-
PDF
- Split View
-
Views
-
Cite
Cite
Capucine Mellon-Duval, Hélène de Pontual, Luisa Métral, Loic Quemener, Growth of European hake (Merluccius merluccius) in the Gulf of Lions based on conventional tagging, ICES Journal of Marine Science, Volume 67, Issue 1, January 2010, Pages 62–70, https://doi.org/10.1093/icesjms/fsp215
Close - Share Icon Share
Abstract
Growth of European hake was estimated from the results of a conventional tagging study in the Gulf of Lions, the first tagging experiment to have been undertaken on the species in Mediterranean waters. In all, 4277 hake 15–40 cm long were tagged and released on the inshore fishing grounds during spring 2006. The overall recapture rate was 6.5% and times-at-liberty ranged from 1 to 717 d. Growth rate in hake varied with size and sex. The estimated growth parameter (von Bertalanffy k) was estimated as double previously published values based on size frequency distribution in the area. Compared with recent growth parameters derived from the tagging experiments in the Bay of Biscay, k was estimated to be slightly lower in the Gulf of Lions. With this faster growth, hake would mature earlier than previously thought: at age 2 for both sexes, instead of at age 3 or 4 as currently accepted for the Mediterranean. Growth rate by sex decreased to a similar level once fish had attained sexual maturity.Mellon-Duval, C., de Pontual, H., Métral, L., and Quemener, L. 2010. Growth of European hake (Merluccius merluccius) in the Gulf of Lions based on conventional tagging. – ICES Journal of Marine Science, 67: 62–70.
Introduction
The European hake (Merluccius merluccius) is both commercially and ecologically important in the ecosystem of the Gulf of Lions (northwestern Mediterranean off southern France, Figure 1). With a broad spatial distribution, from the coast to the continental slope, and a diet based on small pelagic fish (Ferraton et al., 2007), it plays a basic ecological role in the area. Hake are found over a range of environmental conditions, which may influence its population parameters. In the Gulf of Lions, it is a major focus for stock assessment because it is the dominant commercial demersal species (Aldebert and Carries, 1988; Oliver and Massutí, 1995; Aldebert and Recasens, 1996), with catches principally of juveniles. For these reasons, better understanding of its biological parameters is essential.
Map showing the location of the Gulf of Lions (box) in southern France where hake were tagged for the growth study between April and May 2006.
Despite many biological studies on European hake having been carried out, age estimation remains a challenging task (Morales-Nin et al., 1998; Courbin et al., 2007). In the Mediterranean, most growth analyses have been based on the size-at-age data derived from either length frequency analyses (Bouhlal and Ktari, 1975; Orsi-Relini et al., 1989; Recasens, 1992; Aldebert and Recasens, 1995; Morales-Nin and Aldebert, 1997) or interpretation of the otolith macrostructure (Morales-Nin et al., 1998). Unlike many fish species, the otoliths of hake have not been used successfully to construct a growth model because of many difficulties in interpreting patterns of macrostructure (Oliver et al., 1989; Morales-Nin et al., 1998). However, analyses of otolith microincrements have recently enhanced knowledge of hake growth in early life stages both in the Mediterranean (Morales-Nin and Aldebert, 1997; Arneri and Morales-Nin, 2000; Morales-Nin and Moranta, 2004; Belcari et al., 2006) and in the Atlantic (Kacher and Amara 2005; Piñeiro et al., 2008). A first attempt to analyse otolith macrostructure was recently conducted on hake from the Gulf of Lions (Courbin et al., 2007). Another was made on hake taken in the Gulf of Alicante, and a fast-growth hypothesis was tested based on otolith interpretation and length frequency distribution (Garcia-Rodriguez and Esteban, 2002).
Although tag-recapture experiments have been used commonly to study fish growth, the first successful experiment on tagging hake is only recent (de Pontual et al., 2003). Most studies using conventional T-tag techniques have proved to be an effective method for growth analysis. However, because recovery rates are variable and sometimes low, especially for fish, the use of such a method requires substantial tagging effort to gain sufficient data for estimating growth accurately. The first tagging experiments successfully carried out with hake in the Bay of Biscay showed that hake growth was being underestimated when based on direct otolith interpretation (de Pontual et al., 2006).
The growth of hake in the Mediterranean has never been validated. Hake live in many areas of the eastern and western Mediterranean. The literature on age and growth in different areas, published since the 1950s, reports various estimates of growth parameters, growth rates, and otolith interpretation. Most of the studies indicated a von Bertalanffy k parameter of ∼0.1 year−1. However, we are aware of three studies (Alemany and Oliver, 1995; Garcia-Rodriguez and Esteban, 1996, 2002) that have suggested a higher growth rate (k of ∼0.2 year−1). Recent progress on growth estimation of juvenile hake has been made based on interpretation of daily otolith increments (Arneri and Morales-Nin, 2000; Morales-Nin and Moranta, 2004; Belcari et al., 2006). Yet, little is known about the growth of older fish.
Here, we report the results of the first tag-recapture experiment made in the Gulf of Lions, carried out in 2006 to estimate hake growth based on a 2-year period of recapture data. Mean growth rates and individual growth variability are used to examine differences in growth between sexes and by length category. We estimated von Bertalanffy parameters from recaptured fish and compared them with estimates previously obtained in the same area derived from the length frequency analysis (Aldebert and Recasens, 1995). We then compared European hake growth in the Gulf of Lions (Mediterranean) with that in the Bay of Biscay (Atlantic), using published data derived from tagging experiments (de Pontual et al., 2006).
Material and methods
The tagging experiment was performed in the Gulf of Lions from 15 April to 15 May 2006, between 42°15′ and 43°35′N, and 3°00′ and 6°00′E, an area of known high density of hake. Fish were caught in a four-panel bottom trawl (high opening) equipped with a codend specially designed to minimize mortality (de Pontual et al., 2003). Hauls lasted 10–15 min at a speed of 3 knots and were performed on the continental shelf 30–60 m deep. On board, the swimbladder of all hake captured was perforated to enhance survival, then the fish were placed in a tank supplied with flowing seawater.
All fish were tagged using numbered FD-94 or FD-68B (depending on fish length size) Floy T-bar anchor tags inserted in the muscle of the fish below the first dorsal fin. After tagging and measurement (TL, total length to the nearest millimetre), fish were retained in tanks for a period of 30 min to ∼4 h. Releases were made twice per day at different locations, selected to preclude their immediate recapture.
The tagging experiment was advertised in local newspapers, on the radio, and via posters to fisher organizations and in fish markets. A reward of €50 was offered for each tagged fish returned to the laboratory giving the date and location of capture. Such fish were frozen individually, then defrosted for measuring, weighing, and sex determination.
