-
PDF
- Split View
-
Views
-
Cite
Cite
Nicola J. Downey, Michael J. Roberts, Dan Baird, An investigation of the spawning behaviour of the chokka squid Loligo reynaudii and the potential effects of temperature using acoustic telemetry, ICES Journal of Marine Science, Volume 67, Issue 2, March 2010, Pages 231–243, https://doi.org/10.1093/icesjms/fsp237
Close - Share Icon Share
Abstract
Spawning aggregations of chokka squid are influenced by environmental conditions. Acoustic telemetry was used to monitor the behaviour of spawning squid in relation to environmental variability. During the November squid-fishery closed seasons of 2003–2006, hexagonal VR2 receiver arrays were moored on and around active spawning aggregations in Kromme Bay, South Africa. In all, 45 squid were tagged (V9P-6L-S256 transmitters) and released within these arrays. Three presence–absence behaviours were identified: (i) arrival on the spawning site at dawn and departure after dusk, (ii) continuous presence for a number of days, and (iii) presence interrupted by frequent but short periods of absence. Movement between spawning sites was both diurnal and nocturnal. Squid presence at the monitored sites increased after dawn and decreased towards and after dusk. Occasionally, a core aggregation of squid remained on the spawning sites at night. Temperature data at the sites indicated occasional upwelling, and although the role of temperature in the spawning process is not well understood, data suggest that it is linked to the continuation and or interruption of spawning after an aggregation has formed. The initial formation of spawning aggregations appears to be triggered by upwelling events.Downey, N. J., Roberts, M. J., and Baird, D. 2010. An investigation of the spawning behaviour of the chokka squid Loligo reynaudii and the potential effects of temperature using acoustic telemetry. – ICES Journal of Marine Science, 67: 231–243.
Introduction
The main spawning grounds of the chokka squid, Loligo reynaudii, are on the south coast of South Africa between Plettenberg Bay and Port Alfred (Figure 1). The region is characterized by an exposed coast interspersed with log-spiral-shaped embayments, associated with headlands or capes (Birch, 1981). Winds with a strong easterly component, most frequent in summer and autumn, cause upwelling on the western side of capes (Schumann et al., 1982), resulting in a lowering of sea temperature from ∼21 to ∼9°C during intense events. The region also experiences frequent bottom-turbidity events which can last for several days (Dorfler, 2002). Clearly, chokka squid spawn in a highly dynamic environment.
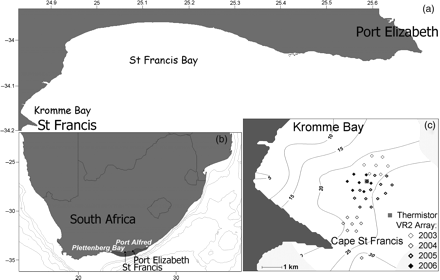
Maps of (a) the study site, Kromme Bay, (b) the main spawning grounds (shaded area) between Plettenberg Bay and Port Alfred, and (c) the positions of the hexagonal VR2 receiver arrays for the years 2003–2006 and the thermistor array overlaid on the bathymetry (contour lines). Latitude is °S, longitude is °E.
Spawning takes place, though sporadically (Augustyn, 1990), throughout the year, and the South African jig fishery, which operates from the ports of St Francis and Port Elizabeth (Figure 1), specifically targets spawning aggregations. Catches are therefore determined to a large extent by the successful formation, the size, and the number of these aggregations.
Commercial catch data on chokka squid reveal fluctuating annual harvests between 2500 and 10 000 t. Such variability in annual catch combined with its unpredictable nature has great financial and socio-economic consequences. During the periods of prolonged good catches, it is tempting, and sometimes necessary, for those involved in the industry to build capacity, but doing so increases the risk factor during periods of poor catches.
A second and far-reaching social-economic consequence of downturns in the squid catch is the suffering of the ∼6600 fishers and their families, leading to an escalation in crime rates in the communities and the surrounding areas during such periods. The unreliable nature of squid catches and the need to manage and forecast squid stocks have been the two major driving forces behind squid research in South Africa for the past 30 years.
Augustyn (1989) was the first to suggest that sea temperature was an important factor influencing the abundance of squid inshore and hence their availability to the jig fishery. He suggested that the favoured temperature range of spawning L. reynaudii is between 14 and 21.5°C and that the distribution of squid inshore is influenced by both temperature and wind. Sauer et al. (1992) thought that this temperature range also governed the geographical extent of the inshore spawning grounds, as outlined in Augustyn (1989). Earlier, Sauer et al. (1991) correlated commercial squid catches and sea temperature and suggested that wind-induced upwelling enhanced daily catches. However, catches decreased outside the temperature range of 11–21°C. The belief then was that the formation of inshore spawning aggregations primarily depended on environmental cues and that it was important to consider other independent variables such as currents, turbidity, oxygen, and swell height. Roberts and Sauer (1994) confirmed the fact that upwelling positively influenced squid catches, but suggested that bottom turbidity was also an important variable influencing spawning aggregations, because it decreases visibility.
Further investigation into the effect of the environment on spawning aggregations was conducted by Roberts (1998). He considered wave height, turbidity, and sea temperature to be the key parameters controlling and determining spawning success. The formation of a spawning aggregation coincidently with an upwelling event led to a conclusion that upwelling may trigger spawning. Schön (2000), using a portable CTD deployed from a commercial fishing vessel, found a positive relationship between wind-induced upwelling and squid catches. The correlation between temperature and catch was generally negative, with larger catches associated with a temperature range of 13–18°C. This statistical approach showed squid catches to be best during strong easterly winds (causing upwelling), sea surface temperatures in the range of 15.0–16.9°C, and zero turbidity. Moreover, upwelling was shown to trigger spawning, whereas high turbidity resulted in the termination of spawning. Dorfler (2002), using a number of case studies, investigated in great detail the effect of turbidity on squid catches. Although the results showed catches decreasing with increased turbidity, the study concluded that it was more likely that an interaction between turbidity and temperature was influencing commercial catches of squid. When temperatures were stable between 14 and 16°C, high turbidity negatively impacted catches, whereas temperatures <10 and >20°C negatively impacted catches, irrespective of the turbidity.
An important characteristic of the research undertaken to date is that it used indirect methods to explain the role of the environment on spawning and hence the catches. This study took a more direct approach, using acoustic telemetry to monitor the individual behaviour of spawning squid in relation to environmental variability.
Material and methods
Four acoustic-telemetry experiments were performed in Kromme Bay (St Francis Bay) during the November 2003–2006 closed seasons for the squid fishery (Figure 1c). Kromme Bay forms part of the main spawning grounds, and there are various levels of fishing activity in the area throughout the year. The closed fishing season was chosen to avoid potential impacts of boat anchors on instrumentation and intense commercial fishing on the spawning process.
All acoustic-telemetry equipment was purchased from Vemco Ltd, Canada. VR2 receivers were deployed in a hexagonal array (Figure 1c), allowing for an area of up to 1.28 km2 to be monitored, depending on the thermal conditions of the water column (unpublished data). Each receiver was deployed 5 m above the seabed using a hollow-core polypropylene rope tensioned with a subsurface buoy. The mooring was anchored to the seabed with a 50-kg weight. In 2004, an additional VR2 receiver was deployed on a spawning site off Cape St Francis (Figure 1c).
