-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander I. Arkhipkin, Vladimir V. Laptikhovsky, Convergence in life-history traits in migratory deep-water squid and fish, ICES Journal of Marine Science, Volume 67, Issue 7, October 2010, Pages 1444–1451, https://doi.org/10.1093/icesjms/fsq103
Close - Share Icon Share
Abstract
The life-history traits of two species of nektonic squid, Onykia ingens and Gonatus antarcticus, and Patagonian toothfish Dissostichus eleginoides, all from the Southwest Atlantic, are reviewed to reveal the extent to which their life cycles have converged. All three follow similar ontogenetic migration, with spawning and egg development in meso-bathypelagic water over the continental slope. Larvae and small juveniles ascend to epipelagic water to feed and grow near the slope. Juveniles and immature adults migrate to the shelf and use it as their feeding grounds. After this period of fast growth on the shelf, the adults descend back to deep water to spawn. The potential advantages that such a life cycle gives to migratory deep-water animals over obligate bathypelagic and benthic fauna are discussed. The most likely cause of ontogenetic migration of juvenile deep-water organisms to the shelf, with the resulting fast growth, is to increase individual fecundity during the adult deep-water phase. Escape from predation and resource utilization at the next trophic level are also examined, but found to be less plausible explanations. Larger, mature animals have higher fecundity, bigger eggs, and larger, better-developed hatchlings, all of which enhance the survival rates of the species at the early stages of their ontogenesis.Arkhipkin, A. I., and Laptikhovsky, V. V. 2010. Convergence in life-history traits in migratory deep-water squid and fish. – ICES Journal of Marine Science, 67: 1444–1451.
Introduction
One of the paradigms of modern ecology is the existence of ecosystems, which are defined as ecological units with interacting environmental components (Calow, 1999). Marine ecosystems usually exchange resources among themselves, creating an ecosystem of the second order, i.e. a meta-ecosystem that is a “set of [local] ecosystems connected by spatial flows of energy, materials and organisms across the ecosystem boundaries” (Loreau et al., 2003). Marine organisms have evolved various life-history strategies to optimize their existence within these meta-ecosystems. Some utilize resources and contribute their production to a single ecosystem, as for example loliginid squid (Loligo gahi) in the Patagonian shelf ecosystem (Arkhipkin et al., 2004). Others travel passively with currents (plankton) or actively swimming (nekton) between spawning, nursery, and feeding grounds located in either neighbour ecosystems (Illex illecebrosus; O'Dor and Coelho, 1993) or distant ecosystems (tuna and billfish; Marti, 1980; Takahashi et al., 2003). Further, many pelagic animals undertake diel migrations between epipelagic and meso-bathypelagic ecosystems (oceanic squid, Nesis, 1977; myctophids, Becker, 1983).
Animals occupying similar ecological niches usually have analogous life-history traits, such as large oceanic plankton-feeders (i.e. basking sharks and baleen whales). The life-history traits of cephalopods and fish often converge despite their belonging to different phyla (molluscs and vertebrates, respectively; Packard, 1972; Rodhouse and Nigmatullin, 1996). The main aim of the present study is to analyse the extent to which the life-history traits are similar in nektonic squid and fish that have evolved analogous life cycles and compete for resources in the same econiches. We have selected abundant deep-water nektonic squid and fish species of the Southwest Atlantic which, during their juvenile and immature ontogenetic phases, live in shallow waters of the Patagonian shelf. Based on our studies and information in the literature, we review the peculiarities of their life cycles and attempt to identify possible ecological advantages of ontogenetic migration of adults from the shelf to the open ocean.
We studied three species. The great hooked squid Onykia (Moroteuthis) ingens (Onychoteuthidae) is abundant throughout the Southern Ocean, but prefers waters of Subantarctic origin. It is a relatively large squid (maximum reported mantle length, ML, 61 cm) found from the surface to the bathypelagic zone (at 1100 m; Jackson et al., 1998). It is commercially unimportant owing to the concentration of ammonia in its flesh, but it is one of the major prey items for shelf and continental slope cetaceans (Clarke, 1980). The gonatid squid Gonatus antarcticus (Gonatidae) likewise has circumantarctic distribution, but lives in the water column from the surface to 1300 m. It reaches 39 cm ML at a maximum recorded age of 352 d (AIA and VVL, unpublished data). It too is abundant but commercially unimportant, mainly because it does not aggregate in schools (Nesis, 1999). Patagonian toothfish Dissostichus eleginoides (Nototheniidae) have a circumAntarctic distribution mostly north of the Antarctic Polar Front (Eastman, 1993). Its range generally coincides with that of O. ingens, living over a wide range of depths from the shelf (50 m) down to the bathypelagic waters of the oceanic basins (3850 m; Froese and Pauly, 2009). It is a large deep-water predator, attaining 2.2 m total length (TL) and living as long as 53 years, and is an important and much-sought resource, currently taken mainly by longliners in deep water. In the Falkland Islands fishery, the annual toothfish catch ranges from 700 to 3000 t (Falkland Islands Government, 2009).
