-
PDF
- Split View
-
Views
-
Cite
Cite
Aimee Lee S. Houde, Dylan J. Fraser, Jeffrey A. Hutchings, Fitness-related consequences of competitive interactions between farmed and wild Atlantic salmon at different proportional representations of wild–farmed hybrids, ICES Journal of Marine Science, Volume 67, Issue 4, May 2010, Pages 657–667, https://doi.org/10.1093/icesjms/fsp272
Close - Share Icon Share
Abstract
Escaped farmed fish possess heritable characteristics that may give them and their wild–farmed hybrid offspring a competitive advantage over wild fish. Limited research has examined whether the results of wild vs. farmed pairwise behavioural contests can predict the change in fitness-related traits of wild fish when exposed to wild–farmed hybrids, or to different proportions of such hybrids, within stream environments. Pairwise aggression tests on North American Atlantic salmon (Salmo salar) revealed that regional farmed salmon and wild–farmed hybrids (F1, F2, and wild backcrosses) were more competitive than wild fish from two divergent populations. The ranking by which hybrids differed in competitive ability from wild fish also depended on the wild population. However, the magnitude of change in fitness-related traits of wild fish, such as mortality, size, and condition, from the same two populations could not be predicted from pairwise test results when replicate groups of wild fish were exposed to different proportions of hybrids (wild:hybrid ratios of 50:50, 70:30, and 85:15) in semi-natural stream environments. Notably, there was greater mortality of both wild and hybrid fish in treatments containing 30% hybrids for both populations; at a composition of 50% hybrids, the mortality of wild fish in one population increased more than it did in the other. The results suggest that for the life stage examined and provided the rate of farmed intrusion and wild–farmed interbreeding remains low (i.e. ≤15% hybrids), the effects of competitive interaction with their farmed counterparts may have comparatively little effect on the mortality of wild populations.Houde, A. S., Fraser, D. J., and Hutchings, J. A. 2010. Fitness-related consequences of competitive interactions between farmed and wild Atlantic salmon at different proportional representations of wild–farmed hybrids. – ICES Journal of Marine Science, 67: 657–667.
Introduction
Captive and wild environments typically differ markedly in selective pressures. Human-mediated selection in captive environments, both intentional and unintentional, may lead to changes in the direction and/or the magnitude of selective pressures relative to those experienced in the wild (Price, 1997; McGinnity et al., 2003, 2009; Frankham, 2008). Generally, behavioural traits are among the first to be unintentionally selected in the captive environment (e.g. increased tameness towards humans or domestication; Hosey, 1997; Price, 1997). Over several generations in captivity, genetically based behavioural changes may occur in organisms which differentiate captive organisms from their wild counterparts (Price, 1997; Frankham, 2008).
Captive organisms, such as commercially bred farmed fish, often escape into the wild and may exhibit behaviours that provide them with a competitive advantage over their wild counterparts. For example, farmed fish tend to be more aggressive and more dominant than wild fish in tank environments (Einum and Fleming, 1997; Fleming and Einum, 1997; Blann and Healey, 2006). However, this relationship may change when conditions are more reflective of the wild environment (Fleming and Einum, 1997). Therefore, the different outcomes when comparing the competitive ability of wild and farmed fish may be attributable to differences in the testing environment. Ideally, to determine the risks that escaped farmed fish pose to the persistence of wild populations, the testing environment should mimic the natural environment (Weber and Fausch, 2003). In addition, early generation (F1) wild–farmed hybrids tend to be more aggressive than wild fish and, depending on the wild population of origin, the aggression levels and dominance rank of F1 hybrids can be intermediate to, or even surpass, those of both farmed and wild fish (Einum and Fleming, 1997; Fleming and Einum, 1997).
Farmed Atlantic salmon (Salmo salar) frequently escape from sea cages and may enter rivers inhabited by wild populations. For example, in eastern North America, escaped farmed salmon have been detected in 87% of monitored rivers within 300 km of aquaculture activity (Morris et al., 2008). Although the reproductive success of farmed salmon can be inferior to that of wild salmon (Fleming et al., 1996; but see Weir et al., 2005), they can interbreed successfully with wild salmon and reduce the latter's fitness, possibly because of a competitive advantage at the juvenile stage (McGinnity et al., 1997, 2003; Fleming et al., 2000). Nevertheless, where there are interactions between farmed and wild salmon, limited research has been devoted to examining the extent to which competitive abilities might differ among wild populations, or between wild fish and multigenerational (i.e. F2 generation or greater) wild–farmed hybrids (McGinnity et al., 2003). It is also not known how the fitness-related traits of wild fish (e.g. mortality, size, condition) might depend on the proportional representation of competitors (e.g. wild–farmed hybrids; Devlin et al., 2004). Such considerations may be especially important for the assessment of risks posed to divergent wild populations in eastern North America resulting from continuous interbreeding with their escaped farmed counterparts, given that many regional rivers contain declining or otherwise endangered wild populations (COSEWIC, 2006; Fraser et al., 2008, in press; Houde et al., 2010).
We undertook two complementary experiments on juvenile Atlantic salmon to test three hypotheses: (i) farmed salmon and multigenerational hybrids of wild–farmed salmon are better competitors than wild salmon; (ii) increasing proportions of hybrid salmon have increasingly negative effects on the fitness-related traits of wild salmon; and (iii) the competitive abilities of hybrid and wild salmon in pairwise contests can be used to predict the magnitude of change in fitness-related traits of wild salmon resulting from competitive interactions with hybrids.
Material and methods
Generation of the crosses
In 2001, grandparental fish were obtained from two wild populations from Nova Scotia, Canada: Stewiacke River (45°08′N 63°22′W; STEW), and Tusket River (43°51′N 65°58′W; TUSK). STEW grandparents (ten females and ten males) were captured as wild parr and raised in captivity until maturity. TUSK grandparents (ten females and ten males) were captured as adults. The farmed strain (FARM) was originally derived from the Saint John River, New Brunswick (45°15′N 66°3′W), and had experienced four generations of selective breeding at the time our study was initiated in 2001 (Glebe, 1998). Pure crosses (STEW, TUSK, and FARM) and F1 hybrid crosses (F1 STEW–FARM and F1 TUSK–FARM) were generated in 2001 at Dalhousie University and were raised under common environmental conditions until maturity to ensure that any behavioural differences observed during the experiment would be attributable to genetic differences and not to environmental differences. Crosses were regenerated in 2005, using the 2001 crosses as parents, and also included F2 hybrids (F2 STEW–FARM and F2 TUSK–FARM: F1 hybrid × F1 hybrid), and wild backcrosses (BC1 STEW–FARM and BC1 TUSK–FARM: wild × F1 hybrid). Details on the generation and rearing of these crosses are described by Houde et al. (2010) and Fraser et al. (in press).
Experiment 1: pairwise differences in competitive ability
Aggression-trough design
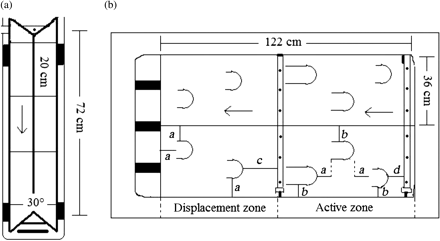
Four aggression troughs were constructed to measure differences in the competitive ability of pairs of juvenile salmon (cf. Einum and Fleming, 1997; Fleming and Einum, 1997; Figure 1). Between August and November 2006, 46–48 wild fry (age 0+ parr) from each wild population (i.e. STEW and TUSK) were chosen randomly from pooled family tanks (11–12 random fry each from four of the available family pooled tanks). These fry were paired with fry randomly chosen from pooled family tanks of their respective wild–farmed hybrids (i.e. F1, F2, and BC1 hybrids), FARM, or from the other wild population (i.e. STEW vs. TUSK). Fry from one cross in each pair were alternately tagged twice on the caudal fin, using a Madajet dental jet inoculator (Mada Medical Products Inc. Carlstadt, NJ, USA) filled with Alcian Blue dye (Davis Diagnostics Limited, Brampton, ON, Canada) at the recommended concentration (of 64–65 mg ml−1) for fish tagging (Kelly, 1967; Hart and Pitcher, 1969). The tagged fry were allowed 24 h to recover and were paired, with length (nearest mm) and weight (nearest 0.01 g) matching as close as possible, with a fry of another cross. Fry were matched for size to minimize the influence of size-based differences in competitive ability (Huntingford et al., 1990).
