-
PDF
- Split View
-
Views
-
Cite
Cite
Karin Hüssy, Why is age determination of Baltic cod (Gadus morhua) so difficult?, ICES Journal of Marine Science, Volume 67, Issue 6, September 2010, Pages 1198–1205, https://doi.org/10.1093/icesjms/fsq023
Close - Share Icon Share
Abstract
The aim of this study was to evaluate the consistency of three methods for assigning annuli in adult Baltic cod otoliths. The methods examined were (i) daily increment patterns, (ii) opacity profiles, and (iii) traditional age reading. Frequency distributions of the distance from the nucleus to the different zones showed that the first annulus of traditional age reading missed the first zone of both increment and opacity methods, but overlapped with the second zone identified by these methods. This pattern did not continue over subsequent zones. Frequency distributions of increment patterns were similar to opacity patterns. However, within individual fish, the co-occurrence of overlap between the two patterns was random. In cases where there was overlap, translucent zone formation started just before the disappearance of visible increments. Overlap in 1 year did not necessarily lead to a consistent pattern the following year, and overlap was not influenced by sex or fish size. The results suggest that otolith opacity in Baltic cod is not associated with seasonal patterns in daily increment structure and that traditional age determination based on otolith opacity yields highly uncertain estimates of age.Hüssy, K. 2010. Why is age determination of Baltic cod (Gadus morhua) so difficult? – ICES Journal of Marine Science, 67: 1198–1205.
Introduction
Since the first description of periodic patterns of opaque and translucent growth structures in otoliths and their relationship with fish age (Reibisch, 1899), the use of this technique has become globally accepted and is used for routine age determination of many fish species (Campana, 2001; Campana and Thorrold, 2001). Translucent zones have traditionally been called “winter rings” and counted to determine the age of a fish (Pannella, 1974; Smedstad and Holm, 1996). In most Northeast Atlantic ecosystems, cod (Gadus morhua) experience more or less pronounced variations in temperature which lead to distinct, annual opacity patterns in otolith macrostructure (Weidman and Millner, 2000; Høie and Folkvord, 2006). Recently, evidence has been found for several stocks that these zones may actually form during summer, but still following an annual pattern (Pilling et al., 2007). At high temperatures, metabolic maintenance costs are high and feeding may be restricted (Jobling, 1988; Björnsson and Steinarsson, 2002). Hence, a temperature effect on growth and deposition may actually be supplemented by a starvation effect.
Typically, stocks from areas with smaller seasonal temperature signals, such as the Faroe Islands and Baltic Sea, show less contrast between opaque and translucent zones (CODYSSEY, 2007). However, in the Faroese stock, annually recurring features give an accurate estimate of the age of the fish (Doering-Arjes et al., 2008). The eastern Baltic cod seems to diverge from the general pattern. Although the amplitude of seasonal temperature variations is greater than for Faroese cod, the visual contrast in their otoliths is low. The unique combinations of prolonged spawning season (MacKenzie et al., 2000; Wieland et al., 2000), the heterogeneous hydrography of the Baltic Sea (Matthäus and Franck, 1992; Schinke and Matthäus, 1998), coupled with interacting seasonal variations in feeding intensity (Bagge, 1981; Maczassek, 2006) and temperature have been hypothesized to result in an optically rather uniform otolith structure lacking a strong seasonal variation in opacity (Hüssy et al., 2009). Also, there are confounding non-annual structures at apparently irregular intervals (Berner, 1968). As a consequence, annual rings are not clearly defined, are not necessarily laid down at regular intervals, and age determination by traditional methods may therefore be subject to serious inconsistencies (Reeves, 2003; ICES, 2006).
These conditions call for rigorous validation of annulus formation. Today, a suite of technologies and methods is available, most of which include examination of biological tags/features in the otolith or mark/recapture (see review in Campana, 2001). No such validation studies yet exist for eastern Baltic cod. Based on the known temperature dependence of otolith accretion (Mosegaard and Titus, 1987; Volk et al., 1990; Otterlei et al., 2002; Hüssy and Mosegaard, 2004) and periodic cycles of increment patterns observed in the otoliths of adult fish, Hüssy et al. (2010) proposed that these patterns could be used for age estimation and hence validation of annulus formation.
