-
PDF
- Split View
-
Views
-
Cite
Cite
Jørgen S. Christiansen, Asbjørn Gildberg, Kjell T. Nilssen, Charlotta Lindblom, Tore Haug, The gastric properties of free-ranging harp (Pagophilus groenlandicus (Erxleben, 1777)) and hooded (Cystophora cristata (Erxleben, 1777)) seals, ICES Journal of Marine Science, Volume 61, Issue 2, 2004, Pages 287–292, https://doi.org/10.1016/j.icesjms.2004.01.002
Close - Share Icon Share
Abstract
The study of trophic relationships in seals is based primarily on prey remains recovered from the digestive tract or scats. Basic data on the gastric properties of seals are scarce and are considered to be important to interpret data from dietary studies of these animals. Hence, we examined the key properties of the gastric chyme post mortem (i.e. temperature, acidity, and the concentration of the proteolytic enzyme pepsin) in free-ranging harp (Pagophilus groenlandicus, n=40) and hooded (Cystophora cristata, n=41) seals. Seals displayed huge inter-individual variations in their gastric properties with ranges in temperature: 23.9–37.9°C, acidity: pH 1.16–7.34, and pepsin concentration: 11–1059 μg ml−1 chyme. The stomach weight and the mean values of gastric parameters revealed, however, significant species-specific differences. The stomach weight relative to body weight of hooded seal exceeded that of harp seal (t=13.77, d.f.=75, p<0.001). Furthermore, the gastric temperature and pepsin concentration were lower for harp (32.8°C and 75 μg ml−1) compared to that for hooded (35.3°C and 344 μg ml−1) seal. The reason for this disparity may be linked to the feeding mode and diet composition displayed by these seal species.
1 Introduction
The diet of seals is essentially reconstructed from analyses of prey remains recovered from gastrointestinal tract or scats (da Silva and Neilson, 1985; Dellinger and Trillmich, 1988; Pierce et al., 1991; Cottrell et al., 1996; Potelov et al., 2000). Very little information exists on the gastric properties and dynamics of the digestive process in seals. Olsen et al. (1996) described the anatomical features of the stomach and gut of harp seals (Pagophilus groenlandicus) whereas Murie and Lavigne (1986) investigated the recovery of fish otoliths from different parts of the digestive tract and, thereby, provided information on gastric evacuation rates and food transit times in phocids. However, most studies have been conducted on relatively few captive seals and data may, therefore, not be representative of animals subjected to natural conditions. The methodological problems involved in the interpretation of diet data from seals were addressed e.g. by Tollit et al. (1997) and Bowen (2000), and in an attempt to overcome some of these problems, telemetric stomach temperature loggers have been employed on captive (Hedd et al., 1996) and free-ranging (Andrews, 1998) animals.
Basic and realistic data on the gastric properties of free-ranging seals are important for designing simulation studies of digestive processes in vitro and interpreting digesta and scat data from in vivo studies of captive seals. Unfortunately, these data are still lacking. Hence, we examined the key properties of the gastric chyme (i.e. temperature, acidity, and the concentration of the proteolytic enzyme pepsin) in free-ranging Arctic seals and related these data to the diet composition displayed by the animals.
2 Material and methods
2.1 The seals
As part of scientific investigations into the physiology and dietary habits of Arctic phocid seals, harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals were sampled in the drift ice along the east coast of Greenland (latitudes 66°N–72°N) in February 2001 (Haug et al., 2002). Seals were killed instantaneously by a rifle shot to the head while they were resting on ice floes and the dead animals were brought on the deck of the RV “Jan Mayen” for dissection and sampling of tissues for various scientific purposes. All seals were sexed, weighed to the nearest kilogram, and standard body length measured to the nearest centimetre (American Society of Mammalogists, 1967). A total of 40 harp (26 males, 14 females) and 41 hooded (18 males, 23 females) seals were examined with respect to the physio-chemical properties of the gastric chyme post mortem.