Aggression trough (left), and two semi-natural stream environments (right). Arrows represent the direction of water flow. Distances: a = 9 cm, b = 5 cm, c = 20 cm, d = 12 cm. (a) Each aggression trough was constructed of timber. A spray bar was attached at one end of the trough, and the other end of the trough was sealed with silicone to maintain a water height of 4 cm. There were 6-cm buffer zones at each end of the trough, and between the buffer zones, screen dividers were placed every 20 cm to form three testing sections per trough. Each testing section had a flow that ranged from 0.9 to 2.5 cm s−1. (b) Each tank was separated into two semi-natural stream environments with a piece of acrylic. Each had a water flow supplied by two spray bars, the primary spray bar resting at the bottom upstream end of the tank and the secondary spray bar placed halfway down the length of the tank and raised 5.5 cm in the water column with plastic supports. Water flowed over an acrylic wall out of the tank. Each environment had hiding areas (i.e. hides) constructed out of pieces of 3″ (7.62 cm) ABS pipe cut in half. In the active zone from the right to the left, the lengths of the hides were 5, 7.5, and 10 cm. The sides of the hides that faced the upstream end were covered with acrylic and sealed with silicone.
Scoring dominance in contest pairs
Each size-matched pair of fry was placed into a testing section of one of the aggression troughs and allowed to acclimate to the trough without food for 48 h on a photoperiod schedule of 10 h of light per day. Each observation period began with a food pellet being dropped into the testing section, followed by visual observations of the pair for 1 min. Two points were awarded to the fry that obtained the food pellet, and one point to the fry that was in the upstream position. The V shape of the trough prevented the fry from being positioned side-by-side during the observation period (Einum and Fleming, 1997), so one fry always had the upstream position. Both position and foraging success correlate with dominance rank (Fausch, 1984). However, more points were awarded to the fry obtaining a food pellet than to a fry holding the upstream position because dominance ranks are more highly correlated with feeding than with the position in a stream (Metcalfe et al., 1989). A further two points were awarded to any fry that initiated attacks, such as a nip or chase (cf. Keenleyside and Yamamoto, 1962) towards the other fry in the pair. Observations of each pair were made every 20 min over a period of 2 h, resulting in a total of seven observations per pair per observation period.
A fry was termed dominant if it had obtained three or more points greater than the score awarded to its competitor. If there was a difference of fewer than three points, the contest was considered unresolved (cf. Einum and Fleming, 1997; Fleming and Einum, 1997). For each pair under observation, all two-sample tests for equality of the proportional amount of time spent feeding and attacking, as well as the proportional amount of time being dominant, were performed in R 2.9.0. (available at http://www.r-project.org/). Statistical significance was set at the level α = 0.05.
Experiment 2: population-level, fitness-related traits of wild fry exposed to different proportions of hybrid fry
Semi-natural stream environment design
Semi-natural stream environments (n = 20) were constructed to simulate the natural environment of salmon fry and to measure fitness-related traits of wild fry exposed to different proportions of hybrid fry at the population level (Figure 1). In the natural environment, fish compete for territories that are energetically favourable in terms of food availability and available refuge (Metcalfe, 1986). A mimic of the natural environment for measuring competition should be a heterogeneous environment with spatial differences in food availability, water flow, and available refuges. Fry tend to prefer mean water velocities of 20–30 cm s−1 and a depth of 20–40 cm in regional rivers (Morantz et al., 1987), and attempts were made to design the semi-natural stream environments with these characteristics. However, owing to logistic constraints, our water velocity and depth were 8 ± 2 cm s−1 and 12.5 cm, respectively. The stream environment was designed based on the assumption that if more food was delivered at the upstream end (Figure 1, primary spray bar location), more fry would prefer to remain in the active zone because they would have direct current, more food, and more hiding spaces relative to the displacement zone. Hiding spaces (hides) of various lengths (5.0, 7.5, and 10.0 cm) were provided, and the longest hides were located in the active zone of the stream environment. Fry would be able to enter the hides from the downstream end and would not be exposed to the water current while in the hide. The stream environments were subject to a photoperiod of natural daylight from July to November in Nova Scotia, Canada.
Treatment levels
Three hybrid treatments containing wild fish exposed to different proportions of hybrids (wild:hybrid ratios of 50:50, 70:30, and 85:15), and one control containing only wild fish were used to measure fitness-related traits for the same wild populations, i.e. STEW and TUSK, in the semi-natural stream environments (Table 1). Each treatment replicate contained a total of 50 fry (density of 113 fry m−2) and the wild:hybrid proportions in the hybrid treatments were chosen to determine an acceptable limit of wild–farmed hybrids in a wild population from a conservation perspective, at this one life stage and one high density. Owing to space limitations, all possible proportions, using all multigenerational hybrids, could not be examined, so we chose to include the most likely hybrids that would be produced in the wild (i.e. F1 hybrids and wild backcrosses) and to equalize the contribution of these hybrid crosses to the hybrid group (i.e. 50% F1 hybrids and 50% wild backcrosses). In addition, one of the control replicates contained 20 wild fry tagged with Alcian Blue dye. This replicate would be used to assess whether tagging had any effect on the size and mortality of wild fry.
Experimental replicates in the semi-natural stream tanks for each population.
| . | Number of fry . | ||
|---|---|---|---|
| Treatment . | Wild fry . | F1 hybrid . | BC1 hybrid . |
| Control 1 | 50 | – | – |
| Control 2t | 30 and 20t | – | – |
| 50:50—1 | 26 | 12t | 12t |
| 50:50—2 | 26t | 12 | 12 |
| 70:30—1 | 36 | 7t | 7t |
| 70:30—2 | 36 | 7t | 7t |
| 70:30—3 | 36 | 7t | 7t |
| 85:15—1 | 42 | 4t | 4t |
| 85:15—2 | 42 | 4t | 4t |
| 85: 15—3 | 42 | 4t | 4t |
| . | Number of fry . | ||
|---|---|---|---|
| Treatment . | Wild fry . | F1 hybrid . | BC1 hybrid . |
| Control 1 | 50 | – | – |
| Control 2t | 30 and 20t | – | – |
| 50:50—1 | 26 | 12t | 12t |
| 50:50—2 | 26t | 12 | 12 |
| 70:30—1 | 36 | 7t | 7t |
| 70:30—2 | 36 | 7t | 7t |
| 70:30—3 | 36 | 7t | 7t |
| 85:15—1 | 42 | 4t | 4t |
| 85:15—2 | 42 | 4t | 4t |
| 85: 15—3 | 42 | 4t | 4t |
t, tagged with Alcian Blue dye. Ratios are given as wild:hybrid.