The aim of the present study is therefore to evaluate whether patterns of increment widths and opacity match, whether they are influenced by conditions the year before, and how they are influenced by fish size and sex. This assessment is then followed up with an evaluation of how these patterns compare with the structures identified as annuli by traditional age readers.
Material and methods
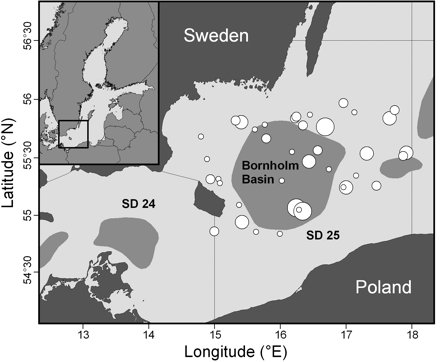
The samples used in this study were the same as those in the study of Hüssy et al. (2010). Cod <40 cm were selected randomly from the Danish Baltic International Trawl Survey from the first quarter of the years 2001 and 2004 in ICES Subdivision (SD) 25 (Figure 1). Fish were measured to the nearest centimetre and weighed (g) on board. Sagittal otoliths were removed, cleaned, and stored individually in labelled paper bags. None of the fish were spawning, confirming their stock origin as eastern Baltic cod, the spawning period of which extends from April to September as opposed to that of western Baltic (SD 22–24) cod, which spawn in February and March (Wieland et al., 2000).
The locations of eastern Baltic cod samples in ICES SD 25, 2001 and 2004 combined. The spawning area in the Bornholm Basin is shown; white bubbles, sampling stations, where the size indicates the number of cod analysed (size ranges 1–5, 6–10, 11–15, and 16–25).
Daily increment patterns
A segment was cut from the central transverse plane of the otolith (ISOMET 1000 Buehler), fixed on a microscope slide with thermoplastic glue (Buehler Thermoplastic Cement no. 40-8100), and ground to the central plane on a rotating disc with abrasive paper (grit 30 to 0.3 µm) to a thickness of ∼200 µm and polished with 1.0 µm alumina paste. Otolith sections were viewed under a microscope (Leica DMLB) and the images digitized (Leica DFC320 camera and Leica IM 50 framegrabber) using a standard set-up (8 bit/channel with a frame of 2048 × 1536 pixels, exposure 100–500 ms).
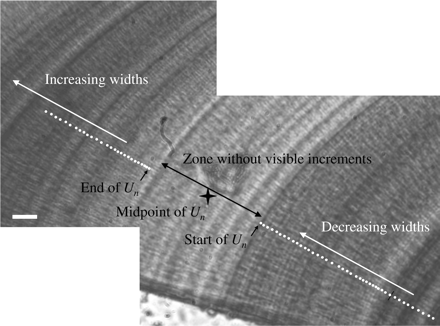
Daily growth increments were examined at a magnification of 0.08 µm pixel−1, based on otolith optical characteristics in the form of a profile of grey values (caliper tool of IMAGE PRO), ranging between 0 (black) and 255 (pure white). The start of an increment was defined as a rising point of inflection between the previously formed opaque zone and the subsequent transparent zone and was calculated from the divergence of individual pixel grey values from the running average. Distance from nucleus on progressing days i was calculated as Distancei = Distancei−1 + Incrementi. Areas of the profile where increments were not visible were treated by recording the width of these zones and calculating the relative otolith radius at which increments were again visible, following the same procedure. This resulted in growth profiles from the centre to the edge with individual increment widths in relation to distance from the nucleus. These sections of the otoliths revealed a series of zones with clearly distinguishable increments, with increasing/decreasing widths in a dome-shaped pattern, interrupted by zones where there was no visible regular increment structure. The width of the zones averaged 115 µm (range 40–250 µm), and hereafter they are referred to as U-zones. The distances from the otolith centre to the midpoints of zones without visible increments is hereafter referred to as U1, U2, and U3, corresponding to the zones formed during the first, second, and third winter and calculated as Un = (Unend − Unstart)/2 (Figure 2).