Tagging
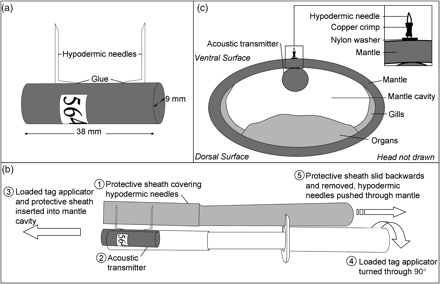
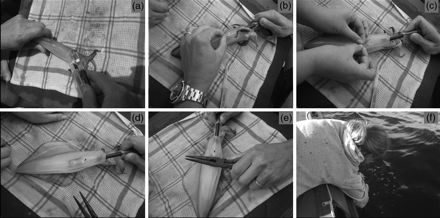
Male and female squid were tagged with V9 acoustic transmitters (Figure 2a). To allow for the attachment of transmitters to the squid, two 18-gauge hypodermic needles were glued to the surface of each transmitter (Figure 2a), their length depending on the sex and the size of the animal being tagged. Hypodermic needles 17 mm long were used for large males, and needles 14 mm long for smaller “sneaker” males and females. Squid were caught on active spawning sites, within the hexagonal array of VR2 receivers, using a jig. The animals were removed from the water, their sex determined, and placed on a damp cloth (Figure 3a). The transmitter was inserted (Figure 3a) using an applicator designed specifically for use on chokka squid (Figure 2b). The applicator, with the hypodermic needles covered, was held sideways initially, then slipped into the mantle cavity (Figure 2b). The apparatus was turned through 90°, the protective applicator sheath removed (Figure 2b), and the hypodermic needles pushed through the mantle (Figure 3b). Nylon washers were then pushed onto the ends of the needles (Figures 2c and 3c) and held in place with copper crimps (Figures 2c and 3d and e). The squid were then placed in a plastic bin full of seawater alongside the boat to recover, or simply held alongside the boat if sea conditions were too rough (Figure 3f). Once the animal had resumed normal fin-beating and swimming, it was released on the capture site. When possible, scuba divers equipped with video cameras followed the animal to the seabed to ensure that it had reintegrated into the spawning aggregation.
The instrumentation used for tagging: (a) attachment of hypodermic needles to an acoustic transmitter, (b) the specially designed tag applicator used to tag L. reynaudii, and (c) the placement of the acoustic transmitter within the mantle of the squid, on the ventral side, to avoid piercing organs with the hypodermic needles.
Attaching a transmitter to a squid: (a) a transmitter is inserted beneath the mantle using a specifically designed applicator; (b) the apparatus is turned through 90°, the protective applicator sheath removed, and the hypodermic needles pushed through the mantle. (c) Nylon washers are pushed onto the ends of the hypodermic needles and (d) a metal cylinder slipped over each hypodermic needle, (e) the metal cylinders are crimped using long-nose pliers, and (f) the squid are held submerged alongside the boat until strong swimming ability is displayed (fin beating). Only then is the animal released on the capture site.
In all, 45 squid were tagged over the four experiments. The transmitters used in 2003 did not have pressure sensors, so only presence–absence data were collected. Transmitters with pressure sensors were used in 2004–2006, providing depth as well as presence–absence information. Details of the transmitters used, the total number of squid tagged, and the number of males and females tagged per acoustic-telemetry experiment, are listed in Table 1. To correct possible time-drift of individual VR2 receiver clocks, VR2 data files were time-corrected using a program created by Dale Webber of Vemco. Data were analysed for presence–absence on the spawning site, position in the water column, and diurnal behaviour patterns.
Dates, details of the transmitters used, and the number of squid tagged during acoustic-telemetry experiments in 2003–2006.
| Year . | Dates of acoustic-telemetry use . | Tag type . | Minimum off-time (s) . | Maximum off-time (s) . | Pressure sensor . | Number of squid tagged . | Males . | Females . |
|---|---|---|---|---|---|---|---|---|
| 2003 | 12–22 November | V8SC-2H-R256 | 10 | 35 | No | 4 | 2 | 2 |
| 2004 | 07–21 November | V9P-6L-S256 | 30 | 90 | Yes | 12 | 6 | 6 |
| 2005 | 14–21 November | V9P-6L-S256 | 20 | 69 | Yes | 23 | 13 | 10 |
| 30 | 90 | |||||||
| 2006 | 16–21 November | V9P-6L-S256 | 30 | 90 | Yes | 6 | 4 | 2 |
| Year . | Dates of acoustic-telemetry use . | Tag type . | Minimum off-time (s) . | Maximum off-time (s) . | Pressure sensor . | Number of squid tagged . | Males . | Females . |
|---|---|---|---|---|---|---|---|---|
| 2003 | 12–22 November | V8SC-2H-R256 | 10 | 35 | No | 4 | 2 | 2 |
| 2004 | 07–21 November | V9P-6L-S256 | 30 | 90 | Yes | 12 | 6 | 6 |
| 2005 | 14–21 November | V9P-6L-S256 | 20 | 69 | Yes | 23 | 13 | 10 |
| 30 | 90 | |||||||
| 2006 | 16–21 November | V9P-6L-S256 | 30 | 90 | Yes | 6 | 4 | 2 |
Dates, details of the transmitters used, and the number of squid tagged during acoustic-telemetry experiments in 2003–2006.
| Year . | Dates of acoustic-telemetry use . | Tag type . | Minimum off-time (s) . | Maximum off-time (s) . | Pressure sensor . | Number of squid tagged . | Males . | Females . |
|---|---|---|---|---|---|---|---|---|
| 2003 | 12–22 November | V8SC-2H-R256 | 10 | 35 | No | 4 | 2 | 2 |
| 2004 | 07–21 November | V9P-6L-S256 | 30 | 90 | Yes | 12 | 6 | 6 |
| 2005 | 14–21 November | V9P-6L-S256 | 20 | 69 | Yes | 23 | 13 | 10 |
| 30 | 90 | |||||||
| 2006 | 16–21 November | V9P-6L-S256 | 30 | 90 | Yes | 6 | 4 | 2 |
| Year . | Dates of acoustic-telemetry use . | Tag type . | Minimum off-time (s) . | Maximum off-time (s) . | Pressure sensor . | Number of squid tagged . | Males . | Females . |
|---|---|---|---|---|---|---|---|---|
| 2003 | 12–22 November | V8SC-2H-R256 | 10 | 35 | No | 4 | 2 | 2 |
| 2004 | 07–21 November | V9P-6L-S256 | 30 | 90 | Yes | 12 | 6 | 6 |
| 2005 | 14–21 November | V9P-6L-S256 | 20 | 69 | Yes | 23 | 13 | 10 |
| 30 | 90 | |||||||
| 2006 | 16–21 November | V9P-6L-S256 | 30 | 90 | Yes | 6 | 4 | 2 |
Environmental data
Temperature data were collected using an array of Star-oddi Starmon mini underwater temperature recorders deployed at depths of 9, 14, 18, 21, and 24 m. The thermistor array recorded temperature hourly. When sea conditions permitted, daily temperature, turbidity, and salinity profiles were obtained using a portable CTD (Seabird 19 Plus). In 2003, divers determined daily visibility. In 2005, an OBS-3A turbidity sensor was deployed 83 cm from the seabed, in the centre of the VR2 receiver array. The OBS-3A recorded turbidity levels hourly. No turbidity data were collected in 2006 because of equipment failure. Hourly wind data recorded at Port Elizabeth airport were obtained from the South African Weather Services for 2003–2006. The data were filtered using an UNH Lanczos filter (weighted 73), and stick-vector plots generated. Wind direction in these plots is orientated away from the axis.
Results
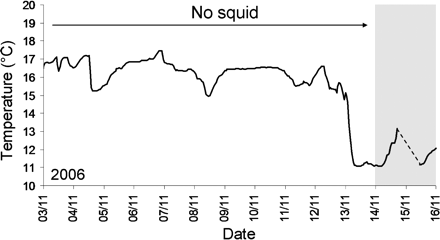
Well-established spawning aggregations were monitored in 2003–2005, but the date and environmental conditions at formation were unknown. In 2006, no squid were found within Kromme Bay until the formation of an aggregation around the 14/15 November (discovered 15 November). Interestingly, that aggregation formed after the bottom temperature dropped from 16.5 to 11.5°C (Figure 4).
Temperature conditions before and during the formation of a spawning aggregation (shaded block) in 2006. The dashed line indicates a period during which no temperature data were recorded. The aggregations studied in 2003–2005 were already well-established upon discovery.
Squid identification codes for all four experiments, tagging details, and a summary of the data collected for each squid are listed in Table 2. To provide a measure of spawning intensity, the number of hours each squid was present on the spawning site is expressed as a percentage of the total number of hours of passive tracking. Day has been taken as 05:01–19:00 and night as 19:01–05:00.