Growth analysis and modelling
We estimated growth rates (length increment ΔL/time-at-liberty ΔT) separately for each sex and for sexes combined. Daily growth rates (cm d−1) were tested for differences by sex, time-at-liberty, and length. Growth rates were examined for four different length-at-release categories (15–19, 20–24, 25–29, and 30–44 cm) to analyse both juvenile and adult stages. The L50 in the Gulf of Lions, the size at which 50% of fish are mature, is currently thought to be 38.0 cm for females and 28.8 cm for males (Recasens et al., 1998). Consequently, the first length category only included juveniles (of both sexes), the second and the third contained juvenile females and maturing males, and the fourth consisted of maturing females (no males were found). The few fish 30–44 cm TL tagged (just 3% of the total) are not considered a representative sample so are generally not analysed further. The growth rates of fish tagged when <30 cm were compared between those that had spent either more or less than 1 year at liberty. More than 1 year at liberty is considered to be a significant time-at-liberty and therefore to give a better understanding of the growth of fish tagged at the same length. Differences between groups were tested for significance using t-tests or ANOVA, after checking normality of the data distribution (Kolmogorov–Smirnov test) and variance homogeneity (Levene test). A t-test for non-homogeneous variance (SPSS, 2008) was used when required. Tukey tests were performed for post hoc comparison between factor modalities. The significance level for all tests was p < 0.05.
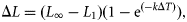
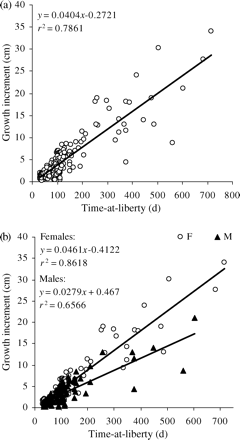
The linear trend (Figure 2) of the relationship between ΔL as a function of ΔT [r2 = 0.78 for sexes combined (p < 0.001), r2 = 0.86 for females (p < 0.001), and r2 = 0.65 for males (p < 0.001)] indirectly indicates that recoveries mainly consisted of fish tagged as juveniles, when growth is fast and almost linear. As a consequence, L∞ might be difficult to estimate because older fish are missing. This is the reason three different von Bertalanffy growth models were fitted, using non-linear regression procedures in SPSS Software 17 (SPSS, 2008). To compare growth estimated in the same area based on the length frequency method (Aldebert and Recasens, 1995), we fixed L∞ in our first model to the value obtained by those authors and only estimated k. In our second model, both L∞ and k were estimated, L∞ being constrained to ranges consistent with the maximum lengths known for both males and females. Finally, in our third model, k was estimated with L∞ fixed at the value obtained in the Bay of Biscay for sexes combined. Confidence intervals (CIs) were estimated by bootstrap for the first two models and asymptotically for the third, because the last method was used in the Atlantic analysis by de Pontual et al. (2006).
Growth increment (ΔL) plotted against time-at-liberty (ΔT) of hake tagged in the Gulf of Lions in 2006. (a) Combined sexes. (b) Separate sexes (F, female; M, male).
Results
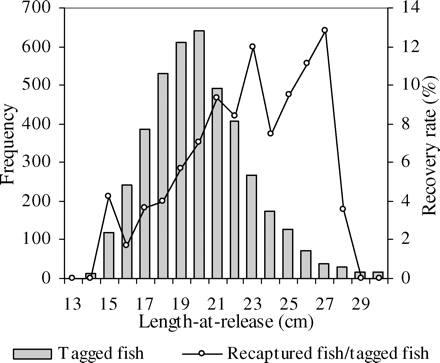
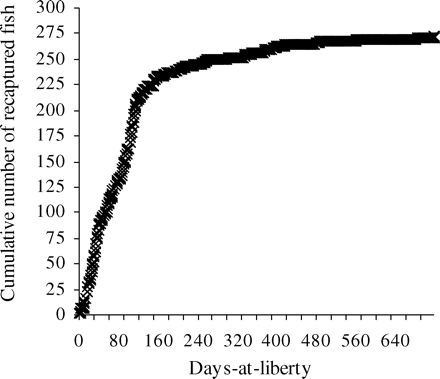
In all, 4277 hake were tagged and released during this first experiment, and to the time of writing (end of August 2008), 280 have been recaptured, corresponding to a recovery rate of 6.5% and time-at-liberty (ΔT) ranging from 1 to 717 d. The date of recovery was available for all but eight hake, and 22 had dubious growth probably attributable to questionable information on the date of recapture and/or errors in measurement. These 30 hake were excluded from the analysis, leaving 250 recoveries for further analysis. We observed increasing recovery rates from 2 to 13% with increasing length-at-release from 15 to 27 cm (Figure 3). At greater length, which corresponded to few tagged fish (3%) that are not depicted in Figure 3, recovery rates oscillated between 0 and 50%. As shown by the cumulative number of recaptures over time (Figure 4), 81% of recoveries were made in the first 5 months. Of the 242 recaptures assigned a sex, 130 were females and 112 were males.
Length frequency distribution of tagged hake (total length, TL) released in the Gulf of Lions in 2006 and the distribution of recovery rates according to fish length-at-release (n = 280). The low number (just 3%) of tagged fish with a total length ≥30 cm is not depicted.
Cumulative number of recoveries (n = 272) from the 2006 tagging experiment in the Gulf of Lions plotted against time-at-liberty.
Fish recovered within 30 d of tagging had null growth and were therefore excluded from subsequent analyses (n = 50). Of the balance of 200 recoveries (71% of the total), 108 were females, with a TL at release of 15–44 cm (mean 21 cm), 85 were males with a TL at release of 15–27 cm (mean 21 cm), and 7 were fish whose sex could not be determined. The TL at recapture ranged from 17 to 57 cm for females and from 16 to 39 cm for males, and the average time-at-liberty was 141 d for females and 121 d for males. Only eight females and six males spent more than 1 year free.
Daily growth rates were normally distributed. Mean growth rates per day, month, and year are given in Table 1 for combined and separated sexes. The mean daily growth rate differed significantly between sexes (t = 3.933, p < 0.001), a feature that could not be explained by either length-at-release or time-at-liberty because mean values of those two factors were similar in males and females.