Experimental replicates in the semi-natural stream tanks for each population.
| . | Number of fry . | ||
|---|---|---|---|
| Treatment . | Wild fry . | F1 hybrid . | BC1 hybrid . |
| Control 1 | 50 | – | – |
| Control 2t | 30 and 20t | – | – |
| 50:50—1 | 26 | 12t | 12t |
| 50:50—2 | 26t | 12 | 12 |
| 70:30—1 | 36 | 7t | 7t |
| 70:30—2 | 36 | 7t | 7t |
| 70:30—3 | 36 | 7t | 7t |
| 85:15—1 | 42 | 4t | 4t |
| 85:15—2 | 42 | 4t | 4t |
| 85: 15—3 | 42 | 4t | 4t |
| . | Number of fry . | ||
|---|---|---|---|
| Treatment . | Wild fry . | F1 hybrid . | BC1 hybrid . |
| Control 1 | 50 | – | – |
| Control 2t | 30 and 20t | – | – |
| 50:50—1 | 26 | 12t | 12t |
| 50:50—2 | 26t | 12 | 12 |
| 70:30—1 | 36 | 7t | 7t |
| 70:30—2 | 36 | 7t | 7t |
| 70:30—3 | 36 | 7t | 7t |
| 85:15—1 | 42 | 4t | 4t |
| 85:15—2 | 42 | 4t | 4t |
| 85: 15—3 | 42 | 4t | 4t |
t, tagged with Alcian Blue dye. Ratios are given as wild:hybrid.
From 12 to 31 July 2006, before the start of the experiment, the experimental fry (Table 1) were chosen randomly from pooled family tanks (80–100 random wild fry and 12–15 random hybrid fry from each of the four or five replicate family pooled tanks) and were held in the stream environments in equal numbers (50 fry per stream environment). The fry were fed an abundance of food pellets (1/8 tsp, four times a day ad libitum), delivered at the upstream end, and allowed to acclimate to the stream environments. On 1 and 2 August, a few experimental fry were randomly selected for bulk tagging with Alcian Blue dye (Table 1), anaesthetized with clove oil, tagged twice on the caudal fin, and allowed to recover with food for 2 d. The mortality 2–3 d after tagging was minimal (∼0.04%). On 4 and 5 August, all experimental fry were measured (to the nearest mm), weighed (nearest 0.01 g), and placed into a stream environment. During the first week after sampling, the fry were fed an excess of food pellets at the upstream end and allowed to recover from the sampling.
From 14 August 2006, 9 d after the experimental set-up, until the end of the experiment on 11 November 2006 (90 d in all), a limited feeding schedule was initiated to mimic the natural environment and to induce competition among the fry within the simulated stream environment (Keenleyside and Yamamoto, 1962; Symons, 1968). The fry were fed 2–3 times at random times during the day and in random areas (i.e. front or back) of the stream environment (Figure 1; primary spray bar and secondary spray bar location, respectively). If the fry were fed at the upstream end, they received 1/8 tsp of food pellets and if they were fed at the downstream end they received only a “sprinkle” of feed to ensure that the most favourable area in the stream environment would remain the upstream end.
Data for fitness-related traits were collected in two ways. First, dead fry were removed from the stream environment daily and checked for tags. Second, fry were sampled monthly for fork length (nearest mm) and weight (nearest 0.01 g); condition was calculated according to Fulton's condition factor as 100 × weight/length3 (Fulton, 1904). In addition, during each of the three sampling periods, tagged fry were retagged with Alcian Blue dye to reduce the possibility of tag loss in small- and fast-growing fish (Kelly, 1967; Herbinger et al., 1990; Thedinga and Johnson, 1995).
Analysis
Survival, length, weight, and condition over time were analysed in R 2.9.0. Statistical significance was set at the level α = 0.05. Proportional survival was analysed using a weighted binomial generalized linear model (glm). Length, weight, and condition were analysed using ANCOVAs (lm). Forward stepwise model selection on the data was used as an initial exploration of significant terms. The available terms for model selection were days, treatment, cross, population, and interactions among these terms. Length, weight, and condition terms from model selection were inserted into a linear mixed-effects model (lme), with a random effect of tank. Although we could not account for repeated measures of individual fry within tanks in our experimental design, we could account for some of the lack of independence in repeated measures by using linear mixed-effects models with tank as a random effect. Non-significant terms were removed one at a time after analysis of deviance (ANOVA) and an F-test (ANOVA) of the generated binomial and linear mixed-effects models, respectively. Non-significant interaction terms were removed first, followed by non-significant main terms.
Results
Experiment 1: pairwise differences in competitive ability
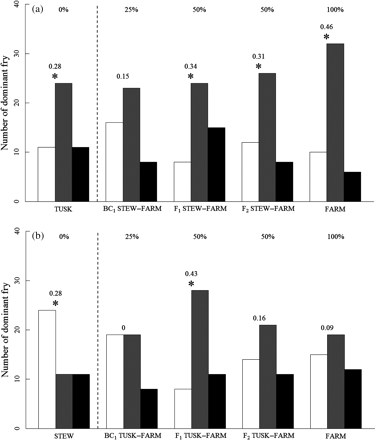
The proportions of STEW contests won by cross, from the highest to the lowest proportional difference, were: FARM > F1 STEW > F2 STEW > TUSK > BC1 STEW > STEW (Figure 2). There were significant differences in the proportion of dominant fry in all STEW pairwise contests except for the STEW vs. BC1 STEW pairing. The proportions of TUSK contests won by cross, from the highest to the lowest proportional difference, were: F1 TUSK > FARM > F2 TUSK > BC1 TUSK = TUSK > STEW (Figure 2). In comparisons involving TUSK, significant differences in the proportions of dominant fry were only detected for the TUSK vs. F1 TUSK and STEW pairings.
Number of dominant fry in the (a) STEW and (b) TUSK pair contests. White bars represent wild fry, grey bars the other cross, and black bars the unresolved contests for the pairs. Percentages reflect the amount of farmed genes in the crosses. Numbers above the bars represent the absolute differences in the proportion of the dominant wild fry and the proportion of the dominant fry of the other cross. Asterisks indicate pairs in which there was a significant difference between the proportion of dominant wild fry and the proportion of dominant fry of the other cross in the pair, using two-sample tests for equality of proportions.
There were more feeding observations than aggressive attack observations (Table 2), and the proportion of feeding observations was significantly different from the proportion of aggressive attack observations (two-sample test for equality of proportions, p < 0.001). STEW fed less than the fry with whom they were paired, whereas TUSK fed just as much as the fry with whom they were paired (two-sample test for equality of proportions, p < 0.001 and p = 0.312, respectively).
Number of feeding observations and aggressive attack observations for cross pairs.
| . | Number of feeding observations . | Number of aggressive attack observations . | ||
|---|---|---|---|---|
| Cross pairs . | Wild . | Competitor . | Wild . | Competitor . |
| STEW vs. | ||||
| TUSK | 5 | 7 | 0 | 0 |
| BC1 STEW–FARM | 0 | 4 | 1 | 2 |
| F1 STEW–FARM | 5 | 10 | 6 | 7 |
| F2 STEW–FARM | 2 | 10 | 0 | 0 |
| FARM | 4 | 26 | 1 | 2 |
| TUSK vs. | ||||
| BC1 TUSK–FARM | 9 | 10 | 2 | 3 |
| F1 TUSK–FARM | 2 | 10 | 1 | 3 |
| F2 TUSK–FARM | 14 | 22 | 1 | 1 |
| FARM | 12 | 5 | 0 | 0 |
| . | Number of feeding observations . | Number of aggressive attack observations . | ||
|---|---|---|---|---|
| Cross pairs . | Wild . | Competitor . | Wild . | Competitor . |
| STEW vs. | ||||
| TUSK | 5 | 7 | 0 | 0 |
| BC1 STEW–FARM | 0 | 4 | 1 | 2 |
| F1 STEW–FARM | 5 | 10 | 6 | 7 |
| F2 STEW–FARM | 2 | 10 | 0 | 0 |
| FARM | 4 | 26 | 1 | 2 |
| TUSK vs. | ||||
| BC1 TUSK–FARM | 9 | 10 | 2 | 3 |
| F1 TUSK–FARM | 2 | 10 | 1 | 3 |
| F2 TUSK–FARM | 14 | 22 | 1 | 1 |
| FARM | 12 | 5 | 0 | 0 |
Number of feeding observations and aggressive attack observations for cross pairs.
