-
PDF
- Split View
-
Views
-
Cite
Cite
Maria João Correia, José Lino Costa, Carlos Antunes, Giulio De Leo, Isabel Domingos, The decline in recruitment of the European eel: new insights from a 40-year-long time-series in the Minho estuary (Portugal), ICES Journal of Marine Science, Volume 75, Issue 6, November-December 2018, Pages 1975–1983, https://doi.org/10.1093/icesjms/fsy073
Close - Share Icon Share
Abstract
In the past three decades the European eel Anguilla anguilla experienced up to 99% decline in recruitment in some parts of its distribution range. In addition to this long-term trend, glass eel recruitment exhibits significant inter-annual variability partially explained by large-scale oceanographic processes as well as local-scale meteorological and hydrological variables. A 40-year long time series of glass eel fishery catch in the Minho estuary, Portugal, was used to assess (a) whether local recruitment followed the general pattern of decline of A. anguilla observed elsewhere, and (b) whether environmental variables may explain inter-annual fluctuations in glass eel recruitment in the Minho estuary. The analysis shows that, in contrast to the majority of coastal systems in northern Europe and the Mediterranean, CPUE of glass eel in the Minho estuary did not exhibit a marked decline between 1974–2015 and never dropped below 20% of the 10-year mean (1974–1983), taken as baseline. The difference between the recruitment trend observed in the Minho river and that reported by WGEEL for wider European geographical scales highlights the need to calculate recruitment indices with a higher geographic resolution to better support the assessment of the status of the European eel population.
Introduction
The European eel Anguilla anguilla (L.) is classified as critically endangered in the IUCN Red List. Following high recruitment levels in the late 1970s, there was a rapid decreasing trend over three decades, with the recruitment level in 2017 estimated at 1.6% for the North Sea and 8.7% elsewhere in the distribution area (ICES, 2017), with respect to 1960–1979 averages. Changes in ocean conditions affecting the survival of larvae, reduction in the number and quality of spawners driven by anthropogenic factors such as habitat loss, pollution, and parasite introductions as well as overexploitation have been pointed out as main drivers of the eel recruitment decline (Friedland et al., 2007; Kettle et al., 2008; Belpaire et al., 2009; ICES, 2017). Quantifying and understanding the mechanisms regulating recruitment is therefore important to improve the management of the eel stock (ICES, 2014).
The European eel stock is assessed annually by the Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL), based on data on recruitment, landings, and eel biomass reported by European Union (EU) countries and some non-EU countries from the Mediterranean (ICES, 2017). Owing to the difficulties in monitoring stock-wide spawner escapement (Dekker, 2002), glass eel (i.e. juvenile) recruitment time-series have often been used as an indicator of the status of the eel stock (ICES, 2014). However, there is a great heterogeneity among the recruitment time-series used to assess the trend of the eel stock, which includes both fishery statistics data and scientific surveys. Moreover, there are inconsistencies in reporting between countries as well as incomplete reporting (Dekker, 2002). The variability of reporting standards and their level of detail and coverage restrict the scope and value of international assessment of the European eel stock (Aranburu et al., 2016).
Eel recruitment is affected by large-scale oceanographic processes (Bonhommeau et al., 2008; Melià et al., 2013; Baltazar-Soares et al., 2014). The newly hatched larvae depend on ocean currents and food availability, during their migration across the North Atlantic from the spawning area in the Sargasso Sea to the North African and European continental shelves. Thereafter, they metamorphose into glass eels and colonize coastal, estuarine, and freshwater habitats (Tesch, 2003). Temperature and ocean productivity in the spawning area have an important effect on feeding, growth, and survival of larvae (Désaunay and Guérault, 1997; Knights, 2003; Bonhommeau et al., 2008). The North Atlantic Oscillation (NAO) index is also correlated with the variation in eel recruitment (Friedland et al., 2007; Kettle et al., 2008; Durif et al., 2011; Arribas et al., 2012) because it forces changes in fronts and currents that affect spawning and larvae migration (Miller et al., 2009). The negative correlation found between the NAO and recruitment usually occurs with time lags of up to 4 years (Bonhommeau et al., 2008, 2009; Kettle et al., 2008; Arribas et al., 2012), which correspond to variations in the duration of larval migration. Local environmental factors also play a significant role in determining the recruitment to estuaries (Domingos, 1992; Arribas et al., 2012; Aranburu et al., 2016), especially rainfall (Domingos, 1992). The increase in the river flow promotes the transport of continental odours into the sea attracting the glass eels that arrive at the continental shelf (Tosi and Sola, 1993). The annual fluctuations in glass eel recruitment are driven by large-scale oceanographic processes and local environmental factors (Elie and Rochard, 1994; Zompola et al., 2008), but a full understanding of their lagged effects on recruitment still needs to be better assessed (Kettle et al., 2008).