Material and methods
The data analysed here on the distribution, size structure, and maturity of O. ingens, G. antarcticus, and Patagonian toothfish were collected by the Falkland Islands Fisheries Department from 1988 to 2009 on board commercial trawlers and from 1999 to 2007 during scientific surveys. Toothfish were additionally collected aboard commercial longliners between 1992 and 2009. Commercial trawlers used various types of bottom nets and fished mainly on the shelf and slope around the Falkland Islands at depths of 35–1400 m (mainly 120–350 m). Longliners fished the Patagonian continental slope at depths of 652–2122 m (mainly 1000–1500 m). The scientific surveys were conducted with bottom, semi-pelagic, and pelagic nets between 25 and 957 m deep. Data were collected every month of the year, and at each sampling station, position, depth, haul duration, catch by species, and biological information were recorded. Toothfish otoliths and squid statoliths were processed to estimate ages and growth rates. Stomach samples were analysed to study feeding spectra at various ontogenetic stages. Gonad samples from maturing and mature fish and squid were used to investigate egg size, potential fecundity, and oogenesis.
Further sampling details for the two squid species are described in Arkhipkin and Roa-Ureta (2005) and Laptikhovsky et al. (2007), and sampling details for toothfish in Arkhipkin et al. (2003) and Laptikhovsky et al. (2006, 2008). Additionally, we used literature data where appropriate.
Results and discussion
Distribution by size and maturity stage
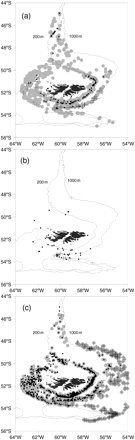
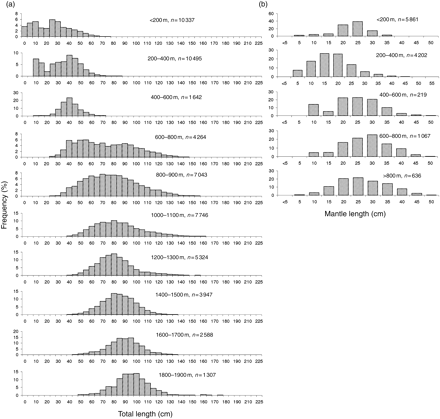
The vertical distributions of squid and toothfish were examined by length frequency analysis at different depths. The smallest O. ingens were observed on the shelf edge and upper parts of the continental slope at depths of 200–400 m (Figures 1 and 2). These juvenile squid were also found on the shelf (<200 m), but were less abundant at depths greater than 200–400 m. Shelf waters were the common habitat of larger immature O. ingens in the size range 20–25 cm ML, and larger O. ingens (>40 cm ML) were taken only in deeper water over the continental slope (>600 m). However, some medium-sized O. ingens of 20–30 cm ML were also found over the slope. The vertical distribution of G. antarcticus was similar to that of O. ingens. The smallest ones (<3–4 cm ML) were caught in epipelagic waters over the slope, juveniles and immatures (5–35 cm ML) on the shelf, and large specimens (>35 cm TL) only in deeper water of the continental slope (Rodhouse et al., 1992; Nesis, 1999). The smallest toothfish (5–15 cm TL) were caught on the shelf at depths <200 m. Modal sizes increased with depth, however, the largest fish being found as deep as 1900 m (90–110 cm TL; Figures 1 and 2).
The spatial distribution of various sizes of squid and fish studied on the Patagonian shelf and slope. (a) Onykia ingens (dots, <10 cm; grey dots, 10–45 cm; plus signs, >45 cm), (b) G. antarcticus (dots, 1–19 cm; plus signs 20–30 cm), and (c) Patagonian toothfish D. eleginoides (dots, <50 cm; grey dots, 50–100 cm; plus signs, >100 cm).