Mean values, standard errors (s.e.) of female (n = 108), male (n = 85), and combined sexes (n = 200) growth rates by day, month, and year in the Gulf of Lions determined by conventional tagging.
| Unit . | Female growth rate . | Male growth rate . | Combined sexes growth rate . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| cm.d−1 | 0.040 | 0.0015 | 0.031 | 0.0015 | 0.036 | 0.001 |
| cm.month−1 | 1.2 | 0.05 | 0.9 | 0.05 | 1.1 | 0.03 |
| cm.year−1 | 14.7 | 0.5 | 11.4 | 0.5 | 13.1 | 0.4 |
| Unit . | Female growth rate . | Male growth rate . | Combined sexes growth rate . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| cm.d−1 | 0.040 | 0.0015 | 0.031 | 0.0015 | 0.036 | 0.001 |
| cm.month−1 | 1.2 | 0.05 | 0.9 | 0.05 | 1.1 | 0.03 |
| cm.year−1 | 14.7 | 0.5 | 11.4 | 0.5 | 13.1 | 0.4 |
The growth rates correspond to tagged fish whose length-at-release and time-at-liberty varied, respectively, from 15 to 44 cm and from 30 to 717 d.
Mean values, standard errors (s.e.) of female (n = 108), male (n = 85), and combined sexes (n = 200) growth rates by day, month, and year in the Gulf of Lions determined by conventional tagging.
| Unit . | Female growth rate . | Male growth rate . | Combined sexes growth rate . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| cm.d−1 | 0.040 | 0.0015 | 0.031 | 0.0015 | 0.036 | 0.001 |
| cm.month−1 | 1.2 | 0.05 | 0.9 | 0.05 | 1.1 | 0.03 |
| cm.year−1 | 14.7 | 0.5 | 11.4 | 0.5 | 13.1 | 0.4 |
| Unit . | Female growth rate . | Male growth rate . | Combined sexes growth rate . | |||
|---|---|---|---|---|---|---|
| . | Mean . | s.e. . | Mean . | s.e. . | Mean . | s.e. . |
| cm.d−1 | 0.040 | 0.0015 | 0.031 | 0.0015 | 0.036 | 0.001 |
| cm.month−1 | 1.2 | 0.05 | 0.9 | 0.05 | 1.1 | 0.03 |
| cm.year−1 | 14.7 | 0.5 | 11.4 | 0.5 | 13.1 | 0.4 |
The growth rates correspond to tagged fish whose length-at-release and time-at-liberty varied, respectively, from 15 to 44 cm and from 30 to 717 d.
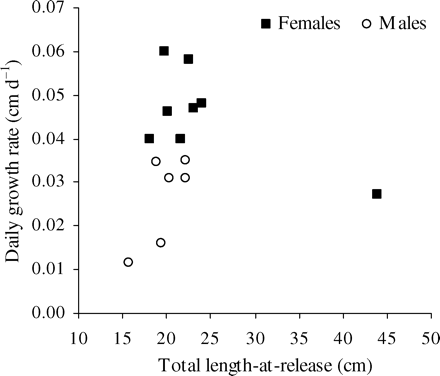
Mean growth rates in relation to time-at-liberty and length-at-release are given separately by sex in Table 2. The mean growth rate of females <30 cm length-at-release was significantly (t = 2.484, p < 0.005) higher in those that spent more than 1 year free than in those that spent less than 1 year free. In males, the reverse was observed, but there the difference was not significant. These growth differences between sexes should be viewed in relation to the maturity already reached at that length for males and not for females. There was a significant difference (t = 4.36, p = 0.001) in growth rate between females and males <30 cm length-at-release that had spent more than 1 year free (Figure 5). The single female tagged at a length >30 cm (44 cm) and recovered more than 1 year later showed a low growth rate compared with other females recovered.
Daily growth rates for hake recaptured after more than 1 year free (n = 14) plotted against total length-at-release.
Mean values, standard error (s.e.), and sample size (n) of female and male daily growth rates (cm d−1) in the Gulf of Lions in terms of time-at-liberty and length-at-release.
| Factor . | Female growth rate . | Male growth rate . | ||||
|---|---|---|---|---|---|---|
| n . | Mean . | s.e. . | n . | Mean . | s.e. . | |
| Length-at-release/time-at-liberty | ||||||
| <30 cm/<1 year | 98 | 0.040 | 0.0016 | 79 | 0.031 | 0.0016 |
| <30 cm/≥1 year | 7 | 0.048 | 0.0029 | 6 | 0.026 | 0.0041 |
| >30 cm/≥1 year | 1 | 0.027 | – | – | – | – |
| Total | 106 | 85 | ||||
| Length-at-release category | ||||||
| 15–19 cm | 29 | 0.040 | 0.0028 | 24 | 0.034 | 0.0031 |
| 20–24 cm | 67 | 0.041 | 0.0018 | 52 | 0.031 | 0.0021 |
| 25–29 cm | 9 | 0.040 | 0.0050 | 9 | 0.026 | 0.0050 |
| 30–44 cm | 3 | 0.026 | 0.0087 | – | – | – |
| Total | 108 | 85 | ||||
| Factor . | Female growth rate . | Male growth rate . | ||||
|---|---|---|---|---|---|---|
| n . | Mean . | s.e. . | n . | Mean . | s.e. . | |
| Length-at-release/time-at-liberty | ||||||
| <30 cm/<1 year | 98 | 0.040 | 0.0016 | 79 | 0.031 | 0.0016 |
| <30 cm/≥1 year | 7 | 0.048 | 0.0029 | 6 | 0.026 | 0.0041 |
| >30 cm/≥1 year | 1 | 0.027 | – | – | – | – |
| Total | 106 | 85 | ||||
| Length-at-release category | ||||||
| 15–19 cm | 29 | 0.040 | 0.0028 | 24 | 0.034 | 0.0031 |
| 20–24 cm | 67 | 0.041 | 0.0018 | 52 | 0.031 | 0.0021 |
| 25–29 cm | 9 | 0.040 | 0.0050 | 9 | 0.026 | 0.0050 |
| 30–44 cm | 3 | 0.026 | 0.0087 | – | – | – |
| Total | 108 | 85 | ||||
Mean values, standard error (s.e.), and sample size (n) of female and male daily growth rates (cm d−1) in the Gulf of Lions in terms of time-at-liberty and length-at-release.