The 40-year-long time-series of glass eel recruitment in the Minho estuary presents a unique opportunity to investigate demographic trends of glass eel recruitment in the most western regions of recruitment above the Gibraltar strait. The glass eel fishery in Portugal was banned in 2000, except for the Minho River, where data from commercial catches are available since 1974. These data have been used to assess trends in glass eel recruitment in recent decades (ICES, 2017); however, this time-series has not been adjusted for variations in fishing effort over time (Maunder and Punt, 2004) and therefore its use as a proxy for abundance of recruits may be inappropriate as changes in fishing effort occurred over the study period. In addition, there is evidence that illegal fishing occurs in most Portuguese estuaries, even after the ban of glass eel fisheries in 2000. Therefore, it is important to assess whether the relative importance of underreported vs. reported fishing has changed over time so that catch-per-unit-effort (CPUE) derived from reported landings can be used as a proxy for glass eel recruitment. The present study has two objectives: (i) to estimate glass eel recruitment trend in Portugal by using a 40-year time series of CPUE data from the Minho estuary; and (ii) to identify possible large-scale oceanographic drivers and local environmental drivers of eel trends in the Minho estuary.
Methods
Data source
The Minho is a relatively large river (300 km river length, 405 m3 s−1 water discharge, 17080 km2 catchment area) that sets the northern border between Portugal and Spain (Figure 1). The fishery operates only in the estuary, up to the tidal limit (20 km upstream from the river mouth), using a stow net, locally known as “tela”. This net is a passive fishing gear that depends entirely on the current of the flood tide to operate, which implies that extreme river flows that cancel the effect of the tide, prevent setting the net, and hence the fishery. The net (2 mm mesh size) has two lateral wings, about 10 m long each (9.5 m on the floatline and 11.5 m on the leadline) and 8 m high. It is kept at the surface by large buoys, anchored to the bottom with irons set at the beginning of the flood and is attached to the boat with its mouth facing the direction of the sea. In the boat, leaning against the mouth of the net is a spotlight to attract the glass eels that are harvested with the help of a hand net. Each fishing season starts at the beginning of the flood tide and last an average of 3 h.
Location of the Portuguese Minho River watershed (Portugal NW).
Aggregated glass eel annual landings (kg) and number of licenses in the fishing seasons between 1973/74 and 2015/16 were provided by the Caminha Port Captaincy official statistics. To standardize the effort data over the period studied, the fishing effort was calculated as the product of the annual number of licenses issued by the Captaincy and the number of new moons in each fishing season. The latter is a better proxy to compute fishing effort with respect to the total number of fishing days per season, as the fishery operates for only 3–4 days before and after the new moon, when eel movement peaks and catches are expected to be higher. CPUE was derived as the ratio between total landings (in metric ton) and effort (i.e. number of licenses × number of new moon phases).
The use of CPUE as an index of abundance is based on the proportionality between CPUE and abundance providing that catchability is constant over time (Maunder et al., 2006). The sources for the catchability variation (fishing efficiency, fish vulnerability to the gear, or the behaviour of the fishermen) were assumed to remain constant over the study period, as the same fishing gear—the “tela” introduced by Spanish fishermen in 1969/1970—and the same fishing operation were used since 1973, when the glass eel fishery started to operate in the Portuguese side of this estuary.
Because underreporting to the Caminha Port Captaincy occurs, daily trade purchase data between 1990 and 2009 (total weight of glass eels and the number of fishermen vendors) were obtained from trusted glass eel buyers, to investigate the reliability of annual landings and how it might affect the CPUE trend. These data, referred to hereafter as “survey data”, were used to calculate the average monthly catch per fisherman and the total yearly catch in the Minho estuary. The latter was then contrasted with Caminha Port Captaincy landing data in the same period 1990–2009 to derive the fraction of the catch not reported to the Caminha Port Captaincy.