Length frequency distributions at different depths of (a) Patagonian toothfish D. eleginoides, and (b) the squid O. ingens on the Patagonian shelf and slope.
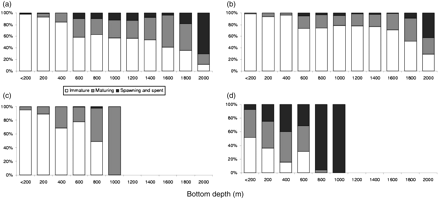
The distributions of squid and toothfish of different maturity stages also varied with depth (Figure 3). For O. ingens, shallow waters were inhabited mainly by juveniles and immature animals, and the proportion of maturing females increased with depth (Figure 3c and d). Mature and spawning female O. ingens were taken only over the continental slope at depths >800 m. It is a common feature for many families of squid that males start maturing much younger than females (Mangold, 1987), so it is unsurprising that mature males were encountered in small numbers even at depths <200 m, although the majority of mature males were taken at depths >800 m. Mating (identified by the presence of spermatangia in females) took place only in deep water. The maturation pattern of G. antarcticus was similar to that of O. ingens. Only small and immature squid were taken over the shelf (our data; Rodhouse et al., 1992; Thompson, 1994), and mature adults only in bathypelagic waters at depths >800 m. Juvenile toothfish exclusively inhabited shallow water (<200 m). Larger immature toothfish were caught in deeper parts of the shelf and upper continental slope (200–400 m), but mature toothfish of both sexes were caught only in deep bathypelagic layers at depths >800 m. Toothfish size increased with depth, and the maximum depth at which they were caught was ∼2000 m (Figure 3a and b). Depth distributions of the maturity stages of females and males were similar, with a slightly greater proportion of maturing and mature females at 800–1000 m.
Bathymetric distribution of immature, maturing, and mature (a) female and (b) male Patagonian toothfish D. eleginoides, and (c) female and (d) male O. ingens on the Patagonian shelf and slope.
Life cycles
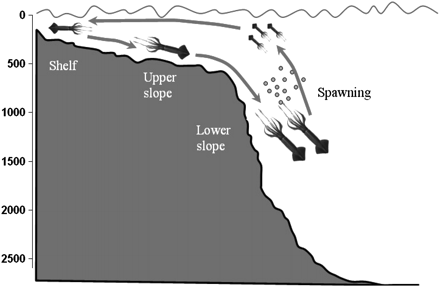
Size and maturity distribution with depth of O. ingens, G. antarcticus, and Patagonian toothfish confirmed their migratory deep-water life cycle in the Southwest Atlantic (Figure 4).
Schematic of bathymetric ontogenetic migrations during the life cycle of the squid O. ingens on the Patagonian shelf and slope (see text for explanation). Depth (m) on y-axis.
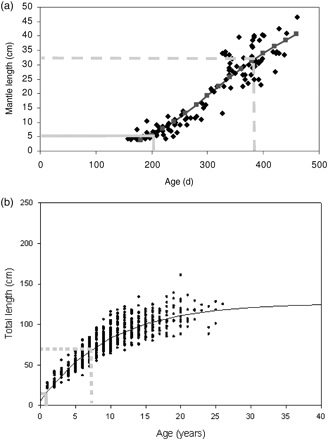
Spawning and spent female O. ingens were recently discovered on the continental slope off Patagonia at depths >800 m (Laptikhovsky et al., 2007), indicative of spawning in deep water. Spawning and spent female O. ingens from the Chatham Rise (New Zealand) have also been sampled by bottom trawl (Jackson and Mladenov, 1994) suggesting that spawning takes place near the seabed. Egg masses of O. ingens have yet to be found, but analogy with the reproductive morphology of other bentho-pelagic squid (Mangold, 1987) suggests that the egg masses should be pelagic. Larvae and juveniles also live in pelagic layers, and young squid start to migrate to near the bottom of the upper slope (200–400 m) at 5 cm ML and ∼200-d old (Figure 5a). While growing, they migrate inshore and attain 32–35 cm ML after ∼6 months. Males then mature, whereas females remain immature. At an age of ∼380 d, O. ingens start to descend to meso-bathypelagic waters, with simultaneous maturation of the females. Spawning takes place once females attain 45–48 cm ML, at an age of 460–480 d. As with all coleoid cephalopods, they die soon after spawning (Laptikhovsky et al., 2007).