| Factor . | Female growth rate . | Male growth rate . | ||||
|---|---|---|---|---|---|---|
| n . | Mean . | s.e. . | n . | Mean . | s.e. . | |
| Length-at-release/time-at-liberty | ||||||
| <30 cm/<1 year | 98 | 0.040 | 0.0016 | 79 | 0.031 | 0.0016 |
| <30 cm/≥1 year | 7 | 0.048 | 0.0029 | 6 | 0.026 | 0.0041 |
| >30 cm/≥1 year | 1 | 0.027 | – | – | – | – |
| Total | 106 | 85 | ||||
| Length-at-release category | ||||||
| 15–19 cm | 29 | 0.040 | 0.0028 | 24 | 0.034 | 0.0031 |
| 20–24 cm | 67 | 0.041 | 0.0018 | 52 | 0.031 | 0.0021 |
| 25–29 cm | 9 | 0.040 | 0.0050 | 9 | 0.026 | 0.0050 |
| 30–44 cm | 3 | 0.026 | 0.0087 | – | – | – |
| Total | 108 | 85 | ||||
| Factor . | Female growth rate . | Male growth rate . | ||||
|---|---|---|---|---|---|---|
| n . | Mean . | s.e. . | n . | Mean . | s.e. . | |
| Length-at-release/time-at-liberty | ||||||
| <30 cm/<1 year | 98 | 0.040 | 0.0016 | 79 | 0.031 | 0.0016 |
| <30 cm/≥1 year | 7 | 0.048 | 0.0029 | 6 | 0.026 | 0.0041 |
| >30 cm/≥1 year | 1 | 0.027 | – | – | – | – |
| Total | 106 | 85 | ||||
| Length-at-release category | ||||||
| 15–19 cm | 29 | 0.040 | 0.0028 | 24 | 0.034 | 0.0031 |
| 20–24 cm | 67 | 0.041 | 0.0018 | 52 | 0.031 | 0.0021 |
| 25–29 cm | 9 | 0.040 | 0.0050 | 9 | 0.026 | 0.0050 |
| 30–44 cm | 3 | 0.026 | 0.0087 | – | – | – |
| Total | 108 | 85 | ||||
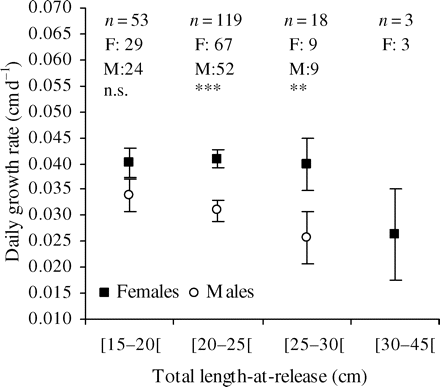
There was no growth difference (p = 0.136) between sexes in the smallest length category (Figure 6), but there were significant differences in the next two length categories up (p < 0.001 and p = 0.045). Female growth rates did not differ significantly between length categories. However, there was a decrease in the largest class, which also had greater variability. A decreasing trend was observed for males through the three size categories, but differences were not significant, even between extreme classes. We noted that the mean growth rates by sex were similar in the largest length class (25–29 cm for males, 30–44 cm for females).
Comparison of daily growth rates (mean ± s.e.) by size category and sex. F, females; M, males; n, number of individuals; n.s., not significant. ** and *** represent significant differences between females and males at α = 0.01 and 0.001, respectively.
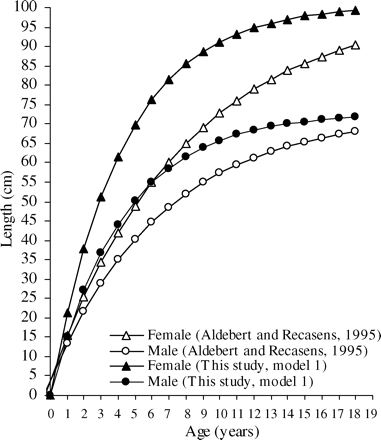
By fitting the von Bertalanffy function to the tagging data, the growth parameter k was first estimated for each sex separately, with fixed L∞ (model 1), based on the earlier estimates of Aldebert and Recasens (1995). With L∞ values of 100.7 cm for females and 72.8 cm for males, k was estimated to be 0.236 ± 0.009 year−1 for females and 0.233 ± 0.023 year−1 for males (Table 3). The corresponding growth models were plotted against those previously estimated by Aldebert and Recasens (1995), where k = 0.124 year−1 and t0 = −0.35 for females, and k = 0.149 year−1 and t0 = −0.383 for males (Figure 7). It is of note that our estimates of k were nearly double the values reported by the earlier authors.
A von Bertalanffy growth model fitted to recapture data (tagging survey 2006) in the Gulf of Lions for female (L∞ = 100.7; k = 0.236 year−1) and male hake (L∞ = 72.8; k = 0.233 year−1) compared with the growth model of Aldebert and Recasens (1995), of female (L∞ = 100.7; k = 0.124 year−1, t0 = −0.350) and male hake (L∞ = 72.8; k = 0.149 year−1, t0 = −0.383).
Hake growth parameters estimated from a tag-recapture experiment in the Gulf of Lions (s.e., standard error; n, sample size), L∞ fixed in models 1 and 3, L∞ constrained in model 2.
| Sex and model . | Method . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L∞ bounds . | L∞ . | s.e. . | k (year−1) . | s.e. . | kmin . | kmax (year−1) . | r2 ANOVA . | r2 parameters . | |
| Females (n = 108) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 100.7 | – | 0.236 | 0.009 | 0.220 | 0.252 | 0.87 | – |
| Model 2 (bootstrap) | 100≤ L∞≤130 | 114.6 | 14.0 | 0.197 | 0.030 | 0.135 | 0.258 | 0.87 | −0.98 |
| Males (n = 85) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 72.8 | – | 0.233 | 0.023 | 0.210 | 0.0256 | 0.70 | – |
| Model 2 (bootstrap) | 70≤ L∞≤100 | 83.0 | 13.6 | 0.192 | 0.044 | 0.103 | 0.281 | 0.70 | −0.95 |
| Both sexes (n = 200) | |||||||||
| Model 3 (asymptotic) | L∞ fixed | 110 | – | 0.178 | 0.005 | 0.168 | 0.187 | 0.78 | – |
| Sex and model . | Method . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L∞ bounds . | L∞ . | s.e. . | k (year−1) . | s.e. . | kmin . | kmax (year−1) . | r2 ANOVA . | r2 parameters . | |
| Females (n = 108) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 100.7 | – | 0.236 | 0.009 | 0.220 | 0.252 | 0.87 | – |
| Model 2 (bootstrap) | 100≤ L∞≤130 | 114.6 | 14.0 | 0.197 | 0.030 | 0.135 | 0.258 | 0.87 | −0.98 |
| Males (n = 85) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 72.8 | – | 0.233 | 0.023 | 0.210 | 0.0256 | 0.70 | – |
| Model 2 (bootstrap) | 70≤ L∞≤100 | 83.0 | 13.6 | 0.192 | 0.044 | 0.103 | 0.281 | 0.70 | −0.95 |
| Both sexes (n = 200) | |||||||||
| Model 3 (asymptotic) | L∞ fixed | 110 | – | 0.178 | 0.005 | 0.168 | 0.187 | 0.78 | – |
Hake growth parameters estimated from a tag-recapture experiment in the Gulf of Lions (s.e., standard error; n, sample size), L∞ fixed in models 1 and 3, L∞ constrained in model 2.