Example of a zone of a Baltic cod otolith without a visible increment, and the measurements used to calculate the midpoint of the zone (star). Bar = 10 µm.
Reader-defined marks
To assess the overlap between increment patterns and traditional age determination, the location of annuli used for the latter was recorded. Otolith sections were viewed under a stereomicroscope (Leica MZ6) at a magnification of 2.8 µm pixel−1 using reflected light in a standardized set-up (IBACS, 2006). Images were digitized using a standard set-up (8 bit/channel with a frame of 2048 × 1536 pixels, exposure 107.9 ms). The midpoint of each zone counted by expert readers as an annulus was marked with the “measurement” tool of IMAGE PRO (v. 5.0). For comparison with daily increment patterns, the ventral axis was selected. Subsequently, the distance of each mark to the nucleus was measured and counted as R1, R2, and R3, referring to these reader-defined annuli as R-zones.
Opacity-derived marks
To obtain an objective measure of annulus location, profiles of opacity were recorded under reflected light along the same growth axis, from nucleus to ventral otolith edge. Profile values range between 0 (black) and 255 (pure white), the lower values associated with less opacity. Therefore, an annulus appears as “dark” owing to its lower opacity. The opacity profiles were then run through the following routine. First, a second-order polynomial function was fitted to the data for each cod individually. Residuals were calculated for each pixel (profile values − function estimate) and smoothed with a running average over 50 µm for the entire profile. The smoothed residuals were then run through a simple analysis, where a negative value was assigned as a translucent ring, and a positive value as the intermittent growth period. To exclude noise, e.g. through cracks in the sample, or bubbles under the otolith section, assignment of a translucent ring was restricted to zones ≥30 µm, corresponding to the smallest zone without daily increments. The location of a translucent ring was then recorded as the distance from the nucleus to the midpoint within each zone of negative residuals, where the midpoint of a zone was assigned as a U-zone. Translucent rings were numbered starting from the nucleus as T1, T2, T3, and T4 (with this procedure, there were four marks in five fish). The zones with low opacity were thereafter referred to as T-zones.
Overlap
Overlap was defined where any translucent ring (T1, T2, T3, or T4) co-occurred within a zone of ±150 µm from the midpoint of an individual zone without visible increments (U1, U2, U3). Hence, overlap does not include possible additional low-opacity zones, but only relates to low-opacity patterns in zones without visible increments. A value of 1 indicates overlap between zones, and 0 no overlap. The 300-µm interval was chosen based on the widest zone without visible increments found in these cod otoliths to allow for some flexibility regarding the opacity values and the low contrast between the growth zones of Baltic cod. In all, 31 and 27 sections with reader-defined annuli, increment patterns, and opacity profiles from centre to edge were prepared for 2001 and 2004, respectively.
Statistical analyses
All statistical analyses were carried out in R (R Development Core Team, 2009). Continuous data were tested for normality and homogeneity of variances using a Shapiro–Wilk normality test and the Bartlett test of homogeneity of variances. For normality and variance homogeneity, data were compared with ANOVA. Non-normally distributed data were tested with the Kolmogorov–Smirnov (from now on KS) two-sample tests, and overlap patterns with G-tests.
Results
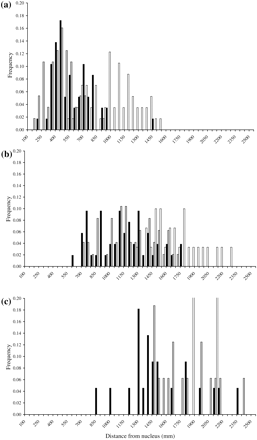
The frequency distributions of distances from the nucleus to zones identified by the three methods differed markedly from each other (Figure 3). There were no significant differences in frequency distributions between years of U-zones (U1, U2, and U3), R-zones (R1 and R2), or T-zones (T1, T2, T3, and T4; KS, all p > 0.05). Data from the two years were therefore combined for all the three methods.
