-
PDF
- Split View
-
Views
-
Cite
Cite
Simone Libralato, Cosimo Solidoro, Comparing methods for building trophic spectra of ecological data, ICES Journal of Marine Science, Volume 67, Issue 3, April 2010, Pages 426–434, https://doi.org/10.1093/icesjms/fsp249
Close - Share Icon Share
Abstract
The distribution of biomass, production, and catches over trophic levels (TLs) of the foodweb has been shown theoretically and empirically to provide powerful insights into ecosystem functioning and the effects of fishing. One approach for building trophic spectra of ecological data is based on smoothing original data and assuming zeroes when no values are available for a TL (smoothing-based method). An alternative method is proposed, based on the distribution of ecological data according to density functions (dispersion-based method), and a systematic review of the different alternatives is presented. Six different methods for building trophic spectra, i.e. the smoothing-based and five alternative forms for dispersion-based (using normal, lognormal, and Weibull distributions, also including shifted lognormal and Weibull with zero at TL 2), were applied to ecological properties (i.e. production, biomass, and catches) derived for 24 foodweb models to test their relative performance. The smoothing-based method suffers from the lack of consistency with original data and from unrealistic emergent properties, such as transfer efficiency. The analysis demonstrates the advantages of the dispersion-based method for overcoming these issues and shows, using transfer efficiencies estimated from the models (flow-based estimates) as a reference, that the normal density distribution function performs better.Libralato, S., and Solidoro, C. 2010. Comparing methods for building trophic spectra of ecological data. – ICES Journal of Marine Science, 67: 426–434.
Introduction
The trophic level (TL) has been a central concept in ecological studies since the seminal work by Lindeman (1942). Originally proposed as the number of energy transfers (levels) from primary producers to a consumer (assigning integer values; i.e. herbivores = 2, consumers of herbivores = 3, etc.), the fractional TL is now computed empirically from information on the diet of a species (Odum and Heald, 1975; Pauly and Watson, 2005). Fractional TL provides insights into energetic pathways (Stergiou and Karpouzi, 2002) and might be used as an empirically based synthetic index for intra- and inter-ecosystem comparisons of species' feeding habits (Badalamenti et al., 2000). Moreover, because TL is positively related to fish size (Jennings et al., 2002a) and fishing is selective with regard to size, there is a relationship between fishing activity and TL. In fact, TL has been suggested as an indicator of fisheries effects on marine communities and has been used successfully in several analyses (Pauly et al., 1998a; Pinnegar et al., 2002; Rochet and Trenkel, 2003; Pauly and Watson, 2005; Piet and Jennings, 2005).
The pyramid of biomasses and production over integer TLs has long been used to represent the ecosystem structure (Lindeman, 1942). More recently, the distribution of ecological properties such as biomass, production, and catches over TLs, termed the trophic spectrum, has been proposed by Gascuel et al. (2005) to provide important insights into the ecological effects of exploitation on marine ecosystems. For instance, the shape of biomass data along TLs (the biomass trophic spectrum) has been used as an indicator of ecosystem structure and functioning (Gascuel et al., 2005), and a modelling approach representing biomass and production as functions of TL (Gascuel et al., 2008; Gascuel and Pauly, in press) highlighted the effects of increasing fishing pressure on the shape of biomass trophic spectra, with distinct, alternate system behaviours resulting when bottom-up or top-down effects dominate.
Notwithstanding recent advances on trophic spectra modelling, the basic study of the trophic spectrum of empirical marine data represents a useful and still novel ecological analysis. The methodology has been used successfully on empirical data on catch (Gascuel et al., 2005), abundance (Bozec et al., 2005), and biomass (Munyandorero, 2006) of marine species, providing information on ecosystem functioning.
Data-based trophic spectrum analyses employ the methodology of Gascuel et al. (2005) to obtain a trophic spectrum from ecological data that is based on a seventh-order weighted smoothing of data previously aggregated by fixed TL interval, using zero values for empty intervals (the smoothing-based method).
Here, we compare a set of alternatives for constructing trophic spectra based also on the dispersion of empirical data based on a different density distribution function (ddf; the dispersion-based method) to test capabilities and limitations of different approaches. Using production, biomass and catches taken from a set of 24 well-documented foodwebs, which we assume to be accurate and unbiased, we construct trophic spectra with alternative methods and compare their properties. Our results provide a basis for discussion of the alternatives and a comparison of their robustness.
Material and methods
Smoothing-based trophic spectra
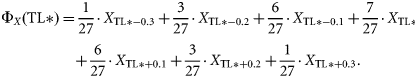
Clearly, at the boundaries of the TL domain (i.e. TL* < TLmin + 0.3 and TL* > TLmax − 0.3), the weighted average is forced to become asymmetrical (not centred) and of lower order. This smoothing-based procedure will therefore provide unreasonable trophic spectra especially at the lower boundary, i.e. TL = 2, and will lack consistency with input values of ecological properties, i.e. the integral of the trophic spectrum might be different from the sum of the input data for constructing it, implying a loss of information. Hence, the smoothing-based method, although considered robust because no assumption is made about the distribution of original variables (Bozec et al., 2005; Gascuel et al., 2005), is poorly defined at the boundaries of the TL domain analysed. More importantly, this weighted average is applied disregarding the discontinuity of the data, including the zero values in the smoothing (Gascuel et al., 2005). However, zero values may result from the fact that field sampling cannot perfectly resolve all TLs, so zeroes might be considered as unknown values (missing information) rather than the result of empirical evidence, and hence be disregarded from the averaging procedure.
Dispersion-based trophic spectra
An alternative procedure is based on the dispersion of data using opportune ddfs. Original data (production, biomass, or catch) recorded for each ith consumer of the ecosystem (Xi) are not discretized to a specific point  of the TL domain, but are distributed over a wider interval assuming that ecological property Xi of the ith consumer is distributed around a central value
of the TL domain, but are distributed over a wider interval assuming that ecological property Xi of the ith consumer is distributed around a central value  with a dispersion σi2.
with a dispersion σi2.
 for each consumer (species i) is computed based on a widely used definition (Odum and Heald, 1975; Pauly and Watson, 2005), as
for each consumer (species i) is computed based on a widely used definition (Odum and Heald, 1975; Pauly and Watson, 2005), as 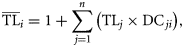
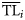 for each species is quantified as the variance of the TLs of its prey (TLj) weighted by the fraction of each prey item in the diet (DCji), and it is defined as
for each species is quantified as the variance of the TLs of its prey (TLj) weighted by the fraction of each prey item in the diet (DCji), and it is defined as 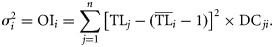
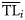 (Christensen et al., 2005).
(Christensen et al., 2005).Different ddfs Xi(TL) have been tested including normal, lognormal, and Weibull distributions. However, only consumers have been included in the trophic spectrum analysis (functional groups with 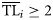 ), so to test the distributions restricted to the same domain of data, lognormal and Weibull distributions with zero shifted to TL = 2 were also considered. In this way, the dispersion-based method for building trophic spectra was applied using five alternative forms for the ddf (Table 1).