2.2 Physio-chemical analyses
During dissection, the stomachs were excised at the oesophageal and pyloric sphincters (Olsen et al., 1996) and removed. The in situ temperature (0.1°C) and acidity (0.01 pH) of the gastric chyme were measured simultaneously by a probe (Radiometer, PHM80 portable pH-meter) inserted into the oesophageal entrance of the stomach. The tip of the probe reached the mucosal lining of the greater curvature of the stomach ahead of the antrum pylori (Olsen et al., 1996). About 10 ml of the gastric chyme from the same area was sampled randomly from 13 harp (11 males, 2 females) and 21 hooded (9 males, 12 females) seals with a pipette and transferred into labelled glass vials. The colour of the chyme was noted and glass vials and stomachs were frozen at −25°C immediately for later laboratory analyses. Single stomachs were examined within 3–4 min after excision and the time elapsed from killing to the end of the examination was registered for each seal.
Pepsin analysis of the gastric chyme was done at 25°C and at pH 3 since this acidity was reported to be optimal for the gastric protease activity in harp seal (Shamsuzzaman and Haard, 1984; Han and Shahidi, 1995). Bovine haemoglobin (2%) was used as substrate, and the enzyme reaction was stopped after incubation for 1 h by addition of TCA to a 5% final concentration. The activity was calculated as μmol tyrosine eq. ml−1 h−1 (Gildberg and Raa, 1983). The specific activity of crystalline swine pepsin (Sigma) at 25°C was found to be 933 U mg−1. We assumed that seal pepsin had the same specific activity as that of swine, and the pepsin concentration was expressed accordingly in μg ml−1 gastric chyme.
2.3 Dietary analyses
The diet composition displayed by individual seals was identified from analyses of the stomach and colon contents as described by Haug et al. (2002). Recovered food items were grouped into one of four dietary categories and weighed, i.e. either fish (i.e. otoliths), crustaceans, cephalopods (i.e. beaks), or a mixture of the prey taxa. Empty stomachs were rinsed in freshwater and weighed (g).
2.4 Data analyses
Stomach weight–body weight relationships were examined for each species by simple linear regression analysis (SLRA) and the slopes (β) of the regression lines were compared using Student's t-test. The potential effect of the time elapsed from killing to the examination of stomach temperature, acidity, and pepsin concentration was tested by SLRA. The interrelationships between species, sex, body weight, stomach weight, temperature, acidity, pepsin concentration, and the weight of stomach and colon contents were examined by SLRA. Mean values of body and stomach weight, temperature, acidity, and pepsin concentration between species and the pepsin concentration for the dietary categories within species were compared by Student's t-test assuming unequal variances. Differences at the p<0.05 level were considered significant (Zar, 1984).
3 Results
3.1 Stomach weight–body weight relationships
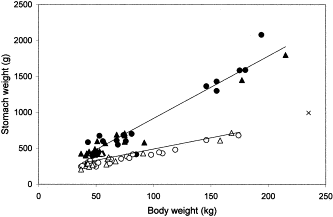
The mean±s.d. body weight did not differ significantly between our samples of harp (69.5±37.6 kg; range: 37–174 kg) and hooded (82.4±50.2 kg; range: 37–215 kg) seals. The stomach of both species was cylindroid with a sharp pyloric bend and the mean weight of empty stomachs differed significantly (p<0.001) between harp and hooded seals (Table 1). Stomach weight (SW, g) increased linearly with body weight (BW, kg) for both species according to the following equations (Figure 1): SWHarp seal = 3.155BW + 132.930 (n=39, s.e.β=0.143, r2=0.928, p<0.001) and SWHooded seal = 8.716BW + 24.825 (n=40, s.e.β=0.378, r2=0.935, p<0.001). The slope of the regression lines differed significantly (t=13.77, d.f.=75, p<0.001). In other words, in this sample, hooded seals had heavier stomachs at a given body weight and displayed a steeper increase in stomach weight with body weight compared to harp seals (Figure 1).