Several local and oceanic variables were used to investigate the influence of large-scale and small-scale factors on glass eel abundance in the Minho estuary. Local environment variables included precipitation (average and cumulative rainfall per year and per fishing season and average seasonal rainfall) and river flow (monthly river flow and average river flow per fishing season) obtained from the Portuguese System of Hydrological Information (http://snirh.pt/) for the river Minho watershed. The influence of oceanic conditions on recruitment was assessed using the NAO and the Sea Surface Temperature (SST) in the Sargasso Sea and in the Portuguese coastal area. Seasonal and annual NAO indices (defined as the difference of normalized sea level pressure between Lisbon, Portugal and Stykkisholmur/Reykjavik, Iceland) were obtained from the National Center for Atmospheric Research (NCAR, USA) database (https://climatedataguide.ucar.edu/climate-data/hurrell-north-atlantic-oscillation-nao-index-station-based). SST for the Sargasso Sea was averaged in the region between 23°–30° N and 48°–74° W, described as the European eel spawning area in the Sargasso Sea (McCleave et al., 1987), and for the Portuguese coast between the 41°–43° N and 11°–9° W, both based on data available on a 1° × 1° grid from ICOADS (http://icoads.noaa.gov).
Data analysis
Recruitment data
The reliability of glass eel landings data provided by the Caminha Port Captaincy was assessed by testing its correlation with the “survey data”. To investigate whether underreporting may have introduced bias in the CPUE, we computed the ratio between glass eel Caminha Port Captaincy landings and the “survey data” obtained from trusted glass eel buyers for the 1990–2009 period, assessed through linear regression whether if this ratio increased or decreased in time and tested the correlation between the two data sets.
As the extreme high river flow in the Minho River may impede the operation of the fishing gear by overcoming the flood tide current, their influence on catches was assessed by analysing the relationship between the annual catch of glass eels and the average seasonal river flow, as well as between the monthly catches of glass eel estimated from the “survey data” and the average monthly river flow.
To assess the viability of using CPUE as an index of glass eel abundance, its independence from the total fishing effort was tested using landing data and survey data. Correlation tests using either the official statistics on landings (CPUE vs. total effort) or the survey data (catch per fishermen per month vs. number of fishing days per month) were also conducted.
The long-term trend of the Minho CPUE time-series was assessed through linear regression, and the simple moving average method was applied to smooth out short-term fluctuations and highlight long-term cycles (Shumway and Stoffer, 2011). To estimate the slopes and breakpoints between phases of increasing and decreasing CPUE, the segment package in R (Muggeo, 2008) was used and the model was selected according to the Akaike Information Criterion. The autocorrelation function of the Minho CPUE time-series was also computed, to assess whether periodic fluctuations and dependence structures/patterns exist in the data (Shumway and Stoffer, 2011).
Finally, the Minho glass eel time-series was standardized (divided by its mean over the reference period 1974–1983, before glass eel recruitment decline) to define a recruitment index for the Minho River, which was graphically compared to the ICES WGEEL recruitment index for the North Sea and elsewhere in Europe (ICES, 2016).
Environmental data
The influence of environmental variables on glass eel recruitment for the period between 1974 and 2016 was assessed using a generalized linear model (GLM) with a normal distribution and identity link function. Logarithmic transformation was applied to the Minho glass eel series (CPUE) to obtain a better approximation to a normal distribution. A stepwise procedure was performed to select the most significant variables using the Akaike Information Criteria (AIC) (stepAIC library MASS in R, R Development Core Team 2006).
Fifteen environmental variables were selected based on previous knowledge on their ability to influence glass eel recruitment (Supplementary Table S1). The number of candidate variables included in the model was first reduced by removing the highly correlated variables. A Spearman correlation analysis was conducted and variables with high correlation coefficient (r > 0.7) were excluded from the analysis (Dormann et al., 2013), to avoid multicollinearity and redundancy of variables. From the candidate variables, only 11 potential explanatory variables were selected to be included in the model: average rain in the winter, spring, summer, and autumn, before the fishing season (rainwi, rainsp, rainsu, rainau), annual and seasonal NAO (NAO, NAOwi, NAOsp, NAOsu, NAOau), and SST in the Sargasso Sea and in the Portuguese coast (SSTss, SSTpc). The oceanographic data (NAO, NAOwi, NAOsp, NAOsu, NAOau, and SSTSS) were included in the stepwise procedure lagged from 1 to 4 years, to investigate the most significant lagged effects of oceanographic conditions on the recruitment of glass eel.
Model diagnosis was investigated through graphical methods. The normality assumption was assessed by analysing the distribution of the model residuals (normal probability plot and histogram), and the homogeneity by plotting residuals against the predicted values (Zuur et al., 2009). To detect influential observations in the model obtained, either outliers or observations with large leverage, or both, the Cook’s distance measure was computed, and the scores obtained were plotted against observations to examine relative discrepancies (Cook and Weisberg, 1982); Cook’s values higher than 0.5 were considered of concern and removed from the model.
