-
PDF
- Split View
-
Views
-
Cite
Cite
Felippe A. Postuma, Maria A. Gasalla, On the relationship between squid and the environment: artisanal jigging for Loligo plei at São Sebastião Island (24°S), southeastern Brazil, ICES Journal of Marine Science, Volume 67, Issue 7, October 2010, Pages 1353–1362, https://doi.org/10.1093/icesjms/fsq105
Close - Share Icon Share
Abstract
The squid Loligo plei concentrates in the southeastern Brazil Bight, where it has traditionally supported small-scale fisheries around São Sebastião Island (SSI). Sea surface temperature (SST), chlorophyll-a (Chl a), windspeed, wave height, rainfall, and lunar phase are related to fishing records and to the results of a survey of local fishers to investigate how they believe environmental variables might affect catches of L. plei. Daily fishery-dependent data over the years 2005–2009 were obtained from a fishing cooperative and were matched with satellite and meteorological forecast data. Generalized linear models were used to explore the significance of environmental variables in relation to variability in catch and catch per unit effort (cpue). Squid are fished with jigs in water shallower than 20 m, generally where SST is warmer and Chl a and windspeed are lower. Cpue and monthly catches decreased from 2005 to 2008, followed by a slight increase in 2009. The correlations between fishery and environmental data relate well to fishers' oceanological knowledge, underscoring the potential of incorporating such knowledge into evaluations of the fishery.Postuma, F. A., and Gasalla, M. A. 2010. On the relationship between squid and the environment: artisanal jigging for Loligo plei at São Sebastião Island (24°S), southeastern Brazil. – ICES Journal of Marine Science, 67: 1353–1362.
Introduction
The relationships between the productivity of commercial squid and climate and/or oceanography have been described for several species and fisheries around the world. Squid life-history plasticity, with fast individual growth rates and rapid rates of turnover at a population level, means that these organisms can respond quickly to environmental or ecosystem change (Rodhouse, 2005; Pecl and Jackson, 2008).
Correlations between environmental variables and resource productivity have been found for many ommastrephid squid, which contribute a relatively large proportion of the world's commercial catch of squid. For example, the recruitment of Illex argentinus in the southern Atlantic correlates with sea surface temperature (SST; Waluda et al., 1999), the abundance of Illex illecebrosus fluctuates seemingly in concert with the North Atlantic Oscillation (NAO; Dawe et al., 2000), and the population structure and the distribution patterns of Illex coindetii in the eastern Ionian Sea are related to the thermohaline circulation (Lefkaditou et al., 2008). Changes in environmental parameters such as SST and chlorophyll also influence the spawning areas of Todarodes pacificus in the Sea of Japan (Sakurai et al., 2000) and the fishing of Ommastrephes bartramii in the northwestern Pacific (Chen et al., 2007; Wei et al., 2009).
For loliginids, there is also evidence of strong links between squid productivity and the environment. For instance, catches of Loligo reynaudii in South Africa have been related to changes in the ecosystem, with SST influencing spawning and mating (Roberts, 1998, 2005), and catches of Loligo opalescens decrease after El Niño Southern Oscillation (ENSO) events in the southern California Bight (Maxwell et al., 2004). In northern Europe, links between the abundance of Loligo forbesi and SST have been identified (Bellido et al., 2001; Pierce and Boyle, 2003; Chen et al., 2006). The oceanography also influences the migration of Patagonian long-finned squid (Loligo gahi), which tends to follow cold water masses (5.5°C), which influence squid maturation directly (Arkhipkin et al., 2004). Correlations of SST and other ocean proxies with catch per unit effort (cpue) are potentially important for developing forecasts to support the management of fisheries generally (Pierce et al., 2008). In terms of the present potential for climate change, such analyses are also relevant at a local level, especially in ecosystems classified as warming hotspots, e.g. the southern Brazilian shelf (Heileman and Gasalla, 2008; Belkin, 2009).
In the southern portion of the Brazilian continental shelf, two loliginids (Loligo plei and Loligo sanpaulensis) exist in considerable concentrations (Perez, 2002; Gasalla et al., 2005; Martins and Perez, 2006, 2007; Rodrigues and Gasalla, 2008). Their importance appears to have been increasing in the fisheries, with increased availability to the market and other members of the ecosystem (Gasalla et al., 2005; Gasalla, 2009). The neritic L. plei is found from the coast of Florida, USA (Hixon et al., 1980), to Rio Grande do Sul, Brazil (Perez et al., 2005). It is a warm temperate species with the southernmost limit of its distribution associated with the influence of the Brazil Current (Haimovici and Perez, 1991; Martins and Perez, 2007). Loligo plei spawns throughout the year, peaking during summer, when fishing fleets take advantage of the spawning concentrations between 40 and 100 m deep (Perez et al., 2002; Rodrigues and Gasalla, 2008). The size structure and maturity of L. plei taken by fisheries may vary seasonally; larger, mature squid concentrate closer to the coast, and smaller immature ones deeper and farther from shore (Martins and Perez, 2007; Rodrigues and Gasalla, 2008).
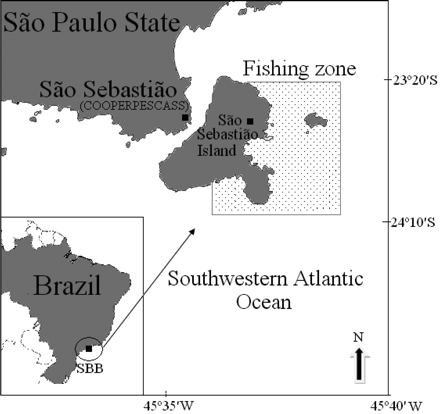
In Brazil, the Loligo plei fishery is concentrated primarily in three coastal states: Rio de Janeiro, around latitude 23°S (Costa and Haimovici, 1990), São Paulo, between 24°10 and 23°40′S, and Santa Catarina, from 26 to 28°S (Perez, 2002; Martins and Perez, 2007). It operates primarily in shallow water between November and March, but the trawl fishery targeting shrimps regularly takes L. plei as bycatch over the shelf throughout the year (Rodrigues and Gasalla, 2008). In terms of small-scale, artisanal squid fisheries, one of the most traditional operates in the central region of the southeastern Brazilian Bight, particularly around nearshore islands off the north coast of São Paulo (Figure 1). São Sebastião Island (SSI) is a focal point of traditional activity, where fishers, including women and children, use home-made jigs (locally referred to as zangarilhos) with handlines to capture the squid. Fishers' income increases during summer when squid and tourists concentrate at the coast (Gasalla, 2005).
Map of the study area in the southeastern Brazil Bight (SBB), Southwest Atlantic Ocean (the area shown with dots is the squid-related fishing zone).
The island is located off the north coast of the state of São Paulo, between 23°40 and 24°10′S and 45°35 and 45°00′W, in the southern Brazilian Bight (Figure 1). The oceanography, in terms of the structure and dynamics of water masses around SSI, seems to be typical of the local continental shelf, with wind-driven intrusion of South Atlantic Central Water (SACW) being the most relevant physical process for the ecosystem (Castro et al., 2008). The SACW, which is carried below the Brazilian Current, is cold and nutrient-rich, stimulating pelagic productivity whenever it reaches the photic zone. Other water masses in the area include shelf water, derived from the upper layers of SACW, and coastal water, which is a result of mixing. During summer, cold water upwells off northeastern and western SSI, shaping a stratified layer ∼10 m deep. The meeting of different water masses when the surface temperature is usually warmer results in a thermocline with a stable water column in between, which is of biological importance (Castro et al., 2008; Saldanha-Corrêa and Gianesella, 2008).