| . | Number of feeding observations . | Number of aggressive attack observations . | ||
|---|---|---|---|---|
| Cross pairs . | Wild . | Competitor . | Wild . | Competitor . |
| STEW vs. | ||||
| TUSK | 5 | 7 | 0 | 0 |
| BC1 STEW–FARM | 0 | 4 | 1 | 2 |
| F1 STEW–FARM | 5 | 10 | 6 | 7 |
| F2 STEW–FARM | 2 | 10 | 0 | 0 |
| FARM | 4 | 26 | 1 | 2 |
| TUSK vs. | ||||
| BC1 TUSK–FARM | 9 | 10 | 2 | 3 |
| F1 TUSK–FARM | 2 | 10 | 1 | 3 |
| F2 TUSK–FARM | 14 | 22 | 1 | 1 |
| FARM | 12 | 5 | 0 | 0 |
| . | Number of feeding observations . | Number of aggressive attack observations . | ||
|---|---|---|---|---|
| Cross pairs . | Wild . | Competitor . | Wild . | Competitor . |
| STEW vs. | ||||
| TUSK | 5 | 7 | 0 | 0 |
| BC1 STEW–FARM | 0 | 4 | 1 | 2 |
| F1 STEW–FARM | 5 | 10 | 6 | 7 |
| F2 STEW–FARM | 2 | 10 | 0 | 0 |
| FARM | 4 | 26 | 1 | 2 |
| TUSK vs. | ||||
| BC1 TUSK–FARM | 9 | 10 | 2 | 3 |
| F1 TUSK–FARM | 2 | 10 | 1 | 3 |
| F2 TUSK–FARM | 14 | 22 | 1 | 1 |
| FARM | 12 | 5 | 0 | 0 |
The differences in points gained among fry pairs was not correlated with differences in fry length (Spearman's rank correlation, ρ = 0.095, n = 420, p = 0.051) or weight (Spearman's rank correlation, ρ = −0.084, n = 420, p = 0.083). In addition, tagging had no effect on the dominant fry ranking within pairs (two-sample test for equality of proportions, p = 0.163).
Experiment 2: population level fitness-related traits of wild fry exposed to different proportions of hybrid fry
Replicate loss and tag loss
One STEW 85:15 replicate and one TUSK 50:50 replicate were lost during the experiment because of an accidental transfer of TUSK–FARM hybrids from the TUSK replicate into the STEW replicate. Tag loss was greatest during the first period before first retagging (i.e. 1 August–9 September, 40 d), and tag loss decreased thereafter (Table 3).
Tag loss in the semi-natural stream tank replicates.
| Treatment . | Initial tags (1 August) . | Tags lost (8 September) . | Tags lost (7 October) . | Tags lost (11 November) . | Total . |
|---|---|---|---|---|---|
| STEW | |||||
| Control 2t | 20 | 5 | 0 | 0 | 5 |
| 50:50—1 | 24 | 4 | 1 | 1 | 6 |
| 50:50—2 | 26 | 0 | 3 | 0 | 3 |
| 70:30—1 | 14 | 2 | 0 | 1 | 3 |
| 70:30—2 | 14 | 3 | 0 | 0 | 3 |
| 70:30—3 | 14 | 0 | 0 | 0 | 0 |
| 85:15—1 | 8 | 2 | 0 | 0 | 2 |
| 85:15—2 | 8 | 1 | 0 | 1 | 2 |
| Total | 128 | 17 | 4 | 3 | 24 |
| TUSK | |||||
| Control 2t | 20 | 3 | 0 | 0 | 3 |
| 50:50—2 | 26 | 5 | 0 | 0 | 5 |
| 70:30—1 | 14 | 1 | 0 | 0 | 1 |
| 70:30—2 | 14 | 0 | 1 | 0 | 1 |
| 70:30—3 | 14 | 3 | 0 | 0 | 3 |
| 85:15—1 | 8 | 1 | 0 | 0 | 1 |
| 85:15—2 | 8 | 1 | 0 | 0 | 1 |
| 85:15—3 | 8 | 0 | 0 | 1 | 1 |
| Total | 112 | 14 | 1 | 1 | 16 |
| Treatment . | Initial tags (1 August) . | Tags lost (8 September) . | Tags lost (7 October) . | Tags lost (11 November) . | Total . |
|---|---|---|---|---|---|
| STEW | |||||
| Control 2t | 20 | 5 | 0 | 0 | 5 |
| 50:50—1 | 24 | 4 | 1 | 1 | 6 |
| 50:50—2 | 26 | 0 | 3 | 0 | 3 |
| 70:30—1 | 14 | 2 | 0 | 1 | 3 |
| 70:30—2 | 14 | 3 | 0 | 0 | 3 |
| 70:30—3 | 14 | 0 | 0 | 0 | 0 |
| 85:15—1 | 8 | 2 | 0 | 0 | 2 |
| 85:15—2 | 8 | 1 | 0 | 1 | 2 |
| Total | 128 | 17 | 4 | 3 | 24 |
| TUSK | |||||
| Control 2t | 20 | 3 | 0 | 0 | 3 |
| 50:50—2 | 26 | 5 | 0 | 0 | 5 |
| 70:30—1 | 14 | 1 | 0 | 0 | 1 |
| 70:30—2 | 14 | 0 | 1 | 0 | 1 |
| 70:30—3 | 14 | 3 | 0 | 0 | 3 |
| 85:15—1 | 8 | 1 | 0 | 0 | 1 |
| 85:15—2 | 8 | 1 | 0 | 0 | 1 |
| 85:15—3 | 8 | 0 | 0 | 1 | 1 |
| Total | 112 | 14 | 1 | 1 | 16 |
Tag loss in the semi-natural stream tank replicates.