Results
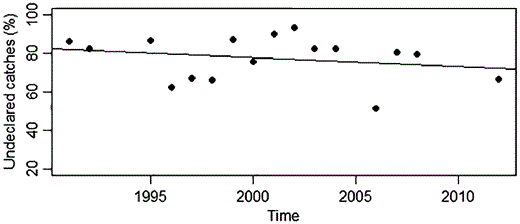
The results indicate that glass eel catches were systematically underreported in the river Minho between 1990 and 2009 (Figure 2), with official statistics on landings being on average one-third of the glass eels traded by trusted buyers. However, no temporal trend was detected in the share of reported catch during the same period (p > 0.05), despite increasing regulation control.
Undeclared catches of glass eel (total landings reported vs. the catches calculated from the “survey data”) in the Minho River between 1991 and 2012. Regression slope coefficient non-significant different from zero (p > 0.05).
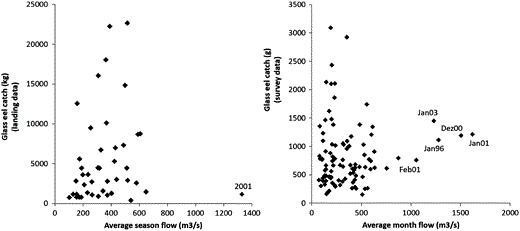
The extreme river flows observed between December 2000 and February 2001 (when 25% of the fishing days registered river flow values above 1500 m3 s−1 and 50% above 1000 m3 s−1), corresponded to low glass eel catches. However, no correlation was observed between the glass eel catch and the river flow (Figure 3) during the study period, indicating that CPUE time-series was not biased by these hydrological conditions.
Relationship between river flow (m3 s−1) and glass eel catches. Annual glass eel catches (kg) vs. average river flow for the 1974–2016 fishing seasons (left). Monthly data for the 1991–2012 fishing period with average glass eel catch (g) per fisherman vs. monthly average river flow (right).
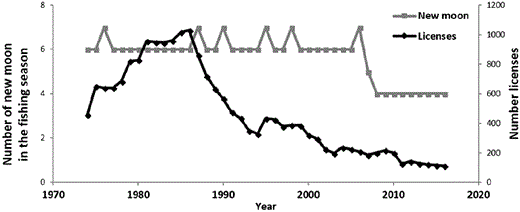
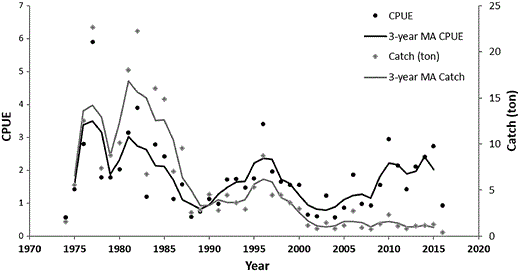
Fishing effort in the Minho River has been decreasing since the 1980s, both for the number of licenses and the length of the fishing period (Figure 4). While total catch mirrored the trend of fishing effort (r = 0.75; t-value = 7.384; 41; p < 0.01) (Figures 4 and 5), CPUE did no exhibit a long-term decline during the same period (slope of the linear regression = −0.02; p > 0.05) (Figure 5). The correlation between CPUE and effort, either in official statistics on landings or in the survey data, was not significant (respectively, r = 0.23, t-value = 1.513, 41, p > 0.1; r = 0.13, t-value = 1.329, 98, p > 0.1), indicating independence between the two variables.
Number of new moons that occurred during the fishing seasons and glass eel fishing licenses issued in the River Minho between 1973/1974 and 2015/2016 fishing seasons.
CPUE (kg/licence × moon) and total catch (tons) of glass eel in the Minho River, between 1973/1974 and 2015/2016 fishing seasons. The 3-year moving average (MA) of the CPUE and of the total catch time-series are also plotted.
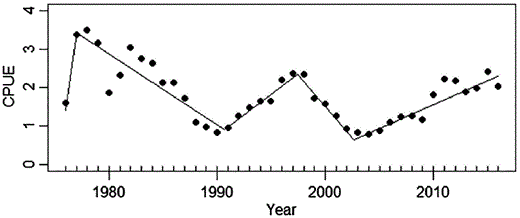
The piecewise regression on the time-series smoothed with a 3-year moving average showed four distinct alternate phases of decreasing and then increasing CPUE, each lasting from few years to a decade or more (Figure 6), whereas total catch continued to decline, coinciding with the reduction in fishing effort (Figures 4 and 5). CPUE was high and decreased between 1977 and 1991, it then increased for 7 years until 1997, decreased again for 5 years until 2002, and recovered since then to 1997 levels. Even though a cyclic trend with amplitude between 10 and 15 years characterizes CPUE, autocorrelation analysis performed on both the original and smoothed data failed to detect any significant period of oscillation (Supplementary Figure S1).