Here, we aim to relate the patterns of catch and cpue of L. plei in the local fishery around SSI with a suite of environmental variables to investigate the major influences on squid catches. In addition, the findings are compared with the results of interviews seeking information on fishers' knowledge of the species–environment relationship.
Methods
Table 1 summarizes the data collected, including fishery-dependent information, environmental data, and face-to-face interviews with local squid fishers.
Data sources, sample size, and other information used in this study.
| Source . | Sample size . | Type of information . | Period . |
|---|---|---|---|
| Squid fishing landings—local fishing cooperative (COOPERPESCASS) | 1 478 | Landings (kg) per vessel, per day | January 2005–March 2009 |
| Satellite database (ANTARES/SeaWiFS) | 527 d | SST and Chl a | March 2005–March 2009 |
| Prediction models (NOAA–NCEP) | 527 d | Windspeed (Ws), wave height (H), and rainfall (Rf) | March 2005–March 2009 |
| Interviews with fishers | 103 | Environmental conditions at the time of fishing, and effort-related parameters | November 2002–March 2009 |
| Source . | Sample size . | Type of information . | Period . |
|---|---|---|---|
| Squid fishing landings—local fishing cooperative (COOPERPESCASS) | 1 478 | Landings (kg) per vessel, per day | January 2005–March 2009 |
| Satellite database (ANTARES/SeaWiFS) | 527 d | SST and Chl a | March 2005–March 2009 |
| Prediction models (NOAA–NCEP) | 527 d | Windspeed (Ws), wave height (H), and rainfall (Rf) | March 2005–March 2009 |
| Interviews with fishers | 103 | Environmental conditions at the time of fishing, and effort-related parameters | November 2002–March 2009 |
Data sources, sample size, and other information used in this study.
| Source . | Sample size . | Type of information . | Period . |
|---|---|---|---|
| Squid fishing landings—local fishing cooperative (COOPERPESCASS) | 1 478 | Landings (kg) per vessel, per day | January 2005–March 2009 |
| Satellite database (ANTARES/SeaWiFS) | 527 d | SST and Chl a | March 2005–March 2009 |
| Prediction models (NOAA–NCEP) | 527 d | Windspeed (Ws), wave height (H), and rainfall (Rf) | March 2005–March 2009 |
| Interviews with fishers | 103 | Environmental conditions at the time of fishing, and effort-related parameters | November 2002–March 2009 |
| Source . | Sample size . | Type of information . | Period . |
|---|---|---|---|
| Squid fishing landings—local fishing cooperative (COOPERPESCASS) | 1 478 | Landings (kg) per vessel, per day | January 2005–March 2009 |
| Satellite database (ANTARES/SeaWiFS) | 527 d | SST and Chl a | March 2005–March 2009 |
| Prediction models (NOAA–NCEP) | 527 d | Windspeed (Ws), wave height (H), and rainfall (Rf) | March 2005–March 2009 |
| Interviews with fishers | 103 | Environmental conditions at the time of fishing, and effort-related parameters | November 2002–March 2009 |
Local landings
Research on and monitoring of the fishery around SSI began in 2002, with visits to fishing communities and landing points for biological sampling. Following a pilot phase, it was considered that the most representative source of information on squid fishery landings was the São Sebastião Fishing Cooperative (COOPERPESCASS), where 80% of the vessels operating around SSI land their catches. Data systematically recorded included daily squid catches per vessel, and the cooperative's administration provided sales records of all squid landed from 2005 to 2009. The data included vessel name (motorized boats and wooden canoes), landing date, and total catch per fishing trip (in kg). Unfortunately, information on the duration of each fishing trip was not available.
During visits to the local cooperative landing site, we identified the squid species as L. plei in all cases. In all, information on 1478 fishing trips, resulting from 527 d of fishing over five seasons (2005, 2005/2006, 2006/2007, 2007/2008, and 2008/2009) were compiled. During 2005, data included only the period January–March. All records were digitized during the visits to the fishing cooperative and combined with related information; each datapoint collected at the cooperative is equivalent to a fishing trip of duration at least 1 d (Table 1).
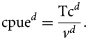
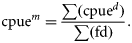
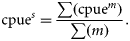
Environmental data
Environmental data were obtained from two sources (Table 1). Satellite data were provided by the ANTARES Project (http://www.dsr.inpe.br/antares), where the variables SST (°C) and chlorophyll-a concentration (Chl a, g mm−3) were obtained. The project's data source is the satellite-based SeaWiFS Program (NASA), which runs twice a day with a resolution of 82 km2. The search area covered a rectangle between latitudes 23°40 and 24°10′S and longitudes 45°35 and 45°00′W. Complementary data on rainfall (Rf, mm3), windspeed (Ws, m s−2), and wave height (H, m) were obtained from modelled forecasts (Global Forecast System, GFS) available from the US National Center for Environmental Prediction of the National Oceanic and Atmospheric Administration (NCEP–NOAA). The GFS runs four times per day out to 384 h, with a resolution of 55 km2 (www.ncep.noaa.gov). Daily data were confronted with fishing records and selected according to the corresponding day of squid fishing activity. Simple monthly means were calculated for the variables. In addition, moon-phase data between 2005 and 2009 were obtained and compared with daily fishing information (DHESP, 2010).
Interviews with fishers
In all, 103 fishers were interviewed at landing points and in fishing communities in the municipalities of São Sebastião and Ilhabela (São Paulo), ∼80% of all the squid fishers in the region (n = 129). Semi-structured questionnaires were given to fishers selected by a “snowball” procedure, in which people from the community and the interviewees themselves identified other people to be interviewed (Bailey, 1982). Semi-structured interviews provided a flexible and informal technique of listening through open questions, allowing more dialogue than structured interviews. Questionnaires addressed squid fishing procedures, recent catches, and information on environmental conditions deemed favourable for squid fishing. The methodology followed the ethno-oceanographic approach detailed in Gasalla and Diegues (in press), and it also included technical and fishing-effort-related questions. The perception of the fishers on the environmental conditions at the time of fishing and on squid stock status and availability (present vs. past proportions) was sought during the interviews.
Data analysis
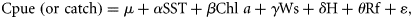
The best fit of the models was assessed using Akaike's information criterion (AIC). When a model involving q parameters was fitted to the data, the AIC was defined as −Lq + 2q, where Lq is the maximum log-likelihood (Akaike, 1974). Wald's statistics (W) and their p-levels were used to test the significance of each GLM regression coefficient (Agresti, 2002).
The significant relationships identified from the GLM, i.e. AIC and W, between environmental variables, and catch and cpue were then further explored using cross-correlation plots, with the aim of determining whether there were time-lagged effects, with a significance level of p < 0.05 for time-lags of months (Shumway and Stoffer, 2000). Statistical analyses were run using STATISTICA, version 9.0 (StatSoft).