The relationship between stomach weight and body weight of harp (Pagophilus groenlandicus, open symbols) and hooded (Cystophora cristata, filled symbols) seals examined in this study. Circles and triangles denote male and female specimens, respectively. One female hooded seal (×) was considered a priori to be an outlier and was excluded from the statistical treatment.
Stomach weights and physio-chemical properties of the gastric chyme of harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals examined in this study. Data of harp vs. hooded seals were analysed by Student's t-test.
| . | Pagophilus groenlandicus . | Cystophora cristata . | p . |
|---|---|---|---|
| Stomach weight, g | |||
| n | 39 | 40 | <0.001 |
| Mean (±s.d.) | 352.1 (123.2) | 742.7 (452.4) | |
| Range | 205–715 | 395–2 080 | |
| Temperature, °C | |||
| n | 39 | 41 | <0.001 |
| Mean (±s.d.) | 32.8 (3.6) | 35.3 (2.1) | |
| Range | 23.9–37.6 | 27.5–37.9 | |
| Acidity, pH | |||
| n | 40 | 41 | n.s. |
| Mean (±s.d.) | 5.64 (1.98) | 5.50 (1.99) | |
| Range | 1.34–7.34 | 1.16–7.10 | |
| Pepsin, μg ml−1 chyme | |||
| n | 13 | 20 | <0.001 |
| Mean (±s.d.) | 75 (64) | 344 (298) | |
| Range | 11–213 | 23–1 059 | |
| . | Pagophilus groenlandicus . | Cystophora cristata . | p . |
|---|---|---|---|
| Stomach weight, g | |||
| n | 39 | 40 | <0.001 |
| Mean (±s.d.) | 352.1 (123.2) | 742.7 (452.4) | |
| Range | 205–715 | 395–2 080 | |
| Temperature, °C | |||
| n | 39 | 41 | <0.001 |
| Mean (±s.d.) | 32.8 (3.6) | 35.3 (2.1) | |
| Range | 23.9–37.6 | 27.5–37.9 | |
| Acidity, pH | |||
| n | 40 | 41 | n.s. |
| Mean (±s.d.) | 5.64 (1.98) | 5.50 (1.99) | |
| Range | 1.34–7.34 | 1.16–7.10 | |
| Pepsin, μg ml−1 chyme | |||
| n | 13 | 20 | <0.001 |
| Mean (±s.d.) | 75 (64) | 344 (298) | |
| Range | 11–213 | 23–1 059 | |
Stomach weights and physio-chemical properties of the gastric chyme of harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals examined in this study. Data of harp vs. hooded seals were analysed by Student's t-test.
| . | Pagophilus groenlandicus . | Cystophora cristata . | p . |
|---|---|---|---|
| Stomach weight, g | |||
| n | 39 | 40 | <0.001 |
| Mean (±s.d.) | 352.1 (123.2) | 742.7 (452.4) | |
| Range | 205–715 | 395–2 080 | |
| Temperature, °C | |||
| n | 39 | 41 | <0.001 |
| Mean (±s.d.) | 32.8 (3.6) | 35.3 (2.1) | |
| Range | 23.9–37.6 | 27.5–37.9 | |
| Acidity, pH | |||
| n | 40 | 41 | n.s. |
| Mean (±s.d.) | 5.64 (1.98) | 5.50 (1.99) | |
| Range | 1.34–7.34 | 1.16–7.10 | |
| Pepsin, μg ml−1 chyme | |||
| n | 13 | 20 | <0.001 |
| Mean (±s.d.) | 75 (64) | 344 (298) | |
| Range | 11–213 | 23–1 059 | |
| . | Pagophilus groenlandicus . | Cystophora cristata . | p . |
|---|---|---|---|
| Stomach weight, g | |||
| n | 39 | 40 | <0.001 |
| Mean (±s.d.) | 352.1 (123.2) | 742.7 (452.4) | |