Piecewise regression fit to the glass eel CPUE time-series from the River Minho smoothed using 3-year moving average.
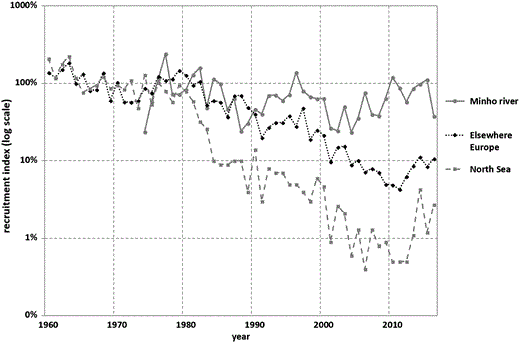
Minho recruitment index never dropped below 20% of the recruitment in the late 1970s to early 1980s, and in 2010, it fully recovered to baseline levels (Figure 7).
Glass eel recruitment index in the River Minho (scaled to the 1973–1984 average) and the WGEEL recruitment index for the North Sea and Elsewhere Europe (ICES, 2016). Logarithmic scale on the y-axis.
The GLM model derived from environmental data accounted for 34% of the observed variation in the glass eel CPUE estimated for the Minho River (Table 1). The highest explanatory power (lowest AIC) was obtained from models including local and oceanographic variables, namely average rain in autumn (rainau) and NAO. The final GLM model retained only three from the 11 candidate explanatory variables: one local—average rain in autumn (rainau)—and two oceanographic—winter NAO (NAOwi) and SST in the Sargasso Sea (SSTss)—both with a 1-year lag. The results of the model indicated a positive effect of autumn rainfall on recruitment, which was the most important variable (Table 1). On the contrary, the other two variables selected—winter NAO and SST in the Sargasso Sea—presented negative effects on recruitment, and were introduced in the model with 1-year lag.
Summary of parameters estimates (a) for recruitment variation (logCPUE) of glass eel in the Minho River from the generalized linear model over the period 1974–2014 and (b) the correspondent analysis of the deviance table (terms added sequentially).
| (a) . | . | Value . | . | SE . | t-value . |
|---|---|---|---|---|---|
| Intercept | 5.713 | 2.297 | 2.487* | ||
| rainAu_lag1 | 4.8 × 10−4 | 2.0 × 10−4 | 2.359** | ||
| NAOwi_lag1 | −2.6 × 10−2 | 1.1 × 10−2 | −2.427* | ||
| SSTss_lag1 | −2.1 × 10−1 | 0.9 × 10−1 | −2.346* |
| (a) . | . | Value . | . | SE . | t-value . |
|---|---|---|---|---|---|
| Intercept | 5.713 | 2.297 | 2.487* | ||
| rainAu_lag1 | 4.8 × 10−4 | 2.0 × 10−4 | 2.359** | ||
| NAOwi_lag1 | −2.6 × 10−2 | 1.1 × 10−2 | −2.427* | ||
| SSTss_lag1 | −2.1 × 10−1 | 0.9 × 10−1 | −2.346* |
| (b) . | df . | Deviance residuals . | df . | Residual deviance . | % explained . |
|---|---|---|---|---|---|
| Null | 40 | 0.834 | |||
| rainAu_lag1 | 1 | 0.108 | 39 | 0.726 | 13.0 |
| NAOwi_lag1 | 1 | 0.093 | 38 | 0.632 | 11.2 |
| SSTss_lag1 | 1 | 0.082 | 37 | 0.550 | 9.8 |
| (b) . | df . | Deviance residuals . | df . | Residual deviance . | % explained . |
|---|---|---|---|---|---|
| Null | 40 | 0.834 | |||
| rainAu_lag1 | 1 | 0.108 | 39 | 0.726 | 13.0 |
| NAOwi_lag1 | 1 | 0.093 | 38 | 0.632 | 11.2 |
| SSTss_lag1 | 1 | 0.082 | 37 | 0.550 | 9.8 |
Explanatory variables: average rain in autumn (rainAu_lag1), North Atlantic Oscillation winter index (NAOwi_lag1), SST at Sargasso Sea (SSTss_lag1), in the previous year.
Significant codes:
p < 0.05;
p < 0.01.