Frequency distributions of distance to the nucleus for the different zones derived from the three different methods by consecutive annuli: (a) first annulus, (b) second annulus, and (c) third annulus. Bar colours: black, opacity profile; white, expert reader-defined annuli; grey, daily increment patterns. In (c), the reader-defined annulus frequencies were shortened for consistency between graphs and the two samples actually correspond to a frequency of 0.5.
Reader-defined annuli and translucent zones
The frequency distribution of T-zones differed considerably from that of R-zones (Figure 3). There were significant differences with respect to both the first annulus (Figure 3a; KS, D = 0.6165, p < 0.001) and the second annulus (Figure 3b; KS, D = 0.7551, p < 0.001), but the distribution of the first reader-defined annulus (R1) corresponded to the second translucent ring (T2, KS, D = 0.247, p = 0.073). This implies that the first zone with lower opacity is not counted as an annulus by traditional age-reading methods. The pattern did not, however, persist over the next age class, because the distributions of T3 and R2 differed significantly (Figure 3c; KS, D = 0.4121, p < 0.05). Therefore, although experts at age reading use opacity patterns for assigning annuli, interpretation of the patterns seems to be very subjective for Baltic cod. Owing to the lack of significance in frequency distributions with reader-defined annuli, overlap with these zones was not examined in further detail.
Reader-defined annuli and zones without visible increments
The frequency distribution of U-zones differed considerably from that of R-zones (Figure 3). The mode of U1 was at 450 µm, smaller than the smallest values of R1 (Figure 3a). This difference was highly significant (KS, D = 0.8224, p < 0.001), as was the difference between U2 and R2 (Figure 3b; KS, D = 0.0.6542, p < 0.001). The distribution of the second zone without visible increments (U2) did not correspond to the first reader-defined annulus (R1; KS, D = 0.3037, p < 0.05), nor did U3 correspond to R2 (KS, D = 0.1458, p = 0.95). This implies that the first winter zone (without visible increments) is missed by traditional age-reading methods. Hence, there is no consistent pattern in assignment of zones between the two methods, and for that reason, overlap between these zones was not examined in further detail.
To evaluate whether it was the presence of the first zone only that influenced age readings, the proportions of otoliths within each age class were evaluated based on counting the seasonal increment patterns (Hüssy et al., 2010) and by traditional age determination methods. In general, traditional age determination seems to have a tendency to underestimate the assumed true age from increment patterns, particularly for the older year classes in that the risk of assigning a wrong (younger) age increases. Apparently, there must be discrepancies other than interpreting the first annulus. The results do demonstrate, however, that there are serious differences between the two methods and that these differences are inconsistent between the two years examined here (Table 1).
Proportion of traditional ages within classes of age estimates from increment patterns for 2001 and 2004, respectively.
| . | . | Traditional age . | ||
|---|---|---|---|---|
| Year . | Increment age . | 1 . | 2 . | 3 . |
| 2001 | 1 | 0.91 | 0.07 | 0.01 |
| 2 | 0.35 | 0.65 | 0.00 | |
| 3 | 0.05 | 0.74 | 0.21 | |
| 2004 | 1 | 0.64 | 0.35 | 0.01 |
| 2 | 0.05 | 0.86 | 0.10 | |
| 3 | 0.00 | 0.50 | 0.50 | |
| . | . | Traditional age . | ||
|---|---|---|---|---|
| Year . | Increment age . | 1 . | 2 . | 3 . |
| 2001 | 1 | 0.91 | 0.07 | 0.01 |
| 2 | 0.35 | 0.65 | 0.00 | |
| 3 | 0.05 | 0.74 | 0.21 | |
| 2004 | 1 | 0.64 | 0.35 | 0.01 |
| 2 | 0.05 | 0.86 | 0.10 | |
| 3 | 0.00 | 0.50 | 0.50 | |
Emboldened values indicate the fields where a value of 1 would indicate total correspondence between the two methods.