Results
Squid fishing around SSI
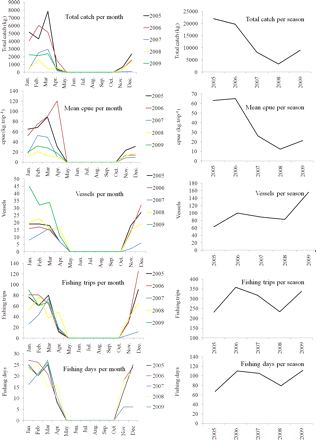
The average cpue of the squid fishery during summer around SSI was 37.5 kg (±51.3 s.d.; range 8.76–120.47 kg) of L. plei per fishing trip. Fishing effort increased between 2006 and 2009 in terms of fishing days and trips. Monthly catches were highest in March 2005 and February 2006, 7857.79 and 6035.59 kg, respectively (Table 2). Catch and cpue tended to peak at the end of each season (January–April; Figure 3). Local availability of L. plei appears to have decreased, however, because both cpues and total catch decreased between 2005/2006 and 2007/2008, though they increased slightly in 2008/2009. The number of fishing trips around SSI ranged from 6 to 120 per month. The number of squid vessels fishing in 2005 was 63, but increased to 156 in the 2009 fishing season (Figure 3).
Monthly information on squid fishing during the period January 2005–March 2009.
| Fishing season . | Month . | Total catch (kg) . | Vessels . | Fishing days . | Fishing trips . | Mean cpue (kg trip−1) . |
|---|---|---|---|---|---|---|
| 2005 | January 2005 | 5 157.91 | 19 | 25 | 77 | 64.85 |
| February 2005 | 4 298.05 | 19 | 20 | 61 | 68.17 | |
| March 2005 | 7 857.79 | 18 | 25 | 80 | 89.16 | |
| April 2005 | 3 929.35 | 7 | 8 | 13 | 30.60 | |
| 21 243.10 | 63 | 78 | 231 | 63.20 | ||
| 2005/2006 | November 2005 | 653.90 | 18 | 13 | 30 | 23.70 |
| December 2005 | 2 381.99 | 27 | 25 | 91 | 31.21 | |
| January 2006 | 4 051.75 | 16 | 27 | 81 | 52.09 | |
| February 2006 | 6 035.59 | 17 | 26 | 81 | 74.75 | |
| March 2006 | 5 171.34 | 15 | 21 | 64 | 89.83 | |
| April 2006 | 1 471.50 | 7 | 9 | 11 | 120.47 | |
| 19 766.07 | 100 | 121 | 358 | 65.34 | ||
| 2006/2007 | November 2006 | 454.76 | 14 | 18 | 29 | 13.47 |
| December 2006 | 1 581.58 | 32 | 24 | 125 | 14.27 | |
| January 2007 | 476.99 | 8 | 16 | 26 | 17.26 | |
| February 2007 | 2 527.61 | 12 | 21 | 44 | 52.59 | |
| March 2007 | 2 971.08 | 16 | 26 | 74 | 49.14 | |
| April 2007 | 208.42 | 7 | 11 | 19 | 11.27 | |
| 8 220.45 | 89 | 116 | 317 | 26.33 | ||
| 2007/2008 | November 2007 | 52.56 | 2 | 6 | 6 | 8.76 |
| December 2007 | 129.35 | 6 | 6 | 12 | 9.99 | |
| January 2008 | 545.79 | 20 | 22 | 52 | 11.33 | |
| February 2008 | 1 617.41 | 23 | 25 | 77 | 20.90 | |
| March 2008 | 425.32 | 16 | 15 | 38 | 12.65 | |
| April 2008 | 604.80 | 16 | 16 | 49 | 10.99 | |
| 3 375.24 | 83 | 90 | 234 | 12.43 | ||
| 2008/2009 | November 2008 | 454.74 | 15 | 18 | 37 | 12.66 |
| December 2008 | 1 428.00 | 19 | 22 | 65 | 17.96 | |
| January 2009 | 2 253.50 | 45 | 24 | 90 | 19.87 | |
| February 2009 | 2 151.70 | 32 | 20 | 61 | 31.98 | |
| March 2009 | 2 386.70 | 34 | 27 | 68 | 28.02 | |
| April 2009 | 316.00 | 11 | 11 | 17 | 18.38 | |
| 8 990.64 | 156 | 122 | 338 | 21.48 | ||
| Total | 61 595.48 | 491 | 527 | 1 478 | 37.33 |
| Fishing season . | Month . | Total catch (kg) . | Vessels . | Fishing days . | Fishing trips . | Mean cpue (kg trip−1) . |
|---|---|---|---|---|---|---|
| 2005 | January 2005 | 5 157.91 | 19 | 25 | 77 | 64.85 |
| February 2005 | 4 298.05 | 19 | 20 | 61 | 68.17 | |
| March 2005 | 7 857.79 | 18 | 25 | 80 | 89.16 | |
| April 2005 | 3 929.35 | 7 | 8 | 13 | 30.60 | |
| 21 243.10 | 63 | 78 | 231 | 63.20 | ||
| 2005/2006 | November 2005 | 653.90 | 18 | 13 | 30 | 23.70 |
| December 2005 | 2 381.99 | 27 | 25 | 91 | 31.21 | |
| January 2006 | 4 051.75 | 16 | 27 | 81 | 52.09 | |
| February 2006 | 6 035.59 | 17 | 26 | 81 | 74.75 | |
| March 2006 | 5 171.34 | 15 | 21 | 64 | 89.83 | |
| April 2006 | 1 471.50 | 7 | 9 | 11 | 120.47 | |
| 19 766.07 | 100 | 121 | 358 | 65.34 | ||
| 2006/2007 | November 2006 | 454.76 | 14 | 18 | 29 | 13.47 |
| December 2006 | 1 581.58 | 32 | 24 | 125 | 14.27 | |
| January 2007 | 476.99 | 8 | 16 | 26 | 17.26 | |
| February 2007 | 2 527.61 | 12 | 21 | 44 | 52.59 | |
| March 2007 | 2 971.08 | 16 | 26 | 74 | 49.14 | |
| April 2007 | 208.42 | 7 | 11 | 19 | 11.27 | |
| 8 220.45 | 89 | 116 | 317 | 26.33 | ||
| 2007/2008 | November 2007 | 52.56 | 2 | 6 | 6 | 8.76 |
| December 2007 | 129.35 | 6 | 6 | 12 | 9.99 | |
| January 2008 | 545.79 | 20 | 22 | 52 | 11.33 | |
| February 2008 | 1 617.41 | 23 | 25 | 77 | 20.90 | |
| March 2008 | 425.32 | 16 | 15 | 38 | 12.65 | |
| April 2008 | 604.80 | 16 | 16 | 49 | 10.99 | |
| 3 375.24 | 83 | 90 | 234 | 12.43 | ||
| 2008/2009 | November 2008 | 454.74 | 15 | 18 | 37 | 12.66 |
| December 2008 | 1 428.00 | 19 | 22 | 65 | 17.96 | |
| January 2009 | 2 253.50 | 45 | 24 | 90 | 19.87 | |
| February 2009 | 2 151.70 | 32 | 20 | 61 | 31.98 | |
| March 2009 | 2 386.70 | 34 | 27 | 68 | 28.02 | |
| April 2009 | 316.00 | 11 | 11 | 17 | 18.38 | |
| 8 990.64 | 156 | 122 | 338 | 21.48 | ||
| Total | 61 595.48 | 491 | 527 | 1 478 | 37.33 |
Monthly information on squid fishing during the period January 2005–March 2009.