Squid ID codes, date of tagging, date of last detection, hours spent at the spawning site, percentage of time spent at the spawning site relative to the total number of tracking days, and the total number of detections recorded for the 45 squid tagged in 2003–2006.
| . | . | . | . | Time spent at spawning site (h) . | Time spent at spawning site as a % of total passive tracking time . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Year and squid code . | Sex . | Date tagged . | Date of last detection . | Day . | Night . | Total . | % time . | Tracking days . | Total number of detections recorded . |
| 2003 | |||||||||
| M1 | Male | 12 November | 15 November | 43 | 30 | 73 | 30 | 10 | 3 899 |
| M2 | Male | 12 November | 15 November | 30 | 17 | 47 | 20 | 10 | 1 921 |
| F1 | Female | 15 November | 22 November | 26 | 24 | 50 | 30 | 7 | 327 |
| F2 | Female | 15 November | 21 November | 2 | 3 | 5 | 3 | 7 | 33 |
| Mean ± s.d. | 25.3 ± 17.1 | 18.5 ± 11.6 | 43.8 ± 28.3 | 21 ± 13 | 1 545 ± 1 775 | ||||
| 2004 | |||||||||
| SM3 | Sneaker male | 07 November | 08 November | 3 | 10 | 13 | 4 | 14 | 40 |
| M4 | Male | 07 November | 11 November | 7 | 7 | 14 | 4 | 14 | 91 |
| M5 | Male | 07 November | 08 November | 13 | 2 | 15 | 4 | 14 | 294 |
| M6 | Male | 08 November | 20 November | 38 | 4 | 42 | 13 | 13 | 721 |
| M7 | Male | 08 November | 09 November | 8 | 2 | 10 | 3 | 13 | 185 |
| M8 | Male | 08 November | 08 November | 2 | 0 | 2 | 1 | 13 | 29 |
| F3 | Female | 07 November | 12 November | 31 | 15 | 46 | 14 | 14 | 814 |
| F4 | Female | 07 November | 10 November | 37 | 12 | 49 | 15 | 14 | 1 167 |
| F5 | Female | 09 November | 09 November | 3 | 0 | 3 | 1 | 12 | 71 |
| F6 | Female | 09 November | 09 November | 5 | 5 | 10 | 3 | 12 | 109 |
| F7 | Female | 09 November | 11 November | 31 | 22 | 53 | 18 | 12 | 1 412 |
| F8 | Female | 09 November | 09 November | 2 | 0 | 2 | 1 | 12 | 31 |
| Mean ± s.d. | 15 ± 14.7 | 6.6 ± 6.9 | 21.6 ± 19.8 | 7 ± 6 | 413.7 ± 489.3 | ||||
| 2005 | |||||||||
| M9 | Male | 14 November | 21 November | 101 | 44 | 145 | 86 | 7 | 3 668 |
| M10 | Male | 14 November | 21 November | 96 | 43 | 139 | 83 | 7 | 2 918 |
| M11 | Male | 14 November | 21 November | 71 | 10 | 81 | 48 | 7 | 1 646 |
| M12 | Male | 15 November | 21 November | 95 | 50 | 145 | 86 | 6 | 3 453 |
| M13 | Male | 16 November | 21 November | 14 | 0 | 14 | 12 | 5 | 165 |
| M14 | Male | 16 November | 21 November | 39 | 14 | 53 | 44 | 5 | 640 |
| M15 | Male | 17 November | 21 November | 58 | 15 | 73 | 76 | 4 | 1 298 (511-depth) |
| M16 | Male | 17 November | 21 November | 61 | 23 | 84 | 88 | 4 | 1 613 |
| M17 | Male | 19 November | 21 November | 21 | 3 | 24 | 50 | 2 | 579 (158-depth) |
| M18 | Male | 19 November | 21 November | 23 | 7 | 30 | 63 | 2 | 345 |
| M19 | Male | 19 November | 21 November | 15 | 2 | 17 | 35 | 2 | 156 |
| M20 | Male | 19 November | 21 November | 36 | 20 | 56 | 78 | 2 | 745 |
| SM21 | Sneaker male | 14 November | 18 November | 32 | 33 | 65 | 39 | 7 | 1 877 |
| F9 | Female | 16 November | 16 November | 2 | 0 | 2 | 2 | 5 | 50 |
| F10 | Female | 14 November | 21 November | 47 | 40 | 87 | 52 | 7 | 982 |
| F11 | Female | 14 November | 21 November | 78 | 27 | 105 | 63 | 7 | 2 650 |
| F12 | Female | 15 November | 21 November | 23 | 14 | 37 | 26 | 6 | 434 |
| F13 | Female | 15 November | 19 November | 33 | 12 | 45 | 31 | 6 | 978 |
| F14 | Female | 15 November | 21 November | 72 | 39 | 111 | 77 | 6 | 2 314 |
| F15 | Female | 16 November | 17 November | 22 | 13 | 35 | 29 | 5 | 863 |
| F16 | Female | 17 November | 21 November | 63 | 9 | 72 | 75 | 4 | 1 424 |
| F17 | Female | 19 November | 21 November | 27 | 18 | 45 | 94 | 2 | 605 (175-depth) |
| F18 | Female | 19 November | 21 November | 30 | 7 | 37 | 77 | 2 | 629 (157-depth) |
| Mean ± s.d. | 46 ± 28.8 | 19.3 ± 15.3 | 65.3 ± 41.8 | 57 ± 26 | 1 305.7 ± 1 059.4 | ||||
| 2006 | |||||||||
| M22 | Male | 16 November | 18 November | 27 | 10 | 37 | 31 | 5 | 489 |
| SM23 | Sneaker male | 17 November | 20 November | 20 | 11 | 31 | 32 | 4 | 403 |
| SM24 | Sneaker male | 17 November | 21 November | 12 | 7 | 19 | 20 | 4 | 209 |
| SM25 | Sneaker male | 17 November | 21 November | 8 | 6 | 14 | 15 | 4 | 130 |
| F19 | Female | 16 November | 17 November | 5 | 4 | 9 | 8 | 5 | 54 |
| F20 | Female | 16 November | 17 November | 7 | 2 | 9 | 8 | 5 | 58 |
| Mean ± s.d. | 13.2 ± 8.6 | 6.7 ± 3.4 | 19.8 ± 11.7 | 19 ± 11 | 223.8 ± 183.2 | ||||
| 2003–2006 | |||||||||
| Mean ± s.d. | 31.5 ± 27 | 14.1 ± 13.4 | 45.7 ± 38.6 | 35 ± 30 | 944.9 ± 1 043.2 | ||||
| . | . | . | . | Time spent at spawning site (h) . | Time spent at spawning site as a % of total passive tracking time . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Year and squid code . | Sex . | Date tagged . | Date of last detection . | Day . | Night . | Total . | % time . | Tracking days . | Total number of detections recorded . |
| 2003 | |||||||||
| M1 | Male | 12 November | 15 November | 43 | 30 | 73 | 30 | 10 | 3 899 |
| M2 | Male | 12 November | 15 November | 30 | 17 | 47 | 20 | 10 | 1 921 |
| F1 | Female | 15 November | 22 November | 26 | 24 | 50 | 30 | 7 | 327 |
| F2 | Female | 15 November | 21 November | 2 | 3 | 5 | 3 | 7 | 33 |
| Mean ± s.d. | 25.3 ± 17.1 | 18.5 ± 11.6 | 43.8 ± 28.3 | 21 ± 13 | 1 545 ± 1 775 | ||||
| 2004 | |||||||||
| SM3 | Sneaker male | 07 November | 08 November | 3 | 10 | 13 | 4 | 14 | 40 |
| M4 | Male | 07 November | 11 November | 7 | 7 | 14 | 4 | 14 | 91 |
| M5 | Male | 07 November | 08 November | 13 | 2 | 15 | 4 | 14 | 294 |
| M6 | Male | 08 November | 20 November | 38 | 4 | 42 | 13 | 13 | 721 |
| M7 | Male | 08 November | 09 November | 8 | 2 | 10 | 3 | 13 | 185 |
| M8 | Male | 08 November | 08 November | 2 | 0 | 2 | 1 | 13 | 29 |
| F3 | Female | 07 November | 12 November | 31 | 15 | 46 | 14 | 14 | 814 |
| F4 | Female | 07 November | 10 November | 37 | 12 | 49 | 15 | 14 | 1 167 |
| F5 | Female | 09 November | 09 November | 3 | 0 | 3 | 1 | 12 | 71 |