| Sex and model . | Method . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L∞ bounds . | L∞ . | s.e. . | k (year−1) . | s.e. . | kmin . | kmax (year−1) . | r2 ANOVA . | r2 parameters . | |
| Females (n = 108) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 100.7 | – | 0.236 | 0.009 | 0.220 | 0.252 | 0.87 | – |
| Model 2 (bootstrap) | 100≤ L∞≤130 | 114.6 | 14.0 | 0.197 | 0.030 | 0.135 | 0.258 | 0.87 | −0.98 |
| Males (n = 85) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 72.8 | – | 0.233 | 0.023 | 0.210 | 0.0256 | 0.70 | – |
| Model 2 (bootstrap) | 70≤ L∞≤100 | 83.0 | 13.6 | 0.192 | 0.044 | 0.103 | 0.281 | 0.70 | −0.95 |
| Both sexes (n = 200) | |||||||||
| Model 3 (asymptotic) | L∞ fixed | 110 | – | 0.178 | 0.005 | 0.168 | 0.187 | 0.78 | – |
| Sex and model . | Method . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| L∞ bounds . | L∞ . | s.e. . | k (year−1) . | s.e. . | kmin . | kmax (year−1) . | r2 ANOVA . | r2 parameters . | |
| Females (n = 108) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 100.7 | – | 0.236 | 0.009 | 0.220 | 0.252 | 0.87 | – |
| Model 2 (bootstrap) | 100≤ L∞≤130 | 114.6 | 14.0 | 0.197 | 0.030 | 0.135 | 0.258 | 0.87 | −0.98 |
| Males (n = 85) | |||||||||
| Model 1 (bootstrap) | L∞ fixed | 72.8 | – | 0.233 | 0.023 | 0.210 | 0.0256 | 0.70 | – |
| Model 2 (bootstrap) | 70≤ L∞≤100 | 83.0 | 13.6 | 0.192 | 0.044 | 0.103 | 0.281 | 0.70 | −0.95 |
| Both sexes (n = 200) | |||||||||
| Model 3 (asymptotic) | L∞ fixed | 110 | – | 0.178 | 0.005 | 0.168 | 0.187 | 0.78 | – |
In our second model (model 2), parameters k and L∞ were both estimated, with L∞ constrained to the respective ranges of 100–130 cm for females and 70–100 cm for males. Standard errors (Table 3) were higher for both sexes than in model 1 owing to the estimation of two parameters instead of one. As in model 1, the estimated k in model 2 was similar between sexes, but lower than in model 1, and because L∞ and k are negatively correlated (Francis, 1988), L∞ estimates for both sexes were also higher in model 2 than in model 1. As von Bertalanffy parameters were estimated from a limited dataset and available fishery data report maximum lengths of 98 cm for females and 60 cm for males (Recasens et al., 1998; Jadaud et al., 2006), we considered model 1 as the most reliable.
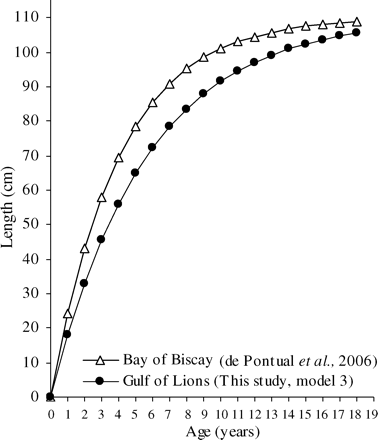
To compare hake growth between the Gulf of Lions and the Bay of Biscay, we fitted a model to sex-combined data, fixing L∞ at 110 cm (de Pontual et al., 2006). The Atlantic value of L∞ for combined sexes was higher than those chosen separately for females (L∞ = 100.7 cm) and males (L∞ = 72.8 cm) in our model 2. However, the only way to compare k in both areas was to retain this large value of L∞ from the Atlantic. In the third model (model 3), k in the Gulf of Lions was estimated at 0.178 ± 0.005 year−1, a value lower than that obtained by de Pontual et al. (2006) in the Bay of Biscay (0.25 ± 0.026 year−1; Table 4, Figure 8).
Comparison of von Bertalanffy growth models in the Bay of Biscay (L∞ = 110; k = 0.25 year−1; n = 15) and the Gulf of Lions (L∞ = 110; k = 0.178 year−1; n = 200) fitted from recapture data for both sexes combined.
Growth parameters for hake in the Gulf of Lions and Bay of Biscay (F, female; M, male; U, undetermined sex; LFA, length frequency analysis; T, tagging; s.e., standard error).
| Sex . | L∞ . | k ± s.e. . | Method . | References . |
|---|---|---|---|---|
| F | 100.7 | 0.124 | LFA | Aldebert and Recasens (1995) |
| F | 100.7 | 0.236 ± 0.007 | T | This study, Model 1 |
| M | 72.8 | 0.149 | LFA | Aldebert and Recasens (1995) |
| M | 72.8 | 0.233 ± 0.011 | T | This study, Model 1 |
| F + M + U | 110 | 0.250 ± 0.026 | T | de Pontual et al. (2006) |
| F + M + U | 110 | 0.178 ± 0.005 | T | This study, Model 3 |
| Sex . | L∞ . | k ± s.e. . | Method . | References . |
|---|---|---|---|---|
| F | 100.7 | 0.124 | LFA | Aldebert and Recasens (1995) |
| F | 100.7 | 0.236 ± 0.007 | T | This study, Model 1 |
| M | 72.8 | 0.149 | LFA | Aldebert and Recasens (1995) |
| M | 72.8 | 0.233 ± 0.011 | T | This study, Model 1 |
| F + M + U | 110 | 0.250 ± 0.026 | T | de Pontual et al. (2006) |
| F + M + U | 110 | 0.178 ± 0.005 | T | This study, Model 3 |
Growth parameters for hake in the Gulf of Lions and Bay of Biscay (F, female; M, male; U, undetermined sex; LFA, length frequency analysis; T, tagging; s.e., standard error).