| Fishing season . | Month . | Total catch (kg) . | Vessels . | Fishing days . | Fishing trips . | Mean cpue (kg trip−1) . |
|---|---|---|---|---|---|---|
| 2005 | January 2005 | 5 157.91 | 19 | 25 | 77 | 64.85 |
| February 2005 | 4 298.05 | 19 | 20 | 61 | 68.17 | |
| March 2005 | 7 857.79 | 18 | 25 | 80 | 89.16 | |
| April 2005 | 3 929.35 | 7 | 8 | 13 | 30.60 | |
| 21 243.10 | 63 | 78 | 231 | 63.20 | ||
| 2005/2006 | November 2005 | 653.90 | 18 | 13 | 30 | 23.70 |
| December 2005 | 2 381.99 | 27 | 25 | 91 | 31.21 | |
| January 2006 | 4 051.75 | 16 | 27 | 81 | 52.09 | |
| February 2006 | 6 035.59 | 17 | 26 | 81 | 74.75 | |
| March 2006 | 5 171.34 | 15 | 21 | 64 | 89.83 | |
| April 2006 | 1 471.50 | 7 | 9 | 11 | 120.47 | |
| 19 766.07 | 100 | 121 | 358 | 65.34 | ||
| 2006/2007 | November 2006 | 454.76 | 14 | 18 | 29 | 13.47 |
| December 2006 | 1 581.58 | 32 | 24 | 125 | 14.27 | |
| January 2007 | 476.99 | 8 | 16 | 26 | 17.26 | |
| February 2007 | 2 527.61 | 12 | 21 | 44 | 52.59 | |
| March 2007 | 2 971.08 | 16 | 26 | 74 | 49.14 | |
| April 2007 | 208.42 | 7 | 11 | 19 | 11.27 | |
| 8 220.45 | 89 | 116 | 317 | 26.33 | ||
| 2007/2008 | November 2007 | 52.56 | 2 | 6 | 6 | 8.76 |
| December 2007 | 129.35 | 6 | 6 | 12 | 9.99 | |
| January 2008 | 545.79 | 20 | 22 | 52 | 11.33 | |
| February 2008 | 1 617.41 | 23 | 25 | 77 | 20.90 | |
| March 2008 | 425.32 | 16 | 15 | 38 | 12.65 | |
| April 2008 | 604.80 | 16 | 16 | 49 | 10.99 | |
| 3 375.24 | 83 | 90 | 234 | 12.43 | ||
| 2008/2009 | November 2008 | 454.74 | 15 | 18 | 37 | 12.66 |
| December 2008 | 1 428.00 | 19 | 22 | 65 | 17.96 | |
| January 2009 | 2 253.50 | 45 | 24 | 90 | 19.87 | |
| February 2009 | 2 151.70 | 32 | 20 | 61 | 31.98 | |
| March 2009 | 2 386.70 | 34 | 27 | 68 | 28.02 | |
| April 2009 | 316.00 | 11 | 11 | 17 | 18.38 | |
| 8 990.64 | 156 | 122 | 338 | 21.48 | ||
| Total | 61 595.48 | 491 | 527 | 1 478 | 37.33 |
| Fishing season . | Month . | Total catch (kg) . | Vessels . | Fishing days . | Fishing trips . | Mean cpue (kg trip−1) . |
|---|---|---|---|---|---|---|
| 2005 | January 2005 | 5 157.91 | 19 | 25 | 77 | 64.85 |
| February 2005 | 4 298.05 | 19 | 20 | 61 | 68.17 | |
| March 2005 | 7 857.79 | 18 | 25 | 80 | 89.16 | |
| April 2005 | 3 929.35 | 7 | 8 | 13 | 30.60 | |
| 21 243.10 | 63 | 78 | 231 | 63.20 | ||
| 2005/2006 | November 2005 | 653.90 | 18 | 13 | 30 | 23.70 |
| December 2005 | 2 381.99 | 27 | 25 | 91 | 31.21 | |
| January 2006 | 4 051.75 | 16 | 27 | 81 | 52.09 | |
| February 2006 | 6 035.59 | 17 | 26 | 81 | 74.75 | |
| March 2006 | 5 171.34 | 15 | 21 | 64 | 89.83 | |
| April 2006 | 1 471.50 | 7 | 9 | 11 | 120.47 | |
| 19 766.07 | 100 | 121 | 358 | 65.34 | ||
| 2006/2007 | November 2006 | 454.76 | 14 | 18 | 29 | 13.47 |
| December 2006 | 1 581.58 | 32 | 24 | 125 | 14.27 | |
| January 2007 | 476.99 | 8 | 16 | 26 | 17.26 | |
| February 2007 | 2 527.61 | 12 | 21 | 44 | 52.59 | |
| March 2007 | 2 971.08 | 16 | 26 | 74 | 49.14 | |
| April 2007 | 208.42 | 7 | 11 | 19 | 11.27 | |
| 8 220.45 | 89 | 116 | 317 | 26.33 | ||
| 2007/2008 | November 2007 | 52.56 | 2 | 6 | 6 | 8.76 |
| December 2007 | 129.35 | 6 | 6 | 12 | 9.99 | |
| January 2008 | 545.79 | 20 | 22 | 52 | 11.33 | |
| February 2008 | 1 617.41 | 23 | 25 | 77 | 20.90 | |
| March 2008 | 425.32 | 16 | 15 | 38 | 12.65 | |
| April 2008 | 604.80 | 16 | 16 | 49 | 10.99 | |
| 3 375.24 | 83 | 90 | 234 | 12.43 | ||
| 2008/2009 | November 2008 | 454.74 | 15 | 18 | 37 | 12.66 |
| December 2008 | 1 428.00 | 19 | 22 | 65 | 17.96 | |
| January 2009 | 2 253.50 | 45 | 24 | 90 | 19.87 | |
| February 2009 | 2 151.70 | 32 | 20 | 61 | 31.98 | |
| March 2009 | 2 386.70 | 34 | 27 | 68 | 28.02 | |
| April 2009 | 316.00 | 11 | 11 | 17 | 18.38 | |
| 8 990.64 | 156 | 122 | 338 | 21.48 | ||
| Total | 61 595.48 | 491 | 527 | 1 478 | 37.33 |
Average cpue, total catch, and fishing effort (in fishing days and number of trips) around SSI, per month and fishing season.
Environmental conditions and artisanal fishing
Squid were fished under a wide range of environmental conditions, with SST ranging from 18 to 29°C and Chl a up to 3.88 g mm−3. The exploratory analysis revealed that catch and cpue were positively correlated linearly with SST and Rf, but negatively with Ws, H, and Chl a (Table 3).