Summary of parameters estimates (a) for recruitment variation (logCPUE) of glass eel in the Minho River from the generalized linear model over the period 1974–2014 and (b) the correspondent analysis of the deviance table (terms added sequentially).
| (a) . | . | Value . | . | SE . | t-value . |
|---|---|---|---|---|---|
| Intercept | 5.713 | 2.297 | 2.487* | ||
| rainAu_lag1 | 4.8 × 10−4 | 2.0 × 10−4 | 2.359** | ||
| NAOwi_lag1 | −2.6 × 10−2 | 1.1 × 10−2 | −2.427* | ||
| SSTss_lag1 | −2.1 × 10−1 | 0.9 × 10−1 | −2.346* |
| (a) . | . | Value . | . | SE . | t-value . |
|---|---|---|---|---|---|
| Intercept | 5.713 | 2.297 | 2.487* | ||
| rainAu_lag1 | 4.8 × 10−4 | 2.0 × 10−4 | 2.359** | ||
| NAOwi_lag1 | −2.6 × 10−2 | 1.1 × 10−2 | −2.427* | ||
| SSTss_lag1 | −2.1 × 10−1 | 0.9 × 10−1 | −2.346* |
| (b) . | df . | Deviance residuals . | df . | Residual deviance . | % explained . |
|---|---|---|---|---|---|
| Null | 40 | 0.834 | |||
| rainAu_lag1 | 1 | 0.108 | 39 | 0.726 | 13.0 |
| NAOwi_lag1 | 1 | 0.093 | 38 | 0.632 | 11.2 |
| SSTss_lag1 | 1 | 0.082 | 37 | 0.550 | 9.8 |
| (b) . | df . | Deviance residuals . | df . | Residual deviance . | % explained . |
|---|---|---|---|---|---|
| Null | 40 | 0.834 | |||
| rainAu_lag1 | 1 | 0.108 | 39 | 0.726 | 13.0 |
| NAOwi_lag1 | 1 | 0.093 | 38 | 0.632 | 11.2 |
| SSTss_lag1 | 1 | 0.082 | 37 | 0.550 | 9.8 |
Explanatory variables: average rain in autumn (rainAu_lag1), North Atlantic Oscillation winter index (NAOwi_lag1), SST at Sargasso Sea (SSTss_lag1), in the previous year.
Significant codes:
p < 0.05;
p < 0.01.
Discussion
The findings from the present study show that glass eel recruitment in Portugal presents a trend different from that reported for the last 40 years at the scale of the European eel distribution area (ICES, 2017). Recruitment of glass eel in the River Minho shows no evidence of a dramatic decline as the one reported for other European rivers, but instead, two phases of decline and two of recovery. The WGEEL recruitment indices from continental North Sea or Elsewhere Europe (continental Atlantic, British Isles, and Mediterranean) (ICES, 2010) show a consistent decline between 1960 and 2016, with <2% of the mean before 1980 in the North Sea and <9% in Elsewhere Europe, in 2015. However, Minho recruitment index never dropped below 20% of the recruitment in the late 1970s to early 1980s, and it fully recovered to baseline levels in 2010. A similar recovery was also exhibited by the recruitment of glass eels to the River Minho in the period 1988–1996, and therefore, it is too early to assess whether the recovery observed in the 2002–2015 period is temporary or not.
The differences observed between the Minho recruitment index and the WGEEL recruitment index, which provides a trend for the population, suggest that the large scale of analysis might be masking the variations occurring throughout the species range. Because the factors affecting recruitment are various and include oceanic causes as well as local conditions, this discrepancy needs to be further investigated. Therefore, new insights on eel recruitment might arise by calculating indices at a higher geographical resolution, rather than only considering the two regions reported by the WGEEL (North Sea and Elsewhere Europe).
A possible explanation for the difference found between global recruitment and the present results may be related to the transatlantic migration routes of the leptocephali towards the continental shelf. Although the mechanisms that regulate the migration of eel larvae are not fully understood, there is evidence that leptocephali drift out from the Sargasso Sea to the African and European coasts forced by the Gulf Stream flow to the northeast Atlantic region (Tesch, 2003). At about 40°N the Gulf Stream splits into two main branches, the North Atlantic Current and the Azores Current, in a complex multi-branched current system, with highly variable patterns over the year. The general circulation of the northeast Atlantic region is determined by the eastward flowing Azores Current in the south (around 33°N) and the equatorward Portugal Current, a branch of the North Atlantic current system (Santos et al., 1995). Along the outer continental shelf and upper continental slope off the west Portuguese coast runs the Iberian poleward current, whose waters have characteristics typical of the sub-tropical waters formed near the Azores (Peliz et al., 2005). These different current systems make the circulation pattern near the Portuguese continental shelf complex and cause great seasonal variability (Peliz et al., 2005), which may favour the arrival of leptocephali through two different routes (Azores current and Portugal current). The strong correlation between the winter warming during particularly strong Iberian poleward currents and low values of the NAO for the preceding months (Varela et al., 2005), in years of higher recruitment in the River Minho, supports the hypothesis of the southern migratory route (Boëtius and Harding, 1985; Baltazar-Soares et al., 2014; Miller et al., 2015), and strengthens the hypothesis of a relationship between the NAO and recruitment in the western and northern Iberian rivers.