| Treatment . | Initial tags (1 August) . | Tags lost (8 September) . | Tags lost (7 October) . | Tags lost (11 November) . | Total . |
|---|---|---|---|---|---|
| STEW | |||||
| Control 2t | 20 | 5 | 0 | 0 | 5 |
| 50:50—1 | 24 | 4 | 1 | 1 | 6 |
| 50:50—2 | 26 | 0 | 3 | 0 | 3 |
| 70:30—1 | 14 | 2 | 0 | 1 | 3 |
| 70:30—2 | 14 | 3 | 0 | 0 | 3 |
| 70:30—3 | 14 | 0 | 0 | 0 | 0 |
| 85:15—1 | 8 | 2 | 0 | 0 | 2 |
| 85:15—2 | 8 | 1 | 0 | 1 | 2 |
| Total | 128 | 17 | 4 | 3 | 24 |
| TUSK | |||||
| Control 2t | 20 | 3 | 0 | 0 | 3 |
| 50:50—2 | 26 | 5 | 0 | 0 | 5 |
| 70:30—1 | 14 | 1 | 0 | 0 | 1 |
| 70:30—2 | 14 | 0 | 1 | 0 | 1 |
| 70:30—3 | 14 | 3 | 0 | 0 | 3 |
| 85:15—1 | 8 | 1 | 0 | 0 | 1 |
| 85:15—2 | 8 | 1 | 0 | 0 | 1 |
| 85:15—3 | 8 | 0 | 0 | 1 | 1 |
| Total | 112 | 14 | 1 | 1 | 16 |
| Treatment . | Initial tags (1 August) . | Tags lost (8 September) . | Tags lost (7 October) . | Tags lost (11 November) . | Total . |
|---|---|---|---|---|---|
| STEW | |||||
| Control 2t | 20 | 5 | 0 | 0 | 5 |
| 50:50—1 | 24 | 4 | 1 | 1 | 6 |
| 50:50—2 | 26 | 0 | 3 | 0 | 3 |
| 70:30—1 | 14 | 2 | 0 | 1 | 3 |
| 70:30—2 | 14 | 3 | 0 | 0 | 3 |
| 70:30—3 | 14 | 0 | 0 | 0 | 0 |
| 85:15—1 | 8 | 2 | 0 | 0 | 2 |
| 85:15—2 | 8 | 1 | 0 | 1 | 2 |
| Total | 128 | 17 | 4 | 3 | 24 |
| TUSK | |||||
| Control 2t | 20 | 3 | 0 | 0 | 3 |
| 50:50—2 | 26 | 5 | 0 | 0 | 5 |
| 70:30—1 | 14 | 1 | 0 | 0 | 1 |
| 70:30—2 | 14 | 0 | 1 | 0 | 1 |
| 70:30—3 | 14 | 3 | 0 | 0 | 3 |
| 85:15—1 | 8 | 1 | 0 | 0 | 1 |
| 85:15—2 | 8 | 1 | 0 | 0 | 1 |
| 85:15—3 | 8 | 0 | 0 | 1 | 1 |
| Total | 112 | 14 | 1 | 1 | 16 |
Survival differences
Of the fry, 19.6% died over the 98 d of the experiment. Some of the removed dead fry (8% of all dead fry) were non-identifiable as tagged or untagged individuals owing to advanced decomposition at the time of discovery. Under the assumption that the non-identifiable dead fry were originally untagged fry, binomial models of survival ∼ days + tag revealed that tagged fry had better survival in the STEW and TUSK tagged controls (analysis of deviance, p < 0.001 for both populations). The increase in survival of tagged fry was small for STEW (3%) and larger for TUSK (17%). Under the alternative assumption that the non-identifiable dead fry were originally tagged fry, identical binomial models revealed that tagged fry had poorer survival (4%) in the STEW tagged controls and better survival (12%) in TUSK tagged controls (analysis of deviance, p < 0.001 for both populations).
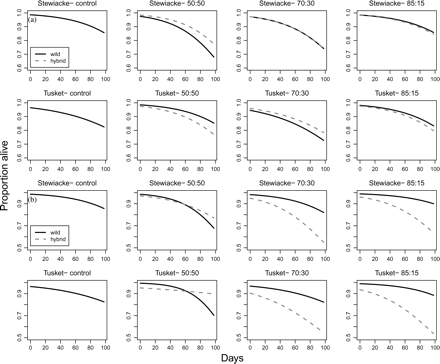
In addition, under the assumption that the non-identifiable dead captures were untagged fry, we detected significant hybrid treatment effects (Table 4, Figure 3). Overall, survival was poorer for both crosses in the 70:30 treatment relative to the 85:15 treatment and control for both populations and for both crosses. There was poorer survival for both crosses in the STEW 50:50 treatment, whereas there was little change in survival for both crosses in the TUSK 50:50 treatment relative to the 85:15 treatment and the control for both crosses. There was no cross effect except for the STEW 50:50 treatment, in which wild STEW fry survived less well than STEW–FARM hybrid fry. Under the alternative assumption that the non-identifiable dead captures were tagged fry, we detected significant hybrid treatment and cross effects (Table 4, Figure 3). There was greater mortality of hybrid fry in 70:30 and 85:15 treatments, and greater mortality of wild fry in 50:50 treatments relative to the control for both populations.
Survival estimates for the treatments over time from the binomial model. Solid lines represent wild fry and dashed lines represent hybrid fry. (a) The assumption is that non-identifiable dead captures were untagged fry. (b) The assumption is that non-identifiable dead captures were tagged fry.
Analysis of deviance of the forward stepwise model on fry survival.
| Terms . | d.f. . | Deviance . | Residual d.f. . | Residual deviance . | Pr(>Chi) . |
|---|---|---|---|---|---|
| Non-identifiable dead captures were untagged fry | |||||
| NULL | – | – | 3 365 | 8 055.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 5 212.2 | ≪0.001 |
| Treatment | 3 | 561.4 | 3 361 | 4 650.8 | ≪0.001 |
| Population | 1 | 62.4 | 3 360 | 4 588.4 | ≪0.001 |
| Cross | 1 | 12.1 | 3 359 | 4 576.3 | ≪0.001 |
| Treatment × population | 3 | 108.2 | 3 356 | 4 468.1 | ≪0.001 |
| Days × population | 1 | 41.2 | 3 355 | 4 426.9 | ≪0.001 |
| Treatment × cross | 2 | 18.4 | 3 352 | 4 408.5 | ≪0.001 |
| Days × treatment | 3 | 22.6 | 3 350 | 4 385.9 | 0.004 |
| Days × treatment × population | 3 | 8.4 | 3 347 | 4 377.5 | ≪0.001 |
| Treatment × population × cross | 3 | 77.6 | 3 344 | 4 299.9 | ≪0.001 |
| Non-identifiable dead captures were tagged fry | |||||
| NULL | – | – | 3 365 | 10 125.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 7 282.2 | ≪0.001 |
| Cross | 1 | 1 669.5 | 3 363 | 5 612.8 | ≪0.001 |
| Treatment | 3 | 372.8 | 3 360 | 5 240.0 | ≪0.001 |
| Population | 1 | 63.9 | 3 359 | 5 176.1 | ≪0.001 |
| Treatment × cross | 2 | 597 | 3 357 | 4 579.1 | ≪0.001 |
| Treatment × population | 3 | 109.4 | 3 354 | 4 469.7 | ≪0.001 |
| Days × population | 1 | 38.9 | 3 353 | 4 430.8 | ≪0.001 |
| Days × treatment | 3 | 18.9 | 3 350 | 4 411.9 | ≪0.001 |
| Days × cross | 1 | 5.8 | 3 349 | 4 406.1 | 0.016 |
| Days × cross × treatment | 2 | 60.3 | 3 347 | 4 345.8 | ≪0.001 |
| Days × treatment × population | 3 | 8.7 | 3 344 | 4 337.2 | 0.034 |
| Treatment × cross × population | 3 | 11 | 3 341 | 4 326.2 | 0.012 |
| Days × cross × treatment × population | 3 | 21.1 | 3 338 | 4 305.1 | ≪0.001 |
| Terms . | d.f. . | Deviance . | Residual d.f. . | Residual deviance . | Pr(>Chi) . |
|---|---|---|---|---|---|
| Non-identifiable dead captures were untagged fry | |||||
| NULL | – | – | 3 365 | 8 055.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 5 212.2 | ≪0.001 |
| Treatment | 3 | 561.4 | 3 361 | 4 650.8 | ≪0.001 |
| Population | 1 | 62.4 | 3 360 | 4 588.4 | ≪0.001 |
| Cross | 1 | 12.1 | 3 359 | 4 576.3 | ≪0.001 |
| Treatment × population | 3 | 108.2 | 3 356 | 4 468.1 | ≪0.001 |
| Days × population | 1 | 41.2 | 3 355 | 4 426.9 | ≪0.001 |
| Treatment × cross | 2 | 18.4 | 3 352 | 4 408.5 | ≪0.001 |
| Days × treatment | 3 | 22.6 | 3 350 | 4 385.9 | 0.004 |
| Days × treatment × population | 3 | 8.4 | 3 347 | 4 377.5 | ≪0.001 |
| Treatment × population × cross | 3 | 77.6 | 3 344 | 4 299.9 | ≪0.001 |
| Non-identifiable dead captures were tagged fry | |||||
| NULL | – | – | 3 365 | 10 125.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 7 282.2 | ≪0.001 |
| Cross | 1 | 1 669.5 | 3 363 | 5 612.8 | ≪0.001 |
| Treatment | 3 | 372.8 | 3 360 | 5 240.0 | ≪0.001 |
| Population | 1 | 63.9 | 3 359 | 5 176.1 | ≪0.001 |
| Treatment × cross | 2 | 597 | 3 357 | 4 579.1 | ≪0.001 |
| Treatment × population | 3 | 109.4 | 3 354 | 4 469.7 | ≪0.001 |
| Days × population | 1 | 38.9 | 3 353 | 4 430.8 | ≪0.001 |
| Days × treatment | 3 | 18.9 | 3 350 | 4 411.9 | ≪0.001 |
| Days × cross | 1 | 5.8 | 3 349 | 4 406.1 | 0.016 |
| Days × cross × treatment | 2 | 60.3 | 3 347 | 4 345.8 | ≪0.001 |
| Days × treatment × population | 3 | 8.7 | 3 344 | 4 337.2 | 0.034 |
| Treatment × cross × population | 3 | 11 | 3 341 | 4 326.2 | 0.012 |
| Days × cross × treatment × population | 3 | 21.1 | 3 338 | 4 305.1 | ≪0.001 |
Analysis of deviance of the forward stepwise model on fry survival.