Analysis of the environmental variables recorded around SSI during summer.
| Variable . | Mean . | Min. . | Max. . | Catchm . | Cpuem . | Catchs . | Cpues . |
|---|---|---|---|---|---|---|---|
| SST | 25.31 | 18.12 | 29.22 | 0.55 (0.01)* | 0.40 (0.01)* | 0.17 (0.87) | 0.71 (0.28) |
| Chl a | 0.76 | 0.00 | 2.49 | −0.55 (0.003)* | −0.35 (0.02)* | −0.71 (0.28) | −0.71 (0.03)* |
| Ws | 3.88 | 0.76 | 8.38 | −0.23 (0.89) | −0.38 (0.38) | 0.01 (0.87) | −0.61 (0.50) |
| H | 1.66 | 0.41 | 3.73 | −0.40 (0.06) | −0.4 (0.03)* | −0.61 (0.39) | −0.86 (0.03)* |
| Rf | 0.72 | 0.00 | 8.36 | 0.52 (0.003)* | 0.26 (0.03)* | 0.26 (0.28) | 0.68 (0.39) |
| Variable . | Mean . | Min. . | Max. . | Catchm . | Cpuem . | Catchs . | Cpues . |
|---|---|---|---|---|---|---|---|
| SST | 25.31 | 18.12 | 29.22 | 0.55 (0.01)* | 0.40 (0.01)* | 0.17 (0.87) | 0.71 (0.28) |
| Chl a | 0.76 | 0.00 | 2.49 | −0.55 (0.003)* | −0.35 (0.02)* | −0.71 (0.28) | −0.71 (0.03)* |
| Ws | 3.88 | 0.76 | 8.38 | −0.23 (0.89) | −0.38 (0.38) | 0.01 (0.87) | −0.61 (0.50) |
| H | 1.66 | 0.41 | 3.73 | −0.40 (0.06) | −0.4 (0.03)* | −0.61 (0.39) | −0.86 (0.03)* |
| Rf | 0.72 | 0.00 | 8.36 | 0.52 (0.003)* | 0.26 (0.03)* | 0.26 (0.28) | 0.68 (0.39) |
Correlation coefficients between the mean values of the environmental variables and Loligo plei fishery-related data for the period 2005–2009 are shown with their respective significance level (p-values).
*Statistically significant (p < 0.05).
Analysis of the environmental variables recorded around SSI during summer.
| Variable . | Mean . | Min. . | Max. . | Catchm . | Cpuem . | Catchs . | Cpues . |
|---|---|---|---|---|---|---|---|
| SST | 25.31 | 18.12 | 29.22 | 0.55 (0.01)* | 0.40 (0.01)* | 0.17 (0.87) | 0.71 (0.28) |
| Chl a | 0.76 | 0.00 | 2.49 | −0.55 (0.003)* | −0.35 (0.02)* | −0.71 (0.28) | −0.71 (0.03)* |
| Ws | 3.88 | 0.76 | 8.38 | −0.23 (0.89) | −0.38 (0.38) | 0.01 (0.87) | −0.61 (0.50) |
| H | 1.66 | 0.41 | 3.73 | −0.40 (0.06) | −0.4 (0.03)* | −0.61 (0.39) | −0.86 (0.03)* |
| Rf | 0.72 | 0.00 | 8.36 | 0.52 (0.003)* | 0.26 (0.03)* | 0.26 (0.28) | 0.68 (0.39) |
| Variable . | Mean . | Min. . | Max. . | Catchm . | Cpuem . | Catchs . | Cpues . |
|---|---|---|---|---|---|---|---|
| SST | 25.31 | 18.12 | 29.22 | 0.55 (0.01)* | 0.40 (0.01)* | 0.17 (0.87) | 0.71 (0.28) |
| Chl a | 0.76 | 0.00 | 2.49 | −0.55 (0.003)* | −0.35 (0.02)* | −0.71 (0.28) | −0.71 (0.03)* |
| Ws | 3.88 | 0.76 | 8.38 | −0.23 (0.89) | −0.38 (0.38) | 0.01 (0.87) | −0.61 (0.50) |
| H | 1.66 | 0.41 | 3.73 | −0.40 (0.06) | −0.4 (0.03)* | −0.61 (0.39) | −0.86 (0.03)* |
| Rf | 0.72 | 0.00 | 8.36 | 0.52 (0.003)* | 0.26 (0.03)* | 0.26 (0.28) | 0.68 (0.39) |
Correlation coefficients between the mean values of the environmental variables and Loligo plei fishery-related data for the period 2005–2009 are shown with their respective significance level (p-values).
*Statistically significant (p < 0.05).
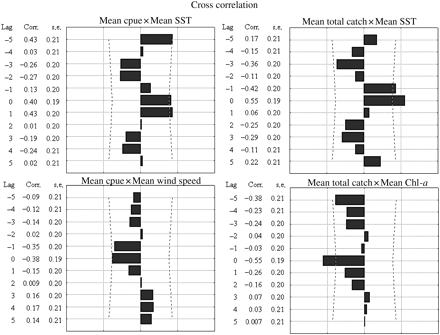
The GLM results showed that the optimal combination of parameters included SST and Chl a in the model fitted to catch, whereas the log-cpue model showed that more factors (Ws, H, Rf, and SST) fitted the variations (Table 4; lower AIC). However, statistically significant models point only towards a relationship of the catch with SST (W = 24.506; p < 0.001) and Chl a (W = 10.508; p < 001), and the log-cpue with SST (W = 11.029; p < 0.001) and windspeed (W = 5.323; p < 0.02). The fishing seasons 2005/2006 and 2007/2008 also showed the best fit as a categorical variable (Table 5). Cross-correlation between cpue and SST showed a positive relationship with a lag of 0–1 months, but negative correlations with windspeed at time-lags of 0–3 months. In terms of catch, SST was positively correlated with lags from −1 to 0 months, and Chl a was negatively correlated with lags of 0 months (Figure 4).
Values of AIC from GLMs fitted to squid catch and log cpue, based on a normal distribution with a logarithmic link function.
| Model . | d.f. . | AIC . |
|---|---|---|
| Catch ∼ Chl a + SST + (Fs) | 6 | 424.5643 |
| Catch ∼ H + Chl a + SST + (Fs) | 7 | 424.6531 |
| Catch ∼ Ws + Chl a + SST + (Fs) | 7 | 425.1190 |
| Catch ∼ Ws + H + Chl a + SST + (Fs) | 8 | 426.3609 |
| Catch ∼ Rf + Chl a + SST + (Fs) | 7 | 426.4823 |
| Catch ∼ H + Rf + Chl a + SST + (Fs) | 8 | 426.5955 |
| Catch ∼ Ws + Rf + Chl a + SST + (Fs) | 8 | 426.9427 |
| Catch ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | 428.3607 |
| Catch ∼ Rf + SST + (Fs) | 6 | 431.6782 |
| log cpue ∼ Ws + H + Rf + SST + (Fs) | 8 | −9.3698 |
| log cpue ∼ Ws + H + Chl a + SST + (Fs) | 8 | −8.5230 |
| log cpue ∼ Ws + H + SST + (Fs) | 7 | −8.5072 |
| log cpue ∼ Ws + Rf + SST + (Fs) | 7 | −7.8019 |
| log cpue ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | −7.4247 |
| log cpue ∼ Rf + SST + (Fs) | 6 | −7.2992 |
| log cpue ∼ SST + (Fs) | 5 | −7.0938 |
| log cpue ∼ Ws + SST + (Fs) | 6 | −6.9620 |
| log cpue ∼ H + Chl a + SST + (Fs) | 7 | −6.4796 |
| log cpue ∼ Chl a + SST + (Fs) | 6 | −6.3750 |
| Model . | d.f. . | AIC . |
|---|---|---|
| Catch ∼ Chl a + SST + (Fs) | 6 | 424.5643 |
| Catch ∼ H + Chl a + SST + (Fs) | 7 | 424.6531 |
| Catch ∼ Ws + Chl a + SST + (Fs) | 7 | 425.1190 |
| Catch ∼ Ws + H + Chl a + SST + (Fs) | 8 | 426.3609 |
| Catch ∼ Rf + Chl a + SST + (Fs) | 7 | 426.4823 |
| Catch ∼ H + Rf + Chl a + SST + (Fs) | 8 | 426.5955 |
| Catch ∼ Ws + Rf + Chl a + SST + (Fs) | 8 | 426.9427 |
| Catch ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | 428.3607 |
| Catch ∼ Rf + SST + (Fs) | 6 | 431.6782 |
| log cpue ∼ Ws + H + Rf + SST + (Fs) | 8 | −9.3698 |
| log cpue ∼ Ws + H + Chl a + SST + (Fs) | 8 | −8.5230 |
| log cpue ∼ Ws + H + SST + (Fs) | 7 | −8.5072 |
| log cpue ∼ Ws + Rf + SST + (Fs) | 7 | −7.8019 |
| log cpue ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | −7.4247 |
| log cpue ∼ Rf + SST + (Fs) | 6 | −7.2992 |
| log cpue ∼ SST + (Fs) | 5 | −7.0938 |
| log cpue ∼ Ws + SST + (Fs) | 6 | −6.9620 |
| log cpue ∼ H + Chl a + SST + (Fs) | 7 | −6.4796 |
| log cpue ∼ Chl a + SST + (Fs) | 6 | −6.3750 |
The symbolic representation of the models follows Wilkinson and Rogers (1973) and Espínola et al. (2010), with ∼ in function of and + main effects. Values are monthly means, d.f. is the degrees of freedom, and (Fs) is the categorical variable fishing season.