), so to test the distributions restricted to the same domain of data, lognormal and Weibull distributions with zero shifted to TL = 2 were also considered. In this way, the dispersion-based method for building trophic spectra was applied using five alternative forms for the ddf (Table 1).
Ddfs used for building trophic spectra based on the dispersion-based method.
| Alternatives for dispersion-based method . | ddf . | Note . |
|---|---|---|
| (1) Normal | 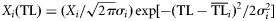 | – |
| (2) Lognormal | 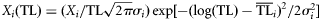 | – |
| (3) Lognormal shifted | 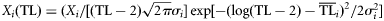 | – |
| (4) Weibull | 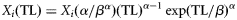 | with 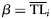 and and 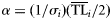 |
| (5) Weibull shifted | 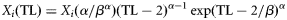 | with 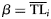 and and 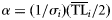 |
| Alternatives for dispersion-based method . | ddf . | Note . |
|---|---|---|
| (1) Normal | 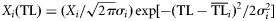 | – |
| (2) Lognormal | 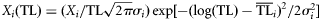 | – |
| (3) Lognormal shifted | 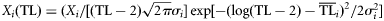 | – |
| (4) Weibull | 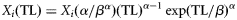 | with 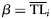 and and 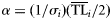 |
| (5) Weibull shifted | 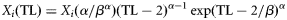 | with 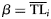 and and 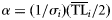 |
The ecological property X (biomass, production, catch) of the ith consumer is distributed around its central value 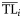 with a dispersion σi2 according to the alternative distribution functions reported above.
with a dispersion σi2 according to the alternative distribution functions reported above. 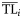 and σi2 are estimated for each consumer species from dietary habits [see Equations (2) and (3)].
and σi2 are estimated for each consumer species from dietary habits [see Equations (2) and (3)].
Ddfs used for building trophic spectra based on the dispersion-based method.
| Alternatives for dispersion-based method . | ddf . | Note . |
|---|---|---|
| (1) Normal | 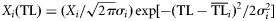 | – |
| (2) Lognormal | 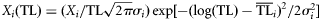 | – |
| (3) Lognormal shifted | 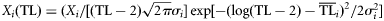 | – |
| (4) Weibull | 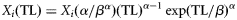 | with 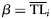 and and 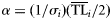 |
| (5) Weibull shifted | 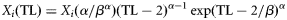 | with 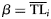 and and 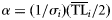 |
| Alternatives for dispersion-based method . | ddf . | Note . |
|---|---|---|
| (1) Normal | 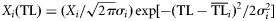 | – |
| (2) Lognormal | 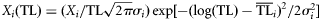 | – |
| (3) Lognormal shifted | 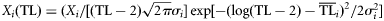 | – |
| (4) Weibull | 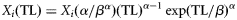 | with 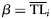 and and 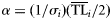 |
| (5) Weibull shifted | 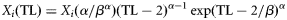 | with 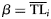 and and 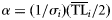 |
The ecological property X (biomass, production, catch) of the ith consumer is distributed around its central value 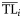 with a dispersion σi2 according to the alternative distribution functions reported above.
with a dispersion σi2 according to the alternative distribution functions reported above. 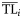 and σi2 are estimated for each consumer species from dietary habits [see Equations (2) and (3)].
and σi2 are estimated for each consumer species from dietary habits [see Equations (2) and (3)].
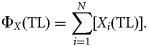
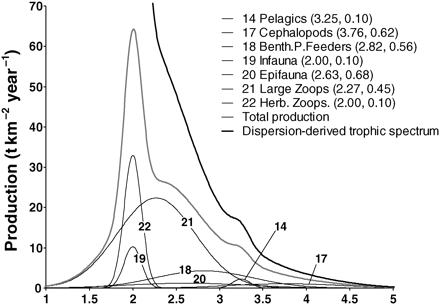
Construction of trophic spectra using the dispersion-based method for the eastern Bering Sea foodweb (NRC, 2003). Trophic spectra for production are built as the sum of productions of all 22 functional groups normally distributed with mean and variance equal to the TL and the OI of the group (in parenthesis), respectively. Only the seven major contributors to total production are reported for reasons of clarity.
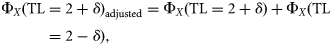
Trophic spectra built with the dispersion-based method using the five ddfs were compared with each other and with the smoothing-based methodology proposed by Gascuel et al. (2005). The dispersion-based method for building the spectra of production, biomass, and catch over TLs, however, addresses the problems of the discontinuity of variables (zero values) that can influence the results irrespective of the distribution assumed for dispersing the data.
Dataset
The trophic spectrum methods were applied to biomass, production, and catch data used in 24 existing foodwebs built using the Ecopath with Ecosim software package, version 5.1 (www.ecopath.org; Christensen and Walters, 2004). The 24 Ecopath foodwebs, selected because they are well-documented, differ widely in terms of ecosystem type and dimension, period represented, fishing pressure, and number of functional groups employed to describe the ecosystem (Table 2). They also have very different pedigree index, which is a measure of the quality of information used to build the model (Christensen et al., 2005). Biomass, production, and catch (here considered as landings plus discards), TL and OI were obtained for all functional groups of each foodweb and used for trophic spectrum analyses. Most of the information (all catches, but also many biomass and production data) is considered as being raw data, but TL and OI were estimated from the model as reported above. All foodwebs were constructed using biomass wet weight and annual rates; biomasses are expressed in g m−2 or t km−2, and flows are in g m−2 year−1 or t km−2 year−1.
Summary of major features of the foodwebs used for comparing trophic spectra.