| Range | 205–715 | 395–2 080 | |
| Temperature, °C | |||
| n | 39 | 41 | <0.001 |
| Mean (±s.d.) | 32.8 (3.6) | 35.3 (2.1) | |
| Range | 23.9–37.6 | 27.5–37.9 | |
| Acidity, pH | |||
| n | 40 | 41 | n.s. |
| Mean (±s.d.) | 5.64 (1.98) | 5.50 (1.99) | |
| Range | 1.34–7.34 | 1.16–7.10 | |
| Pepsin, μg ml−1 chyme | |||
| n | 13 | 20 | <0.001 |
| Mean (±s.d.) | 75 (64) | 344 (298) | |
| Range | 11–213 | 23–1 059 | |
3.2 Physio-chemical properties of the gastric chyme
All stomachs but one were examined between 14 and 150 min post mortem. Linear regression analyses revealed that a time span less than 150 min did not affect the gastric chyme with respect to temperature (harp seal: n=39, r2=0.095, p=0.056; hooded seal: n=41, r2=0.021, p=0.387), acidity (harp seal: n=40, r2=0.001, p=0.915; hooded seal: n=41, r2=0.008, p=0.583), and pepsin concentration (harp seal: n=13, r2=0.010, p=0.758; hooded seal: n=20, r2=0.001, p=0.910). However, for harp seals, stomach temperature tended to decrease gradually with time and temperature data (i.e. 23.3°C) for one male – examined 158 min post mortem – were excluded due to a significant cooling of the stomach.
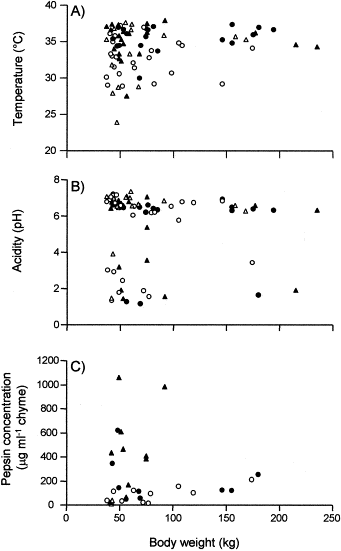
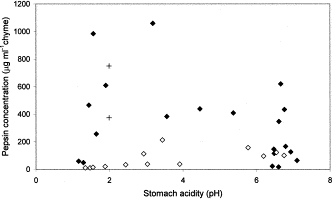
The data showed huge individual variations in the physio-chemical properties of the gastric chyme and we found no straightforward relationship between temperature, acidity, pepsin concentration, body weight, stomach weight, and sex for either species (Figures 2 and 3). The species-specific mean values for temperature, acidity, and pepsin concentration are shown in Table 1. The stomach temperature ranged between 23.9 and 37.9°C and the mean temperature was significantly lower (p<0.001) in harp (32.8°C) than in hooded (35.3°C) seals. The stomach acidities ranged between pH 1.16 and 7.34 and the mean values of pH 5–6 did not differ between species. Finally, the pepsin concentrations of the gastric chyme ranged between 11 and 1059 μg ml−1, with the mean value being significantly lower (p<0.001) for harp (75 μg ml−1) than for hooded (344 μg ml−1) seals (Table 1). There was no clear relationship between acidity and pepsin concentration although the highest pepsin concentrations tended to occur in hooded seal stomachs displaying the lower pH values (Figure 3).
Body weight vs. temperature (A), acidity (B), and pepsin concentration (C) in the gastric chyme of harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals. Symbols are as in Figure 1.
The relationship between pepsin concentration and acidity in the gastric chyme of harp (Pagophilus groenlandicus, open symbols) and hooded (Cystophora cristata, filled symbols) seals. The symbol (+) denotes the same parameters used in an in vitro study (J. S. Christiansen et al., unpubl.).