Age-at-length data and growth curves of (a) the squid O. ingens and (b) Patagonian toothfish D. eleginoides on the Patagonian shelf and slope. Age and length of juvenile immigration to the shelf is marked by solid grey lines, and emigration of subadults from the shelf by dashed grey lines (see text for explanation).
Mating and spawning of G. antarcticus appear to take place in the water column, well off the seabed and far from the surface. We found two spent (possibly brooding) female G. antarcticus in bathypelagic waters at ∼1000 m over bottom depths of 1800–2400 m, and spent males in almost the same location (Laptikhovsky et al., 2007). As the spent female G. antarcticus had remnants of black tissue on their arms, it is also possible that these squid, like other representatives of the family Gonatidae, brood their egg masses for several months until the paralarvae hatch (Seibel et al., 2000). After hatching, the small paralarvae and juveniles inhabit epipelagic waters. In the Southwest Atlantic, they also migrate en masse onto the Falkland Islands shelf, where they grow and are preyed upon by several species of penguin (Thompson, 1994). When the immature adults attain 12–15 cm ML, they emigrate gradually from the shelf to deeper water, mature, and spawn. The complete life cycle of G. antarcticus (with embryogenesis) lasts ∼2 years, similar to that of O. ingens.
Patagonian toothfish spawn at depths >800 m over the eastern part of Burdwood Bank, which is so far the only known spawning ground of toothfish in the Atlantic (Laptikhovsky et al., 2006). The eggs, larvae, and small juveniles (<10 cm TL) develop and grow in epipelagic layers of the Falkland Current over depths of 1000–2500 m (Evseenko et al., 1995; North, 2002). When juveniles attain 10–12 cm TL, they start to migrate towards the Patagonian shelf and are taken at depths <100 m (Figure 5b). The Patagonian shelf and upper slope are major feeding grounds for Patagonian toothfish in the Southwest Atlantic. Immature toothfish remain there for 3–4 years, and then, on reaching 60–70 cm TL, they migrate into deeper water over the Patagonian slope (Laptikhovsky et al., 2008). Adult and mature fish spend the rest of their long lives (up to 53 years; reaching an impressive 2.15 m TL and weights >100 kg) in deep waters of the Patagonian slope and abyssal plains at depths >1000 m.
It is of note that, despite differences in the systematic position and lifespan between the two squid species and toothfish, they have evolved similar life-history traits. Their migratory life cycles are characterized by deep-water spawning, pelagic development of eggs and paralarvae, early migration of juveniles to the shelf, a feeding period on the shelf, and finally migration of adult animals back to deep water to mature and spawn. In the following sections, we discuss the potential advantages development of such a life cycle offers O. ingens, G. antarcticus, and toothfish compared with that of obligate bathypelagic and benthic fauna that share their post-migratory adult habitats in deep water.
Juvenile growth on the shelf
Studies of increment-bearing structures have permitted growth-rate estimates for Patagonian toothfish (Laptikhovsky et al., 2008) and O. ingens (Arkhipkin and Roa-Ureta, 2005). Unfortunately, there were too few data to construct ontogenetic growth curves and to estimate the growth rates of G. antarcticus.
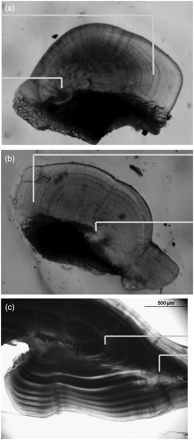
Juvenile toothfish and O. ingens grew faster than their older counterparts. Growth rates of both male and female O. ingens were best described by a Schnute growth curve (Figure 5a), with fastest absolute growth rates during the juvenile period, 5–6 cm ML per month (Arkhipkin and Roa-Ureta, 2005). Statolith microstructure also reflects the ontogenetic shift in squid life. Previously, it was suggested that the boundary between dark and peripheral zones in statolith microstructure corresponded to the shift from a pelagic to a demersal existence (Jackson, 1993). However, analysis of the number of growth increments at the beginning and the end of the part of the life cycle spent on the shelf showed that the dark zone in the statolith is formed during the shelf period of O. ingens ontogenesis (Figure 6a). A similar formation of the dark zone in the statolith microstructure was observed for G. antarcticus (Figure 6b), and it may also correspond to the shelf period of that squid's ontogenesis. Growth of toothfish during ontogenesis was best described by a von Bertalanffy growth curve (Figure 5b). Juvenile toothfish grew at a rate of 12–15 cm TL per year, compared with 1.5–2.5 cm TL per year in adults (Laptikhovsky et al., 2008). Interestingly, otolith microstructure corresponded also with the ontogenetic shift from the shelf to the continental slope. During their life on the shelf, juveniles laid down much wider, more-opaque growth increments than adults in deep water (Figure 6c).