| Sex . | L∞ . | k ± s.e. . | Method . | References . |
|---|---|---|---|---|
| F | 100.7 | 0.124 | LFA | Aldebert and Recasens (1995) |
| F | 100.7 | 0.236 ± 0.007 | T | This study, Model 1 |
| M | 72.8 | 0.149 | LFA | Aldebert and Recasens (1995) |
| M | 72.8 | 0.233 ± 0.011 | T | This study, Model 1 |
| F + M + U | 110 | 0.250 ± 0.026 | T | de Pontual et al. (2006) |
| F + M + U | 110 | 0.178 ± 0.005 | T | This study, Model 3 |
| Sex . | L∞ . | k ± s.e. . | Method . | References . |
|---|---|---|---|---|
| F | 100.7 | 0.124 | LFA | Aldebert and Recasens (1995) |
| F | 100.7 | 0.236 ± 0.007 | T | This study, Model 1 |
| M | 72.8 | 0.149 | LFA | Aldebert and Recasens (1995) |
| M | 72.8 | 0.233 ± 0.011 | T | This study, Model 1 |
| F + M + U | 110 | 0.250 ± 0.026 | T | de Pontual et al. (2006) |
| F + M + U | 110 | 0.178 ± 0.005 | T | This study, Model 3 |
Discussion
Our study has provided a validated growth model for Mediterranean hake, based on tagging data from juveniles and adults of a maximum recapture size 57 cm TL for females and 39 cm TL for males. In von Bertalanffy growth modelling, high values of k obtained regardless of the fitted model indicate that hake are fast-growing. In model 1, the k value is twice that reported in an earlier study on hake from the same area using different methodologies but the same L∞ (Aldebert and Recasens, 1995). Interestingly, the Aldebert and Recasens (1995) estimate of L∞ is within our L∞CIs estimated in model 2, indicating some consistency in estimation. These relatively broad values of CI can be explained by an absence of older hake in the sample. Moreover, our k estimates are close to those obtained in southern areas using other methodologies, in the Balearic Sea (Alemany and Oliver, 1995), in Santa Pola Bay (Garcia-Rodriguez and Esteban, 1996, 1998), and in the Gulf of Alicante (Garcia-Rodriguez and Esteban, 2002; Table 5). In all other Mediterranean areas, values of k reported are ∼0.1 year−1 (Fiorentino et al., 2000), half our estimate made from recoveries.
Growth parameters for hake in the Mediterranean (F, female; M, male).
| Sex . | L∞ (cm) . | k (year−1) . | Programme . | Area . | Source . |
|---|---|---|---|---|---|
| F | 105 | 0.20 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 99.7 | 0.153 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 102–116 | 0.22–0.17 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
| F | 126.9 ± 45.7 | 0.184 ± 0.094 | Fishparm | Balearic Sea | Alemany and Oliver (1995) |
| M | 90 | 0.19 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 73.3 | 0.172 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 86–94 | 0.23–0.27 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
| Sex . | L∞ (cm) . | k (year−1) . | Programme . | Area . | Source . |
|---|---|---|---|---|---|
| F | 105 | 0.20 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 99.7 | 0.153 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 102–116 | 0.22–0.17 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
| F | 126.9 ± 45.7 | 0.184 ± 0.094 | Fishparm | Balearic Sea | Alemany and Oliver (1995) |
| M | 90 | 0.19 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 73.3 | 0.172 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 86–94 | 0.23–0.27 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
Growth parameters for hake in the Mediterranean (F, female; M, male).
| Sex . | L∞ (cm) . | k (year−1) . | Programme . | Area . | Source . |
|---|---|---|---|---|---|
| F | 105 | 0.20 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 99.7 | 0.153 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 102–116 | 0.22–0.17 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
| F | 126.9 ± 45.7 | 0.184 ± 0.094 | Fishparm | Balearic Sea | Alemany and Oliver (1995) |
| M | 90 | 0.19 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 73.3 | 0.172 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 86–94 | 0.23–0.27 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
| Sex . | L∞ (cm) . | k (year−1) . | Programme . | Area . | Source . |
|---|---|---|---|---|---|
| F | 105 | 0.20 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 99.7 | 0.153 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| F | 102–116 | 0.22–0.17 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
| F | 126.9 ± 45.7 | 0.184 ± 0.094 | Fishparm | Balearic Sea | Alemany and Oliver (1995) |
| M | 90 | 0.19 | Elefan | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 73.3 | 0.172 | Fishparm | Santa Paola Bay (Spain) | Garcia-Rodriguez and Esteban (1996, 1998) |
| M | 86–94 | 0.23–0.27 | Elefan | Gulf of Alicante | Garcia-Rodriguez and Esteban (2002) |
Length at the end of the first year of life in the Gulf of Lions was estimated at 15.1 cm for males and 21.2 cm for females (model 1, Figure 7, Table 6), similar in terms of growth rate to that found for females in the Balearic Sea (20.6 cm) by Alemany and Oliver (1995). These values are higher than previous estimates (13.6 cm for males and 15.5 cm for females) for the Gulf of Lions (Aldebert and Recasens, 1995), but consistent with the estimate of Morales-Nin and Aldebert (1997) in the same area (16 cm, sexes combined). However, given the variability in growth rates estimated from recoveries, we did not observe any statistical difference in growth rate between sexes for fish of length <20 cm (Figure 6), i.e. juveniles. Further, our results agree with length estimates based on otolith microstructure analysis of juveniles in other Mediterranean areas, but differ from those from the Atlantic. In the Catalan Sea, seasonal growth rates reported by Morales-Nin and Moranta (2004) yielded an approximated length of 20 cm at the end of the first year. Length at age 1 was estimated to be 16 cm in the central Adriatic (Arneri and Morales-Nin, 2000), 17 cm in the Aegean Sea (Uçkun et al., 2000), and 18 cm in the Tyrrhenian Sea (Belcari et al., 2006). In the Atlantic, reported length-at-age 1 is ∼24 cm (Kacher and Amara, 2005; de Pontual et al., 2006) and 25.3 cm (Piñeiro et al., 2008), values close to that (23.6 cm) reported by Garcia-Rodriguez and Esteban (2002) for the Gulf of Alicante in the Mediterranean, a value based on length frequency analysis.