There is circumstantial evidence that in the westernmost regions of Europe the recruitment of glass eels is higher compared to the North Sea or the Mediterranean, as the glass eel fishery is concentrated in the Atlantic coast of Europe (Dekker, 2003; Knights, 2003). Although it is argued that the highest glass eel densities occur in the Bay of Biscay, it is also acknowledged that the Iberian glass eel fishery is poorly documented (Dekker, 2003). Therefore, given the location of Portugal relative to the Sargasso Sea and the narrow extension of its continental shelf, it is most likely that the country is favourable to the occurrence of high levels of recruitment. Larvae reach the westernmost regions and the Portuguese coast earlier than the far off eastern parts of the distribution range, as evidenced by their earlier time of occurrence, in October. Upon arrival at the continental shelf, leptocephali metamorphose into glass eels and use selective tidal transport for shoreward migration, reacting to continental odour cues and low salinity (Creutzberg, 1961; Tosi and Sola, 1993). The inward progression of glass eels into Portuguese estuaries may probably occur almost readily after their arrival at the continental shelf, while to colonize the North Sea and the Mediterranean continental waters glass eels take longer to move through the extensive continental shelf. To reach those coastal areas, glass eels will be more vulnerable to mortality factors, and can disperse on the extensive continental shelf, resulting in lower glass eel abundance at either the utmost northern or eastern extremities of the species distribution range. In the North Sea, even if leptocephali arrive early at the continental shelf, temperature conditions might hamper colonization, because under low temperatures glass eels avoid brackish (below 2°C) and fresh water (below 6–8°C), and therefore the more north-easterly parts of the ocean might be too cold to allow early entry by glass eels (Tesch, 2003). The abovementioned also explains the spatial differences found in the decline in recruitment in Europe, with regions located at the northern (Scandinavia) and eastern (Mediterranean coastal lagoons) limits of the geographical range showing a larger decrease in recruitment relative to the Atlantic coast (Kettle et al., 2011; Aalto et al., 2016, this study).
The present results show that there is a remarkable difference between official statistics on landings and the survey data gathered from trusted fishermen. However, the ratio between survey and official statistics does not exhibit a temporal trend, and therefore there is no reason to assume different underreporting in the periods not assessed, except after the implementation of the Eel Management Plans in 2012 when it is likely to have diminished due to the control procedures. If this were to be the case, the relative abundance estimated for the last years (between 2012 and 2016) would result in an overestimation of CPUE over the remaining time-series, however, without consequences for the overall trend analysis.
Several authors criticized the use of CPUE as an index of abundance because it requires the assumption that catchability (the coefficient specifying the proportionality between CPUE and abundance) is constant over time (Hampton et al., 2005; Maunder et al., 2006). In this study, CPUE provided a good proxy estimator of glass eel abundance because the efficiency of the gear, the fish vulnerability, or the dynamics of the fishery, the major causes of variations in catchability (Maunder et al., 2006), have not undergone relevant changes in the past 40 years. Although extreme high river flow can hamper the fishery in the River Minho, these environmental conditions are also unlikely to have resulted in the CPUE underestimations in specific years, because the average discharge in River Minho was below the critical values that eliminate the flood tide preventing the fishery (Río-Barja and Rodriguez Lestegás, 1996; Reis et al., 2009). The independence between CPUE and the fishing effort in the Minho estuary also supports its use as an index of glass eel abundance, as pointed out in Gascuel et al. (1995) analyses of glass eel CPUE patterns in French estuaries. There was no evidence that Minho CPUE has been subjected to a changing degree of bias, although some bias might still be inherent to the estimations, implying that the present results should be taken cautiously. However, because fishing operations have remained unchanged, potential bias is constant over time, making the use of CPUE valid in the trend analysis of recruitment.