| Terms . | d.f. . | Deviance . | Residual d.f. . | Residual deviance . | Pr(>Chi) . |
|---|---|---|---|---|---|
| Non-identifiable dead captures were untagged fry | |||||
| NULL | – | – | 3 365 | 8 055.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 5 212.2 | ≪0.001 |
| Treatment | 3 | 561.4 | 3 361 | 4 650.8 | ≪0.001 |
| Population | 1 | 62.4 | 3 360 | 4 588.4 | ≪0.001 |
| Cross | 1 | 12.1 | 3 359 | 4 576.3 | ≪0.001 |
| Treatment × population | 3 | 108.2 | 3 356 | 4 468.1 | ≪0.001 |
| Days × population | 1 | 41.2 | 3 355 | 4 426.9 | ≪0.001 |
| Treatment × cross | 2 | 18.4 | 3 352 | 4 408.5 | ≪0.001 |
| Days × treatment | 3 | 22.6 | 3 350 | 4 385.9 | 0.004 |
| Days × treatment × population | 3 | 8.4 | 3 347 | 4 377.5 | ≪0.001 |
| Treatment × population × cross | 3 | 77.6 | 3 344 | 4 299.9 | ≪0.001 |
| Non-identifiable dead captures were tagged fry | |||||
| NULL | – | – | 3 365 | 10 125.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 7 282.2 | ≪0.001 |
| Cross | 1 | 1 669.5 | 3 363 | 5 612.8 | ≪0.001 |
| Treatment | 3 | 372.8 | 3 360 | 5 240.0 | ≪0.001 |
| Population | 1 | 63.9 | 3 359 | 5 176.1 | ≪0.001 |
| Treatment × cross | 2 | 597 | 3 357 | 4 579.1 | ≪0.001 |
| Treatment × population | 3 | 109.4 | 3 354 | 4 469.7 | ≪0.001 |
| Days × population | 1 | 38.9 | 3 353 | 4 430.8 | ≪0.001 |
| Days × treatment | 3 | 18.9 | 3 350 | 4 411.9 | ≪0.001 |
| Days × cross | 1 | 5.8 | 3 349 | 4 406.1 | 0.016 |
| Days × cross × treatment | 2 | 60.3 | 3 347 | 4 345.8 | ≪0.001 |
| Days × treatment × population | 3 | 8.7 | 3 344 | 4 337.2 | 0.034 |
| Treatment × cross × population | 3 | 11 | 3 341 | 4 326.2 | 0.012 |
| Days × cross × treatment × population | 3 | 21.1 | 3 338 | 4 305.1 | ≪0.001 |
| Terms . | d.f. . | Deviance . | Residual d.f. . | Residual deviance . | Pr(>Chi) . |
|---|---|---|---|---|---|
| Non-identifiable dead captures were untagged fry | |||||
| NULL | – | – | 3 365 | 8 055.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 5 212.2 | ≪0.001 |
| Treatment | 3 | 561.4 | 3 361 | 4 650.8 | ≪0.001 |
| Population | 1 | 62.4 | 3 360 | 4 588.4 | ≪0.001 |
| Cross | 1 | 12.1 | 3 359 | 4 576.3 | ≪0.001 |
| Treatment × population | 3 | 108.2 | 3 356 | 4 468.1 | ≪0.001 |
| Days × population | 1 | 41.2 | 3 355 | 4 426.9 | ≪0.001 |
| Treatment × cross | 2 | 18.4 | 3 352 | 4 408.5 | ≪0.001 |
| Days × treatment | 3 | 22.6 | 3 350 | 4 385.9 | 0.004 |
| Days × treatment × population | 3 | 8.4 | 3 347 | 4 377.5 | ≪0.001 |
| Treatment × population × cross | 3 | 77.6 | 3 344 | 4 299.9 | ≪0.001 |
| Non-identifiable dead captures were tagged fry | |||||
| NULL | – | – | 3 365 | 10 125.6 | – |
| Days | 1 | 2 843.3 | 3 364 | 7 282.2 | ≪0.001 |
| Cross | 1 | 1 669.5 | 3 363 | 5 612.8 | ≪0.001 |
| Treatment | 3 | 372.8 | 3 360 | 5 240.0 | ≪0.001 |
| Population | 1 | 63.9 | 3 359 | 5 176.1 | ≪0.001 |
| Treatment × cross | 2 | 597 | 3 357 | 4 579.1 | ≪0.001 |
| Treatment × population | 3 | 109.4 | 3 354 | 4 469.7 | ≪0.001 |
| Days × population | 1 | 38.9 | 3 353 | 4 430.8 | ≪0.001 |
| Days × treatment | 3 | 18.9 | 3 350 | 4 411.9 | ≪0.001 |
| Days × cross | 1 | 5.8 | 3 349 | 4 406.1 | 0.016 |
| Days × cross × treatment | 2 | 60.3 | 3 347 | 4 345.8 | ≪0.001 |
| Days × treatment × population | 3 | 8.7 | 3 344 | 4 337.2 | 0.034 |
| Treatment × cross × population | 3 | 11 | 3 341 | 4 326.2 | 0.012 |
| Days × cross × treatment × population | 3 | 21.1 | 3 338 | 4 305.1 | ≪0.001 |
Length, weight, and condition differences
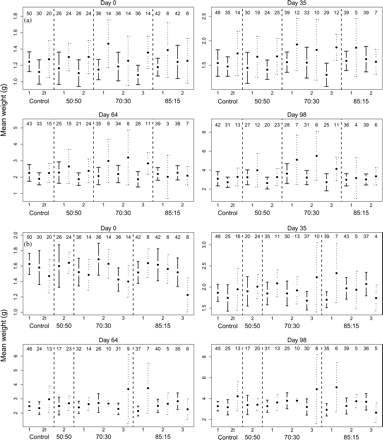
Hybrid treatment had a non-significant effect on length, weight, and condition, but there were cross effects (Table 5). A cross effect on size was expected because there are genetic differences in the growth rates for these crosses raised in common-garden environments (Lawlor, 2003; Fraser et al., in press). Overall, these results suggested that the proportion of hybrids within a stream environment did not affect length and weight. In addition, the length and weight of wild fry in the replicates containing hybrids also did not differ from the length and weight of the wild fry in the controls (weight; Figure 4). In other words, the means of the wild fish within the replicates with hybrids remained within the 95% confidence intervals of the controls during the experimental period. In addition, hybrid treatment had a non-significant effect on condition over time (Table 5).