| Number . | Foodweb . | Ecosystem type and location . | Years . | Functional groups (living) . | Consumers (TL ≥ 2) . | Fishing fleets . | Pedigree index . | Flow-based TE . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Floreana Island, Galapagos | Rocky reefs shallower than 20 m | 2000/2001 | 43 (42) | 39 | 1 | 0.563 | 0.131 | Okey et al. (2004a) |
| 2 | North Central Adriatic Sea | Shelf, 3 miles off the west (or 10 m depth) to 12 miles from the east coast | 1990s | 40 (37) | 36 | 5 | 0.657 | 0.099 | Coll et al. (2007) |
| 3 | South Catalan Sea | Upper slope from 3 miles or 50 to 400 m depth | 1994–2000 | 40 (37) | 36 | 4 | 0.666 | 0.122 | Coll et al. (2006) |
| 4 | Weddell Sea, Antarctica | Southeast shelf of the Weddell Sea, southern Atlantic Ocean | 1980s | 20 (19) | 18 | None | 0.357 | 0.067 | Jarre-Teichmann et al. (1997) |
| 5 | Azores Archipelago | Small shelf around the islands, seamounts and deep oceanic waters | 1997 | 43 (43) | 41 | 13 | – | 0.105 | Guénette and Morato (2001) |
| 6 | Cantabrian Sea | Neritic area of the Cantabrian Sea, from the inner to the outer continental shelf | 1994 | 28 (26) | 25 | 5 | 0.142 | 0.381 | Sanchez and Olaso (2004) |
| 7 | Icelandic fisheries | Shelf area of the northern Atlantic around Iceland | 1997 | 24 (23) | 21 | 14 | 0.295 | 0.140 | Mendy and Buchary (2001) |
| 8 | Newfoundland | From the coast to the 1000 isobath of the ICES Area 2J3KLNO | 1985–1987 | 31 (30) | 29 | 1 | – | 0.169 | Heymans (2003) |
| 9 | Newfoundland | ICES Area 2J3KLNO | 1995–2000 | 45 (44) | 43 | 9 | – | 0.160 | Bundy et al. (2000) |
| 10 | Eastern Bering Sea | Temperate shelf and slope down to 500 m | 1955–1960 | 25 (23) | 22 | 7 | – | 0.170 | Trites et al. (1999) and NRC (2003) |
| 11 | Central North Pacific | Temperate, open ocean | 1990–1998 | 31 (30) | 29 | 9 | – | 0.044 | Cox et al. (2002) |
| 12 | Gulf of Thailand | Tropical shallow coastal area; 10–50 m depth range | 1973 | 40 (39) | 37 | 6 | – | 0.057 | FAO/FISHCODE (2001) and Walters et al. (2005) |
| 13 | North Sea | All area from the Faroe Plateau and the Celtic-Biscay Shelf to the Skagerrak | 1981 | 23 (22) | 21 | 1 | – | 0.116 | Christensen (1995) |
| 14 | Eastern Pacific | Gulf of Alaska and eastern Aleutian Islands, from 50 to 500 m depth | 1963 | 40 (39) | 35 | 1 | – | 0.128 | Guénette and Christensen (2005) |
| 15 | Chesapeake Bay | Temperate, enclosed coastal area | 1950s | 46 (45) | 42 | 16 | 0.471 | 0.126 | Walters et al. (2005) |
| 16 | Northern Gulf of St Lawrence | NAFO 4SR Divisions, areas shallower than 37 m not included | 1980s | 32 (31) | 30 | 1 | 0.651 | 0.172 | Morissette et al. (2003) |
| 17 | Georgia Strait | Temperate narrow basin, average depth 156 m | 1950s | 27 (26) | 24 | 3 | – | 0.112 | Pauly et al. (1998b) and Martell et al. (2002) |
| 18 | Faroe Islands | ICES Area Vb: Faroe Plateau and deep pelagic waters | 1997 | 20 (19) | 18 | 8 | 0.073 | 0.144 | Guénette et al. (2001) |
| 19 | Prince William Sound | Cold temperate coastal area in Alaska (USA) | 1994–1996 | 48 (45) | 42 | 3 | 0.675 | 0.188 | Okey and Pauly (1999) |
| 20 | Mid Atlantic Bight, USA | Temperate continental shelf, from intertidal to shelf break at 200 m | 1995–1998 | 55 (54) | 51 | 1 | 0.415 | 0.165 | Okey (2001) |
| 21 | West Florida Shelf, USA | Subtropical shelf area from intertidal zone to 200 m depth | Late 1990s | 59 (55) | 51 | 11 | 0.623 | 0.117 | Okey et al. (2004b) |
| 22 | South Atlantic States shelf, USA | Subtropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 42 (41) | 37 | 9 | 0.528 | 0.125 | Okey and Pugliese (2001) |
| 23 | Tampa Bay, FL | Tropical open water estuary | – | 52 (51) | 48 | 7 | – | 0.086 | Walters et al. (2005) |
| 24 | South Atlantic States shelf, USA | Tropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 98 (94) | 88 | 10 | 0.499 | 0.192 | T. Okey (unpublished model) |
| Number . | Foodweb . | Ecosystem type and location . | Years . | Functional groups (living) . | Consumers (TL ≥ 2) . | Fishing fleets . | Pedigree index . | Flow-based TE . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Floreana Island, Galapagos | Rocky reefs shallower than 20 m | 2000/2001 | 43 (42) | 39 | 1 | 0.563 | 0.131 | Okey et al. (2004a) |
| 2 | North Central Adriatic Sea | Shelf, 3 miles off the west (or 10 m depth) to 12 miles from the east coast | 1990s | 40 (37) | 36 | 5 | 0.657 | 0.099 | Coll et al. (2007) |
| 3 | South Catalan Sea | Upper slope from 3 miles or 50 to 400 m depth | 1994–2000 | 40 (37) | 36 | 4 | 0.666 | 0.122 | Coll et al. (2006) |
| 4 | Weddell Sea, Antarctica | Southeast shelf of the Weddell Sea, southern Atlantic Ocean | 1980s | 20 (19) | 18 | None | 0.357 | 0.067 | Jarre-Teichmann et al. (1997) |
| 5 | Azores Archipelago | Small shelf around the islands, seamounts and deep oceanic waters | 1997 | 43 (43) | 41 | 13 | – | 0.105 | Guénette and Morato (2001) |
| 6 | Cantabrian Sea | Neritic area of the Cantabrian Sea, from the inner to the outer continental shelf | 1994 | 28 (26) | 25 | 5 | 0.142 | 0.381 | Sanchez and Olaso (2004) |
| 7 | Icelandic fisheries | Shelf area of the northern Atlantic around Iceland | 1997 | 24 (23) | 21 | 14 | 0.295 | 0.140 | Mendy and Buchary (2001) |
| 8 | Newfoundland | From the coast to the 1000 isobath of the ICES Area 2J3KLNO | 1985–1987 | 31 (30) | 29 | 1 | – | 0.169 | Heymans (2003) |
| 9 | Newfoundland | ICES Area 2J3KLNO | 1995–2000 | 45 (44) | 43 | 9 | – | 0.160 | Bundy et al. (2000) |
| 10 | Eastern Bering Sea | Temperate shelf and slope down to 500 m | 1955–1960 | 25 (23) | 22 | 7 | – | 0.170 | Trites et al. (1999) and NRC (2003) |
| 11 | Central North Pacific | Temperate, open ocean | 1990–1998 | 31 (30) | 29 | 9 | – | 0.044 | Cox et al. (2002) |
| 12 | Gulf of Thailand | Tropical shallow coastal area; 10–50 m depth range | 1973 | 40 (39) | 37 | 6 | – | 0.057 | FAO/FISHCODE (2001) and Walters et al. (2005) |
| 13 | North Sea | All area from the Faroe Plateau and the Celtic-Biscay Shelf to the Skagerrak | 1981 | 23 (22) | 21 | 1 | – | 0.116 | Christensen (1995) |
| 14 | Eastern Pacific | Gulf of Alaska and eastern Aleutian Islands, from 50 to 500 m depth | 1963 | 40 (39) | 35 | 1 | – | 0.128 | Guénette and Christensen (2005) |
| 15 | Chesapeake Bay | Temperate, enclosed coastal area | 1950s | 46 (45) | 42 | 16 | 0.471 | 0.126 | Walters et al. (2005) |
| 16 | Northern Gulf of St Lawrence | NAFO 4SR Divisions, areas shallower than 37 m not included | 1980s | 32 (31) | 30 | 1 | 0.651 | 0.172 | Morissette et al. (2003) |
| 17 | Georgia Strait | Temperate narrow basin, average depth 156 m | 1950s | 27 (26) | 24 | 3 | – | 0.112 | Pauly et al. (1998b) and Martell et al. (2002) |
| 18 | Faroe Islands | ICES Area Vb: Faroe Plateau and deep pelagic waters | 1997 | 20 (19) | 18 | 8 | 0.073 | 0.144 | Guénette et al. (2001) |
| 19 | Prince William Sound | Cold temperate coastal area in Alaska (USA) | 1994–1996 | 48 (45) | 42 | 3 | 0.675 | 0.188 | Okey and Pauly (1999) |
| 20 | Mid Atlantic Bight, USA | Temperate continental shelf, from intertidal to shelf break at 200 m | 1995–1998 | 55 (54) | 51 | 1 | 0.415 | 0.165 | Okey (2001) |
| 21 | West Florida Shelf, USA | Subtropical shelf area from intertidal zone to 200 m depth | Late 1990s | 59 (55) | 51 | 11 | 0.623 | 0.117 | Okey et al. (2004b) |
| 22 | South Atlantic States shelf, USA | Subtropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 42 (41) | 37 | 9 | 0.528 | 0.125 | Okey and Pugliese (2001) |
| 23 | Tampa Bay, FL | Tropical open water estuary | – | 52 (51) | 48 | 7 | – | 0.086 | Walters et al. (2005) |
| 24 | South Atlantic States shelf, USA | Tropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 98 (94) | 88 | 10 | 0.499 | 0.192 | T. Okey (unpublished model) |
Summary of major features of the foodwebs used for comparing trophic spectra.