Values of AIC from GLMs fitted to squid catch and log cpue, based on a normal distribution with a logarithmic link function.
| Model . | d.f. . | AIC . |
|---|---|---|
| Catch ∼ Chl a + SST + (Fs) | 6 | 424.5643 |
| Catch ∼ H + Chl a + SST + (Fs) | 7 | 424.6531 |
| Catch ∼ Ws + Chl a + SST + (Fs) | 7 | 425.1190 |
| Catch ∼ Ws + H + Chl a + SST + (Fs) | 8 | 426.3609 |
| Catch ∼ Rf + Chl a + SST + (Fs) | 7 | 426.4823 |
| Catch ∼ H + Rf + Chl a + SST + (Fs) | 8 | 426.5955 |
| Catch ∼ Ws + Rf + Chl a + SST + (Fs) | 8 | 426.9427 |
| Catch ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | 428.3607 |
| Catch ∼ Rf + SST + (Fs) | 6 | 431.6782 |
| log cpue ∼ Ws + H + Rf + SST + (Fs) | 8 | −9.3698 |
| log cpue ∼ Ws + H + Chl a + SST + (Fs) | 8 | −8.5230 |
| log cpue ∼ Ws + H + SST + (Fs) | 7 | −8.5072 |
| log cpue ∼ Ws + Rf + SST + (Fs) | 7 | −7.8019 |
| log cpue ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | −7.4247 |
| log cpue ∼ Rf + SST + (Fs) | 6 | −7.2992 |
| log cpue ∼ SST + (Fs) | 5 | −7.0938 |
| log cpue ∼ Ws + SST + (Fs) | 6 | −6.9620 |
| log cpue ∼ H + Chl a + SST + (Fs) | 7 | −6.4796 |
| log cpue ∼ Chl a + SST + (Fs) | 6 | −6.3750 |
| Model . | d.f. . | AIC . |
|---|---|---|
| Catch ∼ Chl a + SST + (Fs) | 6 | 424.5643 |
| Catch ∼ H + Chl a + SST + (Fs) | 7 | 424.6531 |
| Catch ∼ Ws + Chl a + SST + (Fs) | 7 | 425.1190 |
| Catch ∼ Ws + H + Chl a + SST + (Fs) | 8 | 426.3609 |
| Catch ∼ Rf + Chl a + SST + (Fs) | 7 | 426.4823 |
| Catch ∼ H + Rf + Chl a + SST + (Fs) | 8 | 426.5955 |
| Catch ∼ Ws + Rf + Chl a + SST + (Fs) | 8 | 426.9427 |
| Catch ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | 428.3607 |
| Catch ∼ Rf + SST + (Fs) | 6 | 431.6782 |
| log cpue ∼ Ws + H + Rf + SST + (Fs) | 8 | −9.3698 |
| log cpue ∼ Ws + H + Chl a + SST + (Fs) | 8 | −8.5230 |
| log cpue ∼ Ws + H + SST + (Fs) | 7 | −8.5072 |
| log cpue ∼ Ws + Rf + SST + (Fs) | 7 | −7.8019 |
| log cpue ∼ Ws + H + Rf + Chl a + SST + (Fs) | 9 | −7.4247 |
| log cpue ∼ Rf + SST + (Fs) | 6 | −7.2992 |
| log cpue ∼ SST + (Fs) | 5 | −7.0938 |
| log cpue ∼ Ws + SST + (Fs) | 6 | −6.9620 |
| log cpue ∼ H + Chl a + SST + (Fs) | 7 | −6.4796 |
| log cpue ∼ Chl a + SST + (Fs) | 6 | −6.3750 |
The symbolic representation of the models follows Wilkinson and Rogers (1973) and Espínola et al. (2010), with ∼ in function of and + main effects. Values are monthly means, d.f. is the degrees of freedom, and (Fs) is the categorical variable fishing season.
Wald's statistics (W) and their p-level for the GLM fitted to catch and log cpue (where s.e. is the standard error).
| . | Catch . | log cpue . | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter . | Estimate . | s.e. . | W . | p-value . | Estimate . | s.e. . | W . | p-value . |
| Intercept | −4.693 | 3.175 | 2.184 | 0.139 | −1.172 | 0.534 | 4.806 | 0.028 |
| SST | 0.468 | 0.094 | 24.506 | 0.000* | 0.062 | 0.019 | 11.029 | 0.001* |
| Chl a | −1.815 | 0.558 | 10.583 | 0.001* | −0.029 | 0.123 | 0.056 | 0.813 |
| Ws | 0.119 | 0.220 | 0.293 | 0.588 | −0.075 | 0.033 | 5.323 | 0.021* |
| H | 0.627 | 0.606 | 1.068 | 0.301 | 0.129 | 0.068 | 3.604 | 0.058 |
| Rf | −0.004 | 0.246 | 0.000 | 0.986 | 0.067 | 0.068 | 0.958 | 0.328 |
| 2005–2006 | 0.678 | 0.209 | 10.494 | 0.001* | 0.231 | 0.037 | 39.134 | 0.000* |
| 2006–2007 | −0.529 | 0.246 | 4.642 | 0.031* | −0.072 | 0.042 | 2.962 | 0.085 |
| 2007–2008 | −0.770 | 0.622 | 1.531 | 0.216 | −0.261 | 0.059 | 19.665 | 0.000* |
| 2008–2009 | 0.040 | 0.211 | 0.036 | 0.849 | −0.064 | 0.042 | 2.329 | 0.127 |
| . | Catch . | log cpue . | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter . | Estimate . | s.e. . | W . | p-value . | Estimate . | s.e. . | W . | p-value . |
| Intercept | −4.693 | 3.175 | 2.184 | 0.139 | −1.172 | 0.534 | 4.806 | 0.028 |
| SST | 0.468 | 0.094 | 24.506 | 0.000* | 0.062 | 0.019 | 11.029 | 0.001* |
| Chl a | −1.815 | 0.558 | 10.583 | 0.001* | −0.029 | 0.123 | 0.056 | 0.813 |
| Ws | 0.119 | 0.220 | 0.293 | 0.588 | −0.075 | 0.033 | 5.323 | 0.021* |
| H | 0.627 | 0.606 | 1.068 | 0.301 | 0.129 | 0.068 | 3.604 | 0.058 |
| Rf | −0.004 | 0.246 | 0.000 | 0.986 | 0.067 | 0.068 | 0.958 | 0.328 |
| 2005–2006 | 0.678 | 0.209 | 10.494 | 0.001* | 0.231 | 0.037 | 39.134 | 0.000* |
| 2006–2007 | −0.529 | 0.246 | 4.642 | 0.031* | −0.072 | 0.042 | 2.962 | 0.085 |
| 2007–2008 | −0.770 | 0.622 | 1.531 | 0.216 | −0.261 | 0.059 | 19.665 | 0.000* |
| 2008–2009 | 0.040 | 0.211 | 0.036 | 0.849 | −0.064 | 0.042 | 2.329 | 0.127 |
*Statistically significant (p < 0.05).