Proportion of traditional ages within classes of age estimates from increment patterns for 2001 and 2004, respectively.
| . | . | Traditional age . | ||
|---|---|---|---|---|
| Year . | Increment age . | 1 . | 2 . | 3 . |
| 2001 | 1 | 0.91 | 0.07 | 0.01 |
| 2 | 0.35 | 0.65 | 0.00 | |
| 3 | 0.05 | 0.74 | 0.21 | |
| 2004 | 1 | 0.64 | 0.35 | 0.01 |
| 2 | 0.05 | 0.86 | 0.10 | |
| 3 | 0.00 | 0.50 | 0.50 | |
| . | . | Traditional age . | ||
|---|---|---|---|---|
| Year . | Increment age . | 1 . | 2 . | 3 . |
| 2001 | 1 | 0.91 | 0.07 | 0.01 |
| 2 | 0.35 | 0.65 | 0.00 | |
| 3 | 0.05 | 0.74 | 0.21 | |
| 2004 | 1 | 0.64 | 0.35 | 0.01 |
| 2 | 0.05 | 0.86 | 0.10 | |
| 3 | 0.00 | 0.50 | 0.50 | |
Emboldened values indicate the fields where a value of 1 would indicate total correspondence between the two methods.
Translucent zones and zones without visible increments
As interpretation of opacity patterns for traditional age determination is a highly subjective procedure, an attempt was made to identify zones with less opacity using a purely subjective method. The frequency distribution of these zones (T1–T4) differs from the others in that up to four translucent zones were recorded.
None of the distributions of zone T1 (Figure 3a; KS, D = 0.2574, p = 0.46) or T2 (Figure 3b; KS, D = 0.1394, p = 0.72) differed significantly from the corresponding U-zone distributions. The distribution of T3 and U3 were significantly different (Figure 3c; KS, D = 0.5455, p < 0.01), but those of T4 and U3 did not differ (KS, D = 0.2125, p = 0.98). These results imply that zones of low opacity usually co-occur with zones characterized by an absence of visible increments.
To test this hypothesis of overlap in zones within individual otoliths, an index of overlap was calculated for each U-zone of each otolith. In Table 2, the proportions of fish with and without overlapping zones are shown for each of the three U-zones. Seemingly, there was overlap in approximately half the cod, without any obvious trend with age.
Overlap between zones without increments (U-zones) and low opacity (O-zones), with a value of 0 indicating no overlap, and a value of 1 indicating overlap.
| . | . | . | Overlap . | |
|---|---|---|---|---|
| Year . | Zone . | n . | 0 . | 1 . |
| 2001 | U1 | 29 | 0.48 | 0.52 |
| U2 | 23 | 0.61 | 0.39 | |
| U3 | 11 | 0.73 | 0.27 | |
| 2004 | U1 | 27 | 0.48 | 0.52 |
| U2 | 23 | 0.48 | 0.52 | |
| U3 | 4 | 0.75 | 0.25 | |
| . | . | . | Overlap . | |
|---|---|---|---|---|
| Year . | Zone . | n . | 0 . | 1 . |
| 2001 | U1 | 29 | 0.48 | 0.52 |
| U2 | 23 | 0.61 | 0.39 | |
| U3 | 11 | 0.73 | 0.27 | |
| 2004 | U1 | 27 | 0.48 | 0.52 |
| U2 | 23 | 0.48 | 0.52 | |
| U3 | 4 | 0.75 | 0.25 | |
Overlap between zones without increments (U-zones) and low opacity (O-zones), with a value of 0 indicating no overlap, and a value of 1 indicating overlap.
