and Throckmorton, 1985; Throckmorton, 1985), and the efficiency of the masticatory system (Osborn and Baragar, 1985). A major finding of these static models was that during unilateral clenching, the balancing side joint was loaded more than the working side.
The mandible is composed of elastic materials and it will deform due to loads exerted by the jaw muscles, bite point(s), and joints. Although small, these deformations can cause complex stress and strain patterns in the jaw (see for review: van Eijden, 2000). These stress and strain patterns are assumed to be important factors in the regulation of modeling and remodeling processes. To examine these deformations, the usual modeling technique is finite element analysis, in which the functional components (bone, teeth and cartilage) are divided into many adjoining elements with physical properties describing the material's stress behavior. These models can predict forces and deformations at all sites of the defined system, and can estimate the effect of, for example, the amplitude and direction of muscle action on the jaw deformation or articular stress (Korioth et al., 1992; Korioth and Hannam, 1994; Chen and Xu, 1994; Beek et al., 2000). Finite element models show great promise as a modeling tool. However, to date, these models are static. Their real value is likely to emerge when their analysis is combined with motion.
The dimension of time introduces a desirable element into models of the masticatory system, for the entire spectrum of motional change in various parts of the system is time-dependent, including the signals used to drive the jaw muscles, and the signals derived from peripheral receptors. Very few dynamic simulations have been attempted to date in the masticatory system despite their obvious relevance. The first dynamic model was described by Otten (1988) for the rat. Dynamic models of the human masticatory system were developed soon after that (Ng et al., 1994; Koolstra and van Eijden, 1995; Slager et al., 1997; Langenbach and Hannam, 1999). These models include tendon and muscle fiber components, and are designed to predict jaw motion as a result of muscle activations.
The model developed by Koolstra and van Eijden (1995) has been used to examine, with symmetric muscle action, several aspects of the masticatory system, including the dynamic muscle properties (Koolstra and van Eijden, 1996) and jaw motions (Koolstra and van Eijden, 1997). The three-dimensional capabilities of the system were investigated by unilateral action of the jaw muscles (Koolstra and van Eijden, 1999, 2001). The predicted three-dimensional active envelope of jaw movement was limited in horizontal directions predominantly by the temporomandibular ligaments. The passive tensions of the masticatory muscles influenced its vertical dimension. Most interestingly was the shape of the envelope which was comparable to the Posselt-figure known for the human masticatory system.
The three-dimensional model developed by Langenbach and Hannam (1999) examined the role of the passive muscle tensions during jaw motions. Muscle actions as described for the human masticatory system (Møller, 1966) were used to evoke masticatory cycles. Although the resulting jaw motions strongly resembled human mastication, it became clear that the passive muscle tensions restricted the jaw motions outside the chewing envelope. The joint load distribution found during static clenching (see above) was inverted in the simulation of chewing, i.e., the working side joint was loaded more than the balancing side joint. This reflects the findings that muscle activation patterns differ from symmetric during unilateral clenching (Miller, 1991).
FINAL REMARKS
Due to the examination of a substantial number of species, it can be concluded that we have a widespread view of the anatomy and function of the mammalian masticatory system. The key feature of mammalian mastication is the unilateral occlusal motion. From this pattern, found in primitive mammals, new motor patterns have developed to decrease (e.g., carnivores), or on the contrary, enhance (e.g., herbivores) the asymmetric jaw motion. Another feature of mammals is that the feeding starts with suckling behavior which has to change into mastication. This transition has been examined in only four species, and it seems that two different patterns can be distinguished. In the precocial pig, the transition is relatively gradual, probably because a complex heterogenous activity pattern has to be learned. In the altricial rat and rabbit, the change into the adult masticatory pattern occurs in just 1–2 wk, the rapid transition facilitated by the similarities between the motor patterns of suckling and chewing. One common feature is that the development of occlusion triggers the masticatory motor pattern. This occlusion also plays an essential role in the cycle-to-cycle adjustment of the motor patterns. Among others, muscle spindles and periodontal receptors are the probable sources of this adjustment.
With the progression of research techniques, it becomes clear that many problems remain unsolved. Most mammalian masticatory systems are kinematically and mechanically redundant, i.e., for a desirable motion of a tooth, multiple motor patterns can theoretically be generated all resulting in the same motion. To date, it remains unclear how the central nervous system solves this problem and which criteria influence this process. To encounter the challenge of redundancy, computer models have used mathematical minimization and/or optimization methods with various success. Also, the application of computer models revealed that many basic muscle properties are unknown for the masticatory system. Usually, muscle properties described for the leg muscles are used, and although the type of tissue is similar it is unclear how these properties in the leg muscles relate to those in the masticatory muscles.
The introduction of time into dynamic models creates the opportunity to include signals derived from the central and peripheral nervous systems. In that way, mastication could be modeled as the result of a central pattern generator, adjusted by peripheral feedback, i.e., a centrally controlled system for generating or simulating muscle drive.
From the Symposium Motor Control of Vertebrate Feeding: Function and Evolution presented at the Annual Meeting of the Society for Integrative and Comparative Biology, 3–7 January 2001, at Chicago, Illinois.
E-mail: g.e.langenbach@amc.uva.nl
Fig. 1. Five different mammalian jaw elevator masticatory motor patterns based on EMG recordings. The start of the shown activity patterns coincides with maximum opening. Of the different phases in the chewing cycle the fast closing (FC), the power stroke (PS) and slow opening (SO) are shown. The muscles have been divided into three groups: vertically oriented muscles, triplet 1 and triplet 2. Adapted from Weijs (1994)
Fig. 2. The mean cycles for chewing, nipple attachment/stretch response, and rhythmic sucking in the rat. Cycle starts at the onset of masseter activity. Bar lengths indicate the mean muscle activity duration. Adapted from Westneat and Hall (1992)
Fig. 3. Normalized cycles for sucking, immature chewing and mature chewing in the rabbit. Cycle starts at maximum opening. Bar lengths indicate the mean muscle activity duration. Adapted from Langenbach et al. (1992, 2001)