Estimated length for ages 1–5 from the von Bertalanffy function for females, males, and sexes combined.
| . | Gulf of Lions . | Bay of Biscay . | Gulf of Lions . | |||||
|---|---|---|---|---|---|---|---|---|
| Females . | Males . | Sexes combined . | ||||||
| (1) . | (2) . | (2) . | (1) . | (2) . | (2) . | (3) . | (2) . | |
| . | Model 1 . | Model 2 . | . | Model 1 . | Model 2 . | . | Model 3 . | |
| L∞ | 100.7 | 100.7 | 114.6 | 72.8 | 72.8 | 70.0 | 110 | 110 |
| k | 0.124 | 0.236 | 0.197 | 0.149 | 0.239 | 0.255 | 0.25 | 0.183 |
| Age 1 | 15.5 | 21.2 | 20.5 | 13.6 | 15.1 | 14.5 | 24.3 | 17.9 |
| Age 2 | 25.5 | 37.9 | 37.3 | 21.8 | 27.1 | 26.5 | 43.3 | 32.9 |
| Age 3 | 34.2 | 51.1 | 51.1 | 28.8 | 36.6 | 36.3 | 58.0 | 45.5 |
| Age 4 | 42.0 | 61.5 | 62.5 | 34.9 | 44.1 | 44.5 | 69.5 | 56.0 |
| Age 5 | 48.8 | 69.8 | 71.8 | 40.2 | 50.1 | 51.2 | 78.5 | 64.8 |
| . | Gulf of Lions . | Bay of Biscay . | Gulf of Lions . | |||||
|---|---|---|---|---|---|---|---|---|
| Females . | Males . | Sexes combined . | ||||||
| (1) . | (2) . | (2) . | (1) . | (2) . | (2) . | (3) . | (2) . | |
| . | Model 1 . | Model 2 . | . | Model 1 . | Model 2 . | . | Model 3 . | |
| L∞ | 100.7 | 100.7 | 114.6 | 72.8 | 72.8 | 70.0 | 110 | 110 |
| k | 0.124 | 0.236 | 0.197 | 0.149 | 0.239 | 0.255 | 0.25 | 0.183 |
| Age 1 | 15.5 | 21.2 | 20.5 | 13.6 | 15.1 | 14.5 | 24.3 | 17.9 |
| Age 2 | 25.5 | 37.9 | 37.3 | 21.8 | 27.1 | 26.5 | 43.3 | 32.9 |
| Age 3 | 34.2 | 51.1 | 51.1 | 28.8 | 36.6 | 36.3 | 58.0 | 45.5 |
| Age 4 | 42.0 | 61.5 | 62.5 | 34.9 | 44.1 | 44.5 | 69.5 | 56.0 |
| Age 5 | 48.8 | 69.8 | 71.8 | 40.2 | 50.1 | 51.2 | 78.5 | 64.8 |
(1) Aldebert and Recasens (1995), (2) this study (models 1–3), and (3) de Pontual et al. (2006).
Estimated length for ages 1–5 from the von Bertalanffy function for females, males, and sexes combined.
| . | Gulf of Lions . | Bay of Biscay . | Gulf of Lions . | |||||
|---|---|---|---|---|---|---|---|---|
| Females . | Males . | Sexes combined . | ||||||
| (1) . | (2) . | (2) . | (1) . | (2) . | (2) . | (3) . | (2) . | |
| . | Model 1 . | Model 2 . | . | Model 1 . | Model 2 . | . | Model 3 . | |
| L∞ | 100.7 | 100.7 | 114.6 | 72.8 | 72.8 | 70.0 | 110 | 110 |
| k | 0.124 | 0.236 | 0.197 | 0.149 | 0.239 | 0.255 | 0.25 | 0.183 |
| Age 1 | 15.5 | 21.2 | 20.5 | 13.6 | 15.1 | 14.5 | 24.3 | 17.9 |
| Age 2 | 25.5 | 37.9 | 37.3 | 21.8 | 27.1 | 26.5 | 43.3 | 32.9 |
| Age 3 | 34.2 | 51.1 | 51.1 | 28.8 | 36.6 | 36.3 | 58.0 | 45.5 |
| Age 4 | 42.0 | 61.5 | 62.5 | 34.9 | 44.1 | 44.5 | 69.5 | 56.0 |
| Age 5 | 48.8 | 69.8 | 71.8 | 40.2 | 50.1 | 51.2 | 78.5 | 64.8 |
| . | Gulf of Lions . | Bay of Biscay . | Gulf of Lions . | |||||
|---|---|---|---|---|---|---|---|---|
| Females . | Males . | Sexes combined . | ||||||
| (1) . | (2) . | (2) . | (1) . | (2) . | (2) . | (3) . | (2) . | |
| . | Model 1 . | Model 2 . | . | Model 1 . | Model 2 . | . | Model 3 . | |
| L∞ | 100.7 | 100.7 | 114.6 | 72.8 | 72.8 | 70.0 | 110 | 110 |
| k | 0.124 | 0.236 | 0.197 | 0.149 | 0.239 | 0.255 | 0.25 | 0.183 |
| Age 1 | 15.5 | 21.2 | 20.5 | 13.6 | 15.1 | 14.5 | 24.3 | 17.9 |
| Age 2 | 25.5 | 37.9 | 37.3 | 21.8 | 27.1 | 26.5 | 43.3 | 32.9 |
| Age 3 | 34.2 | 51.1 | 51.1 | 28.8 | 36.6 | 36.3 | 58.0 | 45.5 |
| Age 4 | 42.0 | 61.5 | 62.5 | 34.9 | 44.1 | 44.5 | 69.5 | 56.0 |
| Age 5 | 48.8 | 69.8 | 71.8 | 40.2 | 50.1 | 51.2 | 78.5 | 64.8 |
(1) Aldebert and Recasens (1995), (2) this study (models 1–3), and (3) de Pontual et al. (2006).
We found evidence for different growth patterns between sexes in fish >20 cm TL at the beginning of the second year of life, with lower growth rates in males than females in the size range 20–30 cm. This is consistent with sexual dimorphism for size-at-first-maturity, L50, i.e. 28 and 38 cm for males and females, respectively, in the Gulf of Lions (Recasens et al., 1998). For both sexes, decreasing growth rates coincide with the onset of sexual maturity, which is explained by the portion of the metabolic rate devoted to reproduction rather than somatic growth (West et al., 2001). The mean growth rate of males of 25–30 cm TL is similar to that of females of 30–45 cm TL. Such sexual dimorphism for growth in the second year of life has already been documented by Garcia-Rodriguez and Esteban (1996) for Santa Paola Bay, by Garcia-Rodriguez and Esteban (2002) for the Gulf of Alicante, and by Colloca et al. (2003) for the central Tyrrhenian Sea. At age 2, total lengths are 37.9 cm for females and 27.1 cm for males (model 1, Figure 7); such a female size would correspond to previous estimates for age 3 in the Gulf of Lions (Aldebert and Recasens, 1995; Recasens et al., 1998) or even age 4 or 5 in other Mediterranean areas (Andaloro et al., 1985; Biagi et al., 1998; Tursi et al., 1998; Colloca et al., 2003).