| Number . | Foodweb . | Ecosystem type and location . | Years . | Functional groups (living) . | Consumers (TL ≥ 2) . | Fishing fleets . | Pedigree index . | Flow-based TE . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Floreana Island, Galapagos | Rocky reefs shallower than 20 m | 2000/2001 | 43 (42) | 39 | 1 | 0.563 | 0.131 | Okey et al. (2004a) |
| 2 | North Central Adriatic Sea | Shelf, 3 miles off the west (or 10 m depth) to 12 miles from the east coast | 1990s | 40 (37) | 36 | 5 | 0.657 | 0.099 | Coll et al. (2007) |
| 3 | South Catalan Sea | Upper slope from 3 miles or 50 to 400 m depth | 1994–2000 | 40 (37) | 36 | 4 | 0.666 | 0.122 | Coll et al. (2006) |
| 4 | Weddell Sea, Antarctica | Southeast shelf of the Weddell Sea, southern Atlantic Ocean | 1980s | 20 (19) | 18 | None | 0.357 | 0.067 | Jarre-Teichmann et al. (1997) |
| 5 | Azores Archipelago | Small shelf around the islands, seamounts and deep oceanic waters | 1997 | 43 (43) | 41 | 13 | – | 0.105 | Guénette and Morato (2001) |
| 6 | Cantabrian Sea | Neritic area of the Cantabrian Sea, from the inner to the outer continental shelf | 1994 | 28 (26) | 25 | 5 | 0.142 | 0.381 | Sanchez and Olaso (2004) |
| 7 | Icelandic fisheries | Shelf area of the northern Atlantic around Iceland | 1997 | 24 (23) | 21 | 14 | 0.295 | 0.140 | Mendy and Buchary (2001) |
| 8 | Newfoundland | From the coast to the 1000 isobath of the ICES Area 2J3KLNO | 1985–1987 | 31 (30) | 29 | 1 | – | 0.169 | Heymans (2003) |
| 9 | Newfoundland | ICES Area 2J3KLNO | 1995–2000 | 45 (44) | 43 | 9 | – | 0.160 | Bundy et al. (2000) |
| 10 | Eastern Bering Sea | Temperate shelf and slope down to 500 m | 1955–1960 | 25 (23) | 22 | 7 | – | 0.170 | Trites et al. (1999) and NRC (2003) |
| 11 | Central North Pacific | Temperate, open ocean | 1990–1998 | 31 (30) | 29 | 9 | – | 0.044 | Cox et al. (2002) |
| 12 | Gulf of Thailand | Tropical shallow coastal area; 10–50 m depth range | 1973 | 40 (39) | 37 | 6 | – | 0.057 | FAO/FISHCODE (2001) and Walters et al. (2005) |
| 13 | North Sea | All area from the Faroe Plateau and the Celtic-Biscay Shelf to the Skagerrak | 1981 | 23 (22) | 21 | 1 | – | 0.116 | Christensen (1995) |
| 14 | Eastern Pacific | Gulf of Alaska and eastern Aleutian Islands, from 50 to 500 m depth | 1963 | 40 (39) | 35 | 1 | – | 0.128 | Guénette and Christensen (2005) |
| 15 | Chesapeake Bay | Temperate, enclosed coastal area | 1950s | 46 (45) | 42 | 16 | 0.471 | 0.126 | Walters et al. (2005) |
| 16 | Northern Gulf of St Lawrence | NAFO 4SR Divisions, areas shallower than 37 m not included | 1980s | 32 (31) | 30 | 1 | 0.651 | 0.172 | Morissette et al. (2003) |
| 17 | Georgia Strait | Temperate narrow basin, average depth 156 m | 1950s | 27 (26) | 24 | 3 | – | 0.112 | Pauly et al. (1998b) and Martell et al. (2002) |
| 18 | Faroe Islands | ICES Area Vb: Faroe Plateau and deep pelagic waters | 1997 | 20 (19) | 18 | 8 | 0.073 | 0.144 | Guénette et al. (2001) |
| 19 | Prince William Sound | Cold temperate coastal area in Alaska (USA) | 1994–1996 | 48 (45) | 42 | 3 | 0.675 | 0.188 | Okey and Pauly (1999) |
| 20 | Mid Atlantic Bight, USA | Temperate continental shelf, from intertidal to shelf break at 200 m | 1995–1998 | 55 (54) | 51 | 1 | 0.415 | 0.165 | Okey (2001) |
| 21 | West Florida Shelf, USA | Subtropical shelf area from intertidal zone to 200 m depth | Late 1990s | 59 (55) | 51 | 11 | 0.623 | 0.117 | Okey et al. (2004b) |
| 22 | South Atlantic States shelf, USA | Subtropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 42 (41) | 37 | 9 | 0.528 | 0.125 | Okey and Pugliese (2001) |
| 23 | Tampa Bay, FL | Tropical open water estuary | – | 52 (51) | 48 | 7 | – | 0.086 | Walters et al. (2005) |
| 24 | South Atlantic States shelf, USA | Tropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 98 (94) | 88 | 10 | 0.499 | 0.192 | T. Okey (unpublished model) |
| Number . | Foodweb . | Ecosystem type and location . | Years . | Functional groups (living) . | Consumers (TL ≥ 2) . | Fishing fleets . | Pedigree index . | Flow-based TE . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Floreana Island, Galapagos | Rocky reefs shallower than 20 m | 2000/2001 | 43 (42) | 39 | 1 | 0.563 | 0.131 | Okey et al. (2004a) |
| 2 | North Central Adriatic Sea | Shelf, 3 miles off the west (or 10 m depth) to 12 miles from the east coast | 1990s | 40 (37) | 36 | 5 | 0.657 | 0.099 | Coll et al. (2007) |
| 3 | South Catalan Sea | Upper slope from 3 miles or 50 to 400 m depth | 1994–2000 | 40 (37) | 36 | 4 | 0.666 | 0.122 | Coll et al. (2006) |
| 4 | Weddell Sea, Antarctica | Southeast shelf of the Weddell Sea, southern Atlantic Ocean | 1980s | 20 (19) | 18 | None | 0.357 | 0.067 | Jarre-Teichmann et al. (1997) |