| F6 | Female | 09 November | 09 November | 5 | 5 | 10 | 3 | 12 | 109 |
| F7 | Female | 09 November | 11 November | 31 | 22 | 53 | 18 | 12 | 1 412 |
| F8 | Female | 09 November | 09 November | 2 | 0 | 2 | 1 | 12 | 31 |
| Mean ± s.d. | 15 ± 14.7 | 6.6 ± 6.9 | 21.6 ± 19.8 | 7 ± 6 | 413.7 ± 489.3 | ||||
| 2005 | |||||||||
| M9 | Male | 14 November | 21 November | 101 | 44 | 145 | 86 | 7 | 3 668 |
| M10 | Male | 14 November | 21 November | 96 | 43 | 139 | 83 | 7 | 2 918 |
| M11 | Male | 14 November | 21 November | 71 | 10 | 81 | 48 | 7 | 1 646 |
| M12 | Male | 15 November | 21 November | 95 | 50 | 145 | 86 | 6 | 3 453 |
| M13 | Male | 16 November | 21 November | 14 | 0 | 14 | 12 | 5 | 165 |
| M14 | Male | 16 November | 21 November | 39 | 14 | 53 | 44 | 5 | 640 |
| M15 | Male | 17 November | 21 November | 58 | 15 | 73 | 76 | 4 | 1 298 (511-depth) |
| M16 | Male | 17 November | 21 November | 61 | 23 | 84 | 88 | 4 | 1 613 |
| M17 | Male | 19 November | 21 November | 21 | 3 | 24 | 50 | 2 | 579 (158-depth) |
| M18 | Male | 19 November | 21 November | 23 | 7 | 30 | 63 | 2 | 345 |
| M19 | Male | 19 November | 21 November | 15 | 2 | 17 | 35 | 2 | 156 |
| M20 | Male | 19 November | 21 November | 36 | 20 | 56 | 78 | 2 | 745 |
| SM21 | Sneaker male | 14 November | 18 November | 32 | 33 | 65 | 39 | 7 | 1 877 |
| F9 | Female | 16 November | 16 November | 2 | 0 | 2 | 2 | 5 | 50 |
| F10 | Female | 14 November | 21 November | 47 | 40 | 87 | 52 | 7 | 982 |
| F11 | Female | 14 November | 21 November | 78 | 27 | 105 | 63 | 7 | 2 650 |
| F12 | Female | 15 November | 21 November | 23 | 14 | 37 | 26 | 6 | 434 |
| F13 | Female | 15 November | 19 November | 33 | 12 | 45 | 31 | 6 | 978 |
| F14 | Female | 15 November | 21 November | 72 | 39 | 111 | 77 | 6 | 2 314 |
| F15 | Female | 16 November | 17 November | 22 | 13 | 35 | 29 | 5 | 863 |
| F16 | Female | 17 November | 21 November | 63 | 9 | 72 | 75 | 4 | 1 424 |
| F17 | Female | 19 November | 21 November | 27 | 18 | 45 | 94 | 2 | 605 (175-depth) |
| F18 | Female | 19 November | 21 November | 30 | 7 | 37 | 77 | 2 | 629 (157-depth) |
| Mean ± s.d. | 46 ± 28.8 | 19.3 ± 15.3 | 65.3 ± 41.8 | 57 ± 26 | 1 305.7 ± 1 059.4 | ||||
| 2006 | |||||||||
| M22 | Male | 16 November | 18 November | 27 | 10 | 37 | 31 | 5 | 489 |
| SM23 | Sneaker male | 17 November | 20 November | 20 | 11 | 31 | 32 | 4 | 403 |
| SM24 | Sneaker male | 17 November | 21 November | 12 | 7 | 19 | 20 | 4 | 209 |
| SM25 | Sneaker male | 17 November | 21 November | 8 | 6 | 14 | 15 | 4 | 130 |
| F19 | Female | 16 November | 17 November | 5 | 4 | 9 | 8 | 5 | 54 |
| F20 | Female | 16 November | 17 November | 7 | 2 | 9 | 8 | 5 | 58 |
| Mean ± s.d. | 13.2 ± 8.6 | 6.7 ± 3.4 | 19.8 ± 11.7 | 19 ± 11 | 223.8 ± 183.2 | ||||
| 2003–2006 | |||||||||
| Mean ± s.d. | 31.5 ± 27 | 14.1 ± 13.4 | 45.7 ± 38.6 | 35 ± 30 | 944.9 ± 1 043.2 | ||||
Also given are the means and standard deviations (s.d.) for each year, and for all years combined.
Squid ID codes, date of tagging, date of last detection, hours spent at the spawning site, percentage of time spent at the spawning site relative to the total number of tracking days, and the total number of detections recorded for the 45 squid tagged in 2003–2006.
| . | . | . | . | Time spent at spawning site (h) . | Time spent at spawning site as a % of total passive tracking time . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Year and squid code . | Sex . | Date tagged . | Date of last detection . | Day . | Night . | Total . | % time . | Tracking days . | Total number of detections recorded . |
| 2003 | |||||||||
| M1 | Male | 12 November | 15 November | 43 | 30 | 73 | 30 | 10 | 3 899 |
| M2 | Male | 12 November | 15 November | 30 | 17 | 47 | 20 | 10 | 1 921 |
| F1 | Female | 15 November | 22 November | 26 | 24 | 50 | 30 | 7 | 327 |
| F2 | Female | 15 November | 21 November | 2 | 3 | 5 | 3 | 7 | 33 |
| Mean ± s.d. | 25.3 ± 17.1 | 18.5 ± 11.6 | 43.8 ± 28.3 | 21 ± 13 | 1 545 ± 1 775 | ||||
| 2004 | |||||||||
| SM3 | Sneaker male | 07 November | 08 November | 3 | 10 | 13 | 4 | 14 | 40 |
| M4 | Male | 07 November | 11 November | 7 | 7 | 14 | 4 | 14 | 91 |
| M5 | Male | 07 November | 08 November | 13 | 2 | 15 | 4 | 14 | 294 |
| M6 | Male | 08 November | 20 November | 38 | 4 | 42 | 13 | 13 | 721 |
| M7 | Male | 08 November | 09 November | 8 | 2 | 10 | 3 | 13 | 185 |
| M8 | Male | 08 November | 08 November | 2 | 0 | 2 | 1 | 13 | 29 |
| F3 | Female | 07 November | 12 November | 31 | 15 | 46 | 14 | 14 | 814 |
| F4 | Female | 07 November | 10 November | 37 | 12 | 49 | 15 | 14 | 1 167 |
| F5 | Female | 09 November | 09 November | 3 | 0 | 3 | 1 | 12 | 71 |
| F6 | Female | 09 November | 09 November | 5 | 5 | 10 | 3 | 12 | 109 |
| F7 | Female | 09 November | 11 November | 31 | 22 | 53 | 18 | 12 | 1 412 |
| F8 | Female | 09 November | 09 November | 2 | 0 | 2 | 1 | 12 | 31 |
| Mean ± s.d. | 15 ± 14.7 | 6.6 ± 6.9 | 21.6 ± 19.8 | 7 ± 6 | 413.7 ± 489.3 | ||||
| 2005 | |||||||||
| M9 | Male | 14 November | 21 November | 101 | 44 | 145 | 86 | 7 | 3 668 |
| M10 | Male | 14 November | 21 November | 96 | 43 | 139 | 83 | 7 | 2 918 |
| M11 | Male | 14 November | 21 November | 71 | 10 | 81 | 48 | 7 | 1 646 |
| M12 | Male | 15 November | 21 November | 95 | 50 | 145 | 86 | 6 | 3 453 |
| M13 | Male | 16 November | 21 November | 14 | 0 | 14 | 12 | 5 | 165 |
| M14 | Male | 16 November | 21 November | 39 | 14 | 53 | 44 | 5 | 640 |
| M15 | Male | 17 November | 21 November | 58 | 15 | 73 | 76 | 4 | 1 298 (511-depth) |
| M16 | Male | 17 November | 21 November | 61 | 23 | 84 | 88 | 4 | 1 613 |
| M17 | Male | 19 November | 21 November | 21 | 3 | 24 | 50 | 2 | 579 (158-depth) |
| M18 | Male | 19 November | 21 November | 23 | 7 | 30 | 63 | 2 | 345 |
| M19 | Male | 19 November | 21 November | 15 | 2 | 17 | 35 | 2 | 156 |
| M20 | Male | 19 November | 21 November | 36 | 20 | 56 | 78 | 2 | 745 |
| SM21 | Sneaker male | 14 November | 18 November | 32 | 33 | 65 | 39 | 7 | 1 877 |