3.3 Effects of diet composition
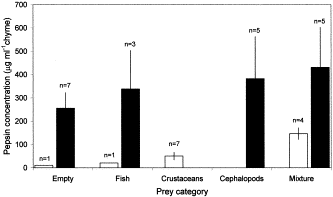
The number of harp and hooded seals pertaining to the different dietary categories is given in Table 2. All stomachs were either completely empty or contained only negligible amounts of food. Hence, prey items were recovered solely from the colon. Two harp and seven hooded seals were completely devoid of food (stomach and colon). There were striking differences in the prey composition displayed by harp and hooded seals. Fish, mainly capelin (Mallotus villosus), polar cod (Boreogadus saida), and eelpouts (Lycodes sp.), were eaten by both seal species. On the other hand, 23 harp seals had consumed planktonic crustaceans only (mainly the hyperiid amphipod Parathemisto libellula) whereas 14 hooded seals had fed exclusively on cephalopods (mainly Gonatus fabricii) (Table 2). We found no relationship between the weight of the colon contents and any of the examined gastric parameters. However, pepsin concentrations of the gastric chyme appeared to be correlated to the prey recovered from the colon (Figure 4). For harp seals, the pepsin concentration tended to increase from animals devoid of food to those feeding on a single prey taxon and a mixed diet. Furthermore, the pepsin concentration of the gastric chyme was significantly higher in animals which had fed on a mixed diet of fish and crustaceans (146.0 μg ml−1, s.e.±25.4, n=4) compared to those specimens which had consumed solely crustaceans (50.7 μg ml−1, s.e.±16.0, n=7) (p=0.008, d.f.=9). The same trend, although not statistically significant due to the high variability in our data, was seen for hooded seals (Figure 4).
The pepsin concentration (mean±s.e.) of the gastric chyme within the different dietary categories found in harp (Pagophilus groenlandicus, open bars) and hooded (Cystophora cristata, filled bars) seals. Stomachs were empty and dietary categories were identified from analysis of the colon contents. The “Empty” category denotes that both stomach and colon were devoid of food.
The number of harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals containing prey in different dietary categories. Stomachs were empty and dietary categories were identified from analysis of the colon contents. The “Empty” category denotes that both stomach and colon were devoid of food.
| Dietary category . | Pagophilusgroenlandicus (n=40) . | Cystophora cristata (n=41) . |
|---|---|---|
| Fish | 5 | 11 |
| Crustaceans | 23 | 0 |
| Cephalopods | 0 | 14 |
| Mixture | 10 | 9 |
| Empty | 2 | 7 |
| Dietary category . | Pagophilusgroenlandicus (n=40) . | Cystophora cristata (n=41) . |
|---|---|---|
| Fish | 5 | 11 |
| Crustaceans | 23 | 0 |
| Cephalopods | 0 | 14 |
| Mixture | 10 | 9 |
| Empty | 2 | 7 |
The number of harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals containing prey in different dietary categories. Stomachs were empty and dietary categories were identified from analysis of the colon contents. The “Empty” category denotes that both stomach and colon were devoid of food.
| Dietary category . | Pagophilusgroenlandicus (n=40) . | Cystophora cristata (n=41) . |
|---|---|---|
| Fish | 5 | 11 |
| Crustaceans | 23 | 0 |
| Cephalopods | 0 | 14 |
| Mixture | 10 | 9 |
| Empty | 2 | 7 |
| Dietary category . | Pagophilusgroenlandicus (n=40) . | Cystophora cristata (n=41) . |
|---|---|---|
| Fish | 5 | 11 |
| Crustaceans | 23 | 0 |
| Cephalopods | 0 | 14 |
| Mixture | 10 | 9 |
| Empty | 2 | 7 |
4 Discussion
4.1 Relationships between body and stomach
The monogastric stomach of harp seals has a mean weight and mean maximum volume of about 640 g and 5.1 l, respectively (Olsen et al., 1996). In other words, the calculated expansion of a harp seal stomach is about 0.8 l per 100 g stomach weight. Our stomach weight data for harp seal are comparable to those of Olsen et al. (1996) (Table 1, Figure 1). Given that the stomach sac of both seal species can be expanded to a similar relative degree, a range in stomach volume of about 1.6–5.7 l for harp and 3.1–16.6 l for hooded seals is suggested. The stomach weight relative to body weight of hooded seals significantly exceeded that of harp seals within the size range examined (Figure 1). Furthermore, the steeper increase in stomach weight with body weight for hooded seals indicates that the stomach develops along different growth trajectories for the two species. Although not quantified, we also noted that the muscular stomach wall of hooded seals appeared thicker and tougher compared to that of harp seals.