| 5 | Azores Archipelago | Small shelf around the islands, seamounts and deep oceanic waters | 1997 | 43 (43) | 41 | 13 | – | 0.105 | Guénette and Morato (2001) |
| 6 | Cantabrian Sea | Neritic area of the Cantabrian Sea, from the inner to the outer continental shelf | 1994 | 28 (26) | 25 | 5 | 0.142 | 0.381 | Sanchez and Olaso (2004) |
| 7 | Icelandic fisheries | Shelf area of the northern Atlantic around Iceland | 1997 | 24 (23) | 21 | 14 | 0.295 | 0.140 | Mendy and Buchary (2001) |
| 8 | Newfoundland | From the coast to the 1000 isobath of the ICES Area 2J3KLNO | 1985–1987 | 31 (30) | 29 | 1 | – | 0.169 | Heymans (2003) |
| 9 | Newfoundland | ICES Area 2J3KLNO | 1995–2000 | 45 (44) | 43 | 9 | – | 0.160 | Bundy et al. (2000) |
| 10 | Eastern Bering Sea | Temperate shelf and slope down to 500 m | 1955–1960 | 25 (23) | 22 | 7 | – | 0.170 | Trites et al. (1999) and NRC (2003) |
| 11 | Central North Pacific | Temperate, open ocean | 1990–1998 | 31 (30) | 29 | 9 | – | 0.044 | Cox et al. (2002) |
| 12 | Gulf of Thailand | Tropical shallow coastal area; 10–50 m depth range | 1973 | 40 (39) | 37 | 6 | – | 0.057 | FAO/FISHCODE (2001) and Walters et al. (2005) |
| 13 | North Sea | All area from the Faroe Plateau and the Celtic-Biscay Shelf to the Skagerrak | 1981 | 23 (22) | 21 | 1 | – | 0.116 | Christensen (1995) |
| 14 | Eastern Pacific | Gulf of Alaska and eastern Aleutian Islands, from 50 to 500 m depth | 1963 | 40 (39) | 35 | 1 | – | 0.128 | Guénette and Christensen (2005) |
| 15 | Chesapeake Bay | Temperate, enclosed coastal area | 1950s | 46 (45) | 42 | 16 | 0.471 | 0.126 | Walters et al. (2005) |
| 16 | Northern Gulf of St Lawrence | NAFO 4SR Divisions, areas shallower than 37 m not included | 1980s | 32 (31) | 30 | 1 | 0.651 | 0.172 | Morissette et al. (2003) |
| 17 | Georgia Strait | Temperate narrow basin, average depth 156 m | 1950s | 27 (26) | 24 | 3 | – | 0.112 | Pauly et al. (1998b) and Martell et al. (2002) |
| 18 | Faroe Islands | ICES Area Vb: Faroe Plateau and deep pelagic waters | 1997 | 20 (19) | 18 | 8 | 0.073 | 0.144 | Guénette et al. (2001) |
| 19 | Prince William Sound | Cold temperate coastal area in Alaska (USA) | 1994–1996 | 48 (45) | 42 | 3 | 0.675 | 0.188 | Okey and Pauly (1999) |
| 20 | Mid Atlantic Bight, USA | Temperate continental shelf, from intertidal to shelf break at 200 m | 1995–1998 | 55 (54) | 51 | 1 | 0.415 | 0.165 | Okey (2001) |
| 21 | West Florida Shelf, USA | Subtropical shelf area from intertidal zone to 200 m depth | Late 1990s | 59 (55) | 51 | 11 | 0.623 | 0.117 | Okey et al. (2004b) |
| 22 | South Atlantic States shelf, USA | Subtropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 42 (41) | 37 | 9 | 0.528 | 0.125 | Okey and Pugliese (2001) |
| 23 | Tampa Bay, FL | Tropical open water estuary | – | 52 (51) | 48 | 7 | – | 0.086 | Walters et al. (2005) |
| 24 | South Atlantic States shelf, USA | Tropical, continental shelf area, from intertidal area to 500 m depth | 1995–1998 | 98 (94) | 88 | 10 | 0.499 | 0.192 | T. Okey (unpublished model) |
Comparison of smoothing- and dispersion-based trophic spectra
The consistency of trophic spectrum methods with original data was evaluated by comparing the integral of smoothing- and dispersion-based trophic spectra with the sum of the ecological property for TL ≥ 2 in the original data.
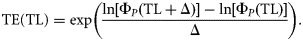
The average of these estimates provided a synthetic measure,  , characteristic of each foodweb (Pauly and Christensen, 1995; Christensen et al., 2005). Average values of
, characteristic of each foodweb (Pauly and Christensen, 1995; Christensen et al., 2005). Average values of  were estimated both based on smoothing-based (
were estimated both based on smoothing-based (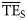 ) and dispersion-based (
) and dispersion-based (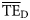 ) trophic spectra to be compared with the
) trophic spectra to be compared with the 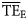 estimated by Ecopath based on flows of matter in the foodweb (Christensen et al., 2005). Although
estimated by Ecopath based on flows of matter in the foodweb (Christensen et al., 2005). Although  is usually reported as an average for 2 ≤ TL ≤ 4 (Pauly and Christensen, 1995), we also included estimates for 2 ≤ TL ≤ 6 to test trophic spectra over a wider range of the TL domain.
is usually reported as an average for 2 ≤ TL ≤ 4 (Pauly and Christensen, 1995), we also included estimates for 2 ≤ TL ≤ 6 to test trophic spectra over a wider range of the TL domain.