Wald's statistics (W) and their p-level for the GLM fitted to catch and log cpue (where s.e. is the standard error).
| . | Catch . | log cpue . | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter . | Estimate . | s.e. . | W . | p-value . | Estimate . | s.e. . | W . | p-value . |
| Intercept | −4.693 | 3.175 | 2.184 | 0.139 | −1.172 | 0.534 | 4.806 | 0.028 |
| SST | 0.468 | 0.094 | 24.506 | 0.000* | 0.062 | 0.019 | 11.029 | 0.001* |
| Chl a | −1.815 | 0.558 | 10.583 | 0.001* | −0.029 | 0.123 | 0.056 | 0.813 |
| Ws | 0.119 | 0.220 | 0.293 | 0.588 | −0.075 | 0.033 | 5.323 | 0.021* |
| H | 0.627 | 0.606 | 1.068 | 0.301 | 0.129 | 0.068 | 3.604 | 0.058 |
| Rf | −0.004 | 0.246 | 0.000 | 0.986 | 0.067 | 0.068 | 0.958 | 0.328 |
| 2005–2006 | 0.678 | 0.209 | 10.494 | 0.001* | 0.231 | 0.037 | 39.134 | 0.000* |
| 2006–2007 | −0.529 | 0.246 | 4.642 | 0.031* | −0.072 | 0.042 | 2.962 | 0.085 |
| 2007–2008 | −0.770 | 0.622 | 1.531 | 0.216 | −0.261 | 0.059 | 19.665 | 0.000* |
| 2008–2009 | 0.040 | 0.211 | 0.036 | 0.849 | −0.064 | 0.042 | 2.329 | 0.127 |
| . | Catch . | log cpue . | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter . | Estimate . | s.e. . | W . | p-value . | Estimate . | s.e. . | W . | p-value . |
| Intercept | −4.693 | 3.175 | 2.184 | 0.139 | −1.172 | 0.534 | 4.806 | 0.028 |
| SST | 0.468 | 0.094 | 24.506 | 0.000* | 0.062 | 0.019 | 11.029 | 0.001* |
| Chl a | −1.815 | 0.558 | 10.583 | 0.001* | −0.029 | 0.123 | 0.056 | 0.813 |
| Ws | 0.119 | 0.220 | 0.293 | 0.588 | −0.075 | 0.033 | 5.323 | 0.021* |
| H | 0.627 | 0.606 | 1.068 | 0.301 | 0.129 | 0.068 | 3.604 | 0.058 |
| Rf | −0.004 | 0.246 | 0.000 | 0.986 | 0.067 | 0.068 | 0.958 | 0.328 |
| 2005–2006 | 0.678 | 0.209 | 10.494 | 0.001* | 0.231 | 0.037 | 39.134 | 0.000* |
| 2006–2007 | −0.529 | 0.246 | 4.642 | 0.031* | −0.072 | 0.042 | 2.962 | 0.085 |
| 2007–2008 | −0.770 | 0.622 | 1.531 | 0.216 | −0.261 | 0.059 | 19.665 | 0.000* |
| 2008–2009 | 0.040 | 0.211 | 0.036 | 0.849 | −0.064 | 0.042 | 2.329 | 0.127 |
*Statistically significant (p < 0.05).
Cross-correlations between significantly correlated environmental variables and the squid fishery during summer.
The artisanal fishery for L. plei is primarily a daylight one, but at night, fishing is influenced by moon phase. Most fishing trips at night (n = 431) were during the full moon, when the best catches (17.6 t) and values of cpue (41.13 kg trip−1) were recorded. Fishing effort and cpue were least during the new moon.
Interviews with fishers and cross-validation
The fishers interviewed were on average ∼40 years old, but they also included children and veterans, with ages ranging from 8 to 80 years. As expected, the fishers were well aware of the best conditions for squid fishing, because traditional knowledge is transmitted across generations. Fishers reported that squid catches were best in sheltered bays, ∼20 m deep. The testimonies provided also showed the importance of warm days and calm, warm, translucent seawater for fishing, reported by 93% of those interviewed. The interviewees also perceived a trend of decreasing squid catches in later years, but in a lesser proportion (66% of interviewees). Such perceptions seem to be in accord with the results of the scientific analysis and proved useful in cross-evaluating the results from different sources (Table 6).
Findings on FOK, and their scientific correspondence.
| Topic . | FOK . | Present scientific findings . |
|---|---|---|
| Squid fishery and a favourable environment | Of those interviewed, 93% said that squid fishing is best in calm, warmer, translucent water | Results showed significant positive relationships between the catch (and cpue) of L. plei and SST, and a significant negative correlation with Chl a and windspeed (Table 3) |
| Catch trends around SSI | Of those interviewed, 66% said that compared with the recent past, the fishery for squid has been decreasing | Results of cpues and total squid catch show a decreasing trend between 2005/2006 and 2007/2008. The slight increase in 2008/2009 did not return them to former levels (Figure 3) |
| Topic . | FOK . | Present scientific findings . |
|---|---|---|
| Squid fishery and a favourable environment | Of those interviewed, 93% said that squid fishing is best in calm, warmer, translucent water | Results showed significant positive relationships between the catch (and cpue) of L. plei and SST, and a significant negative correlation with Chl a and windspeed (Table 3) |
| Catch trends around SSI | Of those interviewed, 66% said that compared with the recent past, the fishery for squid has been decreasing | Results of cpues and total squid catch show a decreasing trend between 2005/2006 and 2007/2008. The slight increase in 2008/2009 did not return them to former levels (Figure 3) |
Findings on FOK, and their scientific correspondence.
| Topic . | FOK . | Present scientific findings . |
|---|---|---|
| Squid fishery and a favourable environment | Of those interviewed, 93% said that squid fishing is best in calm, warmer, translucent water | Results showed significant positive relationships between the catch (and cpue) of L. plei and SST, and a significant negative correlation with Chl a and windspeed (Table 3) |
| Catch trends around SSI | Of those interviewed, 66% said that compared with the recent past, the fishery for squid has been decreasing | Results of cpues and total squid catch show a decreasing trend between 2005/2006 and 2007/2008. The slight increase in 2008/2009 did not return them to former levels (Figure 3) |
| Topic . | FOK . | Present scientific findings . |
|---|---|---|
| Squid fishery and a favourable environment | Of those interviewed, 93% said that squid fishing is best in calm, warmer, translucent water | Results showed significant positive relationships between the catch (and cpue) of L. plei and SST, and a significant negative correlation with Chl a and windspeed (Table 3) |
| Catch trends around SSI | Of those interviewed, 66% said that compared with the recent past, the fishery for squid has been decreasing | Results of cpues and total squid catch show a decreasing trend between 2005/2006 and 2007/2008. The slight increase in 2008/2009 did not return them to former levels (Figure 3) |
Discussion
Squid and the environment
Variability in the abundance of targeted resources can be attributed to many factors, including density-dependence, the environment, and overfishing. For short-lived species such as cephalopods, abundance is notably influenced by oceanographic conditions, largely through their impact on recruitment and growth (Boyle and Rodhouse, 2005). In this study, we showed that L. plei concentrated around SSI between January and April (austral summer) and that catches and cpue generally decreased from 2005 to 2008, followed by a small increase in 2009, although the number of vessels targeting squid increased throughout. The environmental variable most positively correlated with squid productivity, both cpue and catch, was SST, whereas windspeed and Chl a correlated negatively with cpue and catch, respectively. Squid fishing is apparently more productive in warmer water and just after the summer rains, in conditions of less wind, and with translucent water (i.e. less Chl a). Interestingly, all these findings are in accord with fishers' oceanological (=oceanographic) knowledge (FOK; Gasalla and Diegues, in press), confirming our conclusions.