Confidence intervals (error bars) of the mean weight (dots) of fry in (a) STEW and (b) TUSK replicates. Numbers represent the number of observations used in the calculation of the 95% confidence intervals, i.e. twice the standard error of the estimate. Solid confidence intervals are wild fry and dashed confidence intervals are hybrid fry, except for the controls in which the solid confidence intervals are wild non-tagged fry and dashed confidence intervals are tagged wild fry. Note that (i) the gain in untagged fry is attributable to tag loss, (ii) three tagged fry were assigned as untagged fry in (b) 70:30—1 on Day 35 (this mistake was discovered on Day 64), and (iii) there is a gain of one fry in (b) Control 2t from Day 64 to Day 98 (this fry was probably not sampled on Day 64).
F-tests of linear mixed-effects models of length, weight, and condition.
| Terms . | Numerator d.f. . | Denominator d.f. . | F-value . | p-value . |
|---|---|---|---|---|
| Length | ||||
| Intercept | 1 | 3 186 | 135 073.36 | ≪0.001 |
| Days | 1 | 3 186 | 1 532.44 | ≪0.001 |
| Population | 1 | 3 186 | 133.13 | ≪0.001 |
| Cross | 1 | 3 186 | 74.53 | ≪0.001 |
| Days × cross | 1 | 3 186 | 8.07 | 0.005 |
| Population × cross | 1 | 3 186 | 3.95 | 0.047 |
| Weight | ||||
| Intercept | 1 | 3 187 | 8 322.558 | ≪0.001 |
| Days | 1 | 3 187 | 970.271 | ≪0.001 |
| Population | 1 | 3 187 | 43.758 | ≪0.001 |
| Cross | 1 | 3 187 | 30.418 | ≪0.001 |
| Days × cross | 1 | 3 187 | 13.417 | ≪0.001 |
| Condition | ||||
| Intercept | 1 | 3 188 | 59 037.58 | ≪0.001 |
| Days | 1 | 3 188 | 12.85 | ≪0.001 |
| Cross | 1 | 3 188 | 10.12 | 0.002 |
| Days × cross | 1 | 3 188 | 7.48 | 0.006 |
| Terms . | Numerator d.f. . | Denominator d.f. . | F-value . | p-value . |
|---|---|---|---|---|
| Length | ||||
| Intercept | 1 | 3 186 | 135 073.36 | ≪0.001 |
| Days | 1 | 3 186 | 1 532.44 | ≪0.001 |
| Population | 1 | 3 186 | 133.13 | ≪0.001 |
| Cross | 1 | 3 186 | 74.53 | ≪0.001 |
| Days × cross | 1 | 3 186 | 8.07 | 0.005 |
| Population × cross | 1 | 3 186 | 3.95 | 0.047 |
| Weight | ||||
| Intercept | 1 | 3 187 | 8 322.558 | ≪0.001 |
| Days | 1 | 3 187 | 970.271 | ≪0.001 |
| Population | 1 | 3 187 | 43.758 | ≪0.001 |
| Cross | 1 | 3 187 | 30.418 | ≪0.001 |
| Days × cross | 1 | 3 187 | 13.417 | ≪0.001 |
| Condition | ||||
| Intercept | 1 | 3 188 | 59 037.58 | ≪0.001 |
| Days | 1 | 3 188 | 12.85 | ≪0.001 |
| Cross | 1 | 3 188 | 10.12 | 0.002 |
| Days × cross | 1 | 3 188 | 7.48 | 0.006 |
F-tests of linear mixed-effects models of length, weight, and condition.
| Terms . | Numerator d.f. . | Denominator d.f. . | F-value . | p-value . |
|---|---|---|---|---|
| Length | ||||
| Intercept | 1 | 3 186 | 135 073.36 | ≪0.001 |
| Days | 1 | 3 186 | 1 532.44 | ≪0.001 |
| Population | 1 | 3 186 | 133.13 | ≪0.001 |
| Cross | 1 | 3 186 | 74.53 | ≪0.001 |
| Days × cross | 1 | 3 186 | 8.07 | 0.005 |
| Population × cross | 1 | 3 186 | 3.95 | 0.047 |
| Weight | ||||
| Intercept | 1 | 3 187 | 8 322.558 | ≪0.001 |
| Days | 1 | 3 187 | 970.271 | ≪0.001 |
| Population | 1 | 3 187 | 43.758 | ≪0.001 |
| Cross | 1 | 3 187 | 30.418 | ≪0.001 |
| Days × cross | 1 | 3 187 | 13.417 | ≪0.001 |
| Condition | ||||
| Intercept | 1 | 3 188 | 59 037.58 | ≪0.001 |
| Days | 1 | 3 188 | 12.85 | ≪0.001 |
| Cross | 1 | 3 188 | 10.12 | 0.002 |
| Days × cross | 1 | 3 188 | 7.48 | 0.006 |
| Terms . | Numerator d.f. . | Denominator d.f. . | F-value . | p-value . |
|---|---|---|---|---|
| Length | ||||
| Intercept | 1 | 3 186 | 135 073.36 | ≪0.001 |
| Days | 1 | 3 186 | 1 532.44 | ≪0.001 |
| Population | 1 | 3 186 | 133.13 | ≪0.001 |
| Cross | 1 | 3 186 | 74.53 | ≪0.001 |
| Days × cross | 1 | 3 186 | 8.07 | 0.005 |
| Population × cross | 1 | 3 186 | 3.95 | 0.047 |
| Weight | ||||
| Intercept | 1 | 3 187 | 8 322.558 | ≪0.001 |
| Days | 1 | 3 187 | 970.271 | ≪0.001 |
| Population | 1 | 3 187 | 43.758 | ≪0.001 |
| Cross | 1 | 3 187 | 30.418 | ≪0.001 |
| Days × cross | 1 | 3 187 | 13.417 | ≪0.001 |
| Condition | ||||
| Intercept | 1 | 3 188 | 59 037.58 | ≪0.001 |
| Days | 1 | 3 188 | 12.85 | ≪0.001 |
| Cross | 1 | 3 188 | 10.12 | 0.002 |
| Days × cross | 1 | 3 188 | 7.48 | 0.006 |
Linear models of length and weight ∼ days + tag revealed that tagged fry did not have reduced length and weight. Linear models of condition ∼ tag revealed that tagging had no significant effect on fry condition (ANOVA, p > 0.500 for both populations). The initial tag loss was not believed to be a confounding influence on the results because the replicates with low or no tag loss (e.g. Table 3; STEW, 50:50—2 and 70:30—3; TUSK, 70:30—1 and 85:15—1) displayed similar patterns of changing wild and hybrid length and weight means over time compared with replicates with greater tag loss.
Discussion
Pairwise differences in competitive ability
Salmon fry from the wild populations (STEW and TUSK) examined here differed in competitive ability, TUSK being better competitors than STEW. Population-level competitive differences, such as those documented here, have previously been interpreted as adaptive responses to different levels of predator biomass (Rosenau and McPhail, 1987; Swain and Holtby, 1989) and to selection for differential growth rates (higher levels of growth hormone leading to increased appetite and, hence, aggression; Johnsson and Björnsson, 1994; Fleming and Einum, 1997; Fleming et al., 2002). Although TUSK fry grow faster than STEW (Lawlor, 2003; Fraser et al., in press), there is insufficient information on predation or other factors related to growth during juvenile stages to determine whether the aggression differences documented here reflect adaptive responses to different environments.
FARM was either a superior or equal competitor relative to STEW and TUSK, respectively. Other studies that have size-matched pairs of fry have found that farmed fry are better competitors than wild fry (e.g. Einum and Fleming, 1997; Fleming and Einum, 1997; Blann and Healey, 2006; but see Fenderson and Carpenter, 1971; Berejikian et al., 1996). One possible explanation for the equal competitive abilities of FARM and TUSK may be related to growth rate, as discussed above; TUSK has a marginally faster growth rate than FARM (Lawlor, 2003; Fraser et al., in press).