Microstructure of statoliths of the squid (a) O. ingens and (b) G. antarcticus, and (c) the otoliths of Patagonian toothfish D. eleginoides, showing the inner and outer borders of the dark zone (=maximum growth increments), corresponding to immigration to and emigration from the shelf, respectively.
There are several advantages to spending the juvenile phase of life on the Patagonian shelf rather than in the epipelagic waters of the Falkland Current off the continental slope. First, the shelf is ∼2–3°C warmer during the austral summer and autumn than offshore (Arkhipkin et al., 2004). Warmer temperatures accelerate growth rates especially at the juvenile phase. It is thought that a 1°C increase in ambient temperature at the juvenile phase in squid could result in an almost doubling of adult body size (Forsythe, 2004). Juvenile and immature adult O. ingens and G. antarcticus are found over the shelf during summer and autumn, taking advantage of the warmer environment (our data; Thompson, 1994). Juvenile toothfish stay on the shelf for several years, presumably resulting in enhanced growth rates in the warmer water too. Second, there are at least two areas near the Falkland Islands where the Falkland Current meets the shelf and creates local upwelling, resulting in increased productivity. One of these areas is near Beauchene Island, and the other northeast of the Falkland Islands (Arkhipkin et al., 2004). The abundance of prey such as euphausiids, L. gahi, and rock cod Patagonotothen ramsayi in these shelf areas contributes further to supporting the growth of juvenile toothfish and squid.
In summary, it seems that the development of a migratory lifestyle by deep-water organisms taking juvenile stages onto the shelf evolved to support the attainment of adult body size in a relatively short period, compared with the situation for obligatory deep-water organisms with slower growth rates and generally smaller size (Gauldie et al., 1991; Arkhipkin, 2004).
The ecological advantages of large size for deep-water animals
There are several possible evolutionary and ecological advantages of large adult size for the two species of squid and toothfish. One advantage might be in mitigating the intraspecific competition for food that results in resource depletion and cannibalism. This is already partially achieved by the existence of allopatric feeding grounds for juveniles (shelf) and adults (slope and deep-water plains). Larger adult size may additionally move adults to the next trophic level, where they can utilize different resources that would not be accessible if the animals grew more slowly to smaller size.
The diet of toothfish of various sizes and at different depths was studied to reveal the positions of these fish in the trophic webs of the Falkland Islands shelf and slope (Arkhipkin et al., 2003). On the shelf and upper part of the continental slope, juvenile toothfish were active nektonic predators, taking mainly near-bottom fish (P. ramsayi) and squid (L. gahi). Then during its ontogenetic descent to the lower part of the continental slope (500–1000 m deep), the diet was still that of an active near-bottom predator. However, despite their increase in size compared with the shelf ontogenetic phase, immature toothfish seemed to take relatively smaller fish such as the morid A. rostrata, while also feeding on small macrourids and skates. At their deepest (>1000 m), adult toothfish become typical opportunistic predators, feeding mainly on relatively small and relatively inactive fish, squid, and prawn-like crustaceans. Deep-water prawns and crayfish were the main food items of toothfish in many of the deep-water regions they occupy, including the Falkland Islands (mainly Acanthephyra pelagica and Thymops birsteini; Arkhipkin et al., 2003). One possible reason for the apparently decreased hunting by toothfish with depth could be a specific adaptation to maintaining neutral buoyancy in bathypelagic layers over the bottom. Juvenile toothfish inhabiting the shelf and upper slope have much less lipid content in their white muscles and a thinner subcutaneous lipid layer than large toothfish in the bathypelagic habitat (Yukhov, 1982; Oyarzun et al., 1988). Therefore, the need to accumulate more lipid for neutral buoyancy may force toothfish to convert from an active predator on the shelf to a less-active opportunistic feeder on relatively small prey in bathyal waters.