| F9 | Female | 16 November | 16 November | 2 | 0 | 2 | 2 | 5 | 50 |
| F10 | Female | 14 November | 21 November | 47 | 40 | 87 | 52 | 7 | 982 |
| F11 | Female | 14 November | 21 November | 78 | 27 | 105 | 63 | 7 | 2 650 |
| F12 | Female | 15 November | 21 November | 23 | 14 | 37 | 26 | 6 | 434 |
| F13 | Female | 15 November | 19 November | 33 | 12 | 45 | 31 | 6 | 978 |
| F14 | Female | 15 November | 21 November | 72 | 39 | 111 | 77 | 6 | 2 314 |
| F15 | Female | 16 November | 17 November | 22 | 13 | 35 | 29 | 5 | 863 |
| F16 | Female | 17 November | 21 November | 63 | 9 | 72 | 75 | 4 | 1 424 |
| F17 | Female | 19 November | 21 November | 27 | 18 | 45 | 94 | 2 | 605 (175-depth) |
| F18 | Female | 19 November | 21 November | 30 | 7 | 37 | 77 | 2 | 629 (157-depth) |
| Mean ± s.d. | 46 ± 28.8 | 19.3 ± 15.3 | 65.3 ± 41.8 | 57 ± 26 | 1 305.7 ± 1 059.4 | ||||
| 2006 | |||||||||
| M22 | Male | 16 November | 18 November | 27 | 10 | 37 | 31 | 5 | 489 |
| SM23 | Sneaker male | 17 November | 20 November | 20 | 11 | 31 | 32 | 4 | 403 |
| SM24 | Sneaker male | 17 November | 21 November | 12 | 7 | 19 | 20 | 4 | 209 |
| SM25 | Sneaker male | 17 November | 21 November | 8 | 6 | 14 | 15 | 4 | 130 |
| F19 | Female | 16 November | 17 November | 5 | 4 | 9 | 8 | 5 | 54 |
| F20 | Female | 16 November | 17 November | 7 | 2 | 9 | 8 | 5 | 58 |
| Mean ± s.d. | 13.2 ± 8.6 | 6.7 ± 3.4 | 19.8 ± 11.7 | 19 ± 11 | 223.8 ± 183.2 | ||||
| 2003–2006 | |||||||||
| Mean ± s.d. | 31.5 ± 27 | 14.1 ± 13.4 | 45.7 ± 38.6 | 35 ± 30 | 944.9 ± 1 043.2 | ||||
| . | . | . | . | Time spent at spawning site (h) . | Time spent at spawning site as a % of total passive tracking time . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Year and squid code . | Sex . | Date tagged . | Date of last detection . | Day . | Night . | Total . | % time . | Tracking days . | Total number of detections recorded . |
| 2003 | |||||||||
| M1 | Male | 12 November | 15 November | 43 | 30 | 73 | 30 | 10 | 3 899 |
| M2 | Male | 12 November | 15 November | 30 | 17 | 47 | 20 | 10 | 1 921 |
| F1 | Female | 15 November | 22 November | 26 | 24 | 50 | 30 | 7 | 327 |
| F2 | Female | 15 November | 21 November | 2 | 3 | 5 | 3 | 7 | 33 |
| Mean ± s.d. | 25.3 ± 17.1 | 18.5 ± 11.6 | 43.8 ± 28.3 | 21 ± 13 | 1 545 ± 1 775 | ||||
| 2004 | |||||||||
| SM3 | Sneaker male | 07 November | 08 November | 3 | 10 | 13 | 4 | 14 | 40 |
| M4 | Male | 07 November | 11 November | 7 | 7 | 14 | 4 | 14 | 91 |
| M5 | Male | 07 November | 08 November | 13 | 2 | 15 | 4 | 14 | 294 |
| M6 | Male | 08 November | 20 November | 38 | 4 | 42 | 13 | 13 | 721 |
| M7 | Male | 08 November | 09 November | 8 | 2 | 10 | 3 | 13 | 185 |
| M8 | Male | 08 November | 08 November | 2 | 0 | 2 | 1 | 13 | 29 |
| F3 | Female | 07 November | 12 November | 31 | 15 | 46 | 14 | 14 | 814 |
| F4 | Female | 07 November | 10 November | 37 | 12 | 49 | 15 | 14 | 1 167 |
| F5 | Female | 09 November | 09 November | 3 | 0 | 3 | 1 | 12 | 71 |
| F6 | Female | 09 November | 09 November | 5 | 5 | 10 | 3 | 12 | 109 |
| F7 | Female | 09 November | 11 November | 31 | 22 | 53 | 18 | 12 | 1 412 |
| F8 | Female | 09 November | 09 November | 2 | 0 | 2 | 1 | 12 | 31 |
| Mean ± s.d. | 15 ± 14.7 | 6.6 ± 6.9 | 21.6 ± 19.8 | 7 ± 6 | 413.7 ± 489.3 | ||||
| 2005 | |||||||||
| M9 | Male | 14 November | 21 November | 101 | 44 | 145 | 86 | 7 | 3 668 |
| M10 | Male | 14 November | 21 November | 96 | 43 | 139 | 83 | 7 | 2 918 |
| M11 | Male | 14 November | 21 November | 71 | 10 | 81 | 48 | 7 | 1 646 |
| M12 | Male | 15 November | 21 November | 95 | 50 | 145 | 86 | 6 | 3 453 |
| M13 | Male | 16 November | 21 November | 14 | 0 | 14 | 12 | 5 | 165 |
| M14 | Male | 16 November | 21 November | 39 | 14 | 53 | 44 | 5 | 640 |
| M15 | Male | 17 November | 21 November | 58 | 15 | 73 | 76 | 4 | 1 298 (511-depth) |
| M16 | Male | 17 November | 21 November | 61 | 23 | 84 | 88 | 4 | 1 613 |
| M17 | Male | 19 November | 21 November | 21 | 3 | 24 | 50 | 2 | 579 (158-depth) |
| M18 | Male | 19 November | 21 November | 23 | 7 | 30 | 63 | 2 | 345 |
| M19 | Male | 19 November | 21 November | 15 | 2 | 17 | 35 | 2 | 156 |
| M20 | Male | 19 November | 21 November | 36 | 20 | 56 | 78 | 2 | 745 |
| SM21 | Sneaker male | 14 November | 18 November | 32 | 33 | 65 | 39 | 7 | 1 877 |
| F9 | Female | 16 November | 16 November | 2 | 0 | 2 | 2 | 5 | 50 |
| F10 | Female | 14 November | 21 November | 47 | 40 | 87 | 52 | 7 | 982 |
| F11 | Female | 14 November | 21 November | 78 | 27 | 105 | 63 | 7 | 2 650 |
| F12 | Female | 15 November | 21 November | 23 | 14 | 37 | 26 | 6 | 434 |
| F13 | Female | 15 November | 19 November | 33 | 12 | 45 | 31 | 6 | 978 |
| F14 | Female | 15 November | 21 November | 72 | 39 | 111 | 77 | 6 | 2 314 |
| F15 | Female | 16 November | 17 November | 22 | 13 | 35 | 29 | 5 | 863 |
| F16 | Female | 17 November | 21 November | 63 | 9 | 72 | 75 | 4 | 1 424 |
| F17 | Female | 19 November | 21 November | 27 | 18 | 45 | 94 | 2 | 605 (175-depth) |
| F18 | Female | 19 November | 21 November | 30 | 7 | 37 | 77 | 2 | 629 (157-depth) |
| Mean ± s.d. | 46 ± 28.8 | 19.3 ± 15.3 | 65.3 ± 41.8 | 57 ± 26 | 1 305.7 ± 1 059.4 | ||||
| 2006 | |||||||||
| M22 | Male | 16 November | 18 November | 27 | 10 | 37 | 31 | 5 | 489 |
| SM23 | Sneaker male | 17 November | 20 November | 20 | 11 | 31 | 32 | 4 | 403 |
| SM24 | Sneaker male | 17 November | 21 November | 12 | 7 | 19 | 20 | 4 | 209 |
| SM25 | Sneaker male | 17 November | 21 November | 8 | 6 | 14 | 15 | 4 | 130 |
| F19 | Female | 16 November | 17 November | 5 | 4 | 9 | 8 | 5 | 54 |
| F20 | Female | 16 November | 17 November | 7 | 2 | 9 | 8 | 5 | 58 |
| Mean ± s.d. | 13.2 ± 8.6 | 6.7 ± 3.4 | 19.8 ± 11.7 | 19 ± 11 | 223.8 ± 183.2 | ||||
| 2003–2006 | |||||||||
| Mean ± s.d. | 31.5 ± 27 | 14.1 ± 13.4 | 45.7 ± 38.6 | 35 ± 30 | 944.9 ± 1 043.2 | ||||
Also given are the means and standard deviations (s.d.) for each year, and for all years combined.