Results
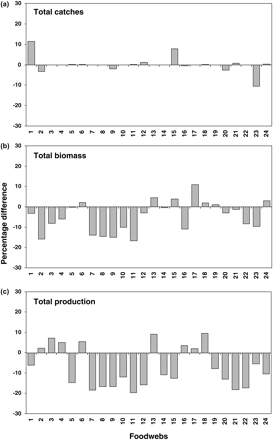
Integrals of the dispersion-based trophic spectra (five alternative forms) do not differ according to the total ecological property of the foodweb, i.e. total catch, total biomass, or total production, because the distribution functions and the eventual adjustment serve to conserve the ecological properties. Conversely, the integrals of spectra built using the smoothing-based method (calculated as reported in Gascuel et al., 2005) are less consistent with regard to original input data (Figure 2). In fact, generally good agreement was observed only when comparing total catches with the integral of smoothing-based trophic spectra for each foodweb (Figure 2a). Notable differences were observed in only three foodwebs, Floreana and Chesapeake Bay (overestimation of total catches in the order of 11.5 and 7.7%, respectively), and Tampa Bay (underestimation of total catches by −10.5%). For total biomass (Figure 2b), the smoothing-based trophic spectra were consistent with total biomass (difference <1%) in only three foodwebs (Azores, Eastern Pacific, and Prince William Sound); underestimated total biomass in 15 cases (maximum −16.8% for the central North Pacific) and overestimated total biomass in six cases (maximum +10.9% for Georgia Strait). This inconsistency of smoothing-based trophic spectra with original input data was even greater for production (Figure 2c), for 16 foodwebs production was underestimated (maximum −19.6% for the central North Pacific; minimum −5.5% for Tampa Bay), and the other eight foodwebs overestimated it (maximum +9.5% for Faroe Islands; minimum +2% Georgia Strait).
Difference between integral of smoothing-based trophic spectra (Xtot’) and total value of input data (Xtot) for ecological properties (a) catch, (b) biomass, and (c) production for the 24 foodwebs analysed and numbered in Table 2. Differences are reported as percentages, calculated as (Xtot' − Xtot)/Xtot.
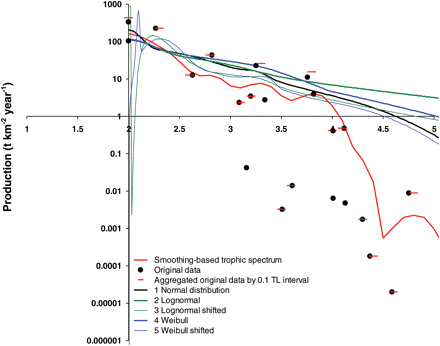
Smoothing and alternative dispersion-based trophic spectra obtained for the 24 foodwebs were compared in terms of their shape and tested by comparing their TE estimates with those obtained from original foodwebs. An example of such comparison is shown in Figure 3, where the production trophic spectra for the eastern Bering Sea foodweb (NRC, 2003) is constructed by employing the different methods (note that logarithmic scaling was used for the y-axis), and original input data are shown. Dispersion-derived trophic spectra are considerably different from smoothing-derived spectra for high values of TL, but these differences have very low absolute values (Figure 3). However, all dispersion-based alternatives are closer to the data than the smoothing-based method for intermediate to low values of TL, where absolute differences are more pronounced. Figure 3 also illustrates the similarity of the behaviour of the dispersion-based trophic spectra, except shifted lognormal and Weibull (alternatives 3 and 5), which show considerable variability for TLs close to 2.
Comparison of different methods for building trophic spectra for production in the eastern Bering Sea (NRC, 2003). The smoothing-derived spectrum is compared with five alternative dispersion-based trophic spectra, each assuming different ddfs for production of consumers (i.e. normal, lognormal, lognormal shifted, Weibull, and Weibull shifted). Original data for the 22 consumer functional groups and data aggregated for the 0.1 TL interval are reported. The y-axis is log-transformed to clarity. Slopes of the alternative spectra permit the calculations of average TE for the foodweb, as listed in Table 3.
Flow-based synthetic measures of TE, as provided by Ecopath (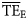 ) for each foodweb, were compared with the average
) for each foodweb, were compared with the average  values estimated based on the smoothing-based and the five dispersion-based trophic spectra,
values estimated based on the smoothing-based and the five dispersion-based trophic spectra, 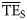 and
and 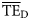 , respectively, by employing Equation (6). We report the results of such comparison for the eastern Bering Sea foodweb in Table 3, which shows that
, respectively, by employing Equation (6). We report the results of such comparison for the eastern Bering Sea foodweb in Table 3, which shows that 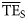 estimates (0.086 and 0.065, using the range 2 ≤ TL ≤ 4 and 2 ≤ TL ≤ 6, respectively) are considerably lower than flow-based
estimates (0.086 and 0.065, using the range 2 ≤ TL ≤ 4 and 2 ≤ TL ≤ 6, respectively) are considerably lower than flow-based 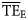 values (0.170 and 0.162, respectively). Conversely,
values (0.170 and 0.162, respectively). Conversely, 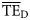 estimates based on lognormal (0.280, 0.378) and Weibull (0.246, 0.225) distributions overestimate the
estimates based on lognormal (0.280, 0.378) and Weibull (0.246, 0.225) distributions overestimate the 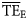 values for the eastern Bering Sea. The shifting of these two ddfs (alternative forms 3 and 5) produce dispersion-based trophic spectra with
values for the eastern Bering Sea. The shifting of these two ddfs (alternative forms 3 and 5) produce dispersion-based trophic spectra with 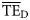 averages more consistent with flow-based estimates (Table 3).
averages more consistent with flow-based estimates (Table 3). 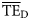 estimates based on normal ddfs (alternative form 1) were 0.197 for 2 ≤ TL ≤ 4 and 0.127 for 2 ≤ TL ≤ 6, so showing the smallest differences with flow-based estimates for both TL ranges (Table 3).
estimates based on normal ddfs (alternative form 1) were 0.197 for 2 ≤ TL ≤ 4 and 0.127 for 2 ≤ TL ≤ 6, so showing the smallest differences with flow-based estimates for both TL ranges (Table 3).
Estimates of average TE for the eastern Bering Sea (NRC, 2003) from flow-based (Ecopath) calculations, from smoothing-derived spectra, and from dispersion-derived trophic spectra using alternative ddfs.
| . | For 2 ≤ TL ≤ 4 . | for 2 ≤ TL ≤ 6 . | ||
|---|---|---|---|---|
| Estimates . | 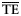 .
. | 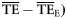 .
. | 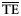 .
. | 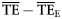 .
. |
Flow-based, 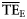 | 0.170 | – | 0.162 | – |
Smoothing-based,  | 0.086 | −0.084 | 0.065 | −0.097 |
Dispersion-based alternatives,  | ||||
| 1 Normal ddf | 0.197 | 0.027 | 0.127 | −0.035 |
| 2 Lognormal | 0.280 | 0.110 | 0.378 | 0.215 |
| 3 Lognormal shifted | 0.188 | 0.018 | 0.268 | 0.106 |
| 4 Weibull | 0.246 | 0.076 | 0.225 | 0.062 |
| 5 Weibull shifted | 0.233 | 0.063 | 0.140 | −0.022 |
| . | For 2 ≤ TL ≤ 4 . | for 2 ≤ TL ≤ 6 . | ||
|---|---|---|---|---|
| Estimates . | 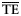 .
. | 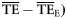 .
. | 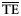 .
. | 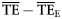 .