Similar tendencies in trophic level positions have been documented for the different ontogenetic stages of O. ingens (Phillips et al., 2003). Juvenile O. ingens on the shelf are active nektonic predators, feeding mainly on planktonic crustaceans and small L. gahi. Immature O. ingens of medium size on the upper part of the continental slope then switched diet to the next trophic level; feeding mainly on adult L. gahi and myctophids. However, large adult O. ingens remain at the same trophic level as medium-sized ones and continue feeding on abundant myctophids at >1000 m. Body consistency changes during the ontogenetic descent of O. ingens to bathyal waters; the muscles in the mantle disintegrate, becoming “watery” and containing more ammonia than those of juveniles inhabiting the shelf (Jackson and Mladenov, 1994). Hence, neither toothfish nor O. ingens take advantage of their large size in deep water to feed at the next trophic level, compared with medium-sized fish on the upper slope.
Another possible ecological advantage to attaining large size in deep waters would be in mitigating predation pressure. However, there are few active predators in the deep waters of the Southern Ocean that can capture even medium-sized toothfish and squid, apart from the sleeper shark (Somniosus pacificus), the meso-bathypelagic giant squid Architeuthis dux, and the colossal squid Mesonychoteuthis hamiltoni. However, large, fully grown toothfish and squid constitute one of the main prey of sperm whales hunting in Antarctic and Subantarctic waters (Clarke, 1980; Yukhov, 1982). Therefore, the large size of these animals acquired during their relatively fast growth period on the shelf does not seem to protect them from predation by giant and colossal squid, deep-water sharks, and sperm whales.
A third ecological advantage of large size might be to allow enhanced fecundity. A larger female can produce more eggs at every spawning event than smaller ones (Murua et al., 2003). Both notothenioid fish and oegopsid squid have evolved embryonization of eggs for their descents to cold deep water and spawning in bathypelagic temperate and Subantarctic zones (following the so-called Thorson–Rass rule, reviewed by Laptikhovsky, 2006). The eggs become larger, accumulate more yolk, so develop for longer. These results in hatchlings that are larger, better developed, more capable of feeding on higher trophic level resources, and better able to escape predation by mesoplanktonic crustaceans and jellyfish. However, larger eggs also result in decreases in potential fecundity and contributions to recruitment, if spawning females of these taxa are the same size as those of taxa with small eggs. To increase the contribution to recruitment after evolving embryonization, a species would need to increase the size of its spawners. One way for deep-water organisms to achieve this is by spending the juvenile ontogenetic stages (characterized by the fastest absolute growth rates) in a warmer, more productive environment (e.g. the temperate shelf) than their bathypelagic and bathybenthic adult habitats.
Interestingly, this type of life cycle has evolved independently in several fish and squid species inhabiting only temperate and subpolar environments. Our analysis and review of the data here and in the literature suggest that the main reason for this may have been to increase potential fecundity. Apart from Patagonian toothfish and squid O. ingens and G. antarcticus from the Southern Ocean and South Atlantic, similar life cycles have evolved in the North Pacific sablefish Anoplopoma fimbria and rockfish Sebastolobus spp. (Vetter and Lynn, 2003; Maloney and Sigley, 2008). Such a life cycle does not seem to be found in deep-water animals living in tropical and subtropical waters. For many of those, the development of larvae and juveniles in warm epipelagic water over the continental slope, e.g. the squid Ancistrocheirus lesueurii (Arkhipkin, 1997) and the fish Aphanopus carbo (Neves et al., 2009), and in the open ocean, e.g. the squid Onychoteuthis banksii (Arkhipkin and Nigmatullin, 1997) and gempylid fish such as Diplospinus multistriatus (Nakamura and Parin, 1993), boosts growth rates and consequently increases the sizes of adults moving to bathypelagic waters. The necessity of migrating to ecologically active tropical shelves with their greater numbers of predators is therefore avoided.
Acknowledgements
We gratefully acknowledge the work of the scientific observers of the Fisheries Department of the Falkland Islands, who collected the samples of toothfish and squid during commercial and research surveys. We also thank Andreas Winter (Fisheries Department, Falkland Islands) for his comments and suggestions on earlier drafts of the manuscript, and the editor and reviewers of the submitted version for their valuable comments.