Presence–absence analysis
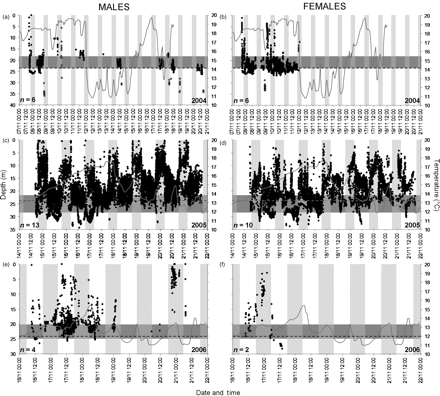
For each year, the presence of tagged squid at the spawning site, bottom temperature, and wind data were plotted against date and time (Figure 5). Turbidity data were not plotted because the levels remained consistently low in all the years that turbidity was recorded, so the factor did not influence behaviour. In 2003, both male squid (M1 and M2) remained at the spawning site for a number of days and nights immediately after tagging (Figure 5a). The presence of females F1 and F2 was sporadic, but overall followed a pattern of being present by day and absent from the site after sunset. M1 and F2 were present at the spawning site for 30% of their total passive tracking time, M2 for 20%, and F2 for 3% (Table 2).
The presence of individually tagged squid (indicated by a black block) on the monitored spawning sites in (a) 2003, (c) 2004, (e and g) 2005, and (h) 2006. White squares indicate the presence of squid on the second, deeper spawning site monitored in 2004. Also shown are hourly bottom-temperature data (at 24 m). Stick vector plots (b, d, f, and i) represent wind data. Wind direction is orientated away from the central axis. Day/night periods are also indicated (night is the area shaded).
In 2004, the presence of tagged males and females appeared to be more erratic (Figure 5c). Males (M5, M6, M7, and M8) and females (F3, F4, F5, F6, F7, and F8) were present most often during daylight. Only one squid (F7) was continuously present for a number of days (∼60 h). Four squid (M4, M6, M7, and F4) were briefly detected by the VR2 receiver moored off Cape St Francis, indicated by the white squares in Figure 5c. Time spent on the spawning site relative to each squid's tracking time varied from 1% (F5 and F8) to 18% (F7), with an average of 7% (Table 2).
In 2005, the general presence–absence pattern was similar to that found in 2003. Males were present for longer continuous periods than females (Figure 5e and g), females appearing to leave the spawning site more often. Again, the periods of absence were usually at night. Spawning activity appears to have been intense throughout the seven-day study, with squid present on the spawning site from 2% (F9) to 94% (F17), average 57%, of their total tracking time (Table 2).
In 2006, just two squid, M22 and SM23, were present for long continuous periods (Figure 5h). Other tagged squid (SM24, SM25, F19, and F20) spent short periods at the spawning site, most often by day. Time spent at the spawning site ranged from 8% (F19 and F20) to 31% (M22), average 19%, of total passive tracking time.
Water-column activity
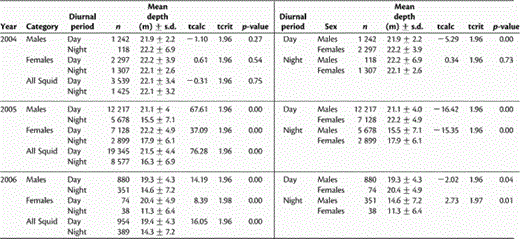
Depth data for all male and female squid tagged in 2004–2006 are presented in Figure 6. As more than one receiver was deployed on the study site, duplicate data, i.e. single detections recorded by more than one VR2 receiver, were removed for statistical purposes, and the total number of successfully detected transmissions for each sex per day and night was calculated. The data for each sex were separated into depth categories, and the percentage of detections recorded in each depth category by day and night plotted (Figure 7). Two-sample, two-tailed t-tests were used to determine significant differences in mean depth by day vs. night for male, female, and all squid combined, as well as mean depth for males vs. females by day and night (Table 3). The bottom temperature is superimposed on Figure 6.
Depth plots of all male and female squid tagged for the years (a and b) 2004, (c and d) 2005, and (e and f) 2006. Also shown are hourly bottom-temperature data (the 24-m mark is indicated by a dashed line), day/night periods (night indicated by vertical shaded areas), and the bottom depth of stations where VR2 receivers were deployed (indicated by horizontally shaded areas). Note that because of the sloping down of the seafloor in an offshore direction and the detection range of the VR2 receivers, it is possible for squid to be detected deeper than the depth range at which a VR2 is deployed.
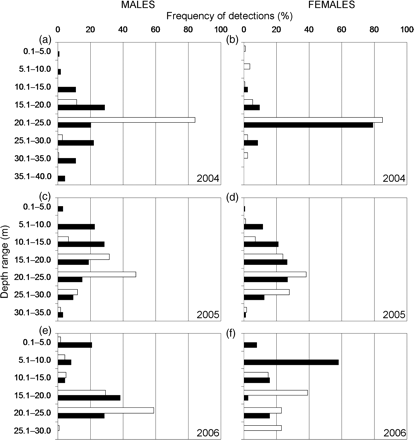
The frequency of detections recorded in different depth categories for males and females for the years (a and b) 2004, (c and d) 2005, and (e and f) 2006. White bars indicate the frequency of detections recorded during daylight and black bars those recorded at night.
The results of two-sample, two-tailed t-tests to determine if significant differences (p < 0.05) exist for (left panel) mean depth by day vs. by night for male, female and all squid, and (right panel) mean depth for males vs. females by day and night.
 |
 |
The results of two-sample, two-tailed t-tests to determine if significant differences (p < 0.05) exist for (left panel) mean depth by day vs. by night for male, female and all squid, and (right panel) mean depth for males vs. females by day and night.
 |
 |
Immediately evident in the depth plots is the diurnal vertical movement in the water column (Figure 6). Both male and female squid moved higher up the water column by night, with males sometimes almost reaching the surface. By day, activity was generally close to the seabed. This daily vertical migration was observed in all three years (2004–2006) for which pressure data were collected.
Table 3 lists the results of the two-sample, two-tailed t-tests. Differences between mean depth by day and night were significant for all three groups (males, females, and all squid combined) in 2005 (males, p = 0.00; females, p = 0.00; all squid, p = 0.00) and 2006 (males, p = 0.00; females, p = 0.00; all squid, p = 0.00), with daytime depth being deeper than night depth. Mean depths for different periods of the 24 h (day and night) were also compared between sexes (Table 3), and the differences were significant in all three years. In 2004, there was a significant difference (p = 0.00) in the mean depth of male and female squid by day, female mean depth being slightly deeper than that of males, but the difference at night was not significant (p = 0.73). In 2005, females were significantly deeper (p = 0.00) than males by both day and night. Female squid in 2006 were significantly deeper than males in terms of mean depth by day (p = 0.04), and the reverse was true for mean depths at night (p = 0.01).
In daylight in 2004, >80% of the detections of both males and females were in the depth range 20.1–25.0 m (Figure 7a and b), but tagged males and females did differ in that females by day were throughout the water column, though at low frequencies. Males only occupied the depth range 15.1–30.0 m. By night, females were most frequently encountered (∼80%) in the same depth range as during the day, but males were scarce though mainly at low frequencies throughout and in the depth range 15.1–30.0 m. In 2005, both males and females (Figure 7c and d) were most commonly found by day at the same depth (20.1–25.0 m), though females tended to be slightly higher in the water column than males. By night, males were more common shallower than females, with the highest frequencies of occurrence at 10.1–15.0 m for males and at 15.1–25.0 m for females. In 2006, males were most common by day (∼60%) in the depth range 20.1–25.0 m (Figure 7e), and at night at 15.1–20.0 m. For the two female squid shown in Figure 7f though, the patterns identified in 2004 and 2005 were not repeated, peak occurrence being 40% by day in the depth range 15.1–20.0 m and ∼60% by night in the depth range 5.1–10.0 m.