The abundance of glass eels in the River Minho is favoured when NAO is in its negative phase in the winter before the beginning of the fishing season. These lagged effects suggest a 1-year delay between the NAO and the arrival of the larvae at the Portuguese coast, which may be correlated with the rainfall regime in Portugal, the strength and direction of ocean currents, and ocean productivity (Ottersen et al., 2001). Similar results have been presented to explain the decline in eel recruitment in the early 1980s (Knights, 2003; Friedland et al., 2007; Kettle et al., 2008; Durif et al., 2011; Baltazar-Soares et al., 2014), with variable time lags. The results obtained in the present study are in accordance with the 1-year larvae migration period already reported (Lecomte-Finiger, 1992; Friedland et al., 2007), contrary to longer durations (1.5–3 years) estimated by other authors (e.g. McCleave et al., 1987; Tesch, 2003; Kettle and Haines, 2006; Bonhommeau et al., 2009; Miller et al., 2015). The inconsistencies found in the duration of larvae migration may be attributed to: different methods of analysis (otolith reading and modelling), variable conditions of the ocean circulation patterns, and distance from the spawning area.
In general, the decrease in recruitment in the late 1980s and early 1990s coincided with a strong and persistent positive phase in the NAO index (Hurrell, 1995). However, the opposite association observed between 2002 and 2004, coinciding with years of weaker NAO index, indicates that this index per se is insufficient to explain the apparent decadal cycle observed in the recruitment time-series from Minho. Moreover, the results obtained contradict previous findings from a recruitment time-series index in the Netherlands, which exhibited a pattern of multi-decadal change, inversely related to the NAO winter index (Friedland et al., 2007).
The results of the present study, indicating a decrease in the abundance of glass eels in the River Minho when SST in the Sargasso Sea increases with a 1-year time lag, are consistent with previous conclusions on the impact of SST changes in the Sargasso Sea. Two important changes result from an increase in SST in the eel spawning area: a decrease in marine production and food availability (Bonhommeau et al., 2008) and a displacement in thermal fronts that may affect the spawning location and increase the retention of leptocephali within the Sargasso Sea gyre (Friedland et al., 2007). These changes may contribute to reducing the number of recruits arriving at continental coasts of Europe and Africa the following year, and therefore result in a 1-year lagged decline in the recruitment of glass eels in the River Minho.
Although there are uncertainties regarding the migration of leptocephali (e.g. migration duration and pathways, spatial synchrony), the migration pattern of glass eel, following their arrival at the European continental shelf, seems to be strongly influenced by continental cues (Tesch, 2003). In fact, in the present study, local rainfall in the autumn, at the beginning of the glass eel fishing season, was the most important variable to explain the abundance of glass eels entering the Minho estuary. Rainfall in the River Minho during the autumn is directly related to variations in river flow, and therefore, intense rainfall increases freshwater discharges (Loureiro et al., 1986) and river plumes in the open sea (Otero et al., 2008). Strong positive relationships between the river flow and glass eel migration were also found in the Mondego (Domingos, 1992) and Guadalquivir estuaries (Arribas et al., 2012), and therefore, larger catchments potentially attract more glass eels through the larger plume of freshwater odour stimuli they create (Tesch, 2003), as is the case with the Severn in the United Kingdom (Aprahamian et al., 2007). The high runoff of some Portuguese rivers and the formation of the western Iberian Buoyant Plume stretched offshore in upwelling-favourable conditions (Peliz et al., 2005) might be an important trigger for the metamorphosis of leptocephali and to modulate the migratory behaviour of glass eels towards continental waters.
The recruitment of European eel to continental waters shows undoubtedly a decline. However, the lack of spatial and temporal synchrony in the decline reported by several studies (Moriarty and Dekker, 1997; ICES, 2008; Kettle et al., 2008), along with such a large and diverse distribution range, suggests the implication of local environmental factors that may influence the success of recruitment to continental waters. The variability of local climate conditions and the regional systems of ocean currents at the continental shelf will determine the level of glass eel recruitment into continental waters, and bring up regional and local scale patterns of abundance, even at the catchment level (Zompola et al., 2008; Pacariz et al., 2014; Bornarel et al., 2018). The narrow Portuguese continental shelf is the foremost continental region that leptocephali find after crossing the Atlantic Ocean and where they first appear. Because of its shortest distance to the Sargasso Sea and the possibility of receiving leptocephali from two different migratory routes, Portugal is likely a place of high recruitment. The regional system of currents and the presence of rivers with high river flow that affect recruitment to the estuaries, together with the shorter period of vulnerability that glass eels encounter in their migration towards the coast, strongly support the Portuguese eel recruitment trend presented here.
Acknowledgements
This study was partially funded by the Fundação para a Ciência e a Tecnologia (FCT), through the PhD Grant attributed to MJ (SFRH/BD/101396/2014) and had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic project UID/MAR/04292/2013 granted to MARE. We thank Mr. Serafim Cubal (Caminha Port Captaincy) for providing the glass eel fishery data and anonymous glass eel buyers for providing the daily trade purchase data. We also thank the three referees who considerably contributed to improve an earlier draft of this manuscript.
References