| . | . | . | Overlap . | |
|---|---|---|---|---|
| Year . | Zone . | n . | 0 . | 1 . |
| 2001 | U1 | 29 | 0.48 | 0.52 |
| U2 | 23 | 0.61 | 0.39 | |
| U3 | 11 | 0.73 | 0.27 | |
| 2004 | U1 | 27 | 0.48 | 0.52 |
| U2 | 23 | 0.48 | 0.52 | |
| U3 | 4 | 0.75 | 0.25 | |
| . | . | . | Overlap . | |
|---|---|---|---|---|
| Year . | Zone . | n . | 0 . | 1 . |
| 2001 | U1 | 29 | 0.48 | 0.52 |
| U2 | 23 | 0.61 | 0.39 | |
| U3 | 11 | 0.73 | 0.27 | |
| 2004 | U1 | 27 | 0.48 | 0.52 |
| U2 | 23 | 0.48 | 0.52 | |
| U3 | 4 | 0.75 | 0.25 | |
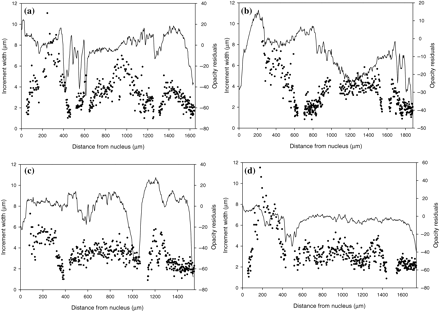
To evaluate whether overlap during the first zone entrains overlap in subsequent years, otoliths with two or more uncountable zones (age ≥2 years) were selected. In Figure 4, examples are shown for four 2-year-old cod with different overlap patterns. Because of the limited number of fish with three U-zones (15 cod), those data were not included in the analysis. There was no significant effect of year (G-test, G = 3.124, d.f. = 3, p = 0.99), so data from the two years are combined in Table 3. No consistent pattern in overlap between age classes was evident, in that the distribution into the four possible categories of overlap patterns was completely equal (χ2 test, χ2 = 0.435, p = 0.99). In those cases where there was an overlap, the translucent zones seemed to be found before the zone without visible increments was laid down, because the relationship between the two zones was T1 = 0.94 × U1 (n = 29, r2 = 0.21, p < 0.05) and T2 = 0.82 × U2 (n = 23, r2 = 0.18, p < 0.05). I conclude that opacity patterns are not useful as indicators of zones without visible increments.
Overlap between zones without increments (U-zones) and low opacity for consecutive ages (U1 and U2).
| U1 . | U2 . | ntotal . | nfemale . | nmale . | Fish size . |
|---|---|---|---|---|---|
| 0 | 0 | 12 | 5 | 7 | 24.4 (7.4) |
| 0 | 1 | 11 | 3 | 8 | 23.7 (7.6) |
| 1 | 0 | 13 | 4 | 9 | 24.3 (5.2) |
| 1 | 1 | 10 | 6 | 4 | 23.4 (7.1) |
| U1 . | U2 . | ntotal . | nfemale . | nmale . | Fish size . |
|---|---|---|---|---|---|
| 0 | 0 | 12 | 5 | 7 | 24.4 (7.4) |
| 0 | 1 | 11 | 3 | 8 | 23.7 (7.6) |
| 1 | 0 | 13 | 4 | 9 | 24.3 (5.2) |
| 1 | 1 | 10 | 6 | 4 | 23.4 (7.1) |
n, number of otoliths, and fish size is the average (s.d.) of fish size (cm). Overlap values: 0, no overlap; 1, overlap.