Previous studies on L. plei off southeastern Brazil indicated that the fleets that target squid operate mainly during summer, catching squid in an advanced reproductive stage. The combination of conditions favourable for survival provided by the physical environment of the shelf results in the persistence of a large group of spawners (Perez, 2002; Rodrigues and Gasalla, 2008). SST was the environmental variable that could best explain the patterns of catch and cpue, likely influencing the population dynamics through effects on recruitment, spawning, seasonality, and the levels of reproductive investment (Rodhouse et al., 1998; Forsythe, 2004; Pierce et al., 2005); similar examples of this include L. reynaudii off South Africa (Roberts, 1998, 2005) and L. pealei and I. illecebrosus in the Northwest Atlantic (Brodziak and Hendrickson, 1999; Dawe et al., 2007).
The results of the cross-correlation involving the variables SST, Chl a, and windspeed showed time-lags of around 0 and 1 months, suggesting that squid respond quickly to changes in the environment. Monitoring SST anomalies in the South Atlantic can provide a means to predict squid recruitment before fishing commences (Waluda et al., 1999). The same has been found for coastal Loligo spp. in Scottish waters (Pierce et al., 2005). The negative correlation between catch and Chl a suggests that the transparency of the water is also a relevant issue for squid fishers. It is our belief that L. plei are attracted by the colour of the zangarilho jigs, so transparent water (lower Chl a) can boost catches simply from the perspective of jig visibility. In South Africa, L. reynaudii spawning stock distribution and abundance vary in accord with the incidence of storms during the spawning season, which reduce underwater visibility on the spawning grounds and negatively impact spawning success (Roberts, 1998).
The association of large catches of L. plei with the full moon matches the situation for L. forbesi (Young et al., 2006) and O. bartramii (Nakamura and Siriraksophon, 1992) in Scotland and the United States, respectively. There, the association was explained by squid daily migration in search of food at the surface by night, facilitating their ability to see their prey (Young et al., 2006). This finding also seems to be coherent with the feeding patterns of L. plei around SSI (Postuma, 2010). The dynamics of ocean currents and windspeed appear to be important factors in cephalopod life cycles because of the vertical migrations throughout the water column (O'Dor et al., 2002). Martins et al. (2006) suggested that food availability on the spawning grounds at Santa Catarina Island, in the south of our study area, may be associated with the intensity of wind, favouring squid, which feed on both pelagic and demersal organisms.
The abundance of L. plei has been associated previously with the increase in primary productivity produced by intrusion of cold SACW (Martins and Perez, 2006, 2007; Rodrigues and Gasalla, 2008). However, from our analysis of L. plei association with warmer surface water, we now suggest a complementary hypothesis. Warmer SSTs, in conjunction with the stratified water column in summer, may provide favourable conditions for reproduction in the warmer surface water, and the squid can meet their food needs by migrating down the water column to the deeper layers associated with the SACW intrusion. There is a strong link between squid abundance and oceanographic conditions during the spawning season. Simultaneous studies (Postuma, 2010) have shown that the fisheries around SSI are directed mainly at mature squid.
Cross-validation of the findings with FOK
The results obtained from the interviews with local fishers cross-validate the scientific analysis of this study, adding robustness to the explanatory model (Gasalla and Diegues, in press). From the fishers' perspective, squid concentration has decreased over the past few years, and the best catches are now made in clear, calm water and warm weather, supporting the scientific findings of our study. This point seems to be an important issue for consideration in future studies of cephalopods, especially of small-scale fisheries where the success of management measures can depend on the extent of fisher inclusion in the process. Moreover, the management of certain stocks and qualitative changes in ecosystems should benefit from the knowledge gleaned from fishers on catch trends, ecology and behaviour of the target species, and other information based on years of practical experience (Berkes et al., 2001).
The seasonal and variable abundance of loliginid squid, strongly associated with the environment, create uncertainties for management and the industry (Roberts, 1998). The sensitivity of cephalopods to fluctuations in oceanographic conditions is an important factor in the stock-assessment process and poses a challenge to fisheries management.
Future perspectives and conclusions
The squid fishery on the shelf around SSI is not currently managed. However, the creation of environmental protection areas is in progress. The importance of managing cephalopod fisheries under an ecosystem approach, including the human dimension, i.e. ecosystem-based fishery management (EBFM) should be emphasized. The operational aspects of applying such an approach to coastal fisheries are not trivial, but in terms of current understanding of cephalopod fisheries, the need is becoming greater (Leslie and McLeod, 2007). The interactions shown by the models presented here should be useful for future EBFM initiatives.
In addition, this contribution should be viewed in light of global research on climate and fisheries interactions, knowing that geographically distant squid species and stocks can fluctuate either in or out of phase rather than being driven by large-scale climate conditions. It would be appropriate, therefore, to evaluate whether the processes responsible for the fluctuations are similar in different regions. Collaborative studies on this issue are needed to improve the management of fisheries under an ecosystem context (Lehodey et al., 2006).
Decreases in squid catches and cpue around SSI during the fishing seasons between 2005 and 2008, with a slight increase in 2009, were associated with varying environmental conditions, mostly SST, windspeed, and Chl a. The relationships corresponded to fishers' perceptions that the ideal squid jigging environment is when the water is warm, calm, and translucent. Future work, using locally collected data, may contribute to better forecasting than is possible at the moment. For this purpose, we suggest that SST, Chl a, rainfall, and windspeed be monitored to develop understanding of their relationship with fishing and the biology of L. plei. Moreover, we stress that it is not only the industrial fisheries that can provide insights into the understanding of changing conditions in the oceans and in the resources contained therein. Systematic monitoring of small-scale fisheries, taken along with such underutilized (but available) information as that from fishing cooperatives, satellite records of sea surface parameters, and fishers' knowledge, would in our opinion be of great value to management, especially in data-poor ecosystems.
Acknowledgements
We thank FAPESP (the São Paulo Research Foundation) for financial support, the fishing cooperative of São Sebastião (COOPERPESCASS) for data, and the Instituto Oceanografico of the University of São Paulo for supporting the research. We also acknowledge the participation of many local fishers during the fieldwork. The contribution was presented at CIAC '09 in Vigo, and the University of Vigo, FAPESP, and the Fisheries Secretary of the Municipality of São Sebastião supported the authors' attendance. Finally, we thank G. Pecl, A. Payne, Y. Sakurai, G. Pierce, and two anonymous reviewers for their valued scientific and editorial comments which helped us improve the final paper substantially.