Diurnal behaviour
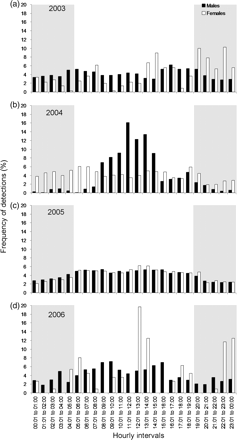
To analyse diurnal patterns at the spawning sites, the percentage of transmissions successfully detected per hour in a typical 24-h period are plotted in Figure 8, separately for males and females each year, using the data from which duplicates had been removed. Overall, for all years, there was a general increase in the percentage of detections recorded towards dawn and a general decrease in detections after dusk (Figure 8a–d), but this trend was most apparent in 2003 and 2006 for males alone and in 2004 and 2005 for males and females. This general increase and subsequent decrease did not continue towards and beyond midday, respectively. Instead, peaks in the percentage of detections recorded by day tended to vary between years.
The frequency of detections recorded by the VR2 receivers during a typical day for the years (a) 2003 (n for males 5820, for females 360), (b) 2004 (n for males 1360, for females 3604), (c) 2005 (n for males 19 102, for females 10 929), and (d) 2006 (n for males 1231, for females 112). Periods of darkness are indicated by shaded areas and daylight by unshaded areas.
Squid–environment dynamics
Bottom temperature data indicated a number of upwelling events, caused by easterly winds, during the experiments in 2003 (Figure 5a and b), 2004 (Figure 5c and d), and 2005 (Figure 5e–g). Surface and bottom temperature differed by up to 7°C during such events, indicating the pressure of intense thermoclines. In 2006 (Figure 5h), bottom temperature remained relatively low, fluctuating between 11 and 15°C. It appears that spawning, inferred by the upward–downward movement close to the seabed evident in Figure 6, was taking place at a variety of temperatures: 11.5–19.6°C (Figure 6a and b), 11.8°C–17.6°C (Figure 6c and d), and 12.3–15.4°C (Figure 6e and f).
The upwelling event in 2003 evolved rapidly and lasted 15 h (Figure 5a). Temperature dropped at a rate of 0.55°C h−1, from 18.8 to 10.5°C, before increasing again just as suddenly. Both males (M1 and M2) remained at the spawning site during the event and were detected by all seven VR2 receivers, indicating extensive movement around the site. As bottom temperature increased, M2 left the spawning site, but M1 remained there for a further 24 h. Female F1 returned to the spawning site on two occasions, remaining for 24 h before moving off. F2 was detected briefly as she swam past or through the VR2 receiver array.
Figures 5c and 6a and b reveal the sudden departure of squid (M4, F3, and F7) from the spawning site as upwelling took place in 2004. That upwelling event, resulting from strong easterly winds (Figure 5d), was also sudden, and the bottom temperature dropped rapidly by 0.7°C h−1. Unlike the upwelling event in 2003, the lower temperature persisted for four days. Before that, three of the 12 squid tagged (F3, F4, and F7) spent considerable time at the spawning site (Figure 5c, Table 2), and all those present appeared to be spawning (Figure 6a and b). For the rest of this study, only one squid (M6) returned to the spawning site (Figure 5c); it appeared on six occasions and frequently moved from the monitored spawning site to a deeper spawning site off Cape St Francis.
Presence–absence data for 2005 (Figure 5e and g) revealed squid to be present throughout the experiment. The drop in temperature resulting from easterly winds (Figure 5f) was far more gradual in 2005 than during the upwelling events of 2003 and 2004, bottom temperature declining at a rate of 0.18°C h−1. The intense pattern of spawning activity, evident from the high percentage of time present on the spawning site relative to passive tracking time for a number of squid (Table 2), continued uninterrupted from the start of upwelling and throughout the event (Figure 6c and d).
In the 2006 experiment, recurrent warming and cooling of bottom water was gradual (Figure 5h), and the temperature change was no faster than 0.14°C h−1. Spawning activity was only evident during the first three days of the 2006 experiment (Figure 6e and f), and squid present after 19 November were higher up in the water column and did not appear to be spawning (Figure 6e). This was probably a consequence of the extremely rough sea conditions in Kromme Bay on 20 and 21 November 2006.
Discussion
Presence–absence behaviour
A previous acoustic-telemetry study conducted in Oyster Bay (O'Dor et al., 1996; Sauer et al., 1997) showed that male squid moved at dawn onto the inshore spawning grounds and circled above egg beds, drawing in females and other males. Males and females formed pairs, then mating and spawning activity commenced, often continuing for a number of hours after sunset. Females and “sneaker” males remained at the spawning site until their departure after dusk. Males left the spawning site by day, possibly moving to other spawning sites in the area, and returned briefly before moving off at dusk. During the Oyster Bay study, although monitoring was continuous, no acoustically tagged males or females were present at the spawning site between 18:00 and 04:00.
The results from the current study differ somewhat from those of the earlier experiment, showing a mixed pattern of presence–absence at the spawning site, not solely related to the day/night periods. Both sexes remained there during some nights (Figure 5), in all years during which the experiments were conducted. No definite pattern of presence at a spawning site was found, but three general presence–absence behaviours were identified. They can be described as (i) the arrival of individual squid at the spawning site at dawn and departure at or shortly after dusk, (ii) the continuous and uninterrupted presence at the spawning site for a number of days, and (iii) presence at the spawning site interrupted by frequent, yet short, periods of absence by day or night. Certain squid displayed only one type of presence–absence behaviour (e.g. F16, Figure 5g), whereas others showed a combination of the behaviour patterns described (e.g. M16, Figure 5e). The presence–absence behaviour of individual squid simultaneously present at the spawning site did not match that of other squid. The driving force of the behaviour was clearly, therefore, unrelated to environmental conditions. It is likely that the overall condition of the animal, e.g. the state of the gonads (ripe, partially spent, or spent), and possibly the reproductive success of a squid at a particular spawning site, probably governed these movements.
Movement between spawning sites
Previous studies on the inshore spawning grounds of chokka squid have shown a great deal of emigration and immigration between spawning sites within an area (Lipiński, 1994; Sauer et al., 1997, 2000; Lipiński et al., 1998). Our results provide further evidence of such movement. In 2004, four squid (M4, M6, M7, and F4) left the monitored spawning site and were shortly thereafter detected at a deeper spawning site off Cape St Francis (Figure 5c), 1.7 km away. On one occasion, M4 made the journey between these two sites at night. Movement between spawning sites is therefore not restricted to daylight.
Water-column activity
After dusk, the dense mating and spawning aggregations broke up, as documented in previous studies (Sauer and Smale, 1993; Sauer et al., 1997; Roberts, 1998; Hanlon et al., 2002). Most of the squid remaining at the spawning sites moved higher up the water column (Figure 6). This distinct pattern of vertical distribution in the water column is best illustrated in Figure 6c and d. Earlier studies showed that squid caught on the spawning grounds at night have more food in their stomachs than squid caught by day (Sauer and Lipiński, 1991). Also the prey spectrum varies between day and night in that L. reynaudii (i.e. cannibalism) dominates the diet of squid caught by day (Sauer and Lipiński, 1991). This indicates opportunistic spawning-site feeding by day and it is most likely the injured or weak squid that are preyed upon. The diet of squid caught at night includes benthic polychaetes and crustaceans as well as midwater prey (Sauer and Lipiński, 1991). Sauer and Smale (1993) observed two principle forms of nocturnal feeding behaviour on the spawning grounds, namely feeding in the water column and what might be benthic feeding. Individual squid disturbed the sand with their arms or with the water jet from their siphon. Another behaviour observed at night (Sauer et al., 1992; Sauer and Smale, 1993; Sauer, 1995) is the deposition of egg strands by lone females, using stored spermatophores for fertilization. In the current study, detections made close to the bottom at night might be a result of male squid feeding on the seabed and female squid both feeding there and depositing egg strands.