Overlap between zones without increments (U-zones) and low opacity for consecutive ages (U1 and U2).
| U1 . | U2 . | ntotal . | nfemale . | nmale . | Fish size . |
|---|---|---|---|---|---|
| 0 | 0 | 12 | 5 | 7 | 24.4 (7.4) |
| 0 | 1 | 11 | 3 | 8 | 23.7 (7.6) |
| 1 | 0 | 13 | 4 | 9 | 24.3 (5.2) |
| 1 | 1 | 10 | 6 | 4 | 23.4 (7.1) |
| U1 . | U2 . | ntotal . | nfemale . | nmale . | Fish size . |
|---|---|---|---|---|---|
| 0 | 0 | 12 | 5 | 7 | 24.4 (7.4) |
| 0 | 1 | 11 | 3 | 8 | 23.7 (7.6) |
| 1 | 0 | 13 | 4 | 9 | 24.3 (5.2) |
| 1 | 1 | 10 | 6 | 4 | 23.4 (7.1) |
n, number of otoliths, and fish size is the average (s.d.) of fish size (cm). Overlap values: 0, no overlap; 1, overlap.
Patterns of increment width (dots) and opacity (lines) in four 2-year-old cod with different overlap sequences over the two zones. The numbers in parenthesis following indicate the distance from the nucleus to the first and second zone without increments: (a) overlap in both years (500 and 1300 µm), (b) no overlap (650 and 1550 µm), (c) overlap in the second but not the first year (450 and 1100 µm), (d) overlap in the first but not the second year (450 and 1500 µm).
To assess whether factors such as size or sex could influence overlap, fish were grouped into four groups: overlap/no overlap in U1, and overlap/no overlap in U2. No significant differences in fish size were found between these groups (Table 3; ANOVA, F = 0.173, d.f. = 45, p = 0.91). There was no significant effect of size on the number of otoliths within each category (G-test, G = 2.701, d.f. = 3, p = 0.95), demonstrating that neither fish size nor sex are the driving forces behind differential overlap in increment and opacity patterns.
Discussion
Translucent zones are formed during winter/spring in the otoliths of most temperate fish species of the northern hemisphere (Beckman and Wilson, 1995), and Atlantic cod are no exception (Smedstad and Holm, 1996; Godø and Michalsen, 2000; Pilling et al., 2007; Høie et al., 2009). Several conditions have been hypothesized as key factors, e.g. somatic growth related to food consumption (Geffen and Nash, 1995; Wright et al., 2002a), reproduction (Morales-Nin et al., 1998), photoperiod (Wright et al., 1992), and environmental temperature (Pilling et al., 2007). However, the underlying mechanisms of zone formation, including regional differences, are still poorly understood (Pilling et al., 2007).
Although only little is known of opacity pattern formation in eastern Baltic cod, it is certain that otolith macrostructure formation does not conform to the general pattern. From the few existing studies, it is apparent that translucent zones form virtually throughout the year (Tokareva, 1963), with the greatest frequency from September to May (Tokareva, 1963; Berner, 1968). However, these patterns are not consistent across years (Mosegaard et al., 1997). Also there seems to be an age effect, in that juvenile and young adult fish initiate and end translucent zones earlier in the year (Tokareva, 1963; Berner, 1968). Until recently, no empirical explanation for these irregular opacity patterns was available. Speculations have been many, including genetic and phenotypic differences associated with temperature adaptation (Kändler, 1949; Berner, 1968).
To evaluate the timing of translucent zone formation and the laying down of reader-defined structures, I used seasonal patterns in daily increment widths, which are closely linked to the environmental temperature and hence may serve as an estimator of time (Hüssy et al., 2010). The few reports examining the co-occurrence of daily increment and opacity patterns show that in species from temperate waters, patterns during annulus formation seem to follow a rule: decreasing increments, eventually with disruption in daily formation, along with a translucent zone in winter/spring (Pannella, 1980; Victor and Brothers, 1982; Wright et al., 2002b). Translucent zones may also be laid down between annuli, but they are usually less prominent, and their increment widths do not differ from adjacent increments (Wright et al., 2002b).
The results of this study have demonstrated that there is no general synchrony in the formation of otolith zones without daily increments related to cold water temperatures and translucent zones. The presence of pattern overlap seems to be random and was not even consistent within individual cod, in that overlap in one year did not necessarily entrain overlap the next year. In those cases where overlap was observed, patterns were slightly out of phase, because translucent zones seemed to be laid down before the zone without visible increments. Neither sex nor fish size had a significant influence on overlap. Kändler (1949) suggested a link between hydrographic conditions in the central Baltic Sea and the vertical distribution and migration patterns of cod, which the results of a recent tagging study seem to support (Hüssy et al., 2010). Hence, I now examine possible mechanisms for these deviations from the general pattern within different life stages.