4.2 The gastric environment of Arctic seals
The prey composition and food consumption of harp and hooded seals varies greatly with season and among regions (Kapel, 2000 and references therein; Nilssen et al., 2000). Seals in this study were sampled in February and most stomachs were empty. The distinct difference in foraging behaviour with harp seals feeding on planktonic crustaceans and hooded seals feeding mainly on cephalopods in deeper waters (Table 2) is in good agreement with other studies on these seal species in late winter (Potelov et al., 2000).
The wide scatter of data concerning stomach temperature, acidity, and pepsin concentration was particularly conspicuous in animals with body weights less than 100 kg (Figure 2) and reflects the highly dynamic processes that take place in the gastric environment of Arctic seals. Very little is known about the digestive capabilities and gastric evacuation rates in seals and most data are obtained from a limited number of captive animals adjusted to a regular and unbalanced diet (e.g. Murie and Lavigne, 1986; Tollit et al., 1997; Mårtensson et al., 1998). The use of telemetric technologies on captive and free-ranging animals may, therefore, facilitate the interpretation of gastrointestinal data from seals.
Both the gastric mean temperature and mean pepsin concentration (Table 1) were significantly lower for harp compared to that for hooded seals. The reason for this disparity between the species is obscure. The digestive process in seals is highly affected by the amount and quality of prey eaten. A green to dark-brown colour of the gastric chyme was seen among hooded seals feeding on cephalopods (pigments from ink) whereas the chyme from animals feeding on fish and crustaceans tended to be yellow to orange (carotenoid pigments). Seasonal feeding cycles and nutritional status of the prey should also be considered in digestive studies on seal, since many forage fish such as capelin contain more digestive enzymes during intensive feeding periods. This may lead to a high degree of autolysis, which again promotes the gastric degradation of prey (Gildberg, 1978). On the other hand, when harp seals feed pelagically in ice-laden waters, stomach temperature may drop temporarily (Hedd et al., 1996) and digestion may be hampered.
Seals were resting on ice floes when killed and the animals were in the later stages of the postprandial phase since the stomachs were empty and the digesta occurred in the colon. An in vitro study showed that pepsin concentrations of 375–750 μg ml−1 gastric juice (37°C and pH∼2) were required to digest whole capelin (Figure 3 and J. S. Christiansen et al., unpubl.). These pepsin values are somewhat higher than the mean values found in the free-ranging harp and hooded seals (Table 1). A down-regulation of the gastric pepsin response, however, is likely to occur when the stomach is emptied. This is consistent with the observation that animals devoid of food also displayed the lowest pepsin concentrations (Figure 4). Therefore, the mean gastric pepsin concentration of harp and hooded seals is likely to be higher in the early stage of the postprandial phase.
A diet rich in energy delays the gastric evacuation of food in mammals (Wisén et al., 1993). Given the uncertainties concerning seasonal fluctuations, the energy densities of the main prey of harp and hooded seals, i.e. Parathemisto libellula, polar cod, capelin, and Gonatus fabricii, have been reported to be about 3.9, 4.4, 8.4, and 6.9 kJ g−1 wet weight, respectively (Mårtensson et al., 1994; Lawson et al., 1998). Consequently, it appears that hooded seals fed on the more energy-rich prey compared to harp seals (Table 2). It may, therefore, be speculated that a prolonged retention of the food bolus in the stomach of hooded seals may have elicited the higher pepsin response for this species (Table 1, Figure 4). Hence, different feeding modes and diets may explain the difference in stomach temperature and pepsin concentration between the two seal species.
We thank the crew on board the RV “Jan Mayen” for technical assistance and two anonymous referees for constructive comments. The study was supported by grant nos. 127546/120 and 133646/120 from The Research Council of Norway.
References
Author notes
Present address: Åbo Akademi University, Environmental and Marine Biology, Akademigatan 1, FIN-20500 Turku/Åbo, Finland.