The significantly shallower night-time depths for males and females (separately and combined) during the 2005 and 2006 experiments indicate the primary nocturnal activity in these instances to be midwater feeding (Table 3). This is reflected in Figure 7e and f, which show the frequency of occurrence in shallower depth ranges increasing at night. The mean depth of females by day was significantly deeper than that of males, for all three years that pressure tags were used (Table 3). The sex ratio on the inshore spawning grounds (∼2:1) is skewed towards males (Augustyn, 1990; Sauer, 1991; Hanlon et al., 2002), and males have to engage in agonistic bouts to gain access to females. It is therefore likely that tagged males were neither paired nor participating in mating and spawning all the time, resulting in their significantly shallower mean depth by day. Females, however, tagged squid included, are likely to be paired and actively spawning when present at a spawning site. This is evident from diving observations by day in which no lone females have been seen either in the water column or depositing egg strands (Sauer and Smale, 1993). The significantly deeper mean depths of females by day in these results correspond to those observations. A comparison of the mean depth of males and females at night provides an indication of the intensity of egg-strand deposition by lone females. Data indicate that it was intense during the 2005 experiment. Figure 7 further illustrates the vertical distribution of tagged males and females in the water column by day and night. Of interest is the distribution of both sexes throughout the water column at night, a result of the two different modes of feeding and the continued spawning activity of females. Sauer (1995) reported seeing squid in the top five metres of the water column during two night dives, an observation not made by day, as well as a few squid throughout the water column.
Aggregation dynamics
In contrast to the results of an earlier telemetry study (O'Dor et al., 1996; Sauer et al., 1997), it appears that a core aggregation of squid remains at the spawning site at night. Approaching dawn, more squid arrive at the spawning site and the size of the aggregation increases, leading to an increase in the percentage of detections recorded by day over the percentage recorded at night (Table 2). The early-morning arrival of squid at the spawning site has been observed in many studies (O'Dor et al., 1996; Sauer et al., 1997; Roberts, 1998) and is confirmed here by the general increase in the number of detections then (Figure 8). The presence of egg beds is likely to be the main factor attracting squid to specific areas. The peaks and troughs in the percentage of detections recorded in the hours between dawn and dusk (Figure 8) are possibly the result of immigration and emigration of tagged squid to and from the spawning sites monitored. Figure 8 shows a general decrease in the number of detections after dusk as a consequence of large-scale emigration from the spawning site. There is evidence to suggest that squid leaving the spawning site either move offshore to feed (Sauer et al., 1997) or to other inshore spawning sites (this study).
It is possible, however, that departures from inshore spawning sites are not all related to feeding. Several studies indicate a “resting” stage in L. reynaudii. Evidence to support this theory includes the occasional presence of spent (Augustyn et al., 1994) and partially spent (Roberts, 1998) squid on the inshore spawning grounds, and individuals spawning several times during their life cycle (Melo and Sauer, 1999), with a peak in spring/early summer (Sauer et al., 1991). Serial spawning is one possible explanation for the long periods of absence from inshore spawning sites, as observed here, as is movement between spawning sites over a number of weeks (Melo and Sauer, 1999).
Squid–environment dynamics
The results obtained in this telemetry investigation further support the notion that temperature plays a role in the spawning behaviour of chokka squid. In the study of Roberts (1998), the formation of a spawning aggregation off the Tsitsikamma coast coincident with a cold-water upwelling event led to the suggestion that upwelling may trigger spawning. The formation of a spawning aggregation in Kromme Bay shortly after upwelling in the 2006 acoustic-telemetry study (Figure 4) certainly reinforces this notion.
Temperature has also been demonstrated to influence inshore squid catches, which are almost completely dependent upon the formation of spawning aggregations. Both Sauer (1991) and Schön (2000) found that catches increased during cold upwelling events, suggesting that a dramatic drop in temperature could be a cue for spawning. In the present study, spawning activity, inferred by vertical movement in Figure 6, was found in the temperature range 11.5–19.6°C.
It seems, however, that despite the outcome of this detailed telemetry study, the exact role of temperature in the spawning process is not yet completely understood. For instance, the sudden upwelling event observed in the 2004 experiment, during which bottom temperatures dropped by ∼7°C, appears to have terminated spawning (Figure 6a and b). This contrasts with the squid behaviour observed during the 2003 experiment, where two males remained at the spawning site during a short, but intense, upwelling event (Figure 5a). Unfortunately, the tags used in the 2003 experiment did not have pressure data sensors, so there is no way of telling whether the two males continued spawning or simply moved higher up in the water column, i.e. above the thermocline, to avoid the cold water. Such behaviour was observed in the 2004 experiment, when one squid moved up the water column coincidently with upwelling (Figure 6b), before leaving the spawning site.
Certainly, from the 2005 pressure data (Figure 6c and d), it can be concluded that gradual changes in temperature have no effect on spawning squid. The absence of spawning after 19 November in the 2006 study is the result of the very rough sea conditions then, and likely not related to temperature.
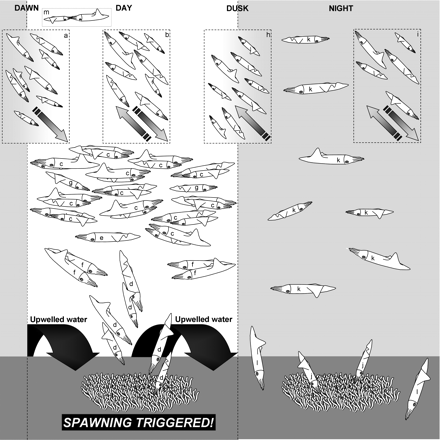
In summary, three general presence–absence behaviours are found at chokka squid spawning sites: (i) arrival at dawn and departure after dusk, (ii) a continuous and uninterrupted presence for a number of days, and (iii) a presence interrupted by frequent but short periods of absence. In contrast to the findings of earlier studies, a core aggregation of squid occasionally remains on active spawning sites at night. As dawn approaches, more squid arrive at the spawning site and the size of the aggregation increases (Figure 9a). By day, the aggregation is dense, comprising mating pairs (Figure 9c), pairs depositing egg strands (Figure 9d), lone consort males (Figure 9e), males engaged in agonistic behaviour (Figure 9f), and lone sneaker males (Figure 9g). Partially spent and spent squid move offshore seemingly to rest, and ripe squid move between spawning sites (Figure 9b). Shortly after dusk, pairs of spawners break apart, and some squid leave the spawning site, moving offshore, presumably to feed or to rest (Figure 9h). It is thought that head-to-head mating takes place offshore (Figure 9m). Those squid remaining at a spawning site at night search for prey throughout the water column (Figure 9k) and in the benthos (Figure 9l), whereas lone females deposit egg strands (Figure 9j). Movement between the spawning sites continues at night (Figure 9i). The initial formation of spawning aggregations, before the deposition of the first egg strand, is probably triggered by upwelling. Our study has also indicated that rapid changes in temperature, such as sudden upwelling events, may disrupt spawning activity for a short time, but further investigation is needed to confirm this. Gradual temperature changes, however, do not disrupt spawning.
Schematic representation of the squid activities at spawning sites by day and night. The process of pair formation, mating, and spawning appears to be triggered by upwelling events. (a) At dawn, a large number of squid arrive at the spawning site and the size of the aggregation increases. (b) Throughout the day, partially spent and spent squid move offshore to rest, and ripe squid move to and from the spawning sites. (c) By day, a dense concentration forms, consisting of paired males and females. (d) Female squid, accompanied by males, deposit egg strands, (e) lone consort males are in evidence, (f) male squid engage in agonistic bouts, and (g) sneaker males are on the scene. (h) After dusk, this dense aggregation breaks down and a number of squid move offshore. (i) A loose aggregation of squid remains at the spawning site, and movement between spawning sites continues. (j) Lone females continue to deposit egg strands, while other squid search for food in (k) the water column and (l) the benthos. It is thought that (m) head-to-head mating and the deposition of spermatophores into the female buccal mass takes place offshore.
Acknowledgements
The work was funded by the South African Squid Management Industrial Association (SASMIA), Marine and Coastal Management (MCM), South Africa's National Research Foundation (NRF), the Nelson Mandela Metropolitan University (NMMU), and the Bayworld Centre for Research and Education (BCRE). We thank Tamaryn Morris, Marcel van den Berg, Rick Harding, Dale Webber, Malcolm Smale, and Warwick Sauer for assistance in the field.