In the Baltic Sea, younger cod are mainly distributed in shallower water, where they experience stronger temperature cycles than at depth (Bagge et al., 1994; Uzars, 1994, Uzars and Plikshs, 2000), with temperatures between 2 and 3°C in February and ca. 15°C in August/September (ICES, 2009). In juvenile fish and coastal areas in general, feeding intensity peaks in late summer (August; Maczassek, 2006), so the seasonal maxima and minima of feeding and temperature cycles are only slightly out of phase, with last food consumption just before the time of lowest temperature. This synchrony explains the overlap in zones without visible increments with translucent zones, and the appearance of translucent bands before the coldest period. However, overlap was found only in about half the samples, perhaps because of the distribution patterns of juvenile cod, which also live in deeper water (Nielsen et al., 1997), where the peaks of maximum/minimum temperatures are later in the year (ICES, 2009). With the present samples, it was unfortunately impossible to resolve this hypothesis.
In adult cod, additional variation may be introduced. Baltic cod mature at 27 cm and an age of 2.2 years (ICES, 2009), so start their summer spawning migrations into the central Bornholm Basin with the onset of colder temperature. With these migrations, the seasonal pattern in feeding changes, both with respect to prey species and quantity of food consumed (Zalachowski et al., 1975; Bagge, 1981; Bagge and Bay, 1987; Bagge et al., 1994; Maczassek, 2006). Maximum food consumption is in May and the seasonal low in summer (Bagge, 1981; Maczassek, 2006). In reproductively active cod, the seasonal maxima and minima in the temperature and feeding cycles are therefore out of phase, explaining the decoupling between zones without increments and transparent zones, but not the inconsistency between years. Recent evidence from electronic data storage tags shows that the temperature response of opacity formation depends on migration behaviour and the timing of the migration (Hüssy et al., 2010). Inconsistency in pattern formation between individuals and years may therefore be attributable to skipped spawning, which is known for Baltic cod (Tomkiewicz et al., 2003). Also, the prolonged spawning season in Baltic cod (from April through September; Wieland et al., 2000) may act to enforce variability between individuals, resulting in the observed lack of a general annual periodicity in transparency patterns.
It is therefore unsurprising that age determination of Baltic cod based on counting annuli is a difficult task and that the estimates of age are subject to inconsistencies (Reeves, 2003; ICES, 2006). The study has shown that the first true annulus is apparently missed by age readers, but that the reader-defined first annulus corresponds to the first translucent zone. Kändler (1949) realized that the first annulus of particularly late-hatched cod may not be clearly defined owing to the small size of the otoliths. Subsequent reader-defined annuli do not overlap with zones lacking increments, and only slightly with translucent zones. All this shows clearly how subjective the interpretation of eastern Baltic cod otoliths can be and supports the need for further scientific evaluation of growth zones and age. Apparently, seasonal patterns in daily increment widths can be used to validate at least the first annulus, but this study has shown that the age readings of older fish are questionable. This poses a serious problem to stock assessment, because even small errors in age determination resulting in over- or underestimation influences the values of fishing mortality and spawning-stock biomass (Reeves, 2003). The results here seemingly call for the development and implementation of new methods of age determination, possibly using increment width analysis, and particularly to validate the true age of Baltic cod.
Acknowledgements
The study was funded by the EU Open Call for Tenders—Reference FISH/2006/15: “ImproveD mEthodology for Baltic COD age Estimation” (DECODE). I thank B. Nielsen, National Institute of Aquatic Resources, Technical University of Denmark, and Hanna Wróblewska, Sea Fisheries Institute, Poland, for preparing the otolith slides, and two anonymous reviewers whose comments helped improve an earlier draft of the manuscript.