The rank change in the competitive abilities of hybrids relative to wild salmon also differed between STEW and TUSK populations. STEW fry were poorer competitors, whereas TUSK fry were just as competitive relative to their respective wild–farmed hybrids (except for the TUSK vs. F1 TUSK–FARM pairing). The rank change in competitive abilities of hybrids was related to the amount of farmed genes for STEW comparisons, whereas the increased competitive ability of F1 TUSK–FARM perhaps may be attributable to heterosis. Heterosis is expected to halve in the second generation because of recombination loss (Falconer and MacKay, 1996) and may have been lost in F2 TUSK–FARM because these hybrids were just as competitive as TUSK. Einum and Fleming (1997) observed similar situations to STEW and TUSK with wild–farmed hybrids from Norway's Imsa and Lone Rivers, respectively. Our results suggest that the intermediate competitive abilities of STEW–FARM hybrids could be attributable to additive genetic variation, whereas the competitive abilities of TUSK–FARM hybrids could be attributable to non-additive genetic factors, e.g. dominance, overdominance, and epistasis.
Population level fitness-related traits in relation to increasing proportions of hybrids
There was a reduction in fitness-related traits in the form of increased mortality but not in the form of decreased body size for certain levels of treatment. In general, the increase in mortality did not follow a gradient of increasing proportions of hybrids, nor was it greater for wild fry than for hybrid fry for all treatments, as predicted and empirically supported in a similar study (Devlin et al., 2004). The only exception was that wild fry had greater mortality than hybrid fry in the STEW 50:50 treatments. Overall, mortality for both wild and hybrid fry was greatest in the treatment with the largest proportion of hybrids, i.e. 50:50 (STEW only) and 70:30 (STEW and TUSK). Mortality may have increased for both types of fry because of competitive displacement by a greater proportion of dominant fry relative to the other two treatments, i.e. 85:15 and the control. Both subdominant wild and hybrid fry may have had limited access to food, leading to increased metabolic demands and other physiological stresses when competing with dominant fry; this may have contributed to their reduced survival (Ruzzante, 1994). These mortality results are subject to the assumption that the non-identifiable dead fry (8% of all dead fry) were originally untagged, which seems more likely than the alternative that the non-identifiable dead fry had been tagged originally. During the sampling periods, for example, the number of untagged fry in some of the replicates was greater than the number of untagged fry present at the beginning of the experiment (see Figure 4 for the numbers).
Population level fitness-related traits in relation to pairwise comparisons
At the population level in semi-natural stream environments, the results from pairwise competitive ability tests could not be used to predict the change in magnitude of fitness-related traits for two different wild populations. For example, the pairwise competitive ability comparisons suggested that both wild populations would achieve similar changes in fitness-related traits in the semi-natural stream environments. In other words, for both wild populations, F1 hybrids were better competitors than their wild counterparts, and BC1 hybrids were just as competitive as their wild counterpart. However, wild fry experienced increased mortality relative to hybrid fry in the STEW 50:50 treatment, whereas this situation was not observed for the TUSK 50:50 treatment. A possible explanation for the difference is that the pairwise competitive ability comparisons were based on ranks and were not a quantitative measure of the change in magnitude of hybrids relative to their wild counterparts. If there had been a greater change in the magnitude of the competitive abilities of STEW–FARM hybrid fry relative to TUSK–FARM hybrid fry, then wild STEW fry should have performed more poorly when exposed to their STEW–FARM hybrids. Collectively, at least for the life stage examined (and the density of fish), there appears to be more at work here than simply the rank difference in competitive ability between wild and hybrids in determining the consequences imposed on wild fish exposed to wild–farmed hybrids.
Potential impacts of the observed interactions between farmed and wild fish
Our results may have a bearing on the potential impacts that differential rates of farmed intrusion could have on contrasting wild populations. Specifically, at a life stage where competitive interactions may have a strong influence on fitness (Mills, 1989), wild populations were not being impacted by the presence of low frequencies of hybrids (≤15%). However, the population growth rate of wild populations may have been negatively affected when hybrids comprised a greater proportion within populations (≥30%) because of the increased mortality of wild fry (except TUSK, which did not experience increased mortality at a hybrid proportion of 50%). The semi-natural stream environments used in our research may have been reasonable representations of the wild environment for the life stage examined. For example, the fry exhibited behaviours in our stream environments analogous to those expected in the wild (cf. Fausch, 1984; Metcalfe et al., 1989). Moreover, during feeding, fry would hold a position from which they would dart up to obtain a food pellet and return to that position before securing another pellet (cf. Gibson, 1993).
Our results also suggest that there should, perhaps, be greater concern for some of the salmon populations exposed to higher frequencies of hybrids in the wild than for others. For example, relative to TUSK, STEW appears to receive greater inputs of FARM (e.g. 33% FARM in 1995 for STEW and ∼1% FARM in 1998 for TUSK, but these are almost certainly underestimates; Morris et al., 2008), potentially leading to greater hybrid production in the STEW population. Furthermore, relative to TUSK, more wild fry died in STEW than the hybrids when exposed to greater presence of hybrids (≥50%). Regardless of the inability to predict the consequences to wild fry in the presence of hybrid fry by simply assessing pairwise competitive abilities, the wild populations experienced increased mortality in the semi-natural stream environments for which the hybrid proportion was ≥30%. In addition to the increased mortality associated with these behavioural interactions, comparatively high (≥30%) proportional representations of hybrid fish might also be of concern because of the probability that many wild genotypes will have been replaced by farmed genotypes through wild–farmed interbreeding. Indeed, the literature suggests than even minimal wild–farmed interbreeding in the wild can have a negative effect on the persistence of wild populations because of genetic alteration, such as the loss of local adaptation and increased risk-taking behaviour in hybrids, and because of other wild–farmed ecological interactions (reviewed in Gross, 1998; Naylor et al., 2005; Jonsson and Jonsson, 2006).
In terms of interactions between farmed and wild salmon in eastern North America, our study is relevant given that farmed Atlantic salmon continue to be detected in many wild populations within a 300-km radius of most aquaculture activity (Morris et al., 2008). Nevertheless, we caution that our research was based on experimentation under semi-natural conditions, and it considered only one life stage (and density). Therefore, it did not fully represent the environmental conditions to which our study populations are normally exposed (e.g. Fraser et al., 2008, in press). Moreover, our work assumed that the fry used in our study, whose parents and grandparents had been reared in a laboratory environment, did not experience any genetic and behavioural adaptation relative to fry normally conceived and reared in the wild (reviewed in Fraser, 2008). Notwithstanding these caveats, the present study represents an appropriate point of departure from which to examine wild–farmed salmon competitive interactions by testing hypotheses using common-garden experimentation, including multiple populations in which salmon of farmed origin intrude on wild-spawning events.
Acknowledgements
The work was supported by the Natural Sciences and Engineering Research Council (Canada) through an undergraduate student research award to ASH, a post-doctoral fellowship to DJF, and a Strategic Grant to JAH. ASH was also supported by an Atlantic Salmon Federation Olin Fellowship. The manuscript was significantly improved by the comments received from two referees. We thank, at Dalhousie University, C. Herbinger, D. Ruzzante, J. Eddington (Aquatron Facility), M. Merrimen, K. Tae, M. Vickaryous, R. Myers, C. Smith, M. Kluge, E. Adams, L. Goodbrand, L. Weir, P. Debes, M. Morris, and W. Blanchard. We also thank P. Amiro (Department of Fisheries and Oceans Nova Scotia Region), Lourdes (Mada Medical Products Inc., New Jersey), and Clive (Mada Medical Products Inc., New Jersey).