In the Bay of Biscay, growth rates estimated from an ensemble of recoveries were 0.039 ± 0.005 cm d−1 (n = 8) for females, 0.029 ± 0.006 cm d−1 (n = 7) for males, and 0.038 ± 0.004 cm d−1 (n = 20) for both sexes combined (de Pontual et al., 2006). Such data suggest that fish tagged in the Gulf of Lions grew at the same rate as those tagged in the Bay of Biscay. However, when only fish that spent a significant time at liberty (>1 year) were considered, tagged fish grew more slowly in the Gulf of Lions (sexes combined, 0.038 ± 0.004 cm d−1, n = 13) than those in the Bay of Biscay (sexes combined, 0.054 ± 0.004 cm d−1, n = 6). A comparable result was obtained off Northwest Iberia (sexes combined, 0.052 ± 0.003 cm d−1) despite the fewer (n = 2) recoveries (Piñeiro et al., 2007). Moreover, k values estimated with a common and fixed L∞ (model 3) are lower in the Gulf of Lions (k = 0.178 ± 0.005 year−1) than in the Bay of Biscay (k = 0.25 ± 0.026 year−1; de Pontual et al., 2006). This result suggests that European hake grow faster in the Atlantic than in the Mediterranean. In both areas, males mature smaller than females, although the values of L50 differ: 37.8 cm for males and 48.8 cm for females in the Bay of Biscay (Lucio et al., 2000). This led to estimates of age-at-first-maturity in this area of 1+ and 2+ for males and females, respectively (de Pontual et al., 2006). Faster growth in the Bay of Biscay could be explained by genetic factors, because the Atlantic and Mediterranean host separate stocks of hake (Cimmaruta et al., 2005), and/or by the effects of environmental factors such as temperature and food availability. The temperature in the Gulf of Lions rarely falls below 13°C, whereas in the Bay of Biscay it can decrease to a minimum range of 8.0–9.5°C (Puillat et al., 2004). A relatively stable thermocline appears in the Gulf of Lions in summer and autumn, and the water column homogenizes in winter. Water temperature over the thermocline may reach 20–25°C and is constant below it, ∼13–13.5°C (Lefevre et al., 1997). Thermal stratification is also observed in summer and autumn in the Bay of Biscay, where the temperature is cooler on average than in the Mediterranean Sea. In areas of cooler bottom temperature (8–10°C), silver hake (Merluccius bilinearis) is not only larger and more abundant, but also grows faster (Steves and Cowen, 2000). However, a recent experiment on captive hake in controlled conditions has suggested that the species could be more eurythermal than previously thought (pers. obs.). Trophic conditions might well be another driving factor for growth. This hypothesis is supported by the greater productivity of Atlantic (Laborde et al., 1999) than Mediterranean waters (Lefevre et al., 1997), and in areas such as Namibia, the high productivity of the waters may explain the fast growth of shallow-water Cape hake (Merluccius capensis; Gordoa et al., 2001).
Comparing our results with those published for different areas is problematic because of the different methodological approaches used (length frequency analysis, otolith macrostructure, tagging, etc.), the different sampling strategies (Orsi-Relini et al., 1991), the uncertainties involved in the otolith age-estimation method, which is both complex and non-validated (Morales-Nin and Aldebert, 1997; Morales-Nin et al., 1998; de Pontual et al., 2006), and confounding factors influencing growth (sex, season, area, etc.). In most studies, although the sexes were identified, lengths were generally pooled and a mean growth rate was given over different length ranges. When we compared growth rates in the Gulf of Lions with those in the Bay of Biscay based on pooled data from recaptured fish, mean growth appeared similar. However, when we separated fish that had spent more or less than 1 year at liberty, there was a significant difference between the two areas. This clearly highlights the need for further work based on validated methodologies and reliable data.
Hake are the demersal fish most sought by trawlers, gillnetters, and longliners in the Mediterranean, and the stocks are assessed regularly by the General Fisheries Commission for the Mediterranean (GFCM). From de Pontual et al. (2006) and the present study, hake are assumed to grow faster than previously estimated. Growth underestimation has clearly been demonstrated, through marked otolith analysis, to result from age-overestimation attributable to biased age estimation (de Pontual et al., 2006). This clearly places in question the reliability of current estimates of stock demographic structure, and a shift towards higher relative frequencies of youngest age groups is expected. Such a bias would most probably impact the stock assessment results of the GFCM (Jadaud et al., 2006). As shown by Bertignac and de Pontual (2007), bias in age estimation strongly impacts absolute levels of fishing mortality (F) and spawning-stock biomass (SSB). In terms of temporal trends, SSB is also affected, whereas F and recruitment are broadly similar to previous estimates. In the Mediterranean Sea, the bulk of hake trawl catches (the most commonly used fishing method) is assumed to be made up of age groups 0 and 1 but, according to the fast-growth hypothesis presented here, would be made up by the recruits of the year. Such a growth rate also produces a shift in the age-at-first-maturity towards younger ages in both sexes, more precisely at 2 instead of 3 or 4 years, as reported previously. As shown by Bertignac and de Pontual (2007), this would result in greater reactivity of the population to both environmental change and new management measures.
In conclusion, our results strongly support the fast-growth hypothesis proposed by Alemany and Oliver (1995) and Garcia-Rodriguez and Esteban (1996, 1998, 2002) in the Mediterranean. Growth depends on sex, and male and female growth rates clearly differ in the Gulf of Lions from the second year of life, when the fish first mature. Despite growth differences between the Bay of Biscay and the Gulf of Lions, hake would mature at about the same age (2 years) in both areas. Hake growth in the Gulf of Lions is some twice as fast as previously published, but slower than in the Bay of Biscay. Combined factors, including temperature, greater production in the area, and genetics, may contribute to explaining the higher growth rate observed in the Atlantic. The maximum length of the hake we recovered in the Gulf of Lions may have introduced some bias against older ages because von Bertalanffy modelling provides an accurate description of growth over the range of lengths to which it has been fitted (Kirkwood, 1983). Such a shortcoming could be solved by tagging large hake. Inconsistency between estimated growth rates, brought to light by other authors (Aldebert, 1993; Fiorentino et al., 2000), strengthens the argument for further research to understand better both hake biology and its complex otolith pattern.
Acknowledgements
We thank all those who participated in the tagging experiment (Ysabelle Cheret, Carole Leite, Hervé Barone, Cyril Przybyla, Patrick Le Gall, Alain Guillou, Aurélie Dessin, and Thibault Geoffroy), and the fishers who returned the tagged fish. We also thank our colleague Serge Mortreux for developing the gear we used, the crew of the RV “L'Europe” for their valued assistance during the MERVIV survey, and Yvonne Aldebert for her frequent encouragement and advice on hake growth. Comments by García Rodríguez, an anonymous reviewer, and the editor were also helpful in improving the manuscript. Finally, we thank Helen McCombie for her review of the English text. The study was funded by the Ifremer–MEDICIS programme.