. |
Flow-based, 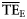 | 0.170 | – | 0.162 | – |
Smoothing-based,  | 0.086 | −0.084 | 0.065 | −0.097 |
Dispersion-based alternatives,  | ||||
| 1 Normal ddf | 0.197 | 0.027 | 0.127 | −0.035 |
| 2 Lognormal | 0.280 | 0.110 | 0.378 | 0.215 |
| 3 Lognormal shifted | 0.188 | 0.018 | 0.268 | 0.106 |
| 4 Weibull | 0.246 | 0.076 | 0.225 | 0.062 |
| 5 Weibull shifted | 0.233 | 0.063 | 0.140 | −0.022 |
Average TE is calculated always for both 2 ≤ TL ≤ 4 and 2 ≤ TL ≤ 6 ranges.
Estimates of average TE for the eastern Bering Sea (NRC, 2003) from flow-based (Ecopath) calculations, from smoothing-derived spectra, and from dispersion-derived trophic spectra using alternative ddfs.
| . | For 2 ≤ TL ≤ 4 . | for 2 ≤ TL ≤ 6 . | ||
|---|---|---|---|---|
| Estimates . | 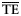 .
. | 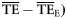 .
. | 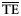 .
. | 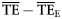 .
. |
Flow-based, 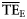 | 0.170 | – | 0.162 | – |
Smoothing-based,  | 0.086 | −0.084 | 0.065 | −0.097 |
Dispersion-based alternatives,  | ||||
| 1 Normal ddf | 0.197 | 0.027 | 0.127 | −0.035 |
| 2 Lognormal | 0.280 | 0.110 | 0.378 | 0.215 |
| 3 Lognormal shifted | 0.188 | 0.018 | 0.268 | 0.106 |
| 4 Weibull | 0.246 | 0.076 | 0.225 | 0.062 |
| 5 Weibull shifted | 0.233 | 0.063 | 0.140 | −0.022 |
| . | For 2 ≤ TL ≤ 4 . | for 2 ≤ TL ≤ 6 . | ||
|---|---|---|---|---|
| Estimates . | 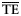 .
. | 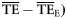 .
. | 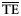 .
. | 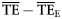 .
. |
Flow-based, 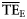 | 0.170 | – | 0.162 | – |
Smoothing-based,  | 0.086 | −0.084 | 0.065 | −0.097 |
Dispersion-based alternatives,  | ||||
| 1 Normal ddf | 0.197 | 0.027 | 0.127 | −0.035 |
| 2 Lognormal | 0.280 | 0.110 | 0.378 | 0.215 |
| 3 Lognormal shifted | 0.188 | 0.018 | 0.268 | 0.106 |
| 4 Weibull | 0.246 | 0.076 | 0.225 | 0.062 |
| 5 Weibull shifted | 0.233 | 0.063 | 0.140 | −0.022 |
Average TE is calculated always for both 2 ≤ TL ≤ 4 and 2 ≤ TL ≤ 6 ranges.
The normal ddf dispersion-based method generally performed better than the alternatives, and only these results (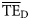 ) will be reported compared with the TE for 2 ≤ TL ≤ 4 from the smoothing-based method (
) will be reported compared with the TE for 2 ≤ TL ≤ 4 from the smoothing-based method (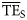 ) for the 24 foodwebs. Regarding all 24 foodwebs,
) for the 24 foodwebs. Regarding all 24 foodwebs, 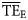 values varied from 0.04 to 0.38 as estimated for the central North Pacific and the Cantabrian Sea, respectively. The mean
values varied from 0.04 to 0.38 as estimated for the central North Pacific and the Cantabrian Sea, respectively. The mean 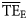 value among the 24 foodwebs was 0.135.
value among the 24 foodwebs was 0.135.
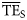 values from smoothing-based trophic spectra varied from 0.038 (Newfoundland 1995–2000) to 0.225 (Prince William Sound), with a mean of 0.099.
values from smoothing-based trophic spectra varied from 0.038 (Newfoundland 1995–2000) to 0.225 (Prince William Sound), with a mean of 0.099. 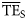 values differed significantly from flow-derived
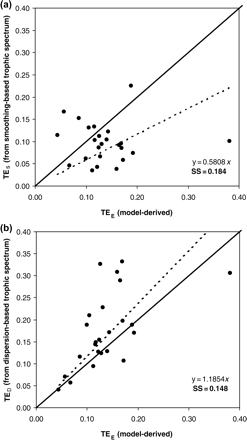
values differed significantly from flow-derived 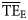 values (Figure 4a; sum of squares of TE differences, SS = 0.184), and those estimated based on smoothing-based trophic spectra systematically underestimated the flow-based values (on average
values (Figure 4a; sum of squares of TE differences, SS = 0.184), and those estimated based on smoothing-based trophic spectra systematically underestimated the flow-based values (on average  values were underestimated by approximately −0.037).
values were underestimated by approximately −0.037).
Comparison of average TE ( ) for foodwebs estimated based on flows (model-derived
) for foodwebs estimated based on flows (model-derived 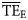 ) and based on trophic spectra methods. Smoothing-based (
) and based on trophic spectra methods. Smoothing-based (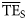 ) and dispersion-based (
) and dispersion-based (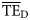 ) trophic spectra are used in (a) and (b), respectively.
) trophic spectra are used in (a) and (b), respectively.
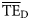 values estimated based on the dispersion-based trophic spectrum method using normal ddf (Figure 4b) varied from 0.057 (Weddell Sea) to 0.332 (Newfoundland 1985–1987), with a mean of 0.176.
values estimated based on the dispersion-based trophic spectrum method using normal ddf (Figure 4b) varied from 0.057 (Weddell Sea) to 0.332 (Newfoundland 1985–1987), with a mean of 0.176. 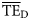 estimates were better related to flow-based
estimates were better related to flow-based 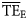 values (SS = 0.148), whereas the average bias was approximately –0.040. The slope of the regression for
values (SS = 0.148), whereas the average bias was approximately –0.040. The slope of the regression for 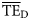 against
against 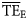 is much closer to 1 than for the
is much closer to 1 than for the 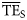 vs.
vs. 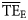 regression (1.12 and 0.58, respectively; Figure 4).
regression (1.12 and 0.58, respectively; Figure 4).
Discussion
Trophic spectra of ecological data, i.e. the continuous distribution of biomass, production, and catch across TLs, are increasingly used as a mean for analysing marine ecosystem structure and functioning. In particular, the effects of exploitation on fish community structure seem to be detectable through trophic spectrum analysis which, therefore, has been used successfully as an ecosystem indicator of fishing impact (Bozec et al., 2005; Moloney et al., 2005). Although the models of trophic spectra might be useful to study and predict the theoretical responses of marine communities to disturbances (Gascuel et al., 2008; Gascuel and Pauly, in press), the analysis of trophic spectra applied directly to ecological data will remain an important tool for detecting erosions in the ecosystem structure. Systematic review of different methods for building trophic spectra is, therefore, useful in increasing the reliability of this type of analysis.
The availability and use of existing foodwebs facilitated the comparison between the trophic spectrum methods using foodwebs as virtual systems: alternative methods for trophic spectrum analysis are therefore compared in terms of their capabilities of representing the virtual system. Moreover, foodwebs permitted comparison of the TE ( ), estimated based on web flows and as an emerging property of trophic spectra. The results, although obtained for foodweb outputs, are also valid for empirical data given that production, biomass, catch, TL, and OI are provided for each species or functional group of the system being analysed (Jennings et al., 2002b).
), estimated based on web flows and as an emerging property of trophic spectra. The results, although obtained for foodweb outputs, are also valid for empirical data given that production, biomass, catch, TL, and OI are provided for each species or functional group of the system being analysed (Jennings et al., 2002b).
Application of smoothing-based trophic spectra (Gascuel et al., 2005) to a set of 24 foodwebs revealed that this method is not always consistent with respect to the original data. Therefore, properties analysed, i.e. the total value of the ecological property calculated from the spectrum (by integration), does not equal the sum of the original data used as input.
Generally, the trophic spectra of catches from the smoothing-based approach were more consistent with input data than biomass and production spectra, which produced biases as large as 20% (Figure 2). The trophic spectra of catch data were biased relative to the input data in ecosystems with a significant proportion of species of low TL species in the catches (Floreana, Chesapeake Bay, and Tampa Bay). For example, catches of sea cucumbers (TL = 2.06) are 2.922 t km−2 year−1, and 70% of the total catch in the Floreana rocky reef foodweb (Okey et al., 2004a); adult oysters (TL = 2.09) constitute 10% of the total catch (1.266 t km−2 year−1) in Chesapeake Bay; and blue crab (TL = 2.65) represents 9.6% of the total catch (0.099 t km−2 year−1) in the Tampa Bay foodweb (Walters et al., 2005). Conversely, the smoothing-based trophic spectrum is accurate when catches are made at a medium–high TL, such as for the central North Pacific (Cox et al., 2002), where target species range from TL = 3.3 (flying squid) to TL = 4.68 (large sharks).
The relatively small bias in the catch spectra and the much larger and more common bias in the biomass and production spectra produced by the smoothing-based approach are attributable to the general absence of low TL functional groups in the catch spectra and the inevitable presence of low TL groups in the other two data types. When ecological data include values for TL close to 2 (lower boundary of the TL domain), the weighted average becomes asymmetrical, so affecting the smoothing-based trophic spectra considerably. This might also explain the difference between the frequency distribution of data and the resulting trophic spectra in applications using empirical data (e.g. Bozec et al., 2005).
The bias of smoothing-based trophic spectra resulted in no correlation with overall model quality, measured in the original foodweb models through the pedigree index, and in weak correlation with biological resolution, i.e. the number of functional groups used to describe the ecological networks. Although positive bias (integral of spectra larger than input data) was more common for ecological networks with few functional groups and negative bias (integral smaller than total input data) for ecological networks described by many functional groups, the correlation was not significant. Clearly, therefore, the smoothing-based trophic spectra can be corrected for consistency with data by rescaling the spectra so as to obtain the integral of the input total value for the ecological property (total catch, total biomass, total production). Therefore, the lack of consistency with original data could be corrected, but the smoothing results in a modified shape of the trophic spectra, with unavoidable implications.
The smoothing- and dispersion-based trophic spectra differed greatly in shape, and this was also demonstrated in substantial differences in their emerging properties, such as TE. Although the most consistent differences were apparently in the higher part of the TL domain in log scales (Figure 3), these differences involve very low absolute values. Conversely, smoothing- and dispersion-based spectra based on different ddfs show consistent absolute differences in the lower part of the TL domain.
The comparative analysis based on  estimates revealed that the shape of the dispersion-based trophic spectra using normal ddfs gave estimated
estimates revealed that the shape of the dispersion-based trophic spectra using normal ddfs gave estimated 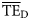 values that were more similar to those quantified on the flow basis (
values that were more similar to those quantified on the flow basis (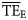 , Ecopath calculations; Christensen et al., 2005). Conversely, smoothing-based trophic spectra provided
, Ecopath calculations; Christensen et al., 2005). Conversely, smoothing-based trophic spectra provided 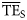 estimates that compared poorly with those obtained from flow measurements (
estimates that compared poorly with those obtained from flow measurements (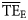 ), suggesting that the poor definition of smoothing at the boundaries of the TL domain might be a critical issue that can be overcome by employing dispersion-based trophic spectra.
), suggesting that the poor definition of smoothing at the boundaries of the TL domain might be a critical issue that can be overcome by employing dispersion-based trophic spectra.
Utilization of normal ddfs and OIs as a measure of dispersion in trophic spectrum analysis might represent two advancements to be evaluated further. Symmetrical distribution of the TL of prey might be a weak assumption, particularly when very few functional group items are represented in the diet of a predator. However, applications using alternative ddfs such as lognormal and Weibull gave poorer results in terms of correct representation of the shape of trophic spectra. Moreover, although normal ddfs call for non-mechanistic adjustments for avoiding properties to be dispersed to unrealistic values (TL < 2), dispersion-based methods performed better with the normal form rather than the non-negative ddf (lognormal and Weibull with zero shifted to TL = 2).
A weakness in the OI, as a measure of dispersion, is that it only represents the dispersion of prey of a given predator and might be a weak measure of the distribution of energy flow, which is the basis of the trophic spectrum continuum. Gascuel et al. (2005) pointed out that the OI might not be a very efficient measure for building trophic spectra because it does not represent a reliable measure of energy dispersion. Despite these considerations, however, our results show that dispersion-based trophic spectra are more consistent with 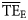 estimates based on flow calculations. It is likely that a better measure of dispersion can be developed, such as one that accounts for (i) errors in the defined diet composition, (ii) dispersion of prey items along TL, and (iii) cascade propagation of this dispersion along the ecological network. By employing OI, the current dispersion-based trophic spectrum method accounts only for the first two sources of variability. Nevertheless, it performed consistently when applied to 24 ecological networks, as measured by the model-derived measures of
estimates based on flow calculations. It is likely that a better measure of dispersion can be developed, such as one that accounts for (i) errors in the defined diet composition, (ii) dispersion of prey items along TL, and (iii) cascade propagation of this dispersion along the ecological network. By employing OI, the current dispersion-based trophic spectrum method accounts only for the first two sources of variability. Nevertheless, it performed consistently when applied to 24 ecological networks, as measured by the model-derived measures of  , indicating that the construction of trophic spectra benefits from accounting for a dispersion measure, even if roughly estimated.
, indicating that the construction of trophic spectra benefits from accounting for a dispersion measure, even if roughly estimated.
Acknowledgements
We thank V. Christensen, M. Coll, and T. Okey for providing the Ecopath with Ecosim models used in this work, and G. Cossarini and D. Moutopoulos for their useful and constructive comments on an early draft of this paper. T. Okey kindly revised the manuscript, providing useful comments and suggestions for improvement. This work has been partially funded by the SESAME project (EC Contract No. GOCE-036949, funded by the Sixth Framework Programme) and by the Centro Euro-Mediterraneo per i Cambiamenti Climatici.